1. Introduction
In clinical chemistry, the development of minimally invasive diagnostic and screening assays based on cell-free nucleic acids has garnered increasing attention. Cell-free DNA, mRNA, and microRNAs (miRNAs) found in blood have emerged as prominent biomarkers in the pursuit of assay innovation. However, a critical challenge inherent to the utilization of blood-based biomarkers is the influence of pre-analytical factors, such as the way the blood sample is handled, including the storage and processing, which might exert pronounced impacts on assay outcomes [
1].
MiRNAs are a class of small non-coding RNA molecules, typically comprising around 22 nucleotides, with pivotal roles in pathophysiological processes. They function by post-transcriptionally regulating gene expression mostly through gene silencing [
2,
3]. MiRNAs play significant roles in several cellular processes including differentiation, proliferation, apoptosis, and tumorigenesis [
4]. Recent research has unveiled the dysregulation of miRNA expression in various disease conditions, including cancer, chronic kidney disease, and autism spectrum disorder [
5,
6,
7]. Notably, investigations carried out on fresh human tissues and formalin-fixed paraffin-embedded (FFPE) tissues have underscored the remarkable stability of miRNAs [
8,
9]. Likewise, studies involving human plasma have indicated the relative stability of plasma miRNAs, bolstering their potential as diagnostic biomarkers [
10]. However, a 2022 study revealed the degradation of certain miRNAs after 24 hours of storage at room temperature [
11].
Among these miRNAs, as stated above, miRNA-451a emerged as a key player in various human diseases [
12,
13,
14,
15]. MiRNA-451a has been implicated in a spectrum of cancers, such as esophageal cancer [
16], gastric cancer [
17], renal cell carcinoma [
18], hepatocellular carcinoma [
19], colorectal cancer [
20], and breast cancer [
21]. As such, miRNA-451a was recently proposed as a potential biomarker and therapeutic target across multiple cancer types [
22]. Considering that most errors in clinical laboratory tests occur during the pre-analytical phase [
23], diligent monitoring and control of pre-analytical factors are paramount to minimizing inaccuracies in clinical assays. Sample type, storage temperature, and time of sample processing can all alter, to varying degree, the expression of miRNAs. Thus, if miRNAs are going to be used in diagnostic assays in the future, it is critical to characterize these pre-analytical factors to generate consistent and accurate conclusions about miRNA expression levels. Given the extensive interest in miRNA-451a as a putative biomarker for disease, this study sought to investigate pre-analytical factors that may exert significant effects on the accurate quantification of plasma miRNA-451a in blood. Here, we provide a set of empirical guidelines which can help minimize misinterpretation of miRNA-451a dysregulation in blood samples.
2. Materials and Methods
2.1. Blood Samples
Blood from healthy donors were obtained from Boys Town National Research Hospital, Omaha, NE, USA. Written informed consent was obtained from all donors prior to blood draw and this study was approved (IRB # 20-14-XP) by the institutional review board of Boys Town National Research Hospital, Omaha, NE, USA. Blood was collected from each donor using standard venipuncture technique into one 10 mL K3EDTA tube (BD vacutainer1, Becton Dickinson, Franklin Lakes, NJ).
2.2. Plasma Separation
Plasma was separated from blood by a previously described method [
24]. Immediately after blood draw, blood was centrifuged at room temperature (RT) at 1600 x g for 10 minutes. The platelet rich plasma layer was then carefully removed without disturbing the buffy coat, and transferred into a new tube, mixed well, and divided into two aliquots. One aliquot was stored at RT, the other at 4ºC. Plasma aliquots were taken at days 0, 1, 2, 3 and 7 and centrifuged at RT at 16000 x g for 10 minutes to remove platelets, residual cells, cell debris, apoptotic bodies, and nuclei prior to RNA extraction.
2.3. Preparation of Artificially Hemolyzed Blood Samples
Immediately after blood draw, a 1 mL aliquot of blood was removed from each donor and placed into an Eppendorf tube and subjected to freezing and thawing to induce hemolysis. The remaining blood from each donor centrifuged two times as described previously to get platelet free plasma. Platelet free plasma from each donor was divided into two 1 mL aliquots. Hemolyzed blood samples were also subjected to the same two step centrifugation protocol to get platelet free hemolyzed plasma. From one aliquot of non-hemolyzed plasma, 50 µL was removed and 50 µL of hemolyzed plasma added and mixed well by vortexing to get a hemolyzed plasma sample. RNA was extracted from both non-hemolyzed and hemolyzed blood samples as described below.
2.4. Plasma RNA Extraction
Manufacturer’s recommended protocol was followed to extract RNA from plasma using RNeasy® mini kit (cat. # 74004, QIAGEN Sciences Inc., Germantown, MD). RNA was eluted in 50 μL of nuclease free water. Total RNA concentration was determined using Qubit™ RNA HS Assay Kit (Life Technologies, Thermo Fisher Scientific Inc.), Qubit™ 4.0 Fluorometer (Life Technologies, Thermo Fisher Scientific Inc.) and stored at -80˚C until cDNA transcription.
2.5. Quantitative Analysis of miRNAs Using ddPCR
Total RNA (10 ng) was transcribed and amplified using TaqMan Advanced cDNA Synthesis Kit (Cat. # A28007, Applied Biosystems) following manufacturer’s recommended protocol. cDNA samples were diluted with 0.1X TE buffer before ddPCR. Complimentary DNA samples were analyzed using TaqMan advanced miRNA assays for miRNA-451a (Assay ID 478107-mir), miRNA-423-5p (Assay ID 478090-mir), and miRNA-199a-3p (Assay ID 477961-mir) (Cat. # A25576, Applied Biosystems). The 20 µL ddPCR reaction mixture contained 10 µL of Bio-Rad 2x ddPCR Supermix for probes, 2.0 µL of diluted cDNA, 1.0 µL of individual miRNA-specific primer-probe mix and reaction volume adjusted to 20 µL by nuclease free water. Droplet digital PCR was performed using Bio-Rad Automated QX200 droplet digital PCR system as previously described [
25].
2.6. Statistical Analysis
Statistical analysis was performed in R [
26]. The R package “rstatix” [
27] was used to run all statistical comparisons and figures were generated using the ggplot2 package [
28]. Pairwise t-tests were used to compare miRNA expression at different time points in a repeated measure experimental design with a Bonferroni adjustment for multiple comparisons. An unpaired student’s t-test was used to compare hemolyzed versus non-hemolyzed plasma miRNA-451a expression. P-values less than 0.05 were considered statistically significant.
3. Results
3.1. MiRNA-451 Levels in Different Fractions of Human Blood
We first sought to quantify levels of miRNA-451a in different components of blood. To begin with, we determined the distribution of miRNA-451a by fractionating blood into 5 distinct components: RBCs, WBCs, platelets, plasma containing platelets, and platelet-free plasma. Subsequently, we extracted total RNA from each fraction and performed miRNA-451a quantification using ddPCR. The total copy number of miRNA-541a was calculated as the sum of all copies in all 5 fractions of blood (RBCs, WBCs, platelets, plasma with platelets, and platelet free plasma). Copy number in each fraction was divided by the total copy number and proportions are reported as a percentage of the total miRNA-451a copies. As depicted in
Table 1, our results reveal that 99.9% of miRNA-451a is predominantly located within the red blood cell fraction. White blood cells contain the second-highest amount, with a percentage of 0.055%. In contrast, platelets, plasma with platelets, and platelet-free plasma exhibit much lower miRNA-451a levels, accounting for 0.012%, 0.007%, and 0.0028%, respectively. The results suggest that most of circulating miRNA-451a is found in RBCs.
3.2. Effect of Hemolysis On Plasma miRNA-451a Levels
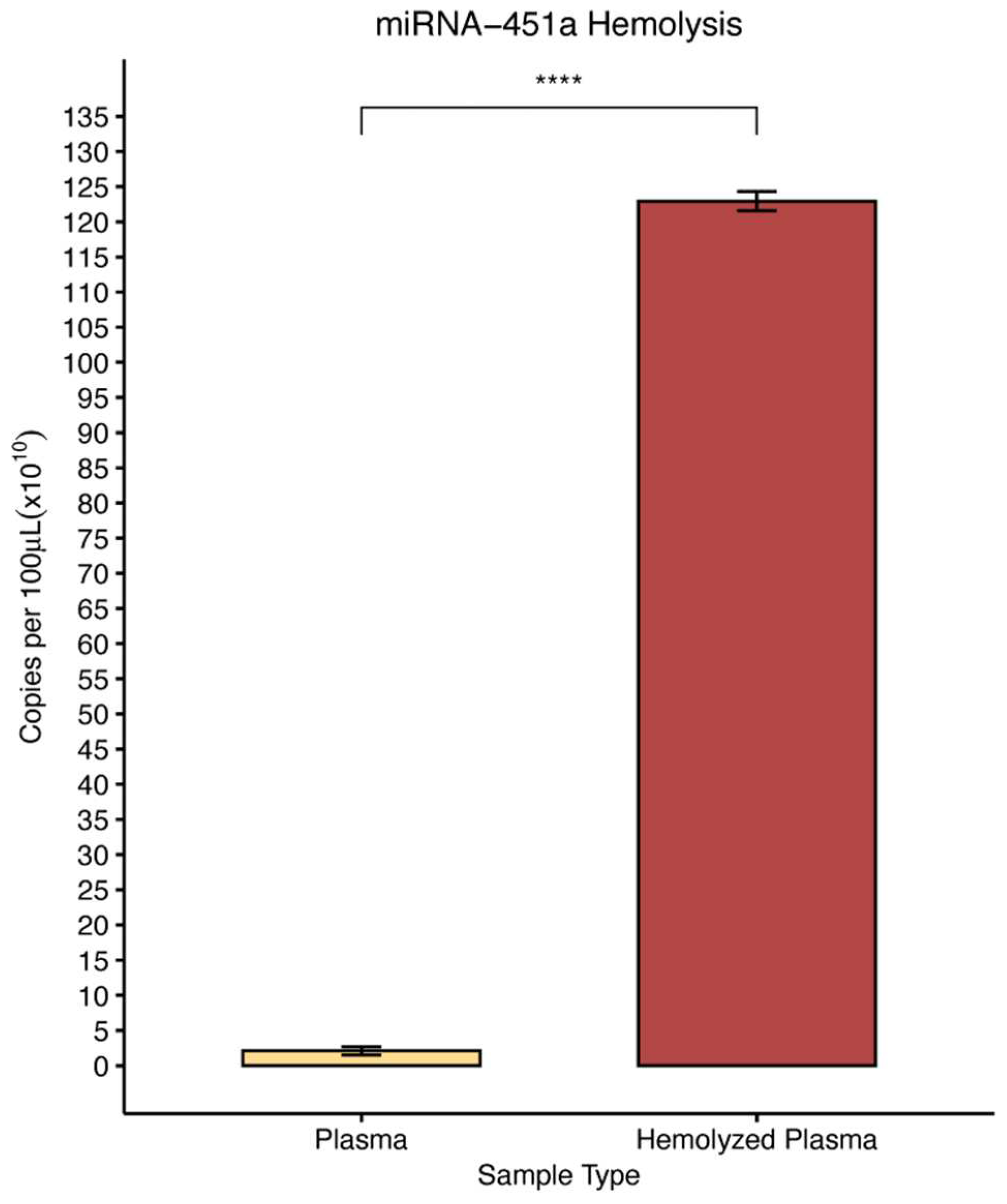
After observing that over 99% of miRNA-451a expression resides in red blood cells, we investigated how hemolysis might influence the amount of miRNA-451a in platelet free plasma (PFP). To simulate hemolysis, we generated spiked platelet free plasma as described above, and we compared levels of miRNA-451a in hemolyzed versus non-hemolyzed PFP using ddPCR. There was a statistically significant 58.5-fold increase in miRNA-451a concentration in hemolyzed samples as compared to non-hemolyzed samples (
Figure 1). Here we suspect that if hemolysis occurs during sample storage or preparation, the levels of miRNA-451a in PFP may significantly change.
3.3. Effect of Storage of Whole Blood and Platelet Rich Plasma at RT or 4ºC on Circulating miRNA-451a Stability
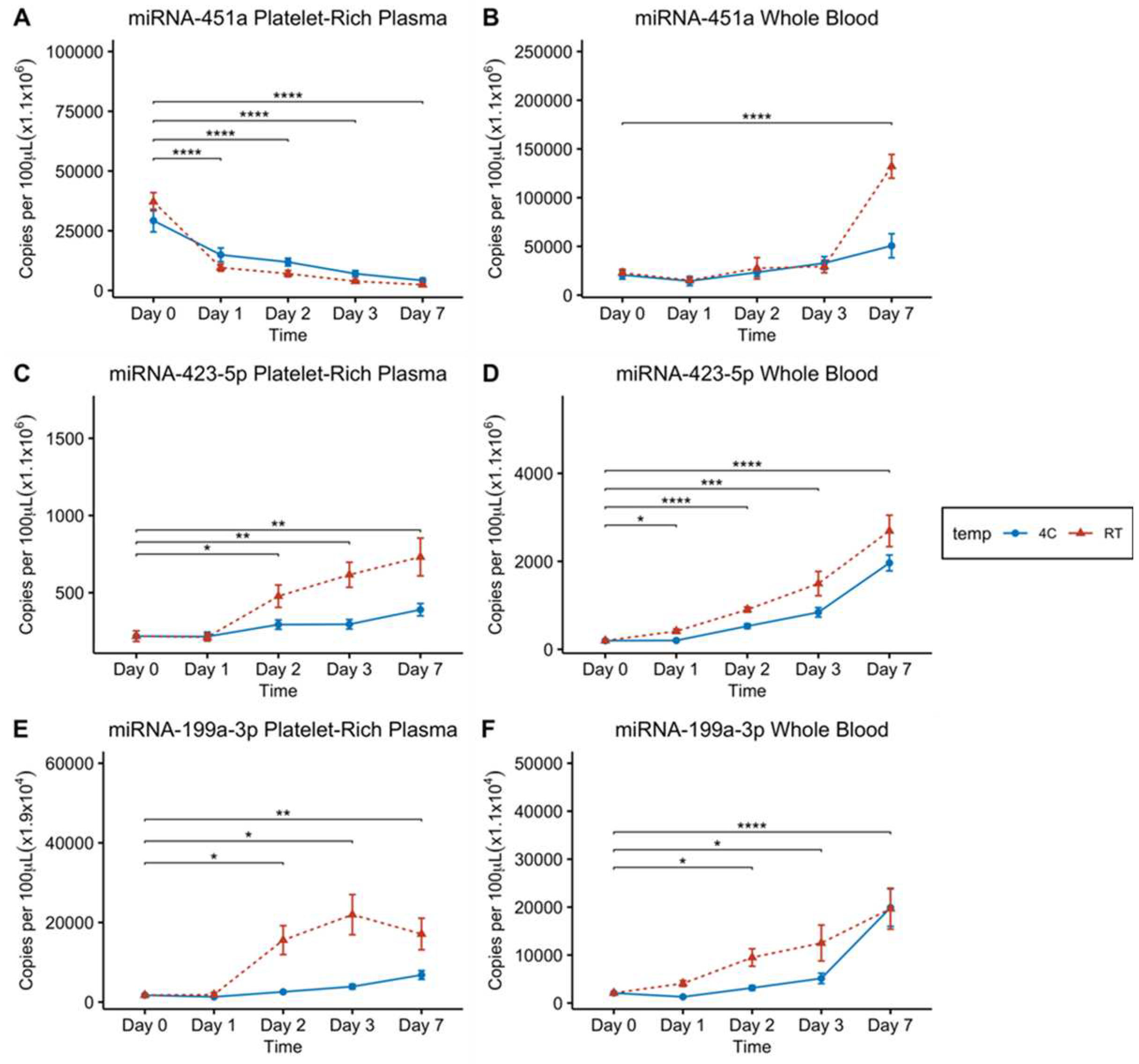
To evaluate the impact of storage conditions on circulating miRNA-451a stability in both whole blood and platelet-rich plasma (PRP), we examined the levels of miRNA-451a at room temperature (RT) or 4oC over a period of seven days in 17 donor samples. We observed that plasma samples stored at RT or 4°C showed a time-dependent decrease in miRNA-451a levels, highlighting the inherent instability of miRNA-451a at RT and 4°C (
Figure 2A). On the other hand, the results in whole blood indicated that within the first day, both storage conditions exhibited a statistically insignificant, minor reduction in miRNA-451a levels (
Figure 2B). However, as time progresses towards days 2 and 3, there was a slight increase in miRNA-451a concentration (
Figure 2B). Notably, after seven days of storage, blood samples kept at RT exhibited a statistically significant 5.7-fold increase, suggesting whole blood samples stored at RT may produce dysregulated miRNA-451a concentrations after 7 days. There was also a significant 2.5-fold increase in miRNA-451a concentration after one week storage at 4°C. Here we observed that in platelet-rich plasma (PRP), miRNA-451a concentration significantly decreases over time, even after just 24 hours if samples are stored at 4°C or RT. Conversely, after 7 days at 4°C or RT whole blood levels of miRNA-451a will actually increase significantly. These observations suggest that perhaps miRNA-451a levels are not stable at 4°C or RT for extended periods of time, and blood samples at these temperatures should be processed within 24 hours.
3.4. Effect of Storage of Whole Blood and Platelet Rich Plasma at RT and 4ºC on Circulating miRNA-423-5p Concentration
After observing inconsistent levels of miRNA-451a at different storage conditions and in different sample types, we wanted to confirm whether this phenomenon was unique to miRNA-451a or perhaps common to other highly abundant miRNAs. To do this we measured the levels of two other highly abundant miRNAs in the blood, miRNA-423-5p and miRNA-199a-3p. MiRNA-423-5p is known for its high abundance in plasma and has been reported as an endogenous control for the quantification of circulating miRNAs in certain cancers [
29]. We conducted an analysis of miRNA-423-5p levels in stored blood and plasma samples derived from 8 donor samples. For plasma samples stored at RT (
Figure 2C), there was no notable change in miRNA-423-5p concentration after one day. However, RT and 4°C concentrations significantly increased after 2 days, and continued increasing through days 3 and 7, where 4°C sample concentrations less increase than their RT counterparts (
Figure 2C). In whole blood samples stored at RT, we observed a significant increase in miRNA-423-5p concentration at all time-points when compared to day 0 measurements (
Figure 2D). Here we observed that miRNA-423-5p showed different changes in expression over time than miRNA-451a, but its levels did increase over time in both 4°C and RT storage conditions. This again indicates that pre-analytical factors of storage temperature and time contribute to variance in miRNA expression measurements with ddPCR.
3.5. Effect of Storage of Whole Blood and Platelet Rich Plasma at RT and 4ºC on Circulating miRNA-199a-3p Concentration
Lastly, in order to further confirm the role of temperature and storage time on PFP and whole blood samples, we measured miRNA-199a-3p levels using ddPCR. MiRNA-199a-3p is another miRNA that is highly abundant in blood and is a promising diagnostic and prognostic marker in glioma [
30]. Eight donor blood samples were used in this analysis as well. As shown in
Figure 2E, plasma stored at RT and 4°C again exhibited a significant increase in miRNA-199a-3p concentration, after 48 hours when compared to day 0 levels. Whole blood stored at both RT and 4°C showed miRNA-199-3p concentrations continuously increasing starting at the 2-day period all the way to 7 days when compared to day 0 (
Figure 2F). Once more there was significant changes in miRNA expression in both PFP and Whole blood samples over the course of 7 days, where we saw increases in miRNA-199a-3p levels as time progressed, but storage at 4°C appears to reduce the magnitude of miRNA-199a-3p increases over time.
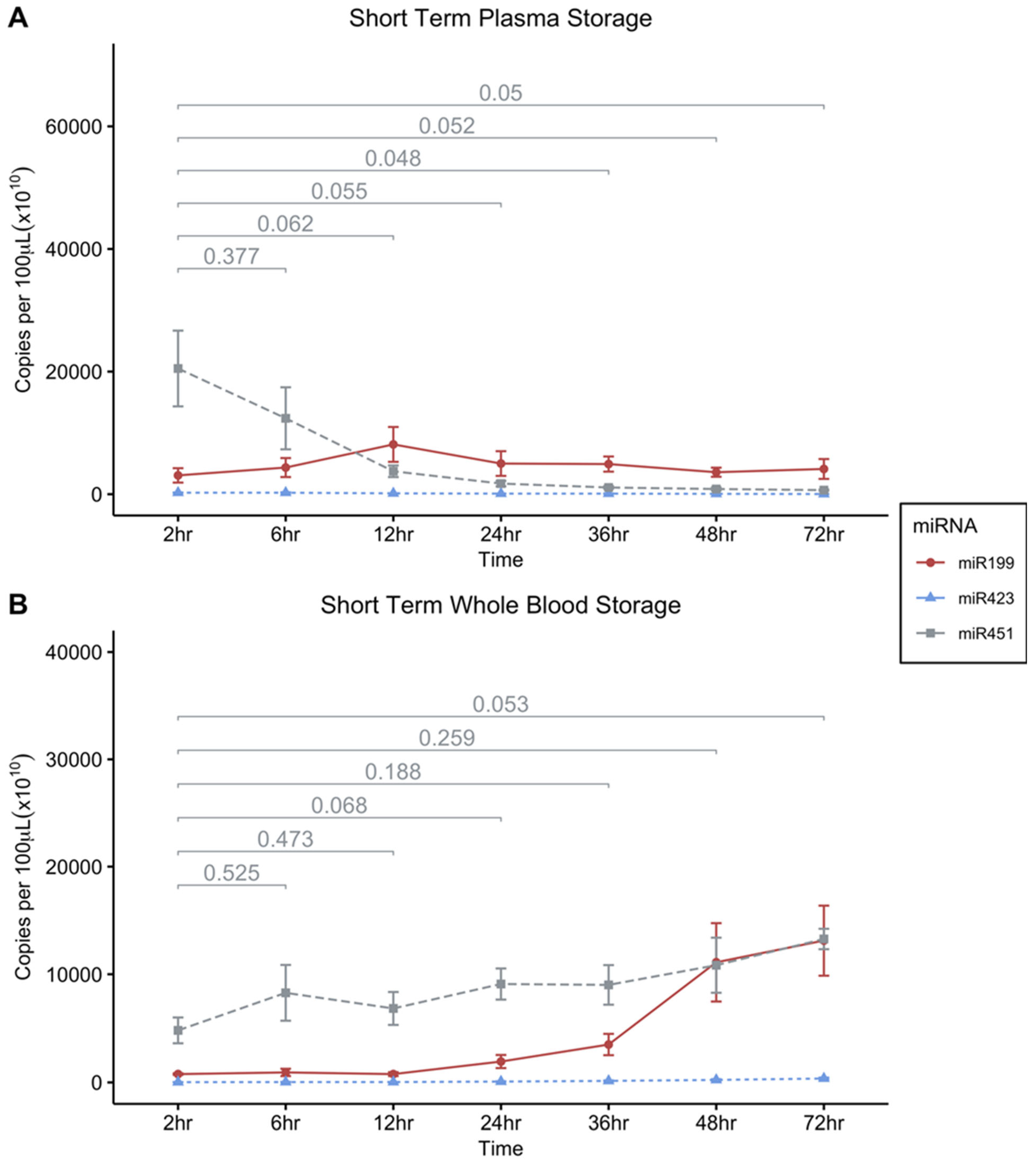
3.6. Effect of Short-Term Storage of Whole Blood and Platelet Free Plasma at RT on Circulating miRNA-451a
Storage of PFP and whole blood at room temperature over the course of 7 days had an influence on miRNA expression levels. We then narrowed the scope of time to smaller increments, post-blood draw to narrow in on an optimal window in which samples could be processed to produce consistent results. To this end, measurements of the stability of miRNA-451a, miRNA-199a-3p, and miRNA-423-5p were taken at room temperature (RT) storage conditions over shorter time increments. We assessed the levels in whole blood or in PFP samples over a 72-hour period (at 2, 6, 12, 24-, 36-, 48-, and 72-hour time points). MiRNA-451a in PFP displayed a decline over the span of 72 hours becoming significantly lower than the 2-hour time point at 36 hours, where the highest concentrations of miRNA-451a were recorded in the first 6 hours after sampling (
Figure 3A).
MiRNA-451a concentration remained relatively stable in whole blood at RT over 72 hours only beginning to approach statistically significant increases from the 2-hour initial measurements after about three days (
Figure 3B). It should be noted that there was a large degree of variation in the observed miRNA levels within time-points in the short-term RT experiments and the trends observed merit further validation.
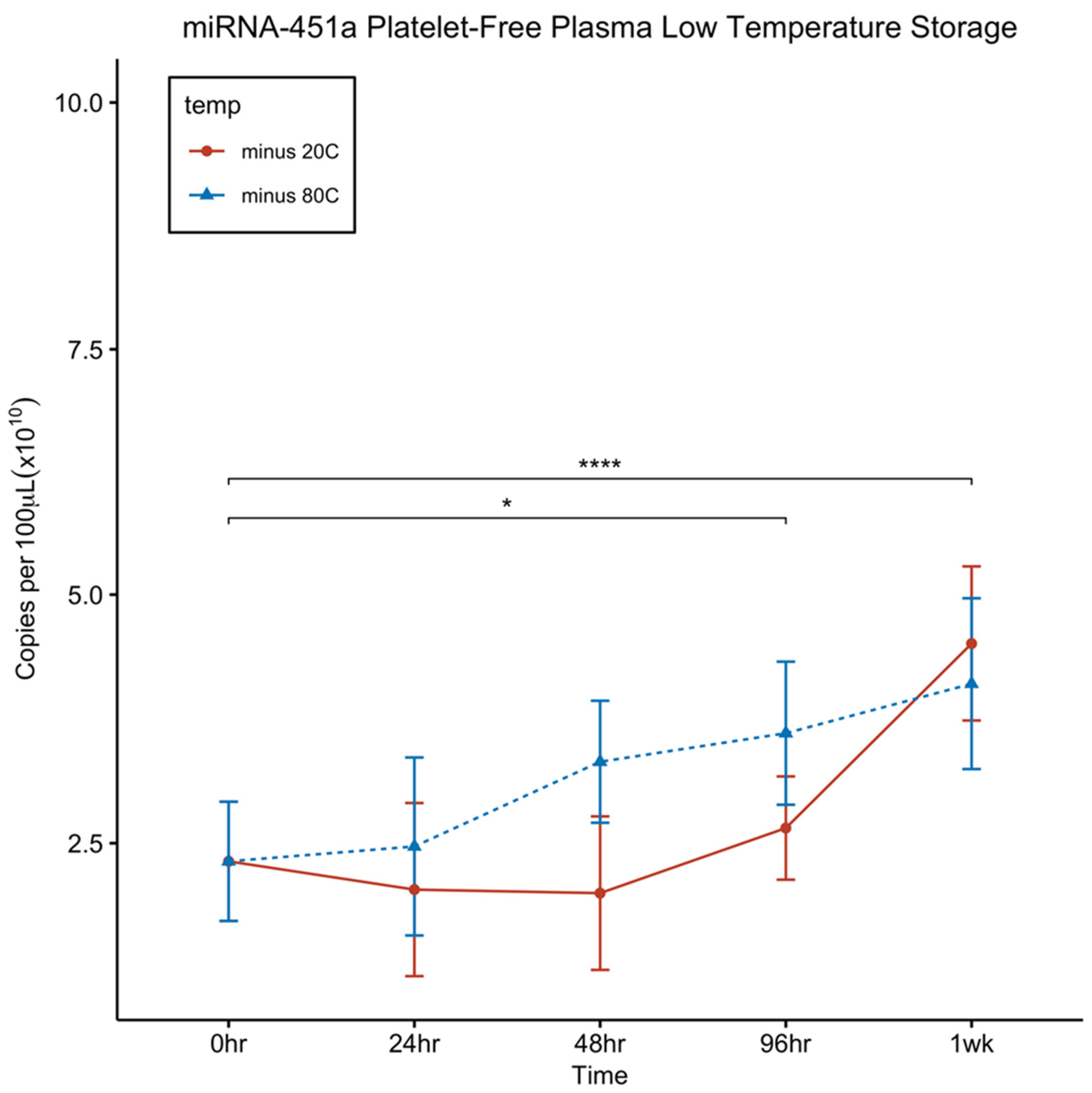
3.7. Effect of -20ºC and -80ºC Storage Conditions on Platelet Free Plasma miRNA-451a Levels
Given the observed instability of miRNA-451a in plasma at room temperature (RT) and 4ºC (
Figure 2A), we conducted a stability study at lower temperatures, specifically at -20ºC and -80ºC, over a period of 7 days. As depicted in
Figure 4, plasma stored at colder temperatures showed no decrease in levels over the entire 7-day duration at -20ºC and -80 ºC. Instead, interestingly, we noted a significant increase in the levels over time, which is counter-intuitive to what would be expected. There was an observed difficulty in RNA extraction due to formation of heavy protein precipitations in the aqueous phase limiting access to get most of the aqueous phase formed during the first time-points. We suspect this may be the reason for the increase in miRNA concentrations at later time-points. Regardless, these findings indicate that both -20ºC and -80ºC are suitable temperatures for the storage of plasma samples intended for miRNA-451a quantification.
4. Discussion
Circulating miRNA-451a exhibits significant potential as a biomarker for diagnosing various diseases, including cancer, chronic kidney disease, and autism spectrum disorder [12 -15]. However, one of the major challenges in utilizing circulating miRNAs for developing novel diagnostic assays is preanalytical variability. This study addresses several preanalytical factors that can impact assay accuracy, such as blood sample hemolysis, the way plasma is separated from blood, sample storage time, and temperature. We investigated the distribution of miRNA-451a in different blood fractions, including RBCs, WBCs, platelets, platelet-rich plasma, and PFP. Based on our results we devised a set of empirically driven suggestions for sample processing to avoid variations in circulating miRNA assays driven by pre-analytical factors above.
Our findings reveal that an overwhelming 99.9% of miRNA-451a is localized within RBCs (
Table 1), confirming earlier work [
31]. In contrast, plasma contains only minute amounts of miRNA-451a compared to RBCs (
Table 1). Consequently, our results demonstrated that plasma samples derived from hemolysis, a pre-analytical factor, exhibited a 58.5-fold increase in miRNA-451a levels as compared to non-hemolyzed plasma (
Figure 1), highlighting the inadvisability of using hemolyzed samples in miRNA-451a-based assays.
Furthermore, our investigation delves into the effects of storage time and temperature on miRNA-451a levels in blood and platelet-rich plasma. When whole blood is stored at room temperature (RT) and 4°C for up to 7 days, miRNA-451a concentration remains stable for the first three days (
Figure 2B). However, by day 7, there was a statistically significant increase in miRNA-451a concentration (
Figure 2B). In contrast, when platelet-rich plasma is stored under the same conditions, there is a consistent statistically significant decrease in miRNA-451a levels (
Figure 2A), indicating that miRNA-451a levels in plasma decrease over time.
The question of why miRNA-451a remains stable in stored whole blood but decline in platelet rich plasma can potentially be explained by the fact that RBCs are capable of releasing exosomes and other extracellular vesicles that contain miRNA-451a into circulation, as previously reported [
32]. Thus, any decrease in miRNA-451a levels that is due to its instability/degradation may be compensated for by what is released from RBCs or RBCs undergoing hemolysis post-blood draw.
In addition to miRNA-451a, we also investigated the stability of two other clinically important miRNAs in plasma, miRNA-199a-3p and miRNA-423-5p. When whole blood is stored at RT and 4°C, the levels of both miRNA-423-5p (
Figure 2D) and miRNA-199a-3p (
Figure 2F) increased over time. In platelet-rich plasma stored at RT, miRNA-423-5p exhibits a statistically significant increase from day 2, whereas storage at 4°C, it shows an increase only at day 7 (
Figure 2C). MiRNA-199a-3p in platelet-rich plasma stored at RT demonstrates a statistically significant increase from day 2, whereas samples stored at 4°C showed a statistically significant increase at days 3 and 7 (
Figure 2E). The increase in the levels of miRNA-423-5p and miRNA-199a-3p in whole blood is likely due to the release of extracellular vesicles from blood cells, which carry a diverse population of miRNAs.
The increase in miRNA-423-5p and miRNA-199a-3p concentrations in platelet-rich plasma can be attributed to the fact that both miRNA-423-5p and miRNA-199a-3p are among the 20 most abundant miRNAs in platelets [
33]. Consequently, the observed increase may result from the release of exosomes from platelets during storage. Additionally, dead cells, apoptotic bodies, and other cellular debris may also release miRNAs into plasma during storage. Therefore, we recommend using platelet-free plasma for measuring circulating cell-free miRNA, achieved through high-speed centrifugation (at 16,000 g for 10 minutes) to remove most platelets, apoptotic bodies, dead cells, and other cellular debris.
Our study design examines the effects of storage at days 1, 2, 3, and 7. Notably, miRNA-451a concentration in plasma significantly decreases at day 1 (
Figure 2A). To investigate short-term storage effects, we conducted another study with whole blood and platelet-free plasma stored at RT. Blood and plasma samples were stored at RT. In whole blood, miRNA-451a level remains stable for 72 hours showing a trend towards significantly increasing (
Figure 3B). However, in platelet-free plasma, a decline in miRNA-451a levels is observed becoming significant at the 24-hour mark (
Figure 3A). These findings underscore the importance of promptly processing plasma samples for miRNA-451a detection.
Since miRNA-451a proves unstable at room temperature and 4°C, we explored the stability of platelet-free plasma at -20°C and -80°C over a 7 day period. Our data shows that miRNA-451a concentration did not decrease at both storage temperatures for up to 7 days (Fig. 4).
5. Conclusions
In conclusion, our study underscores the need for precautions when using miRNA-451a as a biomarker in diagnostic assays. A common perception is that miRNAs are quite stable especially due to secondary structure and interactions with their targeting complex [
34], but here we observe that different pre-analytical analysis factors will yield different miRNA-451a expression levels. Careful monitoring and control of the preanalytical phase are essential during blood collection and processing to prevent hemolysis, which can release miRNA-451a into plasma and compromise assay validity. To eliminate platelets and other cell debris, the recommended approach is the two-step centrifugation protocol previously described [
24]. Additionally, we emphasize the significance of immediate processing of blood samples to obtain plasma and extract RNA. When plasma storage is necessary, particularly for batch processing, it is advisable to store platelet-free plasma at either -20°C or -80°C. Importantly, blood should never be stored at these temperatures, as freezing and thawing can cause hemolysis, leading to increased miRNA-451a concentration in plasma. To maintain the integrity of plasma samples, it is recommended that the latter are devoid of platelets, as the presence of platelets during freezing and thawing may lead to the release of platelet contents and an increase in miRNA-451a concentration.
Author Contributions
MRF: Conceptualization, project supervision, funding acquisition, and writing of original draft. DSC: Methodology, investigation, and review & editing of manuscript. WT: Methodology, software, visualization, validation, formal analysis, and review & editing of manuscript. CJ: Methodology, investigation, and review & editing of manuscript. GK: Methodology, investigation, and review & editing of manuscript. NF: Methodology, investigation, and review & editing of manuscript. AO: Methodology, investigation, and review & editing of manuscript. All the authors have read and approved the final version of the manuscript.
Funding
This research was funded by a Ryan Foundation, grant to MRF.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Boys Town National Research Hospital, Omaha NE, USA (IRB protocol number: # 20-14-XP: Approval date: September 28th, 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article are available from the authors without reservation.
Acknowledgments
We wish to thank Dr. Dominic Cosgrove, Center for Sensory Neuroscience, Boys Town National Research Hospital, Omaha NE USA for his advice and help during this project. We gratefully acknowledge the assistance from Rebecca Cash for IRB application. We want to thank Dan Meehan, Center for Sensory Neuroscience for his help. This research was funded by a research grant from the Ryan Foundation to MRF.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Salvianti F, Gelmini S, Costanza F, Mancini I, Sonnati G, Simi L, Pazzagli M, Pinzani P. The pre-analytical phase of the liquid biopsy. N Biotechnol. 2020 Mar 25;55:19-29. [CrossRef] [PubMed]
- Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006 Oct;11(4):441-50. [CrossRef] [PubMed]
- Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008 Mar;9(3):219-30. [CrossRef] [PubMed]
- Cai Y, Yu X, Hu S, Yu J. A brief review on the mechanisms of miRNA regulation. Genomics Proteomics Bioinformatics. 2009 Dec;7(4):147-54. [CrossRef] [PubMed]
- Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther. 2016 Jan 28;1:15004. [CrossRef] [PubMed]
- Peters LJF, Floege J, Biessen EAL, Jankowski J, van der Vorst EPC. MicroRNAs in Chronic Kidney Disease: Four Candidates for Clinical Application. Int J Mol Sci. 2020 Sep 7;21(18):6547. [CrossRef] [PubMed]
- Schepici G, Cavalli E, Bramanti P, Mazzon E. Autism Spectrum Disorder and miRNA: An Overview of Experimental Models. Brain Sci. 2019 Oct 3;9(10):265. [CrossRef] [PubMed]
- Li J, Smyth P, Flavin R, Cahill S, Denning K, Aherne S, Guenther SM, O’O'Leary JJ, Sheils O. Comparison of miRNA expression patterns using total RNA extracted from matched samples of formalin-fixed paraffin-embedded (FFPE) cells and snap frozen cells. BMC Biotechnol. 2007 Jun 29;7:36. [CrossRef] [PubMed]
- Xi Y, Nakajima G, Gavin E, Morris CG, Kudo K, Hayashi K, Ju J. Systematic analysis of microRNA expression of RNA extracted from fresh frozen and formalin-fixed paraffin-embedded samples. RNA. 2007 Oct;13(10):1668-74. [CrossRef] [PubMed]
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’O'Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008 Jul 29;105(30):10513-8. [CrossRef] [PubMed]
- Kim SH, MacIntyre DA, Sykes L, Arianoglou M, Bennett PR, Terzidou V. Whole Blood Holding Time Prior to Plasma Processing Alters microRNA Expression Profile. Front Genet. 2022 Jan 14;12:818334. [CrossRef] [PubMed]
- Li Z, Li Y, Fu J, Li N, Shen L. Clinical utility of microRNA-451 as diagnostic biomarker for human cancers. Biosci Rep. 2019 Jan 15;39(1):BSR20180653. [CrossRef] [PubMed]
- Ahmed HM, Georgy DB, Meabed MH, Abd El Kareem RM, Botrous OE. The Association Between Plasma MicroRNA-451 Expression Levels and Chronic Kidney Disease in Children with β-Thalassemia Major. Iran J Kidney Dis. 2022 May;16(3):188-194. [PubMed]
- Abdelsalam M, Wahab AM, El Sayed Zaki M, Motawea M. MicroRNA-451 as an Early Predictor of Chronic Kidney Disease in Diabetic Nephropathy. Int J Nephrol. 2020 Aug 14;2020:8075376. [CrossRef] [PubMed]
- Garrido-Torres N, Guzmán-Torres K, García-Cerro S, Pinilla Bermúdez G, Cruz-Baquero C, Ochoa H, García-González D, Canal-Rivero M, Crespo-Facorro B, Ruiz-Veguilla M. miRNAs as biomarkers of autism spectrum disorder: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry. 2023 Feb 3. [CrossRef] [PubMed]
- Xie Z, Chen G, Zhang X, Li D, Huang J, Yang C, Zhang P, Qin Y, Duan Y, Gong B, Li Z. Salivary microRNAs as promising biomarkers for detection of esophageal cancer. PLoS One. 2013;8(4):e57502. [CrossRef] [PubMed]
- Konishi H, Ichikawa D, Komatsu S, Shiozaki A, Tsujiura M, Takeshita H, Morimura R, Nagata H, Arita T, Kawaguchi T, Hirashima S, Fujiwara H, Okamoto K, Otsuji E. Detection of gastric cancer-associated microRNAs on microRNA microarray comparing pre- and post-operative plasma. Br J Cancer. 2012 Feb 14;106(4):740-7. [CrossRef] [PubMed]
- Redova M, Poprach A, Nekvindova J, Iliev R, Radova L, Lakomy R, Svoboda M, Vyzula R, Slaby O. Circulating miR-378 and miR-451 in serum are potential biomarkers for renal cell carcinoma. J Transl Med. 2012 Mar 22;10:55. [CrossRef] [PubMed]
- Li HP, Zeng XC, Zhang B, Long JT, Zhou B, Tan GS, Zeng WX, Chen W, Yang JY. miR-451 inhibits cell proliferation in human hepatocellular carcinoma through direct suppression of IKK-β. Carcinogenesis. 2013 Nov;34(11):2443-51. [CrossRef] [PubMed]
- Ng EK, Chong WW, Jin H, Lam EK, Shin VY, Yu J, Poon TC, Ng SS, Sung JJ. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut. 2009 Oct;58(10):1375-81. [CrossRef] [PubMed]
- Luo J, Zhao Q, Zhang W, Zhang Z, Gao J, Zhang C, Li Y, Tian Y. A novel panel of microRNAs provides a sensitive and specific tool for the diagnosis of breast cancer. Mol Med Rep. 2014 Aug;10(2):785-91. [CrossRef] [PubMed]
- Bai H, Wu S. miR-451: A Novel Biomarker and Potential Therapeutic Target for Cancer. Onco Targets Ther. 2019 Dec 16;12:11069-11082. [CrossRef] [PubMed]
- Plebani, M. Errors in clinical laboratories or errors in laboratory medicine? Clin Chem Lab Med. 2006;44(6):750-9. [CrossRef] [PubMed]
- Chiu RW, Poon LL, Lau TK, Leung TN, Wong EM, Lo YM. Effects of blood-processing protocols on fetal and total DNA quantification in maternal plasma. Clin Chem. 2001 Sep;47(9):1607-13. [PubMed]
- Campomenosi P, Gini E, Noonan DM, Poli A, D’D'Antona P, Rotolo N, Dominioni L, Imperatori A. A comparison between quantitative PCR and droplet digital PCR technologies for circulating microRNA quantification in human lung cancer. BMC Biotechnol. 2016 Aug 18;16(1):60. [CrossRef] [PubMed]
- R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
- H. Wickham. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York, 2016.
- Alboukadel Kassambara (2021). rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R package version 0.7.0. https://CRAN.R-project.
- Guo Y, Zhou X, Gao F, Wang M, Yang Q, Li X, Liu Z, Luo A. MiR-423-5p is a novel endogenous control for the quantification of circulating miRNAs in human esophageal squamous cell carcinoma. Heliyon. 2023 Mar 25;9(4):e14515. [CrossRef] [PubMed]
- Chai C, Song LJ, Yang B, Han SY, Li XQ, Li M. Circulating miR-199a-3p in plasma and its potential diagnostic and prognostic value in glioma. Eur Rev Med Pharmacol Sci. 2016 Dec;20(23):4885-4890. [PubMed]
- Teruel-Montoya R, Kong X, Abraham S, Ma L, Kunapuli SP, Holinstat M, Shaw CA, McKenzie SE, Edelstein LC, Bray PF. MicroRNA expression differences in human hematopoietic cell lineages enable regulated transgene expression. PLoS One. 2014 Jul 16;9(7):e102259. [CrossRef] [PubMed]
- Thangaraju K, Neerukonda SN, Katneni U, Buehler PW. Extracellular Vesicles from Red Blood Cells and Their Evolving Roles in Health, Coagulopathy and Therapy. Int J Mol Sci. 2020 Dec 25;22(1):153. [CrossRef] [PubMed]
- Leng Q, Ding J, Dai M, Liu L, Fang Q, Wang DW, Wu L, Wang Y. Insights Into Platelet-Derived MicroRNAs in Cardiovascular and Oncologic Diseases: Potential Predictor and Therapeutic Target. Front Cardiovasc Med. 2022 Jun 9;9:879351. [CrossRef] [PubMed]
- Pogue AI, Hill JM, Lukiw WJ. MicroRNA (miRNA): sequence and stability, viroid-like properties, and disease association in the CNS. Brain Res. 2014 Oct 10;1584:73-9. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).