Introduction
In advanced Parkinson’s disease (PD), and especially in PD related dementia (PDD), psychotic symptoms are common. Besides being frightening and stressful for the patient and the caregiver, these symptoms also are an independent predictor of mortality [
1]. Clozapine, an atypical antipsychotic drug, can be used to treat psychosis in PD, but this needs specialized monitoring due to the risk of adverse events [
2].
A possible manifestation of psychosis in PD is delusional misidentification syndrome, of which Capgras syndrome (CS) is best known. In CS, the patient believes a person has been replaced by an imposter or duplicate [
3]. The subject of the delusion is usually a close relative or spouse. CS is most often associated with neurodegenerative disease [
4], but can also be found in schizophrenia or psychotic depression. Treatment of this syndrome is challenging [
3].
In some cases, the misidentification is not about a person but an inanimate object or a place. The delusional believe that a place has been duplicated is sometimes also referred to as reduplicative paramnesia [
5]. Here, we present a case of misidentification of a patient’s home in PDD where treatment with rivastigmine rapidly resolved the delusional syndrome. Ethical approval was not obtained for this case report, as all observations were in a clinical setting. Written informed consent to publication was obtained from the patient and her husband.
Case Report
Our patient, a 75-year-old female, was diagnosed with idiopathic Parkinson’s disease at the age of 68. Initially, she presented with bradykinesia, rigidity, gait disorder and a mild tremor in her right hand. No cognitive deficits were found at the time (Mini-Mental State Examination (MMSE) 30/30). She had no history of neurologic or psychiatric disease. She was treated with levodopa/carbidopa 125 mg 3 times daily with good response.
Over the years, the dosage of levodopa/carbidopa was gradually increased to 250 mg 4 times daily and 125 mg levodopa-benserazide extended-release before bedtime. At the age of 73, she started to fall more frequently, leading to fractures in both her wrists. Also, she mentions that her memory is deteriorating.
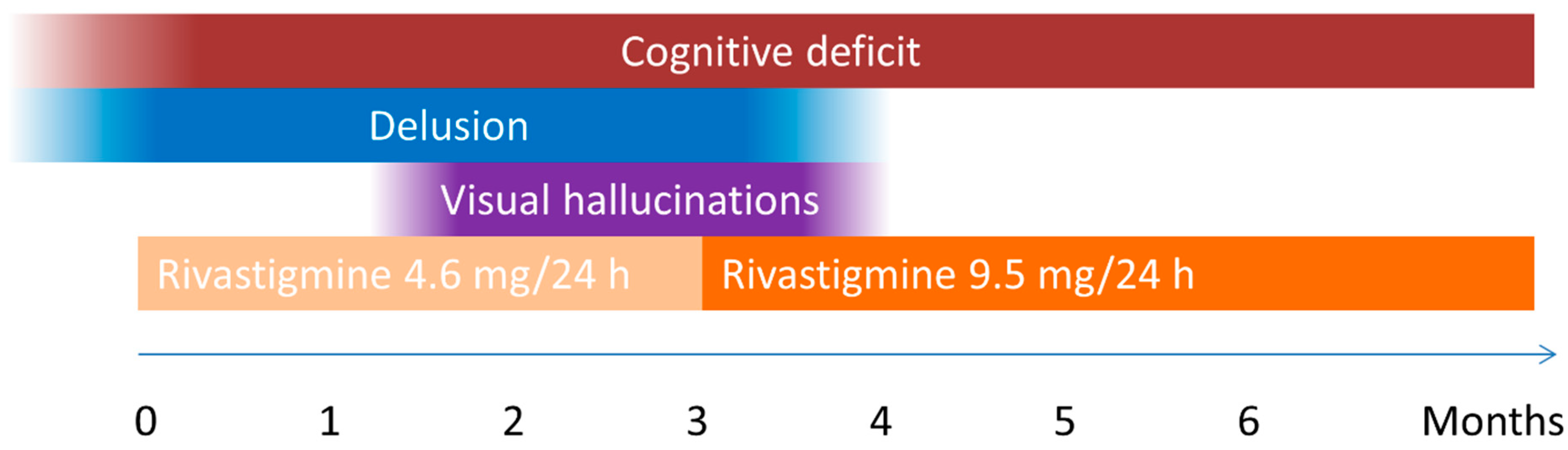
Around the age of 75, she had difficulties remembering which day or year it was and how long she and her husband had been together. Her Montreal Cognitive Assessment (MoCA)-score was 14/30. At this point she developed the conviction that she lived in a duplicate of her real home. She reported that she was in somebody else’s house, although she recognized all her own furniture and belongings. She had no delusions about people, objects or other places. No hallucinations were reported at the time. As the delusion usually occurred around the beginning of the evening, the usual levodopa intake at 4 pm and 8 pm was lowered with 62.5 mg. Also, treatment with a rivastigmine patch 4.6 mg/24 h was started for the her cognitive decline.
After three months, the delusion was still present and she also reported seeing people in her house. At this point, the rivastigmine dose was increased to 9.5 mg/24 h (see
Figure 1 for a timeline of her symptoms). One month after start of the higher dose rivastigmine patches, both the delusion and the visual hallucinations had completely resolved. Her cognition did not improve from this treatment. Her MoCA-score was still 14/30 after three months of treatment with rivastigmine 9.5 mg/24 h. At this time, she was still free of any psychotic symptoms.
Discussion
We here show that misidentification syndrome and visual hallucinations in a patient with PDD were completely resolved under treatment with rivastigmine (given in patches of 9.5 mg/24 h). This case is important for two reasons. In the first place, treatment of misidentification syndromes is challenging, especially in PD patients who are vulnerable to side effects of antipsychotic medication and may lack mental capacities for cognitive behavioral therapy. Secondly, the fact that an increase in available acetylcholine induced such a clear improvement, indicates that the dysfunctional connections were likely to be cholinergic. This knowledge may help to better understand pathophysiology of this type of delusions.
We cannot rule out natural fluctuation of the disease being a factor of importance in our case. Also, the levodopa dose reduction may have played a role. However, as the delusion only resolved after increase in dosage of rivastigmine and not after levodopa dose reduction, we assume that rivastigmine played an important role.
Pandis et al. showed that 40% of the cases with CS with organic etiology show no response to treatment [
6]. In the review of CS in PD patients by Cannes et al., 8/15 cases reported (partial) improvement of the delusion after treatment [
3]. In 7 of these cases treatment consisted of either quetiapine or clozapine, in 4 cases combined with reduction of levodopa dosage. Only in 1 of all 15 case the delusion was treated with a cholinesterase inhibitor (rivastigmine, dose not mentioned), combined with quetiapine [
7]. This partially improved the delusion in that patient. However, in 1 case CS occurred while the patient was already treated with high-dose rivastigmine [
8]. In this case, reduction of levodopa in combination with quetiapine improved the symptoms. We could find only one case in literature where treatment with rivastigmine mildly improved reduplicative paramnesia in PDD [
9]. What is remarkable in our case is that the patient was not treated with antipsychotics but with a cholinesterase inhibitor and the delusion resolved completely.
Our patient also suffered from visual hallucinations (VHs). VHs are a common psychotic symptom in Parkinson’s disease [
10]. Indeed, in the retrospective study of Josephs, all patients with CS and PDD concurrently had VHs, as was the case in our patient [
4]. This might suggest that in these patients, there is a similar underlying pathology for misidentification syndrome and VHs.
PET studies have shown that PDD patients have a widespread reduction of acetylcholine activity in the cerebral cortex, especially in the posterior regions [
11,
12]. Cholinergic loss in the occipital region may contribute to VHs [
13]. As these areas are also involved in recognition of familiar objects and faces, the same denervated areas may be involved in CS and other misidentification syndromes.
Diffusion tensor imaging showed that the absence of an affective response to familiar stimuli might arise from disruption in the inferior fronto-occipital fasciculus (IFOF), which connects the occipital and posterior temporal cortex with the lateral frontal cortex [
14]. This trajectory might also be involved in the occurrence of VHs [
15]. It is also likely that connections with ventral limbic structures, especially the amygdala, play an important role in the affective response in recognition [
16].
A recent meta-analysis based on individual participant data showed a significant effect of cholinesterase inhibitors on delusions in PD [
17]. Our case report supports this finding for delusional misidentification syndrome specifically.
Our case, among others [
18,
19,
20], is in support of the treatment algorithm proposed by Lizarraga et al., which suggests to first consider cholinesterase inhibitors in PDD patients with VHs or delusions, before reducing PD medication and starting treatment with antipsychotics [
21]. However, placebo controlled trials assessing cholinesterase inhibitors for treatment of psychotic symptoms in PDD are needed.
Funding Statement
This research was supported by The Netherlands Organisation for Health Research and Development (ZonMw), grant number 636310010.
Declaration of Interest
None.
References
- L.M.L. De Lau, D. Verbaan, J. Marinus, J.J. van Hilten, Survival in Parkinson’s disease. Relation with motor and non-motor features, Park. Relat. Disord. 20(2014) 613–616. [CrossRef]
- K. Seppi et al., Update on treatments for nonmotor symptoms of Parkinson’s disease—an evidence-based medicine review, Mov. Disord. 34(2019) 180–198. [CrossRef]
- Cannas et al., Capgras syndrome in Parkinson ’ s disease : two new cases and literature review, Neurol. Sci. 38(2017) 225–231. [CrossRef]
- K.A. Josephs, Capgras syndrome and its relationship to neurodegenerative disease, Arch. Neurol. 64(2007) 1762–1766. [CrossRef]
- C.S. Peckins, L. Khorashadi, E.R. Wolpow, A Case of Reduplicative Paramnesia for Home, Cogn. Behav. Neurol. 29(2016) 150–157. [CrossRef]
- Pandis, N. Agrawal, N. Poole, Capgras’ Delusion: A Systematic Review of 255 Published Cases, Psychopathology, 2019, pp. 1–13. [CrossRef]
- S. Medic, D. Kuljic Obradovic, D. Markovic Zigic, Capgras syndrome in Parkinson’s disease: a case report, in Movement Disorders, 2012, p. 26.
- A. Moro, R.P. Munhoz, M. Moscovich, W.O. Arruda, H.A.G. Teive, Delusional misidentification syndrome and other unusual delusions in advanced Parkinson ’ s disease, Park. Relat. Disord. 19(2013) 751–754. [CrossRef]
- J. Pagonabarraga, G. Llebaria, C. García-Sánchez, B. Pascual-Sedano, A. Gironell, and J. Kulisevsky, A prospective study of delusional misidentification syndromes in Parkinson’s disease with dementia, Mov. Disord. 23(2008) 443–448. [CrossRef]
- G. Fénelon, F. Mahieux, R. Huon, M. Ziegler, Hallucinations in Parkinson ’ s disease Prevalence , phenomenology and risk factors, Brain 123(2000) 733–745. [CrossRef]
- R. Hilker et al., Dementia in Parkinson disease: Functional imaging of cholinergic and dopaminergic pathways, Neurology 65(2005) 1716–1722. [CrossRef]
- H. Shimada et al., Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET, Neurology 73(2009) 273–278. [CrossRef]
- E.E. Benarroch, Acetylcholine in the cerebral cortex: Effects and clinical implications, Neurology 75(2010) 659–665. [CrossRef]
- M.A. Bobes et al., Testing the connections within face processing circuitry in Capgras delusion with diffusion imaging tractography, NeuroImage Clin. 11(2016) 30–40. [CrossRef]
- Amad et al., The multimodal connectivity of the hippocampal complex in auditory and visual hallucinations, Mol. Psychiatry 19(2014) 184–191. [CrossRef]
- N. Breen, D. Caine, M. Coltheart, Models of face recognition and delusional misidentification: A critical review, Cogn. Neuropsychol. 17(2000) 55–71. [CrossRef]
- E. d'Angremont, M.J.H. Begemann, T. van Laar, I.E. Sommer, Cholinesterase Inhibitors for Psychotic Symptoms in Alzheimer's Disease, Parkinson's Disease and Dementia with Lewy Bodies: An Individual Participant Data Meta-Analysis, Available at SSRN: https://ssrn.com/abstract=4335150 or http://dx.doi.org/10.2139/ssrn.4335150. [CrossRef]
- A. Scicutella, Rivastigmine Treatment of Othello Syndrome and Post-ECT Delirium in a Patient With Parkinson’s Disease, J. Neuropsychiatry Clin. Neurosci. 27(2015) e90. [CrossRef]
- J.M.P. Rovers, P.L.J. Dautzenberg, J.P. Ter Bruggen, [Rivastigmine als ondersteuning bij het dilemma van de behandeling van hallucinates optredend bij ziekte van Parkinson], Tijdschr. Gerontol. Geriatr. 37(2006) 117–120. [CrossRef]
- J. Bergman, V. Lerner, Successful use of donepezil for the treatment of psychotic symptoms in patients with Parkinson’s disease, Clin. Neuropharmacol. 25(2002) 107–110. [CrossRef]
- K.J. Lizarraga, S.H. Fox, A.P. Strafella, A.E. Lang, Hallucinations, Delusions and Impulse Control Disorders in Parkinson Disease, Clin. Geriatr. Med. 36(2020) 105–118. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).