Submitted:
06 December 2023
Posted:
07 December 2023
You are already at the latest version
Abstract
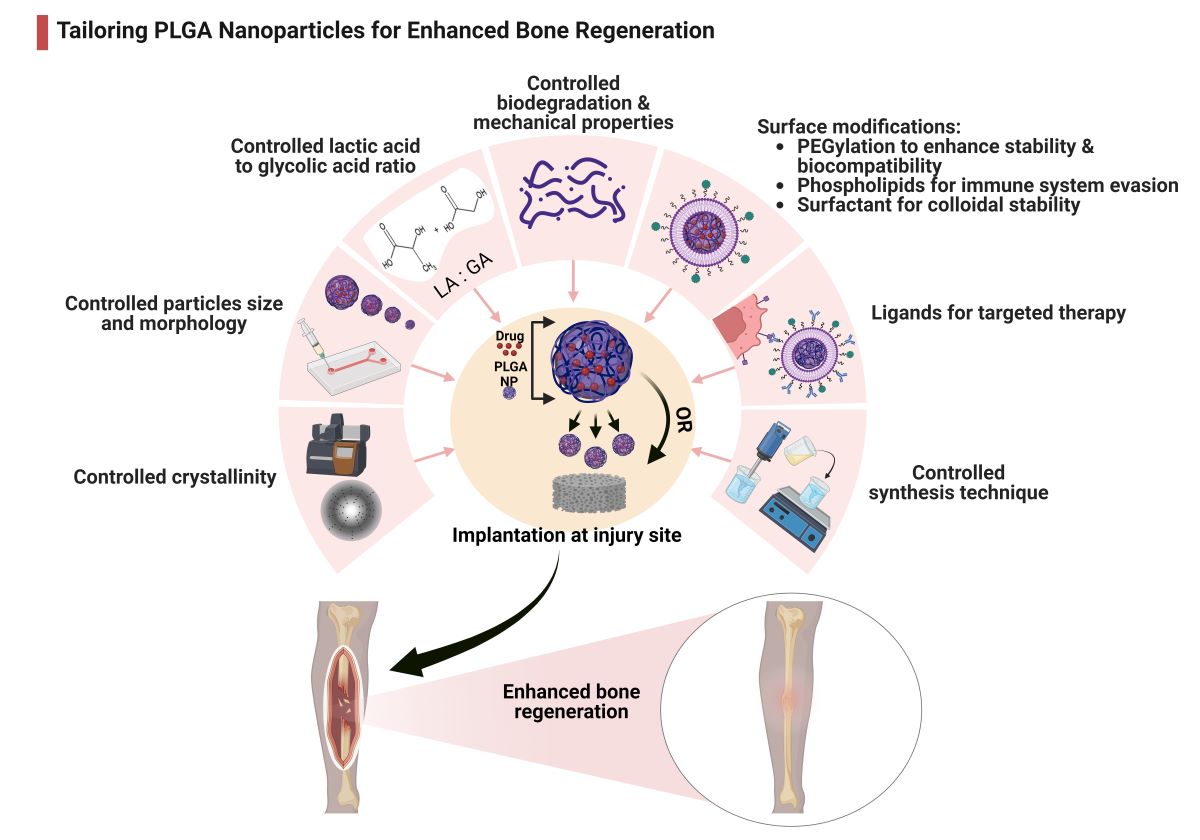
Keywords:
1. Introduction
2. Nanostructured Materials for Enhanced Bone Regeneration
2.1. Nanoparticles Classifications
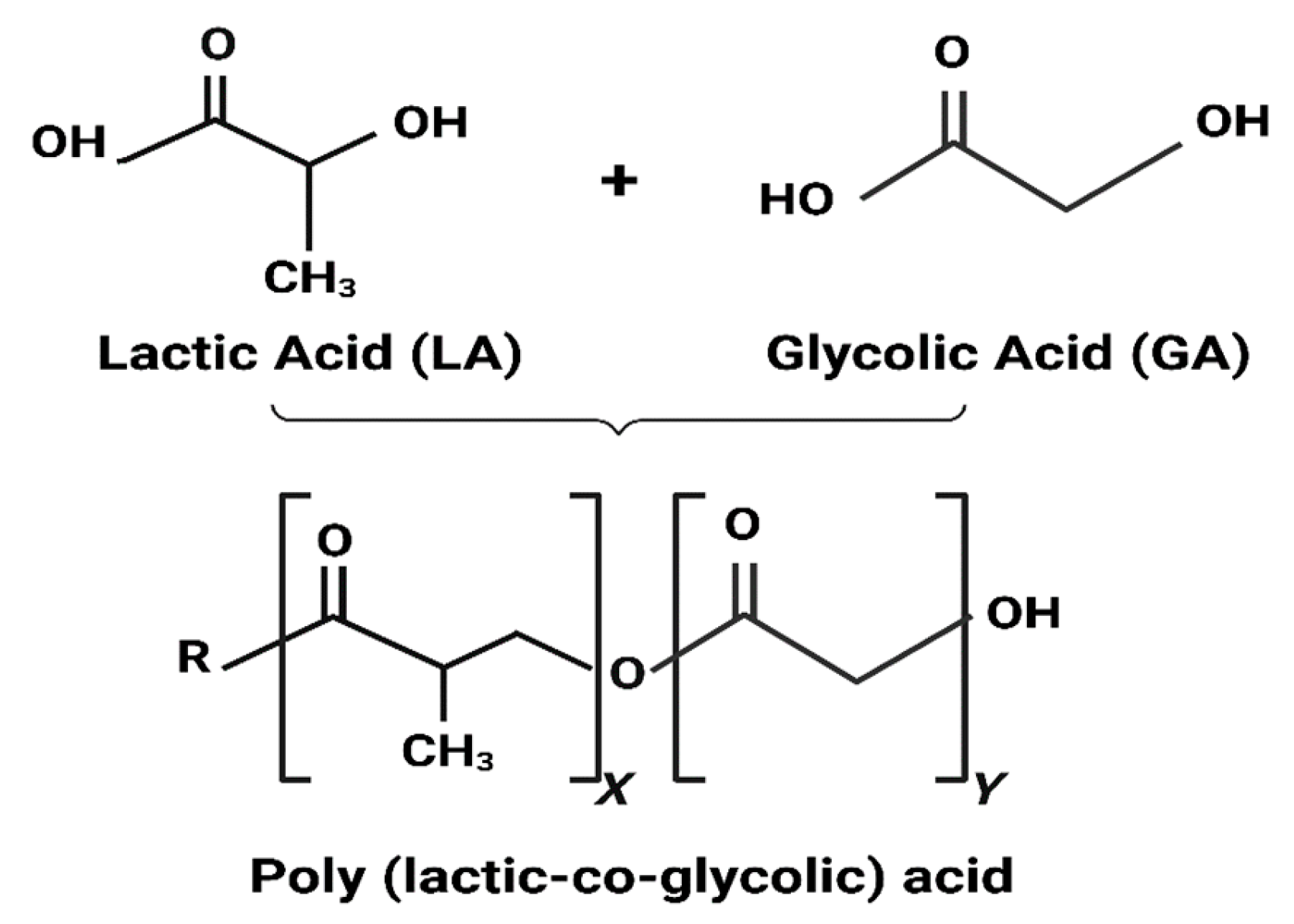
3. PLGA Nanoparticles and Their Properties
3.1. Modulating PLGA Properties for Enhanced Bone Regeneration
3.1.1. PLGA Physicochemical Properties
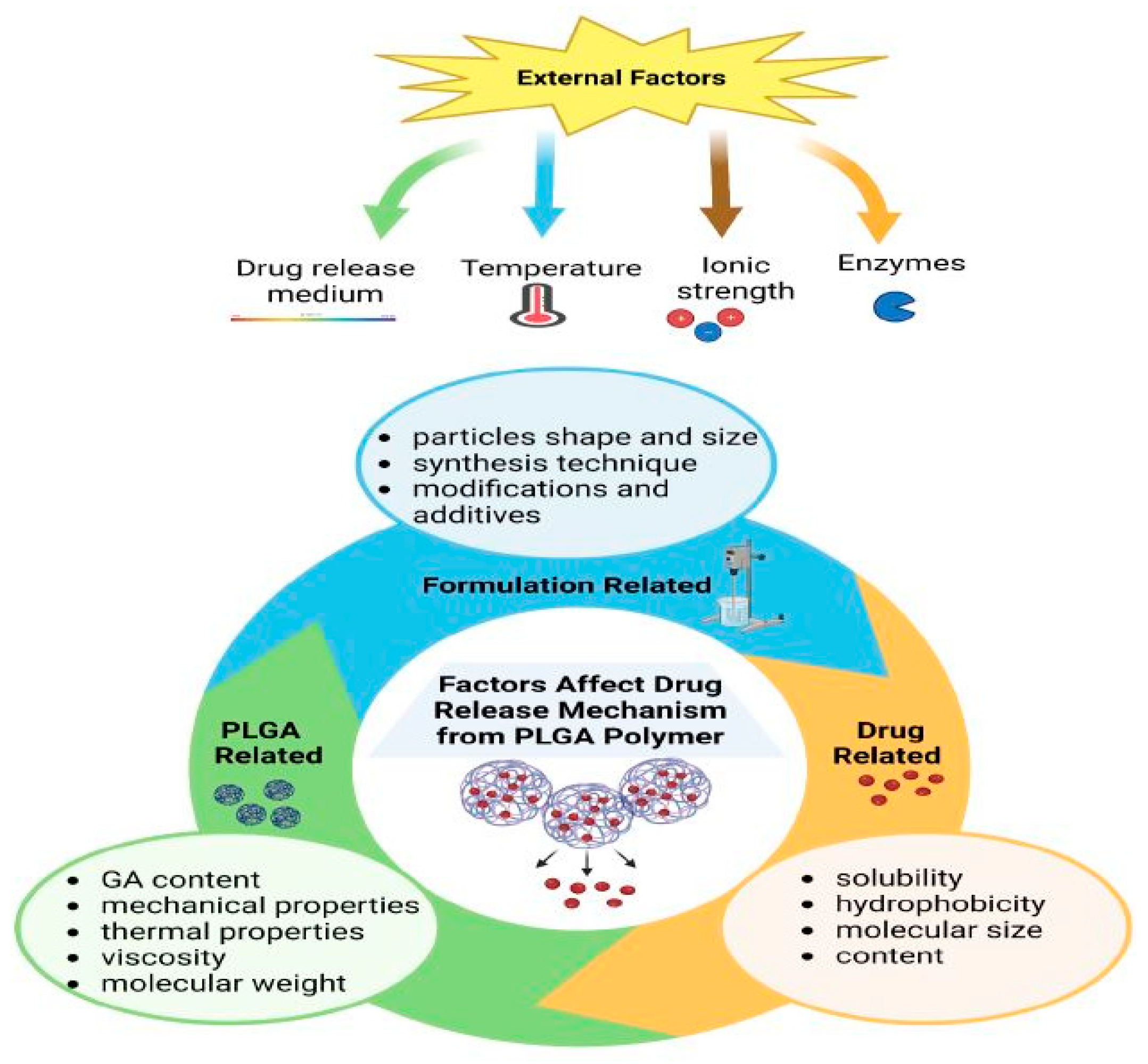
3.1.2. Biodegradation
3.1.3. Mechanical Strength
3.1.4. Particles Size and Morphology
3.2. Surface Modifications
3.2.1. PEGylation
3.2.2. Surfactants
3.2.3. Phospholipids
3.2.4. Surface Ligands
4. PLGA Nanoparticles Therapeutic Uses
5. Techniques of PLGA-Based Particles Preparation
5.1. The Emulsion-Solvent Evaporation Method
5.2. Nanoprecipitation Method
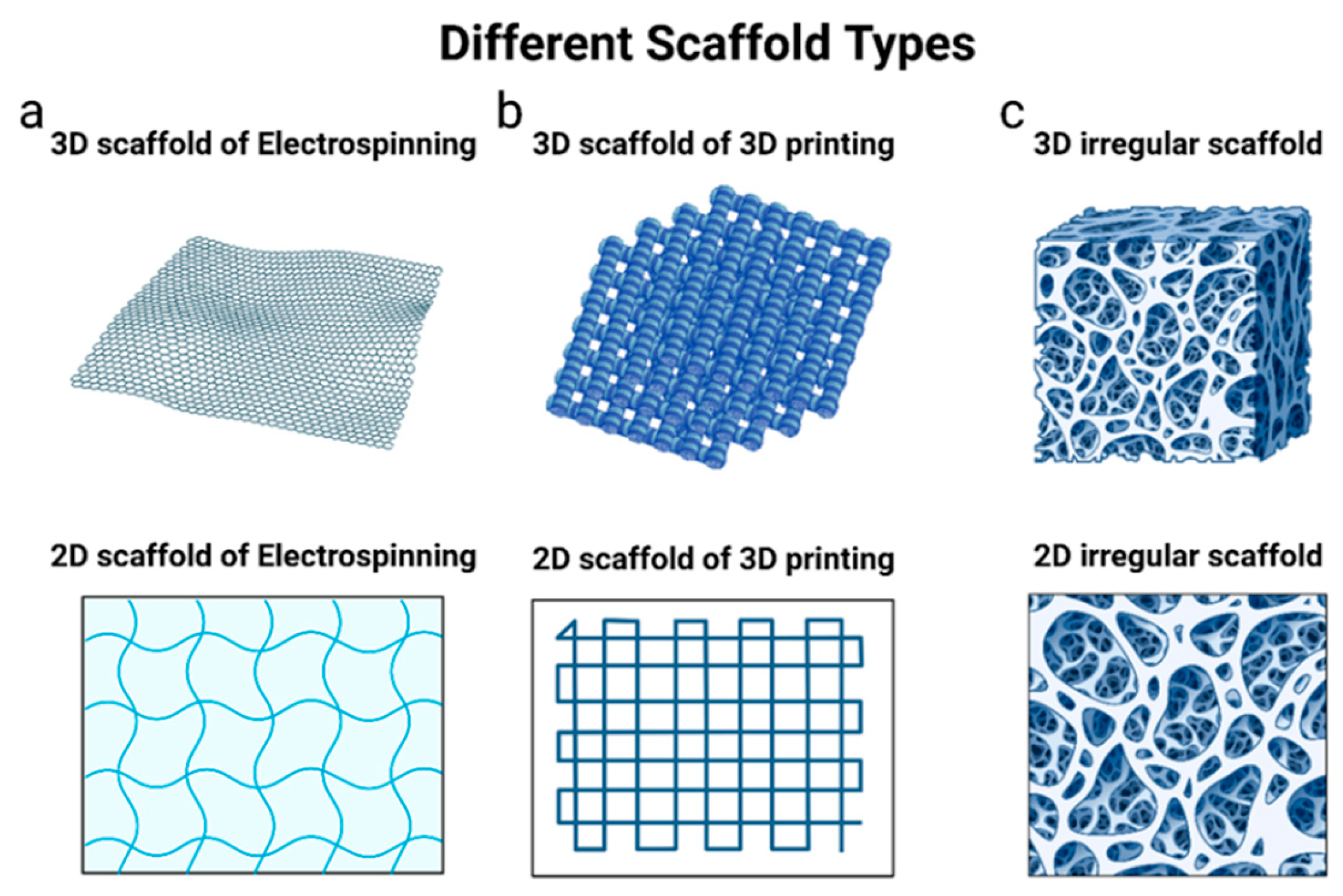
5.3. Electrospinning Method
5.4. 3D Printing Method
5.5. Other Methods
6. Fabrication Forms of PLGA Particles
6.1. PLGA as Nano or Micro Particles
6.2. PLGA Scaffolds
7. PLGA Loaded Bioactive Molecules for Bone Regeneration
7.1. Peptides
7.1.1. BMP-2
7.1.2. Other Proteins
7.2. Drugs
7.3. Ions
8. Cytotoxicity and Safety Evaluation of PLGA Nanoparticles
9. Commercial Products Based on PLGA
| Product name | Composition | Clinical Usage | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Polyglactin 910 (Vicryl suture) | Copolymer of glycolide and lactide | Internal suture | Low friction, easy to handle, fast absorption | Can cause inflammation if remains in skin more than 7 days causing scar tissue or stitch sinuses | [199,205] |
| Coated Vicryl Plus | Copolymer of glycolide and lactide coated with antibiotic agent | Surgical incision suture |
Prevent bacterial infection at surgical site | Low efficacy in oral, breast and cardiac surgeries | [199,206] |
| OsteoScafTM scaffold | PLGA and calcium phosphate | Clot-retention device and osteoconductive support for bone growth | Preserve alveolar bone structure following tooth extraction | low mechanical properties and local acidification of PLGA can lead to clinical failure | [200,207] |
| Biosteon interference screw | Hydroxyapatite particles within a PLLA matrix | Reconstruction of anterior cruciate ligaments and suture anchors for rotator cuff repairs | Osteoconductive material, HA particles improve strength retention, bone-bonding potential and pH buffering during graft healing | Differences in the resorption rates between PLGA and HA particles could induce potential complications | [201,208] |
| Bilok interference screws | β-TCP particles within PLLA matrix | Ligament restoration and suture anchors in rotator cuff repairs | Enhances the structural integrity, faster degradation and increased hydrophilicity | Screw can fracture during insertion or after insertion | [202,209] |
| ActivaScrewTM Interference screw | Proprietary blend of PLGA | Fixation of tissue including ligament, tendon to bone, or a bone-tendon to bone | Easy guided insertion, high strength, after operation screw dimensions slightly change improving screw’s fit, isoelasticity | - Cannot be used in early weight-bearing rehabilitation due to their elasticity - Additional casting is required to maintain reduction and alignment |
[203,210] |
| Milagro Advance Interference Screw |
70% PLGA and 30% β-TCP | Attachment of soft tissue grafts or bone-tendon-bone grafts to the tibia and/or femur during cruciate ligament reconstruction procedure. | Rapid insertion, excellent fixation strength and enhanced bone engagement | Marrow edema around bone tunnels seen 3 months after operation and reduced after 6 months | [211,212] |
| Biosure Regenesorb Interference Screw | β-TCP/PLGA/ calcium sulfate |
Fixing ligaments, tendons, soft tissues or bone-tendon-bone grafts in knee surgery | Open architecture allows bone ingrowth through the screw and attachment to graft, strength | Require special surgical fixation technique | [204,213] |
10. Current Challenges and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhu, G.; Zhao, Z.; Yin, C.; Zhao, Q.; Xu, H.; Wang, J.; Zhang, J.; Zhang, X.; Zhang, Y.; et al. Individualized plasticity autograft mimic with efficient bioactivity inducing osteogenesis. Int. J. Oral Sci. 2021, 13, 14. [Google Scholar] [CrossRef]
- Fillingham, Y.; Jacobs, J. Bone grafts and their substitutes. Bone Joint J. 2016, 98-B, 6–9. [CrossRef]
- Hamandi, F.; Goswami, T. Hierarchical Structure and Properties of the Bone at Nano Level. Bioengineering 2022, 9. [Google Scholar] [CrossRef]
- Feng, X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Curr. Chem. Biol. 2009, 3, 189–196. [Google Scholar] [CrossRef]
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B. Normal Bone Anatomy and Physiology. Clin. J. Am. Soc. Nephrol. 2008, 3. [Google Scholar] [CrossRef] [PubMed]
- Zorlutuna, P.; Vrana, N.E.; Khademhosseini, A. The Expanding World of Tissue Engineering: The Building Blocks and New Applications of Tissue Engineered Constructs. IEEE Rev. Biomed. Eng. 2013, 6, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- Pérez, R.A.; Won, J.-E.; Knowles, J.C.; Kim, H.-W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current concepts in scaffolding for bone tissue engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Leteve, M.; Passuti, N. Current Concepts in Bone Graft Substitutes. New J. Glas. Ceram. 2018, 08, 39–54. [Google Scholar] [CrossRef]
- Bhushan, S.; Singh, S.; Maiti, T.K.; Sharma, C.; Dutt, D.; Sharma, S.; Li, C.; Tag Eldin, E.M. Scaffold Fabrication Techniques of Biomaterials for Bone Tissue Engineering: A Critical Review. Bioengineering 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak, A.; Balawender, K. Structural and Metabolic Changes in Bone. Animals 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yan, J.; Yang, J.; Li, B. Nanomaterials promise better bone repair. Mater. Today 2016, 19, 451–463. [Google Scholar] [CrossRef]
- Lyons, J.G.; Plantz, M.A.; Hsu, W.K.; Hsu, E.L.; Minardi, S. Nanostructured Biomaterials for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Roohani-Esfahani, S.I.; Zreiqat, H. Nanoparticles: A promising new therapeutic platform for bone regeneration? Nanomedicine 2017, 12, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Babuska, V.; Kasi, P.B.; Chocholata, P.; Wiesnerova, L.; Dvorakova, J.; Vrzakova, R.; Nekleionova, A.; Landsmann, L.; Kulda, V. Nanomaterials in Bone Regeneration. Appl. Sci. 2022, 12. [Google Scholar] [CrossRef]
- Bozorgi, A.; Khazaei, M.; Soleimani, M.; Jamalpoor, Z. Application of nanoparticles in bone tissue engineering; a review on the molecular mechanisms driving osteogenesis. Biomater. Sci. 2021, 9, 4541–4567. [Google Scholar] [CrossRef]
- Liang, W.; Ding, P.; Li, G.; Lu, E.; Zhao, Z. Hydroxyapatite nanoparticles facilitate osteoblast differentiation and bone formation within sagittal suture during expansion in rats. Drug Des. Devel. Ther. 2021, 15, 905–917. [Google Scholar] [CrossRef]
- Huang, Z.; He, Y.; Chang, X.; Liu, J.; Yu, L.; Wu, Y.; Li, Y.; Tian, J.; Kang, L.; Wu, D.; et al. A Magnetic Iron Oxide/Polydopamine Coating Can Improve Osteogenesis of 3D-Printed Porous Titanium Scaffolds with a Static Magnetic Field by Upregulating the TGFβ-Smads Pathway. Adv. Healthc. Mater. 2020, 9, 2000318. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, Z.; Pei, X.; Zhang, X.; Cheng, X.; Hu, S.; Gao, X.; Wang, J.; Chen, J.; Wan, Q. ZIF-8-Modified Multifunctional Bone-Adhesive Hydrogels Promoting Angiogenesis and Osteogenesis for Bone Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 36978–36995. [Google Scholar] [CrossRef] [PubMed]
- Anu Mary Ealia, S.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 32019. [Google Scholar] [CrossRef]
- Ng, K.K.; Zheng, G. Molecular Interactions in Organic Nanoparticles for Phototheranostic Applications. Chem. Rev. 2015, 115, 11012–11042. [Google Scholar] [CrossRef] [PubMed]
- Higino, T.; França, R. Drug-delivery nanoparticles for bone-tissue and dental applications. Biomed. Phys. Eng. Express 2022, 8, 42001. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, B.H.; Lashin, M.M.A.; Mahmood, M.A.; Al-Mubaddel, F.S.; Ilyas, N.; Rahman, N.; Sohail, M.; Khan, A.; Abdullaev, S.S.; Khan, R. Organic and inorganic nanomaterials: fabrication, properties and applications. RSC Adv. 2023, 13, 13735–13785. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Sadia, H.; Ali Shah, S.Z.; Khan, M.N.; Shah, A.A.; Ullah, N.; Ullah, M.F.; Bibi, H.; Bafakeeh, O.T.; Khedher, N.B.; et al. Classification, Synthetic, and Characterization Approaches to Nanoparticles, and Their Applications in Various Fields of Nanotechnology: A Review. Catalysts 2022, 12. [Google Scholar] [CrossRef]
- Bhatti, R.; Shakeel, H.; Malik, K.; Qasim, M.; Khan, M.A.; Ahmed, N.; Jabeen, S. Inorganic Nanoparticles: Toxic Effects, Mechanisms of Cytotoxicityand Phytochemical Interactions. Adv Pharm Bull 2022, 12, 757–762. [Google Scholar] [CrossRef]
- Shannahan, J. The biocorona: a challenge for the biomedical application of nanoparticles. 2017, 6, 345–353. [Google Scholar] [CrossRef]
- Tanaka, M.; Izumiya, M.; Haniu, H.; Ueda, K.; Ma, C.; Ueshiba, K.; Ideta, H.; Sobajima, A.; Uchiyama, S.; Takahashi, J.; et al. Current Methods in the Study of Nanomaterials for Bone Regeneration. Nanomaterials 2022, 12. [Google Scholar] [CrossRef]
- Bolhassani, A.; Javanzad, S.; Saleh, T.; Hashemi, M.; Aghasadeghi, M.R.; Sadat, S.M. Polymeric nanoparticles. Hum. Vaccin. Immunother. 2014, 10, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Idrees, H.; Zaidi, S.Z.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials 2020, 10. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, Y. Chitosan-based nanocarriers for encapsulation and delivery of curcumin: A review. Int. J. Biol. Macromol. 2021, 179, 125–135. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Lu, Y.; Cheng, D.; Niu, B.; Wang, X.; Wu, X.; Wang, A. Properties of Poly (Lactic-co-Glycolic Acid) and Progress of Poly (Lactic-co-Glycolic Acid)-Based Biodegradable Materials in Biomedical Research. Pharmaceuticals 2023, 16. [Google Scholar] [CrossRef]
- Loureiro, J.A.; Pereira, M.C. PLGA Based Drug Carrier and Pharmaceutical Applications: The Most Recent Advances. Pharmaceutics 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Jo, A.; Ringel-Scaia, V.M.; McDaniel, D.K.; Thomas, C.A.; Zhang, R.; Riffle, J.S.; Allen, I.C.; Davis, R.M. Fabrication and characterization of PLGA nanoparticles encapsulating large CRISPR–Cas9 plasmid. J. Nanobiotechnology 2020, 18, 16. [Google Scholar] [CrossRef]
- Pantazis, P.; Dimas, K.; Wyche, J.H.; Anant, S.; Houchen, C.W.; Panyam, J.; Ramanujam, R.P. Preparation of siRNA-Encapsulated PLGA Nanoparticles for Sustained Release of siRNA and Evaluation of Encapsulation Efficiency BT - Nanoparticles in Biology and Medicine: Methods and Protocols. In; Soloviev, M., Ed.; Humana Press: Totowa, NJ, 2012; pp. 311–319 ISBN 978-1-61779-953-2.
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polym. 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Astete, C.E.; Sabliov, C.M. Synthesis and characterization of PLGA nanoparticles. J. Biomater. Sci. Polym. Ed. 2006, 17, 247–289. [Google Scholar] [CrossRef] [PubMed]
- Kohno, M.; Andhariya, J. V.; Wan, B.; Bao, Q.; Rothstein, S.; Hezel, M.; Wang, Y.; Burgess, D.J. The effect of PLGA molecular weight differences on risperidone release from microspheres. Int. J. Pharm. 2020, 582, 1–8. [Google Scholar] [CrossRef]
- Ortega-Oller, I.; Padial-Molina, M.; Galindo-Moreno, P.; O’Valle, F.; Jódar-Reyes, A.B.; Peula-García, J.M. Bone Regeneration from PLGA Micro-Nanoparticles. Biomed Res. Int. 2015, 2015, 415289. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly(lactic-co-glycolic acid)-based composite bone-substitute materials. Bioact. Mater. 2021, 6, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C. V; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Essa, D.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. The Design of Poly(lactide-co-glycolide) Nanocarriers for Medical Applications. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Dinarvand, R.; Sepehri, N.; Manoochehri, S.; Rouhani, H.; Atyabi, F. Polylactide-co-glycolide nanoparticles for controlled delivery of anticancer agents. Int. J. Nanomedicine 2011, 6, 877–895. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Liang, M.; Zhang, Z.; Bernaerts, K. V; Zhang, T. Synthesis and crystallization behavior of poly (lactide-co-glycolide). Polymer (Guildf). 2021, 235, 124302. [Google Scholar] [CrossRef]
- Swider, E.; Koshkina, O.; Tel, J.; Cruz, L.J.; de Vries, I.J.M.; Srinivas, M. Customizing poly(lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater. 2018, 73, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Sulaiman, M.; Yuvaraju, P.D.; Galiwango, E.; Rehman, I.U.; Al-Marzouqi, A.H.; Khaleel, A.; Mohsin, S. Biomimetic PLGA/Strontium-Zinc Nano Hydroxyapatite Composite Scaffolds for Bone Regeneration. J. Funct. Biomater. 2022, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ding, J. In vitro degradation of three-dimensional porous poly(d,l-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials 2004, 25, 5821–5830. [Google Scholar] [CrossRef]
- Maadani, A.M.; Salahinejad, E. Performance comparison of PLA- and PLGA-coated porous bioceramic scaffolds: Mechanical, biodegradability, bioactivity, delivery and biocompatibility assessments. J. Control. Release 2022, 351, 1–7. [Google Scholar] [CrossRef]
- Roces, C.B.; Christensen, D.; Perrie, Y. Translating the fabrication of protein-loaded poly(lactic-co-glycolic acid) nanoparticles from bench to scale-independent production using microfluidics. Drug Deliv. Transl. Res. 2020, 10, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Lanao, R.P.F.; Jonker, A.M.; Wolke, J.G.C.; Jansen, J.A.; Van Hest, J.C.M.; Leeuwenburgh, S.C.G. Physicochemical properties and applications of poly(lactic-co-glycolic acid) for use in bone regeneration. Tissue Eng. - Part B Rev. 2013, 19, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wu, J.; Qiao, W.; Zhao, Y.; Wong, K.H.M.; Chu, P.K.; Bian, L.; Wu, S.; Zheng, Y.; Cheung, K.M.C.; et al. Precisely controlled delivery of magnesium ions thru sponge-like monodisperse PLGA/nano-MgO-alginate core-shell microsphere device to enable in-situ bone regeneration. Biomaterials 2018, 174, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Banche-Niclot, F.; Licini, C.; Montalbano, G.; Fiorilli, S.; Mattioli-Belmonte, M.; Vitale-Brovarone, C. 3D Printed Scaffold Based on Type I Collagen/PLGA_TGF-β1 Nanoparticles Mimicking the Growth Factor Footprint of Human Bone Tissue. Polymers (Basel). 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, K.; Azadpour, P.; Moztarzadeh, F.; Urbanska, A.M.; Mozafari, M. Tissue-engineered chitosan/bioactive glass bone scaffolds integrated with PLGA nanoparticles: A therapeutic design for on-demand drug delivery. Mater. Lett. 2015, 138, 16–20. [Google Scholar] [CrossRef]
- Noori Koopaei, M.; Reza Khoshayand, M.; Hossein Mostafavi, S.; Amini, M.; Reza Khorramizadeh, M.; Jeddi Tehrani, M.; Atyabi, F.; Dinarvand, R. Docetaxel Loaded PEG-PLGA Nanoparticles: Optimized Drug Loading, In-vitro Cytotoxicity and In-vivo Antitumor Effect. Iran. J. Pharm. Res. 2014, 12, 819–833. [Google Scholar]
- Abbasnezhad, N.; Zirak, N.; Shirinbayan, M.; Tcharkhtchi, A.; Bakir, F. On the importance of physical and mechanical properties of PLGA films during drug release. J. Drug Deliv. Sci. Technol. 2021, 63, 102446. [Google Scholar] [CrossRef]
- Pannuzzo, M.; Horta, B.A.C.; La Rosa, C.; Decuzzi, P. Predicting the Miscibility and Rigidity of Poly(lactic-co-glycolic acid)/Polyethylene Glycol Blends via Molecular Dynamics Simulations. Macromolecules 2020, 53, 3643–3654. [Google Scholar] [CrossRef]
- Assani, K.; Neidhard-Doll, A.; Goswami, T. Mechanical properties of nanoparticles in the drug delivery kinetics. J. Pharm. Biopharm. Res. 2022, 4, 248–255. [Google Scholar] [CrossRef]
- ENOKI, S.; SATO, M.; TANAKA, K.; KATAYAMA, T. Mechanical Properties of a Single Cancellous Bone Trabeculae Taken From Bovine Femur. Int. J. Mod. Phys. Conf. Ser. 2012, 06, 349–354. [Google Scholar] [CrossRef]
- Gerhardt, L.C.; Boccaccini, A.R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials (Basel). 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.Y.; Ashman, R.B.; Turner, C.H. Young’s modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J. Biomech. 1993, 26, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.-Y.; Roy, M.E.; Tsui, T.Y.; Pharr, G.M. Elastic properties of microstructural components of human bone tissue as measured by nanoindentation. J. Biomed. Mater. Res. 1999, 45, 48–54. [Google Scholar] [CrossRef]
- Fiedler, T.; Belova, I. V; Murch, G.E.; Roether, J.A.; Boccaccini, A.R. Tailoring elastic properties of PLGA/TiO2 biomaterials. Comput. Mater. Sci. 2012, 61, 283–286. [Google Scholar] [CrossRef]
- Park, J.-W.; Hwang, J.-U.; Back, J.-H.; Jang, S.-W.; Kim, H.-J.; Kim, P.-S.; Shin, S.; Kim, T. High strength PLGA/Hydroxyapatite composites with tunable surface structure using PLGA direct grafting method for orthopedic implants. Compos. Part B Eng. 2019, 178, 107449. [Google Scholar] [CrossRef]
- Dong, W.; Huang, X.; Sun, Y.; Zhao, S.; Yin, J.; Chen, L. Mechanical characteristics and in vitro degradation kinetics analysis of polylactic glycolic acid/β-tricalcium phosphate (PLGA/β-TCP) biocomposite interference screw. Polym. Degrad. Stab. 2021, 186, 109421. [Google Scholar] [CrossRef]
- Lai, Y.; Li, Y.; Cao, H.; Long, J.; Wang, X.; Li, L.; Li, C.; Jia, Q.; Teng, B.; Tang, T.; et al. Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials 2019, 197, 207–219. [Google Scholar] [CrossRef]
- Magri, A.M.P.; Fernandes, K.R.; Assis, L.; Kido, H.W.; Avanzi, I.R.; Medeiros, M. da C.; Granito, R.N.; Braga, F.J.C.; Rennó, A.C.M. Incorporation of collagen and PLGA in bioactive glass: in vivo biological evaluation. Int. J. Biol. Macromol. 2019, 134, 869–881. [Google Scholar] [CrossRef]
- Duan, P.; Pan, Z.; Cao, L.; Gao, J.; Yao, H.; Liu, X.; Guo, R.; Liang, X.; Dong, J.; Ding, J. Restoration of osteochondral defects by implanting bilayered poly(lactide-co-glycolide) porous scaffolds in rabbit joints for 12 and 24 weeks. J. Orthop. Transl. 2019, 19, 68–80. [Google Scholar] [CrossRef]
- Sahin, A.; Esendagli, G.; Yerlikaya, F.; Caban-Toktas, S.; Yoyen-Ermis, D.; Horzum, U.; Aktas, Y.; Khan, M.; Couvreur, P.; Capan, Y. A small variation in average particle size of PLGA nanoparticles prepared by nanoprecipitation leads to considerable change in nanoparticles’ characteristics and efficacy of intracellular delivery. Artif. Cells, Nanomedicine, Biotechnol. 2017, 45, 1657–1664. [Google Scholar] [CrossRef]
- Mandl, H.K.; Quijano, E.; Suh, H.W.; Sparago, E.; Oeck, S.; Grun, M.; Glazer, P.M.; Saltzman, W.M. Optimizing biodegradable nanoparticle size for tissue-specific delivery. J. Control. Release 2019, 314, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, C. Tuning the Size of Poly(lactic-co-glycolic Acid) (PLGA) Nanoparticles Fabricated by Nanoprecipitation. Biotechnol. J. 2018, 13, 1700203. [Google Scholar] [CrossRef] [PubMed]
- Amoyav, B.; Benny, O. Controlled and tunable polymer particles’ production using a single microfluidic device. Appl. Nanosci. 2018, 8, 905–914. [Google Scholar] [CrossRef]
- Bao, Y.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Preparation of size-tunable sub-200 nm PLGA-based nanoparticles with a wide size range using a microfluidic platform. PLoS One 2022, 17, e0271050. [Google Scholar] [CrossRef] [PubMed]
- Mares, A.G.; Pacassoni, G.; Marti, J.S.; Pujals, S.; Albertazzi, L. Formulation of tunable size PLGA-PEG nanoparticles for drug delivery using microfluidic technology. PLoS One 2021, 16, e0251821. [Google Scholar] [CrossRef] [PubMed]
- El-Hammadi, M.M.; Arias, J.L. Recent Advances in the Surface Functionalization of PLGA-Based Nanomedicines. Nanomaterials 2022, 12. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug delivery systems based on polyethylene glycol hydrogels for enhanced bone regeneration. Front. Bioeng. Biotechnol. 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Xiao, L.-Q.; Tan, L.; Sun, W.; Wu, T.; Chen, L.-W.; Mei, Y.; Shi, B. Controlled release of simvastatin-loaded thermo-sensitive PLGA-PEG-PLGA hydrogel for bone tissue regeneration: in vitro and in vivo characteristics. J. Biomed. Mater. Res. Part A 2015, 103, 3580–3589. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Wang, Y.-H.; Lin, C.-W.; Lee, T.-C.; Fu, Y.-C.; Ho, M.-L.; Wang, C.-K. Combination of a Bioceramic Scaffold and Simvastatin Nanoparticles as a Synthetic Alternative to Autologous Bone Grafting. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Han, S.; Yang, H.; Ni, X.; Deng, Y.; Li, Z.; Xing, X.; Du, M. Programmed release of vascular endothelial growth factor and exosome from injectable chitosan nanofibrous microsphere-based PLGA-PEG-PLGA hydrogel for enhanced bone regeneration. Int. J. Biol. Macromol. 2023, 253, 126721. [Google Scholar] [CrossRef] [PubMed]
- Heinz, H.; Pramanik, C.; Heinz, O.; Ding, Y.; Mishra, R.K.; Marchon, D.; Flatt, R.J.; Estrela-Lopis, I.; Llop, J.; Moya, S.; et al. Nanoparticle decoration with surfactants: Molecular interactions, assembly, and applications. Surf. Sci. Rep. 2017, 72, 1–58. [Google Scholar] [CrossRef]
- Istikharoh, F.; Sujuti, H.; Mustamsir, E.; Swastirani, A. Preparation and biodegradable properties of hydroxyapatite nanoparticle composite coated with poly lactic-co-glycolic acid/polyvinyl alcohol for bone regeneration. Dent. Med. Probl. 2020, 57, 363–367. [Google Scholar] [CrossRef]
- Yilgor, P.; Hasirci, N.; Hasirci, V. Sequential BMP-2/BMP-7 delivery from polyester nanocapsules. J. Biomed. Mater. Res. Part A 2010, 93A, 528–536. [Google Scholar] [CrossRef]
- Shkodra-Pula, B.; Grune, C.; Traeger, A.; Vollrath, A.; Schubert, S.; Fischer, D.; Schubert, U.S. Effect of surfactant on the size and stability of PLGA nanoparticles encapsulating a protein kinase C inhibitor. Int. J. Pharm. 2019, 566, 756–764. [Google Scholar] [CrossRef]
- Bose, R.J.C.; Lee, S.-H.; Park, H. Lipid-based surface engineering of PLGA nanoparticles for drug and gene delivery applications. Biomater. Res. 2016, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, H.; Li, X.; Su, J.; Qin, L.; Sun, Y.; Guo, C.; Beck-Broichsitter, M.; Moehwald, M.; Chen, L.; et al. Phospholipid-modified poly(lactide-co-glycolide) microparticles for tuning the interaction with alveolar macrophages: In vitro and in vivo assessment. Eur. J. Pharm. Biopharm. 2019, 143, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.; Piras, A.M.; Dash, M. Cell membrane coated nanocarriers - an efficient biomimetic platform for targeted therapy. J. Control. Release 2020, 327, 546–570. [Google Scholar] [CrossRef]
- Tewabe, A.; Abate, A.; Tamrie, M.; Seyfu, A.; Siraj, E.A. Targeted drug delivery — from magic bullet to nanomedicine: Principles, challenges, and future perspectives. J. Multidiscip. Healthc. 2021, 14, 1711–1724. [Google Scholar] [CrossRef]
- Srinivasarao, M.; Low, P.S. Ligand-Targeted Drug Delivery. Chem. Rev. 2017, 117, 12133–12164. [Google Scholar] [CrossRef]
- Kato, K.; Akeda, K.; Miyazaki, S.; Yamada, J.; Muehleman, C.; Miyamoto, K.; Asanuma, Y.A.; Asanuma, K.; Fujiwara, T.; Lenz, M.E.; et al. NF-κB decoy oligodeoxynucleotide preserves disc height in a rabbit anular-puncture model and reduces pain induction in a rat xenograft-radiculopathy model. Eur. Cells Mater. 2021, 42, 90–109. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.C.; Ishida, Y.; Li, K.; Rintanalert, D.; Hatano-sato, K.; Oishi, S.; Hosomichi, J.; Usumi-fujita, R.; Yamaguchi, H.; Tsujimoto, H.; et al. NF-κB Decoy ODN-Loaded Poly(Lactic-co-glycolic Acid) Nanospheres Inhibit Alveolar Ridge Resorption. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef]
- Pal, K.; Laha, D.; Parida, P.K.; Roy, S.; Bardhan, S.; Dutta, A.; Jana, K.; Karmakar, P. An In Vivo Study for Targeted Delivery of Curcumin in Human Triple Negative Breast Carcinoma Cells Using Biocompatible PLGA Microspheres Conjugated with Folic Acid. J. Nanosci. Nanotechnol. 2019, 19, 3720–3733. [Google Scholar] [CrossRef]
- Mukerjee, A.; Ranjan, A.P.; Vishwanatha, J.K. Targeted nanocurcumin therapy using annexin A2 antibody improves tumor accumulation and therapeutic efficacy against highly metastatic breast cancer. J. Biomed. Nanotechnol. 2016, 12, 1374–1392. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, W.; Liu, X.; Huang, W.; Zhu, C.; Xu, Y.; Yang, H.; Bai, J.; Geng, D. Targeting strategies for bone diseases: signaling pathways and clinical studies. Signal Transduct. Target. Ther. 2023, 8, 202. [Google Scholar] [CrossRef]
- Brannon, E.R.; Guevara, M.V.; Pacifici, N.J.; Lee, J.K.; Lewis, J.S.; Eniola-Adefeso, O. Polymeric particle-based therapies for acute inflammatory diseases. Nat. Rev. Mater. 2022, 7, 796–813. [Google Scholar] [CrossRef] [PubMed]
- Yetisgin, A.A.; Cetinel, S.; Zuvin, M.; Kosar, A.; Kutlu, O. Therapeutic Nanoparticles and Their Targeted Delivery Applications. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Operti, M.C.; Bernhardt, A.; Grimm, S.; Engel, A.; Figdor, C.G.; Tagit, O. PLGA-based nanomedicines manufacturing: Technologies overview and challenges in industrial scale-up. Int. J. Pharm. 2021, 605, 120807. [Google Scholar] [CrossRef] [PubMed]
- Jaimes-Aguirre, L.; Morales-Avila, E.; Ocampo-García, B.E.; Medina, L.A.; López-Téllez, G.; Gibbens-Bandala, B. V.; Izquierdo-Sánchez, V. Biodegradable poly(D,L-lactide-co-glycolide)/poly(L-γ-glutamic acid) nanoparticles conjugated to folic acid for targeted delivery of doxorubicin. Mater. Sci. Eng. C 2017. [Google Scholar] [CrossRef]
- Cao, D.; Zhang, X.; Akabar, M.D.; Luo, Y.; Wu, H.; Ke, X.; Ci, T. Liposomal doxorubicin loaded PLGA-PEG-PLGA based thermogel for sustained local drug delivery for the treatment of breast cancer. Artif. Cells, Nanomedicine Biotechnol. 2019. [Google Scholar] [CrossRef]
- He, H.; Markoutsa, E.; Zhan, Y.; Zhang, J.; Xu, P. Mussel-inspired PLGA/polydopamine core-shell nanoparticle for light induced cancer thermochemotherapy. Acta Biomater. 2017. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, P.; Su, W.; Wang, S.; Liao, Z.; Niu, R.; Chang, J. PLGA/polymeric liposome for targeted drug and gene co-delivery. Biomaterials 2010. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Wang, H.; Gong, J.; He, H.; Shin, M.C.; Yang, V.C.; Huang, Y. Low-molecular-weight protamine-modified PLGA nanoparticles for overcoming drug-resistant breast cancer. J. Control. Release 2014, 192, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.J.; Yang, L.J.; Cai, B.T.; Zhu, L.X.; Cao, Y.L.; Wu, G.F.; Zhang, Z.J. Porous nano-hydroxyapatite/collagen scaffold containing drug-loaded ADM–PLGA microspheres for bone cancer treatment. J. Mater. Sci. Mater. Med. 2016. [Google Scholar] [CrossRef]
- Reddy, A.S.; Lakshmi, B.A.; Kim, S.; Kim, J. Synthesis and characterization of acetyl curcumin-loaded core/shell liposome nanoparticles via an electrospray process for drug delivery, and theranostic applications. Eur. J. Pharm. Biopharm. 2019, 142, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Qi, Q.; Mao, Z.; Zhan, X. PLGA nanoparticles introduction into mitoxantrone-loaded ultrasound-responsive liposomes: In vitro and in vivo investigations. Int. J. Pharm. 2017, 528, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Cao, J.Q.; Liu, C.; Zeng, F.; Cheng, H.; Hu, X.Y.; Shao, J.H. Evaluation of rMETase-loaded stealth PLGA/liposomes modified with anti-CAGE scFV for treatment of gastric carcinoma. J. Biomed. Nanotechnol. 2015, 11, 1153–1161. [Google Scholar] [CrossRef]
- Dana, P.; Bunthot, S.; Suktham, K.; Surassmo, S.; Yata, T.; Namdee, K.; Yingmema, W.; Yimsoo, T.; Ruktanonchai, U.R.; Sathornsumetee, S.; et al. Active targeting liposome-PLGA composite for cisplatin delivery against cervical cancer. Colloids Surfaces B Biointerfaces 2020, 196, 111270. [Google Scholar] [CrossRef]
- Cao, X.; Wang, B. Targeted PD-L1 PLGA/liposomes-mediated luteolin therapy for effective liver cancer cell treatment. J. Biomater. Appl. 2021, 36, 843–850. [Google Scholar] [CrossRef]
- Tonbul, H.; Sahin, A.; Tavukcuoglu, E.; Esendagli, G.; Capan, Y. Combination drug delivery with actively-targeted PLGA nanoparticles to overcome multidrug resistance in breast cancer. J. Drug Deliv. Sci. Technol. 2019, 54, 8–13. [Google Scholar] [CrossRef]
- Kumar, S.; Sangwan, P.; Lather, V.; Pandita, D. Biocompatible PLGA-oil hybrid nanoparticles for high loading and controlled delivery of resveratrol. J. Drug Deliv. Sci. Technol. 2015, 30, 54–62. [Google Scholar] [CrossRef]
- Aldawsari, M.F.; Alkholifi, F.K.; Foudah, A.I.; Alqarni, M.H.; Alam, A.; Salkini, M.A.; Sweilam, S.H. Gallic-Acid-Loaded PLGA Nanoparticles: A Promising Transdermal Drug Delivery System with Antioxidant and Antimicrobial Agents. Pharmaceuticals 2023, 16, 1090. [Google Scholar] [CrossRef]
- Gagliardi, A.; Voci, S.; Ambrosio, N.; Fresta, M.; Duranti, A.; Cosco, D. Characterization and Preliminary In Vitro Antioxidant Activity of a New Multidrug Formulation Based on the Co-Encapsulation of Rutin and the α-Acylamino-β-Lactone NAAA Inhibitor URB894 within PLGA Nanoparticles. Antioxidants 2023, 12, 305. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, M.; Kilicarslan, M.; Orhan, K. Effect of process variables on in vitro characteristics of clindamycin phosphate loaded PLGA nanoparticles in dental bone regeneration and 3D characterization studies using nano-CT. J. Drug Deliv. Sci. Technol. 2022, 76, 103710. [Google Scholar] [CrossRef]
- Pudełko, I.; Moskwik, A.; Kwiecień, K.; Kriegseis, S.; Krok-Borkowicz, M.; Schickle, K.; Ochońska, D.; Dobrzyński, P.; Brzychczy-Włoch, M.; Gonzalez-Julian, J.; et al. Porous Zirconia Scaffolds Functionalized with Calcium Phosphate Layers and PLGA Nanoparticles Loaded with Hydrophobic Gentamicin. Int. J. Mol. Sci. 2023, 24, 8400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; He, J.; Zhang, W.; Liu, J. Fabrication of TPGS-Stabilized Liposome-PLGA Hybrid Nanoparticle Via a New Modified Nanoprecipitation Approach: In Vitro and In Vivo Evaluation. Pharm. Res. 2018, 35. [Google Scholar] [CrossRef] [PubMed]
- Zweers, M.L.T.; Engbers, G.H.M.; Grijpma, D.W.; Feijen, J. Release of anti-restenosis drugs from poly(ethylene oxide)-poly(dl-lactic-co-glycolic acid) nanoparticles. J. Control. Release 2006, 114, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Marquette, S.; Peerboom, C.; Yates, A.; Denis, L.; Langer, I.; Amighi, K.; Goole, J. Stability study of full-length antibody (anti-TNF alpha) loaded PLGA microspheres. Int. J. Pharm. 2014, 470, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Thomas Cordonnier, A.S. Protein Encapsulation into PLGA Nanoparticles by a Novel Phase Separation Method Using Non-Toxic Solvents. J. Nanomed. Nanotechnol. 2014, 05. [Google Scholar] [CrossRef]
- Morita, T.; Sakamura, Y.; Horikiri, Y.; Suzuki, T.; Yoshino, H. Protein encapsulation into biodegradable microspheres by a novel S/O/W emulsion method using poly(ethylene glycol) as a protein micronization adjuvant. J. Control. Release 2000, 69, 435–444. [Google Scholar] [CrossRef]
- Pan, H.; Zheng, Q.; Yang, S.; Guo, X. Effects of functionalization of PLGA-[Asp-PEG]n copolymer surfaces with Arg-Gly-Asp peptides, hydroxyapatite nanoparticles, and BMP-2-derived peptides on cell behavior in vitro. J. Biomed. Mater. Res. - Part A 2014, 102, 4526–4535. [Google Scholar] [CrossRef] [PubMed]
- Maji, K.; Dasgupta, S. Hydroxyapatite-Chitosan and Gelatin Based Scaffold for Bone Tissue Engineering. Trans. Indian Ceram. Soc. 2014, 73, 110–114. [Google Scholar] [CrossRef]
- Hu, K.; Xiang, L.; Chen, J.; Qu, H.; Wan, Y.; Xiang, D. PLGA-liposome electrospun fiber delivery of miR-145 and PDGF-BB synergistically promoted wound healing. Chem. Eng. J. 2021, 422. [Google Scholar] [CrossRef]
- Kumar, A.; Madhusudana Rao, K.; Haider, A.; Han, S.S.; Son, T.W.; Kim, J.H.; Oh, T.H. Fabrication and Characterization of Multicomponent Polysaccharide/Nanohydroxyapatite Composite Scaffolds. Polym. Plast. Technol. Eng. 2017, 56, 983–991. [Google Scholar] [CrossRef]
- Kim, T.-H.; Kim, J.-J.; Kim, H.-W. Basic fibroblast growth factor-loaded, mineralized biopolymer-nanofiber scaffold improves adhesion and proliferation of rat mesenchymal stem cells. Biotechnol. Lett. 2014, 36, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Stern, S.T.; Sun, D. PLGA/liposome hybrid nanoparticles for short-chain ceramide delivery. Pharm. Res. 2014, 31, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhang, Y.; Yang, Z.; Zuo, G.; Zhang, Q.; Yao, F.; Wan, Y. Encapsulating doxorubicin-intercalated lamellar nanohydroxyapatite into PLGA nanofibers for sustained drug release. Curr. Appl. Phys. 2019, 19, 1204–1210. [Google Scholar] [CrossRef]
- Ignjatović, N.; Uskoković, V.; Ajduković, Z.; Uskoković, D. Multifunctional hydroxyapatite and poly(D,L-lactide-co-glycolide) nanoparticles for the local delivery of cholecalciferol. Mater. Sci. Eng. C. Mater. Biol. Appl. 2013, 33, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, G.; Qi, F.; Cheng, Y.; Lu, X.; Wang, L.; Zhao, J.; Zhao, B. Enhanced bone regeneration using an insulin-loaded nano-hydroxyapatite/collagen/plgacomposite scaffold. Int. J. Nanomedicine 2018, 13, 117–127. [Google Scholar] [CrossRef]
- Ren, X.; Liu, Q.; Zheng, S.; Zhu, J.; Qi, Z.; Fu, C.; Yang, X.; Zhao, Y. Synergistic delivery of bFGF and BMP-2 from poly(l-lactic-: Co-glycolic acid)/graphene oxide/hydroxyapatite nanofibre scaffolds for bone tissue engineering applications. RSC Adv. 2018, 8, 31911–31923. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Zhang, Z. Porous Chitosan/Nano-Hydroxyapatite Composite Scaffolds Incorporating Simvastatin-Loaded PLGA Microspheres for Bone Repair. Cells Tissues Organs 2018, 205, 20–31. [Google Scholar] [CrossRef]
- Wu, C.; Baldursdottir, S.; Yang, M.; Mu, H. Lipid and PLGA hybrid microparticles as carriers for protein delivery. J. Drug Deliv. Sci. Technol. 2018, 43, 65–72. [Google Scholar] [CrossRef]
- Lu, Y.; Wan, Y.; Gan, D.; Zhang, Q.; Luo, H.; Deng, X.; Li, Z.; Yang, Z. Enwrapping Polydopamine on Doxorubicin-Loaded Lamellar Hydroxyapatite/Poly(lactic- co-glycolic acid) Composite Fibers for Inhibiting Bone Tumor Recurrence and Enhancing Bone Regeneration. ACS Appl. Bio Mater. 2021, 4, 6036–6045. [Google Scholar] [CrossRef]
- Cai, Q.; Qiao, C.; Ning, J.; Ding, X.; Wang, H.; Zhou, Y. A Polysaccharide-based Hydrogel and PLGA Microspheres for Sustained P24 Peptide Delivery: An In vitro and In vivo Study Based on Osteogenic Capability. Chem. Res. Chinese Univ. 2019, 35, 908–915. [Google Scholar] [CrossRef]
- Hu, Z.; Lu, J.; Hu, A.; Dou, Y.; Wang, S.; Su, D.; Ding, W.; Lian, R.; Lu, S.; Xiao, L.; et al. Engineering BPQDs/PLGA nanospheres-integrated wood hydrogel bionic scaffold for combinatory bone repair and osteolytic tumor therapy. Chem. Eng. J. 2022, 446, 137269. [Google Scholar] [CrossRef]
- del Castillo-Santaella, T.; Ortega-Oller, I.; Padial-Molina, M.; O’Valle, F.; Galindo-Moreno, P.; Jódar-Reyes, A.B.; Peula-García, J.M. Formulation, Colloidal Characterization, and In Vitro Biological Effect of BMP-2 Loaded PLGA Nanoparticles for Bone Regeneration. Pharmaceutics 2019, 11, 388. [Google Scholar] [CrossRef]
- Ramanlal Chaudhari, K.; Kumar, A.; Megraj Khandelwal, V.K.; Ukawala, M.; Manjappa, A.S.; Mishra, A.K.; Monkkonen, J.; Ramachandra Murthy, R.S. Bone metastasis targeting: A novel approach to reach bone using Zoledronate anchored PLGA nanoparticle as carrier system loaded with Docetaxel. J. Control. Release 2012, 158, 470–478. [Google Scholar] [CrossRef]
- Kwak, S.; Haider, A.; Gupta, K.C.; Kim, S.; Kang, I.-K. Micro/Nano Multilayered Scaffolds of PLGA and Collagen by Alternately Electrospinning for Bone Tissue Engineering. Nanoscale Res. Lett. 2016, 11, 323. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, X.; Zhao, Y.; Bai, L.; He, Z.; Tong, Q.; Xie, X.; Zhu, H.; Cai, D.; Zhou, Y.; et al. Cryogenic 3D printing of porous scaffolds for in situ delivery of 2D black phosphorus nanosheets, doxorubicin hydrochloride and osteogenic peptide for treating tumor resection-induced bone defects. Biofabrication 2020, 12, 035004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, H.; Huangfu, H.; Zhang, X.; Zhang, H.; Qin, Q.; Fu, L.; Wang, D.; Wang, C.; Wang, L.; et al. 3D printing of bone scaffolds for treating infected mandible bone defects through adjustable dual-release of chlorhexidine and osteogenic peptide. Mater. Des. 2022, 224, 111288. [Google Scholar] [CrossRef]
- Dou, Y.; Huang, J.; Xia, X.; Wei, J.; Zou, Q.; Zuo, Y.; Li, J.; Li, Y. A hierarchical scaffold with a highly pore-interconnective 3D printed PLGA/n-HA framework and an extracellular matrix like gelatin network filler for bone regeneration. J. Mater. Chem. B 2021, 9, 4488–4501. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Gu, P.; Wusiman, A.; Wusiman, A.; Zhang, Y.; Zhang, Y.; Liu, Z.; Liu, Z.; Bo, R.; Bo, R.; et al. Rational Design of PLGA Nanoparticle Vaccine Delivery Systems to Improve Immune Responses. Mol. Pharm. 2019, 16, 5000–5012. [Google Scholar] [CrossRef] [PubMed]
- Maksimenko, O.; Malinovskaya, J.; Shipulo, E.; Osipova, N.; Razzhivina, V.; Arantseva, D.; Yarovaya, O.; Mostovaya, U.; Khalansky, A.; Fedoseeva, V.; et al. Doxorubicin-loaded PLGA nanoparticles for the chemotherapy of glioblastoma: Towards the pharmaceutical development. Int. J. Pharm. 2019, 572, 118733. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, E.; López-Noriega, A.; Thompson, E.; Kelly, H.M.; Cryan, S.A.; O’Brien, F.J. Development of collagen-hydroxyapatite scaffolds incorporating PLGA and alginate microparticles for the controlled delivery of rhBMP-2 for bone tissue engineering. J. Control. Release 2015, 198, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Giottonini, K.Y.; Rodríguez-Córdova, R.J.; Gutiérrez-Valenzuela, C.A.; Peñuñuri-Miranda, O.; Zavala-Rivera, P.; Guerrero-Germán, P.; Lucero-Acuña, A. PLGA nanoparticle preparations by emulsification and nanoprecipitation techniques: effects of formulation parameters. RSC Adv. 2020, 10, 4218–4231. [Google Scholar] [CrossRef]
- Javadzadeh, Y.; Ahadi, F.; Davaran, S.; Mohammadi, G.; Sabzevari, A.; Adibkia, K. Preparation and physicochemical characterization of naproxen–PLGA nanoparticles. Colloids Surfaces B Biointerfaces 2010, 81, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Barichello, J.M.; Morishita, M.; Takayama, K.; Nagai, T. Encapsulation of Hydrophilic and Lipophilic Drugs in PLGA Nanoparticles by the Nanoprecipitation Method. Drug Dev. Ind. Pharm. 1999, 25, 471–476. [Google Scholar] [CrossRef]
- Govender, T. PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. J. Control. Release 1999, 57, 171–185. [Google Scholar] [CrossRef]
- Chatterjee, M.; Chanda, N. Formulation of PLGA nano-carriers: specialized modification for cancer therapeutic applications. Mater. Adv. 2022, 3, 837–858. [Google Scholar] [CrossRef]
- Ren, X.; Liu, Q.; Zheng, S.; Zhu, J.; Qi, Z.; Fu, C.; Yang, X.; Zhao, Y. Synergistic delivery of bFGF and BMP-2 from poly( <scp>l</scp> -lactic- co -glycolic acid)/graphene oxide/hydroxyapatite nanofibre scaffolds for bone tissue engineering applications. RSC Adv. 2018, 8, 31911–31923. [Google Scholar] [CrossRef]
- Li, D.; Sun, H.; Jiang, L.; Zhang, K.; Liu, W.; Zhu, Y.; Fangteng, J.; Shi, C.; Zhao, L.; Sun, H.; et al. Enhanced biocompatibility of PLGA nanofibers with gelatin/nano- hydroxyapatite bone biomimetics incorporation. ACS Appl. Mater. Interfaces 2014, 6, 9402–9410. [Google Scholar] [CrossRef] [PubMed]
- Ghavimi, M.A.; Bani Shahabadi, A.; Jarolmasjed, S.; Memar, M.Y.; Maleki Dizaj, S.; Sharifi, S. Nanofibrous asymmetric collagen/curcumin membrane containing aspirin-loaded PLGA nanoparticles for guided bone regeneration. Sci. Rep. 2020, 10, 18200. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, W.; Liu, Y.; Zhang, K.; Sun, Y.; Lei, P.; Hu, Y. Nano artificial periosteum PLGA/MgO/Quercetin accelerates repair of bone defects through promoting osteogenic − angiogenic coupling effect via Wnt/ β-catenin pathway. Mater. Today Bio 2022, 16, 100348. [Google Scholar] [CrossRef] [PubMed]
- Hatt, L.P.; Wirth, S.; Ristaniemi, A.; Ciric, D.J.; Thompson, K.; Eglin, D.; Stoddart, M.J.; Armiento, A.R. Micro-porous PLGA/ β -TCP/TPU scaffolds prepared by solvent-based 3D printing for bone tissue engineering purposes. Regen. Biomater. 2023. [Google Scholar] [CrossRef] [PubMed]
- Babilotte, J.; Martin, B.; Guduric, V.; Bareille, R.; Agniel, R.; Roques, S.; Héroguez, V.; Dussauze, M.; Gaudon, M.; Le Nihouannen, D.; et al. Development and characterization of a PLGA-HA composite material to fabricate 3D-printed scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2021, 118, 111334. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Sun, J.; Li, Y.; Chen, L.; Chen, C.; Wu, Y.; Wang, Z.; Li, L. Experimental study of rhBMP-2 chitosan nano-sustained release carrier-loaded PLGA/nHA scaffolds to construct mandibular tissue-engineered bone. Arch. Oral Biol. 2019, 102, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Zhao, F.; Yang, Z.; Zhang, W.; Chen, D.; Hang, F.; Chen, X. 3D-Printed Magnesium-Doped Micro-Nano Bioactive Glass Composite Scaffolds Repair Critical Bone Defects by Promoting Osteogenesis, Angiogenesis, and Immunomodulation. 2023. [CrossRef]
- Rasoulianboroujeni, M.; Fahimipour, F.; Shah, P.; Khoshroo, K.; Tahriri, M.; Eslami, H.; Yadegari, A.; Dashtimoghadam, E.; Tayebi, L. Development of 3D-printed PLGA/TiO2 nanocomposite scaffolds for bone tissue engineering applications. Mater. Sci. Eng. C 2019, 96, 105–113. [Google Scholar] [CrossRef]
- Zhao, X.; Han, Y.; Li, J.; Cai, B.; Gao, H.; Feng, W.; Li, S.; Liu, J.; Li, D. BMP-2 immobilized PLGA/hydroxyapatite fibrous scaffold via polydopamine stimulates osteoblast growth. Mater. Sci. Eng. C 2017, 78, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.Y.; Duan, Z.X.; Guo, X.D.; Li, J.F.; Lu, H.W.; Zheng, Q.X.; Quan, D.P.; Yang, S.H. Bone induction by biomimetic PLGA-(PEG-ASP)n copolymer loaded with a novel synthetic BMP-2-related peptide in vitro and in vivo. J. Control. Release 2010, 144, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ren, J.; Ren, T.; Gu, S.; Tan, Q.; Zhang, L.; Lv, K.; Pan, K.; Jiang, X. Bone marrow stromal cells cultured on poly (lactide-co-glycolide)/nano- hydroxyapatite composites with chemical immobilization of Arg-Gly-Asp peptide and preliminary bone regeneration of mandibular defect thereof. J. Biomed. Mater. Res. - Part A 2010, 95, 993–1003. [Google Scholar] [CrossRef]
- Mikos, A.G.; Thorsen, A.J.; Czerwonka, L.A.; Bao, Y.; Langer, R.; Winslow, D.N.; Vacanti, J.P. Preparation and characterization of poly(l-lactic acid) foams. Polymer (Guildf). 1994, 35, 1068–1077. [Google Scholar] [CrossRef]
- Jiang, T.; Yu, X.; Carbone, E.J.; Nelson, C.; Kan, H.M.; Lo, K.W.H. Poly aspartic acid peptide-linked PLGA based nanoscale particles: Potential for bone-targeting drug delivery applications. Int. J. Pharm. 2014, 475, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.; Yang, Y.; Zafar, H.; Wang, D. Tetracycline-grafted mPEG-PLGA micelles for bone-targeting and osteoporotic improvement. Front. Pharmacol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Wang, W.; Ma, L.; Xu, N.; Shi, C.; Xu, G.; He, H.; Pan, W. Alendronate modified mPEG-PLGA nano-micelle drug delivery system loaded with astragaloside has anti-osteoporotic effect in rats. Drug Deliv. 2022, 29, 2386–2402. [Google Scholar] [CrossRef]
- Wang, T.; Guo, S.; Zhang, H. Synergistic Effects of Controlled-Released BMP-2 and VEGF from nHAC/PLGAs Scaffold on Osteogenesis. Biomed Res. Int. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Zhu, H.; Shi, Z.; Cai, X.; Yang, X.; Zhou, C. The combination of PLLA/PLGA/PCL composite scaffolds integrated with BMP-2-loaded microspheres and low-intensity pulsed ultrasound alleviates steroid-induced osteonecrosis of the femoral head. Exp. Ther. Med. 2020, 20, 1–1. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Xie, W.; Gao, W.; Wang, G.; Zeng, L.; Miao, G.; Lei, B.; Lin, Z.; Chen, X. Micro-Nano Bioactive Glass Particles Incorporated Porous Scaffold for Promoting Osteogenesis and Angiogenesis in vitro. Front. Chem. 2019, 7. [Google Scholar] [CrossRef]
- Tian, T.; Hu, Q.; Shi, M.; Liu, C.; Wang, G.; Chen, X. The synergetic effect of hierarchical pores and micro-nano bioactive glass on promoting osteogenesis and angiogenesis in vitro. J. Mech. Behav. Biomed. Mater. 2023, 146, 106093. [Google Scholar] [CrossRef]
- Ehrahimian-Hosseinabadi, M.; Ashrafizadeh, F.; Etemadifar, M.; Venkatraman, S.S. Evaluating and Modeling the Mechanical Properties of the Prepared PLGA/nano-BCP Composite Scaffolds for Bone Tissue Engineering. J. Mater. Sci. Technol. 2011, 27, 1105–1112. [Google Scholar] [CrossRef]
- Mo, X.; Zhang, D.; Liu, K.; Zhao, X.; Li, X.; Wang, W. Nano-Hydroxyapatite Composite Scaffolds Loaded with Bioactive Factors and Drugs for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 1291. [Google Scholar] [CrossRef]
- Canciani, E.; Straticò, P.; Varasano, V.; Dellavia, C.; Sciarrini, C.; Petrizzi, L.; Rimondini, L.; Varoni, E.M. Polylevolysine and Fibronectin-Loaded Nano-Hydroxyapatite/PGLA/Dextran-Based Scaffolds for Improving Bone Regeneration: A Histomorphometric in Animal Study. Int. J. Mol. Sci. 2023, 24, 8137. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Deng, Y.; Ye, Z.; Liang, S.; Tang, Z.; Wei, S. Peptide decorated nano-hydroxyapatite with enhanced bioactivity and osteogenic differentiation via polydopamine coating. Colloids Surfaces B Biointerfaces 2013, 111, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhou, K.; Liu, M.; Guo, X.; Qu, Y.; Cui, W.; Shao, Z.; Zhang, X.; Xu, S. Loading of BMP-2-related peptide onto three-dimensional nano-hydroxyapatite scaffolds accelerates mineralization in critical-sized cranial bone defects. J. Tissue Eng. Regen. Med. 2018, 12, 864–877. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Liu, R.; Gong, Y.; Wang, M.; Huang, Q.; Feng, Q.; Yu, B. Zero-order controlled release of BMP2-derived peptide P24 from the chitosan scaffold by chemical grafting modification technique for promotion of osteogenesis in vitro and enhancement of bone repair in vivo. Theranostics 2017, 7, 1072–1087. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Martin, V.T.; Abdi, M.A.; Chen, L.; Gong, Y.; Yan, Y.; Song, L.; Liu, Z.; Zhang, X.; et al. Sequential Delivery of BMP2-Derived Peptide P24 by Thiolated Chitosan/Calcium Carbonate Composite Microspheres Scaffolds for Bone Regeneration. J. Nanomater. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Zheng, L.; Li, D.; Wang, W.; Zhang, Q.; Zhou, X.; Liu, D.; Zhang, J.; You, Z.; Zhang, J.; He, C. Bilayered Scaffold Prepared from a Kartogenin-Loaded Hydrogel and BMP-2-Derived Peptide-Loaded Porous Nanofibrous Scaffold for Osteochondral Defect Repair. ACS Biomater. Sci. Eng. 2019, 5, 4564–4573. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Jiang, M.; Zhang, M.; Cui, L.; Yang, X.; Wang, X.; Liu, G.; Ding, J.; Chen, X. Biofunctionalized composite scaffold to potentiate osteoconduction, angiogenesis, and favorable metabolic microenvironment for osteonecrosis therapy. Bioact. Mater. 2022, 9, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, L.; Zhou, L.; Chen, C.; Chen, G. Sequential release of vascular endothelial growth factor-A and bone morphogenetic protein-2 from osteogenic scaffolds assembled by PLGA microcapsules: A preliminary study in vitro. Int. J. Biol. Macromol. 2023, 232, 123330. [Google Scholar] [CrossRef]
- An, G.; Zhang, W.-B.; Ma, D.-K.; Lu, B.; Wei, G.-J.; Guang, Y.; Ru, C.-H.; Wang, Y.-S. Influence of VEGF/BMP-2 on the proliferation and osteogenetic differentiation of rat bone mesenchymal stem cells on PLGA/gelatin composite scaffold. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2316–2328. [Google Scholar]
- Lee, H.Y.; Kim, D.-S.; Hwang, G.Y.; Lee, J.-K.; Lee, H.-L.; Jung, J.-W.; Hwang, S.Y.; Baek, S.-W.; Yoon, S. lip; Ha, Y.; et al. Multi-modulation of immune-inflammatory response using bioactive molecule-integrated PLGA composite for spinal fusion. Mater. Today Bio 2023, 19, 100611. [Google Scholar] [CrossRef]
- Kouhi, M.; Fathi, M.; Prabhakaran, M.P.; Shamanian, M.; Ramakrishna, S. Poly L lysine-modified PHBV based nanofibrous scaffolds for bone cell mineralization and osteogenic differentiation. Appl. Surf. Sci. 2018, 457, 616–625. [Google Scholar] [CrossRef]
- Bentmann, A.; Kawelke, N.; Moss, D.; Zentgraf, H.; Bala, Y.; Berger, I.; Gasser, J.A.; Nakchbandi, I.A. Circulating fibronectin affects bone matrix, whereas osteoblast fibronectin modulates osteoblast function. J. Bone Miner. Res. 2010, 25, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Wenzhi, S.; Dezhou, W.; Min, G.; Chunyu, H.; Lanlan, Z.; Peibiao, Z. Assessment of nano-hydroxyapatite and poly (lactide-co-glycolide) nanocomposite microspheres fabricated by novel airflow shearing technique for in vivo bone repair. Mater. Sci. Eng. C 2021, 128, 112299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Xu, Y.; Ji, W.; Zhou, S.; Li, L.; Qiu, L.; Qian, Z.; Wang, X.; Zhang, H. Biomimetic black phosphorus quantum dots-based photothermal therapy combined with anti-PD-L1 treatment inhibits recurrence and metastasis in triple-negative breast cancer. J. Nanobiotechnology 2021, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Mohammapdour, R.; Ghandehari, H. Mechanisms of immune response to inorganic nanoparticles and their degradation products. Adv. Drug Deliv. Rev. 2022, 180, 114022. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Perrault, S.D.; Walkey, C.; Jennings, T.; Fischer, H.C.; Chan, W.C.W. Mediating Tumor Targeting Efficiency of Nanoparticles Through Design. Nano Lett. 2009, 9, 1909–1915. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers (Basel). 2023, 15. [Google Scholar] [CrossRef]
- Semete, B.; Booysen, L.; Lemmer, Y.; Kalombo, L.; Katata, L.; Verschoor, J.; Swai, H.S. In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomedicine Nanotechnology, Biol. Med. 2010, 6, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Irvin-Choy, N.S.; Nelson, K.M.; Gleghorn, J.P.; Day, E.S. Delivery and short-term maternal and fetal safety of vaginally administered PEG-PLGA nanoparticles. Drug Deliv. Transl. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Park, J.S.; Park, M.; Ko, M.Y.; Yi, S.W.; Yoon, J.A.; Yang, S.; Shim, S.H.; Park, K.-H.; Song, H. PLGA nanoparticles with multiple modes are a biologically safe nanocarrier for mammalian development and their offspring. Biomaterials 2018, 183, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Creemers, J.H.A.; Pawlitzky, I.; Grosios, K.; Gileadi, U.; Middleton, M.R.; Gerritsen, W.R.; Mehra, N.; Rivoltini, L.; Walters, I.; Figdor, C.G.; et al. Assessing the safety, tolerability and efficacy of PLGA-based immunomodulatory nanoparticles in patients with advanced NY-ESO-1-positive cancers: a first-in-human phase I open-label dose-escalation study protocol. BMJ Open 2021, 11, e050725. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Qin, L.; Cai, J.; Mei, R.; Qian, H.; Zou, Z. Jug-PLGA-NPs, a New Form of Juglone with Enhanced Efficiency and Reduced Toxicity on Melanoma. Chin. J. Integr. Med. 2022, 28, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xu, C.; Wu, G.; Ye, Q.; Wang, C. Poly(Lactic-co-Glycolic Acid): Applications and Future Prospects for Periodontal Tissue Regeneration. Polymers (Basel). 2017, 9. [Google Scholar] [CrossRef]
- Umbria, R.; Perrini, M.; Montedori, A.; Giani, E.; Rivoiro, C.; Corio, M.; Jefferson, T.; Cerbo, M. Polylactic-Glycolic Acid Absorbable Synthetic Suture (Pgla) Plus Antibacterial : A Systematic Review. 2012, 1–9.
- Araujo-Pires, A.C.; Mendes, V.C.; Ferreira-Junior, O.; Carvalho, P.S.P.; Guan, L.; Davies, J.E. Investigation of a Novel PLGA/CaP Scaffold in the Healing of Tooth Extraction Sockets to Alveolar Bone Preservation in Humans. Clin. Implant Dent. Relat. Res. 2016, 18, 559–570. [Google Scholar] [CrossRef]
- Stryker HA / PLLA Interference Screws Biologically Inspired Fixation. Trauma 2012.
- Murphy, W.L.; Lee, J.-S.; Markel, M.D.; Graf, B.K. Controlled release of biopharmaceutical growth factors from hydroxyapatite coating on bioresorbable interference screws used in cruciate ligament reconstruction surgery 2011.
- Technical Report Bioretec R & D Technical Report Comparison between ActivaScrew TM Interference TCP and Milagro ® Advance Interference Screw : Fixation Strength In-vitro. Bioretec Ltd. 2020, 1, 1–5.
- Di Giunta, A.C.C.; Cassarino, A.M.; Castorina, S.; Luca, T.; Romano, A.M.; Susanna, M.; Costa, D. Arthroscopic Hill-Sachs Filling Technique Using an Absorbable Interference Screw. Arthrosc. Tech. 2022, 11, e2389–e2395. [Google Scholar] [CrossRef]
- Chandrasekhar, H.; Sivakumar; Santhosh Kumar, M.P. Comparison of influence of vicryl and silk suture materials on wound healing after third molar surgery- a review. J. Pharm. Sci. Res. 2017, 9, 2426–2428. [Google Scholar]
- Zhang, Q.; Zhang, C.; Fang, X.; Luo, X.; Guo, J. Biomaterial suture Vicryl Plus reduces wound-related complications. Ther. Clin. Risk Manag. 2018, 14, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Shao, J.; Huang, G.; Zhang, J.; Pan, S. In Vitro and In Vivo Applications of Magnesium-Enriched Biomaterials for Vascularized Osteogenesis in Bone Tissue Engineering: A Review of Literature. J. Funct. Biomater. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, R.; Antoniac, I.; Laptoiu, D.; Antoniac, A.; Grecu, D. Complications related to biocomposite screw fixation in ACL reconstruction based on clinical experience and retrieval analysis. Mater. Plast. 2015, 52, 340–344. [Google Scholar]
- Smith, C.A.; Tennent, T.D.; Pearson, S.E.; Beach, W.R. Fracture of bilok interference screws on insertion during anterior cruciate ligament reconstruction. Arthrosc. J. Arthrosc. Relat. Surg. 2003, 19, e115–e117. [Google Scholar] [CrossRef]
- Heye, P.; Matissek, C.; Seidl, C.; Varga, M.; Kassai, T.; Jozsa, G.; Krebs, T. Making Hardware Removal Unnecessary by Using Resorbable Implants for Osteosynthesis in Children. Children 2022, 9. [Google Scholar] [CrossRef]
- Barber, F.A.; Dockery, W.D.; Hrnack, S.A. Long-Term Degradation of a Poly-Lactide Co-Glycolide/β-Tricalcium Phosphate Biocomposite Interference Screw. Arthrosc. J. Arthrosc. Relat. Surg. 2011, 27, 637–643. [Google Scholar] [CrossRef]
- Frosch, K.H.; Sawallich, T.; Schütze, G.; Losch, A.; Walde, T.; Balcarek, P.; Konietschke, F.; Stürmer, K.M. Magnetic resonance imaging analysis of the bioabsorbable MilagroTM interference screw for graft fixation in anterior cruciate ligament reconstruction. Strateg. Trauma Limb Reconstr. 2009, 4, 73–79. [Google Scholar] [CrossRef]
- Prodromos, C. The Anterior Cruciate Ligament: Reconstruction and Basic Science E-Book; Elsevier Health Sciences, 2017; ISBN 032349739X.
| Classifications | Advantages | Disadvantages | References |
|---|---|---|---|
| Organic nanoparticles: - Micelles - Dendrimers - Liposomes - Polymeric - Protein complexes |
Hydrophilic, non-toxic, biodegradable, easy synthesis process, well-defined structure, changeable size, good surface characteristics, controlled drug delivery | Sensitive to thermal and electromagnetic radiation such as heat and light, more susceptible to change in nature leading to their elimination from the body | [23,24,25] |
| Inorganic nanoparticles: - Silica - Quantum dots - Magnetic nanoparticles - Metal-based nanoparticles (silver, gold, copper, …... etc.) - Ceramics |
- Stability, easy surface functionalization, high surface-to-volume ratio, ultraviolet-visible sensitivity, electrical conductivity. - Catalytic, thermal, and antibacterial properties |
Long-term toxicity, genotoxicity, and oxidation vulnerability may induce an inflammatory response | [26,27] |
| Carbon-based nanoparticles: - Fullerenes - Carbon nanotubes - Graphite, graphene, and graphene oxide - Nano-diamonds |
Electrical conductivity, heat conductivity, good mechanical properties, high stability, high surface area, excellent optical activity | - Can activate ROS-associated signaling pathways, and trigger release of cytokines. - The presence of bio-corona on the surface of carbon-based nanoparticles may alter their activity, biodistribution, pharmacokinetics, cellular uptake, toxicity, and clearance |
[28,29] |
| Intended Use | Active Agent | Targeted Delivery |
PLGA Formulation |
In-vitro/ In-vivo |
Results | References |
|---|---|---|---|---|---|---|
| Anticancer | DOX | Use of FA for targeted delivery against folate receptors | PLGA/DOX/ γPGA/FA NPs |
HeLa cells | PLGA/DOX/γPGA/FA NPs have targeted and pH-dependent release. | [100] |
| DOX | Thermogel | DOX-loaded-liposome fabricated within PLGA-PEG-PLGA thermogel |
4T1 cells (in vivo) BALB/c mice |
The thermogel proved to have a no burst, controlled DOX release in vitro, and enhanced anticancer activity in vitro and in vivo with lesser side effects. | [101] | |
| DOX | Anti-EGFR antibody cetuximab (C) Light-induced chemotherapy (NIR) | DOX/PLGA/PD/ PEG/C core-shell NPs |
UMSCC 22A cells |
The core-shell NPs with photothermal activity and targeting antibody have enhanced and safer chemotherapeutic activity. | [102] | |
| DOX pEGFP DNA solution |
FA | Polymeric-lipsome-loaded-DOX/PLGA nanosphere complexed with pEGFP DNA | MDA-MB-231 cells |
The core-shell nanospheres succeeded in co-delivery of DOX and pEGFP DNA into breast cancer cells. | [103] | |
| DOX | CPPs-LMWP [C24LMWP] | DOX/PLGA/C24-LMWP NP | A549/T, MCF-7/ADR, and 293T |
LMWP delivered DOX/PLGA nanoparticles by targeting the MDR cancer cells overexpressing heparan sulfate proteoglycans. | [104] | |
| DOX (Adriamycin) |
_____ | DOX/PLGA microspheres loaded HA/ collagen scaffold (DOX/ PLGA/HAC) |
BMSC collected from bone marrow of femurs of male Wistar rats (in vivo) male Wistar rats | DOX/PLGA/HAC scaffolds exhibited bone repair activity with no obvious inflammatory signs, as well as enhanced antineoplastic activity. | [105] | |
| Diacetate acetyl curcumin (AC) | _____ | AC/PLGA/liposome | HeLa and HDFa cells |
A new drug delivery system with theranostic applications. | [106] | |
| Mitoxantrone (MXT) | Ultrasound-responsive liposome | MXT/PLGA /Lip |
______ | Sustained release NPs with ultrasound-responsive activity. | [107] | |
| Recombinant methioninase (rMETase) | Single-chain variable fragment (scFV) antibody | scFV/rMETase/PLGA/Lip | SGC-7901 cells |
scFV/PLGA/Lip NPs have higher cellular uptake in gastric cancer cells. scFV/rMETase/PLGA/Lip enhanced the anticancer activity of rMETase. | [108] | |
| Cisplatin | Anti-VEGF antibody Avastin® |
Avatin®/Lip/ PLGA/Cis |
SiHa cells | PLGA forms stable Cis NPs with sustained release. Encapsulating the NP into Avastin® conjugated liposomes enhances its intracellular uptake thus anticancer activity. | [109] | |
| Luteolin (L) | Antibody (PD-L1) |
L/PD-L1/ PLGA/Lip |
HepG2 cell | NPs with improved in vitro release profile, cancer cellular uptake, migration inhibition. | [110] | |
| Paclitaxel and elacridar (ELC) | Transferrin (Tf) | Tf/ PTX-ELC /PLGA NPs |
EMT6/AR1.0 cells |
Co-delivery of PTX and P-gp inhibitors to overcome multidrug resistance and maintain intracellular therapeutic drug levels. | [111] | |
| Antioxidant | Resveratrol | _____ | PLGA-oil nanohybrids (PONHs)/ resveratrol | Normal monkey kidney (Vero) cell |
PONH decreased cytotoxicity and improved scavenging activity of resveratrol in vitro. | [112] |
| Gallic acid (GA) | _____ | GA/PLGA |
S.aureus HaCaT cells |
GA/PLGA NPs with controlled release in vitro, excellent antioxidant activity, good antimicrobial activity against S. aureus, and good biocompatibility. | [113] | |
| Rutin (vitamin P) and NAAA inhibitor (URB894) | _____ | rutin/URB894/ PLGA NPs | C-28 and NCTC-2544 cells | The co-encapsulation of rutin and URB894 in PLGA NPs resulted in synergistic antioxidant activity. | [114] | |
| Antibiotic | Clindamycin | _____ | Clindamycin/ PLGA NPs |
_____ | Formation of sustained clindamycin release up to 3 months. | [115] |
| Gentamicin (gentAOT) | Zirconia scaffolds | gent AOT/ PLGA NPs |
S.aureus Osteoblast-like MG-63 cells |
gentAOT/PLGA NPs adequately inhibited the growth of S. aureus. | [116] | |
| Anti-atherosclerosis | Simvastatin (SIM) | ______ | SIM/PLGA/Lip | RAW 264.7 cells (in vivo) Atherosclerotic model rabbits |
SIM/PLGA/Lip NPs showed increased circulation time and enhanced athero-protective activity. | [117] |
| Anti-restenosis | Dexamethasone (DEX) or Rapamycin (Rap) | ______ | PEO-PLGA/DEX PEO-PLGA/Rap NPs were then coated with gelatin |
_____ | In vitro controlled release of coated NPs. | [118] |
| Method | Advantage | Disadvantage | References |
|---|---|---|---|
| Emulsion-solvent evaporation | Simple, spherical particles | Polydisperse particle sizes, high sheer forces degrade the active agent | [135,136,137] |
| Nanoprecipitation | High yield, reproducible, high encapsulation efficiency of hydrophobic drugs | Polydisperse particle sizes, high sheer forces degrade the active agent | [113,114,117,138] |
| Electrospinning | Easy, forms uniform fibrous scaffolds, multilayered scaffolds | Need electrospinning equipment, 2D nanofibrous membranes | [116,134,139] |
| 3D printing | Adjustable sizes and shapes of the fabricated scaffolds, monodispersed scaffolds | 3D printer is required, not compatible to all types of polymers, drugs may degrade during the drying step | [140,141,142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





