1. Introduction
The recent devasting emergences and re-emergences of infections have reached the highest magnitudes of all times that stimulated immediate global response to action (1). More important was that the mechanisms of co-infections during SARS-CoV-2 pandemic that significantly aggravated the disease, was not understood. In particular, the types of bacterial pathogens involved and their patterns of infection before, during, and post-vaccinations was not clear. To understand these mechanisms and potential co-protection by molecular mimicry, it has become imperative to first determine the frequencies and most common types of co-infections associated to SARS-CoV-2 before, during, and after mass vaccinations.
The World Health Organization (WHO), the European Union, the U.S. Government, and the Centers for Disease Control and Prevention (USA) have prioritized the issue as a threat to human health [8 9]. At present, the annual death estimate is ~3- million humans (4) (5); however, the global cost is expected as USD 3 trillion by 2050, and 10 million additional people could die each year, summing a cumulated over USD 100 trillion(6). The staggering 8.9 million infections, 33,000 deaths, and an annual healthcare cost of €1 billion in the USA and Europe have been a trending dilemma (7–9). In the USA alone, another estimate for antimicrobial-resistant organisms showed at least 2,868,700 infections and 35,900 deaths annually (10). However, total European cost due to community-acquired infections reached 16.8 billion, with 50% of inpatients admitted, mostly senior patients (11). This was a significant rise from 2011 in the annual total cost spending in Europe (10.1 billion pounds), including inpatients, outpatients care, and treatment (12). Despite the significant decline in COVID-19, the global losses in health and wealth of populations remain. Among all types of infections, the healthcare-associated infections (HAI) constitute the highest losses. European countries estimated about 2,609,911 cases and 426,277 claims related to resistance infections alone (13). The WHO) reported a total of 40,000 death cases annually due to nosocomial infection, indicating a rise of 25% in developing countries and by 5-10% in developed countries (14)(15). Unfortunately, in Middle Eastern countries, the effect of healthcare-associated infections by resistant pathogens is not well documented. Limited estimates revealed rates in the regional countries based on the intensity of the problem as followed; Egypt, followed by Lebanon, Syria, Jordan, Iraq, and the Palestinian territories [
19]. The relatively lowest prevalence rates were reported in the Gulf regions. Internal instability affected countries such as Lebanon that was hit by a devastating rate in a ten years survey where carbapenem-resistant
Acinetobacter baumannii was the most common pathogen in pneumonia patients with a mortality rate exceeding 50%(17). The Saudi Ministry of Health (MOH) have launched advanced health clusters system across the country to empower beneficiaries and monitoring communicable and noncommunicable diseases (18). As a result, stricter guidelines and effective control measures revolutionizing the system (19). A recent 10-years surveillance in the Arabian Peninsula (20) indicated the emergence of infections associated with mortality. Another 5-years monitoring resulted in increased susceptibility of nosocomial bacteria at a private tertiary care hospital in Saudi Arabia (21). However, to the best of our knowledge, there is a serious paucity of high quality data on the pre- and post-COVID-19 co-infections.
Co-infection rates before covid-19 vaccination is different in different countries and the data about the rates is limited. In China for example, several studies were conducted with different outcomes on co-infections. Guqin Zhang’s study showed significantly higher rate of bacterial (25.5%) and fungal co-infections (10.9%) (22). Similarly, a study in Jiangsu Province of China, 257 Patients who had confirmed cases of COVID-19 patients showed that 242 (94.2 %) were co-infected with one or more pathogens; however, bacterial co-infections were much higher (23). Furthermore, a Hospital in Beijing, on COVID-19 Patients admitted to ICU, 13 patients had positive 23 BAL samples and 73 sputum positive for bacterial cultures where 56 of respiratory samples (58.3%) were identified to have respiratory bacterial pathogen.(24). European studies showed a lower rate of co-infections than the previous studies. For instance, in Italy, in a non-survivor population 16,654 patients, 11% were had bacterial or fungal co-infections (22). The Miulli General Hospital, Italy examined 233 COVID-19 patients with the age range between 18 to 67 years old; 52 (22.3%) of them had positive co-infection with one or more pathogens.(25). Moreover, a third Italian study investigated the relationship between SARS-CoV-2 and bacterial and fungal co-infections where 35 (57%) were positive for bacterial or fungal infection.(26). However, much higher co-infections were reported in other countries. The frequency of co-infections in some Middle Eastern countries were also high. A Palestinian hospital study on COVID-19 patients showed 51.1% of bacterial co-infection while the rate of fungal co-infection was 48.9%.(27). In India, the mortality within the patients who have had co-infections was 56.7% against and the mortality of 10.6% in total admitted COVID-19 patients. In these co-infections, Gram-negative bacteria were collected from 78% of patients.(28). Another study in India, examined 632 patients, 65 of them (10.3%) had a systemic culture-positive bacterial or fungal coinfection.(29) In a Russia hospital with COVID-19 patients, an increase co-infection tested positive for various bacterial agents was reported among 433 COVID-19 patients (35.96%) (30). Other study on 212 patients, 96 were female and 116 were male, revealed the mortality of 50% who showed fungal and/or bacterial positive cultures in 89 (41.8%) patient (31). A study on 210 patients admitted to ICU with COVID-19 55 patients (26%) had positive sterile body fluid cultures, of which 37 grew bacteria, 7 fungi.(32)
Knowledge of the frequencies and profiles of co-infection after COVID-19 vaccinations is crucial on the evaluation of protection and/or co-infections. It has been well established those co-microbial infections aggravates COVID-19 making poor patient outcomes. High levels of procalcitonin on admission may predict non-survival in critically ill cases in whom bacterial or fungal co-infection is likely(33). Several studies indicated significance of co-infections during SARS-CoV-2 pandemic. However, there is a paucity on high quality data on the evidence of the rates of COVID-19 co-infection after COVID-19 vaccinations. In addition, there are significant variations in the reports rates of co-infections at different geographic regions globally. A study on 1091 hospitalized COVID-19 patients in Saudi Arabia between March 2020 and December 2020 indicated overall 70 fatalities (6.4%). However, of 182 COVID-19 patients admitted to the critical care, 114 patients (62.6%) survived. The in-hospital mortality was 13.4%. In the above study, co-infection was identified in 67/68 (98.5%) non-survivors, mostly with Gram-negative pathogens.(33) Similarly, a study comprise of 76,176 COVID-19 patients estimated the prevalence of bacterial co-infection in 5.62%(34)Furthermore, UK study on respiratory viral co-infections on 6965 patients with SARS-CoV-2 reported 8.4% of co-infections (35). However,55 severe cases and 166 non-severe but COVID-19-positive cases concluded that 221 patients had fungal coinfection.(36). Increased rate of mixed microbial co-infections with SARS-CoV-2 was found on 703 patients with SARS-COV-2 Confirmed cases, 75(10.7%).(37). An intensive care unit study in Iran recorded 15, out of 73 SARS-COV-2 cases, with co-infection with other respiratory pathogens, especially Candida albicans and Klebsiella pneumonia. (38). Recent research identified 46% (89/191) of patient with co-infection.(39). In Spain out of 712 COVID-19 patients, 113 (16%) presented bacterial/fungal coinfections or superinfections, their median age was 73 years.(40) In England, 1% of persons with COVID-19 (2279/223413) had coinfection/secondary infection, of which >65% were bloodstream. Coinfection/secondary bacterial/fungal infections were rare in non-hospitalized and hospitalized persons with COVID-19, varied by ethnicity and age, and were associated with higher and were associated with higher mortality. The most common causative organisms were Escherichia coli, (41). The WHO currently recommends against the prescribing of antimicrobials in mild to moderate COVID-19 cases without clear indication of bacterial infection(42). Ninety-two out of 1,055 (8.7% patients were found to have microbiologically proven respiratory or circulatory tract infections via microbial culture results. Respiratory tract infections were detected as monomicrobial in 44 patients and as polymicrobial in 17 patients, among a total of 61 patients. In addition, 59 (64.1%) patients were male, and 33 (35.9%) were female. Among the microorganisms grown in blood cultures, coagulase-negative staphylococci with a percentage of 31% and Acinetobacter baumannii with a percentage of 27.5% were prominent. In respiratory tract cultures, Acinetobacter baumannii constitutes the majority with a percentage of 33.3%, followed by Staphylococcus aureus and Klebsiella pneumoniae with a percentage of 9.5% each. The most resistant bacteria were A. baumannii, resistant to all antibiotics other than colistin.(43). In a total of 1125 consecutive adults met inclusion criteria, co-infections were microbiologically documented in 102 (9.1%) patients Most frequent microorganisms were Streptococcus pneumoniae (79%), Staphylococcus aureus (6.8%), and Haemophilus influenzae (6.8%). Test positivity was 1% (8/803) for blood cultures, 10.1% (79/780) for pneumococcal urinary antigen test, and 11.4% (15/132) for sputum culture. Patients with PCT higher than 0.2, 0.5, 1, and 2 ng/mL had significantly more co-infections than those with lower levels (p=0.017, p=0.031, p<0.001, and p<0.001, respectively). In multivariate analysis, oxygen saturation ≤94% (OR 2.47, CI 1.57–3.86), ferritin levels <338 ng/mL (OR 2.63, CI 1.69–4.07), and PCT higher than 0.2 ng/mL (OR 1.74, CI 1.11–2.72) were independent risk factors for co-infection at hospital admission owing to COVID-19.(12co-infection in patients hospitalized for COVID-19 is relatively common. (44). Thus, there is no specific trend in the rates of co-infections after COVID-19 vaccination campaign in specific countries.
The molecular mimicry between SARS-CoV-2 and other pathogens is a key factor in understating potential mechanisms of co-infections after vaccination. This is true mostly for respiratory pathogens provoking cytokine storm resembling COVID-19 scenario such as S. aureus (45) and K. pneumoniae (46) which reacts with SARS-CoV-2 spike protein through lipopolysaccharide and induce storm of proinflammatory activity (47,48). Similarly, it has been shown that several other co-infecting pathogens including E. coli and A. baumannii caused pulmonary injury directly associated with cytokine levels in their infection pattern, which in turn were associated with the proliferation of SARS-CoV-2(49). It is known that poliovirus, measles virus, dengue virus, and severe acute respiratory syndrome-related Coronavirus 2 (SARS-CoV-2) have high molecular mimicry at the heptapeptide level with the human proteome(50). Similarly,The proteomes of BCG, Bordetella.pertussis, Corynebacterium .diphtheriae, Clostridium.tetani, Hemophilus.influenzae, Neisseria. meningitidis and Streptococcus.pneumoniae, contain numerous potentially cross-reactive epitopes with SARS-CoV-2(51). Recent study also reported that the incidence of Hepatitis B Virus infection among patients with COVID-19 seems to be lower than the incidence of HBV infection in the overall Chinese population. A hypothesis was proposed recently for this phenomenon that the exhaustion of T lymphocytes may affect HBV-infected patients' ability to respond to other viruses and then reduce the degree of “cytokine storm,” thus culminating in a less severe disease of COVID-19(52).SARS-CoV-2 associated with Helicobacter.pylori in the high burden of intestinal metaplasia.in H. pylori-infected patients is particularly relevant because of the increased expression of SARS-CoV-2 entry receptors ACE2 and TMPRSS2 in the affected gastric mucosa, mainly due to the migration of intestine-specific cell types, including enterocytes, within the gastric lining(53). The viral infection have the ability to dysregulate the immune system which result in autoimmune disease such as multiple sclerosis (MS),systemic lupus erythematosus (SLE) and AutoImmune hepatitis reported in association with COVID-19(54),(55). Thus, despite enormous efforts, the patterns, types, frequency, and mechanisms as well as case fatality rates (CFR) of co-infections before, during, and post-vaccination has not been clear. Thus, the aim of this study was to understand the trending patterns of bacterial co-infections and the frequent types and associated CFRs of each in three phases of the pandemic. This approach has become imperative as a baseline to understand the mechanisms of microbial co-infections in COVID background and the potentials for molecular mimicry in vaccines
2. Materials and Methods
2.1. Microbiological analysis and patients’ demographics
For bacterial co-infection data, microbiological analysis, positive specimens for non-duplicate isolates obtained from clinical infections recovered from hospitals in Ha’il in the periods before, during, and post-vaccinations were collected. A gap period was considered from the time of vaccine administration (December 17 2020) until expected significant antibody titer was obtained (Apr to June 2021) after which time all isolates were considered post-vaccination. All isolates before that date were considered before vaccination. For routine microbiology and standard molecular diagnostic methods, specimens were cultured to confirm primary identifications, preparations of inoculums for storage, and automated testing. Automated testing and ID and susceptibility assays were done on standard diagnostics such as BD Phoenix system (BD Biosciences, Franklin Lakes, NJ, USA) and MicroScan plus (Beckman Coulter, Brea, CA, USA). Laboratory records, hospital medical records, and various sources within hospitals were used for data collection on patients’ demographics. This included COVID-19 zones of isolations, patients outcome records in clinical departments, and the results of regional laboratory for COVID-19 diagnosis.
2.2. Direct multi-gene Molecular Detection of S. aureus lineages by GeneXpert system
GeneXpert diagnostics and characterizations were performed in Cepheid GeneXpert® Dx system using the SA Complete and MRSA assay kits) using manufacturers recommendations and names and codes included in each kit. This system is equipped with multi-gene molecular primers and reagent kits for robust automated direct detection, characterization, and differentiation of different isolates. This test utilizes automated real-time polymerase chain reaction (PCR). Confirmatory susceptibility assays were carried out by culturing. The GeneXpert Dx is all-in-one system that integrates sample purification, nucleic acid amplification, and detection of the target sequence in simple or complex samples using real-time PCR. It consists of an instrument, personal computer, and preloaded software for running tests and viewing the results. A single-use disposable self-contained cartridges with PCR reagents is inserted and inoculated directly with swabs/samples. In addition to avoiding environmental cues that alter the genome, cross-contamination between samples or during specimen collection or processing as well as cross-sequence contaminations in molecular tests are all remote since the cartridge is a disposable, closed, and self-contained kit. A sample processing control (SPC) and a Probe Check Control (PCC) are also included. The SPC is present to control for adequate processing of the target bacteria and to monitor the presence of inhibitor(s) in the PCR reaction. The PCC verifies reagent rehydration, PCR tube filling in the cartridge, probe integrity, and dye stability.
2.3. Statistical Analysis
Collected data was analyzed using Statistical Package for Social Sciences software (IBM SPSS; Version 24 SPSS version 23.0 for Windows (SPSS, Inc., Chicago, IL, USA). Descriptive and stratified analysis were conducted; we present absolute numbers, proportions, and graphical distributions. We conducted exact statistical tests for proportions and show p-values where appropriate (a p-value <0.05 was considered statistically significant).
3. Results
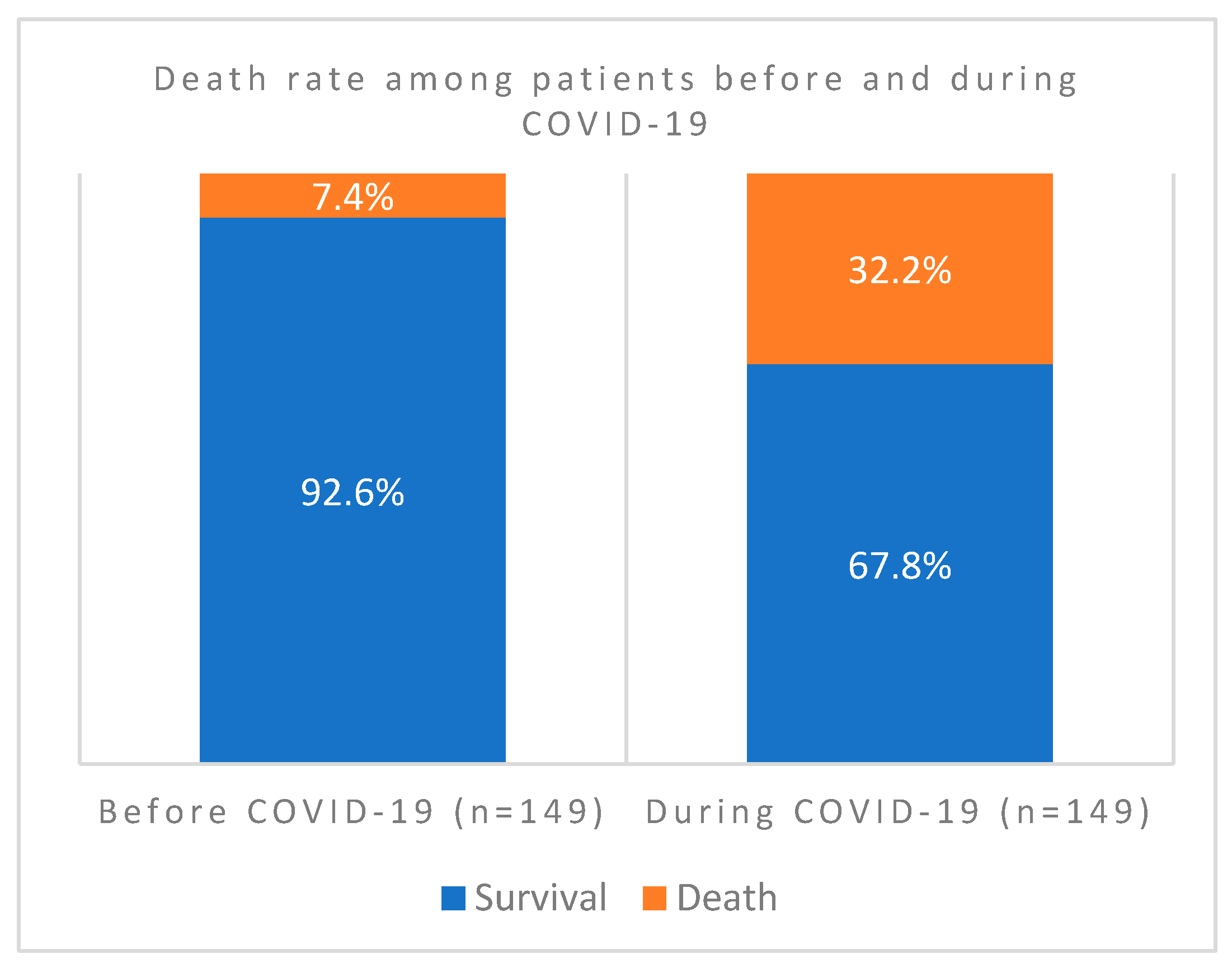
In this comprehensive study, we have investigated 389 cases for clinical profiles, case fatality rates, and patterns of bacterial co-infections before, during, and post-COVID-19 vaccinations. We tried to understand factors that aggravate the disease and the potential mechanisms during host-bacteria-viral interplay. We have screened out all confounding factors that may influence including other existing respiratory syndromes, age and gender specific factors of patients admitted before and during the pandemic. As indicated in
Figure 1., out of the 298 patients screened, COVID case fatality rate during the pandemic was 32.2% compared to only 7% before. The association of case fatality to the pandemic was significantly higher during than that before COVID-19 (
P value = 0.000000075).
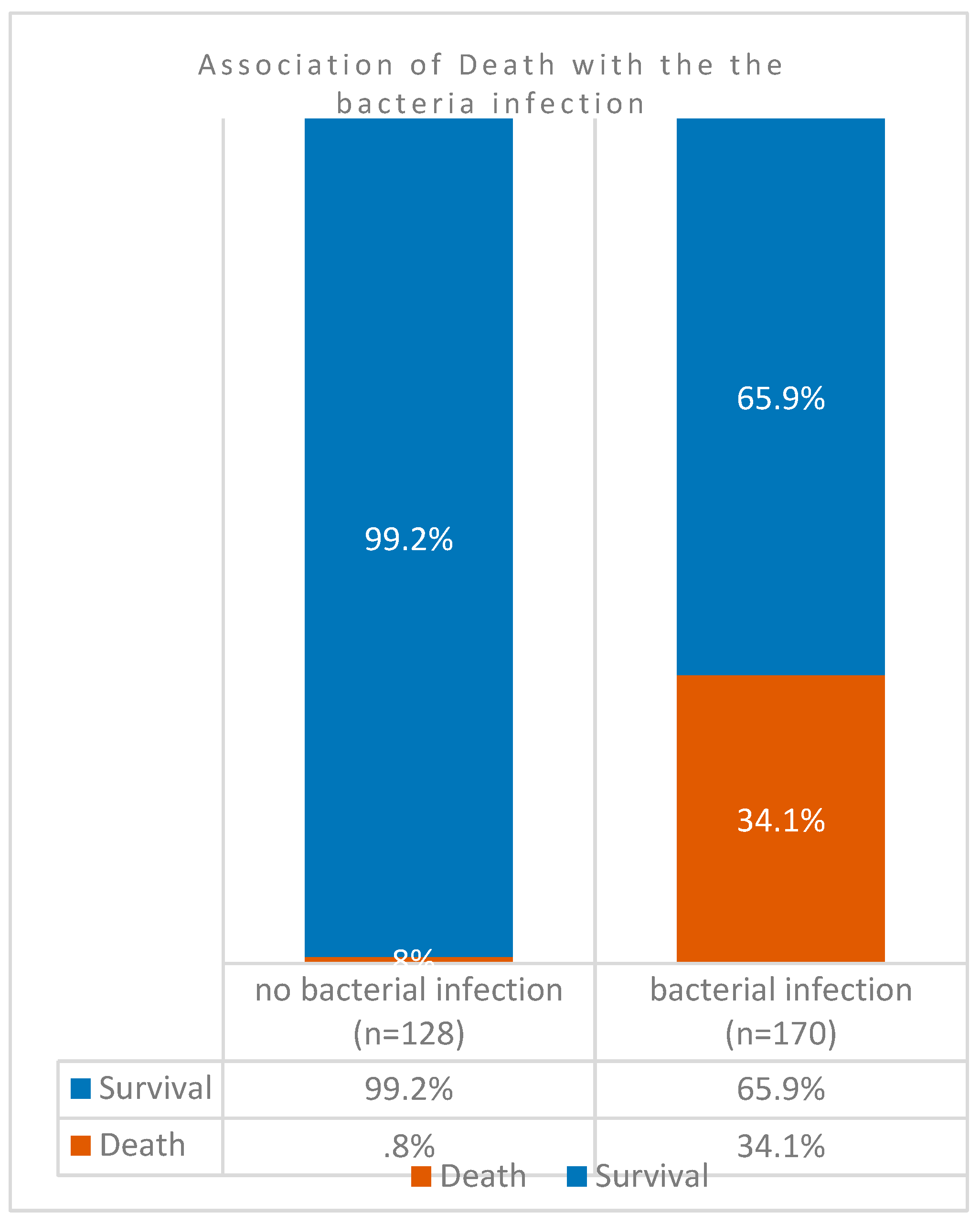
However, in 298 patients, comparison of case fatality rates among co-infected COVID-19 patients (
n = 170) against those without co-infection (
n = 128) indicated that the death rates was significantly higher (34%) in the former group (
Figure 2). Association of mortality and case aggravation to co-infection was significantly higher as indicated by the
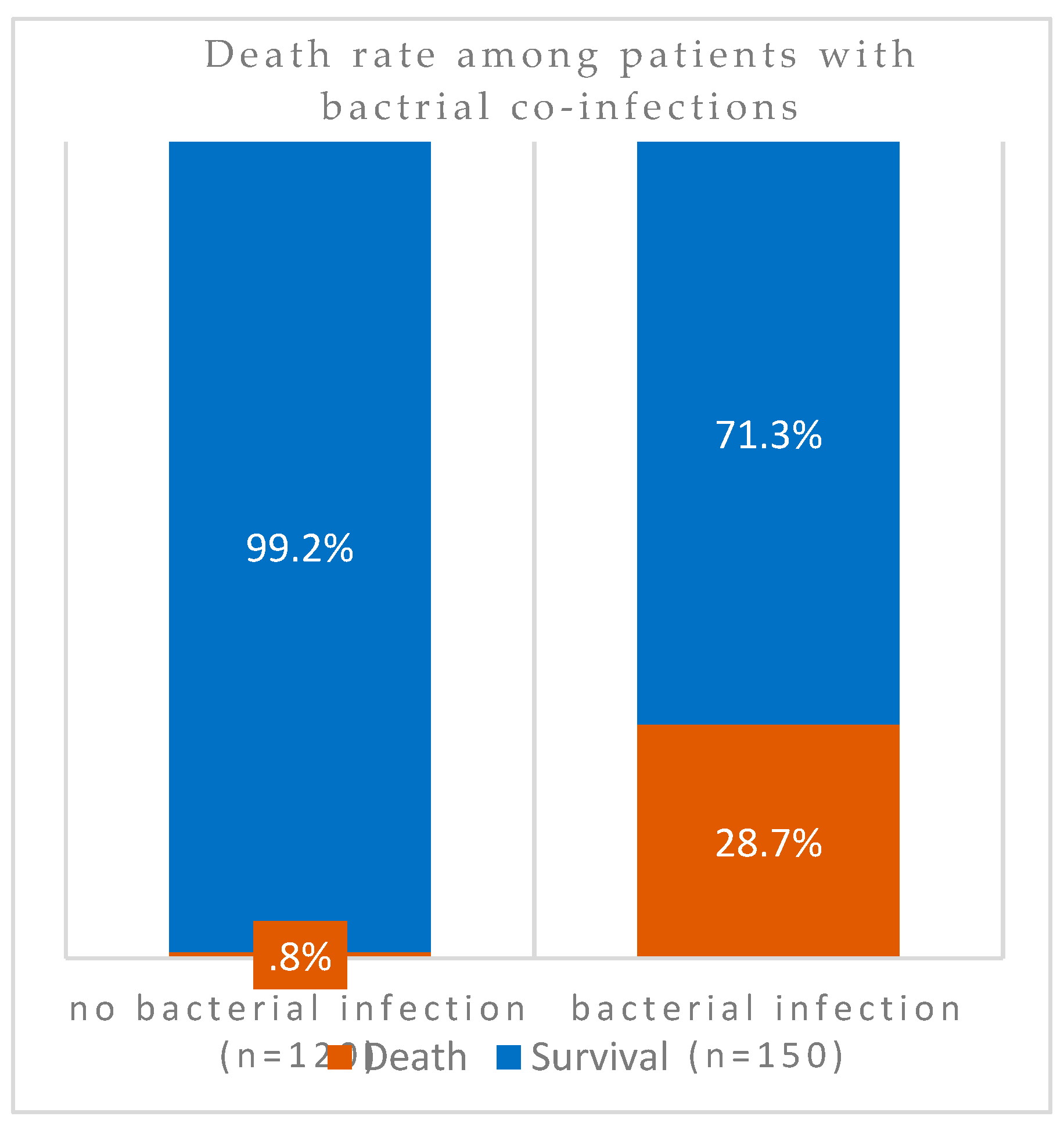
P value = 0.00000000000088). In other words, almost 100% (99%) of patients without SARS-CoV-2 superinfection survived the pandemic. However, exclusion of all patients with Severe Respiratory Distress Syndromes in patients with bacterial co-infections also resulted in higher levels of mortalities (
Figure 3). Among these patients without underlying respiratory syndrome (
n = 270), bacterial infection was associated with a higher death rate as shown by the highly significant value (
P value= 0.00000000076).
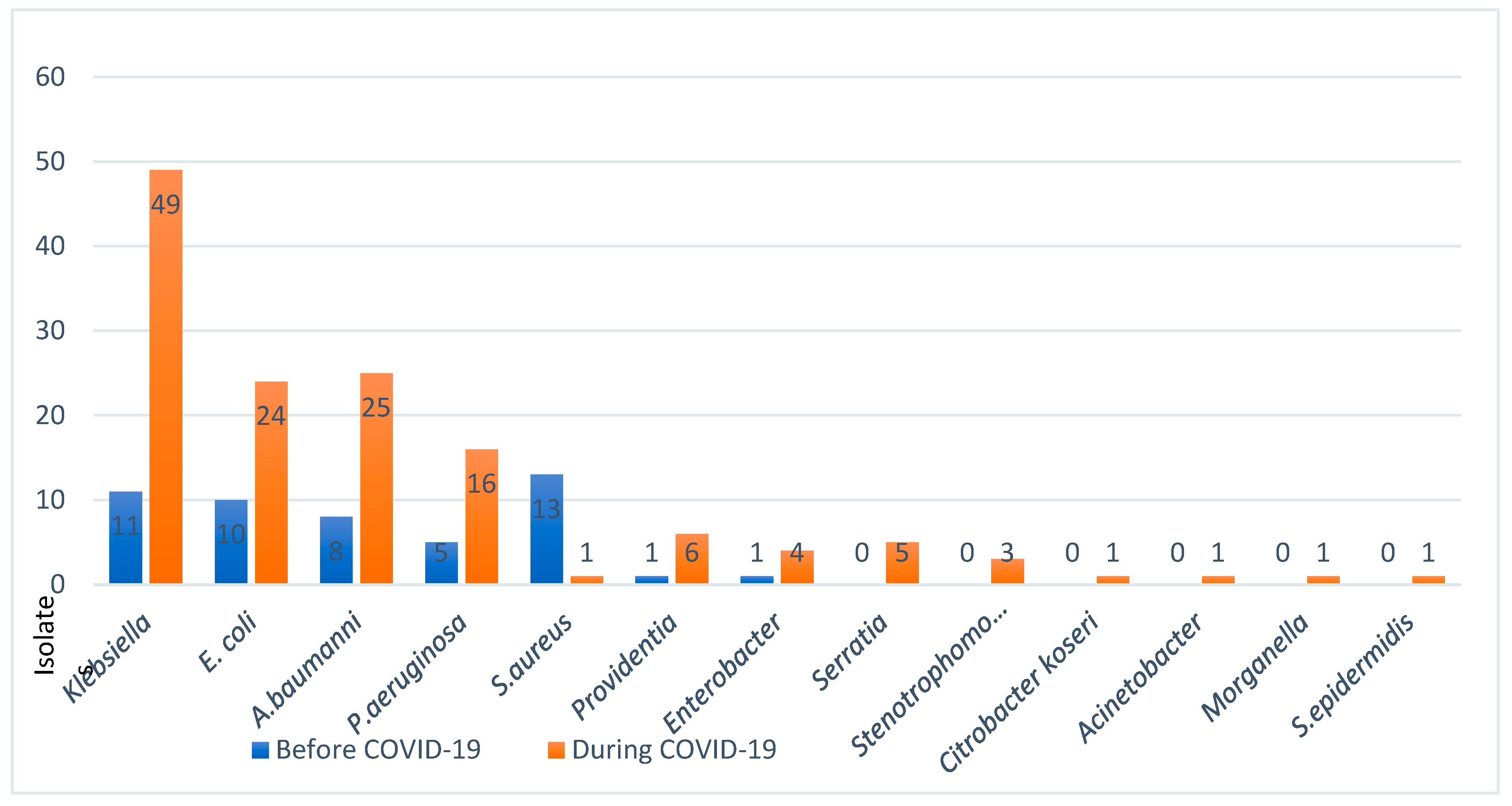
Pathogenic populations of microbial co-infections with SARS-CoV-2 presented with significant changes in their types and profiles. To understand this important factor in host-viral-bacterial interactions, we have examined the trending patterns of infections across three phases of the pandemic i.e., before, during, and after vaccinations. The following frequency of major co-infections were found out of cases (
n=149 before/n=149 during):
Klebsiella pneumonia (n = 11/49 before/during; E. coli n=10/24, A. baumannii n=8/25, and
Ps. Aeruginosa n= 5/16, S. aureus 13/1. The major findings were the significant decline in the rates of Gram-positive species, mainly
Staphylococcus aureus, while a steady increase in a few Gram-negative species was observed during the pandemic (
Figure 4).
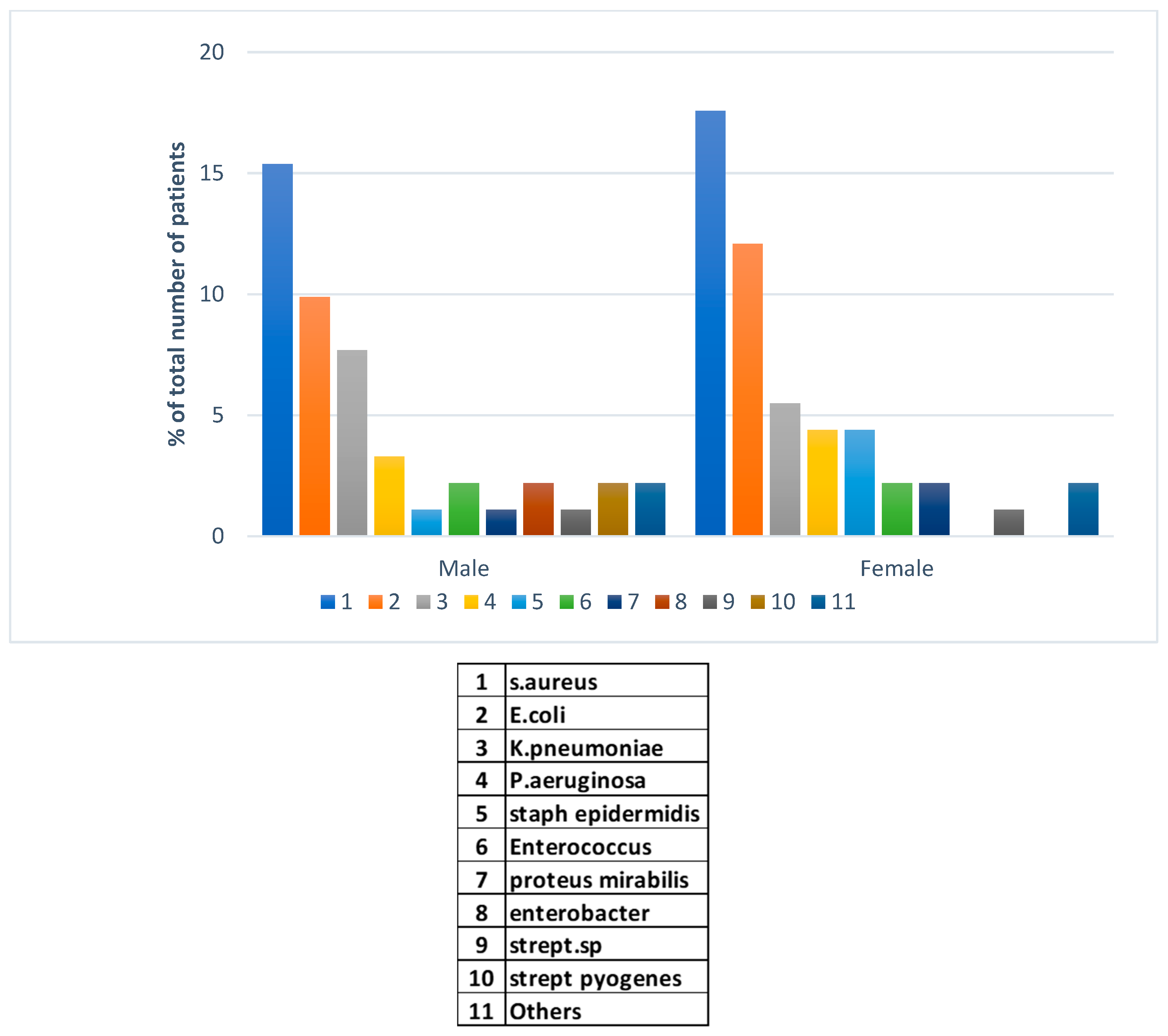
The major finding in this investigation was in the peaks in types of bacterial pathogens co-infecting with SARS-CoV-2 virus during and post-COVID vaccinations. In this region, a 100% of vaccination was achieved during the early stage of the vaccine campaigns consisting of Pfizer, Moderna, and the Oxfora/ AstraZeneca recombinant vaccine. In 91 bacterial co-infection cases post vaccination, we have examined gender-specificity to account for potential differences in susceptibility unlike before the pandemic. Methicillin resistant
S. aureus (MRSA) was the dominant hospital pathogen isolates from cases of infections in both genders post COVID-19 (
Figure 5). This was followed by
E. coli in males and females with the latter gender showing higher rates of isolations in both species.
Klebsiella pneumoniae in the third place was more frequently isolated from male patients post vaccinations. Other Gram-negative and -positive pathogens presented with lower rate of isolations. Much lower frequency of bacterial isolations was reported post-COVID-19 pandemic compared to before and during the pandemic.
4. Discussion
In the current study, we have investigated the factors that exacerbate COVID-19 pandemic fatality rates. By examining patterns of infections across three phases of COVID-19 pandemic i.e., before, during, and post-vaccination, we have also identified missing gaps of potentially novel mechanisms that influenced the patterns of co-infection during host-bacteria-viral interplay. In agreement with the widely reported finding, the higher case fatality rates significantly associated to COVID-19 (32.2%, (P value = 0.000000075) compared to before the pandemic indicated enhanced virulence and epidemicity of the virus. The Lancet Commission on lessons for the future from the COVID-19 pandemic has described the staggering death toll of COVID-19 as both a profound tragedy and a massive global failure at multiple levels (56). However, the global case fatality rate of COVID-19 has decreased by 96.8% during the last years of the pandemic (57). Intriguingly, the unprecedented sharp increase followed by a rapid decline of the pandemic after vaccination has never been witnessed in the modern human history. As with all pandemics, the aftermath SARS-CoV-2 has left several novel observations on its epidemicity, virulence, and mechanisms of co-infections. We have found that while S. aureus dominated before and after vaccinations, Gram-negative pathogens, K. pneumoniae, E. coli, A. baumannii, and Ps. Aeruginosa, peaked in the middle phase during but before vaccinations. It is plausible that these observations provide proof of concepts about two potential mechanisms during and post-vaccination phase. An important gap exists during but before vaccination phase; it is not clear whether both species could have used a common mechanism to elicit cytokine storm or the selective and rapid outgrowth of K. pneumoniae might have suppressed S. aureus. The latter species produces potent exoproteins and excretes several toxins to induce cytokine storm without the need for cell suppression while Gram-negatives use cell-bound LPS which explains the need for cell concentrations. Thus, future vertical studies would gain more insights into the mechanisms of co-infections with SARS-CoV-2.
The uniquely trending pattern of SARS-CoV-2 co-infection with bacterial pathogens in the three phases of the pandemic namely: before, during, and after vaccinations has left a remarkable phenomenon. Although co-infections are widely reported as major aggravators, the mechanisms of how this occurs is poorly understood. The major finding in this study was the sudden drop in the frequency of isolation of
S. aureus lineages during the pandemic pre-vaccinations, whereas a steady increase in limited Gram-negative pathogens was observed at the same time during this phase. Bacterial co-infectors mostly included
Klebsiella pneumonia, E. coli, A. baumannii, and
Ps. Aeruginosa in that order. This was followed by another peak of
S. aureus infections towards the aftermath of the pandemic right after vaccinations dominating all Gram-negative pathogens (
Figure 5).
Staphylococcus aureus is a very well-known superbug that elicited massive cytokine storm leading to serous necrotizing pneumonia outbreak reported in CA-MRSA pandemic a decade ago (58). In addition, recent experimental demonstration proved that
S. aureus provoked cytokine storm in BALB/c mice (45). Nevertheless, recent experimental data from BALB/cJ mice indicated that co-infected mice showed massive immune storm and severe clinical disease leading to higher mortality rate within 48 h of
K. pneumoniae infection. Significantly higher bacterial loads in the lungs were observed, albeit viral loads remained unchanged between co-infected and single-infected mice. (46). It is interesting that these two species provoked cytokine storms during lung necrotizing infections; however, it still remains to be seen whether they both use the same mechanism of induction of major histocompatibility complex (MHC) class II on antigen-presenting cells (APC). A highly significant clue for a common induction is the pattern of co-infection observed in this study. We have observed that all
S. aureus lineages including methicillin sensitive, MRSA, CA-MRSA, as well as animal lineage rates drastically reduced during COVID-19 before vaccinations and then peaked right after vaccinations. If this was a competitive overgrowth by Gram-negatives occupying cytokine induction sites on APC, then it is difficult to explain their sharp declined after vaccination where
S. aureus peaked. It is plausible that there is an element of potential molecular mimicry with Gram-negatives in the vaccines. In support of this, a case of a community-acquired MRSA necrotizing lung infection occurred right after recovery from SARS-CoV-2 infection (59). In addition, evidence demonstrated that SARS-CoV-2 spike protein served as a lipopolysaccharide delivery system and binds to bacterial LPS boosting overzealous storm of proinflammatory activity (47,48). Similarly, it has been shown that several other co-infecting pathogens including
E. coli and
A. baumannii caused pulmonary injury directly associated with cytokine levels in their infection pattern, which in turn were associated with the proliferation of SARS-CoV-2(49). Although there are several scenarios in the molecular mechanisms of co-infections, this study provides clear observations about the coexistence patterns of different co-infecting pathogens in COVID-19 backgrounds.
5. Conclusions
Thus, we have investigated all three phases of SARS-CoV-2 pandemic patterns of infection i.e., before, during, and post-vaccination. In this study, we provide factors that aggravated COVID-19 disease fatality rates including patient gender and bacterial co-infections. The high significant rates of mortality in co-infected patients during COVID-19 indicated influence of bacterial pathogens in patients’ worse outcome. Intrudingly, the positive selection for co-infection by limited Gram-negatives during COVID-19 with concomitant decline in S. aureus followed by peaks of the latter and drastic decline of the former species in post-vaccination phase strongly implied potential element of molecular mimicry in the vaccine component. Future molecular analysis of host-virus-bacterial interplay for identification and characterization of common gene candidate(s) involved in cytokine storm has become imperative since there is a pandemic outbreak of hypervirulent Gram negatives and CA-MRSA necrotizing pneumonias. The project is limited by the lack of wide regional coverage that could provide larger sample sizes for more insights into the mechanisms of pathogenicity and virulence.
Author Contributions
Conceptualization, Kamaleldin Said; Data curation, Kamaleldin Said, Ahmed Alsolami, Khalid Alshammari, Fawwaz Alshammari, Safia Moussa, Mohammed H. Alghozwi, Suliman F. Alshammari, Nawaf F. Alharbi, Amany Khalifa, Madiha Mahmoud and Mohamed E. Ghoniem; Formal analysis, Kamaleldin Said, Ahmed Alsolami, Khalid Alshammari, Fawwaz Alshammari, Safia Moussa, Mohammed H. Alghozwi, Suliman F. Alshammari, Nawaf F. Alharbi, Amany Khalifa, Madiha Mahmoud and Mohamed E. Ghoniem; Funding acquisition, Kamaleldin Said; Investigation, Kamaleldin Said and Ahmed Alsolami; Methodology, Kamaleldin Said, Ahmed Alsolami, Khalid Alshammari, Fawwaz Alshammari, Safia Moussa, Mohammed H. Alghozwi, Suliman F. Alshammari, Nawaf F. Alharbi, Amany Khalifa, Madiha Mahmoud and Mohamed E. Ghoniem; Project administration, Kamaleldin Said; Resources, Kamaleldin Said, Ahmed Alsolami, Khalid Alshammari, Fawwaz Alshammari, Safia Moussa, Mohammed H. Alghozwi, Suliman F. Alshammari, Nawaf F. Alharbi, Amany Khalifa, Madiha Mahmoud and Mohamed E. Ghoniem; Software, Kamaleldin Said, Ahmed Alsolami, Khalid Alshammari, Fawwaz Alshammari, Safia Moussa, Mohammed H. Alghozwi, Suliman F. Alshammari, Nawaf F. Alharbi, Amany Khalifa, Madiha Mahmoud and Mohamed E. Ghoniem; Supervision, Kamaleldin Said and Ahmed Alsolami; Validation, Kamaleldin Said, Ahmed Alsolami, Khalid Alshammari, Fawwaz Alshammari, Safia Moussa, Mohammed H. Alghozwi, Suliman F. Alshammari, Nawaf F. Alharbi, Amany Khalifa, Madiha Mahmoud and Mohamed E. Ghoniem; Visualization, Kamaleldin Said, Ahmed Alsolami, Khalid Alshammari, Fawwaz Alshammari, Safia Moussa, Mohammed H. Alghozwi, Suliman F. Alshammari, Nawaf F. Alharbi, Amany Khalifa, Madiha Mahmoud and Mohamed E. Ghoniem; Writing – original draft, Kamaleldin Said; Writing – review & editing, Kamaleldin Said, Ahmed Alsolami, Khalid Alshammari, Fawwaz Alshammari, Safia Moussa, Mohammed H. Alghozwi, Suliman F. Alshammari, Nawaf F. Alharbi, Amany Khalifa, Madiha Mahmoud and Mohamed E. Ghoniem.
Funding
This research project was funded by Scientific Research Deanship at the University of Ha’il- Saudi Arabia, through project number RG-21074.
Ethical approval and Institutional Review Board Statement
This project (number RG21074) has been reviewed and Approval by the Research Ethical Committee (REC) of the University of Ha’il, dated 22/11/2021 under numbers H-2021-215, File H-2020-632-16160.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
There is no additional data deposited on any other site other than those in this manuscript.
Acknowledgments
We acknowledge the University of Ha’il for the support and encouragement through the Deanship of Scientific Research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Call to Action on Antimicrobial Resistance 2021 [Internet]. [cited 2022 Feb 27]. Available from: https://www.who.int/news/item/30-07-2021-call-to-action-on-antimicrobial-resistance-2021.
- Rello J, Kalwaje Eshwara V, Lagunes L, Alves J, Wunderink RG, Conway-Morris A, et al. A global priority list of the TOp TEn resistant Microorganisms (TOTEM) study at intensive care: a prioritization exercise based on multi-criteria decision analysis. Eur J Clin Microbiol Infect Dis [Internet]. 2019 Feb 4 [cited 2022 Feb 21];38(2):319–23. Available from: https://pubmed.ncbi.nlm.nih.gov/30426331/. [CrossRef]
- Seale AC, Gordon NC, Islam J, Peacock SJ, Scott JAG. AMR Surveillance in low and middle-income settings - A roadmap for participation in the Global Antimicrobial Surveillance System (GLASS). Wellcome Open Res [Internet]. 2017 [cited 2022 Feb 21];2. Available from: https://pubmed.ncbi.nlm.nih.gov/29062918/. [CrossRef]
- Ferreira-Coimbra J, Sarda C, Rello J. Burden of Community-Acquired Pneumonia and Unmet Clinical Needs. Adv Ther [Internet]. 2020 Apr 1 [cited 2022 Feb 9];37(4):1302. Available from: /pmc/articles/PMC7140754/. [CrossRef]
- Global Health Estimates 2016: Deaths by Cause AS by C and by R 2000 2016. GWHO 2018. GHE2016_Deaths_WBInc_2000_2016.xls [Internet]. [cited 2022 Feb 26]. Available from: https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fwww.who.int%2Fhealthinfo%2Fglobal_burden_disease%2FGHE2016_Deaths_WBInc_2000_2016.xls&wdOrigin=BROWSELINK.
- O’Neill J. Tackling drug-resistant infections globally : final report and recommendations / the Review on Antimicrobial Resistance chaired by Jim O’Neill. | Wellcome Collection [Internet]. [cited 2022 Feb 24]. Available from: https://wellcomecollection.org/works/thvwsuba.
- Plachouras D, Kärki T, Hansen S, Hopkins S, Lyytikäinen O, Moro ML, et al. Antimicrobial use in European acute care hospitals: results from the second point prevalence survey (PPS) of healthcare-associated infections and antimicrobial use, 2016 to 2017. Euro Surveill [Internet]. 2018 Nov 15 [cited 2022 Feb 22];23(46). Available from: https://pubmed.ncbi.nlm.nih.gov/30458917/.
- Ricchizzi E, Latour K, Kärki T, Buttazzi R, Jans B, Moro ML, et al. Antimicrobial use in European long-term care facilities: results from the third point prevalence survey of healthcare-associated infections and antimicrobial use, 2016 to 2017. Euro Surveill [Internet]. 2018 Nov 15 [cited 2022 Feb 22];23(46). Available from: https://pubmed.ncbi.nlm.nih.gov/30458913/. [CrossRef]
- Suetens C, Latour K, Kärki T, Ricchizzi E, Kinross P, Moro ML, et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017. Euro Surveill [Internet]. 2018 Nov 15 [cited 2022 Feb 22];23(46). Available from: https://pubmed.ncbi.nlm.nih.gov/30458912/. [CrossRef]
- Centers for Disease Control U. Antibiotic Resistance Threats in the United States, 2019. [cited 2022 Feb 23]; Available from: http://dx.doi.org/10.15620/cdc:82532. [CrossRef]
- Naoum P, Athanasakis K, Kyriopoulos I, Liapikou A, Toumbis M, Kyriopoulos J. Community acquired pneumonia: a cost-of-illness analysis in Greece. Rural Remote Health [Internet]. 2020 Jun 15 [cited 2022 Feb 5];20(2):1–10. Available from: https://www.rrh.org.au/journal/article/5400/. [CrossRef]
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Available from: http://thorax.bmj.com/. [CrossRef]
- Friedrich AW. Control of hospital acquired infections and antimicrobial resistance in Europe: the way to go. Wien Med Wochenschr [Internet]. 2019 Feb 1 [cited 2022 Feb 9];169(Suppl 1):25. Available from: /pmc/articles/PMC6373234/. [CrossRef]
- Olise CC. Fomites: Possible vehicle of nosocomial infections. [cited 2022 Feb 26]; Available from: http://www.alliedacademies.org/public-health-nutrition/.
- Larypoor M, Frsad S. Evaluation of nosocomial infections in one of hospitals of Qom 2008 - Iranian Journal of Medical Microbiology [Internet]. [cited 2022 Feb 26]. Available from: https://ijmm.ir/article-1-194-en.html&sw=.
- Moussally M, Zahreddine N, Kazma J, Ahmadieh R, Kan SS, Kanafan ZA. Prevalence of antibiotic-resistant organisms among hospitalized patients at a tertiary care center in Lebanon, 2010–2018. J Infect Public Health. 2021 Jan 1;14(1):12–6. [CrossRef]
- Kanafani ZA, el Zakhem A, Zahreddine N, Ahmadieh R, Kanj SS. Ten-year surveillance study of ventilator-associated pneumonia at a tertiary care center in Lebanon. J Infect Public Health. 2019 Jul 1;12(4):492–5. [CrossRef]
- Balkhy HH, El-Saed A, Alshamrani MM, Alsaedi A, al Nasser W, el Gammal A, et al. Ten-year resistance trends in pathogens causing healthcare-associated infections; reflection of infection control interventions at a multi-hospital healthcare system in Saudi Arabia, 2007-2016. Antimicrob Resist Infect Control [Internet]. 2020 Jan 30 [cited 2022 Feb 23];9(1). Available from: https://pubmed.ncbi.nlm.nih.gov/32000850/. [CrossRef]
- El-Saed A, Balkhy HH, Alshamrani MM, Aljohani S, Alsaedi A, al Nasser W, et al. High contribution and impact of resistant gram negative pathogens causing surgical site infections at a multi-hospital healthcare system in Saudi Arabia, 2007-2016. BMC Infect Dis [Internet]. 2020 Apr 7 [cited 2022 Feb 23];20(1). Available from: https://pubmed.ncbi.nlm.nih.gov/32264843/. [CrossRef]
- Francis Borgio J, Rasdan AS, Sonbol B, Alhamid G, Almandil NB, Azeez SA. Emerging Status of Multidrug-Resistant Bacteria and Fungi in the Arabian Peninsula. Biology (Basel) [Internet]. 2021 Nov 1 [cited 2022 Feb 23];10(11). Available from: https://pubmed.ncbi.nlm.nih.gov/34827138/. [CrossRef]
- Alhumaid S, al Mutair A, al Alawi Z, Alzahrani AJ, Tobaiqy M, Alresasi AM, et al. Antimicrobial susceptibility of gram-positive and gram-negative bacteria: a 5-year retrospective analysis at a multi-hospital healthcare system in Saudi Arabia. Ann Clin Microbiol Antimicrob [Internet]. 2021 Dec 1 [cited 2022 Feb 23];20(1). Available from: https://pubmed.ncbi.nlm.nih.gov/34118930/. [CrossRef]
- Chen X, Liao B, Cheng L, Peng X, Xu X, Li Y, et al. The microbial coinfection in COVID-19. Available from: https://doi.org/10.1007/s00253-020-10814-6. [CrossRef]
- Zhu X, Ge Y, Wu T, Zhao K, Chen Y, Wu B, et al. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020 Aug 1;285. [CrossRef]
- Yang S, Hua M, Liu X, Du C, Pu L, Xiang P, et al. Bacterial and fungal co-infections among COVID-19 patients in intensive care unit. Microbes Infect. 2021 May 1;23(4–5). [CrossRef]
- Moramarco A, Mastroianni F., Derosa C, Guida P, Tauro L, Laterza M, et al. Co-infections in patients with COVID-19. [cited 2022 Oct 23]; Available from: https://pesquisa.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/resource/pt/covidwho-984617?lang=en.
- Rossato L, Negrão FJ, Simionatto S. Could the COVID-19 pandemic aggravate antimicrobial resistance? Vol. 48, American Journal of Infection Control. Mosby Inc.; 2020. p. 1129–30.
- Naseef HA, Mohammad U, Al-Shami N, Sahoury Y, Abukhalil AD, Dreidi M, et al. Bacterial and fungal co-infections among ICU COVID-19 hospitalized patients in a Palestinian hospital: a retrospective cross-sectional study. F1000Res. 2022 Jan 11;11:30.
- Vijay S, Bansal N, Rao BK, Veeraraghavan B, Rodrigues C, Wattal C, et al. Secondary Infections in Hospitalized COVID-19 Patients: Indian Experience. 2021; Available from: https://doi.org/10.2147/IDR.S299774. [CrossRef]
- Dutta Majumder P. References. Available from: www.ijo.in.
- Sharov KS. Correspondence to. 2020; Available from: www.jogh.org•.
- Silva DL, Lima CM, Magalhães VCR, Baltazar LM, Peres NTA, Caligiorne RB, et al. Fungal and bacterial coinfections increase mortality of severely ill COVID-19 patients. Journal of Hospital Infection. 2021 Jul 1;113:145–54. [CrossRef]
- YAQOOB H, RIZWAN M, GREENBERG D, ARSHAD A, EPELBAUM O, CHANDY D. PREDICTORS AND OUTCOMES OF BACTERIAL AND FUNGAL SUPERINFECTIONS IN CRITICALLY ILL PATIENTS WITH COVID-19. Chest. 2021 Oct;160(4):A591. [CrossRef]
- Alnimr AM, Alshahrani MS, Alwarthan S, AlQahtani SY, Hassan AA, BuMurah NN, et al. Bacterial and Fungal Coinfection in Critically Ill COVID-19 Cases and Predictive Role of Procalcitonin During the First Wave at an Academic Health Center. J Epidemiol Glob Health. 2022 Jun 1;12(2):188–95. [CrossRef]
- Swets MC, Russell CD, Harrison EM, Docherty AB, Lone N, Girvan M, et al. SARS-CoV-2 co-infection with influenza viruses, respiratory syncytial virus, or adenoviruses. Vol. 399, The Lancet. Elsevier B.V.; 2022. p. 1463–4.
- Alshaikh FS, Godman B, Sindi ON, Andrew Seaton R, Kurdi A. Prevalence of bacterial coinfection and patterns of antibiotics prescribing in patients with COVID-19: A systematic review and metaanalysis. Vol. 17, PLoS ONE. Public Library of Science; 2022. [CrossRef]
- Soni S, Namdeo Pudake R, Jain U, Chauhan N. A systematic review on SARS-CoV-2-associated fungal coinfections. Vol. 94, Journal of Medical Virology. John Wiley and Sons Inc; 2022. p. 99–109.
- Zamora-Cintas MI, López DJ, Blanco AC, Rodriguez TM, Segarra JM, Novales JM, et al. Coinfections among hospitalized patients with covid-19 in the first pandemic wave. Diagn Microbiol Infect Dis. 2021 Nov 1;101(3). [CrossRef]
- Rafat Z, Ramandi A, Khaki PA, Ansari S, Ghaderkhani S, Haidar H, et al. Fungal and bacterial co-infections of the respiratory tract among patients with COVID-19 hospitalized in intensive care units. Gene Rep. 2022 Jun 1;27. [CrossRef]
- Sreenath K, Batra P, Vinayaraj E v., Bhatia R, SaiKiran K, Singh V, et al. Coinfections with Other Respiratory Pathogens among Patients with COVID-19. Microbiol Spectr. 2021 Sep 3;9(1). [CrossRef]
- Nebreda-Mayoral T, Miguel-Gómez MA, March-Rosselló GA, Puente-Fuertes L, Cantón-Benito E, Martínez-García AM, et al. Bacterial/fungal infection in hospitalized patients with COVID-19 in a tertiary hospital in the Community of Castilla y León, Spain. Enfermedades infecciosas y microbiologia clinica (English ed). 2022 Apr;40(4):158–65. [CrossRef]
- Gerver SM, Guy R, Wilson K, Thelwall S, Nsonwu O, Rooney G, et al. National surveillance of bacterial and fungal coinfection and secondary infection in COVID-19 patients in England: lessons from the first wave. Clinical Microbiology and Infection. 2021;27(11). [CrossRef]
- Rawson TM, Wilson RC, Holmes A. Understanding the role of bacterial and fungal infection in COVID-19. Vol. 27, Clinical Microbiology and Infection. 2021. [CrossRef]
- Bahceci I, Yildiz IE, Duran OF, Soztanaci US, Kirdi Harbawi Z, Senol FF, et al. Secondary Bacterial Infection Rates Among Patients With COVID-19. Cureus. 2022; [CrossRef]
- Moreno-García E, Puerta-Alcalde P, Letona L, Meira F, Dueñas G, Chumbita M, et al. Bacterial co-infection at hospital admission in patients with COVID-19. International Journal of Infectious Diseases. 2022 May 1;118:197–202. [CrossRef]
- Liao F, Gu W, Fu X, Yuan B, Zhang Y. Community-acquired methicillin-resistant Staphylococcus aureus provoked cytokine storm causing severe infection on BALB/c mice. Mol Immunol. 2021 Dec 1;140:167–74. [CrossRef]
- Villalva C, Patil G, Narayanan S, Chanda D, Ghimire R, Snider T, et al. Klebsiella pneumoniae C o-infection Leads to Fatal Pneumonia in SARS-CoV-2-infected Mice. bioRxiv [Internet]. 2023 Aug 1 [cited 2023 Sep 26]; Available from: /pmc/articles/PMC10418095/. [CrossRef]
- Samsudin F, Raghuvamsi P, Petruk G, Puthia M, Petrlova J, Macary P, et al. SARS-CoV-2 spike protein as a bacterial lipopolysaccharide delivery system in an overzealous inflammatory cascade. J Mol Cell Biol [Internet]. 2023 Sep 1 [cited 2023 Sep 26];14(9). Available from: https://pubmed.ncbi.nlm.nih.gov/36240490/. [CrossRef]
- Petruk G, Puthia M, Petrlova J, Samsudin F, Strömdahl AC, Cerps S, et al. SARS-CoV-2 spike protein binds to bacterial lipopolysaccharide and boosts proinflammatory activity. J Mol Cell Biol [Internet]. 2020 [cited 2023 Sep 26];12(12):916–32. Available from: https://pubmed.ncbi.nlm.nih.gov/33295606/. [CrossRef]
- Chen L, Shen L, Wu W, Guan W, Zhou J, Luo G, et al. Co-infecting pathogens can contribute to inflammatory responses and severe symptoms in COVID-19. J Thorac Dis [Internet]. 2022 Feb 1 [cited 2023 Sep 26];14(2):355–70. Available from: https://pubmed.ncbi.nlm.nih.gov/35280492/. [CrossRef]
- Zhu X, Ge Y, Wu T, Zhao K, Chen Y, Wu B, et al. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020 Aug 1;285. [CrossRef]
- Reche PA. Potential Cross-Reactive Immunity to SARS-CoV-2 From Common Human Pathogens and Vaccines. Front Immunol. 2020 Oct 16;11. [CrossRef]
- Kalligeros M, Karageorgos SA, Shehadeh F, Zacharioudakis IM, Mylonakis E. Systematic Review and Meta-analysis of the Association of Acute Kidney Injury with the Concomitant Use of Vancomycin and Piperacillin-Tazobactam in Children. Antimicrob Agents Chemother. 2019;63(12). [CrossRef]
- Gonzalez I, Lindner C, Schneider I, Morales MA, Rojas A. Inflammation at the crossroads of Helicobacter pylori and COVID-19. Vol. 17, Future Microbiology. Newlands Press Ltd; 2022. p. 77–80. [CrossRef]
- Zamani B, Moeini Taba SM, Shayestehpour M. Systemic lupus erythematosus manifestation following COVID-19: a case report. J Med Case Rep. 2021 Dec 1;15(1). [CrossRef]
- Mekritthikrai K, Jaru-Ampornpan P, Komolmit P, Thanapirom K. Autoimmune Hepatitis Triggered by COVID-19 Vaccine: The First Case From Inactivated Vaccine. ACG Case Rep J. 2022 Jul;9(7):e00811. [CrossRef]
- Sachs JD, Karim SSA, Aknin L, Allen J, Brosbøl K, Colombo F, et al. The Lancet Commission on lessons for the future from the COVID-19 pandemic. Lancet [Internet]. 2022 Oct 8 [cited 2023 Sep 26];400(10359):1224. Available from: /pmc/articles/PMC9539542/. [CrossRef]
- Horita N, Fukumoto T. Global case fatality rate from COVID-19 has decreased by 96.8% during 2.5 years of the pandemic. J Med Virol [Internet]. 2023 Jan 1 [cited 2023 Sep 26];95(1). Available from: /pmc/articles/PMC9874414/. [CrossRef]
- Lee AS, De Lencastre H, Garau J, Kluytmans J, Malhotra-Kumar S, Peschel A, et al. Methicillin-resistant Staphylococcus aureus. Nature Reviews Disease Primers 2018 4:1 [Internet]. 2018 May 31 [cited 2023 Sep 26];4(1):1–23. Available from: https://www.nature.com/articles/nrdp201833.
- McCraw C, Forbush S, Trivedi K. Community-Acquired, Post-COVID-19, Methicillin-Resistant Staphylococcus aureus Pneumonia and Empyema. Cureus [Internet]. 2022 Feb 11 [cited 2023 Sep 26];14(2). Available from: /pmc/articles/PMC8918236/. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).