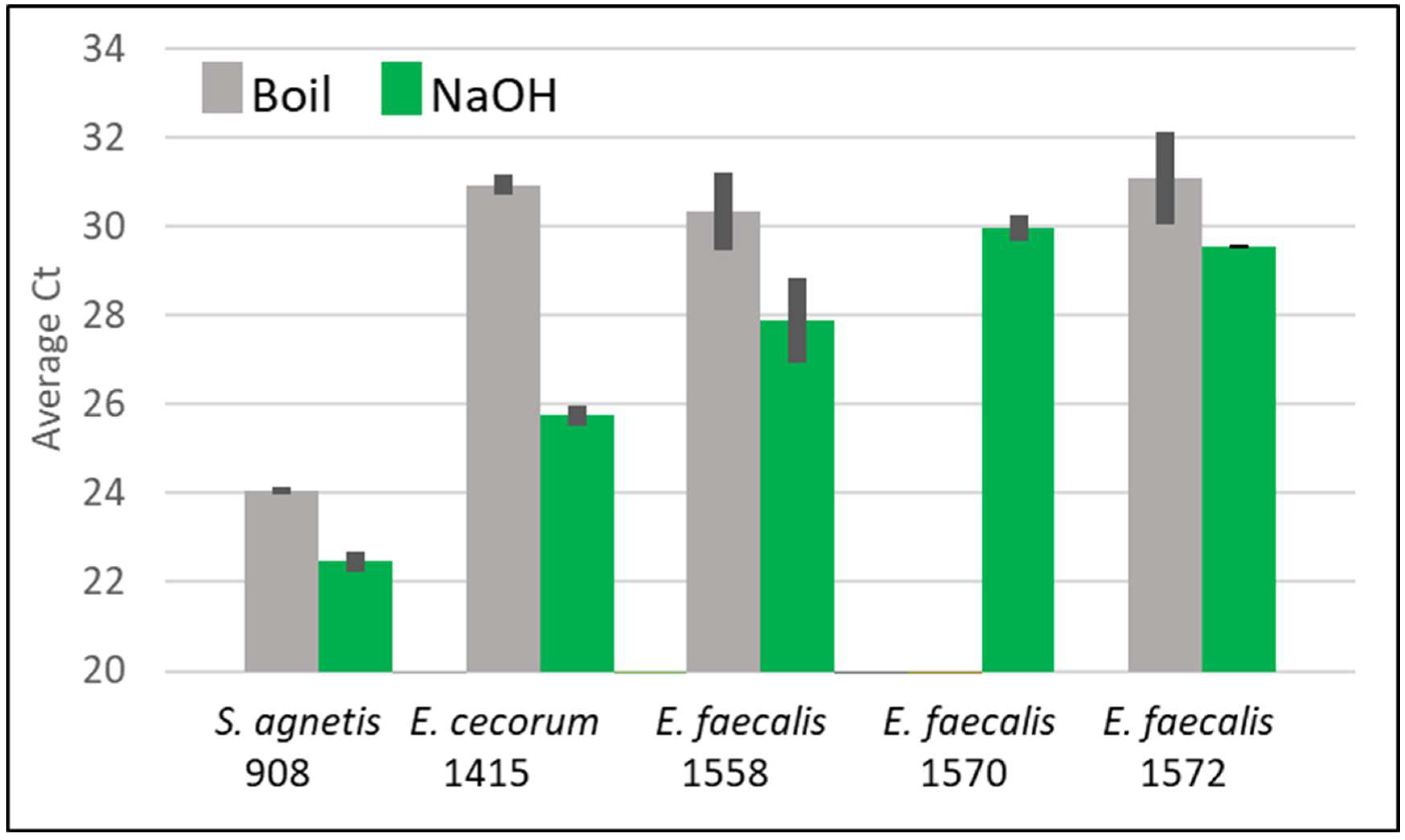
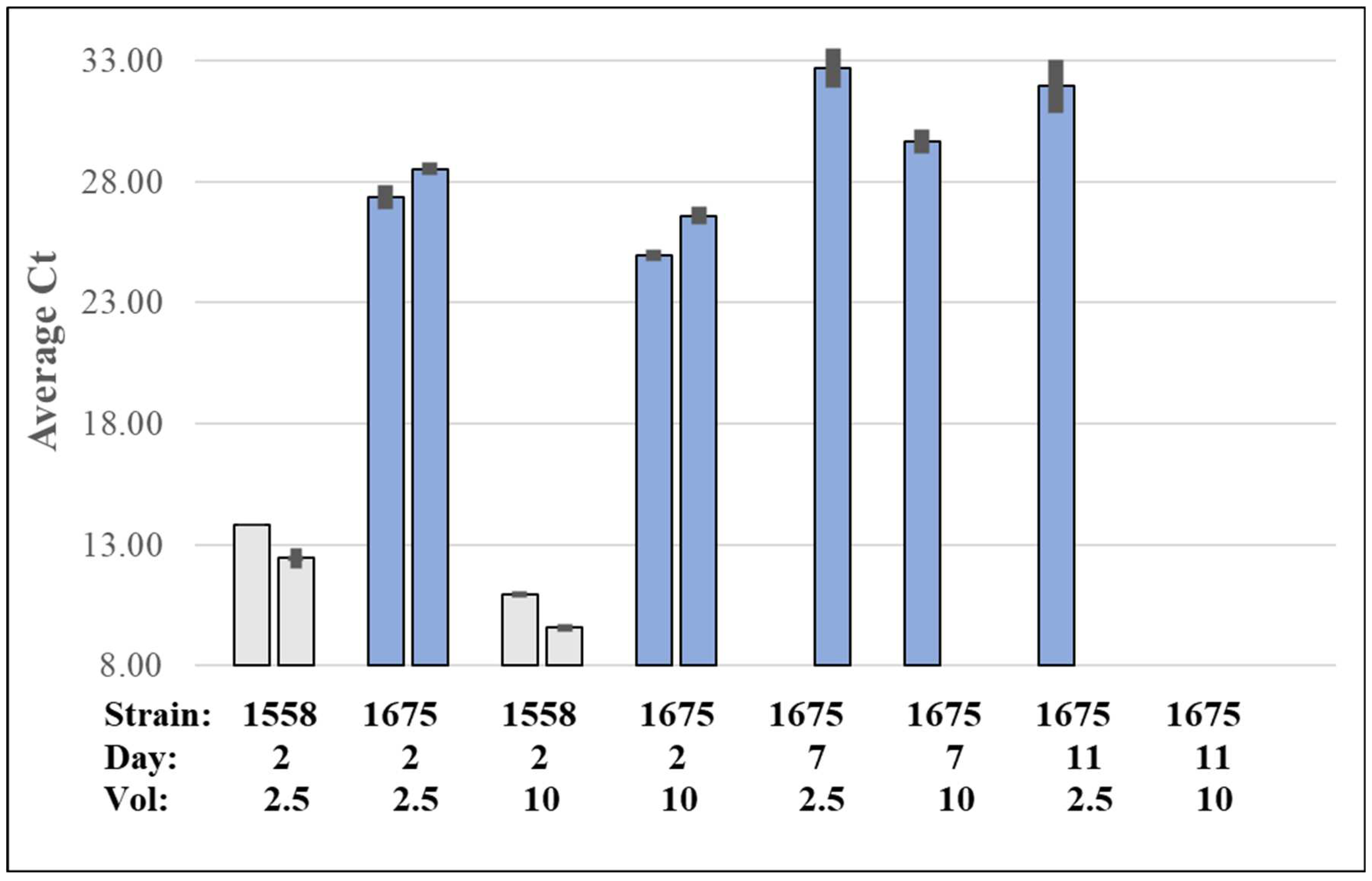
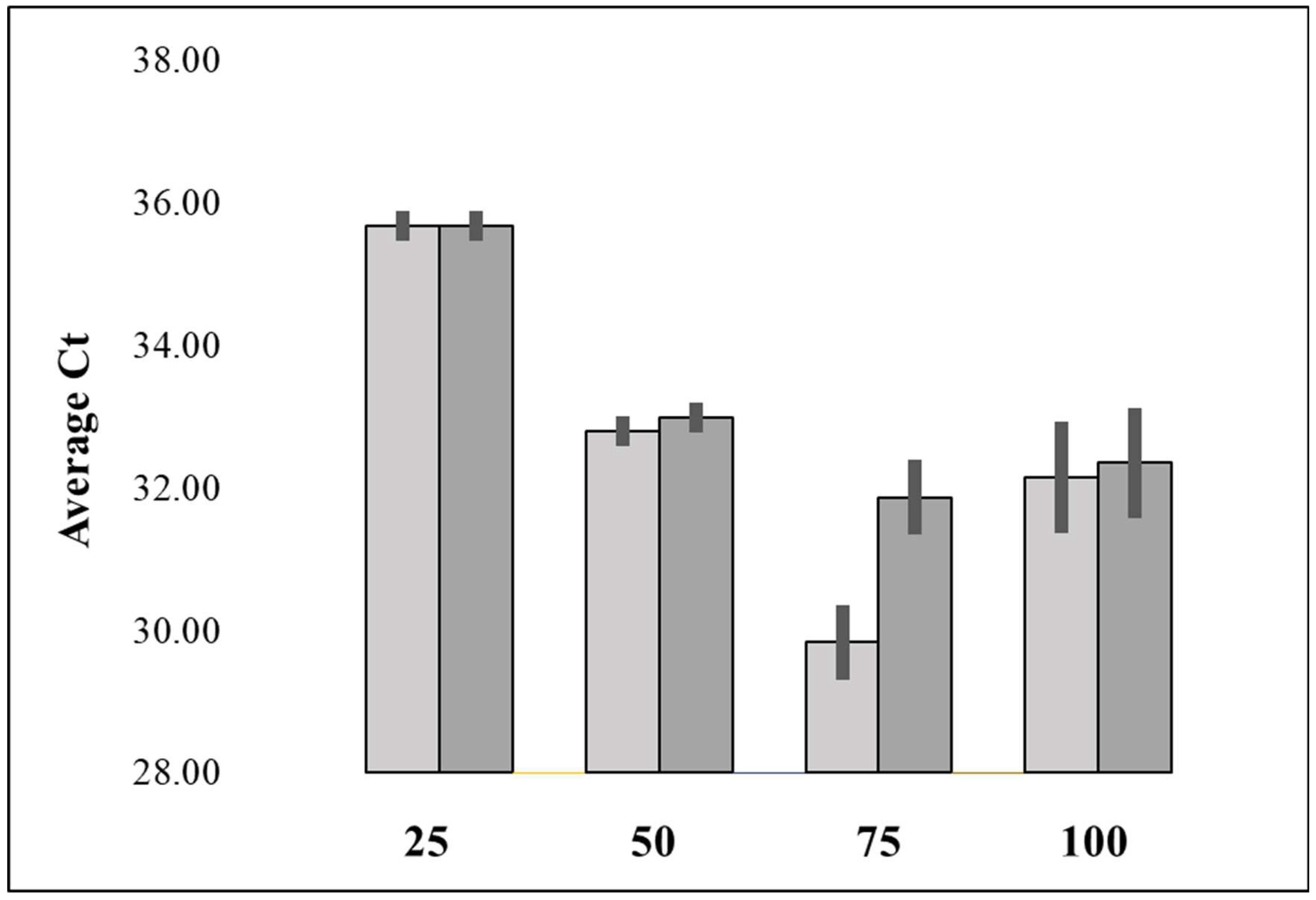
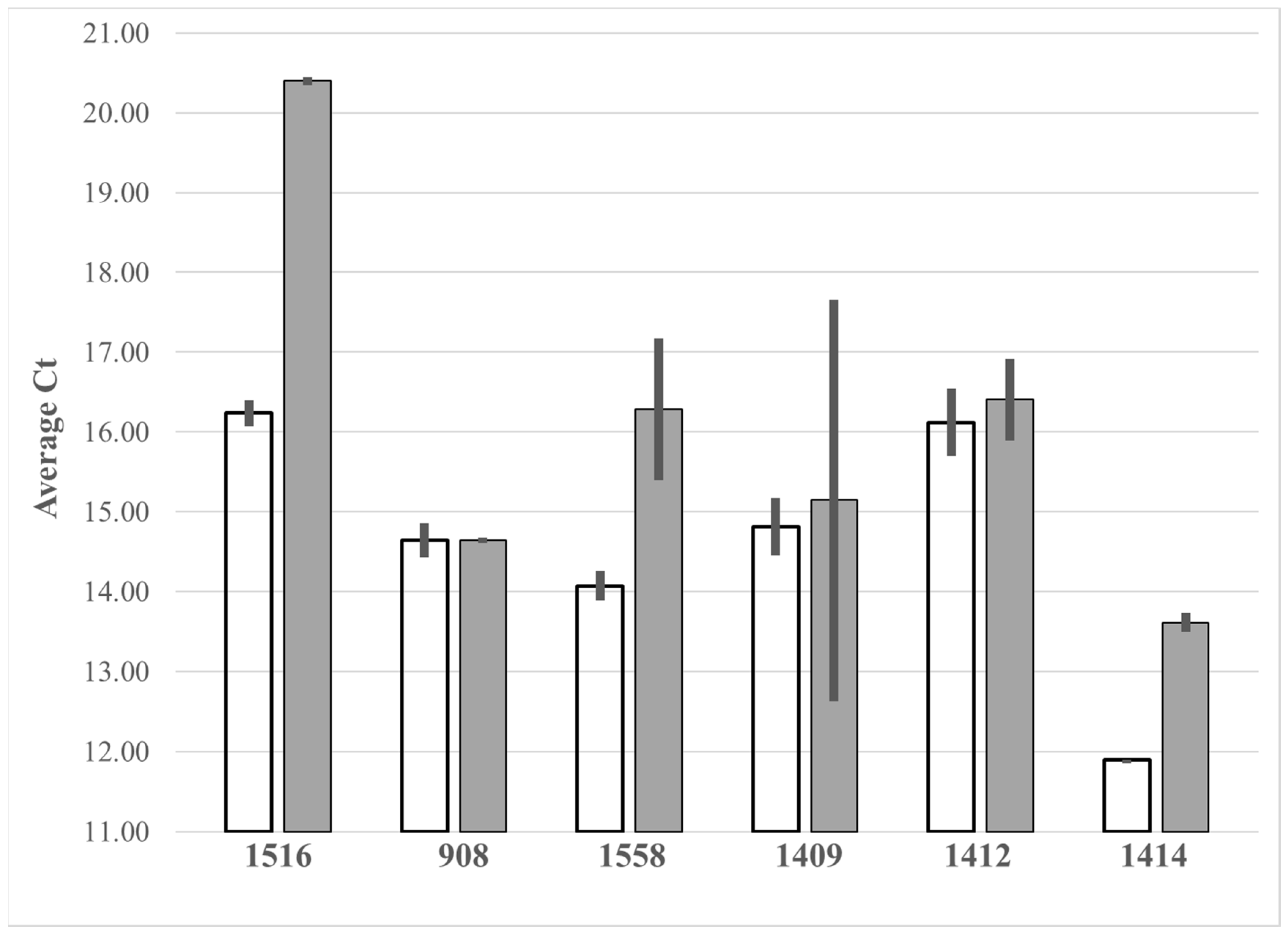
Introduction
Reliable and accurate molecular diagnostics are crucial for the detection of pathogenic bacteria in clinical, food, and environmental samples [
1,
2,
3]. In the study of bacterial genomes, one of the most important steps is the isolation of high-quality genomic DNA. There are various methods and commercial kits available for the rapid extraction of bacterial DNA from bacterial cells for polymerase chain reaction (PCR) [
4,
5,
6,
7]. To identify genomic sequences, the cells need to be lysed by heat treatment, physical, detergent, enzymes, pulverization, pH, chaotropic chemicals, or a combination of these, which is then followed by either chaotropic-based fractionation, phenol extraction, or size exclusion column purification [
8,
9,
10,
11,
12]. Purification of the extracted DNA can involve precipitation or column-based nucleic acid capture. Traditionally, DNA extraction from cultured bacteria often includes expensive, and complicated enzyme combinations or costly hazardous chemicals such as phenol, or chloroform [
13,
14,
15,
16]. These methods vary in effectiveness depending on the application and the matrix from which the cells are obtained [
17]. Although current techniques are well proven to produce high-quality nucleic acids (DNA or RNA), they are time-consuming, labor-intensive, and expensive to implement. In addition, the quantity of genomic DNA that they produce could be insufficient. These limitations become much more significant in settings with limited resources. As a result, the implementation of molecular testing is considerably hampered in many regions of the globe, especially in developing countries [
12]. Therefore, the development of rapid, easy-to-use, and more efficient DNA extraction techniques that do not rely on complex laboratory equipment facilitates the advancement of molecular clinical detection technologies.
Recent years have seen the development of novel approaches for extracting DNA from plants [
18], mouse tails [
19], microbes from sediments [
20,
21], and fungal and oomycetes samples [
22] using NaOH alone or in combination with other chemicals. Therefore, it has been proven that NaOH is capable of effectively lysing a variety of cell types from diverse biological materials within 10 minutes. The extraction would be most effective using NaOH, which would also inactivate nucleases during the extraction process. In most of these treatments, NaOH was administered along with reagents including sodium dodecyl sulfate or sodium acetate [
18,
23], or at elevated temperatures to disrupt cell walls, especially for Gram-positive bacteria [
21,
24]. Hence, these treatments complicate the protocol with additional PCR inhibitors or other time-consuming steps. Consequently, a fast DNA extraction procedure that consistently produces high DNA yields and can be applied to both Gram-positive and Gram-negative bacteria without the use of additional chemicals or enzymatic treatments would be beneficial in terms of saving time and money, while allowing for increased DNA yields.
The goal of this research was to develop an inexpensive technique for rapid lysis of bacteria that can be carried out with minimal laboratory equipment and used for subsequent DNA-based diagnostics. Our impetus was monitoring for specific bacteria in the environment during our lameness trials. In these trials we have shown that bacterial chondronecrosis with osteomyelitis (BCO) lameness can spread through the air from one pen to others [
25,
26]. We had previously used open agar plates for culture-based sampling of air during lameness trials [
25]. However, culture-based sampling is less efficient than qPCR analysis with genus- or species-specific primers. We found that we could use a standard glass impinger to sample air for viable bacterial plate counts in our lameness trial facilities. However, extracting DNAs from these same samples was more problematic, especially for some species of concern in our experiments. We have found that simple alkaline extraction can be effective for liberation of DNA from many Gram positive and negative bacteria. The alkaline extract can either be diluted sufficiently for direct qPCR or the DNA can be directly captured from the alkaline solution using paramagnetic beads. The extraction method was effective for a variety of different environmental samples, including air, soil, sewage water, food, environmental surfaces, and chicken cloacal swabs, as well as broth and plate cultures. The optimized DNA preparation technique could be a substitute for the current expensive protocols, and commercial DNA extraction kits for the detection of various bacterial targets.
Materials and Methods
Reagents and Materials
Purified water was >18 MΩ from a Barnstead™ GenPure™ Pro Water Purification System (Thermo-Fisher Scientific, Waltham, MA, USA). Sterile pure water (SPW) was autoclaved purified water. Stocks of 1 M NaOH were prepared in SPW from solid (S318-3, Thermo-Fisher Scientific). Sterile swabs were autoclaved cotton tip swabs (Puritan LLC, Guilford, ME). Rectal samples were collected with Opti-Swab® Liquid Amies Collection & Transport System (Puritan LLC). Paramagnetic beads were Mag-Bind RXN Pure Plus (Omega Bio-Tek, Norcross, GA, USA), and PureSil-Silica Magnetic Beads (BioChain, Newark, CA, USA). The Mag-Bind RXN Pure Plus are provided in binding buffer. For the PureSil-Silica beads the bead binding buffer (BBB) was 80% isopropanol, 800 mM guanidine isothiocyanate, 8 mM TrisCl pH8, 3.52 mM Na2EDTA (BioChain). The commercial kit for DNA extraction from rectal fecal swabs was PowerLyzer PowerSoil DNA Isolation Kit (Qiagen, Hilden, Germany).
Bacterial Cultures and Growth
Bacterial strains are listed in
Table 1. Bacterial stocks were stored at -80
oC in 40% glycerol. Media included: CHROMagar Orientation (CO) and CHROMagar
Staphylococcus (CS) (DRG International, Inc., Springfield, NJ); tryptic soy broth (TSB) and nutrient broth (NB) (Difco Laboratories, Franklin Lakes, NJ). Difco bacteriological agar was added at 1.5% for solidification, when necessary. Overnight cultures of were diluted 1:100 in broth. Absorbance at 650 nm was used to compute the cell density based on a pre-calibrated formula:
CFU/ml = (A650 x 109) + 106
Statistical Analyses
Student T-Test was used to compare means of Ct values from qPCR, where significant difference was assumed at P<0.05.
Direct Dilution Method
The NaOH lysate was diluted with four volumes of Te, and then 2 µl of the DNA was directly used per 20 µl PCR. The extracts were stored at -20 oC.
Paramagnetic Beads DNA Purification Procedure
NaOH lysate (100 µl) was mixed with either an equal volume of Mag-Bind RXN Pure Plus, or with 95 µl of bead binding buffer and 5 µl of PureSil-Silica beads, at RT in a 0.5 ml microfuge tube. The suspension was mixed by vortexing for 30 seconds, incubated at RT for 5 minutes, and then captured on a magnetic stand. The supernatant was pipetted off and discarded. The captured beads were rinsed twice for 1 minute with 200 µl of 70% ethanol in the stand, followed by supernate removal with a pipet. The 0.5 ml tube was then opened while on the magnetic stand for 5 minutes to air dry. The tube was removed from the magnetic stand. Elution solutions were either Te (10 mM TrisCl 0.1 mM EDTA pH7.5), TE (10 mM TrisCl 1 mM EDTA pH7.5), 10 mM Tris pH 8.0, or SPW, as indicated. The elution volume, as indicated, was added to the 0.5 ml tube, vortexed for 30 seconds, incubated at RT for 5 minutes, and then beads were captured on the magnetic stand. The eluate was then transferred to a new 1.5 ml tube then either stored at -20 oC, or directly assayed by PCR/qPCR.
Results
Air-Sampling during Bacterial Infection Lameness Trial
We had previously found that waving open agar plates for several minutes could capture 50-300 CFUs during our experiments [
25,
28]. The primary airborne bacteria during these experiments is typically
Staphylococcus saprophyticus [
25] while similar sampling on a commercial broiler farm was primarily
Staphylococcus cohnii [
28]. In order to improve the sampling and provide better quantitative data, we evaluated using a sterile impinger containing 20 ml of 0.9% saline, and a battery-operated pipet pump as a portable air sampler for collecting viable bacteria during our BCO lameness trials. With the portable pump and impinger, we found that total CFU increased with the length of time sampled on a given day, and generally increased with day of the experiment implying that the bacterial load in the air was increasing as the birds matured and the bacterial infection spread (
Table 1). As we were interested in using qPCR to determine the prevalence of specific bacterial genera/species, we tried several standard methods for rapid liberation of PCR-ready DNA. These included boiling [
29,
30], sonication [
31], and glass bead-beating [
32]. The qPCR results were either negative or variable even though we had viable bacteria present in the sample (
Table 1). Therefore, we evaluated other methods for liberating PCR-ready DNA from bacterial broth and plate cultures.
Table 1.
Air samples, bacterial counts, extraction method and qPCR results during a 56 day lameness trial in Spring 2021. Samples were collected using the impinger on the indicated day for a given sampling duration (Minutes). For each sample the resuspended pellet was divided for serial dilution for plate counts and for DNA extraction. Total CFU is the computed total viable bacteria in the original sample. Extraction indicates the method used (see text) for that sample where NaOH+MB is NaOH extraction with Magnetic Bead capture and elution. qPCR positive indicates whether that extract produced a specific product for on qPCR-HRM, where NS signifies no specific amplification based on HRM. Avg Ct 16S presents the average Ct (±SEM) from triplicate samples using 8Fx936R 16S primers where ND (Not Determined) indicates species-specific qPCR primers were used.
Table 1.
Air samples, bacterial counts, extraction method and qPCR results during a 56 day lameness trial in Spring 2021. Samples were collected using the impinger on the indicated day for a given sampling duration (Minutes). For each sample the resuspended pellet was divided for serial dilution for plate counts and for DNA extraction. Total CFU is the computed total viable bacteria in the original sample. Extraction indicates the method used (see text) for that sample where NaOH+MB is NaOH extraction with Magnetic Bead capture and elution. qPCR positive indicates whether that extract produced a specific product for on qPCR-HRM, where NS signifies no specific amplification based on HRM. Avg Ct 16S presents the average Ct (±SEM) from triplicate samples using 8Fx936R 16S primers where ND (Not Determined) indicates species-specific qPCR primers were used.
| Day |
Minutes |
Total CFU |
Extraction |
qPCR
positive |
Avg Ct 16S |
| 17 |
3 |
4100 |
Boil |
Yes |
ND |
| 17 |
10 |
8825 |
Boil |
Yes |
ND |
| 20 |
15 |
12205 |
Boil |
NS |
|
| 20 |
30 |
21050 |
Boil |
NS |
|
| 21 |
20 |
4500 |
Bead beating |
NS |
|
| 27 |
20 |
35200 |
Sonication |
NS |
|
| 29 |
20 |
4000 |
Sonication |
NS |
|
| 35 |
20 |
17200 |
Sonication |
NS |
|
| 42 |
20 |
6000 |
NaOH+sodium acetate |
NS |
|
| 42 |
20 |
6600 |
NaOH+MB |
Yes |
23.9±0.03 |
| 44 |
20 |
20000 |
Boil |
NS |
|
| 44 |
20 |
29300 |
NaOH+MB |
Yes |
22.0±0.1 |
| 46 |
20 |
46400 |
Boil |
NS |
|
| 46 |
20 |
20000 |
NaOH+C2mimOAc |
Yes |
20.8±0.1 |
| 48 |
20 |
16000 |
C2mimOAc |
NS |
|
| 48 |
20 |
20000 |
NaOH+MB |
Yes |
23.1±0.1 |
| 50 |
20 |
8000 |
NaOH+MB |
Yes |
24.4±0.2 |
| 50 |
20 |
12000 |
NaOH+MB |
Yes |
20.5±2.4 |
| 52 |
20 |
9000 |
NaOH+MB |
Yes |
23.2±0.6 |
| 52 |
20 |
5000 |
NaOH+MB |
Yes |
23.3±0.6 |
| 54 |
20 |
12200 |
NaOH+MB |
Yes |
23.2±0.5 |
| 54 |
20 |
12500 |
NaOH+MB |
Yes |
17.4±2.0 |
| 56 |
20 |
20000 |
NaOH+MB |
Yes |
19.7±2.4 |
| 56 |
20 |
52900 |
NaOH+MB |
Yes |
21.8±0.3 |
Discussion
The use of 100 mM NaOH to lyse bacteria and extract bacterial DNA from several different environmental samples presents an inexpensive method. Many of these extracts can be directly subjected to qPCR by 5 fold dilution where the final DNA sample is less than or equal to 1/10
th of the qPCR volume. For some samples the sensitivity is improved through capture of the liberated DNA using silica-coated paramagnetic beads. Shi
et al. [
47] determined that DNA has two major binding mechanisms for silica at pH>3: through the phosphate backbone and hydrophobic binding of the DNA bases. Single-stranded nucleic acids bind to silica with greater affinity than double-stranded nucleic acids because the hydrophobic bases are exposed and because single-stranded DNA is more flexible than double-stranded DNA, maximizing contacts. Typically, nucleic acids are driven onto silica using chaotropic salts that dehydrate the nucleic acid and silica, but some amino acid buffers have been also demonstrated to comparably facilitate nucleic acid binding to silica [
48]. We have used a recommended bead binding buffer (BBB- see methods) where a 50:50 mixture of 100 mM NaOH and BBB is pH 12.9. Surprisingly, some DNA will bind to the beads in 100 mM NaOH with no addition of BBB. However, more DNA is recovered from bacterial lysates by addition of at least 0.5:1 BBB to 100 mM NaOH. Presumably alternative binding buffers could also be compatible for DNA capture from high pH solutions.
We compared the alkaline lysis method with different rapid methods for DNA extraction from bacterial cells collected from air samples. These included rapid boiling; sonication; bead-beating; enzyme digestion; and C2minOAc. The 100 mM NaOH protocol was almost always successful for broth bacterial cultures, and somewhat less successful for toothpick samples of bacterial colonies. We had previously used a 10 minute 100
oC treatment for toothpick samples of bacterial colonies with routine success but that suffered from over or under-sampling of the colony [
26,
49]. The 100 mM NaOH extraction seems to be much less susceptible to “over-load”. We also successfully used the 100 mM NaOH extraction protocol to extract DNA from samples from air, garden soil, chicken cloaca, food, environmental surfaces, sewage, pond water, and pig rectal swabs. This technique provides a high degree of reliability, short incubation time, and produces readily amplifiable PCR substrates. NaOH stocks are stable at ambient temperature and the extraction requires no organic solvents, inhibitory compounds, expensive enzymes, or special equipment (shakers, mixers, heaters, etc.). Of note, assessment of animal microbiome is related to animal nutrition and health, and is a research topic of many scientists [
39,
40,
44,
50,
51,
52,
53,
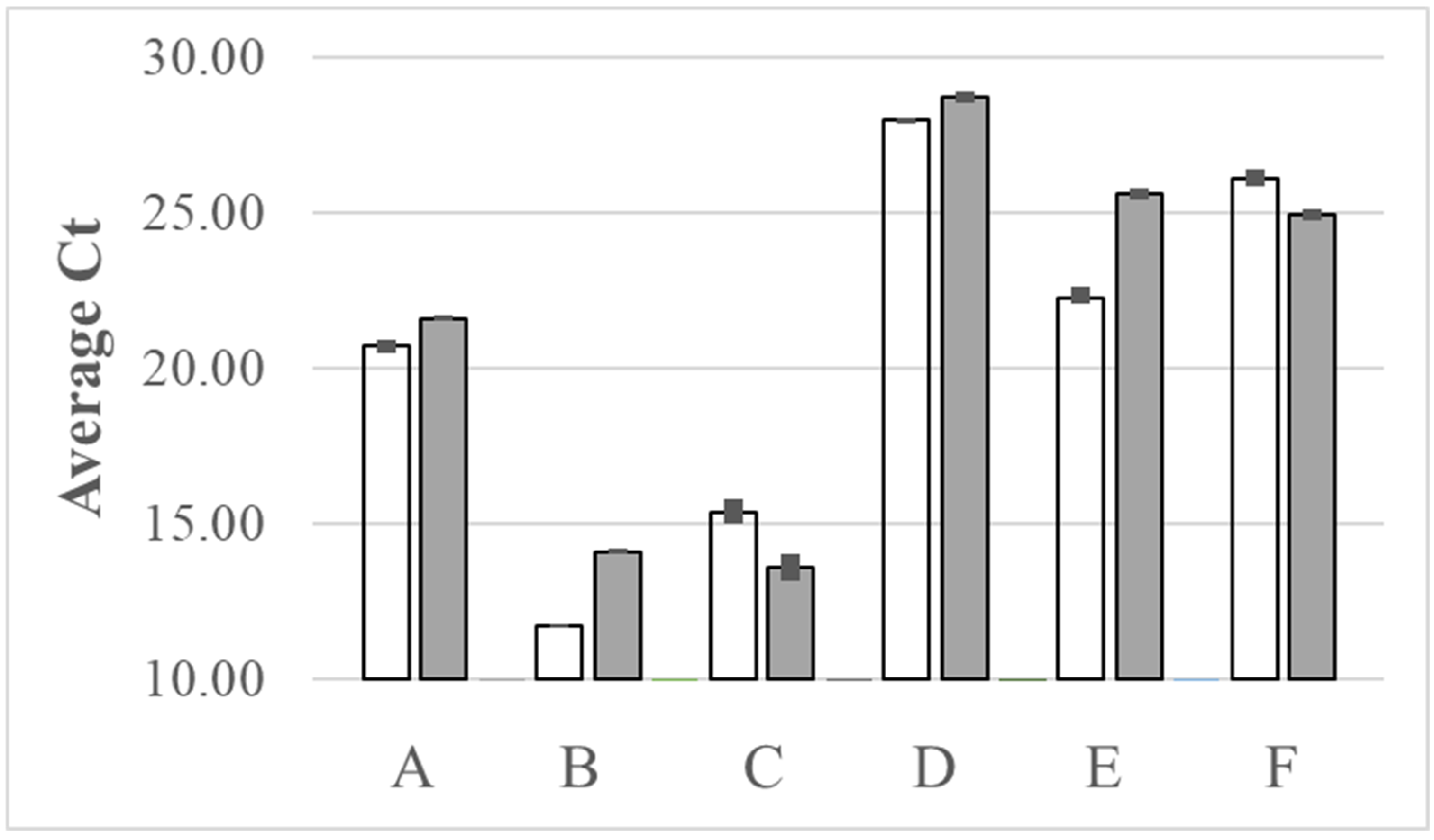
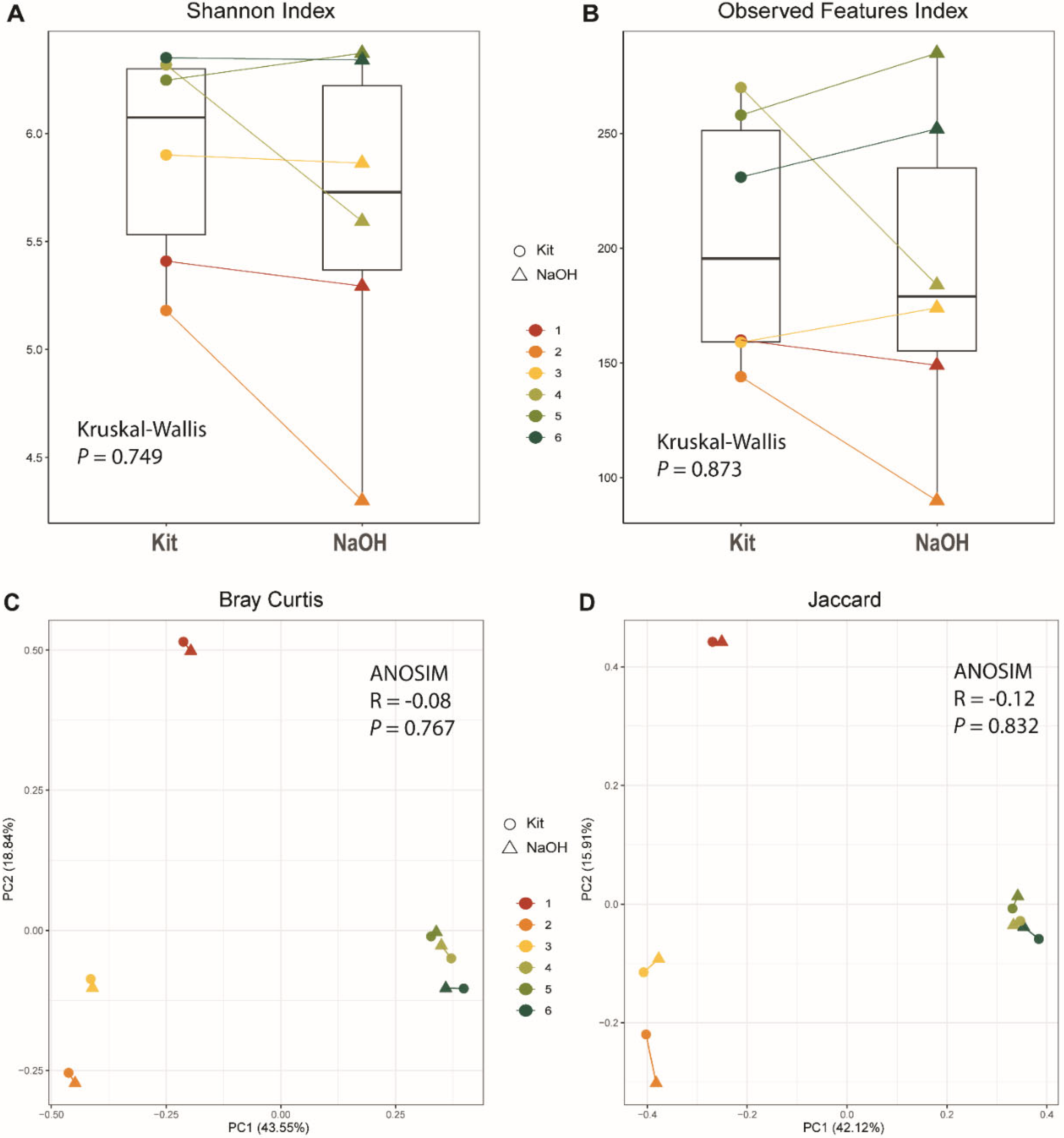
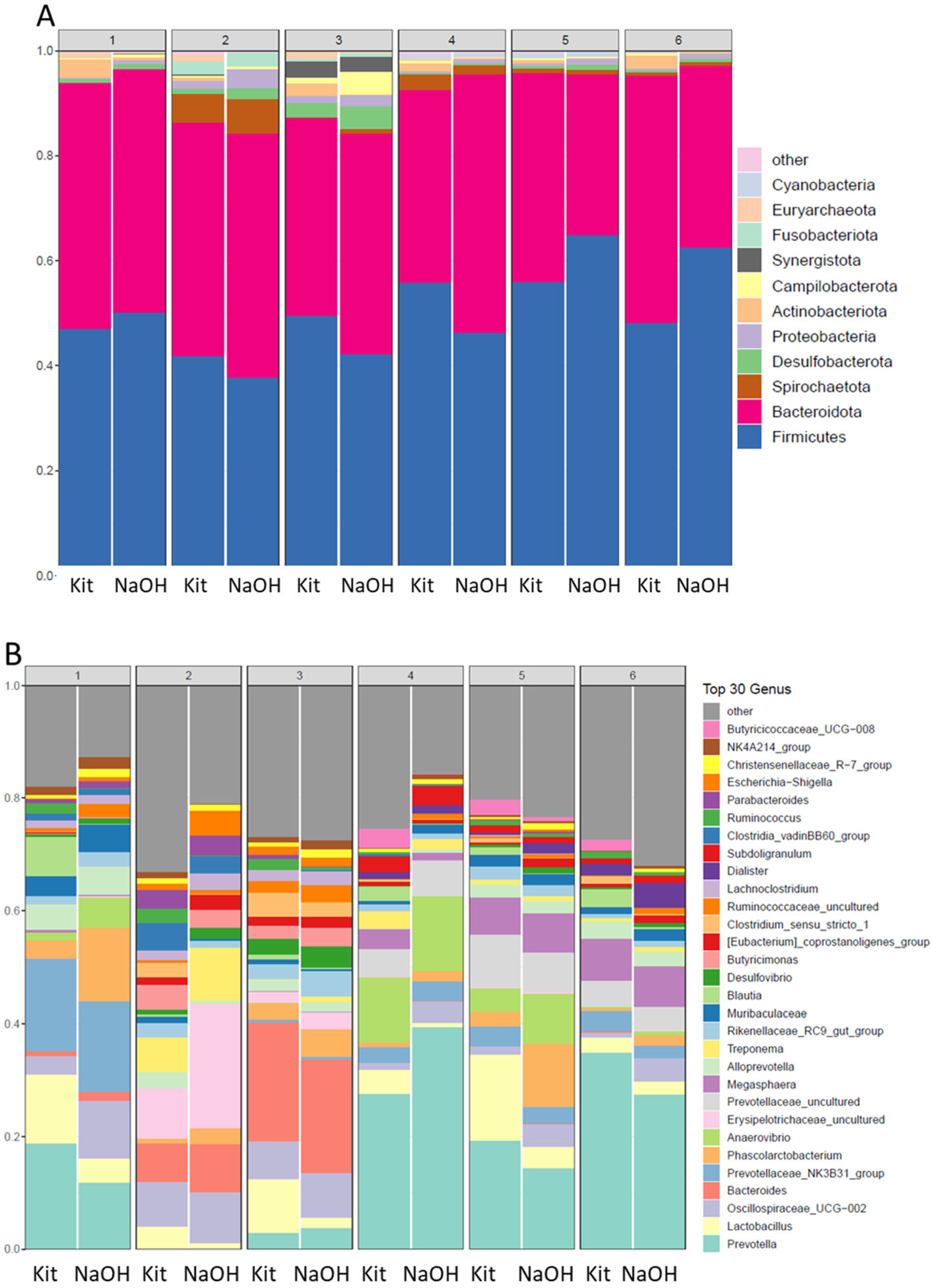
54]. Efficient DNA extraction from specimens such as fecal swabs is critical to reveal microbial composition and structures. Interestingly, the paired swab microbiomes illustrated by the two DNA extraction methods, a commercial kit, or the NaOH extraction, were very similar in terms of community composition, overall alpha and beta diversities. Thus, the inexpensive NaOH-magnetic bead extraction technique could be used to replace the more expensive and time-consuming commercial kit(s), for microbiome studies.
One disadvantage of NaOH DNA extraction might be in converting the dsDNA to ssDNA, which cannot be quantified using the high sensitivity quantification methods, such as fluorimetry. However, the liberated DNA can be easily quantified using qPCR. The NaOH method of DNA extraction has significant importance when extracting DNA from any bacterial species that cannot be cultivated, as might be present in sampling air, soil, sewage or feces. The extracted DNA can be utilized for conventional or quantitative PCR with relative ease, and it may also be useful for other molecular diagnostics such as LAMP or nanopore sequencing. The immediate extract appears to be stable and so are directly applicable to sampling in resource-constrained environments.
In summary, the liberation of genomic DNA from bacteria using 100 mM NaOH followed by purification using paramagnetic beads or a 1:5 dilution in Te offers many benefits over conventional enzymatic techniques and commercial kits. The proposed technique is distinguished by the fact that it requires as few as one incubation step of ten minutes, and no centrifugation. Even with magnetic bead capture the method can be completed in less than one hour. We have routinely scaled up the NaOH extraction and magnetic bead capture to process 100 µl of overnight stationary Staphylococcal cultures to produce 1 ml at 10-30 ng/µl, based on ΔΔCt for qPCR vs control DNA [
37]. Thus, avoiding larger culture volumes, multiple centrifugations, long incubation times, expensive enzymes, and organic solvents [
15]. This simple extraction method combined with an inexpensive air-sampling system, as we have described, should be readily employed in agricultural systems to detect and monitor viral, bacterial, and fungal pathogens for rapid response to critical diseases.
Author Contributions
AS developed the extraction techniques and wrote the initial draft of the manuscript, BZ produced and analyzed the swine microbiome data. AA, JZ and DR secured funding and oversaw the research. DR was responsible for the final draft of the manuscript with contributions from AS, BZ, AA, and JZ. All authors approved the final manuscript.
Acknowledgements
This research was funded by: the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000; and the Ginny Lewis Fund of the Fulbright College of Arts and Sciences at the University of Arkansas.
Conflicts of Interest
The authors declare that they have no competing interests. The funders played no role in the design of the experiments, the interpretation of the data, the writing of this manuscript, or the decision to publish this manuscript.
References
- Kralik, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef]
- Deshmukh, R.A.; Joshi, K.; Bhand, S.; Roy, U. Recent developments in detection and enumeration of waterborne bacteria: a retrospective minireview. Microbiologyopen 2016, 5, 901–922. [Google Scholar] [CrossRef]
- Deurenberg, R.H., E. Bathoorn, M.A. Chlebowicz, N. Couto, M. Ferdous, S. García-Cobos, A.M. Kooistra-Smid, E.C. Raangs, S. Rosema, and A.C. Veloo, Application of next generation sequencing in clinical microbiology and infection prevention. J Biotechnol 2017. 243 16-24.
- Sambrook, J., E.F. Fritsch, and T. Maniatis, Molecular cloning: a laboratory manual. 1989, Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press.
- Maloy, S.R., Experimental techniques in bacterial genetics. 1990: Jones & Bartlett Learning.
- Nelson, J.E.; Krawetz, S.A. Purification of cloned and genomic DNA by guanidine thiocyanate/isobutyl alcohol fractionation. Anal. Biochem. 1992, 207, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Ausubel, F., R. Brent, R. Kingston, D. Moore, J. Seidman, J. Smith, and K. Struhl, Phenol: chloroform extraction. Current Protocols in Molecular Biology. New York, John Wiley & Sons, Inc 1994. 2 3.
- Kolm, C.; Martzy, R.; Brunner, K.; Mach, R.L.; Krska, R.; Heinze, G.; Sommer, R.; Reischer, G.H.; Farnleitner, A.H. A Complementary Isothermal Amplification Method to the U.S. EPA Quantitative Polymerase Chain Reaction Approach for the Detection of Enterococci in Environmental Waters. Environ. Sci. Technol. 2017, 51, 7028–7035. [Google Scholar] [CrossRef]
- Chapela, M.-J.; Garrido-Maestu, A.; Cabado, A.G. Detection of foodborne pathogens by qPCR: A practical approach for food industry applications. Cogent Food Agric. 2015, 1, 1013771. [Google Scholar] [CrossRef]
- Law, J.W.-F.; Ab Mutalib, N.-S.; Chan, K.-G.; Lee, L.-H. Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front. Microbiol. 2015, 5, 770. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C., S. Nogueira, M. Gadanho, and S. Chaves, Chapter 7 - DNA extraction: finding the most suitable method, in Molecular Microbial Diagnostic Methods, N. Cook, M. D'Agostino, and K.C. Thompson, Editors. 2016, Academic Press: San Diego. p. 135-154.
- Martzy, R.; Bica-Schröder, K.; Pálvölgyi, M.; Kolm, C.; Jakwerth, S.; Kirschner, A.K.T.; Sommer, R.; Krska, R.; Mach, R.L.; Farnleitner, A.H.; et al. Simple lysis of bacterial cells for DNA-based diagnostics using hydrophilic ionic liquids. Sci. Rep. 2019, 9, 13994. [Google Scholar] [CrossRef]
- Martzy, R.; Bica-Schröder, K.; Pálvölgyi, M.; Kolm, C.; Jakwerth, S.; Kirschner, A.K.T.; Sommer, R.; Krska, R.; Mach, R.L.; Farnleitner, A.H.; et al. Simple lysis of bacterial cells for DNA-based diagnostics using hydrophilic ionic liquids. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ish-Horowicz, D.; Burke, J. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981, 9, 2989–2898. [Google Scholar] [CrossRef] [PubMed]
- Dyer, D.W.; Iandolo, J.J. Rapid isolation of DNA from Staphylococcus aureus. Appl. Environ. Microbiol. 1983, 46, 283–285. [Google Scholar] [CrossRef]
- Pitcher, D.G.; Saunders, N.A.; Owen, R.J. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 1989, 8, 151–156. [Google Scholar] [CrossRef]
- Reischer, G.H.; Haider, J.M.; Sommer, R.; Stadler, H.; Keiblinger, K.M.; Hornek, R.; Zerobin, W.; Mach, R.L.; Farnleitner, A.H. Quantitative microbial faecal source tracking with sampling guided by hydrological catchment dynamics. Environ. Microbiol. 2008, 10, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qi, M.; Cutler, A.J. A simple method of preparing plant samples for PCR. Nucleic Acids Res. 1993, 21, 4153–4154. [Google Scholar] [CrossRef] [PubMed]
- Truett, G.; Heeger, P.; Mynatt, R.; Truett, A.; Walker, J.; Warman, M.; Meeker, N.D.; Hutchinson, S.A.; Ho, L.; Trede, N.S.; et al. Preparation of PCR-Quality Mouse Genomic DNA with Hot Sodium Hydroxide and Tris (HotSHOT). BioTechniques 2000, 29, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Kouduka, M.; Suko, T.; Morono, Y.; Inagaki, F.; Ito, K.; Suzuki, Y. A new DNA extraction method by controlled alkaline treatments from consolidated subsurface sediments. FEMS Microbiol. Lett. 2012, 326, 47–54. [Google Scholar] [CrossRef]
- Morono, Y., T. Terada, T. Hoshino, and F. Inagaki, Hot-alkaline DNA extraction method for deep-subseafloor archaeal communities. Appl Environ Microbiol 2014. 80 1985-1994.
- Osmundson, T.W., C.A. Eyre, K.M. Hayden, J. Dhillon, and M.M. Garbelotto, Back to basics: An evaluation of N a OH and alternative rapid DNA extraction protocols for DNA barcoding, genotyping, and disease diagnostics from fungal and oomycete samples. Mol Ecol Resour 2013. 13 66-74.
- Park, H.J.; Oh, S.; Vinod, N.; Ji, S.; Noh, H.B.; Koo, J.M.; Lee, S.H.; Kim, S.C.; Lee, K.-S.; Choi, C.W. Characterization of Chemically-Induced Bacterial Ghosts (BGs) Using Sodium Hydroxide-Induced Vibrio parahaemolyticus Ghosts (VPGs). Int. J. Mol. Sci. 2016, 17, 1904. [Google Scholar] [CrossRef]
- Vingataramin, L.; Frost, E.H. A single protocol for extraction of gDNA from bacteria and yeast. BioTechniques 2015, 58, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Alrubaye, A., N.S. Ekesi, A. Hasan, D.A. Koltes, R. Wideman Jr, and D. Rhoads, Chondronecrosis with osteomyelitis in broilers: Further defining a bacterial challenge model using standard litter flooring and protection with probiotics. Poult. Sci. 2020. 99 6474-6480.
- Alrubaye, A.A.; Ekesi, N.S.; Hasan, A.; Elkins, E.; Ojha, S.; Zaki, S.; Dridi, S.; Wideman, R.F.; Rebollo, M.A.; Rhoads, D.D. Chondronecrosis with osteomyelitis in broilers: further defining lameness-inducing models with wire or litter flooring to evaluate protection with organic trace minerals. Poult. Sci. 2020, 99, 5422–5429. [Google Scholar] [CrossRef]
- Baker, G.C.; Smith, J.J.; Cowan, D.A. Review and re-analysis of domain-specific 16S primers. J. Microbiol. Methods 2003, 55, 541–555. [Google Scholar] [CrossRef]
- Ekesi, N.S.; Dolka, B.; Alrubaye, A.A.; Rhoads, D.D. Analysis of genomes of bacterial isolates from lameness outbreaks in broilers. Poult. Sci. 2021, 100, 101148. [Google Scholar] [CrossRef]
- Holmes, D.S.; Quigley, M. A rapid boiling method for the preparation of bacterial plasmids. Anal. Biochem. 1981, 114, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Trkov, M. and G. Avgustin, An improved 16S rRNA based PCR method for the specific detection of Salmonella enterica. Int J Food Microbiol 2003. 80 67-75.
- Zhang, L.; Foxman, B.; Gilsdorf, J.R.; Marrs, C.F. Bacterial genomic DNA isolation using sonication for microarray analysis. BioTechniques 2005, 39, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Teng, F., S.S. Darveekaran Nair, P. Zhu, S. Li, S. Huang, X. Li, J. Xu, and F. Yang, Impact of DNA extraction method and targeted 16S-rRNA hypervariable region on oral microbiota profiling. Sci Rep 2018. 8 16321.
- Zhao, J.; Carmody, L.A.; Kalikin, L.M.; Li, J.; Petrosino, J.F.; Schloss, P.D.; Young, V.B.; LiPuma, J.J. Impact of Enhanced Staphylococcus DNA Extraction on Microbial Community Measures in Cystic Fibrosis Sputum. PLOS ONE 2012, 7, e33127. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, V.P.; Zhang, X.; Morono, Y.; Inagaki, F.; Wang, F. A Modified SDS-Based DNA Extraction Method for High Quality Environmental DNA from Seafloor Environments. Front. Microbiol. 2016, 7, 986. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qi, M.; Cutler, A.J. A simple method of preparing plant samples for PCR. Nucleic Acids Res. 1993, 21, 4153–4154. [Google Scholar] [CrossRef]
- Rudbeck, L.; Dissing, J. Rapid, Simple Alkaline Extraction of Human Genomic DNA from Whole Blood, Buccal Epithelial Cells, Semen and Forensic Stains for PCR. BioTechniques 1998, 25, 588–592. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Rossen, L.; Nørskov, P.; Holmstrøm, K.; Rasmussen, O.F. Inhibition of PCR by components of food samples, microbial diagnostic assays and DNA-extraction solutions. Int. J. Food Microbiol. 1992, 17, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, R.S.; Harkins, D.M.; Nelson, K.E. Advances in Microbiome Research for Animal Health. Annu. Rev. Anim. Biosci. 2021, 9, 289–311. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Woodhams, D.C.; Martino, C.; Allaband, C.; Mu, A.; Javorschi-Miller-Montgomery, S.; Suchodolski, J.S.; Knight, R. Engineering the microbiome for animal health and conservation. Exp. Biol. Med. 2019, 244, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Olm, M.R.; Brown, C.T.; Brooks, B.; Firek, B.; Baker, R.; Burstein, D.; Soenjoyo, K.; Thomas, B.C.; Morowitz, M.; Banfield, J.F. Identical bacterial populations colonize premature infant gut, skin, and oral microbiomes and exhibit different in situ growth rates. Genome Res. 2017, 27, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.; Wilson, J.; Guthrie, A.; Cookson, K.; Vancraeynest, D.; Schaeffer, J.; Moody, R.; Clark, S. New issues and science in broiler chicken intestinal health: Emerging technology and alternative interventions. J. Appl. Poult. Res. 2015, 24, 257–266. [Google Scholar] [CrossRef]
- A Gilbert, J.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tsai, T.; Deng, F.; Wei, X.; Chai, J.; Knapp, J.; Apple, J.; Maxwell, C.V.; Lee, J.A.; Li, Y.; et al. Longitudinal investigation of the swine gut microbiome from birth to market reveals stage and growth performance associated bacteria. Microbiome 2019, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Verrow, S., M. Blair, B. Packard, and W. Godfrey. Gel-Free Size Selection Using SPRIselect For Next Generation Sequencing. [pdf] 2023 [cited 2023 7/28/2023]. Available online: https://ls.beckmancoulter.co.jp/files/appli_note/Gel_Free_Using_SPRIselect.pdf.
- Oberacker, P.; Stepper, P.; Bond, D.M.; Höhn, S.; Focken, J.; Meyer, V.; Schelle, L.; Sugrue, V.J.; Jeunen, G.-J.; Moser, T.; et al. Bio-On-Magnetic-Beads (BOMB): Open platform for high-throughput nucleic acid extraction and manipulation. PLOS Biol. 2019, 17, e3000107. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Shin, Y.K.; Hassanali, A.A.; Singer, S.J. DNA Binding to the Silica Surface. J. Phys. Chem. B 2015, 119, 11030–11040. [Google Scholar] [CrossRef] [PubMed]
- Vandeventer, P.E.; Mejia, J.; Nadim, A.; Johal, M.S.; Niemz, A. DNA Adsorption to and Elution from Silica Surfaces: Influence of Amino Acid Buffers. J. Phys. Chem. B 2013, 117, 10742–10749. [Google Scholar] [CrossRef]
- Al-Rubaye, A.A.K.; Ekesi, N.S.; Zaki, S.; Emami, N.K.; Wideman, R.F.; Rhoads, D.D. Chondronecrosis with osteomyelitis in broilers: Further defining a bacterial challenge model using the wire flooring model. Poult. Sci. 2017, 96, 332–340. [Google Scholar] [CrossRef]
- Gand, M.; Bloemen, B.; Vanneste, K.; Roosens, N.H.C.; De Keersmaecker, S.C.J. Comparison of 6 DNA extraction methods for isolation of high yield of high molecular weight DNA suitable for shotgun metagenomics Nanopore sequencing to detect bacteria. BMC Genom. 2023, 24, 438. [Google Scholar] [CrossRef]
- Wang, X.; Howe, S.; Wei, X.; Deng, F.; Tsai, T.; Chai, J.; Xiao, Y.; Yang, H.; Maxwell, C.V.; Li, Y.; et al. Comprehensive Cultivation of the Swine Gut Microbiome Reveals High Bacterial Diversity and Guides Bacterial Isolation in Pigs. mSystems 2021, 6, e00477–21. [Google Scholar] [CrossRef]
- Trudeau, S.; Thibodeau, A.; Côté, J.-C.; Gaucher, M.-L.; Fravalo, P. Contribution of the Broiler Breeders’ Fecal Microbiota to the Establishment of the Eggshell Microbiota. Front. Microbiol. 2020, 11, 666. [Google Scholar] [CrossRef] [PubMed]
- Rexroad, C.; Vallet, J.; Matukumalli, L.K.; Reecy, J.; Bickhart, D.; Blackburn, H.; Boggess, M.; Cheng, H.; Clutter, A.; Cockett, N.; et al. Genome to Phenome: Improving Animal Health, Production, and Well-Being – A New USDA Blueprint for Animal Genome Research 2018–2027. Front. Genet. 2019, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Sun, C.; Yuan, J.; Yang, N. Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci. Rep. 2017, 7, 45308. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).