Submitted:
03 December 2023
Posted:
05 December 2023
You are already at the latest version
Abstract
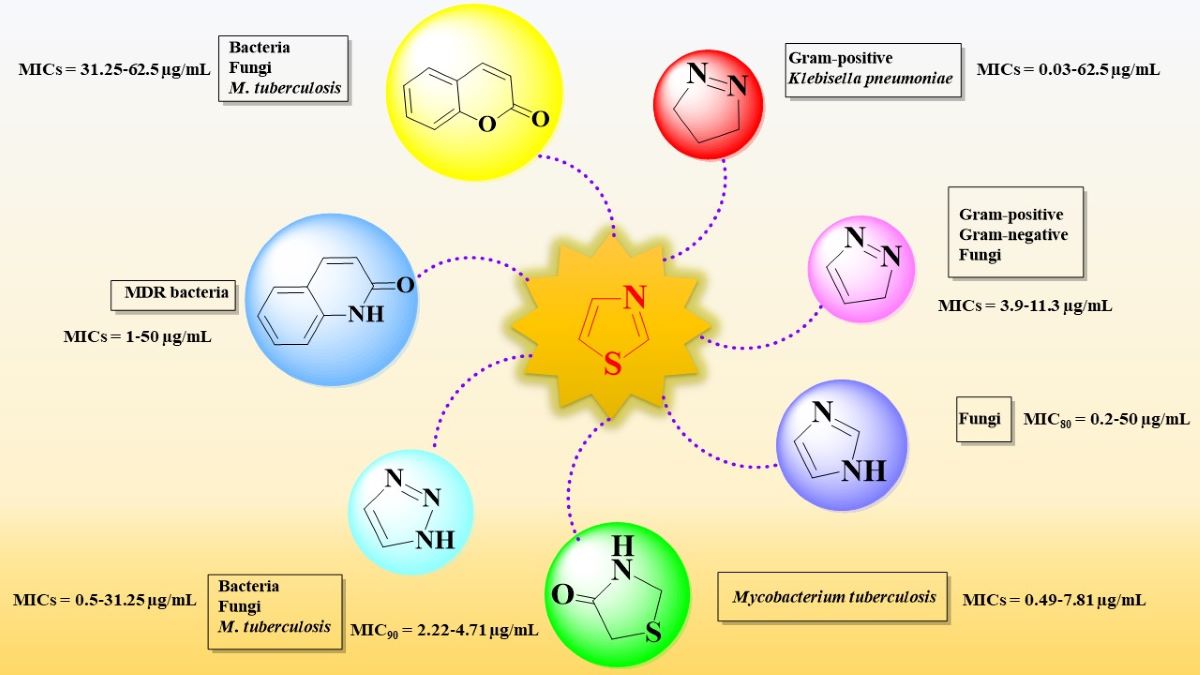
Keywords:
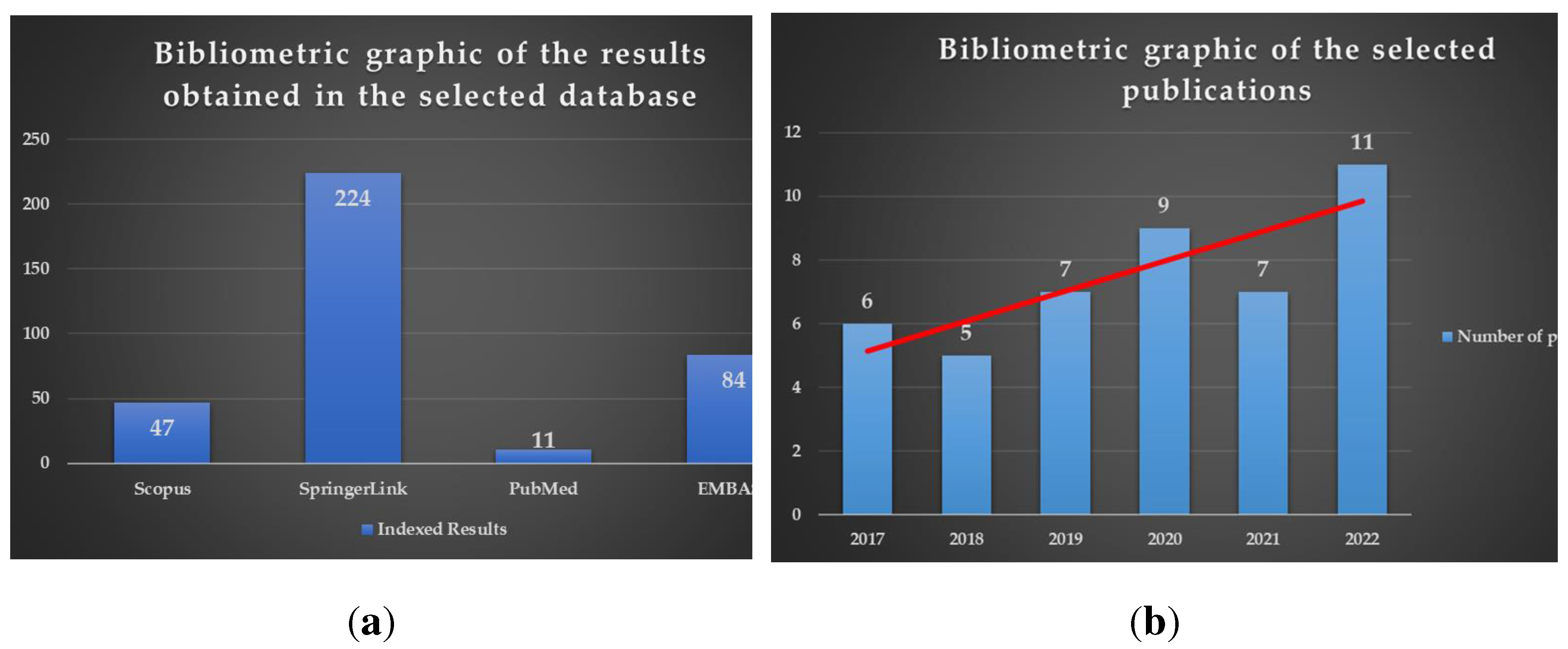
1. Introduction
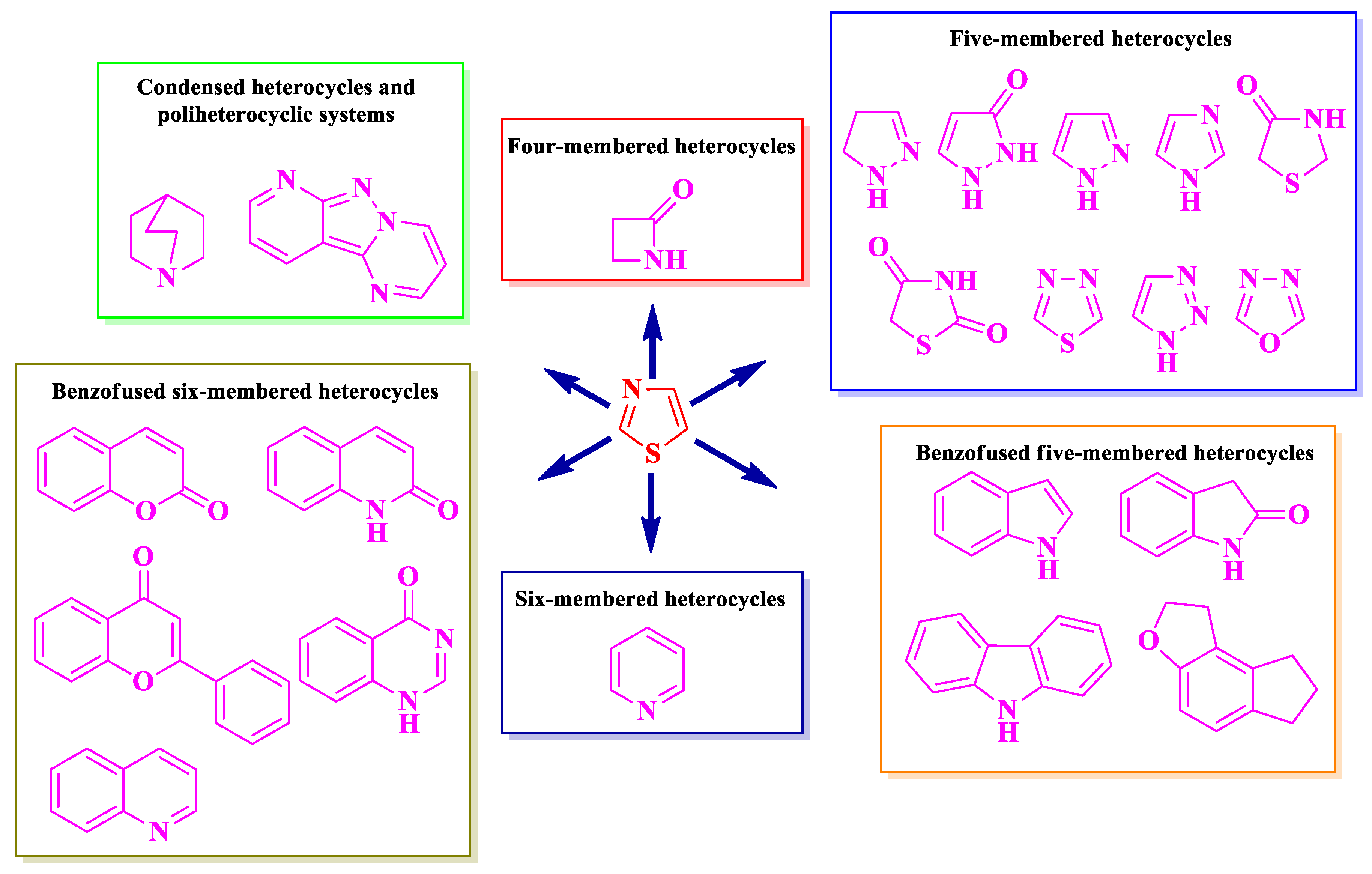
2. Structure-Activity Relationships in Antimicrobial Thiazole-based Hybrid Compounds
2.1. Thiazole Clubbed with Four-Membered Heterocyles
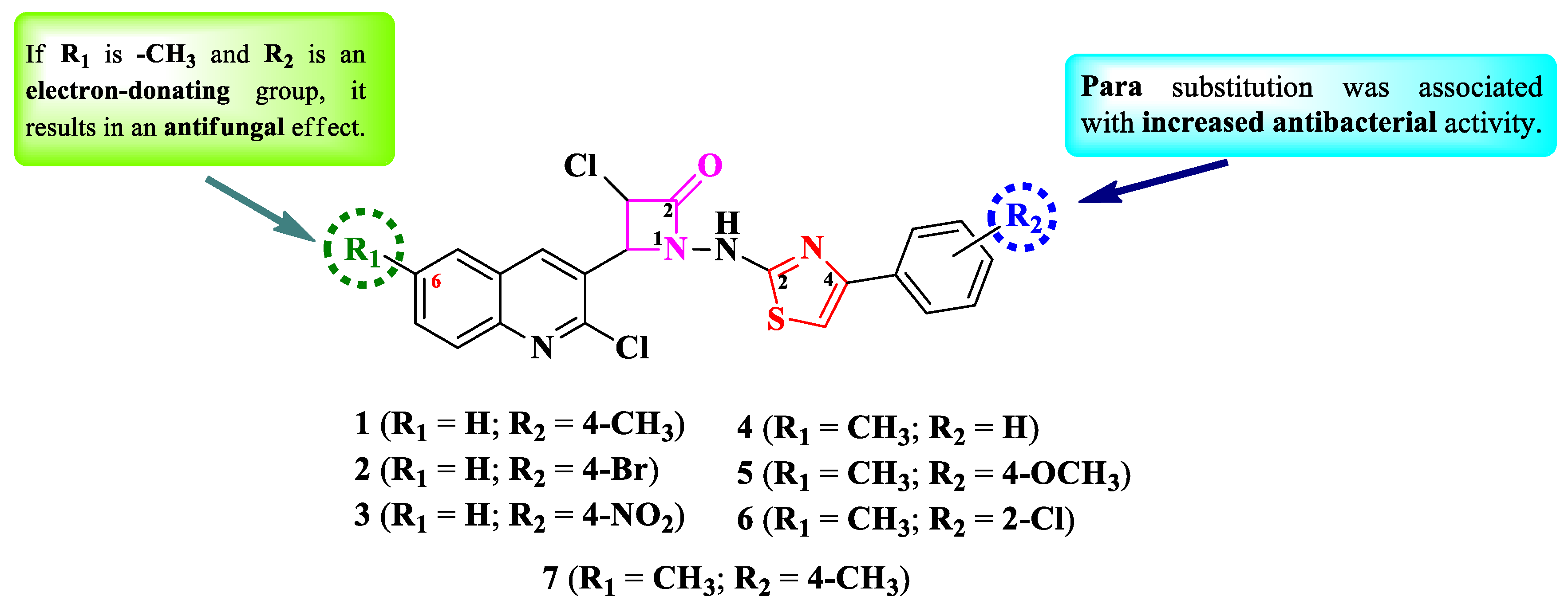
2.1.1. Thiazolyl-Azetidin-2-one Hybrid Compounds
2.2. Thiazole Clubbed with Five-Membered Heterocycles
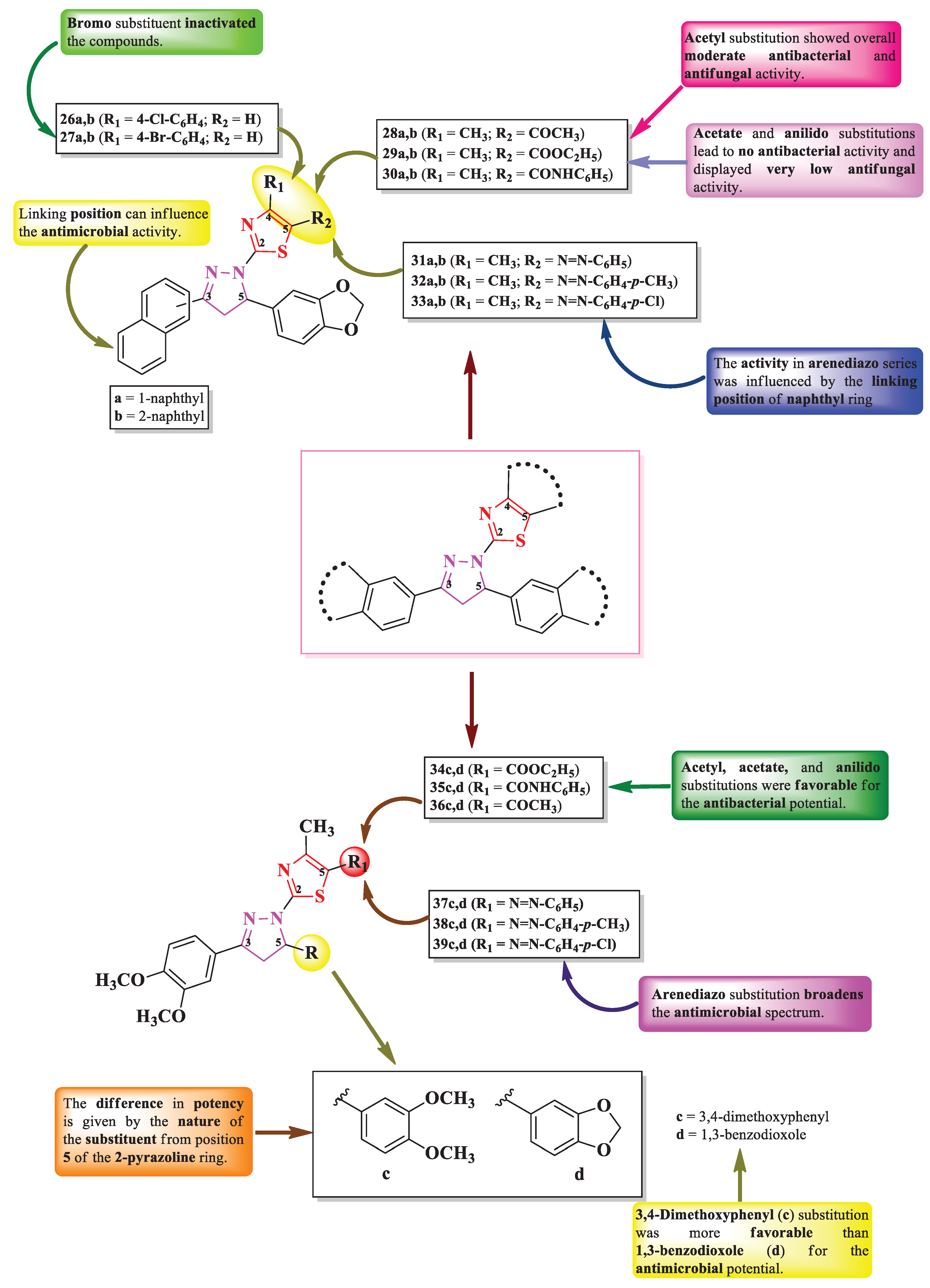
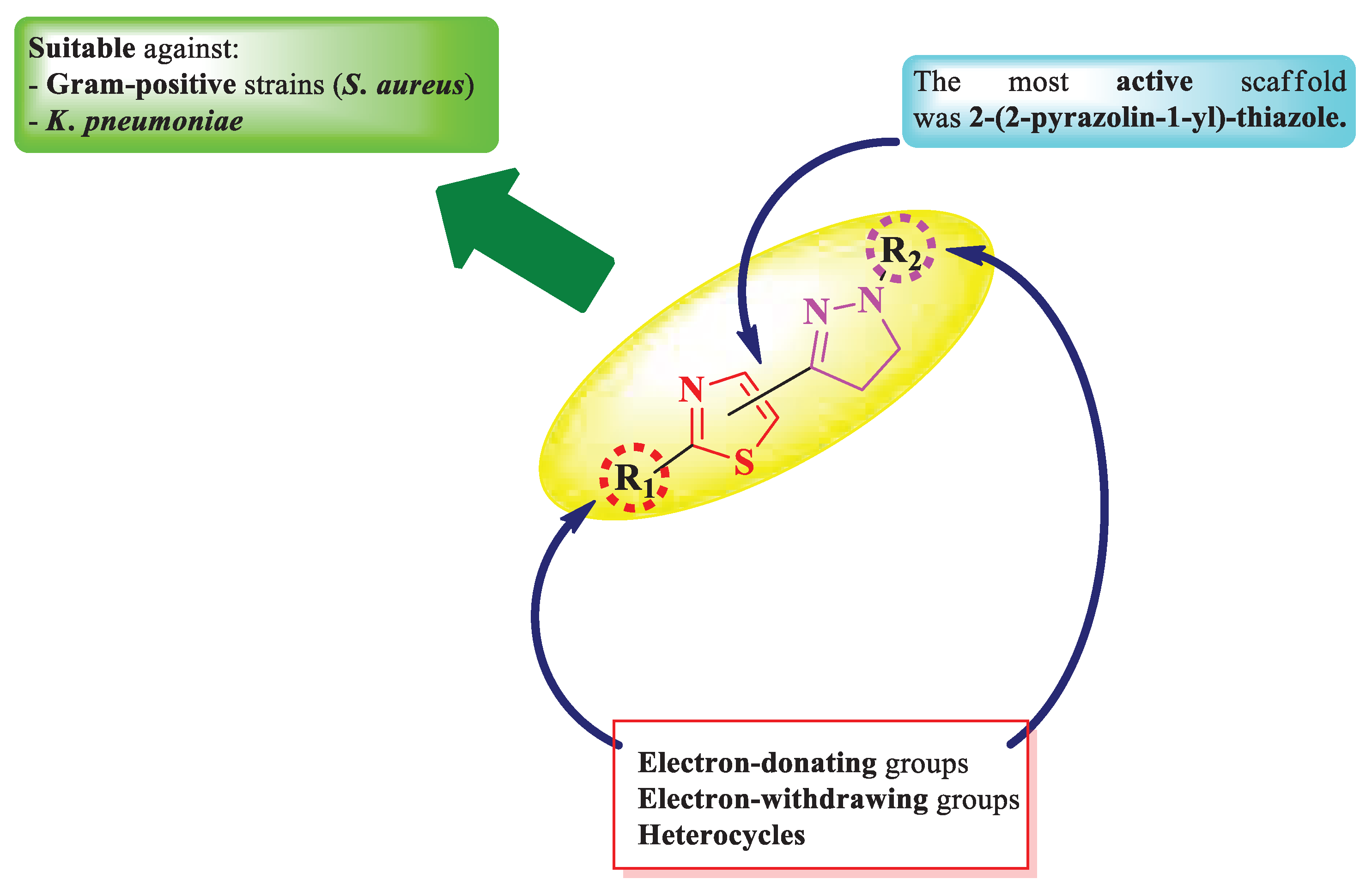
2.2.1. Thiazolyl-2-Pyrazoline Hybrid Compounds
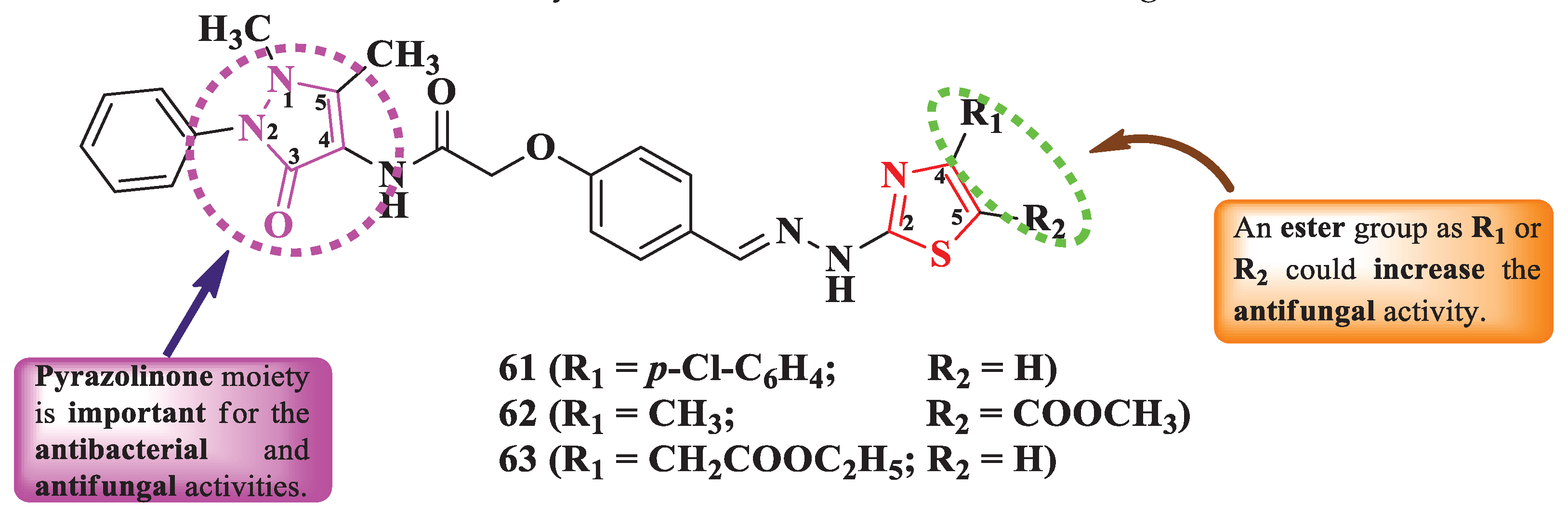
2.2.2. Thiazolyl-Pyrazolin-3-one Hybrid Compounds
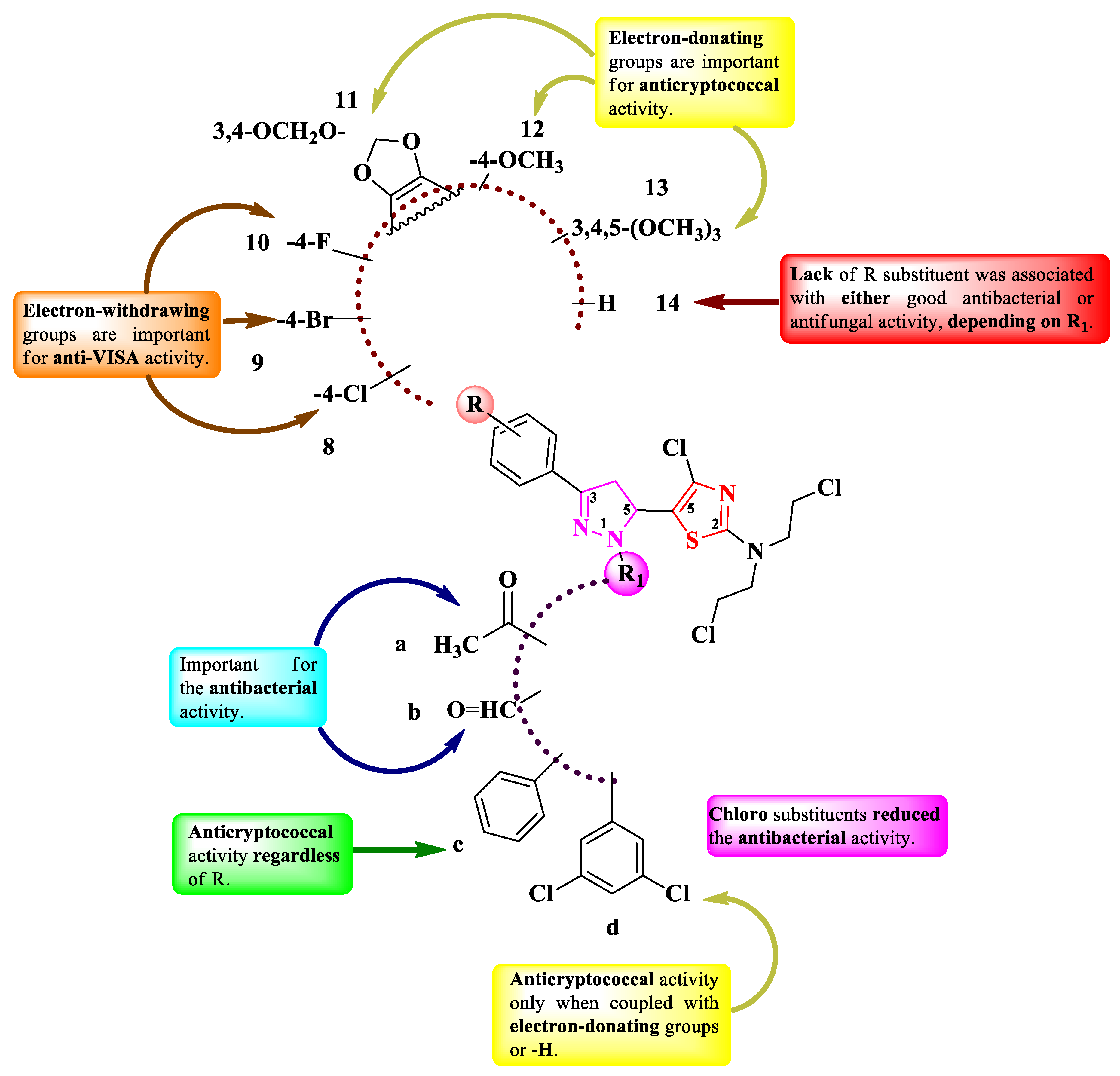
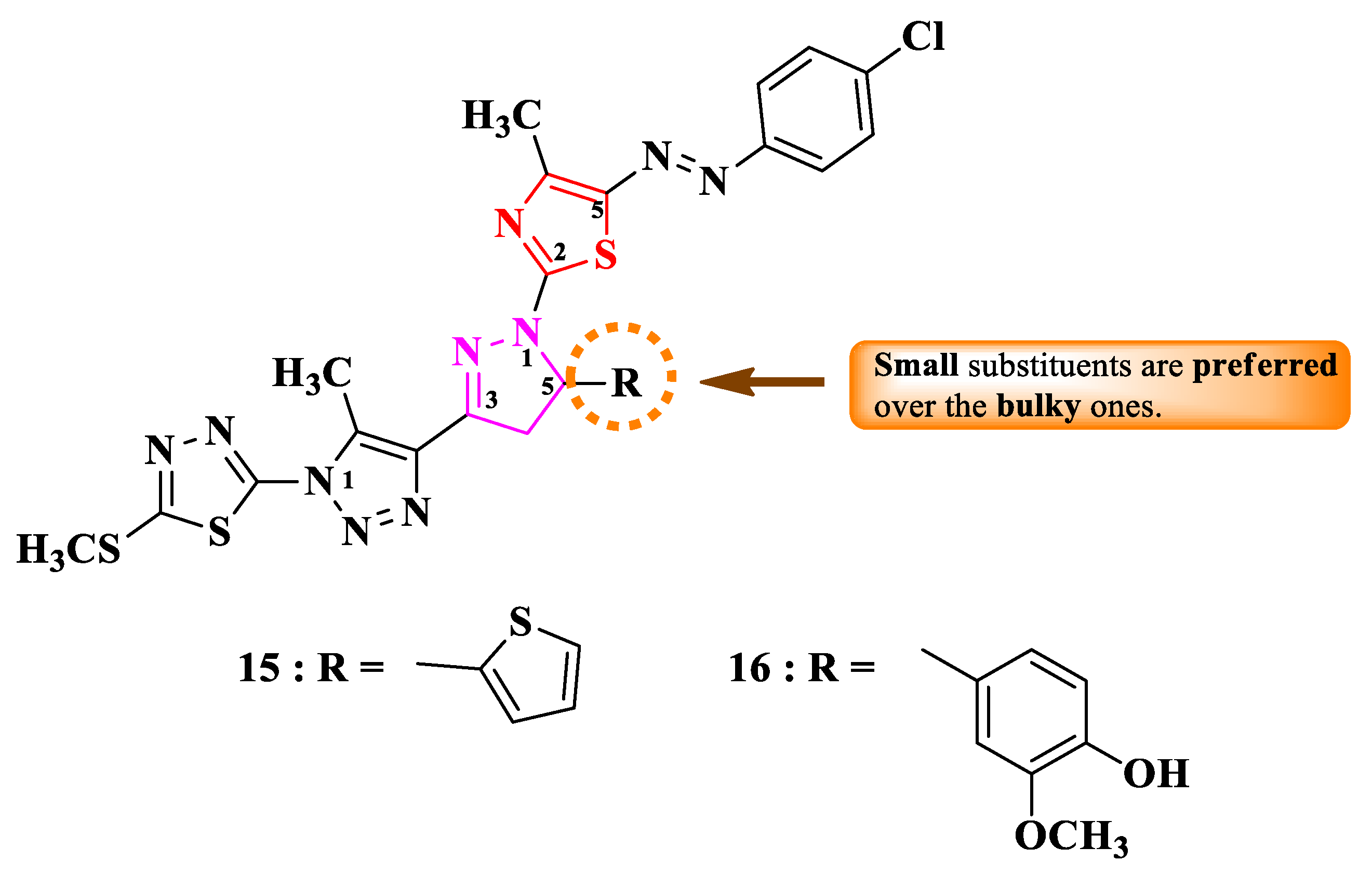
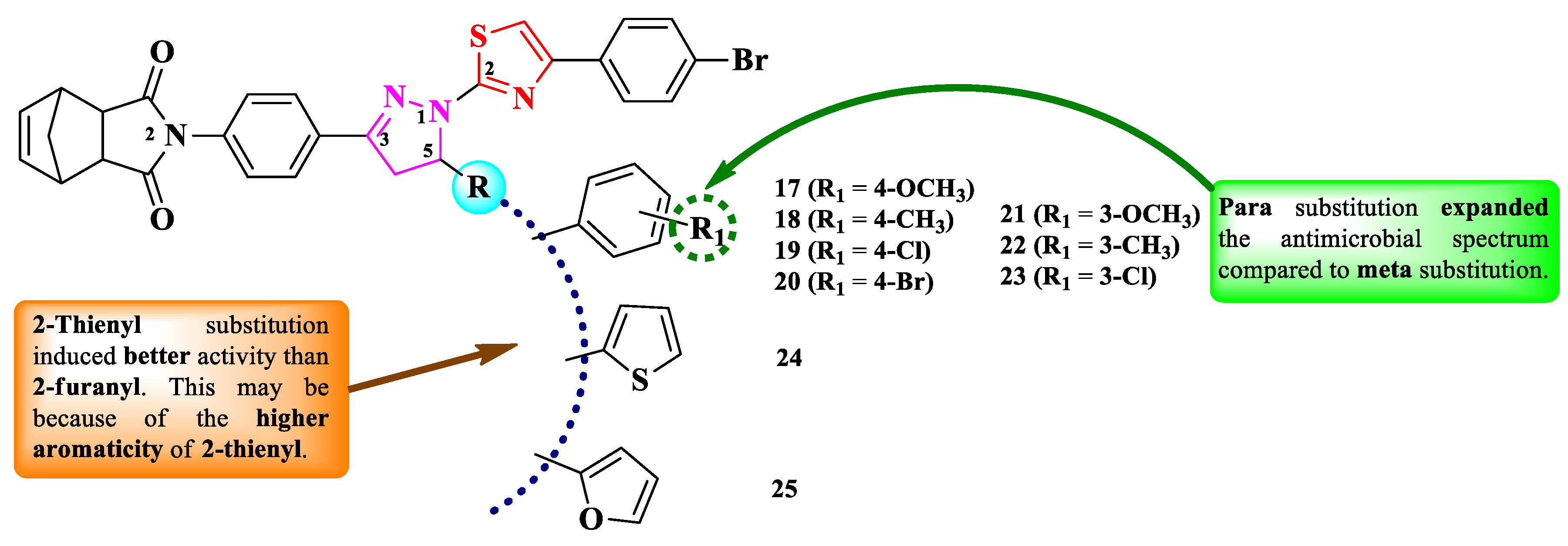
2.2.3. Thiazolyl-Pyrazole Hybrid Compounds
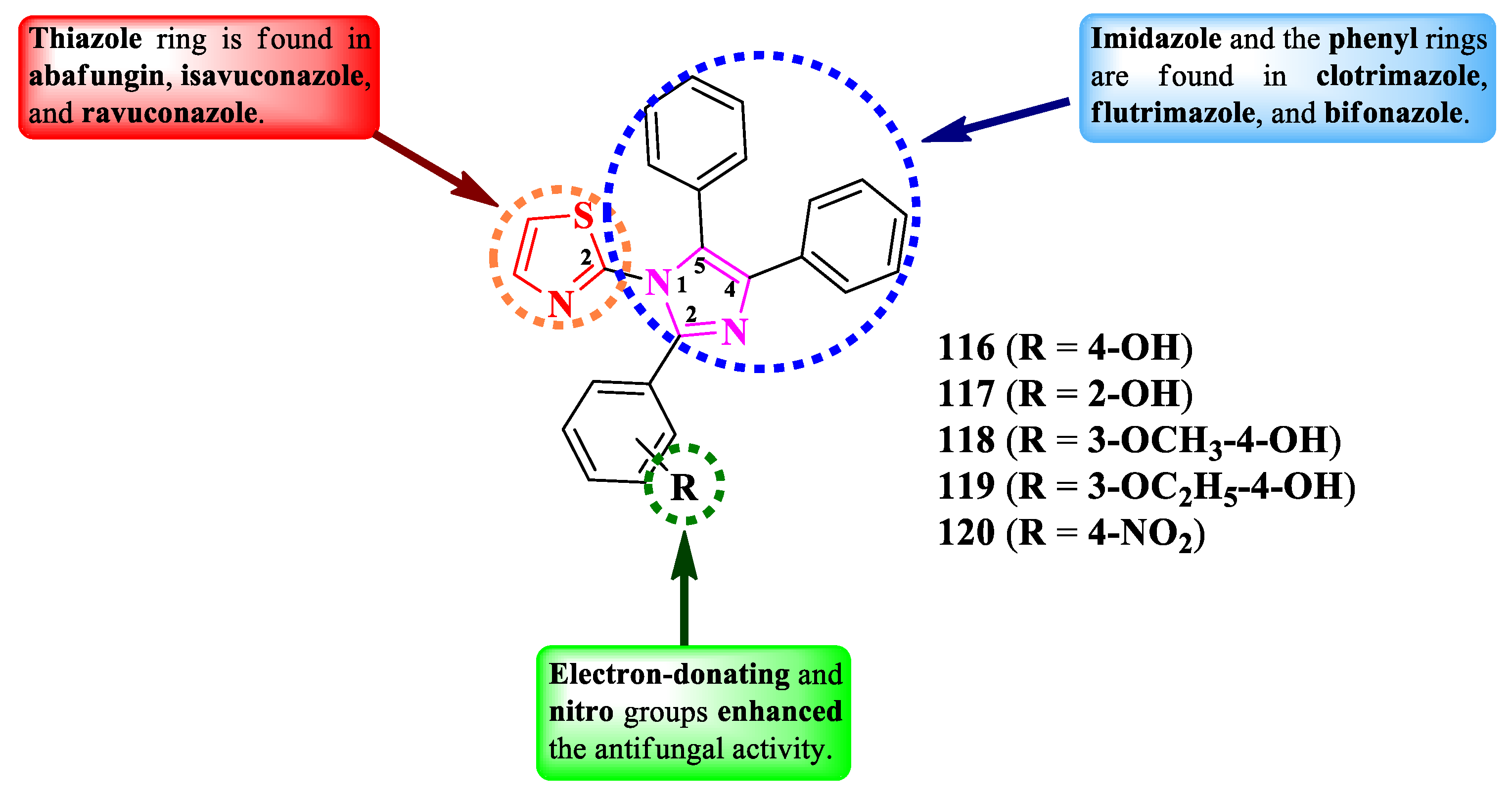
2.2.4. Thiazolyl-Imidazole Hybrid Compounds
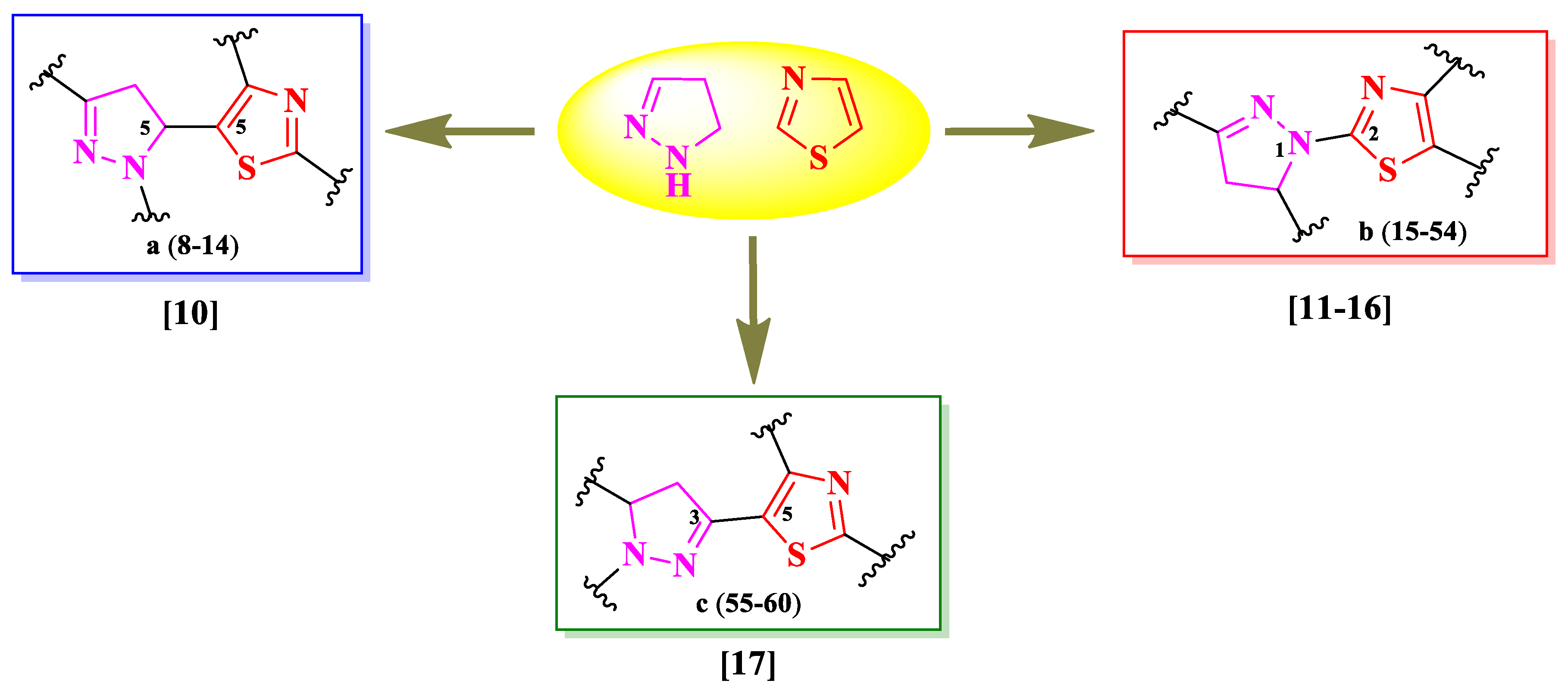
2.2.5. Thiazolyl-Thiazolidin-4-one Hybrid Compounds
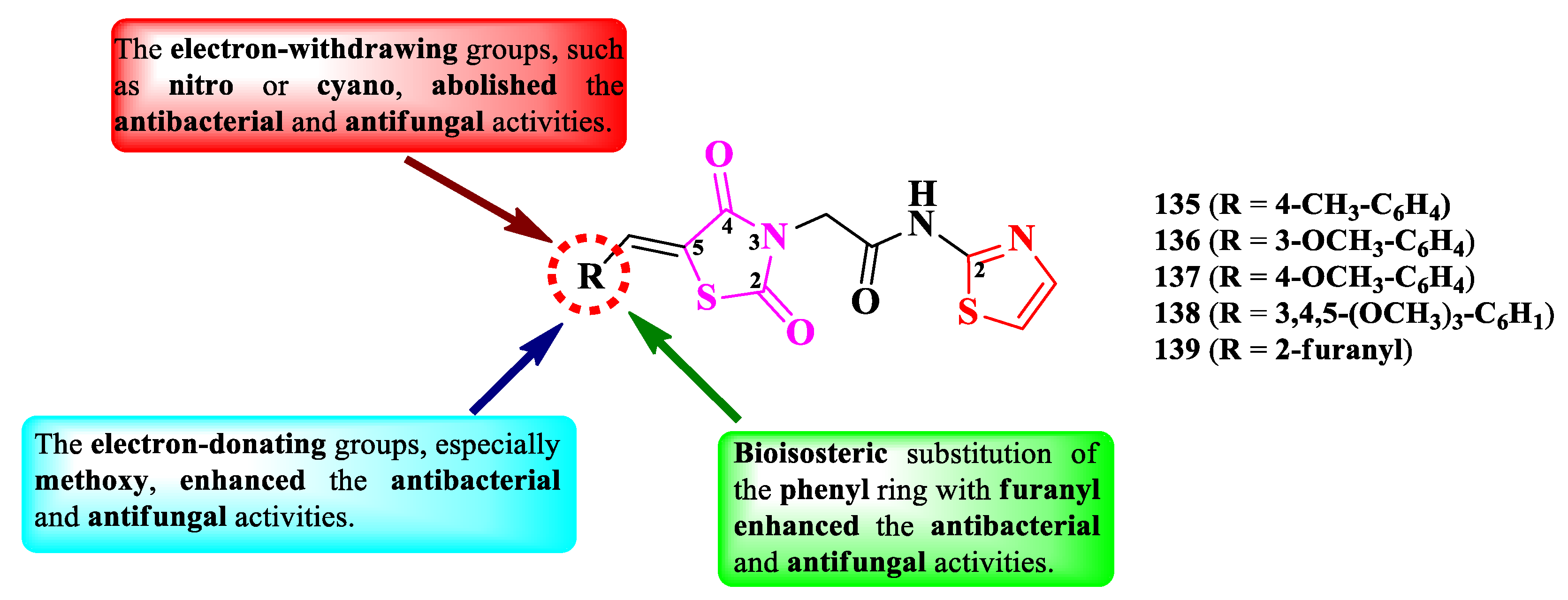
2.2.6. Thiazolyl-Thiazolidindione Hybrid Compounds
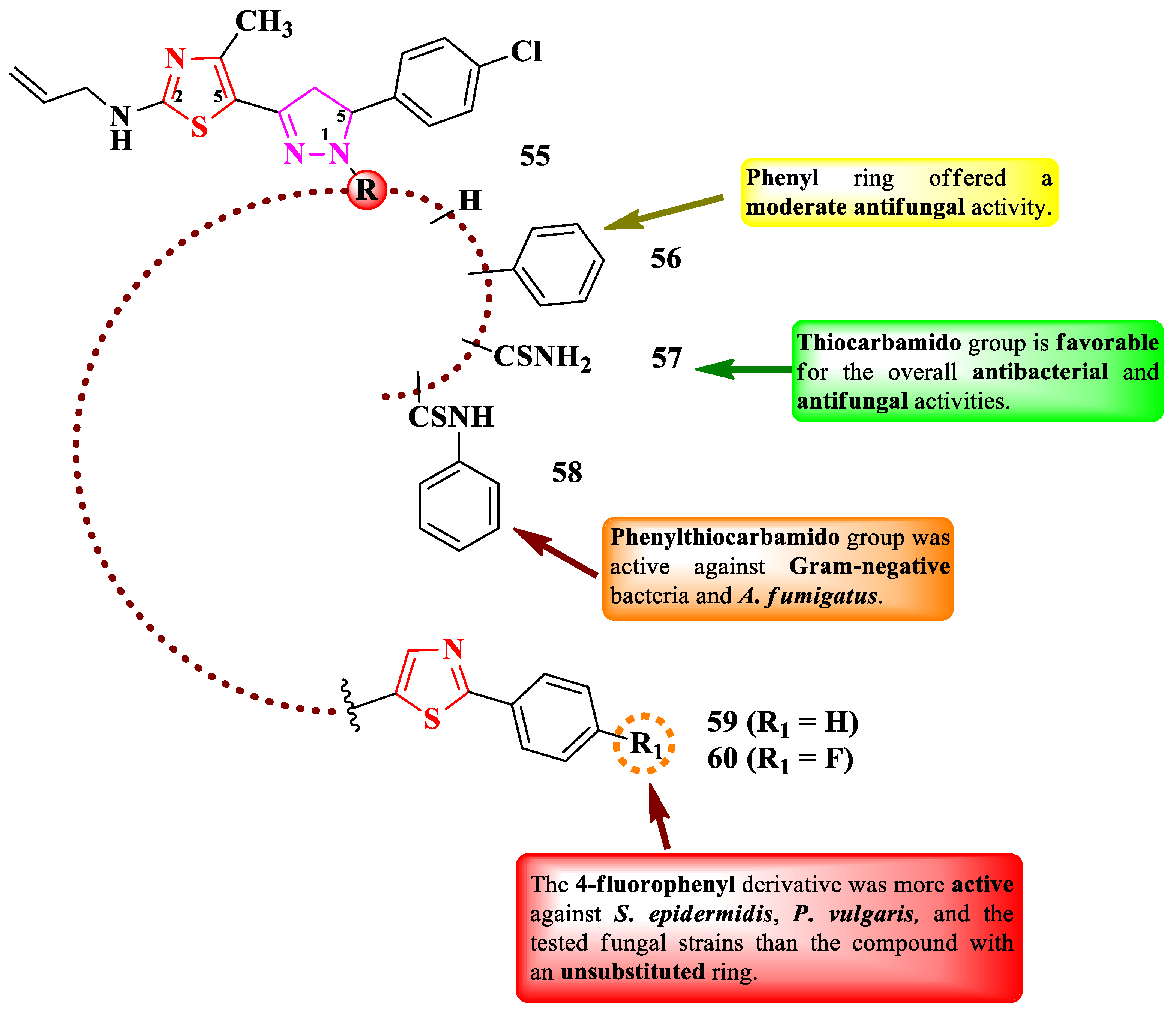
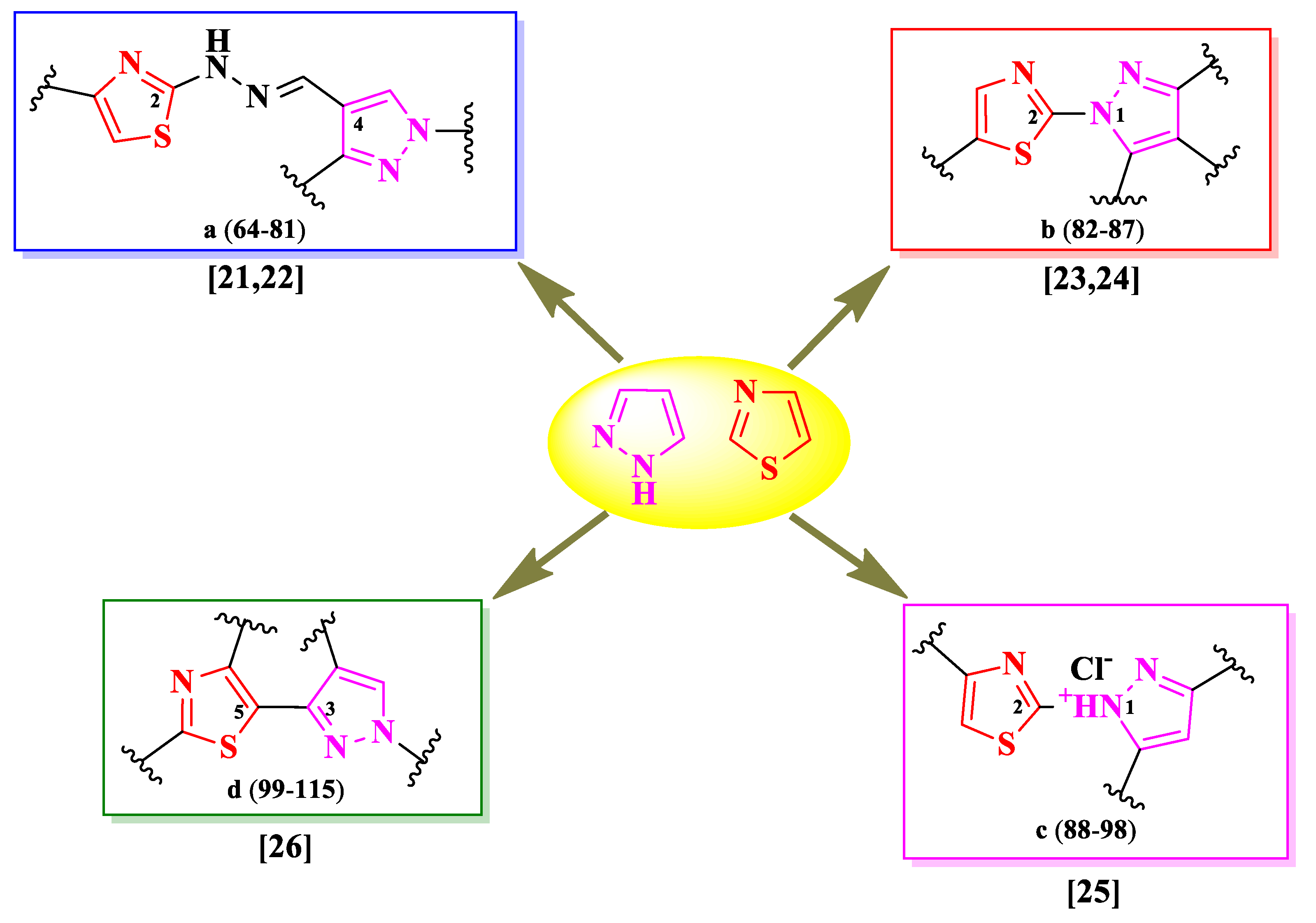
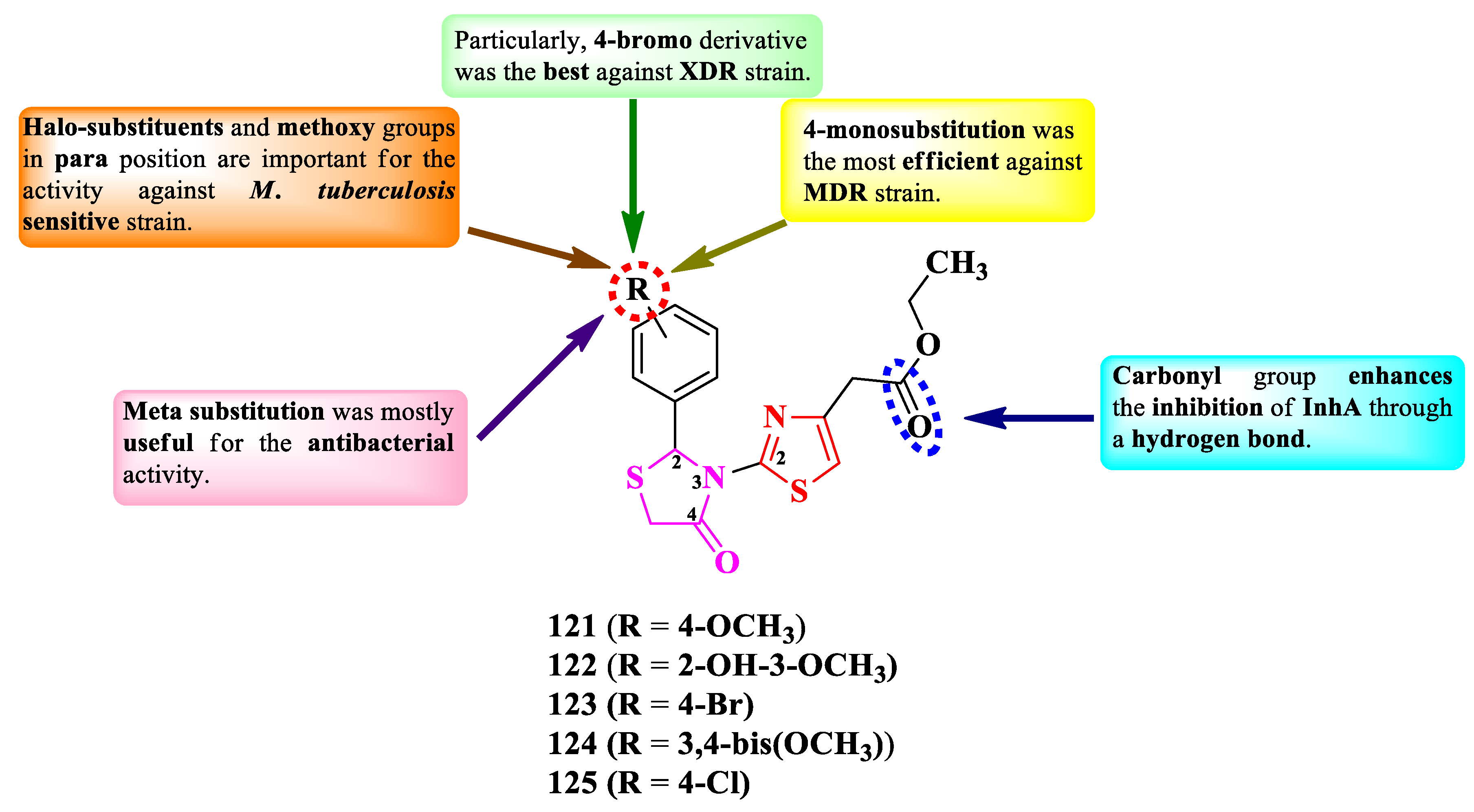
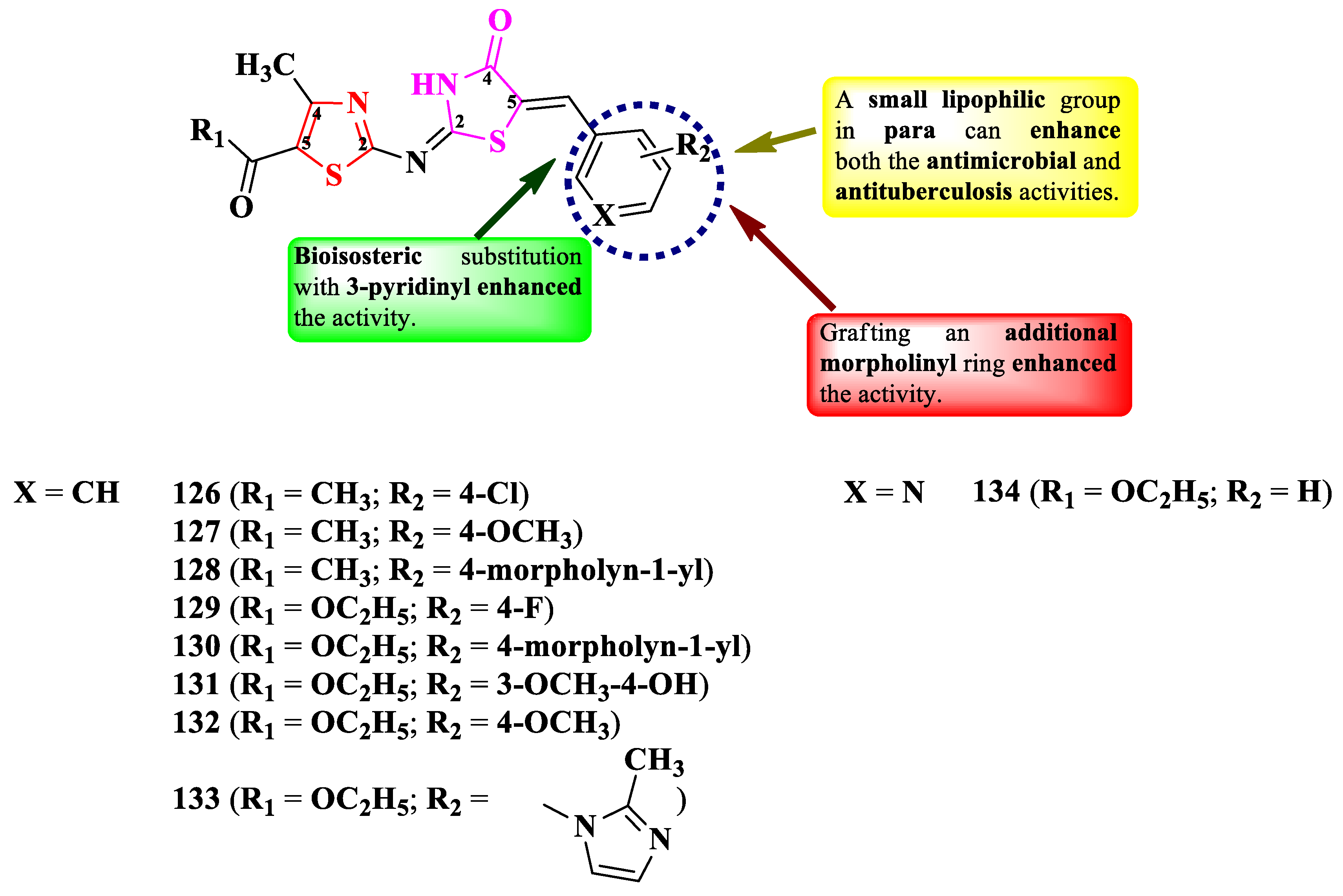
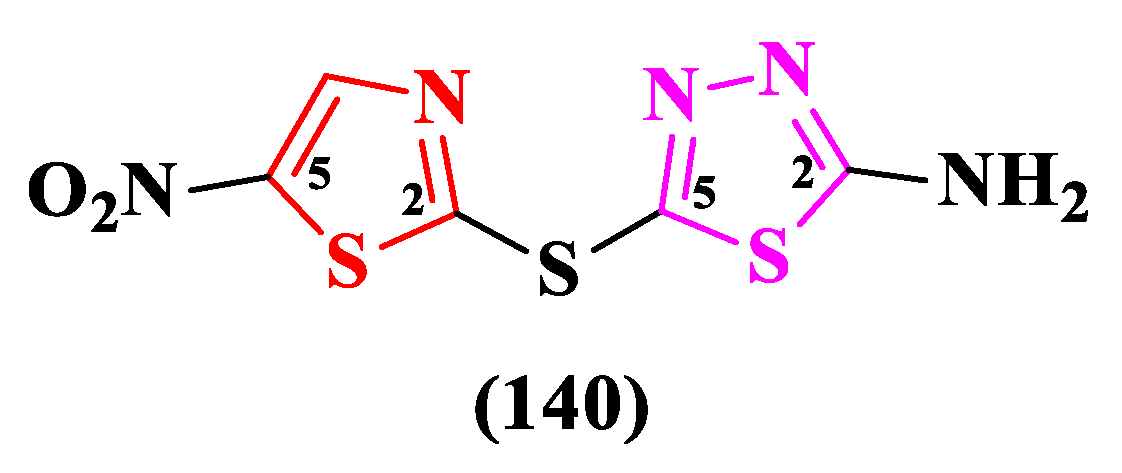
2.2.7. Thiazolyl-1,3,4-Thiadiazole Hybrid Compounds
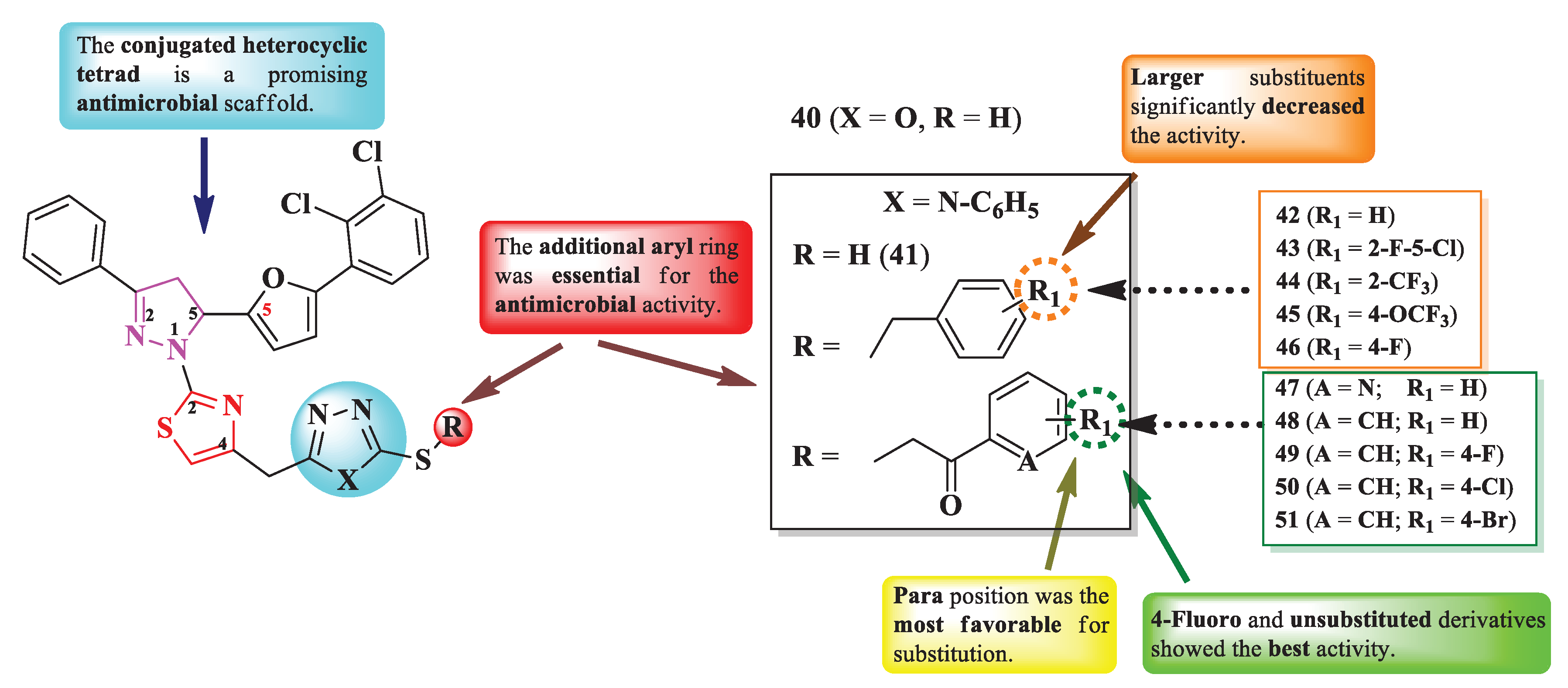
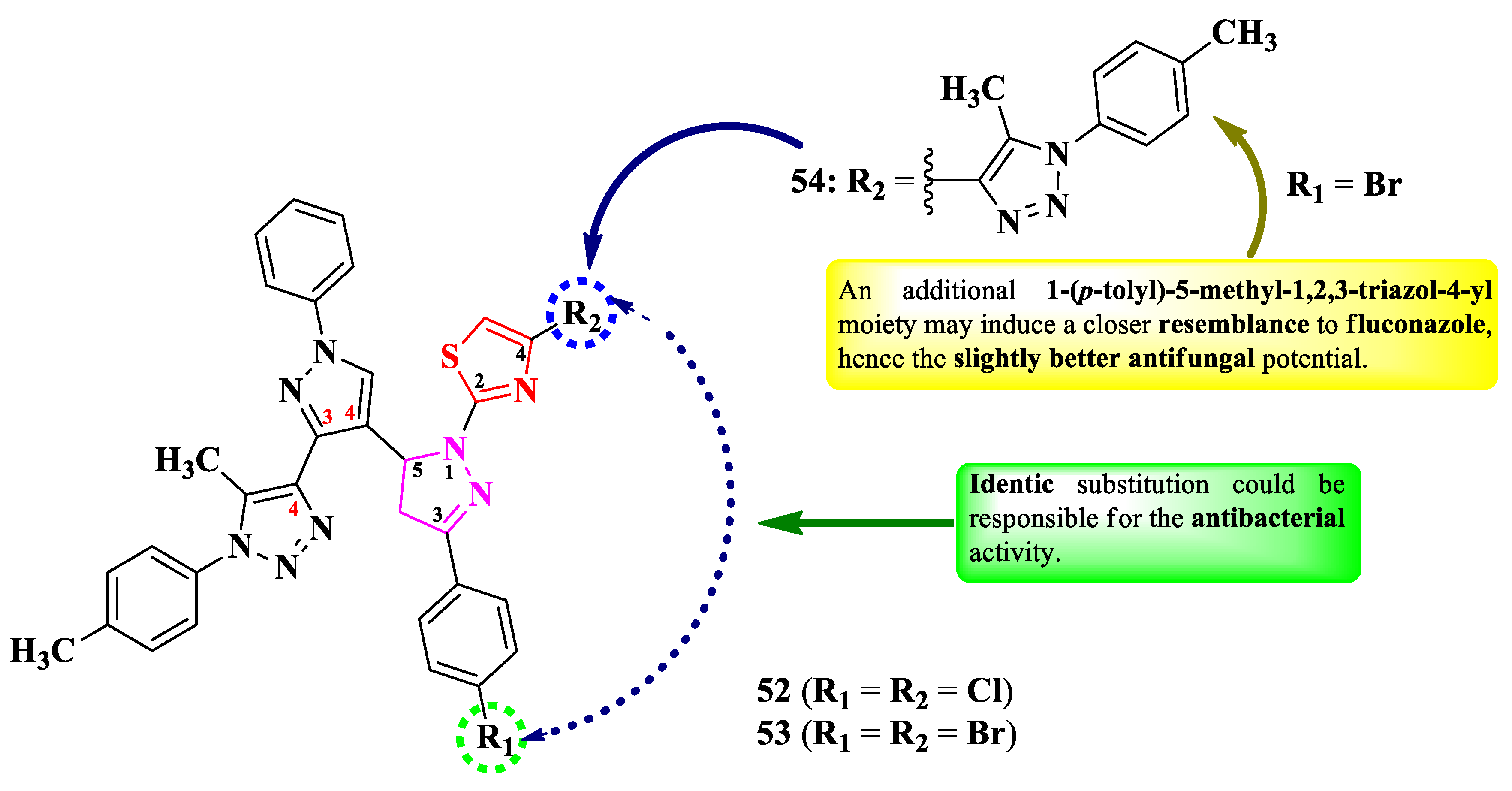
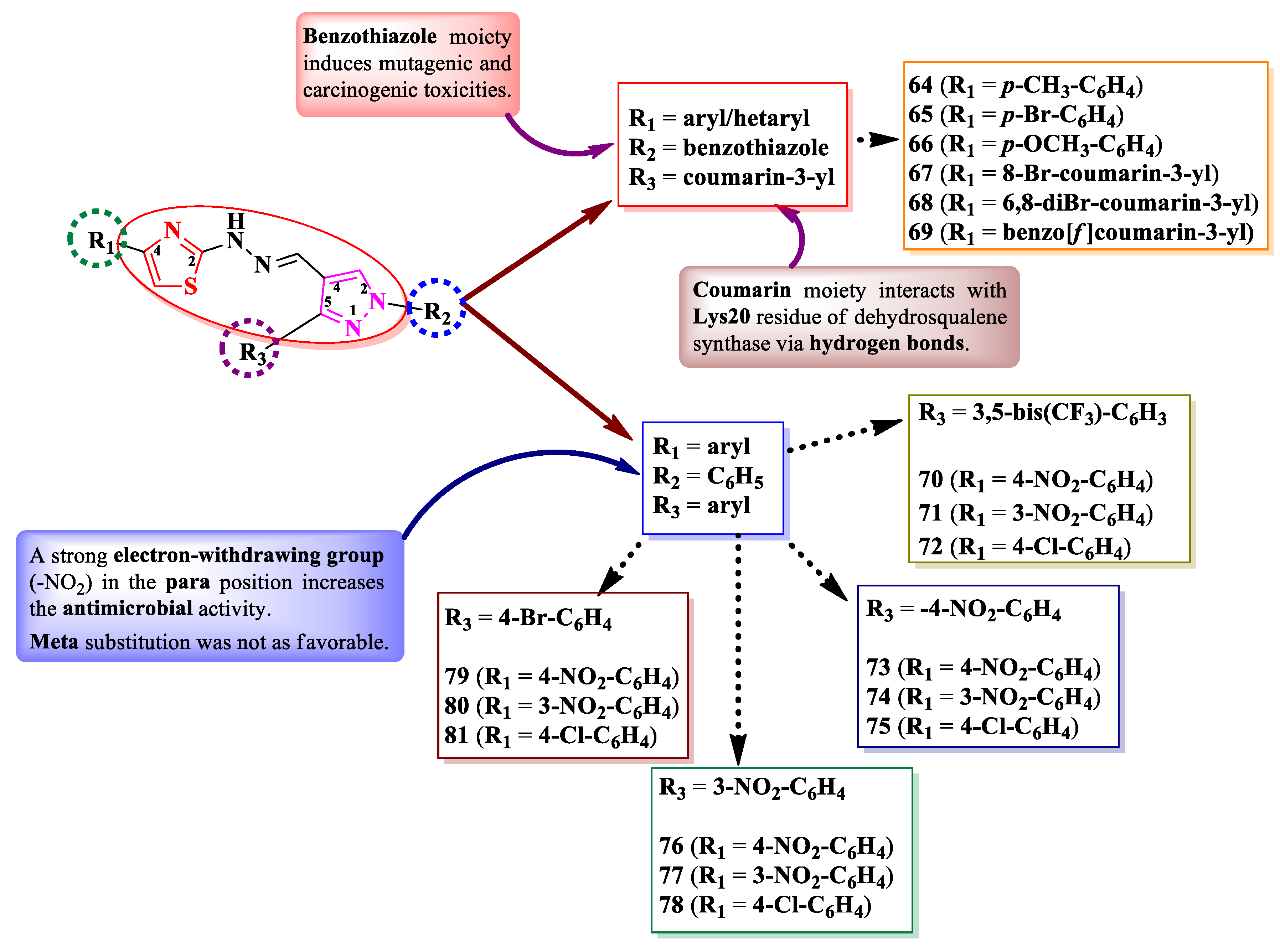
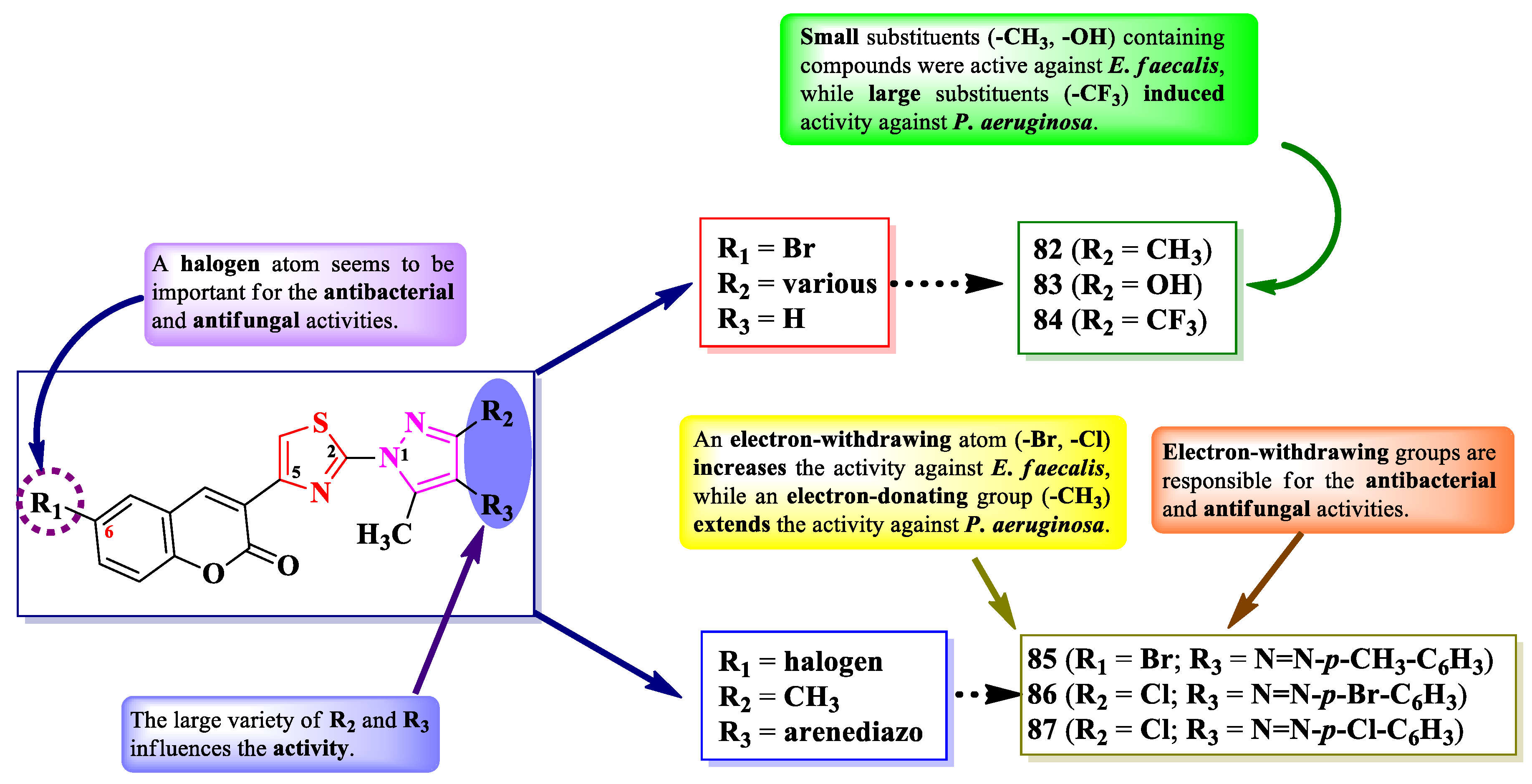
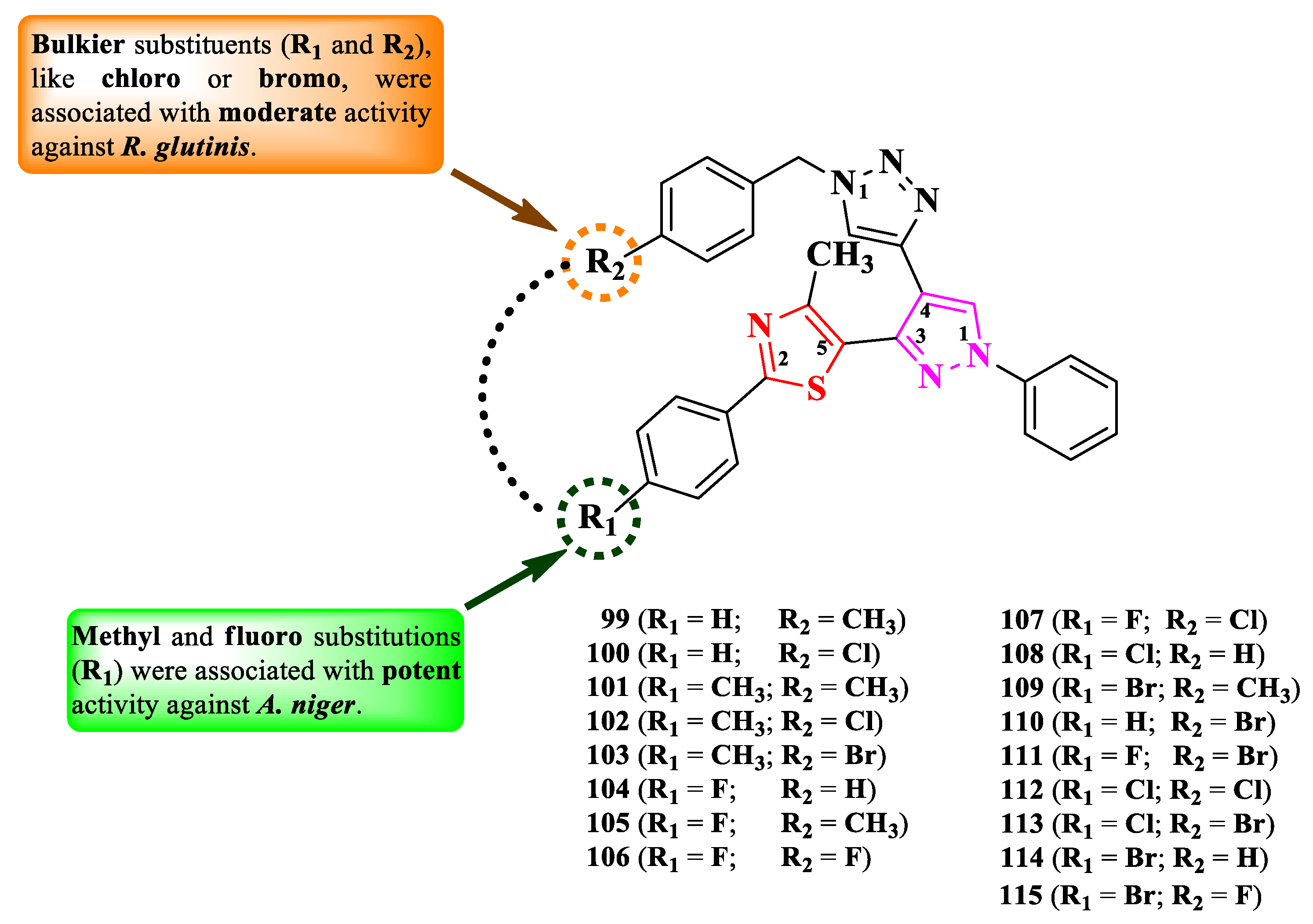
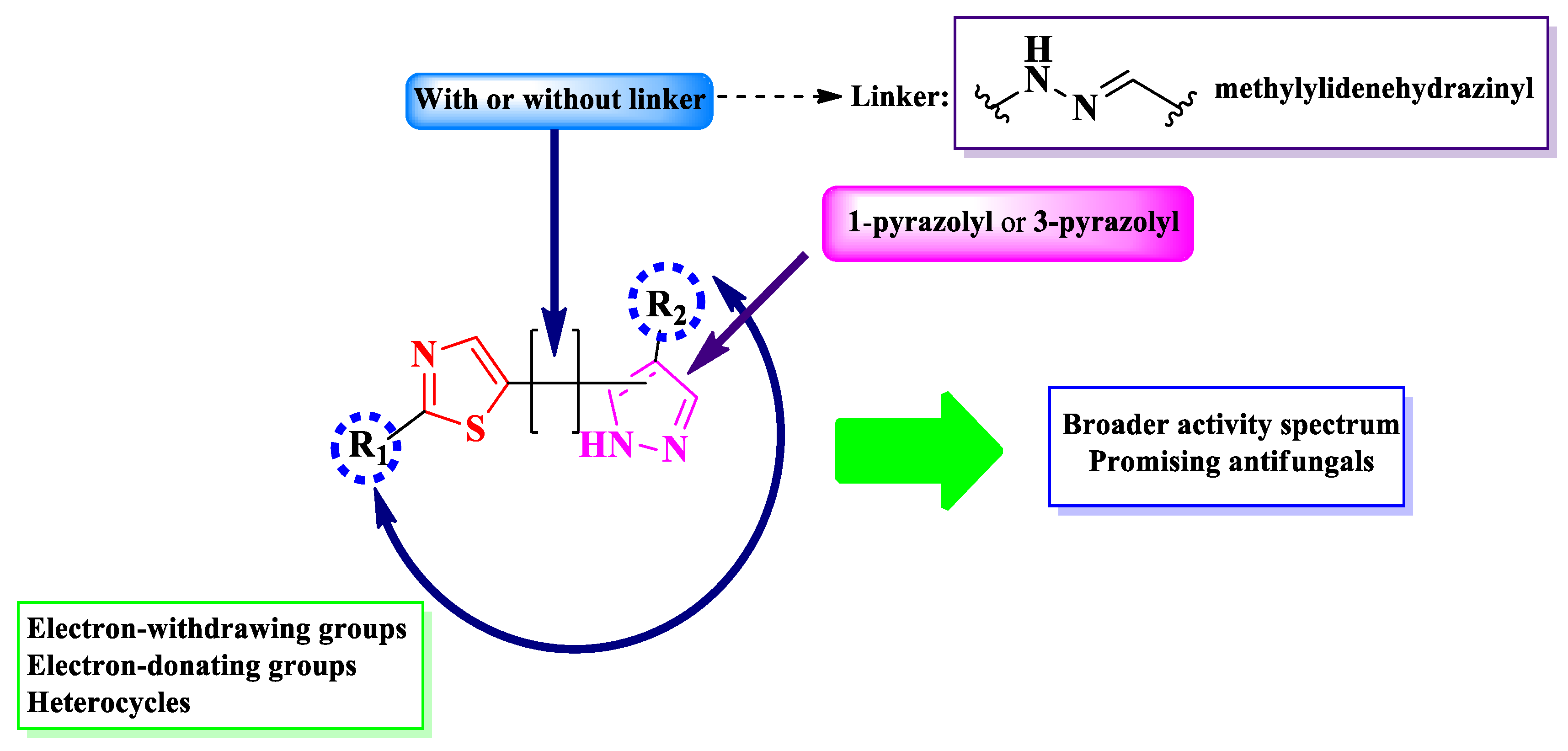
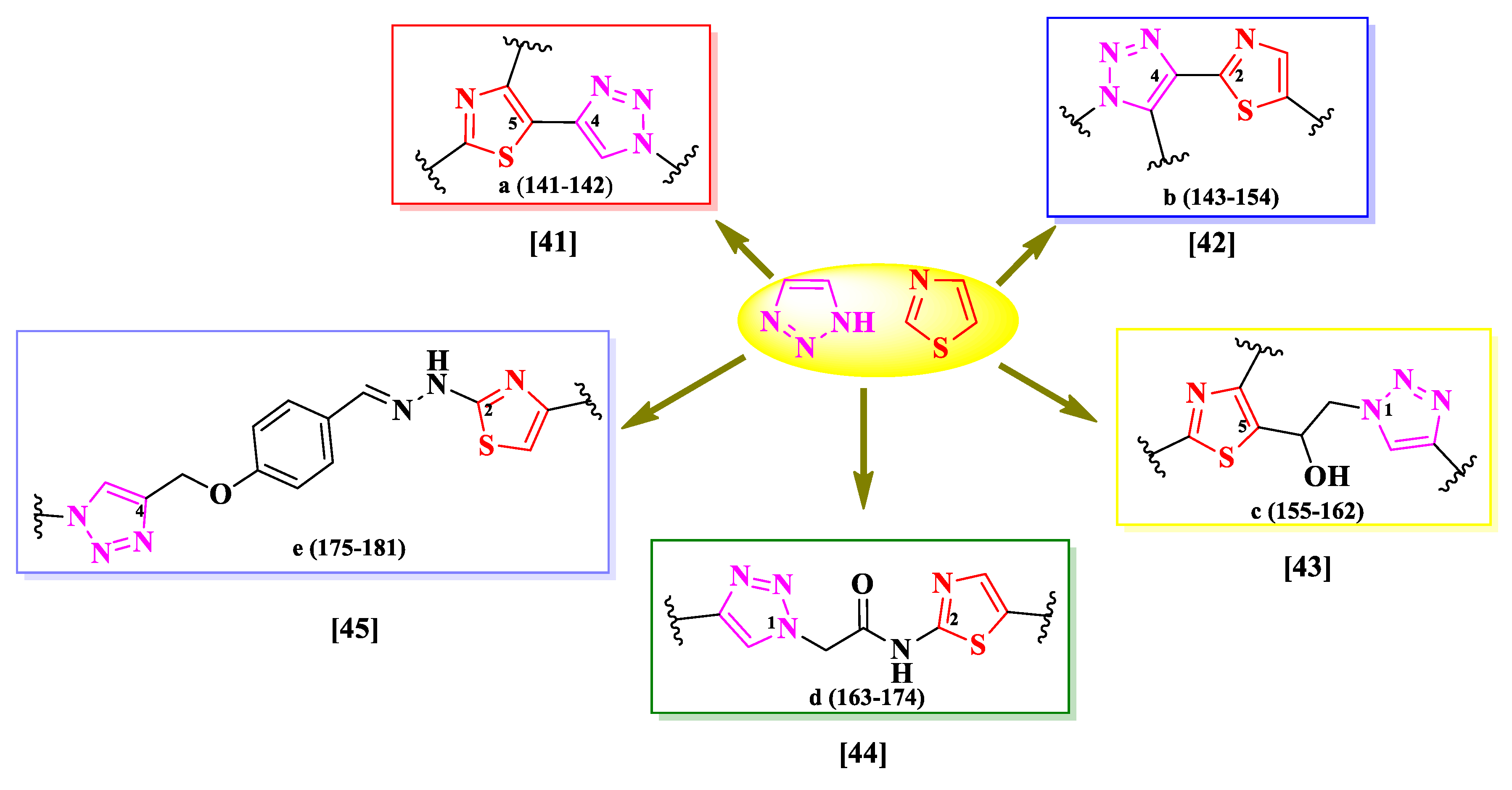
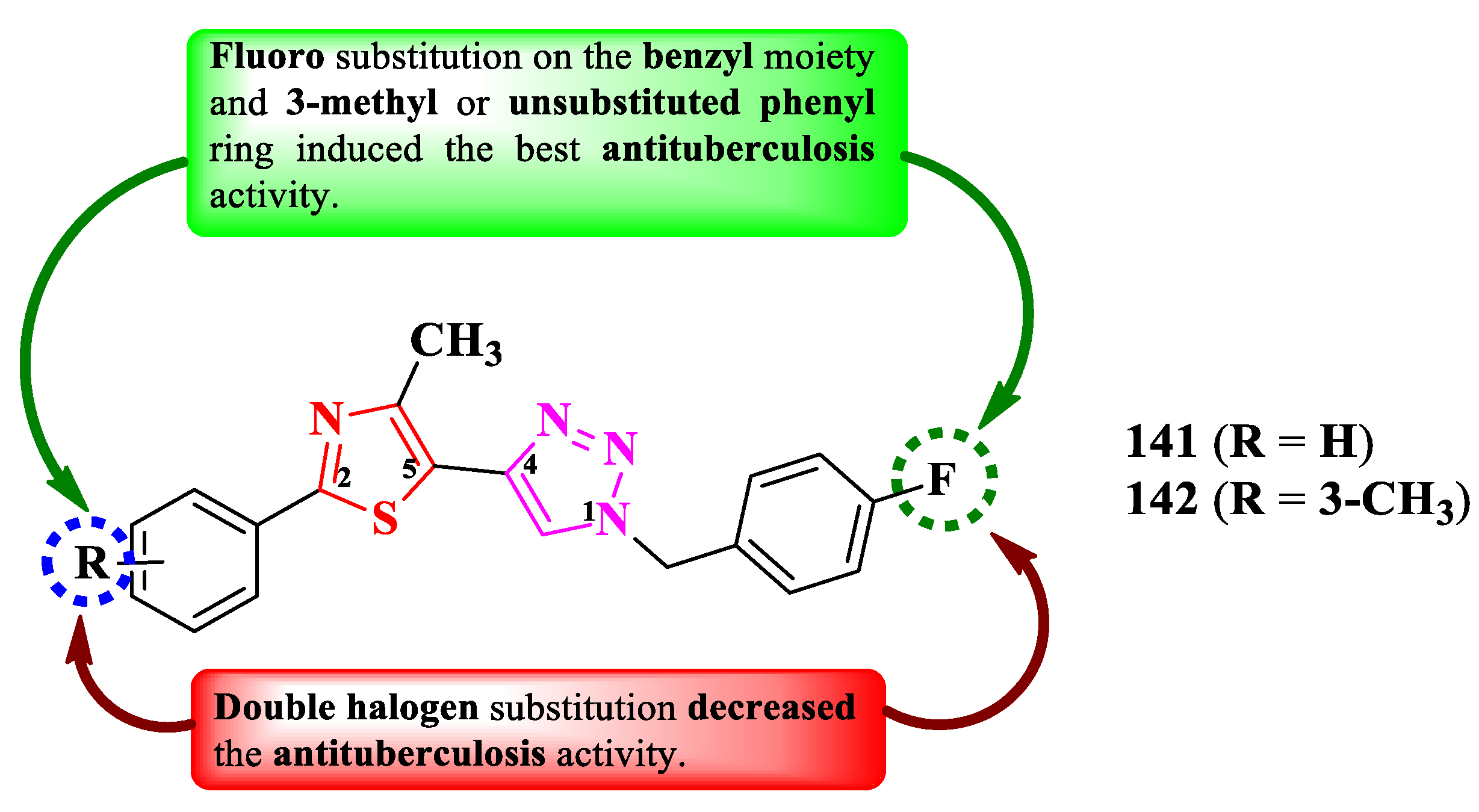
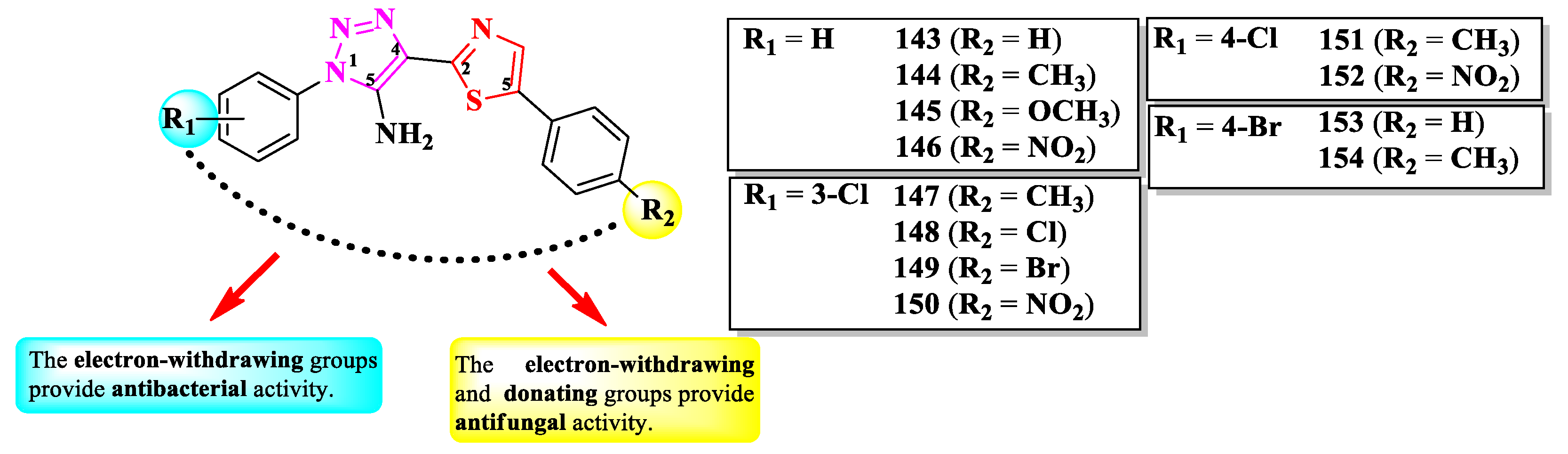
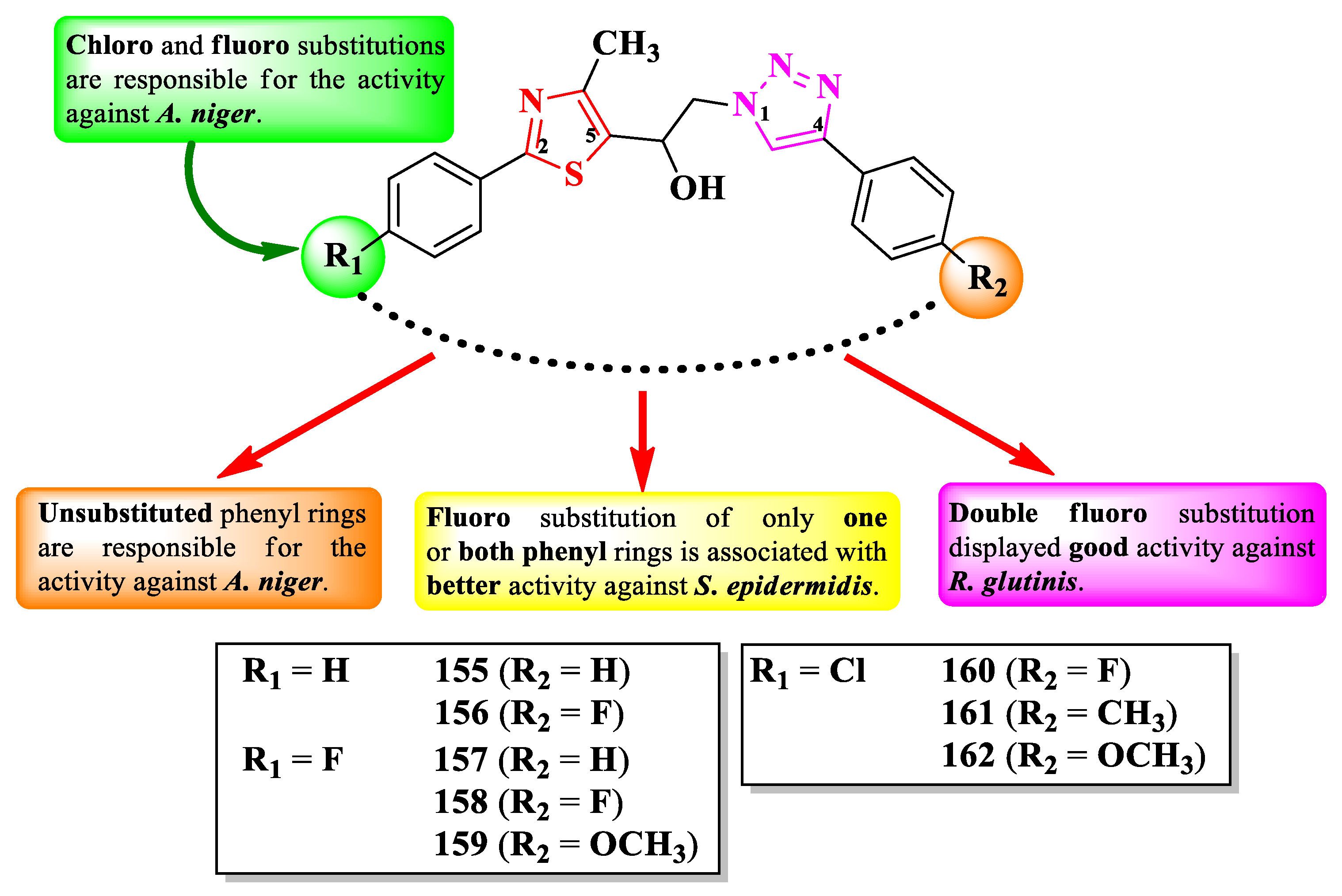
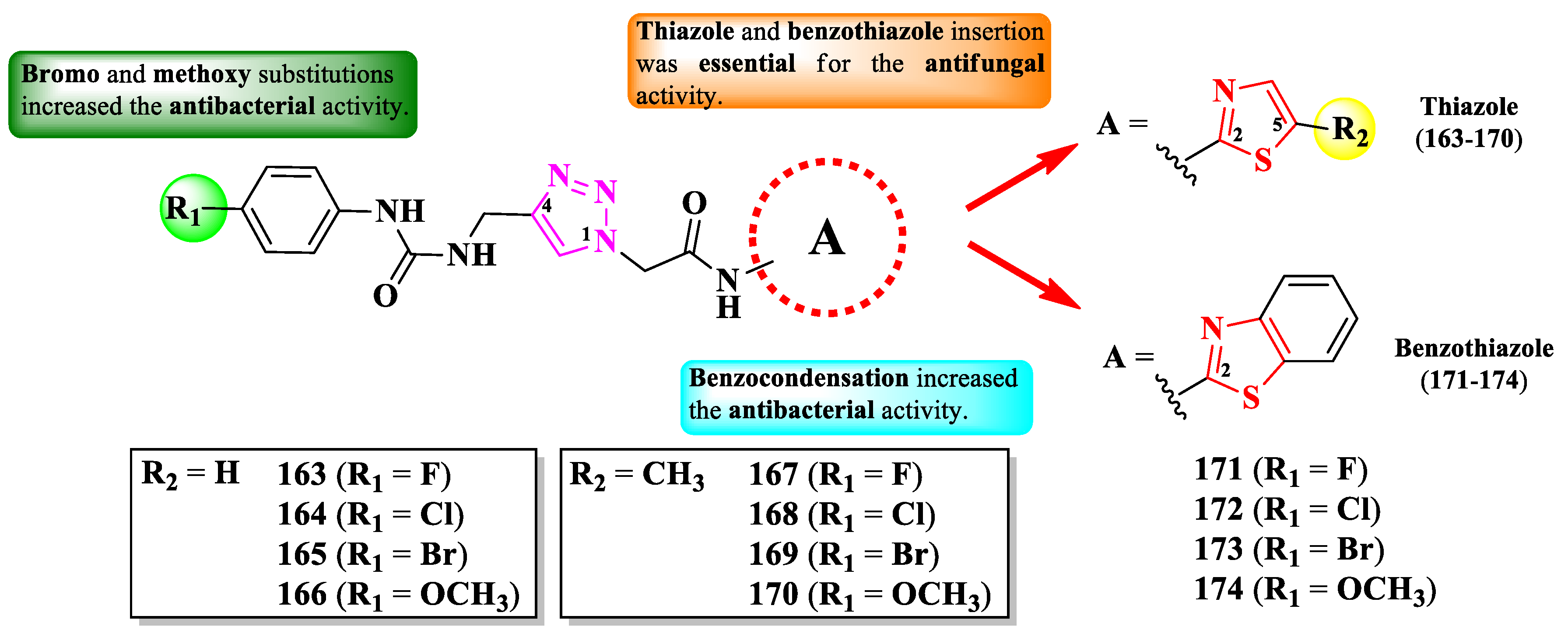
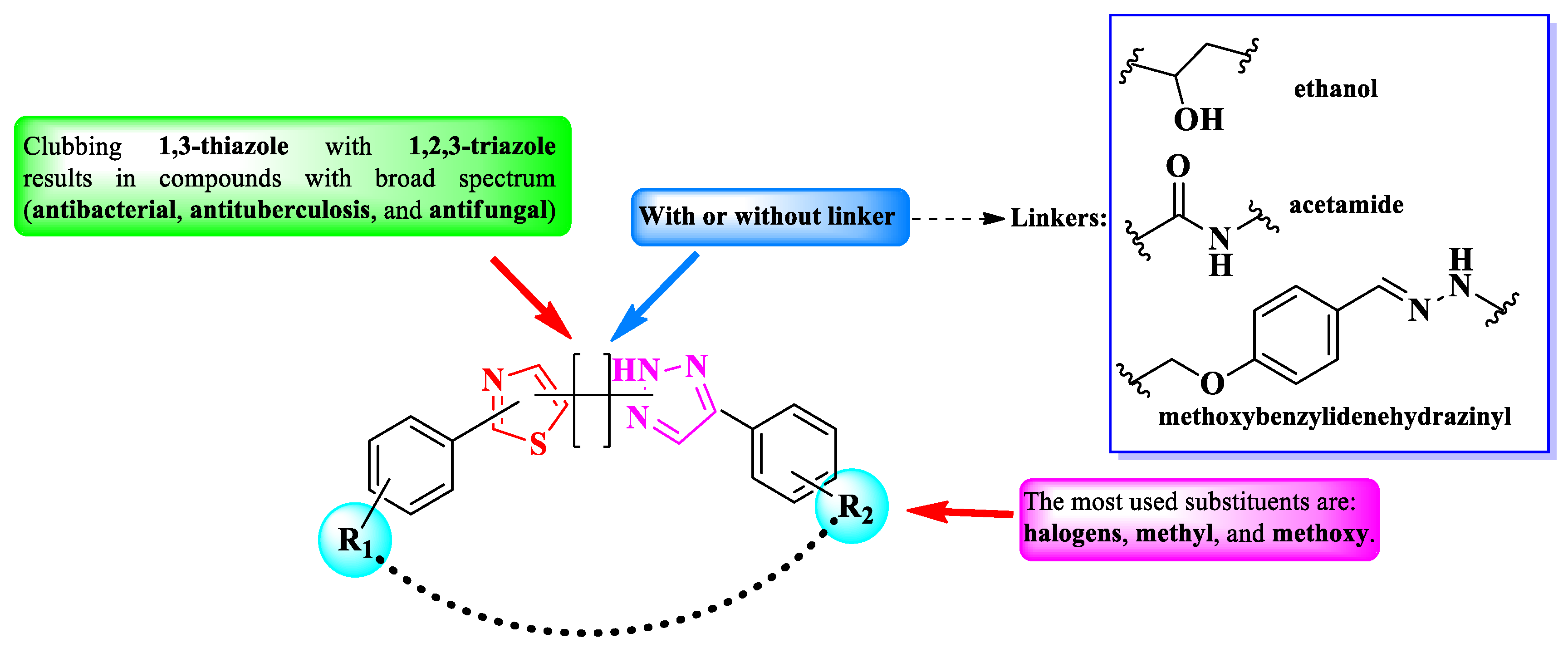
2.2.8. Thiazolyl-1,2,3-Triazole Hybrid Compounds
2.2.9. Thiazolyl-1,3,4-Oxadiazole Hybrid Compounds
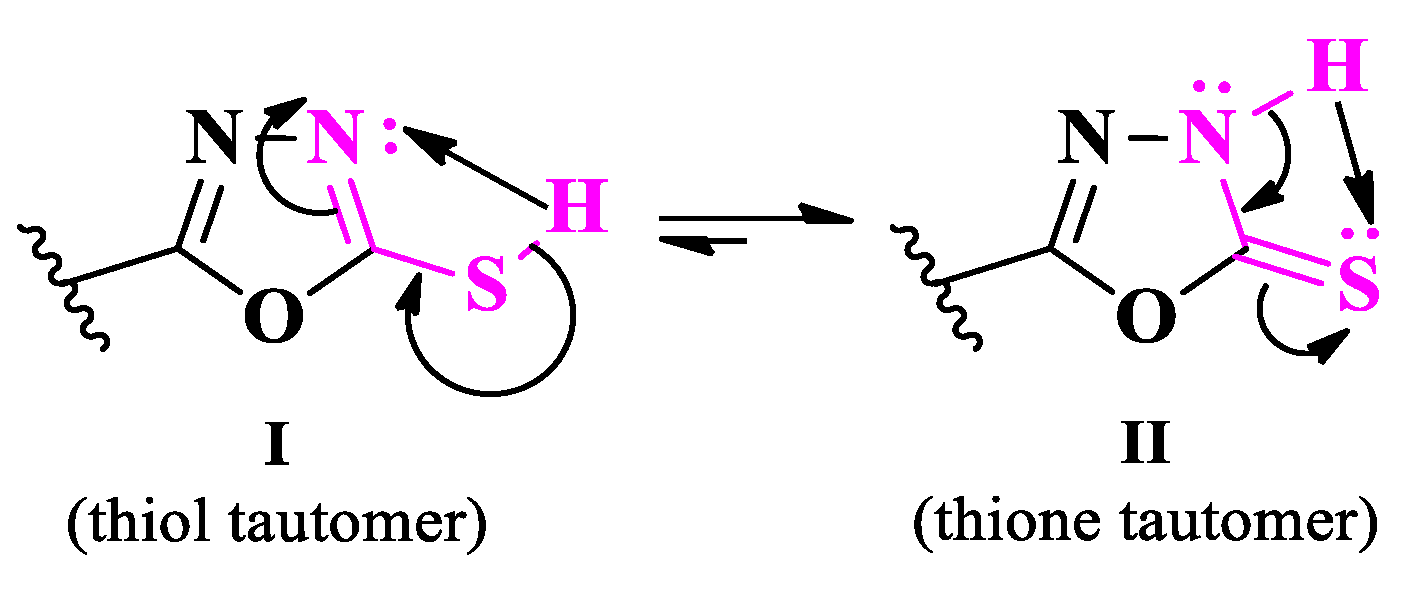
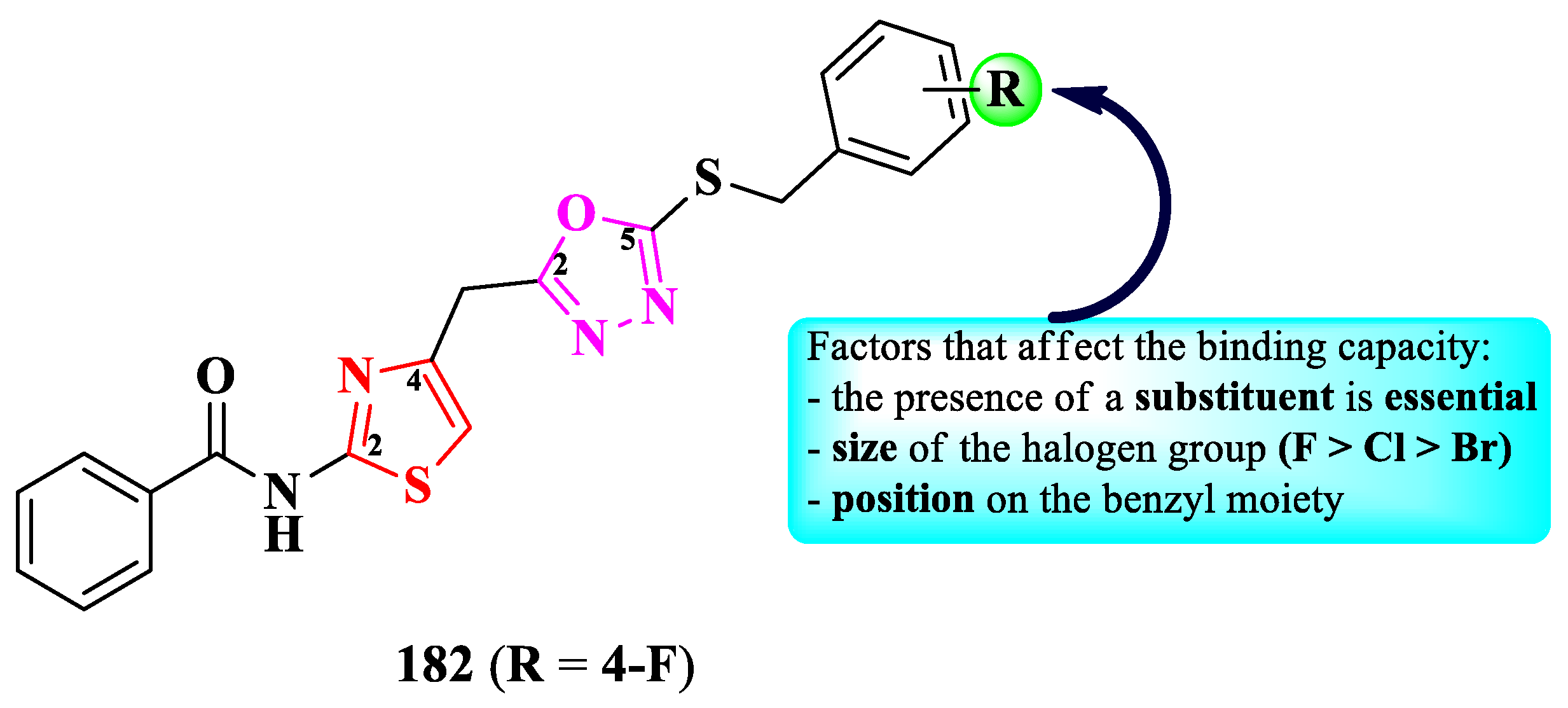
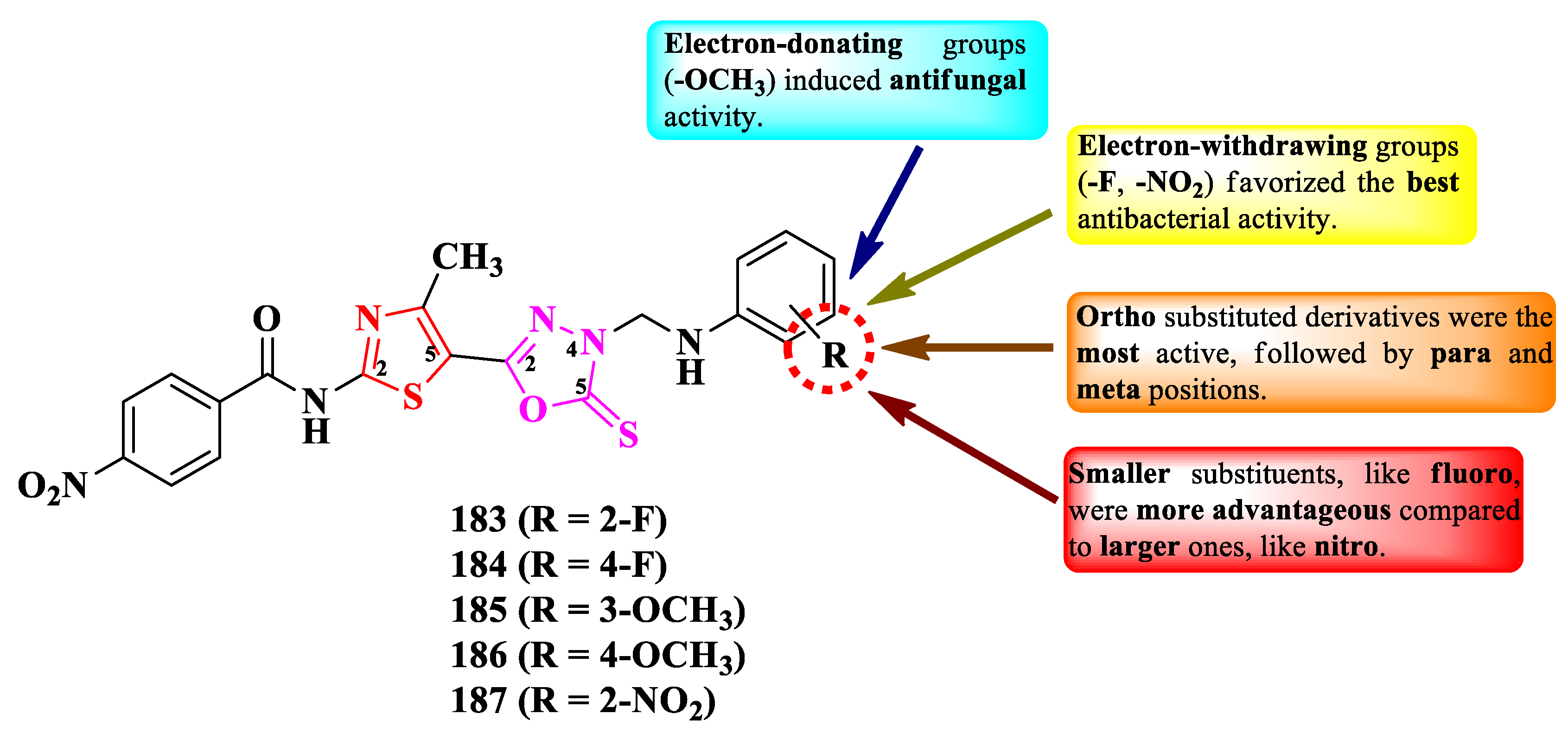
2.3. Thiazole Clubbed with Five-Membered Benzofused Heterocycles
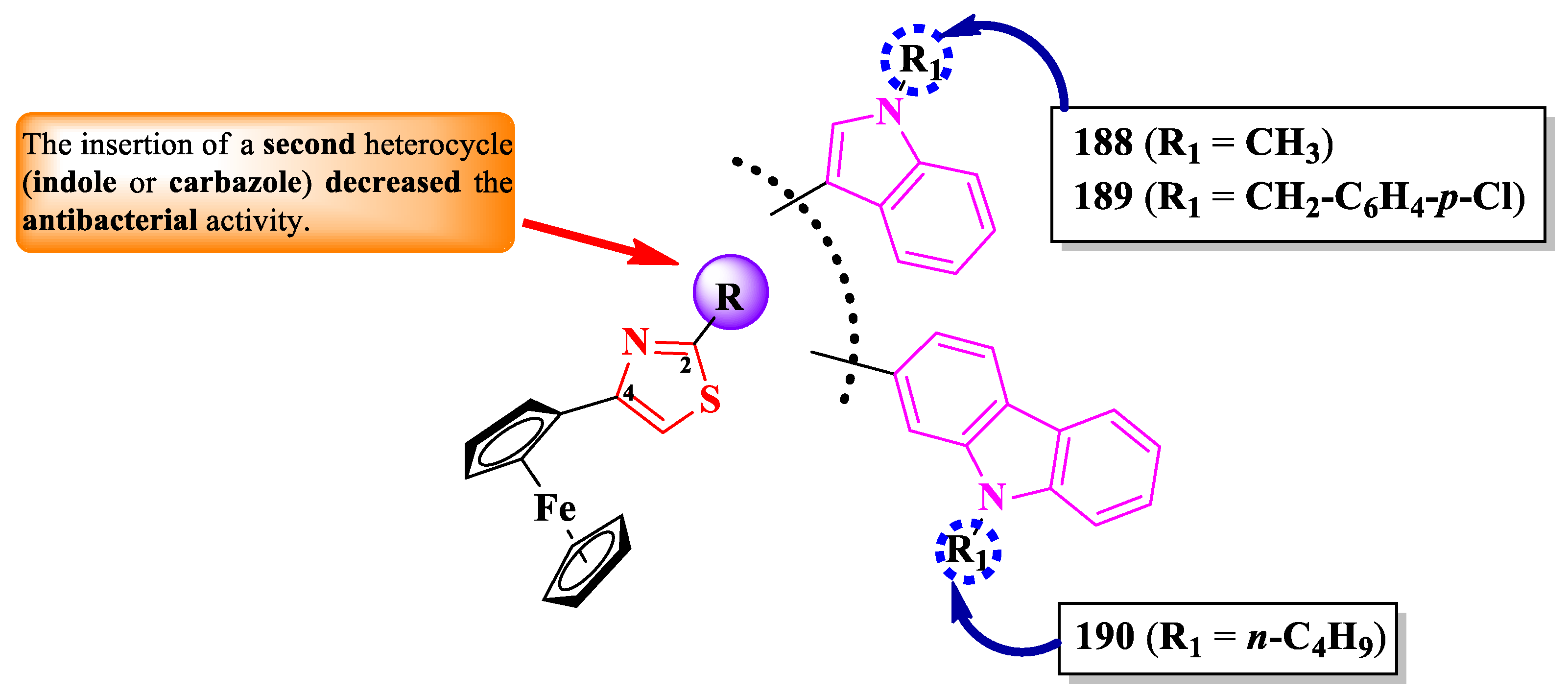
2.3.1. Thiazolyl-Indole and Thiazolyl-Carbazole Hybrid Compounds
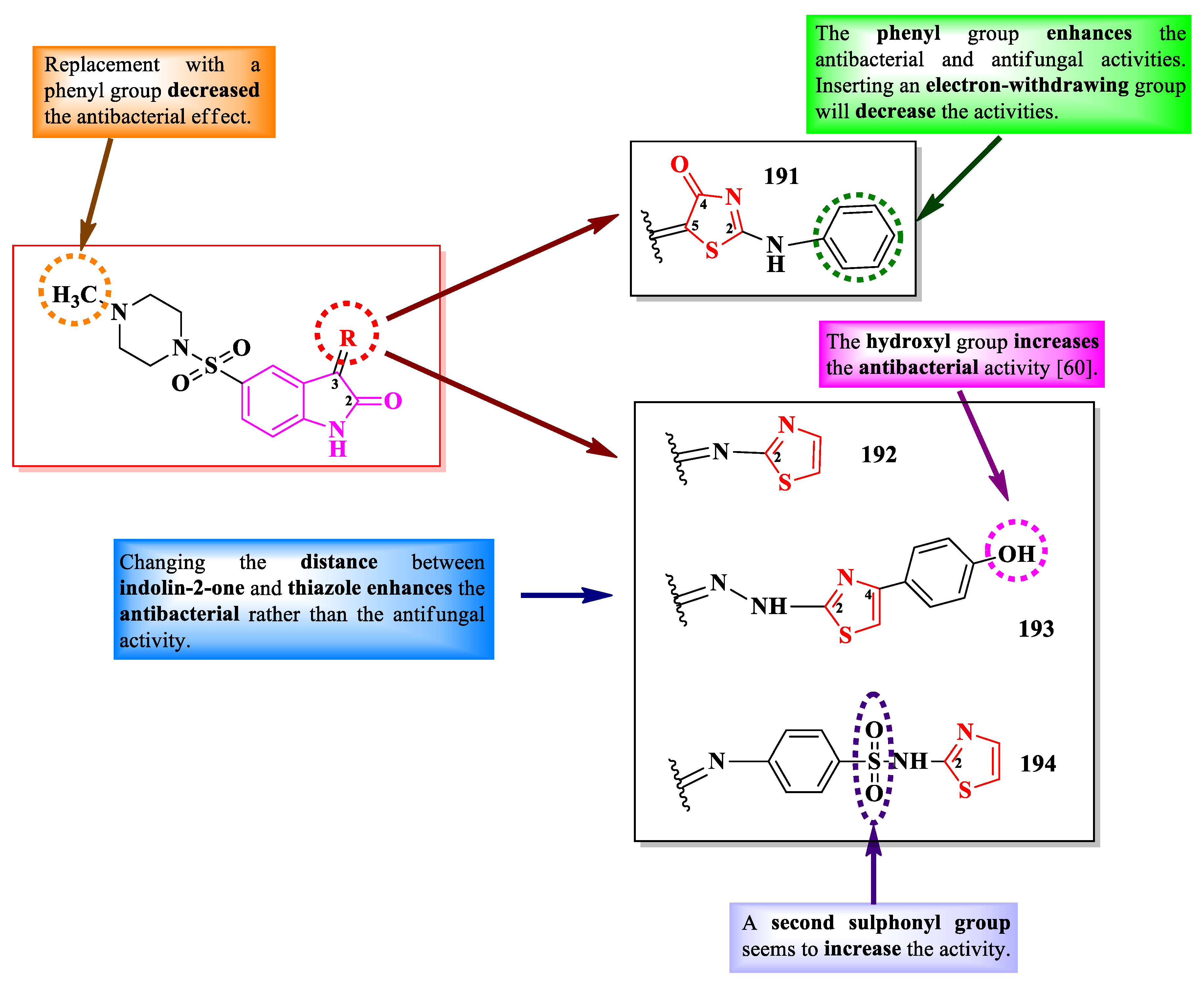
2.3.2. Thiazolyl-Indolin-2-one Hybrid Compounds
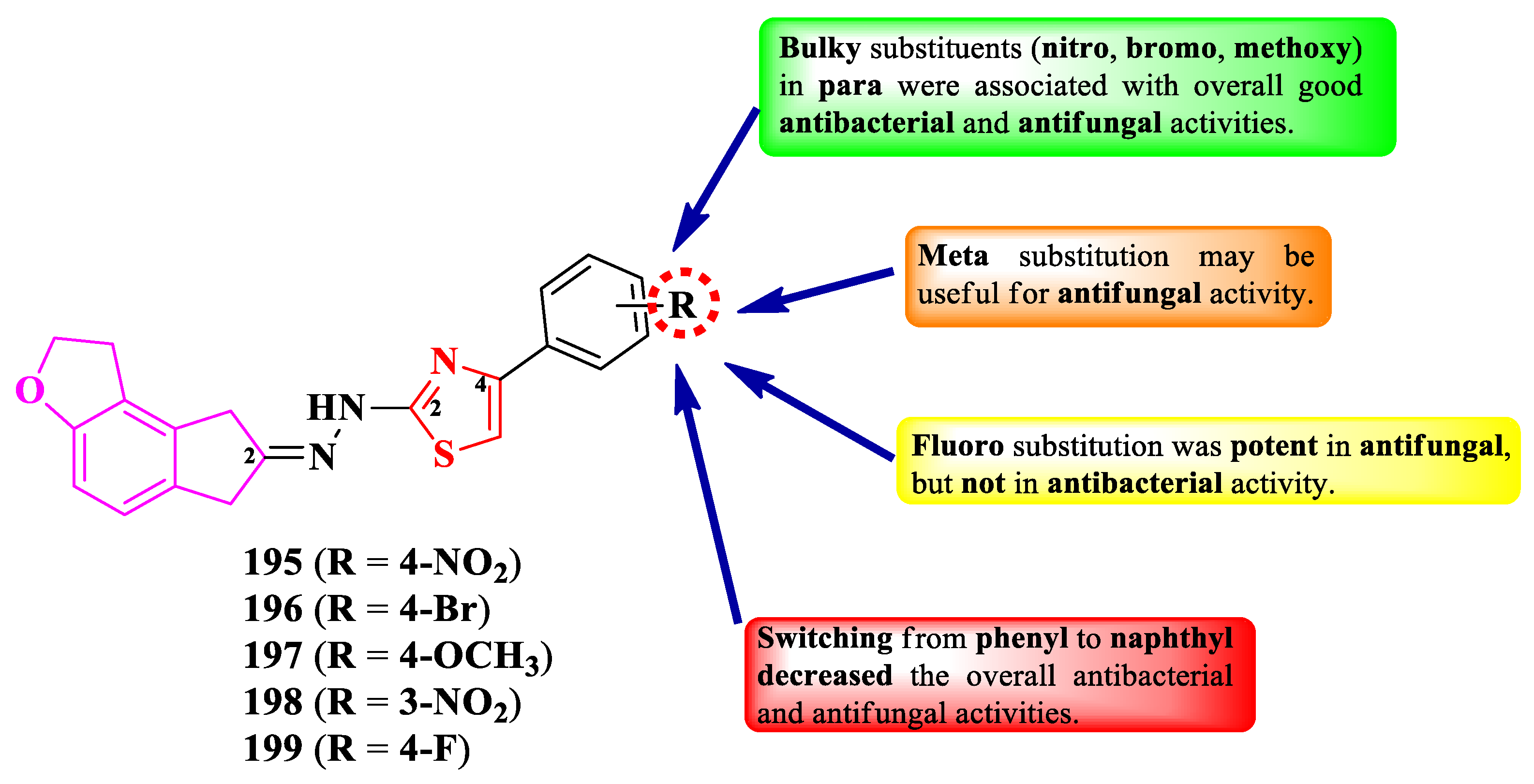
2.3.3. Thiazolyl-Tetrahydroindenofuran Hybrid Compounds
2.4. Thiazole Clubbed with Six-Membered Heterocycles
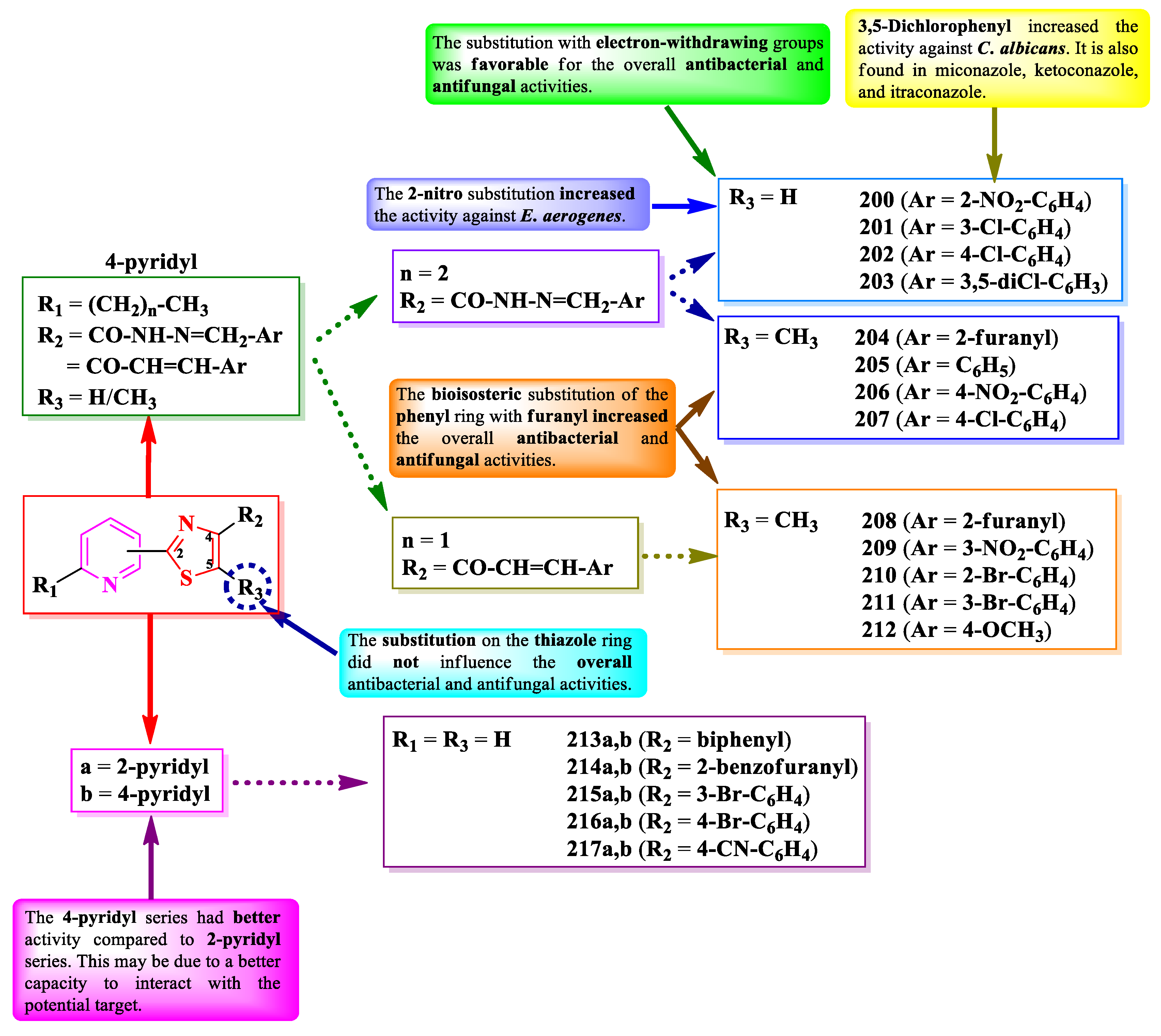
2.4.1. Thiazolyl-Pyridine Hybrid Compounds
2.5. Thiazole Clubbed with Six-Membered Benzofused Heterocycles
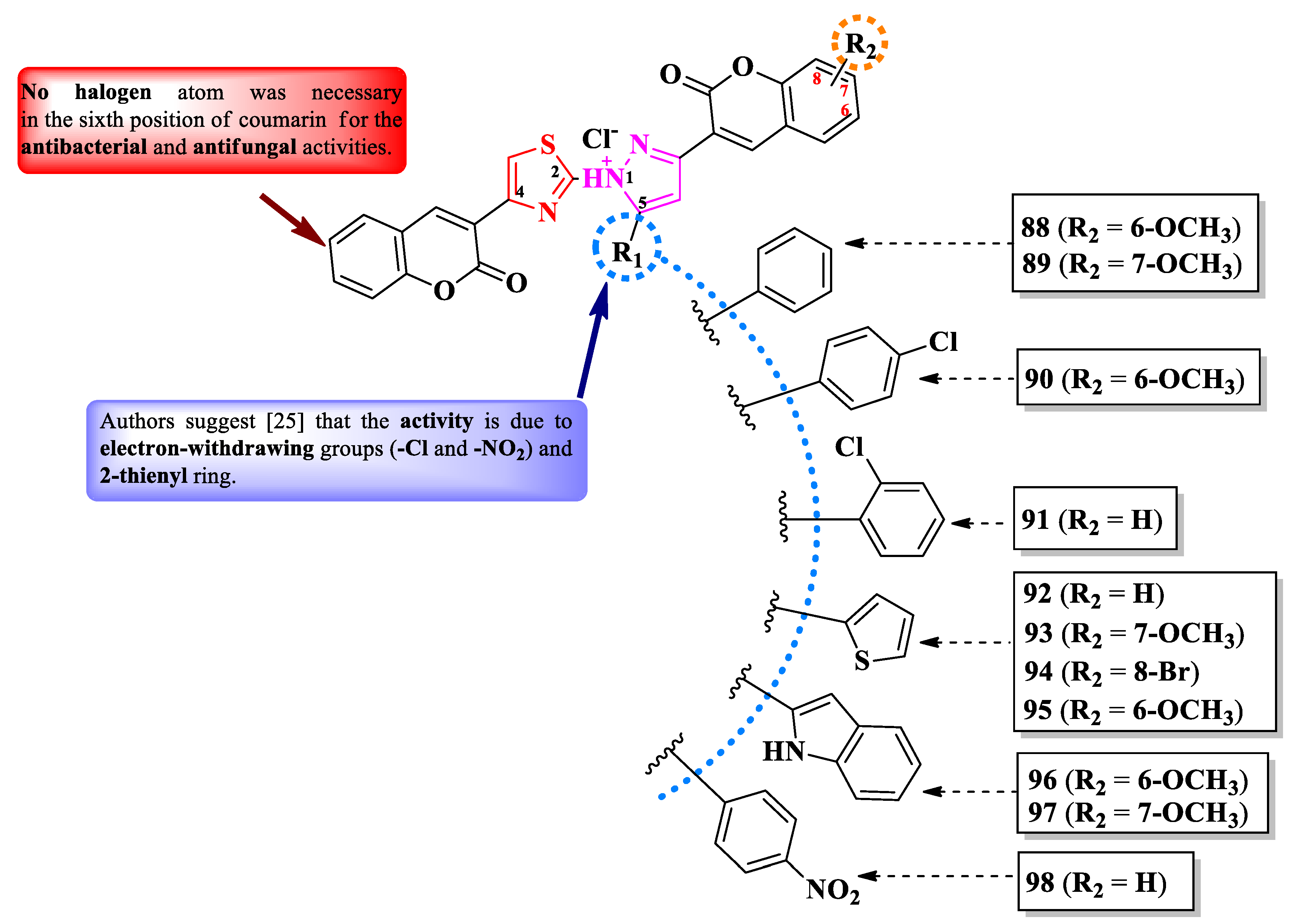
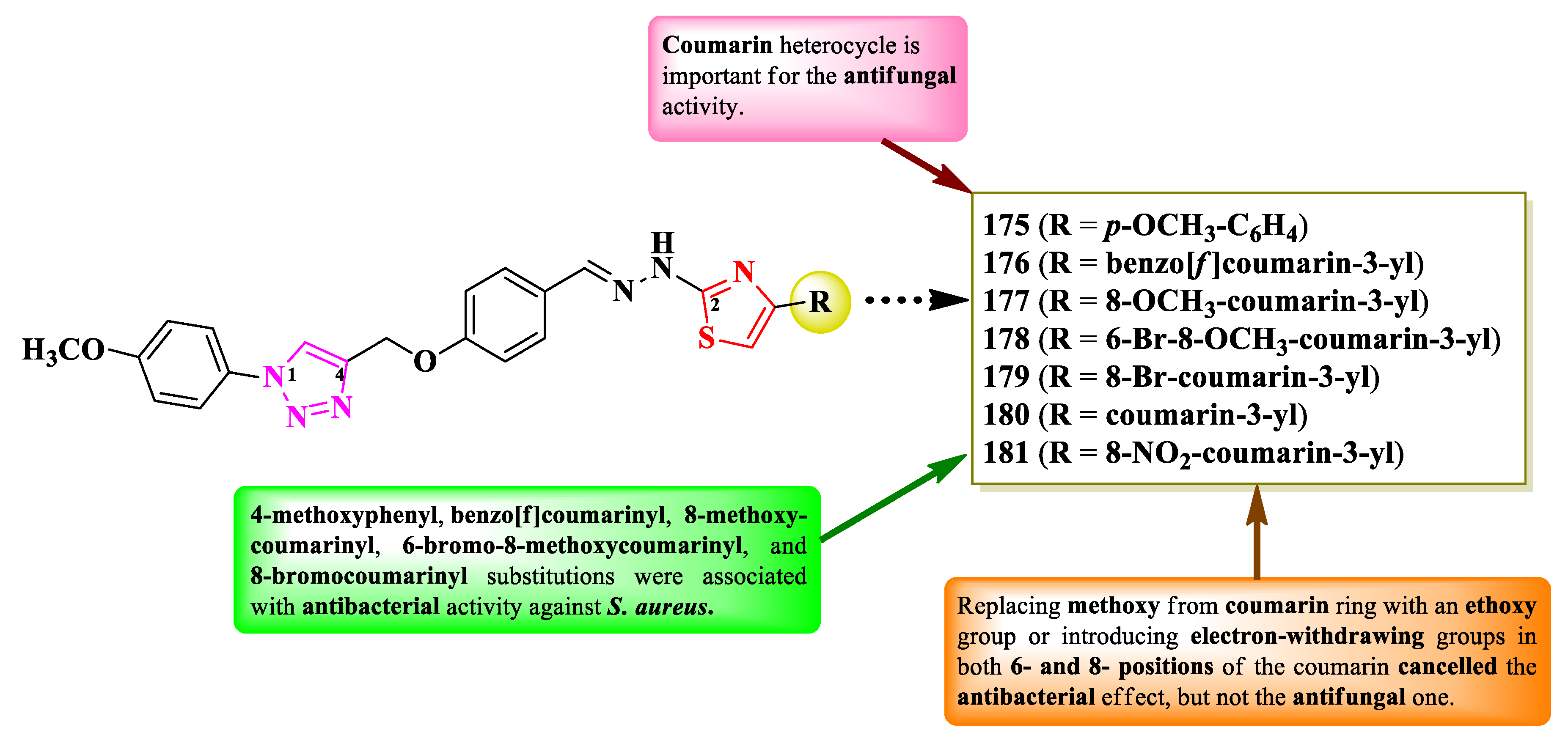
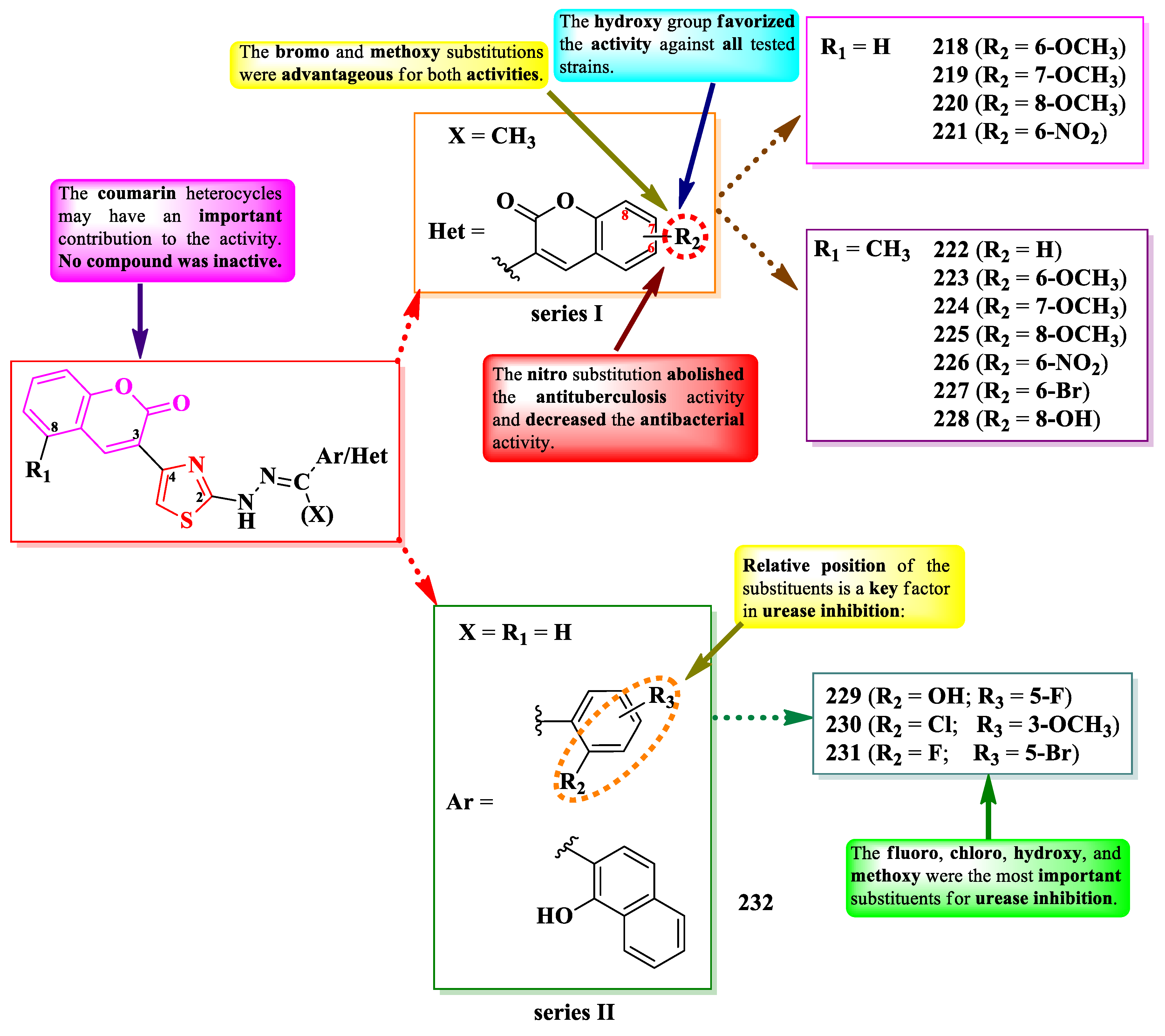
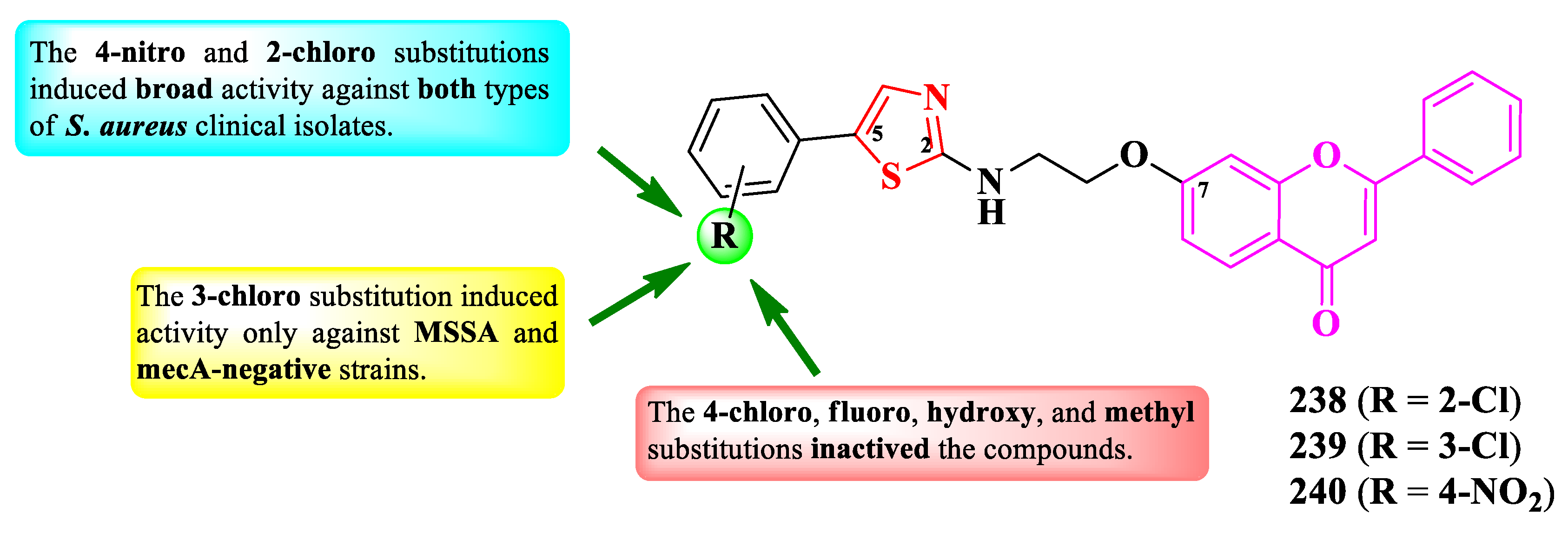
2.5.1. Thiazolyl-Coumarin Hybrid Compounds
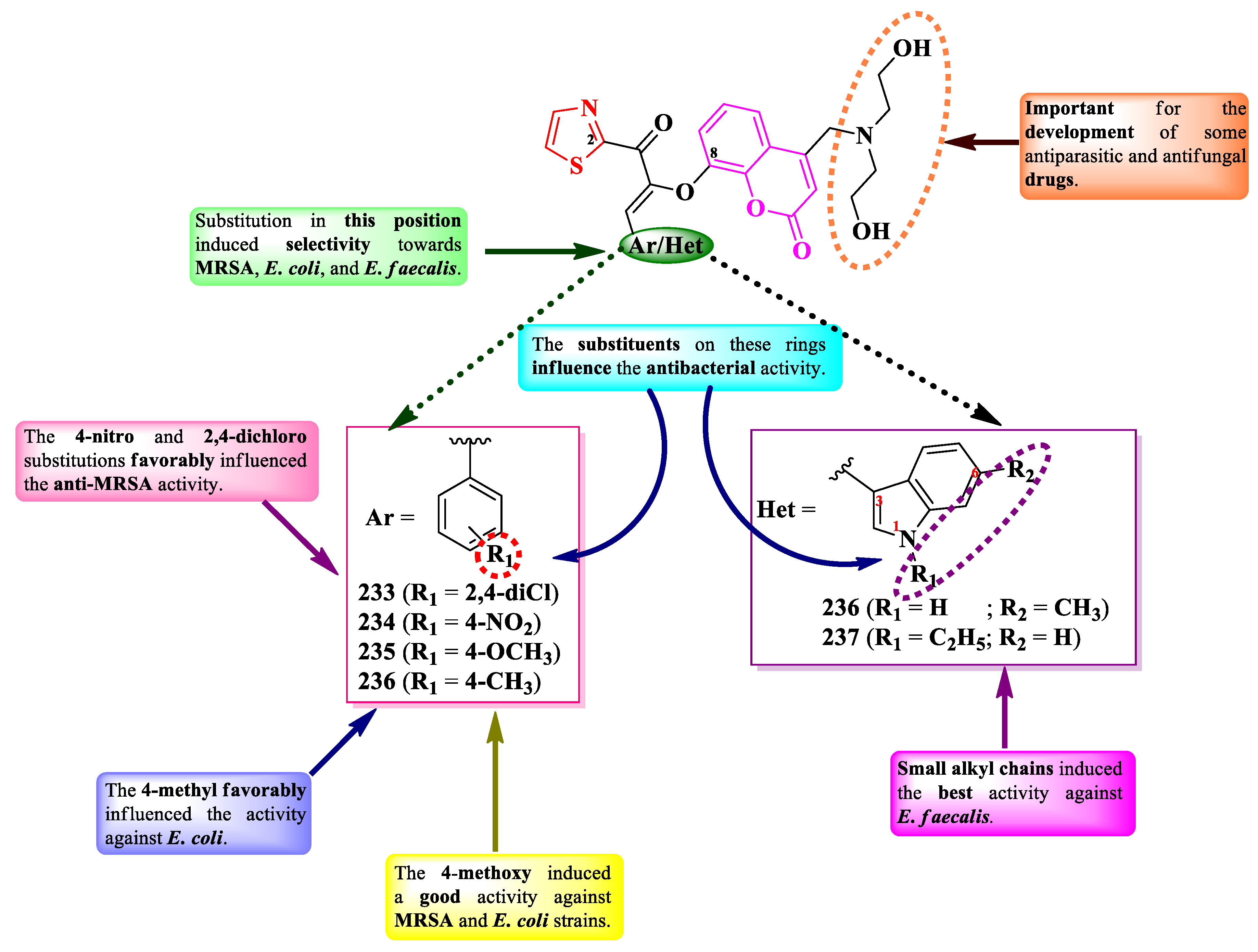
2.5.2. Thiazolyl-Flavone Hybrid Compounds
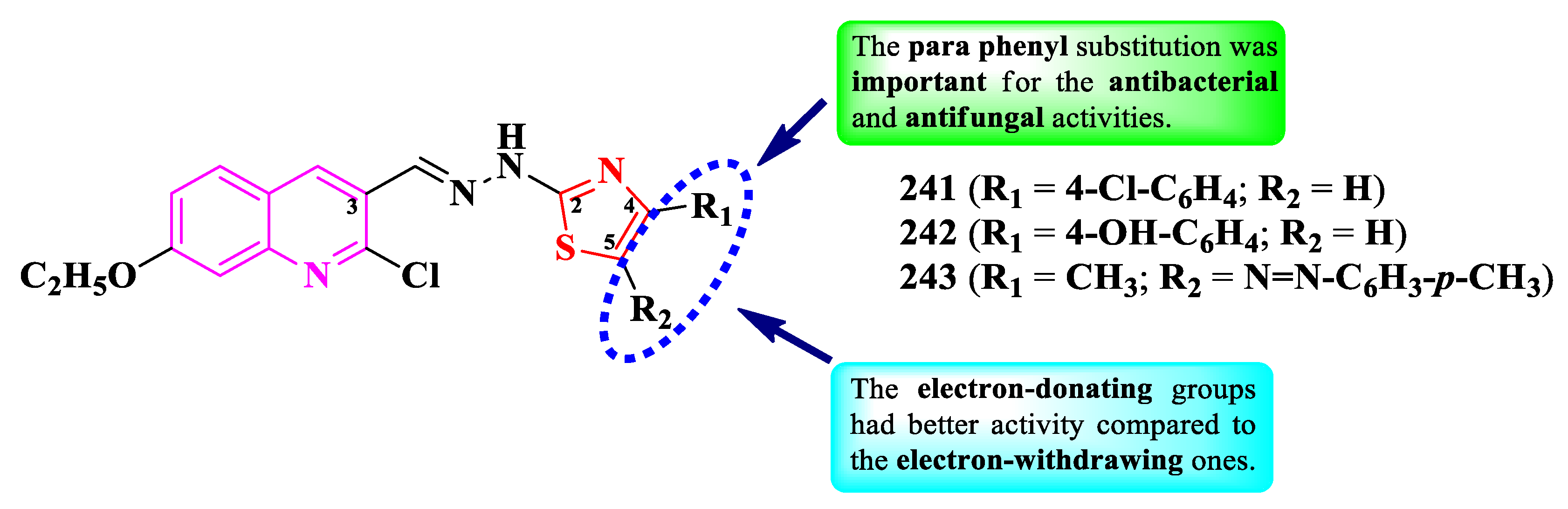
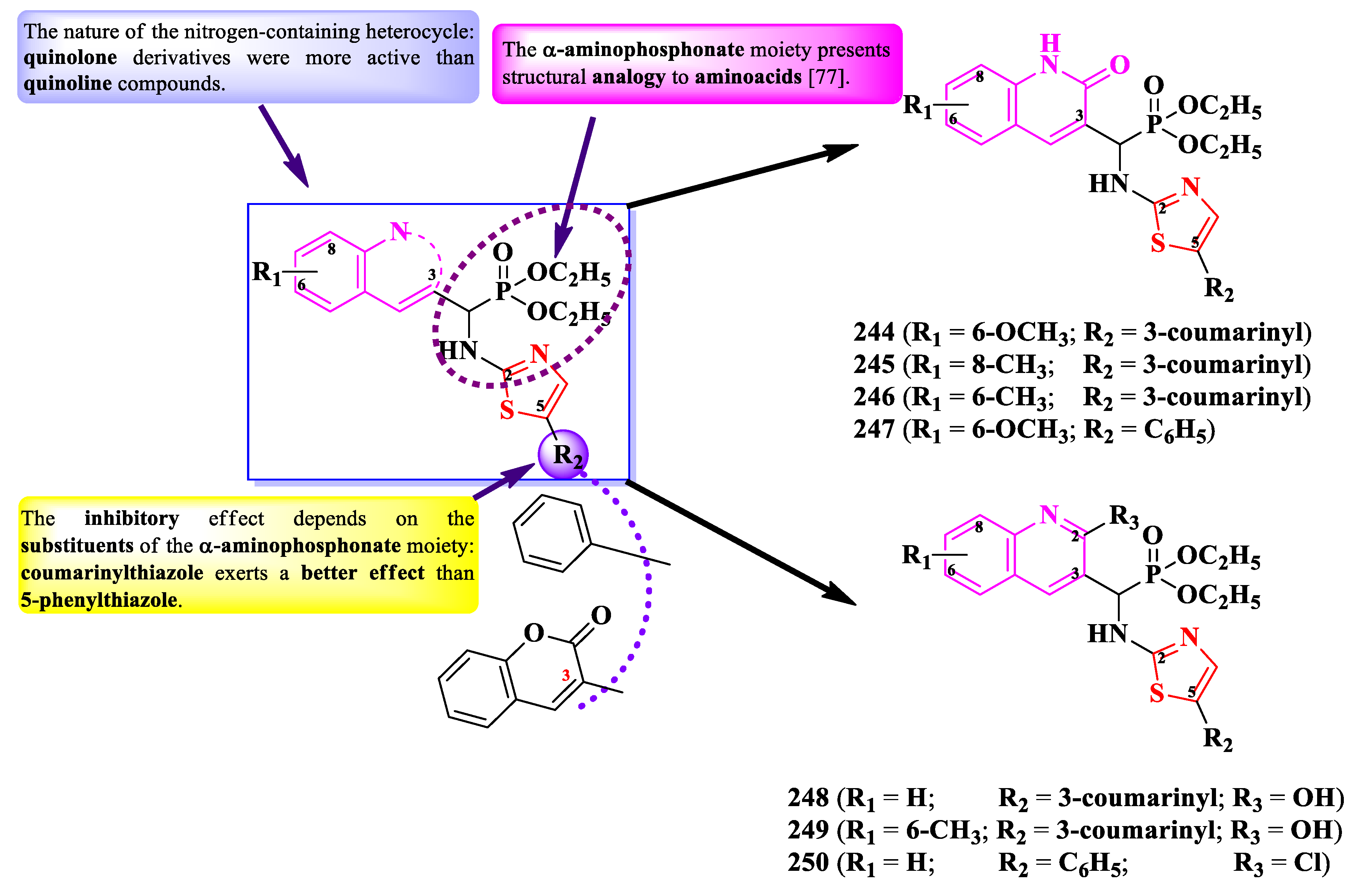
2.5.3. Thiazolyl-Quinoline and Thiazolyl-Quinolone Hybrid Compounds
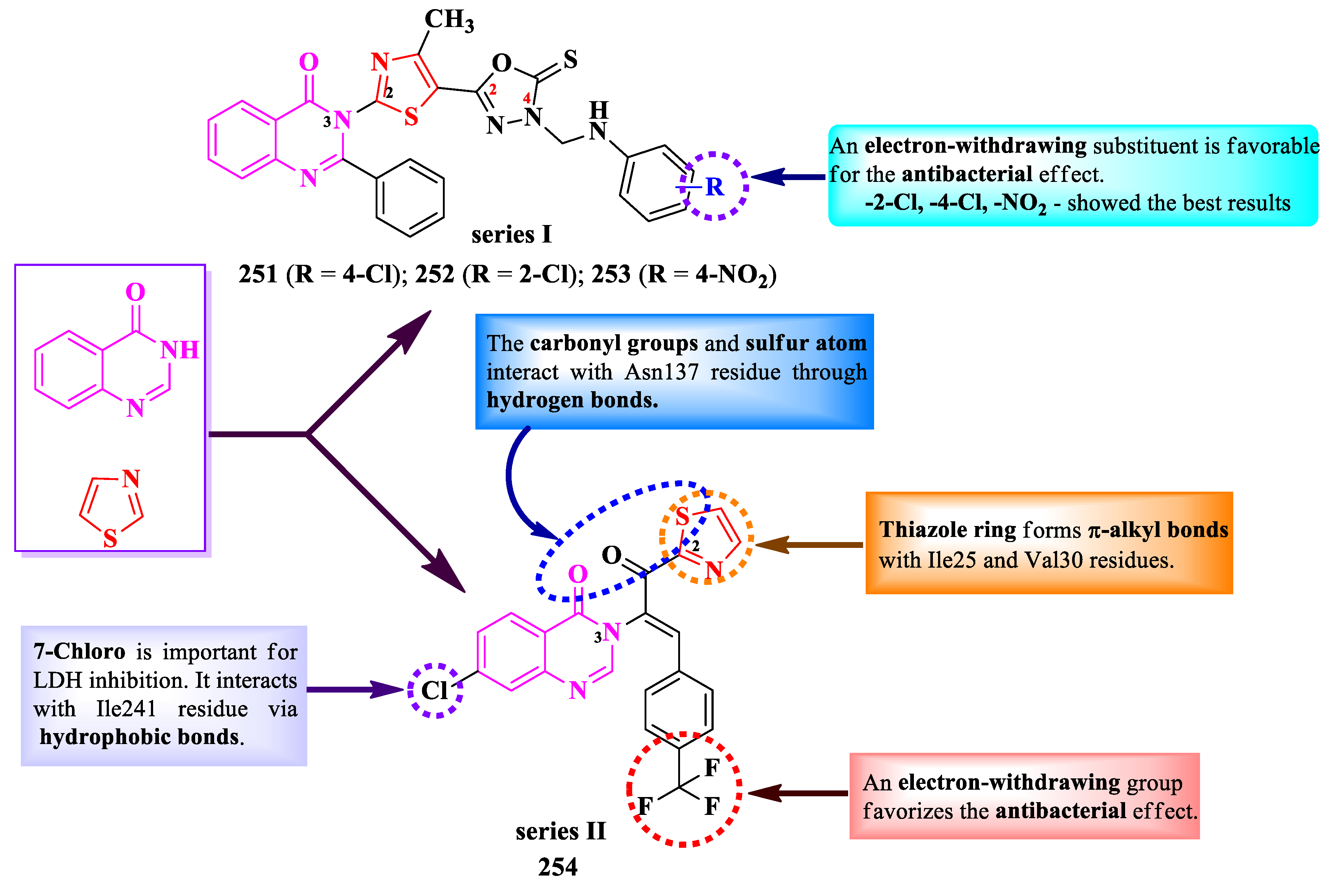
2.5.4. Thiazolyl-Quinazolones Hybrid Compounds
2.6. Thiazole Clubbed with Condensed Heterocycles
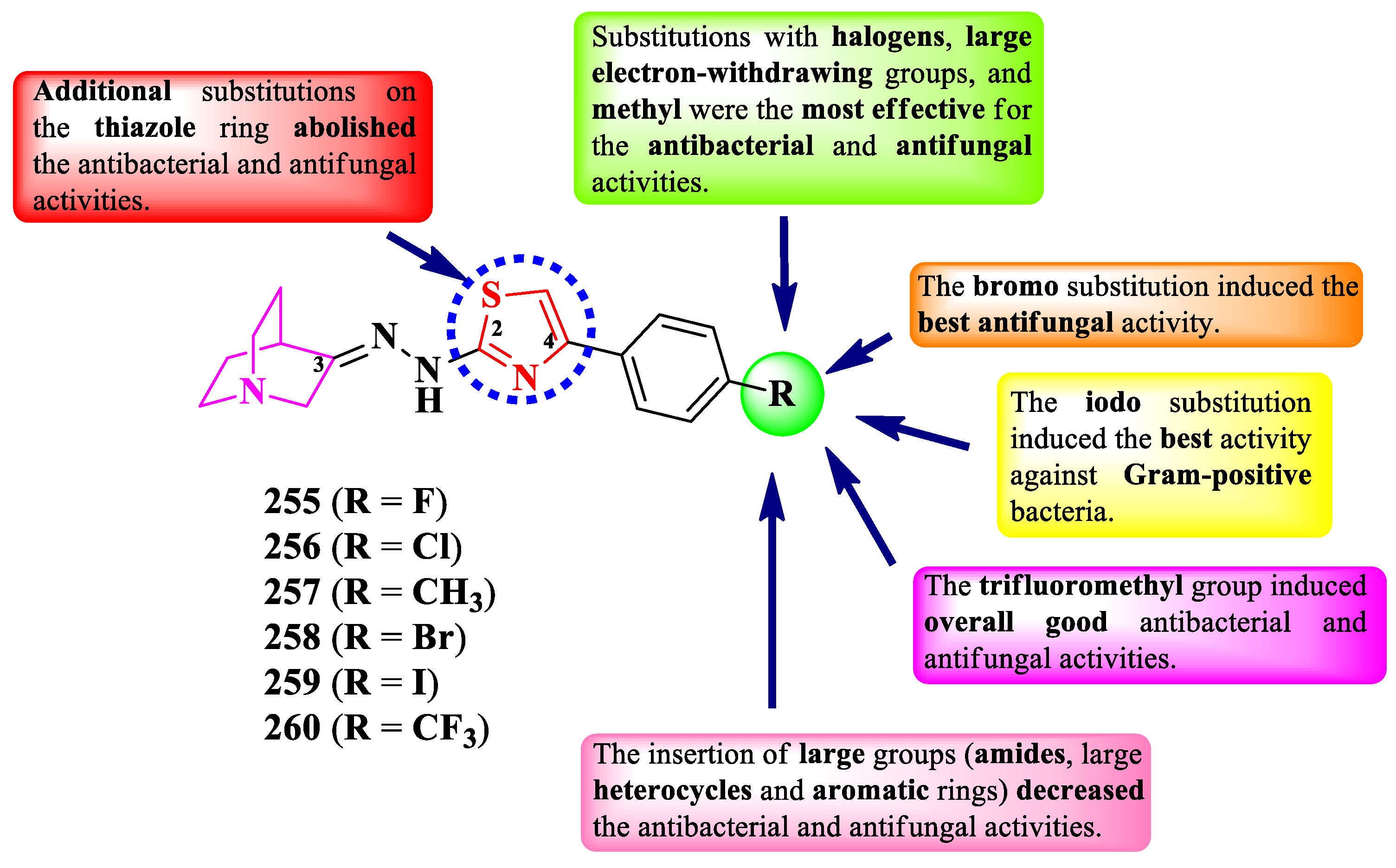
2.6.1. Thiazolyl-Quinuclidine Hybrid Compounds
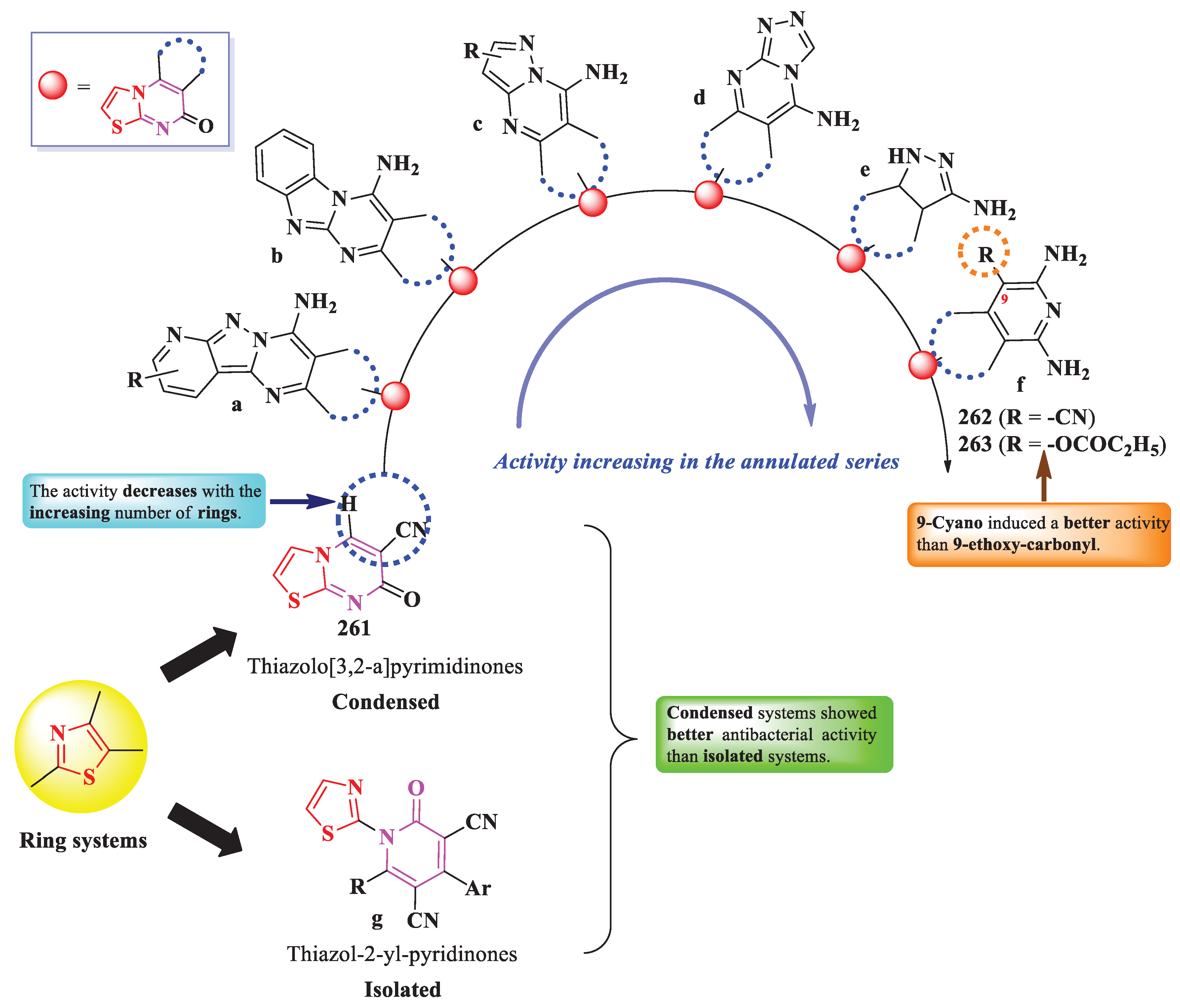
2.6.2. Thiazole Clubbed with Polyheterocyclic Systems
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allel, K.; Day, L.; Hamilton, A.; Lin, L.; Furuya-Kanamori, L.; Moore, C.E.; Van Boeckel, T.; Laxminarayan, R.; Yakob, L. Global antimicrobial-resistance drivers: an ecological country-level study at the human–animal interface. Lancet Planet. Heal. 2023, 7, e291–e303. [Google Scholar] [CrossRef] [PubMed]
- Walesch, S.; Birkelbach, J.; Jézéquel, G.; Haeckl, F.P.J.; Hegemann, J.D.; Hesterkamp, T.; Hirsch, A.K.H.; Hammann, P.; Müller, R. Fighting antibiotic resistance—strategies and (pre)clinical developments to find new antibacterials. EMBO Rep. 2023, 24, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Borcea, A.-M.; Ionuț, I.; Crișan, O.; Oniga, O. An Overview of the Synthesis and Antimicrobial, Antiprotozoal, and Antitumor Activity of Thiazole and Bisthiazole Derivatives. Molecules 2021, 26, 624. [Google Scholar] [CrossRef] [PubMed]
- Mishra, I.; Mishra, R.; Mujwar, S.; Chandra, P.; Sachan, N. A retrospect on antimicrobial potential of thiazole scaffold. J. Heterocycl. Chem. 2020, 57, 2304–2329. [Google Scholar] [CrossRef]
- Petrou, A.; Fesatidou, M.; Geronikaki, A. Thiazole ring—a biologically active scaffold. Molecules 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, H.C. Coumarin-1,2,3-triazole Hybrid Molecules: An Emerging Scaffold for Combating Drug Resistance. Curr. Top. Med. Chem. 2021, 21, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, B.; Almendros, P.; Aragoncillo, C. Highly reactive 4-membered ring nitrogen-containing heterocycles: Synthesis and properties. Curr. Opin. Drug Discov. Dev. 2010, 13, 685–697. [Google Scholar]
- Desai, N.C.; Harsora, J.P.; Monapara, J.D.; Khedkar, V.M. Synthesis, Antimicrobial Capability and Molecular Docking of Heterocyclic Scaffolds Clubbed by 2-Azetidinone, Thiazole and Quinoline Derivatives. Polycycl. Aromat. Compd. 2021, 0, 1–15. [Google Scholar] [CrossRef]
- Kumar, S.; Bawa, S.; Drabu, S.; Kumar, R.; Gupta, H. Biological Activities of Pyrazoline Derivatives -A Recent Development. Recent Pat. Antiinfect. Drug Discov. 2009, 4, 154–163. [Google Scholar] [CrossRef]
- Cuartas, V.; Robledo, S.M.; Vélez, I.D.; Crespo, M. del P.; Sortino, M.; Zacchino, S.; Nogueras, M.; Cobo, J.; Upegui, Y.; Pineda, T.; et al. New thiazolyl-pyrazoline derivatives bearing nitrogen mustard as potential antimicrobial and antiprotozoal agents. Arch. Pharm. 2020; 353. [Google Scholar] [CrossRef]
- Rashdan, H.R.M.; Abdelmonsef, A.H. Towards Covid-19 TMPRSS2 enzyme inhibitors and antimicrobial agents: Synthesis, antimicrobial potency, molecular docking, and drug-likeness prediction of thiadiazole-triazole hybrids. J. Mol. Struct. 2022, 1268, 133659. [Google Scholar] [CrossRef]
- Budak, Y.; Kocyigit, U.M.; Gürdere, M.B.; Özcan, K.; Taslimi, P.; Gülçin, İ.; Ceylan, M. Synthesis and investigation of antibacterial activities and carbonic anhydrase and acetyl cholinesterase inhibition profiles of novel 4,5-dihydropyrazol and pyrazolyl-thiazole derivatives containing methanoisoindol-1,3-dion unit. Synth. Commun. 2017, 47, 2313–2323. [Google Scholar] [CrossRef]
- Mansour, E.; Aboelnaga, A.; Nassar, E.M.; Elewa, S.I. A new series of thiazolyl pyrazoline derivatives linked to benzo[1,3]dioxole moiety: Synthesis and evaluation of antimicrobial and anti-proliferative activities. Synth. Commun. 2020, 50, 368–379. [Google Scholar] [CrossRef]
- Masoud, D.M.; Azzam, R.A.; Hamdy, F.; Mekawey, A.A.I.; Abdel-Aziz, H.A. Synthesis of Some Novel Pyrazoline-Thiazole Hybrids and Their Antimicrobial Activities. J. Heterocycl. Chem. 2019, 56, 3030–3041. [Google Scholar] [CrossRef]
- Bhandare, R.R.; Munikrishnappa, C.S.; Suresh Kumar, G.V.; Konidala, S.K.; Sigalapalli, D.K.; Vaishnav, Y.; Chinnam, S.; Yasin, H.; Al-karmalawy, A.A.; Shaik, A.B. Multistep synthesis and screening of heterocyclic tetrads containing furan, pyrazoline, thiazole and triazole (or oxadiazole) as antimicrobial and anticancer agents. J. Saudi Chem. Soc. 2022, 26, 101447. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Khidre, R.E.; Mohamed, H.A.; El-Hiti, G.A. A Simple Process for the Synthesis of Novel Pyrazolyltriazole and Dihydropyrazolylthiazole Derivatives as Antimicrobial Agents. Arab. J. Sci. Eng. 2017, 42, 2441–2448. [Google Scholar] [CrossRef]
- Bondock, S.; Fouda, A.M. Synthesis and evaluation of some new 5-(hetaryl)thiazoles as potential antimicrobial agents. Synth. Commun. 2018, 48, 561–573. [Google Scholar] [CrossRef]
- Vijesh, A.M.; Isloor, A.M.; Isloor, S.; Shivananda, K.N.; Shyma, P.C.; Arulmoli, T. Synthesis of some new pyrazolone derivatives as potent antimicrobial agents. Der Pharma Chem. 2011, 3, 454–463. [Google Scholar]
- Abu-Melha, S. Molecular modeling and docking studies of new antimicrobial antipyrine-thiazole hybrids. Arab. J. Chem. 2022, 15, 103898. [Google Scholar] [CrossRef]
- Jamwal, A.; Javed, A.; Bhardwaj, V. A review on Pyrazole derivatives of pharmacological potential. J. Pharm. BioSci 2013, 3, 114–123. [Google Scholar]
- Gondru, R.; Sirisha, K.; Raj, S.; Gunda, S.K.; Kumar, C.G.; Pasupuleti, M.; Bavantula, R. Design, Synthesis, In Vitro Evaluation and Docking Studies of Pyrazole-Thiazole Hybrids as Antimicrobial and Antibiofilm Agents. ChemistrySelect 2018, 3, 8270–8276. [Google Scholar] [CrossRef]
- Patil, S.V.; Suryavanshi, M.B.; Nagargoje, D.R.; Kokate, S.V. Synthesis and Antimicrobial Evaluation of Some New Pyrazole Derivatives Containing Thiazole Scaffolds. In Proceedings of the ECSOC-25; MDPI: Basel Switzerland, noiembrie 15 2021; Vol. 17, p. 46.
- Abdel-Aziem, A.; Baaiu, B.S.; Elbazzar, A.W.; Elabbar, F. A facile synthesis of some novel thiazoles, arylazothiazoles, and pyrazole linked to thiazolyl coumarin as antibacterial agents. Synth. Commun. 2020, 50, 2522–2530. [Google Scholar] [CrossRef]
- Kumar, S.; Saini, V.; Maurya, I.K.; Sindhu, J.; Kumari, M.; Kataria, R.; Kumar, V. Design, synthesis, DFT, docking studies and ADME prediction of some new coumarinyl linked pyrazolylthiazoles: Potential standalone or adjuvant antimicrobial agents. PLoS ONE 2018, 13, e0196016. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.O.; Ghodsi, S. Thiazolyl-pyrazole-biscoumarin synthesis and evaluation of their antibacterial and antioxidant activities. Res. Chem. Intermed. 2017, 43, 661–678. [Google Scholar] [CrossRef]
- Nalawade, J.; Shinde, A.; Chavan, A.; Patil, S.; Suryavanshi, M.; Modak, M.; Choudhari, P.; Bobade, V.D.; Mhaske, P.C. Synthesis of new thiazolyl-pyrazolyl-1,2,3-triazole derivatives as potential antimicrobial agents. Eur. J. Med. Chem. 2019, 179, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Kant, V. A Review on Biological Activity of Imidazole and Thiazole Moieties and their Derivatives. Sci. Int. 2013, 1, 253–260. [Google Scholar] [CrossRef]
- Nikalje, A.P.G.; Tiwari, S.V.; Sarkate, A.P.; Karnik, K.S. Imidazole-thiazole coupled derivatives as novel lanosterol 14-α demethylase inhibitors: ionic liquid mediated synthesis, biological evaluation and molecular docking study. Med. Chem. Res. 2018, 27, 592–606. [Google Scholar] [CrossRef]
- Jain, A.K.; Vaidya, A.; Ravichandran, V.; Kashaw, S.K.; Agrawal, R.K. Recent developments and biological activities of thiazolidinone derivatives: A review. Bioorganic Med. Chem. 2012, 20, 3378–3395. [Google Scholar] [CrossRef]
- Othman, D.I.A.; Hamdi, A.; Abdel-Aziz, M.M.; Elfeky, S.M. Novel 2-arylthiazolidin-4-one-thiazole hybrids with potent activity against Mycobacterium tuberculosis. Bioorg. Chem. 2022, 124, 105809. [Google Scholar] [CrossRef] [PubMed]
- Abo-Ashour, M.F.; Eldehna, W.M.; George, R.F.; Abdel-Aziz, M.M.; Elaasser, M.M.; Abou-Seri, S.M.; Abdel Gawad, N.M. Synthesis and Biological Evaluation of 2-Aminothiazole-Thiazolidinone Conjugates as Potential Antitubercular Agents. Future Med. Chem. 2018, 10, 1405–1419. [Google Scholar] [CrossRef]
- Sucheta; Tahlan, S.; Verma, P.K. Biological potential of thiazolidinedione derivatives of synthetic origin. Chem. Cent. J. 2017, 11, 1–29. [Google Scholar] [CrossRef]
- Alegaon, S.G.; U, V.; Alagawadi, K.R.; Kumar, D.; Kavalapure, R.S.; Ranade, S.D.; Priya A, S.; Jalalpure, S.S. Synthesis, Molecular Docking and ADME Studies of Thiazole-Thiazolidinedione Hybrids as Antimicrobial Agents. J. Biomol. Struct. Dyn. 2022, 40, 6211–6227. [Google Scholar] [CrossRef] [PubMed]
- Bhuva, H.; Sahu, D.; Shah, B.; Modi, C.; Patel, M.B. Biological Profile of Thiadiazole. Pharmacologyonline 2011, 1, 528–543. [Google Scholar]
- Stokes, J.M.; Yang, K.; Swanson, K.; Jin, W.; Cubillos-Ruiz, A.; Donghia, N.M.; MacNair, C.R.; French, S.; Carfrae, L.A.; Bloom-Ackerman, Z.; et al. A Deep Learning Approach to Antibiotic Discovery. Cell 2020, 180, 688–702.e13. [Google Scholar] [CrossRef]
- Booq, R.Y.; Tawfik, E.A.; Alfassam, H.A.; Alfahad, A.J.; Alyamani, E.J. Assessment of the Antibacterial Efficacy of Halicin against Pathogenic Bacteria. Antibiotics 2021, 10, 1480. [Google Scholar] [CrossRef]
- Hussain, Z.; Pengfei, S.; Yimin, L.; Shasha, L.; Zehao, L.; Yifan, Y.; Linhui, L.; Linying, Z.; Yong, W. Study on antibacterial effect of halicin (SU3327) against Enterococcus faecalis and Enterococcus faecium. Pathog. Dis. 2022, 80, ftac037. [Google Scholar] [CrossRef]
- Higashihira, S.; Simpson, S.J.; Collier, C.D.; Natoli, R.M.; Kittaka, M.; Greenfield, E.M. Halicin Is Effective Against Staphylococcus aureus Biofilms In Vitro. Clin. Orthop. Relat. Res. 2022, 480, 1476–1487. [Google Scholar] [CrossRef] [PubMed]
- van Gent, M.E.; van der Reijden, T.J.K.; Lennard, P.R.; de Visser, A.W.; Schonkeren-Ravensbergen, B.; Dolezal, N.; Cordfunke, R.A.; Drijfhout, J.W.; Nibbering, P.H. Synergism between the Synthetic Antibacterial and Antibiofilm Peptide (SAAP)-148 and Halicin. Antibiotics 2022, 11, 1–15. [Google Scholar] [CrossRef]
- Asif, M. Pharmacological activities of Triazole analogues as antibacterial, antifungal, antiviral agents. Pharm. Sci. Asia 2017, 44, 59–74. [Google Scholar] [CrossRef]
- Shinde, V.; Mahulikar, P.; Mhaske, P.C.; Chakraborty, S.; Choudhari, A.; Phalle, S.; Choudhari, P.; Sarkar, D. Synthesis and antimycobacterial evaluation of new 5-(1-benzyl-1H-1,2,3-triazol-4-yl)-4-methyl-2-arylthiazole derivatives. Med. Chem. Res. 2019, 28, 805–819. [Google Scholar] [CrossRef]
- Mahale, K.A.; Gosavi, K.S.; Gaikwad, N.D.; Bholay, A.D.; Patil, S.V. Thiazole Substituted [1,2,3] Triazole: Synthesis and Antimicrobial Evaluation. Indian J. Chem. 2022, 61, 640–649. [Google Scholar] [CrossRef]
- Jagadale, S.; Chavan, A.; Shinde, A.; Sisode, V.; Bobade, V.D.; Mhaske, P.C. Synthesis and antimicrobial evaluation of new thiazolyl-1,2,3-triazolyl-alcohol derivatives. Med. Chem. Res. 2020, 29, 989–999. [Google Scholar] [CrossRef]
- Poonia, N.; Lal, K.; Kumar, A.; Kumar, A.; Sahu, S.; Baidya, A.T.K.; Kumar, R. Urea-Thiazole/Benzothiazole Hybrids with a Triazole Linker: Synthesis, Antimicrobial Potential, Pharmacokinetic Profile and in Silico Mechanistic Studies. Mol. Divers. 2022, 26, 2375–2391. [Google Scholar] [CrossRef] [PubMed]
- Gondru, R.; Kanugala, S.; Raj, S.; Ganesh Kumar, C.; Pasupuleti, M.; Banothu, J.; Bavantula, R. 1,2,3-triazole-thiazole hybrids: Synthesis, in vitro antimicrobial activity and antibiofilm studies. Bioorganic Med. Chem. Lett. 2021, 33, 127746. [Google Scholar] [CrossRef] [PubMed]
- Glomb, T.; Świątek, P. Antimicrobial Activity of 1,3,4-Oxadiazole Derivatives. Int. J. Mol. Sci. 2021, 22, 6979. [Google Scholar] [CrossRef] [PubMed]
- Glomb, T.; Szymankiewicz, K.; Świątek, P. Anti-cancer activity of derivatives of 1,3,4-oxadiazole. Molecules 2018, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.M.; Comin, M.; Duarte, T.S.; Foglio, M.A.; De Carvalho, J.E.; Do Carmo Vieira, M.; Formagio, A.S.N. Synthesis, antiproliferative activity and molecular properties predictions of galloyl derivatives. Molecules 2015, 20, 5360–5373. [Google Scholar] [CrossRef] [PubMed]
- Savariz, F.C.; Formagio, A.S.N.; Barbosa, V.A.; Foglio, M.A.; de Carvalho, J.E.; Duarte, M.C.T.; Dias Filho, B.P.; Sarragiotto, M.H. Synthesis, antitumor and antimicrobial activity of novel 1-substituted phenyl-3-[3-alkylamino(methyl)-2-thioxo-1,3,4-oxadiazol-5-yl] β-carboline derivatives. J. Braz. Chem. Soc. 2010, 21, 288–298. [Google Scholar] [CrossRef]
- Athar Abbasi, M.; Raza, H.; Aziz-ur-Rehman; Zahra Siddiqui, S.; Adnan Ali Shah, S.; Hassan, M.; Seo, S.Y. Synthesis of novel N-(1,3-thiazol-2-yl)benzamide clubbed oxadiazole scaffolds: Urease inhibition, Lipinski rule and molecular docking analyses. Bioorg. Chem. 2019, 83, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.C.; Bhatt, N.B.; Joshi, S.B. Synthetic modifications in ethyl 2-amino-4-methylthiazole-5-carboxylate: 3D QSAR analysis and antimicrobial study. Synth. Commun. 2019, 49, 1055–1066. [Google Scholar] [CrossRef]
- Tiperciuc, B.G. Design and development of new azoles heterocycles with biological potential [habilitation thesis]. [Cluj-Napoca: Romania]: “Iuliu Hațieganu” University of Medicine and Pharmacy; 2021. Chapter 1.6.1., Thiazol-5-yl-azoles-5-thiones; pp. 45–7.
- Meanwell, N.A. Chapter Five - A Synopsis of the Properties and Applications of Heteroaromatic Rings in Medicinal Chemistry. In: Advances in Heterocyclic Chemistry; Scriven, E.F. V, Ramsden, C.A.B.T.-A. in H.C., Ed.; Academic Press, 2017; Vol. 123, pp. 245–361 ISBN 0065-2725.
- Patil, S.A.; Patil, S.A.; Ble-González, E.A.; Isbel, S.R.; Hampton, S.M.; Bugarin, A. Carbazole Derivatives as Potential Antimicrobial Agents. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Hanafy, M.S.; Matter, M.A.; Asker, M.S.; Rady, M.R. Production of indole alkaloids in hairy root cultures of Catharanthus roseus L. and their antimicrobial activity. South African J. Bot. 2016, 105, 9–18. [Google Scholar] [CrossRef]
- Yu, H.F.; Qin, X.J.; Ding, C.F.; Wei, X.; Yang, J.; Luo, J.R.; Liu, L.; Khan, A.; Zhang, L.C.; Xia, C.F.; et al. Nepenthe-Like Indole Alkaloids with Antimicrobial Activity from Ervatamia chinensis. Org. Lett. 2018, 20, 4116–4120. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Ding, C.F.; Deng, S.Y.; Gao, W.; Tan, B.Y.; Wu, H.; Guo, Y.; Song, J.F.; Zhang, L.C.; Zhang, R.P.; et al. Monoterpene indole N-oxide alkaloids from Tabernaemontana corymbosa and their antimicrobial activity. Fitoterapia 2022, 158, 105178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, Y.; Li, Y.; Chen, Y. A green synthesis and antibacterial activity of ferrocene-based thiazole derivatives in choline chloride/glycerol eutectic solvent. RSC Adv. 2022, 12, 22054–22059. [Google Scholar] [CrossRef] [PubMed]
- Ashok, D.; Gundu, S.; Aamate, V.K.; Devulapally, M.G. Microwave-assisted synthesis, antioxidant and antimicrobial evaluation of 2-indolinone-based bis-1,2,3-triazole derivatives. Mol. Divers. 2018, 22, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.Y.; Ammar, Y.A.; Abu-Elghait, M.; Salem, M.A.; Assiri, M.A.; Ali, T.E.; Ragab, A. Development of novel indolin-2-one derivative incorporating thiazole moiety as DHFR and quorum sensing inhibitors: Synthesis, antimicrobial, and antibiofilm activities with molecular modelling study. Bioorg. Chem. 2022, 119, 105571. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.W.; Zhao, H.Y.; Zhu, H.; Peng, C.; Zhou, Q.M.; Xiong, L. Novel Indane Derivatives with Antioxidant Activity from the Roots of Anisodus tanguticus. Molecules 2023, 28, 1–10. [Google Scholar] [CrossRef]
- Obafemi, C.A.; Adelani, P.O.; Fadare, O.A.; Akinpelu, D.A.; Famuyiwa, S.O. Synthesis, crystal structure and in vitro antibacterial activity of 2,3a,8b-trihydroxy-3-(thiophen-2-ylcarbonyl)-2-(trifluoromethyl)-2,3,3a, 8b-tetrahydro-4H-indeno[1,2-b]furan-4-one. J. Mol. Struct. 2013, 1049, 429–435. [Google Scholar] [CrossRef]
- Adole, V.A.; More, R.A.; Jagdale, B.S.; Pawar, T.B.; Chobe, S.S. Efficient Synthesis, Antibacterial, Antifungal, Antioxidant and Cytotoxicity Study of 2-(2-Hydrazineyl)thiazole Derivatives. ChemistrySelect 2020, 5, 2778–2786. [Google Scholar] [CrossRef]
- Muluk, M.B.; Phatak, P.S.; Pawar, S.B.; Dhumal, S.T.; Rehman, N.N.M.A.; Dixit, P.P.; Choudhari, P.B.; Haval, K.P. Synthesis, antimicrobial, and antioxidant activities of new pyridyl- and thiazolyl-bearing carbohydrazides. J. Chinese Chem. Soc. 2019, 66, 1507–1517. [Google Scholar] [CrossRef]
- Muluk, M.B.; Ubale, A.S.; Dhumal, S.T.; Rehman, N.N.M.A.; Dixit, P.P.; Kharat, K.K.; Choudhari, P.B.; Haval, K.P. Synthesis, anticancer and antimicrobial evaluation of new pyridyl and thiazolyl clubbed hydrazone scaffolds. Synth. Commun. 2020, 50, 243–255. [Google Scholar] [CrossRef]
- Patil, P.S.; Kasare, S.L.; Badar, A.D.; Kulkarni, R.S.; Dixit, P.P.; Kulkarni, J.A.; Choudhari, P.B.; Haval, K.P. Synthesis, Antimicrobial Evaluation, and Molecular Docking Study of New Thiazole-5-phenylpropenone Derivatives. Russ. J. Gen. Chem. 2020, 90, 1523–1528. [Google Scholar] [CrossRef]
- Eryılmaz, S.; Türk Çelikoğlu, E.; İdil, Ö.; İnkaya, E.; Kozak, Z.; Mısır, E.; Gül, M. Derivatives of Pyridine and Thiazole Hybrid: Synthesis, DFT, Biological Evaluation via Antimicrobial and DNA Cleavage Activity. Bioorg. Chem. 2020, 95. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, M.; Popa, C.V. Pyridine Compounds with Antimicrobial and Antiviral Activities. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Al-Majedy, Y.K.; Kadhum, A.A.H.; Al-Amiery, A.A.; Mohamad, A.B. Coumarins: The antimicrobial agents. Syst. Rev. Pharm. 2016, 8, 62–70. [Google Scholar] [CrossRef]
- Yusufzai, S.K.; Osman, H.; Khan, M.S.; Mohamad, S.; Sulaiman, O.; Parumasivam, T.; Gansau, J.A.; Johansah, N. Noviany Design, characterization, in vitro antibacterial, antitubercular evaluation and structure–activity relationships of new hydrazinyl thiazolyl coumarin derivatives. Med. Chem. Res. 2017, 26, 1139–1148. [Google Scholar] [CrossRef]
- Salar, U.; Qureshi, B.; Khan, K.M.; Lodhi, M.A.; Ul-Haq, Z.; Khan, F.A.; Naz, F.; Taha, M.; Perveen, S.; Hussain, S. Aryl hydrazones linked thiazolyl coumarin hybrids as potential urease inhibitors. J. Iran. Chem. Soc. 2022, 19, 1221–1238. [Google Scholar] [CrossRef]
- Hu, Y.; Hu, C.; Pan, G.; Yu, C.; Ansari, M.F.; Yadav Bheemanaboina, R.R.; Cheng, Y.; Zhou, C.; Zhang, J. Novel chalcone-conjugated, multi-flexible end-group coumarin thiazole hybrids as potential antibacterial repressors against methicillin-resistant Staphylococcus aureus. Eur. J. Med. Chem. 2021, 222, 113628. [Google Scholar] [CrossRef]
- Leonte, D.; Ungureanu, D.; Zaharia, V. Flavones and Related Compounds: Synthesis and Biological Activity. Molecules 2023, 28, 6528. [Google Scholar] [CrossRef]
- Zhao, G.; Lan, D.; Qi, G. Design and development of some thiazole-based flavanoids as novel antibacterial against pathogens causing surgical site infection for possible benefit in bone trauma via inhibition of DNA gyrase. Chem. Biol. Drug Des. 2017, 90, 778–790. [Google Scholar] [CrossRef]
- Kumar, S.; Bawa, S.; Gupta, H. Biological Activities of Quinoline Derivatives. Mini-Reviews Med. Chem. 2010, 9, 1648–1654. [Google Scholar] [CrossRef] [PubMed]
- Ammar, Y.A.; El-Hafez, S.M.A.A.; Hessein, S.A.; Ali, A.M.; Askar, A.A.; Ragab, A. One-Pot Strategy for Thiazole Tethered 7-Ethoxy Quinoline Hybrids: Synthesis and Potential Antimicrobial Agents as Dihydrofolate Reductase (DHFR) Inhibitors with Molecular Docking Study. J. Mol. Struct. 2021, 1242. [Google Scholar] [CrossRef]
- Litim, B.; Djahoudi, A.; Meliani, S.; Boukhari, A. Synthesis and potential antimicrobial activity of novel α-aminophosphonates derivatives bearing substituted quinoline or quinolone and thiazole moieties. Med. Chem. Res. 2022, 31, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Alsibaee, A.M.; Al-Yousef, H.M.; Al-Salem, H.S. Quinazolinones, the Winning Horse in Drug Discovery. Molecules 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Shihory, N.; Khasiya, A.; Pandit, U.; Khedkar, V. Quinazoline clubbed thiazole and 1,3,4-oxadiazole heterocycles: synthesis, characterization, antibacterial evaluation, and molecular docking studies. Phosphorus, Sulfur Silicon Relat. Elem. 2021, 196, 569–577. [Google Scholar] [CrossRef]
- Wang, J.; Ansari, M.F.; Zhou, C.H. Identification of Unique Quinazolone Thiazoles as Novel Structural Scaffolds for Potential Gram-Negative Bacterial Conquerors. J. Med. Chem. 2021, 64, 7630–7645. [Google Scholar] [CrossRef] [PubMed]
- Łączkowski, K.Z.; Landowska, K.; Biernasiuk, A.; Sałat, K.; Furgała, A.; Plech, T.; Malm, A. Synthesis, biological evaluation and molecular docking studies of novel quinuclidinone derivatives as potential antimicrobial and anticonvulsant agents. Med. Chem. Res. 2017, 26, 2088–2104. [Google Scholar] [CrossRef]
- Abdel-Latif, E.; Almatari, A.S.; Abd-ElGhani, G.E. Synthesis and Antibacterial Evaluation of Some New Thiazole-Based Polyheterocyclic Ring Systems. J. Heterocycl. Chem. 2019, 56, 1978–1985. [Google Scholar] [CrossRef]
- Radman Kastelic, A.; Odžak, R.; Pezdirc, I.; Sović, K.; Hrenar, T.; Čipak Gašparović, A.; Skočibušić, M.; Primožič, I. New and Potent Quinuclidine-Based Antimicrobial Agents. Molecules 2019, 24, 1–17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




