Background
Lyme disease (LD), also called Lyme borreliosis, is the most common vector-borne illness in the United States (U.S), Europe, and Asia, with approximately 476,000 estimated new cases diagnosed annually in the U.S. alone [
1]. Cases have increased by 59% in the last year, per the Centers for Disease Control and Prevention (CDC) [
2]. The etiologic agent of LD is the spirochete pathogen
Borrelia burgdorferi (Bb) and the tick vector
Ixodes scapularis, or the deer tick. Bb begins infection at the tick bite site in the host’s skin. A bull’s-eye rash, known as erythema migrans, can appear on the skin due to the spread of Bb from the initial bite site. Symptoms of early-stage disease mimic flu-like symptoms such as fever, headache, fatigue, muscle and joint aches, and swollen lymph nodes [
3]. However, 20-30% of patients do not display the rash, which can lead to misdiagnosis [
4]. Frequent misdiagnoses further exacerbate disease into late-stage LD where Bb travels to distant organs, including the joints, heart, and central nervous system [
4,
5]. This can cause complications including Lyme arthritis, carditis, and meningitis [
5]. Symptoms include arthritis with severe joint pain and swelling, heart palpitations, and inflammation of the brain and spinal cord [
4]. Currently, the only treatment for LD is antibiotics; however, antibiotics are only effective during the early-stage LD [
6]. Also, after treatment and Bb clearance, patients can still develop Post-treatment Lyme Disease Syndrome (PTLDS), a chronic inflammatory disease in patients previously diagnosed with LD [
7]. These complications indicate a need for a human LD vaccine.
Despite efforts to create a vaccine [
8,
9], no effective FDA-approved human LD vaccine exists. The rise in global temperatures has caused the tick population to double in the past two decades [
10]. The CDC predicts that a continued rise in the tick population will increase LD cases [
10]. Additionally, the economic burden of LD is significant; the current annual cost of treatment is estimated to be
$1.3 million per year for the U.S. healthcare system [
3]. A rise in cases will continue to increase costs, whereas a preventative vaccine would provide a long-term solution for preventing LD [
10].
Previously, the FDA approved an LD vaccine, LYMErix, a recombinant OspA protein vaccine adjuvanted with alum [
11]. OspA has been shown to protect against Bb, and other strains of
Borrelia. LYMErix decreased LDA rates by 76% within the first year on the market [
11]. However, there was a reported linkage of OspA-specific serum IgG to an epitope on the human leukocyte function-associated antigen-1 (hLFA1) [
12], which was predicted to cause arthritis in vaccinated patients [
13]. Despite studies concluding that autoimmunity induced by the LYMErix vaccine was insignificant, vaccine sales declined, which resulted in the removal of the vaccine from the market [
10]. Nonetheless, OspA remains a target antigen in LD vaccines, such as in Vanguard crLyme [
14], a dog vaccine, and VLA15, a human vaccine developed by Pfizer currently in phase III clinical trials (NCT05477524) [
8].
To avoid the possible autoimmune effects, we developed a non-OspA LD vaccine with the protective outer surface protein, BBI39 [
15]. BBI39 is an outer surface protein produced on Bb while residing in the tick and early host infection in the skin [
15]. This compares to OspA, which is solely produced while Bb resides in the tick and is downregulated when entering the host [
16]. A previous study found that mice vaccinated with the recombinant protein, BBI39, induced BBI39-specific antibodies, depleted borrelial load, and reduced LD pathogenesis in the heart and joints [
15]. Therefore, we used BBI39 as our target antigen for our rabies virus (RABV) vectored LD vaccine.
To create an effective LD vaccine, a highly immunogenic vaccine vector is crucial. Rabies virus (RABV) vaccine vectors produce a long-lasting humoral immune response [
17]. These vaccine vectors are safe because they are highly attenuated and can be made into inactivated vaccines [
18,
19,
20,
21]. This study utilized the recombinant RABV vector BNSP333, derived from the SAD-B19, an attenuated wildlife rabies vaccine strain [
22]. BNSP333 has been further attenuated by an arginine-to-glutamine mutation at amino acid position 333 of the RABV glycoprotein (G). This mutation further reduces the neurovirulence of the RABV vector and increases its safety profile [
23,
24]. BNSP333 has a simple genome with only five proteins, making it easy to manipulate and allowing for the addition of stably expressed foreign antigens [
25]. The RABV vaccine is highly immunogenic; it induces long-term immunity and a type-1 biased immune response, making it an ideal vector for protection against Bb [
26,
27]. RABV has been used as a vaccine vector against various infectious diseases including SARS-CoV-2 (CoraVax) [
21], Lassa virus (LassaRab) [
28], Ebola virus (FiloRab1) [
29], and Crimean Congo Hemorrhagic Fever Virus [
30]. Some of these vaccines have been tested in nonhuman primates (NHPs) and human studies, further describing the safety and efficacy of this vaccine vector [
31].
In this study, we utilized the attenuated RABV vaccine vector with the borrelial outer surface protein BBI39. We demonstrate that the RABV vector successfully incorporated the chimeric BBI39RVG antigen into the RABV virion. Mice immunized with our candidate vaccine, BNSP333-BBI39RVG, induced high and long-term anti-BBI39 antibody titers with a Th-1 biased immune response, compared to the recombinant protein vaccine. In addition, we studied the adjuvant effects of PHAD-SE. Finally, we show that vaccinated mice inhibit Bb infection during both syringe inoculum and via Bb-infected tick challenge, which is induced by borreliacidal antibodies. We show that the RABV vectored BBI39RVG vaccine is an ideal vaccine candidate against LD.
Materials and Methods:
Borrelia burgdorferi, Cell Lines, Mice, and Ticks
Borrelia burgdorferi strain B31 grown in Barbour-Stoenner-Kelly-H (BSK-H) medium complete (supplemented with 6% rabbit serum) (Sigma-Aldrich B8291) was used in this study. Bacteria were grown at 33°C without CO2 in 5 mL bacterial culture tubes. Ixodes scapularis ticks were maintained in a colony within the Pal lab at University of Maryland. Ticks were subjected to microinjection of Bb to perform challenge experiments with infected ticks. BSR and Vero (CCL81) cells were obtained from ATCC and cultured in 1X DMEM (Corning Cat# 10-013-CV) supplemented with 5% fetal bovine serum (FBS) and 1% penicillin-streptomycin. Cells were maintained at 37°C with 5% CO2. Six- to eight-week-old C3H/HeN mice were purchased from Charles River Laboratories. All animal experiments were performed under the guidelines of the Institutional Animal Care and Use Committee and Institutional Bio-safety Committee of Thomas Jefferson University.
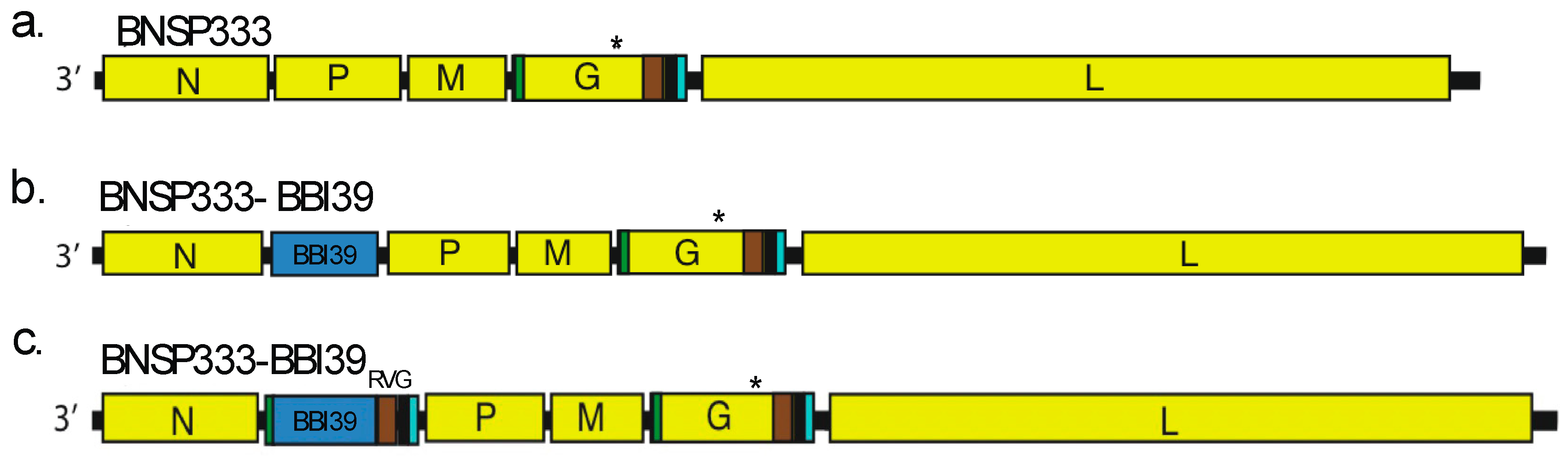
cDNA Molecular Cloning of Vaccine Vectors
We inserted BBI39 and BBI39
RVG, both synthesized by Genscript in pUC57 vectors, in BsiWI and NheI restriction sites of BNSP333 RABV vaccine vector [
20,
32] by T4 Ligation (New England Biolabs catalog #: M0202). This included BNSP333-BBI39 and BNSP333-BBI39
RVG. BBI39
RVG included the final 51 amino acids of the ectodomain (ED51), transmembrane domain (TM), and cytoplasmic domain (CD) of RABV-G, all in the gene synthesis. JM109
E. coli cells were used during molecular cloning under ampicillin resistance. Once plasmids were synthesized, they were sent out for sequencing to Azenta. We utlized forward (5′-GGAGGTCGACTAAAGAGATCTCACATAC-3′) and reverse (5′-TTCTTCAGCCATCTCAAGATCGGCCAGAC) primers for sequencing of BBI39 between RABV-N and RABV-P. WE also used, forward (5′-GTTATGGTGCCATTAAACCGCTG-3′) and reverse (5′-TCTCCAGGATCGATCGAGCATCTT-3′) primers to sequence RABV-G to determine if the 333 mutation was still viable in the glycoprotein before virus recovery.
Recovery of Recombinant Rabies Viruses
Recombinant RABV vectors were recovered on BSR cells in the above-listed conditions. X-tremeGENE 9 transfection reagent (Roche Diagnostics) in Opti-MEM was utilized to transfect BSR cells in 6-well plates with full-length BNSP333 cDNA along with T7 RNA polymerase, RABV nucleoprotein, phosphoprotein, glycoprotein, and polymerse cDNA plasmids. Supernatants were harvested on day 4 post-transfection and overlayed on seeded BSR cells in 12-well plates. After 48 hours, cells were subjected to fix by acetone and staining with a GFP stain against RABV-N (FujiRebio, Cat# 800-092).
Viral Growth, Titration, Purification, and Inactivation
Once viruses were recovered, they were grown on Vero CCL81 cells in viral production serum-free medium (VP-SFM) (Thermo Fisher Scientific) supplemented with 5% Glutamax, 1% penicillin-streptomycin, and 1% HEPES buffer. Cells were infected at a MOI of 0.01 in either T175 flask or 2-stack chambers (Corning). Supernatant collections occurred 5 days post-infection and every 3 days afterward, for a total of 17 days. For titration, RABVs were overlayed on VeroCCL81 cells with a starting dilution of 1:10 and diluted 10-fold in a 96-well plate in triplicate. After 48 hours, cells were fixed with acetone and stained with RABV-N GFP stain to determine the foci forming units (FFU)/mL by fluorescence microscopy.
Viruses were filtered through 0.45 µm PES membrane filters (Nalgene) and concentrated down to 50 mL for purification. Viruses were purified over 20% sucrose cushion and ultracentrifuged at 25,000 rpm in a SW32 rotor for 1.5 hours. Viral particles were resuspended in TEN buffer + 2% sucrose and inactivated with 50 µL per mg of particles with β-propiolactone (BPL, Millipore Sigma, Cat# P5648) in cold culture grade water. The level of inactivation was verified by inoculating Vero CCL81 cells over 3 passages with 10 µg of BPL-inactivated virions.
Immunofluorescence
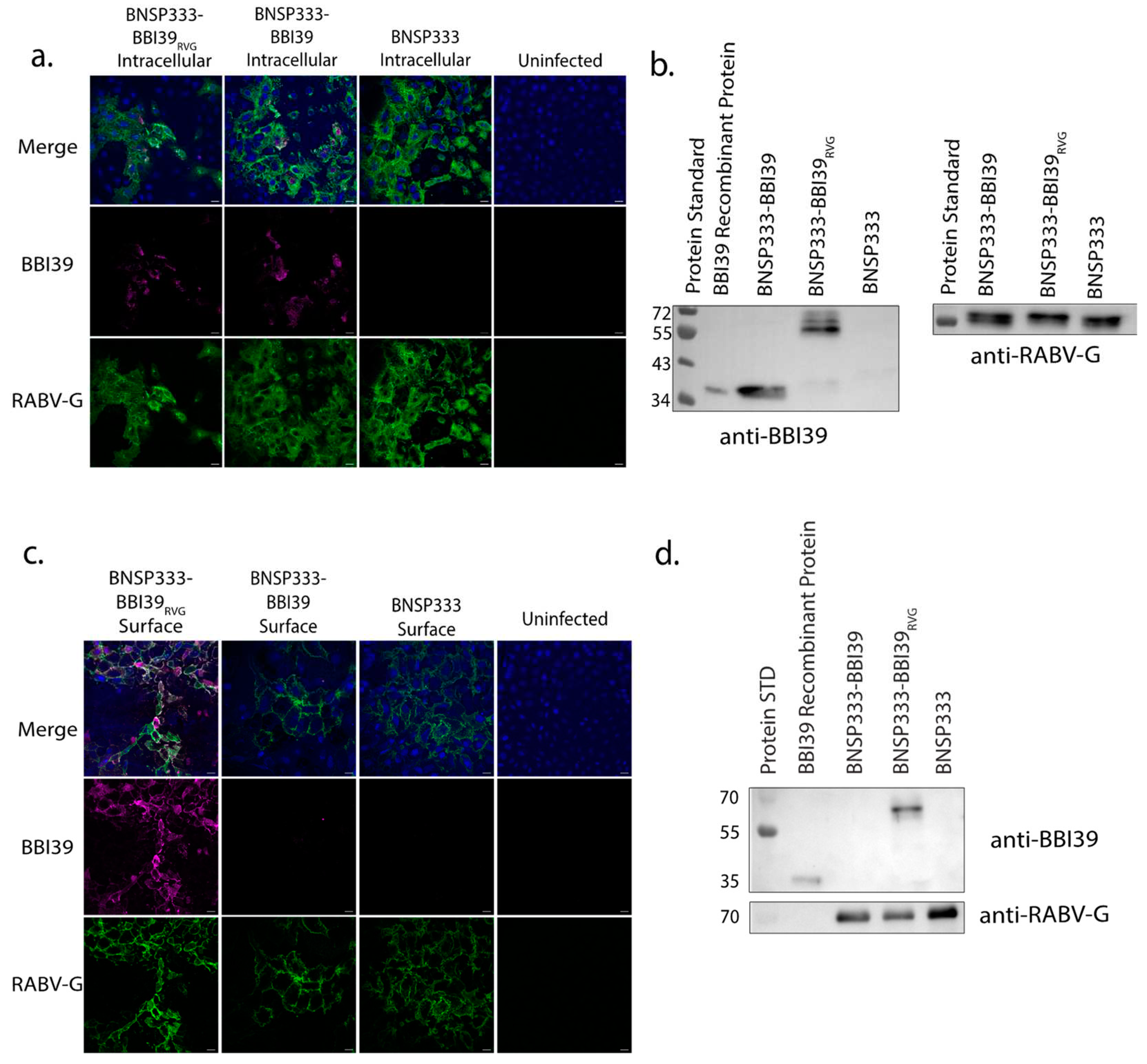
Vero CCL81 cells were seeded on coverslips in 1x DMEM supplemented with 5% FBS and 1% penicillin-streptomycin at 5x105 cells per well. On the same day, cells were infected with BNSP333-BBI39RVG, BNSP333-BBI39, and BNSP333 at an MOI of 0.05. Cells were kept at 34°C for 72 hours and then stained. Cells were fixed in 2% paraformaldehyde (PFA) in 1X PBS for 30 min for surface-stained cells and 15 min for intracellular-stained cells. Then, 2% PFA and 0.01% TritonX were added to intracellular stained cells for another 15 min. After fixing, cells were blocked in PBS with 5% FBS for 1 hour. Following washing with PBS three times, cells were stained with 1:200 primary antibody for 1 hour at room temperature (RT) in PBS with 1% FBS. Primary antibodies included polyclonal mouse anti-BBI39 IgG and human anti-RABV-G 4C12 (provided by Scott Dessain, Lankenau Institute for Medical Research, Wynnewood, PA). Cells were washed with PBS and incubated with secondary antibody in PBS with 1% FBS for 45 min at RT. Secondary antibodies utilized for fluorescent staining were anti-mouse Cy3 and anti-human Cy2. Stained cells were then washed 5 times with PBS and mounted on slides with mounting media containing DAPI (ProLong™ Glass Antifade Mountant, Invitrogen, cat#: P36980). Slides were stored for 24-48 hours in the dark at RT to dry. Slides were visualized with a Nikon A1R confocal microscope. Images were analyzed by Fuji. Red (Cy3) BBI39 staining was changed to magenta by FIJI ImageJ.
Cell Lysate Preparation for Western Blot
For infected cell lysates, 1x106 BSR cells were infected at an MOI of 5 for 72 hours at 34°C in a 6-well plate. Cells were washed twice with cold PBS and 1 mL of RIPA lysis buffer + 1X protease inhibitor (ThermoFisher Halt™ Protease Inhibitor Cocktail 100X, cat#: 78430) was added to lyse infected cells. After 5 minutes of lysis, cells were centrifuged at 14,000 rpm for 1 minute. The supernatant was then subjected to a BCA assay to determine the concentration of the proteins from the cell lysates. Finally, the concentration of cell lysates was adjusted to 10 µg/µL in urea sample buffer containing 2-mercaptoethanol.
Western Blot
Infected cell lysates, recombinant proteins, and purified viral particles were subjected to Western Blot analysis. Lysates and particles were denatured in urea sample buffer and reduced with 2-mercaptoethanol. Samples were boiled for 10 minutes at 95°C. 30 µg of cell lysates, 20 ng of BBI39 recombinant protein, and 1 µg of sucrose purified virions were separated on the gel. Gels were run at 150 V for about 1.5 hours in 1X Laemmli buffer. Proteins were transferred to nitrocellulose membranes for 1 hour at 90 V in 1X Towbin transfer buffer. Electrophoresis of gels and transfer of proteins were done using BioRad western blot equipment. After transfer, membranes were blocked with 5% milk in 1X PBST for 1 hour at RT. Primary antibodies used for probing included polyclonal mouse anti-BBI39 IgG and human anti-RABV-G 4C12 (provided by Scott Dessain, Lankenau Institute for Medical Research, Wynnewood, PA). Blots were probed overnight in 5% BSA in PBS at 4°C. The following day, blots were probed with secondary antibody, which included horseradish peroxidase (HRP)-conjugated anti-mouse (Jackson ImmunoResearch, 115–035–146) at 1:5000 or human IgG (SouthernBiotech, 2040–05) at 1:20,000 diluted in 1X PBST for 1 hour at RT. Proteins were detected with SuperSignal West Dura Chemiluminescent substrate (Thermofisher cat#: A38554) and imaged on a FlourChem R system (ProteinSimple).
Production of Recombinant Proteins for Western Blot, Immunizations, and ELISA
BBI39 was purified using a plasmid from the Utpal Pal laboratory in University Park, Maryland. BBI39 plasmid transformed into JM109 E. coli cells and grew into a 1 L bacterial culture in LB broth with ampicillin. Bacteria were induced with 0.3 mM of ITPG overnight at RT. The following day, bacteria were centrifuged at 4000 rpm for 30 minutes at 4°C. The pellet was resuspended in 20 mL of PBS with 1% TritonX. Then, for further lysis, 0.1 mg/mL of Lysozyme was added to the resuspension. After a 30-minute incubation on ice, bacterial cells were further lysed by sonication for 7 minutes. Lysed bacterial cells were centrifuged at 4000 rpm for 30 minutes at 4°C. BBI39 was purified using Glutathione Agarose (Pierce™, Thermofisher cat#: 16100). The GST tag was removed by 80 units of precision protease (GenScript cat#: Z02799) overnight at 4°C in PCB buffer (50 mM Tris pH 7.0, 150 mM NaCl, 1 mM EDTA, 1 mM DTT, mixed in water). Purification of the protein was further characterized by Western Blot, listed above.
RABV glycoprotein (G) was produced by stripping the glycoprotein from rVSV-ΔG-RABV-G-GFP virions. BEAS-2B cells were infected with rVSV-ΔG-RABV-G-GFP in OptiPRO SFM at an MOI of 0.01. Once all the cells were lysed, supernatants were concentrated and ultracentrifuged through a 20% sucrose cushion at 25,000 rpm for 1.5 hours at 4°C. Viral pellets were then resuspended in β-Octyl-glucopyranoside (OGP) and stripped by ultracentrifugation at 45,000 rpm for 1.5 hours in an SW55Ti rotor at 4°C. Supernatants were collected, frozen in small aliquots, and characterized by Western Blot and ELISA.
Immunizations
Groups of five 6–8-week-old female and male C3H/HeN mice purchased from Charles River Laboratories were immunized intramuscularly (I.M.) with 10 µg of inactivated RABV virions or 1 × 10
4 ffu/mL of live attenuated virus. Five females were used for
Figure 3b,c. Five females and Five males were used for
Figure 2d,e, separately vaccinated from
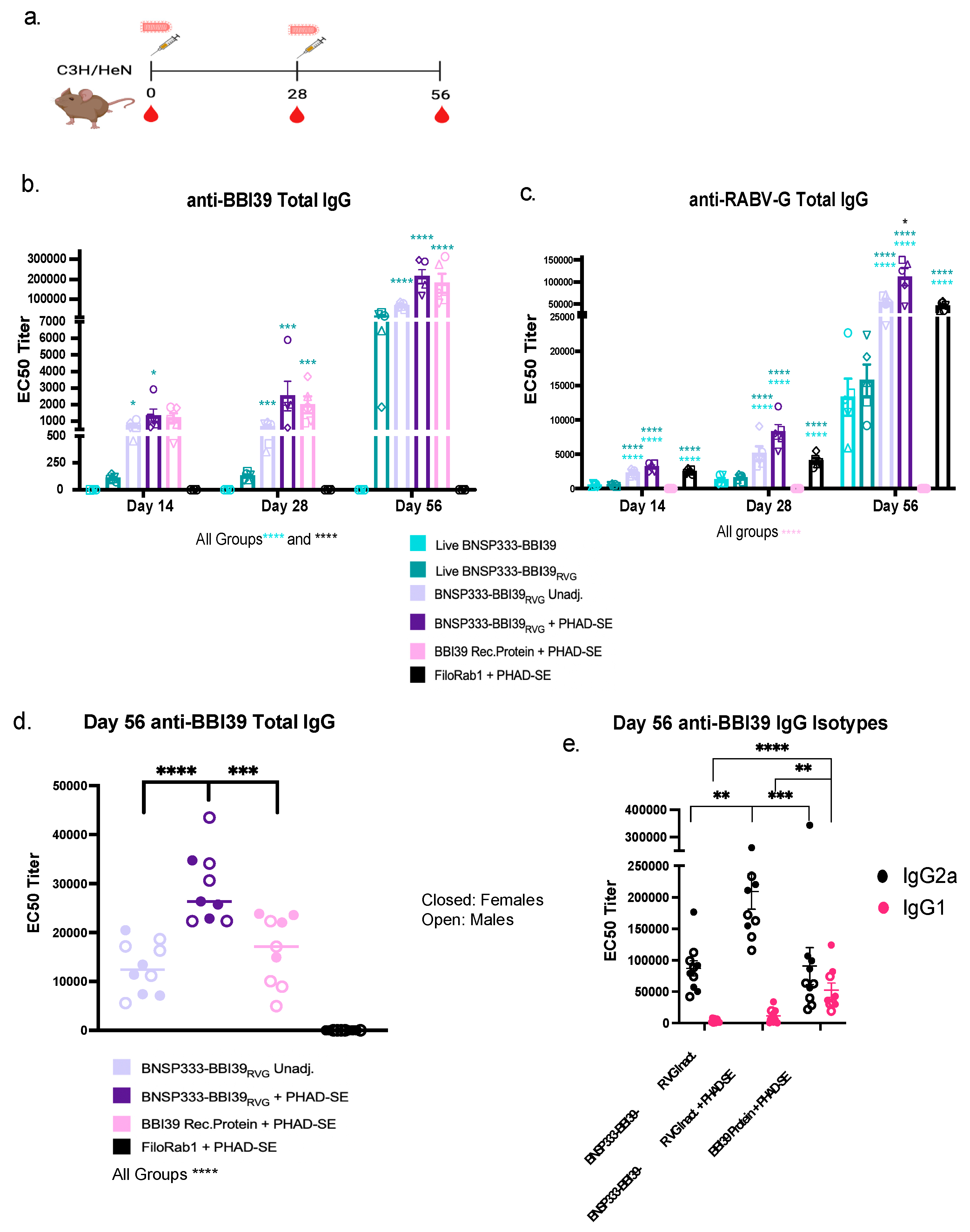
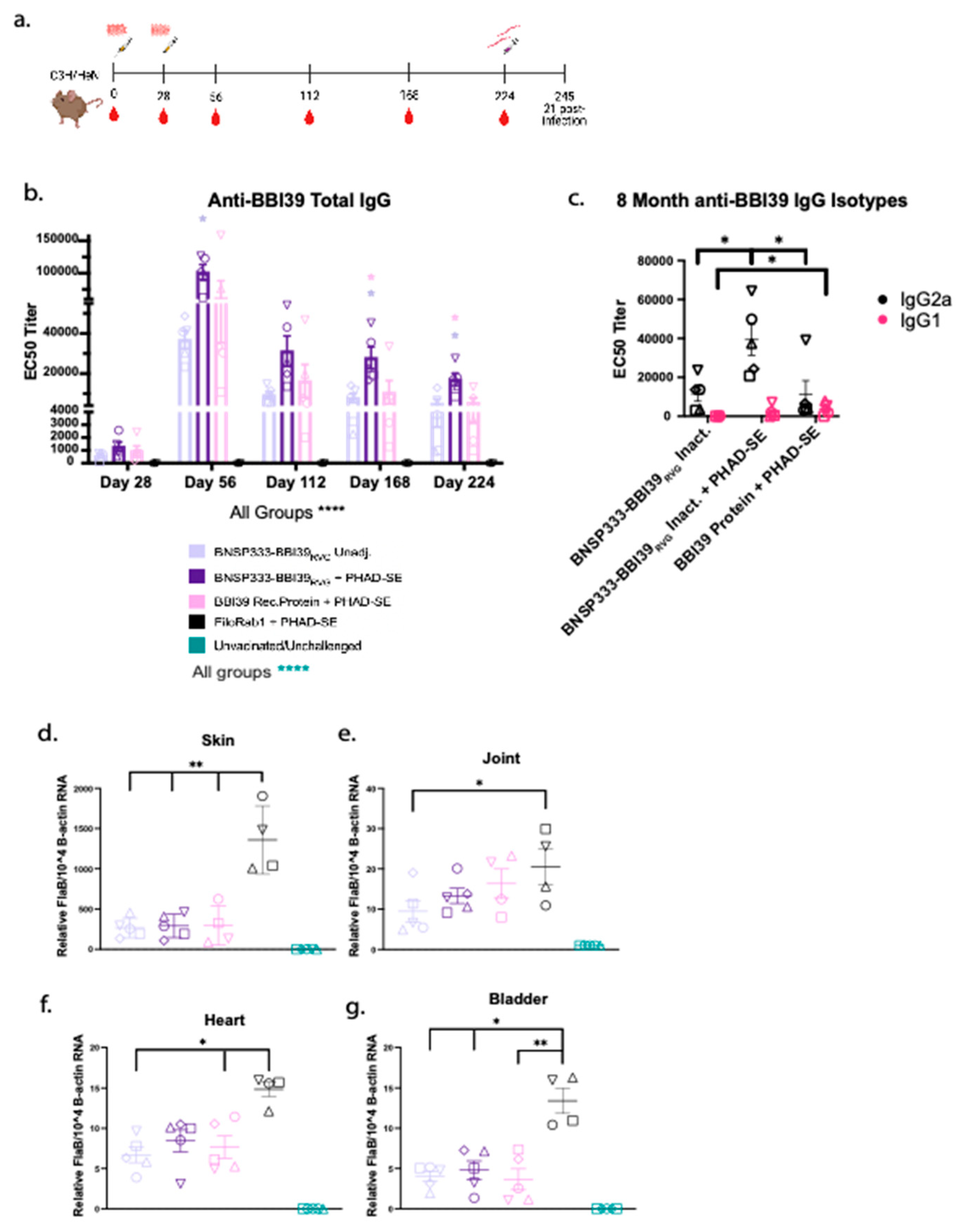
Figure 3b,c. Inactivated vaccines were formulated either unadjuvanted or with 5 µg of synthetic monophosphoryl Lipid-A (MPLA), 3D(6 A)-PHAD, in a squalene oil-in-water emulsion (PHAD-SE) adjuvant. Each immunization contained 100 µL, with 50 µL injected into each hind leg of each mouse. All mice were primed on day 0 and boosted on day 28. Serum for further testing was collected by retroorbital bleeds while mice were under isoflurane anesthesia on days 0, 14, 28, and 56 for short-term experiments with the addition of days 112, 168, and 224 for long-term experiments.
anti-BBI39 and anti-RABV-G ELISA
Total and isotype subclass IgG antibody responses were determined by indirect ELISA. We purified recombinant RABV-G and BBI39, described above, and utilized these proteins to coat Immulon 4 HBX 96-well flat-bottom microtiter plates. Plates were coated with antigens overnight at 4°C with in 15 mM Na2CO3, 35 mM NaHCO3 coating buffer. BBI39 antigen was utilized at 500 ng/well and RABV-G at 50 ng/well. Post-incubation, plates were washed three times with PBS containing 0.05% Tween20 (PBST) and blocked in 5% milk for 2 hours at RT. Plates were washed and primary antibody dilution buffer, containing 0.5% BSA and 0.05% NaN3 in PBST, was added at 100 µL per plate. Mouse sera, at 1:50 starting dilution, was further diluted 3-fold down the plates. Plates were incubated overnight at 4°C with primary antibody. The following day, plates were washed and 100 µL of secondary antibody diluted in PBST was added. Secondary antibodies used in this study were horseradish peroxidase-conjugated goat anti-mouse IgG-Fc (Jackson ImmunoResearch, Cat# 115-005-008); IgG2a (Jackson ImmunoResearch Cat# 115-035-206); or IgG1 (Jackson ImmunoResearch Cat# 115-035-205). All secondary antibodies were diluted to a concentration of 80 ng/mL for BBI39 ELISAs and 25 ng/mL for RABV-G ELISAs. After incubation for 2 hours at RT, plates were washed and then developed with 200 µL/well of o-Phenylenediamine Dihydrochloride substrate (ThermoFisher) for 15 minutes. The reaction was stopped with 3M H2SO4. The optical density (OD) was determined at 490 nm (experimental) and 630 nm (background) on a BioTek ELx800 plate reader on Gen5 software to determine the delta values between the experimental and background readings. ELISA data were analyzed in GraphPad Prism 9 software to determine the EC50 values of antibodies in the mouse sera.
Borreliacidal Assay
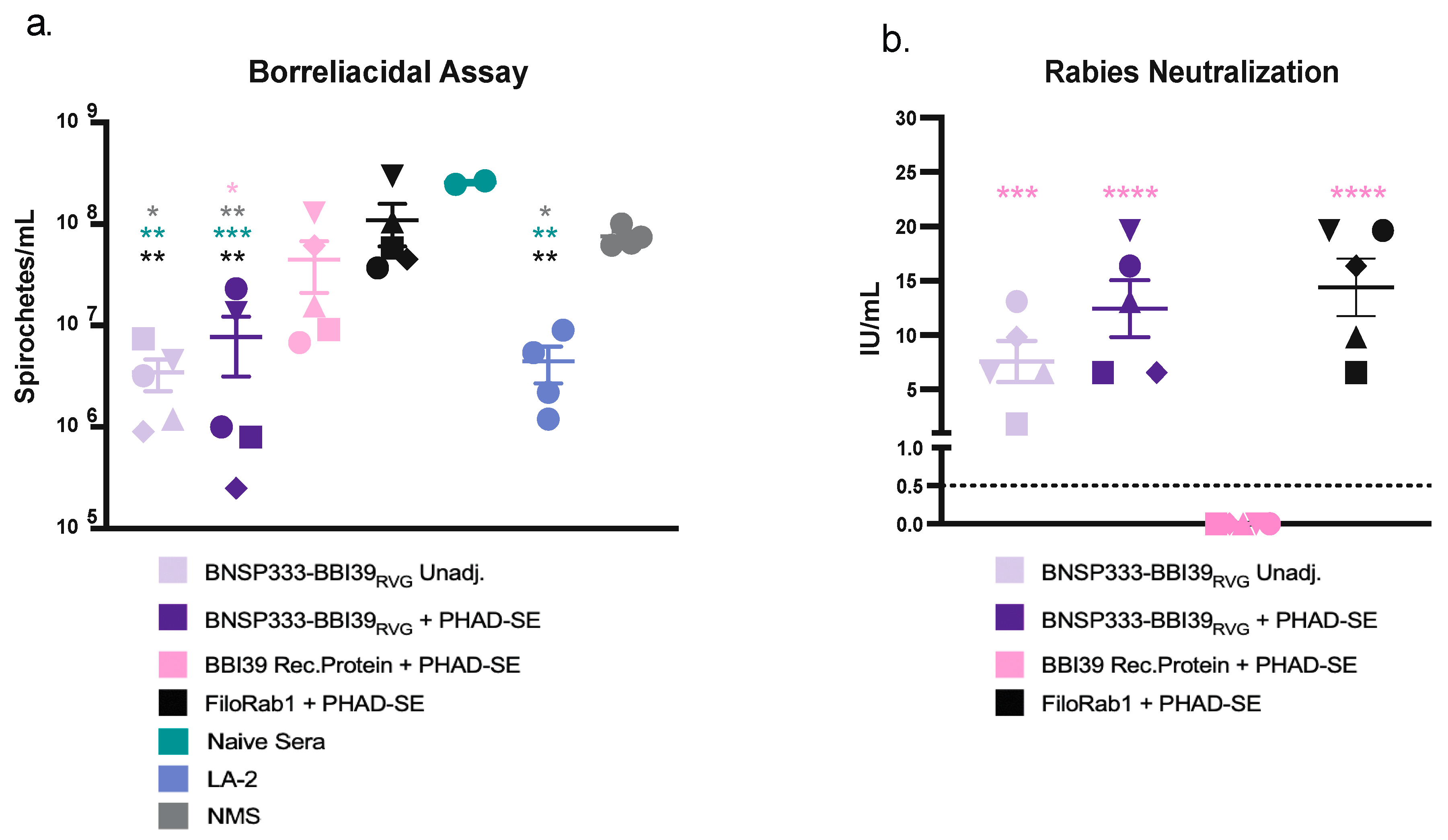
Borrelia burgdorferi, strain B31, was seeded at 1x105 spirochetes/mL in 96-well round-bottom plates with a 1:10 dilution of heat-inactivated mouse sera or 100 ug/mL of LA-2 antibody (Absolute Antibody, Ab01070-3.0) with 1:10 guinea pig complement sera (Sigma S1639) diluted in BSK-H complete media (Sigma-Aldrich B8291) for a total of 100 uL. Mouse sera were heat-inactivated at 56°C for 30 minutes and tested in duplicate. Borrelia was incubated at 33°C with mouse sera for 48 hours in a 96-well round-bottom plate. 1 µL of each well was added to 1 mL of BSK media in Eppendorf tubes. After 7 days, each replicate was counted under a Nikon dark-field microscope with a Petroff-Hausser counting chamber (Hausser Scientific, Cat#: 3900). Borrelial counts were analyzed in GraphPad Prism 9 software to determine if mouse sera inhibited bacterial growth by borreliacidal activity.
RABV Neutralization by Rapid Fluorescent-Focus Inhibition Test (RFFIT)
RFFITs were performed to identify RABV-neutralizing antibodies as described previously [
21]. Mouse sera were heat inactivated at 56°C for 30 minutes. BSR cells were seeded at 25,000 cells/well and cultured in DMEM with 5% FBS and 1% penicillin-streptomycin, in 96-well flat-bottom plates. Individual mouse sera, collected at day 56, were diluted 3-fold with a starting dilution of 1:50. The WHO standard of rabies IgG was used at a starting dilution of 2 international units (IU)/mL. After dilution of sera, CVS11, a challenge virus strain of rabies virus, was added to each well at a titer to achieve 90% infection of BSR cells. The virus and antibody mixture was incubated in a 96-well round-bottom plate for 1 hour at 34°C. After incubation, 105 µL of sera/virus mixture was added to BSR cells and incubated at 34°C for 24 hours. Cells were fixed with 80% acetone and stained with FAD stain against RABV nucleoprotein. Stained cells were assessed for the percentage of viral infection by fluorescent microscopy. The Reed-Muench method was utilized to calculate 50% endpoint titers. Titers were converted to IU/mL by comparison to the WHO standard.
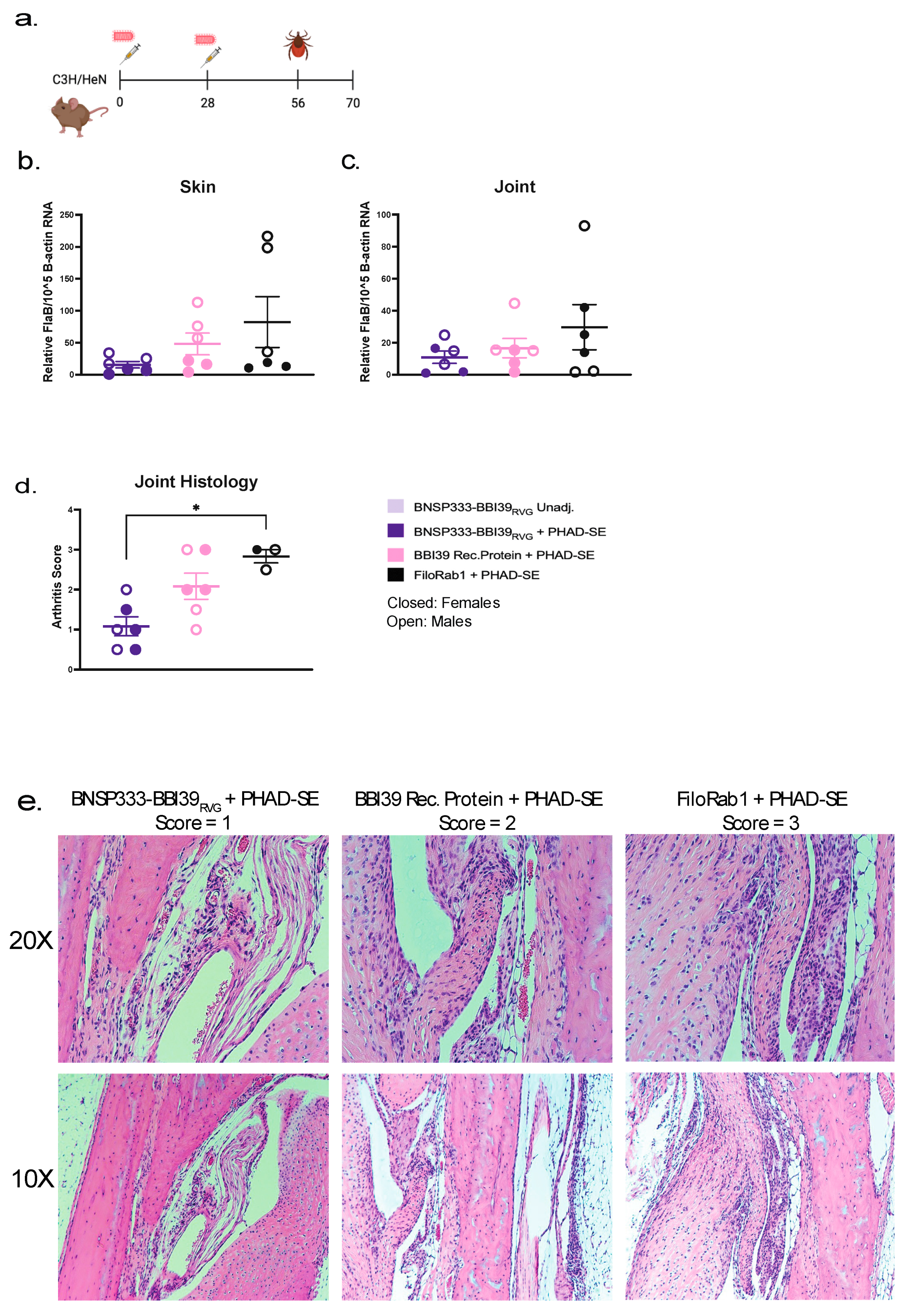
Borrelia burgdorferi Challenge by Needle Injection
Immunized and unimmunized mice were subjected to challenge with
Borrelia burgdorferi, strain B31, by needle route. Mice (
Figure 2d,e) were injected with 1x10
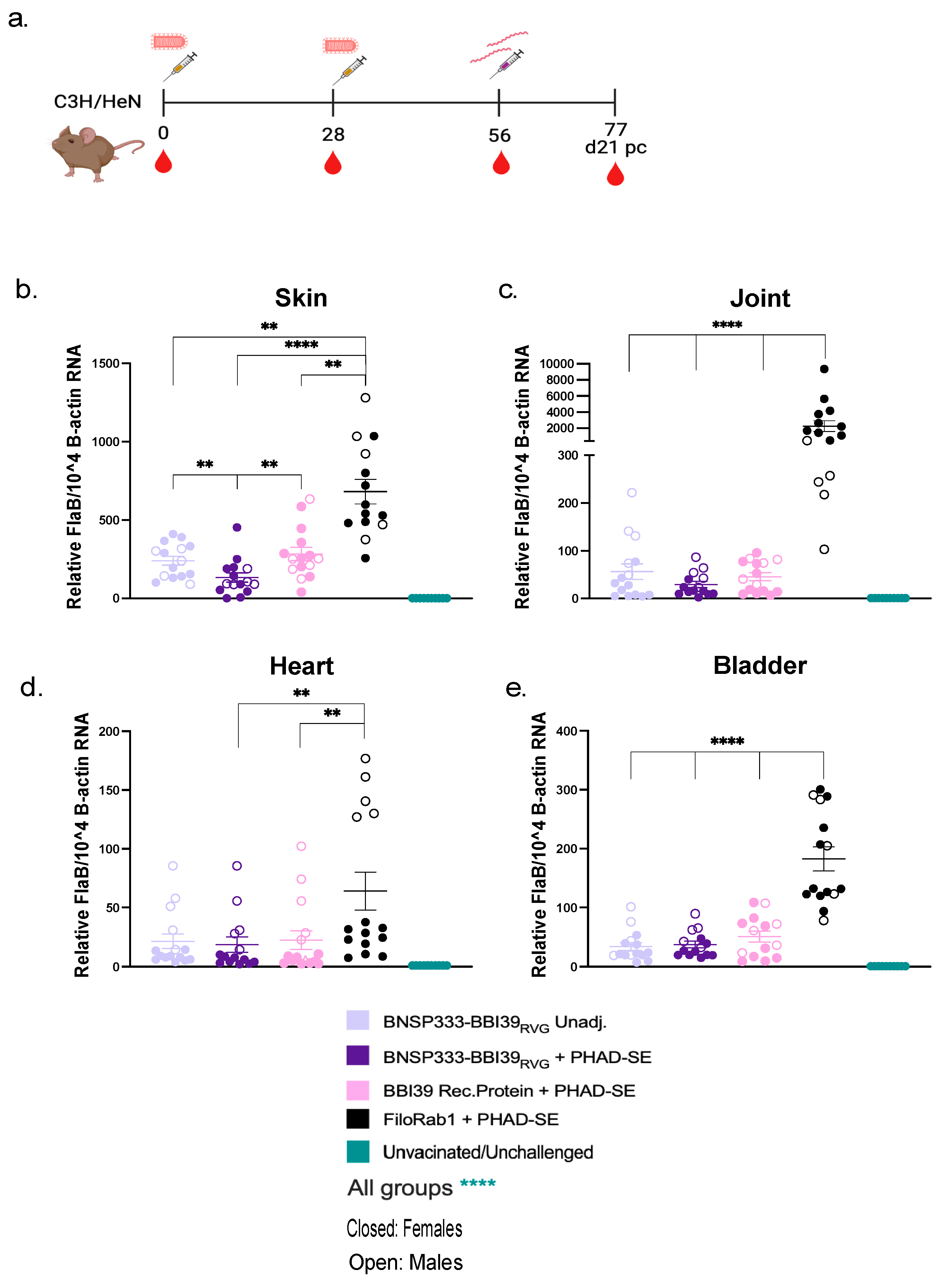
5 spirochetes/100 µL intradermally with an insulin needle. Bacteria were grown in BSK-H complete medium (Sigma-Aldrich B8291) at 33°C before being counted by Nikon dark-field microscopy with a Petroff-Hausser counting chamber (Hausser Scientific, Cat#: 3900). Bacteria were diluted in BSK media in Eppendorf tubes and kept at RT before injection.
21 days post-infection, skin (ear), tibiotarsi joints, heart, and bladders were harvested from infected mice aseptically and were subjected to RNA extraction and qPCR (described below) or for further analysis under dark-field microscopy. Organs were placed in 1 mL BSK-H medium and incubated at 33°C. Every 2 weeks, for 8 weeks, organs were analyzed under dark-field microscopy for qualitative analysis of Borrelia in each organ’s supernatant.
Blood was also collected from challenged mice and subjected to Western Blot analysis to determine whether each mouse was successfully challenged with Borrelia. Borrelia burgdorferi was grown in 50 mL to 1x108 spirochetes/mL. Bacteria were centrifuged at 4000 x g for 20 minutes at 4°C. Borrelial pellets were washed five times with 1 mL of PBS. After each wash, the lysates were centrifuged in Eppendorf tubes at 14,000 x g for 1 minute. Borrelial lysates were subjected to BCA assay to determine the final concentration. Lysates were diluted to a final concentration of 1.5 µg/10 µL in 1X urea sample buffer. Aliquots were frozen at -80°C or used for Western Blot analysis. Lysates were denatured for 10 minutes at 95°C and gels were run and transferred as described above. For primary antibody, individual mouse sera were diluted to 1:1000 in 5% BSA and added to strips of nitrocellulose membrane with Borrelial lysates transferred on each strip. Primary antibody was incubated overnight and further processed as listed above. Individual strips were imaged at the same time to test whether challenged or unchallenged mouse sera responded to borrelial lysates on blots.
Borrelia burgdorferi Challenge by Ixodes scapularis
Groups of 6 mice were immunized as described earlier. On day 56 post-primary immunization, mice were challenged with infected Ixodes scapularis nymphs (5 ticks/mouse). After 3 weeks of infection, mice were euthanized and Bb burden within mouse organs was assessed by qRT-PCR (described below). Skin, heart, and joints were cultured in BSK-H media described above.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
Organs from challenged mice were harvested and placed in 1 mL TRIzol Reagent in 2mL RNase/DNase free Omni tubes (Omni International) which contained beads for homogenization. Bladders were collected in Omni tubes with 1.4 mm ceramic beads (Cat#: 19-627D). Hearts and skin (ear) were collected in Omni tubes with 2.8 ceramic beads (Cat#: 19-628D). Joints were collected in Omni tubes with 2.8 metal beads (Cat#: 19-620D). Tubes were homogenized by Omni Bead Ruptor for 90 seconds. Homogenates were frozen at -80°C for RNA extraction. RNA was extracted from whole organs using TRIzol Reagent phase separation protocol. RNA extraction was done using the PureLink RNA Mini Kit (Ambion). The quantity and quality of RNA extracted was measured using NanoDrop (Thermofisher).
Borrelia was quantified by RT-qPCR. FlaB and mouse B-actin primer probes were designed for use with TaqMan Fast Virus 1 Step Master Mix reagent (ThermoFisher) using 5 µL of extracted RNA from each mouse organ. Primer probes were ordered from ThermoFisher. FlaB was amplified by forward primer (5’-TTGCTGATCAAGCTCAATATAACCA-3’) and reverse primer (5’-GCATCGCTTTCAGGGTCTCAA-3’) with a probe quenched with FAM fluorescent dye (5’-AGAACAGCTGAAGAGCTTGGAATGCAGCCTGCAAAAATTAACACA-3’). Mouse B-actin was amplified by forward primer (5’-AGAGGGAAATCGTGCGTGAC-3’) and reverse primer (5’-ACGGCCAGGTCATCACTATTG-3’) with a probe quenched with VIC fluorescent dye (5’-CAAAGAGAAGCTGTGCTATGTTGCTCTAGACTTCGAGCAGGAGAT-3’). The reaction was set up for a fast-cycling mode with the following cycling protocol: 1 cycle for 5 minutes at 50 °C, 1 cycle for 20 seconds at 95 °C, and 40 cycles of 95 °C for 3 seconds and 60 °C for 30 seconds. The reactions were run on Step One Plus qPCR machine.
Histological Analysis
Joints from challenged mice were collected from each group 3 weeks after infection. Joints were fixed in 4% paraformaldehyde (PFA) and stained with hematoxylin-eosin (H&E) stain. Signs of arthritis were evaluated by as described elsewhere in a blinded manner [
33].
Statistical Analysis
For ELISA, log-transformed 50% effective concentration (EC50) values were determined by plotting against delta OD value (OD [490nm]-OD [630nm]) on GraphPad Prism 9 software. For all statistical analyses, one-way ANOVA with post-hoc Tukey HSD test was performed on log-transformed data.
Discussion
In this study we utilized BBI39 in the RABV vaccine vector, BNSP333. We found that BBI39 can be produced incorporated into the RABV virion with the addition of RVG tail. The incorporation of BBI39 in BNSP333-BBI39
RVG induces anti-BBI39 IgG antibodies in vaccinated mice, however the unincorporated vaccine, BNSP333-BBI39, did not. All RABV vectored vaccines produced anti-RABV-G antibodies. BNSP333-BBI39
RVG, especially with the adjuvant PHAD-SE, can induce a type-1 associated immune response via the change in antibody isotypes. This leads to
Borrelia and RABV neutralization
in vitro. However, the recombinant protein vaccine, although adjuvanted with PHAD-SE, induces a more balanced type1/2 immune responses, which was seen in this study and previous studies [
15,
40]. BNSP333-BBI39
RVG vaccinated mice successfully depleted Bb in syringe and tick challenges, more than the recombinant protein immunized mice, and eliminated LD pathogenesis.
In previous studies, OspA was the most utilized vaccine antigen [
8,
14,
36,
40,
41]. While OspA is protective, it was found to contain a similar epitope to the human leukocyte function-associated antigen-1 (hLFA1), indicating the possibility of autoantibody development LYMErix vaccination [
12]. Although these findings were insignificant, sales in LYMErix decreased dramatically, and the company removed the vaccine from the market in 2002. To prevent the potential for autoantibody development, we utilized BBI39, another surface protein on
Borrelia protective against LD [
15].
Previous platforms utilized to create an LD vaccine include recombinant protein(s) [
8,
14,
15,
36,
41], viral vectors [
42], DNA [
43], and mRNA vaccines [
40,
44]. However, recombinant proteins have been highly utilized for LD vaccine platforms including LYMErix [
41], VLA15 [
8], and Vanguard crLyme [
14]. However, recombinant proteins have low and short-lived immunogenicity requiring adjuvant and multiple inoculations with yearly revaccination [
45]. Viral vaccine vectors, including RABV BNSP333, have been widely studied as efficacious, long-term options [
27,
28,
31], but have rarely been studied as an LD vaccine. One group attempted to use Newcastle disease virus (NDV) as a vaccine vector for OspC [
42], another protective borrelial antigen. However, this vaccine vector amounted to low antibody titers in mice and insignificant depletion of Bb in various organs. Another concern of utilizing a viral vector for a LD vaccine is that the Bb antigen is glycosylated differently by
Borrelia than mammalian cells, how a viral vector is developed. This could possibly change the immune response to a bacterial protein induced by a viral vector [
46]. Nonetheless, we developed an RABV vectored vaccine utilizing BBI39. In this study, we showed that borrelial antigen, BBI39, needs to be incorporated into the RABV virion to elicit high titer anti-BBI39 antibodies. When the borrelial antigen does not include the RVG tail, BBI39 is not incorporated into the RABV virion. Without incorporation, the protein stays inside the cell due to rabies’ non-cytolytic nature and the antigen is not presented to the immune response to elicit antibodies. With the addition of the RVG tail to BBI39
RVG, the antigen is incorporated into the RABV virion, and high titer antibodies are elicited. This protects against Bb and inhibition of pathogenesis of LD. We observed glycosylation of BBI39; however, this antigen still protected against Bb in both syringe and tick challenge. In fact, we saw even greater protection from the viral vector vaccinated mice compared to recombinant protein.
In addition, the long-term efficacy of BNSP333 with BBI39 and other vaccine antigens [
27] demonstrates an ideal platform against LD. The previous study on the BBI39 recombinant protein did not show long-term immunization experiments [
15]; however, our study showed greater wanning immunity of the recombinant protein up to 8 months post-vaccination. In addition to wanning immunity from recombinant proteins, mRNA have shown wanning immunity to various antigens, requiring the need for multiple boosts [
47]. Long-term vaccination and challenge studies were not completed in the mRNA LD vaccine study [
40,
48]. However, we showed with one boost of the BNSP333-BBI39
RVG vaccine, long-term antibody responses were maintained 8-months in vaccinated mice. In addition, a previous study showed that BNSP333 can maintain long-term, up to 1 year, antibody titers and produce antibody-secreting cells in the spleen and bone marrow after only one boost, both with and without adjuvant [
26]. Viral vectors, including RABV, are great candidates in the development a LD vaccine.
Previous studies demonstrated that antibodies are the main correlate of protection to prevent LD [
49,
50]. Type-1 associated antibodies, such as IgG2a are ideal for Bb neutralization [
36,
38]. We saw a greater IgG2a induction and Bb neutralization in vitro from BNSP333-BBI39
RVG + PHAD-SE vaccinated mice than the recombinant protein immunization with adjuvant. Therfore, a viral vector (RABV) with addition of adjuvant provided greater protection against Bb in vaccinated mice compared to recombinant protein vaccinated mice. These data compares to a previous study utilizing PHAD-liposome particle bound with OspA demonstrated high antibody titers, a skew towards Th1 immunity, and borreliacidal effects which resulted in depletion of Bb in ticks [
36]. Although a challenge study was not conducted, these results compare to the immunogenicity and borreliacidal activities in our study. This study also found long-term effects from their vaccine, aligning with our study. However, alum is widely used in the formulation of LD vaccines, including LYMErix [
41] and VLA15 [
8] but, alum is known to induce a type-2 associated immune response [
26], which is not ideal for Bb protection [
51]. The induction of a type-1 associated immune response from the viral vector and adjuvant PHAD-SE is an ideal formulation for developing a successful LD vaccine.
In this study, we utilized a syringe challenge and infected tick challenge. This compares to other studies that only utilized syringe inoculation [
40]. The immune evasion strategies by the tick’s salivary proteins, among other strategies utilized by Bb to enter the host, are not present in a syringe challenge. Although syringe challenge is most feasible when a tick colony is not available, this should be considered when developing other LD vaccines. In our syringe challenge, we identified significant protection against BNSP333-BBI39
RVG. However, in tick challenge, Bb is depleted much less. Therefore, tick challenge is ideal when analyzing the efficacy of a potential LD vaccine.
To further study BNSP333-BBI39RVG as a vaccine candidate, other mouse models of vaccination should be studied, such as C57BL/6 and BALB/C mice, which are less inflammatory mouse models compared to C3H/HeN. This could further analyze differences between adjuvanted and unadjuvanted groups. In addition, non-human primates should be utilized for protective efficacy with this vaccine. Further preclinical testing to determine the inhibition of disease pathogenesis, such as arthritis and carditis, should be completed. This includes long-term challenge experiments or keeping vaccinated and challenged mice for longer periods than 21 days post-challenge. This could show if the lower borrelial burdens in vaccinated mice will continue to prevent LD pathogenesis. Since LD is highly inflammatory, the immune profile of vaccinated and unvaccinated mice, such as cytokine profiles after vaccination and challenge, should also be studied. Greater understanding of the impact on antibody titers and passive transfer experiments will elucidate the necessity of specific antibody titers during Bb infection to prevent LD. Testing this vaccine against other strains of Borrelia such as Borrelia afzelii and Borrelia garinii, which are strains seen in LD patients in Europe and Asia, may determine whether BBI39 vaccination is conserved for these strains. Finally, since we did not see sterile immunity with the BNSP333-BBI39RVG vaccine in either challenge experiments, future research could study different borrelial antigens in combination with the BBI39 vaccine. Targeting the bacteria with different surface proteins could induce higher immunity against Bb. Studied would incorporate the foreign antigen into the RABV virion and use adjuvant PHAD-SE to create another RABV-based Bb vaccine. Other antigens studied for LD vaccines, such as OspA without the hLFA epitope, OspC, and other previously studied Bb or tick antigens could be tested in another vaccine.
In this study, we created a vaccine that targets BBI39 on the surface of Bb. Antibodies elicited against this vaccine contain neutralizing functions against both Bb and RABV, further depleting Bb in vaccinated mice. These results will help further research to develop an effective human LD vaccine.