1. Introduction
Ceramide is an anti-tumor lipid that limits metastatic potential [
1] and evokes cell death [
2,
3,
4] in cancer cells. Extensive studies have supported the development of ceramide analogues and liposomal formulation of short chain C
6-ceramide for cancer therapeutics [
5,
6,
7,
8,
9,
10,
11,
12]. Cationic pyridinium-ceramides including C
6-pyridinium-ceramide (LCL29) [
6] and C
16-pyridinium-ceramide (LCL30) [
7] and B13 [
13] are ceramide analogues. LCL29 and LCL30, both mitochondrial targeting molecules, have been demonstrated as effective anticancer agents in preclinical studies [
7,
14,
15]. B13 is an acid ceramidase inhibitor and shows anti-tumor activities [
13,
16]. In terms of chemical structure, the ceramide analogues exhibit characteristic features of ceramide including an amide bond linked to an acyl chain and functional group. The amide bond is assumed to undergo metabolic degradation, and this should be considered for biological stability in drug development.
We previously demonstrated that a nanoliposomal formulation of short chain C
6-ceramide, named ceramide nanoliposome (CNL), is a necroptosis-inducing reagent in chemotherapy-sensitive and -resistant ovarian cancer cells [
12]. CNL was revealed to target the pore-forming mixed lineage kinase domain-like protein (MLKL), which executes plasma membrane rupture in necroptosis. Pharmacological and genetic approaches showed that CNL targets and activates MLKL independently of RIPK3 phosphorylation, although MLKL was phosphorylated by RIPK3 and in turn converted to an active pore-forming oligomer [
17,
18,
19]. Moreover, C
16-ceramide along with RIPK1 forms a ceramidosome complex that facilitates large membrane pores [
20]. These studies indicated ceramide as a MLKL-activating lipid in necroptosis and suggested the potential of developing mimetics of ceramides as pro-necroptotic reagents.
Pro-apoptotic agents, a major form of chemotherapy, are the main type of anti-cancer treatment [
21]. Genomic heterogeneity–related aberrations in the apoptosis machinery of cancer cells may increase chemotherapy- or apoptosis-resistance, which is a clinical challenge in cancer therapy [
22]. Pro-necroptotic therapy has been proposed to serve as an alternative approach for eradicating apoptosis-resistant and refractory cancer cells [
19,
23,
24]. Ceramide-orchestrated necroptotic signaling [
24,
25,
26,
27] may provide a strategy for developing novel cancer therapy such as pro-necroptotic therapy.
In this study, we developed novel ceramide mimetics and examined their potential as chemotherapeutic reagents. We further investigated their effects on necroptosis in multiple cancer cell lines.
2. Materials and Methods
2.1. Materials
Roswell Park Memorial Institute medium 1640 (RPMI1640), high glucose Dulbecco’s modified Eagle’s medium (DMEM), trypsin, and staurosporine were purchased from Nacalai Tesque (Kyoto, Japan). Fetal bovine serum (FBS) was obtained from Biowest (Nuaillé, France). CellTiter-Glo® 2.0 was purchased from Promega (Madison, WI, USA). RNAiMax, SuperSignal West Dura Extended Duration Substrate, control siRNAs, and human MLKL siRNAs were obtained from Thermo Fisher Scientific (Rockford, IL, USA). Precast 4%–20% gradient polyacrylamide gels and nitrocellulose membranes (0.45 µm pore size) were purchased from Bio-Rad (Hercules, CA, USA). Antibodies for poly ADP-ribose polymerase (PARP) and GAPDH were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Horseradish peroxidase–conjugated antibodies for mouse and rabbit IgG were purchased from Jackson ImmunoResearch (West Grove, PA, USA). A2780, A549, CaCo2, ES2, HeLa, MCF-7, MDA-MB-231, SKOV3, and TOV112D cells were obtained from American Type Culture Collection (Manassas, VA, USA).
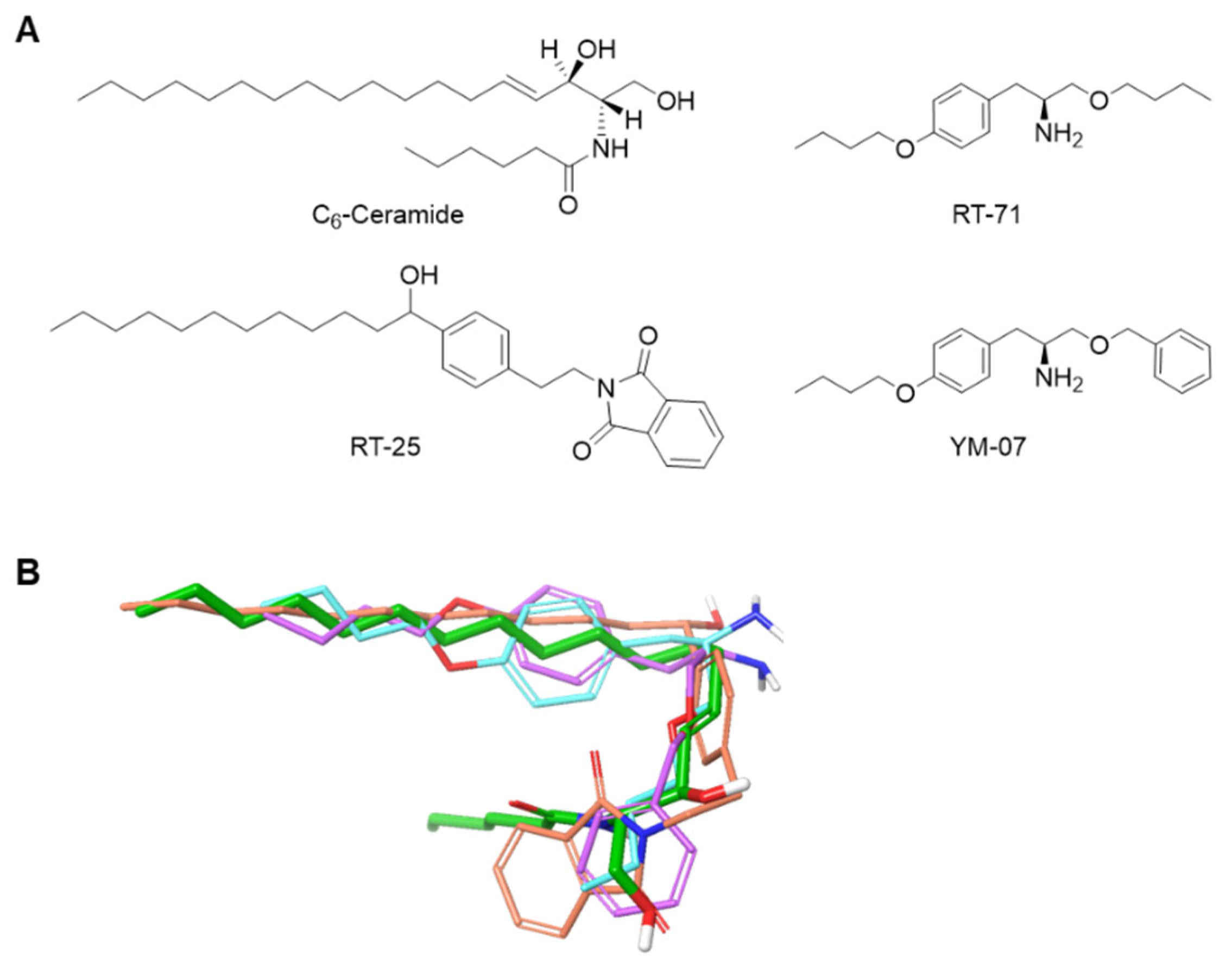
2.2. Compound Design and Synthesis
Compounds including RT-25, RT-71, and YM-07 that mimic the hydrocarbon chain of C
6-ceramide were manually designed. The most stable conformation of C
6-ceramide was then calculated using OMEGA (OpenEye Scientific software, Santa Fe, NM, USA), and the shape of the designed compounds was evaluated using ROCS (OpenEye Scientific software) to confirm that the designed compounds are compatible with the hydrocarbon chain of C
6-ceramide (
Figure 1). The compounds were synthesized following the scheme described in the supporting information, and the structures were determined by
1H-NMR and high-resolution mass spectrometry (
Supplementary Figure S1).
2.3. Cell Culture
A2780, ES-2, and MCF-7 cells were grown in RPMI1640 supplemented with 10% FBS. A549, CaCo2, HeLa, MDA-MB-231, SKOV3, TOV112D and CaCo2 cells were grown in DMEM supplemented with 10% FBS. Cells were maintained at <80% confluence under standard conditions (humidified atmosphere, 95% air, 5% CO2, 37°C). No mycoplasma contamination was observed in the cell lines.
2.4. Cell Viability Assay
Cells plated in 96-well plates (4 × 103 cells/well) were treated with ceramide mimetics for 24 h. CellTiter-Glo®2.0 reagents were added, and the luminescence was measured using a GloMax® Navigator Microplate Luminometer (Promega, Madison, WI, USA).
2.5. Immunoblotting
Cells were washed three times with ice-cold PBS containing 10 mM EDTA and lysed using Laemmli sample buffer supplemented with dithiothreitol. Protein samples (10 µg) were subjected to SDS-PAGE (4%–20% gradient gels) and electrophoretically transferred to nitrocellulose membranes. Membranes were blocked with PBS/0.1% Tween 20 containing 5% nonfat dried milk and washed with PBS/0.1% Tween 20. The membranes were then incubated with primary antibodies for PARP (1:2,000 dilution) or GAPDH (1:1,000 dilution) in PBS/0.1% Tween 20 containing 5% bovine serum albumin. The blots were washed with PBS/0.1% Tween 20 and incubated with secondary antibody conjugated with horseradish peroxidase in PBS/0.1% Tween 20 containing 5% nonfat dried milk. Chemiluminescent signals were detected with SuperSignal West Dura Extended Duration Substrate and ChemiDoc Imaging Systems (Bio-Rad, Hercules, CA, USA).
2.6. Transfection with siRNAs
Cells were transfected with 5 nM siRNAs using RNAiMax in accordance with the manufacturer’s instructions.
2.7. Trypan Blue Exclusion Assay
The floating cells in the medium and cells that remained attached to the plates were counted using a hematocytometer in the presence of 0.2% trypan blue.
2.8. Statistical Analysis
Data presented in bar graphs represent the mean ± SE of independent experiments. Images are representative of at least three independent experiments. Sample sizes for relevant experiments were determined by power analyses conducted during experiment planning (β = 0.2, p = 0.05). Statistical analyses were performed using GraphPad Prism and Instat. Individual t tests were performed for significance assessment of the differences between treatments. A p-value less than 0.05 was considered as significant.
3. Results
To develop necroptosis-inducing reagents, novel ceramide mimetics (RT-25, RT-71, YM-07) were
in silico designed and organically synthesized (
Figure 1 and
Supplementary Figure 1). Our design strategy was to mimic the structural features of ceramide. Compounds were designed with hydrophobic functional groups at both ends and hydrophilic functional groups in the center, like ceramide.
Figure 1.
Design of novel ceramide equivalents. A. Structures of C6-ceramide and the designed molecules. B. 3D structures of RT-25 (orange), RT-71 (blue), and YM-07 (purple) superimposed for the most stable conformation of C6-ceramide (green) using ROCS.
Figure 1.
Design of novel ceramide equivalents. A. Structures of C6-ceramide and the designed molecules. B. 3D structures of RT-25 (orange), RT-71 (blue), and YM-07 (purple) superimposed for the most stable conformation of C6-ceramide (green) using ROCS.
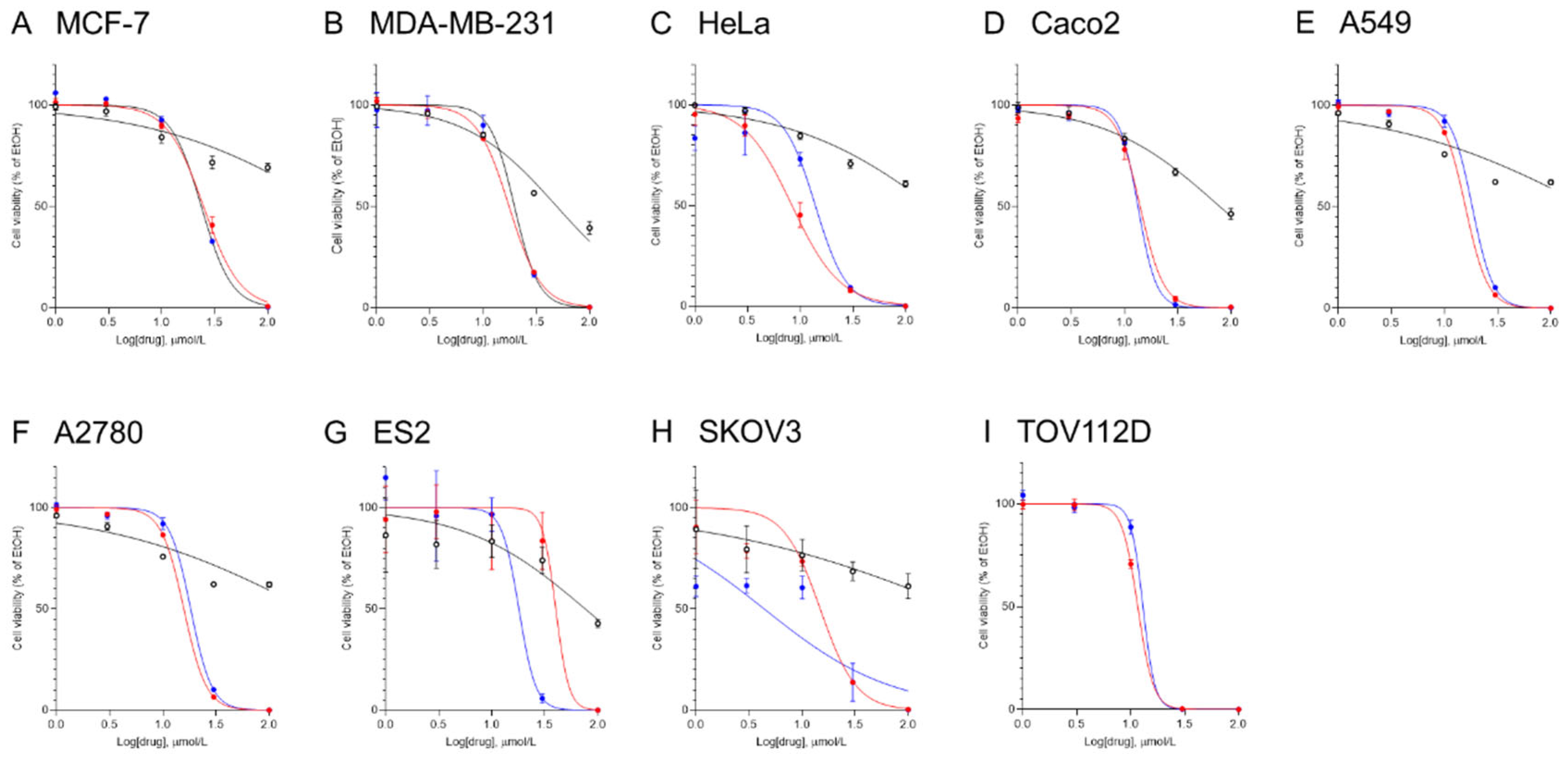
To assess the chemotherapeutic effects of the ceramide mimetics in multiple cancer cell lines, MCF-7, MDA-MB-231, HeLa, CaCo2, A549, A2780, ES2, SKOV3, and TOV112D cells were treated with various concentrations of ceramide mimetics (dissolved in ethanol) for 24 h and then cell viability was determined (
Figure 2). The IC
50 values for ceramide mimetics were calculated from cell viability results (
Table 1). RT-25 had little effect on cell viability. RT-71 and YM-07 both decreased cell viability in a concentration-dependent manner. Potent therapeutic effects of YM-07 were observed in all cancer cell lines. RT71 also showed potent effects in cancer cell lines except ES2 ovarian cancer cells. CNL previously showed chemotherapeutic activities in ovarian cancer cells [
12]. The IC
50 values of both RT-71 and YM-07 were lower than that of CNL.
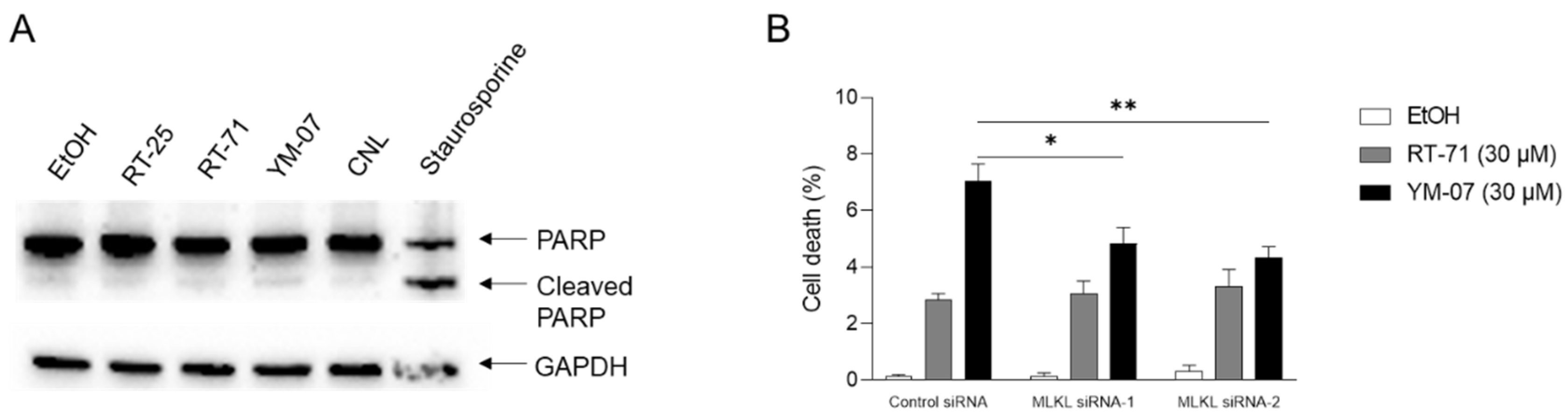
Ceramide is a lipid with pro-apoptotic and pro-necroptotic effects. To investigate the mechanism of cell death induced by the ceramide mimetics, MCF-7 cells were treated with 30 µM ceramide mimetics, 30 µM CNL, or 1 µM staurosporine, a known apoptosis-inducing agent (
Figure 3A). Staurosporine facilitated apoptotic cell death as evidenced by PARP cleavage, whereas ceramide mimetics and CNL failed to induce apoptosis. To examine if ceramide mimetics including RT-71 and YM-07 evoked MLKL-dependent necroptotic cell death, MLKL was knocked-down by RNA interference (
Figure 3B). Treatment with RT-71 or YM-07 induced cell death as determined by trypan blue–positive cell staining. Compared with control siRNA, MLKL siRNA (both siRNAs) significantly suppressed cell death induced by YM-07 but not RT-71. These results suggest that YM-07 exerts pro-necroptotic effects.
4. Discussion
Necroptosis is a promising target for cancer therapeutics. Here we explored ceramide mimetics as potential necroptosis-inducing reagents that exert antitumor effects using multiple cancer cell lines.
Several necroptosis-inducing reagents with a small molecular weight have been identified, including shikonin isolated from the root of
Lithospermum erythrorhizon [
28] and the conventional chemotherapeutic reagent cisplatin. Cisplatin has been shown to exert proapoptotic and necroptotic effects [
29,
30]. Retinoic acid receptor-γ activated by cisplatin has been implicated in RIPK3-MLKL-regulated necroptosis [
30]. These reagents are unlikely to target MLKL, the executioner of necroptosis. Epigenetic loss of RIP3 expression was observed in multiple cancer cell lines [
31], and thus RIPK-activating reagents are assumed not to have potent therapeutic effectiveness. Instead, MLKL-activating reagents are expected to have therapeutic effects. Substantially, RIPK3 expression is little in A549, HeLa, MCF-7, and MDA-MB-231[
31] and novel ceramide mimetic YM-07 similar to CNL also exerts necroptotic cell death in MCF-7 cells. The potential of YM-07 as an MLKL-activating reagent needs to be clarified in further studies.
The molecular mechanisms by which YM-07 exerts pro-necroptotic activity are unknown. In our preliminary studies using a cell-free system, we found that C6-ceramide bound to recombinant human MLKL (manuscript in preparation). Similar to C6-ceramide, YM-07 may also bind and activate MLKL. To develop potent MLKL-activating, necroptosis-inducing reagents, an in vitro interaction and in silico docking simulation between YM-07 and MLKL and the structure-activity correlation for chemically optimizing YM-07 need to be performed.
RT-71 and YM-07 are non-proapoptotic agents. Non-apoptotic cell death is subclassified into non-regulated necrosis and regulated cell death including ferroptosis, necroptosis, pyroptosis, etc [
32]. Though RT-71 exerts non-apoptotic effects on cancer cells, the cell death mechanism is unclear. Pronecroptotic YM-07 might have pronecrotic, pyroptotic and/or ferroptotic activities, since MLKL knockdown failed to fully suppress YM-07-induced cell death. In our preliminary results, YM-07 showed pyroptotic effects in head and neck cancer cell lines. In future studies, we will explore whether YM-07 and RT-71 facilitate regulated necrosis such pyroptosis and ferroptosis. In chronic lymphocytic leukemia cells, CNL induced caspase 3/7-independent necrotic cell death by selectively inhibiting the glycolytic pathway [
33]. Ceramide mimetics are potential to down-regulate aerobic glycolysis [
34,
35,
36] that is considered as a therapeutic target for cancer treatment.
5. Conclusions
Ceramide mimetics (RT-71, YM-07) showed antiproliferative activities against a variety of cancer cells lines. YM-07 may be a potential lead compound for developing necroptosis-inducing, chemotherapeutic reagents. These findings may help establish a molecular basis for ceramide mimetics in necroptotic cell death and provide a foundation for drug discovery for pro-necroptotic cancer therapy.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Supporting information with Supplementary Figure S1.
Author Contributions
Conceptualization, K.Kitatani; methodology, K.Kawai; software, Y.O. R.T., and K.Kawai; validation, Y.O.; formal analysis, Y.O. T.R., and K.N.; investigation, S.S., M.I.., and K.Kitatani; resources, S.S., M.I., Y.K., and K.Kawai; data curation, Y.O., R.T., and K.N.; writing—original draft preparation, K.Kawai and K.Kitatani; writing—review and editing, K.Kawai and K.Kitatani; visualization, K.Kawai and K.Kitatani; supervision, K.Kawai, H.S., Y.K., M.O., M.M., T.N., and K.Kitatani; project administration, K.Kitatani; funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by the JSPS KAKENHI Grants (22K06623 to KK).
Acknowledgments
We thank Risa Togo and Yuya Moriguchi (Setsunan University) for their supports and OpenEye scientific software for providing us a free academic license. We thank Gabrielle White Wolf, PhD, from Edanz (
https://jp.edanz.com/ac) for editing a draft of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kitatani, K.; Usui, T.; Sriraman, S.K.; Toyoshima, M.; Ishibashi, M.; Shigeta, S.; Nagase, S.; Sakamoto, M.; Ogiso, H.; Okazaki, T.; et al. Ceramide limits phosphatidylinositol-3-kinase C2beta-controlled cell motility in ovarian cancer: potential of ceramide as a metastasis-suppressor lipid. Oncogene 2016, 35, 2801–2812. [Google Scholar] [CrossRef]
- Obeid, L.M.; Linardic, C.M.; Karolak, L.A.; Hannun, Y.A. Programmed cell death induced by ceramide. Science 1993, 259, 1769–1771. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A. Functions of ceramide in coordinating cellular responses to stress. Science 1996, 274, 1855–1859. [Google Scholar] [CrossRef]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef]
- Bielawska, A.; Greenberg, M.S.; Perry, D.; Jayadev, S.; Shayman, J.A.; McKay, C.; Hannun, Y.A. (1S,2R)-D-erythro-2-(N-myristoylamino)-1-phenyl-1-propanol as an inhibitor of ceramidase. J Biol Chem 1996, 271, 12646–12654. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.J.; Sundararaj, K.; Koybasi, S.; Phillips, M.S.; Szulc, Z.M.; Bielawska, A.; Day, T.A.; Obeid, L.M.; Hannun, Y.A.; Ogretmen, B. Inhibition of growth and telomerase activity by novel cationic ceramide analogs with high solubility in human head and neck squamous cell carcinoma cells. Otolaryngol Head Neck Surg 2005, 132, 55–62. [Google Scholar] [CrossRef]
- Dindo, D.; Dahm, F.; Szulc, Z.; Bielawska, A.; Obeid, L.M.; Hannun, Y.A.; Graf, R.; Clavien, P.A. Cationic long-chain ceramide LCL-30 induces cell death by mitochondrial targeting in SW403 cells. Mol Cancer Ther 2006, 5, 1520–1529. [Google Scholar] [CrossRef]
- Boddapati, S.V.; D'Souza, G.G.; Erdogan, S.; Torchilin, V.P.; Weissig, V. Organelle-targeted nanocarriers: specific delivery of liposomal ceramide to mitochondria enhances its cytotoxicity in vitro and in vivo. Nano Lett 2008, 8, 2559–2563. [Google Scholar] [CrossRef] [PubMed]
- Barth, B.M.; Cabot, M.C.; Kester, M. Ceramide-based therapeutics for the treatment of cancer. Anticancer Agents Med Chem 2011, 11, 911–919. [Google Scholar] [CrossRef]
- Beckham, T.H.; Lu, P.; Jones, E.E.; Marrison, T.; Lewis, C.S.; Cheng, J.C.; Ramshesh, V.K.; Beeson, G.; Beeson, C.C.; Drake, R.R.; et al. LCL124, a cationic analog of ceramide, selectively induces pancreatic cancer cell death by accumulating in mitochondria. J Pharmacol Exp Ther 2013, 344, 167–178. [Google Scholar] [CrossRef]
- Kester, M.; Bassler, J.; Fox, T.E.; Carter, C.J.; Davidson, J.A.; Parette, M.R. Preclinical development of a C6-ceramide NanoLiposome, a novel sphingolipid therapeutic. Biol Chem 2015, 396, 737–747. [Google Scholar] [CrossRef]
- Zhang, X.; Kitatani, K.; Toyoshima, M.; Ishibashi, M.; Usui, T.; Minato, J.; Egiz, M.; Shigeta, S.; Fox, T.; Deering, T.; et al. Ceramide Nanoliposomes as a MLKL-Dependent, Necroptosis-Inducing, Chemotherapeutic Reagent in Ovarian Cancer. Mol Cancer Ther 2018, 17, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Bielawska, A.; Bielawski, J.; Szulc, Z.M.; Mayroo, N.; Liu, X.; Bai, A.; Elojeimy, S.; Rembiesa, B.; Pierce, J.; Norris, J.S.; et al. Novel analogs of D-e-MAPP and B13. Part 2: signature effects on bioactive sphingolipids. Bioorg Med Chem 2008, 16, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Dahm, F.; Bielawska, A.; Nocito, A.; Georgiev, P.; Szulc, Z.M.; Bielawski, J.; Jochum, W.; Dindo, D.; Hannun, Y.A.; Clavien, P.A. Mitochondrially targeted ceramide LCL-30 inhibits colorectal cancer in mice. Br J Cancer 2008, 98, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Jin, J.; Zhou, H.; Novgorodov, S.A.; Bielawska, A.; Szulc, Z.M.; Hannun, Y.A.; Obeid, L.M.; Hsu, Y.T. Mitochondrially targeted ceramides preferentially promote autophagy, retard cell growth, and induce apoptosis. J Lipid Res 2011, 52, 278–288. [Google Scholar] [CrossRef]
- Canals, D.; Perry, D.M.; Jenkins, R.W.; Hannun, Y.A. Drug targeting of sphingolipid metabolism: sphingomyelinases and ceramidases. Br J Pharmacol 2011, 163, 694–712. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X.; et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Linkermann, A.; Green, D.R. Necroptosis. N Engl J Med 2014, 370, 455–465. [Google Scholar] [CrossRef]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. The role of necroptosis in cancer biology and therapy. Mol Cancer 2019, 18, 100. [Google Scholar] [CrossRef]
- Nganga, R.; Oleinik, N.; Kim, J.; Selvam, S.P.; De Palma, R.; Johnson, K.A.; Parikh, R.Y.; Gangaraju, V.; Peterson, Y.; Dany, M.; et al. Receptor-interacting Ser/Thr kinase 1 (RIPK1) and myosin IIA-dependent ceramidosomes form membrane pores that mediate blebbing and necroptosis. J Biol Chem 2019, 294, 502–519. [Google Scholar] [CrossRef]
- Fridman, J.S.; Lowe, S.W. Control of apoptosis by p53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Yang, Z.; Xie, L.; DeWitt, J.P.; Chen, Y. Cancer therapy in the necroptosis era. Cell Death Differ 2016, 23, 748–756. [Google Scholar] [CrossRef]
- Zhang, X.; Matsuda, M.; Yaegashi, N.; Nabe, T.; Kitatani, K. Regulation of Necroptosis by Phospholipids and Sphingolipids. Cells 2020, 9. [Google Scholar] [CrossRef]
- Parisi, L.R.; Li, N.; Atilla-Gokcumen, G.E. Very Long Chain Fatty Acids Are Functionally Involved in Necroptosis. Cell Chem Biol 2017, 24, 1445–1454 e1448. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.R.; Morrow, L.M.; Visser, M.B.; Atilla-Gokcumen, G.E. Turning the Spotlight on Lipids in Non-Apoptotic Cell Death. ACS Chem Biol 2018. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.R.; Sowlati-Hashjin, S.; Berhane, I.A.; Galster, S.L.; Carter, K.A.; Lovell, J.F.; Chemler, S.R.; Karttunen, M.; Atilla-Gokcumen, G.E. Membrane Disruption by Very Long Chain Fatty Acids during Necroptosis. ACS Chem Biol 2019, 14, 2286–2294. [Google Scholar] [CrossRef]
- Chen, J.; Xie, J.; Jiang, Z.; Wang, B.; Wang, Y.; Hu, X. Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene 2011, 30, 4297–4306. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Kadigamuwa, C.; Choksi, S.; Xu, Q.; Cataisson, C.; Greenbaum, S.S.; Yuspa, S.H.; Liu, Z.G. Role of Retinoic Acid Receptor-gamma in DNA Damage-Induced Necroptosis. iScience 2019, 17, 74–86. [Google Scholar] [CrossRef]
- Koo, G.B.; Morgan, M.J.; Lee, D.G.; Kim, W.J.; Yoon, J.H.; Koo, J.S.; Kim, S.I.; Kim, S.J.; Son, M.K.; Hong, S.S.; et al. Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res 2015, 25, 707–725. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Ryland, L.K.; Doshi, U.A.; Shanmugavelandy, S.S.; Fox, T.E.; Aliaga, C.; Broeg, K.; Baab, K.T.; Young, M.; Khan, O.; Haakenson, J.K.; et al. C6-ceramide nanoliposomes target the Warburg effect in chronic lymphocytic leukemia. PLoS One 2013, 8, e84648. [Google Scholar] [CrossRef]
- Pelicano, H.; Martin, D.S.; Xu, R.H.; Huang, P. Glycolysis inhibition for anticancer treatment. Oncogene 2006, 25, 4633–4646. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Chandel, N.S. Targeting glucose metabolism for cancer therapy. J Exp Med 2012, 209, 211–215. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).