Submitted:
04 December 2023
Posted:
05 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results and discussion
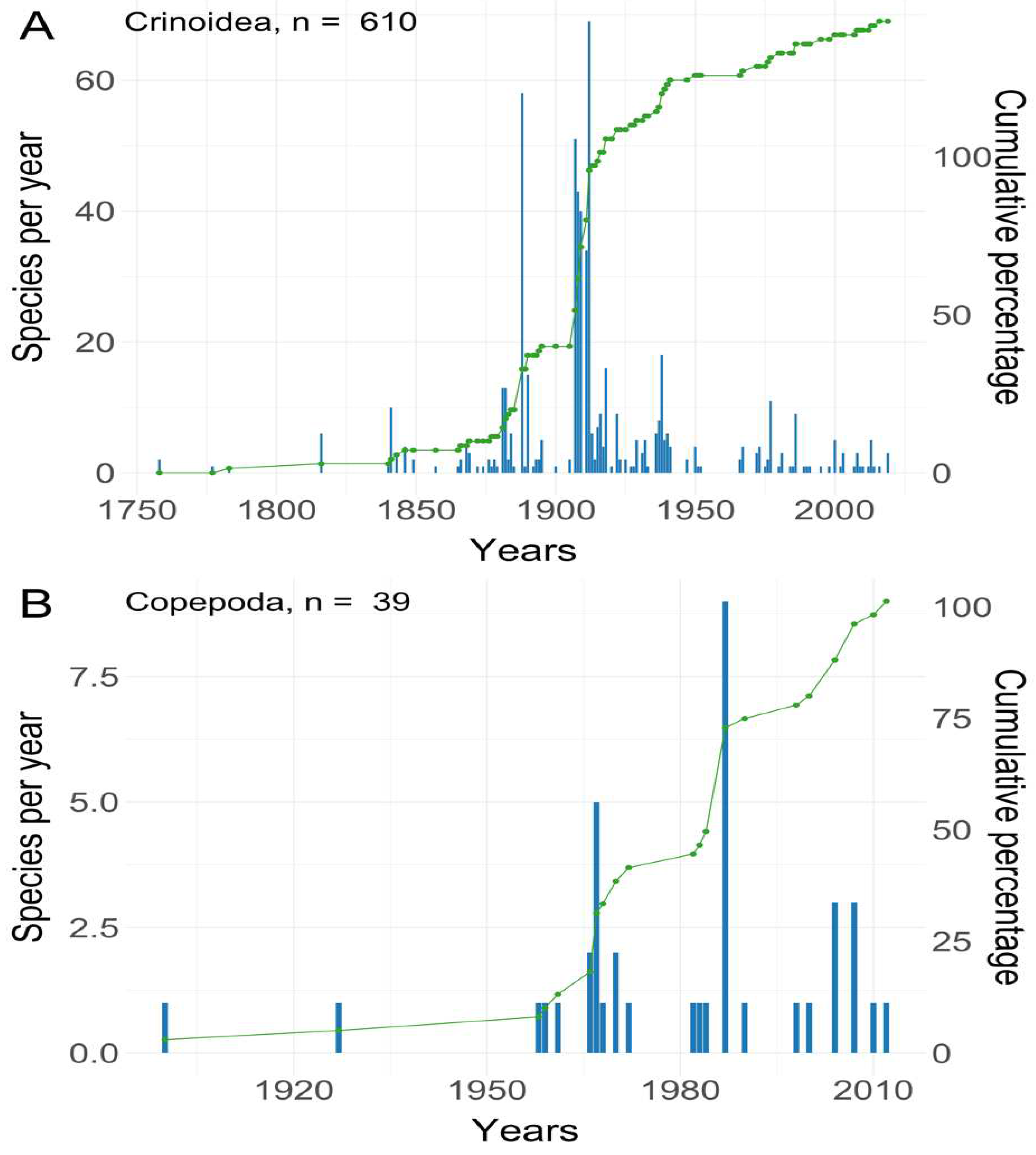
3.1. The history of research
3.2. Sampling methods and challenges
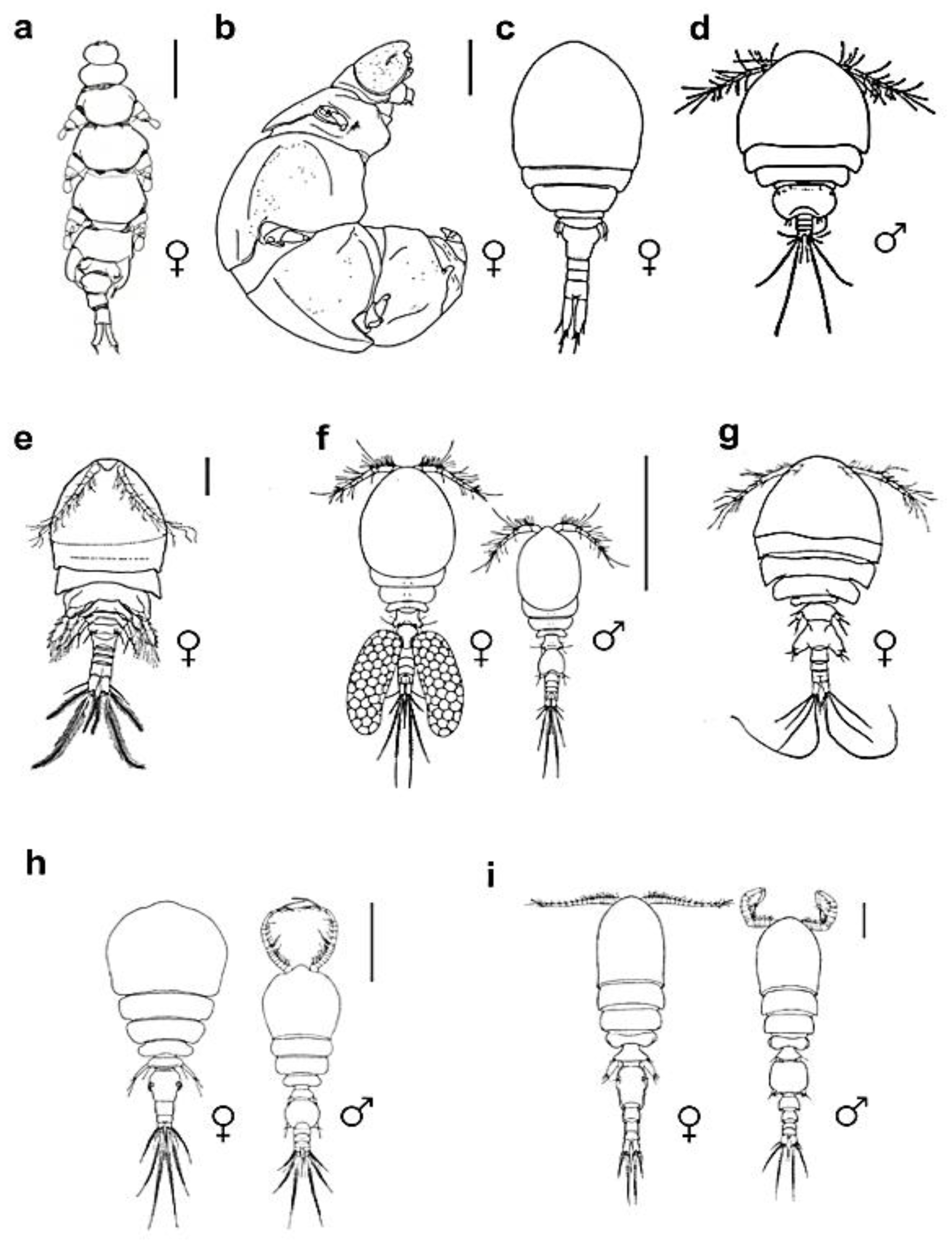
3.3. Diversity and taxonomy of symbiotic copepods
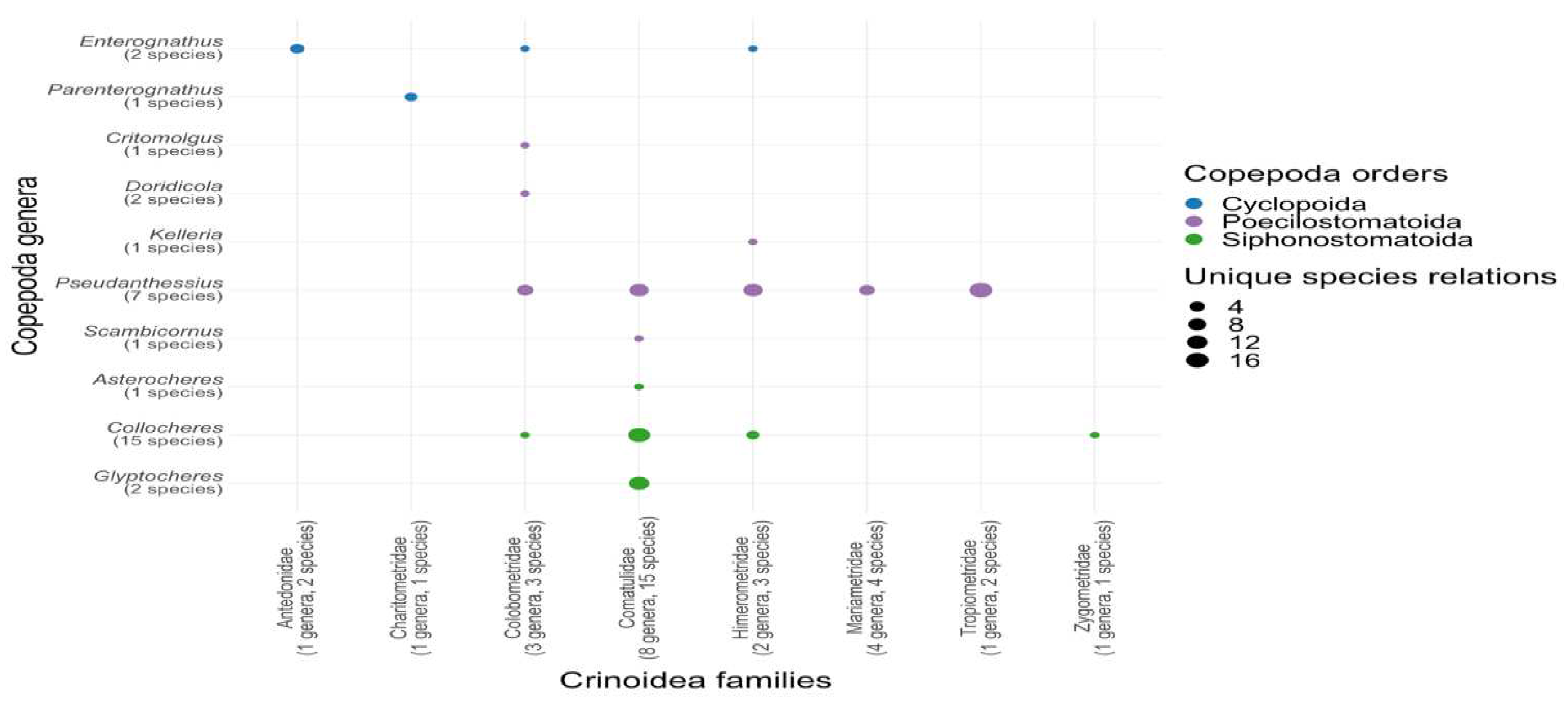
3.4. Specialization in Copepod-Crinoid Symbiosis
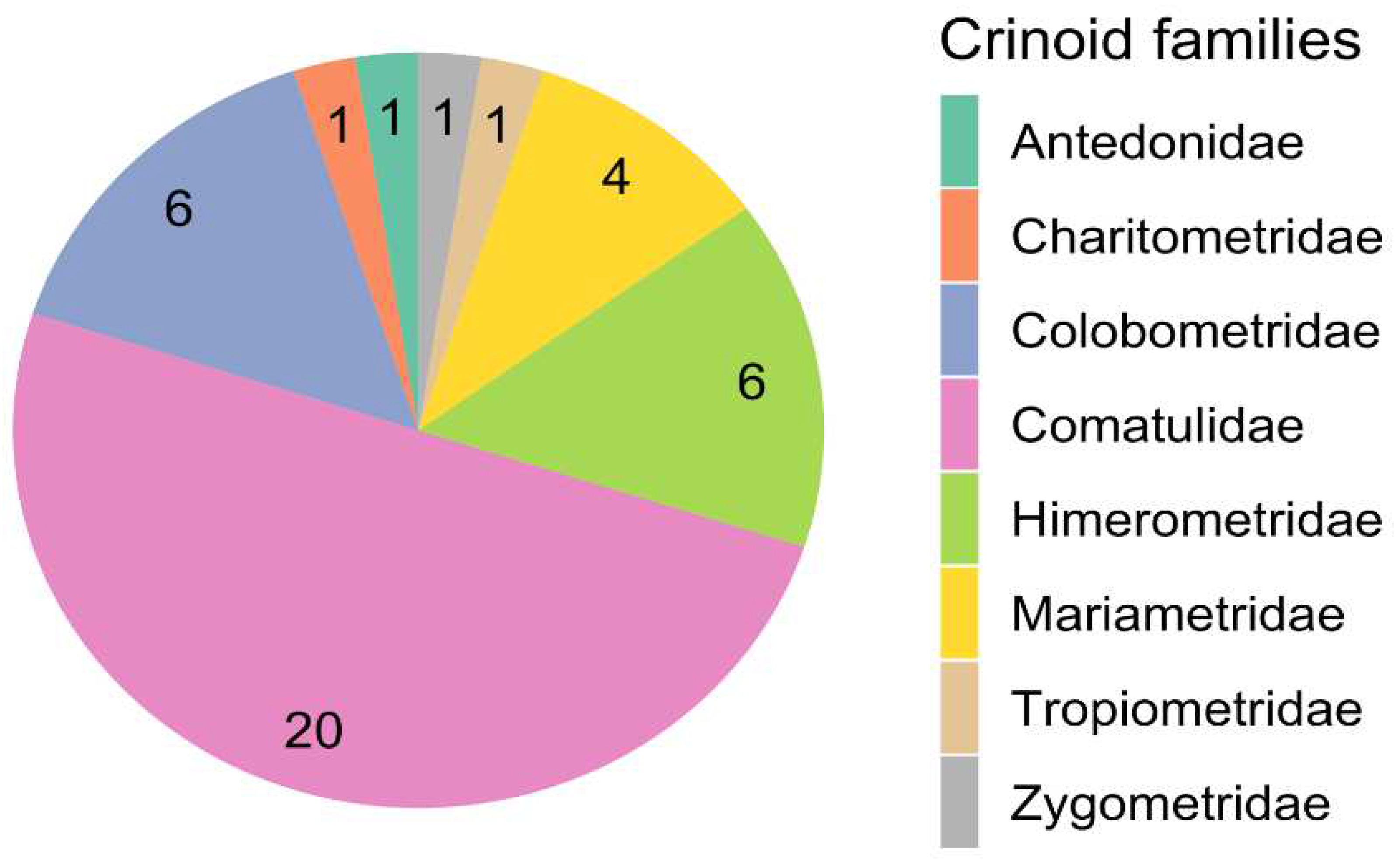
3.5. Distribution of Crinoid-Associated Copepods
3.6. Bathymetric Distribution
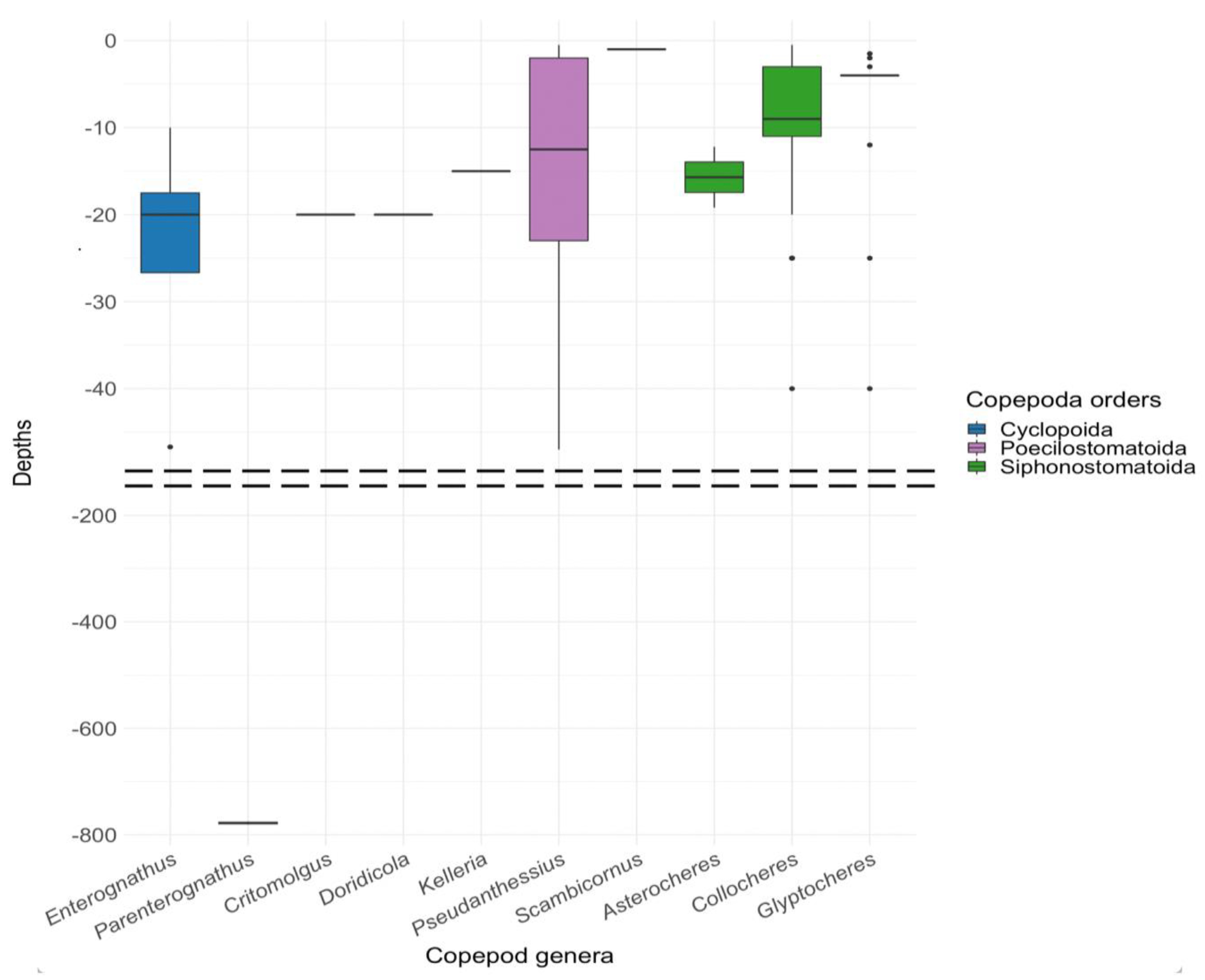
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Copepod | Host species: valid name (and as in original record) | Host abbreviation * | Symbiosis nature abbreviation ** | Site abbreviation ** | Depth (m) | Reference |
|---|---|---|---|---|---|---|
| Cyclopoida | ||||||
| Enterognathidae | ||||||
| Enterognathus comatulae Giesbrecht, 1900 | Antedon bifida (Pennant, 1777) | A | en | GB, IE | Grainger, 1950 | |
| Enterognathus comatulae Giesbrecht, 1901 | Antedon mediterranea (Lamarck, 1816) | A | en | FR, IT | Changeux, Delamare Deboutteville, 1956; Giesbrecht, 1900; Stock, 1959 | |
| Enterognathus inabai Ohtsuka, Shimomura, Kitazawa, 2012 | Lamprometra sp. | M | en | JP | 46.7-46.9 | Ohtsuka, Shimomura, Kitazawa, 2012 |
| Entherognathus lateripes Stock, 1966 | Decametra chadwicki (Clark, 1911) | Col | en | IL | 20 | Stock, 1966 |
| Entherognathus lateripes Stock, 1966 | Oligometra serripinna (Carpenter, 1811) | Col | en | IL | 20 | Stock, 1966 |
| Entherognathus lateripes Stock, 1966 | Heterometra savignii (Müller, 1841) (= Heterometra savignyi (Müller, 1841)) | H | en | IL | 10 | Stock, 1966 |
| Parenterognathus troglodytes Ohtsuka, Kitazawa, Boxshall, 2010 |
Glyptometra crassa (Clark, 1912) |
Ch | en | JP | 775, 780.8-787.1 | Ohtsuka, Kitazawa, Boxshall, 2010 |
| Poecilostomatoida | ||||||
| Kelleriidae | ||||||
| Kelleria gradata Stock, 1967 |
Heterometra savignii (Müller, 1841) (= Heterometra savignyi (Müller, 1841)) |
H | ec | IL | 15 | Stock, 1967 |
| Pseudanthessiidae |
Dichrometra flagellata (Müller, 1841) (= Dichrometra afra Clark, 1912) |
|||||
| Pseudanthessius angularis Humes, Ho, 1970 |
Stephanometra indica (Smith, 1876) (= Stephanometra spicata (Carpenter, 1881)) |
M | ec | MG | 1 | Humes, Ho, 1970 |
| Pseudanthessius angularis Humes, Ho, 1970 |
Anneissia bennetti (Müller, 1841) (= Comanthus bennetti (Müller, 1841)) |
M | ec | MG | 2, 6 | Humes, Ho, 1970 |
| Pseudanthessius comanthi Humes, 1972 | Comanthus wahlbergii (Müller, 1843) | Com | ec | MH | 4, 8 | Humes, 1972 |
| Pseudanthessius comanthi Humes, 1972 |
Oxycomanthus bennetti (Müller, 1841) (= Comanthus bennetti (Müller, 1841)) |
Com | ec | ID | 25 | Humes, 1987 |
| Pseudanthessius comanthi Humes, 1972 |
Heterometra savignii (Müller, 1841) (= Heterometra savignyi (Müller, 1841)) |
Com | ec | AU, ID, PH | 2, 3, 4, 10, 12, 40 | Humes, 1987 |
| Pseudanthessius madrasensis Reddiah, 1968 | Comatulida | ec | IN | Reddiah, 1968 | ||
| Pseudanthessius madrasensis Reddiah, 1968 | Tropiometra afra (Hartlaub, 1890) | T | ec | NC | 1.5, 2, 3 | Humes, 1977 |
| Pseudanthessius madrasensis Reddiah, 1968 | Tropiometra carinata (Lamarck, 1816) | T | ec | MG | 0.5, 1, 1.5, 2, 3, 15 | Humes, Ho, 1970 |
| Pseudanthessius major Stock, 1967 | Cenometra emendatrix (Bell, 1892) | Col | ec | MG | 10, 20 | Stock, 1967 |
| Pseudanthessius major Stock, 1967 | Heterometra africana (Clark, 1911) | H | ec | MG | 17, 18, 25, 29, 34 | Stock, 1967 |
| Pseudanthessius major Stock, 1967 |
Heterometra savignii (Müller, 1841) (= Heterometra savignyi (Müller, 1841)) |
H | ec | IL | 10, 15 | Stock, 1967 |
| Pseudanthessius major Stock, 1967 |
Himerometra robustipinna (Carpenter, 1881) (= Himerometra magnipinna Clark, 1908) |
H | ec | NC | 1 | Humes, 1977 |
| Pseudanthessius major Stock, 1967 |
Dichrometra flagellata (Müller, 1841) (= Dichrometra afra Clark, 1912) |
M | ec | MG | 1, 2, 6 | Stock, 1967 |
| Pseudanthessius major Stock, 1967 |
Lamprometra palmata (Müller, 1841) (= Lamprometra klunzingeri (Hartlaub, 1890)) |
M | ec | MG | 1, 13 | Stock, 1967 |
| Pseudanthessius major Stock, 1967 | Liparometra sp. | M | ec | MG | 15, 23, 27, 35 | Stock, 1967 |
| Pseudanthessius major Stock, 1967 |
Stephanometra indica (Smith, 1876) (= Stephanometra spicata (Carpenter, 1881)) |
M | ec | MG, NC | 2, 3, 13, 17 | Stock, 1967; Humes, 1977 |
| Pseudanthessius minor Stock, 1967 | Heterometra africana (Clark, 1911) | H | ec | MG | 18 | Stock, 1967 |
| Pseudanthessius minor Stock, 1967 |
Dichrometra flagellata (Müller, 1841) (= Dichrometra afra Clark, 1912) |
M | ec | MG | 2 | Stock, 1967 |
| Pseudanthessius minor Stock, 1967 |
Lamprometra palmata (Müller, 1841) (= Lamprometra klunzingeri (Hartlaub, 1890)) |
M | ec | IL, MG | 0.5, 13 | Stock, 1967 |
| Pseudanthessius minor Stock, 1967 | Liparometra sp. | M | ec | MG | 15, 23, 27, 35 | Stock, 1967 |
| Pseudanthessius planus Kim, 2007 |
Himerometra robustipinna (Carpenter, 1881) (= Himerometra magnipinna Clark, 1908) |
H | ec | ID | 2 | Kim, 2007 |
| Pseudanthessius rostellatus Humes, Ho, 1970 |
Phanogenia distincta (Carpenter, 1888) (= Comaster distinctus (Carpenter, 1888)) |
Com | ec | MG | 47 | Humes, Ho, 1970 |
| Rhynchomolgidae | ||||||
| Critomolgus fishelsoni (Stock, 1967) | Oligometra serripinna (Carpenter, 1811) | Col | ec | IL | 20 | Stock, 1967 |
| Doridicola patulus (Humes, 1959) | Cenometra emendatrix (Bell, 1892) | Col | ec | MG | 20 | Humes, Stock, 1973 |
| Doridicola venustus (Humes, 1958) | Cenometra emendatrix (Bell, 1892) | Col | ec | MG | 20 | Humes, Stock, 1973 |
| Synapticolidae | ||||||
| Scambicornus pillaii Stock, 1983 |
Capillaster multiradiatus (Linnaeus, 1758) (= Capillaster multiradiata (Linnaeus, 1758)) |
Com | ec | IL | 1 | Stock, 1983 |
| Siphonostomatoida | ||||||
| Asterocheridae | ||||||
| Asterocheres crinoidicola Humes, 2000 | Comatulida | ec | JM | Kim, 2010 | ||
| Asterocheres crinoidicola Humes, 2000 | Davidaster rubiginosus (Pourtalès, 1869) | Com | ec | BZ | 12.2 | Humes, 2000 |
| Asterocheres crinoidicola Humes, 2000 | Nemaster grandis Clark, 1909 | Com | ec | BZ | 32.2 | Humes, 2000 |
| Asterocheres spinopaulus Johnsson, 1998 | Comatulida | ec | BR | Johnsson, 2002 | ||
| Collocheres amicus Kim, 2007 |
Comanthus briareus (Bell, 1882) (= Comantheria rotula Clark, 1912) |
Com | ec | ID | 17 | Kim, 2007 |
| Collocheres brevipes Shin, Kim, 2004 |
Anneissia solaster (Clark, 1907) (= Comanthus solaster Clark, 1907) |
Com | ec | KP | 25 | Shin, Kim, 2004 |
| Collocheres comanthiphilus Humes, 1987 | Comanthus parvicirrus (Müller, 1841) | Com | ec | NC | 1.5, 5 | Humes, 1987 |
| Collocheres comanthiphilus Humes, 1987 | Comanthus sp. | Com | ec | NC | 1, 3 | Humes, 1987 |
| Collocheres comanthiphilus Humes, 1987 | Comanthus wahlbergii (Müller, 1843) | Com | ec | ID, NC | 0.5, 2, 25 | Humes, 1987 |
| Collocheres comanthiphilus Humes, 1987 |
Oxycomanthus bennetti (Müller, 1841) (= Comanthus bennetti (Müller, 1841)) |
Com | ec | AU, ID, PH | 2, 3, 4, 12, 40 | Humes, 1987 |
| Collocheres humesi Kim, 2007 |
Comanthus briareus (Bell, 1882) (= Comantheria rotula Clark, 1912) |
Com | ec | ID | 17 | Kim, 2007 |
| Collocheres inaequalis Ho, 1982 |
Anneissia japonica (Müller, 1841) (= Comanthus japonica (Müller, 1841), Comanthus japonicus (Müller, 1841)) |
Com | ec | JP | Ho, 1982 | |
| Collocheres inflatiseta Humes, 1987 |
Phanogenia multibrachiata (Carpenter, 1888) (= Comaster multibrachiatus (Carpenter, 1888)) |
Com | ec | ID | 10 | Humes, 1987 |
| Collocheres marginatus Humes, 1987 |
Comaster multifidus (Müller, 1841) (= Comanthina variabilis (Bell, 1882)) |
Com | ec | AU | 9 | Humes, 1987 |
| Collocheres parvus Humes, 1987 | Davidaster rubiginosus (Pourtalès, 1869) | Com | ec | ID | 10 | Humes, 1987 |
| Collocheres prionotus Humes, 1990 | Nemaster grandis Clark, 1909 | Com | ec | ID, MG | 0.5, 1 | Humes, 1990 |
| Collocheres serrulatus Humes, 1987 | Comatulida | Com | ec | ID | 10 | Humes, 1987 |
| Collocheres solidus Shin, Kim, 2004 |
Comanthus briareus (Bell, 1882) (= Comantheria rotula Clark, 1912) |
Com | ec | KP | 25 | Shin, Kim, 2004 |
| Collocheres solidus Shin, Kim, 2004 |
Anneissia solaster (Clark, 1907) (= Comanthus solaster Clark, 1907) |
Com | ec | KP | 25 | Shin, Kim, 2004 |
| Collocheres tamladus Shin, Kim, 2004 | Comanthus parvicirrus (Müller, 1841) | Z | ec | KP | Shin, Kim, 2004 | |
| Collocheres thysanotus Humes, 1987 | Comanthus sp. | Com | ec | AU | 9 | Humes, 1987 |
| Collocheres thysanotus Humes, 1987 | Comanthus wahlbergii (Müller, 1843) | Com | ec | AU | 9 | Humes, 1987 |
| Collocheres titillator Humes, 1987 |
Oxycomanthus bennetti (Müller, 1841) (= Comanthus bennetti (Müller, 1841)) |
Com | ec | ID | 10 | Humes, 1987 |
| Collocheres uncinatus Stock, 1966 |
Comanthus briareus (Bell, 1882) (= Comantheria rotula Clark, 1912) |
Col | ec | IL | 20 | Stock, 1966 |
| Collocheres uncinatus Stock, 1966 |
Anneissia japonica (Müller, 1841) (= Comanthus japonica (Müller, 1841), Comanthus japonicus (Müller, 1841)) |
Com | ec | ID, MG | 0.5, 1, 3 | Humes, 1990 |
| Collocheres uncinatus Stock, 1966 |
Phanogenia multibrachiata (Carpenter, 1888) (= Comaster multibrachiatus (Carpenter, 1888)) |
H | ec | IL | 1, 15 | Stock, 1966 |
| Glyptocheres comanthinae Humes, 1987 |
Comaster multifidus (Müller, 1841) (= Comanthina variabilis (Bell, 1882)) |
Com | ec | ID | 4 | Humes, 1987 |
| Glyptocheres extrusus Humes, 1987 | Davidaster rubiginosus (Pourtalès, 1869) | Com | ec | NC | 1.5 | Humes, 1987 |
| Glyptocheres extrusus Humes, 1987 | Nemaster grandis Clark, 1909 | Com | ec | ID | 25 | Humes, 1987 |
| Glyptocheres extrusus Humes, 1987 | Comatulida | Com | ec | AU, ID, PH | 2, 3, 4, 12, 40 | Humes, 1987 |
| Attribute | Column_name | Description | Units | Attribute_Type |
|---|---|---|---|---|
| Record number | rID | Unique number corresponding to specific occurrence | Integer | |
| Record ID | recordID | A structured code incorporating a concise article reference, region and country observation identifiers, shorthand for the location coordinates, and specific abbreviations for the symbiont and host families, complemented by a distinct number. | Text | |
| Aphia ID of symbiont | aphiaID_Symbiont | Unique number for taxon from WoRMS database | Integer | |
| Kingdom of symbiont | kingdom_Symbiont | Taxonomic rank below Domain | Text | |
| Phylum of symbiont | phylum_Symbiont | Taxonomic rank below Kingdom | Text | |
| Class of symbiont | class_Symbiont | Taxonomic rank below Phylum | Text | |
| Order of symbiont | order_Symbiont | Taxonomic rank below Class | Text | |
| Family of symbiont | family_Symbiont | Taxonomic rank below Order | Text | |
| Genus of symbiont | genus_Symbiont | Taxonomic rank below Family and first element in the Latin binomial name | Text | |
| Specific epithet of symbiont | specificEpithet_Symbiont | Second element in the Latin binomial name | Text | |
| Scientific name authorship of symbiont | scientificNameAuthorship_Symbiont | Third element in the Latin binomial name | Text | |
| Symbiont ID | symbiontID | Reviewed species name | Text | |
| Taxon rank of symbiont | taxonRank_Symbiont | Taxonomic rank information (e.g., genus, species) | Text | |
| Taxonomic status of symbiont | taxonomicStatus_Symbiont | Taxonomic status information (e.g., accepted, unaccepted) | Text | |
| Link of symbiont | link_Symbiont | Link to taxon in WoRMS database | Text | |
| Female Body Length | femaleLength | The length of the female specimen, measured from head to tail | µm | Text |
| Female Body Weight | femaleWeight | The total weight of the female specimen | µm | Text |
| Male Body Length | maleLength | The length of the male specimen, measured from head to tail | µm | Text |
| Male Body Weight | maleWeight | The total weight of the male specimen | µm | Text |
| Aphia ID of host | aphiaID_Host | Unique number for taxon from WoRMS database | Integer | |
| Kingdom of host | kingdom_Host | Taxonomic rank below Domain | Text | |
| Phylum of host | phylum_Host | Taxonomic rank below Kingdom | Text | |
| Class of host | class_Host | Taxonomic rank below Phylum | Text | |
| Order of host | order_Host | Taxonomic rank below Class | Text | |
| Family of host | family_Host | Taxonomic rank below Order | Text | |
| Genus of host | genus_Host | Taxonomic rank below Family and first element in the Latin binomial name | Text | |
| Specific epithet of host | specificEpithet_Host | Second element in the Latin binomial name | Text | |
| Scientific name authorship of host | scientificNameAuthorship_Host | Third element in the Latin binomial name | Text | |
| Host ID | hostID | Reviewed species name | Text | |
| Taxon rank of host | taxonRank_Host | Taxonomic rank information (e.g., genus, species) | Text | |
| Taxonomic status of host | taxonomicStatus_Host | Taxonomic status information (e.g., accepted, unaccepted) | Text | |
| Link of host | link_Host | Link to taxon in WoRMS database | Text | |
| Site ID | siteID | Unique number for locality | Text | |
| Region code | regionCode | Unique number for region | Text | |
| Region | region | Division of the World Ocean (Spalding et al., 2007) | Text | |
| Ocean | ocean | The name of the ocean in which the locality occurs. | Text | |
| Water body | waterBody | The name of the water body in which the locality occurs. | Text | |
| Island | island | The name of the island near which the locality occurs. | Text | |
| Country | country | The name of the country in which the locality occurs. | Text | |
| Country code | countryCode | The standard code (ISO 3166-1-alpha-2) for the country in which the locality occurs. | Text | |
| Locality | locality | Particular area where the taxon was found | Text | |
| Exact Location Description | verbatimLocalition | A comprehensive description of the location from the original article | Text | |
| Geocoordinates | geocoordinates | A combined representation of both latitude and longitude | Degrees Minutes Seconds (DMS) | Text |
| Latitude | latitude | Coordinate that specifies the N–S position of a point on the Earth surface | Degrees Minutes Seconds (DMS) | Text |
| Longitude | longitude | Coordinate that specifies the E–W position of a point on the Earth surface | Degrees Minutes Seconds (DMS) | Text |
| Decimal geocoordinates | decimalGeocoordinates | A combined representation of both latitude and longitude | Decimal degrees, WGS84 | Numeric |
| Decimal latitude | decimalLatitude | Coordinate that specifies the N–S position of a point on the Earth surface | Decimal degrees, WGS84 | Numeric |
| Decimal longitude | decimalLongitude | Coordinate that specifies the E–W position of a point on the Earth surface | Decimal degrees, WGS84 | Numeric |
| Coordinate uncertainty | coordinateUncertaintyInMeters | The horizontal distance from the given decimal latitude and longitude describing the smallest circle containing the whole of the Location. | m | Integer |
| Minimum depth | minimumDepthInMeters | Vertical distance under sea level | m | Integer |
| Maximum depth | maximumDepthInMeters | Vertical distance under sea level | m | Integer |
| Collecting method | collectingMethod | The method of taking sample | Text | |
| Finding method | findingMethod | The method of finding copepods in sample | Text | |
| Type of association | note | Describes the nature of the interaction. | Text | |
| Host interaction site | locationAtHost | The general location or site on the host where the copepod interacts or resides. | Text | |
| Event date | eventDate | Date of sampling. | Date | |
| Year | year | The four-digit year in which the Occurence recorded. Format: yyyy. | Integer | |
| Month | month | The ordinal month in which the Occurence recorded. Format: mm. | Integer | |
| Article ID | articleID | Short reference | Text | |
| Reference | reference | Full reference to article | Text |
References
- Boxshall, G. A.; Halsey, S.H. An Introduction to Copepod Diversity, Part II. The Ray Society, London, 2004, pp. 422-966.
- Bron, J. E.; Frisch, D.; Goetze, E.; Johnson, S. C.; Lee, C. E.; Wyngaard, G. A. Observing copepods through a genomic lens. Front. Zool. 2011, 8(1), 1-15.
- Britayev, T.A.; Beksheneva, L.F.; Deart, Y.V. et al. Structure and variability of symbiotic assemblages associated with feather stars (Crinoidea: Comatulida) Himerometra robustipinna. Oceanology 2016, 56, 666–674.
- Britayev, T.A.; Mekhova, E.S. Assessment of hidden diversity of crinoids and their symbionts in the Bay of Nhatrang, Vietnam. Org. Divers. Evol. 2011, 11, 275–285.
- Burnell, D.J.; ApSimon, J.W. Echinoderm Saponins. In Marine Natural Products: Chemical and Biological Perspectives. Academic Press Inc., pp. 287–379.
- Changeux, J.P.; Delamare Deboutteville, C. Enterognathus comatulae Giesbrecht, 1900. Vie et Milieu 1956, 7(1), 106-107.
- Deheyn, D.D.; Lyskin, S.; Eeckhaut, I. Assemblages of symbionts in tropical shallow-water crinoids and assessment of symbionts' host-specificity. Symbiosis 2006, 42(3), 161-168.
- Fabricius, K. E.; Dale, M. B. Multispecies associations of symbionts on shallow water crinoids of the central Great Barrier Reef. Coenoses 1993, 8, 41–52.
- Fishelson, L. Ecology of northern Red Sea crinoids and their epi- and endozoic fauna. Mar. Biol. 1974, 26(2), 183-192.
- Grainger, J.N.R. Notes on parasitic Crustacea. Ann. Mag. Nat. Hist. 1950, (12)3, 635-638.
- Ho, J.S. Copepoda associated with echinoderms of the Sea of Japan. Rep. Sado Mar. Biol. Stn. Niigata Univ. 1982, 12, 33-61.
- Humes, A.G. Pseudanthessius comanthi n. sp. (Copepoda, Cyclopoida) associated with a crinoid at Eniwetok Atoll. Pac. Sci. 1972, 26(4), 373-380.
- Humes, A.G. Pseudanthessiid copepods (Cyclopoida) associated with crinoids and echinoids (Echinodermata) in the tropical western Pacific Ocean. Smithson. Contrib. Zool. 1977, 243, 1-43.
- Humes, A.G. Copepoda associated with crinoid echinoderms in the western Pacific. Publ. Seto Mar. Biol. Lab. 1987, 32(1-3), 63-108.
- Humes, A.G. Collocheres (Copepoda: Siphonostomatoida) associated with the crinoid Capillaster multiradiatus in the Indo-Pacific. Trans. Am. Microsc. Soc. 1990, 109(1), 61-68.
- Humes, A.G. Asterocheres crinoidicola n. sp., a copepod (Siphonostomatoida: Asterocheridae) parasitic on crinoids in Belize. Syst. Parasitol. 2000, 47(2), 103-110.
- Humes, A.G.; Stock, J.H. A revision of the family Lichomolgidae Kossman, 1877, cyclopoid copepods mainly associated with marine invertebrates. Smithson. Contrib. Zool. 1973, 127, 1-368.
- Humes, A.G.; Ho, J.S. Cyclopoid copepods of the genus Pseudanthessius associated with crinoids in Madagascar. Smithson. Contrib. Zool. 1970, 54, 1-20.
- Ivanenko, V.N.; Hoeksema, B.W.; Mudrova, S.V.; Nikitin, M.A.; Martínez, A.; Rimskaya-Korsakova, N.N.; Berumen, M.L.; Fontaneto, D. Lack of host specificity of copepod crustaceans associated with mushroom corals in the Red Sea. Mol. Phylogenet. Evol. 2018, 127, 770-780. doi: 10.1016/j.ympev.2018.06.024. [CrossRef]
- Huys, R.; Boxshall, G.A. Copepod Evolution. The Ray Society: London, 1991; pp. 468.
- Kim, I.-H.; Hendler, G. Larval Biology of Thaumatopsyllid Copepods Endoparasitic in Caribbean Ophiuroids. J. Crustacean Biol. 2010, 30(2), 206–224.
- Johnsson, R. Asterocherids (Copepoda; Siphonostomatoida) associated with invertebrates from California Reefs: Abrolhos (Brazil). Hydrobiologia 2002, 470, 247-266.
- Kim, I.H. Copepods (Crustacea) associated with marine invertebrates from the Moluccas. Korean J. Syst. Zool., Special Issue 2007, 6, 1-126.
- Kim, I.H. Siphonostomatoid Copepoda (Crustacea) associated with invertebrates from tropical waters. Korean J. Syst. Zool., Special issue 2010, 8, 1-176.
- Korzhavina, O.A.; Grishina, D.Y.; Chen, X.; Fontaneto, D.; Ivanenko, V.N. Diving into Diversity: Copepod Crustaceans in Octocoral Associations. Diversity 2023, 15(11), 1140.
- Martínez, A.; Eckert, E.M.; Artois, T. et al. Human access impacts biodiversity of microscopic animals in sandy beaches. Commun Biol 2020, 3, 175.
- Mikhailov, K.V.; Ivanenko, V.N. Lack of reproducibility of molecular phylogenetic analysis of Cyclopoida. Mol. Phylogenet. Evol. 2019, 139, 106574.
- Mikhailov, K.V.; Ivanenko, V.N. Low support values and lack of reproducibility of molecular phylogenetic analysis of Copepoda orders. Arthrop. Sel. 2021, 30, 39–42.
- Mekhova, E.S.; Britayev, T.A. Feather stars (Crinoidea, Comatulida) of Nhatrang Bay, Vietnam; fauna, habitat and symbionts. In Benthic fauna of the Bay of Nhatrang, Southern Vietnam, vol. 2; Britayev, T.A., Pavlov, D.S., Eds.; KMK Scientific Press: Moscow, 2012; pp. 447–478.
- Messing, C.; Gondim, A.I.; Taylor, K. World List of Crinoidea. Crinoidea. Accessed through: World Register of Marine Species at: https://www.marinespecies.org/aphia.php?p=taxdetails&id=123081 on 2023-08-05.
- Ohtsuka, S.; Kitazawa, K.; Boxshall, G.A. A new genus of endoparasitic copepods (Cyclopoida: Enterognathidae), forming a gall in the calyx of deep-sea crinoids. Zool. Sci. 2010, 27(8), 689-696.
- Ohtsuka, S.; Shimomura, M.; Kitazawa, K. A new species of Enterognathus (Copepoda, Cyclopoida, Enterognathidae) collected from the Seto Inland Sea, western Japan. ZooKeys 2012, 180, 1-8.
- Reddiah, K. Two new Pseudanthessius species (Copepoda - Lichomolgidae) from the Madras harbour. J. Mar. Biol. Assoc. India 1968, (1-2), 320-328.
- Shin, S.; Kim, I.H. Three new species of Collocheres (Copepoda, Siphonostomatoida, Asterocheridae) associated with crinoids and ophiuroids from Korea. Korean J. Biol. Sci. 2004, 8(4), 267-280.
- Stock, J.H. Copepoda associated with Neapolitan invertebrates. Pubblicazione della Stazione Zoologica di Napoli 1959, 31(1), 59-75.
- Stock, J.H. Copepoda associated with invertebrates from the Gulf of Aqaba. 2. Enterognathus n. sp., a new endoparasite of Crinoida (Cyclopoida, Ascidicolidae). Proc. Kon. Ned. Akad. Wet. Amsterdam (C) 1966, 69(2), 211-216.
- Stock, J.H. On Collocheres Canu, 1893, and Leptomyzon Sars, 1915, two synonymous genera of Copepoda. Beaufortia 1966, 13(163), 221-239.
- Stock, J.H. Copepoda associated with invertebrates from the Gulf of Aqaba. 3. The genus Pseudanthessius Claus, 1889 (Cyclopoida, Lichomolgidae). Proc. K. ned. Akad. Wet. (C) 1967, 70(2), 232-248.
- Stock, J.H. Copepoda associated with invertebrates from the Gulf of Aqaba. 4. Two new Lichomolgidae associated with Crinoida. Proc. Kon. Ned. Akad. Wet. Amsterdam, Ser. C, Biol. Sci. 1967, 70(5), 569-578.
- Stock, J.H. A new species of Scambicornus (Copepoda, Cyclopoidea, Sabelliphilidae), associated with an unusual host, the crinoid Capillaster in the Red Sea. In Selected papers on Crustacea. Prof. (Dr.) N. Krishna Pillai felicitation volume; John, P.A., Ed.; Prof. N. Krishna Pillai Farewell Committee: Trivandrum, 1983; pp. 1-207.
- Virgili, R.; Cerrano, C.; Ponti, M. et al. Crinoid diversity and their symbiotic communities at Bangka Island (North Sulawesi, Indonesia). Mar. Biodivers. 2020, 50, 90.
- Walter, T.C.; Boxshall, G. World of Copepods database. Accessed at http://www.marinespecies.org/copepoda on 2022-04-05.
- Wright, J.S.; Fu, R.; Worden, J.R.; Chakraborty, S.; Clinton, N.E.; Risi, C.; Sun, Y.; Yin, L. Rainforest-initiated wet season onset over the southern Amazon. Proc. Natl. Acad. Sci. U.S.A. 2017, 114(32), 8481-8486.
- Zeppilli, D.; Sarrazin, J.; Leduc, D. et al. Is the meiofauna a good indicator for climate change and anthropogenic impacts? Mar Biodiv 2015, 45, 505–535. https://doi.org/10.1007/s12526-015-0359-z. [CrossRef]
- World Register of Marine Species (WoRMS). Available online: https://www.marinespecies.org/ (accessed on 15 December 2022).
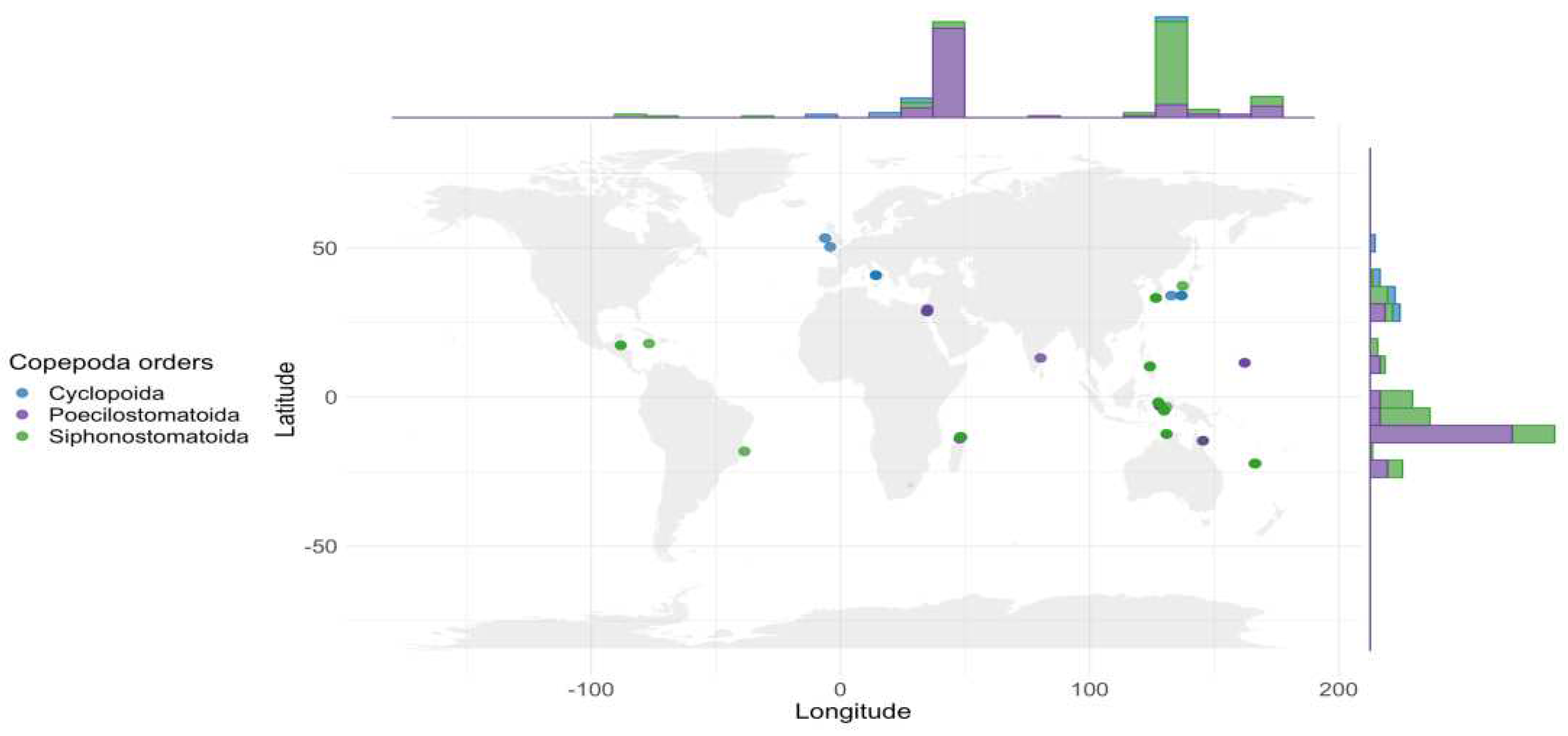
| Region | Country | Reference |
|---|---|---|
| Central Indo-Pacific | Australia | Humes, 1987 |
| Indonesia | Humes, 1987; Humes, 1990; Kim, 2007 | |
| Marshall Islands | Humes, 1972 | |
| New Caledonia | Humes, 1977; Humes, 1987 | |
| Philippines | Humes, 1987 | |
| Temperate Northern Atlantic | France | Changeux, Delamare Deboutteville, 1956 |
| Ireland | Grainger, 1950 | |
| Italy | Giesbrecht, 1900; Stock, 1959 | |
| United Kingdom | Grainger, 1950 | |
| Temperate Northern Pacific | Japan | Ho, 1982; Ohtsuka, Kitazawa, Boxshall, 2010; Ohtsuka, Shimomura, Kitazawa, 2012 |
| Korea | Shin, Kim, 2004 | |
| Tropical Atlantic | Belize | Humes, 2000 |
| Brazil | Johnsson, 2002 | |
| Jamaica | Kim, 2010 | |
| Western Indo-Pacific | India | Reddiah, 1968 |
| Israel | Stock, 1966; Stock, 1967; Stock, 1983 | |
| Madagascar | Humes, 1990; Humes, Ho, 1970; Humes, Stock, 1973; Stock, 1967 |
| Taxa | # of known copepod species | # copepod species | # of copepod records found on crinoids | # crinoid families | # crinoid genera | # crinoid species | Mean of records per copepod species + SE | Mean of host species per copepod species + SE | % copepod species with a single crinoid host |
|---|---|---|---|---|---|---|---|---|---|
| Cyclopoida | |||||||||
| Enterognathidae | 7 | 4 | 11 | 5 | 6 | 7 | 2.75 + 0.85 | 1.75 + 0.48 | 50 |
| Poecilostomatoida | |||||||||
| Kelleriidae | 19 | 1 | 1 | 1 | 1 | 1 | 1 + NA | 1 + NA | 100 |
| Pseudanthessiidae | 61 | 7 | 77 | 6 | 13 | 14 | 13.57 + 5.03 | 3 + 0.93 | 28,57 |
| Rhynchomolgidae | 270 | 3 | 3 | 1 | 2 | 2 | 1 + 0 | 1 + 0 | 100 |
| Synapticolidae | 50 | 1 | 1 | 1 | 1 | 1 | 1 + NA | 1 + NA | 100 |
| Siphonostomatoida | |||||||||
| Asterocheridae | 271 | 19 | 73 | 5 | 12 | 17 | 3.84 + 1.17 | 2 + NA | 63,16 |
| Total | 678 | 35 | 166 | 19 | 35 | 42 |
| Host taxa | # of known crinoid genera | # host crinoid genera (%) | # of known species | # host crinoid species (%) | # records | # of copepod species found on crinoids | # of host species with | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||||
| copepod species | ||||||||||
| Comatulida | ||||||||||
| Antedonidae | 50 | 1 (2%) | 151 | 2 (1.32%) | 5 | 1 | 2 | |||
| Charitometridae | 8 | 1 (12.5%) | 33 | 1 (3.03%) | 2 | 1 | 1 | |||
| Colobometridae | 18 | 3 (16.67%) | 47 | 3 (6.38%) | 11 | 6 | 1 | 2 | ||
| Comatulidae | 23 | 8 (34.78%) | 102 | 16 (15.69%) | 81 | 20 | 7 | 5 | 3 | 1 |
| Himerometridae | 5 | 2 (40%) | 39 | 3 (7.69%) | 18 | 6 | 2 | 1 | ||
| Mariametridae | 7 | 4 (57.14%) | 22 | 5 (22.73%) | 24 | 4 | 1 | 3 | 1 | |
| Tropiometridae | 1 | 1 (100%) | 4 | 2 (50%) | 21 | 1 | 2 | |||
| Zygometridae | 2 | 1 (50%) | 10 | 1 (10%) | 1 | 1 | 1 | |||
| Total | 114 | 21 | 408 | 33 | 163 | 40 | 15 | 10 | 6 | 2 |
| Region | # of localities | # of records | # of symbiontorders | # of symbiontfamilies | # of symbiontgenera | # of symbiontspecies | # of host families | # of host genera | # of host species |
|---|---|---|---|---|---|---|---|---|---|
| Central Indo-Pacific | 21 | 74 | 2 | 2 | 3 | 17 | 4 | 9 | 14 |
| Temperate Northern Atlantic | 4 | 5 | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| Temperate Northern Pacific | 5 | 11 | 2 | 2 | 3 | 6 | 4 | 4 | 5 |
| Tropical Atlantic | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 2 |
| Western Indo-Pacific | 23 | 71 | 3 | 6 | 7 | 13 | 5 | 11 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





