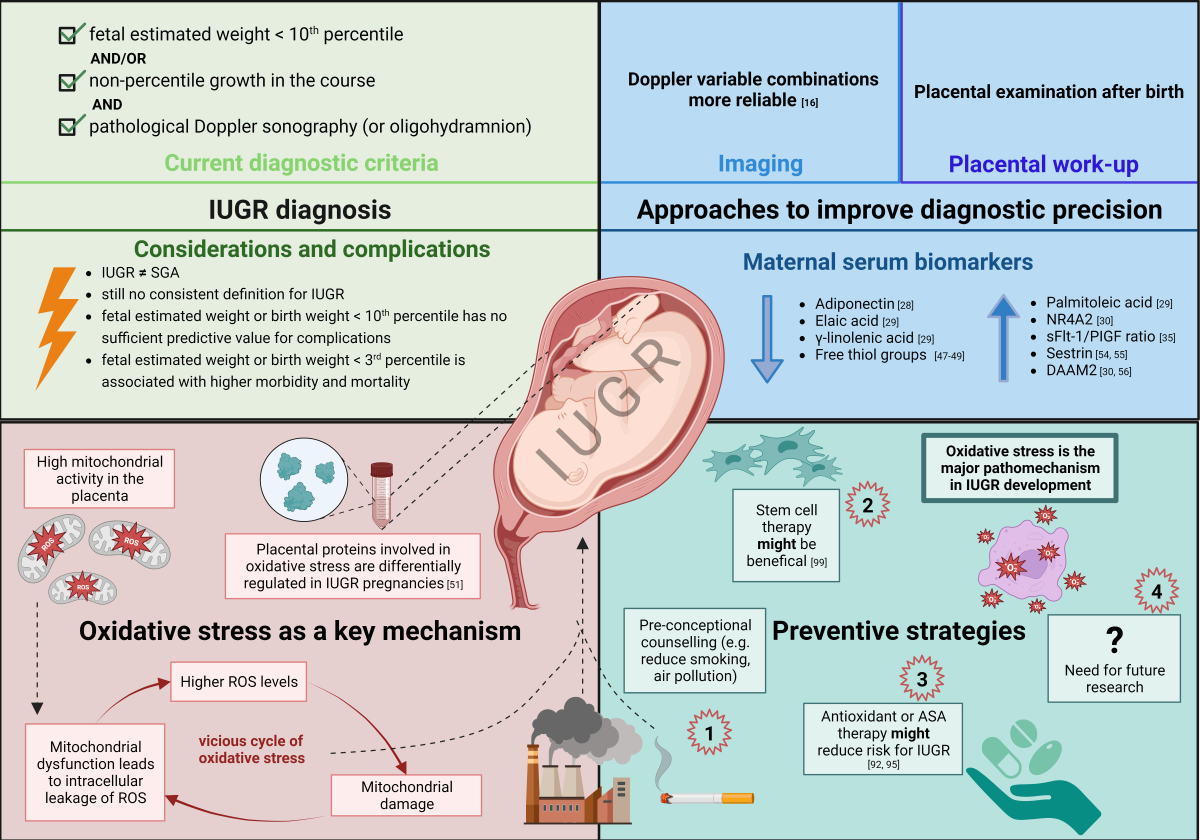
Introduction
Since the first epidemiological associations between low birthweight and elevated risk for coronary heart disease [
1], it has become widely accepted that the underlying clinical condition and its molecular basis strongly affect the frequency and severity of potential neonatal complications and determine the risk for long-term morbidity, rather than birth weight itself [
2,
3,
4]. Most importantly, intrauterine growth restriction (IUGR) and being born small for gestational age (SGA) are two distinct conditions [
5,
6,
7] with different implications for child development.
While being born SGA below the 10
th percentile may be physiological (e.g., when both parents are small), IUGR indicates a pathophysiology during pregnancy [
8]. According to international Delphi consensus, the diagnosis of IUGR in fetuses below the 10
th percentile of estimated fetal weight (EFW) should include both abnormal fetal sizes compared to the respective trajectories and abnormal Doppler findings [
7]. IUGR may also be present in neonates with birth weights above the 10
th percentile [
5].
This review article summarizes approaches to increase the diagnostic accuracy of IUGR and presents current concepts of pathophysiology with a focus on oxidative stress and consecutive inflammatory and metabolic changes. We also discuss clinical implications, the need for identification and interdisciplinary follow-up strategies in children being “at risk” and look at future scientific challenges.
Inconsistent diagnostic criteria of intrauterine growth restriction
IUGR, in recent publications mostly referred to as fetal growth restriction (FGR) [
9,
10], is reliably diagnosed by multiple measurements of fetal size over time only [
9].
When multiple measurements of fetal size are not available, abnormal Doppler findings define whether SGA fetuses below the 10
th percentile should be diagnosed as FGR according to international Delphi consensus [
7]. Nevertheless, some guidelines also still use the presence of EFW or abdominal circumference (AC) below the 10
th percentile of gestational age as sufficient for the diagnosis of IUGR [
10]. Below the 3
rd percentile, there is international agreement that the fetuses should be defined as IUGR even if there is no further evidence of growth crossing percentiles or impaired placental function [
7]. This compromise is based on the fact that below the 3
rd percentile the risk of stillbirth and neonatal morbidity is comparable to fetuses with higher EFW and proven Doppler abnormalities [
7].
In a large cohort study, the combined use of fetal growth trajectories and EFW increased the discrimination rate between fetuses “at risk” for neonatal morbidity and low-risk low-EFW fetuses [
11]. Interestingly, IUGR alone without low EFW was not associated with neonatal morbidity in this study [
11]. However, another study was able to associate also IUGR without low EFW with an increased risk of stillbirths when using different models to define slow fetal growth [
12]. To further assess the risk of neonatal and long-term sequels, the onset of IUGR during pregnancy, the presence of Doppler anomalies in the umbilical and middle cerebral arteries as well as short-term variation (STV) in computerized analysis of CTG (cCTG) and Ductus venosus Doppler anomalies can be used [
7,
13].
Overall, diagnostic criteria for IUGR are still based on ultrasound findings and birth weight and contain some inconsistencies in the distinction between IUGR and SGA. In addition, guidelines for the diagnosis of IUGR focus mainly on intrauterine or early postnatal morbidities and complications, but barely on long-term sequels.
Approaches to increase diagnostic accuracy and identify neonates being “at risk”
A clear definition and diagnosis of IUGR is a prerequisite to reliably identify neonates being “at risk” for perinatal and long-term complications. Disruptive factors such as pre-eclampsia, gestational diabetes, chromosomal disorders, infections, etc. must also be correctly diagnosed and appropriately taken into account. However, as mentioned above, many problems can arise when diagnosing IUGR under everyday conditions, ranging from lack of data to lack of resources to diagnostic challenges. For instance, late-onset IUGR (
>32 weeks of gestation) is much more difficult to detect than early-onset IUGR (<32 weeks of gestation), because the umbilical artery Doppler is mostly normal [
14]. Some IUGR cases remain completely undetected during pregnancy despite sufficient prenatal examinations, especially when the fetus is not absolutely growth restricted within the population based reference chart, but only in relation to its individual growth potential [
15]. Thus, mostly around delivery, numerous studies have been performed to identify additional criteria differentiating IUGR from non-IUGR neonates.
Imaging Studies
Recent studies highlighted that novel Doppler variable combinations such as the cerebral-placental-uterine ratio using the pulsatility indices of the middle cerebral artery, the umbilical artery and the uterine arteries, have a higher predictive accuracy for identifying IUGR than Doppler measurements of only the umbilical artery [
16]. The presence of retrograde blood flow in the aortic isthmus may be an additional indication of an increased risk of perinatal death, stillbirth and respiratory distress syndrome in IUGR patients [
17]. In late onset IUGR, abnormal cerebroplacental ratio indicates hypoxia more sensitively than the pulsatility index of the middle cerebral artery or umbilical artery alone. Moreover, an abnormal cerebroplacental ratio significantly reflects maternal, fetal and composite vascular malperfusion lesions [
18]. Fetal magnetic resonance imaging (f-MRI) is time-consuming and expensive, but safe for the fetus and can help to differentiate between IUGR and SGA [
19]. Placental perfusion can be quantitatively assessed by Intravoxel Incoherent Motion (IVIM) MRI. The perfusion fraction of the placenta was significantly lower in IUGR compared to control placentas [
20]. Diffusion-weighted MRI is able to identify microstructural alterations in IUGR placentas
in vivo [
21].
Placental work-up
Also due to a lack of resources, there is little interest in pathological examination of the placenta after birth in everyday clinical practice. However, postnatal placenta analysis offers the possibility of recognizing cases of IUGR that may have remained undetected during pregnancy. Thus, the child can receive optimal care after birth. By assessing the gross morphology of the placenta, such as shape and size, conclusions can be drawn about the functionality of the organ. Studies have demonstrated a correlation between IUGR and abnormal placental weight, diameter or volume [
22,
23]. Moreover, a reduction in placental surface area has been described [
24]. An overview about antenatal and postnatal placental findings indicative of IUGR is given in Salavati et al. [
15].
In addition to morphological analyses of the placenta, biomarkers from the placenta and amniotic fluid could help to distinguish between healthy pregnancies and pregnancies with IUGR. In a 2022 study [
25], placental samples collected after delivery were analyzed using a metabolomics approach. The authors detected 220 metabolites, of which the concentrations of 179 metabolites were significantly altered in FGR placentas compared with control placentas. 98.3% of the dysregulated metabolites showed lower concentrations in placenta samples from IUGR pregnancies. Placental 3-hydroxybutyric acid, glycine, and phosphatidylcholine with the C42:0 acyl-alkyl radical proved to be the best metabolites for differentiating between IUGR and control placenta samples. However, detection of IUGR was based only on birth weight (< 10
th percentile) in this study [
25].
Maternal and neonatal serum biomarkers
Serum biomarkers are becoming increasingly important in a variety of diseases. As for IUGR, a cohort study demonstrated that the combined use of Doppler measurements and certain metabolites from maternal serum could improve diagnostic precision [
26]. However, it is not recommended to use biomarkers alone as the only diagnostic markers for IUGR. The most interesting biomarkers at present are discussed below.
In 2020, Paules et al. [
27] reported proteomic profiling from maternal EDTA blood samples of pregnancies with late-onset IUGR. Among others, adiponectin was downregulated in IUGR cases, along with other dysregulated proteins that play a role in lipid metabolism [
28]. In maternal blood samples collected between 26 and 37+6 weeks of gestation, Grohmann et al. found lower elaidic acid and gamma-linolenic acid concentrations and higher palmitoleic acid concentrations in IUGR fetuses compared to AGA fetuses. In this study, the authors did not distinguish between early and late onset IUGR [
29].
The group of Tong et al. found that the concentration of Nuclear Receptor Subfamily 4 Group Member 2 (NR4A2) in the maternal circulation was increased in pregnancies with complicated preterm birth (birth before 34 weeks gestation) [
30]. NR4A2 concentrations were even higher in preterm infants with IUGR and preeclampsia than in normal-weight preterm infants with IUGR. Interestingly, the placenta does not appear to be the source of higher maternal circulating NR4A2, as the authors did not detect differential expression of NR4A2 in placental tissue with IUGR or preeclampsia [
31]. For another study, leukocytes from maternal blood during the 1
st trimester were isolated and screened for microRNAs associated to cardiovascular diseases. The authors report that the detection rate for IUGR without preeclampsia raised about 1.52 fold compared to the sole use of routine screening criteria for preeclampsia and/or FGR [
32]. Oluklu et al. determined maternal serum midkine (neurite outgrowth-promoting factor 2) levels and found an increase in pregnancies complicated with IUGR (Oluklu et al., 2022) [
33].
The angiogenetic factors sFlt-1 and PIGF are released from the placenta into maternal circulation and can easily be measured in maternal blood samples. Calculation of the sFlt-1/PIGF ratio is commonly used in diagnosis of preeclampsia [
34]. In cases of IUGR without preeclampsia, elevated sFlt-1/PIGF ratios have been detected as well [
35] and can even be used to help to identify severity stages of IUGR in the early-onset form [
36]. A high ratio is associated with adverse neonatal outcomes such as IUFD and FGR associated with preeclampsia [
37], risk of neonatal intensive care unit admissions and preterm delivery [
38]. The ratio can be useful in predicting fetal outcomes related to prematurity of the baby and growth-related outcomes. The higher the ratio, the higher the risk of complications for both mother and baby [
39]. Pregnancies where FGR has been detected with ultrasound and whose mothers have a high ratio test are at increased risk of adverse fetal outcomes (POP study) [
11]. The ratio test may be used to help distinguish healthy small for gestational age fetuses from those with fetal growth restriction secondary to chronic „placental insufficiency“. The trial of randomized umbilical and fetal flow in Europe-2 (TRUFFLE-2) study is currently evaluating the ratio in late FGR [
40].
Recently, Kirici et al. found pro-inflammatory markers (increased erythrocyte sedimentation rate, high sensitivity C-reactive protein, interleukin-6, and tumor necrosis factor-alpha) to be increased and the anti-inflammatory factor interleukin-10 to be decreased in maternal serum of IUGR pregnancies. However, in this study IUGR was defined as fetal weight below the 10
th percentile [
41].
Moreover, there is growing interest in the measurement of molecules indicative of reactive oxygen species (ROS) production in the maternal and fetal circulation. Both low maternal antioxidants as well as increased amounts of ROS have been found in IUGR pregnancies [
42,
43,
44,
45]. Special interest has been directed towards thiols, ischemia modified albumin levels, Sestrin-2 and disheveled associated activator of morphogenesis 2 (DAAM2).
Free thiol groups are part of the innate antioxidant capacity, scavenging ROS [
46]. Clinical research has shown that levels of free thiols in maternal serum are decreased in pregnancies with IUGR and preeclampsia [
47,
48,
49]. Ischemia modified albumin (IMA) has especially been studied in relation to preeclampsia [
50]. IMA is thought to be created when ROS causes a site-specific alteration of albumin through hydroxyl free radicals [
51,
52]. IMA is indeed increased in fetal cord blood of IUGR neonates. However, it is not elevated in maternal blood, thereby confirming the increased oxidative stress in IUGR neonates, but excluding IMA as a suitable maternal biomarker [
53]. The Sestrin-2 gene is activated under conditions of high oxidative stress and reduces the consecutive damage by increasing antioxidant expression [
54]. Sestrin-2 has been associated with IUGR and adverse outcome when increased in maternal serum [
55]. Finally, DAAM2 was identified by next generation sequencing of circulating cell free RNA in pregnant women, and shown to be associated with placental insufficiency, preterm fetal growth restriction and fetal hypoxia [
30]. Besides its increased expression in maternal serum during pregnancies with growth restriction, a follow-up study showed DAAM2 to be expressed in the placenta throughout pregnancy, and to be dysregulated under growth restricted conditions such as hypoxia [
56]. The identification and characterization of these biomarkers are promising advances for early diagnosis of IUGR. However, their diagnostic sensitivity and specificity still need to be addressed in large clinical trials.
Comprehensive molecular analyses identify oxidative stress as a key mechanism in IUGR
Most of the knowledge about the potential molecular mechanisms of IUGR in humans is based on animal studies. However, the translational relevance of animal models sometimes remains elusive. In the last decade, comprehensive molecular analyses from small amounts of human material have become more affordable. Therefore, it is increasingly feasible to elucidate the molecular basis also from human studies.
Omics techniques allow the comprehensive identification and quantification of differentially expressed gene transcripts, proteins, lipids or other metabolites in IUGR. During routine follow-up of pregnant women or around birth, various biosamples can be collected non-invasively. Recent human studies have been performed in placenta [
57,
58,
59], maternal [
27,
60,
61,
62] or umbilical cord blood [
60,
61] and maternal feces [
62]. Multi-omics analyses help to develop hypotheses of the pathophysiological alterations occurring in the IUGR mother/placenta and the adaptions that take place in the IUGR fetus. As discussed above, biomarker studies indicate a key role of ROS in IUGR pregnancies. In the following, we will therefore focus on the role of oxidative stress and discuss consecutive pro-inflammatory and metabolic changes.
Oxidative Stress in IUGR
Oxidative stress, caused by an increase of reactive oxygen species (ROS) and/or a lack of antioxidant availability and activity, has long been linked to IUGR. Both in cases of IUGR with and without abnormal Doppler findings, e.g., due to maternal malnutrition, maternal and neonatal plasma levels of antioxidants have been shown to be relatively low, whilst oxidant levels are relatively high [
45,
63,
64]. Oxidative stress is generally high in the placenta due to its high mitochondrial activity, which leads to endogenous ROS production. Placental proteomics uncovered that a large amount of the proteins differentially expressed in placentas of late-onset fetal growth restriction pregnancies are involved in the oxidative stress response [
57]. Oxidative stress even appears to precede clinical features, as an increase in maternal urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) concentrations at 12 weeks gestation was associated with an increased risk for giving birth to an infant with a birth weight below the 10
th percentile [
65]. More recently, a cohort study comparing term and preterm SGA infants (defined as birth weight < -2 SD for gestational age) with their controls showed increased serum levels of reactive oxidative metabolites in SGA infants at birth. Moreover, the amount of oxidative stress was inversely correlated with the severity of growth restriction [
66].
The exact origin of oxidative stress in the placenta remains unknown. It is thought to be largely due to inadequate perfusion and metabolic disorders, for which the evidence was well reviewed in several articles by the group of Leslie Myatt [
67,
68,
69]. The presence of oxidative stress has been documented in various cell types involved in pregnancy complications, with recent research also showing increased oxidative stress and dysregulated homeostasis of maternal platelet cells [
70]. On the molecular level, nuclear factor erythroid 2-related factor 2 (NRF2), a transcription factor with a central role in the oxidative stress response through activation of antioxidant and oxidative damage repair agents, was shown to be differentially regulated in preeclampsia with and without IUGR [
71]. This might explain previous reports on upregulation as well as downregulation of NRF2 expression in preeclampsia and IUGR. Although IUGR is in many cases linked to placental pathologies such as preeclampsia, these studies suggest different IUGR ‘endotypes’. Studies in this field are further complicated by the interaction of the different tissues involved (i.e., fetal, maternal and placental structures).
In addition, environmental factors may aggravate oxidative stress. It is well known that smoking is a major risk factor for the development of IUGR. Recently, it has been shown that altered antioxidant defense mechanisms might contribute to this observation [
72]. Similarly, air pollution has been shown to induce oxidative stress in the placenta and alter placental function [
73].
Oxidative Stress and Cellular homeostasis
While the cascade leading to the final accumulation of oxidative stress end products has not yet been fully elucidated, there are several well-known sequels. Oxidative stress causes DNA damage, reduces the genomic stability and alters cell homeostasis and survival.
Genomic instability is one of the nine hallmarks of ageing [
74] and not the only one to be found in placental pathologies. A second hallmark is reduced telomere length which has been shown in placental tissue of IUGR pregnancies [
75]. Third, mitochondrial dysfunction plays a role. Mitochondrial swelling in endothelial cells has been shown in preeclampsia and IUGR derived cells in vitro [
76]. In addition, mitochondrial dysfunction has been shown in IUGR fetuses in several animal models, as reviewed by Pendleton et al., visualizing the consequences of placental dysfunction for the fetus [
77]. Mitochondrial dysfunction in turn leads to intracellular leakage of ROS, thus indicating a vicious circle of oxidative stress. Furthermore, byproducts of lipid peroxidation have been found to induce senescence in placental cells of preeclamptic women [
78]. While cellular senescence is a physiological feature at the end of the placental life span, recent studies have shown that cellular senescence is accelerated in fetal growth restriction and unexplained cases of term stillbirth [
79]. Taken together, these findings indicate that oxidative stress related with IUGR can trigger aging-associated processes and adversely affect cellular homeostasis as well as survival.
Oxidative stress and inflammation: a vicious cycle
In a recent study, human placental explants from healthy pregnancies were collected at term and exposed to different stress conditions including hypoxia (1% oxygen) and oxidative stress (1 mM H
2O
2). Although hypoxia and oxidative stress differentially affected expression and release of damage-associated molecular patterns (DAMPs) and cytokines [
80], both conditions induced a pro-inflammatory profile.
These findings solidified that changes in oxygen concentration can induce sterile placental inflammation, possibly aggravating placental dysfunction and disturbing fetal development in IUGR pregnancy. Recently, the NLRP3 inflammasome was identified as a central pro-inflammatory regulator both in IUGR placental tissue as well as in a mouse model of IUGR [
59]. However, in placental explants of preeclamptic pregnancies, NLRP3 inflammasome activation and IL-1β expression were significantly increased in the subgroup without IUGR only [
81]. In another study, metabolomics were performed in maternal and fetal plasma at delivery. Interestingly, a pro-inflammatory profile could be shown in IUGR fetuses with or without maternal preeclampsia, but was more pronounced in IUGR with preeclampsia [
61].
Follow-up care of children “at risk” after IUGR using the example of neuropediatric sequels
Epidemiological research has revealed that IUGR may have sequels for later health and disease in almost any organ system. A bunch of literature has been published on this association, although there are few studies with strict IUGR criteria [
82]. What needs to be kept in mind is that the adult clinical phenotype of an IUGR born individual is not “fixed” at birth [
83]. Therefore, clinical follow-up strategies are needed to identify children “at risk” for adverse sequels at an early stage in order to enable early intervention and individual support strategies to improve developmental trajectories and ameliorate adverse long-term consequences [
84]. We discuss this concept looking at neuropediatric follow-up care of IUGR-born infants.
Although IUGR children have a higher risk of neurodevelopmental impairment, individual neurocognitive outcomes may vary widely. Sacchi and colleagues performed a systematic review of 60 studies including 52 822 children and found significantly impaired cognitive outcomes in children born IUGR, affecting both preterm and term infants [
85]. Benitez et al. used the Battelle Developmental Inventory (BDI) to assess the developmental progress of 70 children with IUGR at age 6 years. A significant proportion (57,1%) exhibited delays across multiple development domains. Motor and communication skills were most commonly affected [
86]. However, when evaluating neurodevelopmental outcomes, it is critical to distinguish between small gestational age (SGA) and intrauterine growth restriction (IUGR) as stated above. Therefore, Moneith et al. compared developmental outcomes of children born SGA and those born with IUGR. A total of 375 children, which were either SGA or had experienced IUGR were assessed at 3 years of age using the Bayley Scales of Infant Development, the 3
rd edition of the Toddler Development test and the Age and Stage Questionnaire. Children from IUGR pregnancies demonstrated a poorer neurodevelopmental outcome compared to their SGA counterparts [
87]. Furthermore, within the IUGR group, children with an abnormal cerebroplacental ratio (i.e., pulsatility indices of the middle cerebral artery divided by umbilical artery) had poorer neurodevelopmental outcome than children born from pregnancies with abnormal umbilical artery Doppler evidence alone [
87].
Postnatally, cerebral ultrasound sonography is commonly used to monitor brain development in the first weeks of life after IUGR. A recent study by Aisa and colleagues suggests the additional use of 3D ultrasound sonography to detect changes in brain volume as an early indicator of neurodevelopmental disorders [
88]. Further studies on changes of cerebral morphology after IUGR have been reviewed by Dudink et al. [
89]. Thus, IUGR has been associated with reduced total brain volume and significant alterations of grey and white matter in the brain [
89]. Recently, this was confirmed in a magnetic resonance imaging study by Korkalainen and colleagues in 32 children aged 8 to 10 years after IUGR birth. IUGR children had smaller total intracranial volumes compared to children with normal fetal growth. While the authors could not find significant volume differences in grey and white matter, IUGR was associated with alterations of white matter microstructure [
90].
So far, guidelines on neurodevelopmental follow-up care after IUGR are sparse. There is no consent as to early screenings, intervention and support services. Considering the significantly increased risk of neurodevelopmental impairment, neurodevelopmental assessment exceeding the standard follow-up of child development might be indicated, especially in cases of severe growth restriction, reduced postnatal growth or additional pathology (e.g., prematurity, underlying syndrome). Both research on the pathomechanistic sequences as well as studies from the field of health services research on potential benefits of structured follow-up care would be desirable to avoid secondary hits on neurodevelopment after IUGR. Healthcare professionals and parents often still lack information on challenges that IUGR children might face as they grow.
Strategies to prevent or mitigate IUGR
The clinical view- current clinical practice
Ideally, all women should receive detailed counseling before conception, including on the risk of recurrence of IUGR (23% in women with previous IUGR vs. 3% in women without previous IUGR) [
91]. Furthermore, all pregnant women should be examined for possible risk factors (maternal, fetal and uteroplacental) for the occurrence of IUGR as part of a detailed medical history.
In principle, all pregnant women should also be advised to abstain from nicotine. If there is an increased risk of uteroplacental dysfunction with the risk of IUGR, low-dose acetylsalicylic acid (ASA; 100-150 mg/die) should be started prophylactically <16 weeks gestation [
92]. Patients with antiphospholipid syndrome (APS) should be advised before conception and treated with low-dose ASA and low-molecular-weight heparin from early pregnancy onwards [
93].
The research view- future perspectives
Since oxidative stress has increasingly been recognized as a major pathomechanism in the development of IUGR, the question of appropriate prevention approaches arises. Knowledge transfer within the general population on detrimental effects of smoking [
72] or air pollution [
73] on fetal development remains of major importance. In addition, intervention strategies aiming to reduce oxidative stress whenever it is reaching a pathological threshold have been studied. A recent meta-analysis has evaluated the effect of sildenafil citrate on improving pregnancy outcomes in women with IUGR. The use of sildenafil increased birth weight and prolonged pregnancies, but had no positive effect on rates of stillbirth, neonatal deaths and neonatal intensive care unit admissions [
94]. Future studies on the effects of sildenafil on fetal growth could provide more evidence and shed more light on the debate. To date, there is no recommendation for the use of sildenafil in the societies' guidelines. Another large meta-analysis has recently shown that antioxidant therapy might reduce the risk of IUGR when administered after diagnosis of preeclampsia [
95]. However, the included studies were very heterogeneous using different antioxidant compounds. In addition, included studies partially contradict each other with some showing a beneficial effect of one substance whilst other show no effect of the same substance. Similarly, although 10 weeks of supplementation with the anti-inflammatory and antioxidant zinc improved total antioxidant capacity and reduced CRP as an inflammatory parameter, it did not improve the pathological Doppler waves in pregnancies at risk for IUGR [
96]. On a positive note, docosahexaenoic acid (DHA) has been shown to have an anti-inflammatory effect in LPS-induced placental inflammation and to reduce IUGR [
97]. Evidence suggests that antioxidant properties of DHA are mainly based on its ability to enhance mitochondrial functions and biogenesis [
98]. In conclusion, antioxidants might be a therapeutic option to avoid oxidative stress in pregnancy, but the topic needs further study to allow successful implementation in the clinical setting. So far, no consensus has been reached yet as to the properties, timing and dosage of antioxidant therapy.
Beyond antioxidants, there are studies on human umbilical mesenchymal stem cells (hUMSCs) in a therapeutic setting. It has been shown that preeclampsia-pregnancy derived hUMSCs produce more nitric oxide than healthy hUMSCs, thus increasing endogenous oxidative stress levels [
99]. Interestingly, in a rat model for preeclampsia, it could be shown that administrating MSCs from healthy pregnant controls has a beneficial effect on overall fetal growth and inflammation [
100]. This creates an exciting avenue of treatment options that is waiting to be further studied.
Summary and future perspectives
Up to 70% of fetuses below the 10th percentile are constitutional SGA fetuses and have a normal perinatal outcome, whereas IUGR is associated with greater perinatal morbidity and mortality and can be identified by additional abnormalities (pathological Doppler sonography, oligohydramnios, lack of growth in the interval, estimated weight <3rd percentile). An IUGR does not require a weight below the 10th percentile, as even fetuses with an estimated weight above the 10th percentile cannot fulfil their genetically predetermined growth potential due to adverse conditions. Recognizing the latter and those fetuses with a higher mortality and morbidity rate in order to monitor them accordingly and deliver them in good time is still challenging. Various markers have been associated with an existing IUGR; in clinical routine, the Doppler sonographic examination of the fetal vessels and the determination of the sFlt-1/PlGF ratio have been established to date. If there is a corresponding risk of placental insufficiency, ASA should be used preventively. Although epidemiological research has shown that IUGR can have consequences for later health and disease in almost all organ systems, there are few guidelines for follow-up care after IUGR.
In conclusion, it would be desirable to have more established and practicable markers available for a valid assessment of the prenatal situation and the risk of later health consequences. Many recent studies have focused on oxidative stress as an important pathomechanism in IUGR. Further research is needed to translate this theoretical knowledge into the clinical care structure. Since prenatal influences affect the risk of later diseases, awareness should be raised that prenatal and postnatal care of IUGR neonates should be regarded as a continuum.
References
- Barker, D. INFANT MORTALITY, CHILDHOOD NUTRITION, AND ISCHAEMIC HEART DISEASE IN ENGLAND AND WALES. Lancet 1986, 327, 1077–1081. [Google Scholar] [CrossRef]
- Nüsken, K.D.; Schneider, H.; Plank, C.; Trollmann, R.; Nüsken, E.; Rascher, W.; Dötsch, J. Fetal programming of gene expression in growth-restricted rats depends on the cause of low birth weight. Endocrinology 2011, 152, 1327–1335. [Google Scholar] [CrossRef]
- Tzschoppe, A.; Riedel, C.; von Kries, R.; Struwe, E.; Rascher, W.; Dörr, H.G.; Beckmann, M.W.; Schild, R.L.; Goecke, T.W.; Flyvbjerg, A.; et al. Differential effects of low birthweight and intrauterine growth restriction on umbilical cord blood insulin-like growth factor concentrations. Clin Endocrinol (Oxf) 2015, 83, 739–745. [Google Scholar] [CrossRef]
- Olga, L.; Sovio, U.; Wong, H.; Smith, G.; Aiken, C. Association between antenatal diagnosis of late fetal growth restriction and educational outcomes in mid-childhood: A UK prospective cohort study with long-term data linkage study. PLoS Med 2023, 20, e1004225. [Google Scholar] [CrossRef] [PubMed]
- Broere-Brown, Z.A.; Schalekamp-Timmermans, S.; Jaddoe, V.W.V.; Steegers, E.A.P. Deceleration of fetal growth rate as alternative predictor for childhood outcomes: a birth cohort study. BMC Pregnancy Childbirth 2019, 19, 216. [Google Scholar] [CrossRef]
- Musco, H.; Beecher, K.; Chand, K.K.; Colditz, P.B.; Wixey, J.A. Blood biomarkers in the fetally growth restricted and small for gestational age neonate: associations with brain injury. Dev Neurosci 2023, 1. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.; Stampalija, T.; Hecher, K. Diagnosis and management of fetal growth restriction: the ISUOG guideline and comparison with the SMFM guideline. Ultrasound Obstet. Gynecol. 2021, 57, 884–887. [Google Scholar] [CrossRef]
- Fantasia, I.; Zamagni, G.; Lees, C.; Mylrea-Foley, B.; Monasta, L.; Mullins, E.; Prefumo, F.; Stampalija, T. Current practice in the diagnosis and management of fetal growth restriction: An international survey. Acta Obstet. Et Gynecol. Scand. 2022, 101, 1431–1439. [Google Scholar] [CrossRef]
- Lees, C.C.; Stampalija, T.; Baschat, A.; da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.C.; Salomon, L.J.; et al. ISUOG Practice Guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef]
- Martins, J.G.; Biggio, J.R.; Abuhamad, A. Society for Maternal-Fetal Medicine Consult Series #52: Diagnosis and management of fetal growth restriction: (Replaces Clinical Guideline Number 3, April 2012). Am. J. Obstet. Gynecol. 2020, 223, B2–b17. [Google Scholar] [CrossRef]
- Sovio, U.; White, I.R.; Dacey, A.; Pasupathy, D.; Smith, G.C.S. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the Pregnancy Outcome Prediction (POP) study: a prospective cohort study. Lancet 2015, 386, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Gardosi, J.; Hugh, O. Outcome-based comparative analysis of five fetal growth velocity models to define slow growth. Ultrasound 2023. [Google Scholar] [CrossRef]
- Lees, C.C.; Romero, R.; Stampalija, T.; Dall'Asta, A.; DeVore, G.A.; Prefumo, F.; Frusca, T.; Visser, G.H.A.; Hobbins, J.C.; Baschat, A.A.; et al. Clinical Opinion: The diagnosis and management of suspected fetal growth restriction: an evidence-based approach. Am. J. Obstet. Gynecol. 2022, 226, 366–378. [Google Scholar] [CrossRef]
- Baschat, A.A. Planning management and delivery of the growth-restricted fetus. Best Pract. Res.. Clin. Obstet. Gynaecol. 2018, 49, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Salavati, N.; Smies, M.; Ganzevoort, W.; Charles, A.K.; Erwich, J.J.; Plösch, T.; Gordijn, S.J. The Possible Role of Placental Morphometry in the Detection of Fetal Growth Restriction. Front Physiol 2018, 9, 1884. [Google Scholar] [CrossRef]
- MacDonald, T.M.; Hui, L.; Robinson, A.J.; Dane, K.M.; Middleton, A.L.; Tong, S.; Walker, S.P. Cerebral-placental-uterine ratio as novel predictor of late fetal growth restriction: prospective cohort study. Ultrasound Obstet. Gynecol. 2019, 54, 367–375. [Google Scholar] [CrossRef]
- La Verde, M.; Savoia, F.; Riemma, G.; Schiattarella, A.; Conte, A.; Hidar, S.; Torella, M.; Colacurci, N.; De Franciscis, P.; Morlando, M. Fetal aortic isthmus Doppler assessment to predict the adverse perinatal outcomes associated with fetal growth restriction: systematic review and meta-analysis. Arch Gynecol Obs. 2023. [Google Scholar] [CrossRef]
- Shmueli, A.; Mor, L.; Blickstein, O.; Sela, R.; Weiner, E.; Gonen, N.; Schreiber, L.; Levy, M. Placental pathology in pregnancies with late fetal growth restriction and abnormal cerebroplacental ratio. Placenta 2023, 138, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Santacroce, A.; de Bernardo, G.; Alagna, M.G.; Carbone, S.F.; Paternò, I.; Buonocore, G. Magnetic Resonance Imaging in Pregnancy with Intrauterine Growth Restriction: A Pilot Study. Dis Markers 2019, 2019, 4373490. [Google Scholar] [CrossRef]
- Liu, X.L.; Feng, J.; Huang, C.T.; Mei, Y.J.; Xu, Y.K. Use of intravoxel incoherent motion MRI to assess placental perfusion in normal and Fetal Growth Restricted pregnancies on their third trimester. Placenta 2022, 118, 10–15. [Google Scholar] [CrossRef]
- Andescavage, N.; You, W.; Jacobs, M.; Kapse, K.; Quistorff, J.; Bulas, D.; Ahmadzia, H.; Gimovsky, A.; Baschat, A.; Limperopoulos, C. Exploring in vivo placental microstructure in healthy and growth-restricted pregnancies through diffusion-weighted magnetic resonance imaging. Placenta 2020, 93, 113–118. [Google Scholar] [CrossRef]
- Almasry, S.M.; Elfayomy, A.K. Morphometric analysis of terminal villi and gross morphological changes in the placentae of term idiopathic intrauterine growth restriction. Tissue Cell 2012, 44, 214–219. [Google Scholar] [CrossRef]
- Egbor, M.; Ansari, T.; Morris, N.; Green, C.J.; Sibbons, P.D. Pre-eclampsia and fetal growth restriction: how morphometrically different is the placenta? Placenta 2006, 27, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, T.M.; Manwani, R.; Ohadike, C.; Wijesekara, J.; Baker, P.N. The placenta in pre-eclampsia and intrauterine growth restriction: studies on exchange surface areas, diffusion distances and villous membrane diffusive conductances. Placenta 2007, 28, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Bahado-Singh, R.O.; Turkoglu, O.; Yilmaz, A.; Kumar, P.; Zeb, A.; Konda, S.; Sherman, E.; Kirma, J.; Allos, M.; Odibo, A.; et al. Metabolomic identification of placental alterations in fetal growth restriction. J. Matern. -Fetal Neonatal Med. 2022, 35, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Sovio, U.; Goulding, N.; McBride, N.; Cook, E.; Gaccioli, F.; Charnock-Jones, D.S.; Lawlor, D.A.; Smith, G.C.S. A maternal serum metabolite ratio predicts fetal growth restriction at term. Nat Med 2020, 26, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Paules, C.; Youssef, L.; Miranda, J.; Crovetto, F.; Estanyol, J.M.; Fernandez, G.; Crispi, F.; Gratacós, E. Maternal proteomic profiling reveals alterations in lipid metabolism in late-onset fetal growth restriction. Sci Rep 2020, 10, 21033. [Google Scholar] [CrossRef] [PubMed]
- Paules, C.; Dantas, A.P.; Miranda, J.; Crovetto, F.; Eixarch, E.; Rodriguez-Sureda, V.; Dominguez, C.; Casu, G.; Rovira, C.; Nadal, A.; et al. Premature placental aging in term small-for-gestational-age and growth-restricted fetuses. Ultrasound Obstet. Gynecol. 2019, 53, 615–622. [Google Scholar] [CrossRef]
- Grohmann, R.M.; Marçal, V.M.G.; Corazza, I.C.; Peixoto, A.B.; Júnior, E.A.; Nardozza, L.M.M. Maternal Blood Fatty Acid Levels in Fetal Growth Restriction. Rev Bras Ginecol Obs. 2023, 45, 127–133. [Google Scholar] [CrossRef]
- Hannan, N.J.; Stock, O.; Spencer, R.; Whitehead, C.; David, A.L.; Groom, K.; Petersen, S.; Henry, A.; Said, J.M.; Seeho, S.; et al. Circulating mRNAs are differentially expressed in pregnancies with severe placental insufficiency and at high risk of stillbirth. BMC Med. 2020, 18, 145. [Google Scholar] [CrossRef]
- de Alwis, N.; Beard, S.; Binder, N.K.; Pritchard, N.; Kaitu'u-Lino, T.J.; Walker, S.P.; Stock, O.; Groom, K.M.; Petersen, S.; Henry, A.; et al. NR4A2 expression is not altered in placentas from cases of growth restriction or preeclampsia, but is reduced in hypoxic cytotrophoblast. Sci Rep 2021, 11, 20670. [Google Scholar] [CrossRef] [PubMed]
- Hromadnikova, I.; Kotlabova, K.; Krofta, L. First-Trimester Screening for Fetal Growth Restriction and Small-for-Gestational-Age Pregnancies without Preeclampsia Using Cardiovascular Disease-Associated MicroRNA Biomarkers. Biomedicines 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Oluklu, D.; Beser, D.M.; Hendem, D.U.; Kara, O.; Yazihan, N.; Sahin, D. Maternal serum midkine level in fetal growth restriction: a case-control study. J Perinat Med 2023, 51, 396–402. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N Engl J Med 2016, 374, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Dymara-Konopka, W.; Laskowska, M.; Grywalska, E.; Hymos, A.; Błażewicz, A.; Leszczyńska-Gorzelak, B. Similar Pro- and Antiangiogenic Profiles Close to Delivery in Different Clinical Presentations of Two Pregnancy Syndromes: Preeclampsia and Fetal Growth Restriction. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manau, P.; Mendoza, M.; Bonacina, E.; Garrido-Gimenez, C.; Fernandez-Oliva, A.; Zanini, J.; Catalan, M.; Tur, H.; Serrano, B.; Carreras, E. Soluble fms-like tyrosine kinase to placental growth factor ratio in different stages of early-onset fetal growth restriction and small for gestational age. Acta Obstet. Et Gynecol. Scand. 2021, 100, 119–128. [Google Scholar] [CrossRef]
- Verlohren, S.; Brennecke, S.P.; Galindo, A.; Karumanchi, S.A.; Mirkovic, L.B.; Schlembach, D.; Stepan, H.; Vatish, M.; Zeisler, H.; Rana, S. Clinical interpretation and implementation of the sFlt-1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hypertens. 2022, 27, 42–50. [Google Scholar] [CrossRef]
- Lopes Perdigao, J.; Chinthala, S.; Mueller, A.; Minhas, R.; Ramadan, H.; Nasim, R.; Naseem, H.; Young, D.; Shahul, S.; Chan, S.L.; et al. Angiogenic Factor Estimation as a Warning Sign of Preeclampsia-Related Peripartum Morbidity Among Hospitalized Patients. Hypertension 2019, 73, 868–877. [Google Scholar] [CrossRef]
- Dröge, L.A.; Perschel, F.H.; Stütz, N.; Gafron, A.; Frank, L.; Busjahn, A.; Henrich, W.; Verlohren, S. Prediction of Preeclampsia-Related Adverse Outcomes With the sFlt-1 (Soluble fms-Like Tyrosine Kinase 1)/PlGF (Placental Growth Factor)-Ratio in the Clinical Routine. Hypertension 2021, 77, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Mylrea-Foley, B.; Bhide, A.; Mullins, E.; Thornton, J.; Marlow, N.; Stampalija, T.; Napolitano, R.; Lees, C.C. Building consensus: thresholds for delivery in TRUFFLE-2 randomized intervention study. Ultrasound Obstet. Gynecol. 2020, 56, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Kırıcı, P.; Çağıran, F.T.; Kalı, Z.; Tanrıverdi, E.F.; Mavral, N.; Ecin, S.M. Determination of maternal serum pro-inflammatory cytokine changes in intrauterine growth restriction. Eur Rev Med Pharmacol Sci 2023, 27, 1996–2001. [Google Scholar] [CrossRef] [PubMed]
- Biri, A.; Bozkurt, N.; Turp, A.; Kavutcu, M.; Himmetoglu, O.; Durak, I. Role of oxidative stress in intrauterine growth restriction. Gynecol Obs. Invest 2007, 64, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Guvendag Guven, E.S.; Karcaaltincaba, D.; Kandemir, O.; Kiykac, S.; Mentese, A. Cord blood oxidative stress markers correlate with umbilical artery pulsatility in fetal growth restriction. J. Matern. -Fetal Neonatal Med. 2013, 26, 576–580. [Google Scholar] [CrossRef]
- Karowicz-Bilińska, A.; Suzin, J.; Sieroszewski, P. Evaluation of oxidative stress indices during treatment in pregnant women with intrauterine growth retardation. Med Sci Monit 2002, 8, Cr211–Cr216. [Google Scholar] [PubMed]
- Saker, M.; Soulimane Mokhtari, N.; Merzouk, S.A.; Merzouk, H.; Belarbi, B.; Narce, M. Oxidant and antioxidant status in mothers and their newborns according to birthweight. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 141, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Lindgren, A.; Arnadottir, M.; Prytz, H.; Hultberg, B. Thiols as a measure of plasma redox status in healthy subjects and in patients with renal or liver failure. Clin Chem 1999, 45, 1084–1086. [Google Scholar] [CrossRef] [PubMed]
- Ozler, S.; Oztas, E.; Guler, B.G.; Erel, O.; Caglar, A.T.; Ergin, M.; Danisman, N. Dynamic Thiol/Disulfide Homeostasis in Predicting Adverse Neonatal Outcomes in Fetal Growth Restriction. Fetal Pediatr Pathol 2020, 39, 132–144. [Google Scholar] [CrossRef]
- Schoots, M.H.; Bourgonje, M.F.; Bourgonje, A.R.; Prins, J.R.; van Hoorn, E.G.M.; Abdulle, A.E.; Muller Kobold, A.C.; van der Heide, M.; Hillebrands, J.L.; van Goor, H.; et al. Oxidative stress biomarkers in fetal growth restriction with and without preeclampsia. Placenta 2021, 115, 87–96. [Google Scholar] [CrossRef]
- Eroglu, H.; Turgal, M.; Senat, A.; Karakoc, G.; Neselioglu, S.; Yucel, A. Maternal and fetal thiol/disulfide homeostasis in fetal growth restriction. J. Matern. -Fetal Neonatal Med. 2021, 34, 1658–1665. [Google Scholar] [CrossRef]
- Seshadri Reddy, V.; Duggina, P.; Vedhantam, M.; Manne, M.; Varma, N.; Nagaram, S. Maternal serum and fetal cord-blood ischemia-modified albumin concentrations in normal pregnancy and preeclampsia: a systematic review and meta-analysis. J. Matern. -Fetal Neonatal Med. 2018, 31, 3255–3266. [Google Scholar] [CrossRef]
- Lippi, G.; Montagnana, M.; Guidi, G.C. Albumin cobalt binding and ischemia modified albumin generation: an endogenous response to ischemia? Int J Cardiol 2006, 108, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.K.; Roy, D.; Gaze, D.C.; Collinson, P.O.; Kaski, J.C. Role of "Ischemia modified albumin", a new biochemical marker of myocardial ischaemia, in the early diagnosis of acute coronary syndromes. Emerg Med J 2004, 21, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Gölbaşı, C.; Gölbaşı, H.; Kocahakimoğlu Gültekin, C.; Gülseren, V.; Zeytinli Akşit, M.; Bayraktar, B.; Çolak, A.; Taner, C.E. Ischemia modified albumin levels in intrauterine growth restriction: levels are increased in fetal cord blood but not in maternal blood. Ginekol Pol 2022, 93, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, M.; Du, X.; Huang, Z.; Quan, N. Sestrin 2, a potential star of antioxidant stress in cardiovascular diseases. Free Radic. Biol. Med. 2021, 163, 56–68. [Google Scholar] [CrossRef]
- Agaoglu, M.O.; Agaoglu, Z.; Yucel, K.Y.; Ozturk, F.H.; Caglar, T. Evaluation of maternal serum sestrin-2 levels in intrauterine growth restriction. Ir J Med Sci 2023. [Google Scholar] [CrossRef]
- de Alwis, N.; Beard, S.; Binder, N.K.; Pritchard, N.; Kaitu'u-Lino, T.J.; Walker, S.P.; Stock, O.; Groom, K.; Petersen, S.; Henry, A.; et al. DAAM2 is elevated in the circulation and placenta in pregnancies complicated by fetal growth restriction and is regulated by hypoxia. Sci Rep 2021, 11, 5540. [Google Scholar] [CrossRef]
- Gęca, T.; Stupak, A.; Nawrot, R.; Goździcka-Józefiak, A.; Kwaśniewska, A.; Kwaśniewski, W. Placental proteome in late-onset of fetal growth restriction. Mol Med Rep 2022, 26. [Google Scholar] [CrossRef]
- Meng, X.L.; Yuan, P.B.; Wang, X.J.; Hang, J.; Shi, X.M.; Zhao, Y.Y.; Wei, Y. The Proteome Landscape of Human Placentas for Monochorionic Twins with Selective Intrauterine Growth Restriction. Genom. Proteom. Bioinform. 2023. [Google Scholar] [CrossRef]
- Alfian, I.; Chakraborty, A.; Yong, H.E.J.; Saini, S.; Lau, R.W.K.; Kalionis, B.; Dimitriadis, E.; Alfaidy, N.; Ricardo, S.D.; Samuel, C.S.; et al. The Placental NLRP3 Inflammasome and Its Downstream Targets, Caspase-1 and Interleukin-6, Are Increased in Human Fetal Growth Restriction: Implications for Aberrant Inflammation-Induced Trophoblast Dysfunction. Cells 2022, 11. [Google Scholar] [CrossRef]
- Moros, G.; Boutsikou, T.; Fotakis, C.; Iliodromiti, Z.; Sokou, R.; Katsila, T.; Xanthos, T.; Iacovidou, N.; Zoumpoulakis, P. Insights into intrauterine growth restriction based on maternal and umbilical cord blood metabolomics. Sci Rep 2021, 11, 7824. [Google Scholar] [CrossRef]
- Youssef, L.; Simões, R.V.; Miranda, J.; García-Martín, M.L.; Paules, C.; Crovetto, F.; Amigó, N.; Cañellas, N.; Gratacos, E.; Crispi, F. Paired maternal and fetal metabolomics reveal a differential fingerprint in preeclampsia versus fetal growth restriction. Sci Rep 2021, 11, 14422. [Google Scholar] [CrossRef]
- Tao, Z.; Chen, Y.; He, F.; Tang, J.; Zhan, L.; Hu, H.; Ding, Z.; Ruan, S.; Chen, Y.; Chen, B.; et al. Alterations in the Gut Microbiome and Metabolisms in Pregnancies with Fetal Growth Restriction. Microbiol Spectr 2023, 11, e0007623. [Google Scholar] [CrossRef]
- Dede, H.; Takmaz, O.; Ozbasli, E.; Dede, S.; Gungor, M. Higher Level of Oxidative Stress Markers in Small for Gestational Age Newborns Delivered by Cesarean Section at Term. Fetal Pediatr Pathol 2017, 36, 232–239. [Google Scholar] [CrossRef]
- Gupta, P.; Narang, M.; Banerjee, B.D.; Basu, S. Oxidative stress in term small for gestational age neonates born to undernourished mothers: a case control study. BMC Pediatr. 2004, 4, 14. [Google Scholar] [CrossRef]
- Potdar, N.; Singh, R.; Mistry, V.; Evans, M.D.; Farmer, P.B.; Konje, J.C.; Cooke, M.S. First-trimester increase in oxidative stress and risk of small-for-gestational-age fetus. BJOG 2009, 116, 637–642. [Google Scholar] [CrossRef]
- Ashina, M.; Kido, T.; Kyono, Y.; Yoshida, A.; Suga, S.; Nakasone, R.; Abe, S.; Tanimura, K.; Nozu, K.; Fujioka, K. Correlation between Severity of Fetal Growth Restriction and Oxidative Stress in Severe Small-for-Gestational-Age Infants. Int J Environ. Res Public Health 2021, 18. [Google Scholar] [CrossRef]
- Hebert, J.F.; Myatt, L. Placental mitochondrial dysfunction with metabolic diseases: Therapeutic approaches. Biochim Biophys Acta Mol Basis Dis 2021, 1867, 165967. [Google Scholar] [CrossRef]
- Myatt, L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta 2010, 31 Suppl, S66–S69. [Google Scholar] [CrossRef]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem Cell Biol 2004, 122, 369–382. [Google Scholar] [CrossRef]
- Nowaczyk, J.; Poniedziałek, B.; Rzymski, P.; Sikora, D.; Ropacka-Lesiak, M. Platelets in Fetal Growth Restriction: Role of Reactive Oxygen Species, Oxygen Metabolism, and Aggregation. Cells 2022, 11. [Google Scholar] [CrossRef]
- Mundal, S.B.; Rakner, J.J.; Silva, G.B.; Gierman, L.M.; Austdal, M.; Basnet, P.; Elschot, M.; Bakke, S.S.; Ostrop, J.; Thomsen, L.C.V.; et al. Divergent Regulation of Decidual Oxidative-Stress Response by NRF2 and KEAP1 in Preeclampsia with and without Fetal Growth Restriction. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Hoch, D.; Majali-Martinez, A.; Bankoglu, E.E.; Stopper, H.; Glasner, A.; Desoye, G.; Gauster, M.; Hiden, U. Maternal Smoking in the First Trimester and its Consequence on the Early Placenta. Lab Invest 2023, 103, 100059. [Google Scholar] [CrossRef] [PubMed]
- Juan-Reyes, S.S.; Gómez-Oliván, L.M.; Juan-Reyes, N.S.; Islas-Flores, H.; Dublán-García, O.; Orozco-Hernández, J.M.; Pérez-Álvarez, I.; Mejía-García, A. Women with preeclampsia exposed to air pollution during pregnancy: Relationship between oxidative stress and neonatal disease - Pilot study. Sci Total Environ. 2023, 871, 161858. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Li, K.; Xie, C.; Wen, X. Adverse Birth Outcomes and Birth Telomere Length: A Systematic Review and Meta-Analysis. J Pediatr 2019, 215, 64–74.e6. [Google Scholar] [CrossRef] [PubMed]
- Formanowicz, D.; Malińska, A.; Nowicki, M.; Kowalska, K.; Gruca-Stryjak, K.; Bręborowicz, G.; Korybalska, K. Preeclampsia with Intrauterine Growth Restriction Generates Morphological Changes in Endothelial Cells Associated with Mitochondrial Swelling-An In Vitro Study. J Clin Med 2019, 8. [Google Scholar] [CrossRef]
- Pendleton, A.L.; Wesolowski, S.R.; Regnault, T.R.H.; Lynch, R.M.; Limesand, S.W. Dimming the Powerhouse: Mitochondrial Dysfunction in the Liver and Skeletal Muscle of Intrauterine Growth Restricted Fetuses. Front Endocrinol (Lausanne) 2021, 12, 612888. [Google Scholar] [CrossRef] [PubMed]
- Tasta, O.; Swiader, A.; Grazide, M.H.; Rouahi, M.; Parant, O.; Vayssière, C.; Bujold, E.; Salvayre, R.; Guerby, P.; Negre-Salvayre, A. A role for 4-hydroxy-2-nonenal in premature placental senescence in preeclampsia and intrauterine growth restriction. Free. Radic. Biol. Med. 2021, 164, 303–314. [Google Scholar] [CrossRef]
- Kajdy, A.; Modzelewski, J.; Cymbaluk-Płoska, A.; Kwiatkowska, E.; Bednarek-Jędrzejek, M.; Borowski, D.; Stefańska, K.; Rabijewski, M.; Torbé, A.; Kwiatkowski, S. Molecular Pathways of Cellular Senescence and Placental Aging in Late Fetal Growth Restriction and Stillbirth. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Baker, B.C.; Heazell, A.E.P.; Sibley, C.; Wright, R.; Bischof, H.; Beards, F.; Guevara, T.; Girard, S.; Jones, R.L. Hypoxia and oxidative stress induce sterile placental inflammation in vitro. Sci Rep 2021, 11, 7281. [Google Scholar] [CrossRef]
- Silva, G.B.; Gierman, L.M.; Rakner, J.J.; Stødle, G.S.; Mundal, S.B.; Thaning, A.J.; Sporsheim, B.; Elschot, M.; Collett, K.; Bjørge, L.; et al. Cholesterol Crystals and NLRP3 Mediated Inflammation in the Uterine Wall Decidua in Normal and Preeclamptic Pregnancies. Front Immunol 2020, 11, 564712. [Google Scholar] [CrossRef]
- Gaillard, R.; Steegers, E.A.; Tiemeier, H.; Hofman, A.; Jaddoe, V.W. Placental vascular dysfunction, fetal and childhood growth, and cardiovascular development: the generation R study. Circulation 2013, 128, 2202–2210. [Google Scholar] [CrossRef]
- Sehgal, A.; Skilton, M.R.; Crispi, F. Human fetal growth restriction: a cardiovascular journey through to adolescence. J Dev Orig Health Dis 2016, 7, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Chen, Y.; Ye, J.; Ouyang, F.; Jiang, F.; Zhang, J. The optimal postnatal growth trajectory for term small for gestational age babies: a prospective cohort study. J Pediatr 2015, 166, 54–58. [Google Scholar] [CrossRef]
- Sacchi, C.; Marino, C.; Nosarti, C.; Vieno, A.; Visentin, S.; Simonelli, A. Association of Intrauterine Growth Restriction and Small for Gestational Age Status With Childhood Cognitive Outcomes: A Systematic Review and Meta-analysis. JAMA Pediatr 2020, 174, 772–781. [Google Scholar] [CrossRef]
- Benítez Marín, M.J.; Blanco Elena, J.A.; Marín Clavijo, J.; Jiménez López, J.; Lubián López, D.M.; González Mesa, E. Neurodevelopment Outcome in Children with Fetal Growth Restriction at Six Years of Age: A Retrospective Cohort Study. Int J Environ. Res Public Health 2022, 19. [Google Scholar] [CrossRef]
- Monteith, C.; Flood, K.; Pinnamaneni, R.; Levine, T.A.; Alderdice, F.A.; Unterscheider, J.; McAuliffe, F.M.; Dicker, P.; Tully, E.C.; Malone, F.D.; et al. An abnormal cerebroplacental ratio (CPR) is predictive of early childhood delayed neurodevelopment in the setting of fetal growth restriction. Am. J. Obstet. Gynecol. 2019, 221, 273.e1–273.e9. [Google Scholar] [CrossRef]
- Aisa, M.C.; Barbati, A.; Gerli, S.; Clerici, G.; Nikolova, N.; Giardina, I.; Babucci, G.; De Rosa, F.; Cappuccini, B. Brain 3D-echographic early predictors of neuro-behavioral disorders in infants: a prospective observational study. J. Matern. -Fetal Neonatal Med. 2022, 35, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Dudink, I.; Hüppi, P.S.; Sizonenko, S.V.; Castillo-Melendez, M.; Sutherland, A.E.; Allison, B.J.; Miller, S.L. Altered trajectory of neurodevelopment associated with fetal growth restriction. Exp Neurol 2022, 347, 113885. [Google Scholar] [CrossRef]
- Korkalainen, N.; Ilvesmäki, T.; Parkkola, R.; Perhomaa, M.; Mäkikallio, K. Brain volumes and white matter microstructure in 8- to 10-year-old children born with fetal growth restriction. Pediatr Radiol 2022, 52, 2388–2400. [Google Scholar] [CrossRef] [PubMed]
- Ananth, C.V.; Peltier, M.R.; Chavez, M.R.; Kirby, R.S.; Getahun, D.; Vintzileos, A.M. Recurrence of ischemic placental disease. Obstet. Gynecol. 2007, 110, 128–133. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Wright, D.; Poon, L.C.; O'Gorman, N.; Syngelaki, A.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. Aspirin versus Placebo in Pregnancies at High Risk for Preterm Preeclampsia. N Engl J Med 2017, 377, 613–622. [Google Scholar] [CrossRef]
- Mayer-Pickel, K. The Antiphospholipid Syndrome – the Obstetric Point of View. Z. Für Gefäßmedizin 2018, 15, 9–13. [Google Scholar]
- Rakhanova, Y.; Almawi, W.Y.; Aimagambetova, G.; Riethmacher, D. The effects of sildenafil citrate on intrauterine growth restriction: a systematic review and meta-analysis. BMC Pregnancy Childbirth 2023, 23, 409. [Google Scholar] [CrossRef]
- Alves, P.; Fragoso, M.B.T.; Tenório, M.C.S.; Bueno, N.B.; Goulart, M.O.F.; Oliveira, A.C.M. The role played by oral antioxidant therapies in preventing and treating preeclampsia: An updated meta-analysis. Nutr Metab Cardiovasc Dis 2023, 33, 1277–1292. [Google Scholar] [CrossRef]
- Mesdaghinia, E.; Naderi, F.; Bahmani, F.; Chamani, M.; Ghaderi, A.; Asemi, Z. The effects of zinc supplementation on clinical response and metabolic profiles in pregnant women at risk for intrauterine growth restriction: a randomized, double-blind, placebo-controlled trial. J. Matern. -Fetal Neonatal Med. 2021, 34, 1382–1388. [Google Scholar] [CrossRef]
- Bo, Q.; Xie, Y.; Lin, Q.; Fu, L.; Hu, C.; Zhang, Z.; Meng, Q.; Xu, F.; Wang, G.; Miao, Z.; et al. Docosahexaenoic acid protects against lipopolysaccharide-induced fetal growth restriction via inducing the ubiquitination and degradation of NF-κB p65 in placental trophoblasts. J. Nutr. Biochem. 2023, 118, 109359. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Xiao, B.; Cui, D.; Lin, Y.; Zeng, J.; Li, J.; Cao, M.J.; Liu, J. Antioxidant Activity of Docosahexaenoic Acid (DHA) and Its Regulatory Roles in Mitochondria. J Agric Food Chem 2021, 69, 1647–1655. [Google Scholar] [CrossRef]
- Surico, D.; Bordino, V.; Cantaluppi, V.; Mary, D.; Gentilli, S.; Oldani, A.; Farruggio, S.; Melluzza, C.; Raina, G.; Grossini, E. Preeclampsia and intrauterine growth restriction: Role of human umbilical cord mesenchymal stem cells-trophoblast cross-talk. PLoS One 2019, 14, e0218437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Fu, L.; Wang, L.; Lin, L.; Yu, L.; Zhang, L.; Shang, T. Therapeutic benefit of mesenchymal stem cells in pregnant rats with angiotensin receptor agonistic autoantibody-induced hypertension: Implications for immunomodulation and cytoprotection. Hypertens. Pregnancy 2017, 36, 247–258. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).