1. Introduction
However, despite entire efforts invested in efficient developments underlying cancer mortality [2]. With regards mortality rate has been decreased in a past half decade owing to positive outcomes related from killing cancer cells in early cancer diagnosis and better understanding of improved diagnostics techniques, treatments, and advanced tumor biology [3,4]. While conventional cancer therapies have shown some effectiveness, they suffer from various limitations, including non-specific drug distribution, suboptimal pharmacokinetics, and cancer pathologies still often involve surgical intervention, intense radiation, and chemo/hormonal therapeutic drugs, which can destroy healthy cells and cause toxicity [5,6]. Therefore, developing a more effective chemotherapeutic method is crucial. Current therapeutic regimes are limited by cytotoxic drugs due to their lack of affinity for cancer cells and the hydrophobic nature of numerous anticancer drugs, which results in poor cell surface distribution [7,8]. In this context, cancer chemotherapy and detection technologies offer promising tools for reducing side effects, increasing biocompatibility, and improving therapeutic efficacy [9,10]. In prospective, smart nanomaterials, such as polymeric nano particles, liposomes, micelles, dendrimers, have demonstrated the ability to address these limitations by allowing the development of targeted drug delivery systems or active-intracellular delivery into cancerous cells [11]. This drug delivery system is supported by evidence of Nanoparticle-cell surface interactions, attributing lower cytosolic pH (acidic) and elevated redox potential to specific active targeting units [12,13].
Polymeric nanocarriers have garnered considerable attention due to their efficient delivery of bioactive compounds in biomedical applications [14]. These carriers can be further modified to incorporate stimuli-responsive signals, triggered by either endogenous factors like pH, redox potential, or glucose levels, or exogenous factors such as magnetism, light, or ultrasound, enabling precise control over drug release in specific conditions [15–20]. Various strategies have been developed to prepare these stimuli-responsive signals in polymeric nanomaterials, whether synthetic or bio-based polymers. This is especially important to overcome limitations associated with the release of hydrophobic drugs, including poor absorption, limited bioavailability, and formulation challenges [21,22]. Polymer nanoparticles play a significant role in therapeutic settings by safeguarding delicate drugs until they reach their intended delivery site. However, there are numerous physicochemical and biological obstacles that hinder the targeted delivery of cargos, drug solubilization, biocompatibility, and site-specific delivery to cells and tissues, making therapeutic delivery challenging [23,24]. To address these challenges, engineered or smart nanopolymer systems have been developed to possess physicochemical properties that respond to dual stimuli, such as pH/magnetic fields, pH/redox potential, pH/temperature, double pH changes, temperature/reduction, pH and diols, and temperature/enzymes [25,26]. These nanoparticles are designed to undergo chemical alterations in response to various biological stimuli. One of the key challenges in cancer treatment and diagnosis lies in engineered gene/drug delivery systems that can specifically target diseased cells without harming normal healthy tissues/cells, particularly within the tumor microenvironment (TME). Achieving this requires precise and controlled delivery of anticancer agents [27,28]. In contrast, polymer nanoparticles with stimuli-responsive properties offer various approaches, including emulsion polymerization, layer-by-layer assembly, and self-assembly [29], to address these challenges and enhance drug delivery in a targeted manner.
To explore controlled and targeted drug delivery stimuli-responsive biomaterials have emerged as a promising solution, offering the potential for personalized and site-specific cancer therapy. Stimuli-responsive biomaterials are meticulously designed to sense and respond to specific cues within the tumor microenvironment, enabling the precise release of therapeutic agents at the site of action. This targeted drug delivery approach aims to maximize the therapeutic effects on cancer cells while minimizing damage to healthy tissues, ultimately reducing overall toxicity, and enhancing treatment efficacy. By harnessing the unique characteristics of cancer cells and their surrounding microenvironment. Among the diverse range of stimuli-responsive biomaterials, pH, and redox-responsive polymer-nanocomposites have garnered considerable attention for their dual-stimuli responsiveness and potential as future-generation biomaterials. Hence, these novel polymer-nanocomposites are engineered to respond to changes in pH and redox conditions, which are characteristic features of the tumor microenvironment [30]. The slightly acidic pH in tumor tissues, resulting from an increased metabolic rate and inefficient vascularization, serves as a distinctive trigger for pH responsiveness. Simultaneously, the elevated levels of reducing agents, such as glutathione (GSH), within cancer cells offer an opportunity for redox responsiveness. The design principles of pH and redox-responsive polymer-nanocomposites revolve around incorporating pH-sensitive and redox-sensitive elements into the polymer matrix. These elements enable the biomaterials to undergo controlled changes in structure and physicochemical properties in response to changes in pH and redox conditions, facilitating on-demand drug release. As a result, pH and redox-responsive polymer-nanocomposites offer the unique advantage of dual-stimuli responsiveness, further enhancing their precision and effectiveness in drug delivery for cancer therapy.
This review article aims to provide a comprehensive overview of the recent advancements in pH and redox-triggered polymer-nanocomposites for site-specific drug release in cancer therapy. The review will explore the design principles and fabrication methods of these biomaterials, elucidate the mechanisms of their action in response to pH and redox stimuli, and discuss in vitro and in vivo studies that showcase their potential in cancer treatment. Furthermore, the review will analyze the challenges and future perspectives of these innovative biomaterials, underscoring the importance of continued research and development in this exciting field. The emergence of pH and redox-responsive polymer-nanocomposites marks a significant stride towards personalized and targeted cancer therapy, offering hope for improved patient outcomes and a brighter future in cancer treatment.
1.1. Polymeric Nanomaterials
Polymer nanoparticles includes with organic and inorganic nanoparticles [31]. Widely used in theragnostic agents due to valuable significant efficacy and the plethora benefits in cancer treatments [32]. Several subtypes of polymeric NPs are developed for specific triggered drug delivery to tumor. The mainly NPs are classified as polymeric micelles, dendritic polymers, polymeric nanospheres, polymeric conjugate complex [33,34]. The structural and typological diversity of polymeric nanoparticles employed in different applications is shown in
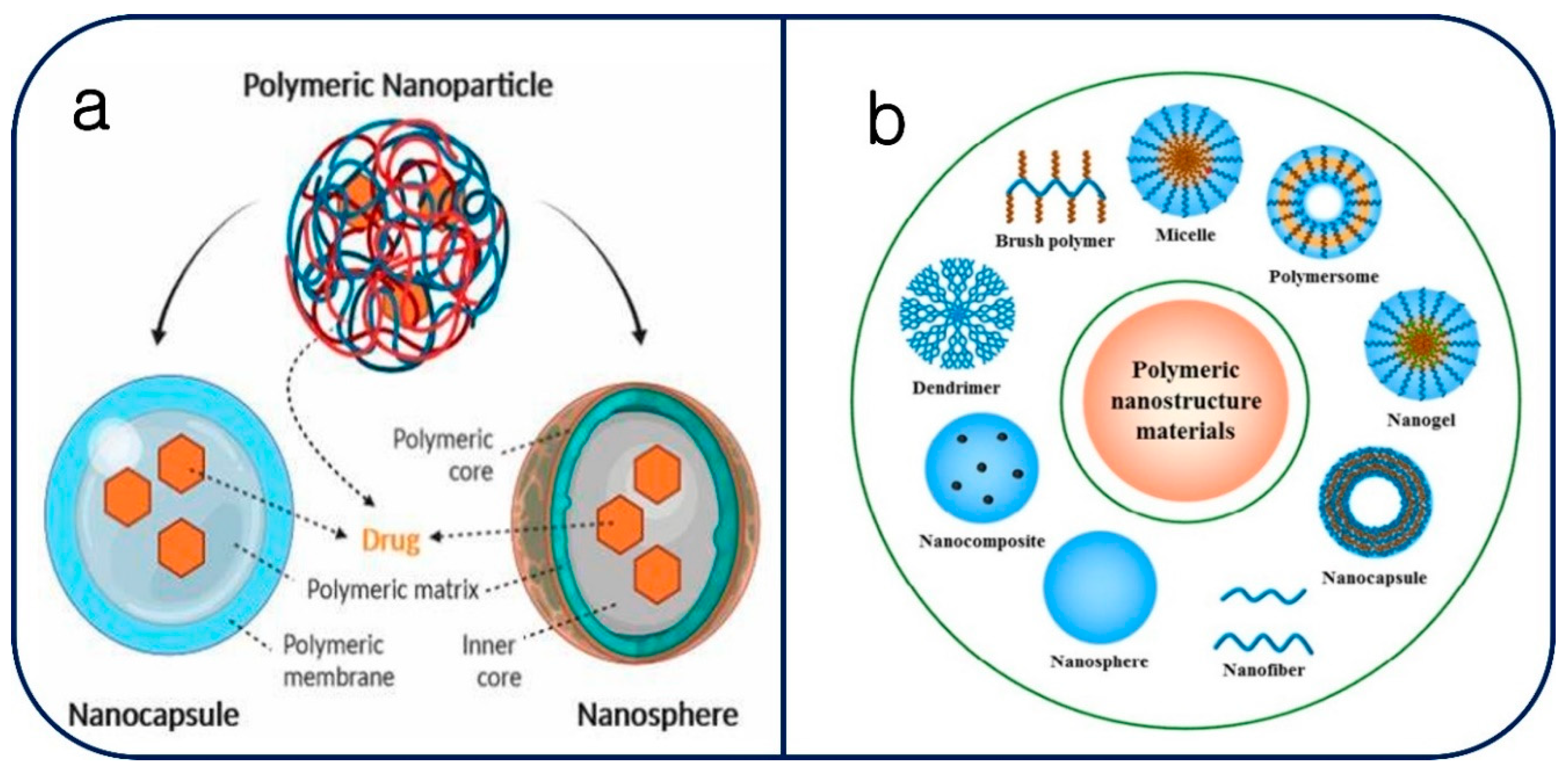
Figure 1. The general structural makeup of polymeric nanoparticles is shown graphically in this section (a) [31]. It provides a crucial comprehension of the fundamental architectural components that these nanoparticles have in common. This covers the polymer matrix as well as any other elements or alterations that support its functional and responsive qualities. The wide variety of polymeric nanoparticles used in various scientific and medical situations is discussed in more detail in section (b) [34]. The structural forms of these nanoparticles include brush polymers, micelles, dendrimers, nanofibers, nanoparticles, polymersomes, and nanogels. Because of their distinct qualities, each of these forms can be customized for a particular need. The advantageous of polymeric composite materials have properties as drug carriers imposed to low solubility drugs solubilization, provides potentials to tumor targets, controlled drug release and ability to reach specific active targeting in tumor diagnosis [35,36]. Considering polymeric micelles are spherically shaped, size ranges from 10 to 100 nm. The simplicity of polymeric micelles formation held via spontaneous self-assembly of hydrophobic and hydrophilic copolymer molecules [37]. Drug encapsulation nano carriers provides physical mixing formation rather than the chemical conjugation. Hydrophobic shell/micelles act as drug reservoirs while hydrophilic shells ensure solubilization of micelles in aqueous solution- [38,39].
The polymer composite nanocarriers must possess.
- (1)
Remain stable in blood until they reach TME
- (2)
Improve the hydrophilic property and delayed recognition in immune system, allowing it for enhanced targets to desired cells/tissues after the reticuloendothelial system (RES) and mononuclear phagocyte system (MPS) surface activity.
- (3)
these are gathered in TMS while allowing to pass through irregular vasculature tumor condition.
- (4)
to respond for stimuli-controlled drug release to loaded therapeutic contents.
- (5)
ability to modifying surface functionalization.
- (6)
tumor-interstitial fluid penetration occurred in TMS.
- (7)
to reach specific active targeting site to carry out drug phenomena [40–42].
The nano particles able to place in tumor Interstitial space allowed for long fluid circulation properties through passive mechanism of which accumulation leads to enhanced surface permeability and retention effect. In case of active targeting, the polymeric nanoparticles need to be modified by such targeting agents in the surface area to enhance tumor efficiency and minimizes caused side effects [43]. polymeric micelles NPs are developed using synthetic polymers or bio polymers. Polyamine, polyether, polyamide, and polyester are known to be Synthetic polymers and polypeptide, polysaccharides, polynucleotide are classified as bio polymers. Depending on their structure, variability and biocompatible enables the flexibility to design engineered polymeric micelles in synthetic polymers. While bio polymers show high-defined structure and considered as more biocompatible nature compared to synthetic materials due to reduced contamination in produced side products [44,45].
2. Role of Tumor Micro-Environment
The formulated Nanoparticles allowed to physicochemical and biological roadblocks which imposes requirements of size, biocompatibility, penetration, surface activity materials for preventing unspecific targets and introducing the specific binding materials to targets. Recently polymeric dual stimuli responsive nano particles acquired more and more attraction towards drug delivery due to their physicochemical unique properties which significantly improve their bioactivity of specific delivered agents for certain diseases tumor treatment [46]. Physicochemical properties exhibit surface ligands based on drug delivery systems in molecular, cellular level to enhancing versatility of multifunctional nano particles [47]. Including these surfer ligands constructed with various internal stimuli and external stimuli factors such as temperature, magnetic, ultrasound, light etc. [48,49] and these studies can demonstrate in both in vivo as well as in vitro. The Physicochemical properties takes place in a simultaneous way at pathological site in intercellular compartment [50]. This kind of compartment system developed to study specific signals from solid tumor microenvironment [51]. In subcellular system tumor targeting, tumor diagnosis and tumor imaging undergoes multifunctional activities with all three-work load done by single move enhancing multimodal approach towards tumor disease [
52]. In addition to this the extracellular environment shows more acidic (pH-6.5) nature in than blood circulation [
53]. Here are a few significant variables that impact the tumor microenvironment's role.
- A
NPs size: The diameter of NP therapeutic should be range from 10-100 nm and with surface combatant either positive or negative charge can lead great clearance to accessibility when dosed into either tumor or circulatory system [54]. Provide emerging treatment of NPs through fenestrations within tumor vasculature permitting for direct-cell access [55]. The lymph compartment of tumor vasculature in mouse model furnished the macromolecules leaking out from blood vessels this is well known as enhanced permeability and retention (EPR) effect [56]. Enables the chemotherapeutic agents as Conjugate polymeric NPs-drug into cancer tissues (ensure deeper penetration) may have favorably biased along with several mechanisms includes particle size [57]. If mentioned size range is correct NPs are restricted from normal vasculature (usually requires size below 2 nm); however, these NPs are still capable to access liver compartment in humans.
- B
NPs surface properties: The NPs depends on high surface-to-volume ratio as compared to larger particles so control of surface properties will be crucial with their behavior in local environment. Usually, NPs surface properties includes with hydrophobic and hydrophilic, pH & charge, PEGylation or other coating, surface functional group (-SH, -NH2, -COOH), surface charge, binding interaction [58–60].
- C
NPs composition: The variety of NPs such as organic NPs (Liposomes, dendrimer, carbon tubes, polymers) and inorganic NPs (QD, magnetic NPs, GNPs, micelles) with dimensions less than few hundred nanometers have been recently shown emerging platforms for diagnostic treatment in drug delivery system. Especially, polymeric micelles, dendrimer, liposomes have been broadly explored for selectively targeting drug delivery of anti-cancer agents. In recent years nano scale composite with RNA (siRNA) as delivery vehicle also been developed for effective therapeutic approach in cancer treatment [61,62].
- D
NPs targeting agents: NPs surrounded with anticancer drugs have been conjugated with different targeting molecules such as aptamers, antibodies, peptides present in Stem cells. Once this encapsulated drug released within cell or enter tumor. Thus, stimulating pH-redox responsive utilized by NPs for sit-specific drug release to enhance accumulation in tumor-site to bring physiological effects by active and passive mechanisms. On condition that destroy only cancer cell before leaving stem cell [63,64].
In addition,
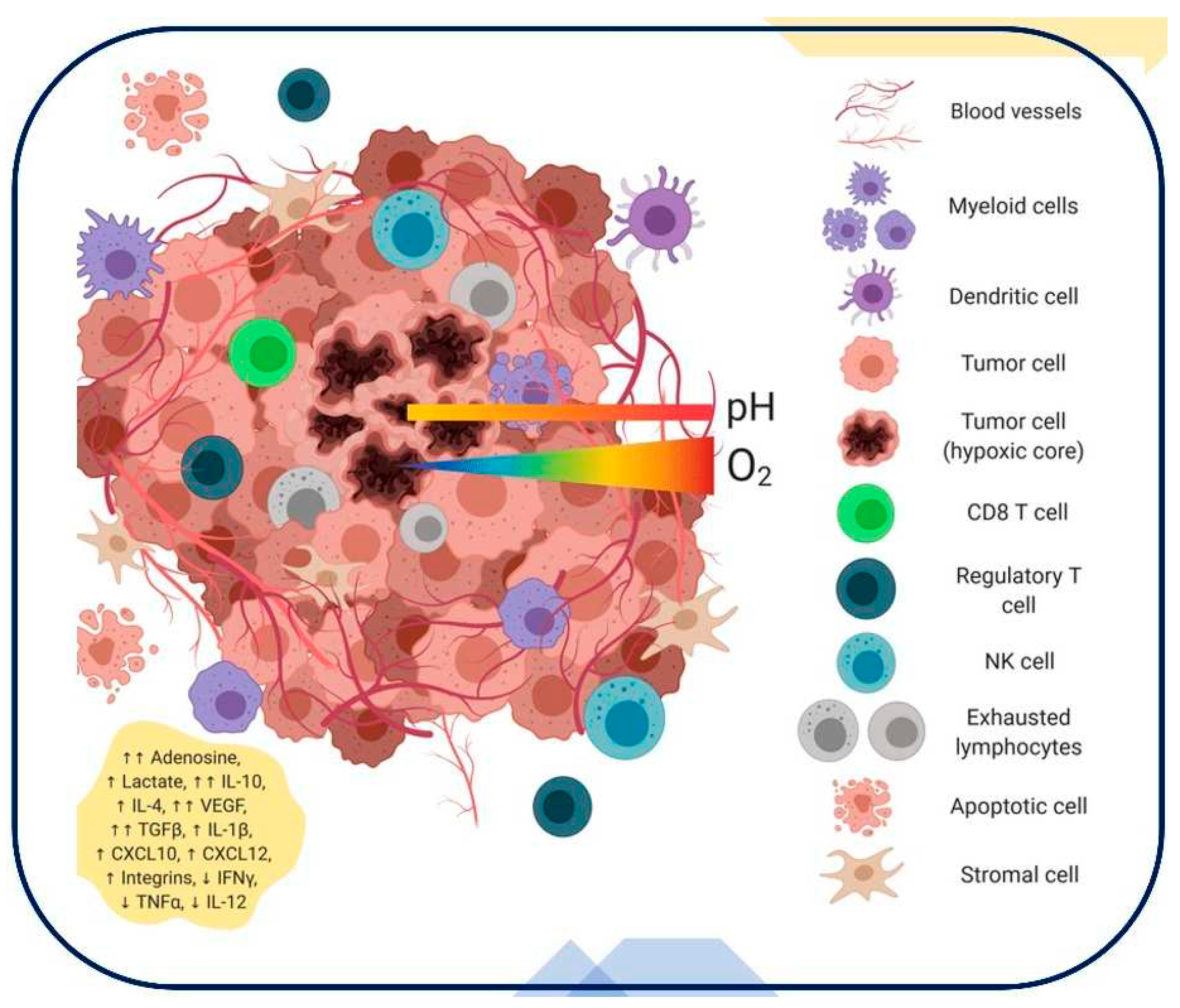
Figure 2. summarizes the main components of the complex and dynamic milieu that makes up the tumor microenvironment within the tumor tissue [62]. The diversity of the tumor microenvironment can be attributed to the varied genetic and phenotypic traits exhibited by tumor cells. Stromal components, such as fibroblasts, extracellular matrix proteins, and other supporting tissues, are also a part of the tumor microenvironment. These components are essential for giving the tumor structural and nutritional support. Since immune cells can play both pro- and anti-tumor functions in the tumor microenvironment, their presence is an important factor. The invasion of immune cells into the tumor tissue is depicted graphically in this figure. The microenvironment surrounding tumors frequently displays aberrant collagen networks and vasculature. The disorder in collagen and blood artery architecture can affect drug delivery effectiveness, metastasis, and tumor growth. Gaining an understanding of these elements and how they interact is essential to appreciating the potential and difficulties that the tumor microenvironment in our experimental setting presents. It offers important new information about the possible interactions between these components and the overall tumor response that may result from our experimental interventions.
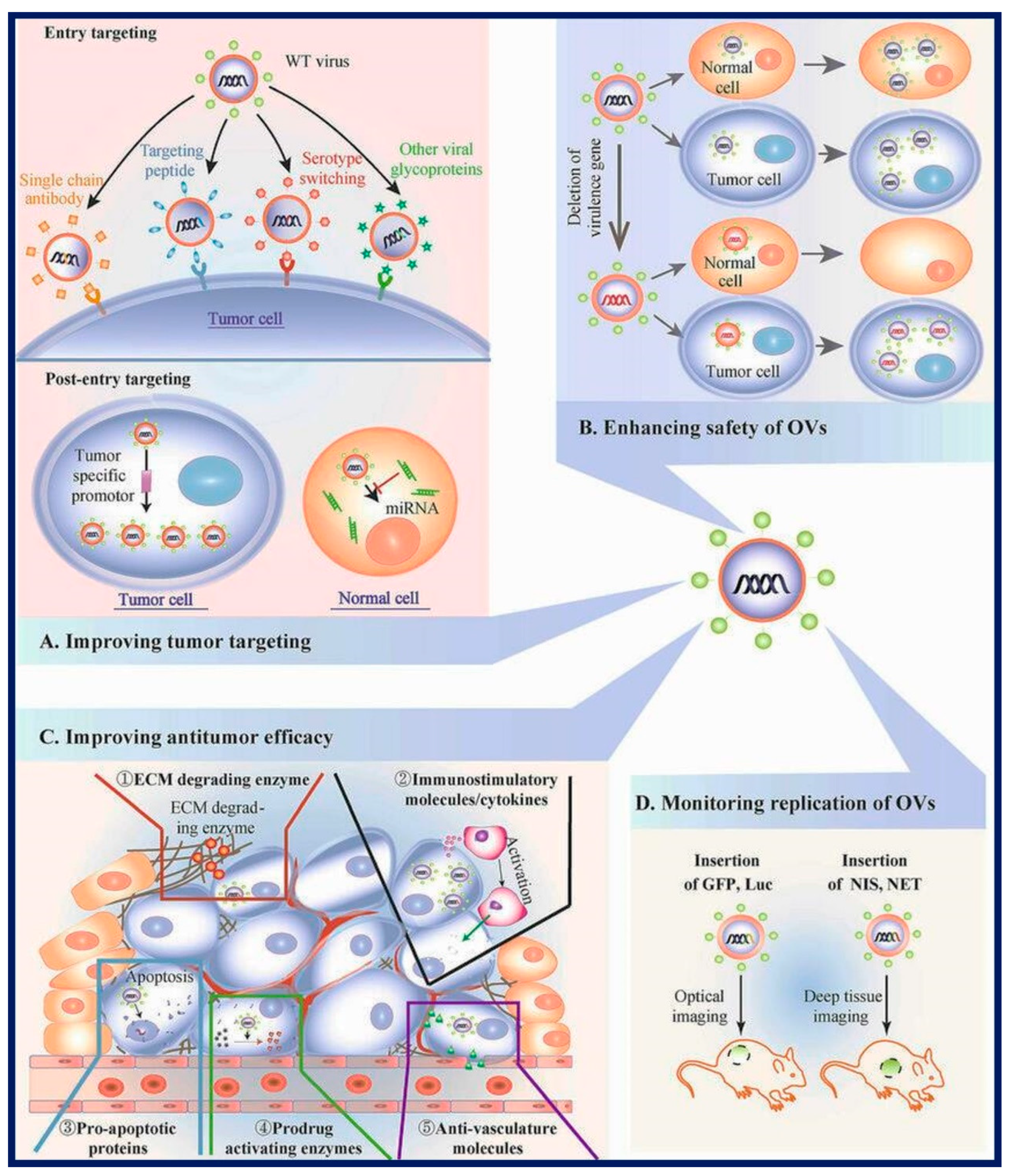
An overview of the genetic tumor microenvironment and its critical role in improving the efficacy of oncolytic viruses (OVs) for cancer therapy is given in
Figure 3 [64]. It clarifies key tactics and elements for maximizing the efficacy of OVs in the treatment of cancer. To increase selectivity for cancer cells, Section (A) discusses methods for better tumor targeting, including serotype switching, tumor-targeted peptides, glycoprotein integration, single-chain antibodies, and tumor-specific promoters. The importance of virulence gene deletion to improve safety in OV treatment is emphasized in Section (B). Strategies to increase antitumor efficacy are covered in Section (C). These strategies include the use of immunostimulatory molecules, suicide genes, ECM-degrading enzymes, and anti-vasculature compounds. The information in Section (D) explains how to use markers like GFP, Rluc, NIS, and NET markers to track the dynamics of OV replication.
2.1. Tumor Redox Micro-Environment
Redox potential marked differences between intracellular and extracellular levels. The synthetic polymers or biopolymers linked or formulated with redox-sensitive bonds which allows to the formation of redox-sensitive materials in the intracellular compartments such as mitochondria, cytosol, and cell nucleus. The glutathione (GSH) level inside tumor microenvironment is range about 0.5×10
-3 M due to elevated concentration of GSH and reductive moieties inside tumor cell. In addition, tumor tissue containing GSH level is four times elevated than the normal tissue. It has also demonstrated that tumor tissues significantly show more reduction conditions (reducing thiol group) and hypoxic than normal cells or healthy cells. Therefore, various redox- responsive micelles nanomaterials are developed with having the ability to enhance trigger the release of their therapeutics agents in respective surface. These are usually located between the hydrophobic and hydrophilic segment with responsive agents such as disulfide bond (-S-S-), thioether bond (-S-), Di selenide bond (-se-ss-) and thiol group (-SH-) also provides the redox responsive site-specific drug release [65–67]. Further developments marked the difference between the GSH level of tumor microenvironment and normal tissues of which this brings promising platform/interest to design prodrugs (polymeric micelles nano particles). Hence disulfide exhibits self-assembled NPs with development of glutathione (GHS) in extra cellular and intra cellular compartment. The oxidized form of thiol group (-SH-) having the ability to generate disulfide linkage formation between peptide molecules in peptide synthesis, this disulfide occurs as side chain or middle of polymeric molecules. It gets cleaved in presence of GHS leads to ensuing drug release property and shows degradation of polymeric micelles [68,69]. The
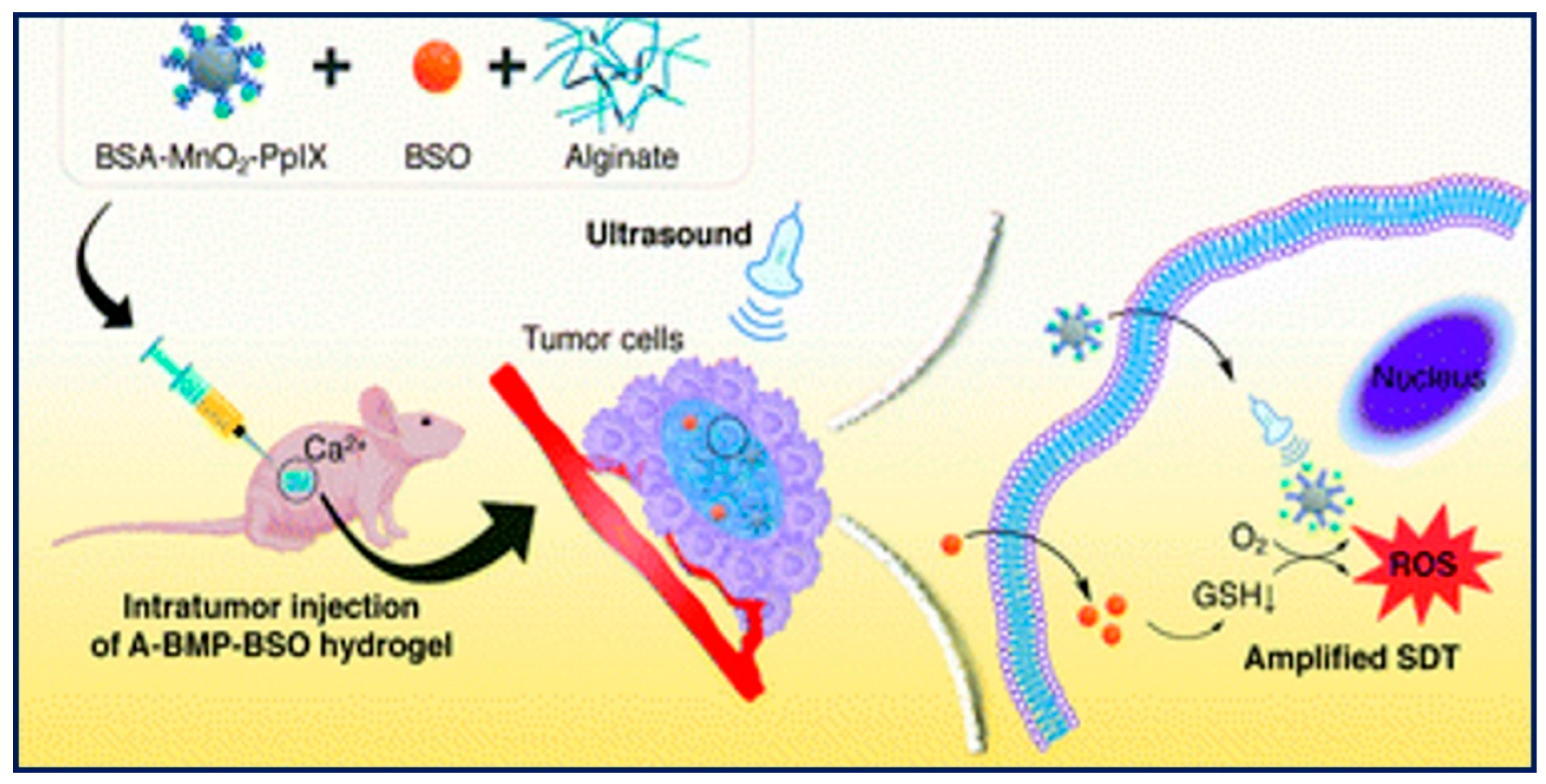
Figure 4 provided a clear schematic depiction of the synthesis and subcutaneous colorectal cancer growth in mouse models. It describes how a Glutathione inhibitor and Composite Hydrogel-mediated Sonodynamic Therapy affect the progression of subcutaneous colorectal cancer in mice models as well as intracellular GSH synthesis.
Sun et al. were reported the redox-responsive micelles for selectively triggered deliver of the drug in tumor microenvironment for the treatment of laryngopharyngeal carcinoma. To enhance the antitumor efficacy of Nanoparticles redox-responsive amphiphilic polymer has been developed by formulating heparosan and deoxycholic acid which fabricated through di- sulphide bond. The polymer micelles get self-assembled with favorable cargoes loading capacity containing doxorubicin (DOX). In addition, self-assembled nanoparticles can be disassembled by reductive cleavage of di-sulphide and triggered drug release capabilities in the intracellular compartment. The heparosan@deoxycholicacid micelles (HSDMs) shows the GSH triggered drug release with nearly 100% release rate. The FaDu Cancerous cell allowed to internalize HSDMs via clathrin-mediated endocytosis. Hence the DOX@HSD of FaDu cancer cells achieve the triggered drug delivery system over normal cells. [70]
Peng Mi et al. have reported that Environment or protection of tumor cell mainly due to reduction and oxidation states of glutathione (GSH) and NADPH/NADP+ which both have different reducing capacities and environment. At molecular level of GHS exhibits high concentrations as compared to NADPH/NADP+ shows subsequent disulfide bonds and excess reactive oxygen species (ROS) reactions leading to reach intracellular high GHS concentration about 10mM, while extracellular environment ranges 2 to 20 μM in the drug delivery system [71]. Recent developments of pH and redox dual-responsive nanohydrogels In vitro has been carried out. The DOX drug release approach up to 95.7% whereas disulfide linkage lead as nanohydrogels redox agent and get degraded using PPT and GSH (reducing agents) it further leads to degradation-trigger drug release. Hence exhibits non-toxicity to HEK 293 cells helping in excellent moves for killing cancerous cell or glioma tumor in cancer therapy [72]
2.2. Tumor pH Micro-Environment
The pH variation provides significant role to internalize the NPs into cells for allowing via vessels for acidified cells. The tumor cells containing pH response signal attributes lesser acidified pH as compared to normal tissues. For effective controlled drug delivery intentionally designed pH responsive polymeric nano materials which changes the cellular level charge and hydrophilicity depending on the pH micro-environment system [73]. Two types of polymers are commonly used cationic and anionic polymer for specific drug delivery. For anionic polymers are more used pH- responsive polymers such as poly (glutamic acid) (PGA), poly (ethyl acrylic acid) (PEAA), poly (methacrylic acid) (PMAA), and poly (acrylic acid) (PAA) [74]. If anion polymeric tumor cells show transition hydrophilic to hydrophobic then pH-decreased which leads to destabilization or deformation of polymers cause swelling or changes in solubility leads drug release and polymer disassembly, hence for cationic changes from hydrophobic to hydrophilic [75]. For example, at lower pH, DOX (anti-cancer drug) loaded with conjugated polymer micelles and cross-linked polymer ion core. The Protonation formulated with Carboxylic groups as nano carrier, at tissue level nano carrier get accelerated and release DOX due to lower electrostatic attractions in both protonated acid and DOX formulation groups [76].
Whereas cationic pH-responsive polymers show positive charges in cellular compartment having the advantage of cellular uptake. It includes poly (β-amino ester), poly (β- amino ester), poly(L-histidine) (PH is) etc. are commonly de-protonated at basic pH level and protonated at acidic pH level. Min et al. Developed MPEG-poly (β-amino ester) polymeric characterized biodegradable and pH-responsive polymers which is useful in cancer treatment, herein hydrophilic PEG with PbAE (biodegradable) results in PEG-PbAE copolymers formed by self-assembly. It provides triggered drug delivery and shows sharp pH dependent biomaterials at tumor level having pH6.4 [77]. Considerable pH variation is repeatedly used for design of suitable stimuli responsive Nanomaterials. To account for abnormally quick metabolism and cells proliferation. Because a great amount of end products developed by tumor tissues and cause cytotoxic effect to neighboring tissue. While acid pH commonly ranges between 5.7– 6.9 [78]. Thus, variety of prodrugs have designed to deliver drug or gene into tumor penetration and get controlled drug release facilities at targeted site in cancer diagnosis [79–81]. In the
Figure 5 outlined the mechanisms of pH activation and micellar self-assembly. The mechanism through which the medication DOX is released by micelles when they self-assemble in response to GSH in the biological system is depicted in
Figure 5A. and schematic representation of the pH activation of nanoparticles in the tumor microenvironment is shown in
Figure 5B. With regards Chang et al. have been developed a polymeric micelle consisting with design copolymer and N- Boc-histidine [82]. The capped N-Boc-histidine enhance the biodegradability as well as biocompatibility of the micelles and Doxorubicin was loaded into micelles (act as anticancer drug). Especially the normal tissues contain pH -7.4, as in cancer microenvironment the acid pH have significantly triggered doxorubicin drug release at pH -6.2. The acid pH polymer nanocarriers released doxorubicin drug at lower circulatory toxicity than free drugs. The anticancer drug should be released in acidic pH microenvironment into tumor cell. Fetch the good intercellular drug release and minimize the extracellular action in tumor diagnosis. In another study, Hu et al. reported reduced cytotoxicity of pH-triggered doxorubicin drug release polymeric micelles due to high internalizations of NPs into tumor [83]. In addition, Yu et al. Develop polymeric micelles contain PbAE, which alter size, surface charge and drug resistant antitumor site [84].
2.3. pH-Redox Tumor Micro-Environment
TME obtained with combined pH and redox dual-stimuli response (1) for tumor extracellular environment can depend on their size, surface properties, and morphology (response to endosomal pH); thus, credit the barriers like tumor accumulation, tumor penetration, blood circulation, and cellular level uptake (2) the intracellular environment response to pH, GSH level, and ROS cleavable moiety in tumor vasculature, induce the rapid drug-release inside tumor cell. However, especially polymeric nano composites have been developed to overcome both extracellular and intracellular barriers to enhance maximum antitumor effect. In this context, (3) internal stimuli-responsive micelle nanomaterials induced by pathophysiological properties between normal and cancer cells, while undergoing dynamical changes with various factors in vivo. So, it shows difficulties to control nanomaterials with respect to precision and speed of response in TME. (4) As for external stimuli the key objectives are supposed to focusing upon how to achieve high-level deeper penetration without harming normal tissues with maximized specificity, efficacy, and selectivity [98–102]. As above discussed, the stimuli response combination, the intracellular environments are characterized by different pH values, meanwhile pH maintained nearly 7.4 in the normal extracellular compartment. Once being surface internalization or enter through endocytosed, the polymeric drug carriers will firstly encounter the early endosome with nearly 6.2 pH, then entering late endosome within about 5.5 pH, thus tumor cell maintains with lower pH environment due to generated ROS (reactive oxygen species) and free radicals (-OH, H2O2, O2) present in TME. while ROS level will be sharply increased 3 times more than normal cells. These high reactive molecules construct the combined dual-sensitive pH-redox polymer prodrug and NPs, which addressing the stability dilemma, reduced size, surface charge reversal to enhance triggered drug release in tumor cells. As one instance, such “titratable” groups like carboxylic acids, amine groups have been introduced into prodrug inducing improved extracellular stability led by prolonged circulation, stealth surface, improved tumor accumulation drug (usually cytotoxic drugs). After entering cancer cell ROS triggers initial drug release and protonation formulated with carboxylic group. In addition, di-sulfide have been employed to ROS which associate the core-crosslink polymeric micelles for preventing the drug leakage while carrying out blood circulation. Fallowed by therapeutic drugs were loaded into NPs materials either covalent binds or non-covalent binds leads to development of polymeric drugs carriers in GSH reduction conditions, thereby enhance the surrounding hydrophilicity and swelling of micellar shell and di-sulfide bond will be cleaved when exposed to abundance of GSH, thereby causing the cellular disassembly of the polymeric micelles structure. Thus, core shell micelles furnished the better DOX drug release in TME by pH-triggered swelling and GHS- triggered disassembly.
Chen et al. reported pH/redox responsive NPs to regulate tumor hypoxia. Here NPs bearing DMMA-modification this usually obtained by tumor acidity (pH) and GSH responsive shell- stacked NPs. The DMMA undergoes surface charge reversal induced the negative charge thick shell and positive charge disulfide-cross-linked core. The NPs entering tumor with negative surface charge and reach prolonged blood circulation under acidic conditions with large size of NPs about ~145 nm. on other side, addressing the stability dilemma, surface charge reversal and size reduction (~40 nm) to enhancing deeper penetration and cellular uptake. Thereby, drug carriers after entering cancer cells induces cytoplasmic GSH rapid DOX release bears de- cross-linking with polypeptide core. The core shell showed DOX release in tumor cell under pH- triggered and GSH-triggered disassembly resulting into antitumor affect [103]. In the
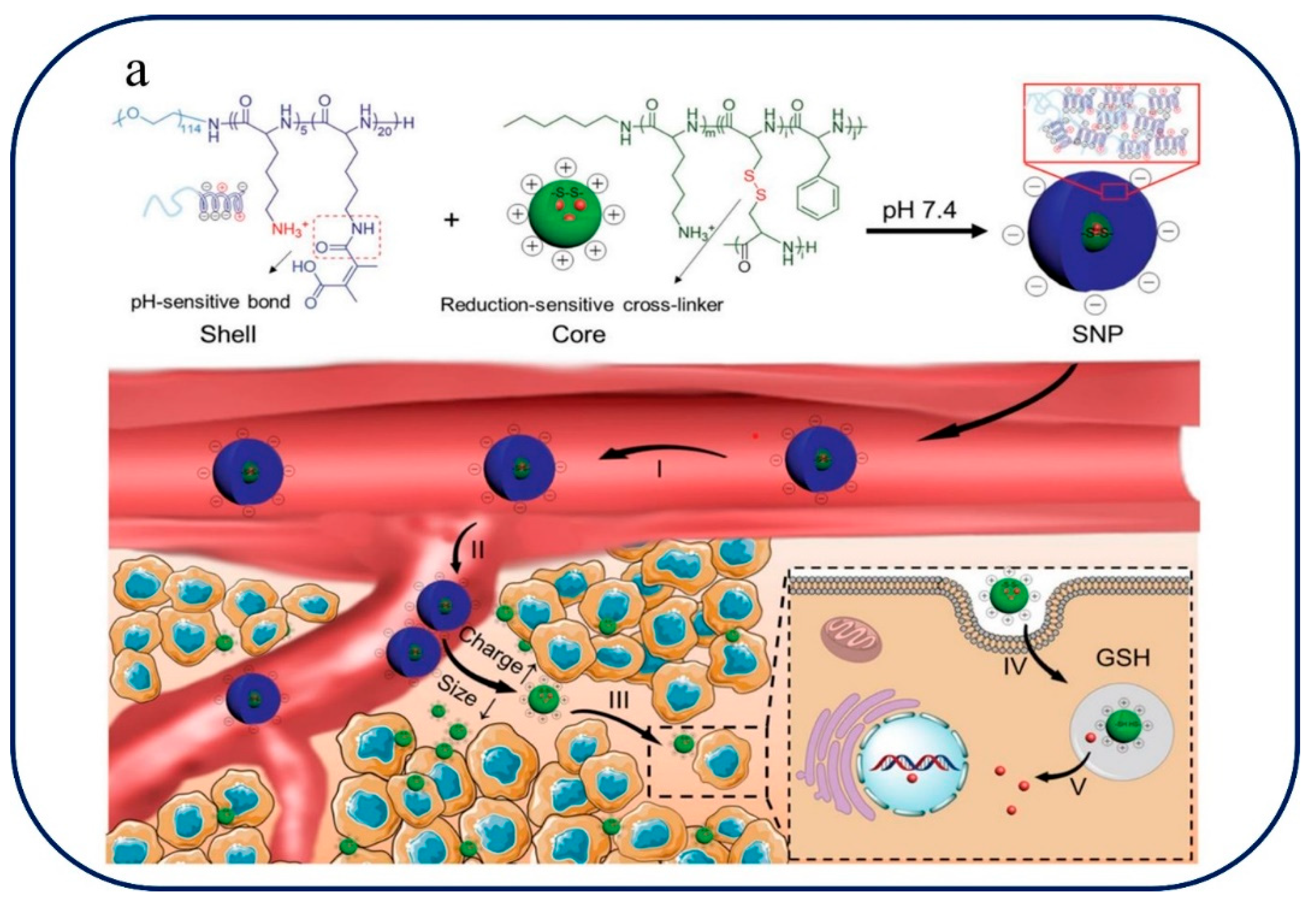
Figure 6 they explain the pH-redox Tumor-Microenvironment-mediated Cascade and Polypeptide Core Self-Assembly for Targeted Drug Delivery. The pH-redox tumor microenvironment-mediated cascade, which is essential for improving drug delivery, is depicted in
Figure 6a. A disulfide-cross-linked polypeptide core and a PEGylated shell are involved in this cascade. PEGylated shells help stabilize nanoparticles and prolong bloodstream circulation, which helps to precisely target tumors. Controlled drug release is made possible by the disulfide-cross-linked polypeptide core, which responds to the redox environment of the tumor.
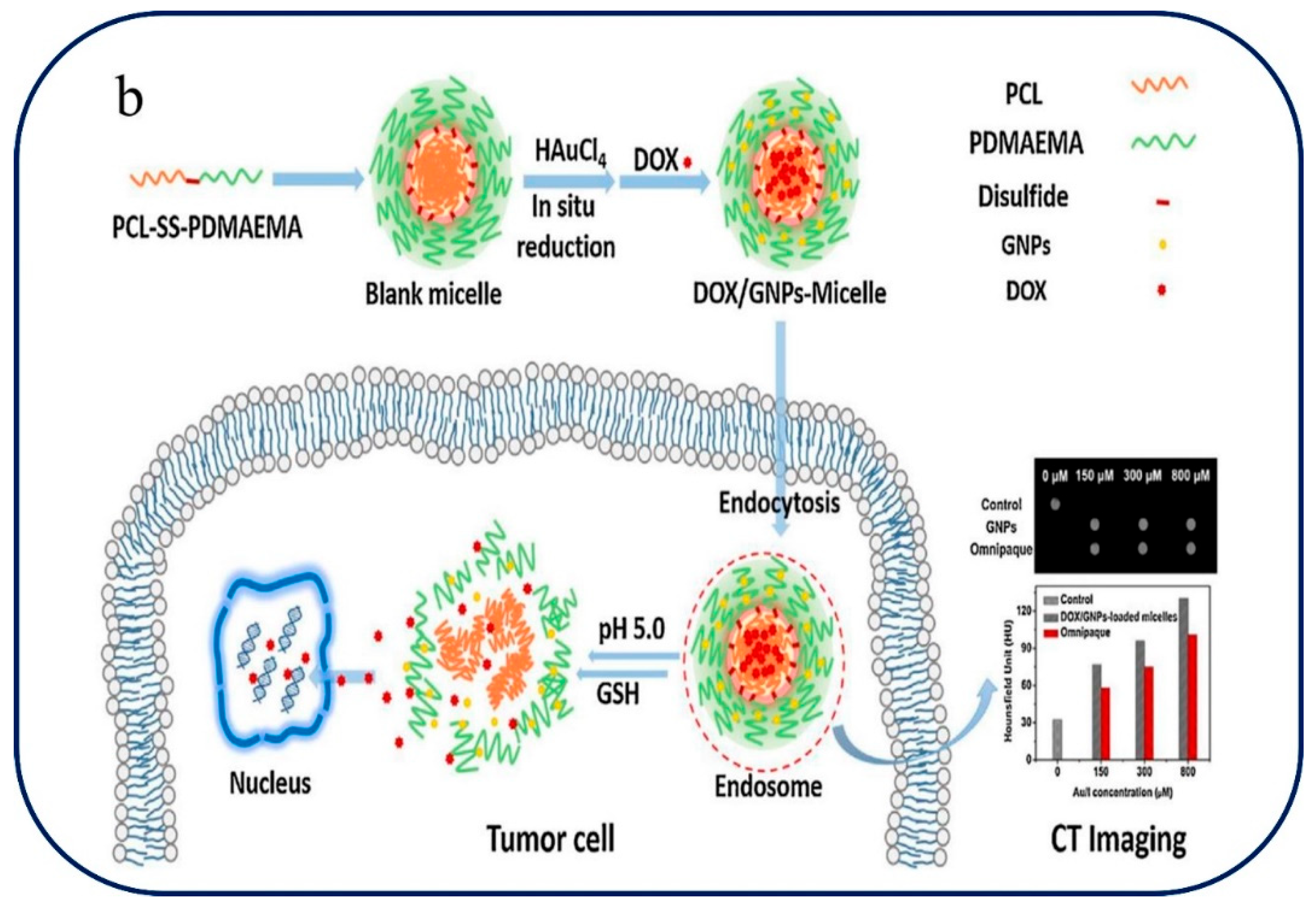
Xiong et al. Have demonstrated the synthesis of pH and redox sensitive micelles for better delivery of DOX and GNPs (gold nano particles). The polymeric micelles consist of amphiphilic copolymer of (PCL-SS-PDMAEMA). The Conjugate PDMAEMA will get protonated and utilizing the acidic conditions, thereby intensifying the hydrophilicity, followed by micellar shell will leads to swelling and di-sulfide bond yield cleavage when exposed to GSH, here cause the disassembly in cellular or sub-cellular compartment. The conjugates NPs loaded with DOX@(PCL-SS-PDMAEMA) @GNPs, thereby core-shell micelles provide better drug deliveries in tumor cell by pH-trigger swelling and GSH-trigger disassembly in the intercellular region in tumor cells [104]. The self-assembly of PCL-SS-PDMAEMA, the polymer at the center of our drug delivery system, is explored in detail in
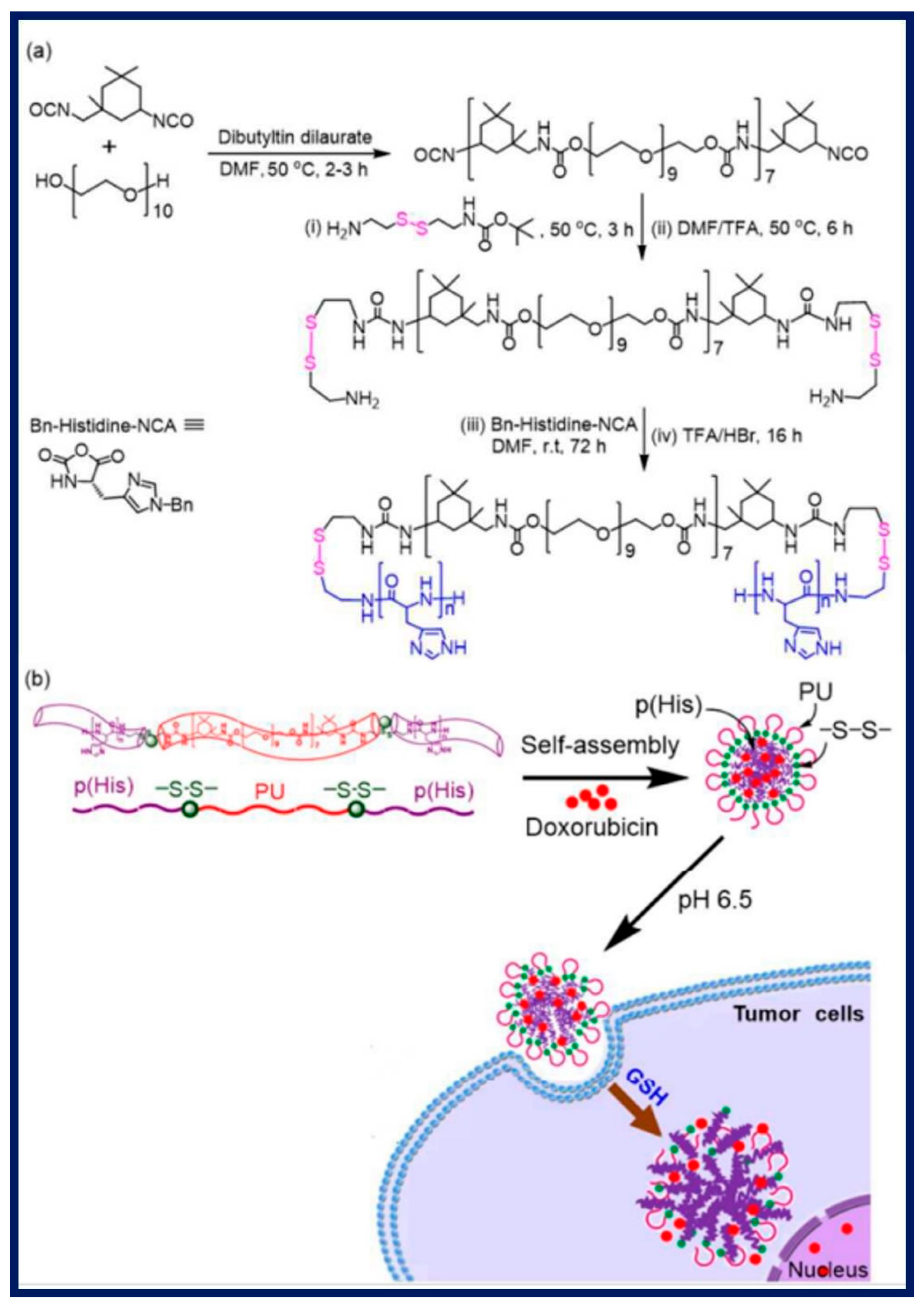
Figure 6b. This core plays a key role in encapsulating therapeutic components that are necessary for cancer treatment and computed tomography (CT) imaging, such as gold nanoparticles and the anticancer medication DOX [104]. Similarly, shi et al. reported four-arm PCL-PEG copolymer provided with PCL as hydrophobic which is conjugate with hydrophilic PEG via di-sulfide bond, this disulfide bond gets degraded in response or when exposed to high level GSH in tumor, resulting rapid DOX release [105]. On drug delivery strategies these conjugated di-sulphide bond reduced upon biological milieu due to unique reversible nature of covalent bond present in the cellular compartment and possess-controlled cleavage drug release provides intracellular-redox potential drug delivery tool.[106]. John et al. reported the synthesis Polymeric micelles like p(His)n–SS–PU–SS–p(His)n triblock induced by carrying out the polymerization, thereby polyurethane nano daisies triggered drug release in tumor cell [107]. A thorough summary of pH and redox-responsive polymeric nanoparticles and their uses in drug administration is provided in
Table 1. The numerous polymeric nanoparticles, their loaded cargo/drugs, the targeted drug release, or delivery methods they use, the intended therapeutic or application, the biological evaluation context, and the relevant references are all compiled in this table.
3. Design Principles and Fabrication Methods
This section delves into the design principles behind pH and redox-responsive polymer-nanocomposites. It explores how these materials respond to change in pH and redox conditions typically found in tumor microenvironments. Various fabrication methods, including nanoprecipitation, emulsion polymerization, and layer-by-layer assembly, are discussed in detail, highlighting their advantages and limitations in creating drug-loaded polymer-nanocomposites with specific responsive properties.
3.1. Design Principles of pH and Redox-Responsive Polymer-Nanocomposites
The design of pH-responsive polymer-nanocomposites is centered on exploiting the acidic nature of the tumor microenvironment. The extracellular pH of solid tumors is generally lower (around 6.5-7.2) than that of healthy tissues (approximately 7.4) [12]. To achieve pH responsiveness, researchers incorporate pH-sensitive moieties into the polymer matrix. Common pH-sensitive groups include weakly acidic functional groups, such as carboxylic acids (-COOH) and sulfonic acids (-SO3H), which undergo ionization in response to pH changes [59,61]. As the pH drops in the tumor microenvironment, these acidic groups become ionized, leading to a charge repulsion effect and subsequent swelling or disintegration of the polymer-nanocomposite, triggering drug release. Redox responsiveness relies on the altered redox potential in cancer cells due to elevated levels of reducing agents, such as glutathione (GSH) [108]. Cancer cells maintain a higher concentration of GSH, which serves as a reducing agent in intracellular redox reactions. Researchers design redox-responsive polymer-nanocomposites by incorporating disulfide (-SS-) linkages or other redox-sensitive motifs. In the reducing environment of cancer cells, the disulfide bonds are cleaved, causing the polymer-nanocomposite to destabilize and release the drug payload [59]. To achieve dual responsiveness, both pH and redox-sensitive elements are combined in the polymer-nanocomposite. This design enables fine-tuning of drug release based on the combined effect of pH and redox conditions in the tumor microenvironment. By strategically incorporating pH-sensitive and redox-sensitive functionalities, the polymer-nanocomposite can respond to two different stimuli, enhancing the precision and control of drug release.
3.2. Fabrication Methods
Nanoprecipitation is a commonly employed technique for fabricating polymer-nanocomposites with high drug loading capacity. Using this technique, a drug and polymer solution are made independently, with the polymer including groups that are sensitive to pH and/or redox. Following the combination of these solutions usually with strong shear or sonication the organic solvent rapidly diffuses into the aqueous phase, causing the spontaneous production of nanoparticles. Numerous medication delivery applications can benefit from the exact control over particle size and drug loading that the nanoprecipitation technique offers. This method's simplicity and scalability allow for the precise control over the release profile and the encapsulation of both hydrophilic and hydrophobic medicines. There are a few restrictions to consider, though. Possible medication instability brought on by contact with organic solvents during manufacture. Restricted ability to regulate the nanoparticles' surface properties and difficulties in attaining consistent drug dispersion [109].
Another flexible technique for creating pH and redox-responsive polymer nanocomposites is emulsion polymerization. This procedure creates a stable emulsion by dispersing monomers, a medication, and an emulsifying agent in an aqueous phase. After that, the emulsion is polymerized using appropriate polymerization techniques or by introducing free radicals. As a result, the polymer-nanocomposites that are produced are usually composed of core-shell structures, where the drug payload is contained within the core. The ability to encapsulate a broad variety of medications and the method's good control over drug loading, surface properties, and particle size are among its benefits. The use of emulsifying chemicals that may affect biocompatibility, the requirement for strict purification procedures, and the possibility of drug degradation during polymerization are limitations. Layer-by-layer assembly is a versatile technique that involves the sequential deposition of alternating layers of oppositely charged polymers or polyelectrolytes onto a substrate. By incorporating pH and redox-sensitive polymers in the multilayer assembly, researchers can design polymer-nanocomposites with tunable responsiveness. Drug molecules can be loaded within the interlayer spaces or encapsulated within one of the layers. Advantages like its precise control over film thickness and drug loading, ability to tailor responsiveness through layer selection, and compatibility with a variety of drugs [29].
Limitations are time-consuming process, may result in relatively thick films, and potential challenges in maintaining film stability in vivo. This design principles and fabrication methods of pH and redox-responsive polymer-nanocomposites play a pivotal role in their application as site-specific drug delivery systems in cancer therapy [110]. By ingeniously engineering these biomaterials to respond to the unique microenvironment of tumors, researchers can enhance therapeutic efficacy while minimizing off-target effects. Nanoprecipitation, emulsion polymerization, and layer-by-layer assembly are promising techniques to create drug-loaded polymer-nanocomposites with specific responsive properties, and ongoing research in this field is expected to lead to the development of more sophisticated and effective dual-stimuli responsive biomaterials for personalized cancer treatment [29].
4. Biocompatibility and Safety
Biocompatibility and safety are critical aspects in the development and clinical translation of biomaterials, especially for applications in drug delivery systems. In this context, pH and redox-responsive polymer-nanocomposites have gained significant interest due to their potential to respond to specific physiological conditions, making them suitable candidates for targeted drug delivery. However, before these materials can be safely used in clinical settings, thorough examination and evaluation of their biocompatibility and potential long-term effects on healthy tissues are essential.
The Biocompatibility refers to the ability of a material to interact favorably with biological systems without causing adverse reactions or toxicity. In the case of pH and redox-responsive polymer-nanocomposites, their interaction with the surrounding biological environment is of utmost importance. pH is a crucial parameter that varies in different tissues and cellular compartments. Target sites can be precisely controlled for drug release thanks to the conformational changes that pH-responsive polymers in nanocomposites can undergo in response to local pH levels. In vitro tests employing cell cultures are carried out to evaluate biocompatibility by monitoring cell survival, growth, and any possible cytotoxicity brought on by these materials [87]. In vivo studies are also performed using animal models to evaluate the tissue response and systemic effects after administration of these polymer-nano-composites Redox-responsive biomaterials can respond to changes in the cellular redox state, which is often altered in disease states [93]. These materials can be designed to release drugs in response to specific intracellular redox conditions. Biocompatibility evaluations for redox-responsive polymer-nanocomposites involve similar in vitro and in vivo studies as those for pH-responsiveness [85–93]. The long-term effects of biomaterials on healthy tissues are crucial to assess to ensure patient safety during extended drug delivery applications. These evaluations require long-term in vivo studies using animal models. To assess the biocompatibility of these materials over time, researchers examine tissue responses, possible inflammatory reactions, and systemic impacts over prolonged periods of time. An important factor in determining the safety of pH and redox-responsive polymer-nanocomposites is preclinical toxicology research. To find possible harmful effects, establish safe dosage ranges for therapeutic usage, and ascertain dose-response correlations, these investigations entail extensive testing in animals. Adhering to regulatory criteria for the conduct of these studies is essential to guarantee a thorough evaluation of the safety of the materials prior to human trials. Creating intelligent biodegradable materials is a crucial factor in ensuring safe medication administration. The ability of a substance to decompose into non-toxic metabolites and finally leave the body is known as biodegradability. This feature is especially crucial for long-term uses because it keeps materials from building up in tissues. To verify the safety profile of these materials, biocompatibility assessments should consider the products of their breakdown. A comprehensive assessment of the biocompatibility and safety of pH and redox-responsive polymer-nanocomposites is necessary for their effective clinical translation as drug delivery systems. By means of preclinical toxicity investigations and thorough assessments of their interactions with healthy tissues, scientists can guarantee the creation of secure biomaterials for precise and regulated medication administration across a range of medical uses.
5. Future Perspectives and Challenges
This section provides an overview of the potential applications of pH and redox-triggered polymer-nanocomposites in cancer therapy as they move closer to clinical trials. It talks about how novel approaches are required to raise drug loading capability, response specificity, and in vivo stability. Additionally, possible synergies with other systems that respond to stimuli and combination therapy are investigated. The ability of pH and redox-responsive polymer-nanocomposites to load drugs is one of their main problems. To maximize the amount of therapeutic chemicals that may be loaded onto these biomaterials without sacrificing their stability and responsiveness, researchers should concentrate on creating novel techniques. Enhanced drug loading efficiencies can be attained by investigating new methods of drug encapsulation and surface modifications. Even if site-specific medication release is possible with pH and redox-responsive polymer-nanocomposites, obtaining even greater response specificity is essential. Future research should aim to design biomaterials that respond only to the precise tumor microenvironment and avoid premature drug release in non-targeted tissues. This could involve incorporating additional stimuli-responsive elements or utilizing advanced targeting ligands. The stability and longevity of polymer-nanocomposites in the complex in vivo environment are vital for their successful clinical translation. Researchers need to address challenges related to biodegradation, immune responses, and potential clearance from the body. Developing stable, long-lasting polymer-nanocomposites will ensure sustained drug release and optimize therapeutic outcomes. The future of cancer therapy lies in personalized medicine. pH and redox-responsive polymer-nanocomposites can be further customized based on individual patient characteristics and specific tumor types. Tailoring drug payloads, stimuli responsiveness, and targeting ligands to match each patient's unique profile could significantly enhance treatment efficacy and minimize side effects.
Despite promising preclinical results, translating pH and redox-triggered polymer-nanocomposites from the laboratory to clinical practice presents challenges. Rigorous testing in large animal models and addressing potential toxicity concerns are essential steps in the journey towards human trials. Cancer cells exhibit substantial heterogeneity, even within the same tumor. Adapting pH and redox-responsive biomaterials to accommodate this diversity poses a significant challenge. Strategies to tackle intra-tumoral variability and ensure efficient drug delivery to all tumor regions need to be explored. The approval process for novel biomaterials in clinical settings involves stringent regulatory guidelines. Demonstrating the safety, efficacy, and long-term effects of pH and redox-responsive polymer-nanocomposites will be critical for gaining regulatory approval and market acceptance. While combining different stimuli-responsive systems or therapies holds promise for enhanced treatment outcomes, it introduces complexities in terms of drug interactions, dosage optimization, and potential adverse effects. Developing effective combination strategies that maximize synergies while minimizing drawbacks is a considerable challenge. For widespread clinical adoption, pH, and redox-responsive polymer-nanocomposites must be scalable and cost-effective. Identifying suitable, readily available raw materials and streamlining the manufacturing processes will be essential to reduce production costs and ensure affordability for patients.
6. Conclusions
In conclusion, pH, and redox-triggered polymer-nanocomposites hold significant promise as future generation dual-stimuli responsive biomaterials for site-specific drug release in cancer therapy. Their ability to precisely target tumor cells while sparing healthy tissues has the potential to revolutionize cancer treatment, offering improved therapeutic outcomes and enhanced patient quality of life. Nevertheless, further research and clinical investigations are essential to overcome the existing challenges and unlock the full potential of these innovative biomaterials in cancer therapeutics.
The development of pH and redox-responsive polymer-nanocomposites as dual-stimuli responsive biomaterials has shown great promise in revolutionizing cancer therapy. However, to fully harness their potential, researchers must address the challenges of drug loading capacity, response specificity, in vivo stability, and regulatory approval. Collaborative efforts between researchers, clinicians, and industry stakeholders are crucial to overcome these obstacles and pave the way for the successful clinical translation of these innovative biomaterials. As advancements continue, pH and redox-responsive polymer-nanocomposites hold immense potential to improve cancer treatment and ultimately contribute to better patient outcomes.
Author Contributions
Shivalingayya, Shivanand P and CPKP contributed to write and revise this manuscript. Both CPKP and JH equally supervised this work. All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Acknowledgments
This research was supported by Basic Science Research Capacity Enhancement Project through Korea Basic Science Institute (National research Facilities and Equipment Center) grant funded by the Ministry of Education. (2019R1A6C1010016) and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1F1A1050130).
References
- Marta Puyol, Joan Seoane, Esther Aguilar, Lisa B. Vozza, Isabel Orbe, Katherine H.Crawford, Ana Fernandez, Freddie Bray, Sonali E. Johnson, and Satish Gopa, WORLD CANCER RESEARCH DAY: A Call to Action for a Coordinated International Research Effort to Prevent, Diagnose, and Treat Cancer, Clin Cancer Res, 27 (2021) 963–966. [CrossRef]
- Smith L, Stiller CA, Aitken JF, Hjalgrim LL, Johannesen T, Lahteenmaki P, McCabe MG, Phillips R, Pritchard-Jones K, Steliarova-Foucher E, Winther JF, Woods RR, Glaser AW, Feltbower RG. International variation in childhood cancer mortality rates from 2001 to 2015: Comparison of trends in the International Cancer Benchmarking Partnership countries. Int J Cancer. 150 (2021) 28-37. [CrossRef]
- Sepideh Parvanian, Seyed Mojtaba Mostafavi, Meysam Aghashiri, Multifunctional nanoparticle developments in cancer diagnosis and treatment, Sensing and Bio-Sensing Research 13 (2017) 81–87. [CrossRef]
- Karim ReFaey,Shashwat Tripathi, BSA; Sanjeet S. Grewal, Adip G. Bhargav, David J. Quinones; Kaisorn L. Chaichana, Samuel O. Antwi, Leslie T. Cooper, Fredric B. Meyer, Roxana. S. Dronca, Robert B. Diasio, Cancer Mortality Rates Increasing vs Cardiovascular Disease Mortality Decreasing in the World: Future Implications, Mayo Clin Proc Inn Qual Out, 5 (2021) 645-653. [CrossRef]
- Thashini Moodley and Moganavelli Singh, Current Stimuli-Responsive Mesoporous Silica Nanoparticles for Cancer, Pharmaceutics 13 (2021) 71. [CrossRef]
- M. Zubair, S. Wang and N. Ali, Advanced Approaches to Breast Cancer Classification and Diagnosis, Front. Pharmacol, 11 (2021). [CrossRef]
- Wen-Qian Li, Han-Fei Guo, Ling-Yu Li, Yong-Fei Zhang, Jiu-Wei Cui, Cancer Medicine. 10 (2021) 4677–4696.
- Milad Ashrafizadeh, Sepideh Mirzaei, Mohammad Hossein Gholami, Farid Hashemi, Amirhossein Zabolian, Mehdi Raei, Kiavash Hushmandi, Ali Zarrabi, Nicolas H. Voelckeri, Amir Reza Arefl, Michael R. Hamblinn, Rajender S. Varma, Saeed Samarghandian, I.J. Arostegi, M. Alzola, Alan Prem Kumar, Vijay Kumar Thakur, Noushin Nabavi, Pooyan Makvandi, Franklin R. Tayy, Gorka Orive, Carbohydrate Polymers, 272 (2021).
- Hashem O. Alsaab, Aljawharah Alqathama, Alanoud S. Al-Hibs, Rami Alzhrani, Khawlah K. Alrabighi, Akram Alwithenani, Atiah H. Almalki and Yusuf S. Althobaiti, Nanomaterials for Antiangiogenic Therapies for Cancer: A Promising Tool for Personalized Medicine, Int. J. Mol.Sci. 22 (2021). [CrossRef]
- Balak Das Kurmi, Preeti Patel, Rishi Paliwal, Shivani Rai Paliwal, Molecular approaches for targeted drug delivery towards cancer: A concise review with respect to nanotechnology, Journal of Drug Delivery Science and Technology 57 (2020). [CrossRef]
- Mahwash Mukhtar, Muhammad Bilal, Abbas Rahdar, Mahmood Barani, Rabia Arshad, Tapan Behl, Ciprian Brisc, Florin Banica and Simona Bungau. Nanomaterials for Diagnosis and Treatment of Brain Cancer: Recent Updates, Chemosensors, 8 (2020). [CrossRef]
- Raghu Solanki, Hadis Rostamabadi, Sunita Patel, Seid Mahdi Jafari, Anticancer nano- delivery systems based on bovine serum albumin nanoparticles: A critical review, International Journal of Biological Macromolecules, 193 (2021) 528–540. [CrossRef]
- Wen-Ying Huang, Chih-Ho Lai, Shin-Lei Peng, Che-Yu Hsu, Po-Hung Hsu, Pei-Yi Chu, Chun-Lung Feng and Yu-Hsin Lin, Targeting Tumor Cells with Nanoparticles for Enhanced Co- Drug Delivery in Cancer Treatment, Pharmaceutics, 13 (2021). [CrossRef]
- Di Chang, Yuanyuan Ma, Xiaoxuan Xu, Jinbing Xie and Shenghong Ju, Stimuli- Responsive Polymeric Nanoplatforms for Cancer Therapy, Stimuli-Responsive Polymeric Nanoplatforms, 9 (2021). [CrossRef]
- Sabya Sachi Das, Priyanshu Bharadwaj, Muhammad Bilal, Mahmood Barani, Abbas Rahdar, Pablo Taboada, Simona Bungau and George Z. Kyzas, Polymers, 12 (2020). [CrossRef]
- Thennakoon M. Sampath Udeni Gunathilake, Yern Chee Ching, Cheng Hock Chuah, Noorsaadah Abd Rahman, Nai-Shang Liou, Recent advances in celluloses and their hybrids for stimuli-responsive drug delivery, International Journal of Biological Macromolecules 158 (2020) 670–688. [CrossRef]
- Dong Liu, Fang Yang, Fei Xiong, and Ning Gu, The Smart Drug Delivery System and Its Clinical Potential, Theranostics, 6 (2016) 1306-1323. [CrossRef]
- Joydeb Majumder & Tamara Minko, Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery, Expert Opinion on Drug Delivery, (2020). [CrossRef]
- Qianqian Sun, Zhao Wang, Bin Liu, Fei He, Shili Gai, Piaoping Yang, Dan Yang, Chunxia Li. Jun Lin, Recent advances on endogenous/exogenous stimuli-triggered nanoplatforms for enhanced chemodynamic therapy, coordination chemistry reviews, 451 (2021). [CrossRef]
- Ghada G. Abdo & Moustafa M. Zaghol & Ashraf Khalil, Recent advances in stimuli- responsive drug release and targeting concepts using mesoporous silica nanoparticles, Emergent Materials, 3 (2020) 407–425. [CrossRef]
- Vanessa T. Chivere, Pierre P. D. Kondiah, Yahya E. Choonara and Viness Pillay, Nanotechnology-Based Biopolymeric Oral Delivery Platforms for Advanced Cancer Treatment, Cancers, 12 (2020). [CrossRef]
- Jayanta Kumar Patra, Gitishree Das, Leonardo Fernandes Fraceto, Estefania Vangelie Ramos Campos, Maria del Pilar Rodriguez-Torres, Laura Susana Acosta-Torres, Luis Armando Diaz-Torres, Renato Grillo, Mallappa Kumara Swamy, Shivesh Sharma, Solomon Habtemariam and Han-Seung Shin, Nano based drug delivery systems: recent developments and future prospects, Patra et al. J Nanobiotechnol, 16 (2018). [CrossRef]
- Qing Zhou, Li Zhang, TieHong Yang, Hong wu, Stimuli-responsive polymeric micelles for drug delivery and cancer therapy, International Journal of Nanomedicine, 13 (2018). [CrossRef]
- S. Moein Moghimi, A. Christy Hunter and J. Clifford Murray, Nanomedicine: current status and future prospects, The FASEB Journal, 19 (2005) 311-330. [CrossRef]
- Magdalena Aflori, Smart Nanomaterials for Biomedical Applications—A Review, Nanomaterials, 11 (2021). [CrossRef]
- Peiyu Zhang, Zhiliang Gao, Jiwei Cui, and Jingcheng Hao, Dual-Stimuli-Responsive Polypeptide Nanoparticles for Photothermal and Photodynamic Therapy, ACS Appl. Bio Mater. (2019). [CrossRef]
- Shareni Jeyamogan, Naveed Ahmed Khan and Ruqaiyyah Siddiqui, Application and Importance of Theranostics in the Diagnosis and Treatment of Cancer, Archives of Medical Research, 52 (2021) 131-142. [CrossRef]
- Imtinungla, Arpita Baruah, Deepti Lourembam, Sunandan Baruah, Nanotechnology in Cancer Detection and Treatment, ADBU-Journal of Engineering Technology, 6 (2017).
- Amit Singh, Meghna Talekar, Thanh-Huyen Tran, Abishek Samanta, Ravi Sundaram, and Mansoor Amiji, Combinatorial Approach in the Design of Multifunctional Polymeric Nano- Delivery Systems for Cancer Therapy, Journal of Materials Chemistry B, 2 (2014). [CrossRef]
- Peiyu Zhang, Zhiliang Gao, Jiwei Cui, and Jingcheng Hao, Dual-Stimuli-Responsive Polypeptide Nanoparticles for Photothermal and Photodynamic Therapy, ACS Appl. Bio Mater. (2019). [CrossRef]
- Aleksandra Zieli ´nska , Filipa Carreiró , Ana M. Oliveira , Andreia Neves , Bárbara Pires , D. Nagasamy Venkatesh , Alessandra Durazzo , Massimo Lucarini , Piotr Eder , Amélia M. Silva , Antonello Santini, and Eliana B. Souto, Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology, Molecules 2020, 25, 3731.
- Hector Katifelis, Maria Gazouli, Cancer-Targeted Nanotheranostics: Recent Advances and Future Perspectives, Cancer Nanotheranostics,(2021) 97-115.
- Cong Luo, Jin Sun, Bingjun Sun, and Zhonggui He, Prodrug-based nanoparticulate drug delivery strategies for cancer therapy, Trends in Pharmacological Sciences, 35 (2014) 556-566. [CrossRef]
- Zhaohui Tanga, Chaoliang Hea, Huayu Tiana, Jianxun Dinga, Benjamin S. Hsiaob, Benjamin Chub and Xuesi Chena, Polymeric Nanostructured Materials for Biomedical Applications, Progress in Polymer Science, 2016, 60, 86-128. [CrossRef]
- Zachary L. Tyrrell, Youqing Shen, Maciej Radosz, Fabrication of micellar nanoparticles for drug delivery through the self-assembly of block copolymers, Progress in Polymer Science 35 (2010) 1128–1143. [CrossRef]
- Stephanie D. Steichen, Mary Caldorera-Moore, Nicholas A. Peppas, A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics, European Journal of Pharmaceutical Sciences, 48 (2013) 416–427. [CrossRef]
- Jin-Wook Choi, Jongil An, Seung-RakSon, Soyern Kim, Jisung Park, Chan Beom Park, Jun Hyup Lee, comb-shaped copolymers for tunable molecular orientation, Reactive functional polymers, 168 (2021). [CrossRef]
- Younsoo Bae, Kazunori Kataoka, Intelligent polymeric micelles from functional poly(ethylene glycol)-poly(amino acid) block copolymers, Advanced Drug Delivery Reviews 61 (2009) 768–784. [CrossRef]
- Enrique Lallana & Ana Sousa-Herves & Francisco Fernandez-Trillo & Ricardo Riguera & Eduardo Fernandez-Megia, Click Chemistry for Drug Delivery Nanosystems, Pharm Res, 29 (2012) 1–34.
- Bing Deng, Ping Ma, and Yan Xie, Reduction-Sensitive Polymeric Nanocarriers in Cancer Therapy: A Comprehensive Review, Nanoscale, (2015). [CrossRef]
- Tushar Date, Vaishnavi Nimbalkar, Jyostna Kamat, Anupama Mittal, Ram I. Mahato, Deepak Chitkara, Lipid-polymer hybrid nanocarriers for delivering cancer therapeutics, Journal of Controlled Release, (2017). [CrossRef]
- Wentao Xia, Zixuan Tao, Bin Zhu, Wenxiang Zhang, Chang Liu, Siyu Chen and Mingming Song, Targeted Delivery of Drugs and Genes Using Polymer Nanocarriers for Cancer Therapy, Int. J. Mol. Sci. 22 (2021). [CrossRef]
- Mohamed F. Attia, Nicolas Anton, Justine Wallyn, Ziad Omran and Thierry F. Vandamme, An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites, Journal of Pharmacy and Pharmacology, 71 (2019) 1185–1198. [CrossRef]
- Shixian Lv, Meilyn Sylvestre, Alexander N. Prossnitz, Lucy F. Yang, and Suzie H. Pun, Design of Polymeric Carriers for Intracellular Peptide Delivery in Oncology Applications, Chemical Reviews, (2020). [CrossRef]
- Huaping Tan and Kacey G. Marra, Injectable, Biodegradable Hydrogels for Tissue Engineering Applications, Materials, 3 (2010) 1746-1767. [CrossRef]
- Di Chang, Yuanyuan Ma, Xiaoxuan Xu, Jinbing Xie and Shenghong Ju, Stimuli- Responsive Polymeric Nanoplatforms for Cancer Therapy, Front. Bioeng. Biotechnol., 9 (2021). [CrossRef]
- Hong Yu Yang, Yi Li, and Doo Sung Lee, Multifunctional and Stimuli-Responsive Magnetic Nanoparticle-Based Delivery Systems for Biomedical Applications, Adv. Therap. 1 (2018). [CrossRef]
- Peng Mi, Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics, Theranostics, 10 (2020) 4557-4588. [CrossRef]
- Sabya Sachi Das, Priyanshu Bharadwaj, Muhammad Bilal, Mahmood Barani, Abbas Rahdar, Pablo Taboada, Simona Bungau and George Z. Kyzas, Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis, Polymers, 12 (2020). [CrossRef]
- Fenghua Meng, Ru Cheng, Chao Deng, and Zhiyuan Zhong, Intracellular drug release nanosystems, materials today, 15 (2012) 436-442. [CrossRef]
- Jinzhi Du, Lucas A. Lane, Shuming Nie, Stimuli-Responsive Nanoparticles for Targeting the Tumor Microenvironment, Journal of Controlled Release, (2015). [CrossRef]
- Lilian E van Vlerken & Mansoor M Amiji, Multi-functional polymeric nanoparticles for tumour-targeted drug delivery, Expert Opin. Drug Deliv. 3 (2006) 205-216. [CrossRef]
- Peter Vaupel, Tumor Microenvironmental Physiology and Its Implications for Radiation Oncology, Seminars in Radiation Oncology, 14 (2004) 198-206. [CrossRef]
- Niko Kimura, Masatoshi Maeki, Yusuke Sato, Yusuke Note, Akihiko Ishida, Hirofumi Tani, Hideyoshi Harashima, and Manabu Tokeshi, Development of the iLiNP Device: Fine Tuning the Lipid Nanoparticle Size within 10 nm for Drug Delivery, ACS Omega, 3 (2018) 5044−5051. [CrossRef]
- Barbara Haley, Eugene Frenkel, Nanoparticles for drug delivery in cancer treatment, Urologic Oncology: Seminars and Original Investigations 26 (2008) 57–64.
- Rajendra Awasthi, Ariane Roseblade, Philip Michael Hansbro, Michael John Rathbone, Kamal Dua, and Mary Bebawy, Nanoparticles in Cancer Treatment: Opportunities and Obstacles, Current Drug Targets, 19 (2018) 1696-1709. [CrossRef]
- Christopher, J. Sunderland, Matthias Steiert, James E. Talmadge, Austin M. Derfus, and Stephen E. Barry, Targeted Nanoparticles for Detecting and Treating Cancer, DRUG DEVELOPMENT RESEARCH, 67 (2006) 70–93. [CrossRef]
- Bing-Huei Chen & Baskaran Stephen Inbaraj, Various physicochemical and surface properties controlling the bioactivity of cerium oxide nanoparticles, Critical Reviews in Biotechnology, 38 (2018) 1003-1024. [CrossRef]
- Marcio J. Tiera, Qin Shi, Françoise M. Winnik and Julio C. Fernandes, Polycation-Based Gene Therapy: Current Knowledge and New Perspectives, Current Gene Therapy, 11 (2011) 288-306. [CrossRef]
- Sara Salatin, Solmaz Maleki dizaj, Ahmad Yari Khosroushahi, Effect of the surface modification, size, and shape on cellular uptake of nanoparticles, Cell Biology International, (2015). [CrossRef]
- Ali Aghebati-Maleki, Sanam Dolati, Majid Ahmadi, Amir Baghbanzhadeh, Milad Asadi, Ali Fotouhi, Mehdi Yousefi, Leili Aghebati-Maleki, Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers, J Cell Physiol. (2019) 1- 11. [CrossRef]
- Fernández, Julián & Luddy, Kimberly & Harmon, Cathal & O'Farrelly, Cliona. (2019). Hepatic Tumor Microenvironments and Effects on NK Cell Phenotype and Function. International Journal of Molecular Sciences. 20. 4131. [CrossRef]
- Frank X. Gu, Rohit Karnik, Andrew Z. Wang, Frank Alexis, Etgar Levy-Nissenbaum, Seungpyo Hong, Robert S. Langer and Omid C. Farokhzad, Targeted nanoparticles for cancer therapy, nanotoday, 2 (2007) 14-21. [CrossRef]
- Qiaoshuai, Lan & Xia, Shuai & Wang, Qian & Huang, Haiyan & Jiang, Shibo & Lu, Lu. (2020). Development of oncolytic virotherapy: from genetic modification to combination therapy. Frontiers of Medicine. 14. [CrossRef]
- Shirin Mollazadeh, Marcin Mackiewicz, Mostafa Yazdimamaghani, Recent advances in the redox-responsive drug delivery nanoplatforms: A chemical structure and physical property perspective, Materials Science & Engineering C 118 (2021). [CrossRef]
- Xiaoshuang Guo, Yuan Cheng, Xiaotian Zhao, Yanli Luo, Jianjun Chen and Wei-En Yuan, Advances in redox-responsive drug delivery systems of tumor microenvironment, Guo et al. J Nanobiotechnol, 16 (2018) 1-10. [CrossRef]
- Xi67yong Zhang, Lu Han, Meiying Liu, Ke Wang, Lei Tao, Qing Wan and Yen Wei, Recent progress and advances in redox- responsive polymers as controlled delivery nanoplatforms, Materials Chemistry Frontiers, (2016). [CrossRef]
- [68]. Sonika Chibh, Avneet Kour, Nitin Yadav, Pankaj Kumar, Pratik Yadav, Virander Singh Chauhan, and Jiban Jyoti Panda, Redox-Responsive Dipeptide Nanostructures toward Targeted Cancer Therapy, ACS Omega, 5 (2020) 3365−3375. [CrossRef]
- Weikai Chen, Chenxi Zhang, Dagui Chen, Yinghua L, Shunli Wu, Can Xu, Li Su and Qin Zhang, Tumor redox microenvironment modulating composite hydrogels for enhanced sonodynamic therapy of colorectal cancer, J. Mater. Chem. B, 2022,10, 1960-1968. [CrossRef]
- Changling Sun, Xiaoying Li, Xiaodong Du, Teng Wang, Redox-responsive micelles for triggered drug delivery and effective laryngopharyngeal cancer therapy, Biomac, (2017). [CrossRef]
- Xiaoshuang Guo, Yuan Cheng, Xiaotian Zhao, Yanli Luo, Jianjun Chen and Wei-En Yuan, Advances in redox-responsive drug delivery systems of tumor microenvironment, Journal of Nanobiotechnology, 16 (2018) 1-10. [CrossRef]
- Yuan-Jia Pan, Yuan-Yuan Chen, Dong-Rui Wang, Chuan Wei, Jia Guo, Da-Ru Lu, Chih- Chang Chu, Chang-Chun Wang, Redox/pH dual stimuli-responsive biodegradable nanohydrogels with varying responses to dithiothreitol and glutathione for controlled drug release, Biomaterials 33 (2012) 6570-6579. [CrossRef]
- Serkan Demirci, Asli Celebioglu, Zeynep Aytacband Tamer Uyar, pH-responsive nanofibers with controlled drug release properties, Polym. Chem., 5 (2014) 2050–2056. [CrossRef]
- Elaref Ratemi, pH-responsive polymers for drug delivery applications, Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications, (2018) 121-141.
- Nayeleh Deirram, Changhe Zhang, Sarah S. Kermaniyan, Angus P. R. Johnston, and Georgina K. Such, pH-Responsive Polymer Nanoparticles for Drug Delivery, Macromol. Rapid Commun., 40 (2019). [CrossRef]
- Jong Oh Kim, Alexander V. Kabanov, and Tatiana K. Bronich, Polymer Micelles with Cross-Linked Polyanion Core for Delivery of a Cationic Drug Doxorubicin, J Control Release., 15 (2009) 197–204. [CrossRef]
- Kyung Hyun Min, Jong-Ho Kim, Sang Mun Bae, Hyeri Shin, Min Sang Kim, Sangjin Park, Hyejung Lee, Rang-Woon Park, In-San Kim, Kwangmeyung Kim, Ick Chan Kwon, Seo Young Jeong a, Doo Sung Lee, Journal of Controlled Release 144 (2010) 259–266.
- Liu, J., Huang, Y., Kumar, A., Tan, A., Jin, S., Mozhi, A., et al. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 32, (2014) 693–710. [CrossRef]
- Du, J., Lane, L. A., and Nie, S. Stimuli-responsive nanoparticles for targeting the tumor microenvironment. J. Control Release 219, (2015) 205–214. [CrossRef]
- Uthaman, Saji & Huh, Kang & Park, In-Kyu. (2018). Tumor microenvironment-responsive nanoparticles for cancer theragnostic applications. Biomaterials Research. 22. [CrossRef]
- Li, Y., Yang, H. Y., and Lee, D. S. Polymer-based and pH-sensitive nanobiosensors for imaging and therapy of acidic pathological areas. Pharm. Res. 33 (2016) 2358–2372. [CrossRef]
- Chang, G., Li, C., Lu, W., and Ding, J. N-Boc-histidine-capped PLGA-PEG- PLGA as a smart polymer for drug delivery sensitive to tumor extracellular pH. Macromol. Biosci., 10 (2010) 1248–1256. [CrossRef]
- Hu, F. Q., Zhang, Y. Y., You, J., Yuan, H., and Du, Y. Z. pH triggered doxorubicin delivery of PEGylated glycolipid conjugate micelles for tumor targeting therapy. Mol. Pharm, 9 (2012) 2469–2478. [CrossRef]
- Yu, Y., Zhang, X., and Qiu, L. The anti-tumor efficacy of curcumin when delivered by size/charge-changing multistage polymeric micelles based on amphiphilic poly(beta-amino ester) derivates. Biomaterials, 35 (2014) 3467–3479. [CrossRef]
- Jinfeng Shi, Yali Ren, Jiaqi Ma, Xi Luo, Jiaxin Li, Yihan Wu, Huan Gu, Chaomei Fu, Zhixing Cao and Jinming Zhang, Novel CD44-targeting and pH/redox-dual- stimuli-responsive core–shell nanoparticles loading triptolide combats breast cancer growth and lung metastasis, Shi et al. J Nanobiotechnol, 19 (2021). [CrossRef]
- Sajini D. Hettiarachchi, Emel Kirbas Cilingir,Heidi Maklouf, Elif S. Seven, Suraj Paudyal, Steven Vanni, Regina M. Grahamb and Roger M. Leblanc, pH and redox triggered doxorubicin release from covalently linked carbon dots conjugates, Nanoscale, 13 (2021). [CrossRef]
- Huanli Sun, Fenghua Meng, Ru Cheng, Chao Deng, Zhiyuan Zhong, Reduction and pH dual-bioresponsive crosslinked polymersomes for efficient intracellular delivery of proteins and potent induction of cancer cell apoptosis, H. Sun et al. / Acta Biomaterialia, 10 (2014) 2159– 2168. [CrossRef]
- Xianglong Hu,Hui Li,Shizhong Luo,Tao Liu, Yanyan Jiang and Shiyong Liu, Thiol and pH dual-responsive dynamic covalent shell cross-linked micelles for triggered release of chemotherapeutic drugs†, Polym. Chem., 4 (2013) 695–706. [CrossRef]
- Wen-Hsuan Chiang,Viet Thang Ho,Wen-Chia Huang,Yi-Fong Huang,Chorng- Shyan Chern,and Hsin-Cheng Chiu, Dual Stimuli-Responsive Polymeric Hollow Nanogels Designed as Carriers for Intracellular Triggered Drug Release, Langmuir, 28 (2012) 15056−15064. [CrossRef]
- Yuan-Jia Pan, Yuan-Yuan Chen, Dong-Rui Wang, Chuan Wei, Jia Guo, Da-Ru Lu, Chih- Chang Chu, Chang-Chun Wang, Redox/pH dual stimuli-responsive biodegradable nanohydrogels with varying responses to dithiothreitol and glutathione for controlled drug release, Y.-J. Pan et al. / Biomaterials, 33 (2012) 6570-6579. [CrossRef]
- Kang Liang, Georgina K. Such, Zhiyuan Zhu, Yan Yan, Hannah Lomas, and Frank Caruso, Charge-Shifting Click Capsules with Dual-Responsive Cargo Release Mechanisms, Adv. Mater. 23 (2011) H273–H277. [CrossRef]
- Remant Bahadur K. C,Bindu Thapa,and Peisheng Xu, pH and Redox Dual Responsive Nanoparticle for Nuclear Targeted Drug Delivery, Mol. Pharmaceutics , 9 (2012) 2719−2729. [CrossRef]
- Xunan Jing,Zhe Zhi,Liming Jin,a Fei Wang,Youshen Wu,Daquan Wang,Kai Yan,Yongping Shao and Lingjie Meng, pH/redox dual-stimuli responsive cross- linked polyphosphazene nanoparticles for multimodal imaging guided chemo- photodynamic therapy, J. Name., (2012) 1-3. [CrossRef]
- Manuela Curcio, Alessandro Paolì, Giuseppe Cirillo, Sebastiano Di Pietro, Martina Forestiero, Francesca Giordano and Francesca Iemma, Combining Dextran Conjugates with Stimuli-Responsive and Folate-Targeting Activity: A New Class of Multifunctional Nanoparticles for Cancer Therapy, Nanomaterials, 11 (2021) 1108. [CrossRef]
- [95]. Jun Chen,Xiaozhong Qiu,Jun Ouyang,|| Jiming Kong,Wen Zhong,and Malcolm M. Q. Xing, pH and Reduction Dual-Sensitive Copolymeric Micelles for Intracellular Doxorubicin Delivery, Biomacromolecules, 12 (2011) 3601–3611. [CrossRef]
- Jia Liu, Xingxin Liu, Ye Yuan, Qilin Li, Bingcheng Chang, Luming Xu, Bo Cai, Chao Qi, Cao Li, Xulin Jiang, Guobin Wang, Zheng Wang, and Lin Wang, Supramolecular Modular Approach towards Conveniently Constructing and Multifunctioning a pH/ Redox Dual Responsive Drug Delivery Nanoplatform for Improved Cancer Chemotherapy, ACS Appl. Mater. Interfaces, (2018). [CrossRef]
- Kun Yu, Xiyao Yang, Lihua He, Rong Zheng, Jie Min, Hongying Su, *, Shaoyun Shan, Qingming Jia, Facile preparation of pH/reduction dual-stimuli responsive dextran nanogel as environment-sensitive carrier of doxorubicin, Polymer, 200 (2020). [CrossRef]
- Cheng R, Meng F, Deng C, Klok HA, Zhong Z. Dual and Multi-Stimuli Responsive Polymeric Nanoparticles for Pro- grammed Site-Specific Drug Delivery. Biomaterials, 34 (2013) 3647−3657. [CrossRef]
- Xiao Fu, Leticia Hosta rigau, Rona Chandrawati, Jiwei Cui, Multi- Stimuli-Responsive Polymer Particles, Films, and Hydrogels for Drug Delivery. Chem. 4 (2018) 2084−2107.
- Guo X, Wei X, Jing Y, Zhou S. Size Changeable Nanocarriers with Nuclear Targeting for Effectively Overcoming Multidrug Resistance in Cancer Therapy. Adv. Mater. 27 (2015) 6450−6456. [CrossRef]
- Jin-Zhi-Du, Hong-Jun-Li and Jung-Wang. Tailor made Dual pH-Sensitive Polymer−Doxorubicin Nanoparticles for Efficient Anticancer Drug Delivery. J. Am. Chem. Soc. 133 (2011) 17560−17563.
- Dai L, Li X, Duan X, Li M, Niu P, Xu H, Cai K, Yang H. A pH/ROS Cascade-Responsive Charge-Reversal Nanosystem with Self-Amplified Drug Release for Synergistic Oxidation-Chemo- therapy. Adv. Sci. 6 (2019).
- Chen, J.; Ding, J.; Wang, Y.; Cheng, J.; Ji, S.; Zhuang, X.; Chen, X. Sequentially Responsive Shell-Stacked Nanoparticles for Deep Penetration into Solid Tumors. Adv. Mater. 29 (2017). [CrossRef]
- Xiong D, Zhang XF, Peng SY, Gu HW, Zhang LJ. Smart pH-sensitive micelles based on redox degradable polymers as DOX/GNPs carriers for controlled drug release and CT imaging. Colloid Surface B. 2018 (163) 29–40.
- H. Shi, M. Xu, J. Zhu, Y. Li, Z. He, Y. Zhang, et al., Programmed co-delivery of platinum nanodrugs and gemcitabine by a clustered nanocarrier for precision chemotherapy for NSCLC tumors, J. Mater. Chem. B 8 (2) (2020) 332–342. [CrossRef]
- Go Saito,Joel A. Swanson, Kyung-Dall Lee, Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities, Advanced Drug Delivery Reviews,55 (2003) 199–215. [CrossRef]
- Johnson V. John, Saji Uthaman, Rimesh Augustine, Hongyu Chen, In-Kyu Park and Il Kim, pH/Redox Dual Stimuli-Responsive Sheddable Nanodaisies for Efficient Intracellular Tumour-Triggered Drug Delivery, Journal of Materials Chemistry B, (2013). [CrossRef]
- M. Ramesh, L. Rajesh Kumar, Anish Khan, Abdullah Mohamed Asiri, 22 - Self-healing polymer composites and its chemistry, self healing composite marker, 2020, 415-427.
- Neelam Chauhan, Yashveer Singh, Engineered polymeric materials/nanomaterials for growth factor/drug delivery in bone tissue engineering applications, Nanoscale Engineering of Biomaterials: Properties and Applications, 2022, 349-396. [CrossRef]
- Arpita Roy, Kalipada Manna, Sagar Pal, Recent advances in various stimuli-responsive hydrogels: from synthetic designs to emerging healthcare applications, Mater. Chem. Front., 2022,6, 2338-2385. [CrossRef]
Figure 1.
(a) Polymeric nanoparticle structure [31] (b) Various Polymeric nanoparticles includes, nanofibers, dendrimers, brush polymers, nanoparticles, polymersomes, micelles, nanocapsuals, and nanogels [34].
Figure 1.
(a) Polymeric nanoparticle structure [31] (b) Various Polymeric nanoparticles includes, nanofibers, dendrimers, brush polymers, nanoparticles, polymersomes, micelles, nanocapsuals, and nanogels [34].
Figure 2.
Tumor Microenvironment Components: Heterogeneous Tumor Cells, Stromal Elements, and Immune Cells in a Dysregulated Vasculature and Collagen Network [62].
Figure 2.
Tumor Microenvironment Components: Heterogeneous Tumor Cells, Stromal Elements, and Immune Cells in a Dysregulated Vasculature and Collagen Network [62].
Figure 3.
Schematic overview of the genetic Tumor micro-environment for Enhancing Oncolytic Virus (OV) Performance in Cancer Therapy. (A) Strategies for Improved Tumor Targeting such as Serotype Switching, Tumor-Targeting Peptides, Glycoproteins from Other Viruses, Single-Chain Antibodies (scAb), Tumor-Specific Promoters, miRNA Target Sequences. (B) Enhancing Safety through Virulence Gene Deletion (C) Amplifying Antitumor Efficacy like, Insertion of Immunostimulatory Molecules/Cytokines, Suicide Genes (Pro-Apoptotic Proteins and Prodrug-Activating Enzymes), ECM-Degrading Enzymes, Anti-Vasculature Molecules. (D) Monitoring OV Replication Dynamics (GFP, Rluc, NIS, NET) [64].
Figure 3.
Schematic overview of the genetic Tumor micro-environment for Enhancing Oncolytic Virus (OV) Performance in Cancer Therapy. (A) Strategies for Improved Tumor Targeting such as Serotype Switching, Tumor-Targeting Peptides, Glycoproteins from Other Viruses, Single-Chain Antibodies (scAb), Tumor-Specific Promoters, miRNA Target Sequences. (B) Enhancing Safety through Virulence Gene Deletion (C) Amplifying Antitumor Efficacy like, Insertion of Immunostimulatory Molecules/Cytokines, Suicide Genes (Pro-Apoptotic Proteins and Prodrug-Activating Enzymes), ECM-Degrading Enzymes, Anti-Vasculature Molecules. (D) Monitoring OV Replication Dynamics (GFP, Rluc, NIS, NET) [64].
Figure 4.
Impact of GSH Inhibitor and Composite Hydrogel-Mediated SDT on Intracellular GSH Synthesis and Subcutaneous Colorectal Cancer Growth in Mouse Models [69].
Figure 4.
Impact of GSH Inhibitor and Composite Hydrogel-Mediated SDT on Intracellular GSH Synthesis and Subcutaneous Colorectal Cancer Growth in Mouse Models [69].
Figure 5.
Mechanism of Micelle Self-Assembly and pH activation. A. Diagram showing how micelles come together on their own, releasing DOX in response to GSH. And B. Schematic view of pH activation of nanoparticles by tumor microenvironment [80].
Figure 5.
Mechanism of Micelle Self-Assembly and pH activation. A. Diagram showing how micelles come together on their own, releasing DOX in response to GSH. And B. Schematic view of pH activation of nanoparticles by tumor microenvironment [80].
Figure 6.
pH-redox Tumor-Microenvironment-mediated Cascade and Polypeptide Core Self-Assembly for Targeted Drug Delivery. (a)pH-redox Tumor-Microenvironment-Mediated Cascade for Optimized Drug Delivery with PEGylated Shell and Disulfide-Cross-Linked Polypeptide Core [103]. (b) polymer PCL-SS-PDMAEMA self-assembly, GNPs and DOX loading and release for cancer chemotherapy and CT imaging [104].
Figure 6.
pH-redox Tumor-Microenvironment-mediated Cascade and Polypeptide Core Self-Assembly for Targeted Drug Delivery. (a)pH-redox Tumor-Microenvironment-Mediated Cascade for Optimized Drug Delivery with PEGylated Shell and Disulfide-Cross-Linked Polypeptide Core [103]. (b) polymer PCL-SS-PDMAEMA self-assembly, GNPs and DOX loading and release for cancer chemotherapy and CT imaging [104].
Figure 7.
(a)Design and Synthesis of pH-Responsive p(L–histidine) n–SS–polyurethane–SS–p(L–histidine) n Triblock Copolymers (n = 25, 35, 50, and 75) for Intracellular Drug Release. (b) Insights view of Self-Assembled Polyurethane Nano daisies Triggered by pH-Responsive p(His) Blocks and Disulfide Bond Cleavage in Response to GSH [107].
Figure 7.
(a)Design and Synthesis of pH-Responsive p(L–histidine) n–SS–polyurethane–SS–p(L–histidine) n Triblock Copolymers (n = 25, 35, 50, and 75) for Intracellular Drug Release. (b) Insights view of Self-Assembled Polyurethane Nano daisies Triggered by pH-Responsive p(His) Blocks and Disulfide Bond Cleavage in Response to GSH [107].
Table 1.
The different pH/redox responsive polymeric nanoparticles, drug delivery strategies and their mechanisms.
Table 1.
The different pH/redox responsive polymeric nanoparticles, drug delivery strategies and their mechanisms.
| Polymeric nano particles |
Cargo/drug |
Drug release / targeting |
Therapy |
Biological evolution |
Reference |
| poly (β-amino esters) |
CD44 |
Controlled drug release |
Breast cancer, lung metastasis |
In vitro |
85 |
| Conjugated (C-dots- HBA-dox) and (C- dots-S–S-dox) |
Doxorubicin |
Controlled drug release |
Cancer chemotherapy |
In vitro |
86 |
| PEG–PAA(SH)–PDEA |
FITC-BSA/CC |
Controlled drug release |
Cancer therapy |
In vitro |
87 |
| PCL-b-P(OEGMA-co- MAEBA) |
Camptothecin or Doxorubicin |
Accelerated drug release |
Cancer chemotherapy |
In vitro |
88 |
| acrylic acid (AAc) and 2- methacryloylethyl acrylate (MEA) |
Doxorubicin |
Rapid drug release |
Anti-cancer treatment |
In vitro |
89 |
| PMAA nanohydrogels |
Doxorubicin |
Controlled drug release |
Anti-cancer treatment |
In vitro |
90 |
| (PDPA) capsules |
Rhodamine B isothiocyanate- labeled OVA |
Cargo release |
-- |
In vitro |
91 |
| Poly (2-(pyridin-2- yldisulfanyl) ethyl acrylate) |
RPDSG/DOX |
Controlled drug release |
Cancer therapy |
In vitro |
92 |
| cross-linked polyphosphazene |
Curcumin and Ce6 |
Controlled drug release |
Cancer therapy |
In vivo and In vitro |
93 |
| DEXssPEGCOOH |
Doxorubicin |
Targeted release |
Cancer therapy |
In vitro |
94 |
| RPAE-PEG |
Doxorubicin |
Controlled drug release |
Cancer therapy |
In vitro |
95 |
| MSNs (DOX@PRMSNs) |
Doxorubicin |
targeting ligands |
Cancer therapy |
In vitro |
96 |
| DOX@Dex-SS nanogel |
Doxorubicin |
Cumulative amount drug release |
Cancer chemotherapy |
In vitro |
97 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).