1. Introduction
The oomycete
Aphanomyces astaci (
Ap. astaci) is an aquatic pathogen responsible for crayfish plague and has devastated freshwater crayfish populations across Europe [
1,
2]. Native to North America, the pathogen was imported into Europe around 1860, carried by non-indigenous crayfish species (NICS) to northern Italy [
3]. North American NICS usually serve as tolerant vectors of
Ap. astaci due to host-pathogen co-evolution [
2]. However, the indigenous crayfish species of Europe possess low to no innate immune defence against the pathogen. To infect a susceptible host, free-swimming microscopic zoospores encyst on the host’s soft tissue, between joints; the pathogen then geminates and hyphae rapidly develop through the host’s tissues, infiltrating the inner organs and killing the host. Upon death of the host, the asexual pathogen produces sporangia (spore balls) causing a massive sporulation event for the zoospores to find a new host [
4]. Local crayfish populations can be lost within weeks [
4,
5]. Tolerant NICS possess a strong innate immune response against
Ap. astaci infection and these North American crayfish species repress
Ap. astaci hyphal growth without killing the parasite, instead acting as mobile vectors of the pathogen [
4,
6,
7]. Since its introduction in Italy, the pathogen has spread across the continent, decimating native European crayfish populations, leading to mortality rates of up to 100% at infected sites [
8,
9]. Due to the continuing introduction of alien crayfish into Europe, the crayfish plague pathogen is currently distributed across all of Europe with different genetic lineages of varying virulence [
1,
9].
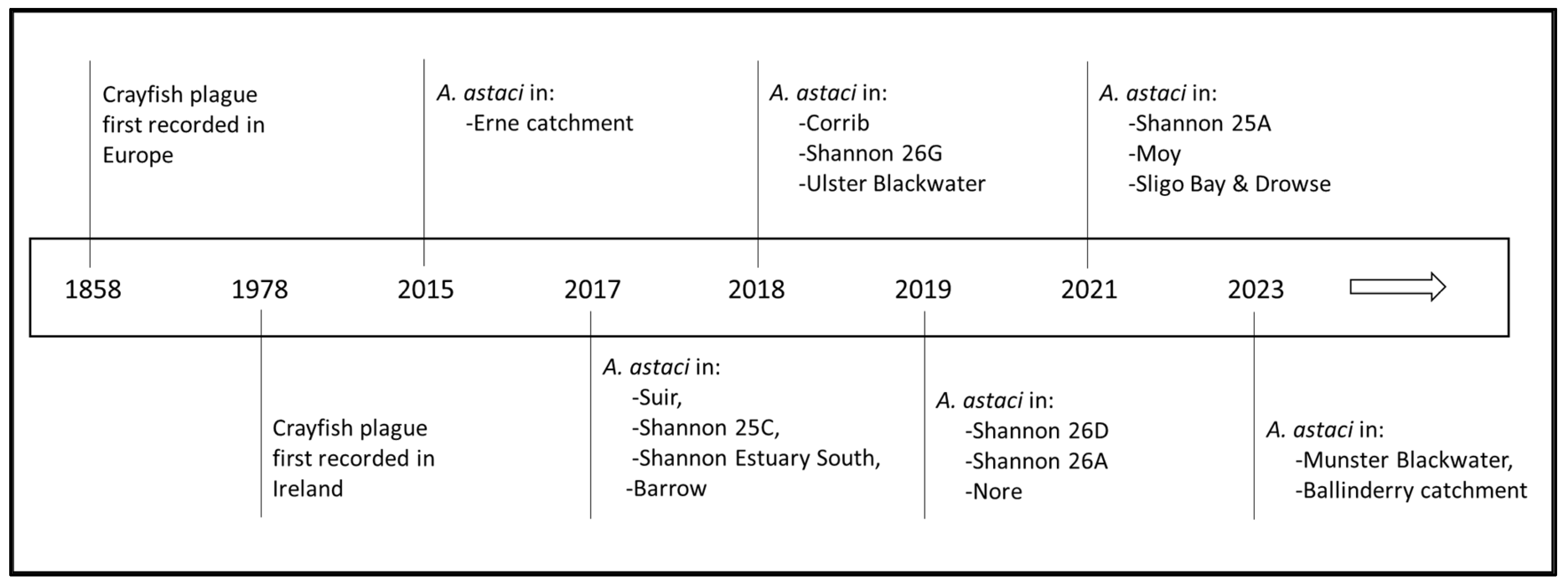
The island of Ireland is governed by Irish and Northern Irish authorities in the south and north-east, respectively. The island is low-lying, with large river-lake catchments in the mainly limestone centre, bounded by hilly edges, generally of acid rock [
10]. The Irish lowlands are home to a single naturalised species of crayfish, the white-clawed crayfish
(Austropotamobius pallipes Lereboullet, 1858). As
A. pallipes is Ireland’s only crayfish species, all other crayfish species are considered NICS in the region. Ireland remained free of crayfish plague and NICS for over 100 years (following European plague outbreaks) with healthy crayfish populations throughout the country and has been referred to as a final stronghold of the species [
11]. A temporally isolated crayfish plague mass mortality occurred in 1987 but was confined to a lake and trail crayfish farm in central Ireland [
12]. The plague was not seen again in Ireland until 2015, when a crayfish mass mortality event on the Erne catchment was confirmed to be caused by
Ap. astaci (
Figure 1) using molecular methods [
13,
14]. In the subsequent years, crayfish mass mortalities have been reported across Ireland, in numerous waterbodies and catchments, and multiple genotypes of the pathogen have been identified. Two common mechanisms of spread are: 1) Spores being shed from latently infected NICS during their moulting phase; 2) when contaminated wetgear, equipment, vehicles, machinery or any wet or submerged items are relocated between waterbodies. As no NICS have ever been reported at sites testing positive for
Ap. astaci, the evidence indicates that the microscopic water mould was introduced into Ireland several times on contaminated wetgear.
In response to the continued outbreaks of crayfish plague, the National Crayfish Plague Survey Programme (NCPSP) was established by the National Parks & Wildlife Service (NPWS), and is tasked with overseeing invertebrate protection and conservation in the Republic of Ireland (hereafter Ireland). The NCPSP is conducted by the Marine Institute, which has responsibility over aquatic pathogens in Ireland. Inland Fisheries Ireland is also coordinating efforts to reduce the spread and have close ties with water users and the angling community. In Northern Ireland, governance lies with the Northern Ireland Environment Agency (NIEA) but they have not established any monitoring programs.
In this review, we detail the introduction and spread of Ap. astaci in Ireland, beginning by summarising the isolated 1987 mass mortality event. We then continue from the next detection in 2015, culminating with the NCPSP report in 2021. We present a comprehensive summary of the sampling efforts, highlighting the sites across various regional catchments where Ap. astaci was positively identified. Attention is given to the diverse genotypes of Ap. astaci detected nationwide. Additionally, we include average cycle threshold (Ct) values and associated errors from environmental DNA (eDNA) sampling in cases where Ap. astaci was detected and data is available. We outline and discuss the measures implemented by these institutions, tasked to manage the spread of Ap. astaci across all of Ireland to protect native species, habitats and ecosystems.
2. Aphanomyces astaci in Ireland
The first incidence of crayfish plague in Ireland occurred in 1987 with no further events recorded until 2015. Since then, outbreaks have been occurring periodically, on an almost annual basis. To date, a total of five reports and articles have been published documenting the known incidences of crayfish plague (
Table 1).
2.1. Historical incidence of crayfish plague in Ireland
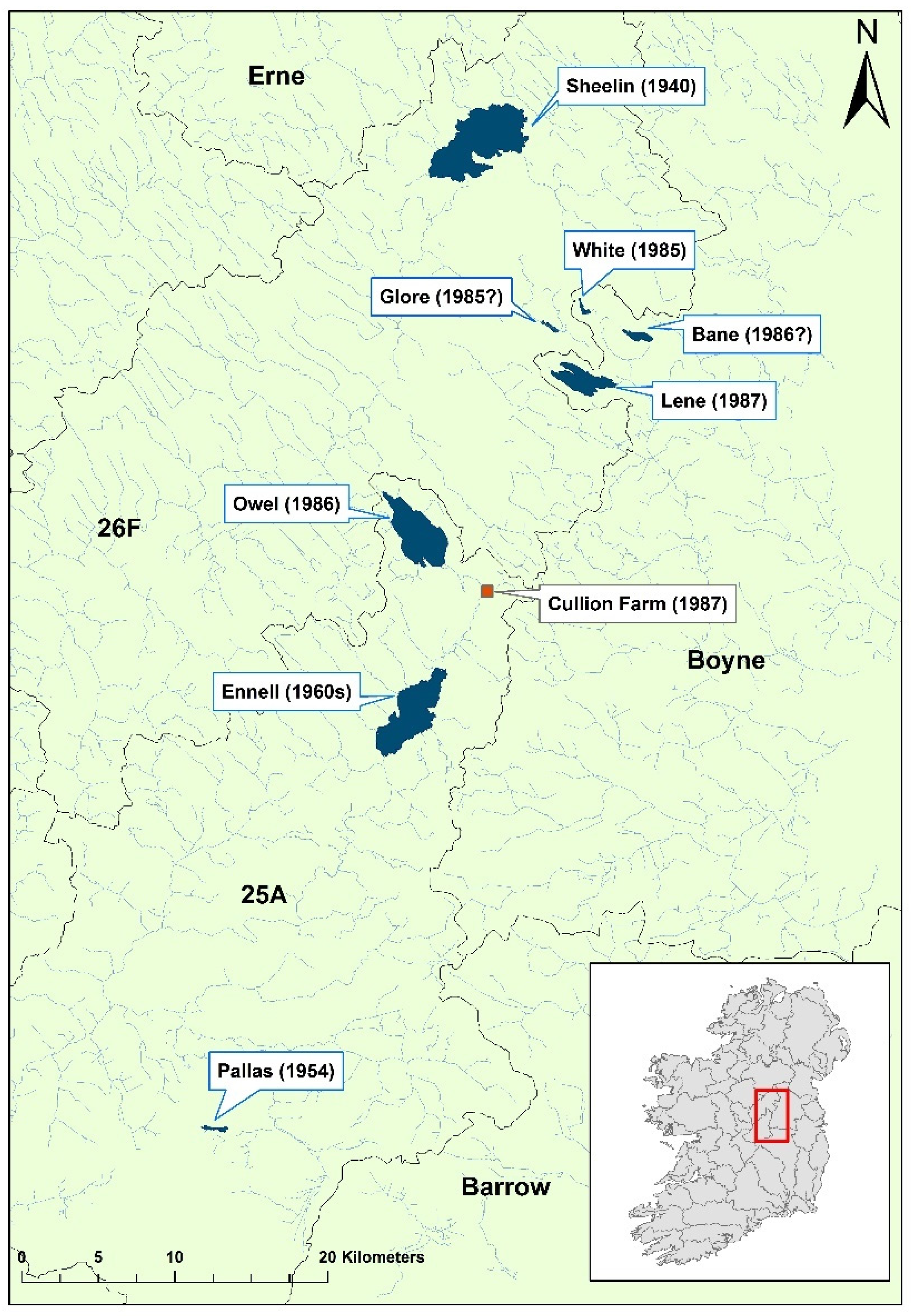
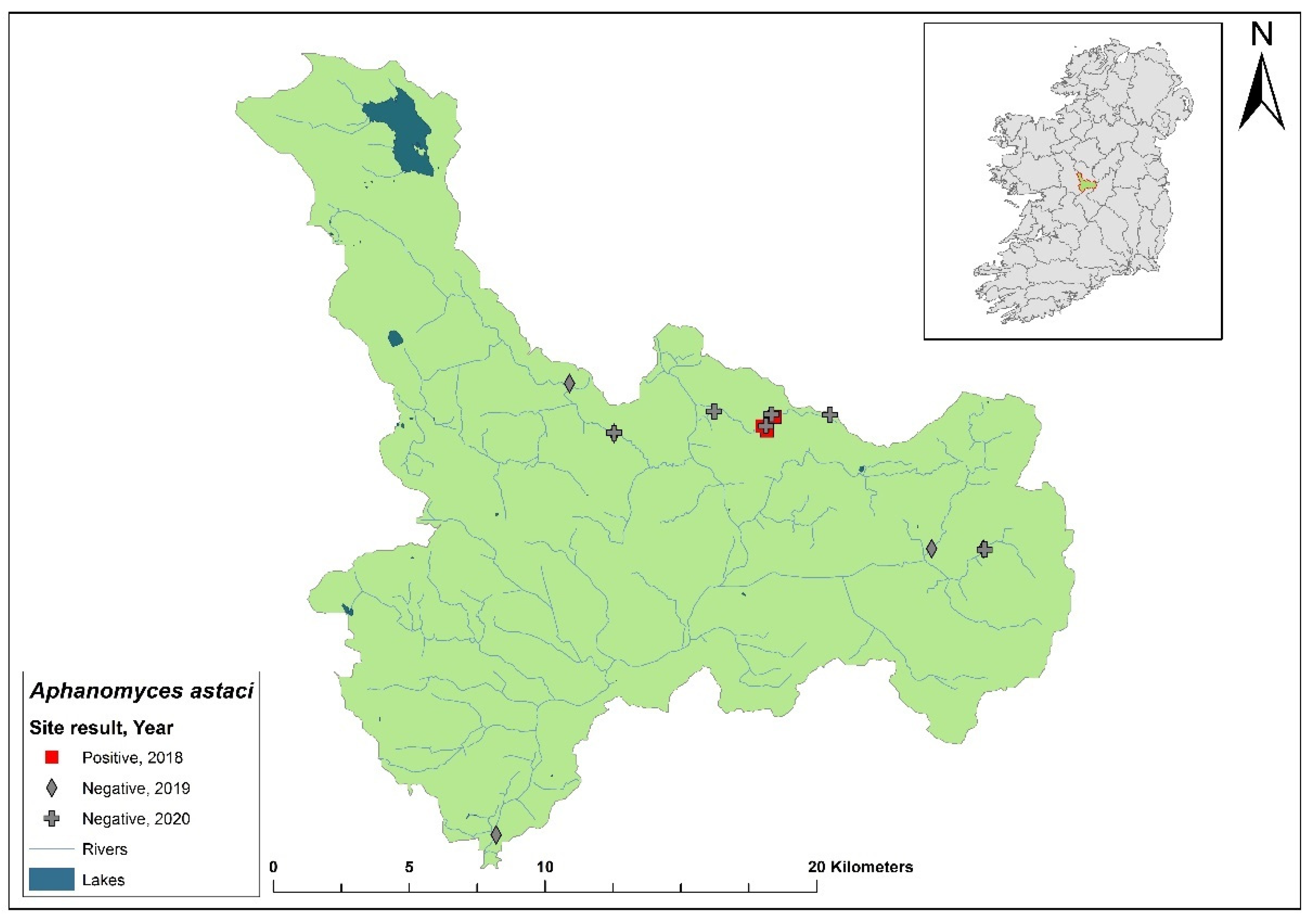
White-clawed crayfish populations were historically abundant in most Irish midland lakes [
12]. However, by 1987, there have been notable disappearances from several sites, such as Lough Sheelin, Pallas Lake, and Lough Ennell, as well as cyclical disappearances and reappearances in others like Upper River Erne (
Figure 2). A 1987 survey of lakes and rivers in central Ireland found that crayfish were absent from White Lake, Lough Glore, and Lough Owel, all of which had previously been known to hold crayfish populations. At the same time, crayfish were still abundant in Lough Lene. In 1987, white-clawed crayfish were harvested from Lough Lene to seed a pilot crayfish farm at Cullion Farm, which indirectly drained into the River Shannon, Ireland’s largest freshwater catchment network. Soon after, crayfish mass mortality events were recorded on both Lough Lene and at the farm. After three weeks, the farm stock was lost entirely and no surviving crayfish could be found in the lake [
12]. Fortunately, the pathogen did not spread down into the Shannon and
Ap. astaci was not detected on the island again for 35 years.
2.2. Recent cases of crayfish plague in Ireland
In 2015, a crayfish mass mortality on the Bruskey river, Co. Cavan, was reported to the National Parks and Wildlife Service. A survey conducted by NPWS removed around 600 dead crayfish from the site and confirmed the animals were infected with
Ap. astaci (see section 3.1) [
13]. Since then, four surveys have been completed for Irish authorities to monitor the presence and spread of
Ap. astaci in Ireland. In total, three reports and one peer-reviewed article have been produced by Irish authorities and the researchers who completed the surveys, and one article was published at the time of the 1988 outbreak (
Table 1). In compiling this review, no projects or reports could be found in relation to crayfish or crayfish plague in Northern Ireland. Neither have the genotypes been determined at either outbreak site in Northern Ireland.
In Ireland, the first crayfish and crayfish plague survey was completed in 2016, assessing the Erne catchment around the initial plague site in 2015. This survey, conducted by Mirimin et al. (2022) from the Marine and Freshwater Research Centre at Atlantic Technological University (ATU), and was the first to implement an eDNA methodology for Ap. astaci in Ireland; eDNA is now the standard method to detect the pathogen in the environment, via filtered water. The following year, in 2017, ATU undertook a comprehensive national crayfish survey. This survey aimed to determine the distribution of crayfish in 17 Special Areas of Conservation across Ireland. It employed a combination of traditional methods, including hand searching, sweep netting, and overnight baited traps at 123 sites. The focus was on A. pallipes, NICS, and potential crayfish mass mortality events. Notably, environmental DNA techniques were not used during the 2017 survey.
Although no mass mortality events were discovered among the 17 SACs during the 2017 survey, at the same time, several reports of crayfish mass mortalities were reported to the NPWS. These mass mortality events were confirmed to be caused by Ap. astaci in each case. In response, the National Crayfish Plague Surveillance Program (NCPSP) was launched for 2018-2019 plague monitoring. During this survey, a pure eDNA approach was utilised to track the spread and prevalence of Ap. astaci nationwide. During the 2018/2019 NCPSP surveys, 608 water samples were taken from across 28 catchments. Post-filtration, eDNA was extracted and analysed for Ap. astaci using qPCR. Dead crayfish collected during the survey were also tested with the same qPCR protocol. Out of the 28 catchments, eight tested positive for Ap. astaci: Suir, Barrow, Shannon 26G, Corrib, Shannon Estuary South, and three other Shannon catchments (26A, 26B and 26D).
The NCPSP program was extended into 2020/2021, during which 738 water samples were collected. Specifically, 168 sites were sampled once, and 30 sites were revisited for repeat sampling. The pathogen was detected in 29 sites, 23 being new site detections. Notably, the 23 positive sites were contained within nine catchments, including three new catchments (Moy, Sligo and Shannon 26C) that were previously free of the crayfish plague. Interestingly, the Shannon 25A catchment tested negative for Ap. astaci using eDNA. However, four dead crayfish from the River Clodiagh within the catchment tested positive for the plague pathogen. Cumulatively, these data show a 59% increase in Ap. astaci positive sites between the 2018/2019 and 2020/2021 survey periods. In 2022, the program was extended for a further four years but no recent data (post-2021) have been released.
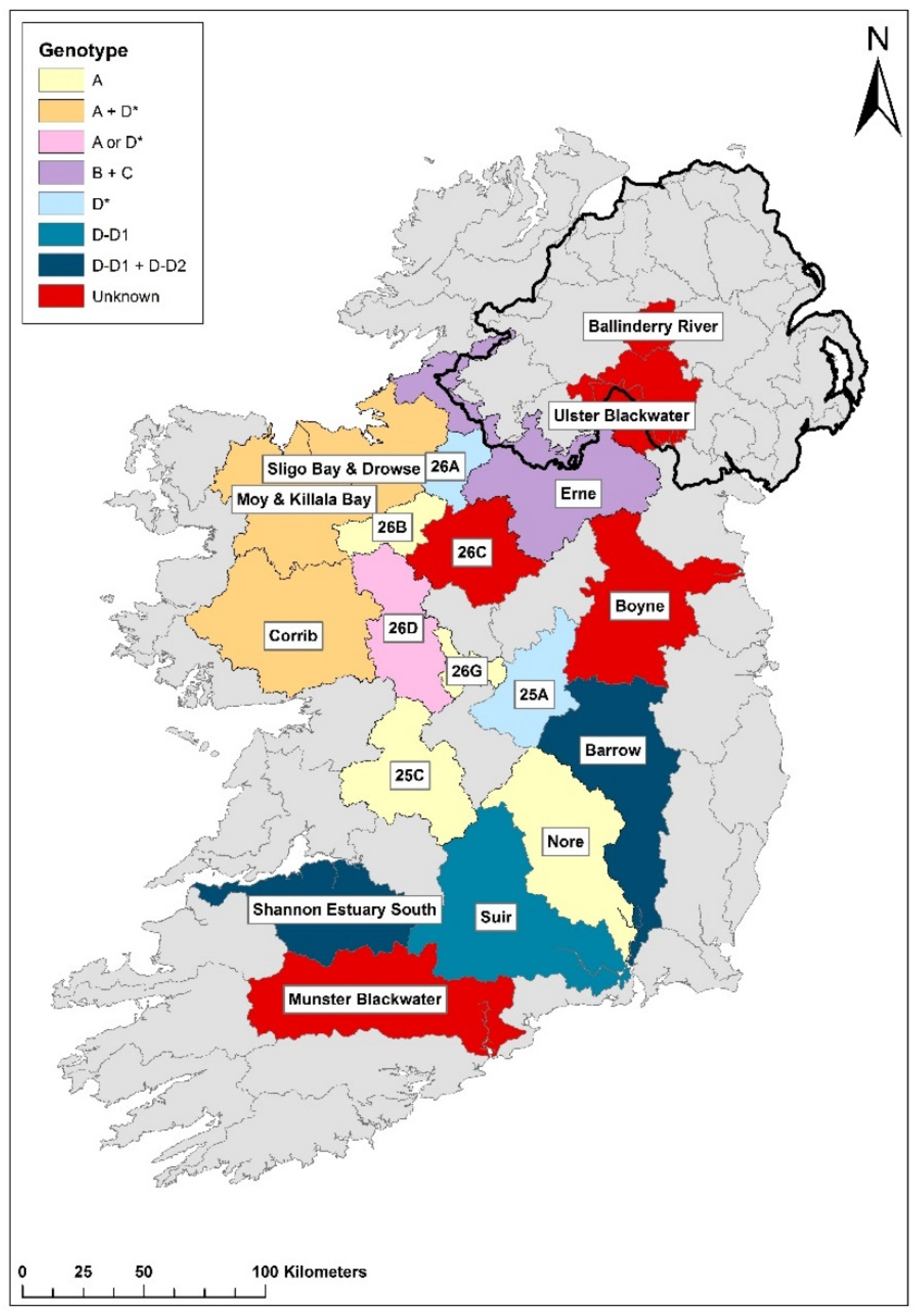
2.3. Multiple Aphanomyces astaci genotypes in Ireland
Europe hosts at least five genetically distinct strains of
Ap. astaci [
2]. Determining the specific genotypes present in Ireland can provide insights into the pathogen's spread and movement within and among water catchments. To determine these specific genotypes in Irish water and from dead crayfish tissue samples, microsatellite markers, genotyping with mitochondrial DNA and a qPCR assay were utilised [
18,
19]. The results from these tests show that all five genotypes present in Europe have been recorded in Ireland (
Figure 3).
The identification of multiple Ap. astaci genotypes in Ireland suggests that the pathogen was introduced multiple times rather than just once. Given that freshwater crayfish are the sole hosts of the pathogen, and considering that no non-indigenous crayfish species (NICS) have been found at or near the infection sites. This pattern implies that the pathogen's spread across Ireland has occurred through a series of distinct introduction events, rather than stemming from a single occurrence.
It is important to recognise that different strains of
Ap. astaci exhibit varying levels of virulence. This implies that distinct strains may pose differing threat levels, with the A genotype being considered less virulent and the B genotype as more virulent [
1,
2,
20]. Controlled infection experiments are yet to be complected for the C and D strains but are essential to fully understand their virulence profiles and potential impact.
2.4. Limitations of the surveys conducted
The reports published on crayfish plague in Ireland lack standardisation and are difficult to compare directly regarding sampling effort. However, the latter two NCPSP reports are more aligned than the initial surveys. The survey conducted by Mirimin et al. in 2016 was most thorough, albeit the smallest, covering an area cantered on the initial outbreak site in the Erne catchment. The Mirimin study included both traditional hand-searching, sweep netting, baited overnight trapping and eDNA analysis. Next, the Gammell et al. (2017) survey was aimed at surveying crayfish abundance and distribution in 17 special areas of conservation in Ireland. Gammel’s report included results for overnight trapping, hand searching and sweep netting, the search of NICS and mass mortality events were included but eDNA samples were not analysed. The two NCPSP survey periods were borne from the experience of the Mirimin et al. (2022) survey. The primary limitation of NCPSP is the lack of conventional surveys and reliance on eDNA to detect Ap. astaci and A. pallipes. The 2018-2019 NCPSP surveys only tested with eDNA while the 2020-2021 NCPSP performed traditional surveys at four sites over two years. However, the NCPSP did sample an impressive number of sites across Ireland.
3. The distribution of Aphanomyces astaci across Ireland
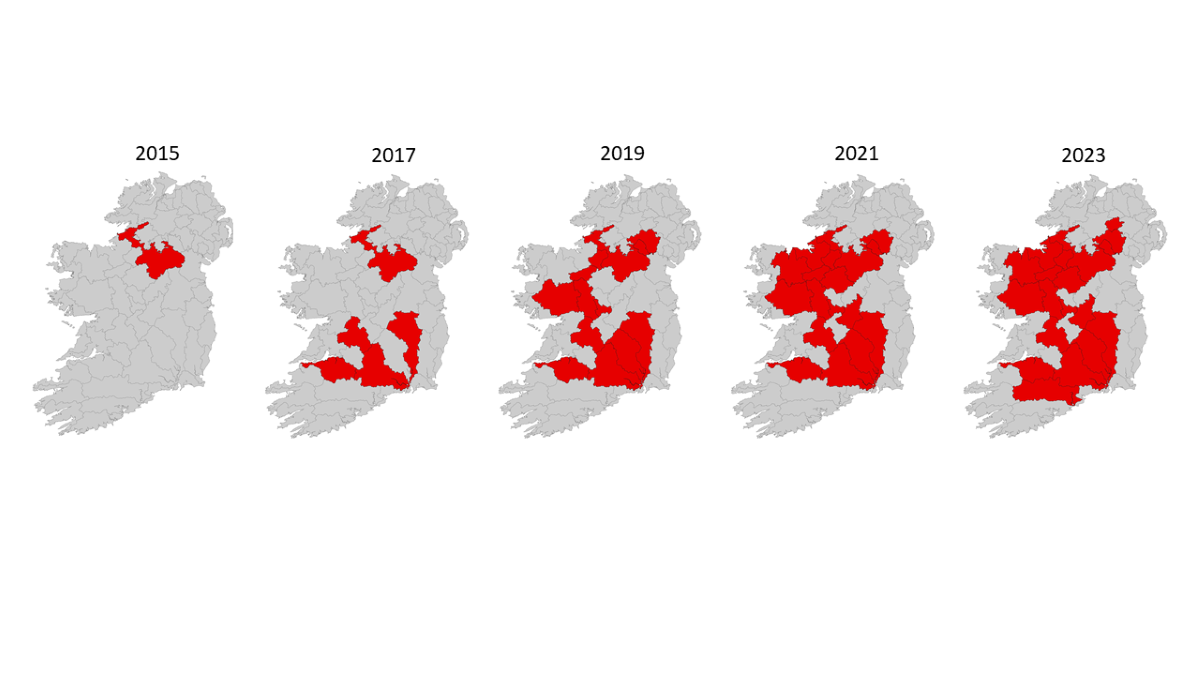
Between 2015 and 2023, 18 water catchments tested positive for
Ap. astaci in Ireland (
Figure 2). However, few details have been made public since the 2021 report was published (in 2022). Since then, additional
Ap. astaci outbreaks have been confirmed in Ireland and Northern Ireland thus we can only mention these outbreaks briefly.
Where data is available, we describe a comprehensive overview of the timeline of detection, the distribution, genotypes and crayfish status within these catchments. We begin with the Erne catchment as it was the first recent site of mass mortality attributed to Ap. astaci. We then alphabetically list the subsequent catchments that tested positive for Ap. astaci at least once between 2015 and 2021. Details for water catchments were provided by the Irish government from the website catchments.ie.
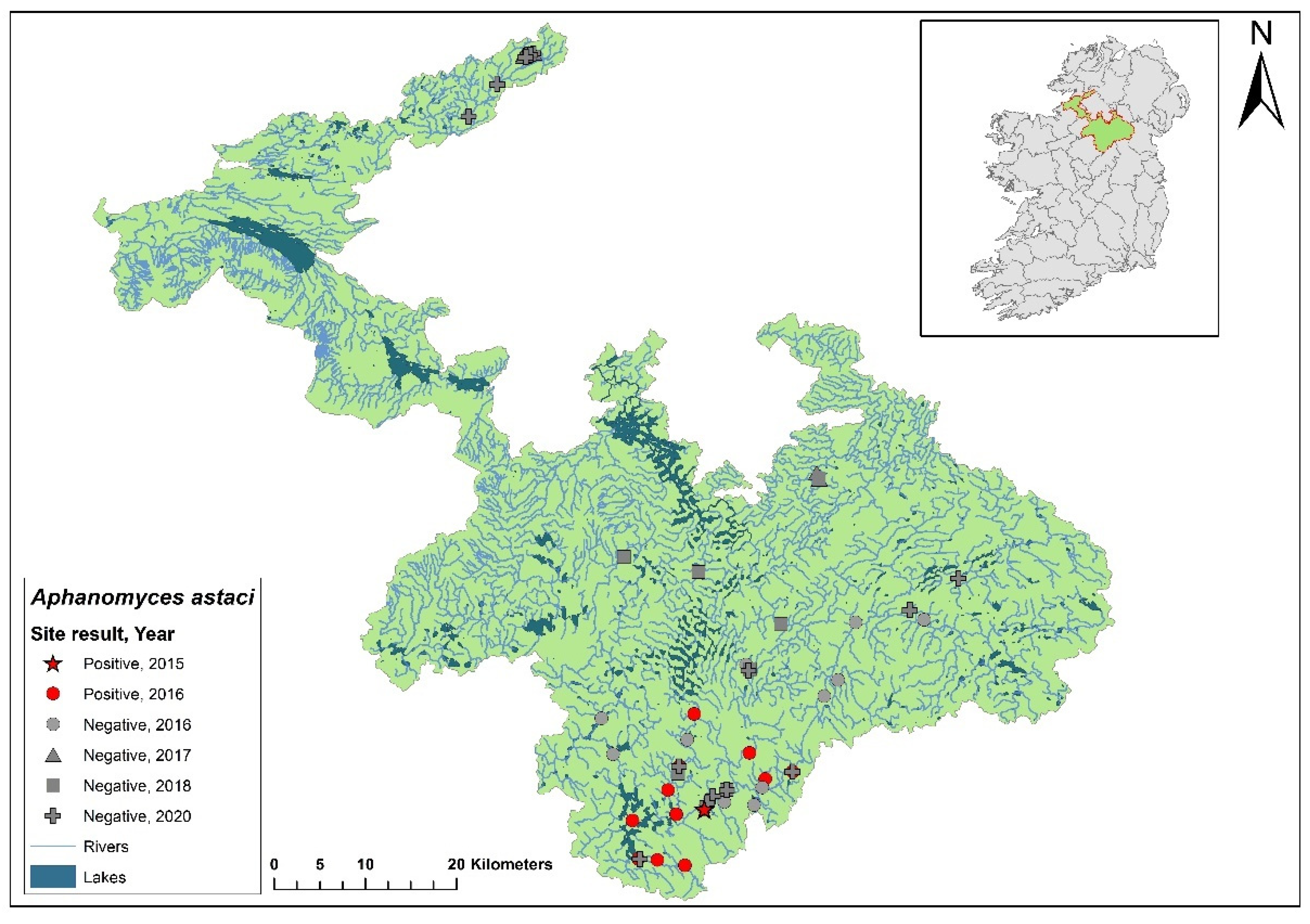
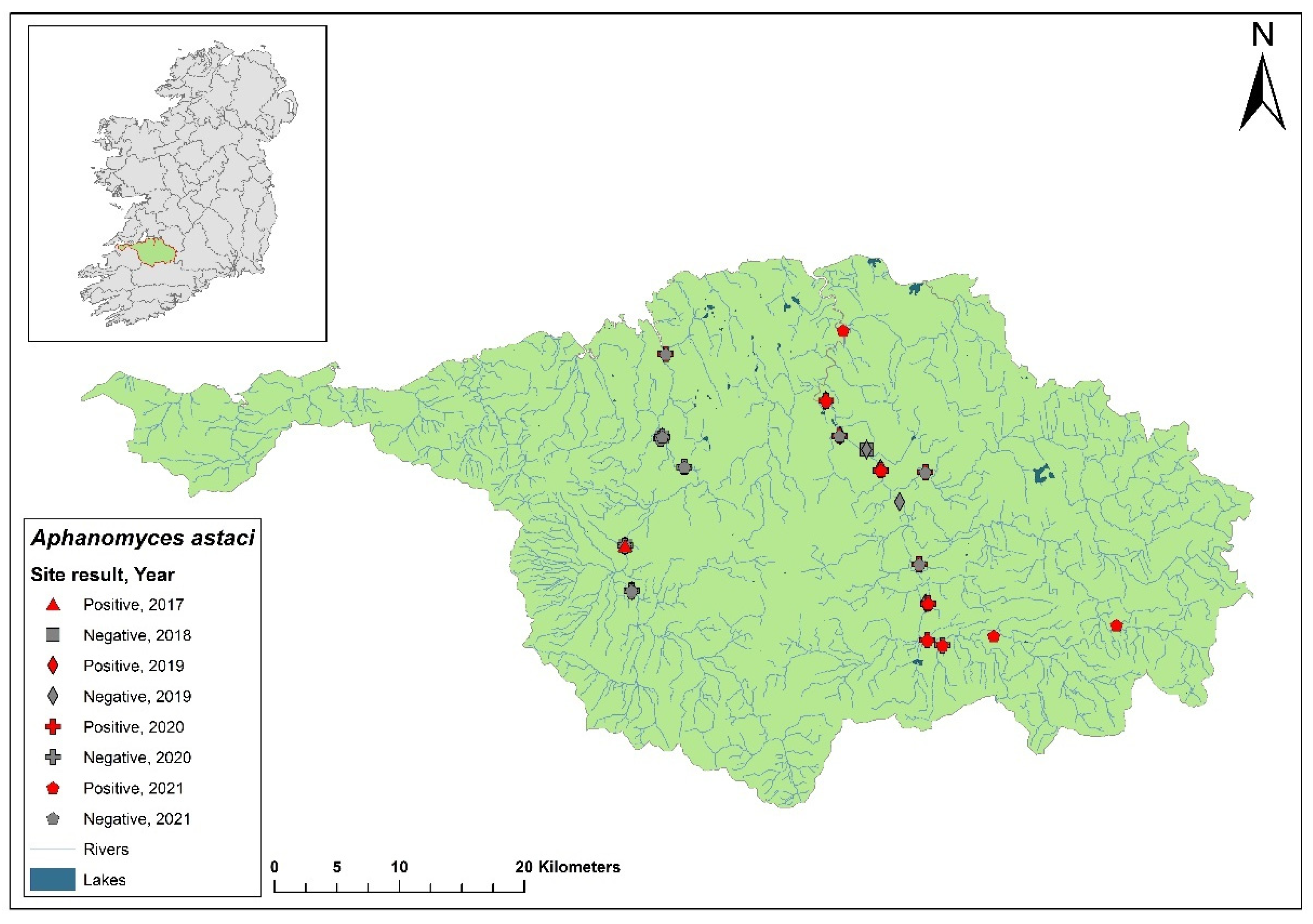
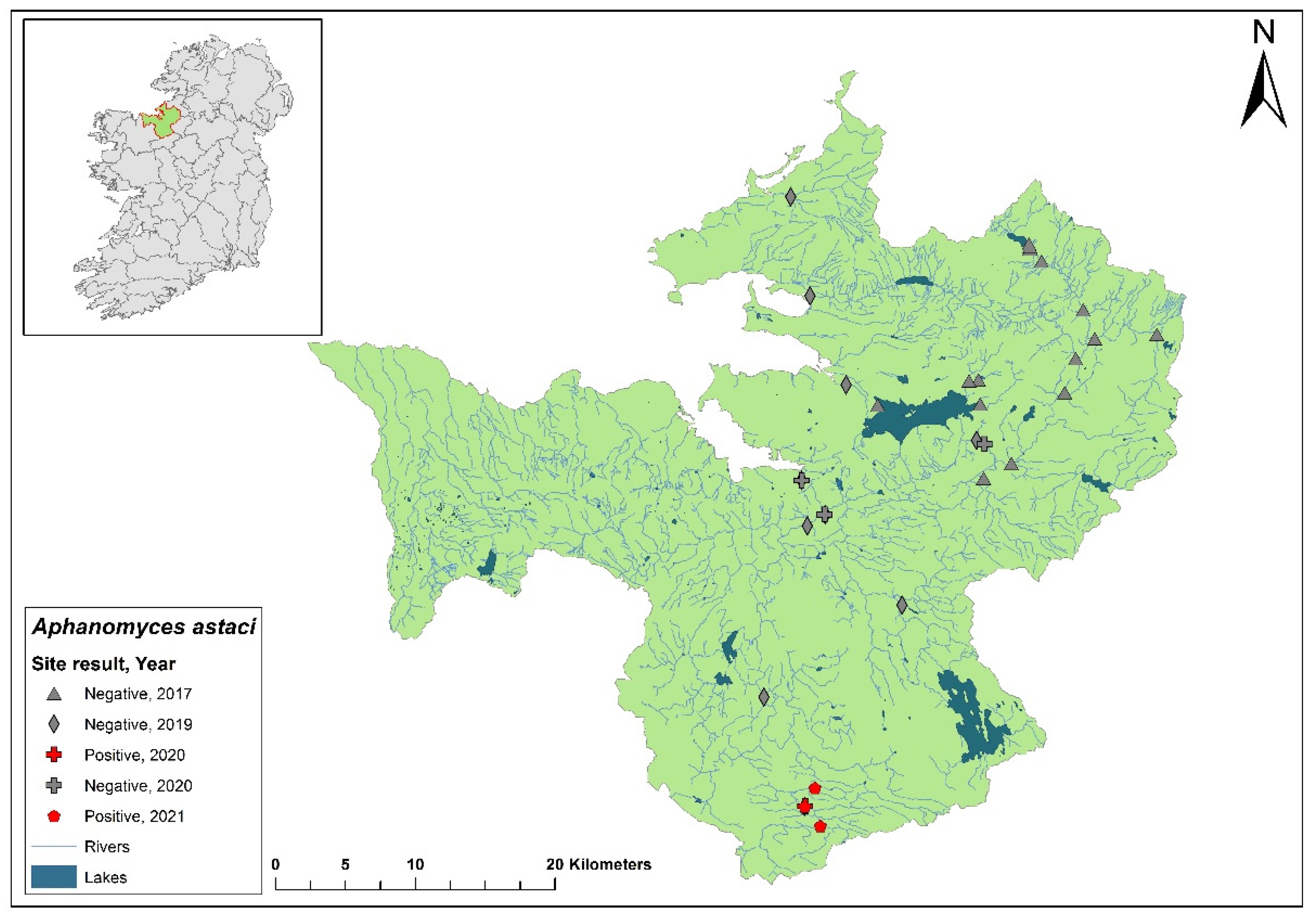
3.1. The Erne catchment
The Erne catchment (ID 36) spans an area of 4,415 km
2 across Ireland (2,512 km
2) and Northern Ireland (1,903 km
2), and freshwater from the catchment enters the sea in Co. Donegal (
Figure 3). The catchment contains 129 rivers, 130 lakes and 66 groundwater bodies. Following the initial crayfish plague outbreak in July 2015, NPWS commissioned a study of the Erne catchment to evaluate local crayfish populations and to gauge the persistence of
Ap. astaci in the area. One year later, the investigation was undertaken by a research team from Atlantic Technological University (formerly Galway-Mayo Institute of Technology) in August 2016. The team employed hand searching, netting or overnight trapping to determine the presence of
A. pallipes and NICS throughout the Erne catchment (
Figure 4).
An established eDNA approach was also adopted to determine the presence of
Ap. astaci in the catchment [
21]. Each of 24 sites were surveyed twice during the survey. On each visit, the team carried out five-minute visual inspections, hand-net searches, and monitored overnight baited traps. Water samples (
n = 163, + 5 negative controls) from all 24 sites were filtered, after which qPCR analysis was performed to detect
Ap. astaci. The limit of detection for determining
Ap. astaci in water samples was determined as Ct 35 (strict positive result equating to 60 target DNA copies/μL) or Ct 40 (relaxed positive result equating to 25 target DNA copies/μL) [
21,
22].
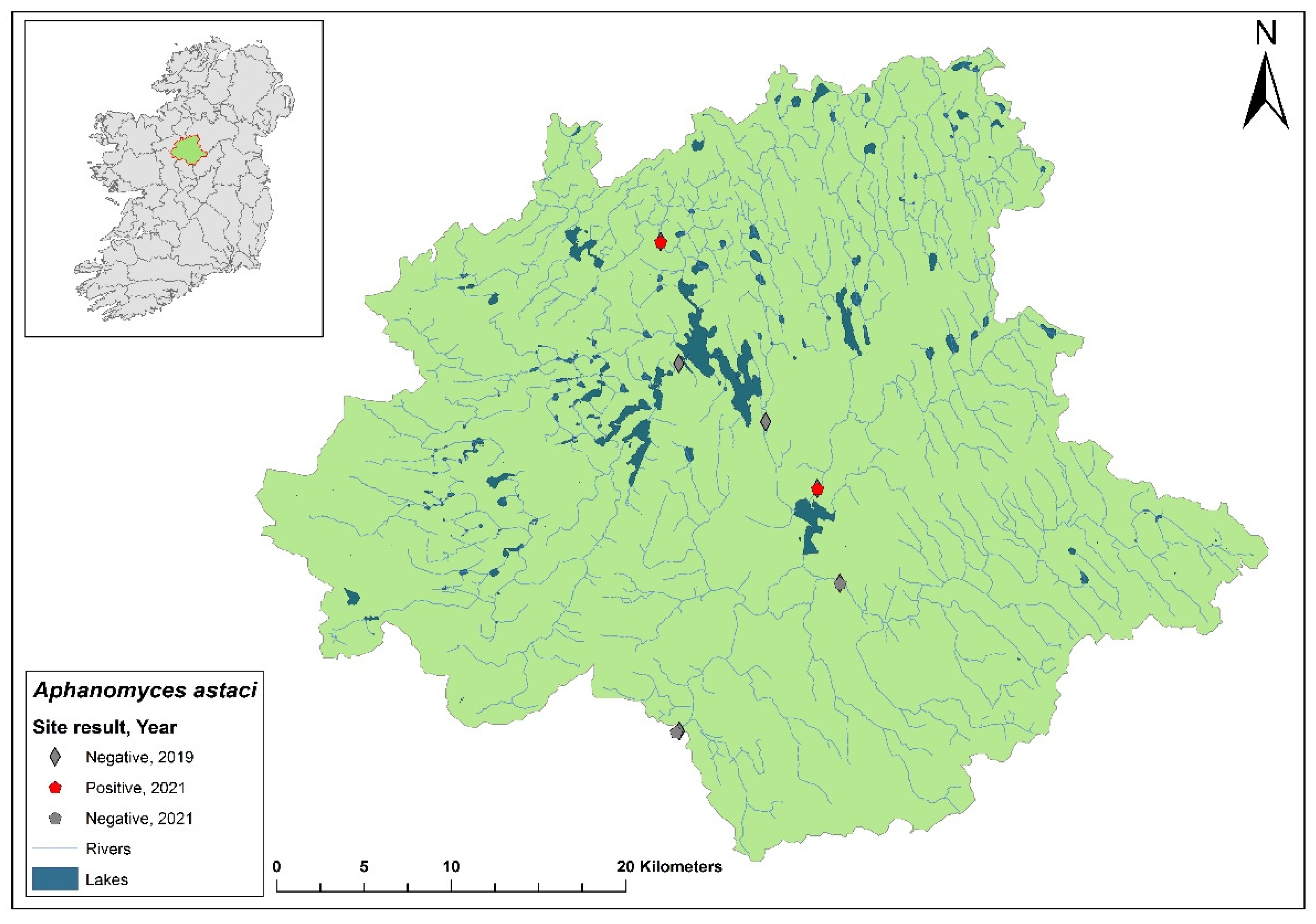
Of the 24 sites tested by eDNA in 2016, 11 were positive for
Ap. astaci, with Ct= 35 - 38. Five of these positive sites contained live, seemingly healthy
A. pallipes crayfish. While the specific genotype of
Ap. astaci wasn't determined during this initial survey, subsequent genotyping of carcasses revealed the presence of the low virulence A genotypes and the C genotypes [
23], as identified via the Makkonen
et al. (2018) microsatellite assay [
19]. The findings from the survey and genotyping analyses show
Ap. astaci's persistence in the catchment both upstream and downstream of the Bruskey River site a year after the first reported outbreak.
As part of the 2018 NCPSP survey, six sites upstream of the 2016 Bruskey river site were assessed by eDNA analysis for the presence of Ap. astaci. All sites were free of the pathogen. A downstream site of the 2015 mass mortality event at Woteraughy Mill (Carrickacleevan) remained free of Ap. astaci and positive for crayfish. During the 2020 survey, 15 sites were tested around the Erne catchment, including sites around the original mass mortality recorded in the Bruskey River. All sites tested negative for Ap. astaci.
In the 2020/2021 NCPSP report, a reanalysis of tissue samples from the 2015 outbreak site and those collected during the 2016 Erne survey was performed using microsatellite genotyping. This analysis identified both B and C genotypes. Concurrently, genotyping was also conducted following the method described by Di Domenico et al., 2021 [
18] which agreed with the result.
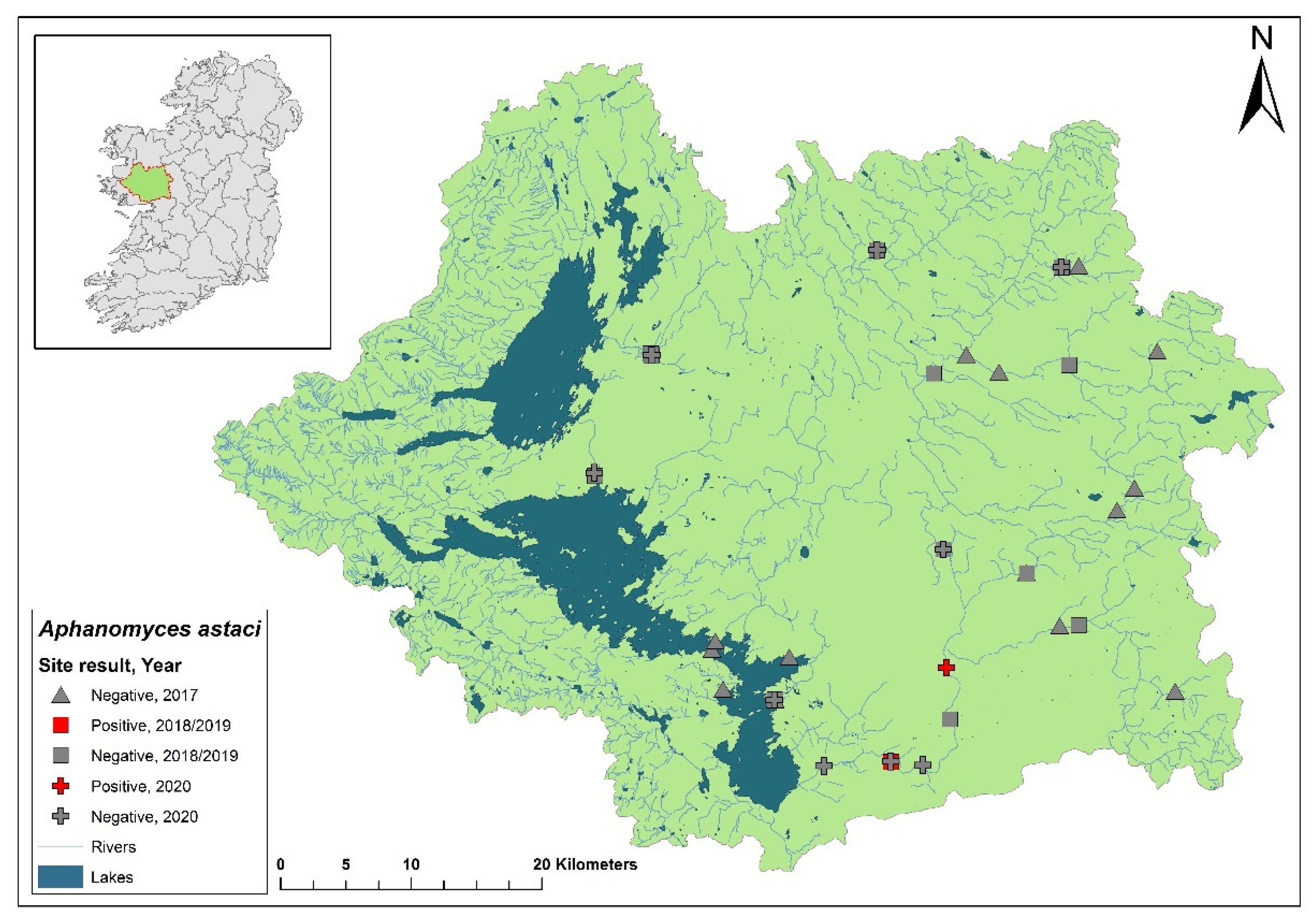
3.2. Barrow catchment
The Barrow catchment (ID 14) spans an area of 3,025 km
2 containing 149 rivers and six groundwaters (
Figure 5). In the 2017 crayfish survey, no evidence of NICS or the crayfish plague was observed at 12 sites on the River Barrow in Co. Carlow. However, in the same year, crayfish mortalities were reported on the river from Royal Oak Bridge. Dead crayfish samples were assessed and all tested positive for
Ap. astaci. During the 2018/2019 NCPSP survey, six sites were tested by qPCR, and one site at Monasterevin Bridge tested positive for
Ap. astaci (Ct= 28 - 33). In 2019, a mortality event was reported in the catchment on the River Slate in Co. Kildare. Tissue samples from crayfish carcases tested positive for
Ap. astaci. Both NCPSP reports showed the same genotyping results with Genotypes D subtypes D2 and D1 for the River Barrow (2017) and Slate (2019) sites, respectively [
16,
17]. In 2020, 24 sites were tested in the catchment and six sites were positive for
Ap. astaci (Ct= 31 – 37) and four were positive for
A. pallipes (Ct= 34 – 38). In 2021, all 24 sites tested negative for
Ap. astaci and
A. pallipes.
3.3. Corrib catchment
The Corrib catchment (ID 30) spans an area of 3,112 km
2, comprising 97 rivers, 31 lakes and 21 groundwater bodies in the west of Ireland (
Figure 6). No evidence of crayfish plague was observed at 14 sites in the 2017 survey. The NCPSP sampled six sites in the Corrib catchment in 2018 and 12 sites in 2019. The report states that one site, in Claregalway on the River Clare, Co. Galway, tested positive for
Ap. astaci Genotypes A in 2018 (Ct= 35) and again in 2019 (Ct= 32 - 36), but with the D genotypes (subtype unknown). Indicating the same site was independently infected two years in a row. In 2020, a site at Corrofin, Co. Clare, tested positive for
Ap. astaci (Ct= 37), but no genotyping was provided in 2020. Additionally,
A. pallipes DNA was detected in the same samples (Ct= 35). Standard ecological sampling was not conducted to confirm the presence of
Ap. astaci in the catchment and this site was not tested in 2021.
3.4. Moy & Killala Bay catchment
The Moy & Killala Bay catchment (ID 34) spans 2,345 km
2 containing 115 rivers, 19 lakes and 37 groundwater bodies in the west of Ireland (
Figure 7). A total of 21 sites on the River Moy were assessed in the 2017 survey and no evidence of plague was observed. Six sites assessed during the first NCPSP survey period also tested negative. However, when assessed in 2020, one site of four at Cloonacannana tested positive for
Ap. astaci (Ct= 36). In 2021, Cloonacannana and two sites, one up- and one downstream, tested positive (Ct= 35 – 36) but no other sites were tested. From the same
Ap. astaci positive samples in 2020 and 2021, positive detections of
A. pallipes were also made via qPCR. Again, indicating a low virulence strain of
Ap. astaci. Genotyping of the 2021 samples indicated evidence for both A and D genotypess from eDNA samples [
17].
3.5. Nore catchment
The Nore catchment (ID 15) spans an area of 2,595 km
2 in south east Ireland and contains 123 rivers, no lakes and 48 groundwaters (
Figure 8). The Nore catchment joins the River Barrow before entering the sea. Ten sites on the Nore showed no evidence of plague during the 2017 survey. Environmental DNA samples for the Nore catchment in 2018 tested negative for
Ap. astaci. However, in 2019, dead crayfish were sampled from Canal Walk on the River Nore in Kilkenny city, all tested positive for
Ap. astaci. Mitochondrial DNA genotyping of the 2019 positive tissue samples identified the A genotype was present [
16]. In 2020, eDNA showed three of 11 sites tested were confirmed positive for
Ap. astaci (Ct= 37 – 38) and
A. pallipes (Ct= 36 – 36) at the same time. The Nore catchment was not sampled in 2021.
3.6. Shannon Estuary South catchment
The Shannon Estuary South catchment (ID 24) spans an area of 2,033 km
2 south of the River Shannon estuary and contains 95 rivers, two lakes and 46 groundwater bodies in the west of Ireland (
Figure 9). Dead crayfish were sampled from the River Deel in the catchment following reports of crayfish mortalities in 2017. All samples tested positive for
Ap. astaci (Genotypes D subtype D2). In 2018, the same sites with additional locations were resampled and all tested negative for
Ap. astaci. Genotyping for the catchment were completed with tissue samples.
In 2019, a crayfish mass mortality event was reported at Castleroberts Bridge on the River Maigue within the catchment (Ct= 37; Genotypes D subtype D1). Two sites, near the mass mortality event and upstream, were assessed for the plague pathogen both were negative. Additional sites on the rivers tested positive (Ct= 27 – 30), the same samples also tested positive for A. pallipes (Ct= 32 - 34).
In 2020, 13 sites in the catchment tested positive for both Ap. astaci (Ct= 27 – 40) and A. pallipes (Ct= 27 – 40), in 2021 six sites tested positive for both Ap. astaci (Ct= 34 – 39) and A. pallipes (Ct= 32 – 37). Of these, four sites consistently tested positive for both over the 2020 and 2021 sampling period. Genotypes D (subtype unknown) was identified at all sites. A site at Askeaton Main Street, was tested in June and November of 2020 and 2021. Both sampling timepoints in 2020 tested positive (June - Ct 36, November - Ct 37) while the same time points tested negative in 2021. From the same samples, A. pallipes was identified at the site in June (Ct= 40) and November (Ct= 38) in 2020 but not 2021. These data imply that Ap. astaci devastated the A. pallipes population at the site between November 2020 and June 2021.
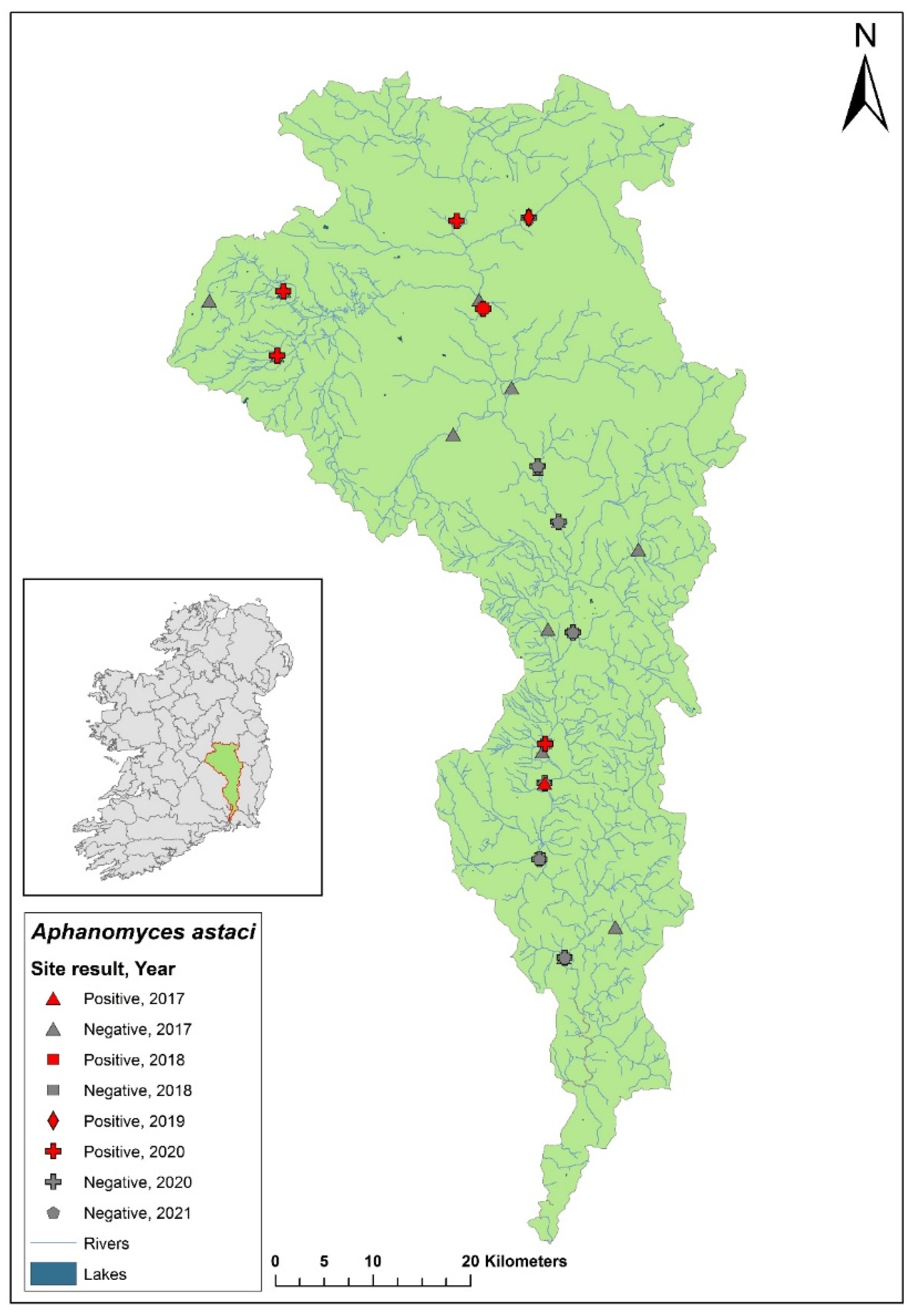
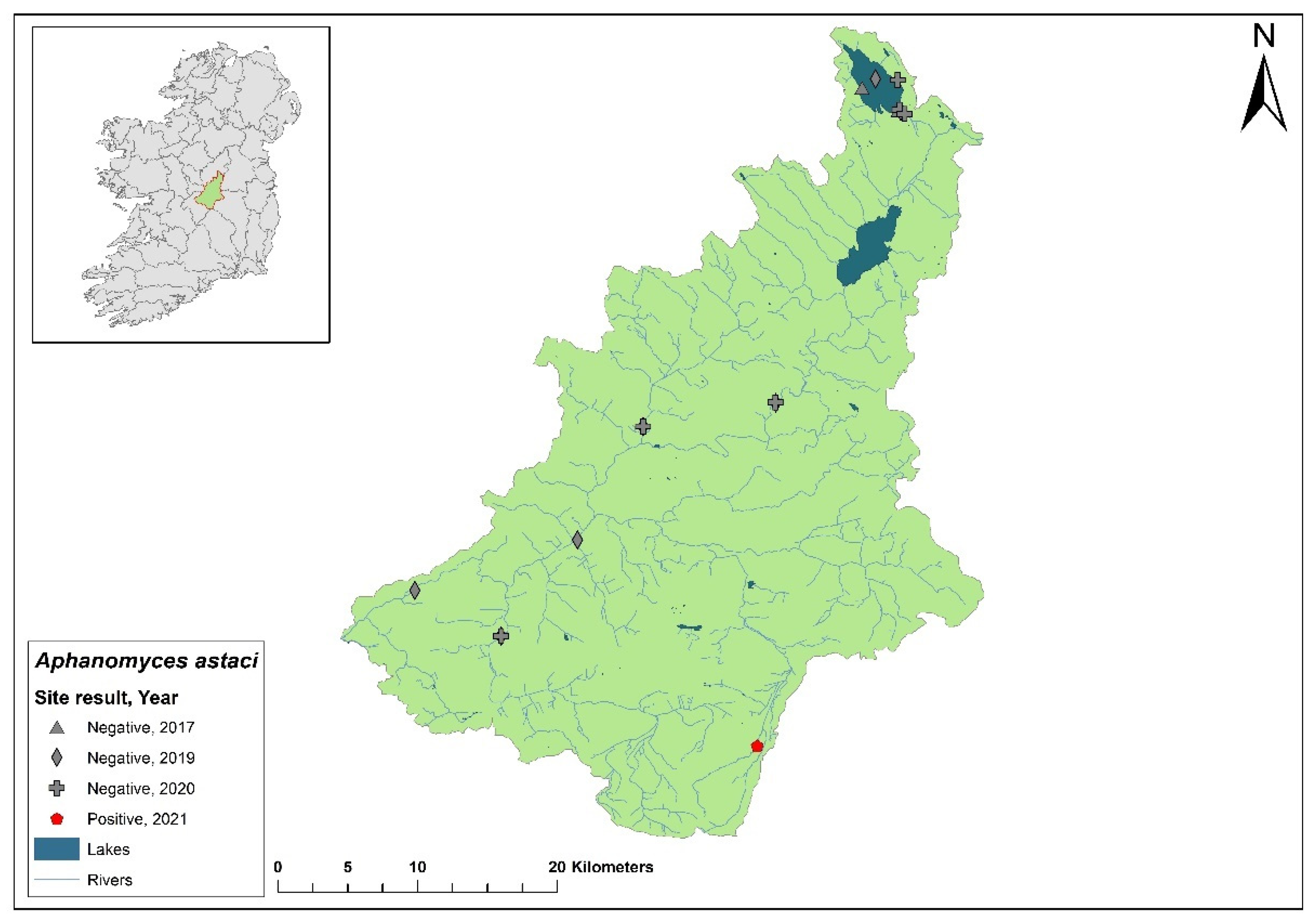
3.7. Lower Shannon (Brosna) 25A catchment
The Lower Shannon catchment (ID 25A) spans an area of 1,248 km
2 containing 62 rivers, four lakes and 32 groundwaters (
Figure 10). In 1987,
Ap. astaci was diagnosed as the cause of a mass mortality event at Lough Owel but no samples remain. No further spread of the pathogen was detected. In 2017, Lough Owel was sampled without any indications of crayfish plague at two sites and had some of the healthiest populations of crayfish across Ireland. In the 2018/2019 NCPSP surveys, seven sites tested negative in the Shannon 25A catchment. Again, in 2020, six sites tested negative. However, in 2021, four dead crayfish were reported in the catchment at Clonaslee on the River Clodiagh, Co. Laois. All four crayfish tested positive for
Ap. astaci and were identified from tissue samples as the D genotypes but the subtype was unknown. No further detection of the of
Ap. astaci has been reported in the catchment. Crayfish were detected in the catchment during the 2018/2019 (Ct= 36 - 39) and 2020/2021 (Ct= 35 – 38) surveys.
3.8. Lower Shannon (Lough Derg) 25C catchment
The Lower Shannon catchment (ID 25C) spans an area of 1,820 km
2 containing 79 rivers, five lakes and 10 groundwater bodies (
Figure 11). In 2017, a research team studying crayfish population genetics from the Marine and Freshwater Research Centre at Atlantic Technological University discovered a crayfish mass mortality event on the River Lorrha in Lorrha village, Co. Tipperary. Dead crayfish samples were taken to the Marine and Freshwater Research Centre, where they tested positive for
Ap. astaci. The Marine Institute later confirmed the diagnosis and identified the A Genotype from tissue samples. The NCPSP assessed six sites in the Shannon 25C catchment in 2018, including Lorrha village, and in 2020 seven sites were assessed in the catchment. All sites following the 2017 mass mortality even were free of
Ap. astaci. Using eDNA,
A. pallipes were detected (Ct= 37-38) in Lorrha village in the 2018/2019 report, and again in 2020 (Ct= 35 – 37). The catchment was not tested in 2021.
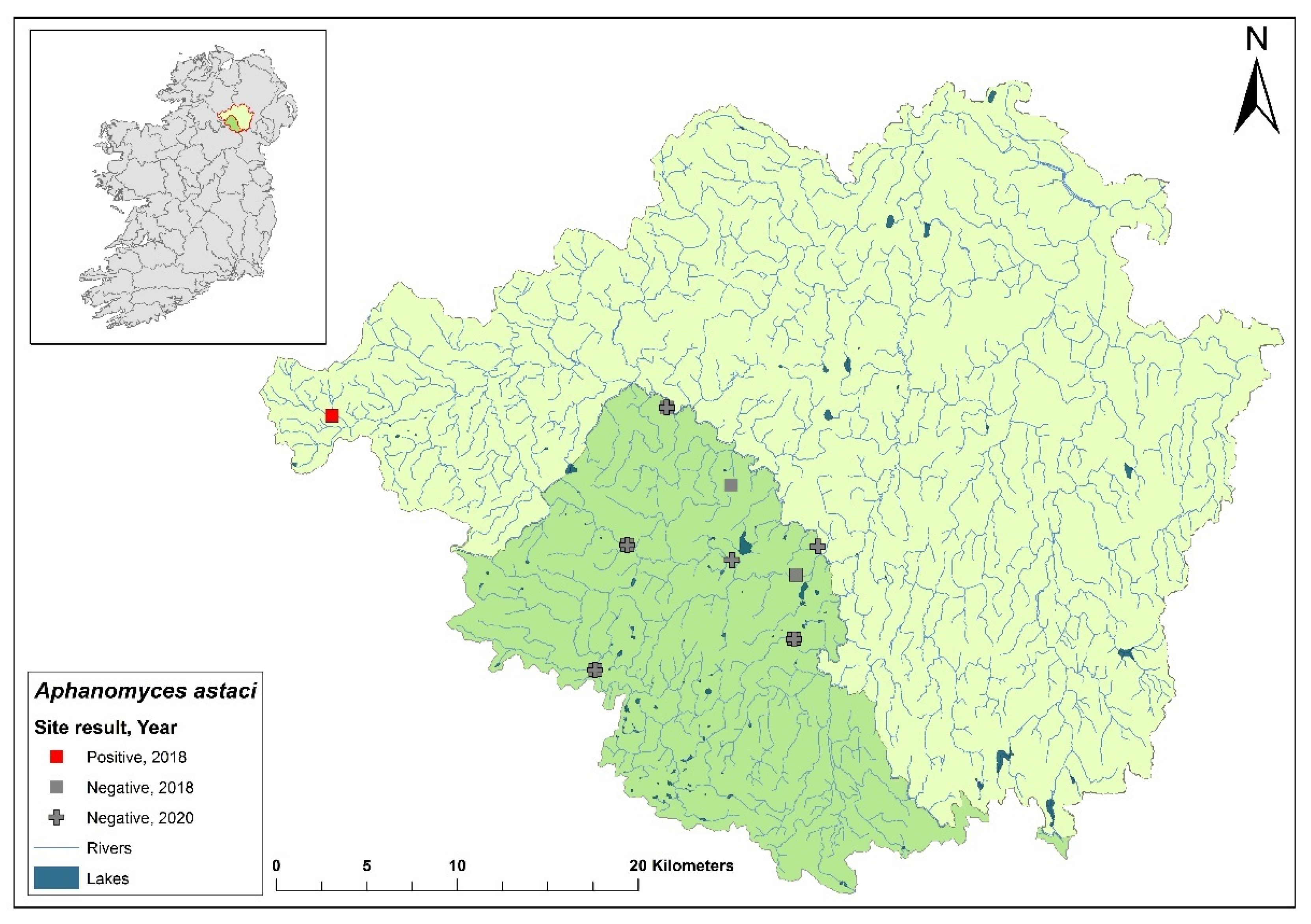
3.9. Ulster Blackwater catchment
The Ulster Blackwater is a cross-border catchment spanning a total area of 1,491 km
2, 1,097 km
2 in Northern Ireland and 393.8 km
2 in Ireland (
Figure 12). In September 2018, dead crayfish were found during a routine field survey at the headwater of the River Blackwater. The specimens tested positive for
Ap. astaci. Following this discovery, there has been no publicised information regarding further crayfish mortalities in the area, nor have there been updates on any subsequent efforts to evaluate the full impact of the crayfish plague in the catchment. Genotyping of the pathogen from these samples has yet to be successfully completed to determine the specific strain of
Ap. astaci involved.
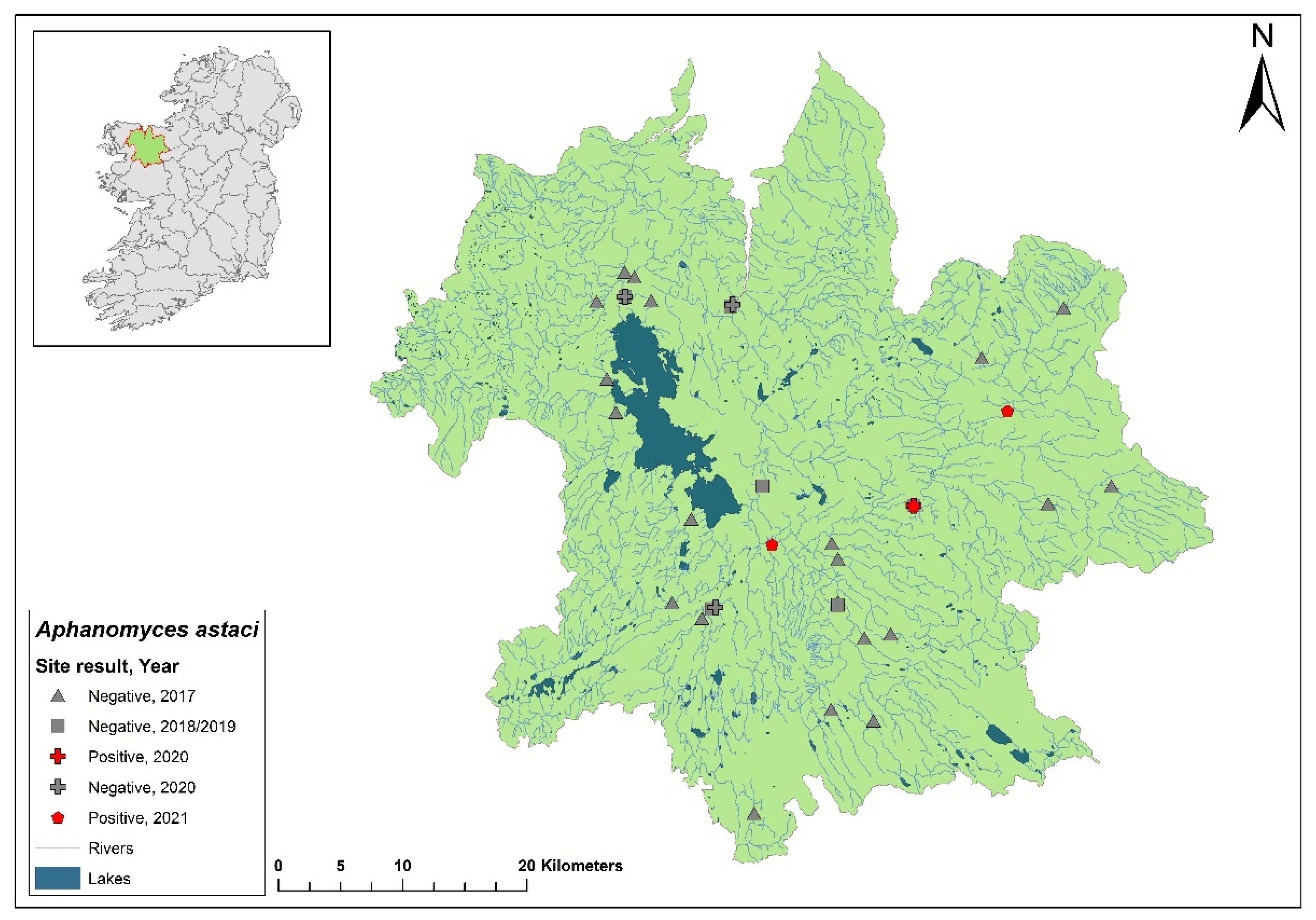
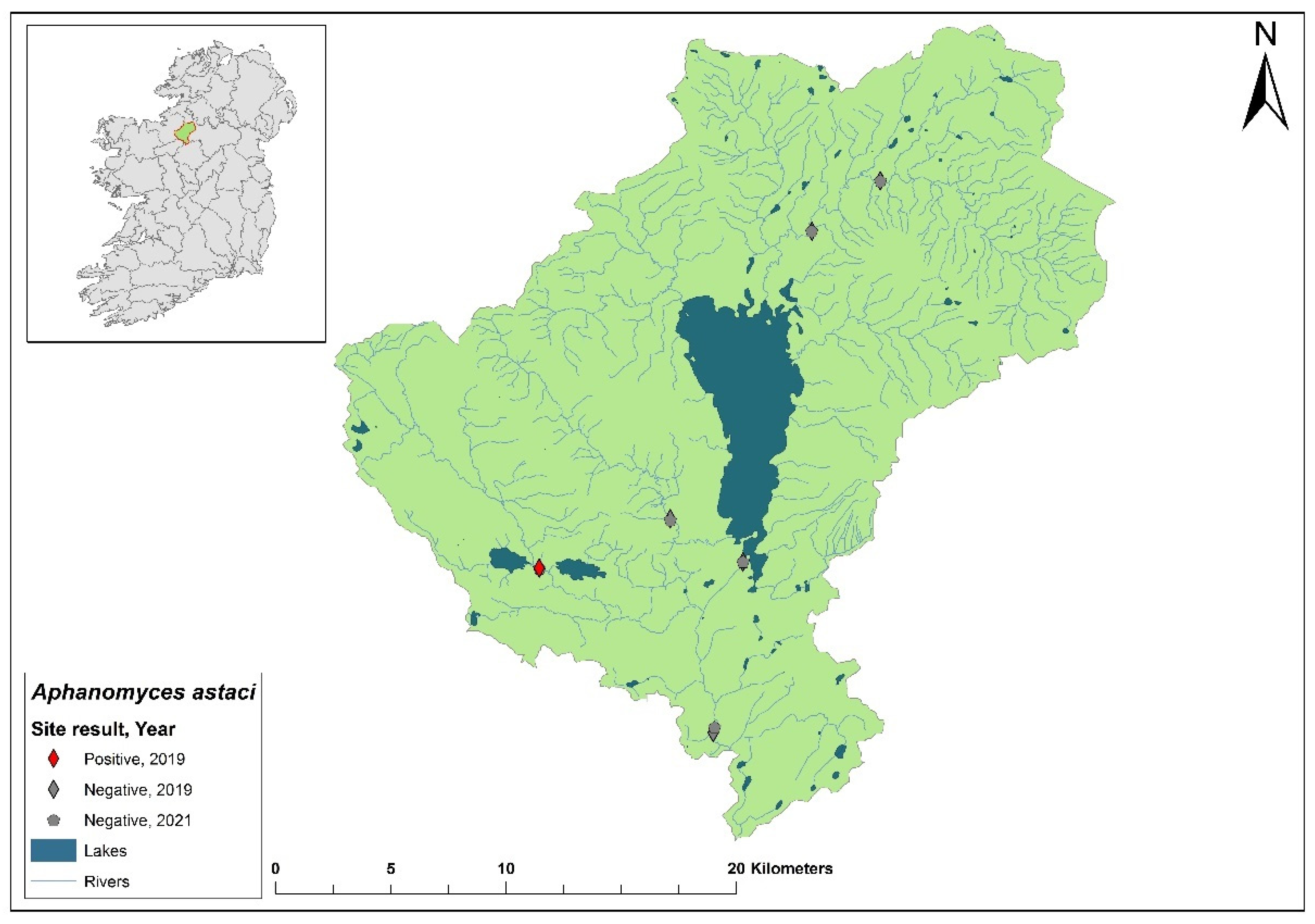
3.10. Upper Shannon (Lough Allen) 26A catchment
The Upper Shannon catchment (ID 26A) spans an area of 604 km
2 containing 25 rivers, eight lakes and 18 groundwaters (
Figure 13). Six samples from the Shannon 26A catchment were sampled in 2019. One sample collected at Ballyfarnon on the Feorish River, Co. Roscommon tested positive for
Ap. astaci (Ct= 35) and was later determined using eDNA samples as the D Genotypes but the subtype was not determined. At the same time, crayfish were detected time by eDNA (Ct= 38 – 40). During the 2020/2021 surveys, all of six sampled sites in the Shannon 26A catchment were negative for
Ap. astaci and two sites tested positive for
A. pallipes by eDNA (Ct= 34 – 35).
3.11. Upper Shannon (Boyle) 26B catchment
The Upper Shannon catchment (ID 26B) spans an area of 674 km
2 containing 28 rivers, 15 lakes and eight groundwater bodies (
Figure 14). Six sites from the Shannon 26B catchment were sampled in 2019. One sample, collected at Cootehall Bridge, Co. Roscommon, tested positive for the pathogen (Ct= 35 – 36), the strain was later classified as the A Genotype from eDNA samples. No sites were tested in 2020. Of the six sites assessed in 2021, four sites remained free of
Ap. astaci. However, two sites, at Bridge West (Ct= 35) and Boyle Footbridge (Ct= 33) tested positive for
Ap. astaci. Moreover,
A. pallipes was also detected at both sites by eDNA (Ct= 37 and -36, respectively).
3.12. Upper Shannon 26C
The Upper Shannon catchment (ID 26C) spans an area of 1,500 km
2 containing 58 rivers, 23 lakes and 15 groundwaters (
Figure 15). In the 2019 survey, six sites were assessed in the Shannon 26C catchment and all tested negative for the pathogen. In 2021, two of the same sites, Drumsna (Ct= 37) and Rinn Marina (Ct= 38) tested positive for
Ap. astaci while two others tested negative. At the same time, eDNA did not detect
A. pallipes at any of the sites in the catchment. Genotyping was not completed.
3.13. Upper Shannon (Suck) 26D catchment
The Upper Shannon catchment (ID 26D) spans an area of 1,598 km
2 containing 58 rivers, one lake and 17 groundwaters (
Figure 16). Six sites were selected for assessment in the Shannon 26D catchment in 2019. One site, Mount Talbot on the River Suck in Co. Roscommon, tested positive for
Ap. astaci (Ct= 36 – 38). However, no crayfish mortalities attributed to crayfish plague have been identified within the catchment. Sampling was not conducted in the catchment in 2020. In 2021, 12 sites were assessed and tested negative for the
Ap. astaci but six were positive for
A. pallipes (Ct= 30 – 37). Genotyping completed for the 2020/2022 NCPSP report placed the positive eDNA sample in either the A or D (subtype unknown) genotypes.
3.14. Shannon 26G catchment
The Upper Shannon catchment (ID 26G) spans an area of 383 km
2 and contains 13 rivers, 12 lakes and one groundwater (
Figure 17). In 2018, reports of crayfish mortalities were reported in the River Al in the Shannon 26G catchment. Three sites were sampled and all tested positive for
Ap. astaci (Ct= 32 – 35). The A Genotype was identified from tissue samples in both NCPSP reports following genotyping. One year later, the sites were inaccessible due to flooding and six other sites in the Shannon 26G catchment were monitored and tested negative for
Ap. astaci. Seven sites were assessed in Shannon 26G catchment in 2020 and were negative for
Ap. astaci. At the same time,
A. pallipes were identified by eDNA at four of six sites tested (Ct= 37 – 40). The catchment was not assessed in 2021.
3.15. Sligo Bay & Drowse catchment
The Sligo Bay & Drowse catchment (ID 35) spans an area of 1,866 km
2 and contains 70 rivers, 18 lakes and 25 ground waters in the north-west of Ireland (
Figure 18). Fifteen sites were monitored in the 2017 survey and no evidence of NICS or crayfish plague were found. Eleven sites were assessed in the 2019 survey and all tested negative for
Ap. astaci. In 2020, one site of four, Gurteen, tested positive for
Ap. astaci (Ct= 35) as well as
A. pallipes (Ct= 33). In 2021, three sites all around Gurteen tested positive for the pathogen (Ct= 30 – 32) and
A. pallipes (Ct= 32 – 36) but no other sites were assessed. The 2021 eDNA samples were placed in the A and D genotypes, indicating evidence for both. It is not clear if the two genotypess were identified in one or more samples.
3.16. Suir catchment
The Suir catchment (ID 16) spans an area of 3,542 km
2 and contains 168 rivers, seven lakes and 43 groundwater bodies in the south of Ireland (
Figure 19). The second recorded outbreak of crayfish plague in Ireland occurred in 2017 on the River Suir, Co. Tipperary. Over a 24 km stretch of the river in the catchment, crayfish losses were estimated to be around 400,000 animals. During the 2017 crayfish survey, 22 sites that were sampled in the Suir catchment, all tested negative for the plague. Of the seven sites assessed in the Suir catchment in 2018, Cahir Bridge on the River Suir tested positive for
Ap. astaci (Ct= 36 – 36), while Carrick-on-Suir tested negative, having tested positive a year prior. Genotyping on tissue samples collected in 2017consistently identified the samples as the D, subtype D1 genotypes. In 2020, one site out of ten sampled in the Suir catchment, River Multeen (Ct= 36), tested positive for
Ap. astaci. The catchment was not assessed in 2021.
3.17. Outbreaks between 2021-2023
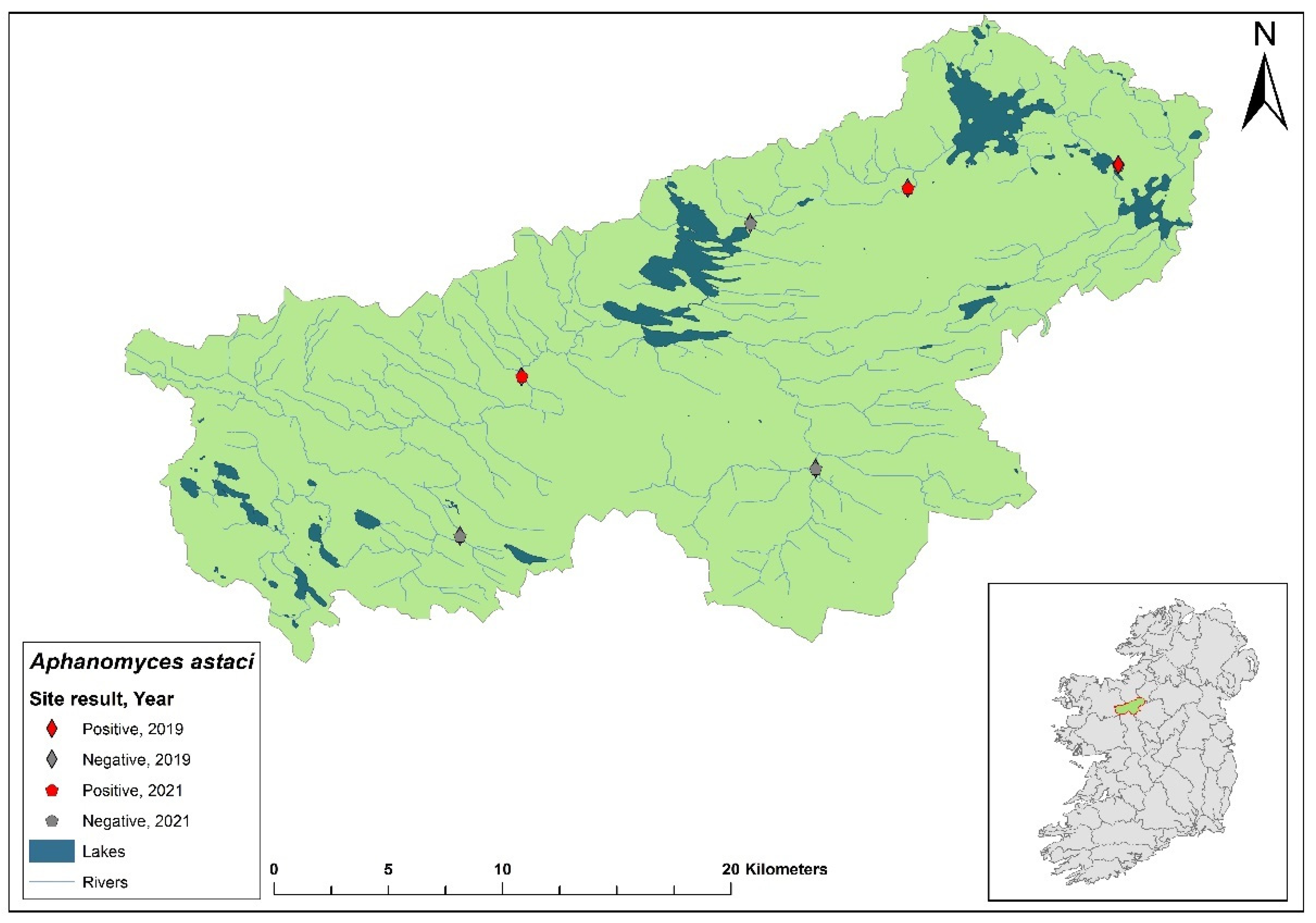
Irish authorities have not released any crayfish plague sampling data since the completion of the NCPSP 2021 survey, and Northern Irish authorities have not released any official data. However, crayfish plague events persist and although none were recorded in 2022, two plague events have been confirmed by authorities in Ireland and Northern Ireland in 2023. In July,
Ap. astaci was detected in the Munster Blackwater in Ireland. In September, a plague event was reported in the Upper Ballinderry River catchment in Northern Ireland (
Figure 3). To date, these two events mark the southernmost and northernmost expansions of
Ap. astaci in Ireland, respectively.
As of October 9th 2023, no record of Ap. astcai has been logged with the National Biodiversity Network Atlas of the United Kingdom of Great Britain and Northern Ireland. At the same time, the National Biodiversity Data Centre in Ireland has logged at least eight independent outbreaks but up to date records have not been kept.
4. Non-indigenous crayfish species
Globally, there are over 600 described species of freshwater crayfish, on the island of Ireland, all but the native
A. pallipes are considered NICS [
10,
24]. Several of these NICS possess traits that make them highly invasive, including rapid growth, large size, and high fecundity, that would enable them to outcompete native
A. pallipes within its Irish range [
2,
25]. Many NICS have been imported and released in Europe, several establishing populations and devastating native crayfish populations and habitats. The spiny-cheek crayfish (
Faxonius limosus), virile crayfish (
F. virilis), signal crayfish (
Pacifastacus leniusculus), red swamp crayfish (
Procambarus clarkii) and marbled crayfish
(Pracambarus fallax f. virginalis) are all established in Europe, compete with native crayfish and can transmit
Ap. astaci [
1].
4.1. No non-indigenous crayfish species found during the 2016-2021 surveys
The 2016 Erne catchment survey assessed the presence of NICS via conventional hand searching, netting or overnight trapping methods and no NICS were found. Likewise, during the 2017 national crayfish survey, no NICS were found using hand searching, netting or overnight traps. Subsequently, the NCPSP developed a multiplex qPCR assay (one reaction detecting 2 or 3 species of NICS each) to test for eight species of NICS, including: signal crayfish (P. leniusculus), noble crayfish (A. astacus), spiny-cheek crayfish (F. limosus), marbled crayfish (P. fallax f. virginalis), red-swamp crayfish (P. clarkii), common yabby (C. destructor), narrow-clawed crayfish (A. leptodactylus) and virile crayfish (F. virilis). However, parameters and validation data of this multiplex assay developed by the Marine Institute have not been published. During the NCPSP monitoring programs, no evidence for NICS was found.
4.2. Legislative changes regarding non-indigenous crayfish species
Ireland implemented legislative changes with the “S.I. No. 354/2018 - European Union (Invasive Alien Species) (Freshwater Crayfish) Regulations 2018 (SI 354/18)” that came into effect on September 18
th, 2018 [
26]. The regulation was designed to mitigate the risk of disease transmission from NICS by prohibiting the trade of five species, including:
F. limosus,
F. virilis,
P. leniusculus,
P. clarkii and
P. fallax f. virginalis, species that are well-established in Europe. The legislation expressly forbids the intentional release of these species into natural habitats and constrains the intentional possession, transportation, sale, breeding, exchange, and ornamental use of live specimens, barring specific exemptions, including research-related activities.
Critical transport stipulations mandate that deceased and live specimens of these crayfish be managed with precautions to impede potential reproduction or escape. Moreover, any transportation of soil or freshwater from areas known to host these species is regulated, ensuring the containment of their spread. However, the testing of soil and water for Ap. astaci by qPCR or other molecular methods is not specified. The legislation cautions against any actions that might inadvertently aid the reproduction, spread, or establishment of these crayfish species, unless explicitly sanctioned. Furthermore, the legislation underscores adherence to eradication measures: landowners or custodians are obligated to comply with any directives from the Minister (for Culture, Heritage and the Gaeltacht) geared towards the control or elimination of these species.
A significant aspect of the legislation is its emphasis on cooperation and accurate reporting; failures to assist, obstructions of authorised personnel, or deliberate misinformation are deemed violations. Penalties and fines for breaching this legislation could reach a maximum of €100,000 and/or imprisonment for up to two years.
4.3. Wild, established non-indigenous crayfish species in Ireland
Many NICS are hosts of
Ap. astaci and can be chronic shedders of
Ap. astaci spores. The first record of established wild NICS in Ireland was reported in 2019 with the presence of a strong population of Common Yabby (
Cherax destructor) in a former quarry in Co. Cork [
27]. The stock was estimated to be present at the site for ten years and no records of
C. destructor have been reported elsewhere in the local area. The species typically requires higher temperatures to survive than are present in Ireland year-round. Moreover,
C. destructor is generally susceptible to
Ap. astaci infection and is unlikely to vector the pathogen [
28]. The new legislation protects native habitats and species from NICS states: “Part 5, Eradication, section 10. (1) Where the Minister confirms the appearance in the State or part thereof of an invasive alien species of crayfish— (a) whose presence in the State was previously unknown… he or she shall… (i) without delay, publish a statement on the internet providing details of the confirmation, and (ii) as soon as practicable but within 3 months of confirmation of the appearance, develop and adopt eradication measures and apply or secure the application of those eradication measures.” Considering
C. destructor is not listed as one of the five species of concern in the legislation, it is unclear whether any action will be taken to eradicate the population.
4.4. The sale of non-indigenous crayfish species in Ireland through the pet trade
In Ireland, NICS have been available for purchase online through the pet trade.
P. fallax f. virginalis, P. clarkii, C. quadricarinatus and Cambarellus patzcuarensis have been recorded for sale in the recent past [
29,
30]. Regardless of the 2018 legislative changes, a search of the popular sales website DoneDeal.ie, on 24/09/2023, showed Dwarf Red Lobster (
C. patzcuarensis) were currently available in Co. Dublin (see S.I. Figure S1; on Oct. 5
th the advert was online for 43 days). It is not likely that
C. patzcuarensis could tolerate the temperate Irish climate and the threat of the species establishing and outcompeting the white-clawed crayfish is low [
31]. Albeit at low levels,
C. patzcuarensis is a recorded vector of
Ap. astaci [
32]. As
C. patzcuarensis is not listed on the restricted crayfish list (SI 354/18), it is unlikely that any action can be taken to intervene in its sale.
5. Management and Mitigation Strategies
5.1. Passive monitoring
Authorities in both Ireland and Northern Ireland appear to have taken a laissez-faire non-intervention approach to the administration of NICS and
Ap. astaci. Research funding related to
Ap. astaci in Ireland has primarily focused on monitoring and determining the spread of the plague pathogen across water catchments; a surveillance programme was established without a mitigation or management programme. Yet, surprisingly, proactive restrictions have not been imposed, such as limiting the movement of wetgear and watercrafts between waterbodies during active mass mortality events. While efforts were taken to publicise the initial plague event and subsequent outbreaks, including a detailed press release by Inland Fisheries Ireland [
13], the emphasis has largely been on passive voluntary preventative measures. “Voluntary bans'' were placed, extended and lifted on several waterbodies.
The data indicate that the voluntary bans were ineffective at curbing the spread of
Ap. astaci, and this measure was criticised by stakeholders, including the angling community [
33,
34]. Stakeholders received advice on the “Check, Clean, Dry” protocol when transitioning between watercourses, and similar literature and videos were disseminated online. Signs informing the public about the crayfish plague and detailing the protocol were prominently placed at high-traffic watercourses. Yet, the continued spread of
Ap. astaci to new catchments suggests these passive measures have been ineffective. Considering the initial four reported crayfish plague cases in Ireland (spanning from Bruskey River [2015] to River Deel [2017]) each presented unique genotypes, this indicates they were independent introductions. This raises the possibility that the initial outreach campaign didn't sufficiently target the international community, particularly tourists engaged in angling and water-based recreational activities in Ireland. This oversight may have inadvertently facilitated the pathogen's rapid succession and multiple introductions.
The legislative change made in 2018 to prohibit the trade of five NICS in the country is arguably the strongest effort made to protect the freshwater environment but is also lacking. Only five species of NICS were legislated for. Considering Ireland only has a single and protected species of freshwater crayfish, the legislation could have extended to all freshwater crayfish species, as interfering with A. pallipes was already prohibited. Neither the established population of C. destructor nor C. patzcuarensis, recently being sold online are listed on the new legislation.
Regarding Northern Ireland, similar efforts to those by southern authorities were made online to advertise the crayfish plague, with the same “Check, Clean, Dry” protocols. To report on the spread of
Ap. astaci across the island of Ireland for this review, personal communication with the Northern Irish authority stated that the genotypes of
Ap. astaci could not be determined at outbreak sites because the infected crayfish tissues were frozen. Although freezing tissues is a standard method of preserving DNA [
35], genotyping
Ap. astaci is a specialist task and should be outsourced to expert and experienced labs at the outset. Regardless, there is little data of effort to monitor the distribution of
Ap. astaci in Northern Ireland and no scientific literature can be found.
6. Perspectives and considerations for the future
6.1. The crayfish plague surveys have not informed crayfish conservation
The rapid spread of crayfish plague in Ireland, impacting numerous water catchments, highlights a critical gap in conservation efforts across Ireland. In the eight years since the 2015 detection of crayfish plague, 18 water catchments have been affected. Neither Irish nor Northern Irish authorities have implemented timely or active measures that stopped the spread of the disease. The National Crayfish Plague Surveillance Program in Ireland is, as the name states, a surveillance programme to monitor Ap. astaci’s distribution. It is important to restate, no effective measures have been implemented to halt the plague’s progression. Regarding Northern Ireland, no program, survey or report could be found on the issue.
Given that the crayfish plague pathogen can eliminate A. pallipes from a site within a few weeks, and considering that outbreaks are typically only reported after a mass mortality event, it's likely that by the time the disease is detected, it has already spread throughout an entire site. Therefore, at the stage of detection following a mortality event, there are no viable mitigation measures or restorative solutions available. However, two immediate actions that could reduce the further spread of Ap. astaci are 1) an immediate prohibition on the public entering the catchment at or near the mortality event site, 2) authorities removing dead and moribund animals from the site to reduce the number of Ap. astaci spores released from infected carcasses. Though these solutions would require careful planning, the occurrences of Ap. astaci already calls for an urgent need for coordinated and rapid actions to prevent further mass mortality events.
6.2. Effective management strategies for crayfish conservation:
As previously discussed, proactive management might involve legislative changes to restrict public access to areas during mass mortality events. Such measures would enable swift semi-isolation of crayfish plague sites, facilitated by field officers setting up clear signage at key access points. Moreover, the removal of deceased and dying crayfish could significantly reduce the release of spores into the water, albeit demanding substantial resources. As an island, Ireland has a unique opportunity to remain free from crayfish plague and NICS. Thus, it's crucial to implement legislation that prohibits the introduction of all live and unprocessed NICS, extending beyond the species already present in Europe. Exceptions should be made only for essential purposes, including scientific research. In the event of a crayfish plague outbreak, caused by Ap. astaci, the pathogen is likely to die out once all host crayfish perish. After such an outbreak, it would be prudent to undertake a thorough eDNA survey across affected water bodies to determine whether Ap. astaci spores remain. Should repeated water tests consistently return negative results, it could then become viable to consider reintroducing A. pallipes into these environments.
6.3. Conventional crayfish sampling
Environmental DNA provides a powerful means to sample a large number of sites for specific species. Although eDNA is a relatively new sampling methodology with extremely powerful detection potential, it possesses inherent weaknesses. These limitations include imperfect sampling, which can occur at any stage of the protocol, contamination, false positive results, false negative results, and biases in sampling and processing (including user bias) [
36]. In a field study testing for coldwater crayfish (
Faxonius eupunctus), positive eDNA detections were shown to increase as one moves further downstream from a population, regardless of abundance [
37]. While another study showed that
P. clarkii crayfish carcasses do not produce detectable levels of eDNA [
38]. These studies indicate that detecting crayfish and their abundances by eDNA alone is challenging and error prone.
During the NCPSP surveys, certain sites provided surprising results. The Upper Shannon 26D catchment is an example where positive Ap. astaci but negative A. pallipes results were obtained by eDNA. Gurteen in the Sligo Bay & Drowse catchment tested positive for Ap. astaci and A. pallipes in 2020, and again in 2021. These are two interesting examples that warrant further investigation. Given the NCPSP's reliance on eDNA to detect A. pallipes, contradictory findings, such as the detection of Ap. astaci without its host, might suggest false positives. In such scenarios, traditional survey methods, including hand searching, sweep netting, and overnight bait trapping, should be employed to verify the presence or absence of A. pallipes. These methods were notably underutilised during the 2018-2021 surveys. Environmental DNA analyses should complement, rather than replace conventional crayfish sampling, and a predetermined number of sites should be designated for traditional sampling to serve as controls, at a minimum.
6.4. Lack of standardisation
The four recently published reports/article analysed in this review span from 2016 to 2021. The initial study, conducted by ATU, incorporated both eDNA testing and traditional survey methods (
Table 1). In contrast, the subsequent study report, also by ATU focused solely on conventional sampling techniques. The 2017 survey omitted eDNA testing, as this was not a requirement specified in the tender (grant) application. Although these two surveys were overseen by different principal investigators, they shared the same sampling team, suggesting a comparable level of effort in data collection. In comparison, the National Crayfish Plague Surveillance Program (NCPSP) surveys demonstrated a higher degree of standardisation. These surveys predominantly utilised eDNA testing. The assays used, including genotyping, were evidently well-optimized, enhancing the reliability and consistency of the findings. However, the NCPSP traded a more thorough sampling approach for a greater coverage, and invested more effort collecting samples from a greater number of sites compared to previous surveys. The value of this trade-off is unclear, during the short summer survey period of the 2017 crayfish survey 127 sites were sampled, that only increased to 168 sites for the annual NCPSP 2021 report period.
6.5. Continued public engagement
Both Irish and Northern Irish authorities have issued press releases regarding crayfish plague events, and these have been covered by local and national newspapers. However, the topic contains several examples of easily misunderstood or confusing vocabulary. For instance, “crayfish plague” can be misunderstood to be a plague of crayfish. Regarding public engagement, the term “voluntary ban” combines two contradictory concepts, is unintuitive and lacks clarity. Future public engagement campaigns should use plain language, avoid technical jargon and provide clear and concise descriptions and explanations. Pictures and diagrams could be used to describe complex concepts. Signs should be transparent regarding the challenges of managing crayfish plague. It is vital for the public to grasp the severe impact of the crayfish plague on the freshwater environment, emphasizing the urgency and significance of this issue.
7. Concluding remark
The spread of the crayfish plague in Ireland presents a significant conservation challenge and it is imperative that Irish and Northern Irish authorities adopt a more proactive and comprehensive multi-faceted approach to crayfish and crayfish plague management. Effective management requires not only immediate action in response to outbreaks but also a long-term strategy incorporating both modern and traditional sampling methods, legislative changes, and public awareness campaigns. The future of Ireland's only crayfish, and the ecosystems they inhabit, hinges on a coordinated and dedicated effort to combat this devastating disease. With greater and concerted efforts, there is hope for the successful conservation and recovery of Ireland's native crayfish populations and the preservation of their important role in freshwater ecosystem.
8. Data sources
The historical plague event was documented first-hand by Reynolds [
12]. Data from four recent surveys completed in 2016, 2017, 2019 and 2021, were retrieved from their respective articles or reports; Mirimin
et al. (2022) [
14]; White-clawed crayfish
Austropotamobius pallipes survey in designated SACs in 2017[
16]; National Crayfish Plague Surveillance program 2018_2019: Report National Crayfish Plague Surveillance Programme 2020-2021 [
17], respectively.
Author Contributions
Conceptualisation, D.J.B.; data curation, D.J.B and R.M.; Mapping via GIS, R.M.; Writing – Original Draft Preparation & writing, D.J.B.; Writing – Review & Editing, D.J.B., R.M., K.T., A.V., J.D.R.
Funding
This review was funded by Fraunhofer Institute for Molecular Biology and Applied Ecology IME, Giessen.
Data Availability Statement
All data were obtained from open access and available field reports and a peer-reviewed research article.
Conflicts of Interest
The authors declare that this review was created in the absence of commercial, financial or other relationships that could might be considered as a conflict of interest.
References
- Jussila, J., Francesconi, C., Theissinger, K., Kokko, H., & Makkonen, J. Is Aphanomyces astaci Loosing its Stamina: A Latent Crayfish Plague Disease Agent From Lake Venesjärvi, Finland, 2021. [CrossRef]
- Jussila, J., Edsman, L., Maguire, I., Diéguez-Uribeondo, J., & Theissinger, K. Money kills native ecosystems: European crayfish as an example. Frontiers in Ecology and Evolution, 2021. 9, 648495. [CrossRef]
- Cornalia, E., Sulla malattia dei gamberi. Atti. Soc. Ital. Sci. Nat., 1860. 2: p. 334-336.
- Cerenius, L., Bangyeekhun, E., Keyser, P., Söderhäll, I., & Söderhäll, K. Host prophenoloxidase expression in freshwater crayfish is linked to increased resistance to the crayfish plague fungus, Aphanomyces astaci. Cell. Microbiol., 2003. 5(5): p. 353-357. [CrossRef]
- Becking, T., Kiselev, A., Rossi, V., Street-Jones, D., Grandjean, F., & Gaulin, E. Pathogenicity of animal and plant parasitic Aphanomyces spp. and their economic impact on aquaculture and agriculture. Fungal Biol. Rev., 2022. 40: p. 1-18. [CrossRef]
- Boštjančić, L.L., Francesconi, C., Rutz, C., Hoffbeck, L., Poidevin, L., Kress, A., Jussila, J., Makkonen, J., Feldmeyer, B., Bálint, M. and Schwenk, K., Host-pathogen coevolution drives innate immune response to Aphanomyces astaci infection in freshwater crayfish: transcriptomic evidence. BMC Genomics, 2022. 23(1), p.600. [CrossRef]
- Strand, D.A., Jussila, J., Viljamaa-Dirks, S., Kokko, H., Makkonen, J., Holst-Jensen, A., Viljugrein, H. and Vrålstad, T., Monitoring the spore dynamics of Aphanomyces astaci in the ambient water of latent carrier crayfish. Vet. Microbiol., 2012. 160(1-2): p. 99-107. [CrossRef]
- Souty-Grosset, C., Haffner, P., Reynolds, J.D., Noel, P.Y. and Holdich, D.M., Atlas of crayfish in Europe. Muséum national d'Histoire naturelle Paris. 2006, (P. 188).
- Makkonen, J., Jussila, J., Kortet, R., Vainikka, A. and Kokko, H., Differing virulence of Aphanomyces astaci isolates and elevated resistance of noble crayfish Astacus astacus against crayfish plague. Dis. Aquat. Org., 2012. 102(2): p. 129-136. [CrossRef]
- Reynolds, J. D. Crayfish in Irish Rivers. In Ireland's Rivers, eds.; Kelly-Quinn, M; Reynolds, J. D.; University College Dublin Press. Dublin, Ireland, 2020; pp. 95-.
- Matthews, M.A. and Reynolds, J.D., A population study of the white-clawed crayfish Austropotamobius pallipes (Lereboullet) in an Irish reservoir. Bio. and Enviro., PRIA JSTOR 1995. (pp. 99-109).
- Reynolds, J.D., Crayfish extinctions and crayfish plague in central Ireland. Biol. Conserv., 1988. 45(4): p. 279-285. [CrossRef]
- Inland Fisheries Ireland, Press Release. Investigation underway into cause of Crayfish Plague on River Bruskey, near Ballinagh, Co Cavan. 2015, Inland Fisheries Ireland: Online. https://tinyurl.com/ycsn72fr.
- Mirimin, L., Brady, D., Gammell, M., Lally, H., Minto, C., Graham, C.T., Slattery, O., Cheslett, D., Morrissey, T., Reynolds, J. and White, S., Investigation of the first recent crayfish plague outbreak in Ireland and its subsequent spread in the Bruskey River and surrounding areas. Knowl. Manag. Aquat., 2022 (423): p. 13. [CrossRef]
- Gammell, M., McFarlane, A., Brady, D., O’Brien, J., Mirimin, L., Graham, C., Minto C., and O’Connor I. White-Clawed crayfish Austropotamobius pallipes survey in designated SACs in 2017. 2018. National Parks & Wildlife Service. Dublin, Ireland. Report ISSN 1393 – 6670.
- White, S., Griffin, B., Swords, F., O’Toole, C., Morrissey, T., Cheslett, D., Geary, M., Brenan, A., Viljamaa-Dirks, S., Panteleit, J., Reynolds, J., Nelson, B., The National Crayfish Plague Surveillance Program, Ireland-2018-2019. 2020 Marine Institute. p. 32.
- Swords, F., Griffin, B., White, S., Cheslett, D., Geary, M., Brenan, A., Coakley, C., Nelson, B., The National Crayfish Plague Surveillance Program, Ireland-2018-2019. Marine Institute. Programme, Ireland – 2020-2021. 2022. p. 38.
- Di Domenico, M., Curini, V., Caprioli, R., Giansante, C., Mrugała, A., Mojžišová, M., Cammà, C. and Petrusek, A., Real-Time PCR assays for rapid identification of common Aphanomyces astaci genotypes. Front. Ecol. Evol., 2021. 9: p. 597585. [CrossRef]
- Makkonen J., Jussila J., Panteleit J., Keller N., Schrimpf A., Theissinger K., Kortet R., Laura M., Sandoval-Sierra J., Diéguez-Uribeondo J. and Kokko H, mtDNA allows the sensitive detection and haplotyping of the crayfish plague disease agent Aphanomyces astaci showing clues about its origin and migration. Parasitol., 2018. 145: 1–9. [CrossRef]
- Francesconi, C., Makkonen, J., Schrimpf, A., Jussila, J., Kokko, H. and Theissinger, K., Controlled infection experiment with Aphanomyces astaci provides additional evidence for latent infections and resistance in freshwater crayfish. Front. Ecol. Evol., 2021. 9: p. 647037. [CrossRef]
- Vrålstad, T., Knutsen, A.K., Tengs, T. and Holst-Jensen, A., A quantitative TaqMan® MGB real-time polymerase chain reaction based assay for detection of the causative agent of crayfish plague Aphanomyces astaci. Vet. Microbiol., 2009. 137(1-2): p. 146-155. [CrossRef]
- Klymus, K.E., Merkes, C.M., Allison, M.J., Goldberg, C.S., Helbing, C.C., Hunter, M.E., Jackson, C.A., Lance, R.F., Mangan, A.M., Monroe, E.M. and Piaggio, A.J., Reporting the limits of detection and quantification for environmental DNA assays. Environ. DNA, 2020. 2(3): p. 271-282. [CrossRef]
- Jussila, J., Vrezec, A., Makkonen, J., Kortet, R. and Kokko, H., Invasive crayfish and their invasive diseases in Europe with the focus on the virulence evolution of the crayfish plague. Biological invasions in changing ecosystems: vectors, ecological impacts, management and predictions. De Gruyter Open, Berlin, 2015: p. 183-211. [CrossRef]
- Crandall, K.A. and Buhay, J.E., Global diversity of crayfish (Astacidae, Cambaridae, and Parastacidae—Decapoda) in freshwater. FADA, 2008: p. 295-301. [CrossRef]
- Holdich, D.M., Reynolds, J.D., Souty-Grosset, C. and Sibley, P.J., A review of the ever increasing threat to European crayfish from non-indigenous crayfish species. Knowl. Manag. Aquat. Ecosyst., 2009. (394-395): p. 11. [CrossRef]
-
European Union (Invasive Alien Species) (Freshwater Crayfish) Regulations 2018 in S.I. No. 354 of 2018. 2018. https://www.irishstatutebook.ie/eli/2018/si/354/made/en/pdf: European Union. 2018.
- Sweeney, P., Reynolds J., Nelson, B. and O’Flynn, C., First recorded population of the Common Yabby (Cherax destructor Clark) (Decapoda, Parastacidae) in the wild in Ireland. Ir. Nat.' J. 39, 2022, 14-19.
- Svoboda, J., Hosts and transmission of the crayfish plague pathogen Aphanomyces astaci: a review. J. Fish Dis., 2017. 40(1): p. 127-140. [CrossRef]
- Faulkes, Z., A bomb set to drop: parthenogenetic Marmorkrebs for sale in Ireland, a European location without non-indigenous crayfish. Manag. Biol. Invasions, 2015. 6(1): p. 111. [CrossRef]
- Faulkes, Z. Slipping past the barricades: the illegal trade of pet crayfish in Ireland. Biol. Environ PRIA, 2017. (Vol. 117, No. 1, pp. 15-23). [CrossRef]
- Chucholl, C., Invaders for sale: trade and determinants of introduction of ornamental freshwater crayfish. Biol. Invasions, 2013. 15: p. 125-141. [CrossRef]
- Mrugała, A., Kozubíková-Balcarová, E., Chucholl, C., Cabanillas Resino, S., Viljamaa-Dirks, S., Vukić, J. and Petrusek, A., Trade of ornamental crayfish in Europe as a possible introduction pathway for important crustacean diseases: crayfish plague and white spot syndrome. Biol. Invasions, 2015. 17: p. 1313-1326. [CrossRef]
- Off the Scale. White-clawed crayfish & crayfish plague, Off the Scale Magazine, November 2017. Available online: https://www.offthescaleangling.ie/the-science-bit/crayfish-plague/ (accessed on 01/09/2023).
- Off the Scale. First crayfish plague outbreak of 2019. Off the Scale Magazine, 21/05/2019. Available online: https://www.offthescaleangling.ie/news/ maigue-crayfish/ (accessed on 01/09/2023).
- Kjær, K.H., Winther Pedersen, M., De Sanctis, B., De Cahsan, B., Korneliussen, T.S., Michelsen, C.S., Sand, K.K., Jelavić, S., Ruter, A.H., Schmidt, A.M. and Kjeldsen, K.K., A 2-million-year-old ecosystem in Greenland uncovered by environmental DNA. Nature, 2022. 612(7939): p. 283-291. [CrossRef]
- Beng, K.C. and Corlett, R.T., Applications of environmental DNA (eDNA) in ecology and conservation: opportunities, challenges and prospects. Biodivers. Conserv., 2020. 29: p. 2089-2121. [CrossRef]
- Rice, C.J., E.R. Larson, and C.A. Taylor, Environmental DNA detects a rare large river crayfish but with little relation to local abundance. Freshw. Biol., 2018. 63(5): p. 443-455. [CrossRef]
- Curtis, A.N. and E.R. Larson, No evidence that crayfish carcasses produce detectable environmental DNA (eDNA) in a stream enclosure experiment. PeerJ, 2020. 8: p. e9333. [CrossRef]
Figure 1.
Timeline of the introduction and spread of Aphanomyces astaci to Europe and the subsequent introductions and spread across water catchments around the island of Ireland.
Figure 1.
Timeline of the introduction and spread of Aphanomyces astaci to Europe and the subsequent introductions and spread across water catchments around the island of Ireland.
Figure 2.
Sites surveyed and affected by during the 1987 crayfish plague mass mortality event. Modified from Reynolds 1988 [
12]. Erne refers to the Erne catchment; Boyne refers to the Boyne catchment; Barrow refers to the Barrow catchment; 25A refers to the Lower Shannon (Brosna) 25A catchment; 26F refers to the Lower Shannon 26F catchment. Areas coloured blue represent lakes.
Figure 2.
Sites surveyed and affected by during the 1987 crayfish plague mass mortality event. Modified from Reynolds 1988 [
12]. Erne refers to the Erne catchment; Boyne refers to the Boyne catchment; Barrow refers to the Barrow catchment; 25A refers to the Lower Shannon (Brosna) 25A catchment; 26F refers to the Lower Shannon 26F catchment. Areas coloured blue represent lakes.
Figure 3.
Distribution of Aphanomyces astaci genotypes across infected catchments in Ireland. Genotypes presented are reported in the 2021 NCPSP report; black line signifies Northern Ireland. Ballinderry River and Munster Blackwater occurred in 2023 but genotyping details has not been published at time of writing (Nov., 2023).
Figure 3.
Distribution of Aphanomyces astaci genotypes across infected catchments in Ireland. Genotypes presented are reported in the 2021 NCPSP report; black line signifies Northern Ireland. Ballinderry River and Munster Blackwater occurred in 2023 but genotyping details has not been published at time of writing (Nov., 2023).
Figure 4.
Sites surveyed for Aphanomyces astaci in the Erne catchment. Red star indicates the initial site of the Bruskey River crayfish plague event in Co. Cavan. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 4.
Sites surveyed for Aphanomyces astaci in the Erne catchment. Red star indicates the initial site of the Bruskey River crayfish plague event in Co. Cavan. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 5.
Sites surveyed for Aphanomyces astaci in the Barrow catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 5.
Sites surveyed for Aphanomyces astaci in the Barrow catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 6.
Sites surveyed for Aphanomyces astaci in the Corrib catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 6.
Sites surveyed for Aphanomyces astaci in the Corrib catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 7.
Sites surveyed for Aphanomyces astaci in the Moy & Killala Bay catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 7.
Sites surveyed for Aphanomyces astaci in the Moy & Killala Bay catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 8.
Sites surveyed for Aphanomyces astaci in the Nore catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 8.
Sites surveyed for Aphanomyces astaci in the Nore catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 9.
Sites surveyed for Aphanomyces astaci in the Shannon Estuary South catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 9.
Sites surveyed for Aphanomyces astaci in the Shannon Estuary South catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 10.
Sites surveyed for Aphanomyces astaci in the Lower Shannon 25A catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 10.
Sites surveyed for Aphanomyces astaci in the Lower Shannon 25A catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 11.
Sites surveyed for Aphanomyces astaci in the Shannon Estuary South catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 11.
Sites surveyed for Aphanomyces astaci in the Shannon Estuary South catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 12.
Sites surveyed for Aphanomyces astaci in the Ulster Blackwater catchment. Darker green indicates the area within Ireland; lighter green indicates area within Northern Ireland; symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 12.
Sites surveyed for Aphanomyces astaci in the Ulster Blackwater catchment. Darker green indicates the area within Ireland; lighter green indicates area within Northern Ireland; symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 13.
Sites surveyed for Aphanomyces astaci in the Upper Shannon 26A catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 13.
Sites surveyed for Aphanomyces astaci in the Upper Shannon 26A catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 14.
Sites surveyed for Aphanomyces astaci in the Upper Shannon 26B catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 14.
Sites surveyed for Aphanomyces astaci in the Upper Shannon 26B catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 15.
Sites surveyed for Aphanomyces astaci in the Upper Shannon 26C catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 15.
Sites surveyed for Aphanomyces astaci in the Upper Shannon 26C catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 16.
Sites surveyed for Aphanomyces astaci in the Upper Shannon 26D catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 16.
Sites surveyed for Aphanomyces astaci in the Upper Shannon 26D catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 17.
Sites surveyed for Aphanomyces astaci in the Upper Shannon 26G catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 17.
Sites surveyed for Aphanomyces astaci in the Upper Shannon 26G catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 18.
Sites surveyed for Aphanomyces astaci in the Sligo Bay & Drowse catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 18.
Sites surveyed for Aphanomyces astaci in the Sligo Bay & Drowse catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 19.
Sites surveyed for Aphanomyces astaci in the Suir catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Figure 19.
Sites surveyed for Aphanomyces astaci in the Suir catchment. Symbols indicate sampling years; red symbols indicate sites positive for Ap. astaci, grey symbols indicate sites negative for Ap. astaci.
Table 1.
Surveys undertaken to detect the presence of Aphanomyces astaci in Ireland between 2015 and 2021.
Table 1.
Surveys undertaken to detect the presence of Aphanomyces astaci in Ireland between 2015 and 2021.
| Report*/Article# title |
Methods |
Conducted
by
|
Year/s |
Reference |
| Crayfish Extinctions and Crayfish Plague in Central Ireland#
|
Conventional survey |
Reynolds |
1987 |
Reynolds
[12] |
Investigation of the first recent crayfish plague outbreak in Ireland
and its subsequent spread in the Bruskey River and surrounding areas#
|
Conventional survey; eDNA |
ATU |
2016 |
Mirimin et al.
[14] |
White-clawed crayfish Austropotamobius pallipes survey in
designated SACs* |
Conventional survey |
ATU |
2017 |
Gammell et al.
[15] |
| The National Crayfish Plague Surveillance Program, Ireland* |
eDNA |
MI for NPWS |
2018 2019 |
White et al.
[16] |
| The National Crayfish Plague Surveillance Programme, Ireland* |
Conventional survey; eDNA |
MI for NPWS |
2020 2021 |
Swords and Griffin et al. [17] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).