Submitted:
30 November 2023
Posted:
30 November 2023
You are already at the latest version
Abstract
Keywords:
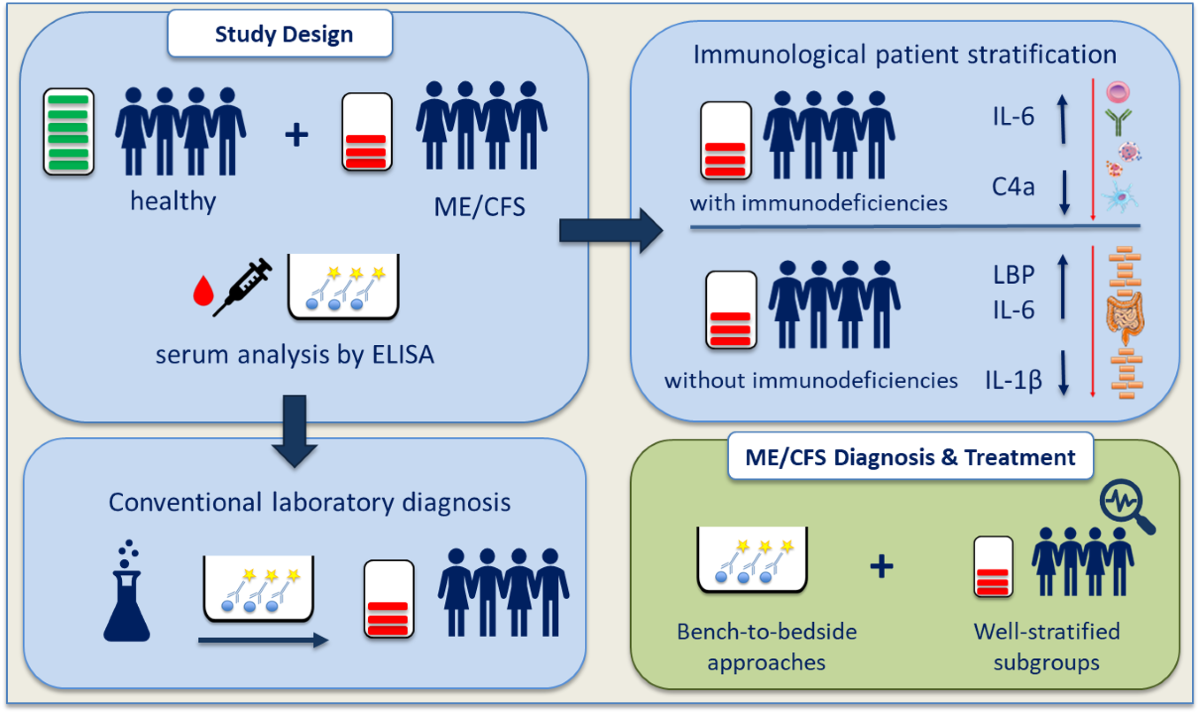
1. Introduction
2. Materials and Methods
2.1. Study Cohort and Sampling Procedure
2.2. Measurement of Disease-Related Parameters
2.3. Statistical Analyses
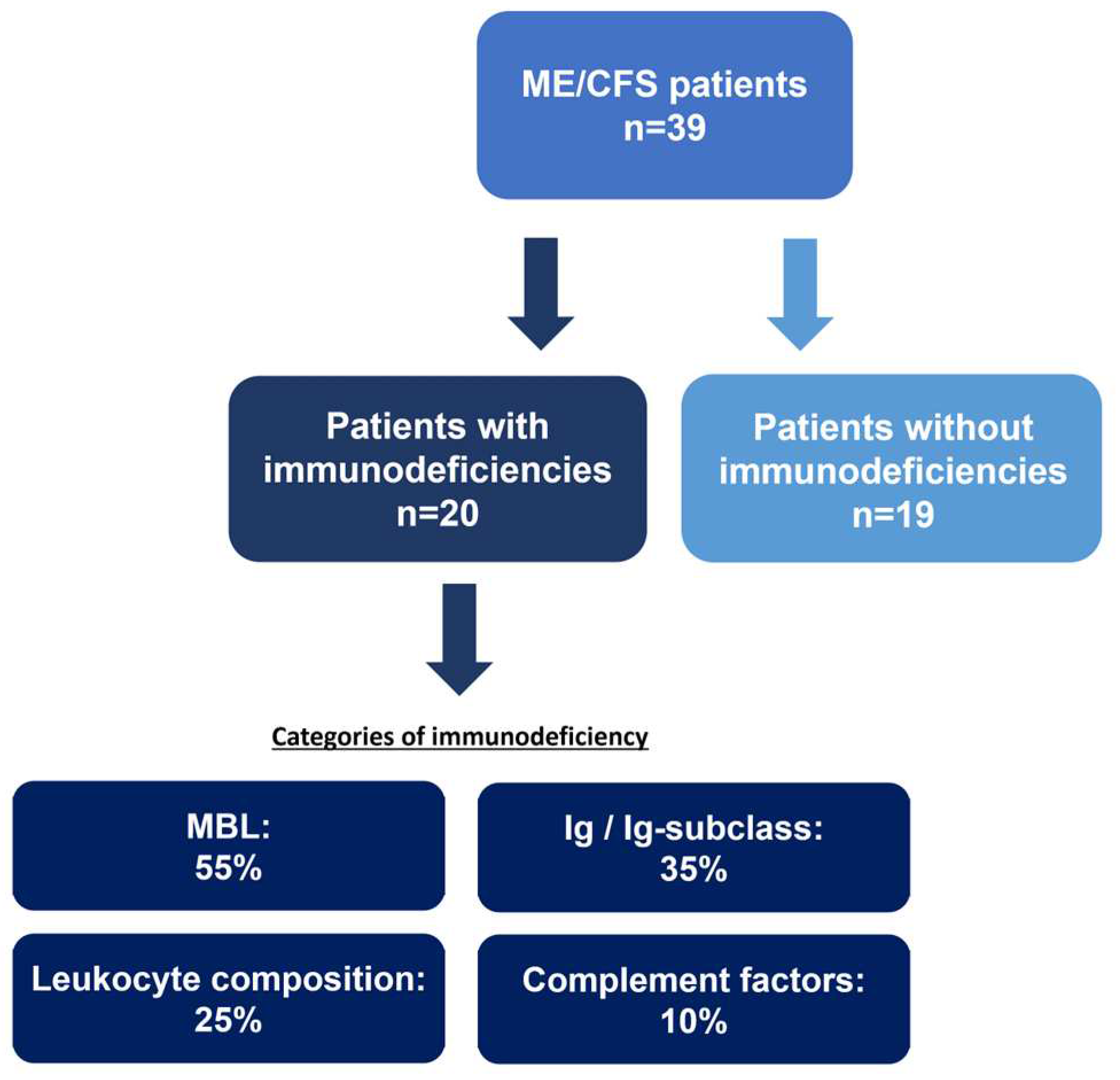
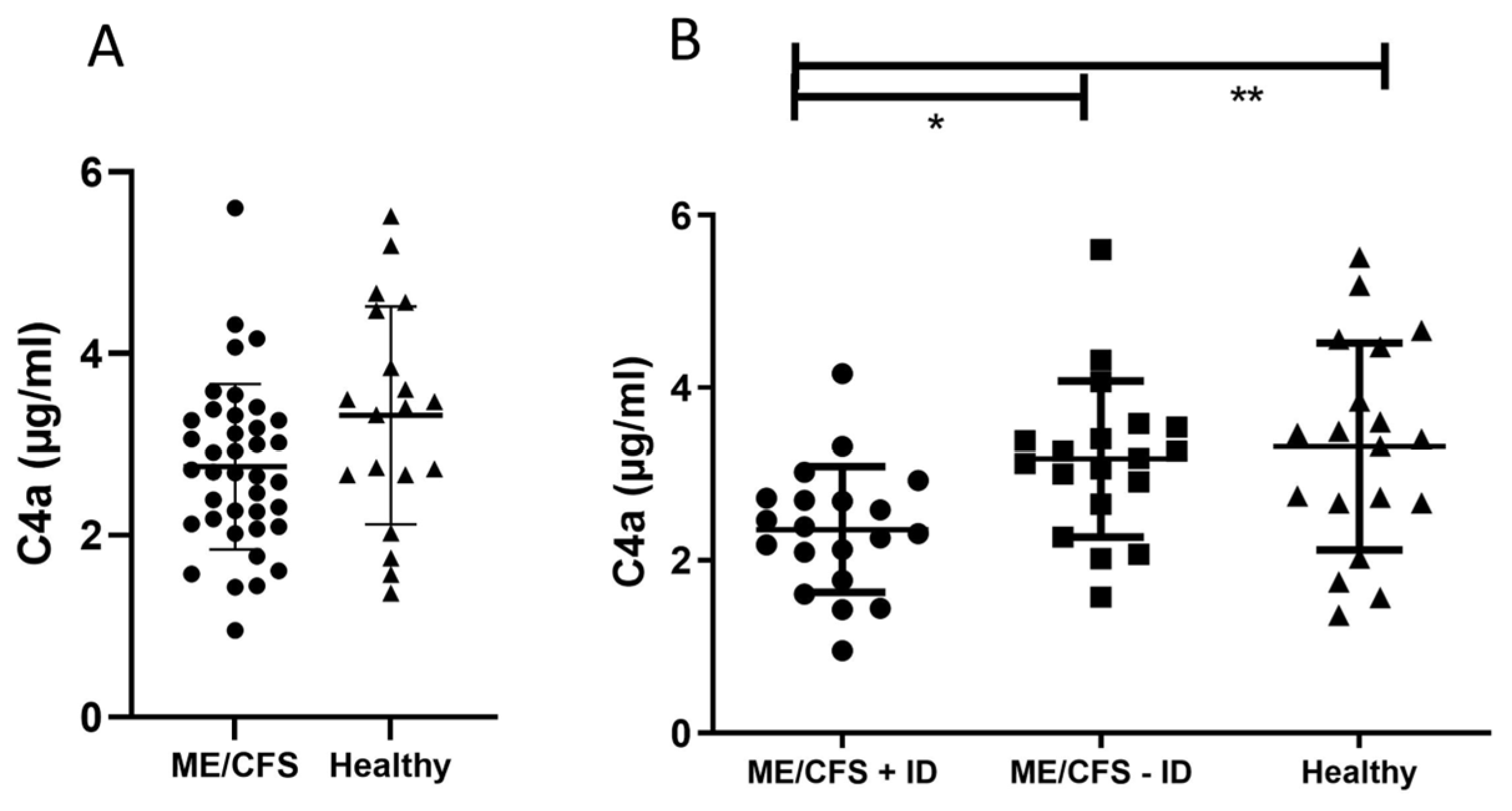
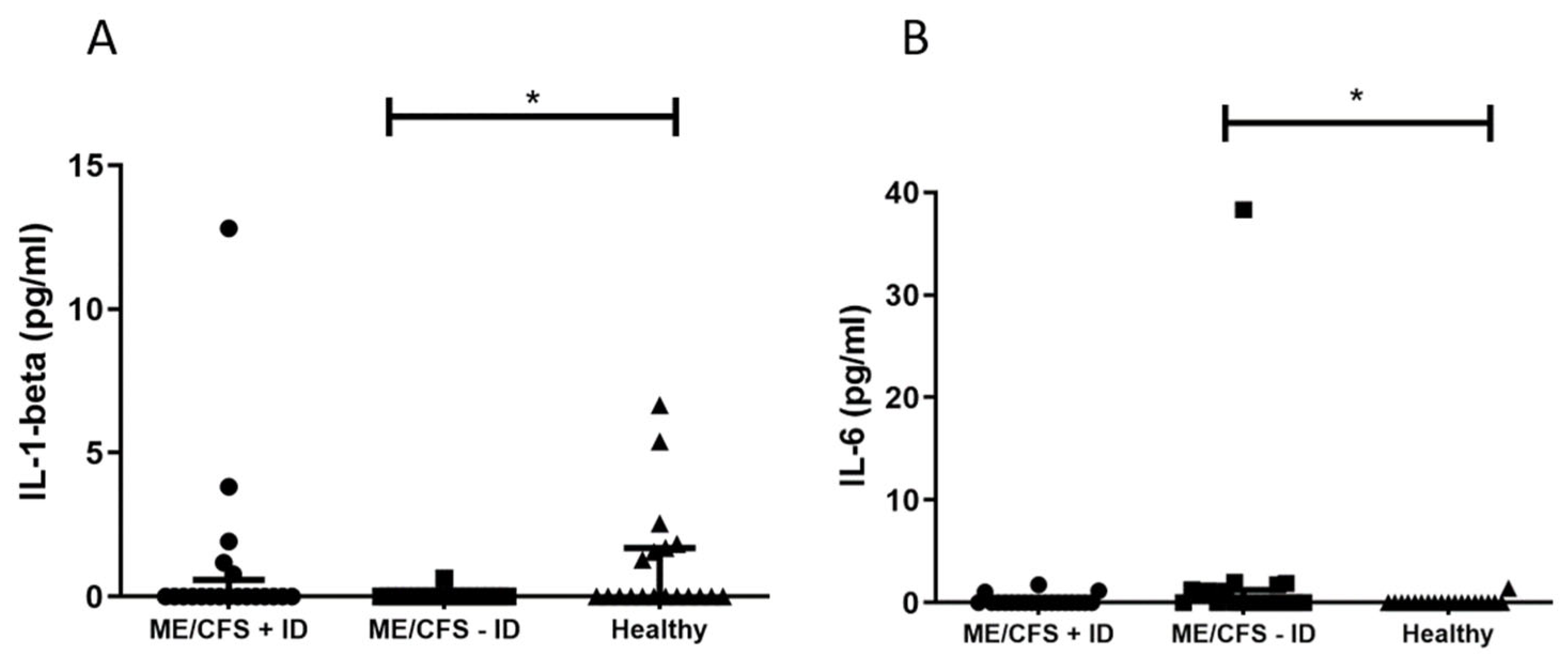
3. Results
3.1. Evaluation of Pro-Inflammatory Immune Mediators
| Study Group | ME/CFS + ID | ME/CFS - ID | Healthy |
| Parameter (unit ± SD) | |||
|
TNF-alpha (pg/ml) LOD: 4 pg/ml |
2.76 (± 2.56) | 7.03 (± 9.08) | 3.63 (± 3.68) |
|
IL-8 (pg/ml) LOD: 2 pg/ml |
10.80 (± 7.78) | 10.68 (± 12.63) | 10.37 (± 8.00) |
|
IL-33 (pg/ml) LOD: 0.9 pg/ml |
35.70 (± 48.23) | 52.13 (± 77.37) | 27.77 (± 35.44) |
|
FGF21 (pg/ml) LOD: 7 pg/ml |
170.50 (± 158.90) | 149.70 (± 116.80) | 155.80 (± 90.14) |
|
ECP (ng/ml) LOD: 39 pg/ml |
11.50 (± 2.66) | 12.77 (± 9.31) | 12.34 (± 4.12) |
|
EDN (ng/ml) LOD: 0.63 ng/ml |
50.11 (± 27.60) | 52.29 (± 29.19) | 41.03 (± 17.78) |
|
Endotoxin-Core IgG (MU/ml) LOD: 0.13 MU/ml |
113.10 (± 219.30) | 77.79 (± 79.75) | 75.97 (± 46.80) |
|
sCD14 (µg/ml) LOD: 6 pg/ml |
12.23 (± 2.00) | 11.99 (± 2.20) | 11.39 (± 1.00) |
|
I-FABP (ng/ml) LOD: 25 pg/ml |
7.86 (± 17.51) | 3.90 (± 7.30) | 11.35 (± 20.29) |
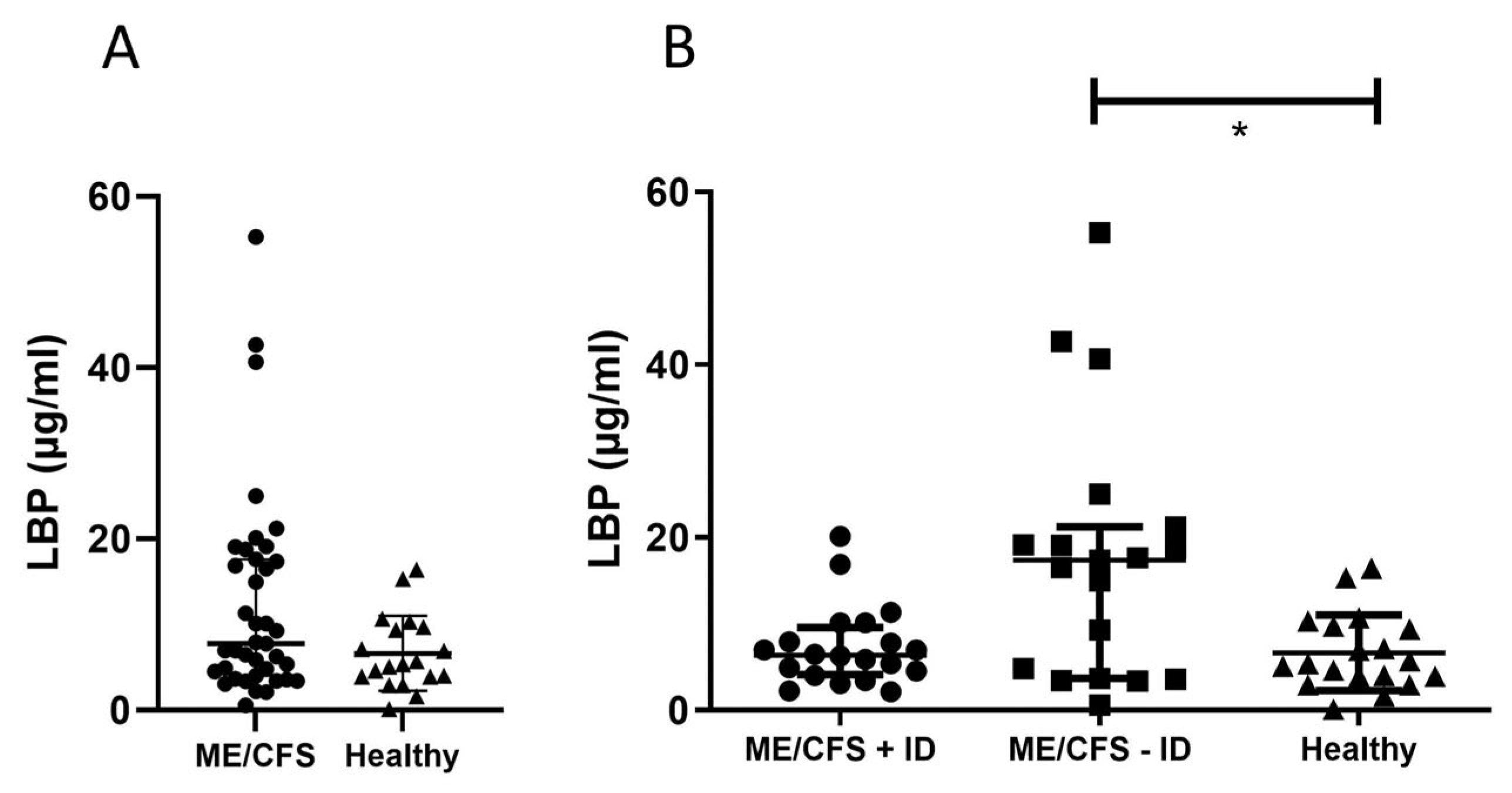
3.2. Immune Marker Related to Enhanced Mast-Cell Activity and Eosinophil Activation
3.3. Biomarker Associated to Mucosal and Intestinal Barrier Integrity
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Kit | Manufacturer | Cat.-Number |
|---|---|---|
| Human IL-1-beta Uncoated ELSIA | ThermoFisher Scientific, Waltham, USA | 88-7261 |
| Human IL-6 Uncoated ELISA Kit | ThermoFisher Scientific, Waltham, USA | 88-7066 |
| Human TNF-alpha Uncoated ELISA | ThermoFisher Scientific, Waltham, USA | 88-7346 |
| Human IL-8 Uncoated ELISA Kit | ThermoFisher Scientific, Waltham, USA | 88-8086 |
| Human IL-33 ELISA Kit | ThermoFisher Scientific, Waltham, USA | BMS2048 |
| Human IFN-gamma Uncoated ELISA | ThermoFisher Scientific, Waltham, USA | 88-7316 |
| BD OptEIA Human C4a ELISA Kit | BD Biosciences, Franklin Lakes, USA | 550947 |
| Fibroblast Growth Factor 21 Human ELISA | BioVendor, Brno, Czech Republic | RD191108200R |
| Human RNASE3/ECP (Ribonuclease A3/Eosinophil cationic Protein) ELISA Kit | Elabscience, Houston, USA | E-EL-H1379 |
| Human EDN (Eosinophil-Derived Neurotoxin) ELISA Kit | Elabscience, Houston, USA | E-EL-H2341 |
| Human LBP ELISA Kit | ThermoFisher Scientific, Waltham, USA | EH297RB |
| EndoCAb IgG, Human, ELISA Kit | Hycult Biotech, Wayne, USA | HK504-IGG |
| Human CD14 ELISA Kit | ThermoFisher Scientific, Waltham, USA | EHCD14 |
| Human FABP-2 (intestinal) ELISA Kit | ThermoFisher Scientific, Waltham, USA | EHFABP2 |
References
- Lutz, L., et al., Evaluation of Immune Dysregulation in an Austrian Patient Cohort Suffering from Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Biomolecules, 2021. 11(9): p. 1359. [CrossRef]
- Lim, E.J., et al., Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Transl Med, 2020. 18(1): p. 100. [CrossRef]
- Tschopp, R., et al., Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A preliminary survey among patients in Switzerland. Heliyon, 2023. 9(5): p. e15595. [CrossRef]
- Ruiz-Pablos, M., et al., Epstein-Barr Virus and the Origin of Myalgic Encephalomyelitis or Chronic Fatigue Syndrome. Frontiers in Immunology, 2021. 12. [CrossRef]
- Komaroff, A.L. and L. Bateman, Will COVID-19 Lead to Myalgic Encephalomyelitis/Chronic Fatigue Syndrome? Frontiers in Medicine, 2021. 7(1132). [CrossRef]
- Altmann, D.M., et al., The immunology of long COVID. Nature Reviews Immunology, 2023. [CrossRef]
- Renz-Polster, H. and C. Scheibenbogen, [Post-COVID syndrome with fatigue and exercise intolerance: myalgic encephalomyelitis/chronic fatigue syndrome]. Inn Med (Heidelb), 2022. 63(8): p. 830-839. [CrossRef]
- Nguyen, T., et al., Novel characterisation of mast cell phenotypes from peripheral blood mononuclear cells in chronic fatigue syndrome/myalgic encephalomyelitis patients. Asian Pac J Allergy Immunol, 2017. 35(2): p. 75-81. [CrossRef]
- Nijs, J., et al., Altered immune response to exercise in patients with chronic fatigue syndrome/myalgic encephalomyelitis: a systematic literature review. Exerc Immunol Rev, 2014. 20: p. 94-116.
- Jonsjö, M.A., et al., The role of low-grade inflammation in ME/CFS (Myalgic Encephalomyelitis/Chronic Fatigue Syndrome) - associations with symptoms. Psychoneuroendocrinology, 2020. 113: p. 104578. [CrossRef]
- Gang, J., et al., Microbiota and COVID-19: Long-term and complex influencing factors. Front Microbiol, 2022. 13: p. 963488. [CrossRef]
- Kedor, C., et al., A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nature Communications, 2022. 13(1): p. 5104. [CrossRef]
- Guenther, S., et al., Frequent IgG subclass and mannose binding lectin deficiency in patients with chronic fatigue syndrome. Hum Immunol, 2015. 76(10): p. 729-35. [CrossRef]
- Jack, D.L., et al., Mannose-binding lectin regulates the inflammatory response of human professional phagocytes to Neisseria meningitidis serogroup B. J Infect Dis, 2001. 184(9): p. 1152-62. [CrossRef]
- Gupta, A. and G.S. Gupta, Status of mannose-binding lectin (MBL) and complement system in COVID-19 patients and therapeutic applications of antiviral plant MBLs. Molecular and Cellular Biochemistry, 2021. 476(8): p. 2917-2942. [CrossRef]
- Ali, Y.M., et al., Lectin Pathway Mediates Complement Activation by SARS-CoV-2 Proteins. Frontiers in Immunology, 2021. 12. [CrossRef]
- Gao, T., et al., Highly pathogenic coronavirus N protein aggravates inflammation by MASP-2-mediated lectin complement pathway overactivation. Signal Transduction and Targeted Therapy, 2022. 7(1): p. 318. [CrossRef]
- Liu, H., et al., Mannan binding lectin attenuates double-stranded RNA-mediated TLR3 activation and innate immunity. FEBS Lett, 2014. 588(6): p. 866-72. [CrossRef]
- Zhou, J., et al., Mannan-Binding Lectin Regulates Inflammatory Cytokine Production, Proliferation, and Cytotoxicity of Human Peripheral Natural Killer Cells. Mediators of Inflammation, 2019. 2019: p. 6738286. [CrossRef]
- Tang, Y., et al., Mannan-binding lectin reduces CpG DNA-induced inflammatory cytokine production by human monocytes. Microbiol Immunol, 2015. 59(4): p. 231-7. [CrossRef]
- Rohrhofer, J., et al., Association between EBV reactivation and development of Long-COVID fatigue. Allergy, 2022. n/a(n/a). [CrossRef]
- König, R.S., et al., The Gut Microbiome in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Front Immunol, 2021. 12: p. 628741. [CrossRef]
- Wirth, K.J. and M. Löhn, Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Comorbidities: Linked by Vascular Pathomechanisms and Vasoactive Mediators? Medicina (Kaunas), 2023. 59(5). [CrossRef]
- Zollner, A., et al., Postacute COVID-19 is Characterized by Gut Viral Antigen Persistence in Inflammatory Bowel Diseases. Gastroenterology, 2022. 163(2): p. 495-506.e8. [CrossRef]
- Sencio, V., et al., Influenza Virus Infection Impairs the Gut's Barrier Properties and Favors Secondary Enteric Bacterial Infection through Reduced Production of Short-Chain Fatty Acids. Infect Immun, 2021. 89(9): p. e0073420. [CrossRef]
- Tugizov, S., Virus-associated disruption of mucosal epithelial tight junctions and its role in viral transmission and spread. Tissue Barriers, 2021. 9(4): p. 1943274. [CrossRef]
- Kim, D.Y., et al., Systematic review of randomized controlled trials for chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Transl Med, 2020. 18(1): p. 7. [CrossRef]
- Fluge, Ø., et al., B-Lymphocyte Depletion in Myalgic Encephalopathy/ Chronic Fatigue Syndrome. An Open-Label Phase II Study with Rituximab Maintenance Treatment. PLoS One, 2015. 10(7): p. e0129898. [CrossRef]
- Fluge, Ø., et al., B-Lymphocyte Depletion in Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Ann Intern Med, 2019. 170(9): p. 585-593. [CrossRef]
- Goudsmit, E.M., et al., Pacing as a strategy to improve energy management in myalgic encephalomyelitis/chronic fatigue syndrome: a consensus document. Disabil Rehabil, 2012. 34(13): p. 1140-7. [CrossRef]
- Rowe, K., Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME) in Adolescents: Practical Guidance and Management Challenges. Adolesc Health Med Ther, 2023. 14: p. 13-26. [CrossRef]
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue, S., P. Board on the Health of Select, and M. Institute of, The National Academies Collection: Reports funded by National Institutes of Health, in Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness. 2015, National Academies Press (US).
- Copyright 2015 by the National Academy of Sciences. All rights reserved.: Washington (DC).
- Galvan-Blasco, P., J. Gil-Serrano, and A. Sala-Cunill, New Biomarkers in Anaphylaxis (Beyond Tryptase). Curr Treat Options Allergy, 2022. 9(4): p. 303-322. [CrossRef]
- Nijs, J., et al., Unravelling the nature of postexertional malaise in myalgic encephalomyelitis/chronic fatigue syndrome: the role of elastase, complement C4a and interleukin-1β. Journal of Internal Medicine, 2010. 267(4): p. 418-435. [CrossRef]
- Polli, A., et al., Exercise-induce hyperalgesia, complement system and elastase activation in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome - a secondary analysis of experimental comparative studies. Scand J Pain, 2019. 19(1): p. 183-192. [CrossRef]
- Sorensen, B., et al., Complement activation in a model of chronic fatigue syndrome. J Allergy Clin Immunol, 2003. 112(2): p. 397-403. [CrossRef]
- Wang, H. and M. Liu, Complement C4, Infections, and Autoimmune Diseases. Frontiers in Immunology, 2021. 12. [CrossRef]
- Kitchens, R.L. and P.A. Thompson, Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res, 2005. 11(4): p. 225-9. [CrossRef]
- Laugerette, F., et al., Postprandial Endotoxin Transporters LBP and sCD14 Differ in Obese vs. Overweight and Normal Weight Men during Fat-Rich Meal Digestion. Nutrients, 2020. 12(6). [CrossRef]
- Hodzic, Z., et al., IL-33 and the intestine: The good, the bad, and the inflammatory. Cytokine, 2017. 100: p. 1-10. [CrossRef]
- Wu, W.H., et al., Interleukin-1β secretion induced by mucosa-associated gut commensal bacteria promotes intestinal barrier repair. Gut Microbes, 2022. 14(1): p. 2014772. [CrossRef]
- Lau, E., et al., The role of I-FABP as a biomarker of intestinal barrier dysfunction driven by gut microbiota changes in obesity. Nutr Metab (Lond), 2016. 13: p. 31. [CrossRef]
- Chelakkot, C., J. Ghim, and S.H. Ryu, Mechanisms regulating intestinal barrier integrity and its pathological implications. Experimental & Molecular Medicine, 2018. 50(8): p. 1-9. [CrossRef]
- Chakaroun, R.M., L. Massier, and P. Kovacs, Gut Microbiome, Intestinal Permeability, and Tissue Bacteria in Metabolic Disease: Perpetrators or Bystanders? Nutrients, 2020. 12(4): p. 1082. [CrossRef]
- Adamowicz, J.L., I. Caikauskaite, and F. Friedberg, Defining recovery in chronic fatigue syndrome: a critical review. Qual Life Res, 2014. 23(9): p. 2407-16. [CrossRef]
- Rowe, K.S., Double-blind randomized controlled trial to assess the efficacy of intravenous gammaglobulin for the management of chronic fatigue syndrome in adolescents. J Psychiatr Res, 1997. 31(1): p. 133-47. [CrossRef]
- Brownlie, H. and N. Speight, Back to the Future? Immunoglobulin Therapy for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Healthcare (Basel), 2021. 9(11). [CrossRef]
- Scheibenbogen, C., et al., Immunoadsorption to remove ß2 adrenergic receptor antibodies in Chronic Fatigue Syndrome CFS/ME. PLOS ONE, 2018. 13(3): p. e0193672. [CrossRef]
- Haberland, A., et al., Aptamer BC 007's Affinity to Specific and Less-Specific Anti-SARS-CoV-2 Neutralizing Antibodies. Viruses, 2021. 13(5). [CrossRef]
- Haberland, A., et al., Aptamer BC 007 - A broad spectrum neutralizer of pathogenic autoantibodies against G-protein-coupled receptors. Eur J Pharmacol, 2016. 789: p. 37-45. [CrossRef]
- Hensley-McBain, T. and J.A. Manuzak, Zonulin as a biomarker and potential therapeutic target in multisystem inflammatory syndrome in children. J Clin Invest, 2021. 131(14). [CrossRef]
- Yonker, L.M., et al., Zonulin Antagonist, Larazotide (AT1001), As an Adjuvant Treatment for Multisystem Inflammatory Syndrome in Children: A Case Series. Crit Care Explor, 2022. 10(2): p. e0641. [CrossRef]
- Scheibenbogen, C., et al., Fighting Post-COVID and ME/CFS - development of curative therapies. Front Med (Lausanne), 2023. 10: p. 1194754. [CrossRef]
- Fulton, C.D.M., et al., Long-term, West Nile virus-induced neurological changes: A comparison of patients and rodent models. Brain Behav Immun Health, 2020. 7: p. 100105. [CrossRef]
- Reynolds, K.J., et al., The economic impact of chronic fatigue syndrome. Cost effectiveness and resource allocation : C/E, 2004. 2(1): p. 4-4. [CrossRef]
| Study Group | ME/CFS + ID | ME/CFS - ID | Healthy |
| Demographic Data | |||
| Mean age in years (± SD) | 41.2 (± 12.6) | 38.4 (± 10.8) | 43.1 (± 13.0) |
| Female sex in percentage (n) | 75 (15) | 84.2 (16) | 73.7 (14) |
| Inclusion criteria | |||
| Immunodeficiency (ID) | yes | no | no |
| ME/CFS (IOM criteria)* | yes | yes | no |
| Exclusion criteria | |||
| Neurological/psychiatric co-morbidities | no | no | yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





