Submitted:
04 December 2023
Posted:
05 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Pastes
2.3. Mechanical, Mineralogical and Microstructural Characterisation of Pastes
3. Results and Discussion
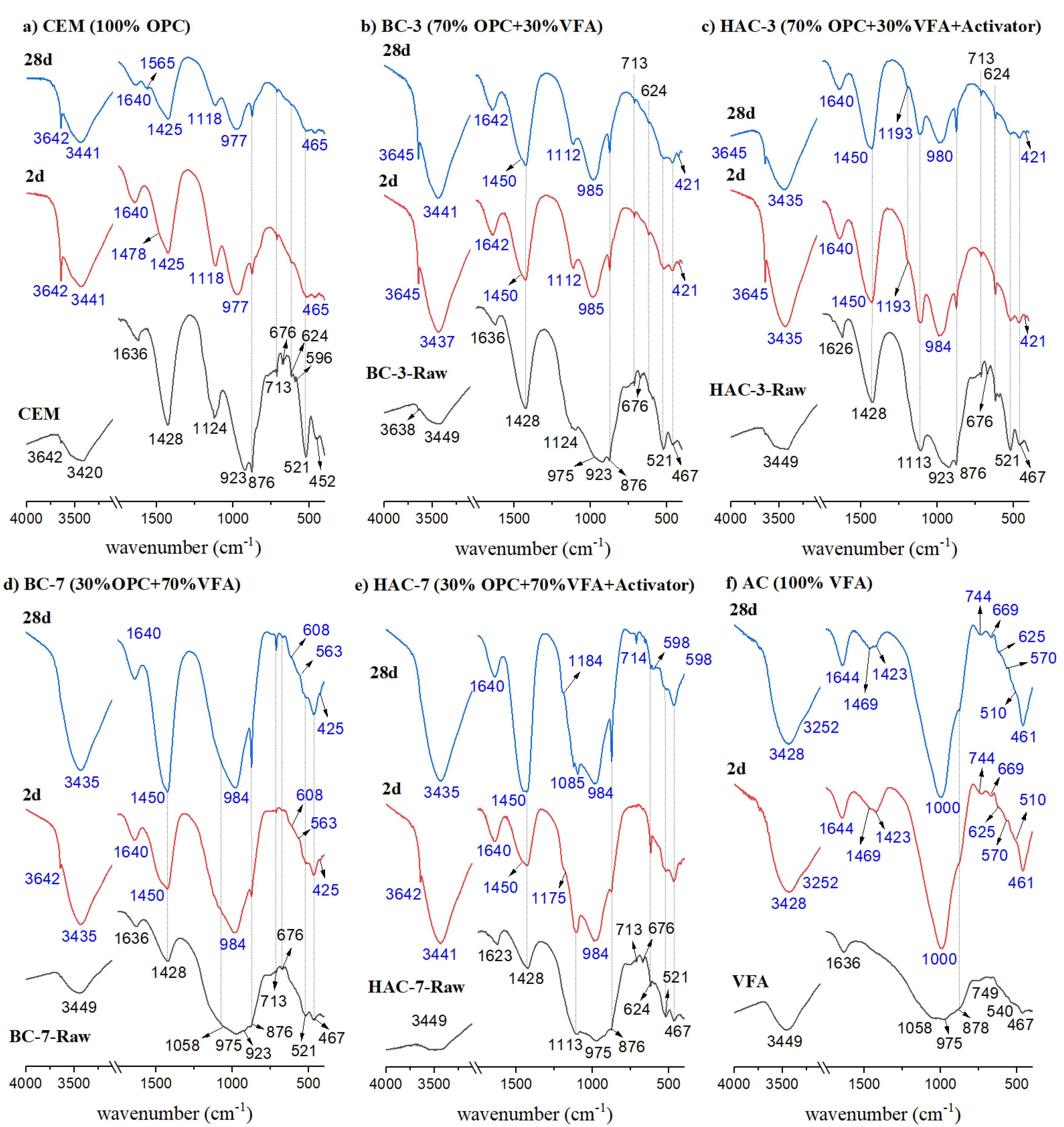
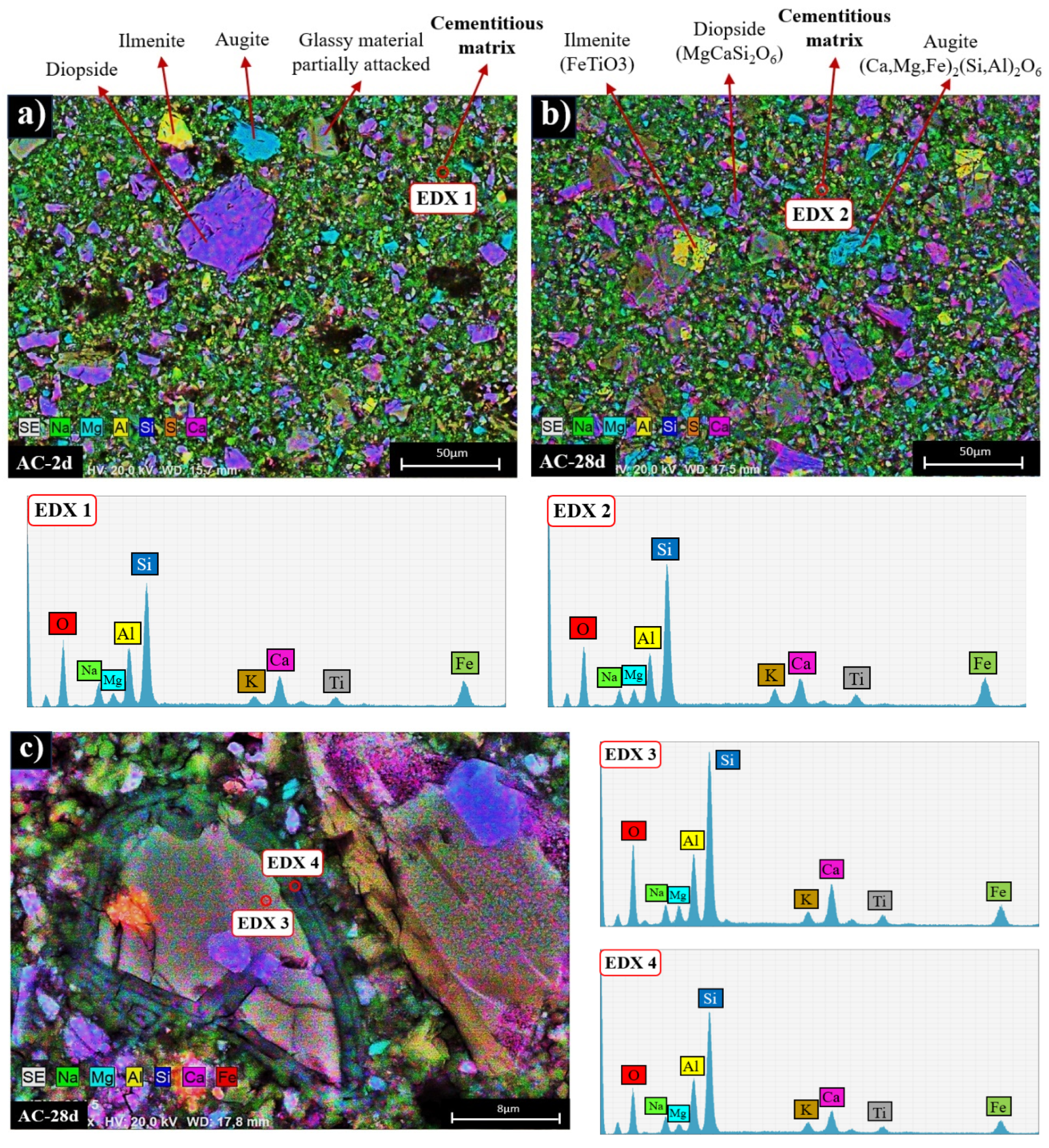
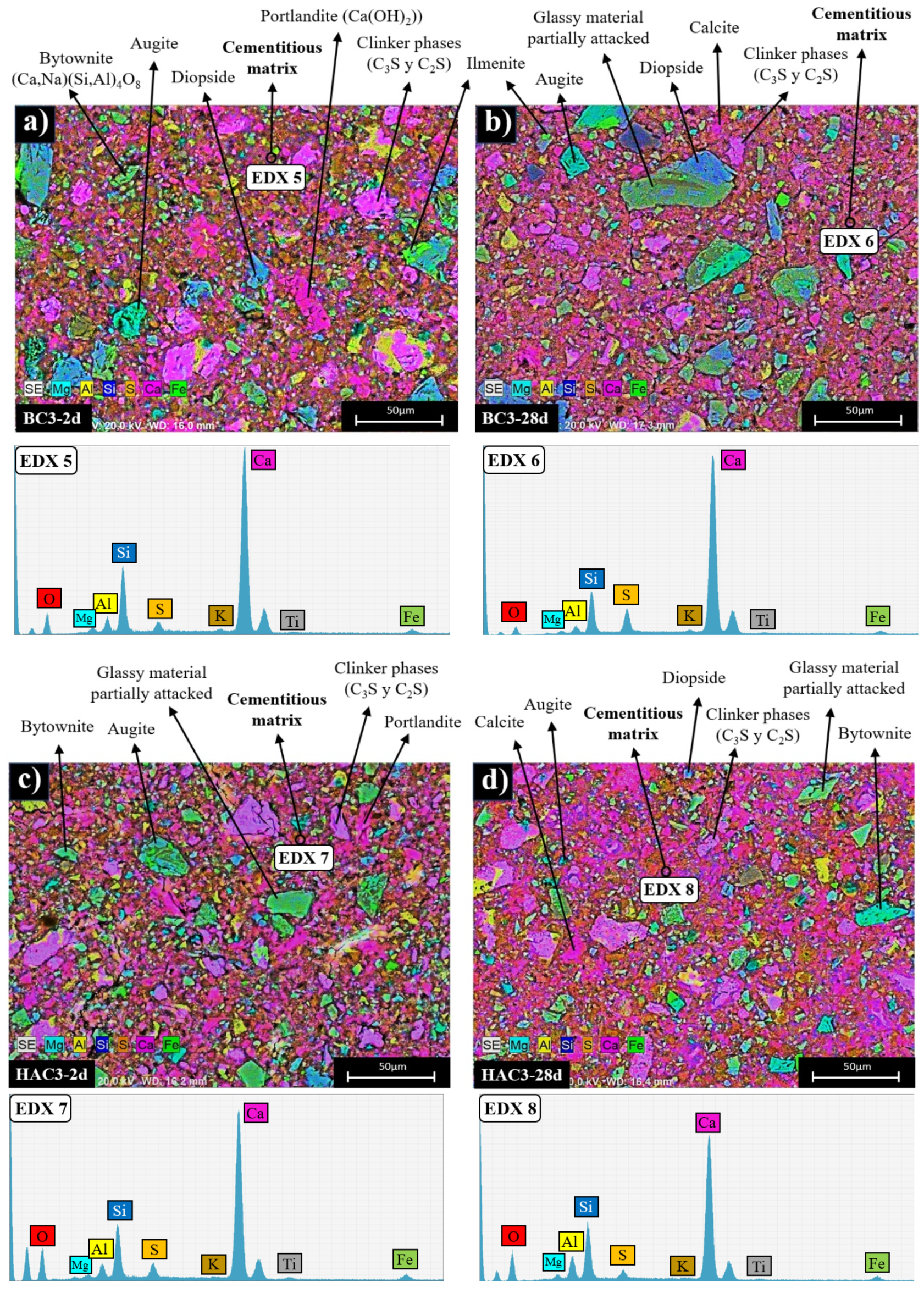
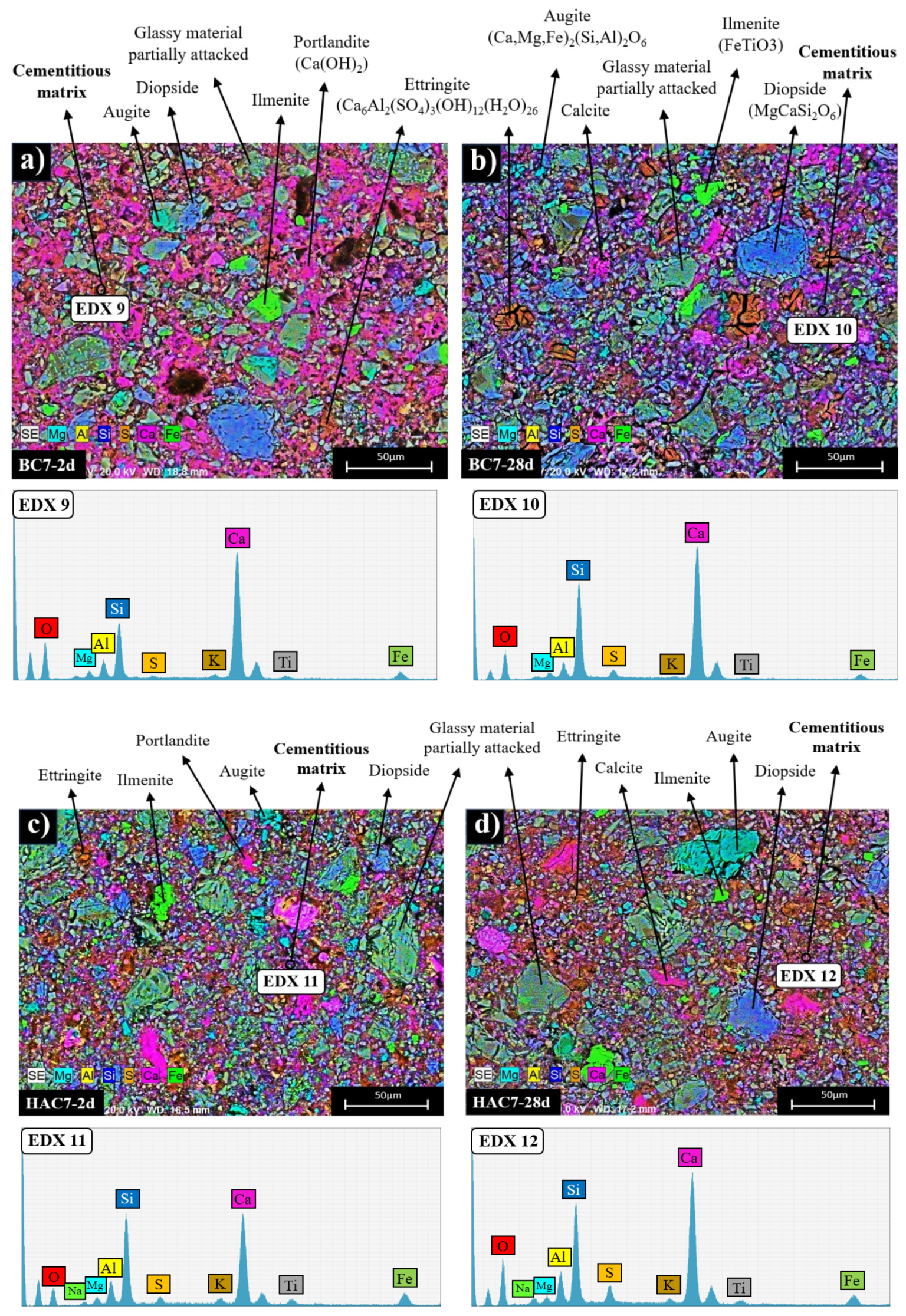
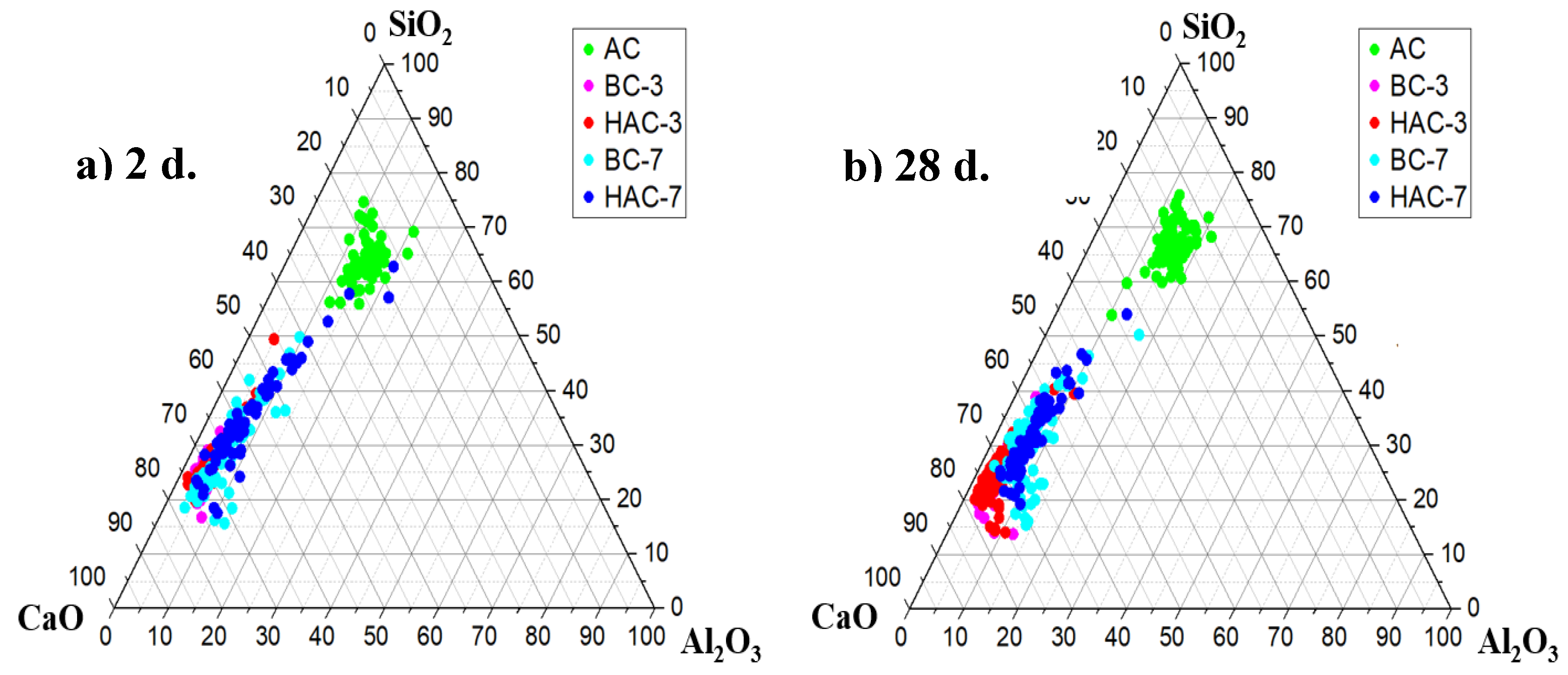
3.2. Reaction Products Characterisation
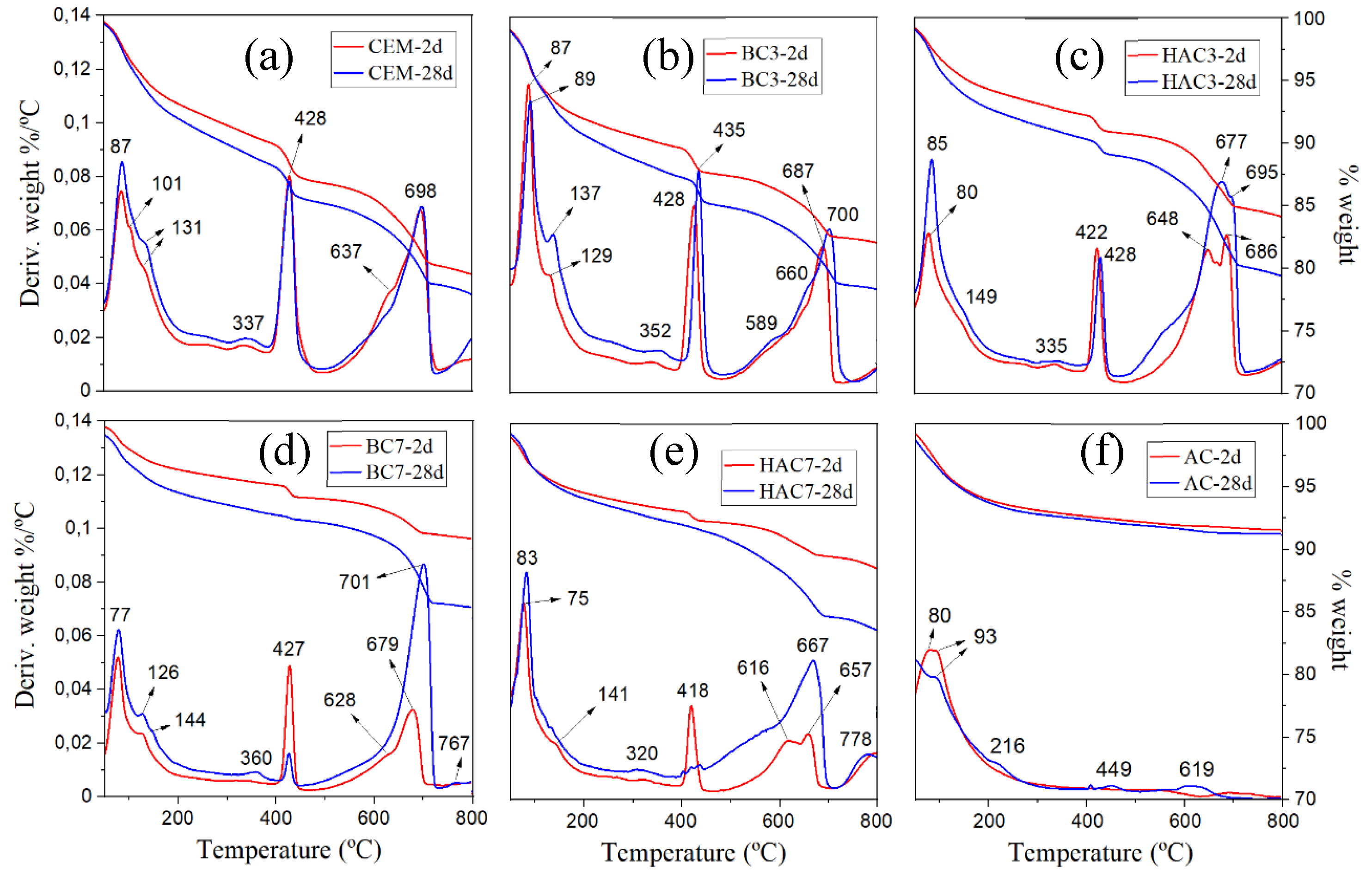
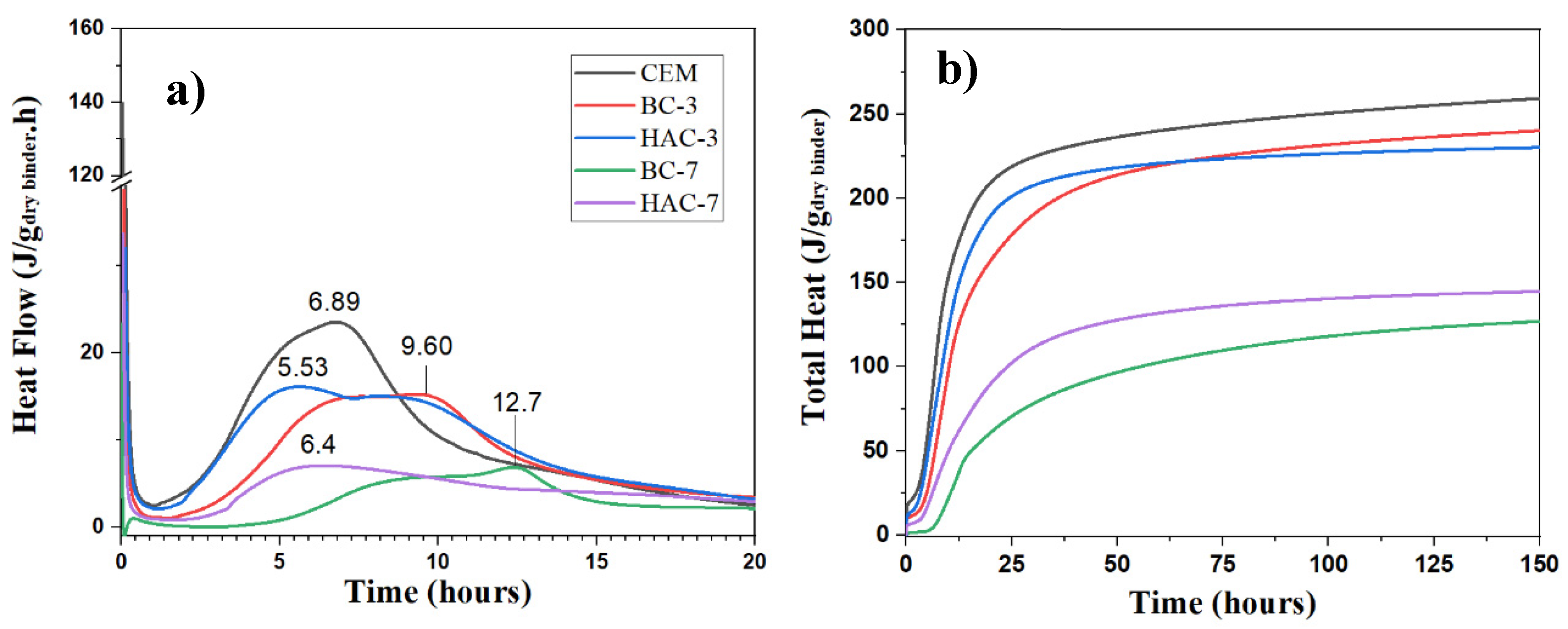
3.2. Hydration Kinetics
4. Conclusions
- In the blended cements (BC-3 and BC-7), volcanic fly ash (VFA) shows pozzolanic behaviour. Compared to the reference cement (CEM), the substitution of cement with VFA increases the induction period in the heat flow curves and decreases the total heat due to dilution effects. This effect is more pronounced at higher VFA contents. The incorporation of VFA leads to a reduction in mechanical strength, especially at early ages, especially with 70% substitution (BC-70). Nevertheless, with the BC-70 blend, the strengths exceed 15 MPa at 2 days and 40 MPa at 28 days in paste, which is considered a good performance given the high level of substitution. Regarding the hydration products formed, the primary hydration product responsible for the good mechanical properties in both cases is a C-(A)-S-H gel, with secondary materials such as AFt and Ca(OH)2 detected. The Ca(OH)2 content decreases with time due to pozzolanic reaction and carbonation.
- In hybrid alkaline cements (HAC-3 and HAC-7), the use of the alkaline activator accelerates the hydration kinetics and generates a higher degree of heat release, increasing the total heat compared to the same mixtures without the activator (BC-3 and BC-7). This phenomenon can be correlated with a greater precipitation of reaction products. The presence of the activator in these cements increases the mechanical strength values, especially at 2 days, where both systems exceed 20 MPa. The HAC-7 system achieves compressive strength values in paste at 28 days similar to those of the CEM system. Reaction products include the formation of a C-(A)-S-H gel, with higher aluminium contents when higher amounts of VFA are used. The presence of AFt, portlandite and calcite is also noted. The amount of portlandite in these systems is low and practically disappears after 28 days. The disappearance of Ca(OH)2 is related to several factors: i) less cement leads to less Ca(OH)2 formation; ii) pozzolanic reaction where Ca(OH)2 reacts with VFA to form hydration products; iii) chemical reaction with the activator (see Eq. 1) to produce in situ alkalinity which accelerates the VFA reaction; and eventually iv) carbonation.
- Alkali activated cements (100% VFA, AC): The activation of these volcanic fly ashes results in a binder with excellent mechanical properties, achieving compressive strengths in excess of 40 MPa after 2 days. The primary reaction product in this case is a gel of the type (N,C)-A-S-H, similar to that obtained when other types of fly ash are activated.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gobierno de Canarias, Informe Comite Científico, Islas Canarias, 2021. https://www3.gobiernodecanarias.org/noticias/la-erupcion-de-la-palma-se-declara-finalizada-tras-85-dias-y-8-horas-de-duracion-y-1-219-hectareas-de-coladas/. https://www3.gobiernodecanarias.org/noticias/wp-content/uploads/2021/12/251221-INFORME-Comit%C3%A9-Cient%C3%ADfico-PDF.pdf.
- Tashima M.M., Soriano L., Borrachero M.V., Monzó J., Payá J., “Towards the valorization of Cumbre Vieja volcanic ash – Production of alternative cements” Constr Build Mater, 370, (2023), 130635, ISSN 0950-0618. [CrossRef]
- Játiva A., Ruales E., Etxeberria M. “Volcanic Ash as a Sustainable Binder Material: An Extensive Review” Materials, 14, (2021), 1302. [CrossRef]
- Olawuyi, B.J.; Olusola, K.O. “Compressive Strength of Volcanic Ash/Ordinary Portland Cement Laterized Concrete” Civ. Eng. Dimens. 12, (2013), 23–28. [CrossRef]
- Siddique R. “Properties of concrete made with volcanic ash” Resour Conserv Recycl, 66, (2012), 40-44, ISSN 0921-3449. [CrossRef]
- Patrick N. Lemougna, Kai-tuo Wang, Qing Tang, A.N. Nzeukou, N. Billong, U. Chinje Melo, Xue-min Cui “Review on the use of volcanic ashes for engineering applications” Resour Conserv Recycl, 137, (2018), 177-190, ISSN 0921-3449. [CrossRef]
- Jackson M.D., Deocampo D., Marra F., Scheetz B. “Mid-Pleistocene pozzolanic volcanic ash in ancient Roman concretes” Geoarchaeology 25(1), (2010), 36–74.
- Palomo A., Monteiro P., Martauz P., Bilek V., Fernandez-Jimenez A., “Hybrid binders: A journey from the past to a sustainable future (opus caementicium futurum)”, Cem. Concr. Res., 2019, nº 105829. [CrossRef]
- ASTM C618, 2023 Edition, March 1, 2023 - Standard Specification for Coal Ash and Raw or Calcined Natural Pozzolan for Use in Concrete.
- Aref M. al-Swaidani, Samira D. Aliyan, N. Adarnaly “Mechanical strength development of mortars containing volcanic scoria-based binders with different fineness” Eng Sci Technol Int J., 19(2), (2016), 970-979, ISSN 2215-0986. [CrossRef]
- Celik K., Jackson M.D., Mancio M., Meral C., Emwas A.-H., Mehta P.K., Monteiro P.J.M. “High-volume natural volcanic pozzolan and limestone powder as partial replacements for portland cement in self-compacting and sustainable concrete” Cem. Concr. Comps. 45, (2014), 136-147, ISSN 0958-9465. [CrossRef]
- Al-Fadala S., Chakkamalayath J., Al-Bahar S., Al-Aibani A., Ahmed S., “Significance of performance based specifications in the qualification and characterization of blended cement using volcanic ash” Constr Build Mater, 144, (2017), 532-540, ISSN 0950-0618. [CrossRef]
- Khandaker M.Anwar Hossain “Blended cement using volcanic ash and pumice” Cem Concr Res, 33(10), (2003), 1601-1605, ISSN 0008-8846. [CrossRef]
- Loredana Contrafatto “Recycled Etna volcanic ash for cement, mortar and concrete manufacturing” Constr Build Mater, 151, (2017), 704-713, ISSN 0950-0618. [CrossRef]
- Bonavetti V. L., Rahhal V. F., Locati F., Irassar E. F., Marfil S., & Maiza P., Pozzolanic activity of argentine vitreous breccia containing mordenite. Mater. Construcc., (2020). 70(337), e208. [CrossRef]
- Medeiros S., Fernandes I., Fournier B., Nunes J., Santos-Silva A., Ramos V., & Soares D., Alkali-silica reaction in volcanic rocks: A worldwide comparative approach. Mater. Construcc., (2022). 72(346), e278. https://doi.org/10.3989/mc.2022.16221. [CrossRef]
- Kupwade-Patil K., De Wolf C., Chin S., Ochsendorf J., Hajiah A. E., Al-Mumin A., Büyüköztürk O., “Impact of Embodied Energy on materials/buildings with partial replacement of ordinary Portland Cement (OPC) by natural Pozzolanic Volcanic Ash” J. Clean. Prod., 177, (2018), 547-554, ISSN 0959-6526. https://doi.org/10.1016/j.jclepro.2017.12.234.Este doi no funciona.
- Kupwade-Patil K., Palkovic S. D., Bumajdad A., Soriano C., Büyüköztürk O. “Use of silica fume and natural volcanic ash as a replacement to Portland cement: Micro and pore structural investigation using NMR, XRD, FTIR and X-ray microtomography” Constr Build Mater, 158, (2018), 574-590, ISSN 0950-0618. [CrossRef]
- Rosales J., Rosales M., Díaz-López J.L., Agrela F., Cabrera M. “Effect of Processed Volcanic Ash as Active Mineral Addition for Cement Manufacture” Materials, 15, (2022), 6305. [CrossRef]
- Djon Li Ndjock B.I., Elimbi A., Cyr M. “Rational utilization of volcanic ashes based on factors affecting their alkaline activation” J. Non-Cryst. Solids, 463, (2017), 31-39, ISSN 0022-3093. [CrossRef]
- Tchakoute H.K., Elimbi A., Yanne E., Djangang C.N. “Utilization of volcanic ashes for the production of geopolymers cured at ambient temperature” Cem. Concr. Compos. 38, (2013), 75–81. [CrossRef]
- Kouamo Tchakoute H., Elimbi A., Diffo Kenne B.B., Mbey J.A., Njopwouo D., “Synthesis of geopolymers from volcanic ash via the alkaline fusion method: Effect of Al2O3/Na2O molar ratio of soda-volcanic ash” Ceram. Int. 39, (2013), 269–276. [CrossRef]
- Lemougna P.N., Chinje Melo U.F., Delplancke M.P., Rahier H. “Influence of the chemical and mineralogical composition on the reactivity of volcanic ashes during alkali activation” Ceram. Int. 40, (2014), 811–820. [CrossRef]
- Djobo J.N.Y., Elimbi A., Tchakouté H.K., Kumar S., “Reactivity of volcanic ash in alkaline medium, microstructural and strength characteristics of resulting geopolymers under different synthesis conditions” J. Mater. Sci. 51, (2016), 10301–10317. [CrossRef]
- Palomo A., Krivenko P., Garcia-Lodeiro I., Kavalerova E., Maltseva O., Fernández-Jiménez A., “A review on alkaline activation: New analytical perspective” Mater. Construcc., 64 [315], (2014), e022.
- Fernández-Jiménez A., Palomo A., Sobrados I., Sanz J., “The role played by the reactive alumina content in the alkaline activation of fly ashes” Microporous Mesoporous Mater., 91, (2006), 111–119. ISSN 1387-1811. [CrossRef]
- Adesina, Adeyemi Damilare “Effect of Green Activators on the Properties of Alkali Activated Materials: A Review” In: SynerCrete'18: Interdisciplinary Approaches for Cement-based Materials and Structural Concrete: Synergizing Expertise and Bridging Scales of Space and Time. RILEM Publications S.A.R.L, (2018), 431-436. ISBN 978-2-35158-20.
- Qu, B., Fernández Jiménez, A., Palomo, A., Martin, A., & Pastor, J. Y. Effect of high temperatures on the mechanical behaviour of hybrid cement. Mater. Construcc., (2020). 70(337), e213. [CrossRef]
- Sidney Diamond “Particle morphologies in fly ash” Cem.Concr. Res., 16(4), (1986), 569-579, ISSN 0008-8846. [CrossRef]
- Tusheng H., Zaibo L., Sanyin Z., Xuguang Z., Xiaoling Q. “Study on the particle morphology, powder characteristics and hydration activity of blast furnace slag prepared by different grinding methods” Constr. Build. Mater., 270, (2021), 121445, ISSN 0950-0618. [CrossRef]
- Alraddadi S., Assaedi H. “Characterization and potential applications of different powder volcanic ash” Journal of King Saud University - Science, 32(7), (2020), 2969-2975, ISSN 1018-3647. [CrossRef]
- Farmer V.C. “The Infrared Spectra of Minerals” in: V.C. Farmer (Ed.), Mineralogical Society, Monograph 4, University of Aberdeen, Aberdeen, UK, (1974).
- Criado M., Palomo A., Fernández-Jiménez A., “Alkali activation of fly ashes. Part 1: Effect of curing conditions on the carbonation of the reaction products” Fuel, 84(16), (2005), 2048-2054. ISSN 0016-2361. [CrossRef]
- Hamidi M., Kacimi L., Cyr M., Clastres P. “Evaluation and improvement of pozzolanic activity of andesite for its use in ecoefficient cement” Constr. Build. Mater. 47, (2013), 1268–1277. [CrossRef]
- Sánchez de Rojas M.I., Frías M., Rivera Lozano J., Escorihuela M.J., Marín F.P. “Research about the pozzolanic activity of waste materials from calcined clay” Mater. Construcc., 51(261), (2001), 45-52, ISSN 0465-2746.
- Garcia-Lodeiro I., Fernández-Jimenez A., Pena P., Palomo A. “Alkaline activation of synthetic aluminosilicate glass” Ceram. Int, 40(4), (2014), 5547-5558, ISSN 0272-8842. [CrossRef]
- Ruiz-Santaquiteria C., Fernández-Jiménez A., Skibsted J., Palomo A. “Clay reactivity: Production of alkali activated cements” Appl. Clay Sci., 73, (2013), 11-16, ISSN 0169-1317.
- Mejía M.J., Rodríguez E., Mejía de Gutiérrez R., Gallego N., “Preparation and characterization of a hybrid alkaline binder based on a fly ash with no commercial value” J. Clean. Prod., 104, (2015), 346-352, ISSN 0959-6526. [CrossRef]
- Cristelo N., Garcia-Lodeiro I., Fernando Rivera J., Miranda T., Palomo A., Coelho J., Fernández-Jiménez A. “One-part hybrid cements from fly ash and electric arc furnace slag activated by sodium sulphate or sodium chloride” J. Build. Eng, 44, (2021), 103298, ISSN 2352-7102. [CrossRef]
- Djobo N., Elimbi A., Tchakouté Kouamo H., Kumar S., “Reactivity of volcanic ash in alkaline medium, microstructural and strength characteristics of resulting geopolymers under different synthesis conditions” J. Mater. Sci., 51(22), (2016), 10301–10317. [CrossRef]
- García Lodeiro I., Fernández-Jimenez A., Palomo A., Macphee D.E “Effect on fresh C-S-H gels of the simultaneous addition of alkali and aluminium” Cem Concr Res, 40(1), (2010), 27-32, ISSN 0008-8846. [CrossRef]
- Garcia-Lodeiro I., Palomo A., Fernández-Jiménez A., Macphee D.E. “Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O–CaO–Al2O3–SiO2–H2O” Cem Concr Res, 41(9), (2011), 923-931, ISSN 0008-8846. [CrossRef]
- Pajares I, Martı́nez-Ramı́rez S, Blanco-Varela M.T “Evolution of ettringite in presence of carbonate, and silicate ions” Cem. Concr. Compos., 25(8), (2003), 861-865, ISSN 0958-9465. [CrossRef]
- Lothenbach B., Durdzinski P., De Weerdt K. “Thermogravimetric Analysys” A practical Guide to Microestructural Analysis of Cementitious Materials, (5), (2016), 178-206. ISBN 978-4987-3867-5.
- Wongkeo W., Thongsanitgarn P., Chindaprasirt P., Chaipanic A., “Thermogravimetry of ternary cement blends” J. Therm. Anal. Calorim. 113, (2013), 1079–1090. [CrossRef]
- Chinh Chu D., Kleib J., Amar M., Benzerzour M., Abriak N. “Determination of the degree of hydration of Portland cement using three different approaches: Scanning electron microscopy (SEM-BSE) and Thermogravimetric analysis (TGA)” Case Studies in Construction Materials, 15, (2021), e00754, ISSN 2214-5095. [CrossRef]
- Kemal Celik, Rotana Hay, Craig W. Hargis, Juhyuk Moon “Effect of volcanic ash pozzolan or limestone replacement on hydration of Portland cement” Constr Build Mater, 197, (2019), 803-812, ISSN 0950-0618. [CrossRef]
- Patrick N. Lemougna, Kai-tuo Wang, Qing Tang, A.N. Nzeukou, N. Billong, U. Chinje Melo, Xue-min Cui “Review on the use of volcanic ashes for engineering applications” Resour Conserv Recycl, 137, (2018), 177-190, ISSN 0921-3449. [CrossRef]
- Ruiz-Agudo E., Kudłacz K., Putnis C.V., Putnis A., Rodriguez-Navarro C. “Dissolution and Carbonation of Portlandite [Ca(OH)2] Single Crystals” Environ. Sci. Technol., 47(19), (2013), 11342-11349. [CrossRef]
- Park S.M., Seo J.H., Lee H.K. “Thermal evolution of hydrates in carbonation-cured Portland cement” Mater. Struct. 51, (2018), 7. [CrossRef]
- Garcia-Lodeiro I., Donatello S., Fernández-Jiménez A., Palomo A., Hydration of Hybrid Alkaline Cement Containing a Very Large Proportion of Fly Ash: A Descriptive Model. Materials 2016, 9(7), 605. 2016. [CrossRef]
- Garcia-Lodeiro I., Palomo A., Fernández-Jiménez A., “Crucial insights on the mix design of alkali-activated cement-based binders”, Handbook of Alkali-Activated Cements, Mortars and Concretes, Editor(s): F. Pacheco-Torgal, J.A. Labrincha, C. Leonelli, A. Palomo, P. Chindaprasirt,Handbook of Alkali-Activated Cements, Mortars and Concretes, Woodhead Publishing, 2015, Pages 49-73,ISBN 9781782422761. [CrossRef]
- Gutteridge W.A, Dalziel J.A.; “Filler cement: The effect of the secondary component on the hydration of Portland cement: Part I. A fine non-hydraulic filler” Cem. Concr. Res., 20(5), (1990), 778-782, ISSN 0008-8846. [CrossRef]
- Bullard J.W., Jennings H.M., Livingston R.A., Nonat A., Scherer G.W., Schweitzer J.S., Scrivener K.L., Thomas J.J. “Mechanisms of cement hydration” Cem. Concr. Res. 41(12), (2011), 1208-1223, ISSN 0008-8846. [CrossRef]
- Andrade Neto J.S., De la Torre A.G., Kirchheim A.P. “Effects of sulfates on the hydration of Portland cement – A review” Constr Build Mater, 279, (2021), 122428, ISSN 0950-0618. [CrossRef]
- Palomo A., Maltseva O., Garcia-Lodeiro I.,Fernández-Jiménez A., “Portland Versus Alkaline Cement: Continuity or Clean Break: “A Key Decision for Global Sustainability”, (2021) Front. Chem. 9:705475. [CrossRef]

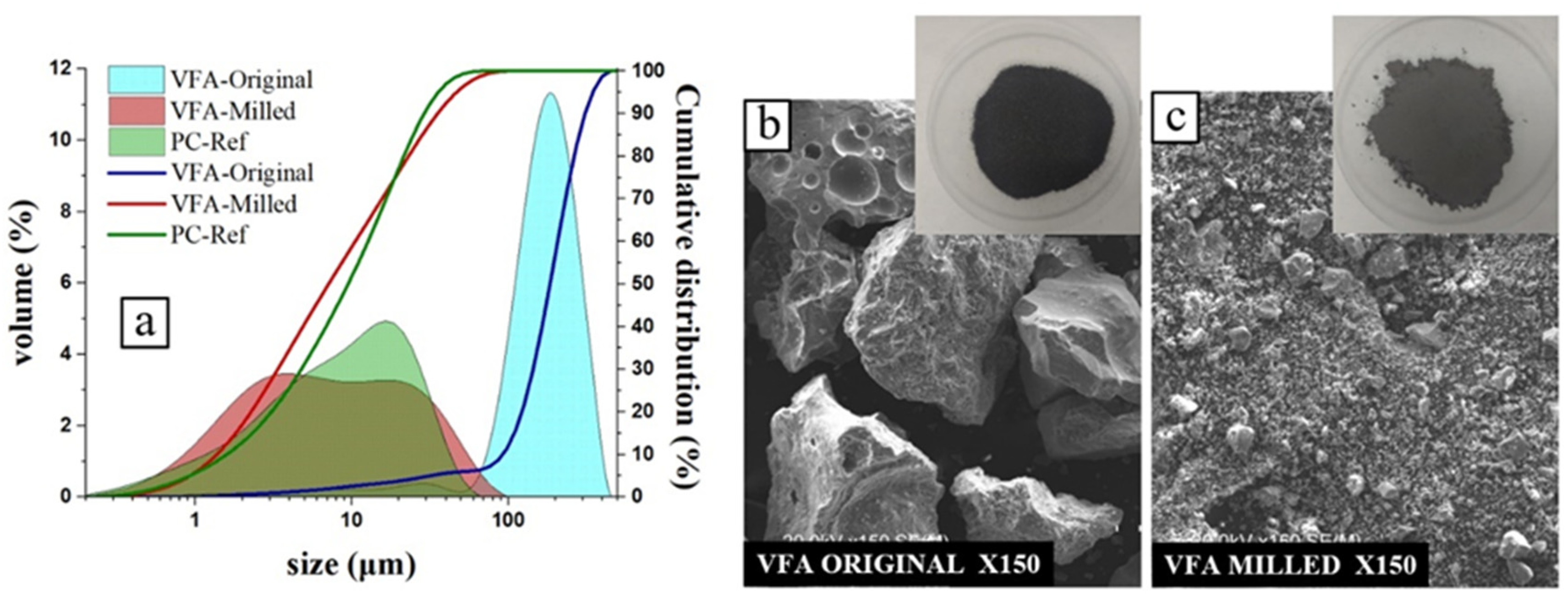
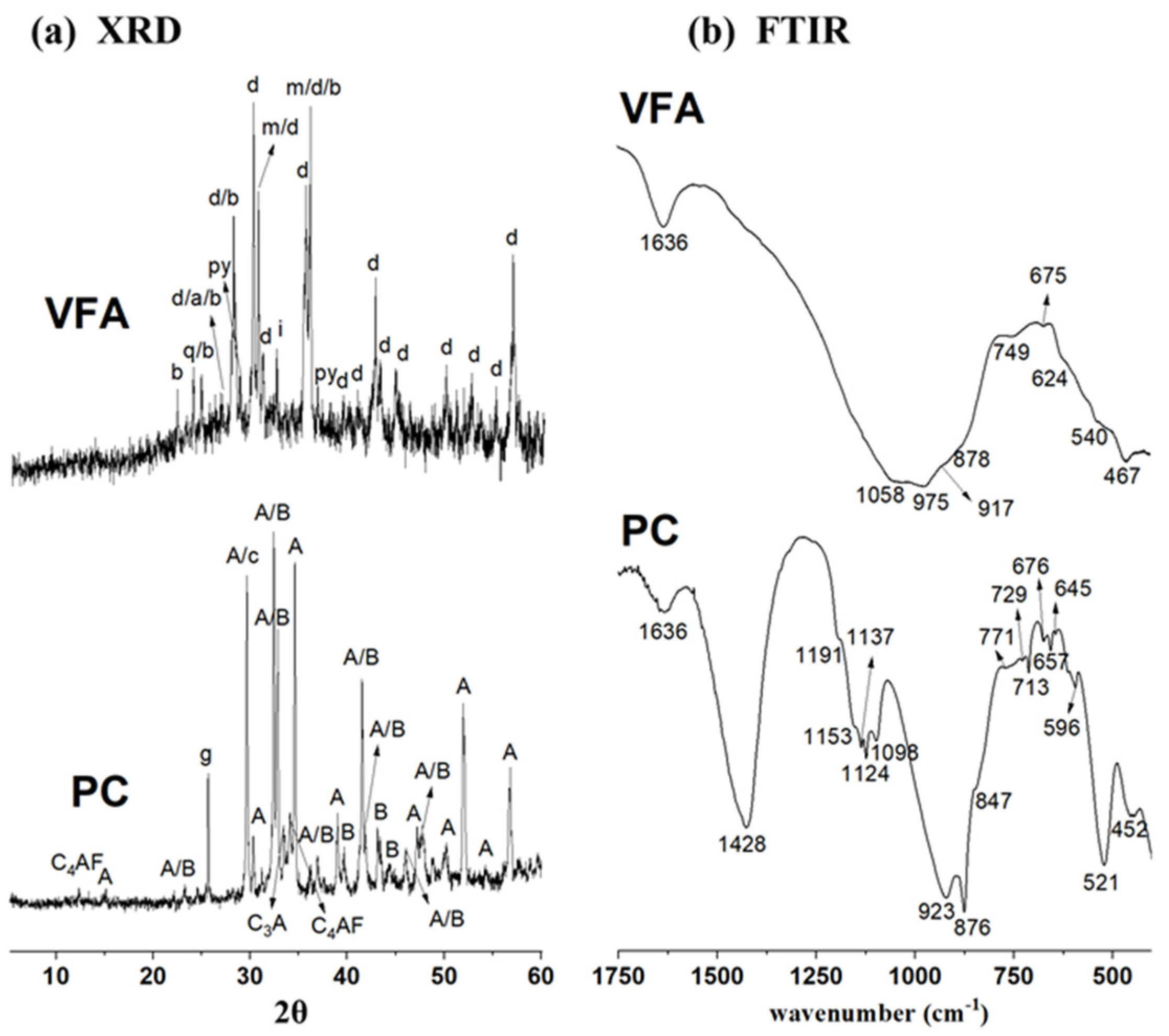
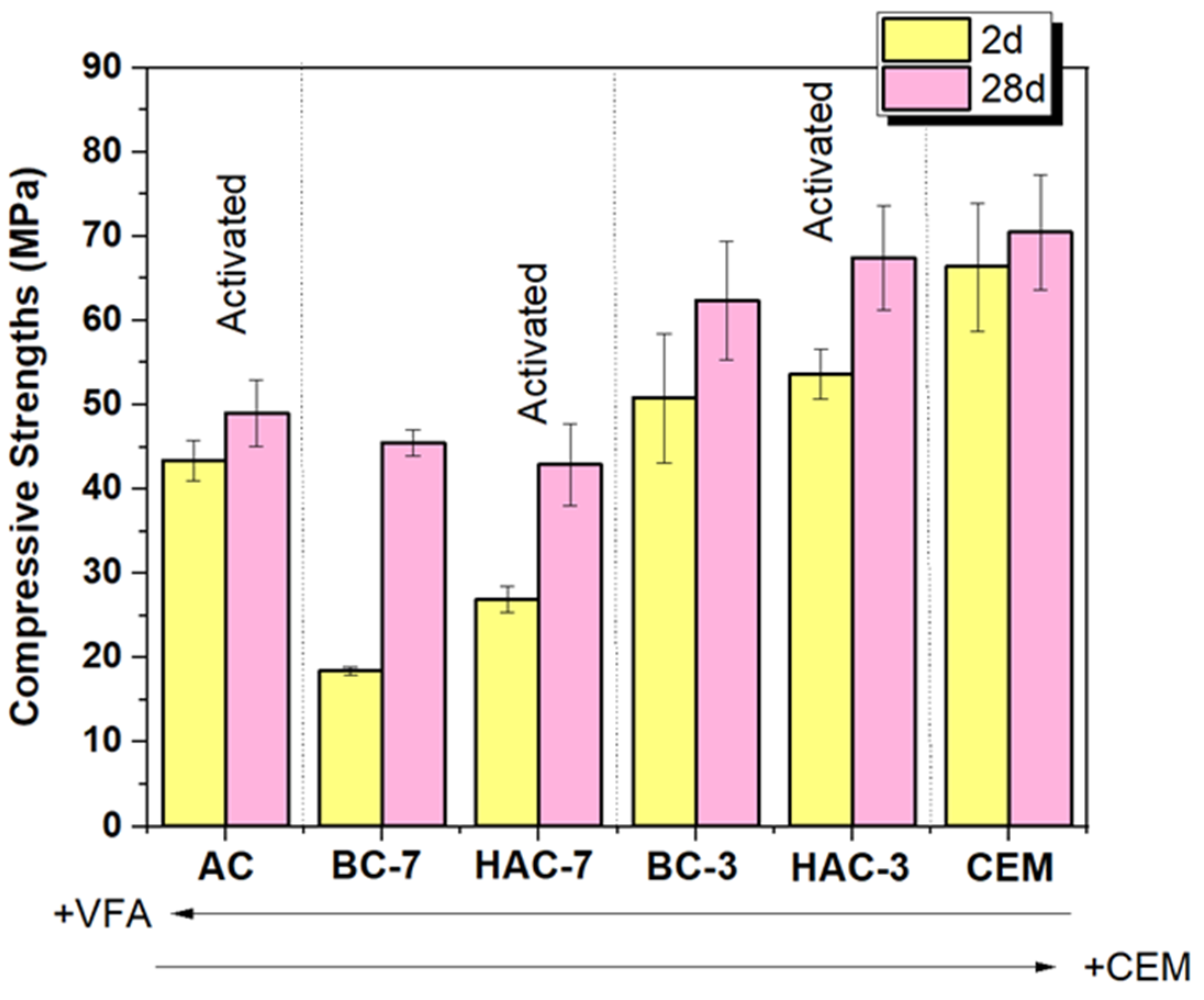
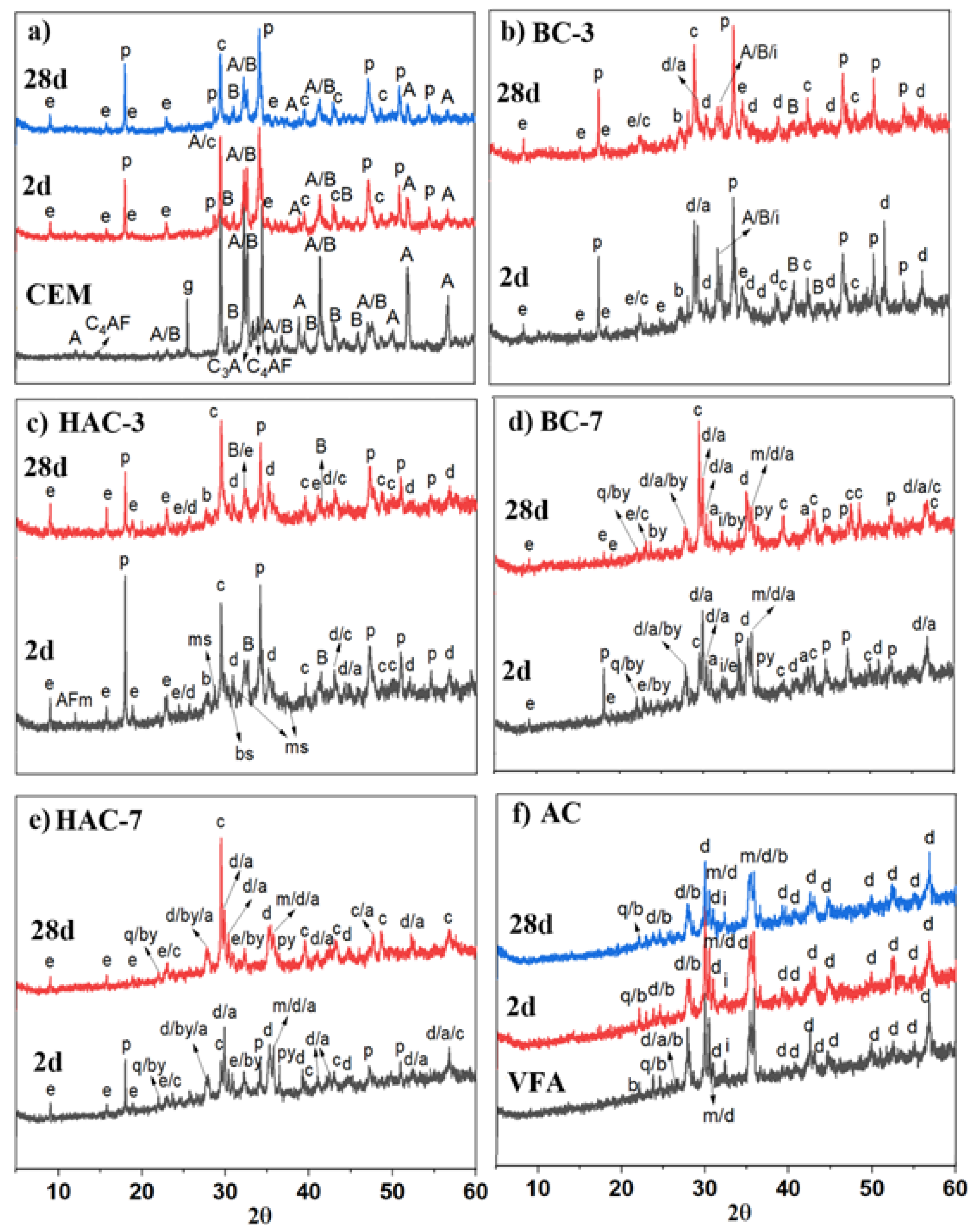
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | TiO2 | Na2O | K2O | P2O5 | SO3 | Others | LoI* | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VFA | 41.56 | 14.49 | 14.73 | 11.92 | 5.57 | 4.03 | 4.21 | 1.75 | 0.81 | 0.19 | 0.74 | - |
| PC | 18.13 | 4.29 | 3.00 | 61.47 | 3.33 | 0.26 | 0.50 | 0.56 | 0.10 | 3.00 | 5.36 | 4.36 |
| CaO Saturated Solution Test Results | |||||
|---|---|---|---|---|---|
| Age | pH | % CaO fixed | |||
| 2 | 12.51±0.01 | 13.46±0.05 | |||
| 28 | 12.32±0.01 | 55.48±3.16 | |||
| HF attack analysis results | |||||
| %SiO2 | %Al2O3 |
SiO2+Al2O3 %Reactive |
Ratio SiO2/Al2O3 |
||
| Initial | Reactive | Initial | Reactive | ||
| 41.56 | 35.73 | 14.49 | 10.70 | 46.43 | 3.34 |
| Name | BINDERS (B) | Activator | Liquid Hydration (L) | L/B | Curing Conditions | |
|---|---|---|---|---|---|---|
| VFA | PC | |||||
| CEM | -- | 100 | -- | Water | 0.3 | 20h. 25 ºC |
| BC-3 | 30 | 70 | -- | Water | 0.3 | 20h. 25 ºC |
| HAC-3 | 30 | 70 | SAc* | Water | 0.3 | 20h. 25 ºC |
| BC-7 | 70 | 30 | -- | Water | 0.3 | 20h. 25 ºC |
| HAC-7 | 70 | 30 | SAc* | Water | 0.3 | 20h. 25 ºC |
| AC | 100 | -- | LAc* | NaOH 8M | 0.4 | 20h- 85ºC |
| Sample | % Portlandite | % Carbonates | ||
|---|---|---|---|---|
| 2d | 28d | 2d | 28d | |
| CEM | 10.27 | 9.86 | 16.36 | 16.36 |
| BC-3 | 7.81 | 8.22 | 12.04 | 14.77 |
| HAC-3 | 5.34 | 4.93 | 15.00 | 21.36 |
| BC-7 | 4.11 | 1.64 | 7.27 | 15.22 |
| HAC-7 | 3.28 | 2.46 | 8.18 | 16.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





