Submitted:
06 December 2023
Posted:
06 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Culture and Experimental Condition
2.2. Comparison of Uptake and Translocation Between Organic Se and Inorganic Se in Wheat Weedlings
2.3. Effects of Aquaporin Inhibition on Uptake of SeMet and SeOMet
2.4. Uptake, transformation and interaction of SeMet and SeOMet in wheat seedlings at different times
2.5. Analysis of Total Se and Se Speciation in Plant Tissues
2.6. Data Analysis
3. Results
3.1. Uptake and Translocation of Different Se Treatments in Wheat Seedlings
3.2. Effects of aquaporin inhibition on uptake of SeMet and SeOMet
3.3. Uptake, translocation and interaction of SeMet and SeOMet in wheat seedlings in different time
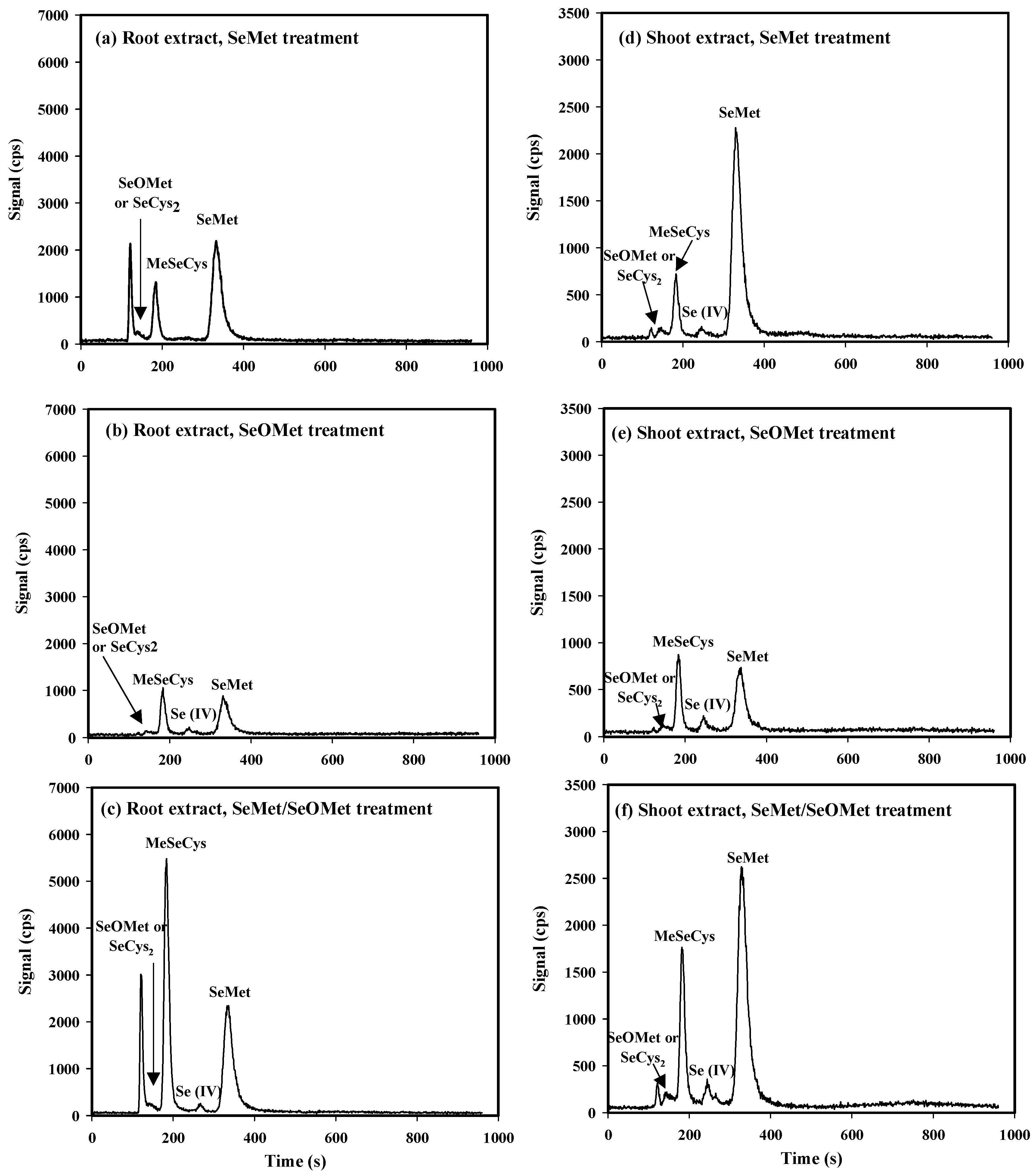
3.4. Transformation of SeMet and SeOMet in Wheat Seedlings
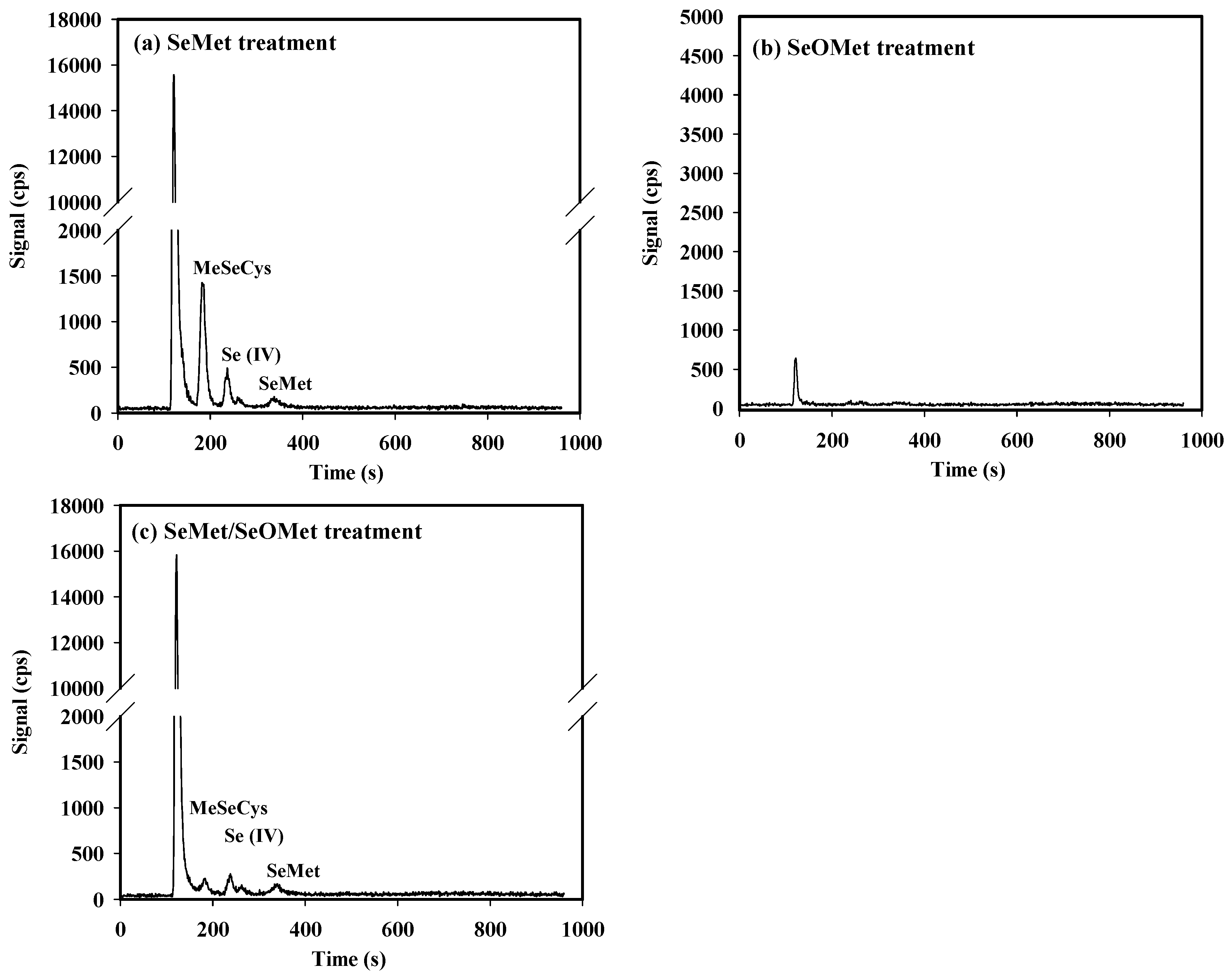
3.5. Se Concentration and Speciation in Xylem Saps
4. Discussion
4.1. Difference of The Uptake and Translocation between Organic Se and Inorganic Se in Wheat Seedlings
4.2. Transformation of SeMet and SeOMet in Wheat Seedlings
4.3. Interaction between SeMet and SeOMet in Wheat Seedlings
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [CrossRef]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Sign. 2011, 14, 1337–1383. [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [CrossRef]
- Haug, A.; Graham, R.D.; Christophersen, O.A; Lyons, G.H. How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microb. Ecol. Health Dis. 2007, 19, 209–228.
- Lv, Y.Y; Yu, T.; Yang, Z.F.; Zhao, W.F.; Zhang, M.; Wang, Q. Constraint on selenium bioavailability caused by its geochemical behavior in typical KaschinBeck disease areas in Aba, Sichuan Province of China. Sci. Total Environ. 2014, 493, 737–749. [CrossRef]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.R.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminen, P.; Hietaniemi, V.; Aspila, P.; Aro, A. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: from deficiency to optimal selenium status of the population. J. Trace Elem. Med. Bio. 2015, 31, 142–147. [CrossRef]
- de Oliveira, A.P.; Naozuka, J.; Landero-Figueroa, J.Á. Effects of Se (IV) or Se (VI) enrichment on proteins and protein-bound Se distribution and Se bioaccessibility in oyster mushrooms. Food Chem. 2022, 383, 132582. [CrossRef]
- Galinha, C.; Sánchez-Martínez, M.; Pacheco, A.M.G.; Freitas, M.D.; Coutinho, J.; Maças, B.; Almeida, A.S.; Pérez-Corona, M.T.; Madrid, Y.; Wolterbeek, H.T. Characterization of selenium-enriched wheat by agronomic biofortification. J. Food Sci. Tech. Mys. 2015, 52, 4236–4245. [CrossRef]
- Trippe III, R.C.; Pilon-Smits, E.A.H. Selenium transport and metabolism in plants: phytoremediation and biofortification implications. J. Hazard. Mater. 2021, 404, 124178. [CrossRef]
- Neal, R.H.; Sposito, G.; Holtzclaw, K.; Traina, S. Selenite adsorption on alluvial soils: soil composition and pH effects. Soil Sci. Soc. Am. J. 1987, 51, 1161–1165. [CrossRef]
- El Mehdawi, A.F.; Jiang, Y.; Guignardi, Z.S.; Esmat, A.; Pilon, M.; Pilon-Smits, E.A.; Schiavon, M. Influence of sulfate supply on selenium uptake dynamics and expression of sulfate/selenate transporters in selenium hyperaccumulator and nonhyperaccumulator Brassicaceae. New Phytol. 2018, 217, 194–205. [CrossRef]
- Wang, M.K.; Peng, Q.; Zhou, F.; Yang, W.X.; Dinh, Q.T.; Liang, D.L. Uptake kinetics and interaction of selenium species in tomato (Solanum lycopersicum L.) seedlings. Environ. Sci. Pollut. R. 2019, 26, 9730–9738. [CrossRef]
- Zhao, X.Q.; Mitani, N.; Yamaji, N.; Shen, R.F.; Ma, J.F. Involvement of silicon influx transporter OsNIP2;1 in selenite uptake in rice. Plant Physiol. 2010, 153, 1871–1877. [CrossRef]
- Zhang, L.H.; Hu, B.; Li, W.; Che, R.H.; Deng, K.; Li, H.; Yu, F.Y.; Ling, H.Q.; Li, Y.J.; Chu, C.C. OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytol. 2014, 201, 1183–1191. [CrossRef]
- Li, H.F.; McGrath, S.P.; Zhao, F.J. Selenium uptake, translocation and speciation in wheat supplied with selenate or selenite. New Phytol. 2008, 178, 92–102. [CrossRef]
- Longchamp, M.; Castrec-Rouelle, M.; Biron, P.; Bariac, T. Variations in the accumulation, localization and rate of metabolization of selenium in mature Zea mays plants supplied with selenite or selenate. Food Chem. 2015, 182, 128-135. [CrossRef]
- Abrams, M.; Burau, R.G.;Zasoski, R.J. Organic selenium distribution in selected California soils. Soil Sci. Soc. Am. J. 1990, 54, 979–982. [CrossRef]
- Li, H.F.; Lombi, E.; Stroud, J.L.; Mcgrath, S.P.; Zhao, F.J. Selenium speciation in soil and rice: influence of water management and Se fertilization. J. Agr. Food Chem. 2010, 58, 11837–11843. [CrossRef]
- Kieliszek, M.; Bła˙zejak, S. Selenium: significance, and outlook for supplementation. Nutriton 2013, 29, 713–718. [CrossRef]
- Kowalska, I.; Smole’n, S.; Czernicka, M.; Halka, M.; Keska, K.; Pitala, J. Effect of selenium form and Salicylic acid on the accumulation of selenium speciation forms in hydroponically grown lettuce. Agriculture 2020, 10, 584. [CrossRef]
- Hu, C.X.; Nie, Z.J.; Shi, H.Z.; Peng, H.Y.; Li, G.X.; Liu, H.Y.; Li, C.; Liu, H.E. Selenium uptake, translocation, subcellular distribution and speciation in winter wheat in response to phosphorus application combined with three types of selenium fertilizer. BMC Plant Biol. 2023, 23, 224. [CrossRef]
- Wang, Q.; Kong, L.X.; Huang, Q.Q.; Li, H.F.; Wan, Y.A. Uptake and translocation mechanisms of different forms of organic selenium in rice (Oryza sativa L.). Front. Plant Sci. 2022, 13, 970480. [CrossRef]
- Zhang, L.H.; Hu, B.; Deng, K.; Gao, X.K.; Sun, G.X.; Zhang, Z.L.; Li, P.; Wang, W.; Li, H.; Zhang, Z.H.; Fu, Z.H; Yang, J.Y; Gao, S.P.; Li, L.G; Yu, F.Y.; Li, Y.J.; Ling, H.Q.; Chu, C.C. NRT1.1B improves selenium concentrations in rice grains by facilitating selenomethinone translocation. Plant Biotechnol. J. 2019, 17, 1058–1068. [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil 2008, 302 , 1–17. [CrossRef]
- Broadley, M.R.; White, P.J.; Bryson, R.J.; Meacham, M.C.; Bowen, H.C.; Johnson, S.E.; Hawkesford, M.J.; McGrath, S.P.; Zhao, F.J.; Breward, N.; Harriman, M.; Tucker, M. Biofortification of UK food crops with selenium. P. Nutr. Soc. 2011, 65, 169–181. [CrossRef]
- Larsen, E.H.; Hansen, M.; Paulin, H.; Sven, M.; Mary, R.; Margaret, R. Speciation and bioavailability of selenium in yeast-based intervention agents used in cancer chemoprevention studies. J. Aoac Int. 2004, 87, 225–232.
- Zhang, L.H.; Shi, W.M.; Wang, X.C. Difference in selenite absorption between high- and low-selenium rice cultivars and its mechanism. Plant Soil 2006, 282, 183–193. [CrossRef]
- Fujii, R.; Deverel, S.J.; Hatfield, D.B. Distribution of selenium in soils of agricultural fields, western San Joaquin Valley, California. Soil Sci. Soc. Am. J. 1988, 52, 1274–1283. [CrossRef]
- Niemietz, C.M.; Tyerman, S.D. New potent inhibitors of aquaporins: silver and gold compounds inhibit aquaporins of plant and human origin. Febs Lett. 2002, 531, 443–447. [CrossRef]
- Huang, Q.Q.; Yu, Y.; Wang, Q.; Luo, Z.; Jiang, R.F.; Li, H.F. Uptake kinetics and translocation of selenite and selenate as affected by iron plaque on root surfaces of rice seedlings. Planta 2015, 241, 907–916. [CrossRef]
- Kikkert, J.; Berkelaar, E. Plant uptake and translocation of inorganic and organic forms of selenium. Arch. Environ. Con. Tox. 2013, 65, 458–465. [CrossRef]
- Wang, M.K.; Dinh, Q.T.; Qi, M.X.; Wang, M.; Yang, W.X.; Zhou, F., Liang, D.L. Radicular and foliar uptake, and xylem- and phloem-mediated transport of selenium in maize (Zea mays L.): a comparison of five Se exogenous species. Plant Soil 2020, 446, 111–123. [CrossRef]
- Xu, H.Z.; Yan, J.P.; Qin, Y.; Xu, J. M.; Shohag, M.J.I.; Wei, Y.Y.; Gu, M.H. Effect of different forms of selenium on the physiological response and the cadmium uptake by rice under cadmium stress. Int. J. Env. Res. Pub. He. 2020, 17, 6991. [CrossRef]
- Hirner, A.; Ladwig, F.; Stransky, H.; Okumoto, S.; Keinath, M.; Harms, A.; Frommer, W.B.; Koch, W. Arabidopsis LHT1 is a high-affinity transporter for cellular aminoacid uptake in both root epidermis and leaf mesophyll. Plant Cell 2006, 18, 1931–1946. [CrossRef]
- Hu, T.; Li, H.F.; Li, J.X.; Zhao, G.S.; Wu, W.L.; Liu, L.P.; Wang, Q.; Guo, Y.B. Absorption and bio-transformation of selenium nanoparticles by wheat seedlings (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 597. [CrossRef]
- Wang, K.; Wang, Y.Q.; Li, K.; Wan, Y.N.; Wang, Q.; Zhuang, Z.; Guo, Y.B.; Li, H.F. Uptake, translocation and biotransformation of selenium nanoparticles in rice seedlings (Oryza sativa L.). J. Nanobiotechnol. 2020, 18, 103. [CrossRef]
- Deng, X.F.; Zhao, Z.Q.; Lv, C.H.; Zhang, Z.Z.; Yuan, L.X.; Liu, X.W. Effects of sulfur application on selenium uptake and seed selenium speciation in soybean (Glycine max L.) grown in different soil types. Ecotoxicol. Environ. Saf. 2021, 209, 111790. [CrossRef]
- Zhou, F.; Yang, W.X.; Wang, M.K.; Miao, Y.X.; Li, Z.; Liang, D.L. Effects of selenium application on Se content and speciation in Lentinula edodes. Food Chem. 2018, 265, 182–188. [CrossRef]
- Maruyama-Nakashita, A. Metabolic changes sustain the plant life in low-sulfur environments. Curr. Opin. Plant Biol., 2017, 39, 144–151. [CrossRef]
- Song, Z.; Shao, H.; Huang, H.; Shen, Y.; Wang, L.; Wu, F.; Han, D.; Song, J.Y.; Jia, H.F. Overexpression of the phosphate transportergene OsPT8 improves the Pi and selenium contents in Nicotiana tabacum. Environ. Exp. Bot. 2017, 137, 158–165. [CrossRef]
- Gong, R.; Ai, C.; Zhang, B.; Cheng, X. Effect of selenite on organic selenium speciation and selenium bioaccessibility in rice grains of two Se-enriched rice cultivars. Food Chem. 2018, 264, 443–448. [CrossRef]
- da Silva, D.F.; Cipriano, P.E.; de Souza, R.R.; Júnior, M.S.; da Silva, R.F.; Faquin, V.; Silva, M.L.D.; Guilherme, L.R.G. Anatomical and physiological characteristics of Raphanus sativus L. submitted to different selenium sources and forms application. Sci. Hortic-Amsterdam 2020, 260, 108839. [CrossRef]
- Xiao, T.T.; Boada, R.; Marini, C.; Mercè, L.; Valiente, M. Influence of a plant biostimulant on the uptake, distribution and speciation of se in se-enriched wheat (Triticum aestivum L. cv. Pinzón). Plant Soil 2020, 455, 409–423. [CrossRef]
- Wang, M.; Ali, F.; Qi, M.X.; Peng, Q.; Wang, M.K.; Ba˜nuelos, G.;Miao, S.Y.; Li, Z.; Dinh, Q.T.; Liang, D.L. Insights into uptake, accumulation, and subcellular distribution of selenium among eight wheat (Triticum aestivum L.) cultivars supplied with selenite and selenate. Ecotoxicol. Environ. Saf. 2021, 207, 111544. [CrossRef]
- Ogra, Y.; Ogihara, Y.; Anan, Y. Comparison of the metabolism of inorganic and organic selenium species between two selenium accumulator plants, garlic and Indian mustard. Metallomics 2017, 9, 61–68. [CrossRef]
- Mazej. D.; Falnoga, I.; Veber, M.; Stibilj, V. Determination of selenium species in plant leaves by HPLC-UV-HG-AFS. Talanta 2006, 68, 558–568. [CrossRef]
- Mazej, D.; Osvald, J.; Stibilj, V. Selenium species in leaves of chicory, dandelion, lamb’s lettuce and parsley. Food Chem. 2008, 107, 75–83. [CrossRef]
- Chen, N.; Zhao, C.H.; Zhang, T.H. Selenium transformation and selenium-rich foods. Food Biosci. 2021, 40, 100875. [CrossRef]
- Ari, B.; Oz, E.; Can, S.Z.; Bakirdere, S. Bioaccessibility and bioavailability of selenium species in Se-enriched leeks (Allium Porrum) cultivated by hydroponically. Food Chem. 2022, 372, 131314. [CrossRef]
- Zhang, K.; Guo, X.; Zhao, Q.; Han, Y.; Zhan, T.; Li, Y.; Tang, C.H.; Zhang, J.M. Development and application of a HPLC-ICP-MS method to determine selenium speciation in muscle of pigs treated with different selenium supplements. Food Chem. 2020, 302, 125371. [CrossRef]
- Rayman, M.P.; Infante, H.G.; Sargent, M. Food-chain selenium and human health: spotlight on speciation. Brit. J. Nutr. 2008, 100, 238–253. [CrossRef]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: current knowledgeand future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484s−1491s. [CrossRef]
- Zhang, L.H.; Chu, C.C. Selenium uptake, transport, metabolism, reutilization, and biofortification in rice. Rice 2022, 15, 30. [CrossRef]
- Kieliszek, M.; Bła˙zejak, S.; Gientka, I.; Bzducha-Wróbel, A. Accumulation and metabolism of selenium by yeast cells. Appl. Microbiol. 2015, 99, 5373–5382. [CrossRef]
- Versini, A.; Di Tullo, P.; Aubry, E.; Bueno, M.; Thiry, Y.; Pannier, F.; Castrec-Rouelle, M. Influence of Se concentrations and species in hydroponic cultures on Se uptake, translocation and assimilation in non-accumulator ryegrass. Plant Physiol. Bioch. 2016, 108, 372–380. [CrossRef]
- Kieliszek, M.; Bła˙zejak, S.; Bzducha-Wróbel, A.; Kot, A.M. Effect of selenium on growth and antioxidative system of yeast cells. Mol. Biol. Rep. 2019, 46, 1797–1808. [CrossRef]
- Dumont, E.; Vanhaecke, F.; Cornelis, R. Selenium speciation from food source to metabolites: a critical review. Anal. Bional. Chem. 2006, 385, 1304–1323. https://doi.org/ 10.1007/s00216-006-0529-8.
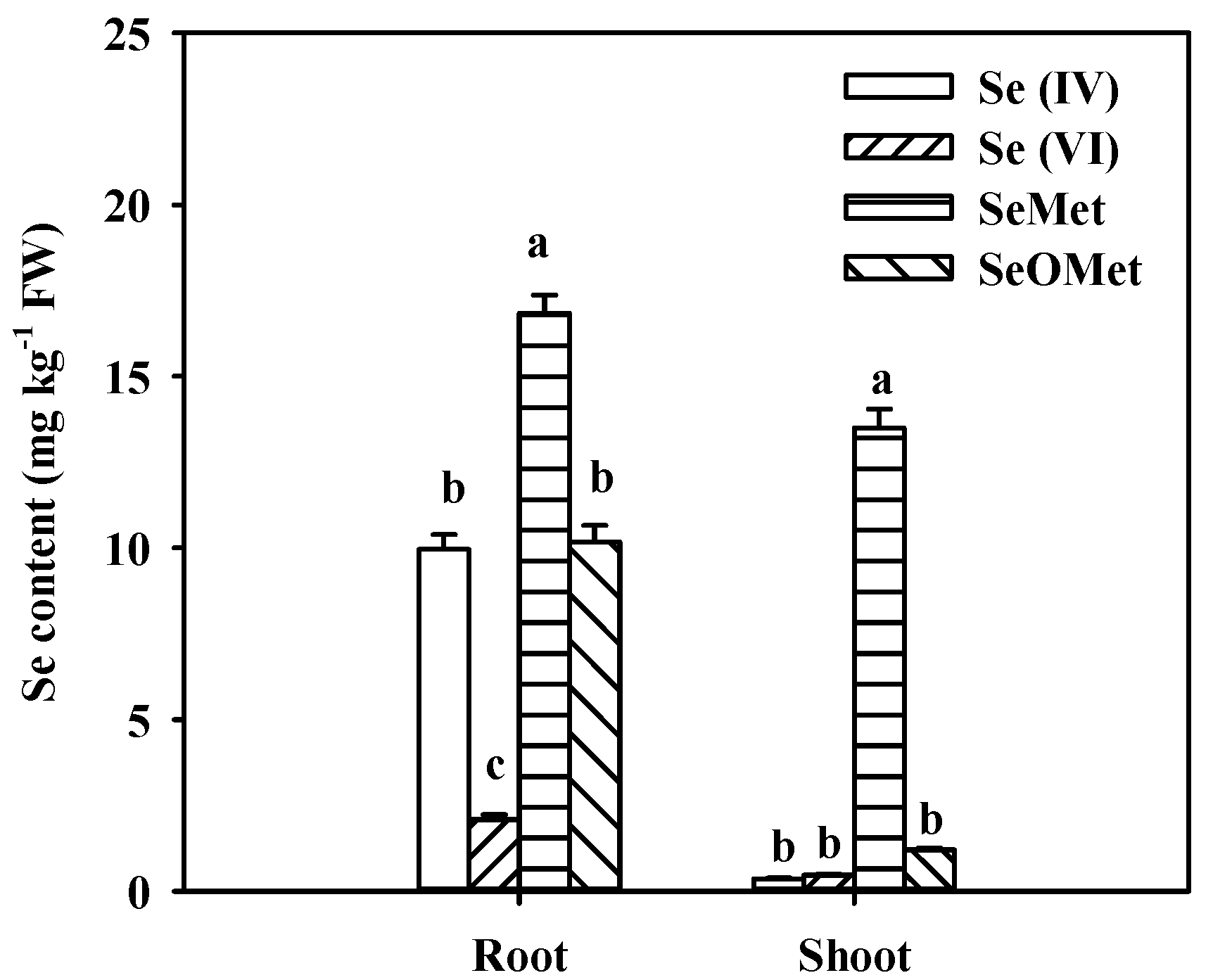
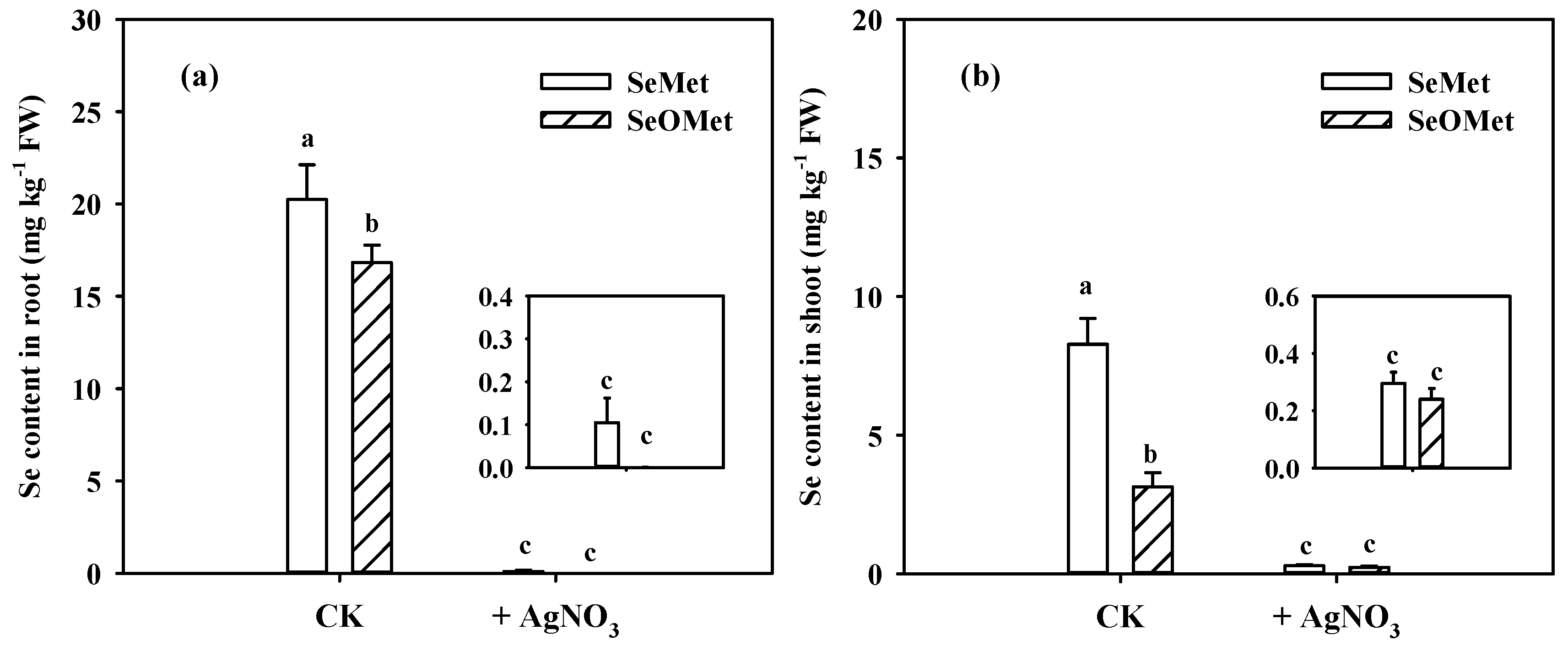
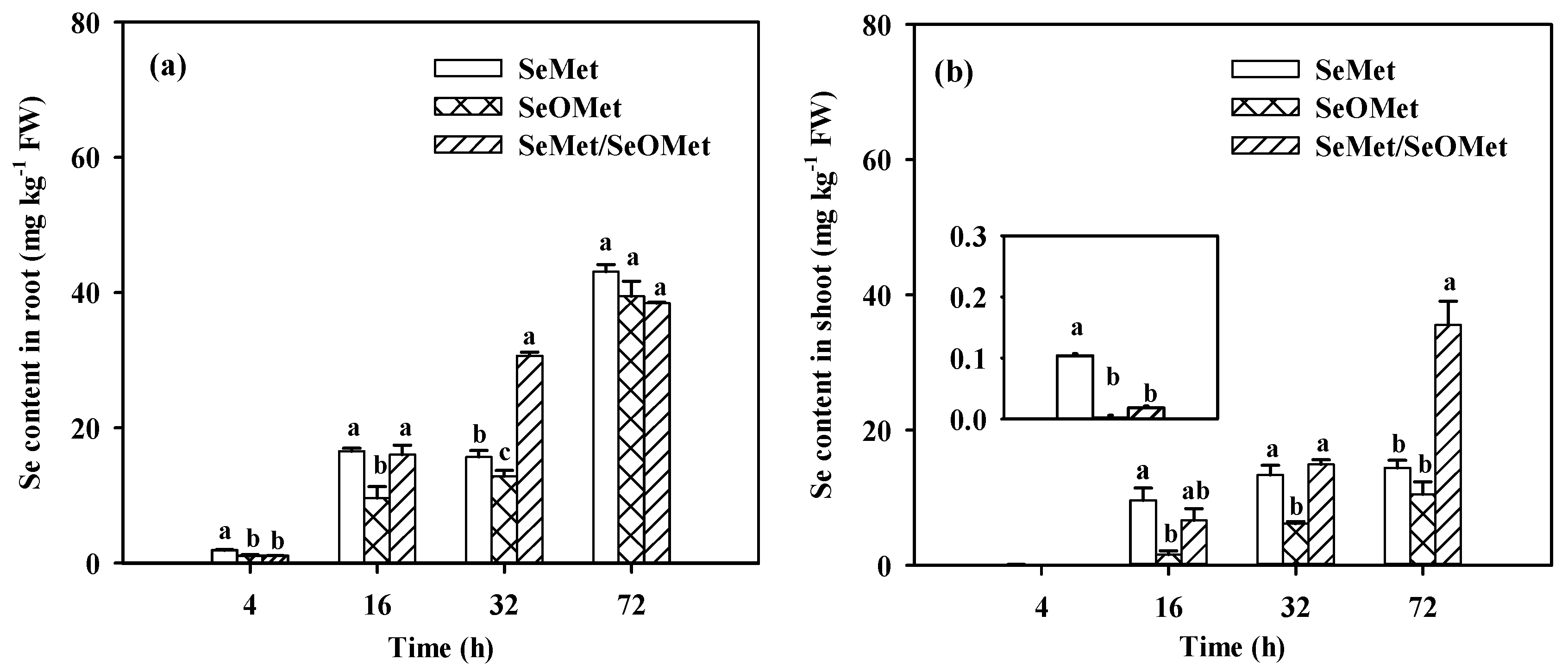
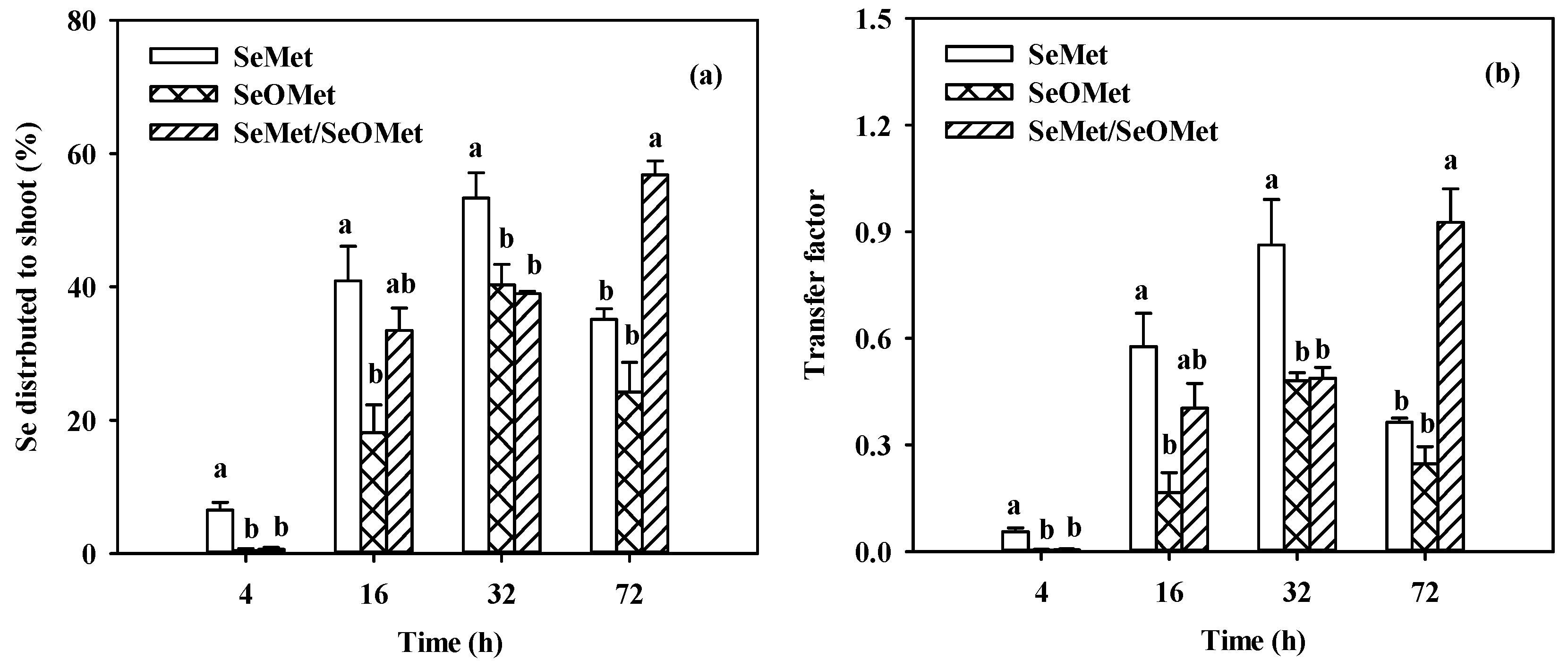
| Se form | Se distribution (%) | Transfer factor | |
|---|---|---|---|
| Root | Shoot | ||
| Se (IV) | 94.0 ± 0.55 a | 6.00 ± 0.63 c | 0.028 ± 0.004 c |
| Se (VI) | 71.3 ± 0.29 b | 28.7 ± 0.34 b | 0.178 ± 0.004 b |
| SeMet | 42.4 ± 1.03 c | 57.6 ± 1.19 a | 0.643 ± 0.038 a |
| SeOMet | 77.1 ± 0.83 b | 22.9 ± 0.96 b | 0.123 ± 0.008 b |
| Treatment | Se species (mg kg-1 FW) | ||||
|---|---|---|---|---|---|
| MeSeCys | Se (IV) | SeMet | SeOMet or SeCys2 | ||
| Root | SeMet | 2.55±0.21 b (6.84%) | 0.04±0.01 a (0.10%) | 8.19±1.14 a (22.0%) | 0.10±0.03 a (0.19%) |
| SeOMet | 1.53±0.16 b (3.65%) | 0.10±0.06 a (0.24%) | 4.33±0.09 a (10.3%) | 0.13±0.03 a (0.30%) | |
| SeMet+SeOMet | 8.34±1.82 a (21.7%) | 0.25±0.01 a (0.66%) | 9.89±0.46 a (25.8%) | 0.56±0.25 a (1.44%) | |
| Shoot | SeMet | 0.68±0.54 a (5.22%) | 0.11±0.07 a (0.86%) | 11.6±1.58 a (87.8%) | 0.29±0.16 a (2.19%) |
| SeOMet | 1.41±0.15 a (11.4%) | 0.22±0.18 a (1.75%) | 3.79±0.81 b (31.2%) | 0.07±0.03 a (0.55%) | |
| SeMet+SeOMet | 2.65±0.41 a (7.60%) | 0.50±0.07 a (1.43%) | 10.6±0.43 a (31.1%) | 0.20±0.01 a (0.58%) | |
| Treatment | Se concentration in xylem sap (mg L-1) | ||
|---|---|---|---|
| MeSeCys | Se (IV) | SeMet | |
| SeMet | 1.29 | 0.27 | 0.16 |
| SeOMet | 0.00 | 0.00 | 0.00 |
| SeMet/SeOMet | 0.13 | 0.15 | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





