Submitted:
30 November 2023
Posted:
30 November 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Chemical Constituents of Essential Oils
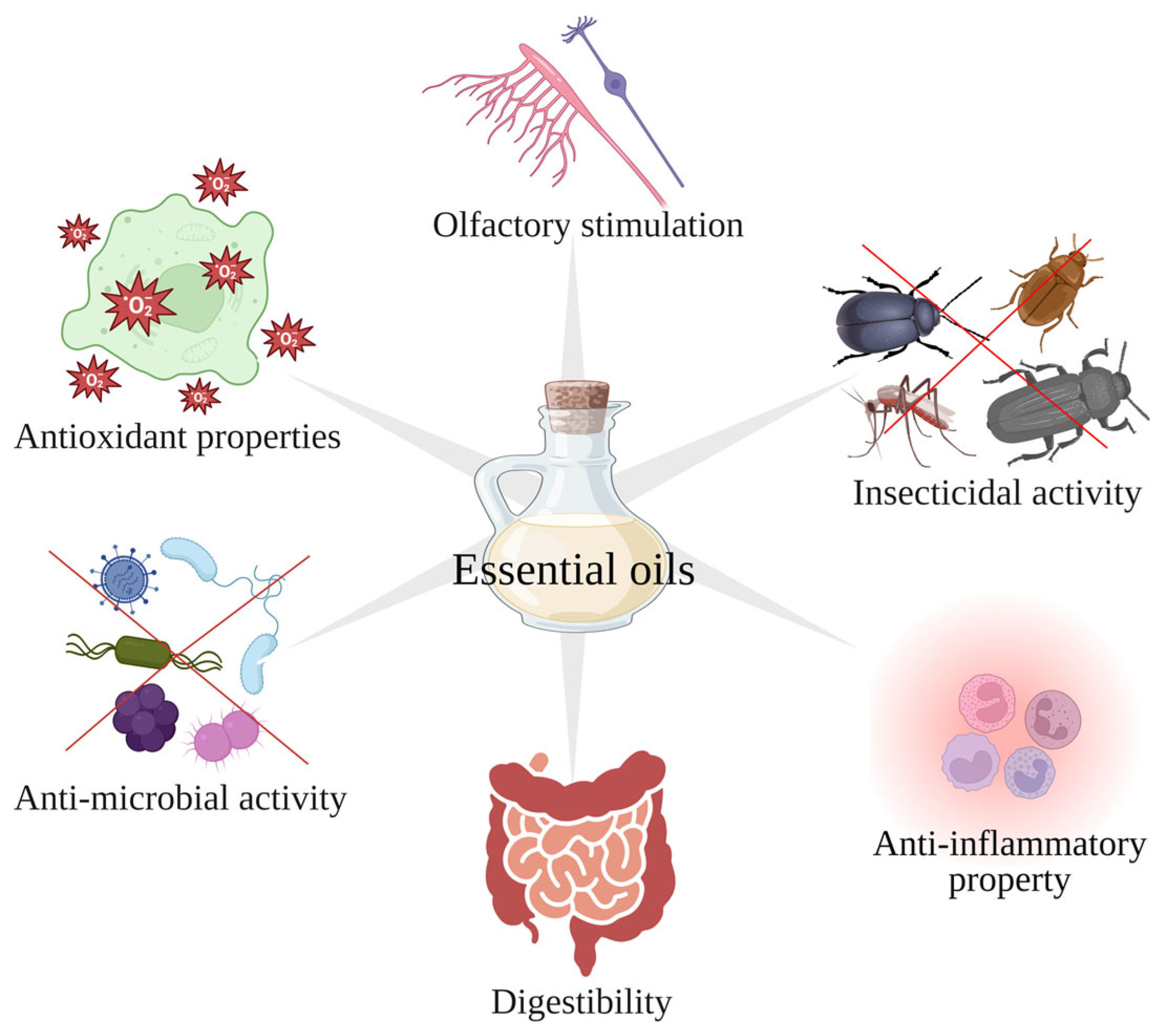
3. Some of the Bioactive Properties of Essential Oils
3.1. Antimicrobial Activity
3.2. Antioxidant Properties
3.3. Anti-Inflammatory Activity
3.4. Insecticidal Activity
4. Effects of EO on the Health Status of Pets and Animals
5. Safety Considerations of Essential Oils
6. Limitations in the Use of Essential Oils
- Comprehensive studies are scarce on the toxicity of EOs, particularly concerning pets and animals, leading to a lack of robust evidence on their potential risks and benefits.
- The effects of EOs can vary significantly among different species of animals. This variability introduces complexity in establishing standardized dosages and safety guidelines applicable across diverse animal groups.
- Determining the appropriate dosage and application methods of EOs for animals is challenging due to factors like body weight, metabolism, and individual sensitivities.
- The chemical composition of EOs could vary based on factors like plant source, extraction method, and storage conditions. This lack of standardization poses challenges in predicting their precise effects on animals and requires careful consideration of each oil’s unique properties.
- Some EOs, even those derived from plants, can pose risks to animals. For instance, ingestion of tea tree oil has led to intoxication in both humans and animals, demonstrating the importance of informed usage.
7. Future Perspectives
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EOs | Essential oils |
| TEO | Thyme EO |
| REO | Rosemary EO |
| NOEO | Natural oregano EO |
| SOEO | synthetic oregano EO |
| TAEO | Thymus algeriensis EO |
| T. algeriensis | Thymus algeriensis |
| L. angustifolia | Lavandula angustifolia |
| F. contracta | Ferulago contracta |
| T. plicata | Thuja plicata |
| J. communis | Juniperus communis |
| M. officinalis | Melissa officinalis |
| C. zeylanicum | Cinnamomum zeylanicum |
| C. citratus | Cymbopogon citratus |
| L. cubeba | Litsea cubeba |
| M. piperita | Mentha piperita |
| S. aromaticum | Syzygium aromaticum |
| O. basilicum | Ocimum basilicum |
| P. graveolens | Pelargonium graveolens |
| S. sclarea | Salvia sclarea |
| T. vulgaris | Thymus vulgaris |
| O. vulgare | Origanum vulgare |
| C. martini | Cymbopogon martini |
| R. officinalis | Rosmarinus officinalis |
| S. pseudointermedius | Staphylococcus pseudointermedius |
| S. aureus | Staphylococcus aureus |
| P. aeruginosa | Pseudomonas aeruginosa |
| B. cinerea | Botrytis cinerea |
| C. albicans | Candida albicans |
| C. famata | Candida famata |
| C. tropicalis | Candida tropicalis |
| T. equinum | Trichophyton equinum |
| M. canis | Microsporum canis |
| C. krusei | Candida krusei |
| C. glabrata | Candida glabrata |
| M. pachydermatis | Malassezia pachydermatis |
| M. otitis | Malassezia otitis |
| HSV–1 | Herpes simplex virus 1 |
| ACE2 | Angiotensin-converting enzyme 2 |
| HAV | Hepatitis A virus |
| SARS-CoV | Severe acute respiratory syndrome coronavirus |
| H. contortus | Haemonchus contortus |
| C. hominivorax | Cochliomyia hominivorax |
| C. suppressalis | Chilo suppressalis |
| C. felis | Ctenocephalides felis |
| D. melanogaster | Drosophila melanogaster |
| R. sanguineus | Rhipicephalus sanguineus |
| NAT | Natural antioxidant feed |
| SOD | Superoxide dismutase |
| GSH-Px | Glutathione peroxidase |
| GR | Glutathione reductase |
| MDA | Malondialdehyde |
| TAC | Total antioxidant capacity |
| CON | Control group |
| TAS | Total antioxidant status |
| TOS | Total oxidant status |
| GSH | Glutathione |
| CAT | Catalase |
| OSI | Oxidative stress index |
| DPPH• | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS •+ | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid |
| H2O2 | Hydrogen peroxide |
| AAE | Ascorbic acid equivalent |
| ROS | Reactive Oxygen Species |
| PUFAs | Polyunsaturated fatty acids |
| CAD | Canine atopic dermatitis |
| CADESI-03 | Canine Atopic Dermatitis Extent and Severity Index-03 |
| AFB1 | Aflatoxin B1 |
| w/v | Weight/Volume |
| mg/mL | Milligram per milliliter |
| mmol/L | Millimoles per liter |
| nmol/mg | Nanomoles per milligram |
| g/kg | Grams per kilogram |
| µg/µL | Microgram per microliter |
| μg cm-2 | Micrograms per square centimeter |
| mg/kg | Milligrams per kilogram |
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericidal concentration |
| MFC | Minimum fungicidal concentration |
| SI | Selectivity index |
| LC50 | Half lethal concentration |
| LD50 | Half lethal dose |
| IC50 | Half maximal inhibitory concentration |
| TC50 | Half-maximal toxic concentration |
| EPA | Environmental Protection Agency |
References
- Possamai, M.C.; dos Santos, I.C.; Silva, E.S.; Gazim, Z.C.; Gonçalves, J.E.; Soares, A.A.; de Melo Germano, R.; Fanin, M.; de Sá, T.C.; Otutumi, L.K. In vitro bacteriostatic activity of Origanum vulgare, Cymbopogon citratus, and Lippia alba essential oils in cat food bacterial isolates. Semin. Cienc. Agrar. 2019, 40, 3107–3122. [Google Scholar] [CrossRef]
- Van Raamsdonk, L.W.; Ozinga, W.A.; Hoogenboom, L.A.; Mulder, P.P.; Mol, J.G.; Groot, M.J.; Van der Fels-Klerx, H.J.; De Nijs, M. Exposure assessment of cattle via roughages to plants producing compounds of concern. Food Chem. 2015, 189, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasut, C.; Sivamaruthi, B.S.; Wongwan, J.; Thiwan, K.; Rungseevijitprapa, W.; Klunklin, A.; Kunaviktikul, W. Effects of Litsea cubeba (Lour.) Persoon essential oil aromatherapy on mood states and salivary cortisol levels in healthy volunteers. Evid. Based Complement. Alternat. Med. 2020, 2020, 4389239. [Google Scholar] [CrossRef] [PubMed]
- Lans, C. Do recent research studies validate the medicinal plants used in British Columbia, Canada for pet diseases and wild animals taken into temporary care? J. Ethnopharmacol. 2019, 236, 366–392. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.I.; Dias-Ferreira, C.; Ribeiro, A.B. Overview of in situ and ex situ remediation technologies for PCB-contaminated soils and sediments and obstacles for full-scale application. Sci. Total Environ. 2013, 445-446, 237–260. [Google Scholar] [CrossRef] [PubMed]
- Zema, D.A.; Calabrò, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef]
- Chirani, M.R.; Kowsari, E.; Teymourian, T.; Ramakrishna, S. Environmental impact of increased soap consumption during COVID-19 pandemic: Biodegradable soap production and sustainable packaging. Sci. Total. Environ. 2021, 2021 796, 149013. [Google Scholar] [CrossRef]
- Teoh, E.S. Secondary metabolites of plants. Med. Orchids Asia. 2015, 5, 59–73. [Google Scholar]
- Štrbac, F.; Krnjajić, S.; Stojanović, D.; Novakov, N.; Bosco, A.; Simin, N.; Ratajac, R.; Stanković, S.; Cringoli, G.; Rinaldi, L. Botanical control of parasites in veterinary medicine. One Health Triad. 2023, 3, 215–222. [Google Scholar]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Tiyajamorn, T.; Bharathi, M.; Chaiyasut, C. A narrative review on the bioactivity and health benefits of alpha-phellandrene. Sci Pharm. 2022, 90, 57. [Google Scholar] [CrossRef]
- Castagna, F.; Palma, E.; Cringoli, G.; Bosco, A.; Nisticò, N.; Caligiuri, G.; Britti, D.; Musella, V. Use of Complementary Natural Feed for Gastrointestinal Nematodes Control in Sheep: Effectiveness and Benefits for Animals. Animals (Basel) 2019, 9, 1037. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, M.Z.; Ismail, H.; Kayani, W.K. Plant Secondary Metabolites: Therapeutic Potential and Pharmacological Properties. In Secondary Metabolites-Trends and Reviews, 1st ed.; Vijayakumar, R., Raja, S., Eds.; IntechOpen: London, United Kingdom, 2022; Volume 1. [Google Scholar] [CrossRef]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Bharathi, M.; Kunaviktikul, W.; Klunklin, A.; Chanthapoon, C.; Chaiyasut, C. Essential oils, phytoncides, aromachology, and aromatherapy-a review. Appl. Sci. 2022, 12, 4495. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. The composition, pharmacological and economic importance of essential oil of Litsea cubeba (Lour.) Pers. Food Sci. Technol. (Campinas) 2022, 42, e35720. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Kesika, P.; Chaiyasut, C. Influence of Probiotic Supplementation on Health Status of the Dogs: A Review. Appl. Sci. 2021, 11, 11384. [Google Scholar] [CrossRef]
- Genovese, A.G.; McLean, M.K.; Khan, S.A. Adverse reactions from essential oil-containing natural flea products exempted from Environmental Protection Agency regulations in dogs and cats. J. Vet. Emerg. Crit. Care (San Antonio) 2012, 22, 470–475. [Google Scholar] [CrossRef]
- Sardi, J.C.O.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J.S. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef]
- Spampinato, C.; Leonardi, D. Candida infections, causes, targets, and resistance mechanisms: traditional and alternative antifungal agents. Biomed. Res. Int. 2013, 2013, 204237. [Google Scholar] [CrossRef]
- Soković, M.D.; Vukojević, J.; Marin, P.D.; Brkić, D.D.; Vajs, V.; Van Griensven, L.J.L.D. Chemical Composition of Essential Oils of Thymus and Mentha Species and Their Antifungal Activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, N.; Fu, Y.J.; Wang, W.; Luo, M.; Zhao, C.J.; Zu, Y.G.; Liu, X.L. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ. Toxicol. Pharmacol. 2011, 32, 63–68. [Google Scholar] [CrossRef]
- Subasinghe, U.; Gamage, M.; Hettiarachchi, D.S. Essential oil content and composition of Indian sandalwood (Santalum album) in Sri Lanka. J. For. Res. 2013, 24, 127–130. [Google Scholar] [CrossRef]
- Hussein, K.A.; Joo, J.H. Chemical composition of neem and lavender essential oils and their antifungal activity against pathogenic fungi causing ginseng root rot. Afr. J. Biotechnol. 2017, 16, 2349–2354. [Google Scholar]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Kos Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; Mayo, B.; Pechová, A.; Petkova, M.; Ramos, F.; Sanz, Y.; Villa, R.E.; Woutersen, R.; Brantom, P.; Chesson, A.; Westendorf, J.; Gregoretti, L.; Manini, P.; Dusemund, B. Safety and efficacy of essential oil, oleoresin and tincture from Zingiber officinale Roscoe when used as sensory additives in feed for all animal species. EFSA J. 2020, 18, e06147. [Google Scholar] [PubMed]
- Daning, D.; Widyobroto, B.; Hanim, C.; Yusiati, L.M. Effect of Galangal (Alpinia galanga) essential oil supplementation on milk production, composition, and characteristics of fatty acids in dairy cows. Adv. Anim. Vet. Sci. 2021, 10, 192–202. [Google Scholar]
- Ruiz-Cano, D.; Sánchez-Carrasco, G.; El-Mihyaoui, A.; B. Arnao, M. Essential Oils and Melatonin as Functional Ingredients in Dogs. Animals 2022, 12, 2089. [Google Scholar] [CrossRef] [PubMed]
- Ibrahium, S.M.; Wahba, A.A.; Farghali, A.A.; Abdel-Baki, A.-A.S.; Mohamed, S.A.A.; Al-Quraishy, S.; Hassan, A.O.; Aboelhadid, S.M. Acaricidal Activity of Tea Tree and Lemon Oil Nanoemulsions against Rhipicephalus annulatus. Pathogens 2022, 11, 1506. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; Mayo, B.; Pechová, A.; Petkova, M.; Ramos, F.; Sanz, Y.; Villa, R.E.; Woutersen, R.; Brantom, P.; Chesson, A.; Schlatter, J.; Schrenk, D.; Westendorf, J.; Manini, P.; Pizzo, F.; Dusemund, B. Safety and efficacy of feed additives consisting of essential oils from the bark and the leaves of Cinnamomum verum J. Presl (cinnamon bark oil and cinnamon leaf oil) for use in all animal species (FEFANA asbl). EFSA J. 2022, 20, e07601. [Google Scholar] [PubMed]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Bampidis, V.; Azimonti, G.; Bastos, M.L.; Christensen, H.; Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; Mayo, B.; Pechová, A.; Petkova, M.; Ramos, F.; Sanz, Y.; Villa, R.E.; Woutersen, R.; Brantom, P.; Chesson, A.; Schlatter, J.; Westendorf, J.; Manini, P.; Dusemund, B. Safety and efficacy of a feed additive consisting of an essential oil from the seeds of Myristica fragrans Houtt. (nutmeg oil) for all animal species (FEFANA asbl). EFSA J, 2023; 21, 0806. [Google Scholar]
- Čmiková, N.; Galovičová, L.; Schwarzová, M.; Vukic, M.D.; Vukovic, N.L.; Kowalczewski, P.Ł.; Bakay, L.; Kluz, M.I.; Puchalski, C.; Kačániová, M. Chemical Composition and Biological Activities of Eucalyptus globulus Essential Oil. Plants (Basel) 2023, 12, 1076. [Google Scholar] [CrossRef] [PubMed]
- Kozuharova, E.; Simeonov, V.; Batovska, D.; Stoycheva, C.; Valchev, H.; Benbassat, N. Chemical composition and comparative analysis of lavender essential oil samples from Bulgaria in relation to the pharmacological effects. Pharmacia 2023, 70, 395–403. [Google Scholar] [CrossRef]
- Keivanfar, L.; Nateghi, L.; Rashidi Nodeh, H. Comparing two different extraction techniques on chemical composition and antioxidant property of three essential oils of Ferulago contracta, Rosmarinus officinalis and Lavendula sublepoidota. J. Food Meas. Charact. 2023, 17, 3579–3591. [Google Scholar] [CrossRef]
- Afkar, S. Assessment of chemical compositions and antibacterial activity of the essential oil of Mentha piperita in response to salicylic acid. Nat. Prod. Res. 2023, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dangol, S.; Poudel, D.K.; Ojha, P.K.; Maharjan, S.; Poudel, A.; Satyal, R.; Rokaya, A.; Timsina, S.; Dosoky, N.S.; Satyal, P.; Setzer, W.N. Essential Oil Composition Analysis of Cymbopogon Species from Eastern Nepal by GC-MS and Chiral GC-MS, and Antimicrobial Activity of Some Major Compounds. Molecules 2023, 28, 543. [Google Scholar] [CrossRef]
- Brah, A.S.; Armah, F.A.; Obuah, C.; Akwetey, S.A.; Adokoh, C.K. Toxicity and therapeutic applications of citrus essential oils (CEOs): A review. Int. J. Food Prop. 2023, 26, 301–26. [Google Scholar] [CrossRef]
- Paiano, R.B.; de Sousa, R.L.M.; Bonilla, J.; Moreno, L.Z.; de Souza, E.D.F.; Baruselli, P.S.; Moreno, A.M. In vitro effects of cinnamon, oregano, and thyme essential oils against Escherichia coli and Trueperella pyogenes isolated from dairy cows with clinical endometritis. Theriogenology 2023, 196, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Najar, B.; Bertelloni, F.; Pistelli, L.; Mancianti, F.; Nardoni, S. Chemical Composition and In Vitro Antimicrobial Efficacy of Sixteen Essential Oils against Escherichia coli and Aspergillus fumigatus Isolated from Poultry. Vet. Sci. 2018, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Tosi, G.; Massi, P.; Pistelli, L.; Mancianti, F. In Vitro Antimicrobial Activity of Essential Oils against Salmonella enterica Serotypes Enteritidis and Typhimurium Strains Isolated from Poultry. Molecules 2019, 24, 900. [Google Scholar] [CrossRef]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Najar, B.; Pistelli, L.; Mancianti, F. Antibacterial and Antifungal Activity of Essential Oils against Pathogens Responsible for Otitis Externa in Dogs and Cats. Medicines 2017, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Pistelli, L.; Mancianti, F. Antimicrobial Activity of Five Essential Oils against Bacteria and Fungi Responsible for Urinary Tract Infections. Molecules 2018, 23, 1668. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, R.; Rajni, E.; Saxena, R. Synergistic, antidermatophytic activity and chemical composition of essential oils against zoonotic dermatophytosis. Russ. J. Bioorganic Chem. 2022, 48, 1338–47. [Google Scholar] [CrossRef]
- Stringaro, A.; Colone, M.; Cecchetti, S.; Zeppetella, E.; Spadaro, F.; Angiolella, L. "In vivo" and "in vitro" antimicrobial activity of Origanum vulgare essential oil and its two phenolic compounds on clinical isolates of Candida spp. Arch. Microbiol. 2022, 205, 15. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.H.; Ye, M.; Wang, K.B.; Fan, L.M.; Su, F.W. Chemical composition and antifungal activity of essential oil from Origanum vulgare against Botrytis cinerea. Food Chem. 2021, 365, 130506. [Google Scholar] [CrossRef] [PubMed]
- Bismarck, D.; Dusold, A.; Heusinger, A.; Müller, E. Antifungal in vitro Activity of Essential Oils against Clinical Isolates of Malassezia pachydermatis from Canine Ears: A Report from a Practice Laboratory. Complement. Med. Res. 2020, 27, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Saab, A.M.; Tundis, R.; Statti, G.A.; Menichini, F.; Lampronti, I.; Gambari, R.; Cinatl, J.; Doerr, H.W. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem. Biodivers. 2008, 5, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, K.J.; Gokila Vani, M.; Wang, C.-S.; Chen, C.-C.; Chen, Y.-C.; Lu, L.-P.; Huang, C.-H.; Lai, C.-S.; Wang, S.-Y. Geranium and Lemon Essential Oils and Their Active Compounds Downregulate Angiotensin-Converting Enzyme 2 (ACE2), a SARS-CoV-2 Spike Receptor-Binding Domain, in Epithelial Cells. Plants 2020, 9, 770. [Google Scholar] [CrossRef] [PubMed]
- Battistini, R.; Rossini, I.; Ercolini, C.; Goria, M.; Callipo, M.R.; Maurella, C.; Pavoni, E.; Serracca, L. Antiviral Activity of Essential Oils Against Hepatitis A Virus in Soft Fruits. Food Environ. Virol. 2019, 11, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.E.; Benincasa, B.I.; Fachin, A.L.; Contini, S.H.T.; França, S.C.; Chagas, A.C.S.; Beleboni, R.O. Essential oils of Citrus aurantifolia, Anthemis nobile and Lavandula officinalis: in vitro anthelmintic activities against Haemonchus contortus. Parasit. Vectors 2018, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Schlieck, T.M.M.; Petrolli, T.G.; Bissacotti, B.F.; Copetti, P.M.; Bottari, N.B.; Morsch, V.M.; da Silva, A.S. Addition of a blend of essential oils (cloves, rosemary and oregano) and vitamin E to replace conventional chemical antioxidants in dog feed: effects on food quality and health of beagles. Arch. Anim. Nutr. 2021, 75, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M. de O e.; Andrade-Wartha, E.R.S.; Carvalho, E.B.T.; Lima, A.; Novoa, A.V.; Mancini-Filho, J. Effect of the aqueous extract of rosemary (Rosmarinus officinalis L.) on oxidative stress in diabetic rats. Rev. Nutr., Campinas 2011, 24, 121–130. [Google Scholar] [CrossRef]
- Xie, Y.J.; Yang, Z.; Cao, D.; Rong, F.; Ding, H.; Zhang, D. Antitermitic and antifungal activities of eugenol and its congeners from the flower buds of Syzgium aromaticum (clove). Ind. Crops Prod. 2015, 77, 780–786. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: their antibacterial properties and potential applications in foods--a review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Peng, Q.Y.; Liu, Y.R.; Ma, Q.G.; Zhang, J.Y.; Guo, Y.P.; Xue, Z.; Zhao, L.H. Effects of oregano essential oil as an antibiotic growth promoter alternative on growth performance, antioxidant status, and intestinal health of broilers. Poult. Sci. 2021, 100, 101163. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.M.; Anderson, G.; Arguelles-Ramos, M.; Ali, A.A.B. Effect of dietary essential oil of oregano on performance parameters, gastrointestinal traits, blood lipid profile, and antioxidant capacity of laying hens during the pullet phase. Front. Anim. Sci. 2022, 3, 1072712. [Google Scholar] [CrossRef]
- Gumus, R.; Gelen, S.U. Effects of dietary thyme and rosemary essential oils on performance parameters with lipid oxidation, water activity, pH, colour and microbial quality of breast and drumstick meats in broiler chickens. Arch. Anim. Breed 2023, 66, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Righi, N.; Deghima, A.; Ismail, D.; Fernandes, P.A.; Baali, F.; Boumerfeg, S.; Baghiani, A.; Coimbra, M.A.; Coelho, E. Chemical composition and in vivo/in silico anti-inflammatory activity of an antioxidant, non-toxic essential oil from Thymus algeriensis Boiss & Reut. S. Afr. J. Bot. 2023, 157, 64–74. [Google Scholar]
- Grando, M.A.; Costa, V.; Genova, J.L.; Rupolo, P.E.; Azevedo, L.B.; Costa, L.B.; Carvalho, S.T.; Ribeiro, T.P.; Monteiro, D.P.; Carvalho, P.L.O. Blend of essential oils can reduce diarrheal disorders and improve liver antioxidant status in weaning piglets. Anim. Biosci. 2023, 36, 119–131. [Google Scholar] [CrossRef]
- Arooj, B.; Asghar, S.; Saleem, M.; Khalid, S.H.; Asif, M.; Chohan, T.; Khan, I.U.; Zubair, H.M.; Yaseen, H.S. Anti-inflammatory mechanisms of eucalyptol rich Eucalyptus globulus essential oil alone and in combination with flurbiprofen. Inflammopharmacology 2023, 31, 1849–1862. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Abebe, W.; Sousa, S.M.; Duarte, V.G.; Machado, M.I.; Matos, F.J. Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. J. Ethnopharmacol. 2003, 89, 277–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, R.; Gao, T.; Hu, Y.; Zhou, J.; Li, C.; Wang, P.; Yang, H.; Xing, W.; Dong, L.; Gao, F. Repeated Inhalation of Peppermint Essential Oil Improves Exercise Performance in Endurance-Trained Rats. Nutrients 2023, 15, 2480. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.T.; Campos, D.R.; Soares, E.F.M.S.; Assis, J.D.; Oliveira, G.F.; Santos, L.O.; Silva, T.M.E.; Silva, M.P.D.; Cid, Y.P.; Scott, F.B.; Comendouros, K. Larvicidal activity in vitro of essential oils against Cochliomyia hominivorax. Vet. Parasitol. 2023, 322, 110020. [Google Scholar] [CrossRef]
- Costa-Júnior, L.M.; Chaves, D.P.; Brito, D.R.B.; Santos, V.A.F.D.; Costa-Júnior, H.N.; Barros, A.T.M. A review on the occurrence of Cochliomyia hominivorax (Diptera: Calliphoridae) in Brazil. Rev. Bras. Parasitol Vet. 2019, 28, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.; P´erez, M.; Romanelli, G.; Blustein, G. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- Dos Santos, J.V.B.; de Almeida Chaves, D.S.; de Souza, M.A.A.; Riger, C.J.; Lambert, M.M.; Campos, D.R.; Moreira, L.O.; Dos Santos Siqueira, R.C.; de Paulo Osorio, R.; Boylan, F.; Correia, T.R.; Coumendouros, K.; Cid, Y.P. In vitro activity of essential oils against adult and immature stages of Ctenocephalides felis felis. Parasitology 2020, 147, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Basij, M.; Sahebzadeh, N.; Shahriari, M.; Panahandeh, S. Insecticidal potential of Ajwain essential oil and its major components against Chilo suppressalis Walker. J. Plant Dis. Prot. 2023, 130, 735–745. [Google Scholar] [CrossRef]
- Pedroso, A.L.; Schonwald, M.K.; Dalla Corte, C.L.; Soares, F.A.A.; Sperança, A.; Godoi, B.; de Carvalho, N.R. Effects of Rosmarinus officinalis L. (Laminaceae) essential oil on adult and larvae of Drosophila melanogaster. Toxicol. Res. (Camb) 2023, 12, 913–921. [Google Scholar] [CrossRef]
- Nardoni, S.; Pistelli, L.; Baronti, I.; Najar, B.; Pisseri, F.; Bandeira Reidel, R.V.; Papini, R.; Perrucci, S.; Mancianti, F. Traditional Mediterranean plants: characterization and use of an essential oils mixture to treat Malassezia otitis externa in atopic dogs. Nat. Prod. Res. 2017, 31, 1891–1894. [Google Scholar] [CrossRef] [PubMed]
- Soares, N.M.M.; Bastos, T.S.; Kaelle, G.C.B.; de Souza, R.B.M.d.S.; de Oliveira, S.G.; Félix, A.P. Digestibility and palatability of the diet and intestinal functionality of dogs fed a blend of yeast cell wall and oregano essential oil. Animals 2023, 13, 2527. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.; Wells, D.L.; Hepper, P.G. The influence of olfactory stimulation on the behaviour of dogs housed in a rescue shelter. Appl. Anim. Behav. Sci. 2005, 91, 143–153. [Google Scholar] [CrossRef]
- Blaskovic, M.; Rosenkrantz, W.; Neuber, A.; Sauter-Louis, C.; Mueller, R.S. The effect of a spot-on formulation containing polyunsaturated fatty acids and essential oils on dogs with atopic dermatitis. Vet. J. 2014, 199, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Low, S.B.; Peak, R.M.; Smithson, C.W.; Perrone, J.; Gaddis, B.; Kontogiorgos, E. Evaluation of a topical gel containing a novel combination of essential oils and antioxidants for reducing oral malodor in dogs. Am. J. Vet. Res. 2014, 75, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Goode, P.; Ellse, L.; Wall, R. Preventing tick attachment to dogs using essential oils. Ticks Tick Borne Dis. 2018, 9, 921–926. [Google Scholar] [CrossRef]
- Monteiro, C.; Ferreira, L.L.; de Paula, L.G.F.; de Oliveira Filho, J.G.; de Oliveira Silva, F.; Muniz, E.R.; Menezes, K.M.F.; de Camargo, F.R.; de Oliveira Nonato, R.; Martins, D.B.; Marreto, R.N.; Borges, L.M.F. Thymol and eugenol microemulsion for Rhiphicephalus sanguineus sensu lato control: Formulation development, field efficacy, and safety on dogs. Vet. Parasitol. 2021, 296, 109501. [Google Scholar] [CrossRef] [PubMed]
- Schlieck, T.M.M.; Petrolli, T.G.; Bissacotti, B.F.; Copetti, P.M.; Bottari, N.B.; Morsch, V.M.; da Silva, A.S. Addition of a blend of essential oils (cloves, rosemary and oregano) and vitamin E to replace conventional chemical antioxidants in dog feed: effects on food quality and health of beagles. Arch. Anim. Nutr. 2021, 75(5), 389–403. [Google Scholar] [CrossRef] [PubMed]
- Batista, L.C.; Cid., Y.P.; De Almeida, A.P.; Prudêncio, E.R.; Riger, C.J.; De Souza, M.A.; Coumendouros, K.; Chaves, D.S. In vitro efficacy of essential oils and extracts of Schinus molle L. against Ctenocephalides felis felis. Parasitology, 2016; 143, 627–638. [Google Scholar]
- Nardoni, S.; Costanzo, A.G.; Mugnaini, L.; Pisseri, F.; Rocchigiani, G.; Papini, R.; Mancianti, F. Open-field study comparing an essential oil-based shampoo with miconazole/chlorhexidine for haircoat disinfection in cats with spontaneous microsporiasis. J. Feline Med. Surg. 2017, 19, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Mugnaini, L.; Nardoni, S.; Pinto, L.; Pistelli, L.; Leonardi, M.; Pisseri, F.; Mancianti, F. In vitro and in vivo antifungal activity of some essential oils against feline isolates of Microsporum canis. J. Mycol Med. 2012, 22, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.L.; Wells, D.L. The influence of olfactory stimulation on the behaviour of cats housed in a rescue shelter. Appl. Anim. Behav. Sci. 2010, 123, 56–62. [Google Scholar] [CrossRef]
- Štrbac, F.; Krnjajić, S.; Stojanović, D.; Ratajac, R.; Simin, N.; Orčić, D.; Rinaldi, L.; Ciccone, E.; Maurelli, M.P.; Cringoli, G.; Bosco, A. In vitro and in vivo anthelmintic efficacy of peppermint (Mentha x piperita L.) essential oil against gastrointestinal nematodes of sheep. Front. Vet. Sci. 2023, 10, 1232570. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Abo-Elmaaty, A.M.A.; Zaglool, A.W.; Mohamed, S.A.M.; Abou-Zeid, S.M.; Farag, M.R.; Alagawany, M.; Di Cerbo, A.; Azzam, M.M.; Alhotan, R.; El-Hady, E. Origanum vulgare Essential Oil Modulates the AFB1-Induced Oxidative Damages, Nephropathy, and Altered Inflammatory Responses in Growing Rabbits. Toxins 2023, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Štrbac, F.; Bosco, A.; Pušić, I.; Stojanović, D.; Simin, N.; Cringoli, G.; Rinaldi, L.; Ratajac, R. The use of essential oils against sheep gastrointestinal nematodes. In Animal Health Perspectives, 1st ed.; Abbas R, Z., Khan, A., Liu, P., Saleemi M, K., Eds.; Unique Scientific Publishers: Faisalabad, Pakistan, 2022; Volume 1. [Google Scholar]
- Vostinaru, O.; Heghes, S.C.; Filip, L. Safety profile of essential oils. In Essential Oils-Bioactive Compounds, New Perspectives and Applications., 1st ed.; de Oliveira, M.S., da Costa, W.A., Silva, S.G., Eds.; IntechOpen: London, United Kingdom, 2020; Volume 1. [Google Scholar] [CrossRef]
- Katiki, L.M.; Chagas, A.C.; Takahira, R.K.; Juliani, H.R.; Ferreira, J.F.; Amarante, A.F. Evaluation of Cymbopogon schoenanthus essential oil in lambs experimentally infected with Haemonchus contortus. Vet. Parasitol. 2012, 186, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Katiki, L.M.; Araujo, R.C.; Ziegelmeyer, L.; Gomes, A.C.P.; Gutmanis, G.; Rodrigues, L.; Bueno, M.S.; Veríssimo, C.; Louvandini, H.; Ferreira, J.F.S.; Amarante, A.F.T. Evaluation of encapsulated anethole and carvone in lambs artificially- and naturally-infected with Haemonchus contortus. Exp. Parasitol. 2019, 197, 36–42. [Google Scholar] [CrossRef]
- Woolf, A. Essential oil poisoning. J. Toxicol. Clin. Toxicol. 1999, 37, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.G.; McLean, M.K.; Khan, S.A. Adverse reactions from essential oil-containing natural flea products exempted from Environmental Protection Agency regulations in dogs and cats. J. Vet. Emerg. Crit. Care (San Antonio). 2012, 22, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Lans, C.; Turner, N.; Khan, T. Medicinal plant treatments for fleas and ear problems of cats and dogs in British Columbia, Canada. Parasitol. Res. 2008, 103, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Veerakumari, L. Botanical anthelmintics. Asian J. Sci. Technol. 2015, 6, 1881–1894. [Google Scholar]
- Prakash, P.; Radha Kumar, M.; Pundir, A.; Puri, S.; Prakash, S.; Kumari, N.; Thakur, M.; Rathour, S.; Jamwal, R.; Janjua, S. Documentation of commonly used ethnoveterinary medicines from wild plants of the high mountains in Shimla District, Himachal Pradesh, India. Horticulturae 2021, 7, 351. [Google Scholar] [CrossRef]
- Borges, D.G.L.; Borges, F.D.A. Plants and their medicinal potential for controlling gastrointestinal nematodes in ruminants. Nematoda 2016, 3, e92016. [Google Scholar] [CrossRef]
- de Aquino Mesquita, M.; E Silva Júnior, J.B.; Panassol, A.M.; de Oliveira, E.F.; Vasconcelos, A.L.; de Paula, H.C.; Bevilaqua, C.M. Anthelmintic activity of Eucalyptus staigeriana encapsulated oil on sheep gastrointestinal nematodes. Parasitol. Res. 2013, 112, 3161–3165. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Bouquillon, S.; Fauconnier, M.-L. Encapsulation of Essential Oils for the Development of Biosourced Pesticides with Controlled Release: A Review. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef] [PubMed]
- Junkuszew, A.; Milerski, M.; Bojar, W.; Szczepaniak, K.; Le Scouarnec, J.; Tomczuk, K.; Dudko, P.; Studzińska, M.B.; Demkowska-Kutrzepa, M.; Bracik, K. Effect of various antiparasitic treatments on lamb growth and mortality. Small Rumin. Res. 2015, 123, 306–313. [Google Scholar] [CrossRef]

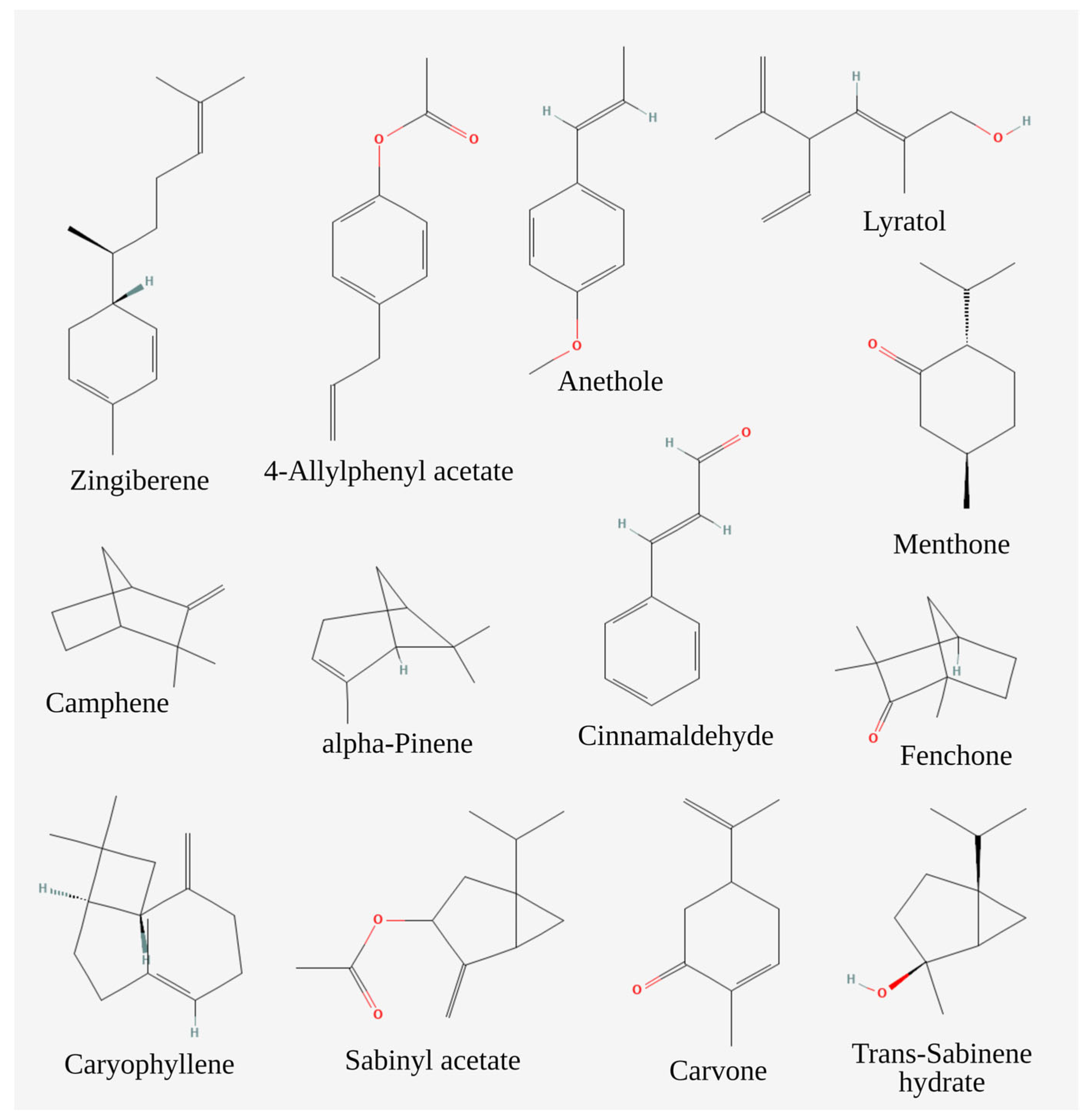
| Phytocompounds (Concentration in %) | Source of EOs | Ref. |
|---|---|---|
| Geranial (35.07%) | Lippia alba | [1] |
| Neral (27.8%) | ||
| Trans-caryophyllene (6.72%) | ||
| Geranial/α-citral (42.88%) | Cymbopogon citratus | [1] |
| β-Citral (32.15%) | ||
| Myrcene (9.82%) | ||
| Carvacrol (18.97%) | Origanum vulgare | [1] |
| Trans-sabinene hydrate (17.75%) | ||
| Terpinen-4-ol (7.57%) | ||
| Thymol (48.9%) | Thymus vulgaris | [19] |
| p-Cymene (19.0%) | ||
| γ-Terpinene (4.1%) | ||
| Carvacrol (3.5 %) | ||
| β-Caryophyllene (3.5 %) | ||
| Carvacrol (12.8%) | Thymus tosevii | |
| α-Terpinyl acetate (12.3%) | ||
| cis-Myrtanol (11.2%) | ||
| Thymol (10.4%) | ||
| Menthol (37.4%) | Mentha piperita | [19] |
| Menthyl acetate (17.4%) | ||
| Menthone (12.7%) | ||
| Limonene (6.9 %) | ||
| Carvone (49.5%) | Mentha spicata | |
| Menthone (21.9%) | ||
| Limonene (5.8 %) | ||
| 1,8-Cineole (26.54%) | Rosmarinus officinalis L. | [20] |
| α-Pinene (20.14%) | ||
| Camphor (12.88%) | ||
| Camphene (11.38%) | ||
| β-Pinene (6.95%) | ||
| cis-α-Santalol (39%) | Santalum album | [21] |
| cis-β-Santalol (17.38%) | ||
| β-Curcumen-12-ol (9.71%) | ||
| n-Hexadecanoic acid (78.25%) | Azadirachta indica | [22] |
| Tetradecanoic acid (7. 24%) | ||
| Silane, triethylfluoro- (3.96%) | ||
| 1,6-Octadien-3-ol, 3,7-dimethyl- (41.74%) | Lavandula angustifolia | [22] |
| Silane, triethylfluoro- (36.71%) | ||
| Bicyclo [2.2.1] heptan-2-one, 1,7,7-trimethyl-, (+)- (6.91%) | ||
| a-Zingiberene (36.78%) | Zingiber officinale | [23] |
| b-Sesquiphellandrene (10.25%) | ||
| ar-Curcumene (9.51%) | ||
| a-Farnesene (6.84%) | ||
| Camphene (3.80%) | ||
| b-Bisabolene (3.65%) | ||
| 1,8-Sineol (24.38%) | Alpinia galanga | [24] |
| cis-β-Farnesene (12.19%) | ||
| β-Pinene (8.48%) | ||
| Phenol, 4-(2-propenyl)-, acetate (6.01%) | ||
| (S)-4-(1-Acetoxyallyl) phenyl acetate (5.66%) | ||
| Trans-Anethole* | Pimpinella anisum | [25] |
| γ-Himachalene* | ||
| Linalool* | Ocimum basilicum | |
| 1,8-Cineole* | ||
| Methyl eugenol* | ||
| Limonene* | Citrus bergamia | [25] |
| Linalyl acetate* | ||
| γ-Terpinene* | ||
| Linalool* | ||
| Eugenol* | Cinnamomum zeylanicum | |
| Cinnamyl acetate* | ||
| Terpinen-4-ol* | Malaleuca alternifolia | |
| α-Terpineol* | ||
| 1,8-Cineole* | ||
| α-Terpinene* | ||
| γ-Terpinene* | ||
| Eugenol* | Syzygium aromaticum | [25] |
| β-Caryophyllene* | ||
| 1,8-Cineole* | ||
| α-Pinene* | ||
| Anethole* | Foeniculum vulgare | |
| Fenchone* | ||
| Geranial* | Zingiber officinale | |
| Neral* | ||
| β-Caryophyllene* | Hypericum perforatum | |
| α-Pinene* | ||
| Linalyl acetate* | Lavandula angustifolia | [25] |
| Linalool* | ||
| Terpinen-4-ol* | ||
| Ocimene* | ||
| Geranial* | Cymbopogon citratus | |
| Neral* | ||
| 1,8-Cineole* | Thymus mastichina | |
| Linalool* | ||
| Menthol* | Mentha piperita | [25] |
| Menthone* | ||
| α-Thuyone* | Rosmarinus officinalis | |
| α-Pinene* | ||
| Camphene* | ||
| Camphor* | ||
| Lyratol* | Artemisia vulgaris | [25] |
| 1,8-cineole* | ||
| α-Thuyone* | Salvia officinalis | |
| Camphor* | ||
| 1,8-Cineole* | ||
| α-Humulene* | ||
| Carvacrol* | Satureja montana | [25] |
| p-Cymene* | ||
| 1,8-Cineole* | Thymus vulgaris | |
| β-Phellandrene* | ||
| Camphor* | ||
| Terpinene (52.24%) | Melaleuca alternifolia | [26] |
| Dihydro-α-terpineol (5.97) | ||
| Diterpene (2.87%) | ||
| (L)-alpha-terpineol (18.32%) | Citrus limon | |
| Alpha-terpinol (13.43%) | ||
| Trans-4-thujanol (9.64%) | ||
| α- Terpinolene (5.81%) | ||
| Citral propylene glycol acetal (5.73%) | ||
| Geranial propylene glycol acetal (4.00%) | ||
| α-Terpineol acetate (3.60%) | ||
| (E)-Cinnamaldehyde (69.0 %) |
Cinnamomum verum J. Presl (Bark oil) |
[27] |
| Eugenol (6.43%) | ||
| b-Caryophyllene (6.33%) | ||
| Linalool (5.02%) | ||
| Eugenol (79.0%) |
Cinnamomum verum J. Presl (Leaf oil) |
|
| Eugenyl acetate (2.71%) | ||
| Benzyl benzoate (3.54%) | ||
| (E)-Cinnamaldehyde (0.86%) | ||
| Sabinene* | Myristica fragrans Houtt. | [28] |
| a-Pinene (pin-2(3)- ene) * | ||
| Myristicin* | ||
| b-Pinene (pin-2(10) ene) * | ||
| 4-Terpineno* | ||
| Limonene* | ||
| c-Terpinene* |
| Study subjects | Essential oil | Dose and duration | Results | Ref. |
|---|---|---|---|---|
| Dogs with otitis | Mixture 1: Citrus paradisi (0.5%), Salvia sclarea (0,5%), Ocimum basilicum (0.5%), Rosmarinus officinalis (1%). Mixture 2: Citrus limon (1%), R. officinalis (1 %), Anthemis nobilis (0.5 %), S. sclarea (0.5%). Mixture 3: S. sclarea (1%), Lavandula hybrida (1%), R. officinalis (1 %). Mixture 4: C. limon (1%), R. officinalis (0.5%), C. paradisi (1%), A. nobilis (0.5 %). Mixture 5: Thymus vulgaris (0.5 %), A. nobilis (1%), C. paradisi (0.5%), L. hybrida (1%). | 200 μL of oil mix per ear once daily for 2 weeks | Mixture 2 showed better improvement in canine otitis. | [66] |
| Dogs | Diet 1: Control; Diet 2: 1.5 kg/ton of yeast cell wall and oregano EO (1.5 YCO); Diet 3: 3.0 kg/ton of yeast cell wall and oregano EO (3.0 YCO). |
1.5 kg/ton YCO or 3.0 kg/ton YCO twice a day for 20 days | Dogs treated with the YCO blend showed signs of enhanced intestinal function. Beneficial bacterial diversity was increased. The concentrations of histamine, phenol, and ammonia were reduced. | [67] |
| Dogs | EOs of Lavandula angustifolia, Anthennis nobilis, Cymbopogon citrates and Mentha piperita. | *EO was diffused using an oil burner into the dogs’ places for 4 h per day for five consecutive days. After 2 days of break, the next EO was used. | L. angustifolia and A. nobilis EOs improved the behaviors and relaxation of dogs in the rescue shelter. | [68] |
| Dogs with CAD | PUFAs: (6 mg/mL of a-linolenic and 30 mg/mL of linoleic acid); EOs (neem oil, rosemary extract, lavender oil, clove oil, tea tree oil, oregano extract, peppermint extract and cedar bark extract) * |
Dogs <10, 10 to 20, and 20 to 40 kg received 0.6, 1.2, and 2.4 mL once a week for 8 weeks. | The topical preparation containing PUFAs and EOs ameliorates the clinical signs of CAD and is safe for dogs. | [69] |
| Dogs | Placebo or active gel** containing EO compounds (menthol and thymol) and polyphenolic antioxidants (phloretin and ferulic acid). | 12 mm of gel/side of mouth. Twice daily for 4 weeks. |
A daily application of tested formulation following an initial dental cleaning reduced halitosis in dogs. | [70] |
| Dogs | EOs of turmeric and orange (negative control) | 2.5% (v/v) of turmeric and orange EOs diluted in water with a 1% coco glucoside excipient. Ten sprays per day for 28 days. | Dogs treated with turmeric EO showed a significantly reduced percentage of ticks attached to their legs or bellies compared to controls. | [71] |
| Dogs infested with ticks | Microemulsion containing 0.5 mg/ mL of thymol and 0.5 mg/ mL of eugenol. | Each dog was sprayed with 10 mL of microemulsion /kg daily for 3 days. | The microemulsion reduced the number of tick larvae in dogs and reduced the larval hatching. The microemulsion was stable and safe. |
[72] |
| Dogs | A mixture of EOs of 6% clove, 2% rosemary, 1% oregano, 3.3% vitamin E, and 87.7% soybean oil (vehicle). | 1% of test mixture in dry feed. The control and test groups had 380 g of feed daily for 28 days. Then, the animals were swapped with a 15-day washout period. | Improved the antioxidant status of the study subjects. | [73] |
| Cats infected with Microsporum canis (dermatophytosis) | Shampoo containing Thymus serpyllum (2%), O. vulgare (5%), and R. officinalis (5%), and itraconazole. | Oral itraconazole (5 mg/kg/day) for 1 week, every 2 weeks for at least 6 weeks. Washed twice a week using 5 mL of shampoo during treatment. | The treatment was effective, and shampoo could be an alternative cat dermatophytosis treatment. | [75] |
| Cats infected with Microsporum canis (dermatophytosis) | EO mixture containing 2% Thymus serpillum, 5% O. vulgare, and 5% R. officinalis in sweet almond oil | EO mixture was applied to the lesion for one month. Itrafungol® (5 mg/kg/day) for 1 week, washout period for 1 week (3 cycles)-served as efficacy control. | Five out of seven cats that received EO treatment showed recovery. No adverse effects were observed in any of the treated cats. | [76] |
| Sheep | EO of Mentha piperita diluted in sunflower oil at 1: 4.5 ratio. | Regular diet (barley and maize grains) with 150 mg/kg EO. Diet and 3.8 mg/kg of albendazole (Positive control). Diet and 50 mL of sunflower oil/ animal (negative control). For 14 days. | EO of M. piperita has potent anthelmintic efficacy. It could be used to control gastrointestinal nematodes in sheep. | [78] |
| Rabbit | O. vulgare EO* | Control, AFB1 group (0.3 mg AFB1/kg diet), OEO group (1 g OEO/kg diet), and Combination group (1 g OEO/kg + 0.3 mg AFB1/kg diet) for 8 weeks. | OEO supplementation improved the harmful effects of AFB1. Improved the antioxidant levels, Decreased the inflammation, and reversed oxidative DNA damage in rabbits. |
[79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





