Submitted:
30 November 2023
Posted:
30 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
Chemicals
Cell culture
Sulphorhodamine B Assay (SRB)
AnnexinV-FITC apoptosis assay
Generation of reactive oxygen species
Fluorescence microscopy
Mitochondrial membrane potential
Seahorse XFe-24 metabolic flux analysis
Western Blot Analysis
In vivo tumor model
Statistical Analysis
3. Results
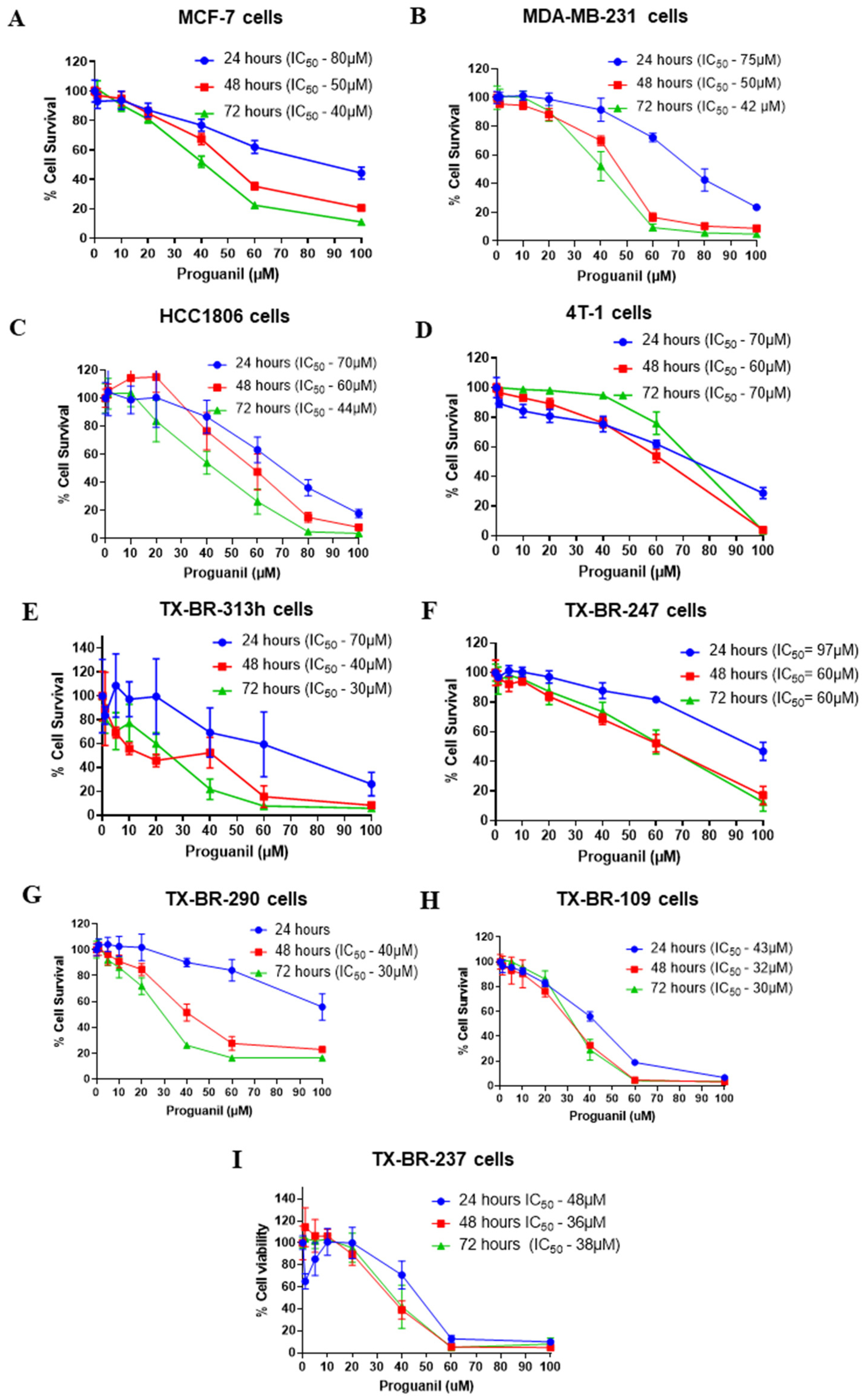
3.1. Proguanil has anti-proliferative effects on breast cancer cells
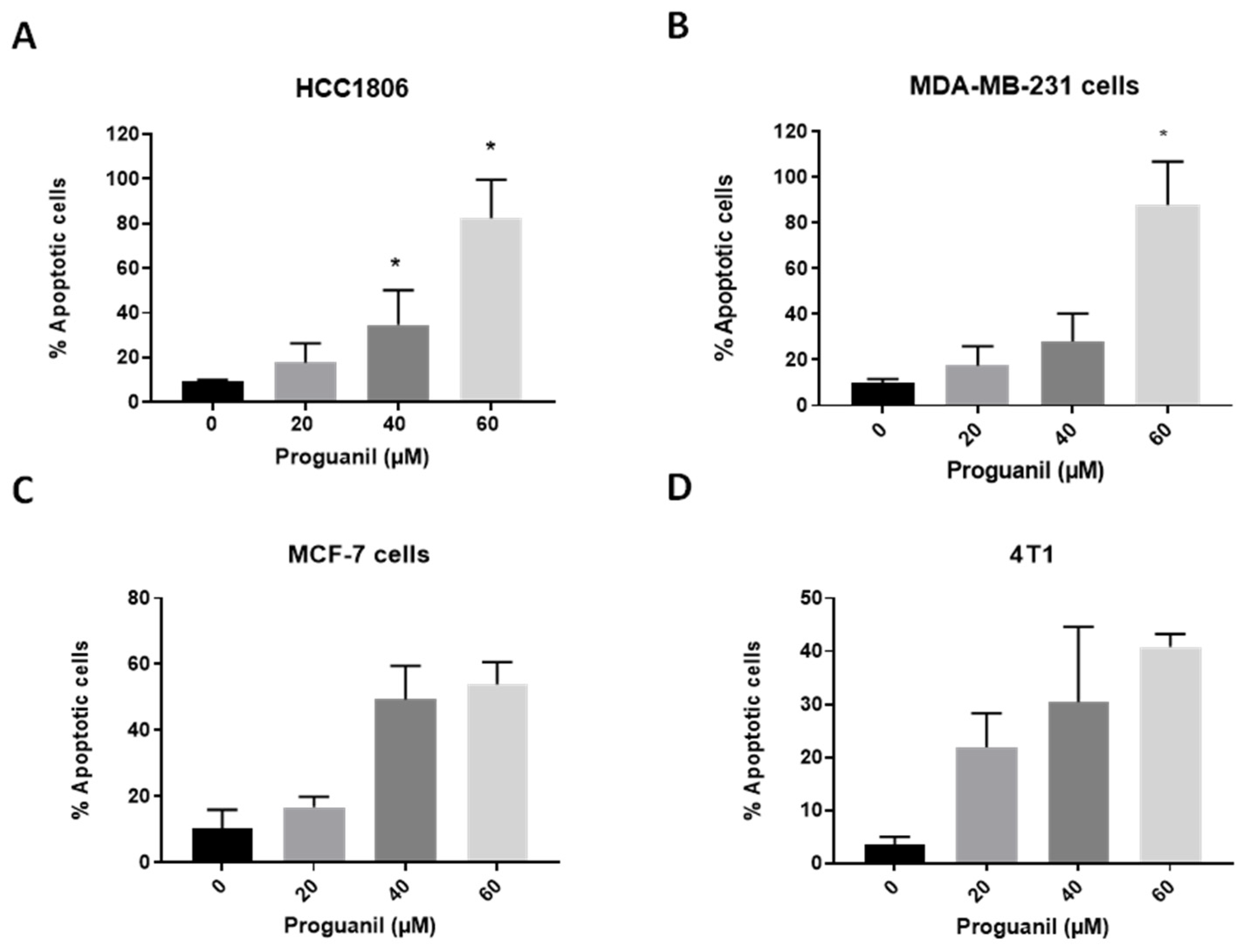
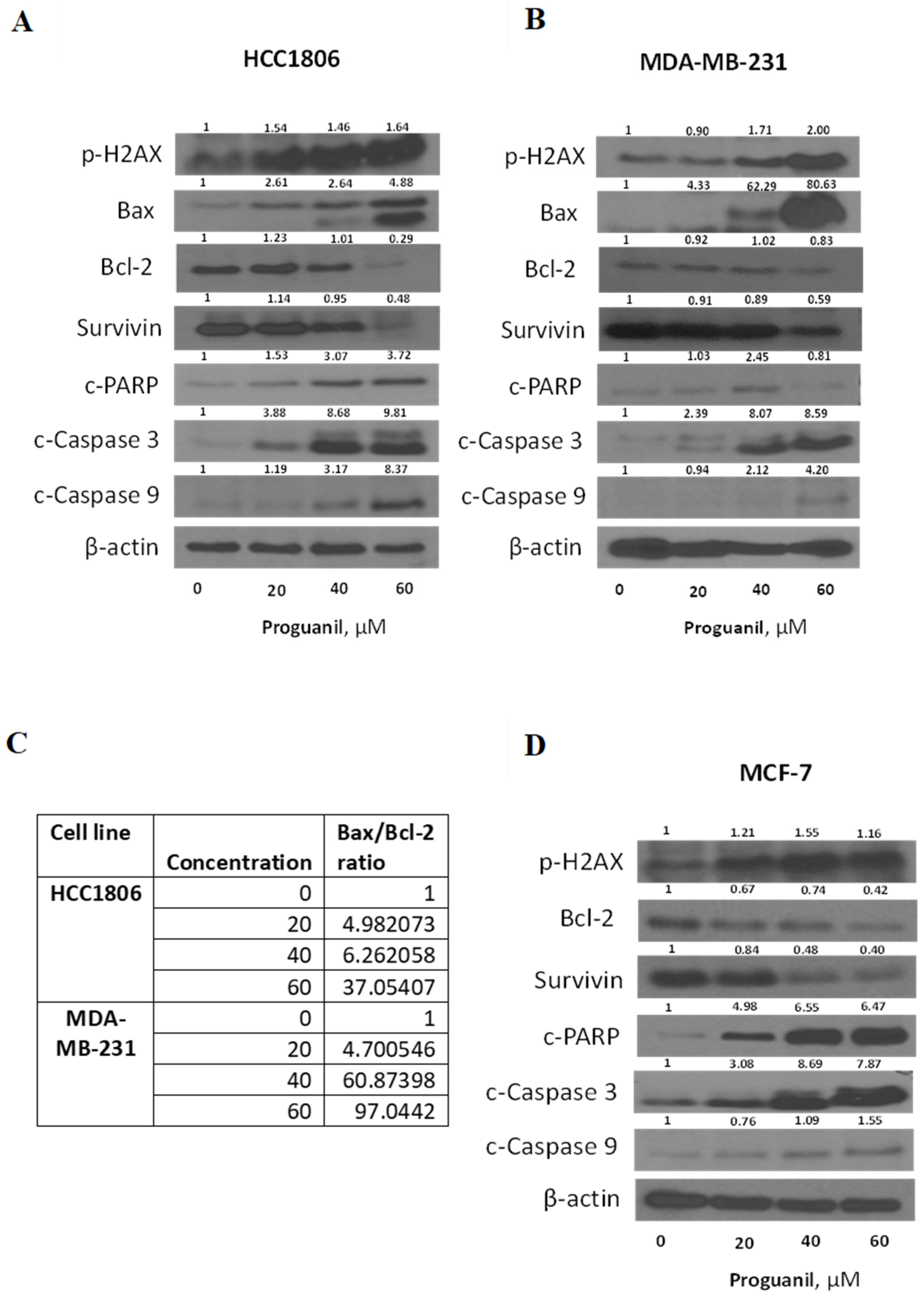
3.2. Proguanil triggers apoptosis in breast cancer cells
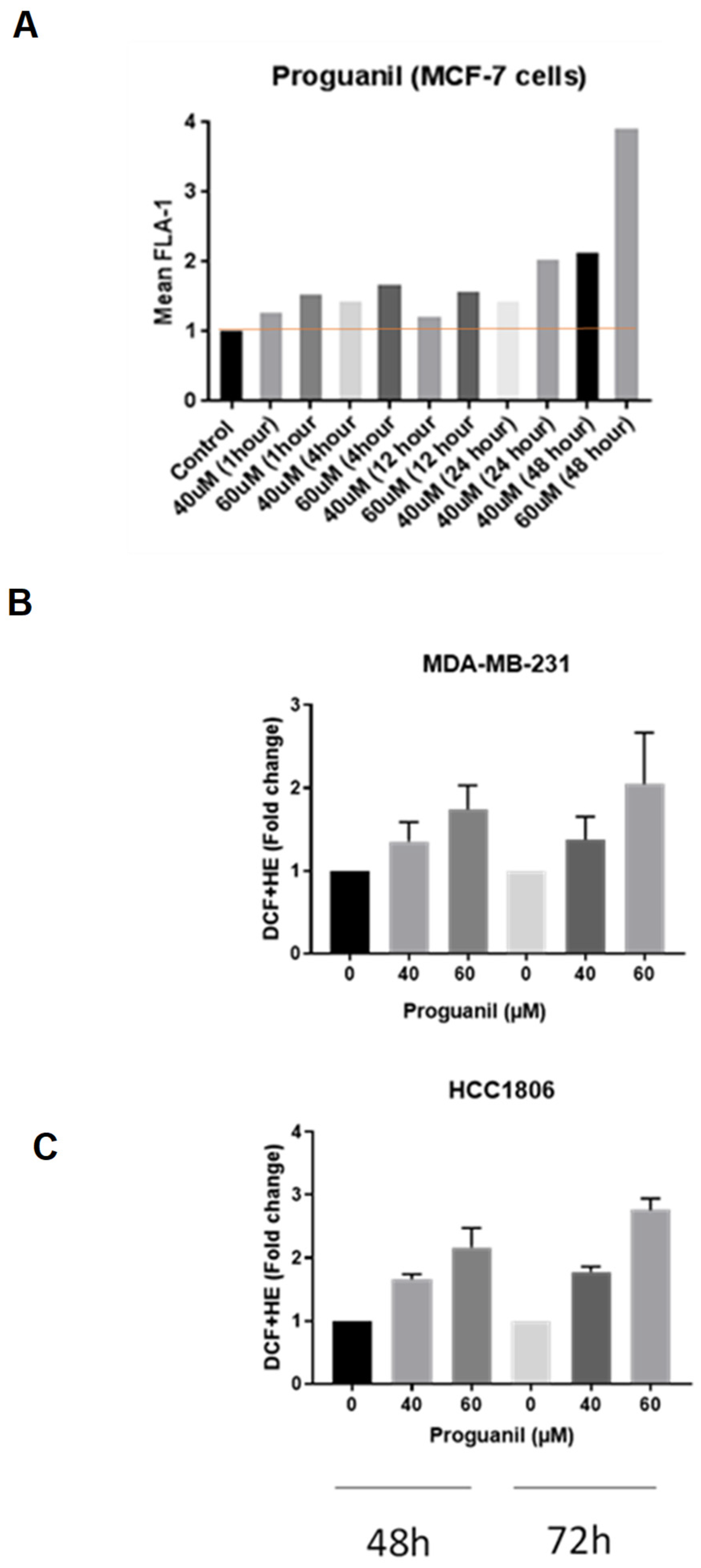
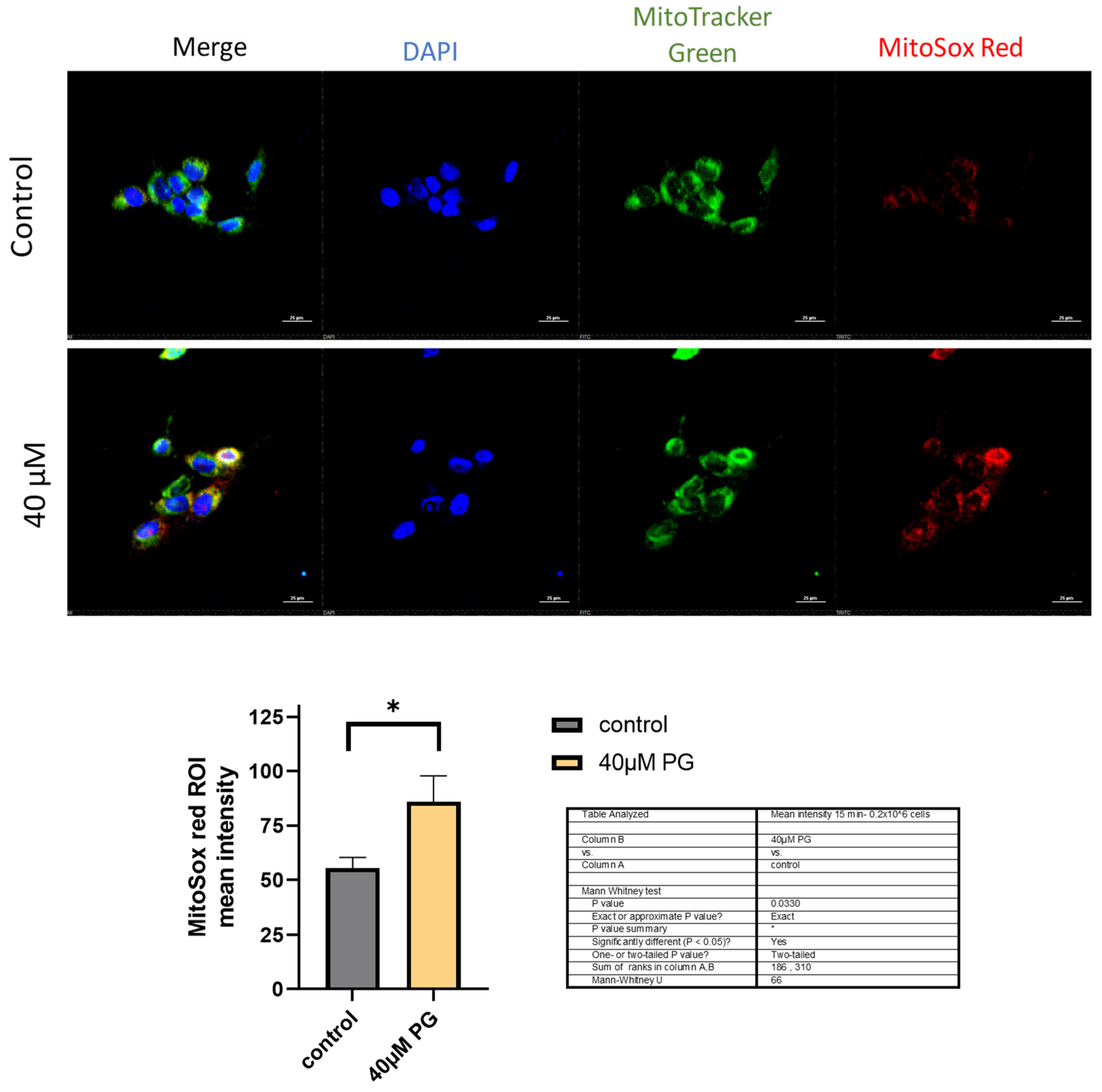
3.3. Proguanil causes the generation of mitochondrial ROS in breast cancer cells
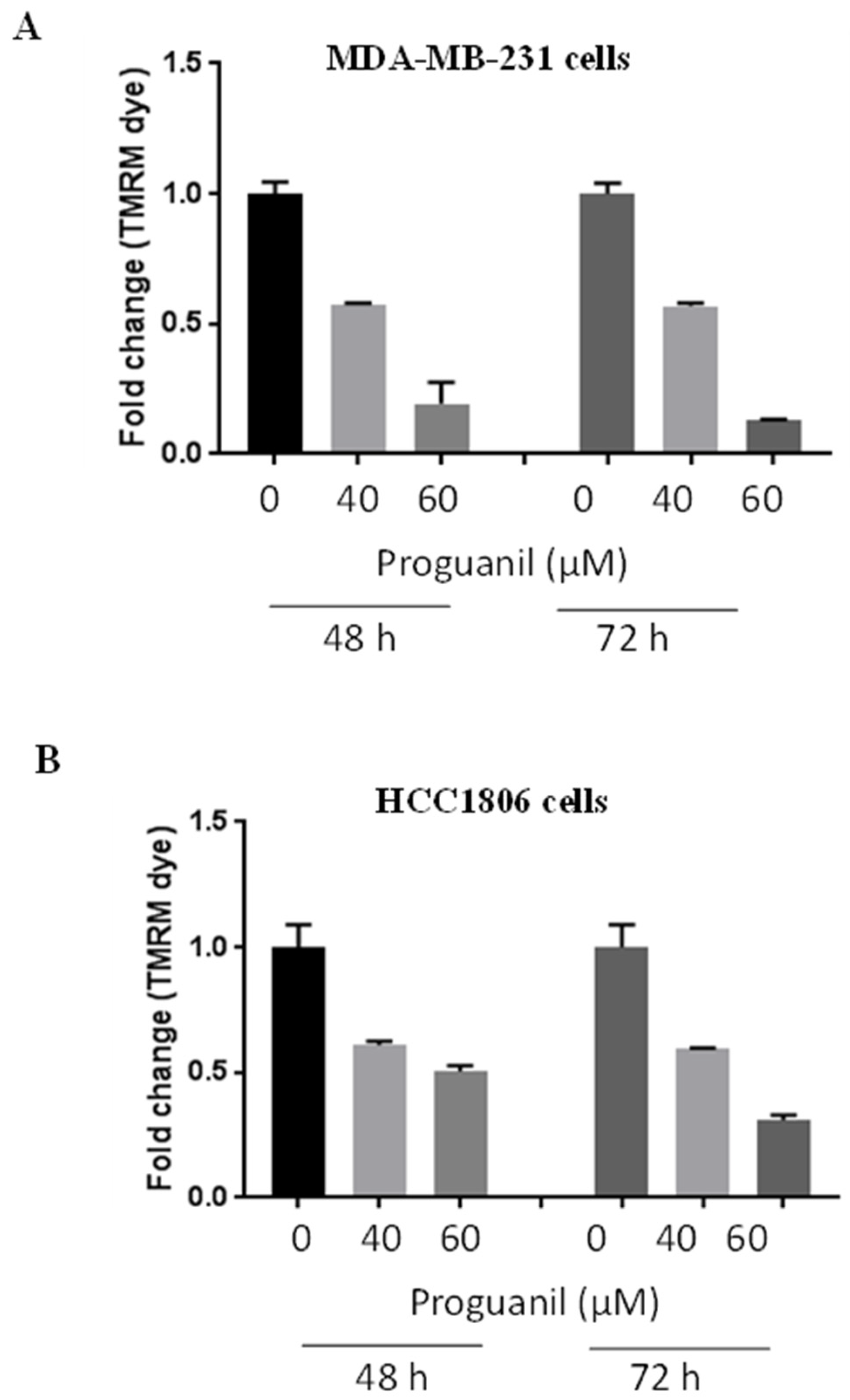
3.4. Proguanil disrupts mitochondrial membrane potential in MDA-MB-231 and HCC1806 cells
3.5. Proguanil treatment activates the caspase-3 cascade in breast cancer cells
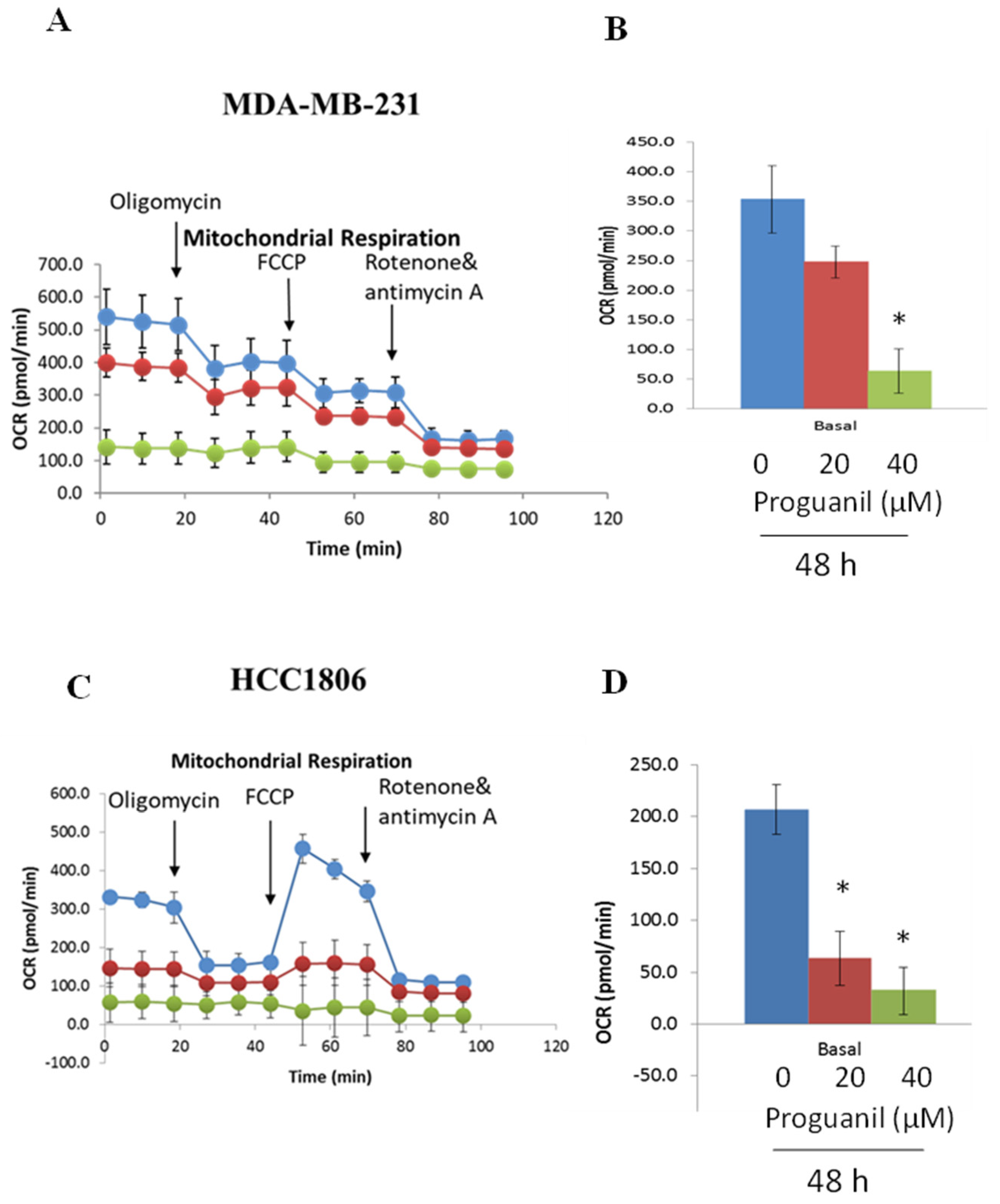
3.6. Proguanil inhibits oxidative phosphorylation of breast cancer cells
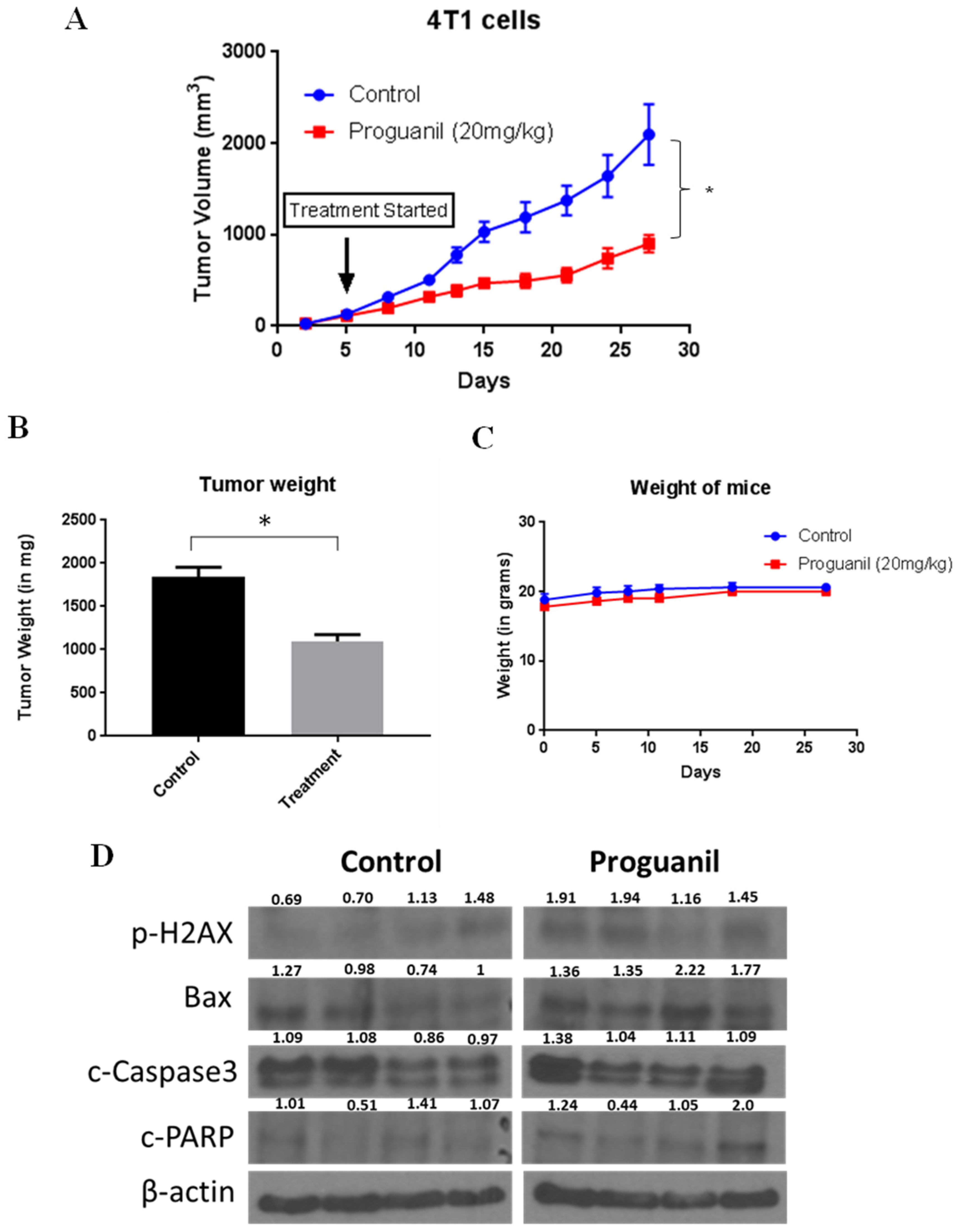
3.7. Proguanil inhibits the growth of orthotopically implanted 4T1 breast tumors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of abbreviations
- 4T1: murine breast cancer cell line
- ATCC: American Type Culture Collection
- Bax: Bcl-2-associated X protein
- bcl-2: B-cell lymphoma 2 protein
- Balb/c: Balb/c mice
- DCFDA: 6-carboxy-2, 7-dichlorodihydrofluorescein diacetate
- DHE: dihydroethidium
- DMEM: Dulbecco's Modified Eagle Medium
- FBS: Fetal Bovine Serum
- HCC1806: human breast carcinoma cell line
- ITS: Insulin-Transferrin-Selenous acid
- MCF-7: human breast carcinoma cell line
- MDA-MB-231: human breast carcinoma cell line
- PSN: Penicillin-Streptomycin-Neomycin
- SRB: Sulphorhodamine B assay
- STR: Short Tandem Repeats
- TMRM: tetramethyl rhodamine
- TX-BR: Patient-derived cells
- ROS: Reactive Oxygen Species”
References
- Sun, Y.S., et al., Risk Factors and Preventions of Breast Cancer. Int J Biol Sci, 2017. 13(11): p. 1387-1397. [CrossRef]
- Kabir, S. and M.A. Ahad. Parametric Study of Malignant Female Breast Tumor Size, Position and Orientation on EIM Parameters. in 2020 SoutheastCon. 2020. IEEE. [CrossRef]
- Pantziarka, P., et al., The Repurposing Drugs in Oncology (ReDO) Project. Ecancermedicalscience, 2014. 8: p. 442. [CrossRef]
- de Sá Junior, P.L., et al., The roles of ROS in cancer heterogeneity and therapy. Oxidative medicine and cellular longevity, 2017. 2017. [CrossRef]
- Gupta, S.C., et al., Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxidants & redox signaling, 2012. 16(11): p. 1295-1322. [CrossRef]
- Yang, H., et al., The role of cellular reactive oxygen species in cancer chemotherapy. Journal of Experimental & Clinical Cancer Research, 2018. 37(1): p. 1-10. [CrossRef]
- Ray, P.D., B.-W. Huang, and Y. Tsuji, Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cellular signalling, 2012. 24(5): p. 981-990. [CrossRef]
- Mügge, A., The role of reactive oxygen species in atherosclerosis. Zeitschrift fur Kardiologie, 1998. 87(11): p. 851-864. [CrossRef]
- Chio, I.I.C. and D.A. Tuveson, ROS in cancer: the burning question. Trends in molecular medicine, 2017. 23(5): p. 411-429. [CrossRef]
- Ishikawa, K., et al., ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science, 2008. 320(5876): p. 661-664. [CrossRef]
- Trachootham, D., J. Alexandre, and P. Huang, Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nature reviews Drug discovery, 2009. 8(7): p. 579-591. [CrossRef]
- Wu, W.-S., The signaling mechanism of ROS in tumor progression. Cancer and Metastasis Reviews, 2006. 25(4): p. 695-705. [CrossRef]
- Wang, J. and J. Yi, Cancer cell killing via ROS: to increase or decrease, that is the question. Cancer biology & therapy, 2008. 7(12): p. 1875-1884. [CrossRef]
- Toler, S.M., D. Noe, and A. Sharma, Selective enhancement of cellular oxidative stress by chloroquine: implications for the treatment of glioblastoma multiforme. Neurosurgical focus, 2006. 21(6): p. 1-4. [CrossRef]
- Renschler, M.F., The emerging role of reactive oxygen species in cancer therapy. European Journal of Cancer, 2004. 40(13): p. 1934-1940. [CrossRef]
- Hecht, F., et al., The role of oxidative stress on breast cancer development and therapy. Tumor biology, 2016. 37(4): p. 4281-4291. [CrossRef]
- Gurer-Orhan, H., et al., The Role of Oxidative Stress Modulators in Breast Cancer. Curr Med Chem, 2018. 25(33): p. 4084-4101. [CrossRef]
- Brown, N.S. and R. Bicknell, Hypoxia and oxidative stress in breast cancer Oxidative stress-its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast cancer research, 2001. 3(5): p. 1-5. [CrossRef]
- WANG, X., et al., The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochemical Journal, 1998. 333(2): p. 291-300. [CrossRef]
- Wiseman, H. and B. Halliwell, Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochemical Journal, 1996. 313(Pt 1): p. 17. [CrossRef]
- Yokomizo, A., et al., Cellular levels of thioredoxin associated with drug sensitivity to cisplatin, mitomycin C, doxorubicin, and etoposide. Cancer Research, 1995. 55(19): p. 4293-4296.
- Johar, R., et al., Role of reactive oxygen species in estrogen dependant breast cancer complication. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents), 2016. 16(2): p. 190-199. [CrossRef]
- Jones, R., Jr., T.N. Pullman, and et al., The therapeutic effectiveness of large doses of paludrine in acute attacks of sporozoite-induced vivax malaria, Chesson strain. J Clin Invest, 1948. 27(3 Pt1): p. 51-5. [CrossRef]
- Kaneko, A., et al., Intrinsic efficacy of proguanil against falciparum and vivax malaria independent of the metabolite cycloguanil. J Infect Dis, 1999. 179(4): p. 974-9. [CrossRef]
- Crowther, A. and A. Levi, Proguanil—the isolation of a metabolite with high antimalarial activity. British Journal of Pharmacology and Chemotherapy, 1953. 8(1): p. 93. [CrossRef]
- Fidock, D.A., T. Nomura, and T.E. Wellems, Cycloguanil and its parent compound proguanil demonstrate distinct activities against Plasmodium falciparum malaria parasites transformed with human dihydrofolate reductase. Mol Pharmacol, 1998. 54(6): p. 1140-7. [CrossRef]
- Skinner-Adams, T.S., et al., Cyclization-blocked proguanil as a strategy to improve the antimalarial activity of atovaquone. Communications biology, 2019. 2(1): p. 1-14. [CrossRef]
- Looareesuwan, S., et al., Malarone (atovaquone and proguanil hydrochloride): a review of its clinical development for treatment of malaria. Malarone Clinical Trials Study Group. The American journal of tropical medicine and hygiene, 1999. 60(4): p. 533-541. [CrossRef]
- Marra, F., J.R. Salzman, and M.H. Ensom, Atovaquone–proguanil for prophylaxis and treatment of malaria. Annals of Pharmacotherapy, 2003. 37(9): p. 1266-1275. [CrossRef]
- Boggild, A.K., et al., Atovaquone-proguanil: report from the CDC expert meeting on malaria chemoprophylaxis (II). The American journal of tropical medicine and hygiene, 2007. 76(2): p. 208-223. [CrossRef]
- Srivastava, I.K. and A.B. Vaidya, A mechanism for the synergistic antimalarial action of atovaquone and proguanil. Antimicrobial agents and chemotherapy, 1999. 43(6): p. 1334-1339. [CrossRef]
- Mounkoro, P., T. Michel, and B. Meunier, Revisiting the mode of action of the antimalarial proguanil using the yeast model. Biochem Biophys Res Commun, 2021. 534: p. 94-98. [CrossRef]
- Gupta, N. and S.K. Srivastava, Atovaquone: An Antiprotozoal Drug Suppresses Primary and Resistant Breast Tumor Growth by Inhibiting HER2/beta-Catenin Signaling. Mol Cancer Ther, 2019. 18(10): p. 1708-1720. [CrossRef]
- Bridges, H.R., et al., Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J, 2014. 462(3): p. 475-87. [CrossRef]
- Bridges, H.R., et al., Molecular features of biguanides required for targeting of mitochondrial respiratory complex I and activation of AMP-kinase. BMC biology, 2016. 14(1): p. 1-11. [CrossRef]
- Lea, M.A. and H. Kim, Effects of Biguanides on Growth and Glycolysis of Bladder and Colon Cancer Cells. Anticancer research, 2018. 38(9): p. 5003-5011. [CrossRef]
- Wu, Y., et al., Proguanil synergistically sensitizes ovarian cancer cells to olaparib by increasing DNA damage and inducing apoptosis. International journal of medical sciences, 2022. 19(2): p. 233. [CrossRef]
- Barbieri, F., et al., Inhibition of Chloride Intracellular Channel 1 (CLIC1) as Biguanide Class-Effect to Impair Human Glioblastoma Stem Cell Viability. Front Pharmacol, 2018. 9: p. 899. [CrossRef]
- Xiao, D., et al., Inhibitory role of proguanil on the growth of bladder cancer via enhancing EGFR degradation and inhibiting its downstream signaling pathway to induce autophagy. Cell Death & Disease, 2022. 13(5): p. 1-14. [CrossRef]
- Scholar, E., Proguanil, in xPharm: The Comprehensive Pharmacology Reference, D.B.B. S.J. Enna, Editor. 2007. p. 1-4.
- Baggish, A.L. and D.R. Hill, Antiparasitic agent atovaquone. Antimicrob Agents Chemother, 2002. 46(5): p. 1163-73. [CrossRef]
- Verma, K., et al., AKR1C3 Inhibitor KV-37 Exhibits Antineoplastic Effects and Potentiates Enzalutamide in Combination Therapy in Prostate Adenocarcinoma CellsAKR1C3 Inhibitor for CRPC Treatment. Molecular cancer therapeutics, 2018. 17(9): p. 1833-1845. [CrossRef]
- Pramanik, K.C., et al., CBP-Mediated FOXO-1 Acetylation Inhibits Pancreatic Tumor Growth by Targeting SirTAcetylation of FOXO-1 by Capsaicin Causes Apoptosis. Molecular cancer therapeutics, 2014. 13(3): p. 687-698. [CrossRef]
- Choi, A.R., et al., Anti-malarial Drugs Primaquine and Chloroquine Have Different Sensitization Effects with Anti-mitotic Drugs in Resistant Cancer Cells. Anticancer Res, 2016. 36(4): p. 1641-8.
- Efferth, T., et al., The anti-malarial artesunate is also active against cancer. Int J Oncol, 2001. 18(4): p. 767-73. [CrossRef]
- Kim, J.H., et al., Co-treatment with the anti-malarial drugs mefloquine and primaquine highly sensitizes drug-resistant cancer cells by increasing P-gp inhibition. Biochem Biophys Res Commun, 2013. 441(3): p. 655-60. [CrossRef]
- Xie, F., et al., Preclinical evidence of synergism between atovaquone and chemotherapy by AMPK-dependent mitochondrial dysfunction. Eur J Pharmacol, 2021. 907: p. 174256. [CrossRef]
- Mudassar, F., et al., Targeting tumor hypoxia and mitochondrial metabolism with anti-parasitic drugs to improve radiation response in high-grade gliomas. J Exp Clin Cancer Res, 2020. 39(1): p. 208. [CrossRef]
- Chen, Q., et al., Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem, 2003. 278(38): p. 36027-31. [CrossRef]
- Pramanik, K.C., S.R. Boreddy, and S.K. Srivastava, Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PloS one, 2011. 6(5): p. e20151. [CrossRef]
- Wen, J.-J. and N.J. Garg, Mitochondrial complex III defects contribute to inefficient respiration and ATP synthesis in the myocardium of Trypanosoma cruzi–infected mice. Antioxidants & redox signaling, 2010. 12(1): p. 27-37. [CrossRef]
- Murphy, M.P., How mitochondria produce reactive oxygen species. Biochemical journal, 2009. 417(1): p. 1-13. [CrossRef]
- Tao, K., et al., Imagable 4T1 model for the study of late stage breast cancer. BMC cancer, 2008. 8(1): p. 1-19. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




