Submitted:
05 December 2023
Posted:
06 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
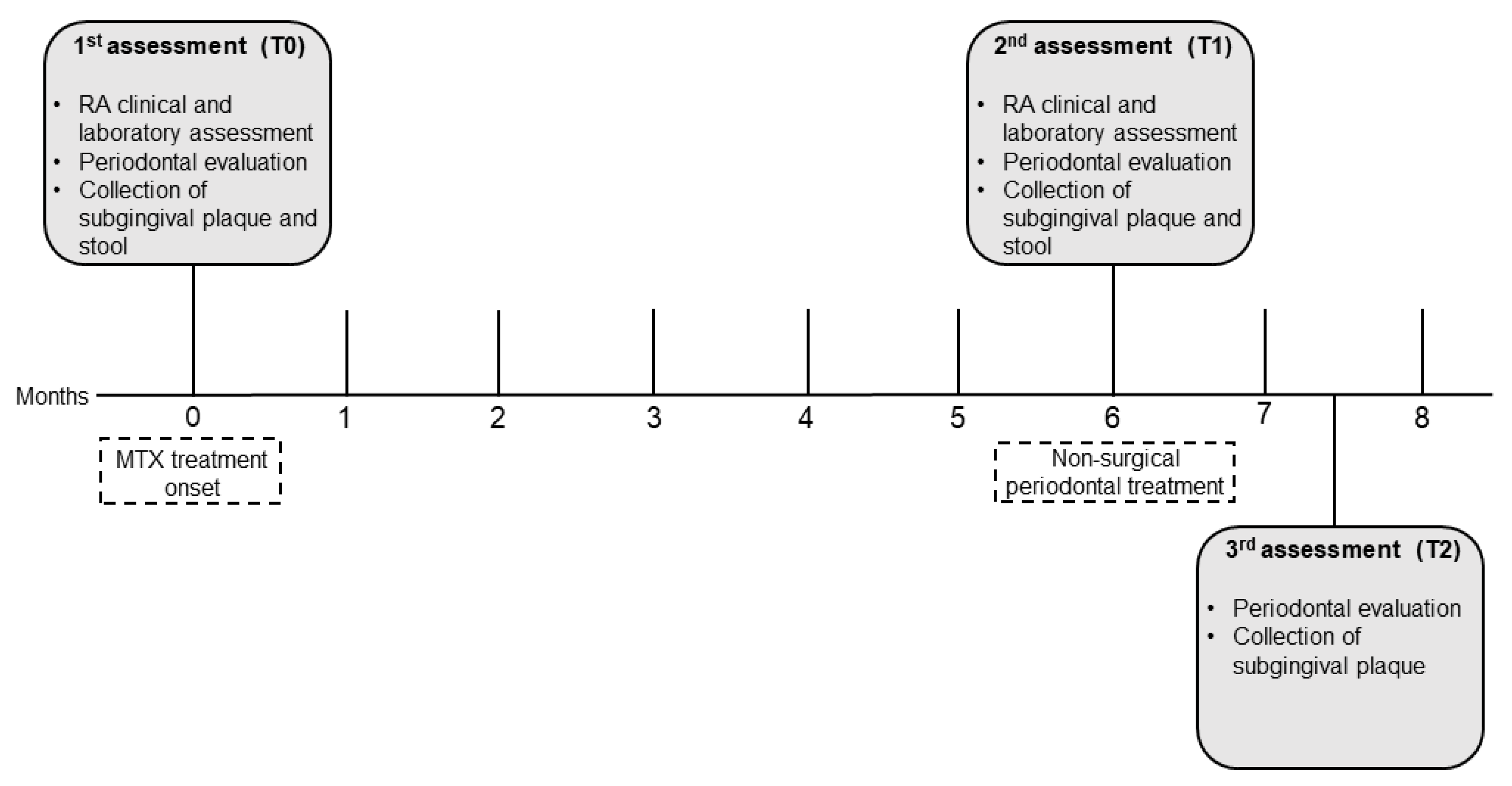
2.1. Study design, setting, and ethical clearance
2.2. Participants and RA diagnostic criteria
2.3. Data assessment and collection
2.4. Clinical and laboratory RA assessment
2.5. Periodontal evaluation and treatment
2.6. Subgingival plaque and stool samples for microbiome analysis
2.6.1. DNA extraction and 16S rRNA amplicon library preparation and sequencing
2.6.2. Read processing and taxonomic classification
2.6.3. Data analysis
2.7. Statistical analysis
3. Results
3.1. Clinicodemographic data
3.2. NSPT modified periodontal parameters
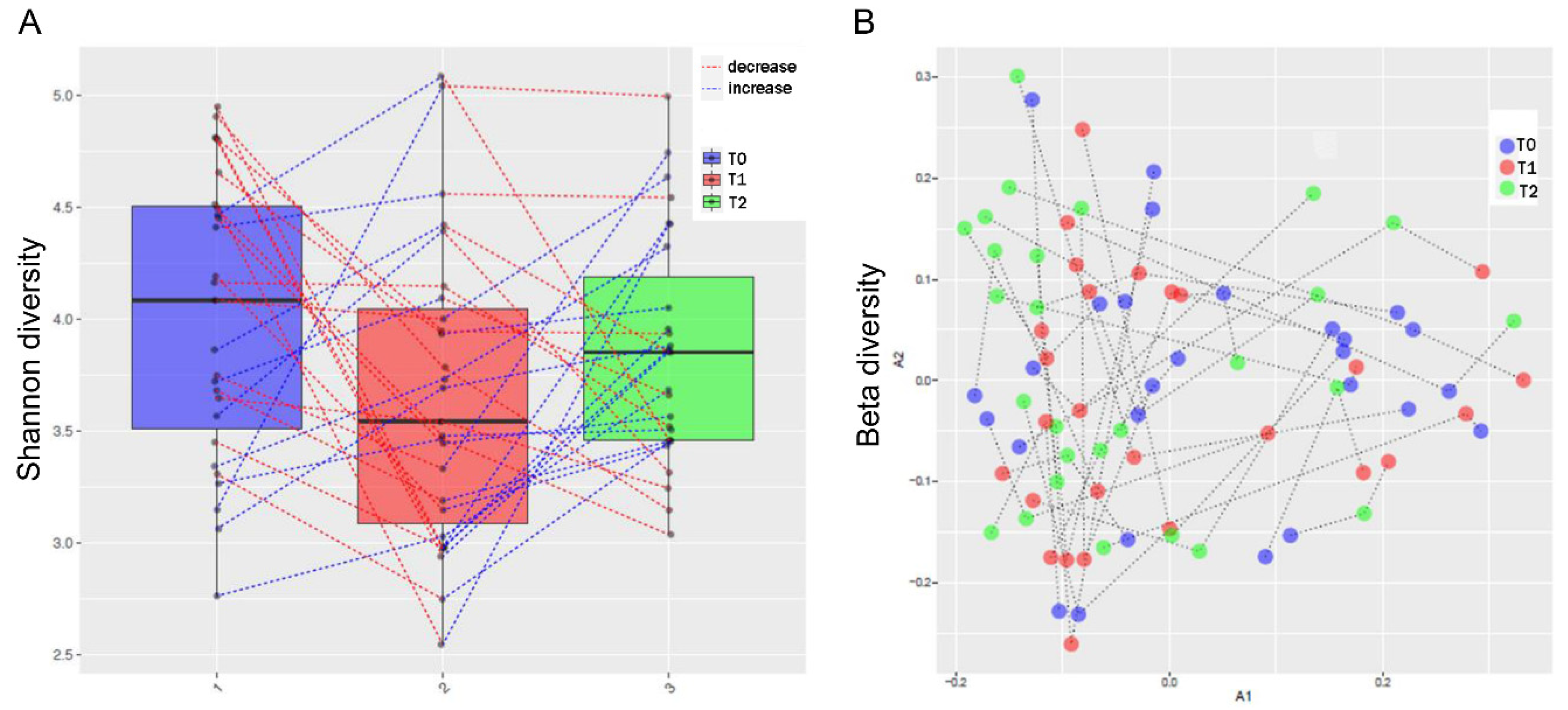
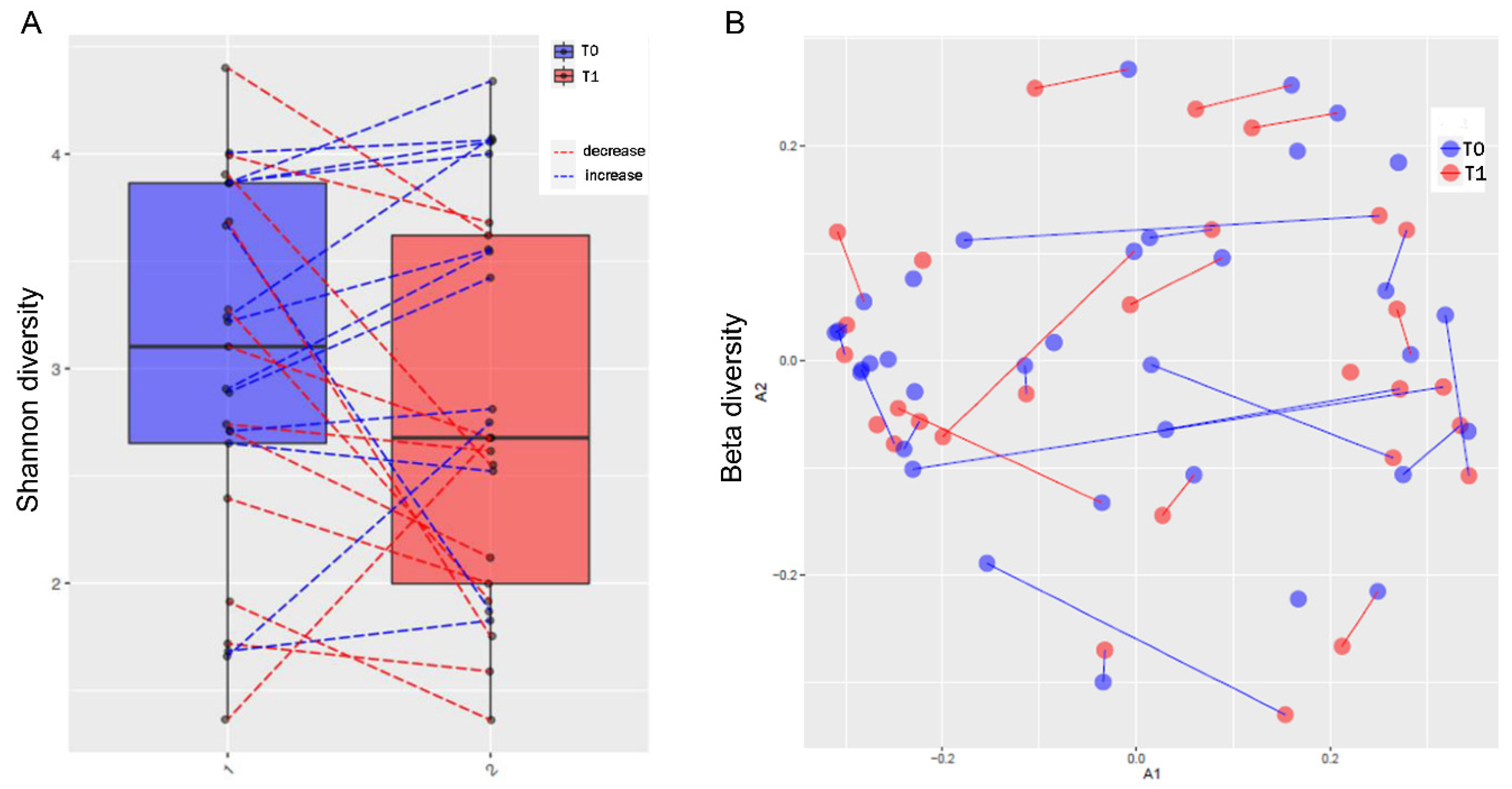
3.3. MTX and NSPT affected subgingival microbial richness and diversity
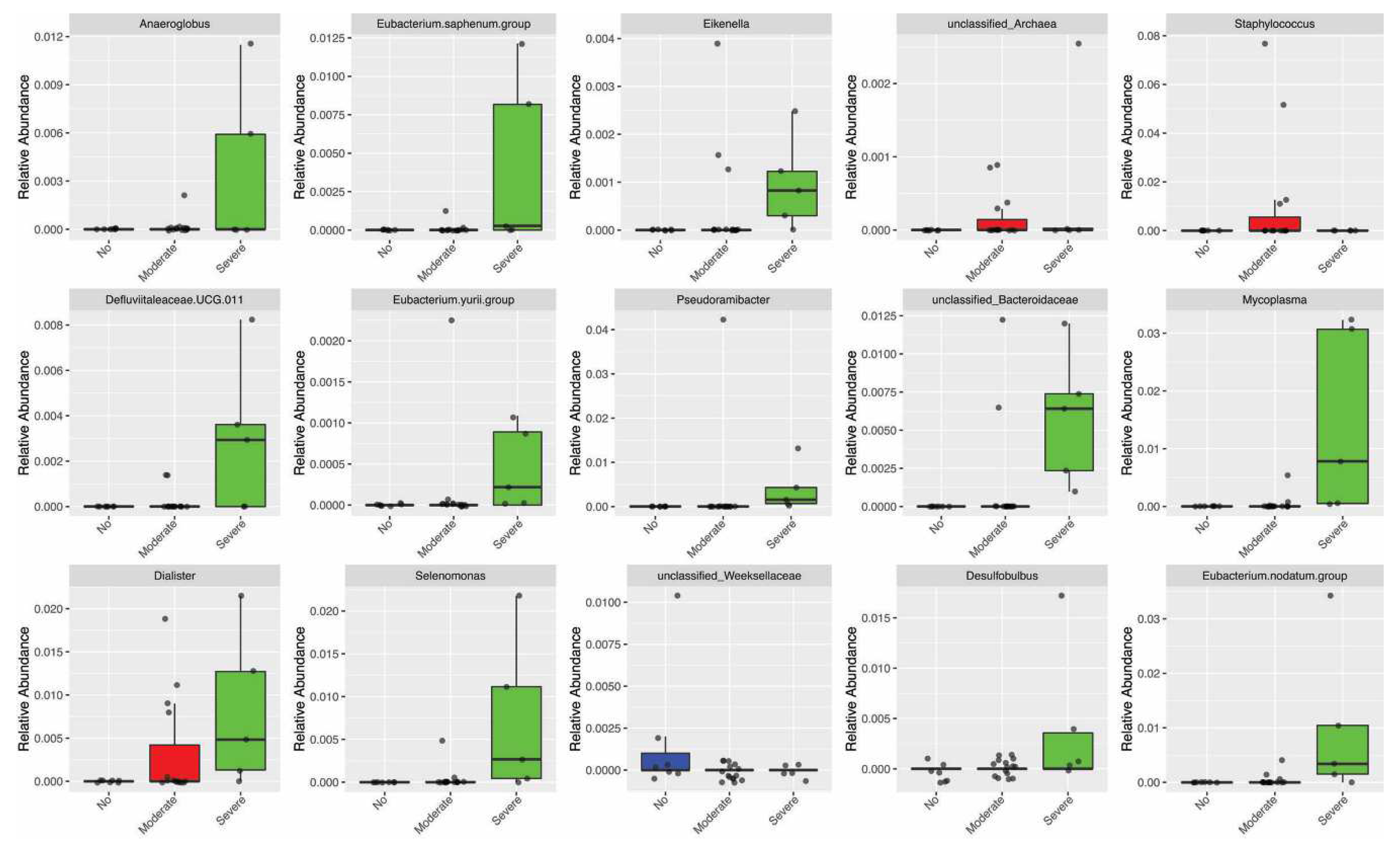
3.4. Periodontitis influenced the relative abundance of different genera in the oral microbiota
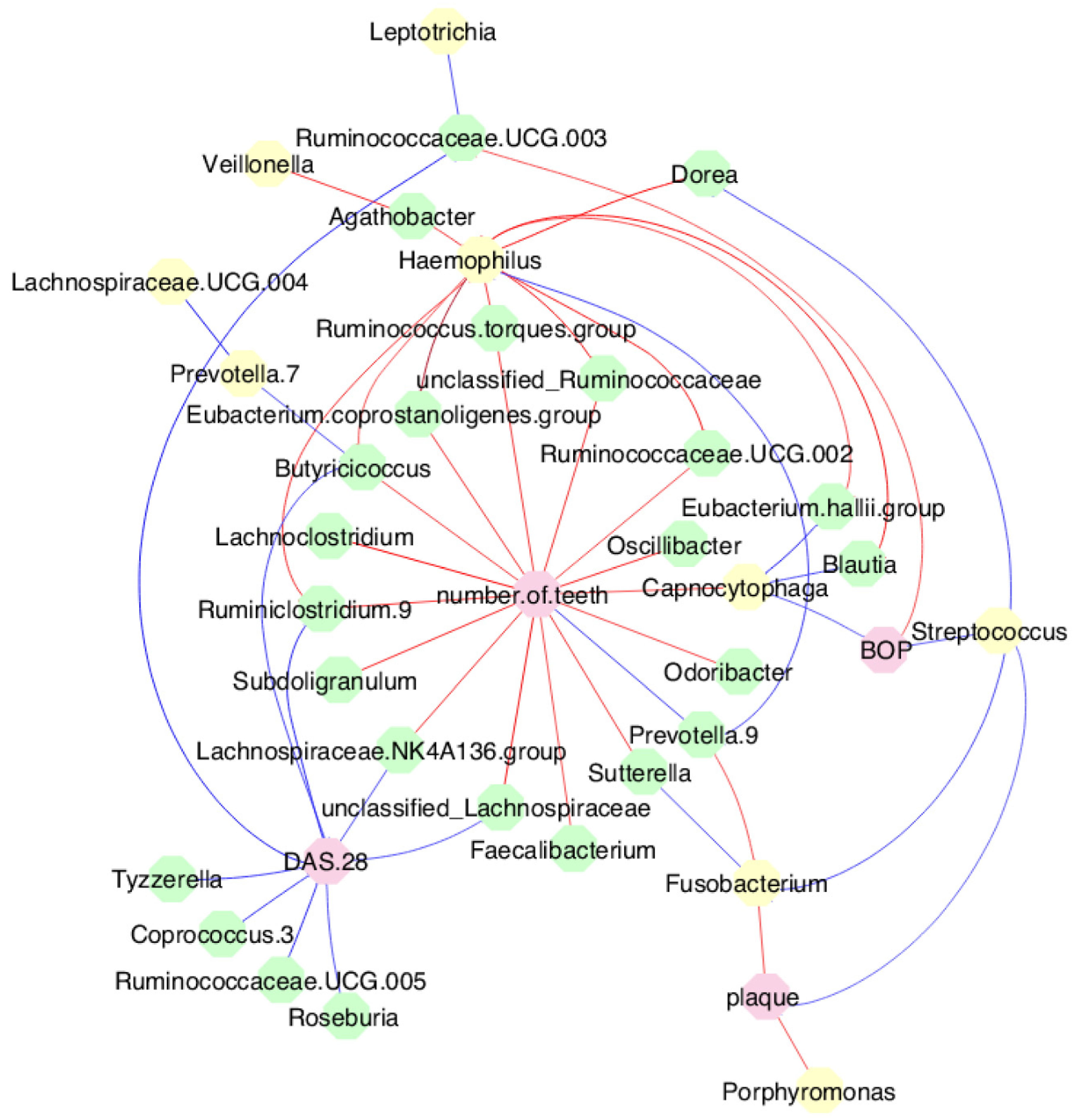
s3.5. MTX treatment and gut microbiome diversity
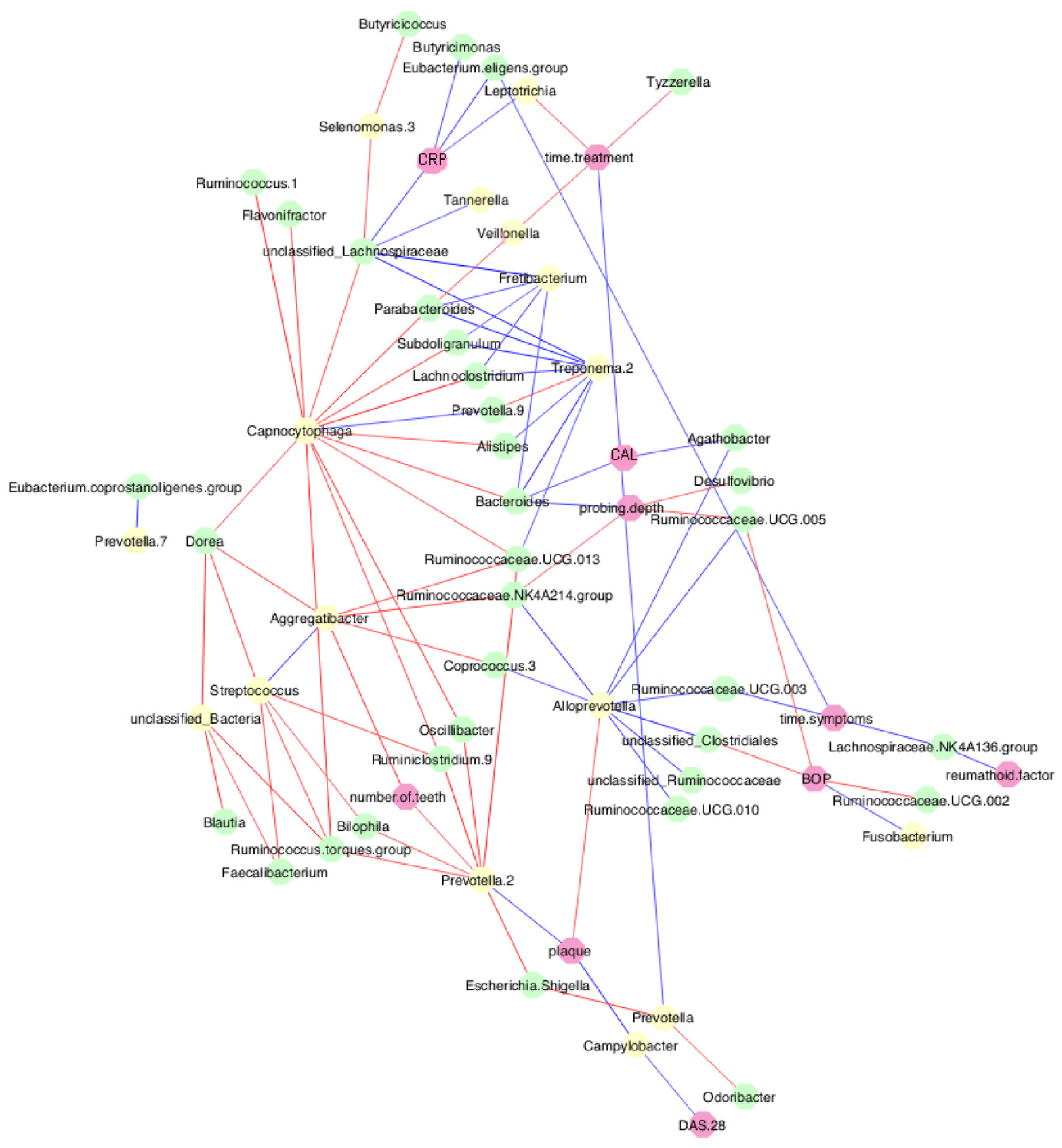
3.6. Periodontal parameters and RA activity influenced the oral and gut microbiota
3.7. MTX treatment defined new associations of periodontal and RA parameters with oral and gut bacteria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
References
- Potempa J, Mydel P, Koziel J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat Rev Rheumatol. 2017;13(10):606-620. [CrossRef]
- Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, Kavanaugh A, McInnes IB, Solomon DH, Strand V, Yamamoto K. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. [CrossRef]
- Maeda Y, Takeda K. Role of Gut microbiota in rheumatoid arthritis. J Clin Med. 2017;6(6):60. [CrossRef]
- Bolstad AI, Fevang BS, Lie SA. Increased risk of periodontitis in patients with rheumatoid arthritis: a nationwide register study in Norway. J Clin Periodontol. 2023;50(8):1022-1032. [CrossRef]
- Krutyhołowa A, Strzelec K, Dziedzic A, Bereta GP, Łazarz-Bartyzel K, Potempa J, Gawron K. Host and bacterial factors linking periodontitis and rheumatoid arthritis. Front Immunol. 2022;13:980805. [CrossRef]
- Farquharson D, Butcher JP, Culshaw S. Periodontitis, Porphyromonas, and the pathogenesis of rheumatoid arthritis. Mucosal Immunol. 2012;5(2):112-120. [CrossRef]
- Ebbers M, Lübcke PM, Volzke J, Kriebel K, Hieke C, Engelmann R, Lang H, Kreikemeyer B, Müller-Hilke B. Interplay between P. gingivalis, F. nucleatum and A. actinomycetemcomitans in murine alveolar bone loss, arthritis onset and progression. Sci Rep. 2018;8(1):15129. [CrossRef]
- Zhang X, Zhang D, Jia H, Feng Q, Wang D, Liang D, Wu X, Li J, Tang L, Li Y, Lan Z, Chen B, Li Y, Zhong H, Xie H, Jie Z, Chen W, Tang S, Xu X, Wang X, Cai X, Liu S, Xia Y, Li J, Qiao X, Al-Aama JY, Chen H, Wang L, Wu QJ, Zhang F, Zheng W, Li Y, Zhang M, Luo G, Xue W, Xiao L, Li J, Chen W, Xu X, Yin Y, Yang H, Wang J, Kristiansen K, Liu L, Li T, Huang Q, Li Y, Wang J. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat Med. 2015;21(8):895-905. [CrossRef]
- Corrêa JD, Fernandes GR, Calderaro DC, Mendonça SMS, Silva JM, Albiero ML, Cunha FQ, Xiao E, Ferreira GA, Teixeira AL, Mukherjee C, Leys EJ, Silva TA, Graves DT. Oral microbial dysbiosis linked to worsened periodontal condition in rheumatoid arthritis patients. Sci Rep. 2019;9(1):8379. [CrossRef]
- Beyer K, Zaura E, Brandt BW, Buijs MJ, Brun JG, Crielaard W, Bolstad AI. Subgingival microbiome of rheumatoid arthritis patients in relation to their disease status and periodontal health. PLoS One. 2018;13(9):e0202278. [CrossRef]
- Eriksson K, Fei G, Lundmark A, Benchimol D, Lee L, Hu YOO, Kats A, Saevarsdottir S, Catrina AI, Klinge B, Andersson AF, Klareskog L, Lundberg K, Jansson L, Yucel-Lindberg T. Periodontal health and oral microbiota in patients with rheumatoid arthritis. J Clin Med. 2019;8(5):630. [CrossRef]
- Sun Y, Chen Q, Lin P, Xu R, He D, Ji W, Bian Y, Shen Y, Li Q, Liu C, Dong K, Tang YW, Pei Z, Yang L, Lu H, Guo X, Xiao L. Characteristics of gut microbiota in patients with rheumatoid arthritis in Shanghai, China. Front Cell Infect Microbiol. 2019;9:369. [CrossRef]
- Chu XJ, Cao NW, Zhou HY, Meng X, Guo B, Zhang HY, Li BZ. The oral and gut microbiome in rheumatoid arthritis patients: a systematic review. Rheumatology (Oxford). 2021;60(3):1054-1066. [CrossRef]
- du Teil Espina M, Gabarrini G, Harmsen HJM, Westra J, van Winkelhoff AJ, van Dijl JM. Talk to your gut: the oral-gut microbiome axis and its immunomodulatory role in the etiology of rheumatoid arthritis. FEMS Microbiol Rev. 2019;43(1):1-18. [CrossRef]
- Mikuls TR, Thiele GM, Deane KD, Payne JB, O’Dell JR, Yu F, Sayles H, Weisman MH, Gregersen PK, Buckner JH, Keating RM, Derber LA, Robinson WH, Holers VM, Norris JM. Porphyromonas gingivalis and disease-related autoantibodies in individuals at increased risk of rheumatoid arthritis. Arthritis Rheum. 2012;64(11):3522-3530. [CrossRef]
- Konig MF, Abusleme L, Reinholdt J, Palmer RJ, Teles RP, Sampson K, Rosen A, Nigrovic PA, Sokolove J, Giles JT, Moutsopoulos NM, Andrade F. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med. 2016;8(369):369ra176. [CrossRef]
- Schwenzer A, Quirke AM, Marzeda AM, Wong A, Montgomery AB, Sayles HR, Eick S, Gawron K, Chomyszyn-Gajewska M, Łazarz-Bartyzel K, Davis S, Potempa J, Kessler BM, Fischer R, Venables PJ, Payne JB, Mikuls TR, Midwood KS. Association of distinct fine specificities of anti-citrullinated peptide antibodies with elevated immune responses to Prevotella intermedia in a subgroup of patients with rheumatoid arthritis and periodontitis. Arthritis Rheumatol. 2017;69(12):2303-2313. [CrossRef]
- Martu MA, Solomon SM, Sufaru IG, Jelihovschi I, Martu S, Rezus E, Surdu AE, Onea RM, Grecu GP, Foia L. Study on the prevalence of periodontopathogenic bacteria in serum and subgingival bacterial plaque in patients with rheumatoid arthritis. Rev Chim 2017;68(8):1946-1950. [CrossRef]
- Möller B, Kollert F, Sculean A, Villiger PM. Infectious triggers in periodontitis and the gut in rheumatoid arthritis (RA): a complex story about association and causality. Front Immunol. 2020;11:1108. [CrossRef]
- Giollo A, Fuzzi E, Doria A. Methotrexate in early rheumatoid arthritis: is the anchor drug still holding? Autoimmun Rev. 2022;21(4):103031. [CrossRef]
- Bodkhe R, Balakrishnan B, Taneja V. The role of microbiome in rheumatoid arthritis treatment. Ther Adv Musculoskelet Dis. 2019;11:1759720X19844632. [CrossRef]
- Lübcke PM, Ebbers MNB, Volzke J, Bull J, Kneitz S, Engelmann R, Lang H, Kreikemeyer B, Müller-Hilke B. Periodontal treatment prevents arthritis in mice and methotrexate ameliorates periodontal bone loss. Sci Rep. 2019;9(1):8128. [CrossRef]
- de Arruda JAA, Corrêa JD, Singh Y, Oliveira SR, Machado CC, Schneider AH, Medeiros JD, Fernandes GR, Macari S, Barrioni BR, Santos MS, Duffles LF, Nakaya HTI, Fukada SY, Graves DT, Cunha FQ, Silva TA. Methotrexate promotes recovery of arthritis-induced alveolar bone loss and modifies the composition of the oral-gut microbiota. Anaerobe. 2022;75:102577. [CrossRef]
- Knottnerus A, Tugwell P. 2008. STROBE--a checklist to Strengthen the Reporting of Observational Studies in Epidemiology. J Clin Epidemiol 61(4):323. [CrossRef]
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, Hawker G. 2010. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62(9):2569-2581. [CrossRef]
- Mitchell KL, Pisetsky DS. Early rheumatoid arthritis. Curr Opin Rheumatol. 2007;19(3):278-283. [CrossRef]
- Oliveira SR, de Arruda JAA, Schneider AH, Carvalho VF, Machado CC, Corrêa JD, Moura MF, Duffles LF, de Souza FFL, Ferreira GA, Costa FO, Abreu LG, Taba Júnior M, Fukada SY, de Oliveira RDR, Louzada-Júnior P, Cunha FQ, Silva TA. Are neutrophil extracellular traps the link for the cross-talk between periodontitis and rheumatoid arthritis physiopathology? Rheumatology (Oxford). 2021;61(1):174-184. [CrossRef]
- Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320(13):1360-1372. [CrossRef]
- Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Clin Periodontol. 2018;45 Suppl 20:S149-S161. [CrossRef]
- Eke PI, Page RC, Wei L, Thornton-Evans G, Genco RJ. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83(12):1449-1454. [CrossRef]
- Klindworth A, Pruesse E, Schweer T, Peplies J, Quast C, Horn M, Glöckner FO. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41(1):e1. [CrossRef]
- de Paula ACL, Medeiros JD, Fernandes GR, da Silva VL, Diniz CG. Microbiome of industrialized Minas frescal cheese reveals high prevalence of putative bacteria: a concern in the one health context. LWT. 2021;139:110791. [CrossRef]
- Parada AE, Needham DM, Fuhrman JA. Every base matters: assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ Microbiol. 2016;18(5):1403-1414. [CrossRef]
- Apprill A, McNally S, Parsons R, Weber L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat Microb Ecol. 2015;75:129-137. [CrossRef]
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: high-resolution sample inference from illumina amplicon data. Nat Methods. 2016;13(7):581-583. [CrossRef]
- Pires DEV, Oliveira FS, Correa FB, Morais DK, Fernandes GB. TAG.ME: taxonomic assignment of genetic markers for ecology. bioRxiv. 2018;263293. [CrossRef]
- Dray S, Dufour AB. The ade4 package: implementing the duality diagram for ecologists. J Stat Soft. 2007;22(4):1-20. [CrossRef]
- Drost HG. 2018. Philentropy: information theory and distance quantification with R. Journal of Open Source Software. 2018;3(26):765. [CrossRef]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [CrossRef]
- Maciejewski M, Sands C, Nair N, Ling S, Verstappen S, Hyrich K, Barton A, Ziemek D, Lewis MR, Plant D. Prediction of response of methotrexate in patients with rheumatoid arthritis using serum lipidomics. Sci Rep. 2021;11(1):7266. [CrossRef]
- Zaragoza-García O, Castro-Alarcón N, Pérez-Rubio G, Guzmán-Guzmán IP. DMARDs-Gut microbiota feedback: implications in the response to therapy. Biomolecules. 2020;10(11):1479. [CrossRef]
- Han M, Zhang N, Mao Y, Huang B, Ren M, Peng Z, Bai Z, Chen L, Liu Y, Wang S, Huang S, Cheng Z. The potential of gut microbiota metabolic capability to detect drug response in rheumatoid arthritis patients. Front Microbiol. 2022;13:839015. [CrossRef]
- Nayak RR, Alexander M, Deshpande I, Stapleton-Gray K, Rimal B, Patterson AD, Ubeda C, Scher JU, Turnbaugh PJ. Methotrexate impacts conserved pathways in diverse human gut bacteria leading to decreased host immune activation. Cell Host Microbe. 2021;29(3):362-377.e11. [CrossRef]
- Kawamoto D, Borges R, Ribeiro RA, de Souza RF, Amado PPP, Saraiva L, Horliana ACRT, Faveri M, Mayer MPA. Oral dysbiosis in severe forms of periodontitis is associated with gut dysbiosis and correlated with salivary inflammatory mediators: a preliminary study. Front Oral Health. 2021;2:722495. [CrossRef]
- Corrêa JD, Calderaro DC, Ferreira GA, Mendonça SM, Fernandes GR, Xiao E, Teixeira AL, Leys EJ, Graves DT, Silva TA. Subgingival microbiota dysbiosis in systemic lupus erythematosus: association with periodontal status. Microbiome. 2017;5(1):34. [CrossRef]
- Scher JU, Ubeda C, Equinda M, Khanin R, Buischi Y, Viale A, Lipuma L, Attur M, Pillinger MH, Weissmann G, Littman DR, Pamer EG, Bretz WA, Abramson SB. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 2012;64(10):3083-3094. [CrossRef]
- Sun J, Zheng Y, Bian X, Ge H, Wang J, Zhang Z. Non-surgical periodontal treatment improves rheumatoid arthritis disease activity: a meta-analysis. Clin Oral Investig. 2021;25(8):4975-4985. [CrossRef]
- Äyräväinen L, Leirisalo-Repo M, Kuuliala A, Ahola K, Koivuniemi R, Meurman JH, Heikkinen AM. Periodontitis in early and chronic rheumatoid arthritis: a prospective follow-up study in Finnish population. BMJ Open. 2017;7(1):e011916. [CrossRef]
- Kordtabar S, Aghaie M, Fakhari E, Vakili MA. Periodontal condition in patients with rheumatoid arthritis: effect of anti-rheumatic Drugs. J Dent (Shiraz). 2019;20(3):190-194. [CrossRef]
- Jung GU, Han JY, Hwang KG, Park CJ, Stathopoulou PG, Fiorellini JP. Effects of conventional synthetic disease-modifying antirheumatic drugs on response to periodontal treatment in patients with rheumatoid arthritis. Biomed Res Int. 2018;2018:1465402. [CrossRef]
- Zhang J, Xu C, Gao L, Zhang D, Li C, Liu J. Influence of anti-rheumatic agents on the periodontal condition of patients with rheumatoid arthritis and periodontitis: a systematic review and meta-analysis. J Periodontal Res. 2021;56(6):1099-1115. [CrossRef]
- Balta MG, Papathanasiou E, Blix IJ, Van Dyke TE. Host modulation and treatment of periodontal disease. J Dent Res. 2021;100(8):798-809. [CrossRef]
- Belstrøm D, Grande MA, Sembler-Møller ML, Kirkby N, Cotton SL, Paster BJ, Holmstrup P. Influence of periodontal treatment on subgingival and salivary microbiotas. J Periodontol. 2018;89(5):531-539. [CrossRef]
- Liu G, Luan Q, Chen F, Chen Z, Zhang Q, Yu X. Shift in the subgingival microbiome following scaling and root planing in generalized aggressive periodontitis. J Clin Periodontol. 2018;45(4):440-452. [CrossRef]
- Schulz S, Porsch M, Grosse I, Hoffmann K, Schaller HG, Reichert S. Comparison of the oral microbiome of patients with generalized aggressive periodontitis and periodontitis-free subjects. Arch Oral Biol. 2019;99:169-176. [CrossRef]
- Greenwood D, Afacan B, Emingil G, Bostanci N, Belibasakis GN. Salivary microbiome shifts in response to periodontal treatment outcome. Proteomics Clin Appl. 2020;14(3):e2000011. [CrossRef]
- Martu MA, Luchian I, Mares M, Solomon S, Ciurcanu O, Danila V, Rezus E, Foia L. The effectiveness of laser applications and photodynamic therapy on relevant periodontal pathogens (Aggregatibacter actinomycetemcomitans) associated with immunomodulating anti-rheumatic drugs. Bioengineering (Basel). 2023;10(1):61. [CrossRef]
- Schwarzberg K, Le R, Bharti B, Lindsay S, Casaburi G, Salvatore F, Saber MH, Alonaizan F, Slots J, Gottlieb RA, Caporaso JG, Kelley ST. The personal human oral microbiome obscures the effects of treatment on periodontal disease. PLoS One. 2014;9(1):e86708. [CrossRef]
- Ziebolz D, Pabel SO, Lange K, Krohn-Grimberghe B, Hornecker E, Mausberg RF. Clinical periodontal and microbiologic parameters in patients with rheumatoid arthritis. J Periodontol. 2011;82(10):1424-1432. [CrossRef]
| Variables | n (%) |
|---|---|
| Age, median (min-max) | 51 (22–70) |
| Sex | |
| Female | 33 (89.2) |
| Male | 4 (10.8) |
| RA | |
| Early | 22 (59.5) |
| Established | 15 (40.5) |
| Tobacco smoking | |
| Never | 22 (59.5) |
| Still | 8 (21.6) |
| Stopped | 7 (18.9) |
| Alcohol consumption | |
| Never | 31 (83.8) |
| Still | 2 (5.4) |
| Stopped | 4 (10.8) |
| RF (IU/mL), median (min-max) | 15.00 (0–1890) |
| ACPA (IU/mL), median (min-max) | 69.75 (0–340) |
| CRP (mg/dL), median (min-max) | 0.77 (0–14) |
| VAS (mm), median (min-max) | 60 (0–100) |
| ESR (mm/h), median (min-max) | 18.00 (2–85) |
| DAS 28, median (min-max) | 4.99 (0.63–8.06) |
| Disease activity | |
| Remission | 7 (18.9) |
| Low | 6 (16.2) |
| Moderate | 11 (29.7) |
| High | 13 (35.1) |
| MTX treatment * (n, %) | |
| Failure | 16 (43.2) |
| Responsive | 17 (45.9) |
| Adverse events | 2 (5.4) |
| NA | 2 (5.4) |
| Time of treatment α, median (min-max) | 3 (0–408) |
| Periodontitis ¶ (n, %) | |
| No (periodontal health and gingivitis) | 10 (27.0) |
| Yes (stages I, II, III, IV) | 27 (73.0) |
| Periodontitis ∞ (n, %) | |
| None | 10 (27.0) |
| Mild | - |
| Moderate | 21 (56.8) |
| Severe | 6 (16.2) |
| Variables | T0, n = 37 | T1, n = 32 | T2, n = 28 | p value |
|---|---|---|---|---|
| Probing depth (mm), median (min-max) | 1.92 (0.86-3.16) | 1.70 (0.64-3.70) | 1.55 a,b (0.52-2.98) | <0.001 § |
| CAL (mm), median (min-max) | 2.05 (1.06-5.15) | 1.89 (0.83-7.14) | 1.83 a (0.78-6.92) | 0.026 § |
| BOP (%), median (min-max) | 9.00 (0-53) | 24.00 a (6-85) | 10.00 b (0-35) | <0.001 § |
| Plaque index (%), median (min-max) | 42.00 (0-100) | 37.50 (6-92) | 18.00 a,b (3-50) | <0.001 § |
| Periodontitis (n, %) | ||||
| None | 10 (27.0) | 8 (21.6) | 11 (29.7) | <0.05 ¶ |
| Mild | 0 (0.0) | 1 (2.7) | 0 (0.0) | |
| Moderate | 21 (56.8) | 17 (45.9) | 13 a,b (35.1) | |
| Severe | 6 (16.2) | 6 (16.2) | 4 (10.8) | |
| NA | - | 5 (13.6) | 9 (24.4) | |
| DAS 28, median (min-max) | 4.99 (0.63-8.06) | 4.49 (0.14-7.76) | - | 0.082 § |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





