1. Introduction
Infectious diseases are the main cause of death among older adults worldwide [
1], and the risk of all-cause mortality increased after infectious disease [
2,
3]. Infections have adverse effects on older adults, and increase their susceptibility and frailty, and also endanger their health [
2,
3]. Therefore, it is crucial to develop optimal strategies for preventing infections and improving the health of older adults.
Malnutrition is a significant healthcare burden worldwide [
4] and, in hospitalized older patients, can worsen prognosis and quality of life by increasing the morbidity and mortality risks [
5] and decreasing the treatment response. Furthermore, malnutrition increases both the re-hospitalization rate and health expenditure [
6,
7,
8,
9], and infections further contribute to malnutrition.
Kidney dysfunction is a major cause of malnutrition. Furthermore, aging and comorbidities, such as diabetes and cardiovascular diseases, which confer additional nutritional risk, may synergistically further the progression of malnutrition [
10,
11,
12,
13]. Compared to the general population, patients with kidney dysfunction experience significant immune dysregulation, which increases their risks of infectious diseases, morbidity, and mortality [
10,
11,
12,
13].
The Global Leadership Initiative on Malnutrition (GLIM) criteria have been proposed recently by representatives of the world's leading clinical nutrition societies [
14], and several cohort studies have successfully applied these criteria in various patient categories and clinical settings. The GLIM criteria have been validated against various semi-gold standard comparators [
15]. A significant association between GLIM-defined malnutrition and mortality has been demonstrated in patients with a wide range of clinical conditions [
16,
17,
18,
19,
20,
21,
22,
23]. However, the GLIM criteria need to be validated in different patient populations and disease states. Furthermore, no study has assessed whether GLIM-defined malnutrition with kidney dysfunction is associated with a higher risk of mortality in patients hospitalized with infectious disease.
In this study, we aimed to investigate whether older patients hospitalized with infectious disease and more severe grades of GLIM-defined malnutrition had an increased mortality risk. Moreover, we determined whether patients with both malnutrition and kidney dysfunction had a higher mortality risk than those with normal kidney function and without malnutrition. To test these hypotheses, we examined the association between malnutrition severity (none, moderate, or severe) and mortality, as well as the malnutrition status stratified by kidney function and mortality in older patients who were hospitalized due to infectious diseases.
2. Materials and Methods
2.1. Participants
This retrospective cohort study enrolled patients aged 65 years or more who were admitted to Aichi Medical University Hospital with any severe infections between April 2019 and March 2020. Severe infections were defined as any infection requiring hospital-treatment [
24]. Upon admission, all new patients were screened for malnutrition using the Malnutrition Universal Screening Tool (MUST) [
25], for younger patients, and the Mini-Nutritional Assessment-Short Form (MNA-SF) [
26], for older patients. Patients with a MUST score ≥2 were deemed to be at risk of malnutrition and were referred to the hospital's nutrition support team [
25], whereas an MNA-SF score ≤11 indicated the need for subsequent nutritional assessment [
26]. Nutritional assessment was undertaken by a team of physicians, nurses, dietitians, and pharmacists within 5 days of admission. However, not all patients could be assessed due to diverse reasons, such as being absent for medical procedures or examinations or having a short hospitalization period. Patients without nutritional assessment data related to the GLIM criteria were excluded from the study. The study adhered to the principles of the Declaration of Helsinki and was approved by the Ethics Review Committee of Aichi Medical University Hospital (No. 2021e136). Although written consent was not obtained due to the retrospective nature of the study, patients were given the right to withdraw from the study through an opt-out notice that was posted on the hospital's website.
2.2. Data Collection
Data was collected retrospectively from medical records. Baseline information collected at admission included age, sex, BMI, laboratory data such as serum albumin, serum C-reactive protein (CRP) levels, and glomerular filtration rate (eGFR = 194×Scr
−1.094×Age
−0.287×0.739 [if female] [
27]); comorbidities (e.g., chronic obstructive pulmonary disease, autoimmune disease, cancer, diabetes mellitus, or cardiovascular disease); and mortality data (time and cause of death).
The GLIM criteria consist of three phenotypic and two etiologic criteria. To diagnose malnutrition, at least one phenotypic and one etiologic criterion must be fulfilled [
28]. In the present study, we used the parameters of the GLIM criteria and their respective thresholds that were used in a recently published study (
Table 1) [
29].
For evaluating weight loss, the participants were requested to report their weight change within the last 3–6 months. BMI was calculated as body weight (kg) divided by the square of the patient’s height (m). To determine reduced muscle mass, the calf circumference (CC; in cm) of their right leg was measured while in the supine position, with the knee flexed at a 90° angle. We used the previously validated CC cutoff values for Japanese patients (30 and 29 cm for men and women, respectively), as previously reported [
30,
31]. During the nutritional assessment visit, the patient was interviewed and their medical record was checked to determine whether their food intake was reduced or there was any issue with food assimilation. The criterion was considered fulfilled if the reduction in oral intake exceeded 50% of the energy requirement for more than a week, it exceeded 2 weeks, or the patient had a chronic gastrointestinal condition that adversely affected food assimilation or absorption, such as short bowel syndrome, pancreatic insufficiency, esophageal strictures, gastroparesis or chronic diarrhea. The disease burden or inflammation criterion was defined as the presence of acute inflammatory diseases, such as major infection, burns, trauma or closed head injury, or the presence of a comorbidity involving chronic or recurrent mild to moderate inflammation, such as malignant disease, chronic obstructive pulmonary disease, congestive heart failure, chronic renal disease, or CRP levels >5 mg/L. For patients diagnosed with malnutrition, three phenotypic criteria were used to assess the severity of malnutrition, in accordance with the diagnostic method of the GLIM criteria [
28]: (i) weight loss >10%, (ii) severely low BMI, or (iii) a severe deficit in muscle mass. However, the GLIM criteria did not provide any specific BMI cutoff values to distinguish between moderate and severe malnutrition in Asians [
30]. To address this issue, we used 17.0 and 17.8 kg/m
2 as cutoff values for severely low BMI in younger and older adult populations, respectively, as previously reported [
30]. Furthermore, although the recently published guidance of GLIM recommended reference values for CC for the assessment of the muscle mass phenotypic criterion [
32], no definite reference was provided. Therefore, at our institution, we used CC values that indicated severe muscle mass loss of <27 and <26 cm for men and women, respectively. These values were 10% lower than the corresponding values that indicate muscle mass loss in the sarcopenia diagnostic criteria [
30]. Using these cutoff values, we found that the prevalence of malnutrition according to the GLIM criteria was similar in patients with severely reduced muscle mass and other phenotypic criteria [
30]. Thus, we considered these as reasonable cutoff values for severely reduced muscle mass.
2.3. Outcomes
The primary outcome of interest was the all-cause mortality rate. The observational period was defined as the time from baseline to the incidence of death or the last visit to our hospital before November 30, 2021, whichever occurred first.
2.4. Statistical Analysis
We conducted a study to examine the correlation between malnutrition severity (none, moderate, or severe) and mortality, as well as the association of malnutrition status stratified by kidney function with mortality in patients who were hospitalized due to infectious diseases. Nutritional status was classified into three groups based on the GLIM criteria: no, moderate, and severe malnutrition. We compared the baseline characteristics of these groups using analysis of variance or the chi-square test.
The cumulative probabilities of mortality were calculated using the Kaplan–Meier method and the log-rank test. The association between nutritional status and all-cause mortality was evaluated using unadjusted and multivariable Cox proportional hazards models. Multivariable Cox proportional hazards models included the following covariates: Model 1, adjusted for GLIM criteria (without malnutrition, moderate malnutrition, and severe malnutrition), age (years) at baseline and sex; Model 2, adjusted for the covariates in Model 1 as well as eGFR categories (eGFR <30, eGFR 30–59, and eGFR ≥60 mL/min/1.73m2); and Model 3, adjusted for the covariates in Model 2 and comorbidities (chronic obstructive pulmonary disease, autoimmune disease, cancer, diabetes mellitus, or cardiovascular disease).
Furthermore, we stratified nutritional status into two categories (no malnutrition or malnutrition [moderate or severe]) and the eGFR level into three categories (<30, 30–59, ≥60 mL/min/1.73m
2) as reported previously [
33]. To analyze the impact of malnutrition and kidney function on an individual's health, we divided the data into six categories based on nutritional status (two groups) and eGFR level (three groups). To determine the impact of these factors, we employed multivariable Cox proportional hazards models, which were adjusted for age, sex, comorbidities, and the aforementioned six categories. The proportional hazard assumption was assessed by Schoenfeld residuals.
The level of statistical significance was set at P<0.05. All statistical analyses were performed using JMP version 14.0.0 (SAS Institute, Cary, NC, USA) and STATA version 13.0 (StataCorp, College Station, TX, USA).
3. Results
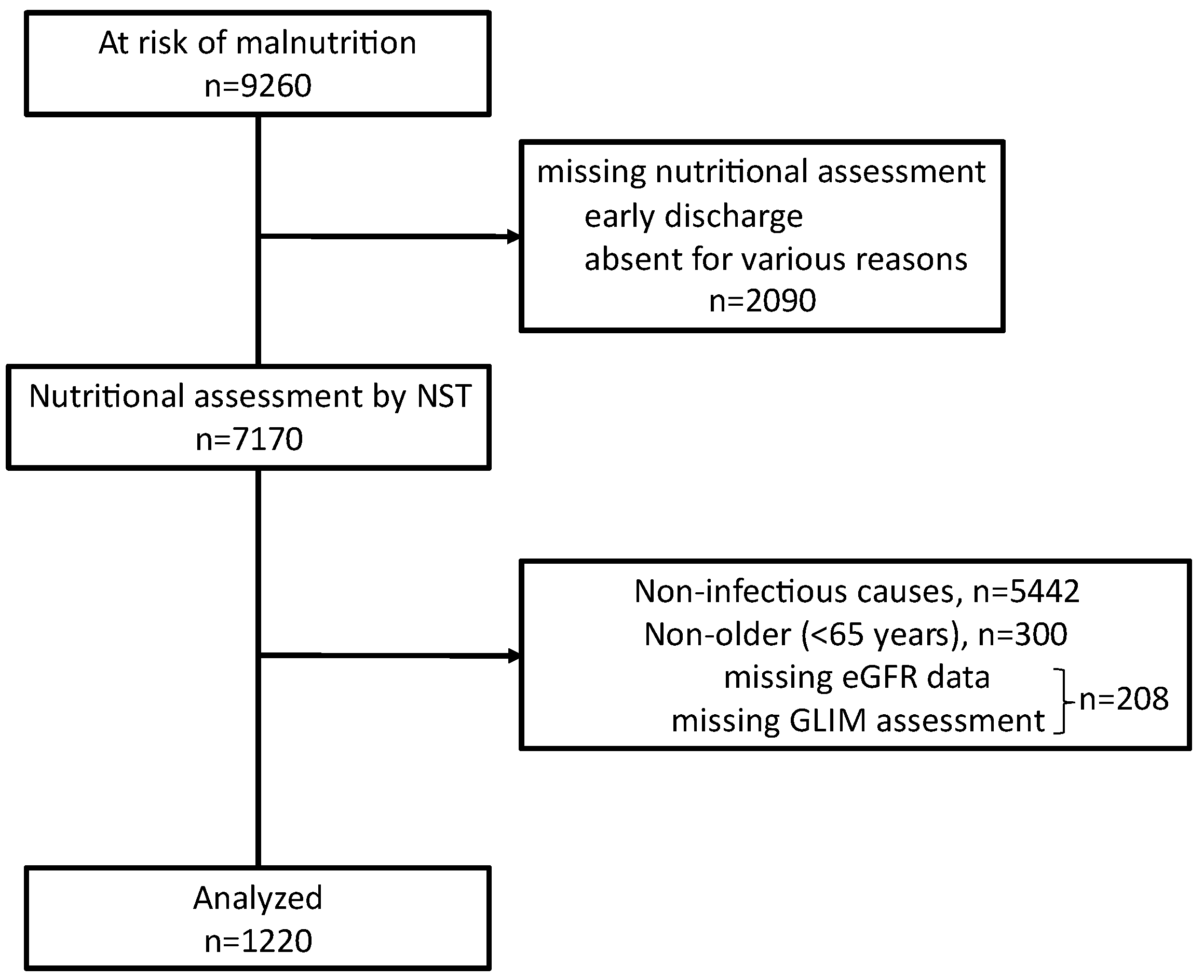
During the study period, through nutritional screening performed during admission, 9260 patients were identified as at risk for malnutrition. A nutritional support team assessed 7170 patients for their nutritional needs. After excluding 208 patients who did not undergo nutritional assessment using the GLIM criteria or lacked related eGFR data, 5442 patients with non-infectious causes of hospitalization, and 300 patients who were younger than 65 years, we included 1220 older patients (age ≥65 years) who were hospitalized due to severe infectious diseases (
Figure 1).
Figure 1.
Flow diagram of inclusion and exclusion of study participants.
Figure 1.
Flow diagram of inclusion and exclusion of study participants.
Table 2 presents the baseline characteristics of the study cohort. In this cohort, 37.6% (459), 32.2% (393), and 30.2% (368) of participants had no, moderate, and severe malnutrition, respectively. Compared to those without malnutrition, patients with severe malnutrition were more likely to be older, have lower BMI, and suffer from diabetes mellitus and pneumonia as a cause of hospitalization (
P < 0.05).
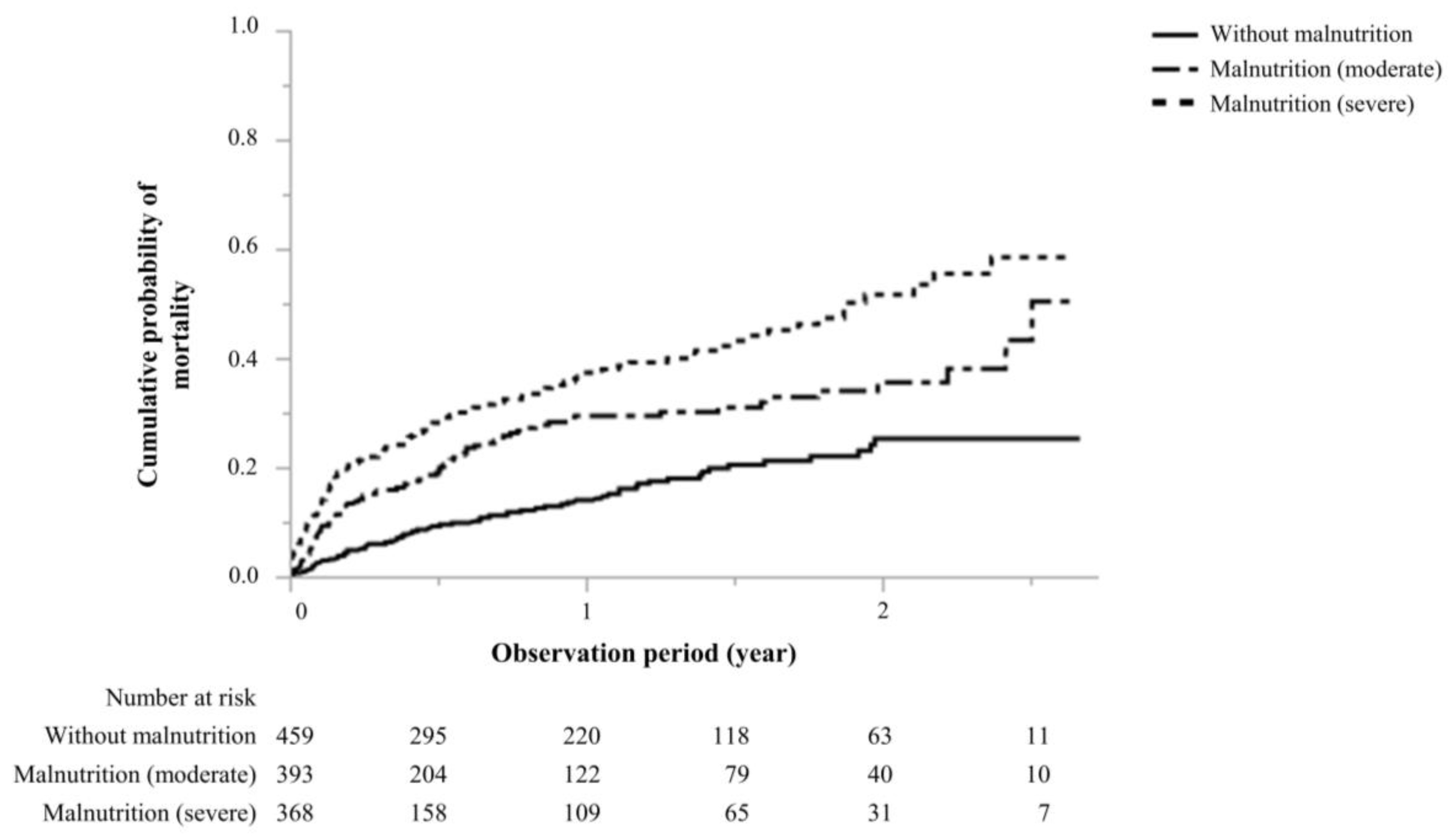
During a median observational period of 0.60 (0.10–1.37) years, the study showed that the number of 273 (22.4%) deaths observed in patients without malnutrition, with moderate malnutrition, and with severe malnutrition were 66 (14.4%), 91(23.2%), and 116 (31.5%), respectively (Table2). Infection-related deaths were observed in 237 patients; these included 52, 82, and 103 deaths in patients without malnutrition, with moderate malnutrition, and with severe malnutrition, respectively. The mortality rates were 146.5, 307.1, and 470.0 per 1,000 person-years in the groups without malnutrition, with moderate malnutrition, and with severe malnutrition, respectively. Compared to patients without malnutrition, the cumulative probability of malnutrition was significantly higher in patients with moderate and severe malnutrition (log-rank test,
P<0.001,
Figure 2).
Figure 2.
Cumulative probability of mortality.
Figure 2.
Cumulative probability of mortality.
According to an unadjusted model, patients with moderate and severe malnutrition had a significantly higher mortality risk than those without malnutrition. The unadjusted hazard ratios (HR) for patients without malnutrition, with moderate malnutrition, and severe malnutrition were 1.00 (reference), 1.95 (95% confidence interval [CI], 1.42–2.68), and 2.97 (2.20–4.03), respectively (
Table 3).
After adjusting for covariates, the study revealed that moderate and severe malnutrition were independently associated with a higher mortality risk compared to no malnutrition. The adjusted hazard ratios (aHR) for patients without malnutrition, with moderate malnutrition, and with severe malnutrition were 1.00 (reference), 1.76 (1.28–2.42), and 2.44 (1.78–3.33), respectively, according to the adjusted Model 3 (
Table 4).
Furthermore, the multivariable models revealed that advanced age (adjusted HR = 1.05, 95% CI: 1.03–1.06 per 1 year;
P<0.001), male sex (adjusted HR = 1.50, 95% CI: 1.16–1.94; P = 0.002), and reduced eGFR of 30–59 mL/min/1.73m
2 (compared to eGFR ≥60 mL/min/1.73m
2, adjusted HR = 1.51, 95% CI: 1.11–2.05;
P = 0.008) and <30 (compared to eGFR ≥60 mL/min/1.73m
2, adjusted HR = 3.04, 95% CI: 2.28–4.04;
P<0.001), as well as the presence of diabetes mellitus (adjusted HR = 1.40, 95% CI: 1.06–1.85;
P = 0.018) and COPD (adjusted HR = 2.93, 95% CI: 1.79–4.79;
P<0.001), were associated with higher mortality rates (
Table S1)
3.1. Association of Malnutrition with Kidney Function and Mortality
Table 4 displays the results of both unadjusted and multivariate analyses between mortality and malnutrition/kidney function. The observations indicate that malnutrition combined with low eGFR are independently linked to mortality, even after adjusting for age, sex, and comorbidities, such as chronic obstructive pulmonary disease, autoimmune disease, malignancy, diabetes mellitus, and cardiovascular disease. The patients with malnutrition and low eGFR (eGFR <30 mL/min/1.73m
2) exhibited the highest mortality rate (aHR 7.35; 95% CI, 4.57–11.8), compared to those without malnutrition and normal eGFR (eGFR ≥60 mL/min/1.73m
2).
4. Discussion
Our study, conducted among older patients hospitalized with infections, found that those with GLIM-defined malnutrition had a higher risk of all-cause mortality. In addition, compared to those who had normal kidney function and no malnutrition, patients with both malnutrition and kidney dysfunction had an even higher mortality risk. Our findings suggest that early assessment of nutritional status using the GLIM criteria, along with kidney function, could be a simple yet effective way to identify infection-related hospitalized patients with a higher risk of death. This is the first study to examine the relationship between nutritional status, based on the GLIM criteria and stratified by kidney function, and clinically relevant outcomes in hospitalized patients with infections. Our results provide valuable insights into the predictors of mortality in patients with infectious diseases.
Previous research in older adults has shown that postoperative infections, such as bloodstream infection, surgical site infection, urinary tract infection, pneumonia, and two, three, or multiple types of infection are associated with low survival rates and with increased risk of mortality after 1 year [
34]. Furthermore, another recent study [
35] added to the growing body of evidence on this topic by conducting a retrospective cohort study. The study included 79 666 adults aged ≥65 years who were admitted to hospitals in Taiwan in 2011 due to urinary tract infection, pneumonia, sepsis, cellulitis, cholecystitis, peritonitis, endocarditis, or meningitis. This previous study assessed four categories of infection episodes: infrequent, increasing, decreasing, and frequent. After adjusting for age, sex, and comorbidities, Lin et al. found an increased risk of all-cause mortality for frequent (adjusted hazard ratio 2·96 [95% CI 2·82–3·11]); increasing (2·15 [2·09–2·22]); and decreasing infections (1·85 [1·80–1·91]), compared with the risk in infrequent infections. However, this past study did not evaluate whether malnutrition was associated with mortality.
Limited data are available on the relationship between GLIM malnutrition and mortality in patients with infectious diseases. One previous retrospective cohort study analyzed 109 intensive care unit (ICU) patients infected with COVID-19 and assessed their malnutrition based on GLIM criteria. The study found that GLIM malnutrition was frequently observed in critically ill COVID-19 patients and was strongly associated with in-hospital mortality and length of stay in the ICU [
36]. Although the study results were consistent with our findings, it should be noted that the sample size was small, and it is unclear whether GLIM criteria can be applied to patients with all infectious diseases.
Regarding the association between kidney dysfunction and infection, one national vital statistics data in Japan [
37], including 273237 dialysis patients has shown increased mortality from infectious diseases, particularly from sepsis, in patients undergoing dialysis compared with the general population. The results were compatible with our study, however, it was unknown whether this finding can be generalizable to non-dialysis patients.
Patients with infection-related hospitalization, GLIM malnutrition, and kidney dysfunction have a high risk of mortality due to different mechanisms. The first plausible mechanism is the close relationship between infectious disease and malnutrition, which create a vicious circle and make each other worse, leading to the spread of infectious diseases [
38]. Malnourished individuals have weaker immune function, which increases their risk of infection and the severity of the infection. This often leads to a prolonged treatment period and a higher likelihood of complications. In contrast, many infectious diseases can cause reduced appetite and indigestion, which can exacerbate moderate malnutrition and make individuals more susceptible to new infections. This vicious cycle is particularly common in older adults [
39].
Previous evidence suggests that patients with kidney impairment experience altered immune responses, including impaired T cell, B cell, and neutrophil function [
40,
41]. Higher age, diabetes, and cardiovascular disease are contributing factors for increased infection susceptibility in people with kidney impairment [
42]. In addition, several mechanisms that are thought to impair immune function have been hypothesized, including the accumulation of uremic toxins [
43], increased oxidative stress [
44], endothelial dysfunction [
28], low-grade inflammation [
46], and mineral and bone disorders [
47].
Therefore, patients with GLIM-defined malnutrition and kidney dysfunction have a higher mortality risk.
The present study has some limitations that need to be considered. Firstly, as it was a retrospective, single-center cohort study, the results may not be generalizable to patients with infectious diseases in different care settings. Secondly, we only collected data on nutritional status at the time of admission; therefore, we do not know how the nutritional status changed during the observation period. Therefore, the clinical impact of nutritional status changes after admission for infectious disease should be evaluated through well-designed studies. Thirdly, the study did not evaluate any confounder of cognitive and physical functions and functional status which might be crucial for the older population. Therefore, further studies must assess these confounders to obtain a better understanding. Fourthly, the precise severity and frequency of infection events during follow-up period could not be evaluated in the present study, and therefore should be interpreted cautiously. Finally, we could not precisely evaluate patients with kidney dysfunction in terms of whether they had acute kidney injury or stable chronic kidney disease and what the cause of kidney dysfunction was present. Thus, further studies are required to obtain this information.
5. Conclusions
The present retrospective cohort study indicated that GLM-defined malnutrition was associated with increased all-cause mortality in older patients hospitalized due to infectious disease. Additionally, patients with malnutrition and kidney dysfunction had an even higher mortality risk. This information is important for clinicians to identify patients who may have a higher risk of mortality and to pay special attention to malnourished patients with kidney dysfunction. Further studies should be conducted to determine the impact of nutritional interventions on patients with infectious diseases.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Predictors of mortality.
Author Contributions
Conceptualization, M.Y.; methodology, M.Y.; software, M.Y.; validation, T.I., and Y.I.; formal analysis, T.I.; investigation, Y.I., K.M., and N.M.; resources, Y.I., K.M, and N.M.; data curation, M.Y., Y.I., K.M., and N.M.; writing—original draft preparation, M.Y.; writing—review and editing, M.Y., and N.M.; visualization, M.Y.; supervision, N.M. and Y.I.; project administration, N.M.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study adhered to the principles of the Declaration of Helsinki and was approved by the Ethics Review Committee of Aichi Medical University Hospital (No. 2021e136).
Informed Consent Statement
Although written consent was not obtained due to the retrospective nature of the study, patients were given the right to withdraw from the study through an opt-out notice that was posted on the hospital's website.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors thank all the collaborators from our nutrition support teams at Aichi Medical University Hospital for their dedicated clinical work.
Conflicts of Interest
The authors report no conflicts of interest.
References
- Ramírez-Soto, M.C. Long-term, all-cause mortality risk after infection episodes in older adults. Lancet Healthy Longev 2023, 4, e452–e454. [Google Scholar] [CrossRef]
- Nielsen, S.L.; Lassen, A.T.; Gradel, K.O.; Jensen, T.G.; Kolmos, H.J.; Hallas, J.; Pedersen, C. Bacteremia is associated with excess long-term mortality: A 12-year population-based cohort study. J Infect 2015, 70, 111–126. [Google Scholar] [CrossRef]
- Dick, A.; Liu, H.; Zwanziger, J.; Perencevich, E.; Furuya, E.Y.; Larson, E.; Pogorzelska-Maziarz, M.; Stone, P.W. Long-term survival and healthcare utilization outcomes attributable to sepsis and pneumonia. BMC Health Serv Res 2012, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.I.T.D.; Perman, M.I.; Waitzberg, D.L. Hospital malnutrition in Latin America: A systematic review. Clin Nutr 2017, 36, 958–967. [Google Scholar] [CrossRef]
- Tannou, T.; Koeberle, S.; Manckoundia, P.; Aubry, R. Multifactorial immunodeficiency in frail elderly patients: Contributing factors and management. Med Mal Infect 2019, 49, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Lo Buglio, A.; Quiete, S.; Pellegrino, G.; Dobrakowski, M.; Kasperczyk, A.; Kasperczyk, S.; Vendemiale, G. Comparison of three nutritional screening tools with the new glim criteria for malnutrition and association with sarcopenia in hospitalized older patients. J Clin Med 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Guenter, P.; Abdelhadi, R.; Anthony, P.; Blackmer, A.; Malone, A.; Mirtallo, J.M.; Phillips, W.; Resnick, H.E. Malnutrition diagnoses and associated outcomes in hospitalized patients: United States, 2018. Nutr Clin Pract 2021, 36, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, L.L.; Kashiwagi, D.T.; Brantley, S.; Scheurer, D.; Varkey, P. Nutrition in the hospitalized patient. J Hosp Med 2013, 8, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Inciong, J.F.B.; Chaudhary, A.; Hsu, H.S.; Joshi, R.; Seo, J.M.; Trung, L.V.; Ungpinitpong, W.; Usman, N. Hospital malnutrition in northeast and Southeast Asia: A systematic literature review. Clin Nutr ESPEN 2020, 39, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, J.; Matsushita, K. Clinical epidemiology of infectious disease among patients with chronic kidney disease. Clin Exp Nephrol 2019, 23, 437–447. [Google Scholar] [CrossRef]
- Chang, C.H.; Fan, P.C.; Kuo, G.; Lin, Y.S.; Tsai, T.Y.; Chang, S.W.; Tian, Y.C.; Lee, C.C. Infection in advanced chronic kidney disease and subsequent adverse outcomes after dialysis initiation: A nationwide cohort study. Sci Rep 2020, 10, 2938. [Google Scholar] [CrossRef]
- Syed-Ahmed, M.; Narayanan, M. Immune dysfunction and risk of infection in chronic kidney disease. Adv Chronic Kidney Dis 2019, 26, 8–15. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Cederholm, T.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Cuerda, C.; Cupisti, A.; Sabatino, A.; Schneider, S.; Torreggiani, M.; et al. Nutritional status and the risk of malnutrition in older adults with chronic kidney disease – Implications for low protein intake and nutritional care: A critical review endorsed by ERN-ERA and ESPEN. Clin Nutr 2023, 42, 443–457. [Google Scholar] [CrossRef]
- Rondel, A.L.M.A.; Langius, J.A.E.; de van der Schueren, M.A.E.; Kruizenga, H.M. The new ESPEN diagnostic criteria for malnutrition predict overall survival in hospitalised patients. Clin Nutr 2018, 37, 163–168. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R. Validity and feasibility of the global leadership initiative on malnutrition diagnostic concept in older people: A literature review from August 2021 to August 2022. Curr Opin Clin Nutr Metab Care 2023, 26, 23–31. [Google Scholar] [CrossRef]
- Balcı, C.; Tufan, G.; Özdemir, N.; Aksoy, S.; Öksüzoğlu, Ö.B.; Zengin, N.; Kars, A.; Halil, M. GLIM criteria as a valid tool for nutrition assessment and mortality prediction in treatment-naïve patients with cancer. Nutr Clin Pract 2023, 38, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Chong, F.; Huo, Z.; Li, N.; Liu, J.; Xu, H. GLIM-defined malnutrition and overall survival in cancer patients: A meta-analysis. JPEN J Parenter Enteral Nutr 2023, 47, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Guo, G.; Cui, B.; Li, Y.; Sun, M.; Li, C.; Wang, X.; Mao, L.; Hui, Y.; Fan, X.; et al. Malnutrition according to the Global Leadership Initiative on Malnutrition criteria is associated with in-hospital mortality and prolonged length of stay in patients with cirrhosis. Nutrition 2023, 105, 111860. [Google Scholar] [CrossRef] [PubMed]
- Joaquín, C.; Alonso, N.; Lupón, J.; Gastelurrutia, P.; Pérez-Monstesdeoca, A.; Domingo, M.; Zamora, E.; Socias, G.; Ramos, A.; Bayes-Genis, A.; et al. Nutritional status according to the GLIM criteria in patients with chronic Heart Failure: Association with prognosis. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Shirai, Y.; Momosaki, R.; Kokura, Y.; Kato, Y.; Okugawa, Y.; Shimizu, A. Validation of Asian body mass index cutoff values for the classification of malnutrition severity according to the global leadership initiative on malnutrition criteria in patients with chronic obstructive pulmonary disease exacerbations. Nutrients 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Torralvo, F.J.; Pérez-Del-Río, V.; García-Olivares, M.; Porras, N.; Abuín-Fernández, J.; Bravo-Bardají, M.F.; García-de-Quevedo, D.; Olveira, G. Global subjective assessment and mini nutritional assessment short form better predict mortality than GLIM malnutrition criteria in elderly patients with hip fracture. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Díaz, G.; T D Correia, M.I.; Gonzalez, M.C.; Reyes, M. The global leadership initiative on malnutrition criteria for the diagnosis of malnutrition in patients admitted to the intensive care unit: A systematic review and meta-analysis. Clin Nutr 2023, 42, 182–189. [Google Scholar] [CrossRef]
- MacDonell, S.O.; Moyes, S.A.; Teh, R.; Dyall, L.; Kerse, N.; Wham, C. Is the utility of the GLIM criteria used to diagnose malnutrition suitable for bicultural populations? Findings from life and living in advanced age cohort study in New Zealand (LiLACS NZ). J Nutr Health Aging 2023, 27, 67–74. [Google Scholar] [CrossRef]
- Sipilä PN, Lindbohm JV, Batty GD, Heikkilä N, Vahtera J, Suominen S, Väänänen A, Koskinen A, Nyberg ST, Meri S, et al. Severe Infection and Risk of Cardiovascular Disease: A Multicohort Study. Circulation. 2023, 147, 1582–1593. [CrossRef]
- Stratton, R.J.; Hackston, A.; Longmore, D.; Dixon, R.; Price, S.; Stroud, M.; King, C.; Elia, M. Malnutrition in hospital outpatients and inpatients: Prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (“MUST”) for adults. Br J Nutr 2004, 92, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci 2001, 56, M366–M372. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Maeda, K.; Fujimoto, Y.; Nonogaki, T.; Ishida, Y.; Ohta, R.; Shimizu, A.; Ueshima, J.; Nagano, A.; Fukushima, R. Prognostic implications of the global leadership initiative on malnutrition criteria as a routine assessment modality for malnutrition in hospitalized patients at a university hospital. Clin Nutr 2023, 42, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Ishida, Y.; Nonogaki, T.; Mori, N. Reference body mass index values and the prevalence of malnutrition according to the Global Leadership Initiative on Malnutrition criteria. Clin Nutr 2020, 39, 180–184. [Google Scholar] [CrossRef]
- Maeda, K.; Koga, T.; Nasu, T.; Takaki, M.; Akagi, J. Predictive accuracy of calf circumference measurements to detect decreased skeletal muscle mass and European Society for Clinical Nutrition and Metabolism-defined malnutrition in hospitalized older patients. Ann Nutr Metab 2017, 71, 10–15. [Google Scholar] [CrossRef]
- Barazzoni, R.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Higashiguchi, T.; Shi, H.P.; Bischoff, S.C.; Boirie, Y.; Carrasco, F.; Cruz-Jentoft, A.; et al. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition (GLIM) diagnosis of malnutrition. Clin Nutr 2022, 41, 1425–1433. [Google Scholar] [CrossRef]
- Oguri, M.; Ishii, H.; Yasuda, K.; Sumi, T.; Takahashi, H.; Murohara, T. Combined prognostic value of malnutrition using GLIM criteria and renal insufficiency in elderly heart failure. ESC Heart Fail 2022, 9, 704–711. [Google Scholar] [CrossRef] [PubMed]
- O'Brien, W.J.; Gupta, K.; Itani, K.M.F. Association of Postoperative Infection With Risk of Long-term Infection and Mortality. JAMA Surg 2020, 155, 61–68. [Google Scholar] [CrossRef]
- Lin, H.Y.; Hsiao, F.Y.; Huang, S.T.; Chen, Y.C.; Lin, S.W.; Chen, L.K. Longitudinal impact of distinct infection trajectories on all-cause mortality of older people in Taiwan: a retrospective, nationwide, population-based study. Lancet Healthy Longev 2023, 4, e508–e516. [Google Scholar] [CrossRef]
- Nicolau, J.; Ayala, L.; Sanchís, P.; Olivares, J.; Dotres, K.; Soler, A.G.; Rodríguez, I.; Gómez, L.A.; Masmiquel, L. Influence of nutritional status on clinical outcomes among hospitalized patients with COVID-19. Clin Nutr ESPEN 2021, 43, 223–229. [Google Scholar] [CrossRef]
- Wakasugi, M.; Kawamura, K.; Yamamoto, S.; Kazama, J.J.; Narita, I. High mortality rate of infectious diseases in dialysis patients: A comparison with the general population in Japan. Ther Apher Dial 2012, 16, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Schaible, U.E.; Kaufmann, S.H.E. Malnutrition and infection: Complex mechanisms and global impacts. PLOS Med 2007, 4, e115. [Google Scholar] [CrossRef]
- Katona, P.; Katona-Apte, J. The interaction between nutrition and infection. Clin Infect Dis 2008, 46, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Litjens, N.H.R.; van Druningen, C.J.; Betjes, M.G.H. Progressive loss of renal function is associated with activation and depletion of naive T lymphocytes. Clin Immunol 2006, 118, 83–91. [Google Scholar] [CrossRef]
- Montecino-Rodriguez, E.; Berent-Maoz, B.; Dorshkind, K. Causes, consequences, and reversal of immune system aging. J Clin Invest 2013, 123, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Cheikh Hassan, H.I.; Tang, M.; Djurdjev, O.; Langsford, D.; Sood, M.M.; Levin, A. Infection in advanced chronic kidney disease leads to increased risk of cardiovascular events, end-stage kidney disease and mortality. Kidney Int 2016, 90, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Pletinck, A.; Glorieux, G.; Schepers, E.; Cohen, G.; Gondouin, B.; Van Landschoot, M.; Eloot, S.; Rops, A.; Van de Voorde, J.; De Vriese, A.; et al. Protein-bound uremic toxins stimulate crosstalk between leukocytes and vessel wall. J Am Soc Nephrol 2013, 24, 1981–1994. [Google Scholar] [CrossRef] [PubMed]
- Sack, M.N.; Fyhrquist, F.Y.; Saijonmaa, O.J.; Fuster, V.; Kovacic, J.C. Basic Biology of Oxidative Stress and the Cardiovascular System: Part 1 of a 3-Part Series. J Am Coll Cardiol 2017, 70, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Yoo, T.H.; Hwang, Y.; Lee, G.H.; Kim, B.; Jang, J.; Yu, H.T.; Kim, M.C.; Cho, J.Y.; Lee, C.J.; et al. Indoxyl sulfate (IS)-mediated immune dysfunction provokes endothelial damage in patients with end-stage renal disease (ESRD). Sci Rep 2017, 7, 3057. [Google Scholar] [CrossRef] [PubMed]
- Sela, S.; Shurtz-Swirski, R.; Cohen-Mazor, M.; Mazor, R.; Chezar, J.; Shapiro, G.; Hassan, K.; Shkolnik, G.; Geron, R.; Kristal, B. Primed peripheral polymorphonuclear leukocyte: A culprit underlying chronic low-grade inflammation and systemic oxidative stress in chronic kidney disease. J Am Soc Nephrol 2005, 16, 2431–2438. [Google Scholar] [CrossRef]
- Ishigami, J.; Jaar, B.G.; Rebholz, C.M.; Grams, M.E.; Michos, E.D.; Wolf, M.; Kovesdy, C.P.; Uchida, S.; Coresh, J.; Lutsey, P.L.; et al. Biomarkers of mineral and bone metabolism and 20-year risk of hospitalization with infection: The atherosclerosis risk in communities study. J Clin Endocrinol Metab 2017, 102, 4648–4657. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).