Submitted:
06 December 2023
Posted:
07 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
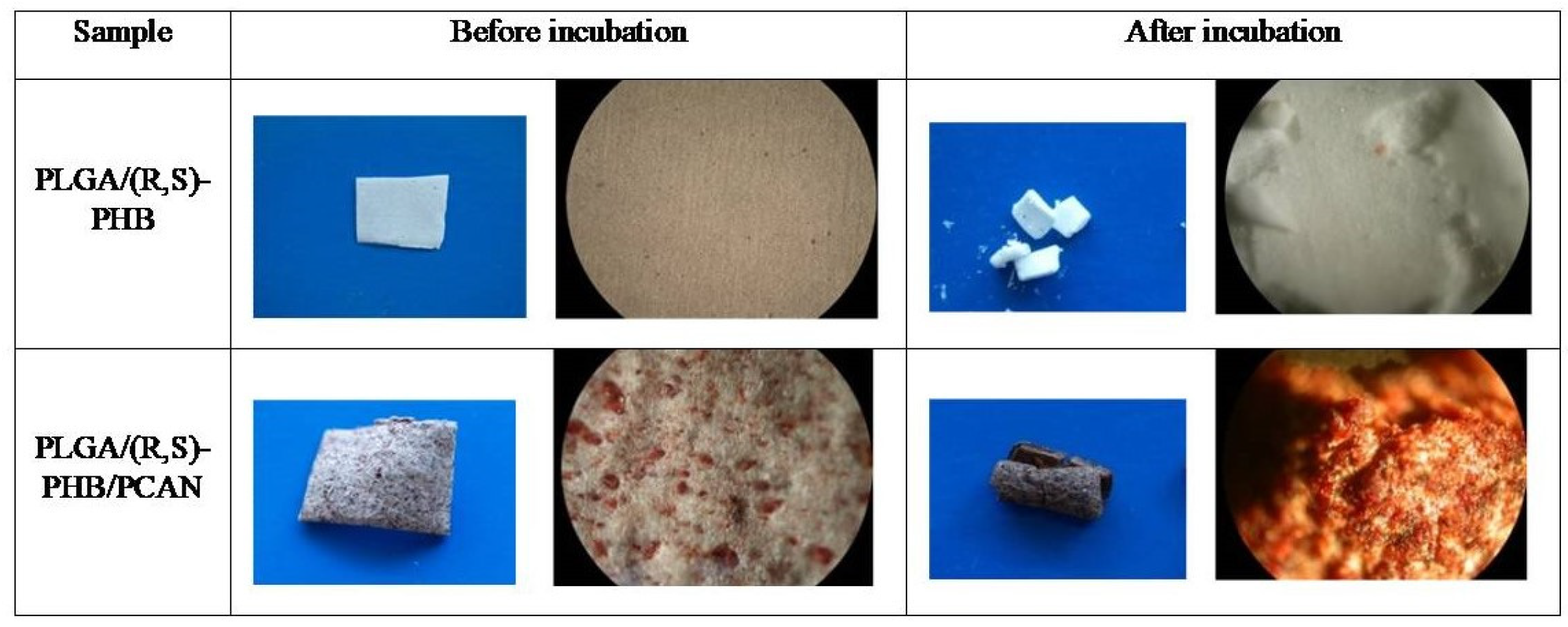
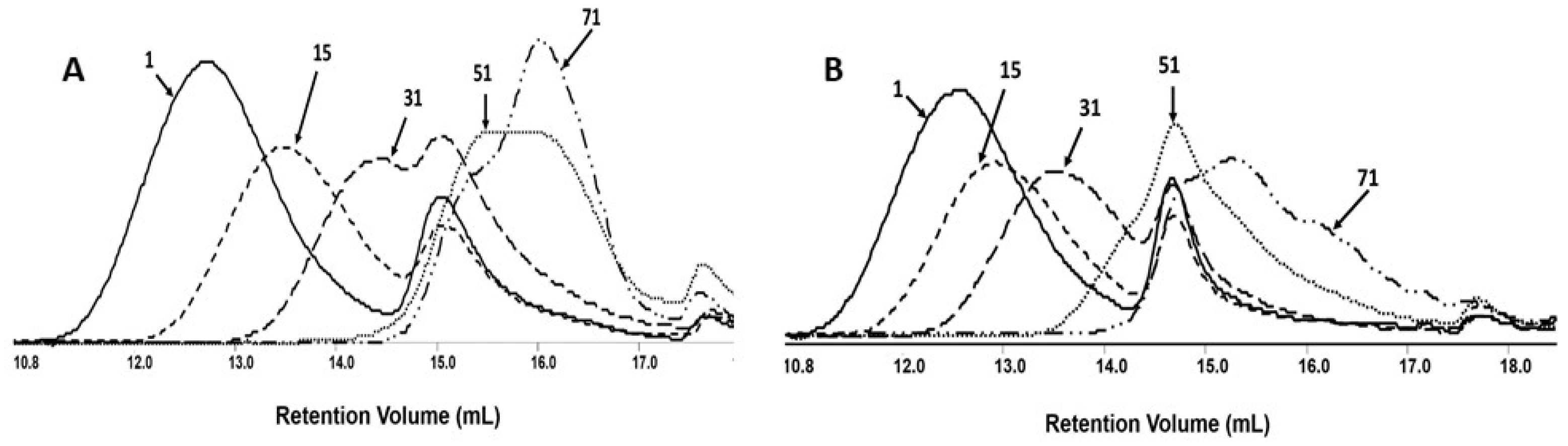
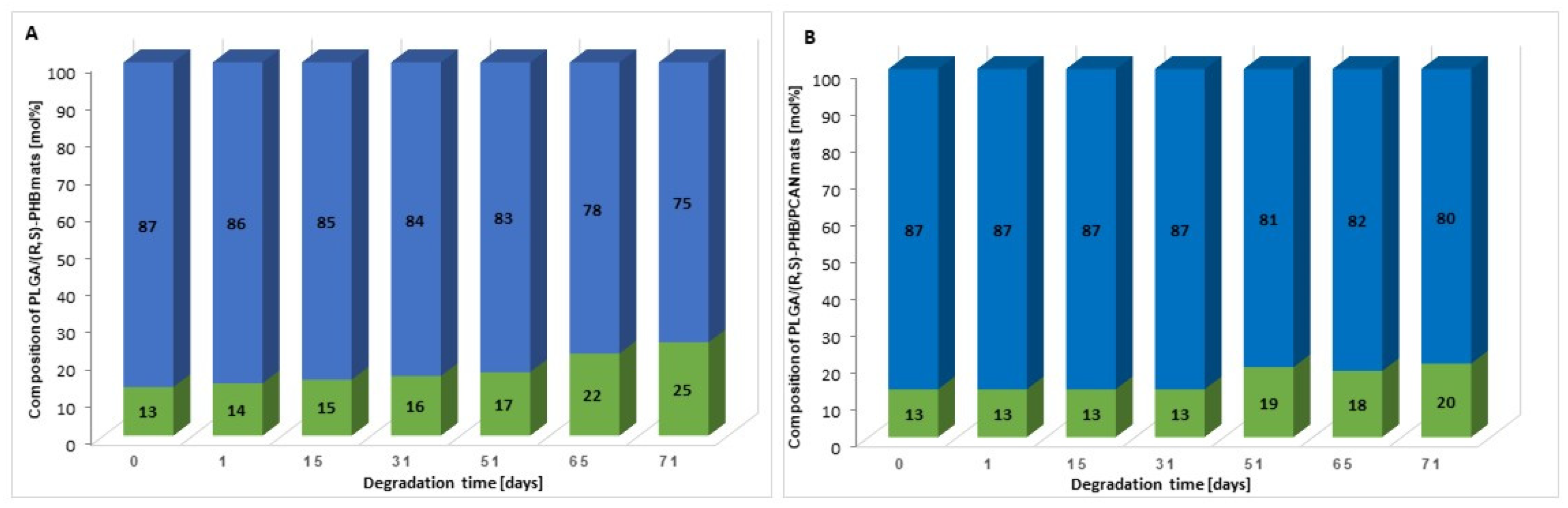
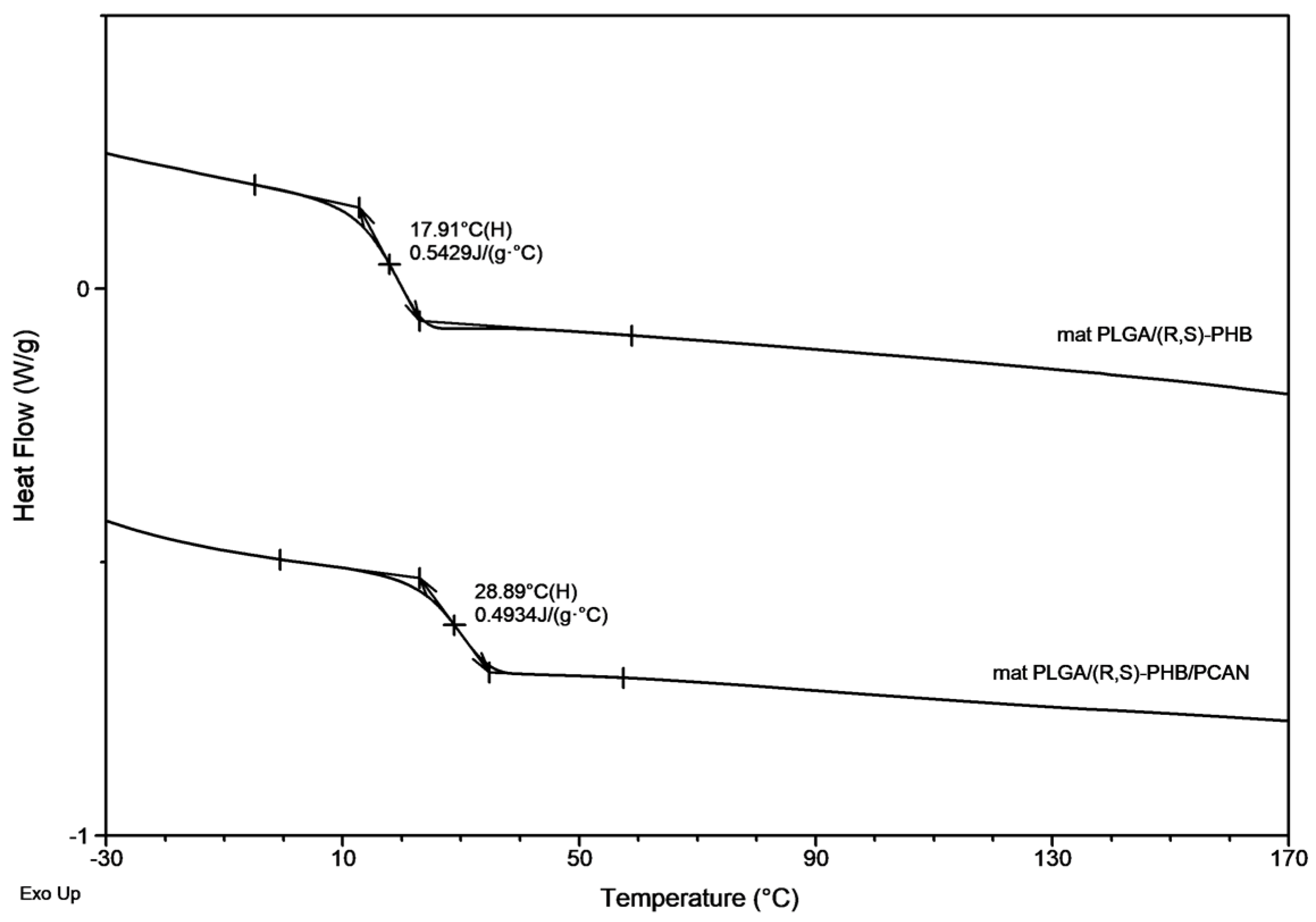
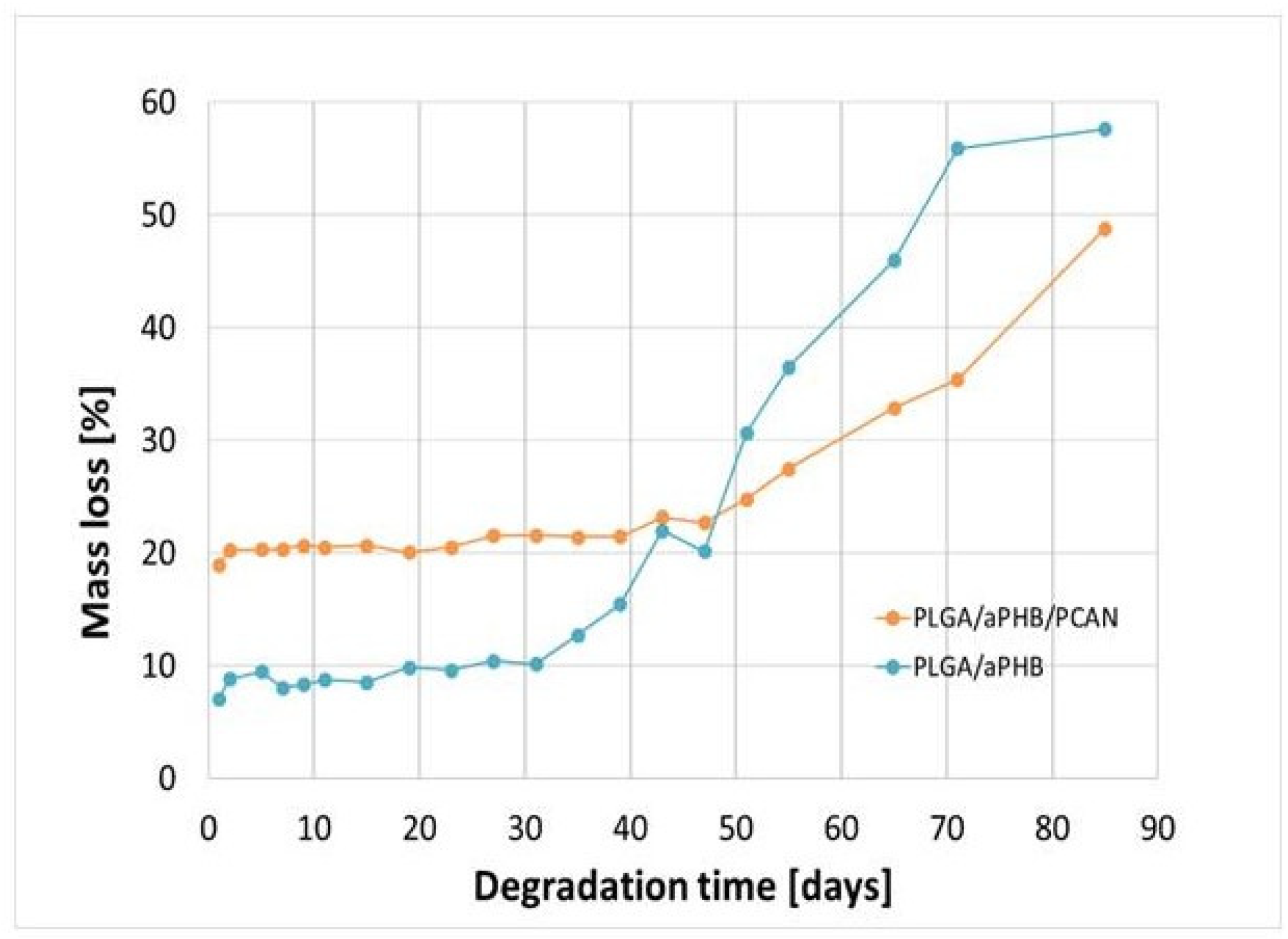
2.1. Degradation of PLGA/(R,S)-PHB and PLGA/(R,S)-PHB/PCAN electrospun nonwoven
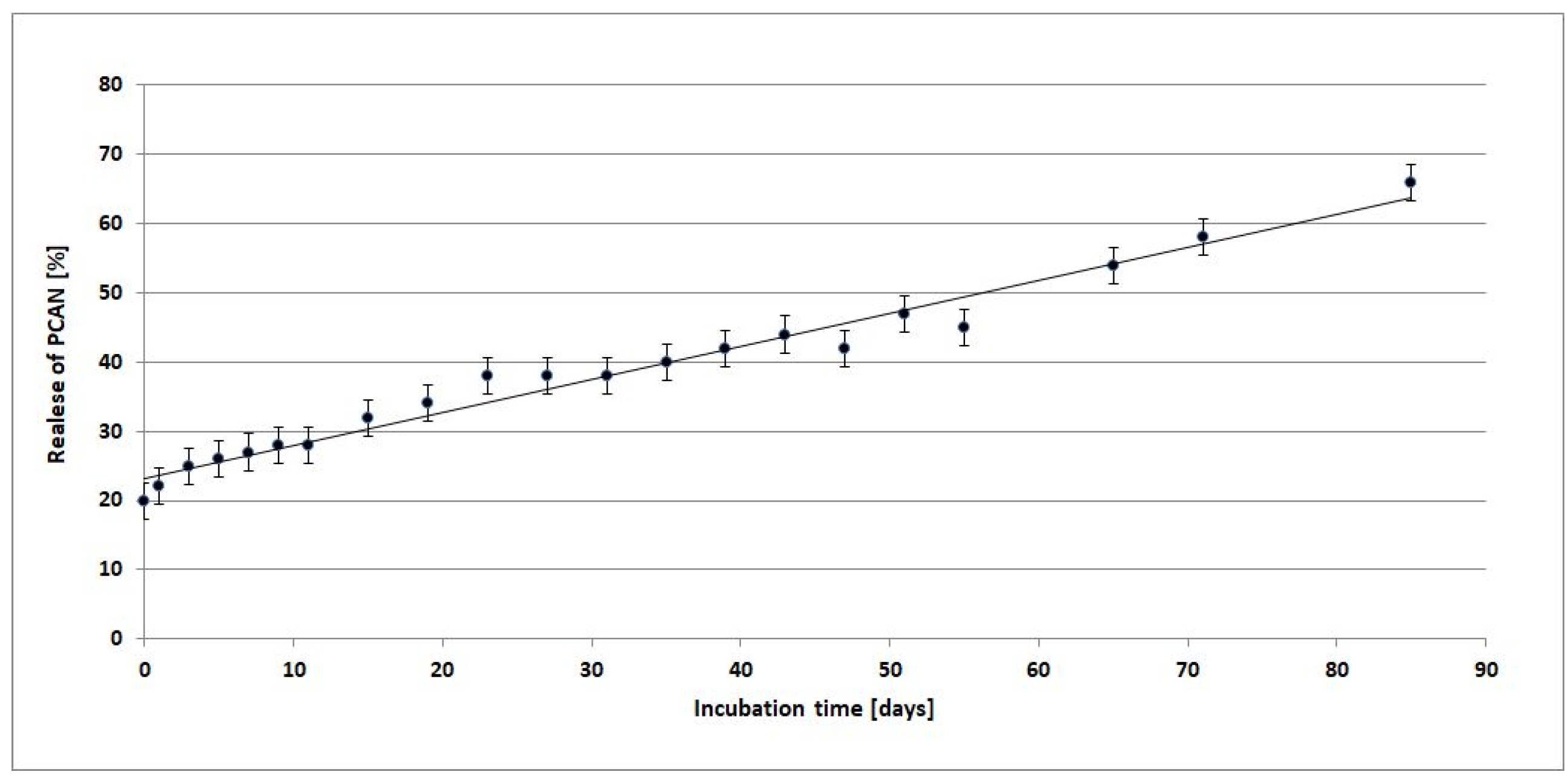
2.2. Release study of PCAN from PLGA/(R,S)-PHB and PLGA/(R,S)-PHB/PCAN electrospun nonwoven
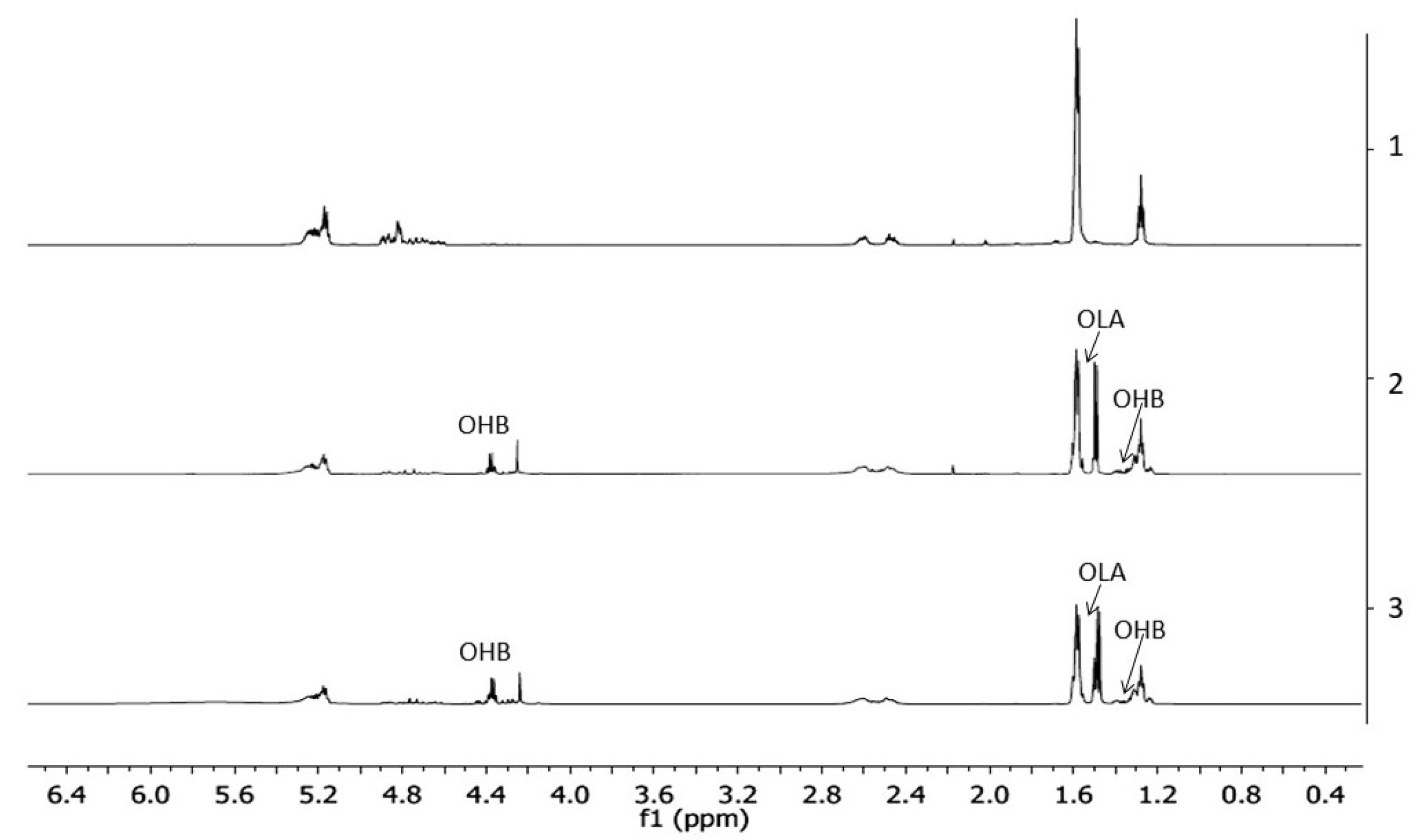
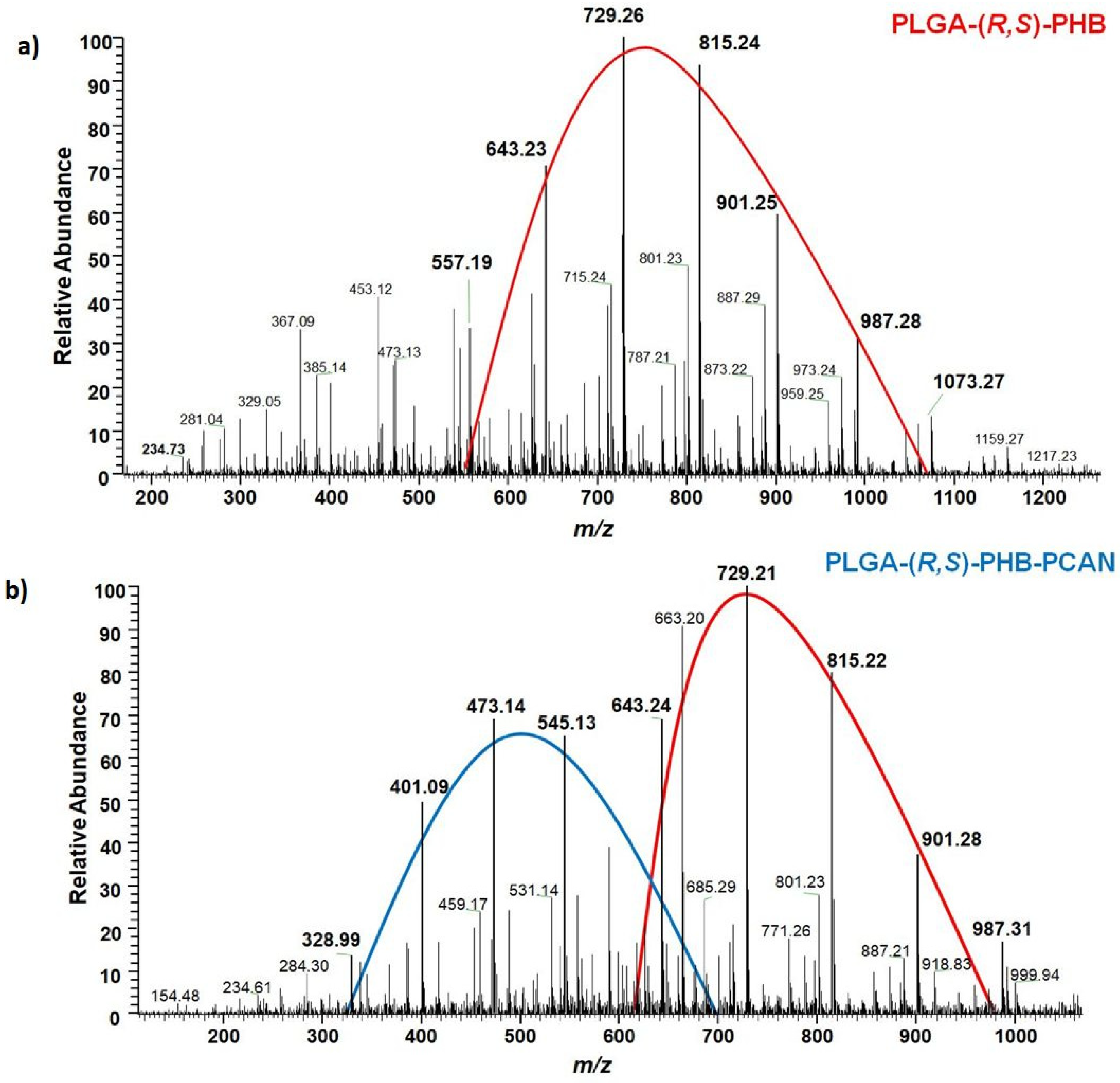
2.3. ESI-MS study of the degradation products released from PLGA/(R,S)-PHB and PLGA/(R,S)-PHB/PCAN electrosun nonwoven
2.4. Cytocompatibility test (MTT assay)
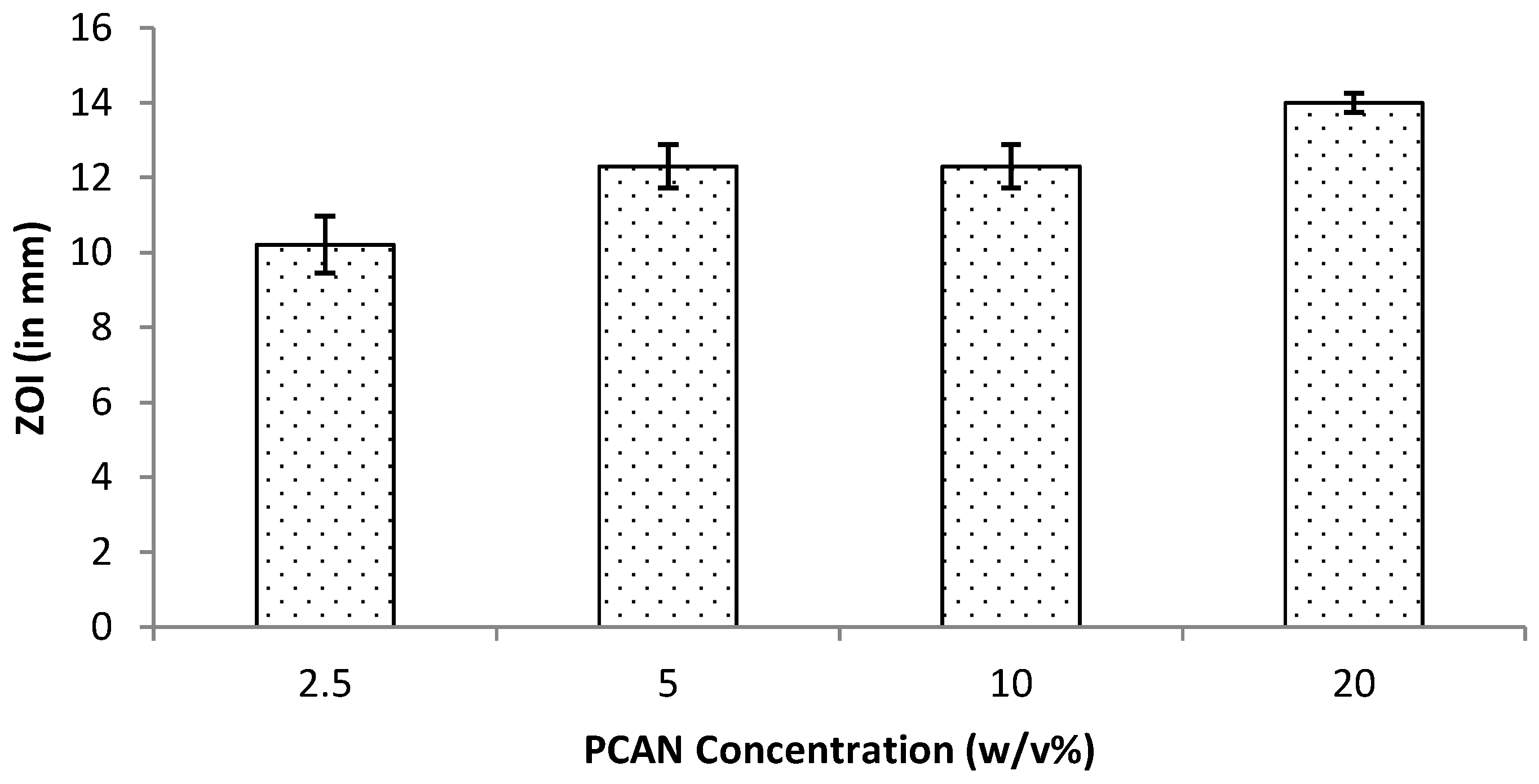
2.5. Antibacterial activity (Disc Diffusion Assay, DDA)
3. Materials and Methods
3.1. Materials
3.2. Electrospinning experiment
3.3. Hydrolytic degradation under laboratory condition
3.4. Biological studies
3.4.1. Cytocompatibility study (In vitro MTT assay)
3.4.2. Antibacterial activity (Disc Diffusion Assay, DDA)
3.5. Measurements
3.5.1. Polarized Optical Microscopy (POM)
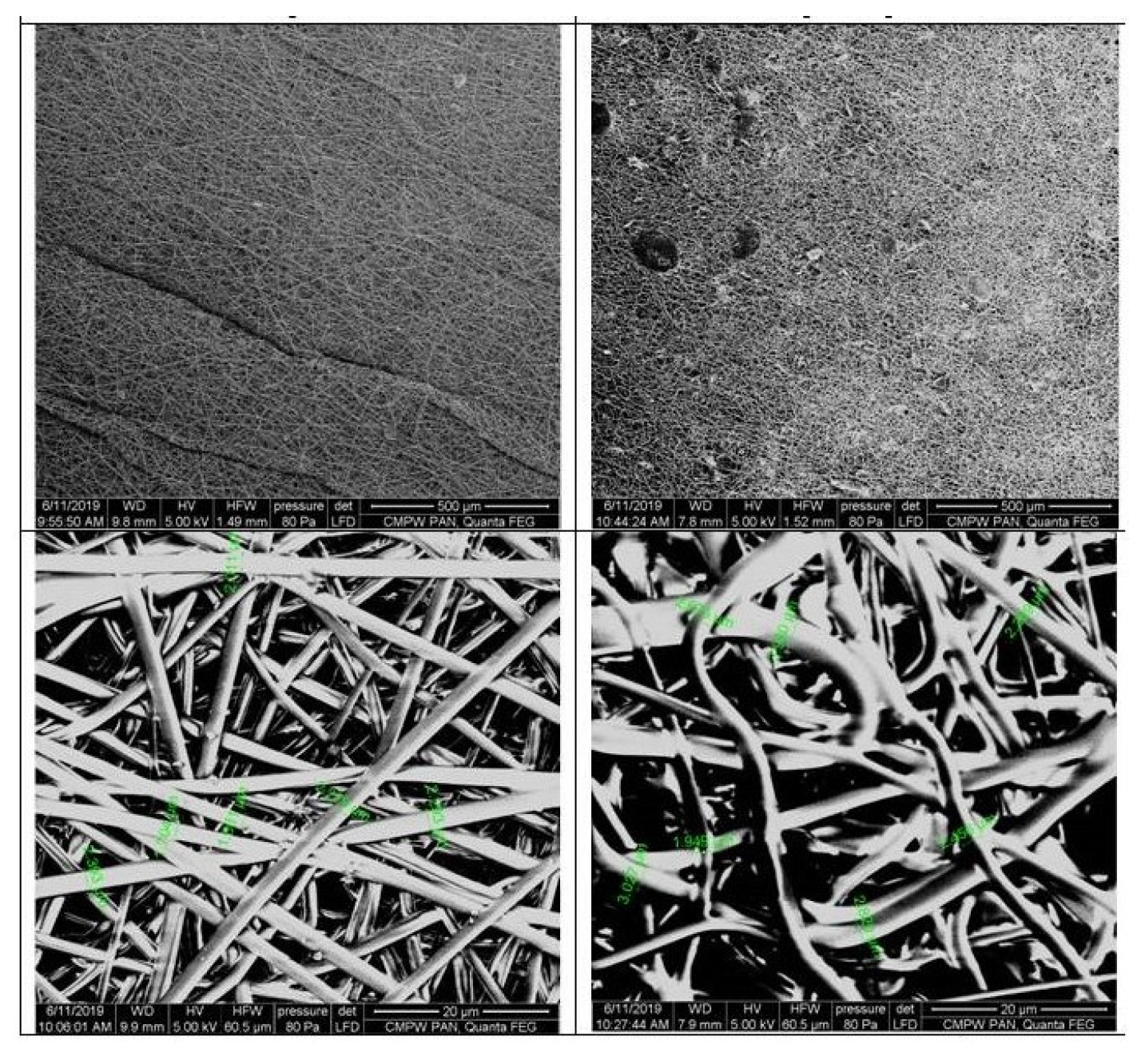
3.5.2. Scanning Electron Microscopy (SEM)
3.5.3. Gel Permeation Chromatography (GPC) analysis
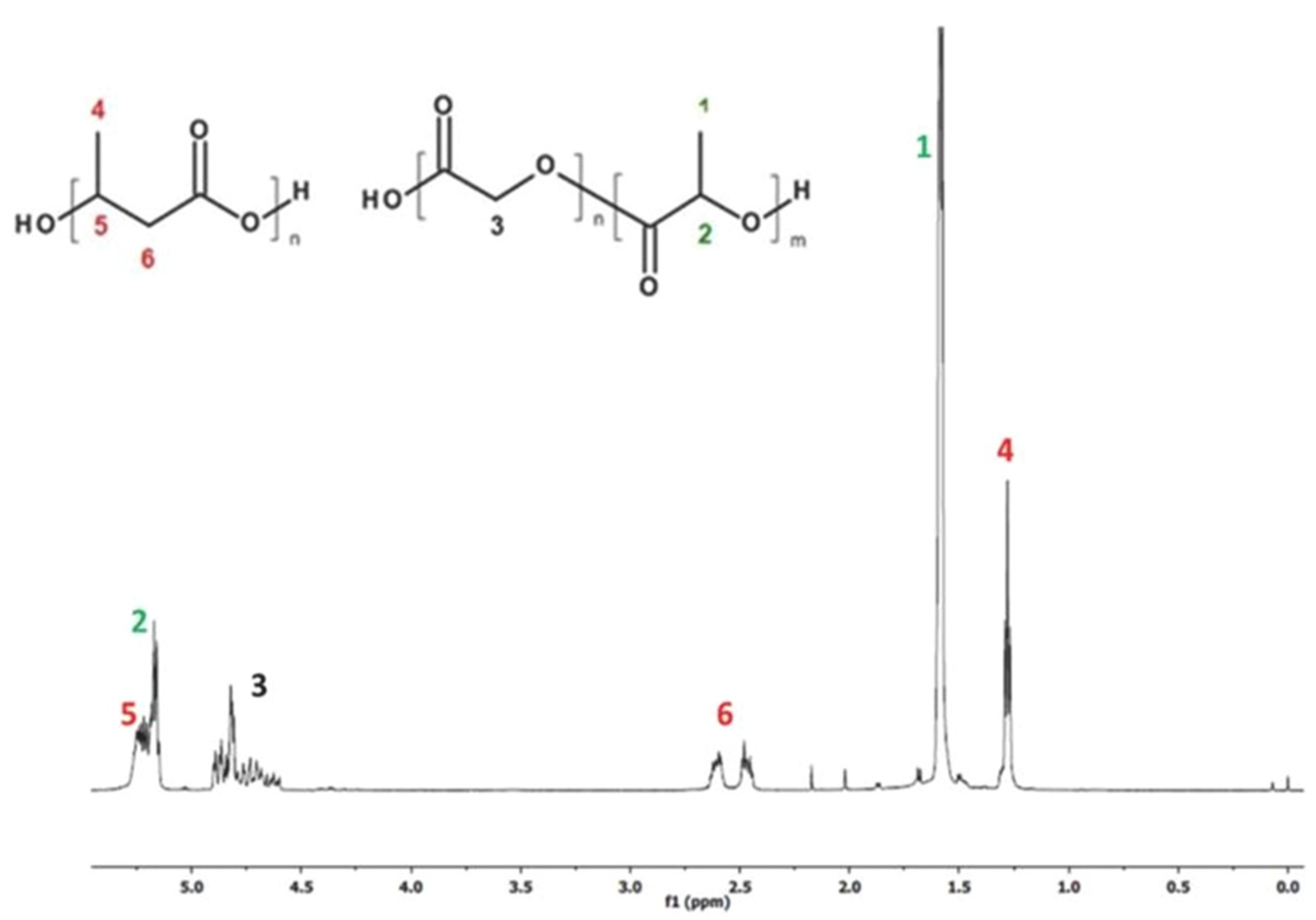
3.5.4. Nuclear magnetic resonance 1H-NMR spectroscopy
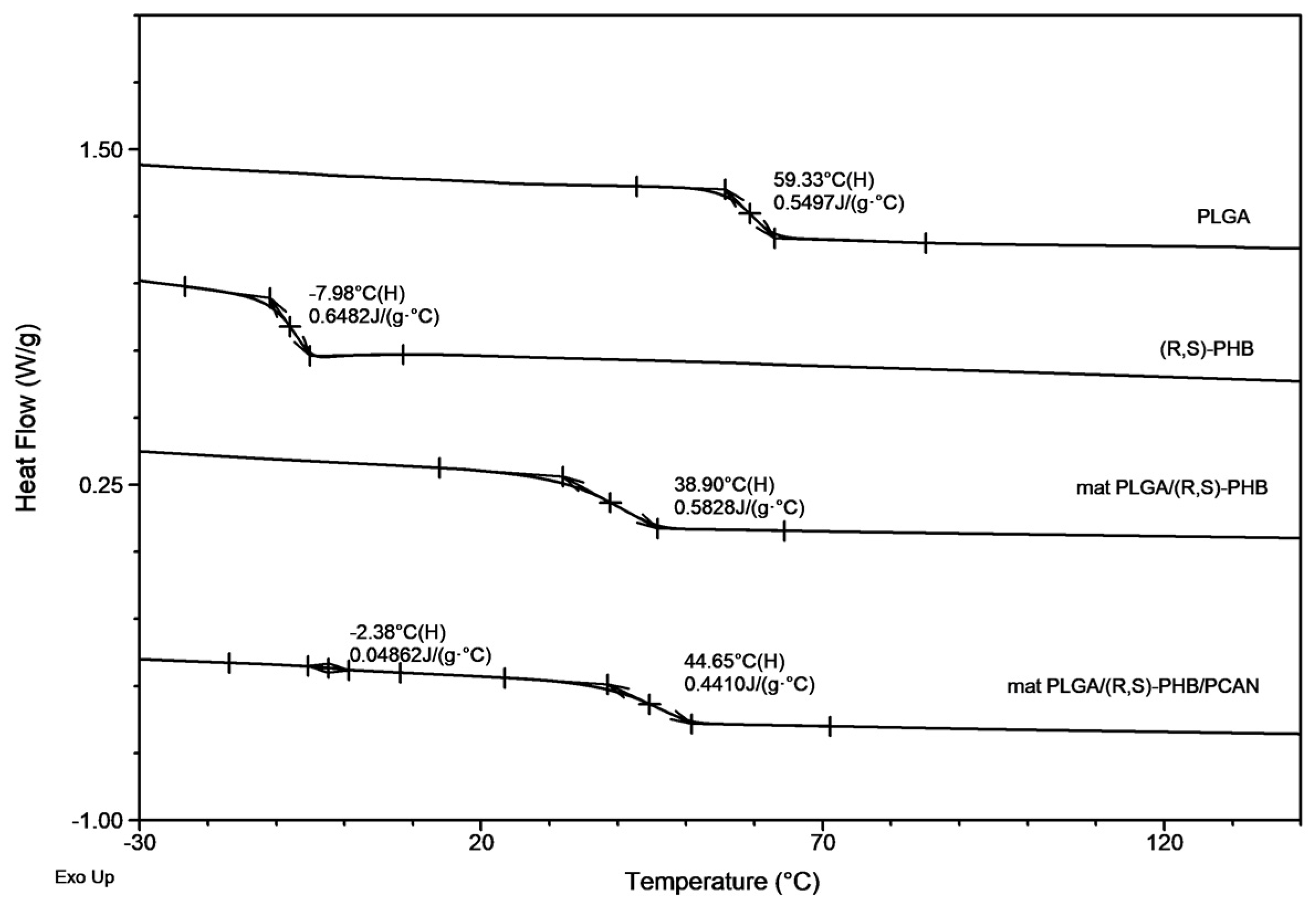
3.5.5. DSC analysis
3.5.6. Electrospray ionization mass spectrometry (ESI-MSn) analysis
3.5.7. Determination of the PCAN release profile by Ultraviolet–visible spectroscopy (UV–VIS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anselmo, A.C.; Mitragotri, S. An overview of clinical and commercial impact of drug delivery systems. J. Control. Release 2014, 190, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Zięba, M.; Chaber, P.; Duale, K.; Martinka Maksymiak, M.; Basczok, M.; Kowalczuk, M.; Adamus, G. Polymeric carriers for delivery systems in the treatment of chronic periodontal disease. Polymers 2020, 12, 1574. [Google Scholar] [CrossRef] [PubMed]
- Zamani, F.; Jahanmard, F.; Ghasemkhah, F.; Amjad-Iranagh, S.; Bagherzadeh, R.; Amani-Tehran, M.; Latifi, M. Chapter 7—Nanofibrous and nanoparticle materials as drug-delivery systems. In Micro and Nano Technologies, Nanostructures for Drug Delivery; Andronescu, E. Grumezescu, A.M. Eds.; Elsevier, 2017, pp. 239–270, ISBN 9780323461436.
- Swain, G.P.; Patel, S.; Gandhi, J.; Shah, P. Development of moxifloxacin hydrochloride loaded in-situ gel for the treatment of periodontitis: In-vitro drug release study and antibacterial activity. J. Oral Biol. Craniofac Res. 2019, 9, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Khan, G.; Patel, R.R.; Yadav, S.K.; Kumar, N.; Chaurasia, S.; Ajmal, G.; Mishra, P.K.; Mishra,B. Development, optimization and evaluation of tinidazole functionalized electrospun poly(ε-caprolactone) nanofiber membranes for the treatment of periodontitis. RSC Adv. 2016, 6, 100214–100229. [Google Scholar] [CrossRef]
- Hamed, R.; Abu Rezeq, A.; Tarawneh, O. Development of hydrogels, oleogels, and bigels as local drug delivery systems for periodontitis. Drug Dev. Ind. Pharm. 2018, 44, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Sun, Y.; Chen, Y.; Liu, Y.; Tang, C.; Qu, X. Injectable adhesive hydrogel through a microcapsule cross-link for periodontitis treatment. ACS Appl. Bio Mater. 2019, 2, 5985–5994. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.A.; Bircha, N.P.; Schiffman, J.D. Designing electrospun nanofiber mats to promote wound healing—a review. J. Mater. Chem. B 2013, 1, 4531–4541. [Google Scholar] [CrossRef] [PubMed]
- Rydz, J.; Sikorska, W.; Kyulavska, M.; Christova, D. Polyester-based (bio)degradable polymers as environmentally friendly materials for sustainable development. Int. J. Mol. Sci. 2015, 16, 564–596. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Pr’eat, V. PLGA-based nanoparticles: an overview of biomedical applications. JCR 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Do, M.P.; Neut, C.; Delcourt, E.; Seixas Certo, T.; Siepmann, J.; Siepmann, F. In situ forming implants for periodontitis treatment with improved adhesive properties. Eur. J. Pharm. Biopharm. 2014, 88, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.-Y.; Chen, D.W.; Hu, C.-C.; Hsieh, Y.-T.; Liu, S.-J.; Chan, E.-C. In vitro and in vivo investigation of drug-eluting implants for the treatment of periodontal disease. AAPS Pharm. Sci. Tech. 2011, 12, 1110–1115. [Google Scholar] [CrossRef]
- Ahuja, A.; Ali, J.; Rahman, S. Biodegradable periodontal intrapocket device containing metronidazole and amoxycillin: formulation and characterisation. Die Pharmazie 2006, 61, 25–29. [Google Scholar] [PubMed]
- Fang, J.; Niu, H.; Lin, T.; Wang, X. Applications of electrospun nanofibers. Chin. Sci. Bull. 2008, 53, 2265–2286. [Google Scholar] [CrossRef]
- Wang, P.; Mele, E. Effect of antibacterial plant extracts on the morphology of electrospun poly(lactic acid) fibres. Materials 2018, 11, 923. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.Y.; Wen, J.; Yim, E.K.F.; Leong, K.W. Sustained release of proteins from electrospun biodegradable. Biomacromolecules 2005, 6, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Gizaw, M. Electrospun fibers as a dressing material for drug and biological agent delivery in wound healing applications. Bioengineering 2018, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Kayser, O.; Kolodziej, H. Antibacterial activity of extracts and constituents of Pelargonium sidoides and Pelargonium reniforme. Planta Med. 1997, 6, 508–510. [Google Scholar] [CrossRef]
- Savickiene, N. , Jekabsone, A., Raudone, L., Abdelgeliel, A. S., Cochis, A., Rimondini, L.; Viškelis, P. Efficacy of proanthocyanidins from Pelargonium sidoides root extract in reducing P. gingivalis viability while preserving oral commensal S. salivarius. Materials 2018, 11, 1499. [Google Scholar] [CrossRef] [PubMed]
- Zięba, M.; Włodarczyk, J.; Gupta, A.; Pastusiak, M.; Chaber, P.; Janeczek, H.; Musioł, M.; Sikorska, W.; Kaczmarczyk, B.; Radecka, I.; Kowalczuk, M.; Savickas, A.; Savickiene, N.; Adamus, G. Bioresorbable electrospun mats of poly(D,L)-lactide/poly[(R,S)-3-hydroxybutyrate] blends for potential use in the treatment of difficult-to-heal skin wounds. Eur. Polym. J. 2021, 147, 110334. [Google Scholar] [CrossRef]
- Dobrzynski, P.; Kasperczyk, J.; Janeczek, H.; Bero, M. Synthesis of biodegradable copolymers with the use of low toxic zirconium compounds. 1. Copolymerization of glycolide with L-Lactide initiated by Zr(Acac)4. Macromolecules 2001, 34, 5090–5098. [Google Scholar] [CrossRef]
- Kurcok, P.; Śmiga,M. ; Jedliński, Z. β-Butyrolactone polymerization initiated with tetrabutylammonium carboxylates: A novel approach to biomimetic polyester synthesis. J. Polym. Sci. Part A: Pol. Chem. 2002, 40, 2184–2189. [Google Scholar] [CrossRef]
- Rydz, J.; Sikorska, W.; Musioł, M.; Janeczek, H.; Włodarczyk, J.; Misiurska-Marczak, M.; Łęczycka, J.; Kowalczuk, M. 3D-Printed polyester-based prototypes for cosmetic applications—Future directions at the forensic engineering of advanced polymeric materials. Materials 2019, 12, 994. [Google Scholar] [CrossRef] [PubMed]
- Maruccia, E.; Ferrari, S.; Bartoli, M.; Lucherini, L.; Meligrana, G.; Pirri, C.F.; Saracco, G.; Gerbaldi, C. Effect of thermal stabilization on PAN-derived electrospun carbon nanofibers for CO2 capture. Polymers 2021, 13, 4197. [Google Scholar] [CrossRef] [PubMed]
- Achille, C.; Sundaresh, S.; Chu, B.; Hadjiargyrou, M. Cdk2 silencing via a DNA/PCL electrospun scaffold suppresses proliferation and increases death of breast cancer cells. PLoS ONE 2012, 7, e52356. [Google Scholar] [CrossRef] [PubMed]
- Musioł, M.; Sikorska, W.; Adamus, G.; Janeczek, H.; Kowalczuk, M.; Rydz, J. (Bio)degradable polymers as a potential material for food packaging: studies on the (bio)degradation process of PLA/(R,S)-PHB rigid foils under industrial composting conditions. Eur. Food Res. Technol. 2016, 242, 815–823. [Google Scholar] [CrossRef]
- Zarzycki, R.; Modrzejewska, Z.; Nawrotek, K. Drug release from hydrogel matrice. Ecol. Chem. Eng. S. 2010, 17, 117–136. [Google Scholar]
- Mannino, G.; Chinigò, G.; Serio, G.; Genova, T.; Gentile, C.; Munaron, L.; Bertea, C.M. Proanthocyanidins and where to find them: A meta-analytic approach to investigate their chemistry, biosynthesis, distribution, and effect on human health. Antioxidants 2021, 10, 1229. [Google Scholar] [CrossRef]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83, Erratum in: Microbiol. Mol. Biol. Rev. 2012, 76, 496. PMID: 21372320; PMCID: PMC3063353. [Google Scholar] [CrossRef]
- Preshaw, P.M. ; Detection and diagnosis of periodontal conditions amenable to prevention. BMC Oral Health 2015, 15 (Suppl 1), S5. [Google Scholar] [CrossRef]
- Alkimavičienė, E.; Pušinskaitė, R.; Basevičienė, N.; Banienė, R. , Savickienė, N. and Pacauskienė, I.M. Efficacy of Proanthocyanidins in Nonsurgical Periodontal Therapy. International dental journal 2023, 73, 195–204, Epub 2022 Sep 24. PMID: 36167610; PMCID: PMC10023589.. [Google Scholar] [CrossRef]
- Cos, P.; De Bruyne, T.; Hermans, N.; Apers, S.; Berghe, D.V.; Vlietinck, A.J. Proanthocyanidins in health care: current and new trends. Curr Med Chem. 2004, 11, 1345–1359. [Google Scholar] [CrossRef] [PubMed]
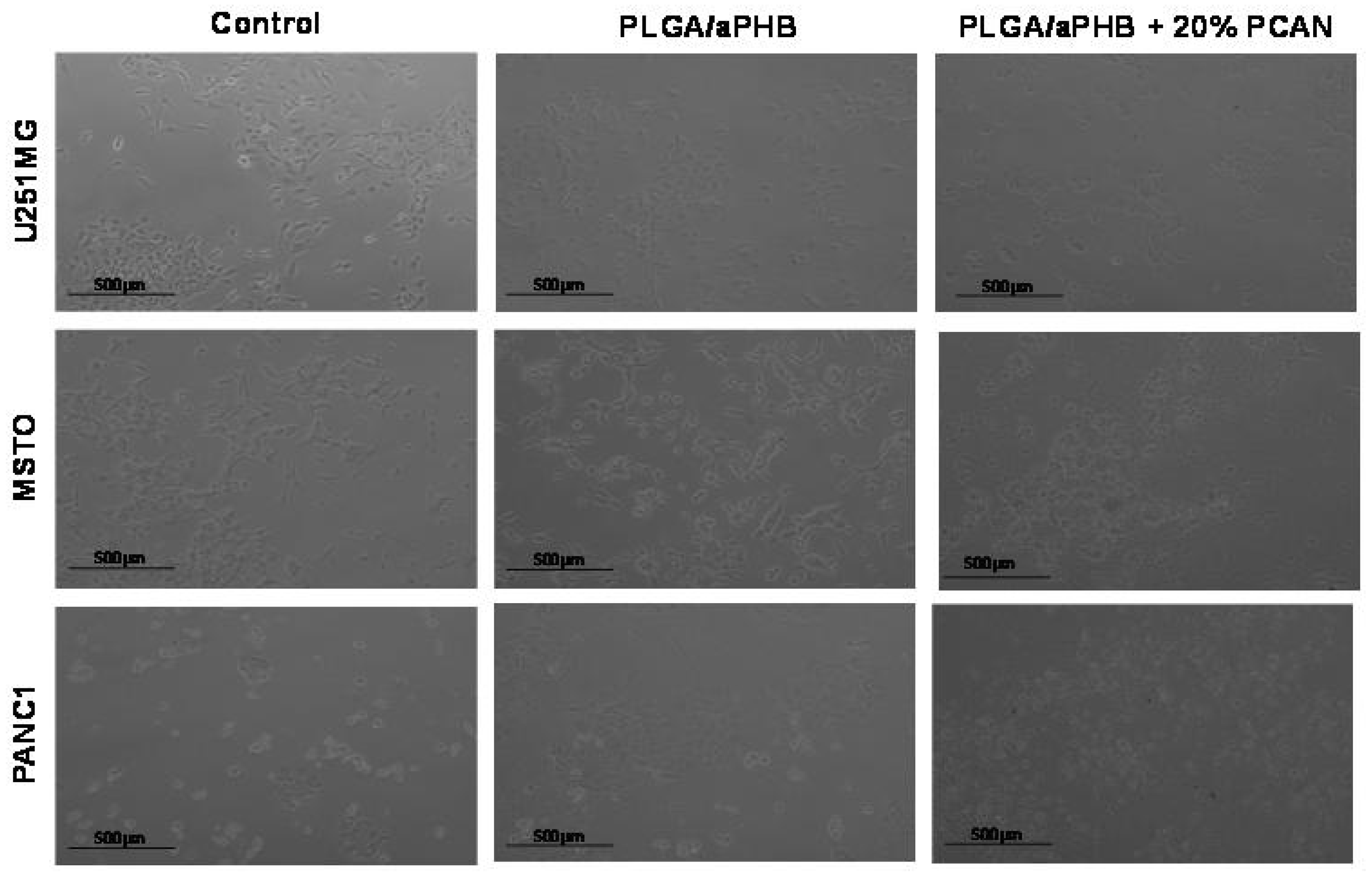
| %Cell viability | PLGA/(R,S)-PHB | PLGA/(R,S)-PHB/PCAN |
|---|---|---|
| U251MG1 | 64.50 ± 1.88% | 34.19 ± 3.38% |
| MSTO 1 | 90.91 ± 6.54% | 70.43 ± 3.66% |
| PANC 1 1 | 88.62 ± 7.49% | 23.73 ± 5.77% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





