Submitted:
28 November 2023
Posted:
01 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Laboratory Analysis
2.2.1. Blood Analysis
2.2.2. Microbiome analysis
2.3. Bioinformatic and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
References
- Margaritelis, N.V.; Veskoukis, A.S.; Paschalis, V.; Vrabas, I.S.; Dipla, K.; Zafeiridis, A.; Kyparos, A.; Nikolaidis, M.G. Blood reflects tissue oxidative stress: a systematic review. Biomarkers. 2015, 20, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Lauber, C.L.; Czarnecki-Maulden, G.; Pan, Y.; Hannah, S.S. Effects of the Dietary Protein and Carbohydrate Ratio on Gut Microbiomes in Dogs of Different Body Conditions. mBio 2017, 24, 8–e01703. [Google Scholar] [CrossRef] [PubMed]
- Barry-Heffernan, C.; Ekena, J.; Dowling, S.; Pinkerton, M.E.; Viviano, K. Biomarkers of oxidative stress as an assessment of the redox status of the liver in dogs. J Vet Intern Med. 2019, 33, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, M.; Shirzad, H.; Rafieian, S.; Rafieian-Kopaei, M. Silybum marianum: Beyond Hepatoprotection. J Evid Based Complementary Altern Med. 2015, 20, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Martello, E.; Perondi, F.; Bisanzio, D.; Lippi, I.; Meineri, G.; Gabriele, V. Antioxidant Effect of a Dietary Supplement Containing Fermentative S-Acetyl-Glutathione and Silybin in Dogs with Liver Disease. Vet Sci. 2023, 8, 10–131. [Google Scholar] [CrossRef] [PubMed]
- Perondi, F.; Bisanzio, D.; Adami, R.; Lippi, I.; Meineri, G.; Cutrignelli, M.I.; Massa, S.; Martello, E. The effect of a diet supplement containing S-acetyl-glutathione (SAG) and other antioxidant natural ingredients on glutathione peroxidase in healthy dogs: a pilot study. Italian Journal of Animal Science 2023, 22, 589–593. [Google Scholar] [CrossRef]
- Marchegiani, A.; Fruganti, A.; Spaterna, A.; Dalle Vedove, E.; Bachetti, B.; Massimini, M.; Di Pierro, F.; Gavazza, A.; Cerquetella, M. Impact of Nutritional Supplementation on Canine Dermatological Disorders. Vet Sci. 2020, 7, 38. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Sechi, S.; Canello, S.; Guidetti, G.; Fiore, F.; Cocco, R. Behavioral disturbances: an innovative approach to monitor the modulatory effects of a nutraceutical diet. J. Vis. Exp. 2017, 119. [Google Scholar]
- Sechi, S.; Di Cerbo, A.; Canello, S.; Guidetti, G.; Chiavolelli, F.; Fiore, F.; Cocco, R. Effects in dogs with behavioural disorders of a commercial nutraceutical diet on stress and neuroendocrine parameters. Vet. Rec. 2017, 180, 18. [Google Scholar] [CrossRef]
- Krawczyk, M.; Grąt, M.; Barski, K.; Ligocka, J.; Antczak, A.; Kornasiewicz, O.; Skalski, M.; Patkowski, W.; Nyckowski, P. , et al. 1000 liver transplantations at the Department of General, Transplant and Liver Surgery, Medical University of Warsaw--analysis of indications and results. Pol Przegl Chir. 2012, 84, 304–12. [Google Scholar] [CrossRef]
- De Almeida Andrade, F.; Schlechta Portella, C.F. Research methods in complementary and alternative medicine: an integrative review. J Integr Med. 2018, 16, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Pilla, R.; Suchodolski, J.S. The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front Vet Sci. 2020, 14, 6–498. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, S.; Wu, J.; Ye, T.; Wang, S.; Wang, P.; Xing, D. Butyrate-producing bacteria, and the gut-heart axis in atherosclerosis. Clinica chimica acta; international journal of clinical chemistry. 2020, 507, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Shalaby, N.; Samocha-Bonet, D.; Kaakoush, N. O.; Danta, M. The Role of the Gastrointestinal Microbiome in Liver Disease. Pathogens (Basel, Switzerland) 2023, 12, 1087. [Google Scholar] [CrossRef]
- Chassaing, B.; Etienne-Mesmin, L.; Gewirtz, A.T. Microbiota-liver axis in hepatic disease. Hepatology. 2014, 59, 328–339. [Google Scholar] [CrossRef]
- Konturek, P.C.; Harsch, I.A.; Konturek, K.; Schink, M.; Konturek, T.; Neurath, M.F.; Zopf, Y. Gut–Liver Axis: How Do Gut Bacteria Influence the Liver? Med. Sci. 2018, 6, 79. [Google Scholar] [CrossRef] [PubMed]
- Minemura, M.; Shimizu, Y. Gut microbiota and liver diseases. World J Gastroenterol. 2015, 14, 1691–702. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of omega-3 fatty acids on the gut microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Desdouets, C. Oxidative stress promotes pathologic polyploidization in non-alcoholic fatty liver disease. J. Clin. Investig. 2015, 125, 981–992. [Google Scholar]
- Wan, M.L.Y.; El-Nezami, H. Targeting gut microbiota in hepatocellular carcinoma: Probiotics as a novel therapy. Hepatobiliary Surg. Nutr. 2018, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- National Research Council; Division on Earth and Life Studies; Board on Agriculture and Natural Resources; Subcommittee on Dog and Cat Nutrition; Committee on Animal Nutrition. https://nap.nationalacademies.org/catalog/10668.
- Laflamme, D.P. Development and validation of a body condition score system for dogs. Canine Pract. 1997, 22, 10–15. [Google Scholar]
- FEDIAF Nutritional Guidelines for Complete and Complementary Pet Food, (europeanpetfood.org).
- Marchegiani, A.; Fruganti, A.; Gavazza, A.; Mangiaterra, S.; Candellone, A.; Fusi, E.; Rossi, G.; Cerquetella, M. Evidences on Molecules Most Frequently Included in Canine and Feline Complementary Feed to Support Liver Function. Vet Med Int. 2020, 9, 9185759. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic. Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable, and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Help with greengenes gg_13_8 reference otus - Other Bioinformatics Tools - QIIME 2 Forum.
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knigh, R. Quantitative and qualitative diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef]
- (https://www.microbiomeanalyst.ca/).
- Gommeren, K.; Desmas, I.; Garcia, A.; Bauer, N.; Moritz, A.; Roth, J.; Peeters, D. Inflammatory cytokine and C-reactive protein concentrations in dogs with systemic inflammatory response syndrome. Journal of veterinary emergency and critical care (San Antonio, Tex.: 2001). 2018, 28, 9–19. [Google Scholar] [CrossRef]
- Torrente, C.; Manzanilla, E. G.; Bosch, L.; Fresno, L.; Rivera Del Alamo, M.; Andaluz, A.; Saco, Y.; Ruiz de Gopegui, R. Plasma iron, C-reactive protein, albumin, and plasma fibrinogen concentrations in dogs with systemic inflammatory response syndrome. Journal of veterinary emergency and critical care (San Antonio, Tex.: 2001). 2015, 25, 611–619. [Google Scholar] [CrossRef]
- Pradhan, S.C.; Girish, C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006, 124, 491–504. [Google Scholar]
- Trappoliere, M.; Caligiuri, A.; Schmid, M.; Bertolani, C.; Failli, P.; Vizzutti, F.; Novo, E.; di Manzano, C.; Marra, F.; Loguercio, C.; Pinzani, M. Silybin, a component of sylimarin, exerts anti-inflammatory and anti-fibrogenic effects on human hepatic stellate cells. J Hepatol. 2009, 50, 1102–11. [Google Scholar] [CrossRef]
- Verma, S.; Thuluvath, P.J. Complementary and alternative medicine in hepatology: review of the evidence of efficacy. Clin Gastroenterol Hepatol. 2007, 5, 408–16. [Google Scholar] [CrossRef] [PubMed]
- Magdalan, J.; Ostrowska, A.; Piotrowska, A.; Izykowska, I.; Nowak, M.; Szelag, A.; Dziegiel, P. Failure of benzylpenicillin, N-acetylcysteine and silibinin to reduce alpha-amanitin hepatotoxicity. In Vivo 2009, 23, 393–9. [Google Scholar] [PubMed]
- Sgorlon, S.; Stefanon, B.; Sandri, M.; Colitti, M. Nutrigenomic activity of plant derived compounds in health and disease: Results of a dietary intervention study in dog. Res. Vet. Sci. 2016, 109, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Vandeweerd, J.M.; Cambier, C.; Gustin, P. Nutraceuticals for canine liver disease: assessing the evidence. Vet Clin North Am Small Anim Pract. 2013, 43, 1171–9. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C. N.; Dalli, J.; Colas, R. A.; Winkler, J. W.; Chiang, N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochimica et biophysica acta. 2015, 1851, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; Byrne, C.D. Omega-3 fatty acids and non-alcoholic fatty liver disease: Evidence of efficacy and mechanism of action. Mol Aspects Med. 2018, 64, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Jump, D.B.; Lytle, K.A.; Depner, C.M.; Tripathy, S. Omega-3 polyunsaturated fatty acids as a treatment strategy for non-alcoholic fatty liver disease. Pharmacol Ther. 2018, 181, 108–25. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Kusakabe, M.; Imanishi, I.; Hisano, T.; Fukamachi, T.; Taguchi, N.; Iyori, K.; Hsiao, Y. H. A randomised, double-blinded, controlled trial to determine the efficacy of combined therapy of oclacitinib and marine oil extract PCSO-524 in dogs with atopic dermatitis. Veterinary dermatology. 2023, 34, 523–531. [Google Scholar] [CrossRef]
- Tu, T.; Li, B.; Li, X.; Zhang, B.; Xiao, Y.; Li, J.; Qin, F.; Liu, N.; Sun, C.; Liu, Q.; Zhou, S. Dietary ω-3 fatty acids reduced atrial fibrillation vulnerability via attenuating myocardial endoplasmic reticulum stress and inflammation in a canine model of atrial fibrillation. Journal of cardiology. 2022, 79, 194–201. [Google Scholar] [CrossRef]
- Meineri, G.; Cocolin, L.; Morelli, G.; Schievano, C.; Atuahene, D.; Ferrocino, I. Effect of an Enteroprotective Complementary Feed on Faecal Markers of Inflammation and Intestinal Microbiota Composition in Weaning Puppies. Veterinary sciences. 2023, 10, 434. [Google Scholar] [CrossRef]
- Schauf, S.; Nakamura, N.; Castrillo, C. Effect of Calsporin (Bacillus subtilis C-3102) addition to the diet on faecal quality and nutrient digestibility in healthy adult dogs. J. Appl. Anim. Nutr. 2019, 7. [Google Scholar]
- de Lima, D.C.; Souza, C.M.M.; Nakamura, N.; Mesa, D.; de Oliveira, S.G.; Félix, A.P. Dietary supplementation with Bacillus subtilis C-3102 improves gut health indicators and fecal microbiota of dogs. Anim. Feed. Sci. Technol. 2020, 270, 114672. [Google Scholar] [CrossRef]
- Panasevich, M.R.; Daristotle, L.; Quesnell, R.; Reinhart, G.A.; Frantz, N.Z. Altered fecal microbiota, IgA, and fermentative end-products in adult dogs fed prebiotics and a nonviable Lactobacillus acidophilus. J Anim Sci. 2021, 99, skab347. [Google Scholar] [CrossRef] [PubMed]
- Pinna, C. , Vecchiato, C.G.; Bolduan, C.; Grandi, M.; Stefanelli, C.; Windisch, W.; Zaghini, G.; Biagi, G. Influence of dietary protein and fructooligosaccharides on fecal fermentative end-products, fecal bacterial populations, and apparent total tract digestibility in dogs. BMC veterinary research. 2018, 14, 106. [Google Scholar]
- Van den Abbeele, P.; Duysburgh, C.; Rakebrandt, M.; Marzorati, M. Dried yeast cell walls high in beta-glucan and mannan-oligosaccharides positively affect microbial composition and activity in the canine gastrointestinal tract in vitro. Journal of animal science. 2020, 98, skaa173. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Grieshop, C.M.; Flickinger, E.A.; Bauer, L.L.; Healy, H.P.; Dawson, K.A.; Merchen, N.R.; Fahey, G.C.j.r. Supplemental fructooligosaccharides and mannanoligosaccharides influence immune function, ileal and total tract nutrient digestibilities, microbial populations and concentrations of protein catabolites in the large bowel of dogs. J. Nutr. 2002, 132, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Flickinger, E.A.; Schreijen, E.M.; Patil, A.R.; Hussein, H.S.; Grieshop, C.M.; Merchen, N.R.; Fahey, G.C. J.r. . Nutrient digestibilities, microbial populations, and protein catabolites as affected by fructan supplementation of dog diets. J. Anim. Sci. 2003, 81, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- Ephraim, E.; Brockman, J. A.; Jewell, D. E. A Diet Supplemented with Polyphenols, Prebiotics and Omega-3 Fatty Acids Modulates the Intestinal Microbiota and Improves the Profile of Metabolites Linked with Anxiety in Dogs. Biology, 2022, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rahman, S. U.; Huang, Y.; Zhang, Y.; Ming, P.; Zhu, L.; Chu, X.; Li, J.; Feng, S.; Wang, X.; Wu, J. Green tea polyphenols decrease weight gain, ameliorate alteration of gut microbiota, and mitigate intestinal inflammation in canines with high-fat-diet-induced obesity. The Journal of nutritional biochemistry. 2020, 78, 108324. [Google Scholar] [CrossRef]
- Scarsella, E.; Cintio, M.; Iacumin, L.; Ginaldi, F.; Stefanon, B. Interplay between Neuroendocrine Biomarkers and Gut Microbiota in Dogs Supplemented with Grape Proanthocyanidins: Results of Dietary Intervention Study. Animals: an open access journal from MDPI. 2020, 10, 531. [Google Scholar] [CrossRef]
- Wang, W.; Zhai, T.; Luo, P.; Miao, X.; Wang, J.; Chen, Y. Beneficial effects of silibinin on serum lipids, bile acids, and gut microbiota in methionine-choline-deficient diet-induced mice. Frontiers in nutrition. 2023, 10, 1257158. [Google Scholar] [CrossRef] [PubMed]
- AlShawaqfeh, M. K.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J. A.; Steiner, J. M.; Serpedin, E.; Suchodolski, J. S. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS microbiology ecology. 2017, 93, 10. [Google Scholar] [CrossRef] [PubMed]
- Crost, E.H.; Tailford, L.E.; Le Gall, G.; Fons, M.; Henrissat, B.; Juge, N. Utilisation of mucin glycans by the human gut symbiont Ruminococcus gnavus is strain dependent. PLoS One. 2013, 8, e76341. [Google Scholar] [CrossRef] [PubMed]
- Caspani, G.; Kennedy, S.; Foster, J.A.; Swann, J. Gut microbial metabolites in depression: Understanding the biochemical mechanisms. Microb. Cell. 2019, 6, 454–481. [Google Scholar] [CrossRef]
- Sandri, M.; Dal Monego, S.; Conte, G.; Sgorlon, S.; Stefanon, B. Raw meat-based diet influences faecal microbiome and end products of fermentation in healthy dogs. BMC Vet. Res. 2017, 13, 65. [Google Scholar] [CrossRef]
- Gilley, S.P.; Ruebel, M.L.; Sims, C.; Zhong, Y.; Turner, D.; Lan, R.S.; Pack, L.M.; Piccolo, B.D.; Chintapalli, S.V.; Abraham, A.; Bode, L.; Andres, A.; Shankar, K. Associations between maternal obesity and offspring gut microbiome in the first year of life. Pediatr Obes. 2022, 17, e12921. [Google Scholar] [CrossRef]
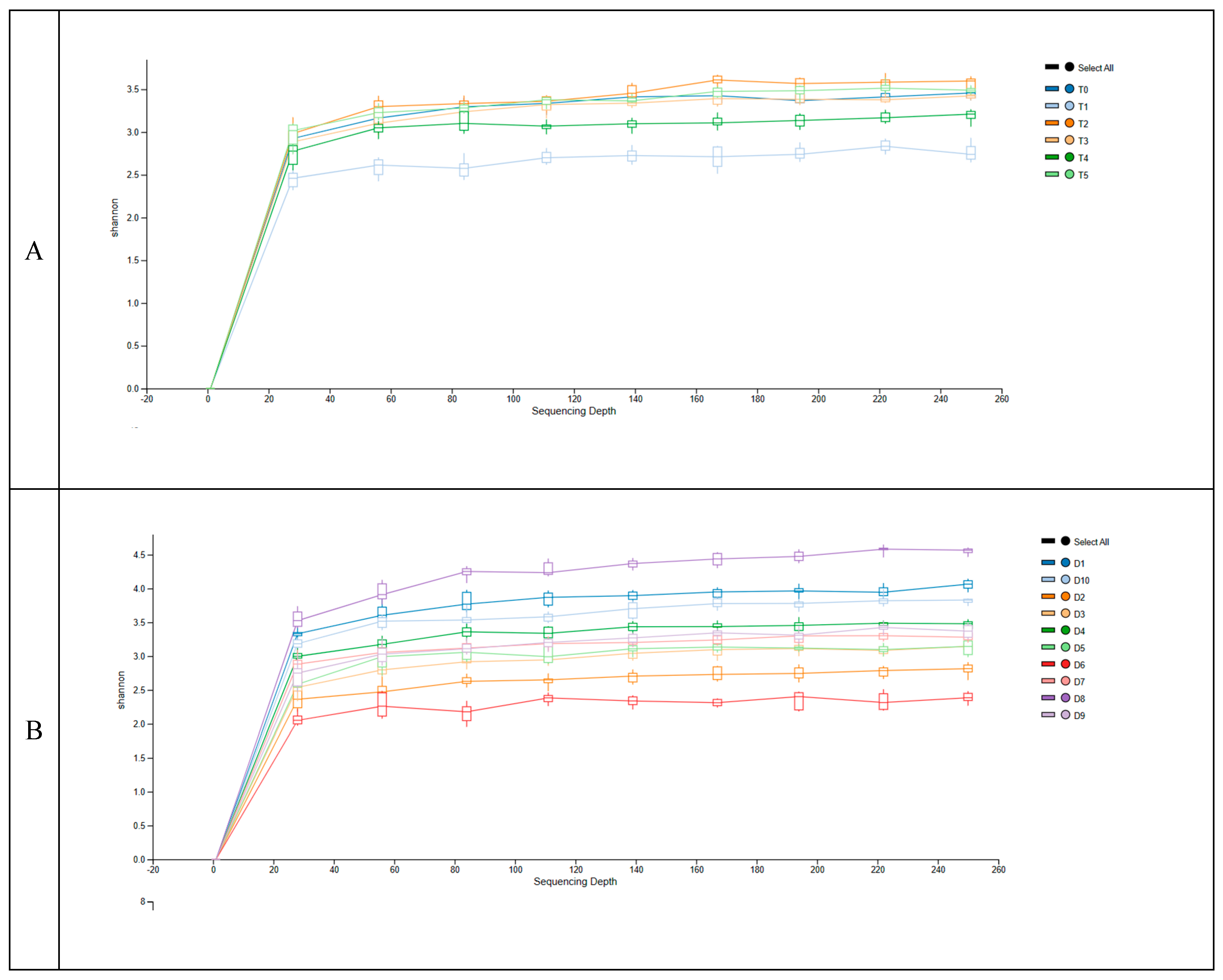
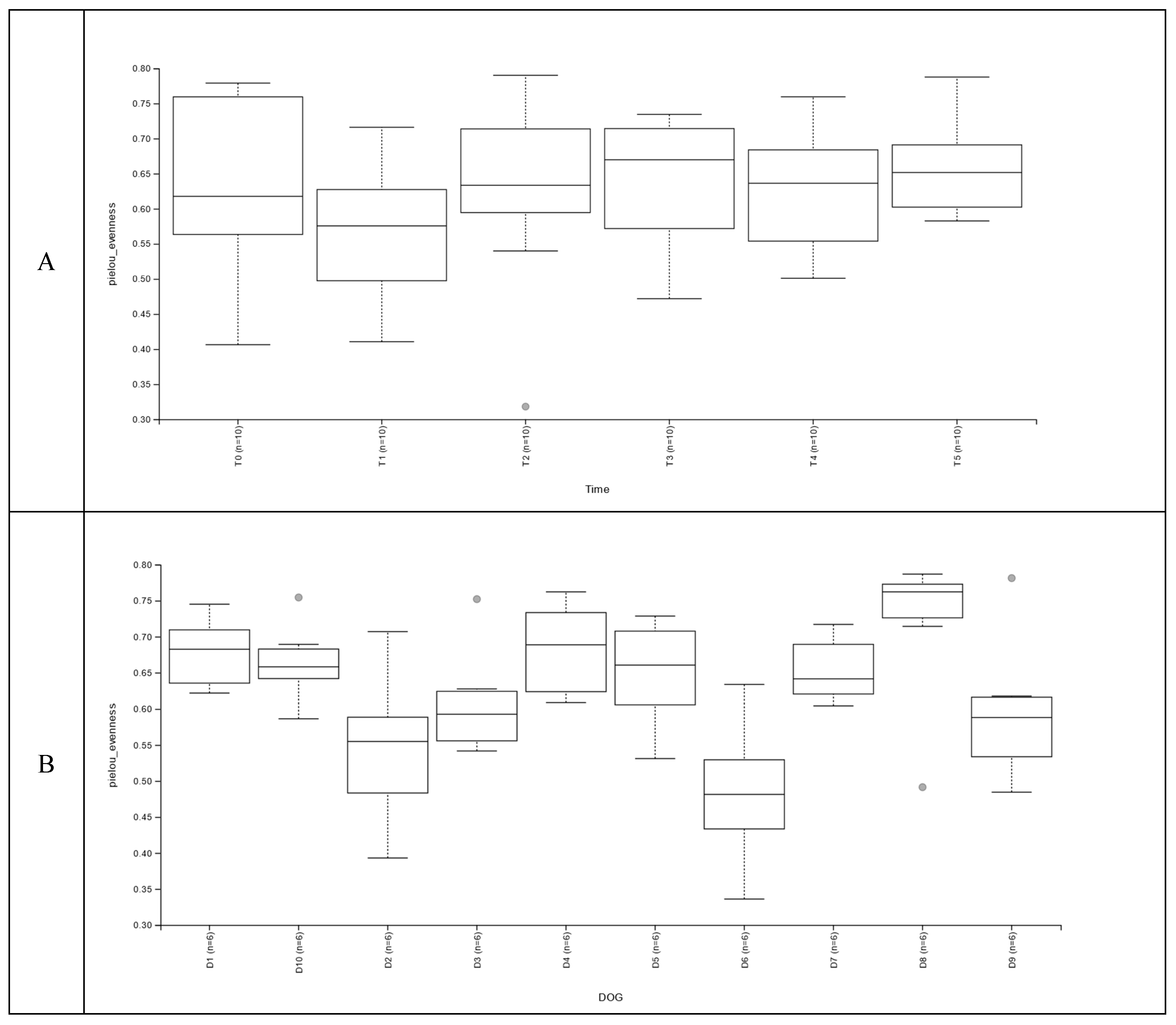
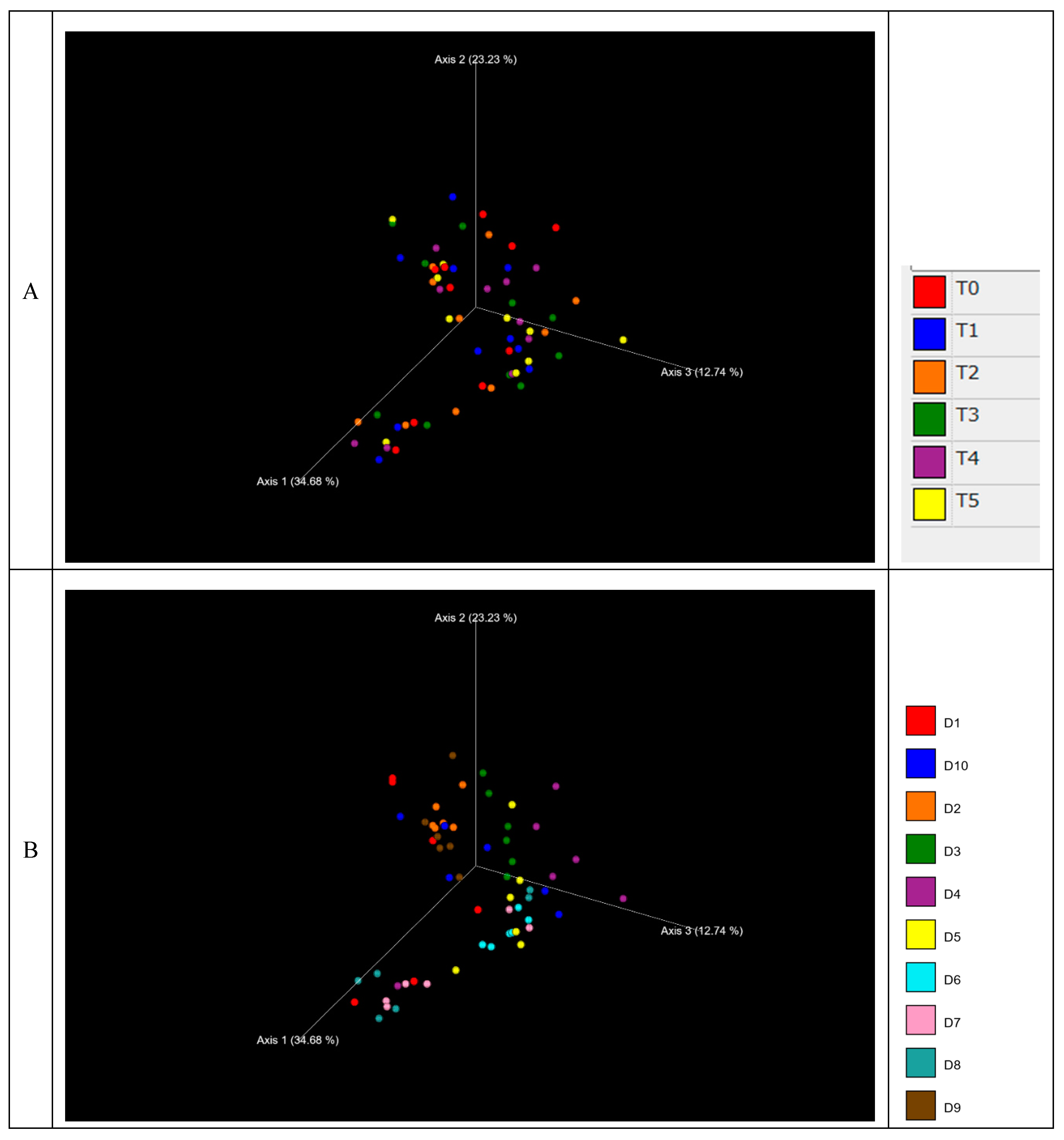
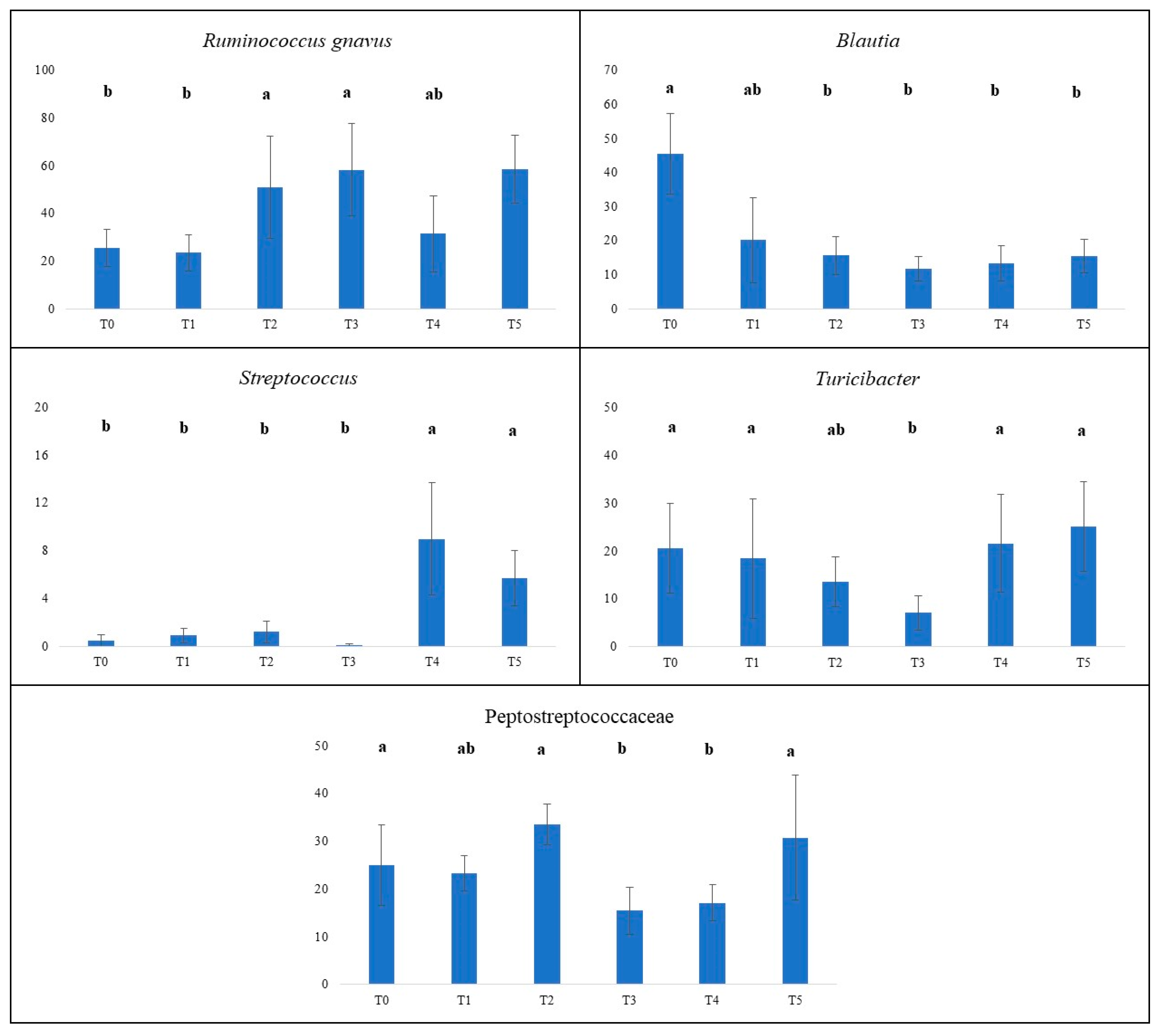
| Dog number | Age | Sex | Breed | Weight (kg) | BCS |
|---|---|---|---|---|---|
| D1 | 12 | F | American staffordshire terrier | 30 | 7 |
| D2 | 10 | F | Dachshund | 9 | 8 |
| D3 | 8 | M | English Bulldog | 30 | 8 |
| D4 | 8 | M | Shar Pei | 35,5 | 7 |
| D5 | 12 | M | Staffordshire bull terrier | 22,1 | 7 |
| D6 | 11 | F | Beagle | 17,5 | 8 |
| D7 | 13 | M | Bullterrier | 38,1 | 7 |
| D8 | 10 | F | Dalmatian | 28,8 | 7 |
| D9 | 9 | F | German shepherd | 34,8 | 6 |
| D10 | 8 | M | Bassethound | 30,5 | 8 |
| Diet | Amount |
|---|---|
| Sylibum marianum | 2.5% |
| Choline chloride | 0.8% |
| Bacillus subtilis | 6% |
| Fructoligosaccharides | 0.5% |
| Beta glucans from barley | 0.5% |
| Betaine | 0.9% |
| n-3 polyunsaturated fatty acids (n-3 PUFA) in fish oil | 6% |
| Vitamin B12 | 0.00002% |
| Vitamin C | 0.15% |
| Alpha tocopherol | 3% |
| Zinc | 0.9% |
| Magnesium | 1.6% |
| Arginine | 0.4% |
| Taurine | 2% |
| Hydrolysed proteins | 24% |
| Maltodextrine | 48.74998% |
| Time | AST UI/l |
ALT UI/l |
ALP UI/l |
GGT UI/l |
Glucose mg/100ml |
Trygl mg/100ml |
Total bilirubin mg/100ml |
CRP mg/l |
|---|---|---|---|---|---|---|---|---|
| 23-66 | 21-102 | 20-156 | 1.2-6.0 | 65-110 | 20-112 | 0.1-0.5 | ||
| T0 | 49.5 a | 107.2 ns | 302.1 a | 6.3 ns | 106.4 a | 162.7 ns | 0.6 a | 1.2 a |
| T1 | 32.8 ab | 82.4 ns | 246.0 ab | 6.2 ns | 108.6 a | 119.1 ns | 0.4 ab | 0.9 a |
| T2 | 31.0 b | 91.2 ns | 242.9 ab | 6.3 ns | 97.4 ab | 150.6 ns | 0.4 ab | 0.8 ab |
| T3 | 34.3 ab | 91.6 ns | 208.6 b | 6.2 ns | 92.9 b | 110.5 ns | 0.3 b | 0.6 b |
| T4 | 36.0 ab | 96.0 ns | 213.4 b | 5.4 ns | 93.9 b | 114.6 ns | 0.3 b | 0.6 b |
| T5 | 40.5 ab | 91.1 ns | 204.6 b | 5.2 ns | 91.2 b | 103.1 ns | 0.3 b | 0.6 b |
| RMSE | 2.1 | 7.4 | 27.5 | 0.4 | 2.8 | 9.9 | 0.0 | 4.5 |
| Time | Total mmol/l |
Lactic % |
Acetic % |
Propionic % |
Isobutyric % |
Butyric % |
Isovaleric % |
|---|---|---|---|---|---|---|---|
| 23-66 | 21-102 | 20-156 | 1.2-6.0 | 65-110 | 20-112 | 0.1-0.5 | |
| T0 | 213.9 ab | 2.1 ab | 66.7 ns | 15.9 ns | 3.3 ns | 3.5 ns | 8.5 ns |
| T1 | 173.5 b | 0.4 b | 66.9 ns | 20.9 ns | 3.9 ns | 2.5 ns | 5.4 ns |
| T2 | 227.1 ab | 1.4 ab | 69.6 ns | 16.4 ns | 4.0 ns | 4.5 ns | 4.1 ns |
| T3 | 249.7 a | 2.6 a | 72.1 ns | 14.0 ns | 3.8 ns | 4.2 ns | 3.4 ns |
| T4 | 225.1 ab | 1.9 ab | 66.6 ns | 17.4 ns | 4.6 ns | 3.2 ns | 6.3 ns |
| T5 | 210.0 ab | 2.5 a | 67.4 ns | 16.7 ns | 4.3 ns | 4.1 ns | 5.0 ns |
| RMSE | 8.9 | 0.3 | 1.8 | 1.3 | 0.4 | 0.3 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





