Submitted:
04 December 2023
Posted:
05 December 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
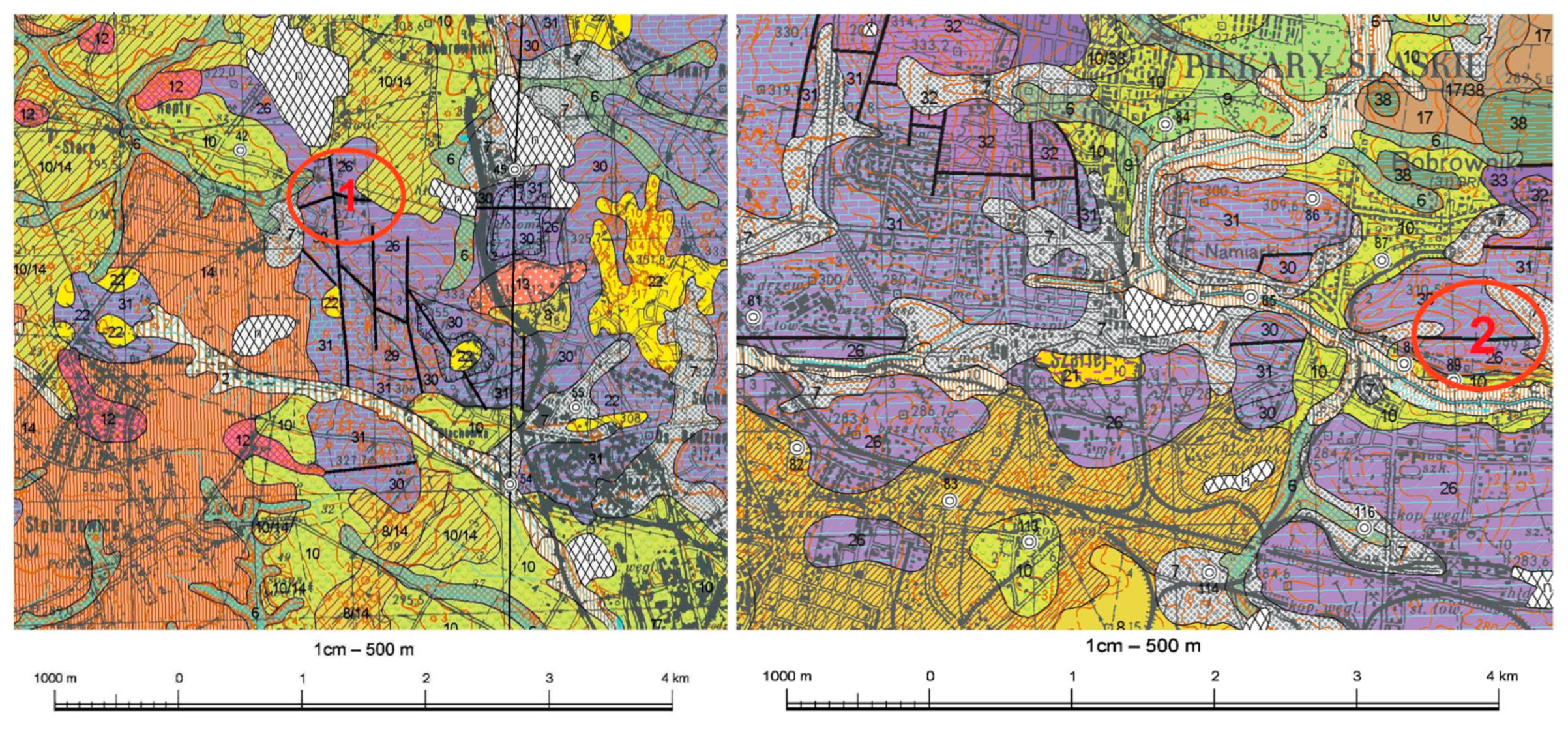
2.1. Materials
2.2. Methods
3. Results
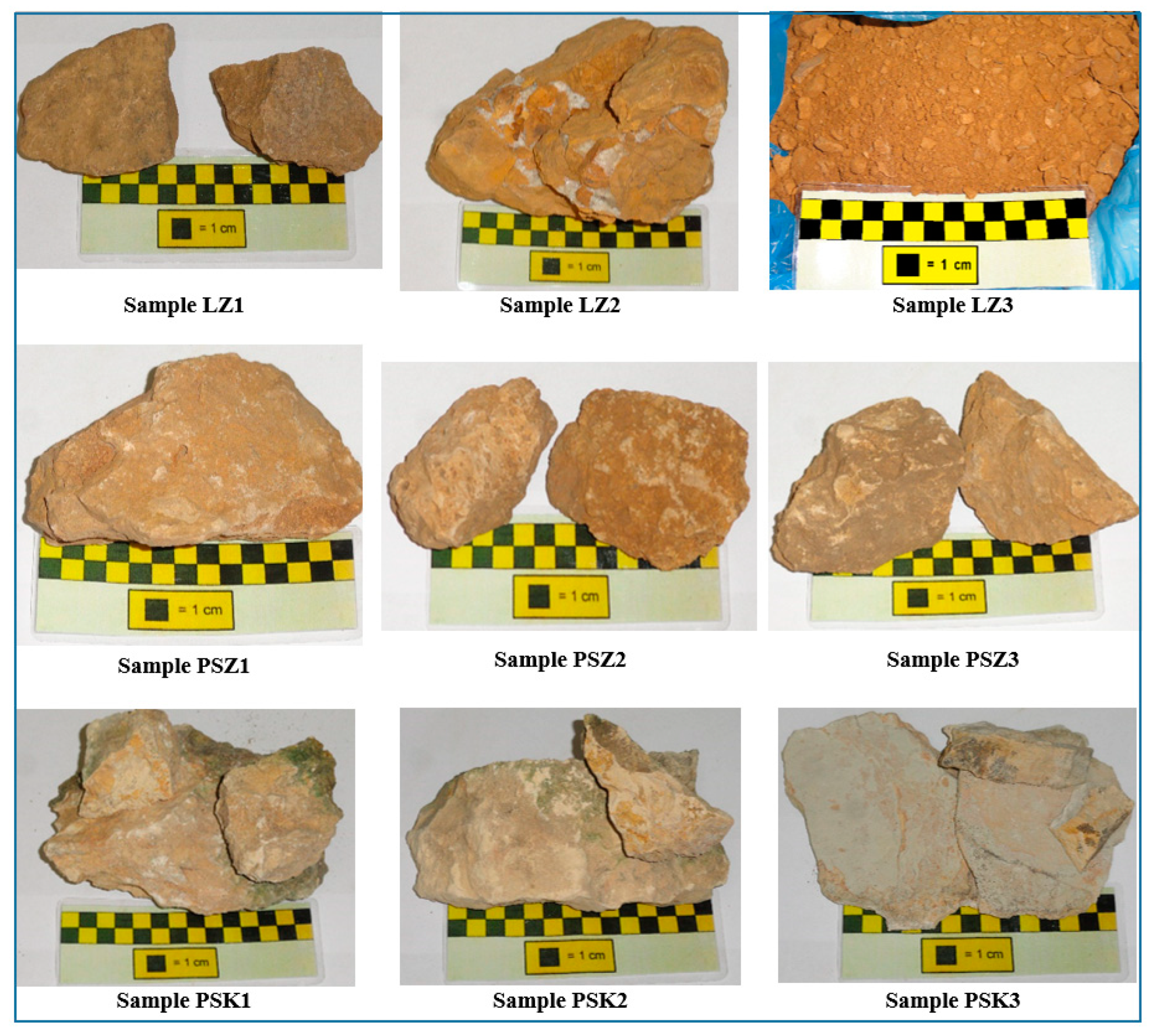
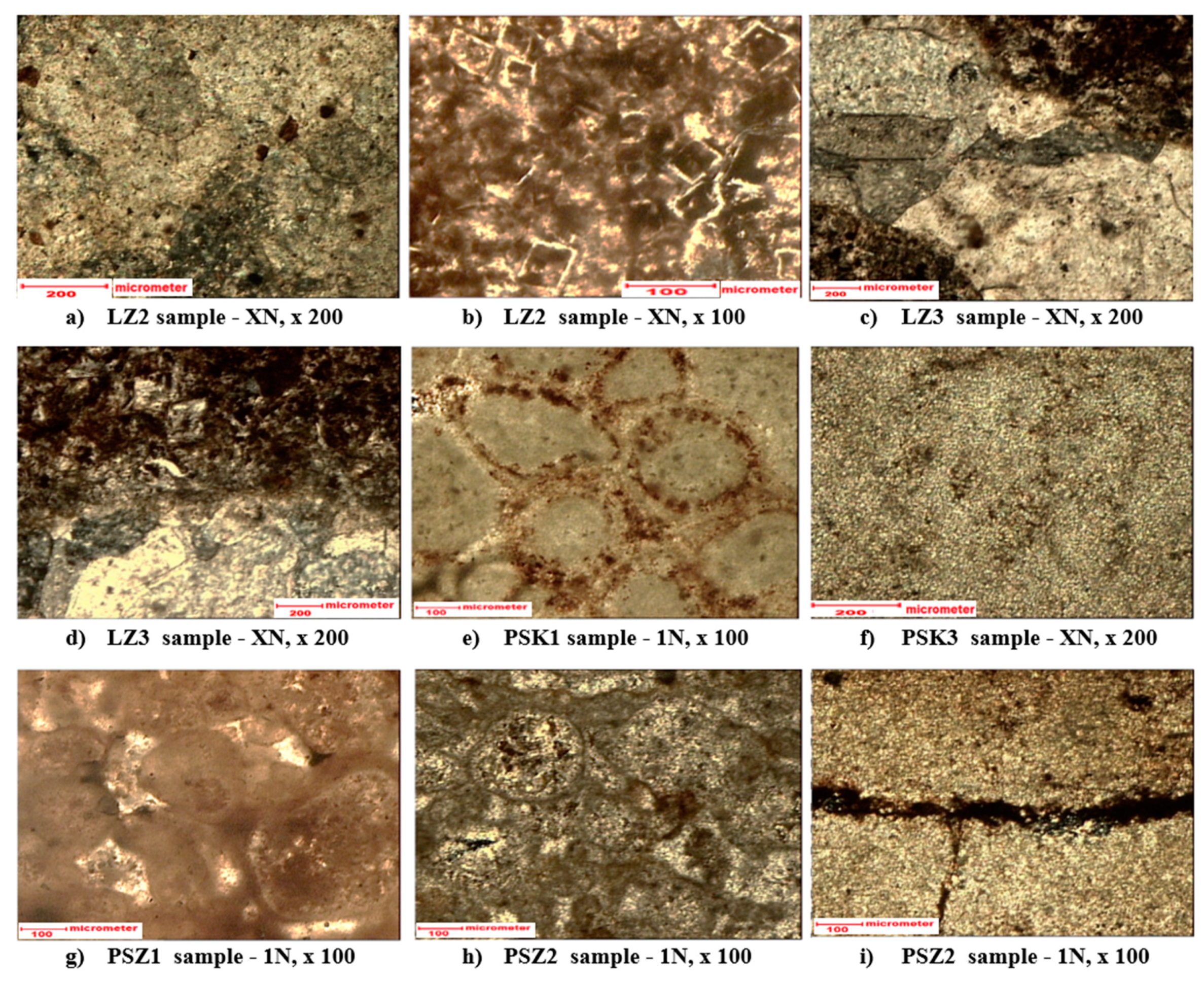
3.1. Mineralogical and petrographic characteristics of samples
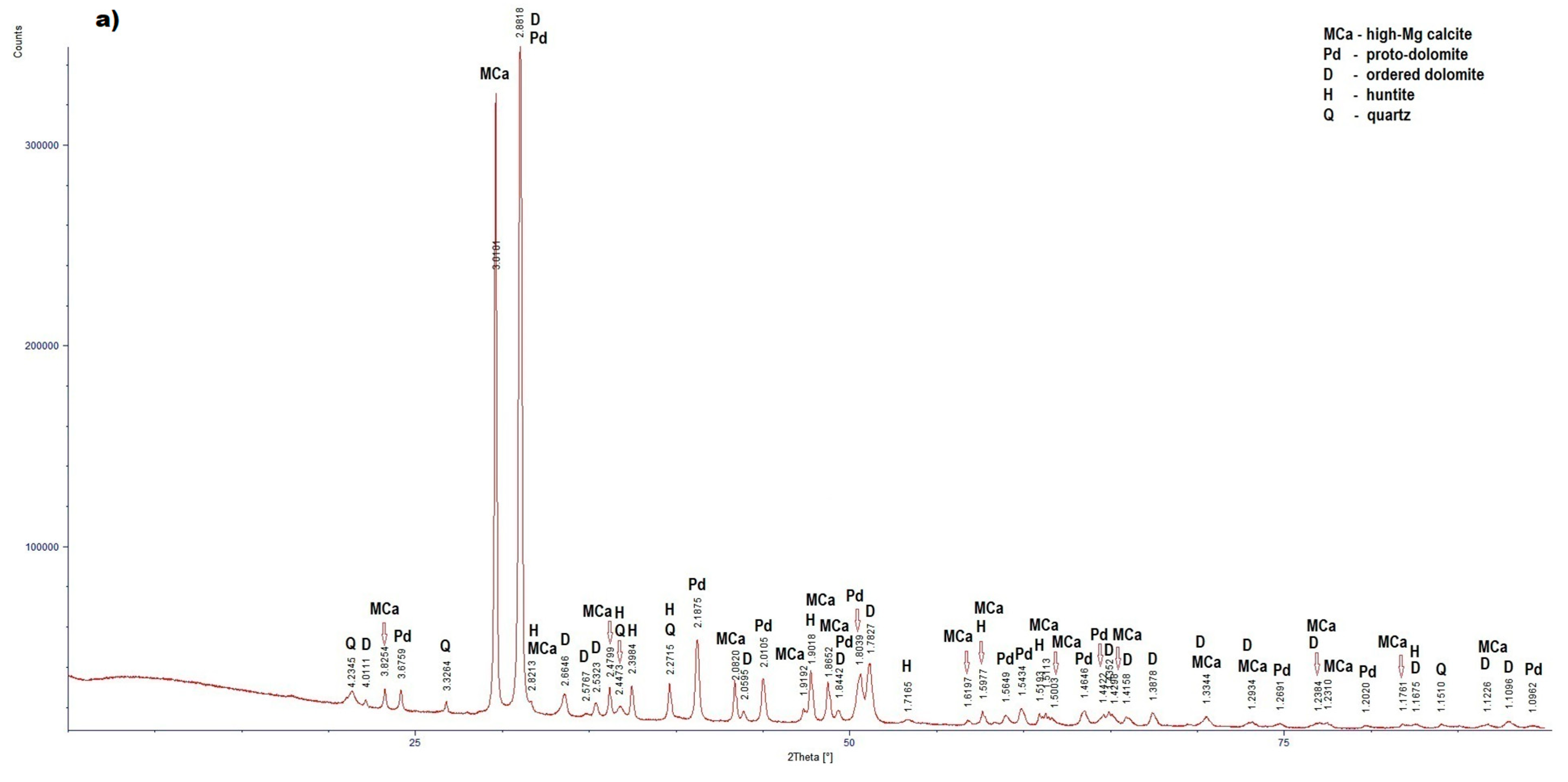
3.2. Results of X-Ray Diffraction
3.3. Results of X-Ray Fluorescence
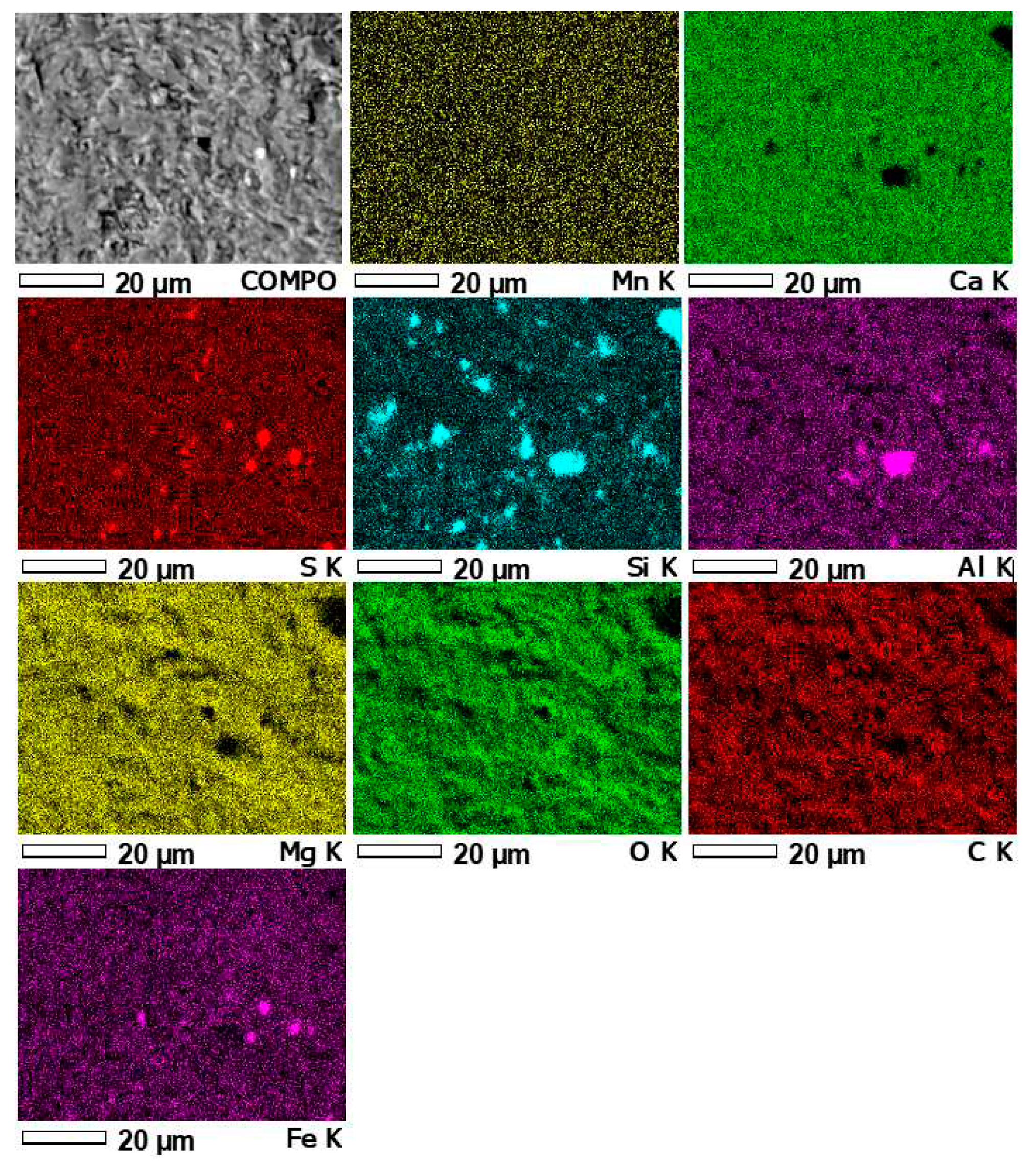
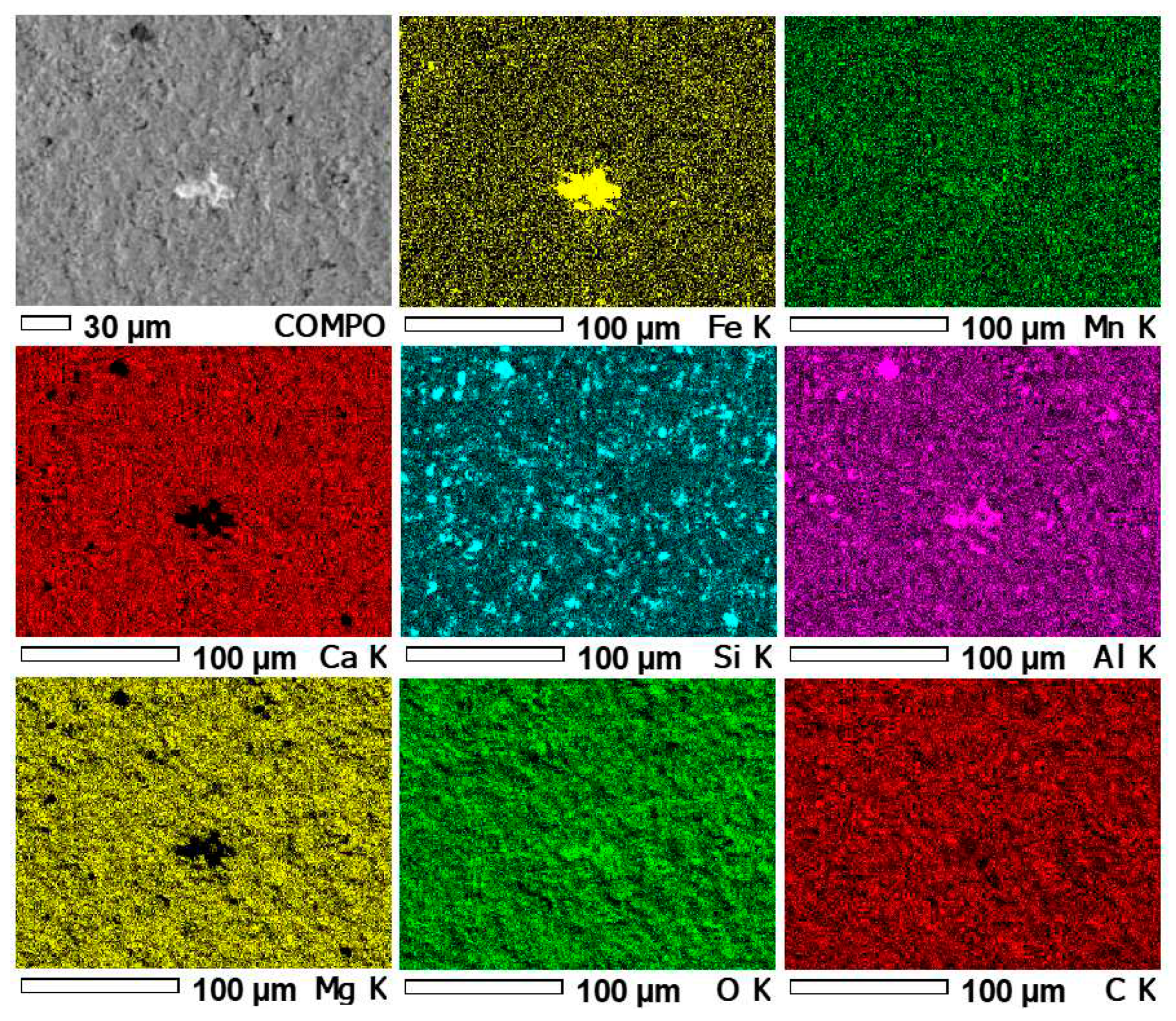
3.4. Results of microprobe measurements
4. Discussion
4.1. Mineral phases and the trace elements of the Triassic dolomites
4.2. Dolomites’ formation during sedimentary and diagenetic processes
5. Conclusions
- The results of the research indicate that the studied carbonate rocks are lime dolomites according to the Chilingar classification. Three of them in Pettijohn classification reprezents calcareous dolomites.
- The analyzed rocks apart from the dolomite phases, contain also calcite phases, including the dominant high-magnesium calcite.
- The results of X-Ray diffraction showed that five carbonate phases with different magnesium content occur in the examined rocks: low-magnesium calcite, high-magnesium calcite, proto-dolomite, ordered dolomite and huntite.
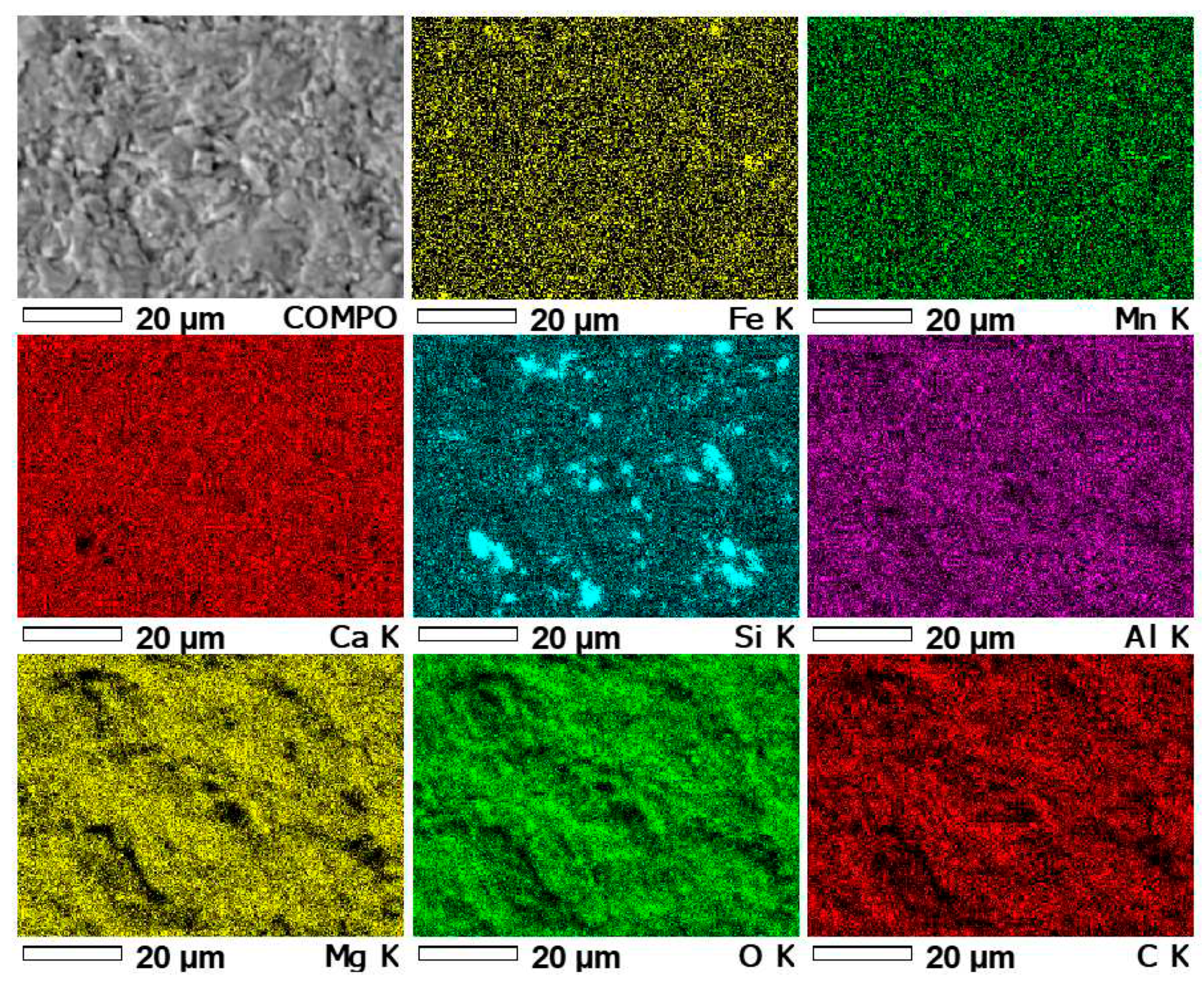
- X-Ray Fluorescence (XRF) results showed the presence of elements, not only those typical of carbonate minerals, but also elements such as: Al, Si, Fe, K, Na, Ti, Cr, Cd, Br, Cr, As, Mn, Zr, Co, Ni, Cu, Zn, Pb and Cl. Some of them may occur in minerals formed during diagenetic processes.
- The results of the microprobe measurements showed that there are two types of dolomite in the studied rocks: proto-dolomite and ordered dolomite. Proto-dolomite is characterized by a reduced magnesium content compared to the stoichiometric value for dolomite, but higher than that of high-magnesium calcite, while ordered dolomite has a Mg content close to the stoichiometric value.
- The results of microprobe measurements have shown that high-magnesium calcite has a Mg content higher than "pure" (low-magnesium) calcite, but lower than proto-dolomite.
- During microprobe measurements the magnesium-calcium carbonate phase was determined. The Mg content of this phase is higher than in ordered dolomite, but lower than typical for huntite. That’s why this mineral was named as de-huntite. The reduced content of Mg in this phase is probably the effect of dehuntization – the proces of advance stage of diagenesis.
- The achievement of the project is the identification of carbonate phases rich in magnesium, which have not been studied so far in the formation of the Tarnowice Unit, such as: high-magnesium calcite, proto-dolomite, ordered dolomite and de-huntite.
- On the basis of the obtained data, a theory connected with the formation of the studied rocks was formed. According to the data the rocks of the Tarnowice Unit are the sediments of the drying, shallow, epicontinental sea. They were re-flooded by seawater during the regressions of the Upper Muschelkalk period.
- The original sediments of the Tarnowice Unit were probably limestones which were dolomitized during metasomatic processes. This may be evidenced by the presence of carbonate phases that differ in terms of magnesium content.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matysik, M. Reefal environments and sedimentary processes of the Anisian Karchowice Beds In Upper Silesia, Southern Poland. Annales Societatis Geologorum Poloniae 2010, 80, 123–145. [Google Scholar]
- Senkowiczowa, H. Possibilities of formalizing the lithostratigraphic division of the Middle and Upper Triassic of the Silesian-Cracow Upland. Geological Quarterly 1980, 24, 787–804. [Google Scholar]
- Niedźwiedzki, R. Lithostratigraphy of the Górażdże Formation of the Dziewkowice Information in Opole Silesia. Geological and Mineralogical Works of Wrocław University LXXI 2000, Wroclaw.
- Niedźwiedzki, R.; Szulc, J.; Zarankiewicz, M. Stone treasures of the Saint Anne Land. Geological Guide 2013. Available online: www.annaland.pl.
- Stanienda, K. Effects of dolomitization processes in the Triassic limestone of Tarnów Opolski Deposit; Silesian University of Technology Press: Gliwice, Poland, 2011; ISBN 978-83-7880-071-2. [Google Scholar]
- Stanienda, K. Diagenesis of the Triassic limestone from the Opole Silesia in the aspect of magnesian calcite presence; Silesian University of Technology Press: Gliwice, Poland, 2013; ISBN 978-83-7335-872-0. [Google Scholar]
- Stanienda, K. Huntite in the Triassic limestones of Opolski Silesia. Mineral Resources Management 2013, 9, 79–98. [Google Scholar]
- Stanienda, K. Mineral phases in carbonate rocks of the Gogolin Beds from the area of Opole Silesia. Mineral Resources Management 2014, 30, 17–42. [Google Scholar]
- Stanienda, K. Carbonate phases rich in magnesium in the Triassic limestones of the East part of Germanic Basin. Carbonates and Evaporites 2016, 31, 387–405. [Google Scholar] [CrossRef]
- Stanienda, K. Mineral phases in carbonate rocks of the Górażdże Beds from the area of Opole Silesia. Mineral Resources Management 2016, 32, 67–92. [Google Scholar] [CrossRef]
- Stanienda-Pilecki, K. Carbonate minerals with magnesium in Triassic Terebratula limestone in the term of limestone with magnesium application as a sorbent in desulfurization of flue gases. Archives of Mining Sciences 2017, 62, 459–482. [Google Scholar] [CrossRef]
- Stanienda-Pilecki, K. Magnesium calcite in Muschelkalk limestones of the Polish part of the Germanic Basin. Carbonates and Evaporites 2018, 33, 801–821. [Google Scholar] [CrossRef] [PubMed]
- Stanienda-Pilecki, K. The Use of Limestones Built of Carbonate Phases with Increased Mg Content in Processes of Flue Gas Desulfurization. Minerals 2021, 11, 1044. [Google Scholar] [CrossRef]
- Szulc, J. International Workshop – Field Seminar The Muschelkalk- Sedimentary Environments, Facies and Diagenesis- Excursion Guidebook and Abstracts; Kraków: Opole, Poland, 1990; pp. 1–32. [Google Scholar]
- Szulc, J. Early alpinie tectonics and lithofacies succesion in the Silesian part of the Muschelkalk Basin. In Muschelkalk; Hagdorn H., Seilacher A., Eds. Goldschneck: Stuttgart, Germany, 1993; pp. 19–28. [Google Scholar]
- Szulc, J. Middle triassic evolution of the Northern Peri-Tethys area is influenced by early opening of the Tethys Ocean. Annales Societatis Geologorum Poloniae 2000, 70, 1–48. [Google Scholar]
- Szulc, J. Stratigraphy and correlation with Tethys and other Germanic subbasins. In International Workshop on the Triassic of Southern Poland. In Proceedings of the Pan-European Correlation of Epicontinental Triassic 4th Meeting, Fieldtrip Guide, 3–8 September 2007; pp. 26–28. [Google Scholar]
- Szulc, J. Triassic of the Silesia-Cracow area. In Proceedings of the Materials of the 42nd Speleological Symposium, Kraków, Poland; 9–10 2008. [Google Scholar]
- Biernat, S. Reamble: Wilanowski S., Lewandowski J. (2016) Detailed geological map of Poland. Sheet 910 – Bytom (M-34-50-D); Polish Geological Institute, National Research Institute: Poland, 1954. [Google Scholar]
- Chilingar, G.V. Classification of limestones and dolomites on basis of Ca/Mg ratio. J. Sed. Petrol. 1957, 27, 187–189. [Google Scholar]
- Pettijohn, F.J. Sedimentary rocks. III ed.; New York, NY, USA, 1975. [Google Scholar]
- Boggs S., Jr. Petrology of sedimentary rocks. Second Edition.; Cambridge University Press: London, UK, 2010. [Google Scholar]
- Mackenzie, F.T. , Andersson, A.J. The Marine carbon system and ocean acidification during Phanerozoic Time. Geochemical Perspectives 2013, 2. [Google Scholar]
- Mavromatis, V. , Götschl, K.E., Grengg, C., Konrad, F., Purgstaller, B., Dietzel, M. Barium partitioning in calcite and aragonite as a function of growth rate. Geochimica et Cosmochimica Acta 2018, 237, 65–78. [Google Scholar] [CrossRef]
- Morse, J.W. , Mackenzie, F.T. Geochemistry of sedimentary carbonates. Elsevier 1990, 33, 707. [Google Scholar]
- Morse, J.W. , Andersson, A.J., Mackenzie, F.T. Initial responses of carbonate-rich shelf sediments to rising atmospheric pCO2 and ‘‘ocean acidification’’: Role of high Mg-calcites. Geochimica et Cosmochimica Acta 2006, 70, 5814–5830. [Google Scholar] [CrossRef]
- Polański, A. Basics of geochemistry; Geological Publishing House: Warsaw, Poland, 1988. [Google Scholar]
- Tucker, M. E. , Wright, V. P. Carbonate sedimentology; Blackwell Scientific Publications: Oxford London, Edinburgh Boston Melbourne, 1990; pp. 366–372. [Google Scholar]
- Zhang, S.; Zhou, R.; DePaolo, D.J. The Seawater Sr/Ca ratio in the past 50 Myr from Bulk Carbonate Sediments Corrected for Diagenesis; Elsevier.
- Zhang, S. , DePaolo, D.J. Equilibrium calcite-fluid Sr/Ca partition coefficient from marine sediment and pore fluids. Geochimica et Cosmochimica Acta 2020, 289, 33–46. [Google Scholar] [CrossRef]
- Zhong, S. , Mucci, A. Calcite and aragonite precipitation from seawater solutions of various salinities: Precipitation rates and overgrowth compositions. Chemical Geology 1989, 78, 283–299. [Google Scholar] [CrossRef]
- Makowski, H. Historical Geology; Geological Publishing House: Warsaw, Poland, 1977. [Google Scholar]
- Senkowiczowa, H. , Szyperko-Śliwczyńska, A. Stratigraphy and palaeography of the Triassic. Geological Institute 1972, 252. [Google Scholar]
- Czermiński, J. (Ed.) Geological Structure of Poland, Volume II, Catalog of Fossils, Part 2, Mesozoic; Geological Publishing House: Warsaw, Poland, 1970. [Google Scholar]
- Bujoczek, M.; Gładysz, K.; Stępień, A. Tarnowskie Góry Underground - a living organism on a selected fragment of the mine of the Fryderyk Mine (Blachówka – Western – Urban Glückhilfe). Project Report; Tarnowskie Góry Cave Mountain Club: Poland, 2013. [Google Scholar]
- Migaszewski, Z. Origin of the dolomites. Geological Quarterly 1990, 34, 202. [Google Scholar]
- Migaszewski, Z. Current views on the genesis of dolomites. Geological Quarterly 1990, 3, 111–117. [Google Scholar]
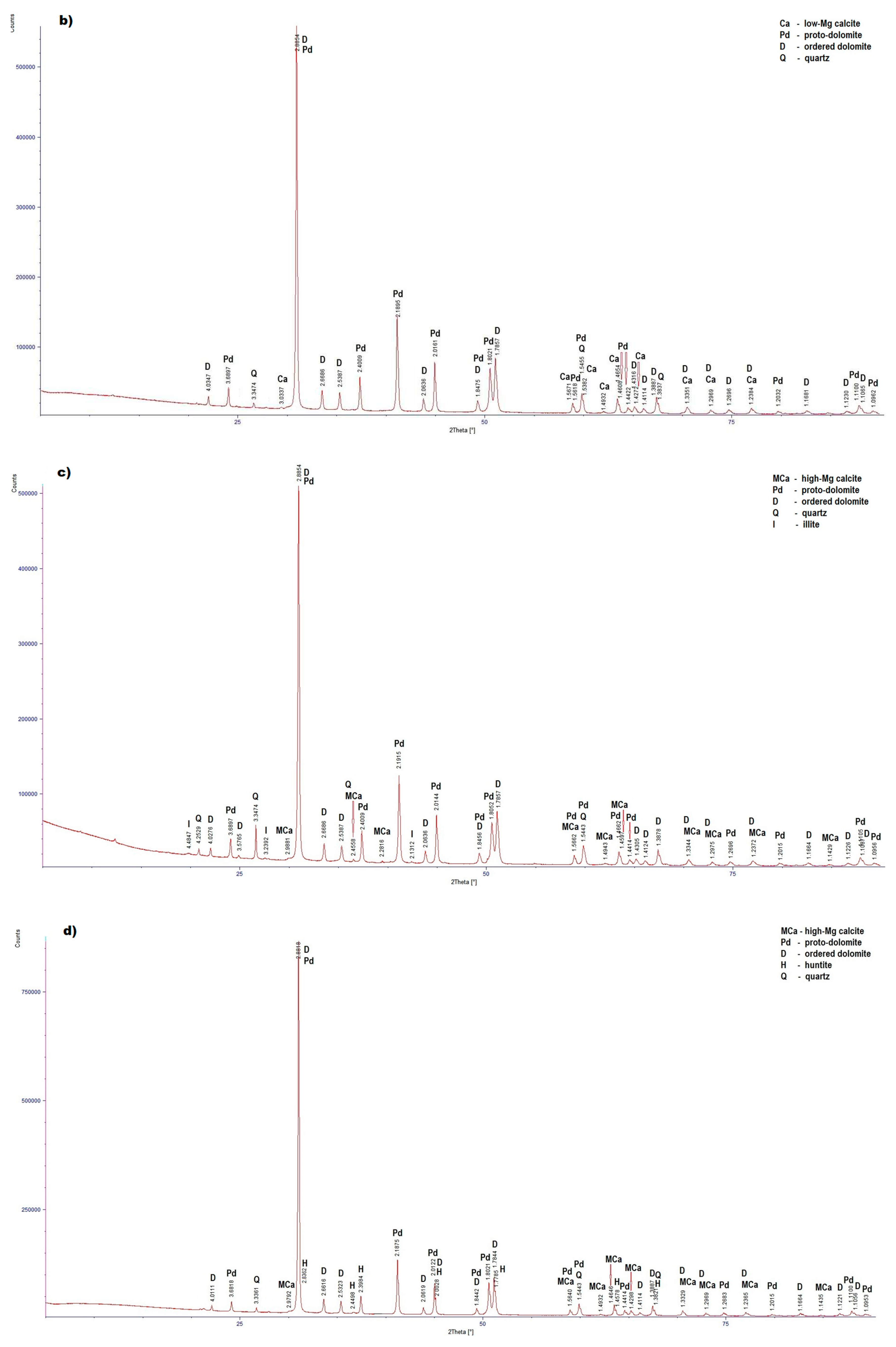
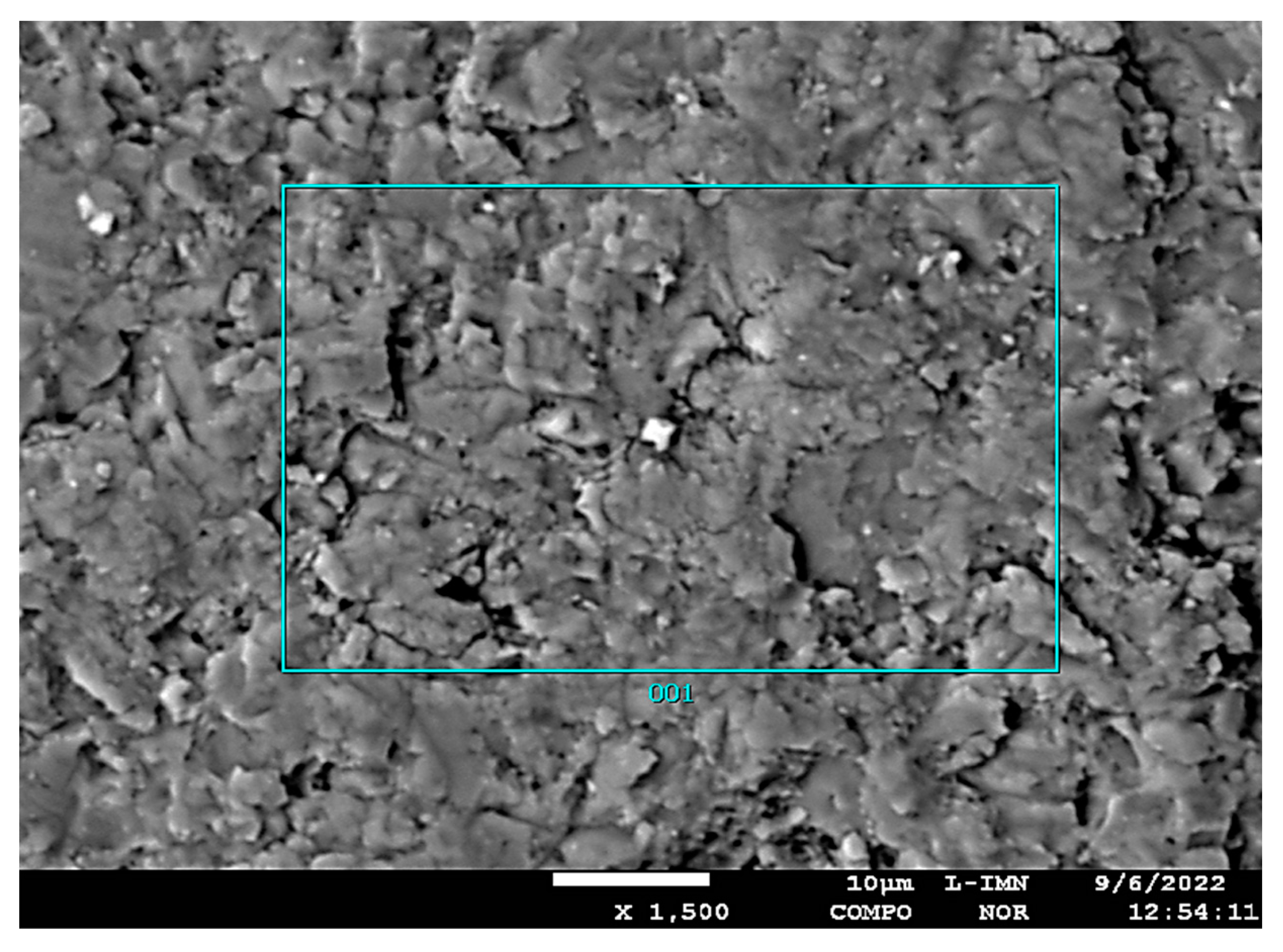
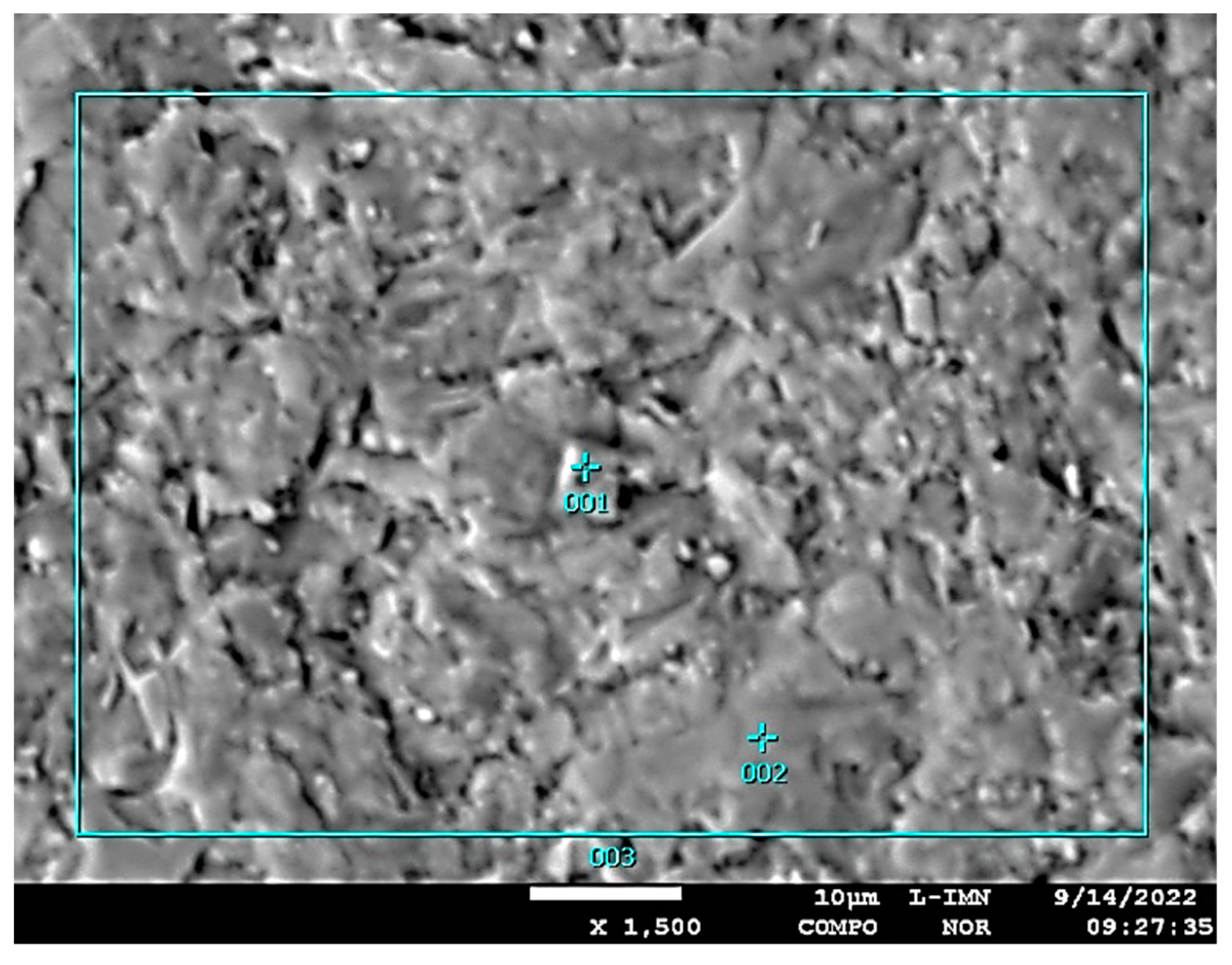


| Type of Sample | Type of Mineral | dhkl- index of the lattice intervals of exposed parallel lattice planes system Ǻ intensity of diffraction lines |
|---|---|---|
|
LZ2 (Figure4a) |
High-Mg calcite | 3.8254 (12), 3,0101 (100), 2,8213 (2), 2.4799 (14), 2.0820 (16), 1.9182 (7), 1.9018 (21), 1.8652 (23), 1.6197 (4), 1.5977 (11), 1.5193 (6), 1.5113 (3), 1.5003 (3), 1.4298 (8), 1.3344 (3), 1,2934 (4), 1.2384 (1), 1.2310 (1), 1.1761 (3), 1.1226 (3) |
| Proto-dolomite | 3.6759 (4), 2.8818 (100), 2.1875 (30), 2.0105 (15), 1.8442 (5), 1.8039 (17), 1.5649 (4), 1.5434 (10), 1.4646 (7), 1.4422 (3), 1.2691 (3), 1.2020 (1), 1.0962 (2) | |
| Ordered dolomite | 4.0111 (1), 2.8818 (100), 2.6648 (4), 2.5767 (3), 2.5323 (2), 2.0595 (3), 1.8442 (5), 1.7827 (21), 1.4352 (3), 1.4158 (3), 1.3878 (9), 1.3344 (4), 1.2934 (2), 1.2384 (3), 1.1675 (2), 1.1226 (2), 1.1096 (8) | |
| Huntite | 2,8213 (100); 2,4473 (9); 2,3984 (10); 2,2715 (5); 1,9018 (1); 1,7165 (3); 1,5977 (10); 1,5193 (1); 1,5113 (1); 1,1675 (1); | |
| Quartz | 4,2345 (19); 3,3264 (100); 2,4473 (7); 2,2715 (7); 1,1510 (2) | |
|
LZ3 |
High-Mg calcite |
3,8468 (8); 3,0284 (100); 2,7931 (2); 2,4860 (14); 2,2787 (21); 2,0886 (16); 1,9052 (21); 1,8685 (23); 1,3344 (3); 1,2975 (4); 1,2384 (1); 1,1236 (3) |
| Proto-dolomite | 3.6957 (4), 2.8854 (100), 2.4041 (13), 2.1915 (30), 2.0161 (15), 1.8475 (5), 1.8039 (17), 1.5662 (3), 1.5465 (10), 1.4654 (7), 1.4440 (3), 1.2704 (3), 1.2020 (1), 1.1105 (8), 1.0962 (2) | |
| Ordered dolomite | 4.0276 (1), 2.8854 (100), 2.6686 (4), 2.5387 (2), 2.0636 (3), 1.8475 (5), 1.4305 (3), 1.,4131 (1), 1.3894 (8), 1.3344 (3), 1.2975 (2), 1.2384 (3), 1.1681 (2), 1.1236 (2), 1.1073 (8) | |
| Quartz | 4,2529 (19); 3,3425 (100); 2,4558 (7); 2,2787 (7); 1,5412 (10); 1,3844 (6) |
|
|
PSK1 (Figure4b) |
Low-Mg calcite |
3.0337 (100), 1.5671 (1), 1.5382 (9), 1.4932 (5), 1.4654 (2), 1.4272 (5), 1.3351 (3), 1.2969 (5), 1.2384 (2) |
| Proto-dolomite | 3.6897 (4), 2.8854 (100), 2.4009 (13), 2.1895 (30), 2.0161 (15), 1.8475 (5), 1.8021 (17), 1.5618 (3), 1.5455 (10), 1.4608 (7), 1.4422 (3), 1.2696 (3), 1.2032 (1), 1.1100 (8), 1.0962 (2) | |
| Ordered dolomite | 4.0347 (1), 2.8854 (100), 2.6686 (4), 2.5387 (2), 2.0636 (3), 1.8475 (5), 1.7857 (21), 1,4316 (3), 1.4114 (1), 1.3887 (8), 1.3351 (3), 1.2969 (2), 1.2384 (3), 1.1681 (2), 1.1230 (2), 1.1065 (8) | |
| Quartz | 3.3474 (100), 1.5455 (10), 1.3837 (6) |
|
|
PSK3 (Figure4c) |
High-Mg calcite |
2.9881 (100), 2.4558 (14), 2.2816 (21), 1.5662 (1), 1.4933 (3), 1.4662 (3), 1.4597 (2), 1.3344 (3), 1.2975 (4), 1.2372 (1), 1.1429 (4) |
| Proto-dolomite | 3.6897 (4), 2.8854 (100), 2.4009 (13), 2.1915 (30), 2.0144 (15), 1.8456 (5), 1.8052 (17), 1.5662 (3), 1.5443 (10), 1.4662 (7), 1.4414 (3), 1.2696 (3), 1.2015 (1), 1.1105 (8), 1.0956 (2) | |
| Ordered dolomite | 4.0276 (1), 3.5710 (4), 2.8854 (100), 2.6686 (4), 2.5387 (2), 2.0636 (3), 1.8456 (5), 1,.7857 (21), 1.4305 (3), 1.4124 (1), 1.3878 (8), 1.3344 (3), 1.2975 (2), 1.2372 (3). 1.1664 (2), 1.1226 (2), 1.1087 (8) | |
| Quartz | 4.2529 (19), 3.3474 (100), 2.4558 (7), 1.5443 (10) | |
| Illite | 4.4847 (100), 3.2392 (65), 2.1312 (31) | |
|
PSZ1 (Figure4d) |
High-Mg calcite |
2.9792 (100), 1.5640 (1), 1.4932 (3), 1.4646 (3), 1.4298 (8), 1.3329 (3), 1.2969 (4), 1.2365 (1), 1.1435 (4) |
| Proto-dolomite | 3.6818 (4), 2.8818 (100), 2.1875 (30), 2.0122 (15), 1.8442 (5), 1.8021 (17), 1.5640 (3), 1.5443 (10); 1.4414 (3), 1.2683 (3), 1.2015 (1), 1.1100 (8); 1.0953 (2) | |
| Ordered dolomite | 4.0111 (1), 2,8818 (100), 2.6616 (4), 2.5323 (2), 2.0619 (3), 2.0028 (15); 1.8442 (5), 1.7844 (21), 1.4114 (1); 1.3887 (8), 1.3329 (3), 1.2969 (2), 1.2365 (3), 1.1664 (2), 1.1221 (2); 1.1056 (8) | |
| Huntite | 2.8362 (100), 2.4498 (9), 2.3984 (10), 2.0028 (7), 1.7785 (15), 1.4578 (2), 1.3821 (1) | |
| Quartz | 3.3361 (100), 1.5443 (10), 1.3821 (6) |
|
|
PSZ3 |
High-Mg calcite | 3.0101 (100), 1,5627 (1), 1.5382 (9), 1.4895 (3), 1.4578 (3), 1.4262 (8), 1.3269 (3), 1.2946 (4), 1.2352 (1), 1.1435 (4) |
| Proto-dolomite | 3.6681 (4), 2.8688 (100), 2.3928 (13), 2.1829 (30), 2.0066 (15), 1.8410 (5), 1.7978 (17), 1.5627 (3), 1.5403 (10), 1.2670 (3), 1.2015 (1), 1.0944 (2) | |
| Ordered dolomite | 3.9947 (1), 2.8688 (100), 2.6545 (4), 2.5224 (2), 2.0578 (3), 1.8410 (5), 1.7797 (21), 1.4396 (3), 1.4089 (1), 1.3861 (8), 1.3320 (3), 1.2946 (2), 1.2352 (3), 1.1655 (2), 1.1211 (2), 1.1082 (8) | |
| Quartz | 3.3264 (100), 1.5403 (10), 1.3804 (6) |
| Element | Unit | Detection limit | Sample number | |||||
| LZ2 | LZ3 | PSK1 | PSK3 | PSZ1 | PSZ2 | |||
| Main elements | ||||||||
| C | mass% | 0.02437 | 6.53 | 8.06 | 8.16 | 7.69 | 8.45 | 8.36 |
| O | mass% | 0.15465 | 52.54 | 55.35 | 56.54 | 56.54 | 56.34 | 56.39 |
| Na | mass% | 0.01456 | 0.62 | 0.24 | 0.13 | 0.10 | 0.14 | 0.13 |
| Mg | mass% | 0.00861 | 7.23 | 9.48 | 10.95 | 10.50 | 10.78 | 10.98 |
| Al | mass% | 0.00161 | 0.33 | 0.18 | 0.26 | 0.70 | 0.15 | 0.15 |
| Si | mass% | 0.00276 | 3.42 | 1.22 | 1.31 | 2.78 | 0.83 | 0.72 |
| K | mass% | 0.00139 | 0.05 | 0.02 | 0.08 | 0.19 | 0.03 | 0.03 |
| Ca | mass% | 0.00454 | 18.95 | 22.66 | 21.80 | 20.66 | 21.75 | 21.80 |
| Mn | mass% | 0.00142 | 0.78 | 0.45 | 0.06 | 0.04 | 0.09 | 0.06 |
| Fe | mass% | 0.00148 | 8.39 | 2.18 | 0.33 | 0.51 | 1,12 | 0,84 |
| Trace elements | ||||||||
| F | ppm | 577,45 | BDL | BDL | 776 | 475 | BDL | BDL |
| P | ppm | 4.03 | 222 | 55 | 64 | 87 | 94 | 69 |
| S | ppm | 6.98 | 891 | 359 | 1491 | 544 | 719 | 869 |
| Cl | ppm | 37.72 | 564 | 500 | 613 | 531 | 620 | 633 |
| Ti | ppm | 24.43 | 113 | 68 | 100 | 292 | 70 | 56 |
| Cr | ppm | 15,87 | 22 | 7 | 13 | 27 | BDL | BDL |
| Co | ppm | 15.25 | 31 | BDL | 19 | 23 | BDL | BDL |
| Ni | ppm | 8.08 | 26 | 26 | 17 | 15 | 20 | 18 |
| Cu | ppm | 7.5 | 22 | 12 | 362 | 545 | 16 | 17 |
| Zn | ppm | 6.6 | 6803 | 290 | 5 | 9 | 1248 | 3455 |
| As | ppm | 16,8 | BDL | BDL | BDL | BDL | 23 | 41 |
| Rb | ppm | 3.8 | BDL | BDL | 5 | 9 | BDL | BDL |
| Br | ppm | 5.07 | BDL | 13 | BDL | BDL | 5 | 5 |
| Ba | ppm | 66.5 | BDL | BDL | 99 | 233 | BDL | BDL |
| Sr | ppm | 4.2 | 50 | 46 | 117 | 81 | 81 | 78 |
| Cd | ppm | 15.05 | BDL | BDL | BDL | BDL | 29 | 37 |
| Pb | ppm | 13.27 | 2818 | 74 | 73 | 36 | 151 | 178 |
| Sample number | Content of basic elements [%] | |||||||
|---|---|---|---|---|---|---|---|---|
| Ca | Mg | Fe | Mn | Si | Al | Na | K | |
| LZ2 | 18.95 | 7.23 | 8.39 | 0.78 | 3.42 | 0.33 | 0.62 | 0.05 |
| LZ3 | 22.66 | 9.48 | 2.18 | 0.45 | 1.22 | 0.33 | 0.24 | 0.02 |
| PSK1 | 21.80 | 10.95 | 0.33 | 0.06 | 1.31 | 0.26 | 0.13 | 0.08 |
| PSK3 | 20.66 | 10.50 | 0.51 | 0.04 | 2.78 | 0.70 | 0.10 | 0.19 |
| PSZ1 | 21.75 | 10.78 | 1.12 | 0.09 | 0.83 | 0.15 | 0.14 | 0.03 |
| PSZ2 | 21.80 | 10.98 | 0.84 | 0.06 | 0.72 | 0.15 | 0.13 | 0.03 |
| Content of basic oxides [%] | ||||||||
| CaO | MgO | Fe2O3 | MnO | SiO2 | Al2O3 | Na2O | K2O | |
| LZ2 | 26.51 | 11.99 | 11.99 | 1.01 | 7.32 | 0.62 | 0.84 | 0.06 |
| LZ3 | 31.70 | 15.72 | 3.12 | 0.58 | 2.61 | 0.62 | 0.32 | 0.02 |
| PSK1 | 30.50 | 18,16 | 0.47 | 0.08 | 2.80 | 0.49 | 0.17 | 0.09 |
| PSK3 | 28.91 | 17.41 | 0.73 | 0.05 | 5.95 | 1.32 | 0.13 | 0.23 |
| PSZ1 | 30.43 | 17.87 | 1.60 | 0.12 | 1.77 | 0.28 | 0,19 | 0.04 |
| PSZ2 | 30.50 | 18.21 | 1.20 | 0.07 | 1.54 | 0.28 | 0.17 | 0.04 |
| MgCO3 content and Ca/Mg ratio | ||||||||
| Ca [%] | CaO [%] | Mg [%] | MgO [%] | MgCO3 [%] | Ca/Mg | |||
| LZ2 | 18.95 | 26,51 | 7.23 | 11.99 | 25.78 | 2.62 | ||
| LZ3 | 22.66 | 31.70 | 9.48 | 15.72 | 33.80 | 2.39 | ||
| PSK1 | 21.80 | 30.50 | 10.95 | 18.16 | 39.04 | 1.99 | ||
| PSK3 | 20.66 | 28.91 | 10.50 | 17.41 | 37.43 | 1.97 | ||
| PSZ1 | 21.75 | 30.43 | 10.78 | 17.87 | 38.42 | 2.02 | ||
| PSZ2 | 21.80 | 30.50 | 10.98 | 18.21 | 39.15 | 1.98 | ||
| Specimen name | Sample number | Ca/Mg | MgO [%] | MgCO3 [%] | Rock name in the Chilingar G.V. (1957) classification | Rock name in the Pettijohn F.J. (1975) classification |
|---|---|---|---|---|---|---|
| Rocks of the Lazarówka Quarry | LZ2 | 2.62 | 11.99 | 25.31 | Lime Dolomite | Calcerous dolomite |
| LZ3 | 2.39 | 15.72 | 33.18 | Lime Dolomite | Calcerous dolomite | |
| Rocks from the area of Piekary Śląskie City | PSK1 | 1.99 | 18.16 | 38.33 | Lime Dolomite | Lime Dolomite |
| PSK3 | 1.97 | 17.41 | 36.75 | Lime Dolomite | Lime Dolomite | |
| PSZ1 | 2.02 | 17.87 | 37.74 | Lime Dolomite | Calcerous dolomite | |
| PSZ2 | 1.98 | 18.21 | 38.43 | Lime Dolomite | Lime Dolomite |
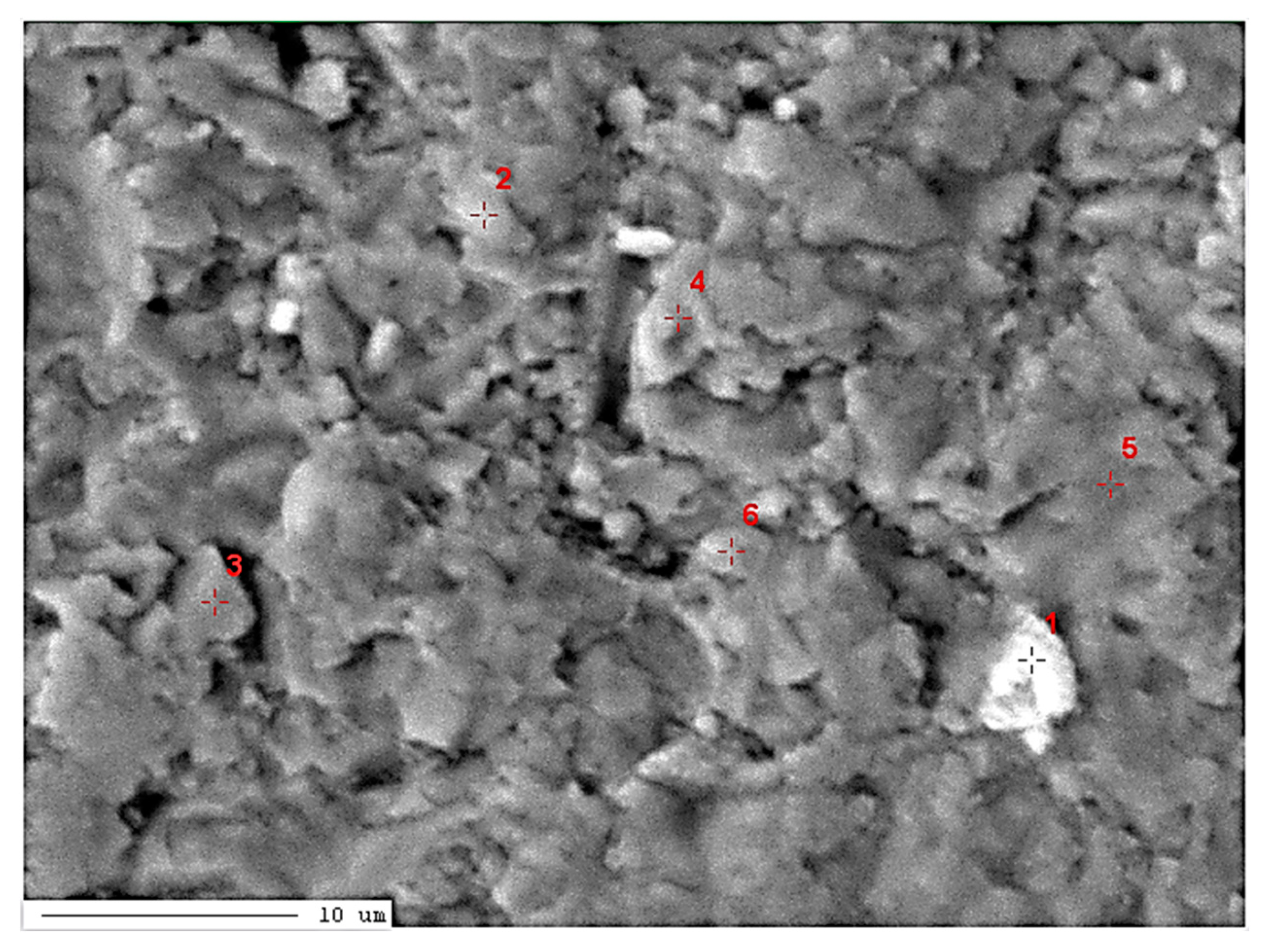
| Point Number | Element | Total [mass%] | MgO [%] |
||||||||
| C | O | Mg | Si | Al | Ca | Fe | Mn | S | |||
| Content [mass%] | |||||||||||
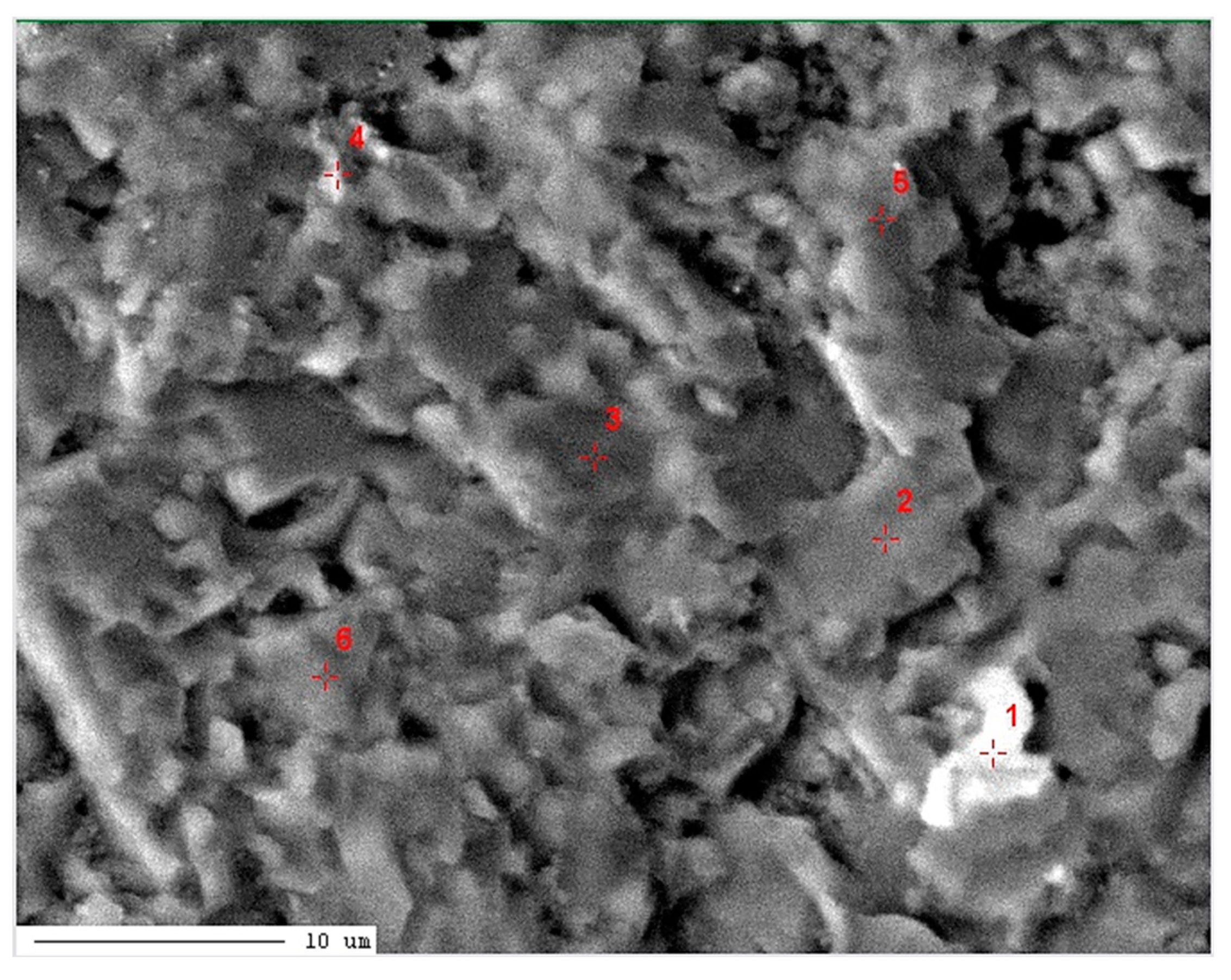
| 1 / low-Mg calcite (Ca0.95,Mg0.05)CO3 |
10.58 | 52.10 | 1.20 | 0.74 | 0.05 | 25.44 | 1.47 | 1.05 | 7.37 | 100.00 | 2.00 |
| 2 / high-Mg calcite (Ca0.57,Mg0.43)CO3 |
10.93 | 54.19 | 10.37 | BDL | 0.07 | 20.96 | 2.87 | 0.60 | 0.01 | 100.00 | 17.28 |
| 3 / high-Mg calcite (Ca0.69,Mg0.31)CO3 |
12.07 | 55.99 | 7.47 | 0.01 | 0.06 | 21.29 | 2.65 | 0.46 | BDL | 100.00 | 12.45 |
| 4 / high-Mg calcite (Ca0.57,Mg0.43)CO3 |
11.90 | 53.47 | 10.41 | 0.05 | 0.05 | 21.96 | 1.89 | 0.25 | 0.02 | 100.00 | 17.35 |
| 5 / proto-dolomite (Ca0.53,Mg0.47)CO3 |
11.20 | 53.49 | 11.39 | 0.05 | 0.08 | 20.92 | 2.25 | 0.61 | 0.01 | 100.00 | 18.98 |
| 6 / proto-dolomite (Ca0.51,Mg0.49)CO3 |
11.29 | 53.44 | 11.71 | 0.01 | 0.11 | 20.69 | 2.17 | 0.56 | 0.02 | 100.00 | 19.52 |
| Point Number | Element | Total [mass%] | MgO [%] |
|||||||
| C | O | Mg | Si | Al | Ca | Fe | Mn | |||
| [mass%] | ||||||||||
| 1 / huntite (Ca0.41,Mg0.59)CO3 |
7.9 | 51.06 | 14.21 | 1.03 | 0.44 | 24.86 | 0.06 | 0.43 | 100.00 | 23.68 |
| Point Number | Element | Total [mass%] | MgO [%] |
|||||||
| C | O | Mg | Si | Al | Ca | Fe | Mn | |||
| [mass%] | ||||||||||
| 1 / Fe-Si oxyhydroxide |
3.01 | 16.90 | 0.63 | 41.18 | 0.05 | 2.65 | 35.24 | 0.34 | 100.00 | 1.05 |
| 2 / proto-dolomite (Ca0.41,Mg0.59)CO3 |
11.41 | 55.23 | 11.77 | 0.13 | 0.05 | 21.34 | 0.06 | 0.01 | 100.00 | 19.62 |
| 3 / high-Mg calcite (Ca0.41,Mg0.59)CO3 |
11.33 | 52.39 | 9.53 | 0.38 | 6.00 | 20.21 | 0.15 | 0.01 | 100.00 | 15.88 |
| 4 / proto-dolomite (Ca0.41,Mg0.59)CO3 |
11.00 | 54.63 | 11.16 | 1.29 | 0.61 | 21.15 | 0.14 | 0.02 | 100.00 | 18.60 |
| 5 / high-Mg calcite (Ca0.41,Mg0.59)CO3 |
11.34 | 56.15 | 8.54 | 0.64 | 0.16 | 21.62 | 1.51 | 0.04 | 100.00 | 14.23 |
| 6 / proto-dolomite (Ca0.41,Mg0.59)CO3 |
11.35 | 56.15 | 10.96 | 0.13 | 0.07 | 21.30 | 0.03 | 0.01 | 100.00 | 18.27 |
| Point Number | Element | Total [mass%] | MgO [%] |
|||||||
| C | O | Mg | Si | Al | Ca | Fe | Mn | |||
| [mass%] | ||||||||||
| Contents of elements in micro-area 1 (Figure11) | ||||||||||
| 1 / huntite (Ca0.41,Mg0.59)CO3 |
7.71 | 51.44 | 15.24 | 1.14 | 0.12 | 24.12 | 0.20 | 0.03 | 100.00 | 25.40 |
| 2 / huntite (Ca0.41,Mg0.59)CO3 |
5.37 | 44.11 | 17.76 | 0.28 | 0.24 | 32.00 | 0.16 | 0.08 | 100.00 | 29.60 |
| Total area / huntite (Ca0.41,Mg0.59)CO3 |
7.43 | 52.12 | 14.24 | 0.60 | 0.11 | 25.11 | 0.28 | 0.11 | 100.00 | 23.73 |
| Contents of elements in micro-area 2 (Figure12) | ||||||||||
| 1 / huntite (Ca0.42,Mg0.58)CO3 |
9.57 | 57.17 | 13.62 | BDL | 0.09 | 18.80 | 0.69 | 0.06 | 100.00 | 22.70 |
| 2 / huntite (Ca0.31,Mg0.69)CO3 |
6.12 | 47.93 | 16.50 | 0.02 | 0.09 | 29.17 | 0.09 | 0.08 | 100.00 | 27.50 |
| 3 / huntite (Ca0.49,Mg0.61)CO3 |
4,50 | 42.67 | 14.63 | 0.30 | 0.13 | 33.83 | 3.78 | 0.16 | 100.00 | 24.38 |
| Total area / huntite (Ca0.43,Mg0.57)CO3 |
7.60 | 52.00 | 13.76 | 0.53 | 0.16 | 25.00 | 0.83 | 0.12 | 100.00 | 22.93 |
| Point Number | Element | Total [mass%] | MgO [%] |
|||||||
| C | O | Mg | Si | Al | Ca | Fe | Mn | |||
| Content [mass%] | ||||||||||
| 1 / low-Mg calcite (Ca0.98,Mg0.02)CO3 |
10.09 | 55.47 | 0.60 | 0.02 | 0.02 | 33.72 | 0.07 | 0.01 | 100.00 | 1.00 |
| 2 / proto-dolomite (Ca0.51,Mg0.49)CO3 |
12.65 | 55.20 | 11.78 | BDL | 0.02 | 20.32 | BDL | 0.03 | 100.00 | 19.63 |
| 3 / high-Mg calcite (Ca0.68,Mg0.32)CO3 |
11.52 | 55.82 | 7.66 | 0.30 | 0.08 | 20.53 | 3.93 | 0.16 | 100.00 | 12.77 |
| 4 / proto-dolomite (Ca0.52,Mg0.48)CO3 |
11.82 | 55.57 | 11.52 | 0.05 | 0.02 | 20.86 | 0.15 | 0.01 | 100.00 | 19.20 |
| 5 / proto-dolomite (Ca0.51,Mg0.49)CO3 |
11.31 | 56.14 | 11.75 | BDL | 0.01 | 20.63 | 0.13 | 0.03 | 100.00 | 19.58 |
| 6 / proto-dolomite (Ca0.51,Mg0.49)CO3 |
13.05 | 56.67 | 11.68 | 0.03 | 0.04 | 18.41 | 0.08 | 0.04 | 100.00 | 19.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





