Submitted:
05 December 2023
Posted:
06 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment
2.2. Relative Root Length
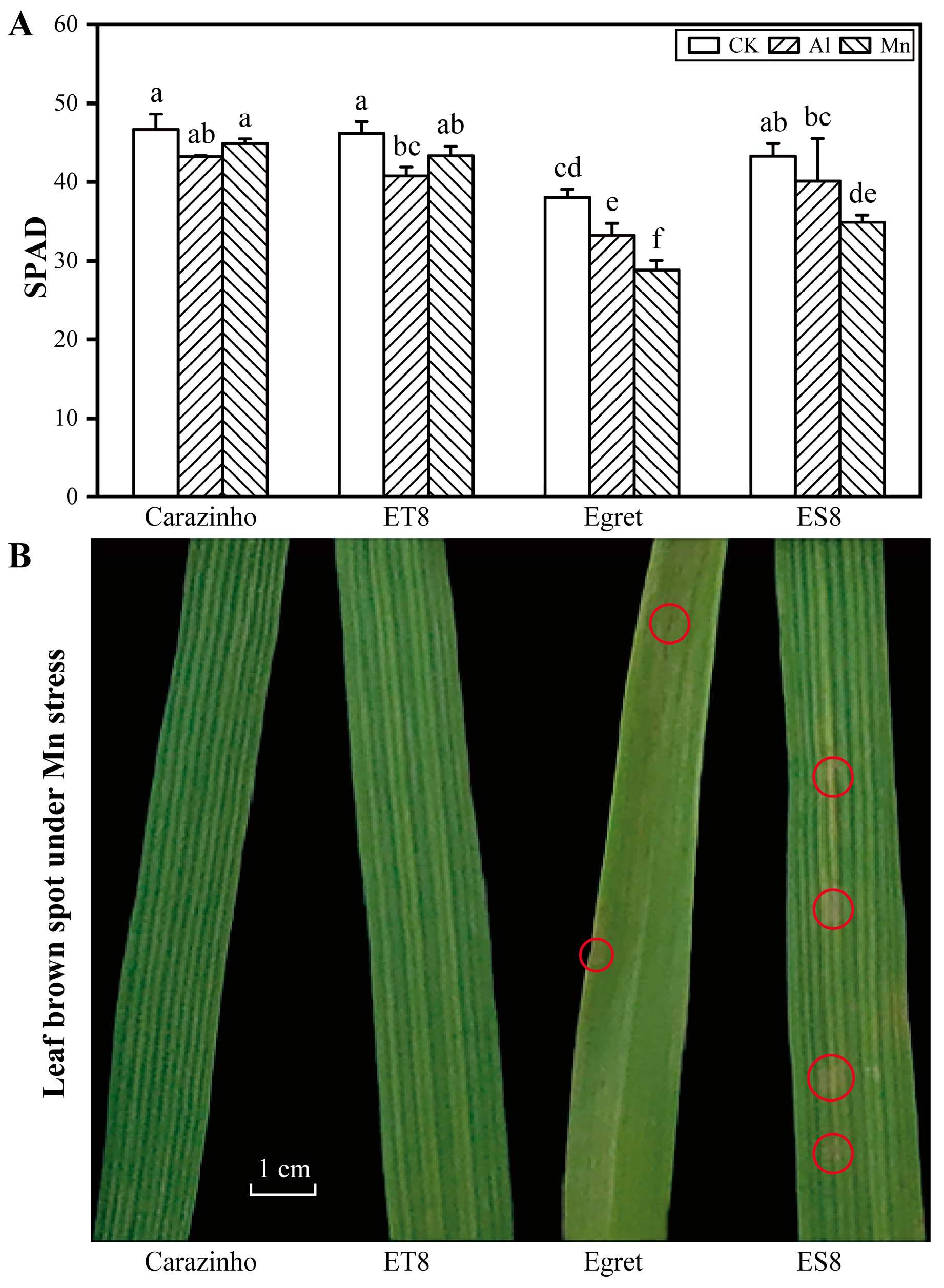
2.3. SPAD and Brown Spots
2.4. Metal Ions Accumulation and Subcellular Distribution
2.5. RNA Extraction, Library Preparation, Sequencing, and Read Mapping
2.6. Differential Expression and Functional Enrichment Analysis.
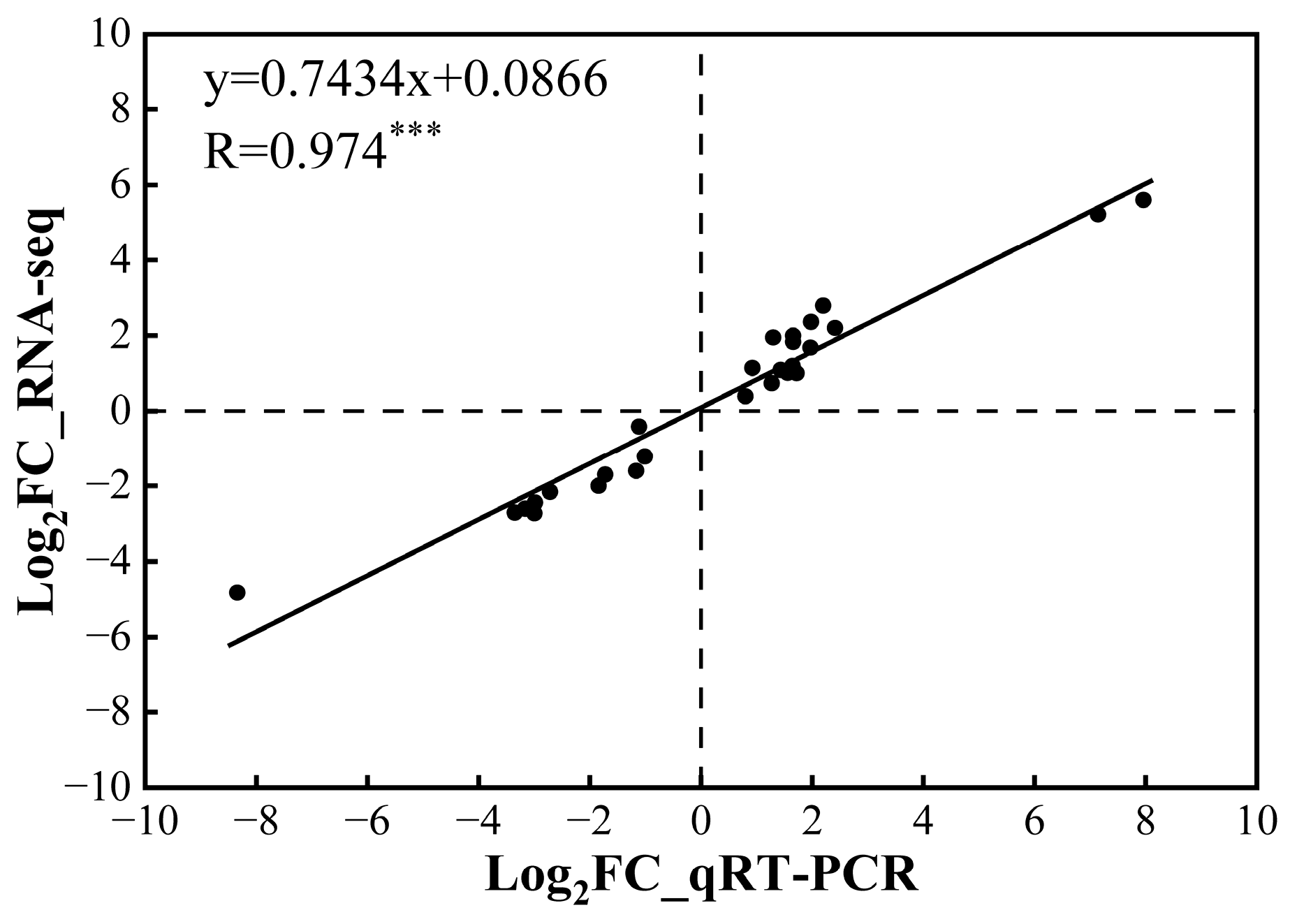
2.7. Quantitative Real-Time PCR (qRT-PCR) Validation.
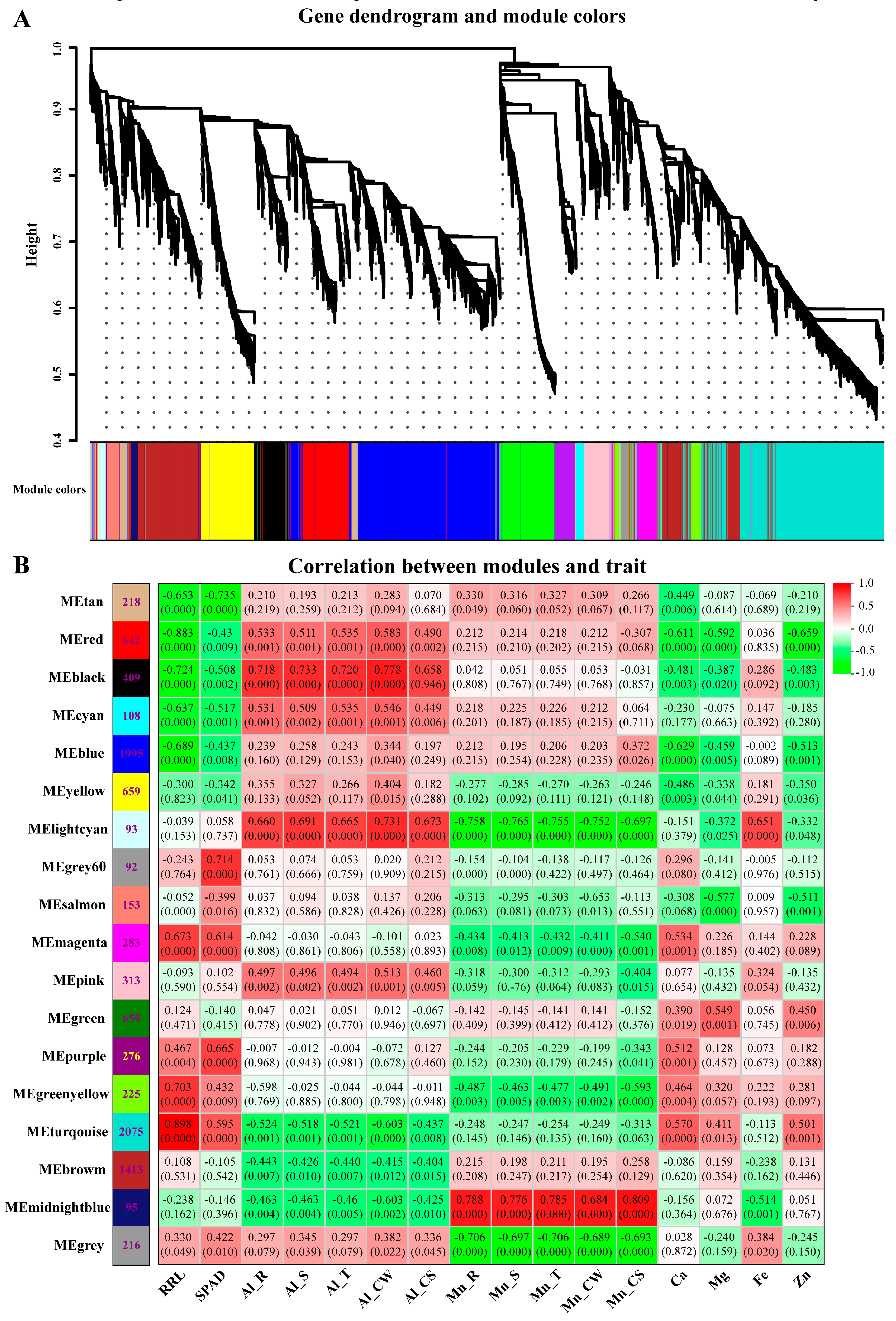
2.8. Weighted Gene Co-Expression Network Analysis (WGCNA)
2.9. Statistical Analysis
3. Results
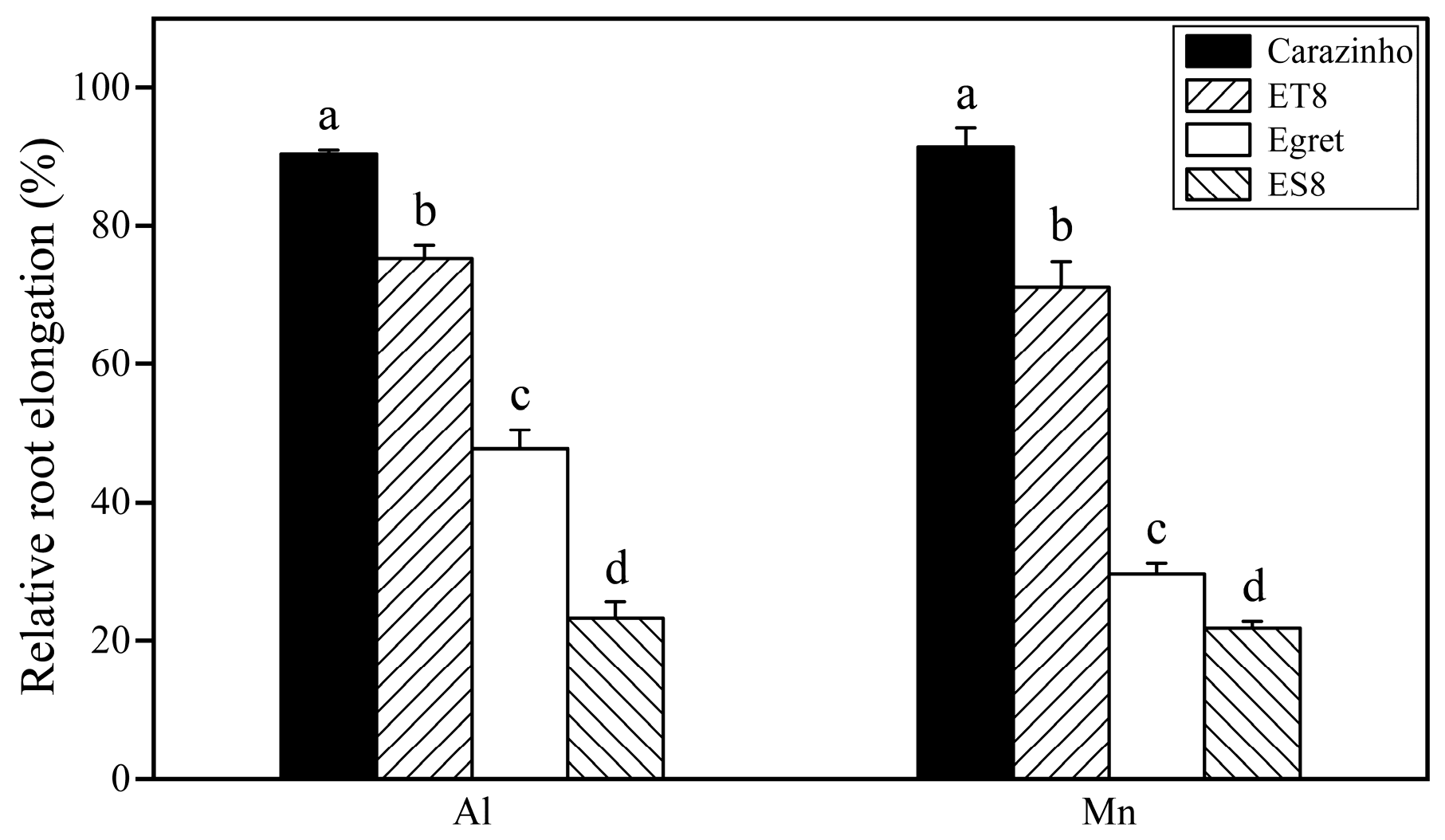
3.1. Tolerance of Wheat Varieties to Al and Mn toxicity
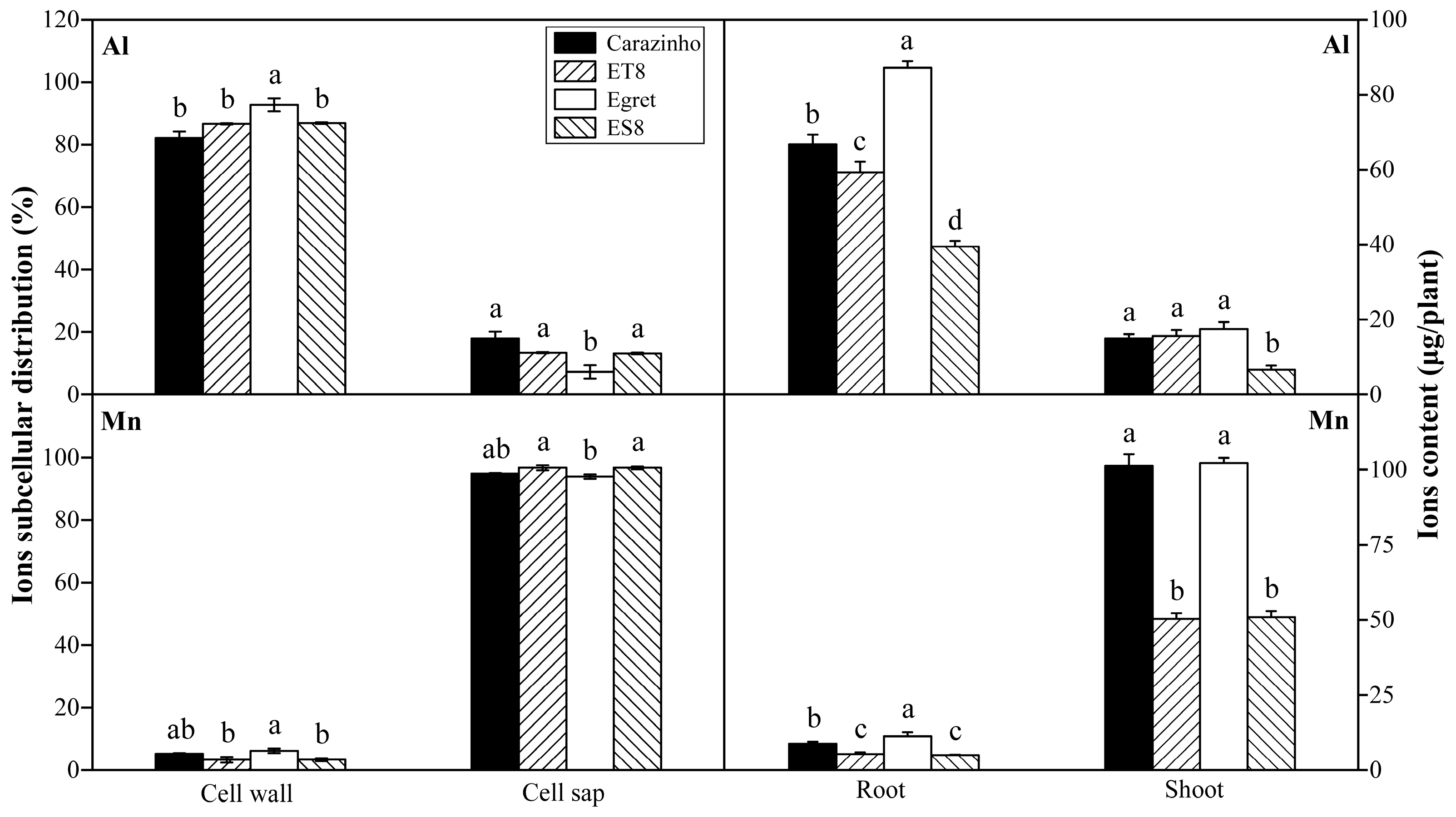
3.2. Al and Mn Subcellular Distribution in Root Cell and Contents in Plants
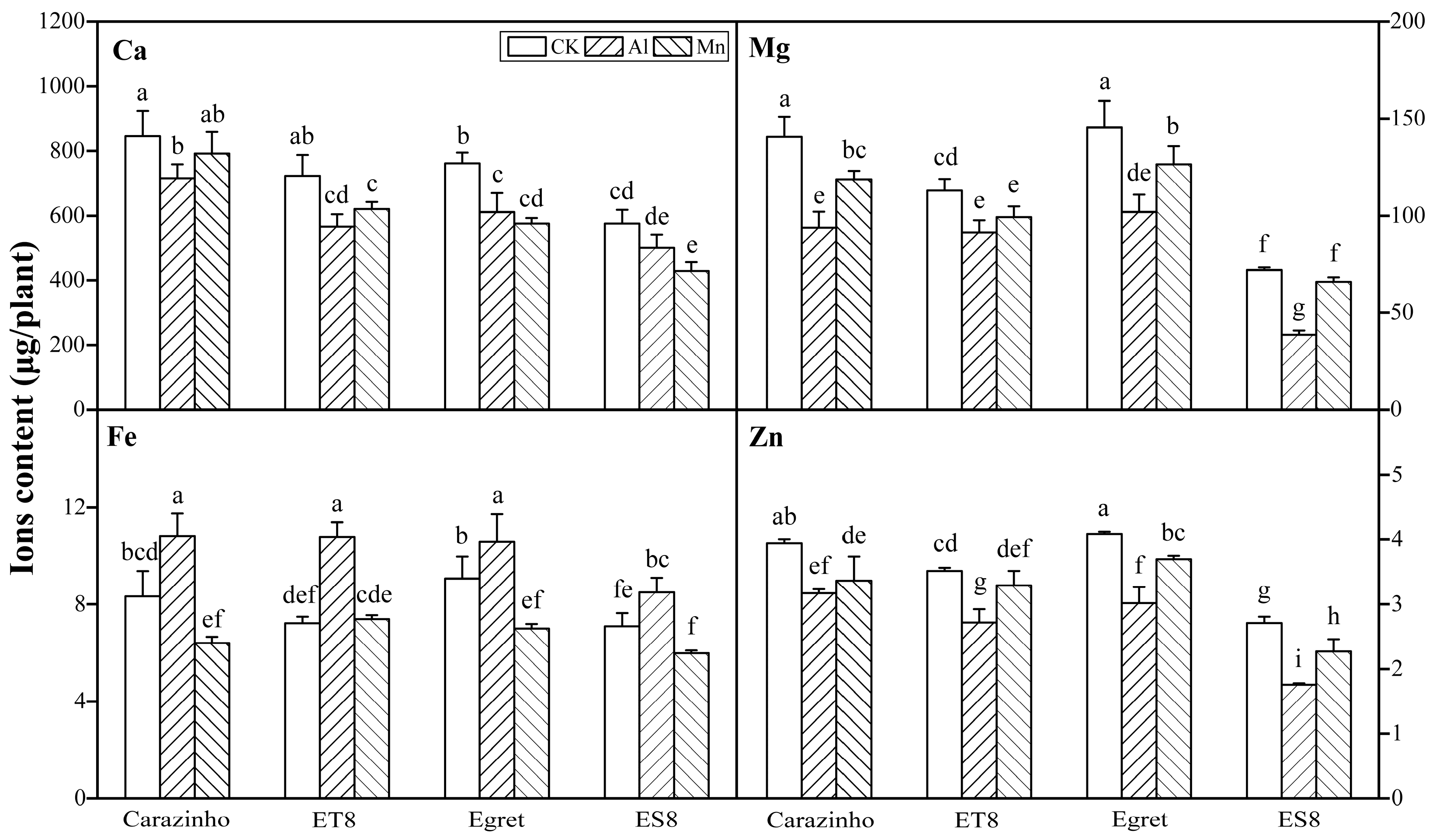
3.3. Al and Mn Effectiveness on Four Metal Ions Contents
3.4. Wheat Root Transcriptome Profiling in Response to Al and Mn
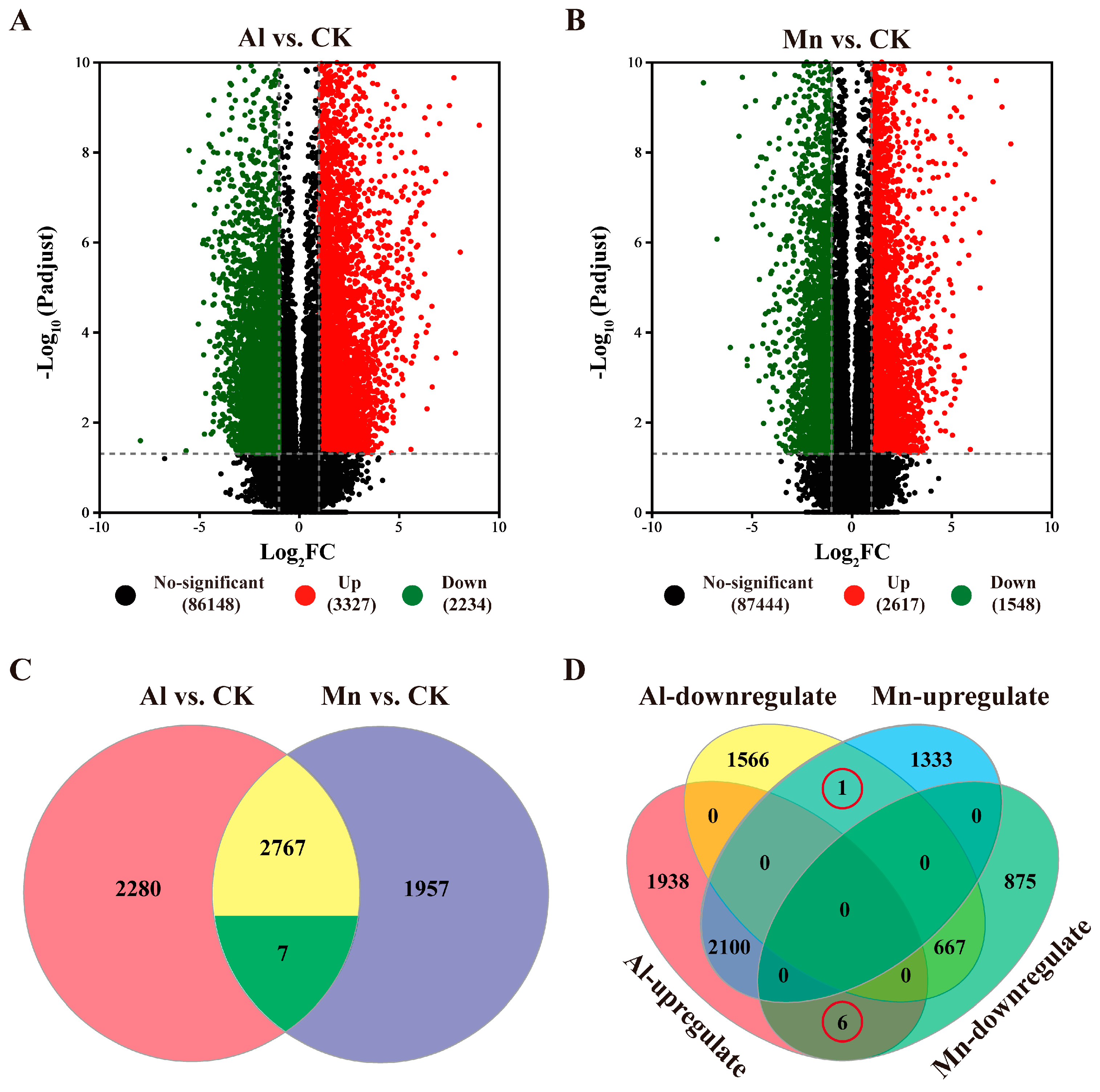
3.5. Identification of Differentially Expressed Genes in Response to Al and Mn Toxicity
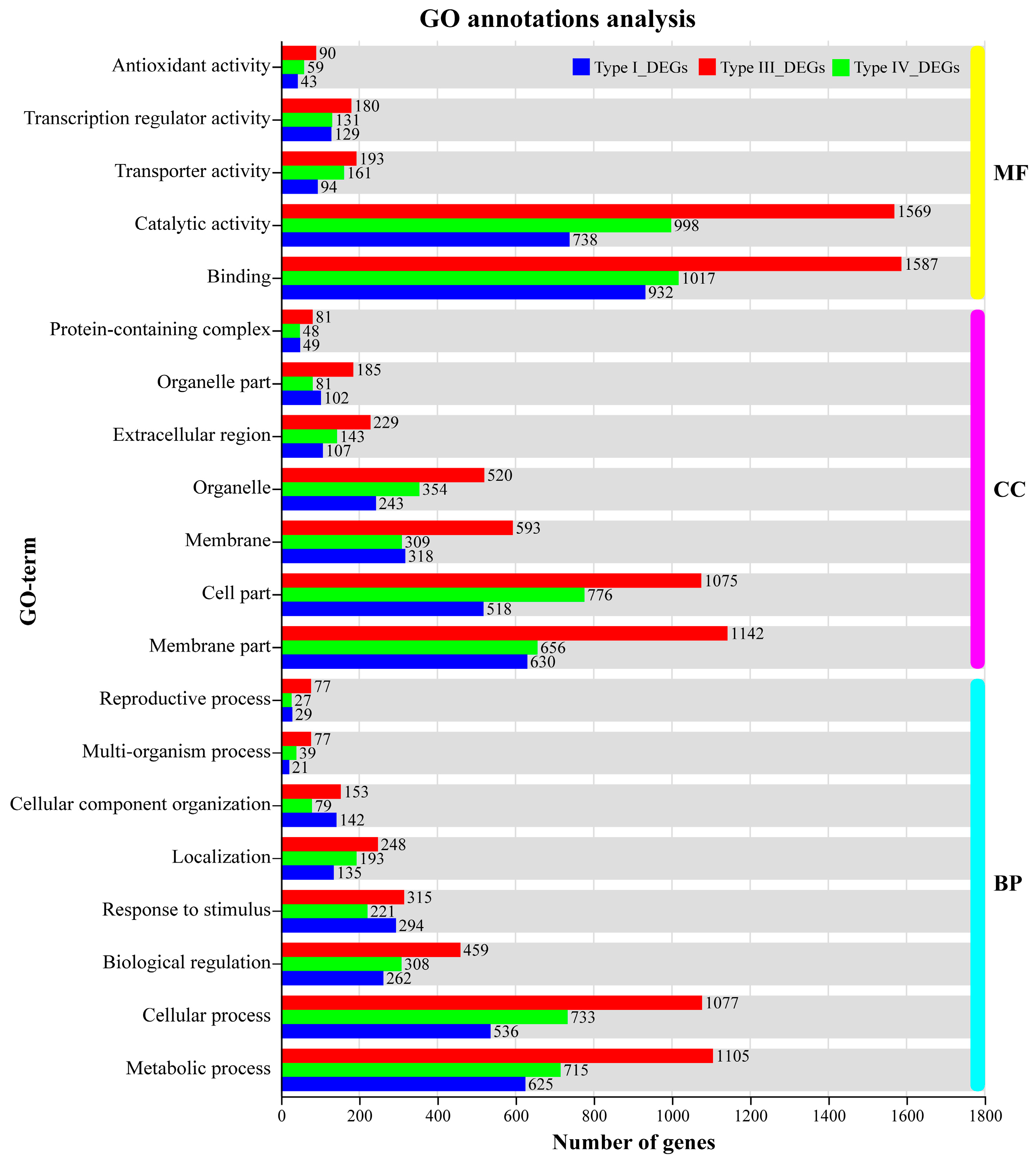
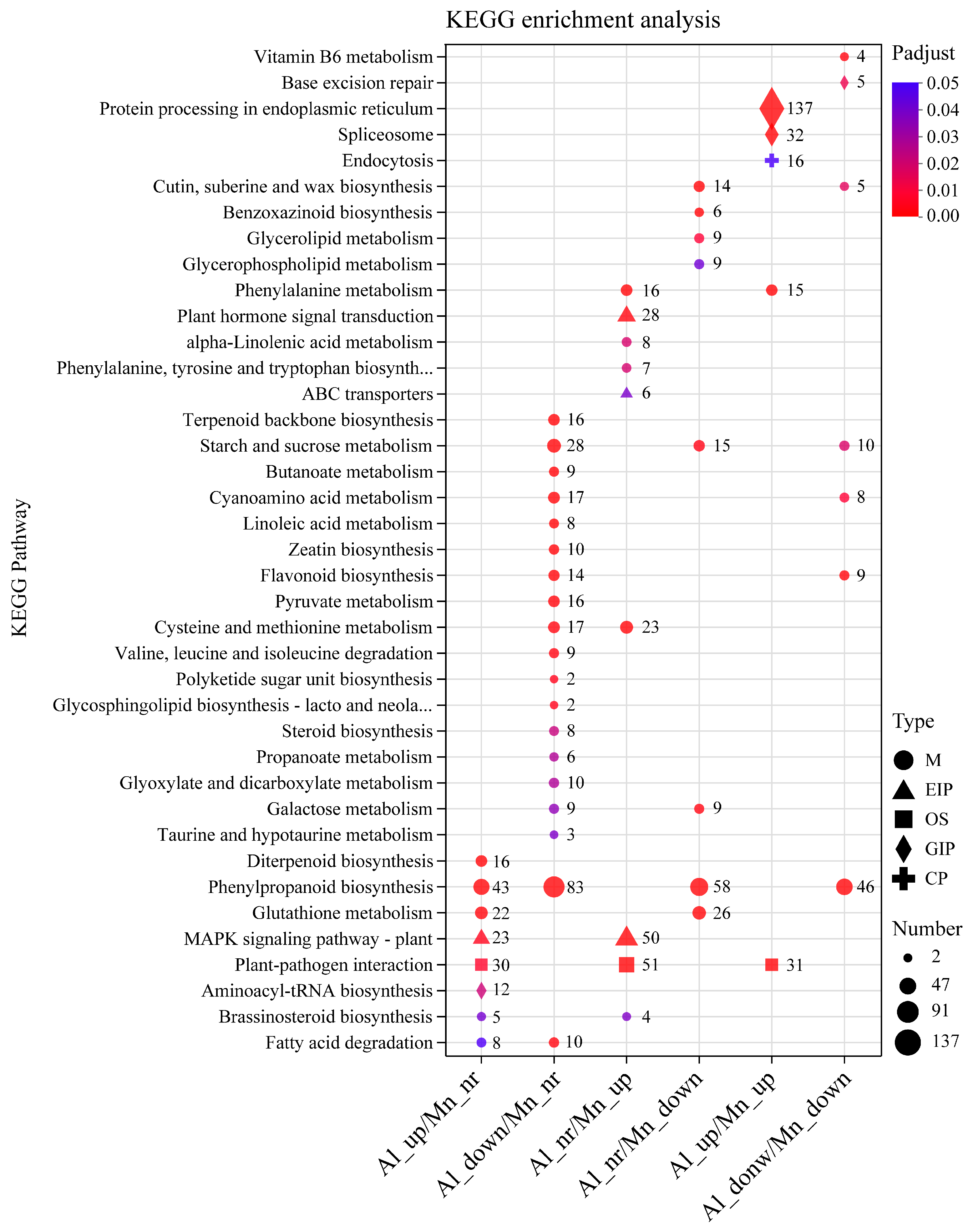
3.6. GO Functional Annotations and KEGG Pathway Analysis
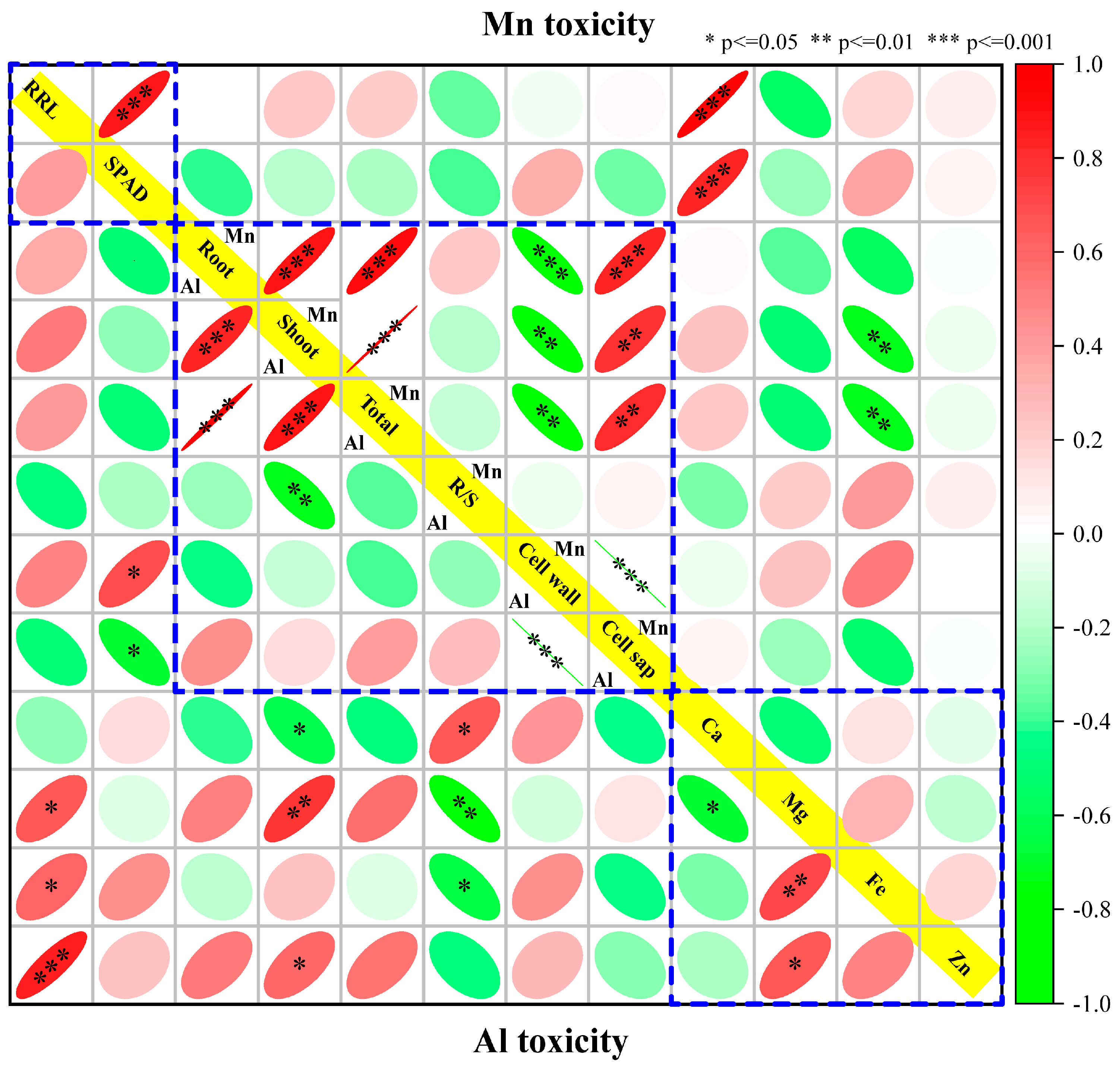
3.7. Correlations between Physiological Traits and Expressed Module Eigengenes
4. Discussion
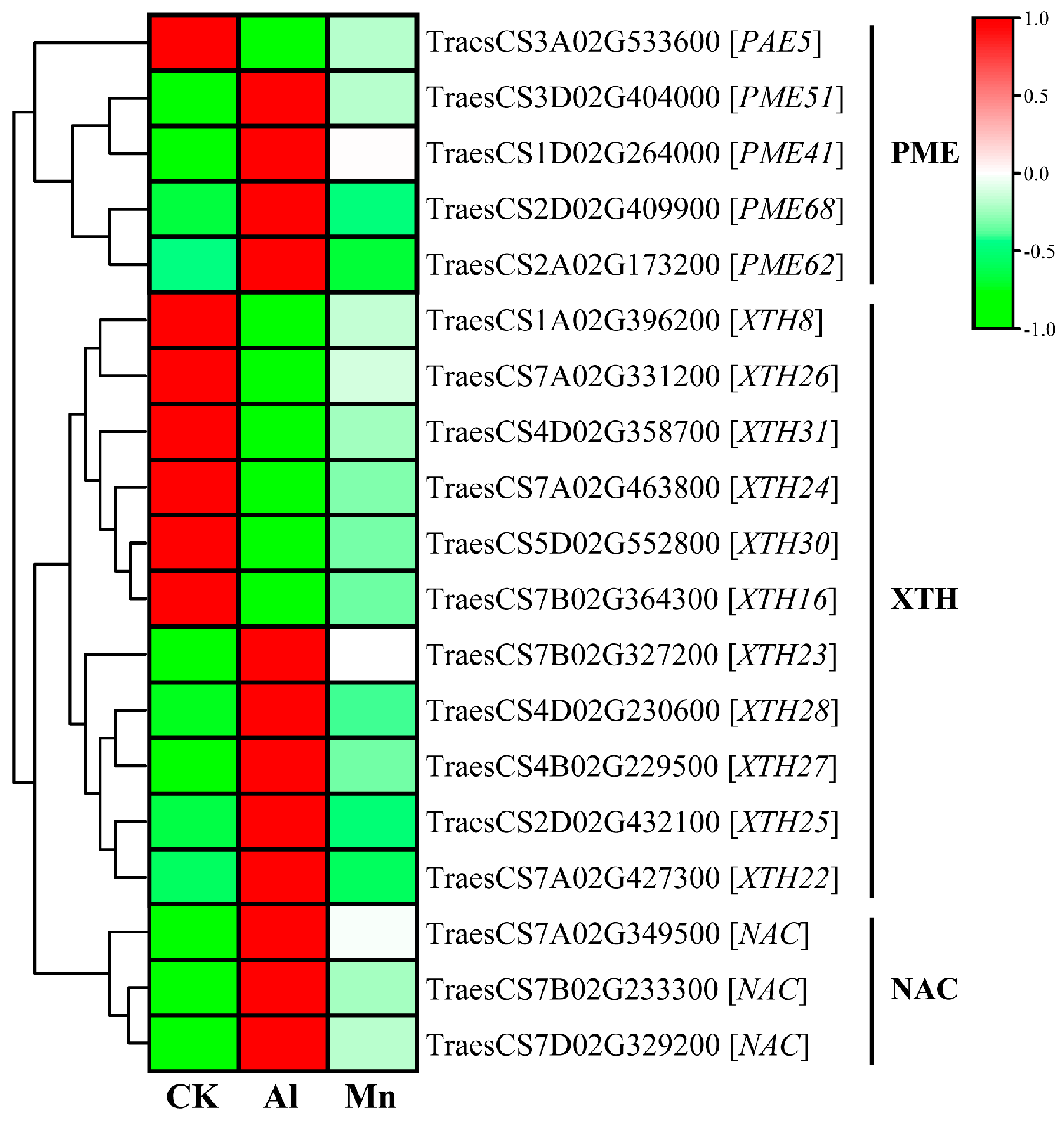
4.1. Wall Cell Biogenesis and Macromolecule Metabolism Respond Exclusively to Al Stress
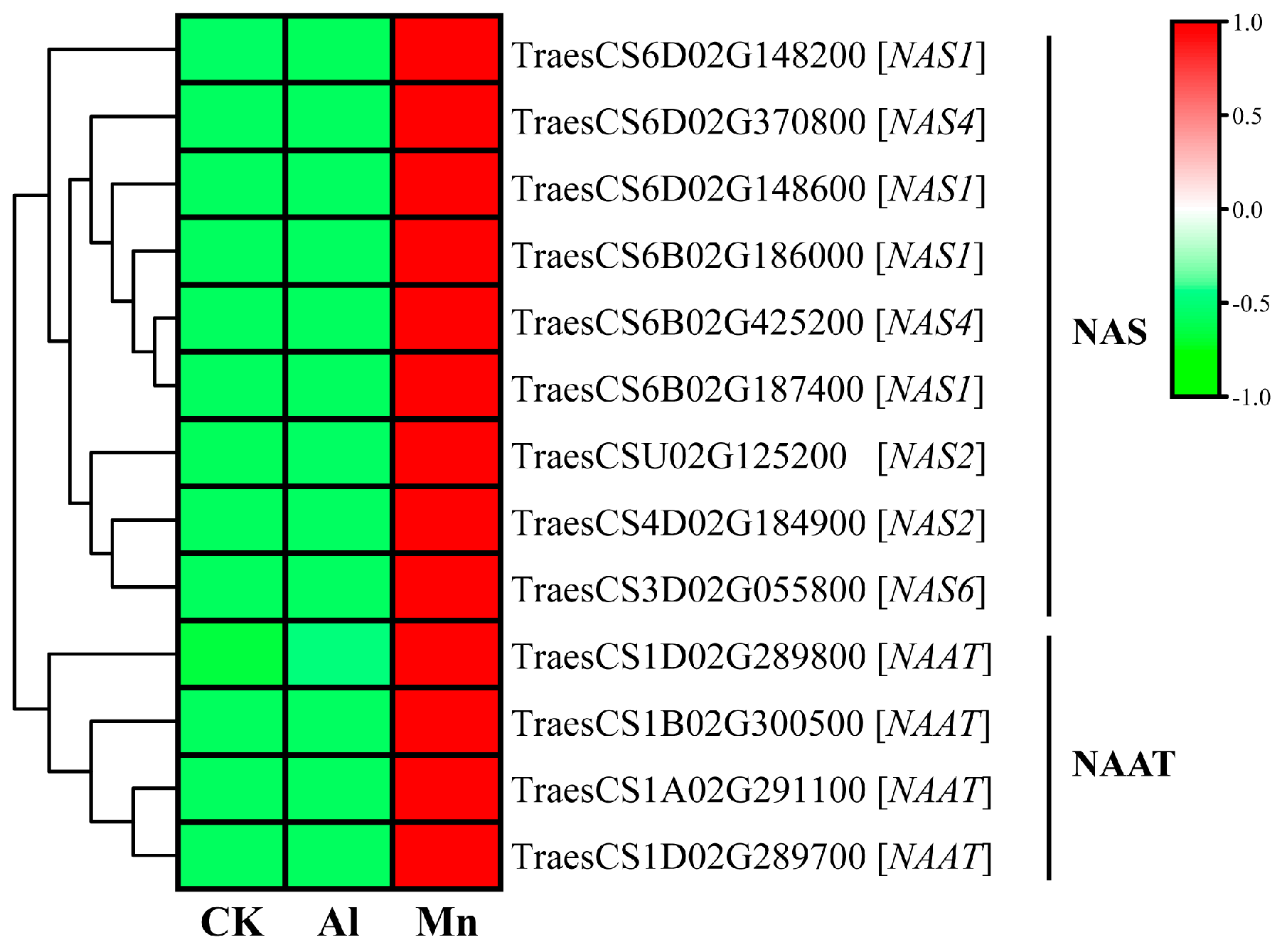
4.2. Nicotianamine Synthesis Responds Exclusively to Mn Stress
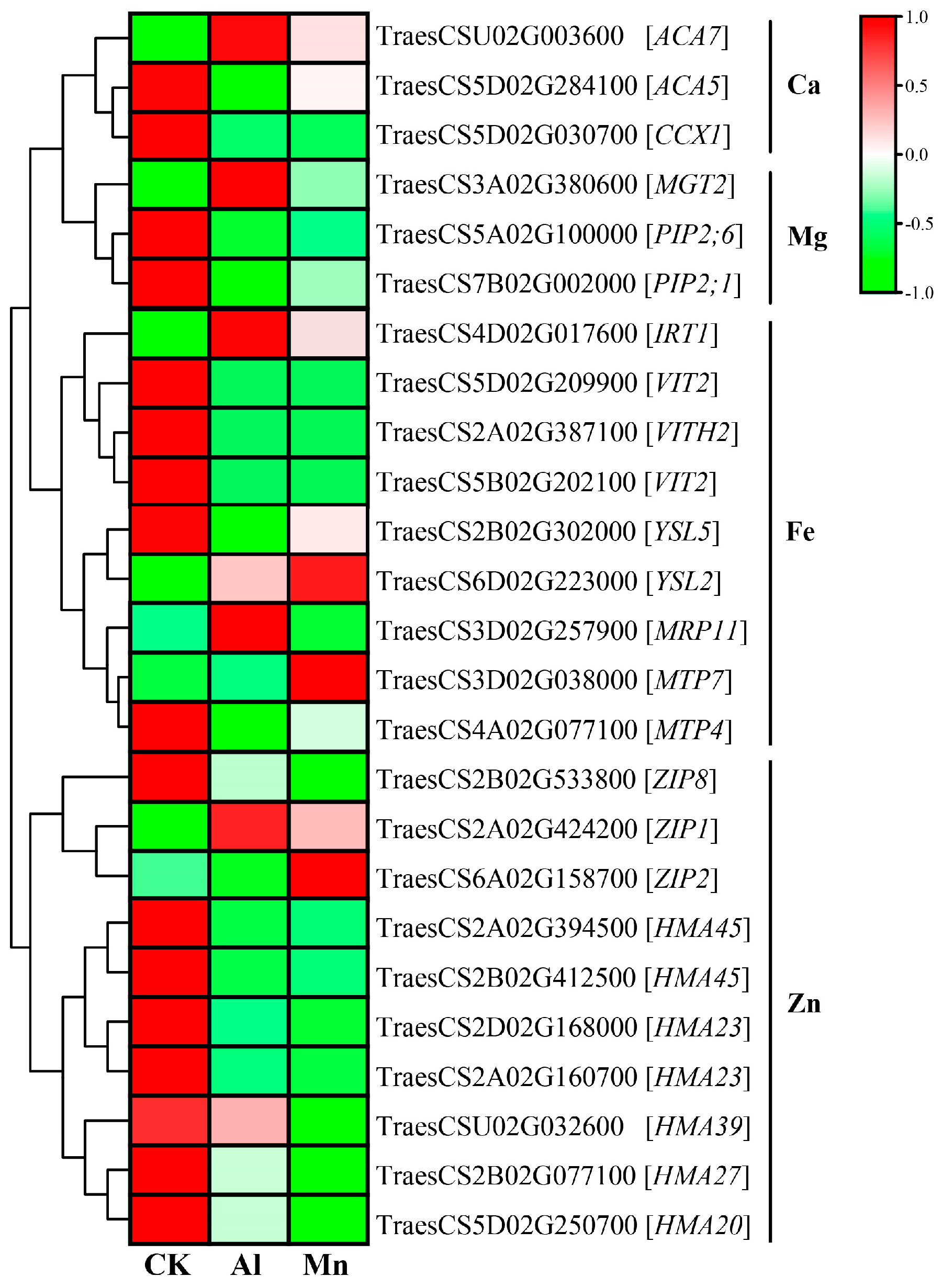
4.3. Metal Ions Transportation and Accumulation under Al and Mn Stress
4.4. Phenylpropanoid Biosynthesis under Al and Mn Stresses
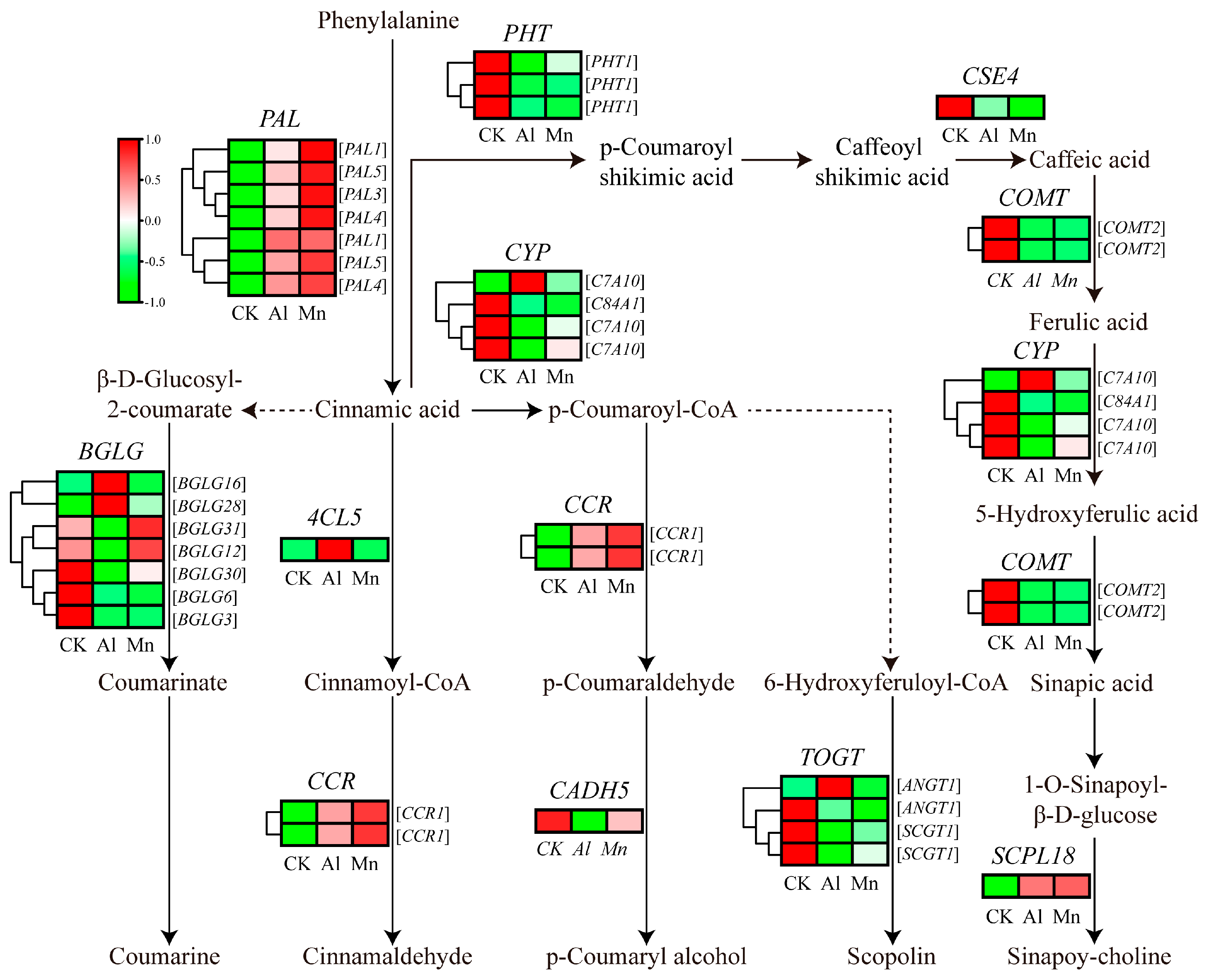
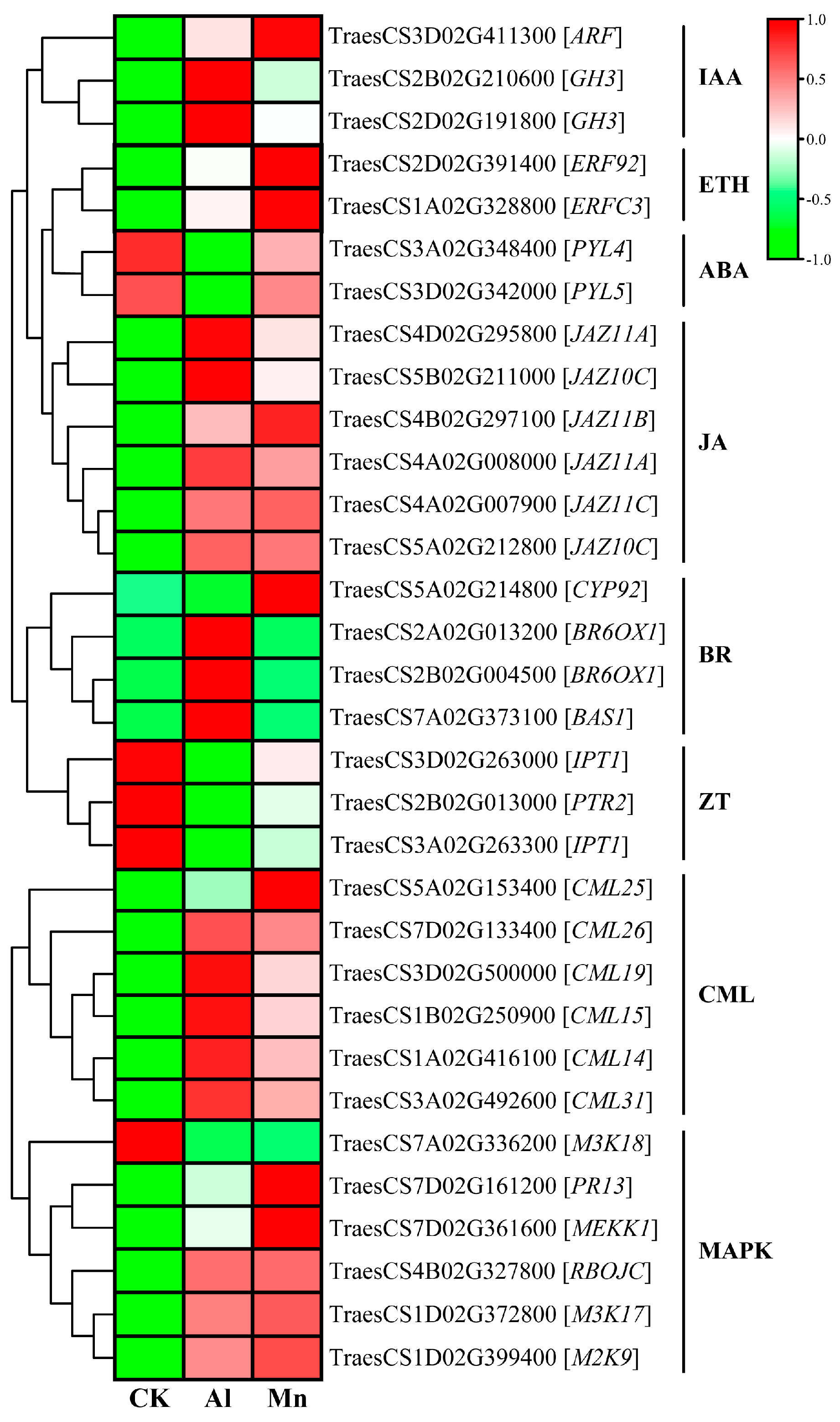
4.5. Signaling in Root under Al and Mn Stresses
4.6 Transcription Factors Regulate Wheat Tolerance to Al and Mn Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kinraide, T.B. Toxicity factors in acidic forest soils: attempts to evaluate separately the toxic effects of excessive Al3+ and H+ and insufficient Ca2+ and Mg2+ upon root elongation. Euro. J. Soil Sci. 2003, 54, 323–333. [Google Scholar] [CrossRef]
- Kochian, L.V.; Hoekenga, O.A.; Pineros, M.A. How do crop plants tolerate acid soils? - Mechanisms of aluminum tolerance and phosphorous efficiency. Ann. Rev. Plant Bio. 2004, 55, 459–493. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Cai, S.; Shah, J.M.; Zhang, G. The combined treatment of Mn and Al alleviates the toxicity of Al or Mn stress alone in barley. Acta Physio. Plant. 2016, 38. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, W.; Sun, L.; Tian, J.; Liao, H. Proteomic analysis reveals growth inhibition of soybean roots by manganese toxicity is associated with alteration of cell wall structure and lignification. J. Proteomics 2016, 143, 151–160. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Suzuki, T.; Fujita, M. Polyamines-induced aluminum tolerance in mung bean: A study on antioxidant defense and methylglyoxal detoxification systems. Ecotoxicology 2017, 26, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, M.; Chen, J.; Yang, S.; Chen, J.; Xue, Y. Comparative transcriptome analysis reveals complex physiological response and gene regulation in peanut roots and leaves under manganese toxicity stress. Inter. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Morita, A.; Yanagisawa, O.; Maeda, S.; Takatsu, S.; Ikka, T. Tea plant (Camellia sinensis L.) roots secrete oxalic acid and caffeine into medium containing aluminum. Soil Sci. Plant Nutr. 2011, 57, 796–802. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, L.; Liu, P.; Liu, G.; Tian, J.; Liao, H. Malate synthesis and secretion mediated by a manganese-enhanced malate dehydrogenase confers superior manganese tolerance in Stylosanthes guianensis. Plant Physi. 2015, 167, 176. [Google Scholar] [CrossRef]
- Chen, W.; Tang, L.; Wang, J.; Zhu, H.; Jin, J.; Yang, J.; Fan, W. Research advances in the mutual mechanisms regulating response of plant roots to phosphate deficiency and aluminum toxicity. Inter. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Kochian, L.V.; Pineros, M.A.; Liu, J.; Magalhaes, J.V. Plant adaptation to acid soils: The molecular basis for crop aluminum resistance. Ann.Rev. Plant Bio. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Wang, X.; Ai, S.; Liao, H. Deciphering interactions between phosphorus status and toxic metal exposure in plants and rhizospheres to improve crops reared on acid soil. Cells 2023, 12. [Google Scholar] [CrossRef]
- Lytle, C.M.; Lytle, F.W.; Smith, B.N. Use of XAS to determine the chemical speciation of bioaccumulated manganese in Potamogeton pectinatus. J. Enviro. Quality 1996, 25, 311–316. [Google Scholar] [CrossRef]
- Che, J.; Zhao, X.Q.; Shen, R.F. Molecular mechanisms of plant adaptation to acid soils: A review. Pedosphere 2023, 33, 14–22. [Google Scholar] [CrossRef]
- Wang, Y.; Li, R.; Li, D.; Jia, X.; Zhou, D.; Li, J.; Lyi, S.M.; Hou, S.; Huang, Y.; Kochian, L.V.; et al. NIP1; 2 is a plasma membrane-localized transporter mediating aluminum uptake, translocation, and tolerance in Arabidopsis. PNAS 2017, 114, 5047–5052. [Google Scholar] [CrossRef]
- Wang, P.; Wan, N.; Horst, W.J.; Yang, Z.B. From stress to responses: aluminium-induced signalling in the root apex. J. Exp. Bot. 2023, 74, 1358–1371. [Google Scholar] [CrossRef] [PubMed]
- Leskova, A.; Javot, H.; Giehl, R.F.H. Metal crossroads in plants: modulation of nutrient acquisition and root development by essential trace metals. J. Exp. Bot. 2022, 73, 1751–1765. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Moraes, L.A.C.; Navroski, D. Lime and Micronutrients Interaction in soybean genotypes adapted to tropical and subtropical conditions. Comm. Soil Sci. Plant Analy. 2017, 48, 792–800. [Google Scholar] [CrossRef]
- Rao, K.S.; Rao, B.S.; Vishnuvardhan, D.; Prasad, K.V. Alteration of superhelical state of DNA by aluminium (Al). Biochem. Biophysi. Acta 1993, 1172, 17–20. [Google Scholar] [CrossRef]
- Watanabe, T.; Osaki, M. Mechanisms of adaptation to high aluminum condition in native plant species growing in acid soils: A review. Comm. Soil Sci. Plant Analy. 2002, 33, 1247–1260. [Google Scholar] [CrossRef]
- Zishiri, R.M.; Mutengwa, C.S.; Tandzi, L.N.; Manyevere, A. Growth response and dry matter partitioning of quality protein Maize (Zea mays L.) genotypes under aluminum toxicity. Agronomy-Basel 2022, 12. [Google Scholar] [CrossRef]
- Khabaz-Saberi, H.; Rengel, Z.; Wilson, R.; Setter, T.L. Variation of tolerance to manganese toxicity in Australian hexaploid wheat. J. Plant Nutri. Soil Sci. 2010, 173, 103–112. [Google Scholar] [CrossRef]
- Yang, Z.B.; You, J.F.; Xu, M.Y.; Yang, Z.M. Interaction between aluminum toxicity and manganese toxicity in soybean (Glycine max). Plant Soil 2009, 319, 277–289. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Chen, R.F.; Shen, R.F. Coadaptation of plants to multiple stresses in acidic soils. Soil Sci. 2014, 179, 503–513. [Google Scholar] [CrossRef]
- Khabaz-Saberi, H.; Rengel, Z. Aluminum, manganese, and iron tolerance improves performance of wheat genotypes in waterlogged acidic soils. J. Plant Nutr. Soil Sci. 2010, 173, 461–468. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, H.; Zhou, C.; Bao, Y.; Xu, X.; Chen, Y.; Shen, Z.; Chen, C. Transcriptomic, epigenomic and physiological comparisons reveal key factors for different manganese tolerances in three Chenopodium ambrosioides L. populations. Plant Physiol. Biochem. 2023, 201. [Google Scholar] [CrossRef]
- Wei, Y.; Han, R.; Xie, Y.; Jiang, C.; Yu, Y. Recent advances in understanding mechanisms of plant tolerance and response to aluminum toxicity. Sustainability 2021, 13. [Google Scholar] [CrossRef]
- Sasaki, T.; Yamamoto, Y.; Ezaki, B.; Katsuhara, M.; Ahn, S.J.; Ryan, P.R.; Delhaize, E.; Matsumoto, H. A wheat gene encoding an aluminum-activated malate transporter. Plant J 2004, 37, 645–653. [Google Scholar] [CrossRef]
- Li, J.Y.; Liu, J.P.; Dong, D.K.; Jia, X.M.; McCouch, S.R.; Kochian, L.V. Natural variation underlies alterations in Nramp aluminum transporter (NRAT1) expression and function that play a key role in rice aluminum tolerance. PNAS. 2014, 111, 6503–6508. [Google Scholar] [CrossRef]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. FASTP: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: a fast spliced aligner with low memory requirements. Nature Meth. 2015, 12, 357–U121. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nature Biotech. 2015, 33, 290. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Gen. Bio. 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucl. Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network analysis and visualization of proteomics data. J. Prot. Res. 2019, 18, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lu, L.; Yu, Y.; Liu, L.; Hu, Y.; Ye, Y.; Jin, C.; Lin, X. Decreasing methylation of pectin caused by nitric oxide leads to higher aluminium binding in cell walls and greater aluminium sensitivity of wheat roots. J. Exp. Bot. 2016, 67, 979–989. [Google Scholar] [CrossRef]
- Rocha, J.; Ciceron, F.; de Sanctis, D.; Lelimousin, M.; Chazalet, V.; Lerouxel, O.; Breton, C. Structure of Arabidopsis thaliana FUT1 reveals a variant of the gt-b class fold and provides insight into xyloglucan fucosylation. Plant Cell 2016, 28, 2352–2364. [Google Scholar] [CrossRef]
- Zhu, X.F.; Shi, Y.Z.; Lei, G.J.; Fry, S.C.; Zhang, B.C.; Zhou, Y.H.; Braam, J.; Jiang, T.; Xu, X.Y.; Mao, C.Z.; et al. XTH31, encoding an in vitro XEH/XET-active enzyme, regulates aluminum sensitivity by modulating in Vivo XET action, cell wall xyloglucan content, and aluminum binding capacity in Arabidopsis. Plant Cell 2012, 24, 4731–4747. [Google Scholar] [CrossRef]
- Becnel, J.; Natarajan, M.; Kipp, A.; Braam, J. Developmental expression patterns of Arabidopsis XTH genes reported by transgenes and genevestigator. Plant Mol. Bio. 2006, 61, 451–467. [Google Scholar] [CrossRef]
- Yokoyama, R.; Nishitani, K. A comprehensive expression analysis of all members of a gene family encoding cell-wall enzymes allowed us to predict cis-regulatory regions involved in cell-wall construction in specific organs of arabidopsis. Plant Cell Physiol. 2001, 42, 1025–1033. [Google Scholar] [CrossRef]
- Tao, Y.; Wan, J.X.; Liu, Y.S.; Yang, X.Z.; Shen, R.F.; Zhu, X.F. The NAC transcription factor ANAC017 regulates aluminum tolerance by regulating the cell wall-modifying genes. Plant Physiol. 2022, 189, 2517–2534. [Google Scholar] [CrossRef]
- Yang, J.L.; Zhu, X.F.; Peng, Y.X.; Zheng, C.; Li, G.X.; Liu, Y.; Shi, Y.Z.; Zheng, S.J. Cell wall hemicellulose contributes significantly to aluminum adsorption and root growth in Arabidopsis. Plant Physiol. 2011, 155, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Tsuyama, T.; Kawai, R.; Shitan, N.; Matoh, T.; Sugiyama, J.; Yoshinaga, A.; Takabe, K.; Fujita, M.; Yazaki, K. Proton-dependent coniferin transport, a common major transport event in differentiating xylem tissue of woody plants. Plant Physiol. 2013, 162, 918–926. [Google Scholar] [CrossRef]
- Larsen, P.B.; Geisler, M.J.; Jones, C.A.; Williams, K.M.; Cancel, J.D. ALS3 encodes a phloem-localized ABC transporter-like protein that is required for aluminum tolerance in Arabidopsis. Plant J. 2005, 41, 353–363. [Google Scholar] [CrossRef]
- Huang, C.F.; Yamaji, N.; Mitani, N.; Yano, M.; Nagamura, Y.; Ma, J.F. A bacterial-type ABC transporter is involved in aluminum tolerance in rice. Plant Cell 2009, 21, 655–667. [Google Scholar] [CrossRef]
- Seregin, I.V.; Kozhevnikova, A.D. Nicotianamine: A key player in metal homeostasis and hyperaccumulation in plants. Inter. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Gautam, T.; Jan, I.; Batra, R.; Singh, K.; Pandey, R.; Sharma, P.K.; Balyan, H.S.; Gupta, P.K. Further studies on nicotianamine aminotransferase (NAAT) genes involved in biofortification in bread wheat (Triticum aestivum L.). Plant Gene 2023, 33. [Google Scholar] [CrossRef]
- Han, D.; Zhang, Z.; Ni, B.; Ding, H.; Liu, W.; Li, W.; Chai, L.; Yang, G. Isolation and functional analysis of MxNAS3 involved in enhanced iron stress tolerance and abnormal flower in transgenic Arabidopsis. J. Plant Inter. 2018, 13, 433–441. [Google Scholar] [CrossRef]
- Yang, G.; Li, J.; Liu, W.; Yu, Z.; Shi, Y.; Lv, B.; Wang, B.; Han, D. Molecular cloning and characterization of MxNAS2, a gene encoding nicotianamine synthase in Malus xiaojinensis, with functions in tolerance to iron stress and misshapen flower in transgenic tobacco. Sci. Horti. 2015, 183, 77–86. [Google Scholar] [CrossRef]
- Millaleo, R.; Reyes- Diaz, M.; Ivanov, A.G.; Mora, M.L.; Alberdi, M. Manganese as essential and toxic element for plants: Transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 2010, 10, 470–481. [Google Scholar] [CrossRef]
- Araki, R.; Murata, J.; Murata, Y. A novel barley yellow stripe 1-like transporter (HvYSL2) localized to the root endodermis transports metal-phytosiderophore complexes. Plant Cell Physiol. 2011, 52, 1931–1940. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Xia, J.; Ma, J.F. OsYSL6 is involved in the detoxification of excess manganese in rice. Plant Physiol. 2011, 157, 1832–1840. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Masuda, H.; Bashir, K.; Inoue, H.; Tsukamoto, T.; Takahashi, M.; Nakanishi, H.; Aoki, N.; Hirose, T.; Ohsugi, R.; et al. Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J. 2010, 62, 379–390. [Google Scholar] [CrossRef]
- Xia, J.; Yamaji, N.; Kasai, T.; Ma, J.F. Plasma membrane-localized transporter for aluminum in rice. PNAS. 2010, 107, 18381–18385. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-F.; Yamaji, N.; Chen, Z.; Ma, J.F. A tonoplast-localized half-size ABC transporter is required for internal detoxification of aluminum in rice. Plant J. 2012, 69, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Peng, A.; Li, Y.; Zuo, H.; Li, P.; Wang, J.; Yu, K.; Liu, C.; Zhao, S.; Wan, X.; et al. Tea plant roots respond to aluminum-induced mineral nutrient imbalances by transcriptional regulation of multiple cation and anion transporters. BMC Plant Bio. 2022, 22. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.J.; Yokosho, K.; Yamaji, N.; Fujii-Kashino, M.; Ma, J.F. Functional characterization of two half-size ABC transporter genes in aluminium-accumulating buckwheat. New Phytol. 2017, 215, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.B.; Cancel, J.; Rounds, M.; Ochoa, V. Arabidopsis ALS1 encodes a root tip and stele localized half type ABC transporter required for root growth in an aluminum toxic environment. Planta 2007, 225, 1447–1458. [Google Scholar] [CrossRef]
- Shao, J.F.; Yamaji, N.; Shen, R.F.; Ma, J.F. The key to Vn homeostasis in plants: Regulation of Mn transporters. Trends Plant Sci. 2017, 22, 215–224. [Google Scholar] [CrossRef]
- Alejandro, S.; Hoeller, S.; Meier, B.; Peiter, E. Manganese in plants: From acquisition to subcellular allocation. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Milner, M.J.; Seamon, J.; Craft, E.; Kochian, L.V. Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. J. Exp. Bot. 2013, 64, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, Y.; Bashir, K.; Nakanishi, H.; Nishizawa, N.K. OsNRAMP5, a major player for constitutive iron and manganese uptake in rice. Plant Sign. Behav. 2012, 7, 763–766. [Google Scholar] [CrossRef]
- Moustaka, J.; Ouzounidou, G.; Baycu, G.; Moustakas, M. Aluminum resistance in wheat involves maintenance of leaf Ca2+ and Mg2+ content, decreased lipid peroxidation and Al accumulation, and low photosystem II excitation pressure. Biometals 2016, 29, 611–623. [Google Scholar] [CrossRef]
- Quinteiro Ribeiro, M.A.; de Almeida, A.A.F.; Mielke, M.S.; Gomes, F.P.; Pires, M.V.; Baligar, V.C. Aluminum effects on growth, photosynthesis, and mineral nutrition of cacao genotypes. J. Plant Nutr. 2013, 36, 1161–1179. [Google Scholar] [CrossRef]
- Dou, C.; Fu, X.; Chen, X.; Shi, J.; Chen, Y. Accumulation and interaction of calcium and manganese in Phytolacca americana. Plant Sci. 2009, 177, 601–606. [Google Scholar] [CrossRef]
- Rengel, Z.; Zhang, W.H. Role of dynamics of intracellular calcium in aluminium-toxicity syndrome. New Phytol. 2003, 159, 295–314. [Google Scholar] [CrossRef]
- Hauck, M.; Paul, A.; Gross, S.; Raubuch, M. Manganese toxicity in epiphytic lichens: chlorophyll degradation and interaction with iron and phosphorus. Envir. Exp. Bot. 2003, 49, 181–191. [Google Scholar] [CrossRef]
- Yang, J.L.; You, J.F.; Li, Y.Y.; Wu, P.; Zheng, S.J. Magnesium enhances aluminum-induced citrate secretion in rice bean roots (Vigna umbellata) by restoring plasma membrane H+-ATPase activity. Plant Cell Physiol. 2007, 48, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Hare, A.; Comparini, D.; Bouteau, F.; Kawano, T. Zinc-dependent protection of tobacco and rice cells from aluminum-induced superoxide-mediated cytotoxicity. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Xie, J.; Wen, W.; Li, J.; Zhou, P.; An, Y. Interaction of zinc and IAA alleviate aluminum-induced damage on photosystems via promoting proton motive force and reducing proton gradient in alfalfa. BMC Plant Bio. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Boursiac, Y.; Lee, S.M.; Romanowsky, S.; Blank, R.; Sladek, C.; Chung, W.S.; Harper, J.F. Disruption of the vacuolar calcium-ATPases in Arabidopsis results in the activation of a salicylic acid-dependent programmed cell death pathway. Plant Physiol. 2010, 154, 1158–1171. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Babourina, O.; Rengel, Z. Role of magnesium in alleviation of aluminium toxicity in plants. J. Exp. Bot. 2011, 62, 2251–2264. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Feng, Y.; Luo, S.; Cheng, L.; Tong, W.; Lu, X.; Li, Y.; Zhang, P. The aquaporin MePIP2;7 improves MeMGT9-mediated Mg2+ acquisition in cassava. J. Integr. Plant Bio. 2023. [Google Scholar] [CrossRef]
- Chen, Z.C.; Yamaji, N.; Motoyama, R.; Nagamura, Y.; Ma, J.F. Up-regulation of a magnesium transporter gene OsMGT1 is required for conferring aluminum tolerance in rice. Plant Physiol. 2012, 159, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Punshon, T.; Lanzirotti, A.; Li, L.; Alonso, J.M.; Ecker, J.R.; Kaplan, J.; Guerinot, M.L. Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 2006, 314, 1295–1298. [Google Scholar] [CrossRef]
- Pittman, J.K. Managing the manganese: molecular mechanisms of manganese transport and homeostasis. New Phytol. 2005, 167, 733–742. [Google Scholar] [CrossRef]
- Cao, J. Molecular evolution of the vacuolar iron transporter (VIT) family genes in 14 plant species. Genes 2019, 10. [Google Scholar] [CrossRef]
- Montanini, B.; Blaudez, D.; Jeandroz, S.; Sanders, D.; Chalot, M. Phylogenetic and functional analysis of the Cation Diffusion Facilitator (CDF) family: improved signature and prediction of substrate specificity. BMC Genomics 2007, 8. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhou, P.; Xiao, Q.; Shi, D. Effects of foliar application of organic acids on alleviation of aluminum toxicity in alfalfa. J. Plant Nutr. Soil Sci. 2014, 177, 421–430. [Google Scholar] [CrossRef]
- Chang, J.D.; Huang, S.; Wiseno, I.; Sui, F.Q.; Feng, F.; Zheng, L.; Ma, J.F.; Zhao, F.-J. Dissecting the promotional effect of zinc on cadmium translocation from roots to shoots in rice. J. Exp. Bot. 2023. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, H.; Hu, Y.; Xu, D.; Zheng, S.; Su, S.; Yang, Q. De novo transcriptome assembly and metabolomic analysis of three tissue types in Cinnamomum cassia. Chin. Herb. Med. 2023, 15, 310–316. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Izbianska, K.; Arasimowicz-Jelonek, M.; Deckert, J. Phenylpropanoid pathway metabolites promote tolerance response of lupine roots to lead stress. Ecoto. Environ. Safety 2014, 110, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Feduraev, P.; Skrypnik, L.; Riabova, A.; Pungin, A.; Tokupova, E.; Maslennikov, P.; Chupakhina, G. Phenylalanine and tyrosine as exogenous precursors of wheat (Triticum aestivum L.) secondary metabolism through PAL-associated pathways. Plants-Basel 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Tao, F.; Li, W. Characterisation of manganese toxicity tolerance in Arabis paniculata. Plant Diversity 2021, 43, 163–172. [Google Scholar] [CrossRef]
- Jia, Y.; Li, X.; Liu, Q.; Hu, X.; Li, J.; Dong, R.; Liu, P.; Liu, G.; Luo, L.; Chen, Z. Physiological and transcriptomic analyses reveal the roles of secondary metabolism in the adaptive responses of Stylosanthes to manganese toxicity. BMC Genomics 2020, 21. [Google Scholar] [CrossRef]
- Lei, Y.; Korpelainen, H.; Li, C. Physiological and biochemical responses to high Mn concentrations in two contrasting Populus cathayana populations. Chemosphere 2007, 68, 686–694. [Google Scholar] [CrossRef]
- Liu, P.; Huang, R.; Hu, X.; Jia, Y.; Li, J.; Luo, J.; Liu, Q.; Luo, L.; Liu, G.; Chen, Z. Physiological responses and proteomic changes reveal insights into Stylosanthes response to manganese toxicity. BMC Plant Bio. 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Su, L.; Lv, A.; Wang, S.; Huang, B.; An, Y. Gene Expression Analysis of Alfalfa Seedlings Response to Acid-Aluminum. Inter. J. Genom. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, L.; Liang, X.; Dai, P.; Zhang, Y.; Li, B.; Lin, X.; Sun, C. Enhancement of polyphenolic metabolism as an adaptive response of lettuce (Lactuca sativa) roots to aluminum stress. Environ. Poll. 2020, 261. [Google Scholar] [CrossRef]
- Ezaki, B.; Higashi, A.; Nanba, N.; Nishiuchi, T. An S-adenosyl Methionine Synthetase (SAMS) gene from Andropogon virginicus L. confers aluminum stress tolerance and facilitates epigenetic gene regulation in Arabidopsis thaliana. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Su, L.; Lv, A.; Wen, W.; Fan, N.; Li, J.; Gao, L.; Zhou, P.; An, Y. MsMYB741 is involved in alfalfa resistance to aluminum stress by regulating flavonoid biosynthesis. Plant J. 2022, 112, 756–771. [Google Scholar] [CrossRef]
- Choudhury, S.; Mazumder, M.K.; Moulick, D.; Sharma, P.; Tata, S.K.; Ghosh, D.; Ali, H.M.; Siddiqui, M.H.; Brestic, M.; Skalicky, M.; et al. A computational study of the role of secondary metabolites for mitigation of acid soil stress in cereals using dehydroascorbate and mono-dehydroascorbate reductases. Antioxidants 2022, 11. [Google Scholar] [CrossRef]
- Wang, P.; Yu, W.; Zhang, J.; Rengel, Z.; Xu, J.; Han, Q.; Chen, L.; Li, K.; Yu, Y.; Chen, Q. Auxin enhances aluminium-induced citrate exudation through upregulation of GmMATE and activation of the plasma membrane H+ -ATPase in soybean roots. Ann. Bot. 2016, 118, 933–940. [Google Scholar] [CrossRef]
- Hou, N.; You, J.; Pang, J.; Xu, M.; Chen, G.; Yang, Z. The accumulation and transport of abscisic acid insoybean (Glycine max L.) under aluminum stress. Plant Soil 2010, 330, 127–137. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Su, H.; Guo, L.; Zhang, J.; Li, Y.; Xu, J.; Zhang, X.; Guo, Y.D.; Zhang, N. Jasmonate and aluminum crosstalk in tomato: Identification and expression analysis of WRKYs and ALMTs during JA/Al-regulated root growth. Plant Physio. Biochem. 2020, 154, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.B.; He, C.; Ma, Y.; Herde, M.; Ding, Z. Jasmonic acid enhances Al-induced root growth inhibition. Plant Physiol. 2017, 173, 1420–1433. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Liu, J.; Yu, G.; Lei, L.; Jiang, X. Moderate Mn accumulation enhances growth and alters leaf hormone contents in the hyperaccumulator Celosia argentea Linn. Environ. Exp. Bot. 2021, 191. [Google Scholar] [CrossRef]
- Wu, Q.; Lin, X.; Li, S.; Liang, Z.; Wang, H.; Tang, T. Endophytic Bacillus sp. AP10 harboured in Arabis paniculata mediates plant growth promotion and manganese detoxification. Ecotox. Environ. Safety 2023, 262. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, P.; Bai, Z.; Herde, M.; Ma, Y.; Li, N.; Liu, S.; Huang, C.-F.; Cui, R.; Ma, H.; et al. Calmodulin-like protein CML24 interacts with CAMTA2 and WRKY46 to regulate ALMT1-dependent Al resistance in Arabidopsis thaliana. New Phytol. 2022, 233, 2471–2487. [Google Scholar] [CrossRef]
- Zhou, F.; Singh, S.; Zhang, J.; Fang, Q.; Li, C.; Wang, J.; Zhao, C.; Wang, P.; Huang, C.F. The MEKK1-MKK1/2-MPK4 cascade phosphorylates and stabilizes STOP1 to confer aluminum resistance in Arabidopsis. Mol. Plant 2023, 16, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Bataller, S.; Villacastin, A.J.; Shen, Q.J.; Bergman, C. Comparative Nutritional Assessment and Metabolomics of a WRKY Rice Mutant with Enhanced Germination Rates. Agronomy-Basel 2023, 13. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Yu, Y.; Liu, W.; Lu, L.; Jin, C.; Lin, X. Nitric oxide alleviates aluminum-induced oxidative damage through regulating the ascorbate-glutathione cycle in roots of wheat. J. Integr. Plant Bio. 2015, 57, 550–561. [Google Scholar] [CrossRef]
- Srivastava, S.; Dubey, R.S. Nitric oxide alleviates manganese toxicity by preventing oxidative stress in excised rice leaves. Acta Physiol. Plant. 2012, 34, 819–825. [Google Scholar] [CrossRef]
- Li, J.B.; Luan, Y.S.; Liu, Z. Overexpression of SpWRKY1 promotes resistance to Phytophthora nicotianae and tolerance to salt and drought stress in transgenic tobacco. Physiol. Plant. 2015, 155, 248–266. [Google Scholar] [CrossRef]
- Sun, K.; Wang, H.; Xia, Z. The maize bHLH transcription factor bHLH105 confers manganese tolerance in transgenic tobacco. Plant Sci. 2019, 280, 97–109. [Google Scholar] [CrossRef]
- Tao, Y.; Wu, Q.; Huang, J.; Fang Shen, R.; Zhu, X.F. The upstream regulation of the root cell wall when Arabidopsis thaliana in response to toxic metal ions focusing on Al. Plant Sig. Behav. 2023, 18. [Google Scholar] [CrossRef]
- Li, C.X.; Yan, J.Y.; Ren, J.Y.; Sun, L.; Xu, C.; Li, G.X.; Ding, Z.J.; Zheng, S.J. A WRKY transcription factor confers aluminum tolerance via regulation of cell wall modifying genes. J. Integr. Plant Bio. 2020, 62, 1176–1192. [Google Scholar] [CrossRef]
- Ding, Z.J.; Yan, J.Y.; Xu, X.Y.; Li, G.X.; Zheng, S.J. WRKY46 functions as a transcriptional repressor of ALMT1, regulating aluminum-induced malate secretion in Arabidopsis. Plant J. 2013, 76, 825–835. [Google Scholar] [CrossRef]
- Iuchi, S.; Koyama, H.; Iuchi, A.; Kobayashi, Y.; Kitabayashi, S.; Kobayashi, Y.; Ikka, T.; Hirayama, T.; Shinozaki, K.; Kobayashi, M. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. PNAS. 2007, 104, 9900–9905. [Google Scholar] [CrossRef]
- Yamaji, N.; Huang, C.F.; Nagao, S.; Yano, M.; Sato, Y.; Nagamura, Y.; Ma, J.F. A Zinc Finger Transcription Factor ART1 regulates multiple genes implicated in aluminum tolerance in rice. Plant Cell 2009, 21, 3339–3349. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, X.; Yang, C.; Cheng, Y.; Cai, Z.; Nian, H.; Ma, Q. GsERF1 enhances Arabidopsis thaliana aluminum tolerance through an ethylene-mediated pathway. BMC Plant Bio. 2022, 22. [Google Scholar] [CrossRef] [PubMed]
- Sawaki, Y.; Iuchi, S.; Kobayashi, Y.; Kobayashi, Y.; Ikka, T.; Sakurai, N.; Fujita, M.; Shinozaki, K.; Shibata, D.; Kobayashi, M.; et al. STOP1 regulates multiple genes that protect arabidopsis from proton and aluminum toxicities. Plant Physiol. 2009, 150, 281–294. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ohyama, Y.; Kobayashi, Y.; Ito, H.; Iuchi, S.; Fujita, M.; Zhao, C.-R.; Tanveer, T.; Ganesan, M.; Kobayashi, M.; et al. STOP2 activates transcription of several genes for Al- and low pH-tolerance that are regulated by STOP1 in Arabidopsis. Mol. Plant 2014, 7, 311–322. [Google Scholar] [CrossRef]
- Wu, W.; Lin, Y.; Chen, Q.; Peng, W.; Peng, J.; Tian, J.; Liang, C.; Liao, H. Functional conservation and divergence of soybean GmSTOP1 members in proton and aluminum tolerance. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
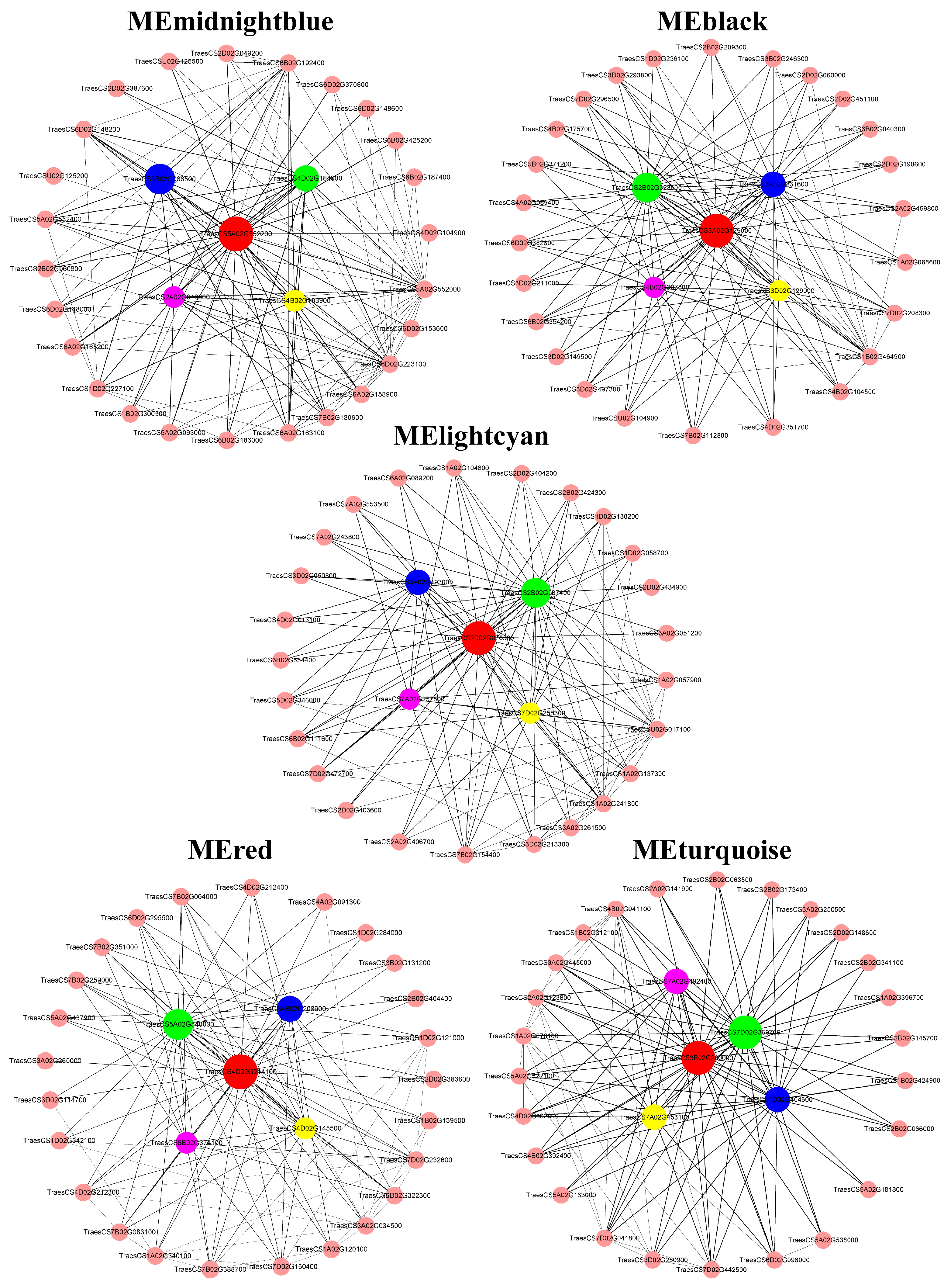
| Module | Gene_id |
Connec tivity |
Kme value |
Fold change | Gene description | |
| Al/CK | Mn/CK | |||||
| midnightblue | TraesCS5A02G552000 | 50.146 | 0.986 | 0.892 | 124.101 | Nicotianamine synthase |
| TraesCS3B02G068500 | 50.064 | 0.986 | 0.830 | 329.475 | Nicotianamine synthase | |
| TraesCS4D02G184900 | 50.032 | 0.994 | 0.694 | 53.203 | Nicotianamine synthase | |
| TraesCS4B02G183900 | 49.729 | 0.987 | 0.429 | 178.986 | Nicotianamine synthase | |
| TraesCS2A02G049900 | 49.645 | 0.985 | 0.486 | 343.721 | Nicotianamine synthase | |
| black | TraesCS3A02G129000 | 109.335 | 0.975 | 2.330 | 1.080 | ABC transporter C family member 3-like |
| TraesCS2B02G323600 | 105.743 | 0.969 | 3.924 | 1.598 | RING-type E3 ubiquitin transferase; zinc finger of C3HC4-type | |
| TraesCS3A02G231600 | 105.472 | 0.970 | 6.459 | 1.459 | Homeobox-leucine zipper protein HOX3 | |
| TraesCS3D02G129900 | 104.621 | 0.966 | 2.711 | 1.007 | ABC subfamily C transporter | |
| TraesCS4B02G307300 | 104.457 | 0.969 | 7.454 | 1.341 | Intracellular trafficking, secretion, and vesicular transport | |
| lightcyan | TraesCS2D02G070500 | 33.891 | 0.967 | 1.597 | 0.592 | Inorganic ion transport; ascorbate peroxidase; Glutathione metabolism |
| TraesCS2B02G087400 | 32.281 | 0.955 | 1.508 | 0.604 | Inorganic ion transport; ascorbate peroxidase; Glutathione metabolism | |
| TraesCS3A02G493000 | 30.610 | 0.944 | 1.497 | 0.660 | Function unknown hypothetical protein TRIUR3 | |
| TraesCS7D02G258300 | 29.417 | 0.943 | 1.921 | 0.560 | Protein processing in endoplasmic reticulum; hydrolase | |
| TraesCS7A02G257500 | 29.376 | 0.941 | 1.797 | 0.594 | TCP family transcription factor; hydrolase | |
| red | TraesCS4D02G214100 | 226.124 | 0.982 | 5.440 | 4.174 | Positive regulation of mRNA splicing via spliceosome |
| TraesCS5A02G548000 | 225.290 | 0.980 | 40.422 | 35.575 | BAG family molecular chaperone regulator 6; IQ calmodulin-binding motif | |
| TraesCS4B02G208900 | 225.095 | 0.975 | 14.932 | 12.665 | Nucleotide exchange factor Fes1B | |
| TraesCS4D02G145500 | 223.512 | 0.965 | 403.199 | 310.521 | Hsp20/alpha crystallin family | |
| TraesCS6B02G374100 | 222.531 | 0.970 | 81.004 | 57.046 | Hsp20/alpha crystallin family | |
| turquoise | TraesCS7D02G369700 | 748.196 | 0.989 | 0.301 | 0.412 | Peroxidase P7; Phenylpropanoid biosynthesis |
| TraesCS3B02G280000 | 728.892 | 0.985 | 0.434 | 0.626 | Proton-transporting V-type ATPase | |
| TraesCS1D02G404600 | 727.511 | 0.984 | 0.346 | 0.525 | Plant-type secondary cell wall biogenesis; Fasciclin-like arabinogalactan protein | |
| TraesCS7A02G453100 | 727.024 | 0.980 | 0.260 | 0.448 | Function unknown | |
| TraesCS7A02G492400 | 722.960 | 0.986 | 0.307 | 0.517 | Auxin efflux carrier component; Intracellular trafficking, secretion, and vesicular transport | |
| TFs family | Al_up | Al_down | Al_nr | Total | |||
| Mn_up | Mn_nr | Mn_down | Mn_nr | Mn_up | Mn_down | ||
| MYB | 30 | 30 | 9 | 24 | 14 | 13 | 120 |
| WRKY | 35 | 21 | 0 | 3 | 54 | 1 | 114 |
| AP2 | 16 | 34 | 0 | 12 | 20 | 3 | 85 |
| bHLH | 7 | 6 | 11 | 8 | 21 | 4 | 57 |
| NAC | 8 | 19 | 4 | 3 | 4 | 7 | 45 |
| HSF | 25 | 5 | 0 | 2 | 2 | 0 | 34 |
| bZIP | 0 | 17 | 5 | 1 | 1 | 9 | 33 |
| HB | 2 | 16 | 1 | 2 | 0 | 3 | 24 |
| GRAS | 5 | 7 | 0 | 3 | 8 | 0 | 23 |
| B3 | 3 | 6 | 0 | 6 | 1 | 1 | 17 |
| LBD | 3 | 3 | 0 | 3 | 3 | 5 | 17 |
| Dof | 2 | 4 | 0 | 2 | 2 | 1 | 11 |
| MIKC | 3 | 4 | 0 | 0 | 2 | 1 | 10 |
| C2H2 | 4 | 3 | 0 | 2 | 0 | 0 | 9 |
| SRS | 6 | 3 | 0 | 0 | 0 | 0 | 9 |
| DBB | 2 | 0 | 2 | 1 | 0 | 1 | 6 |
| GATA | 0 | 2 | 0 | 2 | 0 | 1 | 5 |
| M_type | 0 | 2 | 0 | 1 | 1 | 0 | 4 |
| ARF | 1 | 3 | 0 | 0 | 0 | 0 | 4 |
| ZF-HD | 3 | 0 | 0 | 0 | 0 | 0 | 3 |
| GRF | 0 | 0 | 0 | 2 | 0 | 0 | 2 |
| BES1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| HD-ZIP | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| RAV | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| EIL | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| TCP | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| CAMTA | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| FAR1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| NF-YA | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Total | 156 | 190 | 32 | 77 | 135 | 50 | 640 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





