1. Introduction
Lung, in its functional capacity as a physical barrier of inhaled/airborne toxicants, is a direct target of adverse impact of chronic cigarette smoking (1, 2). The barrier function of lung epithelial cells allows gas exchange, protects against environmental insults and microbial pathogens, regulates ion and fluid transport, among others. Thus, the structural integrity of lung epithelium is a critical feature for normal lung function, and its disruption and dysfunction contribute to several serious diseases (3-5).
Chronic smoking is a major risk factor for several lung diseases, including chronic obstructive pulmonary disease (COPD), cancers, and other diseases (6). Several studies have shown that chronic smoking disrupts lung barrier function, which results in altered solute and macromolecule transport and infiltration of immune cells. These early events promote an unresolved pro-inflammatory environment, which progresses to a sustained chronic inflammatory state and eventually culminates into smoking-related diseases (1, 2, 4).
Cigarette smoke is a dynamic aerosol consisting of particulate and gas vapor phases. To date over 9,000 chemicals and toxicants have been identified in cigarette smoke (7), and 93 are established as Harmful and Potentially Harmful Constituents by the US FDA (8). Among these toxicants, Group 1 carcinogens (e.g., cadmium) (9) and respiratory toxicants (e.g., acrolein) (8), are reported to cause oxidative stress, lung injury and disease in susceptible individuals (6). Alternate tobacco products including orally consumed smokeless tobacco products, electronic nicotine delivery systems (ENDS) and heated tobacco products (HTPs), are not combusted and hence are chemically far less complex than cigarette smoke, and their health effects are an active area of research (10-12).
The modified risk tobacco product provisions, under the Food Drug & Cosmetics Act, may provide a valuable tool in the effort to promote public health by reducing morbidity and mortality associated with tobacco use (13). While the modified risk tobacco product application (MRTPA) does not necessarily require epidemiological evidence, several lines of scientific evidence may be required for evaluation of health risks at the individual and population levels (13). Smoking-induced diseases typically manifest after several decades of sustained smoking, and no surrogate biomarkers for these diseases are reported to date (14, 15).
In the absence of epidemiology, biomarkers of potential harm (BoPH), which are mechanistic, can be valuable interim tools for predicting health risks (16). Since the adverse effects of cigarette smoking persist for several months to years, BoPH, in a clinical study setting, inform of early improvements in biological responses following cessation or switching to candidate modified risk tobacco products. Among the broad range of methods to assess lung function, spirometry is a prominent one. Spirometric outputs such as forced expiratory volume 1 (FEV1) and Forced Vital Capacity (FVC) are well-established measures of lung function and extensively used as diagnostic markers of COPD. However, it typically takes several months to detect significant improvements in lung function following smoking cessation or switching to non-combustible tobacco products (17, 18). Therefore, other measures of lung physiology that are responsive to changes in smoking status relatively rapidly would be valuable tools in the assessment of candidate modified risk tobacco products.
One lung function measure that consistently differentiates smokers and non-smokers is lung permeability. Changes in lung barrier function in cigarette smokers have been quantified previously by 99mTC-DTPA imaging method (19). Smokers consistently exhibit increased lung permeability as demonstrated by the clearance of inhaled 99mTC-DTPA from their lungs, relative to non-smokers. Lung barrier function, as measured by the 99mTC-DTPA clearance method, returns rapidly to normal levels in smokers upon cessation (20), and is unaffected by exposure to nicotine alone (21).
The overall goal of this clinical study is to further qualify lung permeability by the 99mTC-DTPA clearance test as a BoPH for evaluation of tobacco products. The objective of this method development study was to assess the technical and logistical feasibility of conducting 99mTC-DTPA clearance test over several study visits among groups of different types of tobacco product consumers.
2. Materials and Methods
2.1. Lung Permeability Clinical Study design
This method development study was planned as a single-center, 4-group, 22-day ambulatory study at Celerion, Lincoln, NE, that aimed to enroll 24 generally healthy, asymptomatic male and female adult subjects per the eligibility criteria (). Following a Screening Visit, subjects were enrolled in to one of the following tobacco-use groups: smoker (SMK), moist snuff consumer (MSC), vaper (VAP), or non-tobacco consumer (NTC). Enrolled subjects completed an initial visit (Test Visit 1), in which blood samples were collected for biomarker analyses. This was followed with three additional visits (Test Visits 2, 3 and 4). During these Test Visits, study subjects completed three
99mTC-DTPA clearance tests, which were performed at one-week intervals (
Figure S1).
Due to the stringent inclusion and exclusion criteria (), recruitment of vapor consumers became challenging, and we were only able to enroll one subject into the VAP group. Therefore, no results from the VAP group are reported in this manuscript.
2.2. Ethical conduct of the study
The study was conducted under the approval of an accredited Institutional Review Board (Chesapeake, NE).
Clinicaltrials.gov Identifier: NCT06105424. This method development study was performed in compliance with FDA regulations as described in the Code of Federal Regulations (CFR) 21 Parts 50 and 56, Department of Health and Human Services regulations as described in 45 CFR 46, guidelines resulting from the International Council for Harmonisation (ICH) E6 Good Clinical Practice (GCP) which are consistent with the Declaration of Helsinki as adopted in 2008. Prior to initiation of any study-specific procedures, subjects received a copy of the Informed Consent Form (ICF) that summarized, in non-technical terms, the purpose of the study, the procedures to be carried out, and the potential hazards. Study procedures at Screening were initiated only after obtaining the signed informed consent.
2.3. Study subjects
Six SMK, 5 MSC and 6 NTC completed all the test visits and test procedures, and data from those evaluations are presented. Briefly, the inclusion/exclusion criteria (described in ), were that the subjects were generally healthy, between 30-50 years of age. More specifically, the SMK group were smokers who exclusively smoked 10-20 combustible, filtered cigarettes per day for at least 3 years prior to screening; the MSC group exclusively consumed ≥1 can of moist snuff per week for at least 6 months, and the NTC group did not use any tobacco/nicotine products for at least 5 years. No investigational product was administered in this study and all subjects continued their usual lifestyle and tobacco use (SMK and MSC only) through discharge from the study.
2.4. Safety evaluations
Several safety evaluations were performed prior to performing lung permeability tests. Full physical, oral, and nasopharyngeal examinations were performed at the Screening Visit. Vital signs were measured after subjects were seated for 5 minutes (blood pressure, pulse rate, and oral temperature) at Screening and at the beginning of each Test Visit. Blood pressure and pulse rate were additionally measured at the conclusion of each Test Visit (or upon early termination). Single 12-lead electrocardiogram were conducted at Screening. Clinical laboratory assessments consisted of serum chemistry, hematology, and urinalysis. Adverse Event (AE) information was collected throughout the study.
Spirometry was performed at Screening Visit before and after the administration of bronchodialator (albuterol) as a part of safety and was used as a measure of normal lung function.
2.5. Biomarkers of exposure
Exhaled carbon monoxide (ECO), blood nicotine and cotinine concentrations were measured as biomarkers of tobacco exposure. Plasma nicotine and cotinine were analyzed by Celerion Global Bioanalytical Services using liquid chromatography/mass spectrometry (LC/MS/MS) by validated analytical methods at each study visit per applicable sections of Good Laboratory Practice regulations (22). A urine cotinine screen was performed at screening. An ECO measurement was performed at Screening, Test Visit 1, and prior to the lung permeability procedure at Test Visits 2, 3, and 4.
2.6. Lung permeability assessment
The principle of this method is that the disruption of lung epithelial integrity results in increased permeability to solutes, and clearance of inhaled 99mTC-DTPA from lungs as measured by scintigraphy (19). Because of the injury from cigarette smoking, lung epithelial integrity is compromised, and smokers’ lungs are described as “leaky.” Consequently, the rate of disappearance of the labeled solute is faster compared non-smokers.
Subjects in the SMK and MSC group were required to abstain from tobacco products for 8-10h prior to lung permeability assessment. Thus, this study focused on chronic effects of tobacco use on lung permeability. All study subjects were trained to use an incentive spirometer and provided one to take home to practice deep, deep breaths that were required for the inhalation of aerosol for the lung permeability test.
2.7. Radiolabeled probe preparation
Individual doses of 99mTC-DTPA were prepared by Cardinal Health (Lincoln, Nebraska) on the morning of each Test Visit. The 99mTc-DTPA was diluted into sterile physiologic saline solution and ~3 mL of solution containing ~40 mCi was then loaded into a shielded Venti-Scan™ IV Radioaerosol dministration System (Biodex) and nebulized using an air flow rate of 10 liters/minute, producing a mean particle size of 0.5 microns. The radioactivity was measured by Cardinal Health prior to delivery, and a dose calibrator was used by the imaging technician to further ensure the appropriate amount of radioactivity was administered. The subject breathes the aerosol for 3 minutes, depositing approximately 1 mCi to the lungs and a 0.3 rem effective dose equivalent (assuming a 5-hour voiding schedule). The remainder of the 99mTC-DTPA that is not retained in the lungs either remains in the nebulizer or is filtered out and disposed per radiation safety regulations.
2.8. Scintigraphy
The study subjects were aligned in front of the gamma camera in a supine position for posterior imaging. Following the acquisition of a pre-dose image, a nose clip was put in place and the subject inhaled the aerosolized 99mTC-DTPA for approximately for 3 minutes while lying supine in front of a gamma camera to acquire sufficient activity (determined from gamma camera counts/minute) in the lung for imaging. Scintigraphy monitoring of thoracic radioactivity was performed immediately following inhalation of the radio-aerosol and consisted of dynamic acquisition of 60 images at 1-minute intervals. Using computer analysis, rectangular regions of interest (right lung) were used to determine the lung retention as a fraction of the initial decay corrected counts. The technician administering the test ensured that the images acquired were of high quality. Each image was reviewed by a qualified radiologist who was blinded to subject information.
2.9. Image analysis
The time to reach one-half of the peak intensity (T1/2) was determined for the total right lung by using the Digirad (Poway, CA) imaging software and reported as the “Linear Fit”.
2.10. Statistical Methods
Lung permeability T1/2 values were listed by subject and summarized by study groups and test visit using descriptive statistics (n, mean and standard deviation [SD]). Mean graphs for 99mTC-DTPA T1/2 versus test visit are also presented. The effects of tobacco user groups (TUG) for the effect of repeated visits lung permeability testing (LPT) on the lung permeability measurements were investigated by least squares regression model, in which LP is the response variable. TUG and LPT are the independent variables. In addition, the variations of TUG, LPT and subject-to-subjects were estimated by Bayesian Variance Decomposition method, using JMP software (v10).
3. Results
3.1. Demographics
We sought to collect lung permeability test data from 6 subjects from each of smoker, moist snuff and non-tobacco consumer groups. In this study, generally healthy individuals who met the study inclusion exclusion criteria were enrolled. Among the enrolled were 8 smokers (SMK), 5 moist snuff consumers (MSC), and 7 non-tobacco consumers (NTC)) (
Table 1). Eighteen (18) subjects completed the study and three were discontinued (
Supplemental Table S1). As indicated in Materials and Methods section, although a single vapor consumer also completed the study, those data are not included. Thus, data from 6 SMK (3 each male and females), 5 MSC (all male) and 6 NTC (4 females and 2 males) are presented in this manuscript. Since moist snuff is predominantly consumed by males, that group consisted of only male subjects. Overall, a majority of study subjects were Caucasian (90%) and two subjects were African American (10%); one subject belonged to Hispanic or Latino ethnicity.
Table 1. Study demographics. Majority of the subjects are male and white.
3.2. Adverse Events (AEs)
There were no Serious Adverse Events or subject discontinuations due to AEs in this study. A total of 18 mild AEs were reported by 9 (43%) subjects, with 3 subjects in the SMK, 4 subjects in the MSC, and 2 subjects in the NTC groups. Five (24%) subjects reported throat-related AEs including sore throat (2), burning sensation in throat (1), tender throat (1) (all in MSC); and one subject with itchy throat (SMK). The burning sensation in throat was considered to be study-related, tender throat and one sore throat event to be possibly related, and itchy throat and one sore throat event to be not related. Headache was reported by 2 (10%) subjects, both in the SMK. One subject received acetaminophen for headache resolution. The PI considered both headache events to be not related to study procedure. All AEs were mild in severity and resolved without any further follow up.
3.3. Spirometry
As a part of safety evaluations, spirometry was performed to ensure subjects exhibited normal lung function. Overall, FEV1% change ranged from -7 to +12%, with minimal changes noted in the MSC. Changes in FEV1: FVC ratio from pre- to post-bronchodilator time points were generally small across all groups (data not shown).
3.4. Biomarkers of exposure
Blood nicotine and cotinine were used as biomarkers of exposure for tobacco use, whereas ECO was used as a marker of combustion product use (cigarette smoking) (
Table 2). Thus, smokers are expected to exhibit measurable levels of ECO, nicotine and cotinine, whereas moist snuff users to exhibit measurable levels of nicotine and cotinine, but not to ECO. The non-tobacco users should have only baseline exposure to these three biomarkers. Accordingly, subjects in the MSC, and NTC groups had lower ECO levels relative to SMK. Nicotine and cotinine levels were below the level of quantification in NTC across the test visits. SMK and MSC showed readily detectable and comparable levels of cotinine (251-301 ng/ml range) attesting to their tobacco use status. Mean blood nicotine levels were in the range of 2.3- 5.1 ng/ml in SMK and MSC due to overnight abstinence from tobacco.
Table 2: Biomarkers of exposure: Tobacco biomarkers of exposure were measured at each test visit to verify the compliance of the subjects with the assigned tobacco use. ECO was measured in the exhaled breath and nicotine and cotinine were measured in blood of subjects who abstained from tobacco use (SMK and NTC). BLQ, below the level of quantification.
3.5. 99mTC-DTPA Lung Clearance
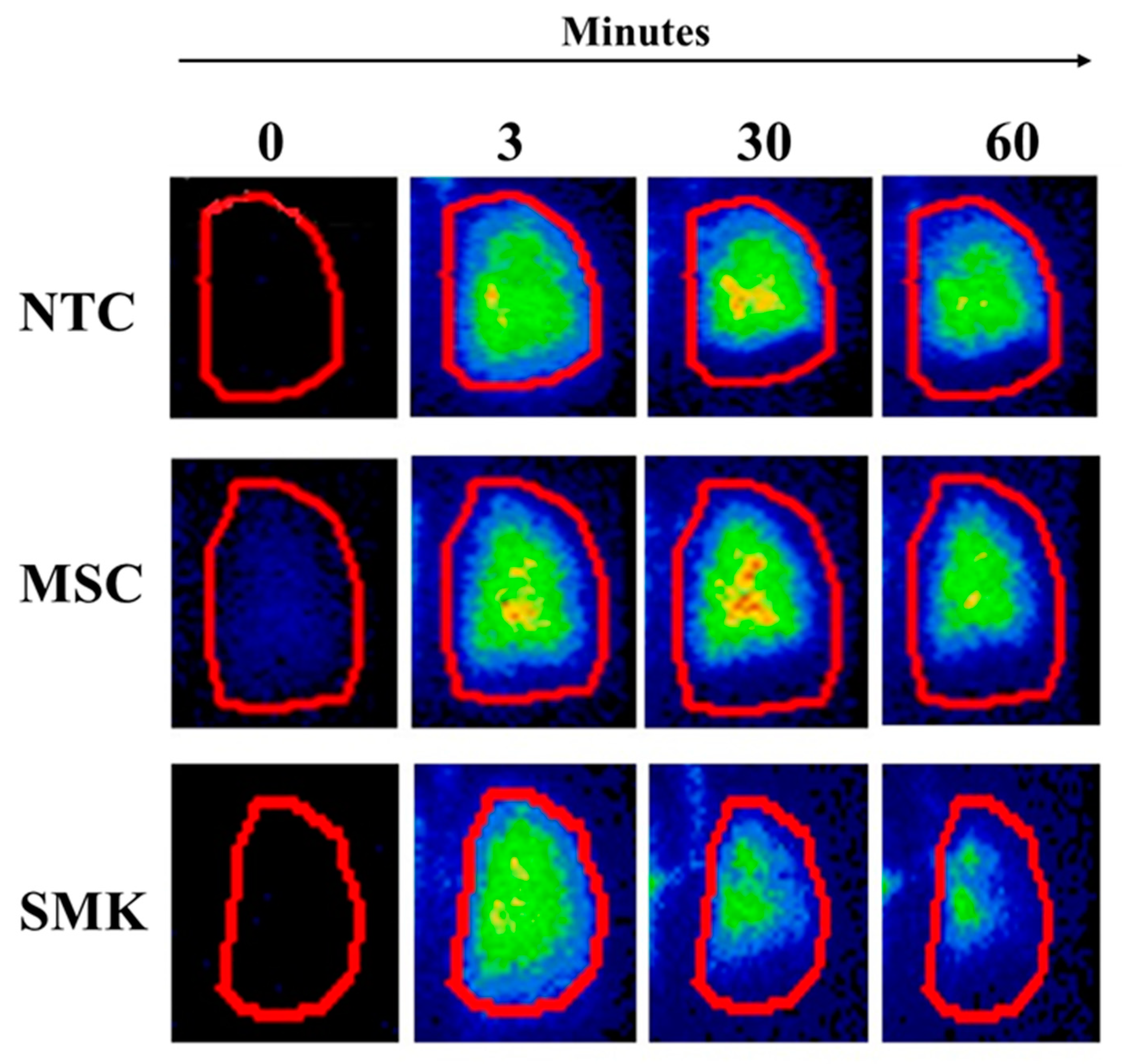
The lung permeability test was performed as a marker of lung epithelial barrier function using lung clearance of inhaled
99mTc-DTPA aerosol. In this method development study, we determined the logistical feasibility of assessing this BoPH in SMK, MSC and NTC groups. Smokers exhibited faster clearance (shorter T
1/2) of the inhaled probe compared to MSC and NTC at all test time points of scintigraphy, indicating altered lung permeability in SMK (
Figure 1). Mean lung permeability T
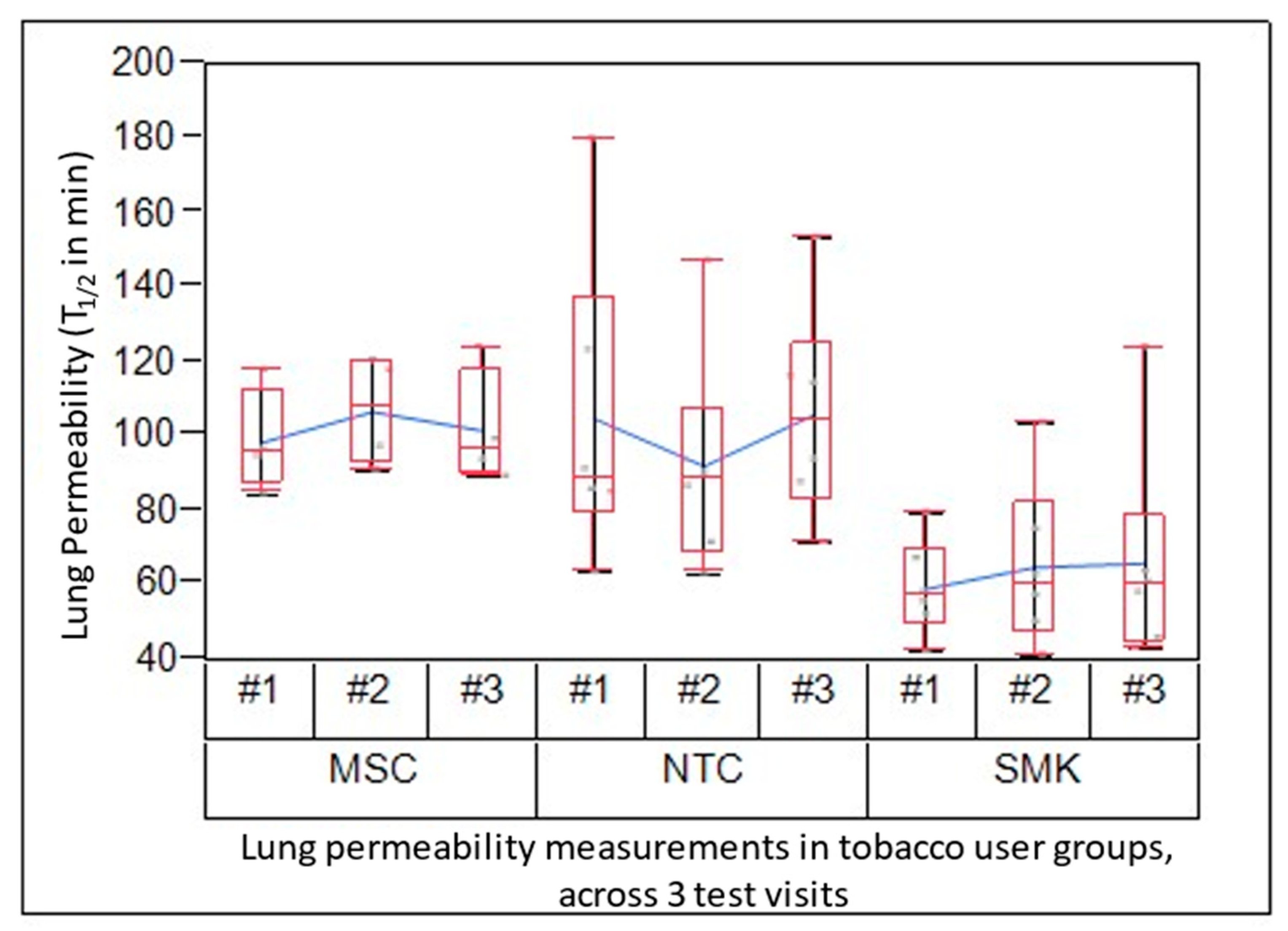
1/2 values in SMK, MSC and NTC were measured at Day 1 (Test Visit 1 baseline), Day 7 (Test Visit 2) and Day 14 (Test Visit 3). The combined lung clearance values of SMK ranged from 59 to 66 minutes. The mean lung clearance in NTC and MSC was similar (93 to 106 minutes NTC; 96 to 107 minutes MSC) (
Figure 2;
Table 3). The combined mean lung permeability values for SMK were 63.4 ± 20.9 min, while they were higher for NTC (101.4 ± 32.4 min) and MSC (99.4 ±15.4 min).
The effect of TUG was highly significant (p<0.0001), while LPT was not significant. Most of the variation was due to subject-to-subject (72%), while LPT only accounted for 4% variation (“measurement error” or repeatability). The TUG contributed to 24% variation, which indicated there were differences between groups.
These findings suggest that the variability of the test method among all subjects was minimal and not significant, across all the 3 test visits. Thus, lung permeability can be assessed reproducibly across multiple time points in tobacco consumers.
Table 3: 99mTC-DTPA clearance from lung in groups of SMK, MSC and NTC: Half-life values (min) of lung clearance of inhaled 99mTC-DTPA are presented. Group mean and standard deviation values at each visit are presented, as well as combined mean ± standard deviation for the group from all three study visits is also provided.
1Combined mean is the average of all individual data points from the three visits in each cohort; § n=7; *n=4. Due to a technical issue during the LP scan, data from one MSC subject on Test Visit 3 was not obtained.
4. Discussion
With a goal to develop and qualify short-term BoPH that inform of lung function for the evaluation of candidate modified risk tobacco products, we have assessed lung permeability in this pilot study. Here we show the technical and logistical feasibility of assessing lung permeability by 99mTC-DTPA inhalation technique in groups of tobacco consumers across several study visits. Further, lung permeability is significantly higher in smokers, relative to moist snuff consumers, indicating that it is a useful test in differentiating the adverse effects of combustible tobacco products from candidate modified risk tobacco products which could reduce harm from cigarette smoking.
Biomarkers are useful tools for estimating the exposure from Harmful and Potentially Harmful Constituents (HPHCs) and informing of the effect/potential harm from the exposure to toxins. While several types of BoPH have been advocated, there are limited biomarkers that directly assess the adverse effects of smoking on lung function (23). Spirometry is a simple and widely used biomarker for measuring lung function and in the diagnosis and staging of COPD (24). Sustained smoking cessation is key for any desired improvements in lung function. The spirometry measures, e.g., FEV1, do not differ between asymptomatic smokers and nonsmokers. However, smoking cessation slows the rate of decline in FEV1 (18, 25). In general, lung function, particularly spirometry indices, take several months to recover following cessation, and factors such as duration of smoking, age, baseline lung function and others contribute to the recovery of lung function (18, 25). For example, a 1-year randomized clinical trial detected no significant effect on spirometry indices (FEV1, FVC and FEV1/FVC) in smokers who quit smoking, reduced cigarette consumption and used e-cigarettes, or who failed to reduce smoking (17). In this study, we employed spirometry as a part of safety assessments, and it did not differ among the study subjects regardless of their tobacco product use.
Assessment of lung permeability by the 99mTC-DTPA inhalation technique is minimally invasive and is well tolerated. This method is an established nuclear medicine technique and involves a very low energy probe with a very short biological half life 2.5 h (26). The study-related AEs were mild and resolved with minimal care. Nevertheless, a need to balance the exposure of study subjects to radiation, although minimal, warrants additional consideration.
The qualification of BoPH is a rigorous process that requires consideration of several criteria (14, 16). Two criteria known as “experiment” and “temporality” are applicable to the relationship of lung permeability to the status of smoking (14, 16). Altered lung permeability has been demonstrated in smokers, detected under acute and chronic smoking, and is reversible upon smoking cessation (20, 27). Partial improvements in lung permeability under smoking abstinence or switching to non-combustible tobacco products could be due to dual use and/or the relatively short study duration (20, 28). Lung permeability measurements in this study showed little variation within the subject across three different visits, across the three groups, attesting to the repeatability of this method. In agreement with our findings, Morrison et al. demonstrated that lung permeability measurements showed minimal variability in two separate study visits (29).
Consistent differences in lung permeability between smokers and non-smokers have been demonstrated (19), and lung permeability returned to normal levels in smokers who abstained for short periods of time. Further, detectable improvements in lung permeability were reported in smokers who switched to Eclipse, an early model combustion-free tobacco heating product, for two weeks indicating that reducing exposure to smoke toxicants improves lung function (28); it should be noted that statistical significance was not achieved in the Eclipse study due to non-compliance of study subjects and/or a need for longer duration of exclusive switch to this product.
Some earlier reports have investigated the applicability of the 99mTC-DTPA inhalation technique for diagnosis/prognosis diseases such as idiopathic fibrosis and silicosis (30-34). Because of extensive biochemical and inflammatory changes involved in the progression of lung diseases, some of which are irreversible, lung tissue undergoes profound remodeling, particularly at the advanced stages (2, 3). As a result, other tools (such as spirometry and other biomarkers) that are proximal to the clinical disease state are deemed more useful in disease prognosis and diagnosis (35).
Mechanistically, disruption of lung barrier function (permeability) is an early indicator of lung injury, and cigarette smoke-induced oxidative stress is a driver of enhanced lung permeability in smokers (6). Oxidative stress caused by HPHCs such as acrolein and crotanaldehyde are hypothesized to disrupt lung epithelial integrity (36-39). Morrison et al. have demonstrated increase lung permeability (lung clearance of 99mTC-DTPA) in smokers under tobacco abstinence for 12h (chronic) and 1h after smoking (acute), which was accompanied by increases in markers of inflammation and oxidative stress (27). However, epithelial injury by ozone in normal subjects failed to show an increase in lung permeability and oxidative stress, while detecting increased neutrophil flux in lungs (40). At the cellular level, cigarette smoke HPHC toxicants such as acrolein have been shown to disrupt tight junctions and increase cellular permeability (41, 42). Cadmium, which is also a HPHC, impaired epithelial integrity through disruption of tight junctions (43). Therefore, the lung permeability BoPH may be viewed as an early clinical risk marker (16).
Among the limitations, keeping with the primary objective of method development, this study was not powered to determine statistically significant differences among various experimental parameters. However, the lung permeability measurement itself accounts for minimal variability (“measurement error” or repeatability). Discernable differences in lung permeability between the three TUG were evident. Second, a technical problem prevented using the scan data for one subject on one test visit; although resolved, it underscores the need to ensure and verify the correct function of instruments prior to use in a clinical study setting. Further qualification of this BoPH and its utilization as a clinical risk marker will require mechanistic investigations into altered alveolar permeability using cellular models and a demonstration of robust improvements upon smoking cessation and/or switching to candidate reduced risk in adequately powered studies.
In summary, lung permeability can be used as a BoPH, along with other biomarkers described previously (44, 45) for differentiating the effects of cigarette smoking from the use of oral and inhaled alternate tobacco products that do not combust. Our results demonstrate that this BoPH can be measured in a clinical setting repeatedly and can be used to differentiate TUGs.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
GLP conceived the study. GLP and PM designed research; PM, BJ, and SBW planned and supervised the study, and PC analyzed data; PM and GLP interpreted results of the study; PM, SBW and GLP drafted the manuscript, revised, and finalized.
Funding
This work is funded by RAI Services Company.
Conflicts of Interest:
P. Makena: S. Baxter-Wright and P. Chen are employees of RAI Services Company. RAI Services Company is a wholly owned subsidiary of Reynolds American, Inc., which is a wholly owned subsidiary of British American Tobacco plc. G. L. Prasad is a former employee of RAI Services Company, and currently works as an independent consultant. The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Tam A, Wadsworth S, Dorscheid D, Man SF, Sin DD. The airway epithelium: more than just a structural barrier. Ther Adv Respir Dis. 2011;5(4):255-73. [CrossRef]
- Thorley AJ, Tetley TD. Pulmonary epithelium, cigarette smoke, and chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007;2(4):409-28.
- Camelo A, Dunmore R, Sleeman MA, Clarke DL. The epithelium in idiopathic pulmonary fibrosis: breaking the barrier. Front Pharmacol. 2014;4:173. [CrossRef]
- De Rose V, Molloy K, Gohy S, Pilette C, Greene CM. Airway Epithelium Dysfunction in Cystic Fibrosis and COPD. Mediators Inflamm. 2018;2018:1309746. [CrossRef]
- Strzelak A, Ratajczak A, Adamiec A, Feleszko W. Tobacco Smoke Induces and Alters Immune Responses in the Lung Triggering Inflammation, Allergy, Asthma and Other Lung Diseases: A Mechanistic Review. International Journal of Environmental Research and Public Health. 2018;15(5):1033. [CrossRef]
- Centers for Diseases Control and Prevention. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Atlanta (GA)2010.
- Rodgman A, Perfetti TA. The Chemical Components of Tobacco and Tobacco Smoke. 2 ed: CRC Press; 2013, 2013. 2332 p. 25 February.
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Tobacco Products. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke; Established List. In: U.S. Department of Health and Human Services, editor. Federal Register2012. p. 20034-7.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. A review of human carcinogens. 100 E: Personal habits and indoor combustions Lyon, France2012. 598 p.
- Forster M, Fiebelkorn S, Yurteri C, Mariner D, Liu C, Wright C, et al. Assessment of novel tobacco heating product THP1.0. Part 3: Comprehensive chemical characterisation of harmful and potentially harmful aerosol emissions. Regul Toxicol Pharmacol. 2018;93:14-33.
- National Academies of Sciences E, Medicine. Public Health Consequences of E-Cigarettes. Stratton K, Kwan LY, Eaton DL, editors. Washington, DC: The National Academies Press; 2018. 774 p.
- US Department of Health and Human Services. About Electronic Cigarettes (E-Cigarettes). In: US Department of Health and Human Services, editor. Atlanta, GA: Centers for Disease Control and Prevention; 2022.
- U.S. Department of Health and Human Services FaDA, Center for Tobacco Products. Modified Risk Tobacco Product Applications. Draft Guidance. In: U.S. Department of Health and Human Services, editor. 2012.
- Medicine) IIo. Evaluation of biomarkers and surrogate endpoints in chronic disease. Washington, DC: The National Academies Press.; 2010.
- Medicine). IIo. Scientific Standards for Studies on Modified Risk Tobacco Products. Washington, DC: The National Academies Press.; 2012.
- Chang CM, Cheng YC, Cho TM, Mishina EV, Del Valle-Pinero AY, van Bemmel DM, et al. Biomarkers of Potential Harm: Summary of an FDA-Sponsored Public Workshop. Nicotine Tob Res. 2019;21(1):3-13. [CrossRef]
- Cibella F, Campagna D, Caponnetto P, Amaradio MD, Caruso M, Russo C, et al. Lung function and respiratory symptoms in a randomized smoking cessation trial of electronic cigarettes. Clin Sci (Lond). 2016;130(21):1929-37. [CrossRef]
- Pride, NB.Smoking cessation: effects on symptoms, spirometry and future trends in COPD. Thorax. 2001;56(suppl 2):ii7-ii10.
- Jones JG, Minty BD, Lawler P, Hulands G, Crawley JC, Veall N. Increased alveolar epithelial permeability in cigarette smokers. Lancet. 1980;1(8159):66-8. [CrossRef]
- Minty BD, Jordan C, Jones JG. Rapid improvement in abnormal pulmonary epithelial permeability after stopping cigarettes. Br Med J (Clin Res Ed). 1981;282(6271):1183-6. [CrossRef]
- Minty BD, Royston D, Jones JG, Hulands GH. The effect of nicotine on pulmonary epithelial permeability in man. Chest. 1984;86(1):72-4. [CrossRef]
- Food and Drug Administration H. Guidance for Industry on Bioanalytical Method Validation; Availability In: Services HaH, editor.: Federal Register; 2001. p. 28526-7.
- Institute of Medicine 2001. Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. Washington, DC: The National Academies Press.; 2001.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). SPIROMETRY FOR HEALTH CARE PROVIDERS. 2010.
- Willemse BW, Postma DS, Timens W, ten Hacken NH. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur Respir J. 2004;23(3):464-76.
- Radiopaedia. Tc-99m DTPA. Available online: https://radiopaedia.org/articles/tc-99m-dtpa?lang=us.
- Morrison D, Rahman I, Lannan S, MacNee W. Epithelial permeability, inflammation, and oxidant stress in the air spaces of smokers. Am J Respir Crit Care Med. 1999;159(2):473-9. [CrossRef]
- Stewart JC, Hyde RW, Boscia J, Chow MY, O’Mara RE, Perillo I, et al. Changes in markers of epithelial permeability and inflammation in chronic smokers switching to a nonburning tobacco device (Eclipse). Nicotine Tob Res. 2006;8(6):773-83. [CrossRef]
- Morrison D, Skwarski K, Millar AM, Adams W, MacNee W. A comparison of three methods of measuring 99mTc-DTPA lung clearance and their repeatability. Eur Respir J. 1998;11(5):1141-6.
- Effros RM, Mason GR, Mena I. 99mTc-DTPA aerosol deposition and clearance in COPD, interstitial disease, and smokers. J Thorac Imaging. 1986;1(2):54-60.
- Kanazawa, M.[Permeability changes in bronchiolo-alveolar epithelium]. Nihon Kyobu Shikkan Gakkai Zasshi. 1989;27(5):556-60.
- Karacavus S, Intepe YS. The role of Tc-99m DTPA aerosol scintigraphy in the differential diagnosis of COPD and asthma. Clin Respir J. 2015;9(2):189-95. [CrossRef]
- Mogulkoc N, Brutsche MH, Bishop PW, Murby B, Greaves MS, Horrocks AW, et al. Pulmonary (99m)Tc-DTPA aerosol clearance and survival in usual interstitial pneumonia (UIP). Thorax. 2001;56(12):916-23. [CrossRef]
- Seven A, Sengül R, Sahin G, Candan G, Esen N, Celikoğlu F, et al. Permeability of the respiratory membrane in healthy, non-smoking controls and patients with sarcoidosis and chronic obstructive lung disease. Biochem Soc Trans. 1993;21 ( Pt 3)(3):309s. [CrossRef]
- Goh NS, Desai SR, Anagnostopoulos C, Hansell DM, Hoyles RK, Sato H, et al. Increased epithelial permeability in pulmonary fibrosis in relation to disease progression. Eur Respir J. 2011;38(1):184-90.
- Aoshiba K, Nagai A. Oxidative stress, cell death, and other damage to alveolar epithelial cells induced by cigarette smoke. Tob Induc Dis. 2003;1(3):219-26.
- Budinger GR, Mutlu GM, Urich D, Soberanes S, Buccellato LJ, Hawkins K, et al. Epithelial cell death is an important contributor to oxidant-mediated acute lung injury. Am J Respir Crit Care Med. 2011;183(8):1043-54. [CrossRef]
- Davidovich N, DiPaolo BC, Lawrence GG, Chhour P, Yehya N, Margulies SS. Cyclic stretch-induced oxidative stress increases pulmonary alveolar epithelial permeability. Am J Respir Cell Mol Biol. 2013;49(1):156-64. [CrossRef]
- Facchinetti F, Amadei F, Geppetti P, Tarantini F, Di Serio C, Dragotto A, et al. Alpha,beta-unsaturated aldehydes in cigarette smoke release inflammatory mediators from human macrophages. Am J Respir Cell Mol Biol. 2007;37(5):617-23.
- Morrison D, Rahman I, MacNee W. Permeability, inflammation and oxidant status in airspace epithelium exposed to ozone. Respir Med. 2006;100(12):2227-34. [CrossRef]
- Olivera D, Knall C, Boggs S, Seagrave J. Cytoskeletal modulation and tyrosine phosphorylation of tight junction proteins are associated with mainstream cigarette smoke-induced permeability of airway epithelium. Exp Toxicol Pathol. 2010;62(2):133-43. [CrossRef]
- Rounds S, Lu Q. Cigarette smoke alters lung vascular permeability and endothelial barrier function (2017 Grover Conference Series). Pulm Circ. 2018;8(3):2045894018794000. [CrossRef]
- Cao X, Lin H, Muskhelishvili L, Latendresse J, Richter P, Heflich RH. Tight junction disruption by cadmium in an in vitro human airway tissue model. Respir Res. 2015;16(1):30. [CrossRef]
- Prasad GL, Jones BA, Chen P, Gregg EO. A cross-sectional study of biomarkers of exposure and effect in smokers and moist snuff consumers. Clin Chem Lab Med. 2016;54(4):633-42. [CrossRef]
- Tran QT, Arimilli S, Scott E, Chen P, Prasad GL. Differences in biomarkers of inflammation and immune responses in chronic smokers and moist snuff users. Cytokine. 2021;137:155299.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).