Submitted:
29 November 2023
Posted:
05 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Source and composition of TM meal
2.2. Experimental diets
2.3. Fish and experimental design
2.4. Fish sampling
2.5. Growth performance and nutrient utilization
2.6. Biochemical Analysis
2.6.1. Proximate composition of diets and tissues
2.6.2. Total lipids, lipid classes and FA analyses
2.7. Statistical analysis
3. Results
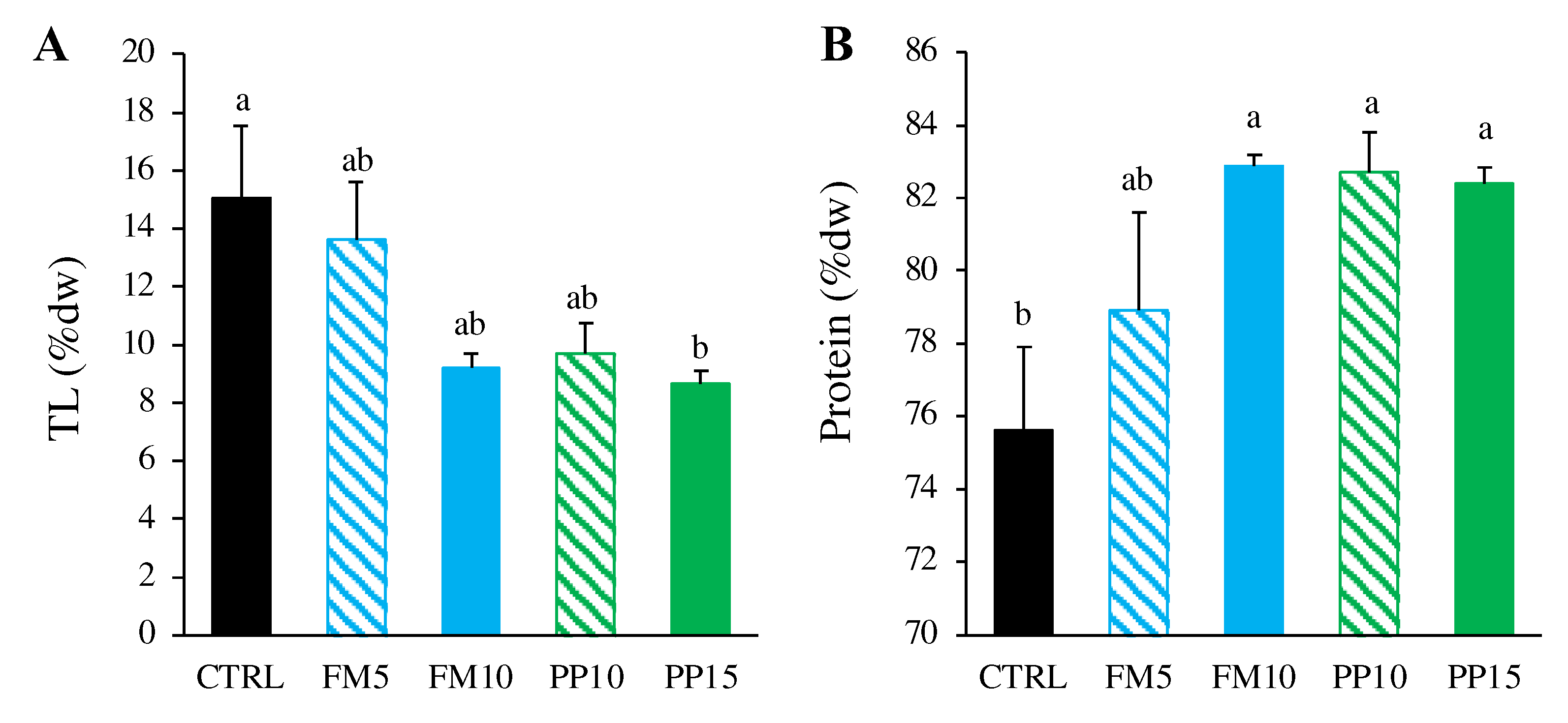
3.1. Growth performance and proximate composition
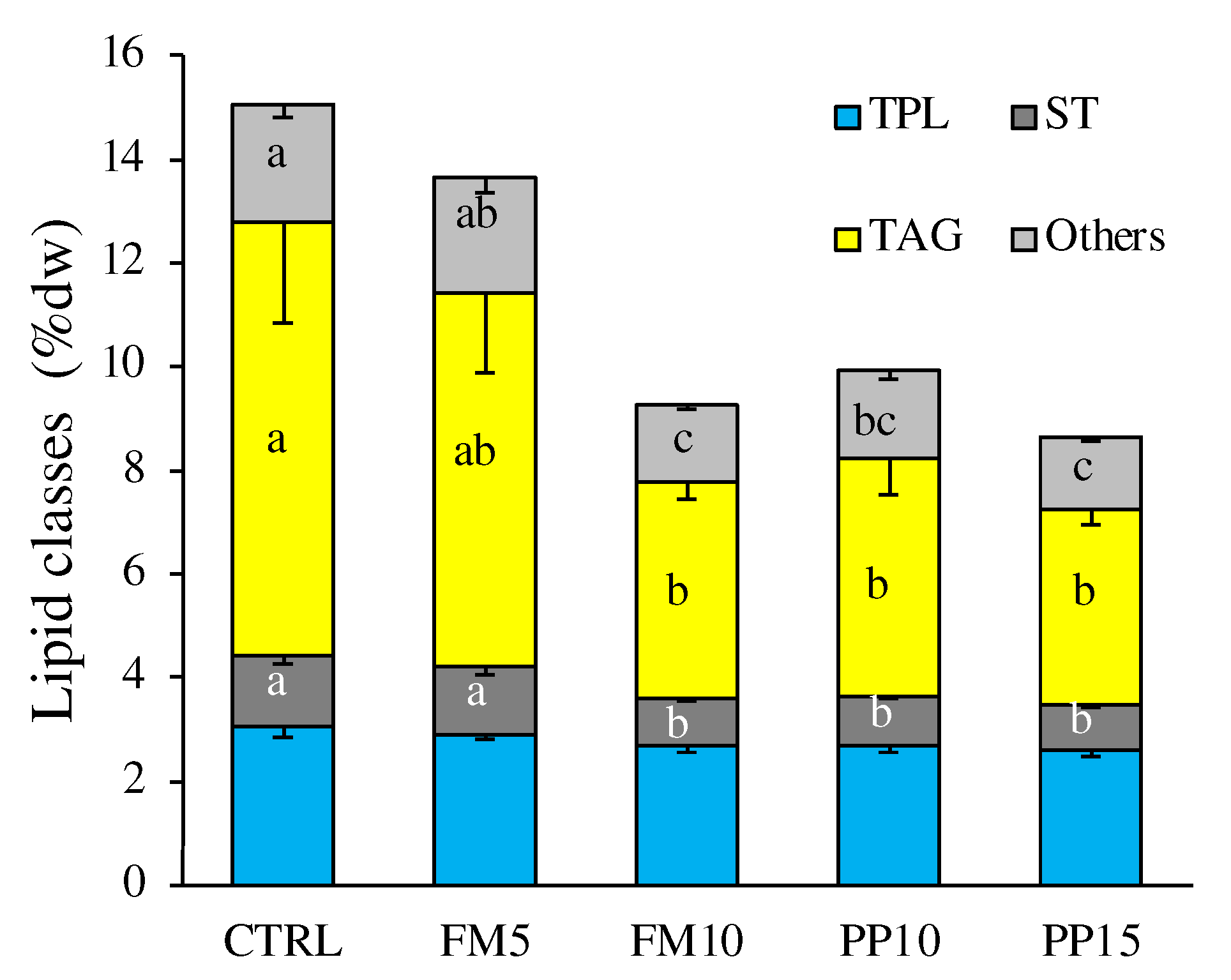
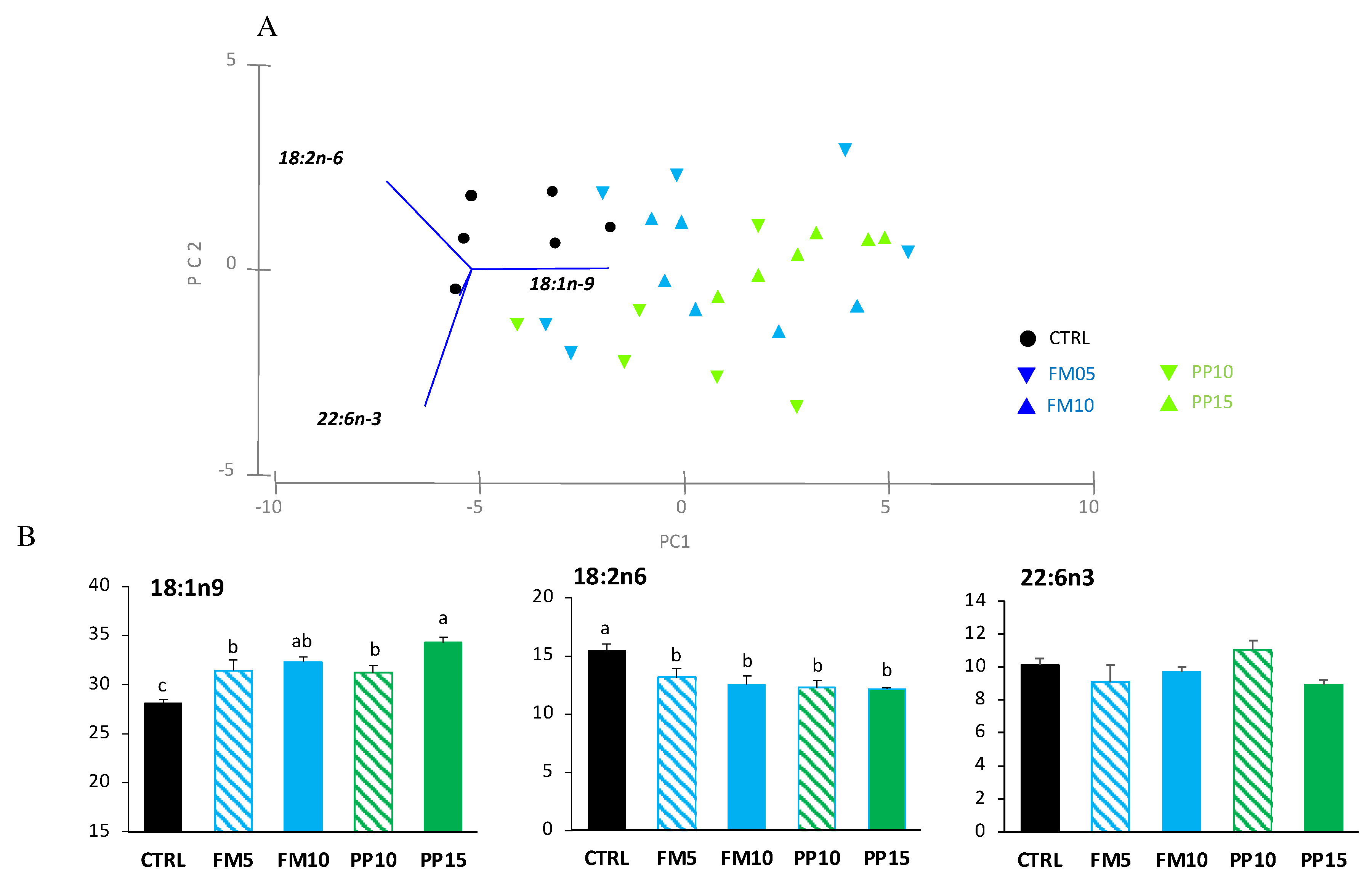
3.2. Lipid classes and fatty acid profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation. 2022. [CrossRef]
- Nijdam, D.; Rood, T.; Westhoek, H. The price of protein: Review of land use and carbon footprints from life cycle assessments of animal food products and their substitutes. Food Policy 2012, 37, 760-770. [CrossRef]
- Hardy, R.W. Utilization of plant proteins in fish diets: effects of global demand and supplies of fishmeal. Aquaculture Research 2010, 41, 770-776. [CrossRef]
- Montero, D.; Benitez-Dorta, V.; Caballero, M.J.; Ponce, M.; Torrecillas, S.; Izquierdo, M.; Zamorano, M.J.; Manchado, M. Dietary vegetable oils: Effects on the expression of immune-related genes in Senegalese sole (Solea senegalensis) intestine. Fish & Shellfish Immunology 2015, 44, 100-108. [CrossRef]
- Bakke-McKellep, A.M.; Penn, M.H.; Salas, P.M.; Refstie, S.; Sperstad, S.; Landsverk, T.; Ringø, E.; Krogdahl, Å. Effects of dietary soyabean meal, inulin and oxytetracycline on intestinal microbiota and epithelial cell stress, apoptosis and proliferation in the teleost Atlantic salmon (Salmo salar L.). British Journal of Nutrition 2007, 97, 699-713. [CrossRef]
- Cottrell, R.S.; Blanchard, J.L.; Halpern, B.S.; Metian, M.; Froehlich, H.E. Global adoption of novel aquaculture feeds could substantially reduce forage fish demand by 2030. Nature Food 2020, 1, 301-308. [CrossRef]
- Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. EDIBLE INSECTS future prospects fo food and feed security; 2013; Volume 171.
- Quang Tran, H.; Van Doan, H.; Stejskal, V. Environmental consequences of using insect meal as an ingredient in aquafeeds: A systematic view. Reviews in Aquaculture 2022, 14, 237-251. [CrossRef]
- Gasco, L.; Finke, M.; Huis, A. Can diets containing insects promote animal health? Journal of Insects as Food and Feed 2018, 4, 1-4. [CrossRef]
- Finke, M.D. Complete nutrient composition of commercially raised invertebrates used as food for insectivores. Zoo Biology 2002, 21, 269-285. [CrossRef]
- Hua, K. A meta-analysis of the effects of replacing fish meals with insect meals on growth performance of fish. Aquaculture 2021, 530, 735732. [CrossRef]
- Marco, M.; Martinez, S.; Hernandez, F.; Madrid, J.; Gai, F.; Rotolo, L.; Belforti, M.; Bergero, D.; Katz, H.; Dabbou, S.; et al. Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: Apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Animal Feed Science and Technology 2015, 209. [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Potential and challenges of insects as an innovative source for food and feed production. Innovative Food Science & Emerging Technologies 2013, 17, 1-11. [CrossRef]
- Koutsos, L.; McComb, A.; Finke, M. Insect Composition and Uses in Animal Feeding Applications: A Brief Review. Annals of the Entomological Society of America 2019, 112, 544-551. [CrossRef]
- Gasco, L.; Henry, M.; Piccolo, G.; Marono, S.; Gai, F.; Renna, M.; Lussiana, C.; Antonopoulou, E.; Mola, P.; Chatzifotis, S. Tenebrio molitor meal in diets for European sea bass (Dicentrarchus labrax L.) juveniles: Growth performance, whole body composition and in vivo apparent digestibility. Animal Feed Science and Technology 2016, 220, 34-45. [CrossRef]
- Alfiko, Y.; Xie, D.; Astuti, R.T.; Wong, J.; Wang, L. Insects as a feed ingredient for fish culture: Status and trends. Aquaculture and Fisheries 2022, 7, 166-178. [CrossRef]
- Fabrikov, D.; Barroso, F.G.; Sánchez-Muros, M.J.; Hidalgo, M.C.; Cardenete, G.; Tomás-Almenar, C.; Melenchón, F.; Guil-Guerrero, J.L. Effect of feeding with insect meal diet on the fatty acid compositions of sea bream (Sparus aurata), tench (Tinca tinca) and rainbow trout (Oncorhynchus mykiss) fillets. Aquaculture 2021, 545, 737170. [CrossRef]
- Iaconisi, V.; Marono, S.; Parisi, G.; Gasco, L.; Genovese, L.; Maricchiolo, G.; Bovera, F.; Piccolo, G. Dietary inclusion of Tenebrio molitor larvae meal: Effects on growth performance and final quality treats of blackspot sea bream (Pagellus bogaraveo). Aquaculture 2017, 476, 49-58. [CrossRef]
- Jeong, S.-M.; Khosravi, S.; Yoon, K.-Y.; Kim, K.-W.; Lee, B.-J.; Hur, S.-W.; Lee, S.-M. Mealworm, Tenebrio molitor, as a feed ingredient for juvenile olive flounder, Paralichthys olivaceus. Aquaculture Reports 2021, 20, 100747. [CrossRef]
- Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Józefiak, D. Black Soldier Fly Full-Fat Larvae Meal as an Alternative to Fish Meal and Fish Oil in Siberian Sturgeon Nutrition: The Effects on Physical Properties of the Feed, Animal Growth Performance, and Feed Acceptance and Utilization. Animals 2020, 10. [CrossRef]
- Mastoraki, M.; Mollá Ferrándiz, P.; Vardali, S.C.; Kontodimas, D.C.; Kotzamanis, Y.P.; Gasco, L.; Chatzifotis, S.; Antonopoulou, E. A comparative study on the effect of fish meal substitution with three different insect meals on growth, body composition and metabolism of European sea bass (Dicentrarchus labrax L.). Aquaculture 2020, 528, 735511. [CrossRef]
- Piccolo, G.; Iaconisi, V.; Marono, S.; Gasco, L.; Loponte, R.; Nizza, S.; Bovera, F.; Parisi, G. Effect of Tenebrio molitor larvae meal on growth performance, in vivo nutrients digestibility, somatic and marketable indexes of gilthead sea bream (Sparus aurata). Animal Feed Science and Technology 2017, 226, 12-20. [CrossRef]
- Basto, A.; Valente, L.M.P.; Conde-Sieira, M.; Soengas, J.L. Central regulation of food intake is not affected by inclusion of defatted Tenebrio molitor larvae meal in diets for European sea bass (Dicentrarchus labrax). Aquaculture 2021, 544, 737088. [CrossRef]
- Iaconisi, V.; Bonelli, A.; Pupino, R.; Gai, F.; Parisi, G. Mealworm as dietary protein source for rainbow trout: Body and fillet quality traits. Aquaculture 2018, 484, 197-204. [CrossRef]
- Villalta, M.; Estévez, A.; Bransden, M.P.; Bell, J.G. The effect of graded concentrations of dietary DHA on growth, survival and tissue fatty acid profile of Senegal sole (Solea senegalensis) larvae during the Artemia feeding period. Aquaculture 2005, 249, 353-365. [CrossRef]
- Borges, P.; Oliveira, B.; Casal, S.; Dias, J.; Conceição, L.; Valente, L.M.P. Dietary lipid level affects growth performance and nutrient utilisation of Senegalese sole (Solea senegalensis) juveniles. British Journal of Nutrition 2009, 102, 1007-1014. [CrossRef]
- Carballo, C.; Berbel, C.; Guerrero-Cózar, I.; Jiménez-Fernández, E.; Cousin, X.; Bégout, M.L.; Manchado, M. Evaluation of different tags on survival, growth and stress response in the flatfish Senegalese sole. Aquaculture 2018, 494, 10-18. [CrossRef]
- AOAC, C. Official methods of analysis of the Association of Analytical Chemists International. Official Methods: Gaithersburg, MD, USA 2005.
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry 1957, 226, 497-509. [CrossRef]
- Olsen, R.E.; Henderson, R.J. The rapid analysis of neutral and polar marine lipids using double-development HPTLC and scanning densitometry. Journal of Experimental Marine Biology and Ecology 1989, 129, 189-197. [CrossRef]
- Christie, W.W. Lipid Analysis: Isolation, Separation, Identification, and Structural Analysis of Lipids; Amer Oil Chemists Society: 2003.
- Hachero-Cruzado, I.; Rodriguez-Rua, A.; Roman-Padilla, J.; Ponce, M.; Fernandez-Diaz, C.; Manchado, M. Characterization of the genomic responses in early Senegalese sole larvae fed diets with different dietary triacylglycerol and total lipids levels. Comp. Biochem. Physiol. Part D Genomics Proteomics 2014, 12, 61-73. [CrossRef]
- Rodiles, A.; Herrera, M.; Hachero-Cruzado, I.; Ruiz-Jarabo, I.; Mancera, J.M.; Cordero, M.L.; Lall, S.P.; Alarcón, F. Tissue composition, blood biochemistry and histology of digestive organs in Senegalese sole (Solea senegalensis) juveniles fed diets containing different plant protein ingredients. Aquaculture Nutrition 2014, 21. [CrossRef]
- Sokal, R.; Rohlf, F. Biometry : the principles and practice of statistics in biological research / Robert R. Sokal and F. James Rohlf; 2013.
- Underwood, A.J. Experiments in ecology: their logical design and interpretation using analysis of variance; Cambridge university press: 1997.
- Gasco, L.; Biasato, I.; Enes, P.; Gai, F. Potential and challenges for the use of insects as feed for aquaculture. 2023; pp. 465-492.
- Fabrikov, D.; Sánchez-Muros, M.J.; Barroso, F.G.; Tomás-Almenar, C.; Melenchón, F.; Hidalgo, M.C.; Morales, A.E.; Rodriguez-Rodriguez, M.; Montes-Lopez, J. Comparative study of growth performance and amino acid catabolism in Oncorhynchus mykiss, Tinca tinca and Sparus aurata and the catabolic changes in response to insect meal inclusion in the diet. Aquaculture 2020, 529, 735731. [CrossRef]
- Bruni, L.; Pastorelli, R.; Viti, C.; Gasco, L.; Parisi, G. Characterisation of the intestinal microbial communities of rainbow trout (Oncorhynchus mykiss) fed with Hermetia illucens (black soldier fly) partially defatted larva meal as partial dietary protein source. Aquaculture 2018, 487, 56-63. [CrossRef]
- Terova, G.; Rimoldi, S.; Ascione, C.; Gini, E.; Ceccotti, C.; Gasco, L. Rainbow trout (Oncorhynchus mykiss) gut microbiota is modulated by insect meal from Hermetia illucens prepupae in the diet. Reviews in Fish Biology and Fisheries 2019, 29, 465-486. [CrossRef]
- Henry, M.A.; Gai, F.; Enes, P.; Peréz-Jiménez, A.; Gasco, L. Effect of partial dietary replacement of fishmeal by yellow mealworm (Tenebrio molitor) larvae meal on the innate immune response and intestinal antioxidant enzymes of rainbow trout (Oncorhynchus mykiss). Fish & Shellfish Immunology 2018, 83, 308-313. [CrossRef]
- Shiau, S.-Y.; Yu, Y.-P. Dietary supplementation of chitin and chitosan depresses growth in tilapia, Oreochromis niloticus×O. aureus. Aquaculture 1999, 179, 439-446. [CrossRef]
- Belforti, M.; Gai, F.; Lussiana, C.; Renna, M.; Malfatto, V.; Rotolo, L.; De Marco, M.; Dabbou, S.; Schiavone, A.; Zoccarato, I.; et al. Tenebrio Molitor Meal in Rainbow Trout (Oncorhynchus Mykiss) Diets: Effects on Animal Performance, Nutrient Digestibility and Chemical Composition of Fillets. Italian Journal of Animal Science 2015, 14, 4170. [CrossRef]
- Sankian, Z.; Khosravi, S.; Kim, Y.-O.; Lee, S.-M. Effects of dietary inclusion of yellow mealworm (Tenebrio molitor) meal on growth performance, feed utilization, body composition, plasma biochemical indices, selected immune parameters and antioxidant enzyme activities of mandarin fish (Siniperca scherzeri) juveniles. Aquaculture 2018, 496, 79-87. [CrossRef]
- Jeong, S.-M.; Khosravi, S.; Mauliasari, I.R.; Lee, S.-M. Dietary inclusion of mealworm (Tenebrio molitor) meal as an alternative protein source in practical diets for rainbow trout (Oncorhynchus mykiss) fry. Fisheries and Aquatic Sciences 2020, 23, 12. [CrossRef]
- Zacour, A.C.; Silva Me Fau - Cecon, P.R.; Cecon Pr Fau - Bambirra, E.A.; Bambirra Ea Fau - Vieira, E.C.; Vieira, E.C. Effect of dietary chitin on cholesterol absorption and metabolism in rats. 1992.
- Hirano, S. Chitin Biotechnology Applications. In Biotechnology Annual Review, El-Gewely, M.R., Ed.; Elsevier: 1996; Volume 2, pp. 237-258.
- Zacour, A.C.; Silva, M.E.; Cecon, P.R.; Bambirra, E.A.; Vieira, E.C. Effect of Dietary Chitin on Cholesterol Absorption and Metabolism in Rats. Journal of Nutritional Science and Vitaminology 1992, 38, 609-613. [CrossRef]
- Kılınç, A.; Teke, M.; Önal, S.; Telefoncu, A. Immobilization of Pancreatic Lipase on Chitin and Chitosan. Preparative Biochemistry & Biotechnology 2006, 36, 153-163. [CrossRef]
- Hansen, J.Ø.; Penn, M.; Øverland, M.; Shearer, K.D.; Krogdahl, Å.; Mydland, L.T.; Storebakken, T. High inclusion of partially deshelled and whole krill meals in diets for Atlantic salmon (Salmo salar). Aquaculture 2010, 310, 164-172. [CrossRef]
- Campos, C.; Valente, L.M.P.; Borges, P.; Bizuayehu, T.; Fernandes, J.M.O. Dietary lipid levels have a remarkable impact on the expression of growth-related genes in Senegalese sole (Solea senegalensis Kaup). Journal of Experimental Biology 2010, 213, 200-209. [CrossRef]
- Basto, A.; Calduch-Giner, J.; Oliveira, B.; Petit, L.; Sá, T.; Maia, M.R.G.; Fonseca, S.C.; Matos, E.; Pérez-Sánchez, J.; Valente, L.M.P. The Use of Defatted Tenebrio molitor Larvae Meal as a Main Protein Source Is Supported in European Sea Bass (Dicentrarchus labrax) by Data on Growth Performance, Lipid Metabolism, and Flesh Quality. Frontiers in Physiology 2021, 12.
- Brodtkorb, T.; Rosenlund, G.; Lie, Ø. Effects of dietary levels of 20:5n-3 and 22:6n-3 on tissue lipid composition in juvenile Atlantic salmon, Salmo salar, with emphasis on brain and eye. Aquaculture Nutrition 1997, 3, 175-187. [CrossRef]
- Morais, S.; Castanheira, F.; Martinez-Rubio, L.; Conceição, L.E.C.; Tocher, D.R. Long chain polyunsaturated fatty acid synthesis in a marine vertebrate: Ontogenetic and nutritional regulation of a fatty acyl desaturase with Δ4 activity. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2012, 1821, 660-671. [CrossRef]
- Borges, P.; Reis, B.; Fernandes, T.J.R.; Palmas, Â.; Castro-Cunha, M.; Médale, F.; Oliveira, M.B.P.P.; Valente, L.M.P. Senegalese sole juveniles can cope with diets devoid of supplemental fish oil while preserving flesh nutritional value. Aquaculture 2014, 418-419, 116-125. [CrossRef]


| TM | Diets | |||||||
| CTRL | FM5 | FM10 | PP10 | PP15 | ||||
| Ingredients (% dw) | ||||||||
| Fish meal LT94 1 | - | 30.00 | 26.50 | 23.20 | 30.00 | 30.00 | ||
| Squid meal 2 | - | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | ||
| CPSP90 3 | - | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | ||
| Krill meal 4 | - | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| Wheat gluten 5 | - | 8.70 | 8.70 | 8.70 | 6.80 | 5.90 | ||
| Soybean protein concentrate 6 | - | 12.00 | 12.00 | 12.00 | 9.90 | 8.90 | ||
| Pea protein concentrate 7 | - | 9.00 | 9.00 | 9.00 | 7.10 | 6.20 | ||
| Wheat flour 8 | - | 12.10 | 12.00 | 11.60 | 11.40 | 10.20 | ||
| Tenebrio molitor meal 9 | - | 0.00 | 5.00 | 10.00 | 10.00 | 15.00 | ||
| Fish oil 10 | - | 5.40 | 5.40 | 5.40 | 5.00 | 4.00 | ||
| Soybean oil 11 | - | 3.00 | 1.60 | 0.30 | 0.00 | 0.00 | ||
| Soy lecithin 12 | - | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| Methionine 13 | - | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | ||
| Lysine 14 | - | 1.20 | 1.20 | 1.20 | 1.20 | 1.20 | ||
| Betaine 15 | - | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| Choline chloride 16 | - | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | ||
| Digestive system improver 17 | - | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | ||
| Vitamins and minerals premix 18 | - | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | ||
| Vitamin C 19 | - | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | ||
| Guar gum 20 | - | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | ||
| Proximate composition (% dw) | ||||||||
| Moisture | 7.78 | 6.49 | 6.16 | 6.18 | 7.29 | 6.50 | ||
| Ash | 2.92 | 8.07 | 7.68 | 7.37 | 8.03 | 8.17 | ||
| Protein | 43.77 | 54.03 | 53.81 | 53.68 | 52.95 | 52.73 | ||
| Lipid | 35.05 | 15.59 | 15.46 | 16.51 | 16.16 | 17.11 | ||
| Diets | ||||||||
| TM | CTRL | FM5 | FM10 | PP10 | PP15 | |||
| Lipid classes (%TL) | ||||||||
| Lyso-phosphatidylcholine | 0.29 | 0.84 | 1.12 | 1.08 | 1.25 | 1.20 | ||
| Sphingomyelin | 0.41 | 0.43 | 0.26 | 0.50 | 0.39 | 0.65 | ||
| Phosphatidylcholine | 1.94 | 4.97 | 5.71 | 5.26 | 5.44 | 5.13 | ||
| Phosphatidylserine | 0.36 | 1.52 | 2.57 | 2.51 | 2.07 | 1.91 | ||
| Phosphatidylinositol | 0.38 | 1.93 | 1.35 | 1.62 | 1.49 | 1.83 | ||
| Phosphatidylethanolamine | 2.54 | 2.11 | 2.90 | 2.51 | 2.19 | 2.57 | ||
| Diacylglycerol | 3.01 | 3.19 | 3.23 | 3.85 | 3.48 | 3.11 | ||
| Sterols | 7.36 | 9.65 | 10.94 | 10.20 | 10.37 | 9.70 | ||
| Free fatty acids | 34.58 | 13.53 | 17.29 | 19.16 | 19.12 | 20.09 | ||
| Triacylglycerol | 43.45 | 47.08 | 42.77 | 41.90 | 43.24 | 42.59 | ||
| Sterol esters | 4.10 | 6.22 | 6.19 | 5.19 | 5.65 | 5.61 | ||
| Fatty acids (% TFA) | ||||||||
| 14:0 | 4.18 | 1.69 | 1.92 | 2.16 | 2.31 | 2.63 | ||
| 16:0 | 17.98 | 17.91 | 18.40 | 19.13 | 19.33 | 19.48 | ||
| 18:0 | 2.55 | 5.19 | 4.94 | 4.81 | 4.73 | 4.45 | ||
| Total Saturated FA | 24.72 | 26.19 | 26.45 | 27.25 | 27.44 | 27.56 | ||
| 16:1n-7 | 2.82 | 3.41 | 3.56 | 3.74 | 3.93 | 3.89 | ||
| 18:1n-9 | 51.75 | 17.96 | 22.46 | 26.90 | 26.64 | 30.44 | ||
| 18:1n-7 | 0.10 | 2.37 | 2.08 | 1.87 | 1.90 | 1.66 | ||
| 20:1n-9 | 0.10 | 1.52 | 1.39 | 1.29 | 1.37 | 1.18 | ||
| 22:1n-11 | nd | 0.96 | 0.89 | 0.79 | 0.93 | 0.80 | ||
| Total Monounsaturated FA | 55.86 | 27.39 | 31.75 | 36.02 | 36.33 | 39.48 | ||
| 18:2n-6 | 18.00 | 19.54 | 16.65 | 13.89 | 12.13 | 12.89 | ||
| 20:4n-6 | 0.00 | 1.10 | 1.06 | 1.01 | 1.00 | 0.83 | ||
| 22:5n-6 | nd | 0.79 | 0.76 | 0.73 | 0.74 | 0.59 | ||
| Total n-6 Polyunsaturated FA | 18.00 | 22.05 | 19.13 | 16.25 | 14.41 | 14.82 | ||
| 18:3n-3 | 0.29 | 2.42 | 1.85 | 1.30 | 1.13 | 0.99 | ||
| 18:4n-3 | nd | 0.77 | 0.71 | 0.63 | 0.71 | 0.61 | ||
| 20:5n-3 | nd | 5.73 | 5.38 | 4.81 | 5.24 | 4.50 | ||
| 22:5n-3 | nd | 0.91 | 0.99 | 0.94 | 0.98 | 0.82 | ||
| 22:6n-3 | nd | 13.05 | 12.37 | 11.62 | 12.37 | 10.01 | ||
| Total n-3 Polyunsaturated FA | 0.29 | 23.61 | 22.00 | 19.94 | 21.12 | 17.49 | ||
| CTRL | FM5 | FM10 | PP10 | PP15 | p-value | |
| Initial body weight (IBW, g) | 219.2 ± 5.3 | 213.7 ± 4.7 | 215.1 ± 5.2 | 214.2 ± 5.3 | 210.8 ± 4.6 | 0.979 |
| Final body weight (FBW, g) | 288.6 ± 6.5 | 284.5 ± 5.8 | 288.6 ± 6.1 | 283.8 ± 6.2 | 288.3 ± 5.5 | 0.887 |
| Specific Growth Rate (SGR, % d-1) | 0.30 ± 0.01b | 0.32 ± 0.01ab | 0.33 ± 0.01ab | 0.31 ± 0.01b | 0.35 ± 0.01a | 0.012 |
| Feed Conversion Ratio (FCR) | 1.76 ± 0.20 | 1.58 ± 0.14 | 1.61 ± 0.02 | 1.71 ± 0.17 | 1.42 ± 0.23 | 0.426 |
| Protein efficiency ratio (PER) | 1.00 ± 0.08 | 1.11 ± 0.08 | 1.09 ± 0.08 | 1.04 ± 0.08 | 1.26 ± 0.08 | 0.299 |
| CTRL | FM5 | FM10 | PP10 | PP15 | P(diet) | |||||
| 14:0 | 3.14±0.21 | 3.46±0.19 | 3.42±0.07 | 3.40±0.16 | 3.69±0.09 | 0.122 | ||||
| 15:0 | 0.45±0.018 | 0.38±0.026 | 0.41±0.03 | 0.46±0.03 | 0.40±0.01 | 0.092 | ||||
| 16:0 | 17.29±0.29 | 17.44±0.32 | 16.88±0.31 | 16.69±0.30 | 16.89±0.15 | 0.351 | ||||
| 18:0 | 3.93±0.38 | 3.92±0.27 | 3.72±0.37 | 3.39±0.24 | 3.25±0.19 | 0.347 | ||||
| 20:0 | 0.20±0.02 | 0.19±0.01 | 0.19±0.01 | 0.19±0.01 | 0.19±0.00 | 0.974 | ||||
| 22:0 | 0.18±0.012 | 0.16±0.01 | 0.14±0.01 | 0.14±0.01 | 0.16±0.01 | 0.095 | ||||
| 24:0 | 0.10±0.02 | 0.15±0.039 | 0.11±0.02 | 0.13±0.02 | 0.11±0.03 | 0.640 | ||||
| Total Saturated FA | 25.29±0.75 | 25.70±0.46 | 24.89±0.56 | 24.41±0.55 | 24.69±0.32 | 0.396 | ||||
| 16:1n-9 | 0.78±0.03b | 0.90±0.05ab | 1.02±0.07a | 1.09±0.05a | 1.05±0.03a | 0.002 | ||||
| 16:1n-7 | 4.94±0.18 | 5.54±0.33 | 5.23±0.12 | 5.33±0.17 | 5.48±0.11 | 0.306 | ||||
| 18:1n-9 | 28.09±0.37c | 31.44±1.11b | 32.28±0.54ab | 31.18±0.80b | 34.24±0.59a | 0.000 | ||||
| 18:1n-7 | 3.34±0.06 | 3.19±0.09 | 3.11±0.05 | 3.19±0.08 | 3.01±0.10 | 0.103 | ||||
| 20:1n-11 | 0.15±0.01 | 0.13±0.01 | 0.15±0.00 | 0.15±0.00 | 0.15±0.01 | 0.069 | ||||
| 20:1n-9 | 1.38±0.06 | 1.44±0.05 | 1.44±0.05 | 1.435±0.07 | 1.68±0.19 | 0.172 | ||||
| 20:1n-7 | 0.20±0.01 | 0.20±0.00 | 0.19±0.01 | 0.19±0.01 | 0.18±0.01 | 0.566 | ||||
| 22:1n-11 | 0.51±0.042 | 0.46±0.03 | 0.49±0.03 | 0.54±0.02 | 0.54±0.04 | 0.391 | ||||
| 22:1n-9cis | 0.27±0.015 | 0.27±0.02 | 0.27±0.01 | 0.28±0.01 | 0.27±0.02 | 0.986 | ||||
| 24:1n-9 | 0.31±0.061 | 0.33±0.03 | 0.34±0.03 | 0.35±0.02 | 0.26±0.06 | 0.658 | ||||
| Total Monounsaturated FA | 40.00±0.56c | 43.92±1.37ab | 44.52±0.48ab | 43.74±0.87b | 46.85±0.47a | 0.000 | ||||
| 18:2n-6 | 15.47±0.57a | 13.16±0.76b | 12.57±0.73b | 12.26±0.66b | 12.12±0.13b | 0.001 | ||||
| 18:3n-6 | 0.10±0.01 | 0.10±0.01 | 0.08±0.00 | 0.10±0.01 | 0.09±0.00 | 0.252 | ||||
| 20:2n-6 | 1.18±0.065ab | 1.21±0.06a | 1.03±0.03ab | 0.94±0.04b | 0.94±0.02b | 0.000 | ||||
| 20:3n-6 | 0.16±0.007 | 0.15±0.01 | 0.16±0.01 | 0.16±0.01 | 0.14±0.01 | 0.480 | ||||
| 20:4n-6 | 0.65±0.07 | 0.66±0.09 | 0.62±0.06 | 0.76±0.10 | 0.61±0.05 | 0.698 | ||||
| 22:4n-6 | 0.27±0.014 | 0.26±0.03 | 0.25±0.01 | 0.26±0.02 | 0.21±0.01 | 0.269 | ||||
| 22:5n-6 | 0.74±0.03 | 0.70±0.07 | 0.71±0.02 | 0.74±0.05 | 0.61±0.02 | 0.226 | ||||
| Total n-6 Polyunsaturated FA | 18.57±0.57a | 16.24±1.00ab | 15.42±0.81b | 15.22±0.72b | 14.71±0.14b | 0.001 | ||||
| 18:3n-3 | 1.39±0.06a | 1.09±0.05b | 1.03±0.07b | 1.01±0.08b | 0.91±0.05b | 0.000 | ||||
| 18:4n-3 | 0.24±0.02ab | 0.18±0.01b | 0.22±0.02ab | 0.25±0.02a | 0.21±0.01ab | 0.035 | ||||
| 20:3n-3 | 0.56±0.021a | 0.55±0.02a | 0.45±0.01b | 0.41±0.02b | 0.39±0.02b | 0.000 | ||||
| 20:4n-3 | 0.35±0.02ab | 0.29±0.01b | 0.33±0.02ab | 0.36±0.03a | 0.31±0.01ab | 0.034 | ||||
| 20:5n-3 | 0.36±0.05ab | 0.25±0.03b | 0.30±0.03ab | 0.40±0.06a | 0.31±0.02ab | 0.024 | ||||
| 22:5n-3 | 2.34±0.31 | 1.83±0.15 | 2.24±0.06 | 2.34±0.32 | 1.96±0.12 | 0.260 | ||||
| 22:6n-3 | 10.13±0.41 | 9.13±1.00 | 9.73±0.29 | 11.05±0.58 | 8.91±0.33 | 0.140 | ||||
| Total n-3 Polyunsaturated FA | 15.41±0.80 | 13.33±1.16 | 14.32±0.28 | 15.83±0.89 | 13.00±0.48 | 0.061 | ||||
| n-3PUFA/n-6PUFA | 0.83±0.04 | 0.82±0.06 | 0.94±0.05 | 1.05±0.07 | 0.88±0.03 | 0.048 |
| CTRL | FM5 | FM10 | PP10 | PP15 | P(diet) | |||||
| 14:0 | 2.30±0.16 | 2.36±0.16 | 1.86±0.16 | 1.99±0.16 | 2.16±0.16 | 0.190 | ||||
| 15:0 | 0.48±0.0a | 0.47±0.02ab | 0.40±0.02b | 0.40±0.02ab | 0.40±0.02b | 0.009 | ||||
| 16:0 | 17.79±0.19 | 18.11±0.19 | 17.97±0.19 | 18.08±0.19 | 18.43±0.19 | 0.223 | ||||
| 18:0 | 3.54±0.12b | 3.63±0.12ab | 4.10±0.12a | 3.82±0.12ab | 3.90±0.12ab | 0.035 | ||||
| 20:0 | 0.25±0.01 | 0.25±0.01 | 0.25±0.01 | 0.26±0.01 | 0.26±0.01 | 0.457 | ||||
| 22:0 | 0.19±0.01b | 0.19±0.01ab | 0.18±0.01ab | 0.17±0.01b | 0.18±0.01ab | 0.035 | ||||
| 24:0 | 0.08±0.02 | 0.10±0.02 | 0.12±0.02 | 0.10±0.02 | 0.07±0.02 | 0.657 | ||||
| Total Saturated FA | 24.65±0.33 | 25.11±0.33 | 24.88±0.33 | 24.83±0.33 | 25.39±0.33 | 0.568 | ||||
| 16:1n-9 | 0.47±0.01b | 0.54±0.01a | 0.55±0.01a | 0.56±0.01a | 0.58±0.01a | 0.000 | ||||
| 16:1n-7 | 4.79±0.15 | 4.76±0.15 | 4.18±0.15 | 4.40±0.15 | 4.35±0.15 | 0.031 | ||||
| 18:1n-9 | 25.33±0.36c | 26.88±0.36b | 27.29±0.36ab | 27.31±0.36ab | 28.62±0.36a | 0.000 | ||||
| 18:1n-7 | 2.80±0.05a | 2.70±0.05ab | 2.56±0.05bc | 2.58±0.05bc | 2.43±0.05c | 0.000 | ||||
| 20:1n-11 | 0.21±0.00a | 0.20±0.00ab | 0.19±0.00ab | 0.201±0.005ab | 0.19±0.00b | 0.023 | ||||
| 20:1n-9 | 1.51±0.03 | 1.45±0.03 | 1.40±0.03 | 1.43±0.03 | 1.36±0.03 | 0.084 | ||||
| 20:1n-7 | 0.16±0.01 | 0.16±0.01 | 0.15±0.01 | 0.15±0.01 | 0.15±0.01 | 0.860 | ||||
| 22:1n-11 | 0.76±0.02a | 0.71±0.02ab | 0.62±0.02b | 0.69±0.02ab | 0.64±0.022b | 0.002 | ||||
| 22:1n-9cis | 0.29±0.01 | 0.29±0.01 | 0.26±0.01 | 0.28±0.01 | 0.27±0.01 | 0.157 | ||||
| 24:1n-9 | 0.40±0.05 | 0.22±0.05 | 0.41±0.05 | 0.43±0.05 | 0.41±0.05 | 0.046 | ||||
| Total Monounsaturated FA | 36.72±0.55 | 37.89±0.55 | 37.61±0.55 | 38.05±0.55 | 39.01±0.55 | 0.100 | ||||
| 18:2n-6 | 13.50±0.26a | 12.86±0.26a | 11.69±0.26b | 11.15±0.26b | 11.25±0.26b | 0.000 | ||||
| 18:3n-6 | 0.14±0.01 | 0.14±0.01 | 0.14±0.01 | 0.13±0.01 | 0.12±0.01 | 0.117 | ||||
| 20:2n-6 | 0.66±0.01abc | 0.68±0.01a | 0.67±0.01ab | 0.62±0.01bc | 0.62±0.01c | 0.001 | ||||
| 20:3n-6 | 0.17±0.01 | 0.16±0.01 | 0.17±0.01 | 0.18±0.01 | 0.16±0.01 | 0.341 | ||||
| 20:4n-6 | 0.82±0.04b | 0.83±0.04b | 1.01±0.04a | 0.98±0.04ab | 0.96±0.04ab | 0.008 | ||||
| 22:4n-6 | 0.28±0.01ab | 0.29±0.01b | 0.30±0.01a | 0.30±0.01ab | 0.27±0.01b | 0.009 | ||||
| 22:5n-6 | 0.61±0.02 | 0.63±0.02 | 0.70±0.02 | 0.69±0.02 | 0.66±0.02 | 0.061 | ||||
| Total n-6 Polyunsaturated FA | 16.19±0.25a | 15.60±0.25b | 14.68±0.25c | 14.05±0.25c | 14.03±0.25c | 0.000 | ||||
| 18:3n-3 | 1.85±0.05a | 1.60±0.05b | 1.35±0.05c | 1.32±0.05c | 1.20±0.05c | 0.000 | ||||
| 18:4n-3 | 0.64±0.03a | 0.57±0.03ab | 0.49±0.03b | 0.52±0.03b | 0.46±0.03b | 0.001 | ||||
| 20:3n-3 | 0.36±0.01a | 0.35±0.01a | 0.32±0.01ab | 0.29±0.01b | 0.28±0.01b | 0.000 | ||||
| 20:4n-3 | 0.44±0.01a | 0.41±0.01ab | 0.39±0.01b | 0.39±0.01b | 0.37±0.01b | 0.001 | ||||
| 20:5n-3 | 1.69±0.05a | 1.43±0.05ab | 1.46±0.05b | 1.57±0.05b | 1.40±0.05b | 0.001 | ||||
| 22:5n-3 | 3.76±0.10a | 3.50±0.10ab | 3.54±0.10ab | 3.54±0.10ab | 3.28±0.10b | 0.043 | ||||
| 22:6n-3 | 13.04±0.45ab | 12.93±0.45b | 14.77±0.45ab | 14.92±0.45a | 14.05±0.45ab | 0.012 | ||||
| Total n-3 Polyunsaturated FA | 21.79±0.44 | 20.80±0.44 | 22.32±0.44 | 22.56±0.44 | 21.07±0.44 | 0.045 | ||||
| n-3PUFA/n-6PUFA | 1.35±0.04bc | 1.33±0.040c | 1.52±0.04a | 1.61±0.04a | 1.50±0.04ab | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





