Submitted:
30 November 2023
Posted:
04 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Study Rationale:
3. Study Objectives:
3.1. Primary Objective:
- To analyze the effectiveness of the antidepressant switch strategy to vortioxetine for the improvement of sexual dysfunction (measured as Total SALSEX Score) after 3 months of follow-up in patients with poor tolerance or risk of treatment discontinuation (the risk of discontinuation is defined as a score ≥ 2 in item 5 of the SALSEX).
3.2. Secondary Objectives:
- □
- To study the individual tolerance and risk of treatment discontinuation using the PSRSexDQ-SALSEX questionnaire at baseline and to the endpoint.
- □
- To determine differences in SD between males and females at baseline and to the endpoint.
- □
- To determine differences in SD between different age groups at baseline and to the endpoint.
- □
- To determine differences in SD between different levels of severity of depression at baseline and to the endpoint.
- □
- To determine differences in SD between different dosages of vortioxetine (10-20 mg)
4. Methodology
4.1. Design
4.2. Study Subjects
4.2.1. Sample Size Calculation
4.2.2. Inclusion Criteria:
- □
- Patients who showed at least moderate intensity in the total score of SALSEX ≥6 (including ≥ 2 in item 5, tolerance of sexual dysfunction).
- □
- Patients with normal sexual function prior to taking antidepressants (Normal sexual function was defined as an absence of habitual dysfunctions of sufficient intensity to cause subjective discomfort in the patient in the areas of desire, orgasm or sexual arousal that would require specialized attention, with previous regular, satisfactory sexual and/or autoerotic practices).
- □
- Patients sexually active treated with an antidepressant for at least 2 months. This time requirement was included to avoid false negatives, as some symptoms do not appear until after this period (loss of sexual desire or erectile/vaginal lubrication dysfunction).
- □
- Previous antidepressant-related sexual dysfunction. Patients were switched to vortioxetine only if there were symptoms of sexual dysfunction that were considered associated with the previous antidepressant.
- □
- Treatment exclusively with antidepressants used within approved label (including SSRIs, SNRIs). Combined treatment with benzodiazepines at low clinical doses was permitted (less than 20 mg clorazepate or equivalent).
- □
- Patients with at least partial response with a maximum score on the Clinical Global Impression Scale of Depression (CGI-D) ≤ 3- mild depression.
4.2.3. Exclusion Criteria:
- □
- SD prior to starting administration of the antidepressant. (Only a mild decrease in libido before starting antidepressant treatment was permitted, as this is considered a symptom of depression itself, although worsening of libido because of treatment was considered as an adverse effect).
- □
- Combination of the antidepressant with antipsychotic drugs or mood stabilizers.
- □
- Use of hormones or any other medication with known capacity to interfere in sexual relationships (antiepileptic drugs, H2 antagonists, recent introduction of contraceptives as concomitant therapy, β-blockers, opiates, and antihypertensive drugs).
- □
- Medically significant intercurrent diseases clearly affecting sexual function.
4.2.4. Switching Procedures:
4.2.5. Sites
4.3. Variables
4.3.1. Primary Variable:
- □
- The Severity of global SD was measured using the SALSEX total score (scoring from 0= no sexual dysfunction to 15= maximum sexual dysfunction) at baseline and to the endpoint. The severity of each individual dimension of sexual functioning (reduced sexual desire, delay of orgasm, anorgasmia and arousal difficulties such as erectile dysfunction or vaginal lubrication) was measured with a Likert scale (0= no SD; 3= maximum SD) of the items 1-4 of the SALSEX questionnaire) at baseline and at endpoint visit.
4.3.2. Secondary Variables:
- □
- To study the individual acceptance of SD and risk of treatment discontinuation, we used the score of the item 5 of the SALSEX questionnaire (0= no risk of discontinuation; 3= maximum risk) at baseline and at endpoint visit.
- □
- To determine differences in SD that varied across the severity of depression we used the Clinical Global Impression-Improvement (CGI-I) scale for depression, and the CGI-S for sexual functioning at baseline and at endpoint visit.
4.4. Data Collection and Analysis
4.4.1. Measurement Scales
PRSexDQ- SALSEX Questionnaire:
- ○
- No SD: Total score of 0 or 1, only if item 1 is evaluated as a slight loss of libido (which is equivalent to a score of 1 on that item).
- ○
- SD present: Total score of 2-15, or total score of 1 if any item except for item 1 (desire) is scored 1.
- □
- Mild SD: 1-5 points, provided no item scores ≥2 points and item 5 (tolerability) is not >1.
- □
- Moderate SD: 6-10 points, provided that no item scores ≥3 points or else <6 points if any item = 2 and provided that item 5 (tolerability) is not >2.
- □
- Severe SD: 11-15 or <11 points if any item = 3 or whenever item 5 (tolerability) = 3.
Clinical Global Impression Scale, severity subscale, applied to sexual dysfunction (CGI-S-SD) to assess the DS severity at baseline
Clinical Global Impression Scale, improvement subscale, applied to sexual dysfunction (CGI-I-SD) to assess the clinical effectiveness of the intervention, administered in the follow-up visit (performed within 3 months after the baseline visit)
Clinical Global Impression scale, severity subscale (CGI-S), applied to the psychiatric disease for which the antidepressant treatment is administered, to assess severity at baseline
Clinical Global Impression scale, improvement subscale, CGI-I) to assess the clinical effectiveness of the intervention, administered in the follow-up visit (performed within 3 months after the baseline visit)
Adverse Events Assessments:
4.4.2. Visits
- □
- Baseline visit (V1): treatment initiation switch if prompted by poorly tolerated SD
- □
- Visit 2 (V2) conducted within 3 months of follow-up after after switching antidepressant at baseline visit
4.4.3. Treatment
4.4.4. Ethical and Legal Considerations
Patient Information
Confidentiality
5. Data Analysis
5.1. Evaluation Criteria and Data Management
5.2. Evaluation Criteria
Evaluations Performed:
6. Project Development Stages
7. Results
| n=74 | |
| Age (years) | 43.1 ± 12.65 |
| < 30 , n (%) | 14 (18.9) |
| 30-40, n (%) | 21 (28.4) |
| 40-50, n (%) | 17 (23.0) |
| > 50, n (%) | 22 (29.7) |
| Gender (Males), n (%) | 40 (54.1) |
| Gender (Females), n (%) | 34 (45.9) |
| Duration of treatment (months) (Baseline) | 19.53 ± 37.27 |
| Duration of treatment (meses) (Follow-up) | 3.21 ± 1.17 |
| Dosage (mg/d) (Follow-up) | 13.11±4.03 |
| SALSEX Total Score at Baseline | 10.32 ± 2.73 |
| SALSEX Total Score at Follow-up | 3.78 ± 3.68 |
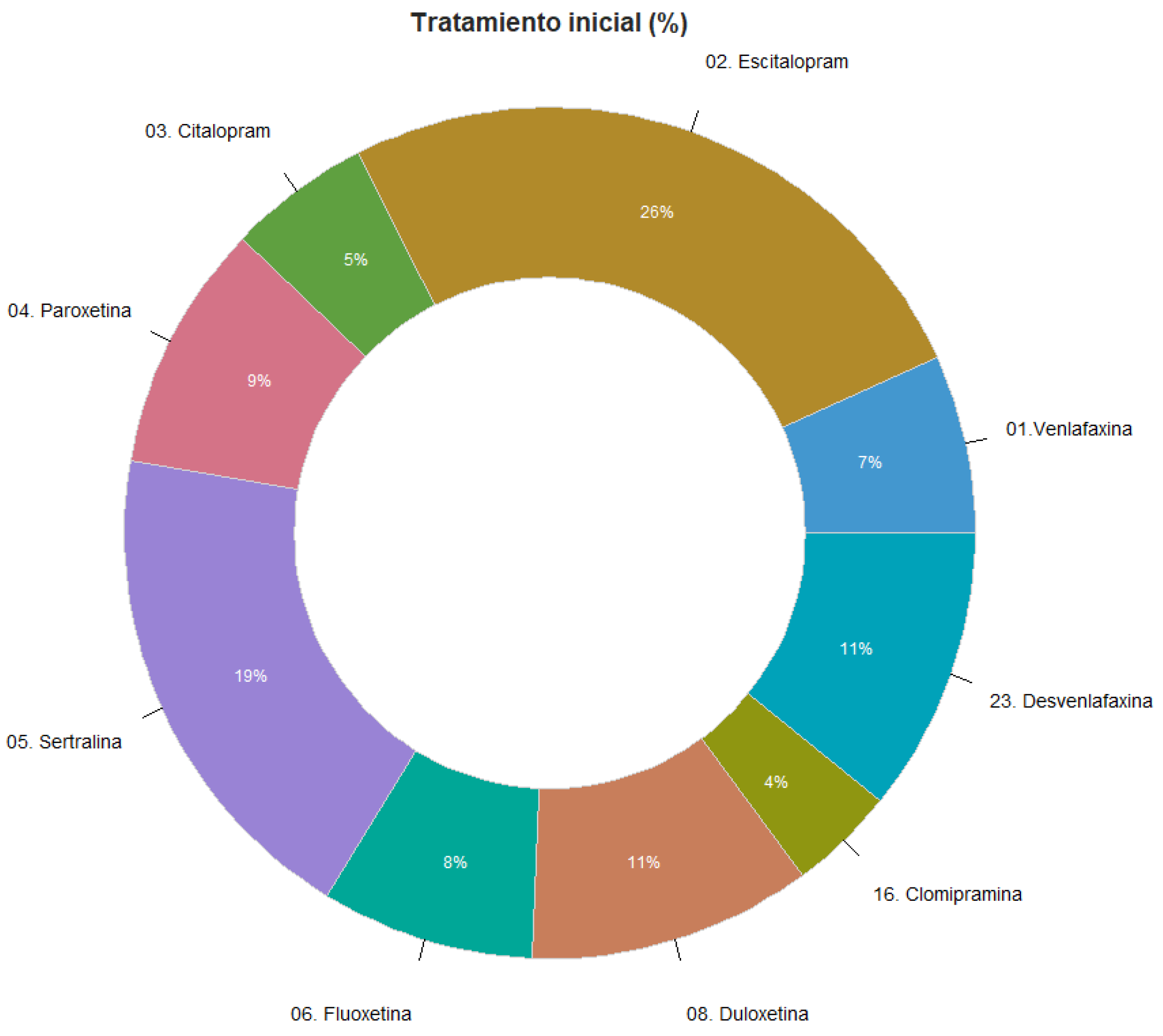
7.1. Previous Antidepressant Treatment at Baseline
7.2. SALSEX Scale Total Score of Sexual Dysfunction.
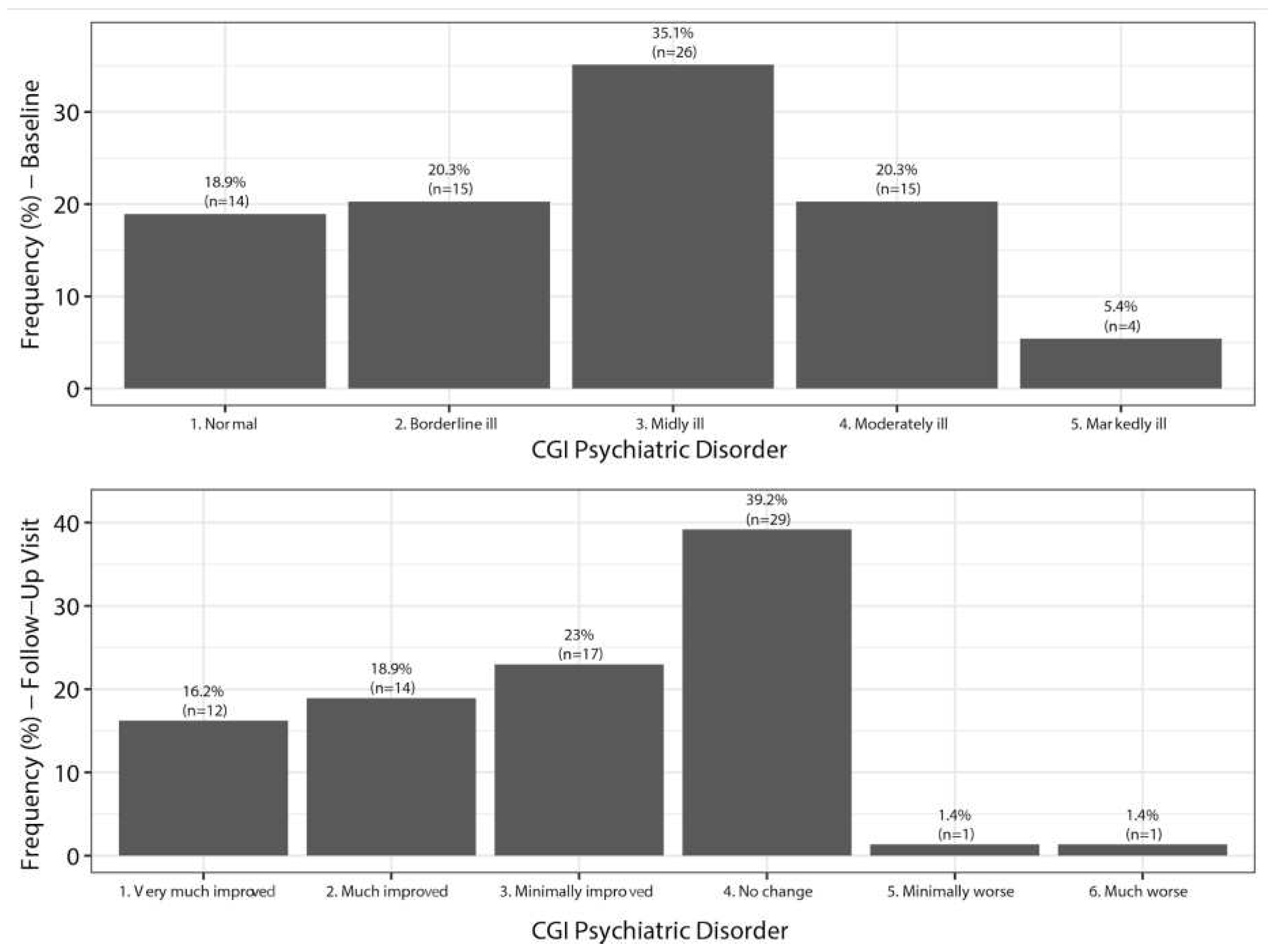
7.3. Clinical Global Impression of Severity of Depression (CGI-D)
7.4. Clinical Global Impression of Severity of Sexual Dysfunction (CGI-SEx)
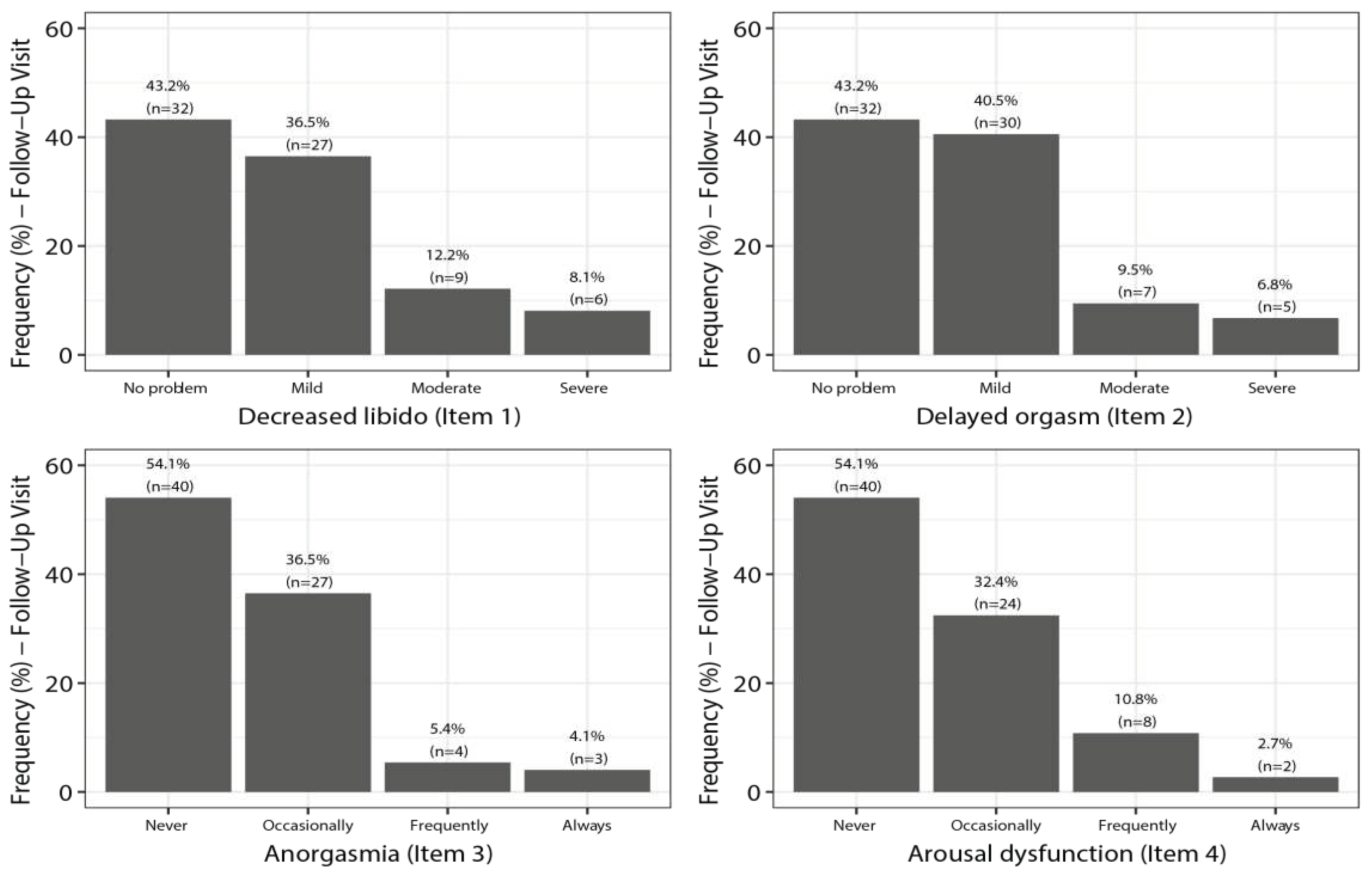
7.5. SALSEX Scale by Items from Baseline to Follow-Up
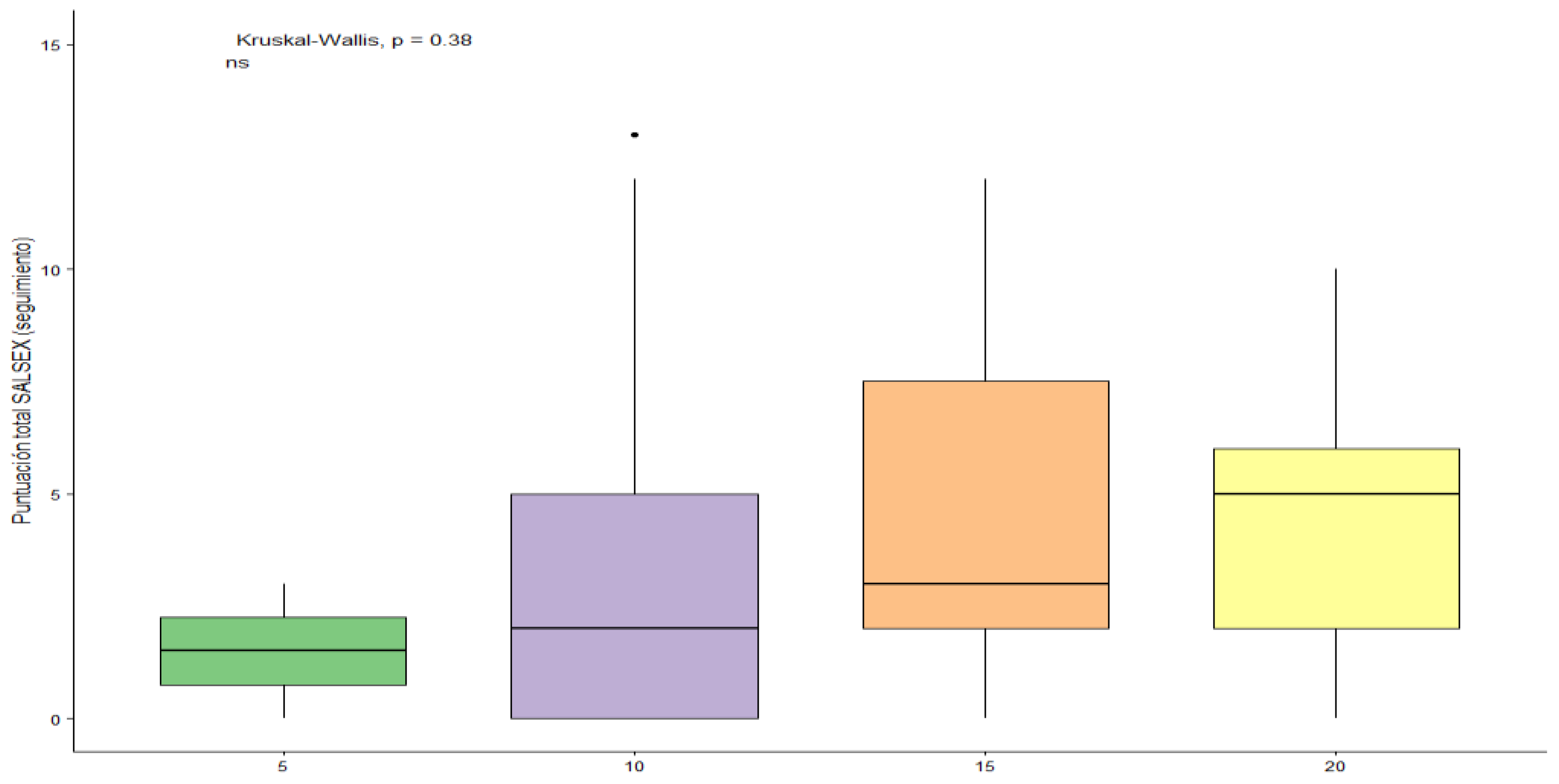
7.6. Sexual Dysfunction and Dosages of Vortioxetine
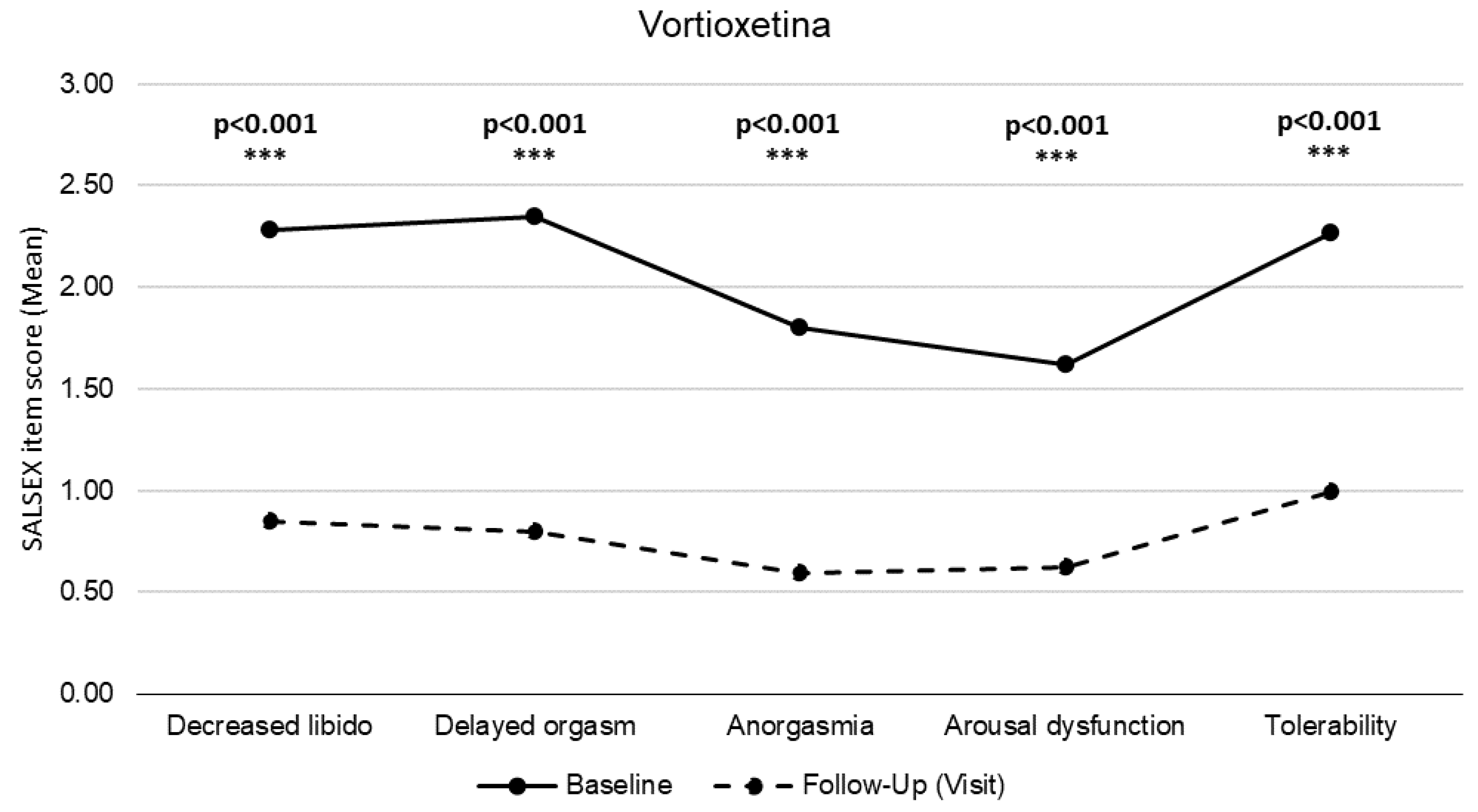
7.7. Clinical Outcomes after Switching to Vortioxetine
8. Discussion
8.1. Value of the Study
8.2. Limitations
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baldwin, D.S.; Foong, T. Antidepressant Drugs and Sexual Dysfunction. Br J Psychiatry 2013, 202, 396–397. [CrossRef]
- Serretti, A.; Chiesa, A. Treatment-Emergent Sexual Dysfunction Related to Antidepressants: A Meta-Analysis. J Clin Psychopharmacol 2009, 29, 259–266. [CrossRef]
- Montejo AL, Calama J, Rico-Villademoros F, Montejo L, González-García N, Pérez J; SALSEX Working Study Group. A Real-World Study on Antidepressant-Associated Sexual Dysfunction in 2144 Outpatients: The SALSEX I Study. Arch Sex Behav. 2019 Apr;48(3):923-933. [CrossRef]
- Montejo, A.L.; Montejo, L.; Baldwin, D.S. The Impact of Severe Mental Disorders and Psychotropic Medications on Sexual Health and Its Implications for Clinical Management. World Psychiatry 2018, 17, 3–11. [CrossRef]
- Alvarez, E.; Perez, V.; Artigas, F. Pharmacology and Clinical Potential of Vortioxetine in the Treatment of Major Depressive Disorder. Neuropsychiatr Dis Treat 2014, 10, 1297–1307. [CrossRef]
- Abler, B.; Seeringer, A.; Hartmann, A.; Grön, G.; Metzger, C.; Walter, M.; Stingl, J. Neural Correlates of Antidepressant-Related Sexual Dysfunction: A Placebo-Controlled fMRI Study on Healthy Males Under Subchronic Paroxetine and Bupropion. Neuropsychopharmacology 2011, 36, 1837. [CrossRef]
- Clayton, A.H.; Croft, H.A.; Handiwala, L. Antidepressants and Sexual Dysfunction: Mechanisms and Clinical Implications. Postgrad Med 2014, 126, 91–99. [CrossRef]
- Montejo, A.L.; Prieto, N.; de Alarcón, R.; Casado-Espada, N.; de la Iglesia, J.; Montejo, L. Management Strategies for Antidepressant-Related Sexual Dysfunction: A Clinical Approach. J Clin Med 2019, 8, 1640. [CrossRef]
- Montejo, A.L.; Montejo, L.; Navarro-Cremades, F. Sexual Side-Effects of Antidepressant and Antipsychotic Drugs. Curr Opin Psychiatry 2015, 28, 418–423. [CrossRef]
- Montejo, A.L.; Rico-Villademoros, F. Psychometric Properties of the Psychotropic-Related Sexual Dysfunction Questionnaire (PRSexDQ-SALSEX) in Patients with Schizophrenia and Other Psychotic Disorders. J Sex Marital Ther 2008, 34, 227–239. [CrossRef]
- McGahuey, C.A.; Gelenberg, A.J.; Laukes, C.A.; Moreno, F.A.; Delgado, P.L.; McKnight, K.M.; Manber, R. The Arizona Sexual Experience Scale (ASEX): Reliability and Validity. J Sex Marital Ther 2000, 26, 25–40. [CrossRef]
- Clayton, A.H.; McGarvey, E.L.; Clavet, G.J. The Changes in Sexual Functioning Questionnaire (CSFQ): Development, Reliability, and Validity. Psychopharmacol Bull 1997, 33, 731–745.
- Montejo-González, A.L.; Llorca, G.; Izquierdo, J.A.; Ledesma, A.; Bousoño, M.; Calcedo, A.; Carrasco, J.L.; Ciudad, J.; Daniel, E.; De la Gandara, J.; et al. SSRI-Induced Sexual Dysfunction: Fluoxetine, Paroxetine, Sertraline, and Fluvoxamine in a Prospective, Multicenter, and Descriptive Clinical Study of 344 Patients. J Sex Marital Ther 1997, 23, 176–194. [CrossRef]
- Montejo, A.L.; Llorca, G.; Izquierdo, J.A.; Rico-Villademoros, F. Incidence of Sexual Dysfunction Associated with Antidepressant Agents: A Prospective Multicenter Study of 1022 Outpatients. Spanish Working Group for the Study of Psychotropic-Related Sexual Dysfunction. J Clin Psychiatry 2001, 62 Suppl 3, 10–21.
- Clayton, A.H.; Warnock, J.K.; Kornstein, S.G.; Pinkerton, R.; Sheldon-Keller, A.; McGarvey, E.L. A Placebo-Controlled Trial of Bupropion SR as an Antidote for Selective Serotonin Reuptake Inhibitor-Induced Sexual Dysfunction. J Clin Psychiatry 2004, 65, 62–67. [CrossRef]
- FDA Selective Serotonin Reuptake Inhibitors (SSRIs) Information. [(accessed on 01 November 2023)]; Available online: https://www.fda.gov/drugs/information-drug-class/selective-serotonin-reuptake-inhibitors-ssris-information.
- Bonierbale, M.; Lançon, C.; Tignol, J. The ELIXIR Study: Evaluation of Sexual Dysfunction in 4557 Depressed Patients in France. Curr Med Res Opin 2003, 19, 114–124. [CrossRef]
- Montejo, A.L.; Deakin, J.F.W.; Gaillard, R.; Harmer, C.; Meyniel, F.; Jabourian, A.; Gabriel, C.; Gruget, C.; Klinge, C.; MacFayden, C.; et al. Better Sexual Acceptability of Agomelatine (25 and 50 Mg) Compared to Escitalopram (20 Mg) in Healthy Volunteers. A 9-Week, Placebo-Controlled Study Using the PRSexDQ Scale. J Psychopharmacol 2015, 29, 1119–1128. [CrossRef]
- Montejo, A.L.; Prieto, N.; Terleira, A.; Matias, J.; Alonso, S.; Paniagua, G.; Naval, S.; Parra, D.G.; Gabriel, C.; Mocaër, E.; et al. Better Sexual Acceptability of Agomelatine (25 and 50 Mg) Compared with Paroxetine (20 Mg) in Healthy Male Volunteers. An 8-Week, Placebo-Controlled Study Using the PRSEXDQ-SALSEX Scale. J Psychopharmacol 2010, 24, 111–120. [CrossRef]
- Jacobsen, P.L.; Mahableshwarkar, A.R.; Serenko, M.; Chan, S.; Trivedi, M.H. A Randomized, Double-Blind, Placebo-Controlled Study of the Efficacy and Safety of Vortioxetine 10 Mg and 20 Mg in Adults with Major Depressive Disorder. J Clin Psychiatry 2015, 76, 575–582. [CrossRef]
- Jacobsen, P.L.; Mahableshwarkar, A.R.; Palo, W.A.; Chen, Y.; Dragheim, M.; Clayton, A.H. Treatment-Emergent Sexual Dysfunction in Randomized Trials of Vortioxetine for Major Depressive Disorder or Generalized Anxiety Disorder: A Pooled Analysis. CNS Spectr 2016, 21, 367–378. [CrossRef]
- Jacobsen, P.; Zhong, W.; Nomikos, G.; Clayton, A. Paroxetine, but Not Vortioxetine, Impairs Sexual Functioning Compared With Placebo in Healthy Adults: A Randomized, Controlled Trial. J Sex Med 2019, 16, 1638–1649. [CrossRef]
- Jacobsen PL, Mahableshwarkar AR, Chen Y, Chrones L, Clayton AH. Effect of Vortioxetine vs. Escitalopram on Sexual Functioning in Adults with Well-Treated Major Depressive Disorder Experiencing SSRI-Induced Sexual Dysfunction. J Sex Med. 2015 Oct;12(10):2036-48. [CrossRef]
- Jacobsen, P.L.; Nomikos, G.G.; Zhong, W.; Cutler, A.J.; Affinito, J.; Clayton, A. Clinical Implications of Directly Switching Antidepressants in Well-Treated Depressed Patients with Treatment-Emergent Sexual Dysfunction: A Comparison between Vortioxetine and Escitalopram. CNS Spectr 2020, 25, 50–63. [CrossRef]
- Callegari, C.; Ielmini, M.; Caselli, I.; Lucca, G.; Isella, C.; Diurni, M.; Pettenon, F.; Poloni, N. Paroxetine versus Vortioxetine for Depressive Symptoms in Postmenopausal Transition: A Preliminary Study. Psychopharmacol Bull 2019, 49, 28–43.
- Mahableshwarkar, A.R.; Jacobsen, P.L.; Serenko, M.; Chen, Y.; Trivedi, M.H. A Randomized, Double-Blind, Placebo-Controlled Study of the Efficacy and Safety of 2 Doses of Vortioxetine in Adults with Major Depressive Disorder. J Clin Psychiatry 2015, 76, 583–591. [CrossRef]
- Alam, M.Y.; Jacobsen, P.L.; Chen, Y.; Serenko, M.; Mahableshwarkar, A.R. Safety, Tolerability, and Efficacy of Vortioxetine (Lu AA21004) in Major Depressive Disorder: Results of an Open-Label, Flexible-Dose, 52-Week Extension Study. Int Clin Psychopharmacol 2014, 29, 36–44. [CrossRef]
- Sánchez-Sánchez F, Ponce-Buj B, Montejo-González AL, Sipán-Sarrión Y, Gimeno-Marqués A, Merino-Gámez A. Repercusión de vortioxetina sobre la función sexual frente a otros antidepresivos [Impact of vortioxetine on sexual function compared to other antidepressants]. Semergen. 2023 Jun 15;49(7):101997. Spanish. [CrossRef]
- Garnock-Jones, K.P. Vortioxetine: A Review of Its Use in Major Depressive Disorder. CNS Drugs 2014, 28, 855–874. [CrossRef]
- Clayton, A.H.; Montejo, A.L. Major Depressive Disorder, Antidepressants, and Sexual Dysfunction. J Clin Psychiatry 2006, 67 Suppl 6, 33–37.
- Montejo, A.L.; Becker, J.; Bueno, G.; Fernández-Ovejero, R.; Gallego, M.T.; González, N.; Juanes, A.; Montejo, L.; Pérez-Urdániz, A.; Prieto, N.; et al. Frequency of Sexual Dysfunction in Patients Treated with Desvenlafaxine: A Prospective Naturalistic Study. J Clin Med 2019, 8, 719. [CrossRef]
- Kennedy, S.H.; Rizvi, S.; Fulton, K.; Rasmussen, J. A Double-Blind Comparison of Sexual Functioning, Antidepressant Efficacy, and Tolerability between Agomelatine and Venlafaxine XR. J Clin Psychopharmacol 2008, 28, 329–333. [CrossRef]
- Clayton, A.H.; Reddy, S.; Focht, K.; Musgnung, J.; Fayyad, R. An Evaluation of Sexual Functioning in Employed Outpatients with Major Depressive Disorder Treated with Desvenlafaxine 50 Mg or Placebo. J Sex Med 2013, 10, 768–776. [CrossRef]
- Deardorff, W.J.; Grossberg, G.T. A Review of the Clinical Efficacy, Safety and Tolerability of the Antidepressants Vilazodone, Levomilnacipran and Vortioxetine. Expert Opin Pharmacother 2014, 15, 2525–2542. [CrossRef]
- D’Agostino, A.; English, C.D.; Rey, J.A. Vortioxetine (Brintellix): A New Serotonergic Antidepressant. Pharmacy and Therapeutics 2015, 40, 36.
- Baldwin, D.S.; Chrones, L.; Florea, I.; Nielsen, R.; Nomikos, G.G.; Palo, W.; Reines, E. The Safety and Tolerability of Vortioxetine: Analysis of Data from Randomized Placebo-Controlled Trials and Open-Label Extension Studies. J Psychopharmacol 2016, 30, 242–252. [CrossRef]
- De Luca, R.; Bonanno, M.; Manuli, A.; Calabrò, R.S. Cutting the First Turf to Heal Post-SSRI Sexual Dysfunction: A Male Retrospective Cohort Study. Medicines (Basel) 2022, 9, 45. [CrossRef]
- Kelliny, M.; Croarkin, P.E.; Moore, K.M.; Bobo, W.V. Profile of Vortioxetine in the Treatment of Major Depressive Disorder: An Overview of the Primary and Secondary Literature. Ther Clin Risk Manag 2015, 11, 1193–1212. [CrossRef]
- Takeda A Randomized, Double-Blind, Parallel Group, Placebo- and Active-Controlled, Phase 4 Study Evaluating the Effect of Vortioxetine 10 and 20 Mg/Day vs Paroxetine 20 Mg/Day on Sexual Functioning in Healthy Subjects; clinicaltrials.gov, 2018 [(accessed on 02 November 2023)]. Available online: https://clinicaltrials.gov/study/NCT02932904.
- Mattingly, G.; Brunner, E.; Chrones, L.; Lawrence, D.F.; Simonsen, K.; Ren, H. Effectiveness of Vortioxetine for Major Depressive Disorder in Real-World Clinical Practice: US Cohort Results from the Global RELIEVE Study. Front Psychiatry 2023, 13, 977560. [CrossRef]


| Characteristic | Males (n=40) |
Females (n=34) |
P-Value | |
| Age (y.o) | 41.28 ± 13.60 | 45.18 ± 11.26 | 0.188 | t=1.329 |
| < 30 n (%) | 11 (27.5) | 3 (8.8) | ||
| 30-40, n (%) | 10 (25.0) | 11 (32.4) | ||
| 40-50, n (%) | 7 (17.5) | 10 (29.4) | ||
| > 50, n (%) | 12 (30.0) | 10 (29.4) | ||
| Treatment duration at baseline (months) | 14.55 ± 24.02 | 25.38 ± 48.22 | 0.129 | W=819.5 |
| Treatment duration ( baseline-follow-up) | 3.28 ± 1.41 | 3.15 ± 0.86 | 0.900 | W=399 |
| Vortioxetine dosage (mg/d) (at follow-up)) | 12.5 ± 4.08 | 13.82 ± 3.9 | 0.151 | W=802 |
| Total score SALSEX - Baseline | 9.68 ± 3.16 | 11.09 ± 1.9 | 0.021 | t=2.372 |
| Total score SALSEX – Follow-up | 2.85 ± 2.8 | 4.88 ± 4.29 | 0.021 | t=2.367 |
| No Dysfunction | Mild | Moderate | Severe | |||||
| Baseline | F-up | Baseline | F-up | Baseline | F-up | Baseline | F-up | |
| Libido decreased | 4.1 | 43.2 | 10.8 | 36.5 | 37.8 | 12.2 | 47.3 | 8.1 |
| Orgasm delayed | 1.4 | 43.2 | 12.2 | 40.5 | 36.5 | 9.5 | 50.0 | 6.8 |
| Never | Sometimes | Often | Allways | |||||
| Baseline | F-up | Baseline | F-up | Baseline | F-up | Baseline | F-up | |
| Anorgasmia | 6.8 | 54.1 | 24.3 | 36.5 | 51.4 | 5.4 | 17.6 | 4.1 |
| Arousal difficulties | 9.5 | 54.1 | 28.4 | 32.4 | 52.7 | 10.8 | 9.5 | 2.7 |
| No sex dysfunction | Good | Moderate | Poor | |||||
| Baseline | F-up | Baseline | F-up | Baseline | F-up | Baseline | F-up | |
| Acceptability of SD | 1.4 | 34.8 | 10.8 | 39.1 | 47.3 | 18.8 | 40.5 | 7.2 |
| No sex dysfunction | MIld | Moderate | Severe | |||||
| Baseline | F-up | Baseline | F-up | Baseline | F-up | Baseline | F-up | |
| Libido decreased | ||||||||
| No problem | 21,4 | 100,0 | 14,3 | - | 35,7 | - | 28,6 | - |
| Moderate/severe | - | 4,5 | 9,1 | 27,3 | 31,8 | 40,9 | 59,1 | 27,3 |
| Orgasm delayed | ||||||||
| No problem | 7,1 | 100,0 | 7,1 | - | 21,4 | - | 64,3 | - |
| Moderate/severe | - | - | 22,7 | 45,5 | 13,6 | 31,8 | 63,6 | 22,7 |
| Never | Sometimes | Often | Allways | |||||
| Baseline | F-up | Baseline | F-up | Baseline | F-up | Baseline | F-up | |
| Anorgasmia | ||||||||
| No problem | 21,4 | 100,0 | 21,4 | - | 35,7 | - | 21,4 | - |
| Moderate/severe | 4,5 | 9,1 | 27,3 | 59,1 | 45,5 | 18,2 | 22,7 | 13,6 |
| Arousal difficulties | ||||||||
| No problem | 28,6 | 100,0 | 42,9 | - | 28,6 | - | - | - |
| Moderate/severe | - | 9,1 | 22,7 | 45,5 | 63,6 | 36,4 | 13,6 | 9,1 |
| No sex dysfunction | Good | Moderate | Poor | |||||
| Baseline | F-up | Baseline | F-up | Baseline | F-up | Baseline | F-up | |
| Acceptability of SD | ||||||||
| No problem | - | 100,0 | 14,3 | - | 35,7 | - | 50,0 | - |
| Poor | - | - | 9,1 | 18,2 | 50,0 | 59,1 | 40,9 | 22,7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





