2. Raw Materials for the Production of Titanium Dioxide
The mineral sources for the production of titanium dioxide are usually titanium-bearing ores: rutiles, ilmenites and lucoxenes. Rutiles are the richest ores - they contain from 93 to 96% titanium dioxide (TiO
2), ilmenites - from 44 to 70%, and lucoxene concentrates can contain up to 90% TiO
2. Only 5% of all the mined titanium ore goes directly to the production of titanium [
5].
At present, more than 300 deposits of titanium minerals have been discovered in the world, including 70 igneous, 10 lateritic, and more than 230 placer deposits. Of these, 90 deposits have been explored according to industrial categories, mainly placer deposits. Primary (igneous) deposits contain about 69%, carbonatite weathering crusts - 11.5%, placer deposits - 19.5% of the world's titanium reserves. Within this group, more than 82% are in ilmenite, less than 12% in anatase, and 6% in rutile [
6,
7,
8].
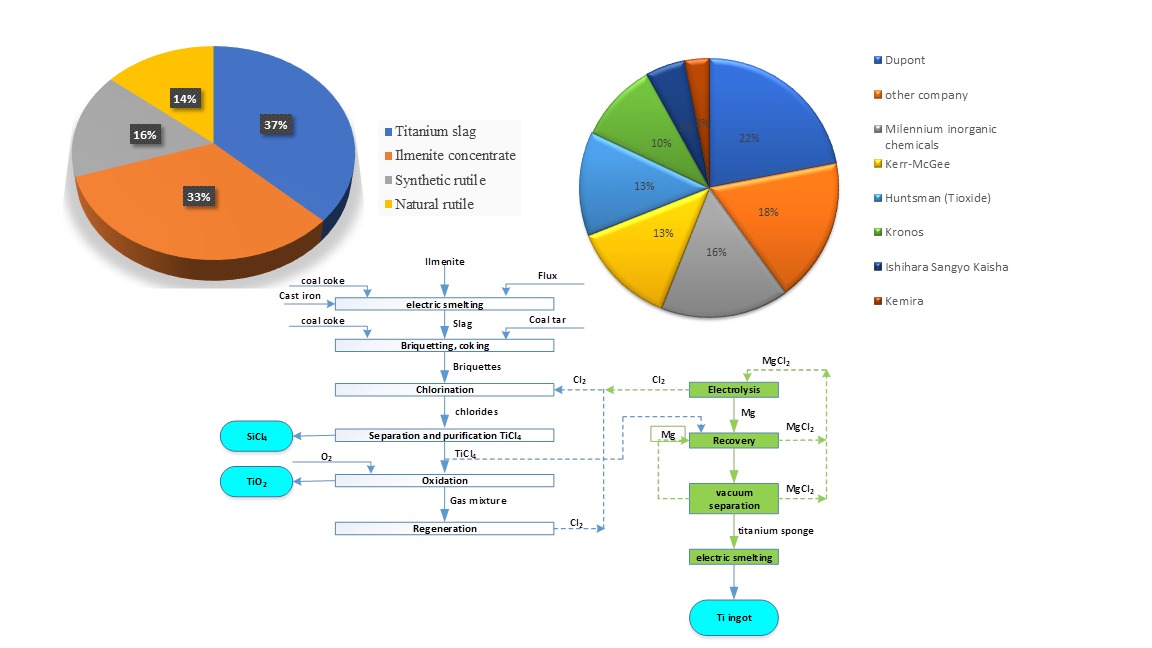
As mentioned above, there are two main industrial technologies for producing pigment titanium dioxide - sulfate and chloride. Raw materials for these technologies are shown in
Figure 1 [
9].
The main raw material for these technologies is high-titanium slag and/or rutile obtained from ilmenite concentrate.
The most common method for producing titanium slag is reduction melting in electric furnaces at a temperature of 1600–1700 °C, during which iron oxide is reduced to metal. The main melting product, titanium slag, contains 75–85% TiO2. The second product is pig iron, which is used as a raw material in steel production.
Titanium slag can be used to produce rutile with a titanium dioxide content of 92–96%. The industrial production of synthetic rutile consists of two stages: reduction smelting and acid leaching, which generates a huge amount of liquid waste - 2 t/t TiO2.
Ilmenite-magnetite and ilmenite-hematite ores of primary deposits form the basis of the mineral resource base of the titanium industry in Canada, China and Norway. Deposits in the weathering crusts of carbonatites are known and developed only in Brazil. In other countries, the main reserves of titanium minerals are located in alluvial, mainly complex deposits. Modern and ancient coastal-marine and accompanying dune placers are of the greatest industrial importance. The length of each placer is small - from hundreds of meters to several kilometers.
3. Global Titanium Dioxide Companies
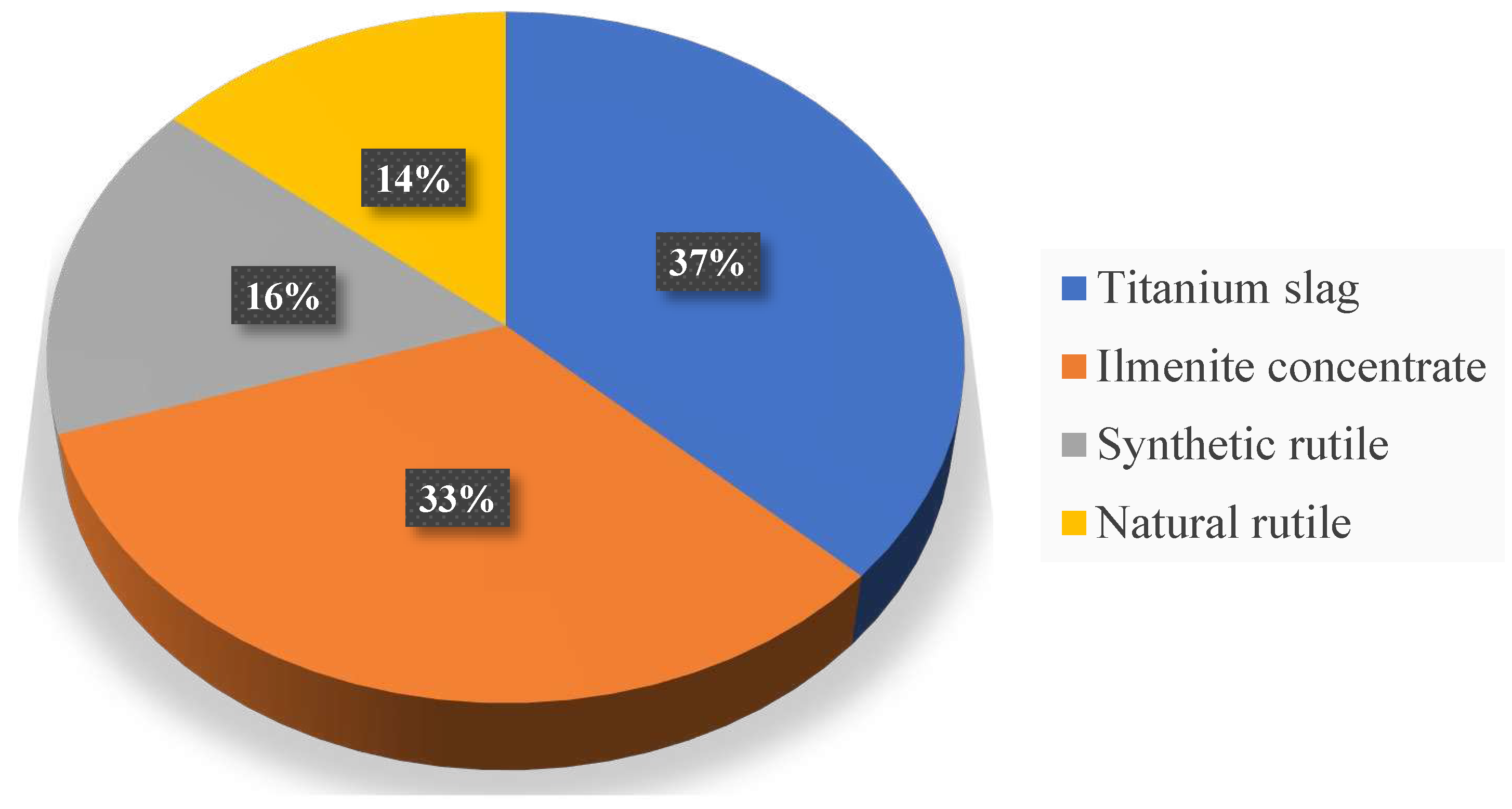
The largest producer of pigment titanium dioxide is E.I. du Pont de Nemours & Co. Inc. (Dupont). Over the past 10 years, its share has increased from 22 to 24% of the world production of this product. The company owns plants in the USA (3 plants), Mexico and Taiwan with a total capacity of 1,000 thousand tons/year, which operate using chloride technology [
10].
Plants of Millennium Inorganic Chemicals Inc. located in USA (2 plants), UK, France (2 plants) and Australia. The production uses both sulfate (total capacity 182 thousand tons/year) and chloride technology (350 thousand tons/year). In January 1998, the company commissioned two new sulphate plants in France, then completed the modernization of the chloride plant in Stallingbaraf (UK), increasing its capacity from 109 thousand tons/year to 150 thousand tons/year. Millennium Chemicals is currently considering a 10% to 20% increase in ultra-fine titanium dioxide production capacity at its plant in Tan, France [
11].
Tioxide (a subsidiary of Huntsman Corp.) (
Figure 2) owns 6 plants with sulfate technology (total capacity - 456 thousand tons/year), located in the UK, Spain, Italy, Malaysia and South Africa, and one plant with chloride technology (100 thousand tons/year) in the UK (Greatham).
Kronos Inc. (subsidiary of NL Industries Inc.) owns 4 plants with sulphate technology in Germany, Canada and Norway with a total capacity of 24 thousand tons/year and 3 plants with chloride technology in Germany, Canada and Belgium with a total capacity of 230 thousand tons/year. [
12]
Kemira Pigments OU produces pigment titanium dioxide at three plants: in the USA, Finland and the Netherlands. In 1998, the company invested $6 million to increase the capacity of the sulphate plant in Pori (Finland) to 120,000 tons/year.
Kerr-McGee operates two of its facilities in Hamilton, USA, which use chloride technology, and also uses Bayer's manufacturing facilities in Germany and Belgium. In 1999, work was completed to expand the capacity of the plant in Hamilton, as a result of which they increased from 150 to 178 thousand tons/year [
13].
Sachtleben Chemie, a subsidiary of Metallgesellschaft AG, operates a factory in Duisburg (Germany) and produces mainly anatase titanium dioxide for synthetic glass fibers, as well as titanium dioxide for the food and pharmaceutical industries.
The Polish company Zaklady Chemiczne operates the only enterprise for the production of rutile pigment titanium dioxide using sulfate technology with a capacity of 36 thousand tons/year, using Norwegian ilmenite concentrate and Canadian titanium slag [
14].
The Czech company Precheza AS owns an enterprise with a capacity of 27 thousand tons per year in Prevov (Czech Republic), producing anatase titanium dioxide.
Slovenia has the only enterprise for the production of rutile titanium dioxide with a capacity of 34,000 tons/year.
China is one of the world's largest producers and consumers of titanium dioxide products. In addition, demand for TiO2 for paints and coatings is likely to increase in the construction industry. According to China's National Bureau of Statistics, China's construction industry generated about 7.3 trillion yuan in added value in 2020.
In November 2021, Asian Paints announced plans to invest US$127 million in a plant in Gujarat, India to expand paint production capacity from 130,000 kiloliters to 250,000 kiloliters over the next two to three years.
In addition, the plastics industry is expected to grow in China and India. The Chinese plastics industry is growing at a fast pace due to the availability of cheaper raw materials and huge demand from developing countries.
About 7.95 million metric tons of plastic products were produced in December 2021, compared to 7.32 million metric tons in November 2021, according to China's National Bureau of Statistics.
According to the Plastics Export Promotion Council (PLEXCONCIL), plastics exports from India increased by 55% to $3,417 million (cumulative) from April to June 2021, compared to $2,211 million in April-June 2020.
The Asia-Pacific region has also seen a significant increase in demand for beauty products, leading to increased use by teenagers and increased awareness of hygiene, which is expanding the researched market.
Therefore, such trends are likely to drive titanium dioxide demand in the region during the 2022-2027 forecast period. [
15]
4. Combined Technologies for the Production of Titanium Dioxide
4.1. Processing of Ilmenite Concentrate by Sulfate Method
Ilmenite concentrate serves as a raw material in the sulfate technology for the production of titanium dioxide, which was introduced into industry in 1931 to produce anatase, and in 1941 to produce rutile [
16].
In this method, titanium-containing ore is dissolved in sulfuric acid, and forms solutions of titanium, iron and other metal sulfates. Then, as a result of a series of chemical reactions, including chemical reduction, purification, precipitation, washing and calcination, a basic titanium dioxide with the required particle size is formed. The crystal structure (anatase or rutile form) is controlled during the process of nucleation and calcination [
17].
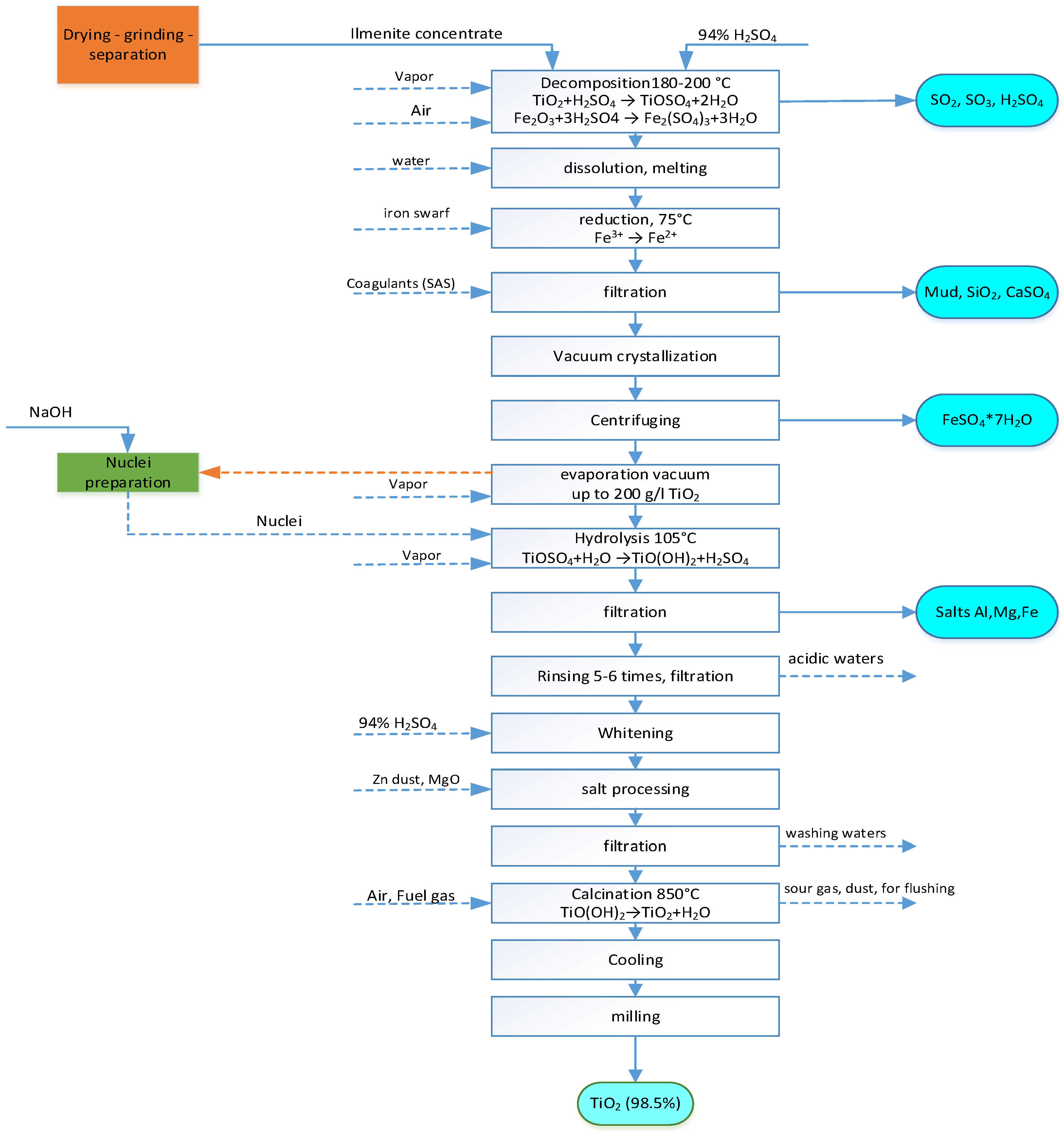
The technology for the production of titanium dioxide by this method is based on the treatment of ilmenite (a natural mixture of various oxides, mainly tetravalent Ti and trivalent Fe) with sulfuric acid.
Sulfate technology requires slightly altered ilmenites, where the content of oxide iron in the mineral is less than or equal to the content of ferrous iron (otherwise, the concentrates will not dissolve in sulfuric acid). These requirements are met by ilmenite concentrates obtained from primary deposits or concentrates from placers of close demolition. According to the sulphate technology, the iron contained in ilmenite concentrates is not used, and significant wastes of ferrous sulphate require disposal.
At the first stage of the process, ilmenite is crushed, dried, and then decomposed with concentrated sulfuric acid. The degree of decomposition of the concentrate is 96-97%. The result is a mixture of titanium sulfate and sulfates of iron (II) and (III), which is cooled and diluted with water to a certain concentration. Then, ferric iron is reduced to ferrous iron in a solution of titanyl sulfate with metallic iron. The resulting solution is settled and fed to black filtration. Ferrous vitriol is crystallized in the filtered solution upon cooling and separated from the mother liquor in centrifuges. The by-product of production (seven-water iron sulphate) is calcined to obtain monohydrate (FeSO4 * H2O) and crushed, and the titanyl sulfate solution is evaporated to a standard concentration and sent to the next stage of the process, which is hydrolysis.
The hydrolysis of a solution of titanium sulfate salts is carried out by the method of introducing nuclei (they are prepared by precipitating Ti(OH)4 from solutions of titanium sulfate with sodium hydroxide). In the process of hydrolysis, amorphous flakes of titanium dioxide hydrate are released, which have a high adsorption capacity, especially with respect to Fe3+ salts, for this reason, at the previous stage, trivalent iron is reduced to ferrous. The process proceeds according to the summary equation:
This process produces a large amount (in terms of monohydrate ~2 tons per 1 ton of TiO2) of diluted 20–22% hydrolyzed sulfuric acid contaminated with iron sulfate, 1–2% titanyl sulfate and several percent of other sulfates. This acid is also a production waste. A possible direction of hydrolytic acid utilization is evaporation to a concentration of 55% and its subsequent use for superphosphate production.
By varying the hydrolysis conditions (concentration, duration of stages, number of nuclei, acidity, etc.), it is possible to achieve the yield of hydrolyzate particles with desired properties, depending on the intended application. During hydrolysis, up to 95-96% of titanium is precipitated, and the resulting metatitanic acid sorbs a significant amount of SO3.
During the final stage of the process, metatitanic acid is subjected to filtration in two stages, in which it is washed from chromophore impurities and bleached. After adding the necessary components, the titanium dioxide hydrate paste is calcined in rotating drum calcining furnaces 40–60 m long. As a result, water is first removed from it (at 200–300 °C), followed by SO3 removal (at 500–800 °C) and a neutral (pH=7) product - titanium dioxide is obtained at 850-900 °C.
At this stage, by varying the drying temperature and using additives (such as zinc oxide, titanium chloride) and other methods, it is possible to carry out rutilization (that is, the rearrangement of titanium oxide into a rutile modification). The calcined product is crushed in two stages and transferred to surface treatment, which is carried out with certain chemicals to give the titanium dioxide pigment certain consumer properties. The processed titanium dioxide pigment is dried and transferred to micro-grinding, after which it is packaged and sent to the warehouse.
The approximate consumption of basic materials for the production of 1 ton of titanium dioxide from ilmenite concentrates by the sulfuric acid method is: ilmenite concentrate containing 42% TiO
2 - 3.1 tons; sulfuric acid (monohydrate) - 4-4.5 tons; iron shavings - 0.24 tons [
18,
19]. The schematic diagram of obtaining TiO
2 by the sulfate method is shown in
Figure 2.
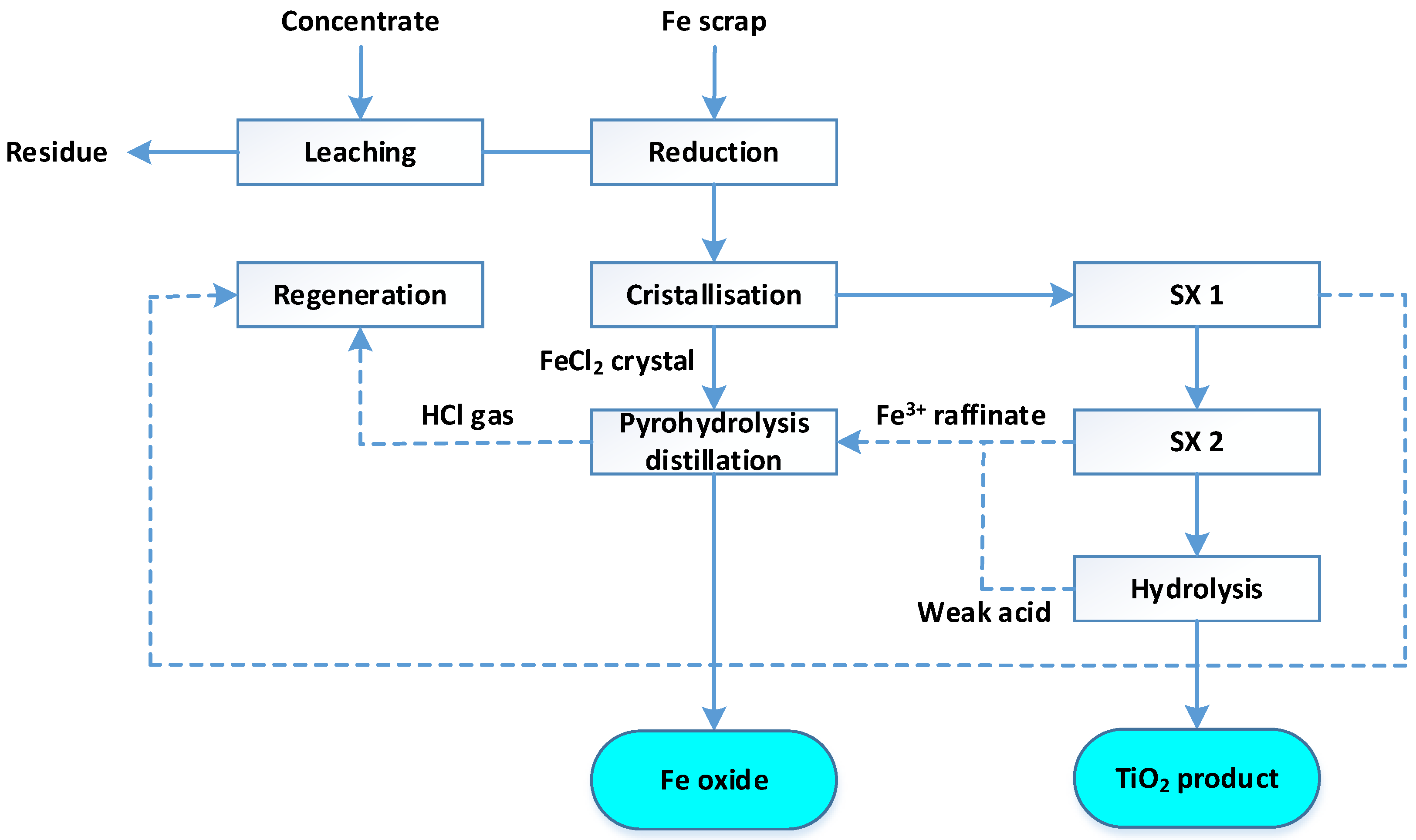
It should be noted that titanium slag obtained during the ore reduction smelting of ilmenite concentrates can also serve as a source of obtaining TiO2 by the sulfate method. In particular, a plant in Canada (Quebec, near Sorel) operates on slag (70% TiO2).
When titanium dioxide is obtained from slags by the sulfuric acid method, solutions after titanium sulfate leaching containing 190 g/l TiO
2 and a small amount of iron hydrolyze more easily than when processing ilmenite concentrates. The resulting hydrolytic acid contains ~9 g/l of iron, which facilitates its regeneration (
Figure 3).
The main advantages of this technology are low capital costs and flexibility in the raw material. he disadvantages are high energy consumption, different quality of the obtained pigment, a large amount of hardly realizable waste, for example, when ilmenite is processed according to a sulfate scheme per ton of titanium dioxide, a large amount of diluted 20-22% hydrolysis sulfuric acid is formed (in terms of monohydrate ~ 2 t per 1 t of TiO2), contaminated with 2-3 t of iron sulfate, 1-2% titanyl sulfate and several percent of other sulphates.
Despite the shortcomings, about 40% of all pigment in the world is obtained using sulfate technology.
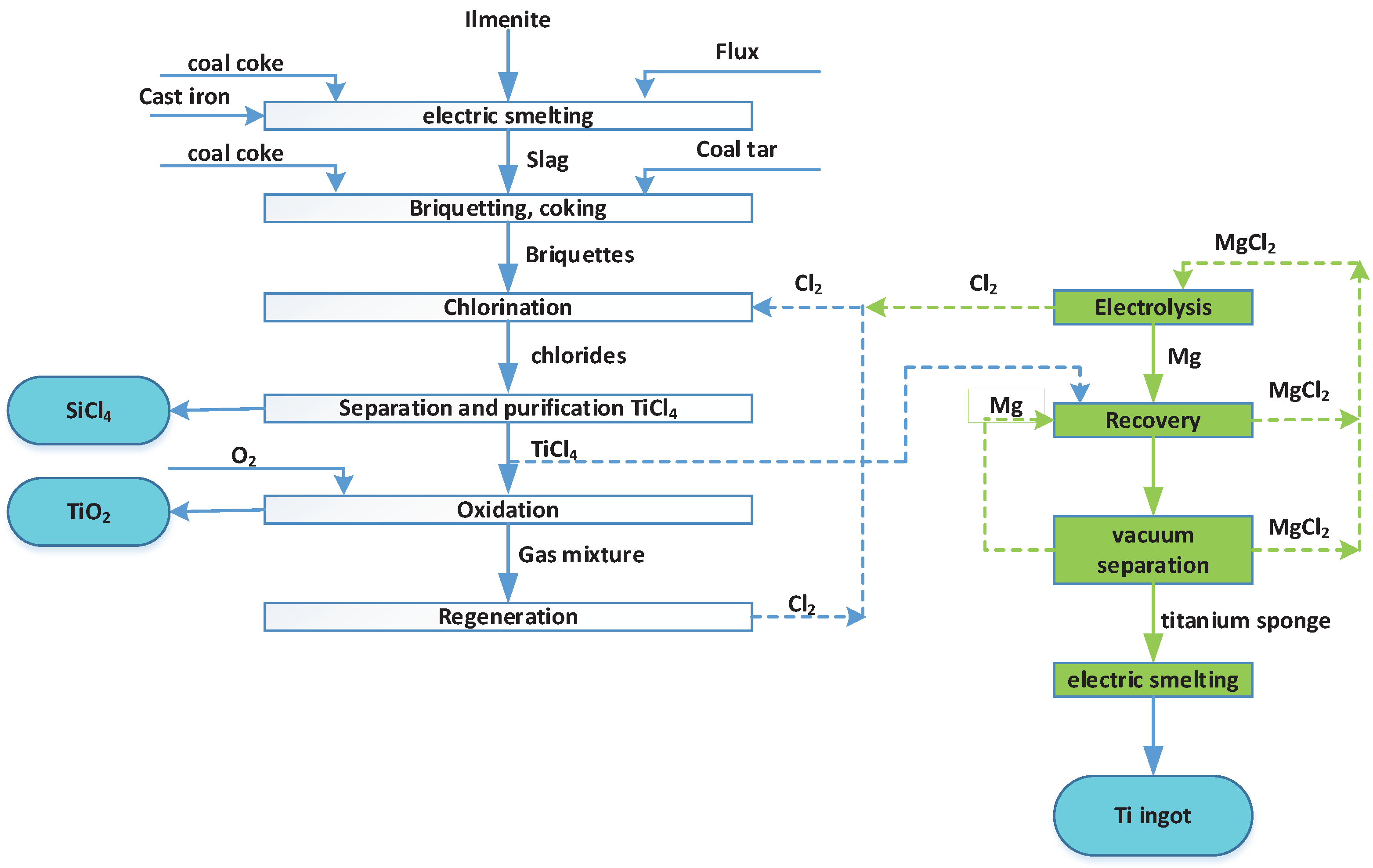
4.2. Processing of Ilmenite Concentrates by the Chloride Method
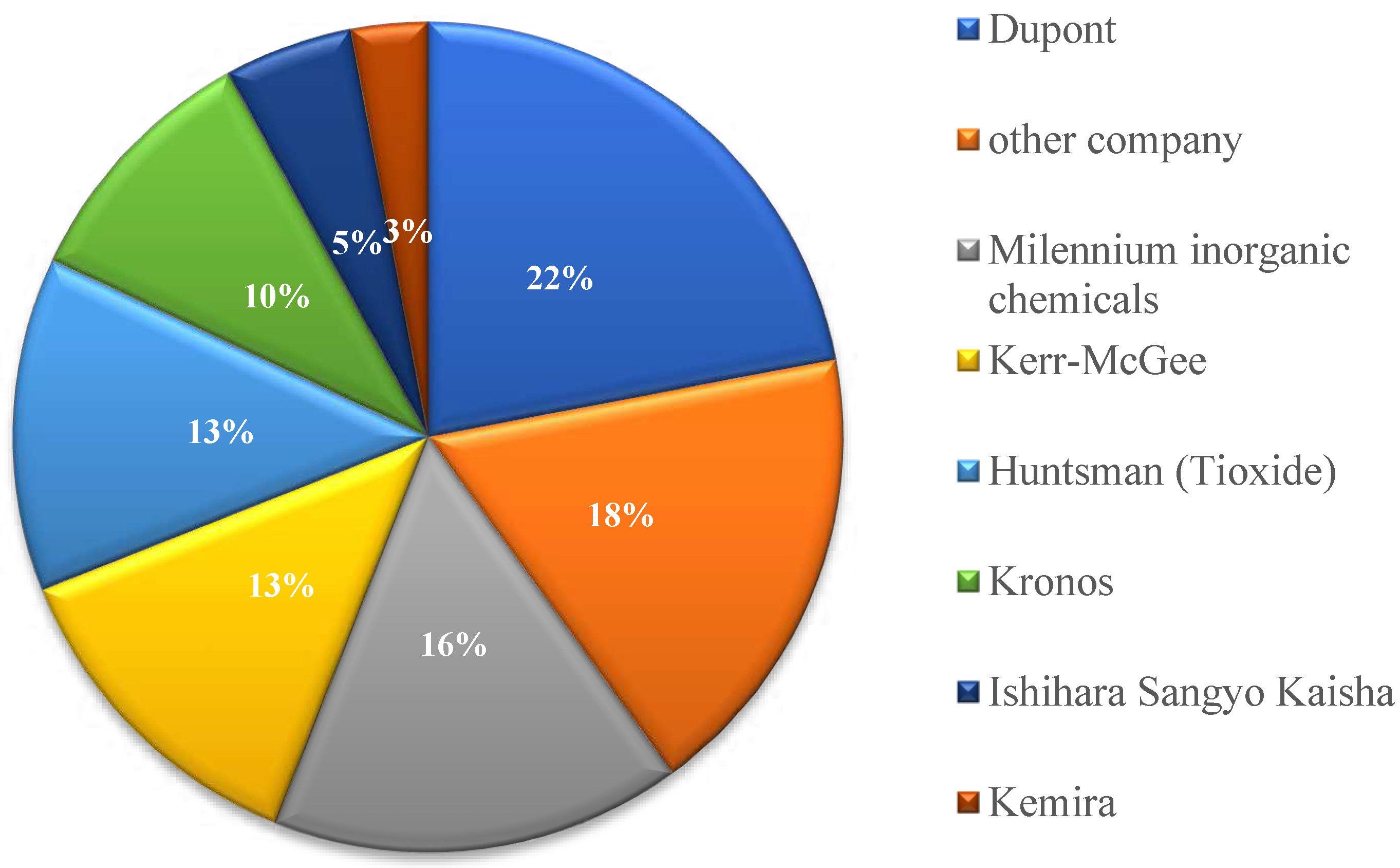
The chloride method for producing titanium dioxide was developed by DuPont; a pilot plant was put into operation in 1948, and in 1958 the chloride technology was introduced on an industrial scale.
The technology consists in the fact that natural or synthetic rutile, reacting with carbon (coke, petroleum coke, etc.) and chlorine gas at high temperature, forms titanium tetrachloride vapor, which after purification is oxidized by oxygen at 1300-1800 °C to titanium dioxide [
20].
Since titanium tetrachloride also serves as an intermediate product in the production of metallic titanium, the production of titanium dioxide by the chloride method is adjacent to titanium metallurgy (
Figure 4).
When TiO
2 is obtained by the chloride method, titanium concentrate reacts with chlorine gas under reduced pressure, resulting in the formation of titanium tetrachloride TiCl
4 and impurities of metal chlorides, which are then removed.
TiO2 can then be obtained from titanium tetrachloride by one of the following methods:
hydrolysis of aqueous solutions of titanium tetrachloride (with subsequent heat treatment of the precipitate);
vapor-phase hydrolysis of titanium tetrachloride (based on the interaction of titanium tetrachloride vapor with water vapor). The process is usually carried out at a temperature of 900-1000 °C;
heat treatment of tetrachloride (combustion in a stream of oxygen). Industrial methods for the production of titanium pigments by hydrolysis have not yet been developed. Their common drawback is an unsatisfactory solution to the problem of using hydrochloric acid or hydrogen chloride formed during hydrolysis, which cannot be returned for reuse in the production of titanium dioxide.
The combustion of titanium tetrachloride with oxygen proceeds according to the reaction:
The released chlorine can be returned to production to produce titanium tetrachloride, which is why the combustion method is used in industry.
The oxidation step in the chloride process allows better control of the particle distribution curve and crystal structure. The result is titanium dioxide with high hiding power and thinning power.
During combustion, it is necessary to maintain the temperature at a constant level in order to obtain particles of the same size, and also to regulate the period of time during which the formed particles are under thermal influence. It is the combustion conditions that determine the structural modification of the resulting titanium dioxide. Often this method produces a product unsuitable for the production of paints and varnishes, since it contains more than 0.5% chlorine. Therefore, the process is carried out on burners of a special design, ensuring the maintenance of the reaction temperature within the specified limits and keeping the combustion products for a certain time.
In order to obtain a monodisperse product, the presence time of titanium dioxide in the high temperature zone should not exceed 0.01–5 s. Depending on the combustion conditions of TiCl4, titanium dioxide has a rutile or anatase structure. Mixing the initial reagents at 400 °C leads to the formation of anatase with a particle size of 0.5-1 μm. Preheating them to 1000 °C during combustion gives a product containing up to 60% rutile.
The combustion of titanium tetrachloride with an admixture of silicon tetrachloride (0.5-4%) leads to a decrease in the particle size of titanium dioxide. Silicon tetrachloride also contributes to the reduction of the so-called photoactivity of titanium dioxide. Furthermore, the addition of aluminum chloride (1-5%) to the combustion products accelerates the transition of anatase to rutile. The proportion of rutile in the finished product depends on the concentration of seed crystals that appear in the first stage of the reaction.
Titanium pigments obtained by burning titanium tetrachloride contain up to 0.6% of adsorbed chlorine. An aqueous suspension of such a product has a pH>7 and is not suitable for the preparation of paints. Desorption of chlorine from the pigment is carried out by calcining it at 300-900 °C, the chlorine impurity content is reduced to 0.1%. Such a product has an aqueous extract pH of 5-6.8 and is suitable for the manufacture of paints and enamels but requires surface treatment with silicon and aluminum compounds. The processing with various combinations of components allows you to achieve optimal properties for each specific application of the finished product [
21,
22,
23,
24].
Compared to the sulphate method, the chloride method is more environmentally friendly and advanced due to the possibility to carry out the process in a continuous mode, which implies full automation of production. However, it is selective to raw materials, and due to the use of chlorine and high temperatures it requires the use of corrosion-resistant equipment. In both methods, the intermediate product is clusters of titanium dioxide crystals, which must then be separated to impart optimal optical properties. Several methods exist for modifying titanium dioxide, including surface treatment with silicon and aluminum oxides.
Environmental problems, such as: unresolved issue of using chlorine and hydrogen compounds in the production of titanium dioxide by chloride method, a large amount of solid waste in the processing of ores with low titanium content, contamination of wastewater and the need for their treatment, are an important factor that somewhat hinders the development of the titanium dioxide market. Environmental protection in developed countries is a dominant factor in the development of titanium dioxide production by one or another method.
According to experts' estimates, in the cost of production of titanium dioxide using raw materials with high TiO2 content the cost of this raw material is: in chloride method - 43%, in sulfate method - 28%. When using raw materials with low TiO2 content, the similar indicators are 20 and 14%, respectively.
4.3. Processing of Titanomagnetites and Titanium Slags by Hydrochloric Acid Technology
Hydrochloric acid leaching is one of the most frequently used well-proven methods of modernization of titanium-containing raw materials [
25,
26,
27].
The paper [
28] proposes a method for extracting iron, titanium, vanadium, and chromium from high-chromium vanadium titanomagnetite concentrates. This process includes several stages: partial reduction of concentrates, magnetic separation, hydrochloric acid leaching of titanium-containing tailings, and alkaline treatment of leach cakes with HCl. With partial reduction, vanadium and chromium are predominantly concentrated in titanium tailings. Then magnetic separation is carried out to separate the iron-containing concentrate with a total iron content of 94.57%. During acid treatment, 90.8% of vanadium and 93.4% of chromium were extracted into the solution, while the loss of titanium was less than 0.3%. Then, with alkali treatment, up to 96.3% silicon was extracted into solution, and a titanium-rich slag with a purity of 93.39% was obtained. The total extraction of iron, titanium, vanadium and chromium under experimental conditions was 88.3%, 93.7%, 81.7% and 84.4%, respectively.
The paper [
29] Partial reduction experiments were carried out in a temperature-controlled muffle furnace (± 5 °C). 120 g of titanomagnetite concentrate was first mixed with pulverized coal and a small amount of Na
2CO
3. The presence of Na
2CO
3 facilitates the carbon gasification reaction and induces the growth of metallic iron particles. The mixture was placed in a sealed silicon carbide crucible, and then it was heated to the desired temperatures in a muffle furnace. After the completion of the reduction experiments, the reduced samples were immediately cooled with water to avoid re-oxidation, and then the resulting intermediate was ground for 30 min. Next the crushed samples were separated by a magnetic separator. The resulting titanium concentrate concentrated vanadium and chromium quite well.
Leaching experiments were carried out in an autoclave. Titanium concentrate was first diluted with hydrochloric acid solution at certain S:L ratios. The autoclave was kept at a temperature for a certain time and then rapidly cooled. The suspension was filtered and the cake was washed with distilled water after leaching. Then it was leached with a dilute solution of NaOH-174.6 g/l, S:L 1:3 at 80 °C for 1 hour. The suspension was filtered and the residue was washed and then dried at 110 °C, after which the resulting intermediate product was calcined at 750 °C for 2 h, with the formation of a titanium-rich slag. According to [
30], when titanium concentrate is leached with hydrochloric acid, silicon is in the form of amorphous hydrated silicon dioxide, which readily dissolves in an alkaline solution of sodium hydroxide [
31].
Compared to existing technologies, the new method has several advantages:
(a) alternative process with higher recovery of iron, titanium, vanadium and chromium;
(b) vanadium and chromium are controlled so that they are concentrated in the titanium concentrate by partial reduction, thereby avoiding the process of melting, converting and calcining, which are carried out at high temperatures;
(c) appears to be more environmentally friendly to some extent, since vanadium and chromium in the reduced samples exist in the V3+ and Cr3+ forms, whereas after simple alkaline treatment, these metals show themselves as V4+ and Cr3+ due to the presence of Fe2+ and small amounts of Fe3+ and their respective redox potentials.
The obtained titanium-rich slag contains 93.39% TiO2, 0.27% CaO and 1.62% MgO, which meets the basic requirements of the chlorination process. However, the amount of non-ferrous metals (V2O5 and Cr2O3) containing 0.54% is considered too high for the chlorination process and does not meet the quality of pigmented titanium dioxide.
The method is proposed [
32,
33] for the enrichment of titanium slag obtained after electric smelting at 1500–1600 °C or reduction roasting at 900–1000 °C, followed by separation of iron, with a composition, wt. %: TiO
2 35-40; Fetotal 5-15; SiO
2 5-15; MgO 2-12; MnO 1-5; CaO
2-10; Al
2O
3 5-15; Cr
2O
3 0.5-5. This method consists in the fact that the slag is subjected to treatment with hydrochloric acid with a concentration of 12-18% at S:L=1:8÷10 (with a total two-fold excess in stoichiometry), followed by processing of the solid product after washing 3-5% sodium hydroxide solution at S:L=1:4÷6. Optimal conditions for leaching time 2 hours and temperature 106-110 °C. The resulting product is hydrated titanium dioxide TiO
2·H
2O with X-ray amorphous structure. In order to convert the product to the rutile form, it is calcined at 800-900 °C.
4.4. Carbothermal Processing Scheme
The technological scheme of processing using carbothermal influence on oxide mineral mixtures is a highly effective way of thermochemical processing of oxide raw materials and is alternative to the chloride or sulfate method [
34,
35,
36]. It allows to achieve effective separation of titanium and silicon components.
It is shown that it is possible to regulate the composition and, consequently, a number of technically important properties of the obtained products. Among the most important results of vacuum carbothermal treatment of leucoxene 50% concentrate should be highlighted the obtaining of nanolaminate materials based on the Ti
3SiC
2 carbide-silicide phase [
34,
37,
38,
39].
Carbothermal reduction of oxide components of leucoxenes by activated carbon was carried out at temperatures up to 1800 K in the range of gas medium pressure from deep vacuum (10-3 Pa) to atmospheric pressure (105 Pa).
The initial composition of leucoxene concentrate %: SiO2 46-52, TiO2 45-50, Al2O3 2-4, Fe2O3 1-3;
It is shown that the carbothermal process allows the targeted formation of submicron ß-SiC and nanolaminate Ti
3SiC
2 phases as a result of two competing reactions:
Silicifying agent in both cases is gaseous SiO. At the initial and intermediate stages of the carbothermal process, SiO is generated as a result of the reduction of silicon dioxide with carbon and lower titanium oxides. The possibility of such interactions under the conditions of a carbothermal process was confirmed by thermodynamic calculations and in experiments with model systems that were carried out in earlier works [
40,
41].
The main reactions of silicon gasification with the formation of SiO are given below:
At the final stages of the carbothermal process, when the conversion of silicon from the oxide to the carbide form is almost complete, the source of SiO is silicon carbide SiC, which can reduce titanium oxides according to the following reactions:
The presence of free carbon in the reaction mixture, as well as an increased CO content in the gas phase, suppresses the silicification of titanium carbide (reaction 5), which leads to a shift of the carbothermal process toward the formation of predominantly submicron ß-SiC. Thus, the initial carbon concentration and the mode of removal of gaseous products (SiO and CO) from the reaction zone are the key factors allowing to regulate the final composition of the products.
As a result, it is shown that during the carbothermic processing of leucoxene raw materials at gas pressures below atmospheric pressure and at an activated carbon concentration in the initial charge from 11 to 17 wt.%, a deep separation of titanium- and silicon-containing components of leucoxene raw materials is ensured.
The composition of the product obtained after carbothermic treatment of leucoxene concentrate %: SiO2 0.4-36, TiO2 55.0-95.4, Al2O3 0.1-3.6, Fe2O3 1.2-4.6;
The disadvantage of this method is that the technology requires high temperatures, therefore, more electricity is consumed. It requires additional processes for post-treatment of titanium dioxide from impurities of silicon, iron and other elements. With the data obtained, there are large discrepancies in the results of the analysis for titanium dioxide and silicon.
4.5. Nitric Acid Opening of Titanium Slags
According to [
42], the slag was crushed to a particle size of less than 50 μm, subjected to magnetic separation to remove metal inclusions, and treated with a 30% nitric acid solution at a temperature of 95 °C, S:L=1:5.5 for 1 hour. The resulting pulp was filtered and a cake containing hydrated titanium and silicon dioxide was separated.
The cake obtained after leaching based on titanium dioxide and silicon was treated three times in 5% NaOH solution at a temperature of 95 °C, S:L=1:5 for 1 hour. The cake was additionally treated with a 5% nitric acid solution to remove sodium impurities. In this case, the extraction of SiO2 from the cake was 97%, and the loss of TiO2 during pulp filtration was 2%. The result was a titanium concentrate composition, wt. %: TiO2 85.4; SiO2 7.82; Al2O3 2.04; MgO 0.40; Fe2O3 2.16; Na2O 0.24.
4.6. Processing of Titanium Slag by Autoclave Alkali Leaching (Cracking or Opening)
In [
43] titanium slag of composition, wt. %: 92.5 TiO
2; Fe total 0.90; MnO 2.82; Al
2O
3 2.17; CaO 0.84; SiO
2 0.64; MgO 0.41 was treated in an autoclave with a sodium hydroxide solution with a concentration of 10 mol/kg H2O at a mass ratio S:L=1:4 and a temperature of 220 °C for 4 h. The pulp was cooled, filtered, washed and dried at 80°C. The obtained Na
4Ti
3O
8 based intermediates were leached in hydrochloric acid solution at pH 0.2 and S:L=1:5, sedimented for 5 h and separated from the acidic solution. As a result, titanium dioxide containing 98.4-99.4 % TiO2 was obtained.
4.7. Alkaline Leaching Process
A technology was proposed for processing slags containing 8–17% TiO
2 [
44] to obtain products that meet the titanium content requirements for the production of pigment titanium dioxide and metallic titanium. It includes two directions. To obtain anosovite concentrate used in the production of pigment by the sulfuric acid method, titanium slag is kept at a temperature of 1300 °C with the addition of a modifier during cooling for four hours. After crushing and grinding, enriched by gravity or anosovite flotation, the sludge is leached with sulfuric acid and alkali. To obtain titanium metal using chlorine technology, the slag is additionally oxidized in the melt, or during annealing with a modifier, then, after crushing and grinding, a rutile concentrate is released.
A new process for the production of titanium dioxide from titanium slag by decomposition with sodium or potassium hydroxide has been proposed [
45,
46,
47]. Ilmenite is decomposed in a concentrated solution of KOH or NaOH at atmospheric pressure and an intermediate product with a high titanium content and a low iron content is obtained.
The decomposition of ilmenite in a concentrated alkaline solution of KOH leads to the formation of potassium titanate (K
4Ti
3O
8) and iron oxide, which proceeds according to the following reaction:
Phase transformation of potassium titanate was carried out by hydrolysis of potassium titanate in an acidic solution of pH 2.0 at 25°C for 60 minutes.
The resulting hydrated titanium dioxide was calcined at a temperature of 400 °С to form crystallization anatase TiO
2. Under such conditions, approximately 95-98% Ti is recovered from titanium slag, in addition, the purity of TiO
2 is 99.3% (
Figure 5).
A similar process of caustic leaching for the production of titanium dioxide from titanium slag is also found in [
48,
49].
Finely ground titanium slag (-61+51 μm) was subjected to interaction with 10 M NaOH with a ratio of S:L 1:4 at a temperature of 220°C for 4 hours. In this case, almost complete dissolution of TiO2 in the form of Na4Ti3O8 was achieved. Titanium dioxide with a rutile structure was obtained by acidification with hydrochloric acid at a temperature of 100 °C, in the pH range of 1.2. Wherein, the purity of TiO2 was 99%.
Compared to other titanium slag recycling processes, the alkaline leaching process is relatively mild and high recoveries are obtained at relatively low temperatures and atmospheric pressure. Consequently, the energy consumption of this technology is lower than existing technologies.
In [
50] proposed a method of enrichment of titanium slag obtained by electric smelting of titanomagnetite concentrate of Tymlai deposit (Kazakhstan), chemical composition, wt. %: TiO
2 52.0; FeO 2.08; SiO
2 15.32; Al
2O
3 8.4; CaO 1.38; MgO 11.8; MnO 0.97; V
2O
5 0.055; Cr
2O
3 0.032; C 4.24. The titanium slag was ground to a particle size of 45 μm. The fusion of titanium slag with sodium hydroxide was carried out at a ratio of TiO
2:NaOH = 1:2 and a temperature of 850 °C. The influence of temperature on the process of aqueous leaching of sinter was carried out in the temperature range of 25-75 °С at the ratio S:L = 1:3.8.
After separating the main amount of alkali, the precipitate was washed twice at a ratio of S:L=1:2.
An increase in the content of titanium dioxide in the resulting intermediate product is possible due to the extraction of impurities into the solution during acid treatment. The influence of the concentration of the solution with hydrochloric acid on the degree of leaching of the titanium middling product was carried out in the concentration range of 63-187 g/dm3 at S:L=1:5 at a temperature of 95 °C for 1.5 hours. Wherein, titanium concentrate of composition wt.% was obtained: TiO2 85.8; FeO 2.26; SiO2 7.66; Al2O3 0.045; Na2O 0.033; CaO 0.041; MgO 0.052; humidity 4.
The silicon content in titanium slag supplied for titanium sponge production should not exceed 3.5%. In this regard, the obtained product based on titanium dioxide had to be purified from silicon. After that the studies on the influence of duration of the process of desiliconization of rutile concentrate and obtaining titanium dioxide of increased frequency were carried out. Wherein, the impact of alkali occurred at a temperature above 90 °C and for the first hour of alkaline treatment silicon almost completely passes into solution. As a result of the conducted researches the optimal conditions of desiliconization of rutile concentrate were determined: ratio S:L=1:6, concentration of sodium hydroxide solution 14-15 g/dm3, temperature of the process 90-95 °С, duration 1.5-2.0 h.
Under optimal conditions, a conditioned rutile concentrate was obtained with the composition wt.%: TiO
2 91-92; FeO 2.3-2.5; SiO
2 1.7-2.0; Al
2O
3 0.008-0.01; CaO 0.06-0.08; MgO 0.07-0.08; Cr
2O
3 0.04-0.06; humidity 4, while titanium dioxide, according to X-ray phase analysis, is represented by a rutile monophase [
50], p. 26.
4.8. Leeds Process
In early 2009, researchers from Leeds University (UK) developed a new environmentally friendly, less time-consuming and cheaper process to produce pigmented TiO2.
The new process consists of three main stages:
1. Roasting of complex ore minerals in air at a temperature of 800-900 °C in the presence of alkali in order to change their chemical structure.
2. Processing with a stream of warm water, followed by leaching of impurities with acid and obtaining by-products of industrial use.
3. The residue after calcination is exposed to the action of chlorine, which is required 20 times less compared to the volumes normally required in industrial conditions.
The Leeds process provides a pigmented TiO2 yield of about 97% (the industry average is currently 85%). The increased yield of the finished product will allow companies to significantly reduce production costs as well as waste disposal costs. The process also utilizes excess heat and carbon dioxide. The carbon dioxide is used to regenerate the alkali. According to the developers of this method, the technology can be improved to increase the TiO2 yield up to 99%. Additionally, the process can be used to separate TiO2 from ores with different degrees of enrichment.
Leeds specialists are working in an industrial partnership with Millennium Inorganic Chemicals to refine the technology to commercial use.
Currently, the world's capacity for the production of titanium dioxide by the chloride method exceeds the capacity of the sulfate method and continues to grow. In the CIS, about 97% of the total volume of titanium dioxide is produced by the sulphate method followed by hydrolysis and calcination (JSC Sumykhimprom and CJSC Crimean Titan, Ukraine). Vapor-phase hydrolysis of titanium tetrachloride is used at OJSC Solikamsk Magnesium Plant (Russia, Solikamsk, Perm Territory), and was used until recently at AVISMA (branch of OJSC VSMPO-AVISMA Corporation, Berezniki, Perm Territory). JSC "Khimprom" (Volgograd) used the method of processing (combustion) of titanium tetrachloride by the plasma-chemical method [
31,
32].
The advantages of the chloride method of pigment production over the sulfate method are in much smaller amount of waste to be neutralized, slightly higher quality of the product, as well as lower specific capital investments, which are 60-75% of investments in the sulfuric acid method.
In spite of the fact that more expensive raw material - rutile - is used for the chloride method, the cost of 1 kg of pigmented titanium dioxide obtained by the chloride method is ultimately less than by the sulfate technology.
Since in chloride technology there are high requirements to initial titanium raw materials limiting the content of impurities, in recent years, as an alternative, hydrometallurgical methods of obtaining pigmented titanium dioxide have been developed. Many of them consist in acid leaching of titanium-rich raw materials.
4.9. Methods for the Processing of Titanium Slag by Preliminary Roasting and Its Further Leaching with Various Reagents
Other studies are based on the roasting of low titanium slag with various alkaline reagents.
Roasting is one of the common metallurgical processes used to separate metals in the processing of raw ores or intermediate products such as titanium slag. Alkaline firing is widely used. The role of alkali roasting is twofold: first, to chemically separate titanium dioxide from silicon compounds and other impurities; secondly, soluble sodium metal salts are formed during alkaline roasting, which can be removed in subsequent aqueous leaching steps. Elemental impurities such as aluminum and silicon form readily soluble compounds that are washed out, while iron, magnesium and calcium form insoluble compounds that are removed by acid leaching.
When titanium slag is sintered with sodium hydroxide, titanium, silicon, vanadium and aluminum form titanates, vanadates, silicates, aluminates, which dissolve in water, while titanium remains in the precipitate, which dissolves in sulfuric or hydrochloric acid with further isolation of titanic acid. The resulting product is converted into a white titanium dioxide pigment by calcination [
51,
52].
A new technology has been developed for the industrial production of high-quality pigment titanium dioxide [
53], composition, wt. %: TiO
2 78.5; Fetotal 7.73; Al
2O
3 2.36; CaO 0.66; MgO 5.57; MnO 0.30; SiO2 2.75; Cr
2O
3 0.21. This technology includes alkaline roasting, leaching with a solution of hydrochloric acid with a concentration of 2 mol/dm
3 and calcining the titanic acid to produce pigmentary titanium dioxide.
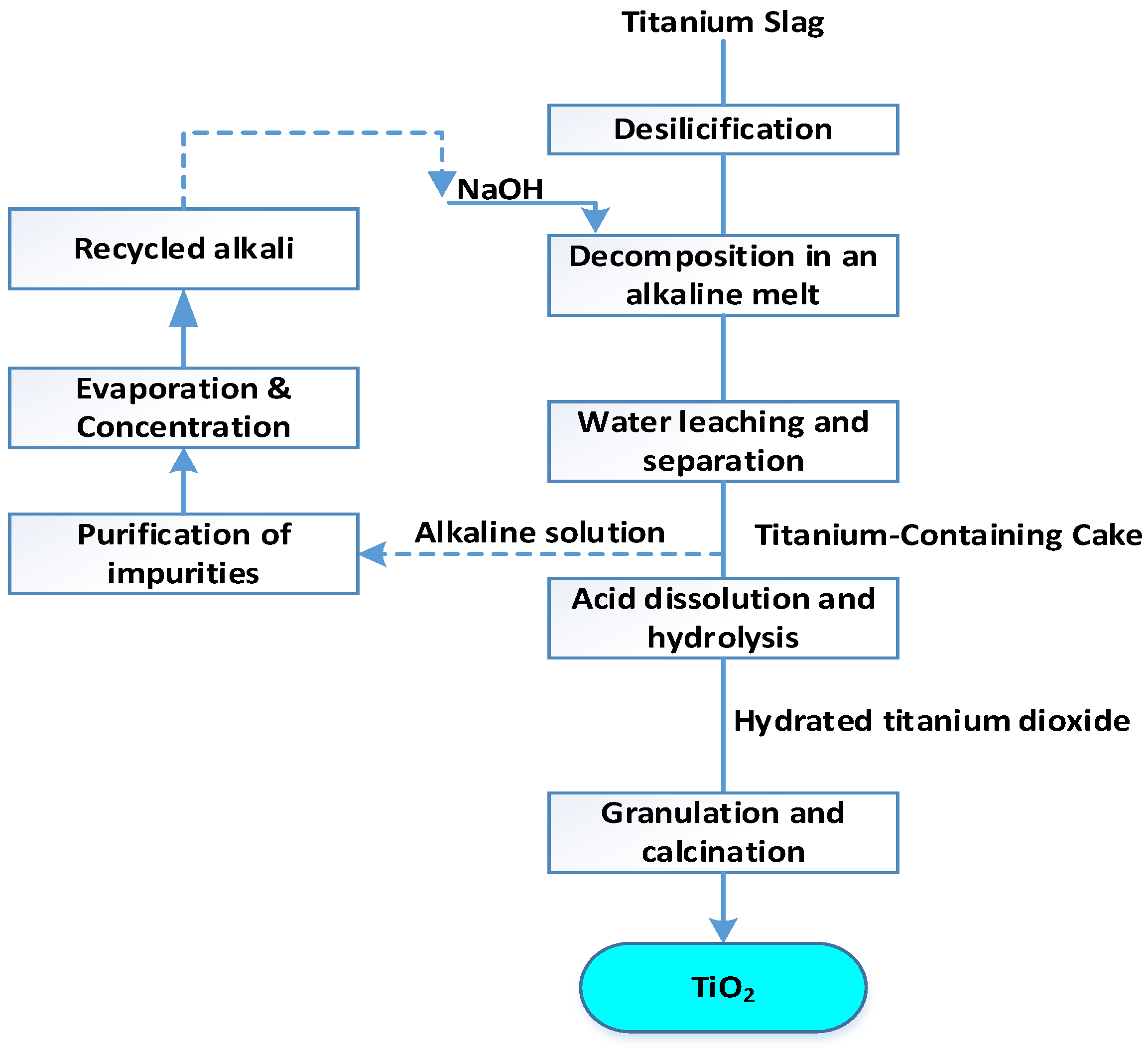
The most interesting technology is the production of pigment titanium dioxide from slag from the processing of titanomagnetite concentrate [
54,
55]. According to the technological scheme (
Figure 6), titanium slag decomposes in an alkaline melt of sodium hydroxide with the formation of sodium titanate, silicate and aluminate at a temperature of 500 °C for 60 minutes and in a ratio of alkali:slag = 1:1.
The resulting cake is leached with water at a temperature of 50 °C, the ratio of S:L=1:5, for 20 minutes. Vanadium, partially aluminum, silicon and manganese pass into the solution. The resulting solution is returned to the slag roasting after purification from impurities and concentration. The washed titanium-containing precipitate is dissolved in a 20% sulfuric acid solution to obtain a titanium oxosulfate solution.
After purification, the sulfate solution is thermally hydrolyzed and titanic acid H2TiO3 is precipitated, which is washed until the main impurities are removed. This is followed by calcination at 800 °C to obtain pigmentary titanium dioxide.
The disadvantage of this method is the long duration of the process, the low degree of extraction of titanium dioxide and the high content of silicon and iron in the final product.
4.10. Processing of High-Titanium Concentrates of Titanomagnetite Ores
The complexity of processing titanomagnetite ores and concentrates is that they are refractory and difficult to recover. When dealing with such materials, it is imperative to establish conditions for primary solid-phase iron reduction, ensuring that the rate of iron oxide reduction surpasses the rate of slag formation and charge melting.
An important aspect of the problem is also the search for solutions for the hydrochemical enrichment of slag to a condition (≥ 80% TiO2) suitable for chlorination or the production of pigment titanium dioxide.
During reduction-metallizing firing, titanium oxide is partially reduced with the formation of lower oxides. This results in the formation of titanium slag. The lower oxides of titanium dissolve ilmenite well, which makes it difficult to restore residual amounts of iron. In addition, lower titanium oxides are very refractory; slags with a high content of the latter are characterized by an increased melting point. All this indicates that the processes of reduction of titanomagnetite concentrates should be carried out in such a way as to separate the processes of iron reduction and the formation of a slag melt.
This problem can be solved by organizing a two-stage method for producing titanium slags. At the first stage of the process, the solid-phase reduction of iron oxides is carried out, and at the second stage, the process of melting the pre-reduced material with the separation of slag and alloy. An analogue of this technology is the ITmk-3 iron production process developed by Kobe Steel, Ltd (Japan), in which the agglomerated raw material is reduced in a rotary furnace, and then the metal pellets are separated from the slag on a screen. The disadvantage of the ITmk-3 method is the use of lime and silicate fluxes with the formation of refractory slags that impede the coagulation of iron and impoverish the slag in titanium.
Due to the difficulty of reducing and refractoriness, high-titanium titanomagnetites cannot be melted in blast furnaces, and their direct electrothermal reduction melting is associated with process instability, melt boiling, poor separation of cast iron from slag, etc. Melt instability and effervescence are the result of intense reduction of FeO in the liquid phase with the release of a large amount of CO gas, which swells viscous titanium-containing slags. The necessary conditions for obtaining stable slags can be created only with a controlled recovery period that occurs at the solid stage before the development of the slag formation process.
In particular, soda is added to the reduction charge [
56,
57,
58], which is both a reduction catalyst and a flux that reduces the viscosity of the slag. The introduction of sodium oxide into the mixture leads to the formation of low-melting sodium titanates (Na2TiO3 with a melting point of 1030 °C; Na
2Ti
2O
5 – 985 °C; Na
2Ti
3O
7 – 1128 °C). The influence of soda additives (1.5-2.5%) on phase transformations during the solid-phase reduction of a poor (3% TiO2) titanomagnetite concentrate with hydrogen in the temperature range of 700-1200 °C was studied [
58]. It is shown that at the final stage of the process (i.e. at 1200 °C) Na
2O is consumed mainly for the binding of SiO
2 into aluminosilicates with the displacement of FeO, MgO, CaO from the silicate phase, which leads to an acceleration of the reduction of iron, and an excess of Na2O forms sodium titanates with TiO
2. To increase the reducibility of titanomagnetite, it is recommended to pre-calcinate the concentrate to hematite, while mixing alkaline additives into the charge as a flux and reduction catalyst [
59].
In this work [
60,
61], a two-stage technology for the reduction melting of titanium magnetite concentrate (TMC) with soda additives was developed, both without oxidation and with preliminary oxidation of TMC to hematite. The optimal technological parameters were determined: the consumption of special coke 8% or the ratio C/Fe= 0.103; consumption of Na
2O - 3%; solid-phase reduction temperature 1250 °C, exposure 50 min; melting temperature 1650 °C, exposure - 35 min. Both options have almost equal and high rates: iron yield from TMC ~ 55%, slag yield 23.3-25.8%, content in carbon-free slag, wt %: Fe=1.0-1.6; TiO
2=62.7-61.9; Na
2O-5.85. The output of TiO
2 in the slag is 89.6-94.1%, the degree of sublimation of Na
2O is 56.5% in the first and 51.9% in the second. Cast iron contains, %: 5.51 C; 0.36 Ti; 0.35 Mn; 0.04 Si; 0.23 V. The output of vanadium in cast iron is 53.0%.