1. Introduction
Quinoa (Chenopodium quinoa Willd.) is a gluten-free grain, widely cultivated in South America, especially in the Andes region, and consumed for thousands of years by local populations. Its cultivation has spread in some European countries, as well as Australia and China, and has been increasingly used in the diet and has an incomparable nutritional value [
1,
2], with outstanding levels of protein, dietary fiber, vitamins, minerals, balanced concentration of essential amino acids, among many other benefits [
3,
4].
Furthermore, another interesting food, this one from Central America, is chia (Salvia hispanica), a protein-rich seed also having a high fiber content, widely used by the Aztec and Mayan civilizations as food and which also contains vitamins and minerals [
5,
6,
7]. One of the most outstanding characteristics of chia is its ability to form a viscous mucilage in contact with water, which can be exploited in the development of food, nutraceutical, and pharmaceutical formulations [
5,
8].
Both quinoa and chia offer a great perspective in the food market and are currently part of the effort to develop new fiber-rich food products [
9,
10,
11]. The inclusion of quinoa and chia as a source of fiber in nutraceutical and pharmaceutical products is still a challenge to be faced. However, for the development of formulations containing quinoa, it is necessary to extract saponins from the grains, due to the bitter residue in the oral mucosa [
12]. On the other hand, chia has a pleasant sweetness in contact with water or in the presence of any other food and the high nutritional value of this seed can be complemented if incorporated into a formulation containing quinoa [
13].
Therefore, given the growing interest in including more fiber in the diet, a practical form of consumption would be a supplement containing quinoa and chia in the form of chewable tablets. It is worth remembering that the dietary fiber content in quinoa is from 3.0-10.7% and chia is from 18.0-30.0% [
14,
15,
16].
One point that will be explored in the present study is the ability of chia to act as a binder for large amounts of quinoa to form a granule. Chia can form mucilage in contact with water and has a viscous liquid characteristic that can be an excellent binder for formulations that require the wet granulation step. On the other hand, to produce the pharmaceutical form of tablets, whose active ingredients are in high proportion and the flow is not adequate, it is necessary to include the wet granulation step, which is a process of aggregating smaller particles into larger particles, by using, for this purpose, a binding agent [
17,
18]. To obtain granules, it is important to determine the granulation point, by establishing the binding agent and the volume of liquid to be added. They must also present acceptable properties and characteristics to promote the excellent flow of the granulate so that tablets with adequate compressibility and compactability can subsequently be produced [
17,
19].
A tool that has been explored to determine the granulation point of pharmaceutical formulations is wet powder rheometry [
18,
20]. The equipment is the Mixer Torque Rheometer (MTR) which, by simple programming the method and weighing 15-22 grams of each sample, is capable of indicating the granulation point by the relation of torque (Nm) versus Binder Ratio (mg.mL). At the end of the experiment, a curve is generated indicating the volume of liquid to be used to obtain a granulate [
21,
22]. The physical characterization of solid particles (powders and granules) is essential for the mixing process (system homogenization), especially if the material is an integral part of the formulation. Tests such as flow properties, true density, and mechanical resistance can bring excellent results on the behavior and performance of granules.
Considering the gap in the scientific literature regarding the use of chia as a binder in nutraceutical and pharmaceutical formulations, the objective of the present work was to study, using torque rheometry, whether it is feasible to use chia, in powder form, as a binder for the production of quinoa granules, to obtain a formulation made of fibers for consumption.
2. Materials and Methods
2.1. Material
Quinoa grains and chia seeds were purchased from the Brazilian market (Taeq, São Paulo, Brazil) and processed to carry out the characterization study until the production of chewable tablets. Furthermore, microcrystalline cellulose - Avicel® PH101 (DuPont, Wilmington, USA) and purified water were used in the study.
2.2. Methods
2.2.1. Quinoa Washing, Drying and Grinding
For eliminating saponins from the grains, quinoa (350 grams) was submerged in a stainless-steel container with purified water in a ratio of 1:7 m/v (quinoa: water) for 48 hours at room temperature, with replacement of the water every 12 hours. After this treatment, the grains were washed twice (in the same proportion) with purified water at 40 ºC. Afterward, washing was completed with the same proportion of water at room temperature. Subsequently, the grains were put into an oven (Fabbe-Primar, São Paulo, Brazil) for drying at 50 ºC for 6 hours, followed by grinding for 3-5 minutes in an IKA A 11 Basic mill (IKA, Staufen, Germany) until obtaining a fine powder. The powder obtained was subjected to further drying in an oven for 3 hours. Humidity was monitored using a Moisture Analyzer (Mettler Toledo, Greifensee, Switzerland) considering a residual water content between 1-3%.
2.2.2. Chia Grinding
The chia seed was ground for 3-5 minutes in an IKA A 11 Basic mill (IKA, Staufen, Germany) until a fine powder was obtained. The powder obtained was dried in an oven (Fabbe-Primar, São Paulo, Brazil) at 50 ºC for 1 hour. Humidity was monitored using a Moisture Analyzer (Mettler Toledo, Greifensee, Switzerland) considering a residual water content between 1-3%.
2.2.3. Particle Size Analysis
The determination of the particle size of ground quinoa and chia was carried out using the laser diffraction Particle Size Analyzer 1090 (Cilas, Orleans, France). For both samples, the Fraunhofer method was used [
23] with operation in dry mode using the following parameters: ultrasound of 10 seconds, obscuration of 21%, frequency of 20 Hertz (Hz), pressure of 500 millibars (mb), measurement and cleaning time of 30 and 15 seconds for quinoa and chia, respectively. To complement it, SPAN was calculated, an independent model, which helps provide data on sample homogeneity [
18]. The results were obtained from the average of six measurements, calculated by the SizeExpert
® software.
2.2.4. Powder Flow Assessment
The flow of ground quinoa and chia samples was analyzed according to the methodology described by Da Silva et al., [
24].
2.2.5. True density
Tests for quinoa and chia powders and granules were carried out to evaluate the true density of the material by applying the methodology described by Da Silva et al., [
18]. The sample amount did not exceed two-thirds of the Microcell volume (4.5 cm3) and the calculation was carried out in g/cm3.
2.3. Design of Experiments
A fractional experimental design was used (
Table 1 and
Table 2), with the aid of the Statistica 13.0 software (Dell Inc., United States), to quantitatively evaluate the quinoa, the diluent (microcrystalline cellulose PH101) and the chia binder in the composition of the formulations. The experiments were performed in triplicate.
2.4. Mixer Torque Rheometer Equipment
The Mixer Torque Rheometer MTR (Caleva, Dorset, England) was investigated in the studies by Belem & Ferraz, 2020 and Da Silva et al., 2022 [
18,
25]. The methodology applied herein was multiple additions, 25 points with successive additions of 1 mL water, mixing time (60 seconds), data collection time (20 seconds), and weight of each sample (15 grams) (
Supplementary Materials: Video S1). At the end of the experiment, a curve was generated indicating the liquid/solid ratio for each experiment.
2.5. Statistical Analysis
The results were statistically analyzed using Statistica 13.0 software (Dell Inc., United States). An analysis of variance (ANOVA) was applied with a significance level of 5% (p = <0.05) and Pareto and response surface graphs were generated.
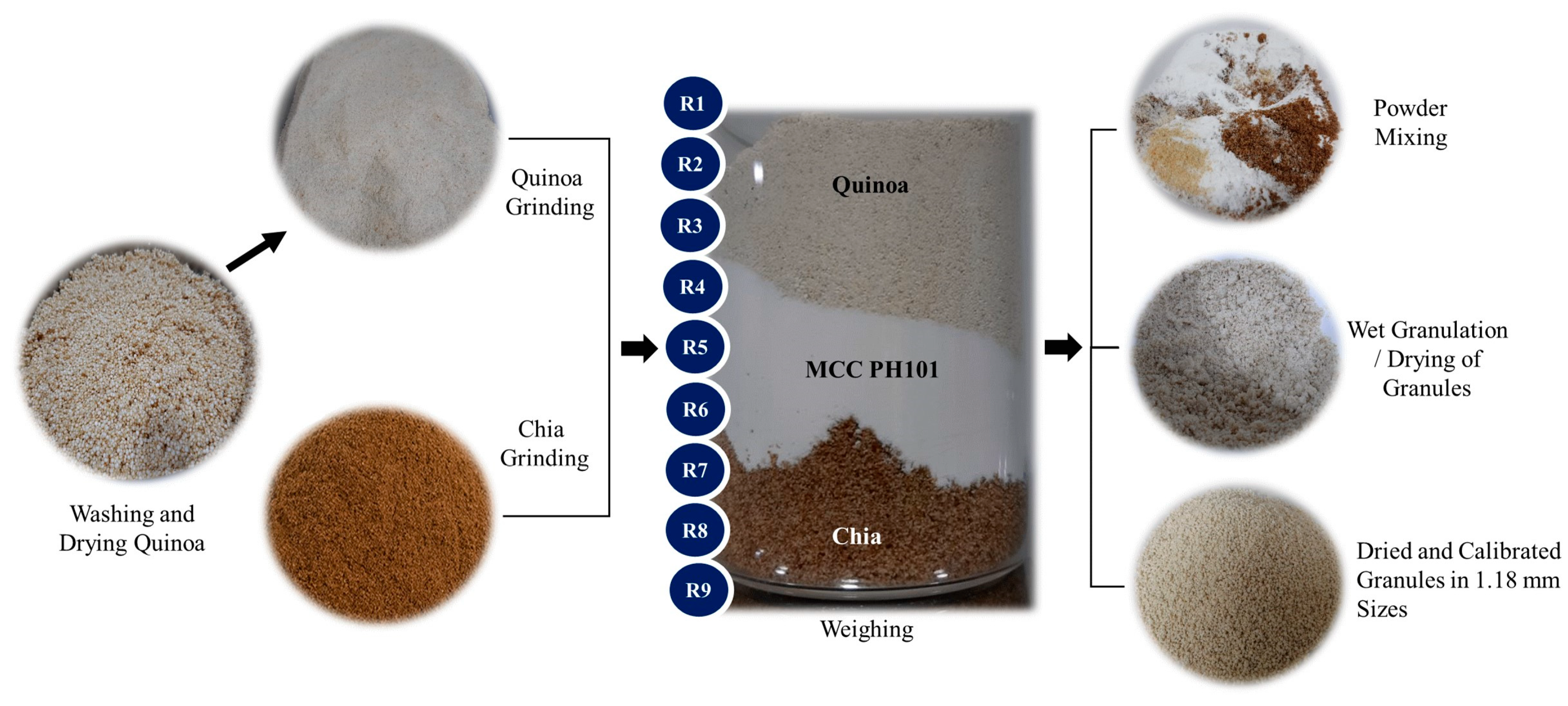
2.6. Granule Production
From the rheometry results, granules of the 9 formulations were produced according to the procedure described by Da Silva et al. (2022) [
18], with sufficient drying time to obtain a residual moisture of 1–3% (Moisture Analyzer - Mettler Toledo, Greifense, Switzerland).
2.6.1. Granules Characterization
The granules were characterized by their flow properties, true density, optical microscopy [
18], particle size distribution, and granule resistance [
26].
2.7. Production of Chewable Tablets
The tablets were produced on a Lemaq rotary compression machine - LM08D Mini Express (Lemaq, São Paulo, Brazil) in a 16 mm punch. R8 (high amount of quinoa) was selected with approximately 800 mg of quinoa per tablet. During the process, parameters such as average weight, hardness, thickness, diameter, friability, and disintegration time were monitored according to specifications required by the American Pharmacopoeia [
27].
3. Results and Discussion
3.1. Particle Size of Quinoa and Chia
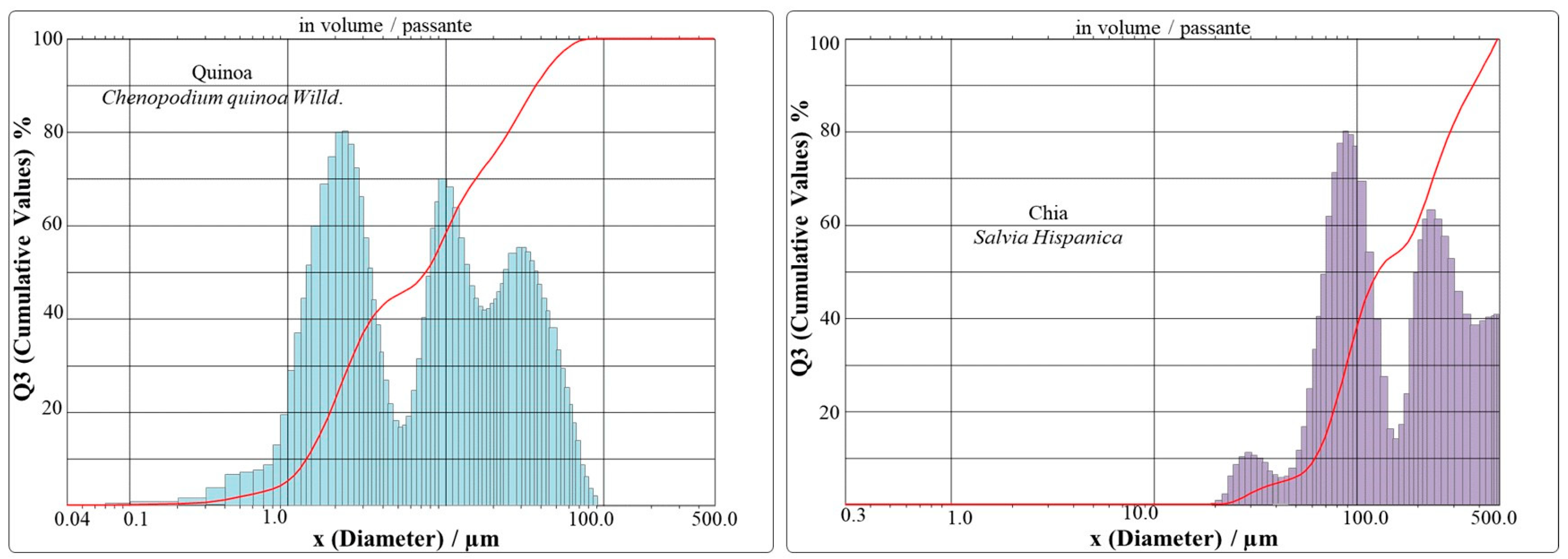
The average particle size of quinoa was 13.50 μm and the distribution curve demonstrated a possible agglomeration, with a bimodal and polydisperse behavior. On the other hand, chia showed a broad particle distribution curve (66.84-377.38 μm) with an average size of 186.50 μm. Both quinoa and chia indicated a heterogeneous type of material (
Figure 1 and
Table 3), which is when the material presents a wide range of particle sizes, with chia being the one that most represents the homogeneous system with the result closest to the SPAN (=1). This perspective of homogeneity is considered appropriate when values are closer to 1 [
28]. However, the equipment has a scale limitation and chia may have been harmed due to the reading range during the analysis. However, as our results indicate, 90% of the particles are below 377.38 µm. This may be due to the fibrous material characteristic of obovoidal to ellipsoidal shape with a rounded base and apex of chia seeds. When it was processed (ground), the irregular shape became a characteristic of the powder with many fibrous furrows remaining [
13].
The characteristics of pharmaceutical powders are essential to determine the specifications and ensure that they follow the product batch to batch. One of the main points is to determine the particle size, especially the input that makes up the largest amount in the formulation. In this case, in very small particles, wet granulation will certainly be part of the production process, because the influence of small particles in the direct compression step can considerably affect the compressibility and compactability of the powder [
29].
3.2. Flow Properties and True Density of Powders
In
Table 4, we demonstrate the results of flow properties and true density. According to the specifications of the American Pharmacopoeia [
27], the values are not indicative of adequate flow to produce formulations with excellent flow capacity, compactability, and compressibility.
In addition to determining the particle size characteristics of a material, we must ensure good flow properties of the formulation. Based on the results presented in
Table 4, it is possible to state that the process of producing chewable tablets by direct compression is not viable. Regarding true density, quinoa presents higher values when compared to chia. When a material has a low-density characteristic, especially when present in large amounts in the formulation, it is likely that these properties will face challenges about compactability and compressibility, especially when dealing with fibrous material such as quinoa [
29,
30].
3.3. Mixer Torque Rheometry (MTR)
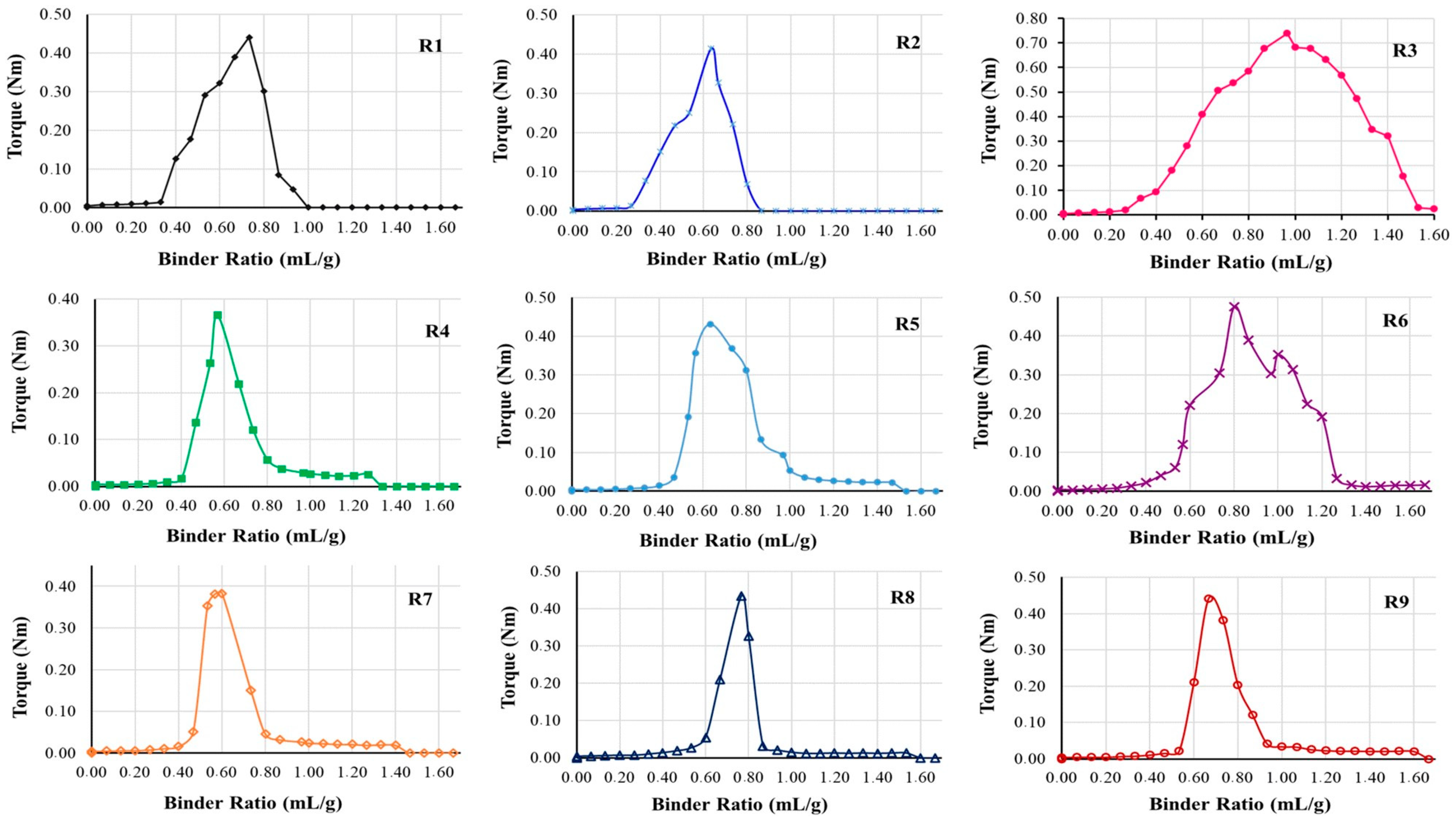
When observing the rheological profiles, the torques differed considerably, as did the amount of water used for each experiment. When using chia as a binder in formulations with a high fiber content (quinoa), a viscous dispersion behavior occurs that was capable of acting as a binder, forming, regardless of the concentration used, the aggregation of the particles of all planned formulations (
Figure 2 and
Table 5).
In each experiment, as the syringe dispensed water into the powder mixture in the rheometer compartment, a swelling process occurred which, in the case of chia, the powder in contact with water formed a viscous dispersion (mucilage) helping to aggregate the particles.
Quinoa particle size differs from chia and MCC PH101 (average 42.52 μm) [
18]. For this reason, each rheological profile showed a different behavior in both torque and water volume. When it comes to quinoa powder, a study showed that small particle sizes had a greater swelling capacity, that is, the original volume increased [
9]. We observed that this behavior corroborated the study carried out herein. On the other hand, although quinoa particles are smaller than those of MCC PH101, Formulations R3 and R6 absorbed the highest amount of water and this is a characteristic of cellulose, present in higher concentrations in these two cases.
MCC PH101 is widely applied in wet granulation and is known to aid granule strength [
31]. When evaluating the rheological profiles (
Figure 2), it is possible to observe that the peaks with the highest torques are those having a high concentration of MCC PH101 (R3 and R6). However, for formulations having a greater amount of MCC PH101, it is not necessary to add more binder to obtain more resistant granules, as chia fulfills its role even in a small proportion. From what can be seen, regardless of the concentration of chia used, the minimum amount (approximately 2%) is sufficient to form mucilage and agglutinate the particles to reach the capillary phase.
3.3.1. Statistical Analysis
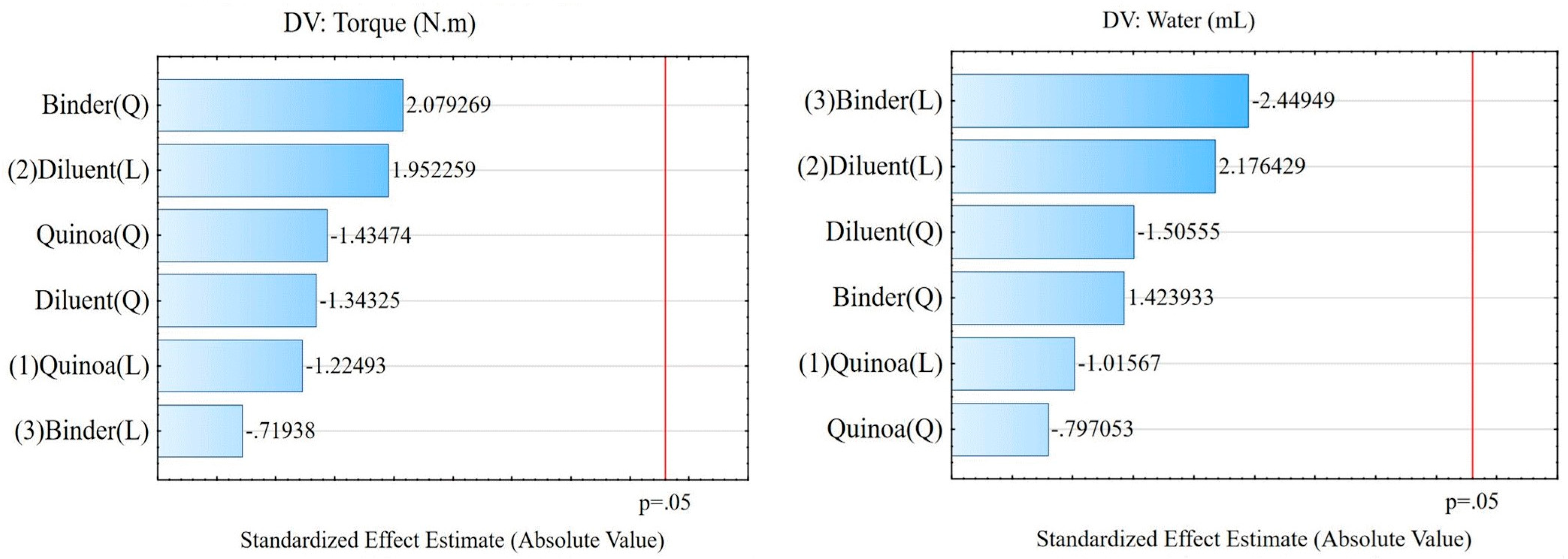
From ANOVA, the model without two-way interactions generated the results for comparing the variables, with values of p>0.050 for Torque N.m (p=0.885 for quinoa, p= 0.817 for diluent and p=0.796 for binder) and Ratio mL/g (p=0.879 for quinoa, p=0.765 for diluent and p= 0.645 for binder). To measure the relationships between torque and ratio variables and what they represent, the quantification of the correlation coefficient (R2) indicated for Torque (N.m) an R2=0.92304 and for Ratio (mL.g) an R2=0.84599. As a result, the model explained that there is no influence of the amount of quinoa, diluent, and binder on the Torque and Ratio responses, as demonstrated in the Pareto graphs presented in
Figure 3.
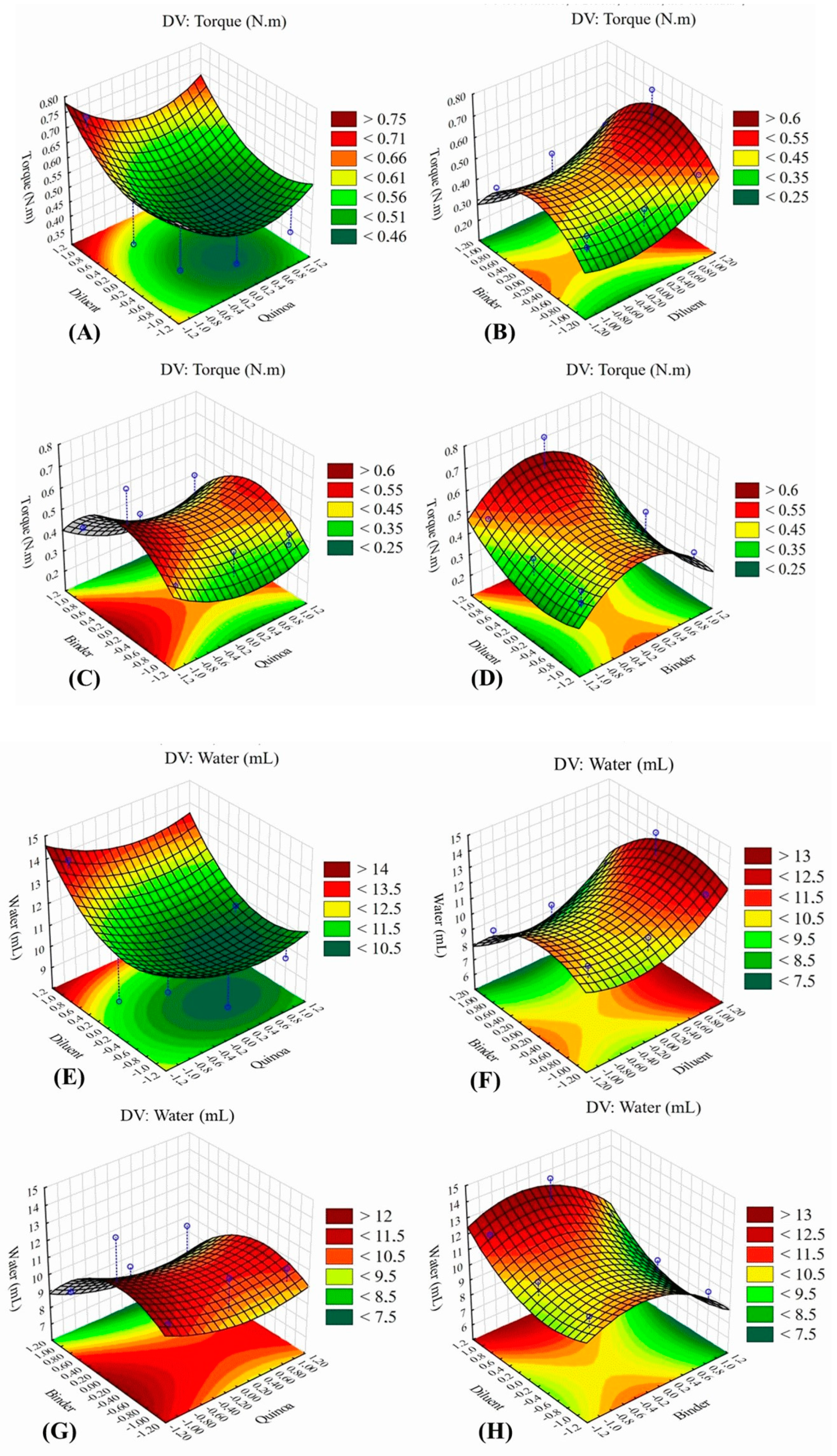
However, the response surface graphs demonstrated that the diluent concentration had an impact on the amount of water required to obtain the granulation point. As can be seen in
Figure 4, both for torque (N.m) and water (mL), the results indicated that an increase in the concentration of microcrystalline cellulose in the formulation seems to be able to influence the torque and volume of water required to reach the granulation point. For the water response (mL), it is possible to observe that the diluent required a greater volume of water to reach the granulation point when the proportion of quinoa was lower. On the other hand, for the chia binder, it was observed a well-distributed water demand, regardless of the amount of quinoa used.
The volume of water determined at the granulation point (
Table 5) was lower for lower concentrations of the diluent, that is, microcrystalline cellulose seems to condition the amount of water required for granulating the mixtures, as indicated in
Figure 4. The use of such an excipient in granules and tablets is interesting since it is a material that contributes to substantially improving the cohesiveness and compactability characteristics of formulations [
32].
It is important to highlight that for chia, even higher concentrations of powder are used, and the water demand to reach the granulation point was not impacted, remaining practically the same (
Table 5). This aspect is very interesting because the binder does not require greater amounts of water, which is desirable in wet granulation. It is worth noting that all water added to the formulation must be later removed by drying, that is, the smaller the amount of water, the shorter the drying time required.
One of the highlights of this work is the fact that the concentration of quinoa in the formulations had no effect on the torque and amount of water for reaching the granulation point. Furthermore, it should be noted that to supplement fiber in the diet, it is necessary to use high amounts thereof, thus the obtaining of granules with a large amount of quinoa will enable the large-scale production of tablets having high doses of dietary fiber.
3.4. Granule Production
After the study using MTR, the formulations were prepared individually as illustrated in
Figure 5.
During the process of producing granulates, it was observed that formulations containing chia in high concentrations (R2, R3, and R4) were more difficult to pass through the sieve after adding water and this may be due to the formation of excess mucilage. Visually, it seems that it is not necessary to add high concentrations of chia to obtain the granulation point.
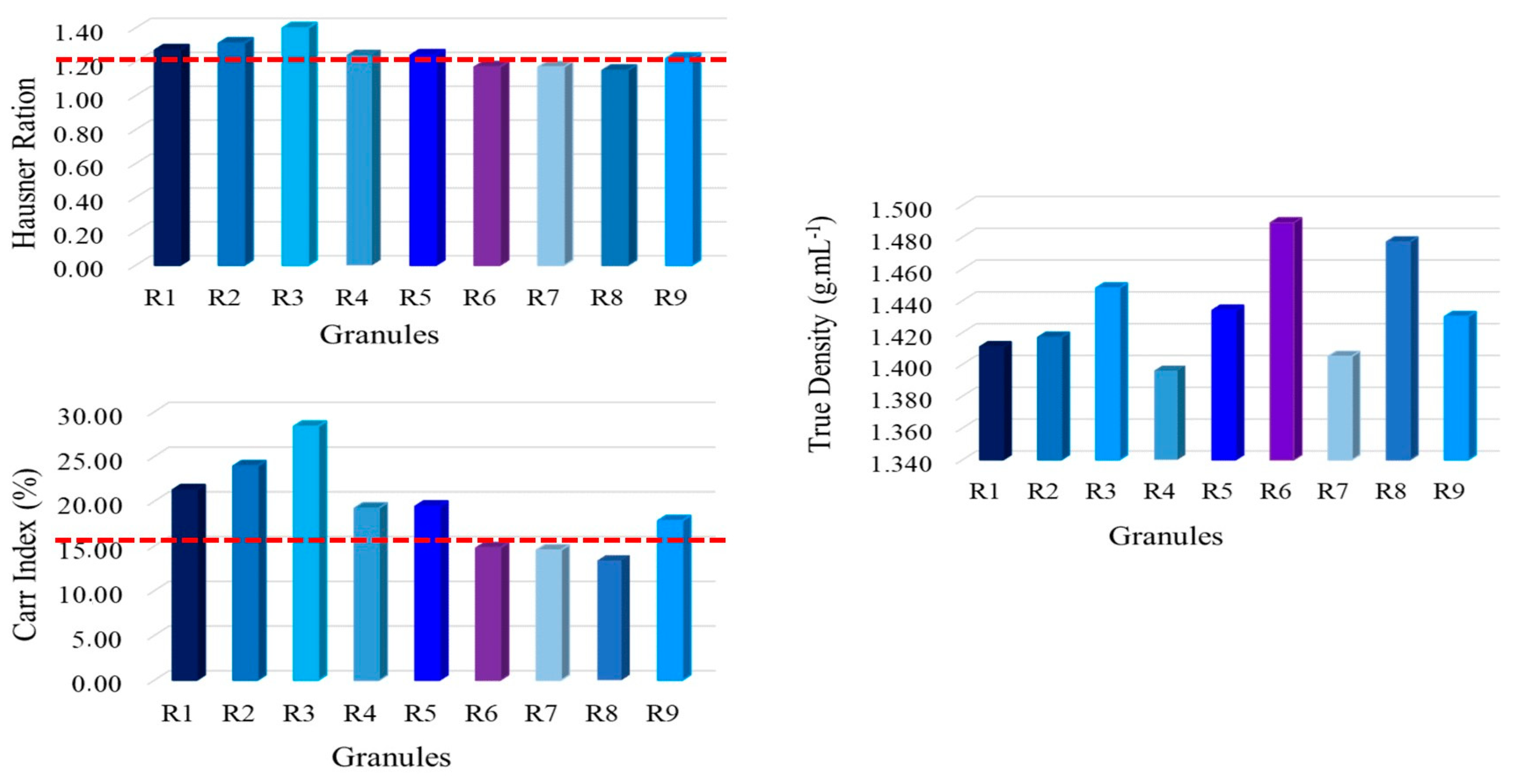
3.5. Physical Characterization of Granules
3.5.1. Granule Flow and True Density
The granule flow and true density results of the formulations are presented in
Figure 6.
For a tablet formulation to have adequate compressibility and compactability, the flow mustn't negatively impact the process. In this sense, an essential critical attribute is the cohesion of the formulation, considered significant in the manufacturing process of any solid pharmaceutical form [
30,
33]). When the flow of a material is considered suitable for formulation development, it means that in the compressibility stage, the particles will have an affinity to adhere to each other, mainly due to the size and shape of the particles being one of the factors that most contribute to this behavior [
30,
34].
The formulations that exhibited exceptional flow properties, with a value of < 15%, were R8, R7, and R6, respectively. The other formulations have a reasonable flow rate or assistance is required, which in both cases can be solved with the addition of excipients that help with the fluidity of the powder, for example, a rheology promoter such as silicon dioxide [
35], a diluent to aid filling and compressibility such as microcrystalline cellulose, lactose or co-processed products [
36], and lubricants such as magnesium stearate [
37].
The true density of the formulations is similar and R4 stood out with a lower density among them, which could be due to the lack of balance in microcrystalline cellulose and the presence of chia in high amounts in the mixture, corroborating that chia has a low density as shown in
Table 4.
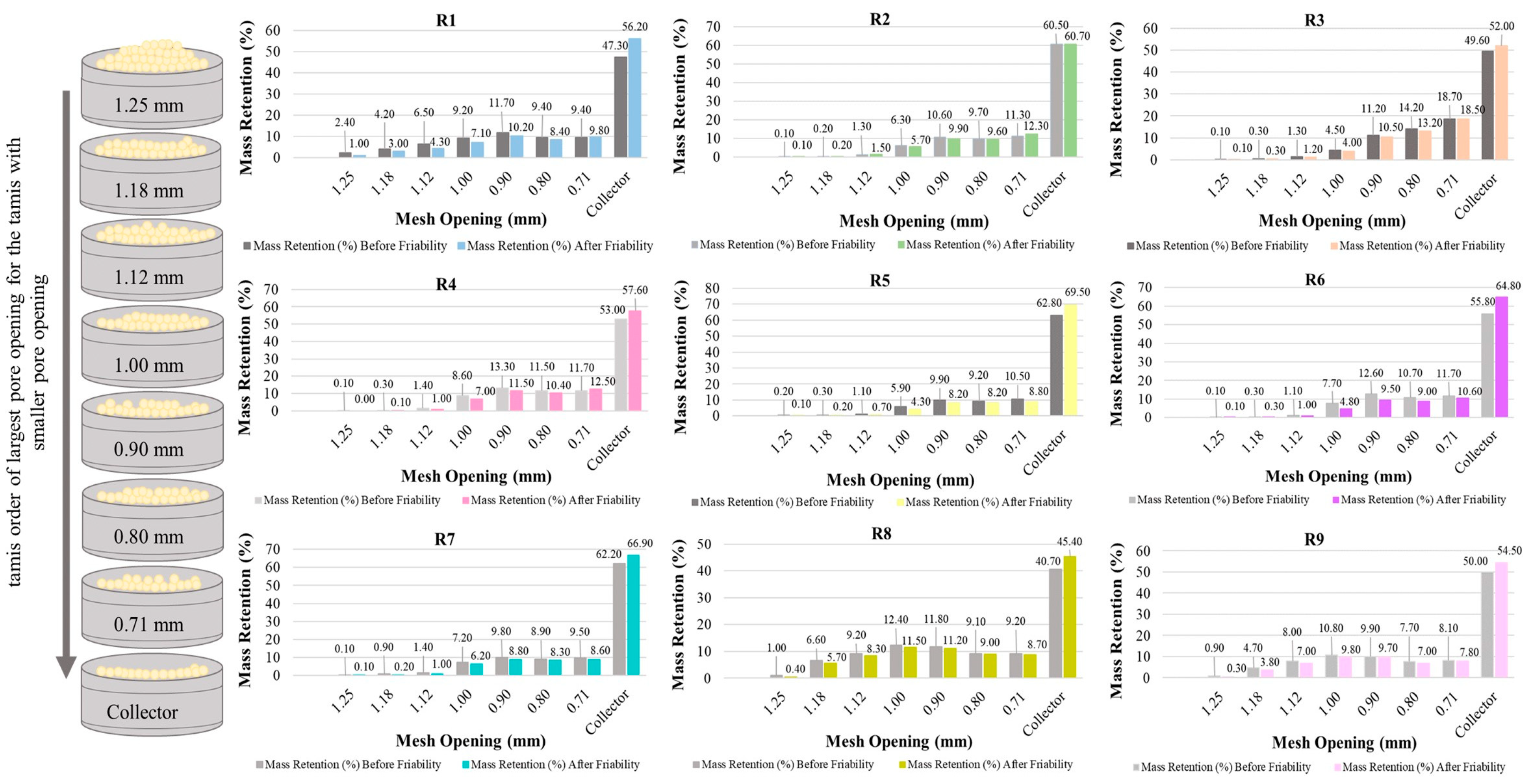
3.5.2. Granulometric Distribution and Granule Strength
The granulometric distribution of the formulations is presented in
Figure 7, where it is possible to verify that R8, R1, and R9 were those that stood out concerning the efficient production of a granulate, presenting a better distribution in the range of 1.18 to 0.8 mm, which represents a suitable size for the granules.
Another important characteristic of the granulate is its resistance shown in
Figure 7 and
Table 6, where it is possible to verify that R2, R3, R9, and R8 have greater resistance. However, it is important to emphasize that R2 did not stand out in particle size distribution, thus being considered an inappropriate formulation.
It is interesting to note that R8 contains 73.17% quinoa with only 2.44% chia as a binder, which makes it a standout among the formulations with the best particle size.
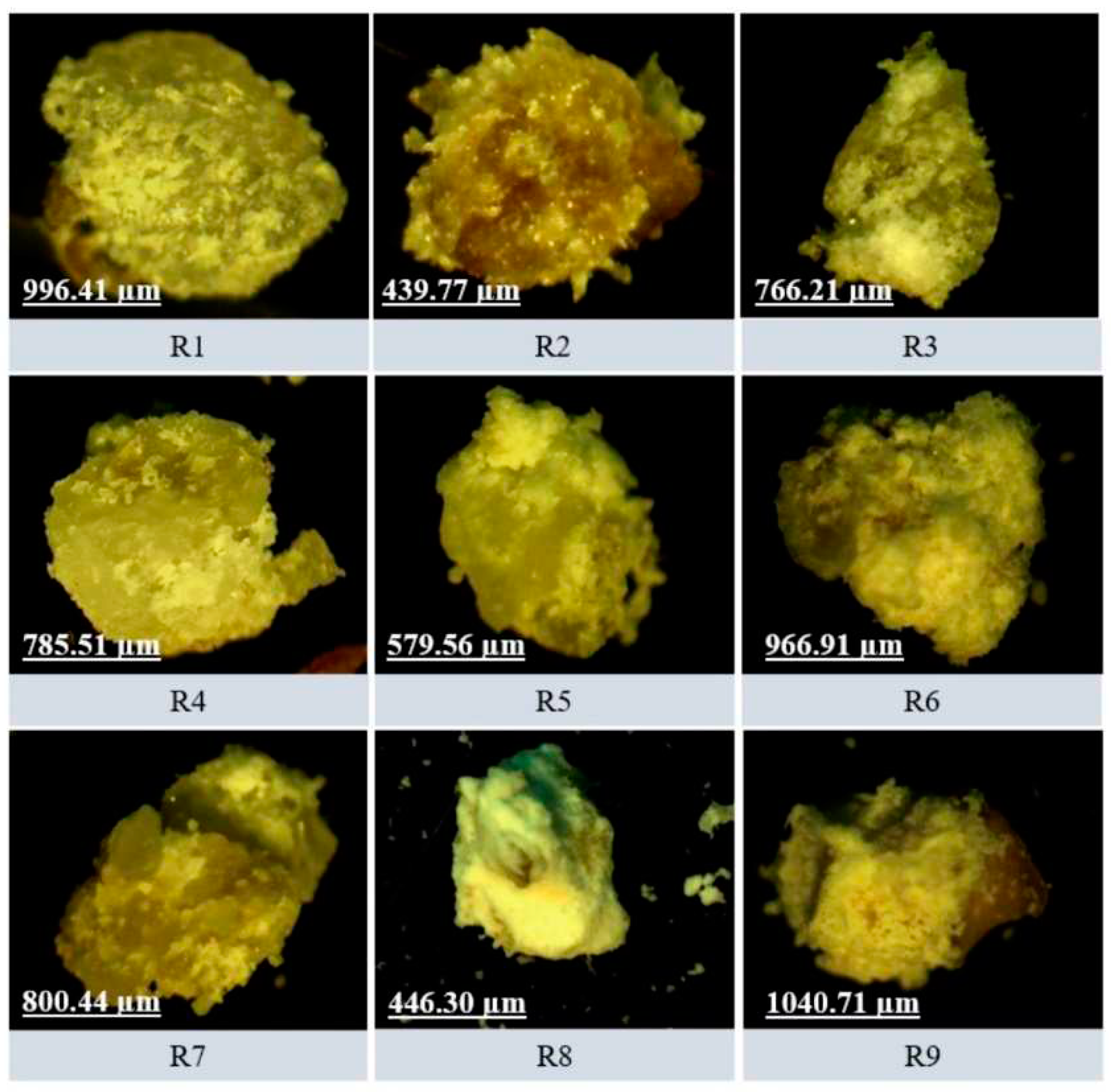
3.5.3. Optical Microscopy
The diameter values of the granules for each formulation are represented in
Figure 8. As it is a fibrous material, we can observe that there are no spherical granules but rather irregular ones, with a variable polydispersity index, which is possibly a characteristic of this type of material [
38]. It is noted that the granules are heterogeneous and have a wide size range varying from 439.00 to 1000.00 µm in average size.
Given the results presented, we noted that it is necessary to work with wet granulation for the fact that quinoa is an integral part of the formulation. As a result, the characteristics of the quinoa powder observed in
Table 4 and
Figure 3 (poor flow and small particle size), in a direct compression process, would not enable efficient production, since the cohesion of the powder would not be sufficient to form tablets. The investigation into wet powder rheometry using chia as a binder highlighted the significant role chia plays in the granulation of powders. Although some granulated formulations still require assistance with the addition of an excipient to avoid compromising the flow, as a whole, chia seems to be an excellent binder for formulations containing fibers. By using chia acting as a binder in quinoa, the formulation acquires qualities rich in fiber content, making it suitable for daily consumption as recommended by health authorities.
3.6. Production of Chewable Tablets
Our results showed that the transformation of quinoa and chia powders into granules was able to significantly improve their flow characteristics and, among the formulations, we selected R8, which contains a greater amount of quinoa and a lower concentration of chia for binding (
Figure 9). This demonstrates that a higher concentration of chia is not necessary to aggregate particles from a considerable volume of quinoa. By using just 2% chia, we can obtain excellent physical properties of the granulate. In this way, it was possible to enable the production of a batch of chewable tablets which, during the production process, all parameters were measured and controlled as described in the American Pharmacopoeia [
27].
5. Conclusions
Given the rheological studies conducted herein, it is possible to verify that chia (Salvia hispanica) can act efficiently as a binder, allowing the obtaining of granules with a high concentration of quinoa, which can then be used in tablet formulations for use as a dietary fiber supplement.
Chia has an interesting attribute of aggregating quinoa particles, and the chia-quinoa combination has fibrous characteristics that can increase the fiber content for consumption. To aggregate the particles, chia mucilage efficiently promotes the granulation point of formulations with varying concentrations of quinoa, which means that chia can be an excellent material binder that is an integral part of a formulation, regardless of the concentration applied.
Chia can be considered a dispersing agent to bind a fibrous material, such as quinoa. Both quinoa and chia can be used to develop chewable tablets.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Video S1: Wet Granulation Using Chia as a Binder to Form Quinoa Granulate.
Author Contributions
Conceptualization: R. P. S. (Rosana Pereira da Silva), F. J. V. R. (Fanny Judhit Vereau Reyes); Data curation: R. P. S. (Rosana Pereira da Silva), F. J. V. R. (Fanny Judhit Vereau Reyes); Formal Analysis: R. P. S. (Rosana Pereira da Silva); Funding acquisition: Not applicable; Investigation: R. P. S. (Rosana Pereira da Silva), F. J. V. R. (Fanny Judhit Vereau Reyes); Methodology: R. P. S. (Rosana Pereira da Silva), F. J. V. R. (Fanny Judhit Vereau Reyes), J. E. S. P. (Julia Estevam da Silva Pestana); Supervision: H. G. F. (Humberto Gomes Ferraz); Visualization: R. P. S. (Rosana Pereira da Silva); Writing—original draft preparation: R. P. S. (Rosana Pereira da Silva); Writing—review and editing: R. P. S. (Rosana Pereira da Silva), F. J. V. R. (Fanny Judhit Vereau Reyes), J. S. P. D. (Josiane Souza Pereira Daniel), J. E. S. P. (Julia Estevam da Silva Pestana), S. A. P. (Samara de Almeida Pires), H. G. F. (Humberto Gomes Ferraz). All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific subsidies from funding agencies in the public, commercial, or non-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The processed data required to reproduce these results are included in the Materials and Methods section. Raw data are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Angeli, V.; Silva, P.M.; Massuela, D.C.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium quinoa Willd.): An Overview of the Potentials of the "Golden Grain" and Socio-Economic and Environmental Aspects of Its Cultivation and Marketization. Foods 2020, 9, 216. [CrossRef]
- Hinojosa, L.; Leguizamo, A.; Carpio, C.; Munoz, D.; Mestanza, C.; Ochoa, J.; Castillo, C.; Murillo, A.; Villacréz, E.; Monar, C.; et al. Quinoa in Ecuador: Recent Advances under Global Expansion. Plants (Basel) 2021, 10, 298. [CrossRef]
- Jancurová, M.; Minarovicová, L.; Dandár, A. Quinoa - a Review. Czech J. Food Sci. 2009, 27, 71-79. [CrossRef]
- Alonso-Miravalles, L.; O'Mahony, J.A. Composition, Protein Profile and Rheological Properties of Pseudocereal-Based Protein-Rich Ingredients. Foods 2018, 7, 73. [CrossRef]
- Segura-Campos, M.R.; Ciau-Solís, N.; Rosado-Rubio, G.; Chel-Guerrero, L.; Betancur-Ancona, D. Chemical and Functional Properties of Chia Seed (Salvia hispanica L.) Gum. Int. J. Food Sci. 2014, 2014, 241053. [CrossRef]
- Da Silva, B.P.; Dias, D.M.; Moreira, M.E.D.; Toledo, R.C.L.; da Matta, S.L.P.; Della Lucia, C.M.; Martino, H.S.D.; Pinheiro-Sant'Ana, H.M. Chia Seed Shows Good Protein Quality, Hypoglycemic Effect and Improves the Lipid Profile and Liver and Intestinal Morphology of Wistar Rats. Plant Foods Hum. Nutr. 2016, 71, 225-230. [CrossRef]
- Ding, Y.; Lin, H.W.; Lin, Y.L.; Yang, D.J.; Yu, Y.S.; Chen, J.W.; Wang, S.Y.; Chen, Y.C. Nutritional composition in the chia seed and its processing properties on restructured ham-like products. J. Food Drug Anal. 2018, 26, 124-134. [CrossRef]
- Felisberto, M.H.F.; Wahanik, A.L.; Gomes-Ruffi, C.R.; Clerici, M.T.P.S.; Chang, Y.K.; Steel, C.J. Use of chia (Salvia hispanica L.) mucilage gel to reduce fat in pound cakes. Lwt-Food Sci. Technol. 2015, 63, 1049-1055. [CrossRef]
- Cotovanu, I.; Batariuc, A.; Mironeasa, S. Characterization of Quinoa Seeds Milling Fractions and Their Effect on the Rheological Properties of Wheat Flour Dough. Appl. Sci. (Basel) 2020, 10, 7225. [CrossRef]
- Muñoz-Pabon, K.S.; Roa-Acosta, D.F.; Hoyos-Concha, J.L.; Bravo-Gómez, J.E.; Ortiz-Gómez, V. Quinoa Snack Production at an Industrial Level: Effect of Extrusion and Baking on Digestibility, Bioactive, Rheological, and Physical Properties. Foods 2022, 11, 3383. [CrossRef]
- Afzal, I.; Ul Haq, M.Z.; Ahmed, S.; Hirich, A.; Bazile, D. Challenges and Perspectives for Integrating Quinoa into the Agri-Food System. Plants (Basel) 2023, 12, 3361. [CrossRef]
- Graf, B.L.; Rojas-Silva, P.; Rojo, L.E.; Delatorre-Herrera, J.; Baldeon, M.E.; Raskin, I. Innovations in Health Value and Functional Food Development of Quinoa (Chenopodium quinoa Willd.). Compr. Rev. Food Sci. Food Saf. 2015, 14, 431-445. [CrossRef]
- Vera-Cespedes, N.; Muñoz, L.A.; Rincón, M.Á.; Haros, C.M. Physico-Chemical and Nutritional Properties of Chia Seeds from Latin American Countries. Foods 2023, 12, 3013. [CrossRef]
- Maradini Filho, A.M.; Pirozi, M.R.; Borges, J.T.d.S.; Sant'Ana, H.M.P.; Chaves, J.B.P.; Coimbra, J.S.D.R. Quinoa: Nutritional, functional, and antinutritional aspects. Crit. Rev. Food Sci. Nutr. 2017, 57, 1618-1630. [CrossRef]
- Fernandes, S.S.; Salas-Mellado, M.d.l.M. Addition of chia seed mucilage for reduction of fat content in bread and cakes. Food Chem. 2017, 227, 237-244. [CrossRef]
- Goyat, J.; Passi, S.J.; Suri, S.; Dutta, H. Development of Chia (Salvia Hispanica, L.) and Quinoa (Chenopodium Quinoa, L.) Seed Flour Substituted Cookies- Physicochemical, Nutritional and Storage Studies. Curr. Res. Nutr. Food. Sci. 2018, 6, 757-769. [CrossRef]
- Suresh, P.; Sreedhar, I.; Vaidhiswaran, R.; Venugopal, A. A comprehensive review on process and engineering aspects of pharmaceutical wet granulation. Chem. Eng. J. 2017, 328, 785-815. [CrossRef]
- Da Silva, R.P.; Fante, A.S.; Silva, A.R.P.; Pereira, F.L.S.; Gutierrez, Y.L.R.; Ferraz, H.G. Wet powder rheometry: The best conditions for wet granulation using diluent and binder in calcium carbonate samples. Powder Technol. 2022, 397, 117087. [CrossRef]
- Otsuka, T.; Kuroiwa, Y.; Sato, K.; Yamashita, K.; Hakomori, T.; Kimura, S.; Iwao, Y.; Itai, S. Use of Mixer Torque Rheometer to Clarify the Relationship between the Kneading States of Wet Mass and the Dissolution of Final Product in High Shear Granulation. Chem. Pharm. Bull. 2018, 66, 554-561. [CrossRef]
- Ibrahim, M.A.; Zayed, G.M.; Alsharif, F.M.; Abdelhafez, W.A. Utilizing mixer torque rheometer in the prediction of optimal wet massing parameters for pellet formulation by extrusion/spheronization. Saudi Pharm. J. 2019, 27, 182-190. [CrossRef]
- Zhang, S.Y.; Lamberto, D.J. Development of New Laboratory Tools for Assessment of Granulation Behavior During Bulk Active Pharmaceutical Ingredient Drying. J. Pharm. Sci. 2014, 103, 152-160. [CrossRef]
- Kuhs, M.; Moore, J.; Kollamaram, G.; Walker, G.; Croker, D. Predicting optimal wet granulation parameters for extrusion-spheronisation of pharmaceutical pellets using a mixer torque rheometer. Int. J. Pharm. 2017, 517, 19-24. [CrossRef]
- Di Stefano, C.; Ferro, V.; Mirabile, S. Comparison between grain-size analyses using laser diffraction and sedimentation methods. Biosyst. Eng. 2010, 106, 205-215. [CrossRef]
- Da Silva, R. P.; Kawai, G. S. D.; Andrade, F. R. D. D.; Bezzon, V. D. N.; Ferraz, H. G. Characterisation and traceability of calcium carbonate from the seaweed Lithothamnium calcareum. Solids, 2021, 2 (2), 192–211. [CrossRef]
- Belem, B.R.; Ferraz, H.G. Rheological profile in mixer torque rheometer of samples containing furazolidone and different binders. Chem. Eng. Res. Des. 2020, 160, 533-539. [CrossRef]
- Issa, M.G.; Pessole, L.; Takahashi, A.I.; Andreo, N.; Ferraz, H.C. Physicochemical and dissolution profile characterization of pellets containing different binders obtained by the extrusion-spheronization process. Braz. J. Pharm. Sci. 2012, 48, 379-388. [CrossRef]
- United States Pharmacopeia Convention. The United States pharmacopeia: The national formulary, 1th ed.; United States Pharmacopeial Convention: Rockville, Maryland, 2022.
- Horiba Scientific. A Guidebook to Particle Size Analysis; Horiba Instruments: Irvine, Ca, 2017.
- Wünsch, I.; Finke, J.H.; John, E.; Juhnke, M.; Kwade, A. The influence of particle size on the application of compression and compaction models for tableting. Int. J. Pharm. 2021, 599, 120424. [CrossRef]
- Leung, L.Y.; Mao, C.; Srivastava, I.; Du, P.; Yang, C.Y. Flow Function of Pharmaceutical Powders Is Predominantly Governed by Cohesion, Not by Friction Coefficients. J Pharm Sci 2017, 106, 1865-1873. [CrossRef]
- Hiremath, P.; Nuguru, K.; Agrahari, V.; Narang, A.S.; Badawy, S.I.F. Chapter 8 - Material Attributes and Their Impact on Wet Granulation Process Performance. Academic Press: 2019; pp. 263-315.
- Tank, D.; Karan, K.; Gajera, B.; Dave, R.H. Investigate the effect of solvents on wet granulation of microcrystalline cellulose using hydroxypropyl methylcellulose as a binder and evaluation of rheological and thermal characteristics of granules. Saudi Pharm. J. 2018, 26, 593-602. [CrossRef]
- Jones-Salkey, O.; Chu, Z.; Ingram, A.; Windows-Yule, C.R.K. Reviewing the Impact of Powder Cohesion on Continuous Direct Compression (CDC) Performance. Pharmaceutics 2023, 15, 1587. [CrossRef]
- Chattoraj, S.; Sun, C.C. Crystal and Particle Engineering Strategies for Improving Powder Compression and Flow Properties to Enable Continuous Tablet Manufacturing by Direct Compression. J. Pharm. Sci. 2018, 107, 968-974. [CrossRef]
- Tadauchi, T.; Yamada, D.; Koide, Y.; Yamada, M.; Shimada, Y.; Yamazoe, E.; Ito, T.; Tahara, K. Improving the Powder Properties of an Active Pharmaceutical Ingredient (Ethenzamide) with a Silica Nanoparticle Coating for Direct Compaction into Tablets. Powders 2022, 1, 231-242. [CrossRef]
- Schönfeld, B.V.; Westedt, U.; Wagner, K.G. Compression Modulus and Apparent Density of Polymeric Excipients during Compression-Impact on Tabletability. Pharmaceutics 2022, 14, 913. [CrossRef]
- Morin, G.; Briens, L. The Effect of Lubricants on Powder Flowability for Pharmaceutical Application. Aaps PharmSciTech 2013, 14, 1158-1168. [CrossRef]
- Luo, Y.; Ni, F.T.; Guo, M.Z.; Liu, J.; Chen, H.; Zhang, S.T.; Li, Y.L.; Chen, G.; Wang, G. Quinoa starch microspheres for drug delivery: preparation and their characteristics. Food Science and Technology 2022, 42, e126421. [CrossRef]
Figure 1.
Histogram of the particle size distribution of quinoa and chia samples.
Figure 1.
Histogram of the particle size distribution of quinoa and chia samples.
Figure 2.
Calculated mean (duplicate) of multiple additions in MTR for different proportions of quinoa, MCC PH101 (diluent), and chia (binder).
Figure 2.
Calculated mean (duplicate) of multiple additions in MTR for different proportions of quinoa, MCC PH101 (diluent), and chia (binder).
Figure 3.
Pareto graphs that represented the analysis of variance of torque and ratio parameters.
Figure 3.
Pareto graphs that represented the analysis of variance of torque and ratio parameters.
Figure 4.
Response surface graphs that indicated the effect on Torque (N.m) with diluent-quinoa (A), the effect on Torque (N.m) with binder-diluent (B), the effect on Torque (N.m) with binder-quinoa (C), the effect on Torque (N.m) with the diluent-binder (D). Likewise, the response surface graphs that indicated the effect on Water (mL) with diluent-quinoa (E), the effect on Water (mL) with binder-diluent (F), the effect on Water (mL) with quinoa-binder (G), the effect on Water (mL) with the binder-diluent (H).
Figure 4.
Response surface graphs that indicated the effect on Torque (N.m) with diluent-quinoa (A), the effect on Torque (N.m) with binder-diluent (B), the effect on Torque (N.m) with binder-quinoa (C), the effect on Torque (N.m) with the diluent-binder (D). Likewise, the response surface graphs that indicated the effect on Water (mL) with diluent-quinoa (E), the effect on Water (mL) with binder-diluent (F), the effect on Water (mL) with quinoa-binder (G), the effect on Water (mL) with the binder-diluent (H).
Figure 5.
Design for granulate production.
Figure 5.
Design for granulate production.
Figure 6.
Granule flow (Carr Index %, Hausner Ration) and true density of the formulations determined in the DoE.
Figure 6.
Granule flow (Carr Index %, Hausner Ration) and true density of the formulations determined in the DoE.
Figure 7.
Granule resistance tests were carried out on sieves before and after the friability of the formulations.
Figure 7.
Granule resistance tests were carried out on sieves before and after the friability of the formulations.
Figure 8.
The average size of the granules in optical microscopy with 3.0 and 4.0x objectives, which represent an increase of 30 to 40x, respectively. The amplitude of the captured image (in width and height) varies from 1500 to 2500 µm.
Figure 8.
The average size of the granules in optical microscopy with 3.0 and 4.0x objectives, which represent an increase of 30 to 40x, respectively. The amplitude of the captured image (in width and height) varies from 1500 to 2500 µm.
Figure 9.
Chewable tablets (R8) produced by wet granulation from the rheometer study (MTR).
Figure 9.
Chewable tablets (R8) produced by wet granulation from the rheometer study (MTR).
Table 1.
Variables and respective levels (-1, 0, +1) used in the fractional factorial experimental design. Amounts are described in parts.
Table 1.
Variables and respective levels (-1, 0, +1) used in the fractional factorial experimental design. Amounts are described in parts.
| Variable |
Level |
| -1 |
0 |
1 |
| Quinoa |
20 |
40 |
60 |
| Microcrystalline Cellulose PH101 |
10 |
20 |
30 |
| Chia |
2 |
15 |
20 |
Table 2.
Test matrix with fractional factorial of (33-1) with 9 formulations. Amounts in parts with their respective weighing values in grams and percentage.
Table 2.
Test matrix with fractional factorial of (33-1) with 9 formulations. Amounts in parts with their respective weighing values in grams and percentage.
| Run |
Quinoa |
Microcrystalline cellulose PH101 |
Chia |
| Part |
grams |
% |
Part |
grams |
% |
Part |
Grams |
% |
| 9 |
60 |
8.18 |
54.55 |
30 |
4.09 |
27.27 |
20 |
2.73 |
18.18 |
| 3 |
20 |
4.62 |
30.77 |
30 |
6.92 |
46.15 |
15 |
3.46 |
23.08 |
| 8 |
60 |
10.98 |
73.17 |
20 |
3.66 |
24.39 |
2 |
0.37 |
2.44 |
| 6 |
40 |
8.33 |
55.55 |
30 |
6.25 |
41.67 |
2 |
0.42 |
2.78 |
| 5 |
40 |
8.00 |
53.33 |
20 |
4.00 |
26.67 |
15 |
3.00 |
20.00 |
| 4 |
40 |
8.57 |
57.14 |
10 |
2.14 |
14.29 |
20 |
4.29 |
28.57 |
| 2 |
20 |
5.00 |
33.33 |
20 |
5.00 |
33.33 |
20 |
5.00 |
33.33 |
| 1 |
20 |
9.38 |
62.50 |
10 |
4.69 |
31.25 |
2 |
0.94 |
6.25 |
| 7 |
60 |
10.59 |
70.59 |
10 |
1.76 |
11.76 |
15 |
2.65 |
17.65 |
Table 3.
Values of d10%, d50%, and d90%, mean and ± standard deviation, and span of quinoa and chia samples obtained from dry particle size analysis.
Table 3.
Values of d10%, d50%, and d90%, mean and ± standard deviation, and span of quinoa and chia samples obtained from dry particle size analysis.
| Samples |
d10
|
d50
|
d90
|
Average |
Span |
| Quinoa powder |
1.31 ± 0.01 |
7.09 ± 0.30 |
37.60 ± 0.19 |
13.50 ± 0.07 |
5.12 |
| Chia powder |
66.84 ± 2.15 |
135.30 ± 3.47 |
377.38 ± 5.52 |
186.50 ± 3.66 |
2.29 |
Table 4.
Flow of samples: apparent density (g.mL-1), compressed density (g.mL-1), Carr Index (IC%), Hausner’s Ratio (RH), and true density (g.mL-1) ± standard deviation.
Table 4.
Flow of samples: apparent density (g.mL-1), compressed density (g.mL-1), Carr Index (IC%), Hausner’s Ratio (RH), and true density (g.mL-1) ± standard deviation.
| Samples |
Apparent density |
Compressed density |
Carr Index |
Hausner`s Ratio |
True density |
| Quinoa powder |
0.50 ± 0.05 |
0.82 ± 0.01 |
39.02 ± 0.01 |
1.64 ± 0.11 |
1.519 ± 0.001 |
| Chia powder |
0.44 ± 0.01 |
0.59 ± 0.01 |
25.42 ± 0.02 |
1.34 ± 0.01 |
1.330 ± 0.010 |
Table 5.
Average maximum torque (Nm), liquid/solid ratio (mL/g), and water volume at the granulation point of the experiments and ± standard deviation.
Table 5.
Average maximum torque (Nm), liquid/solid ratio (mL/g), and water volume at the granulation point of the experiments and ± standard deviation.
| Run |
Average Torque (N.m) |
Average Binder Ratio (mg/mL) |
Average Water Volume (mL) |
| R1 |
0.440 ± 0.051 |
0.733 ± 0.200 |
11.00 |
| R2 |
0.415 ± 0.036 |
0.634 ± 0.034 |
9.00 |
| R3 |
0.739 ± 0.013 |
0.967 ± 0.034 |
14.00 |
| R4 |
0.366 ± 0.006 |
0.567 ± 0.034 |
9.00 |
| R5 |
0.431 ± 0.005 |
0.634 ± 0.034 |
10.00 |
| R6 |
0.475 ± 0.087 |
0.800 ± 0.067 |
12.00 |
| R7 |
0.383 ± 0.025 |
0.600 ± 0.067 |
10.00 |
| R8 |
0.435 ± 0.060 |
0.767 ± 0.167 |
11.00 |
| R9 |
0.441 ± 0.017 |
0.667 ± 0.067 |
10.00 |
Table 6.
Particle size distribution in the range of 1.18-0.80 mm before and after the friability test.
Table 6.
Particle size distribution in the range of 1.18-0.80 mm before and after the friability test.
| Granules |
Mass Retention Before Friability (%) |
Mass Retention
After Friability (%) |
Difference in Mass
Retention (%) |
| R1 |
41.00 |
33.00 |
8.00 |
| R2 |
28.10 |
26.90 |
1.20 |
| R3 |
31.50 |
29.20 |
2.30 |
| R4 |
35.10 |
30.00 |
5.10 |
| R5 |
26.40 |
21.60 |
4.80 |
| R6 |
32.40 |
24.60 |
7.80 |
| R7 |
28.20 |
24.50 |
3.70 |
| R8 |
48.10 |
45.70 |
3.40 |
| R9 |
40.59 |
37.30 |
3.29 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).