1. Introduction
Nowadays, chronic diseases are constantly increasing, causing 71 % of deaths worldwide [
1]. Although the pharmaceutical industry is trying to satisfy the needs in the treatment of diseases, people are becoming more aware of the importance of consuming functional foods for the prevention of diseases. The term "functional foods" refers to novel foods formulated to contain substances (dietary fiber, phytochemicals, probiotics, etc.) that may have health-promoting value, in concentrations that are both safe and sufficiently high to provide the intended benefits [
2]. In addition to fruits and vegetables as the best-known source of functional ingredients, there has been an increasing trend in recent years to use waste streams from the food industry. The food industry generates large amounts of by-products or waste that can be used in a sustainable way to produce a bio-based product, including functional products that meet ESG (economic, social, and environmental) criteria. The use of by-products to develop new high-value functional products contributes to the growing trend to move from a linear to a circular bioeconomy, which means a transition from an unsustainable "take-make-use-dispose" approach to a sustainable 4R approach based on "reduce-reuse-recycle-recover" [
3,
4,
5].
Biscuits offer good potential for the development of new functional formulations, as they are well accepted by consumers worldwide due to their pleasant taste, low price, easy availability, low moisture content, and relatively long shelf life [
6,
7]. Since biscuits are a carb-rich food product, recent research suggests that it is quite possible to obtain a nutritionally high-value functional biscuit by replacing one part of wheat flour with other ingredients originated from various vegetables (pumpkin or carrot powder [
8]) and fruit (banana flour [
9], rosehip powder [
10]) or from waste streams of their processing such as grape pomace [
11], blueberry pomace [
12], apple pomace [
13], olive pomace [
14] or beetroot pomace [
15].
The process of developing new functional products is complex and requires numerous investigations. In the development of functional products such as biscuits, the influence of the functional ingredients on the dough rheological properties of the dough, the technological and physical parameters, the baking tests and the sensory evaluation of the final product must be monitored [
16,
17,
18]. All of these requirements must be met in order for the new functional product to find its way to the customer and for the manufacturer to make the production and marketing of such a product economically viable.
Grape pomace (GP) is the solid residue that remains in large quantities (20-30 % of processed grape or 17 kg per L wine) after the processing of grapes (
Vitis vinifera L.) [
17,
19], one of the most widely grown fruits in the world with an annual production of 75 million tons (period 2012-2021, [
20]). GP offers numerous recycling opportunities but is usually disposed of unplanned in the environment, with negative effects on the ecosystem. Although it has a rich chemical composition, it is most often mentioned in the context of being a natural source of dietary fibers, and phenolic compounds that have antioxidant, antiproliferative, and anti-inflammatory properties [
21,
22,
23]. To date, various studies have shown that different grape pomace extracts have a significant ability to inhibit the proliferation of human lung cancer cells, human breast adenocarcinoma cells, murine melanoma cells [
24], and human colorectal cancer cells [
25] and human hepatocarcinoma cells [
26].
Since phenolic compounds are sensitive to external influences (oxygen, air, light, temperature), their extraction process from the matrix and the form in which they are added to the functional product can influence the properties of the functional product. One of the ways to protect the bioactive phenolic components of grape pomace is encapsulation. In this process, the sensitive core is protected from harmful external influences by a layer of coating material [
21]. The choice of coating material depends on the type of functional ingredient to be protected and the product properties into which the encapsulated beads are to be incorporated [
27]. Some of the commonly used coating materials for the encapsulation of phenolic compounds are gum arabic, maltodextrin [
21], sodium alginate [
28], and chitosan [
29] .
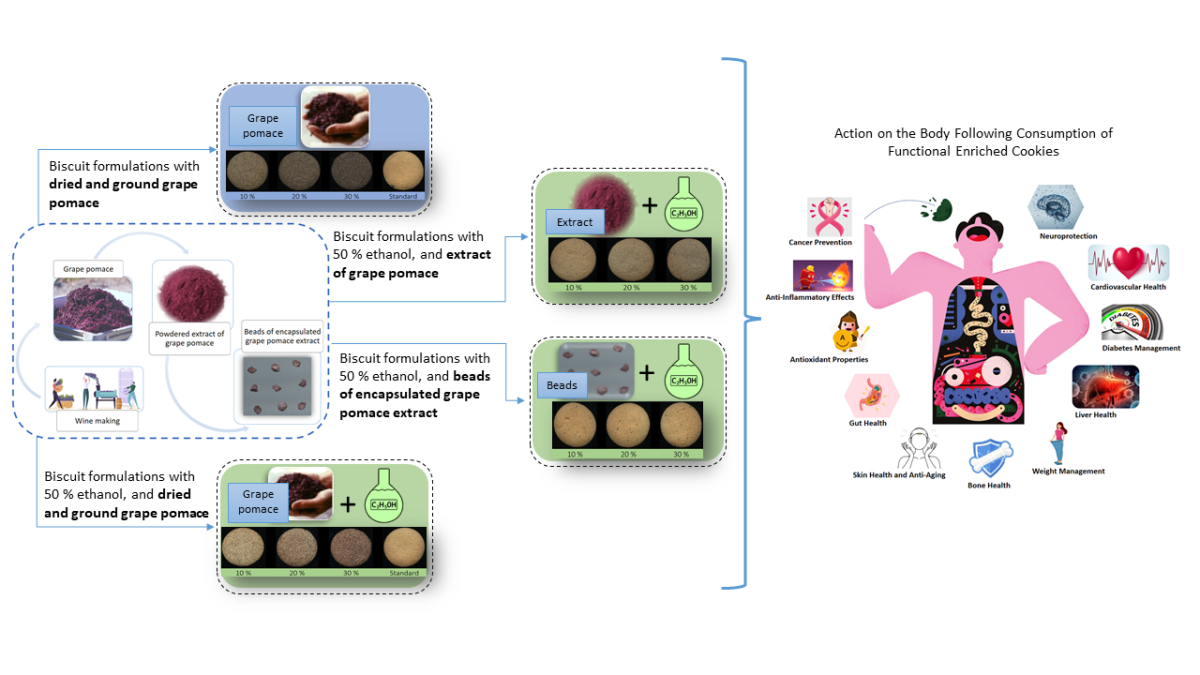
The aim of this study was to prepare a functional biscuit with phenolic compounds derived from a winery by-product and added in various forms, such as grape pomace (GP), grape pomace extract (GPE) and encapsulated grape pomace extract in the form of alginate-based beads (BGPE), and to study the influence of the functional ingredients on the physical, chemical, sensory, textural, antioxidant and antiproliferative properties of the functional biscuit. As far as we know, there are no comparable comprehensive studies comparing the influence of the form of the functional ingredients on the properties of functional biscuits in one place.
2. Materials and Methods
2.1. Chemicals and Reagents
Folin and Ciocalteu’s phenol reagent was purchased from CPA chem (Bogomilovo, Bulgaria); 96% ethanol (p.a.) from Lab Expert (Shenzhen, Guangdong, China). Sodium carbonate anhydrous (p.a.); sodium nitrite; sodium hydroxide; were obtained from Gram Mol (Zagreb, Croatia). Alginic acid sodium salt from brown algae (low viscosity); stand-ard gallic acid monohydrate (98+% A. C. S. reagent); 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ) for spectrophotometric det. of Fe (≥ 98.0%); 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), and ammonium persulfate were purchased from Sigma Aldrich (Saint Louis, USA). Trolox, dimethyl sulfoxide (DMSO) and iron(III)chloride hexahydrate (99+%, for analysis), were purchased from Acros Organics (Thermo Fischer Scientific, USA). From Alfa Aesar GmbH & Co KG (Kandel, Germany) aluminum chloride hexahydrate was obtained, from Carlo Erba Reagents GmbH (Emmendingen, Germany) and 37% hydrochloric acid and from Honeywell (Seelze, Germany). Dulbecco′s Modified Eagle′s Medium (DMEM) containing 2mMglutamine and fetal bovine serum was prepared by GIBCO (EU), whereas trypan blue and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were pur-chased from Sigma Aldrich (MERCK, Darmstadt, Germany). Phosphate-buffered saline (PBS) was purchased from Capricorn Scientific (Ebsdorfergrund, Germany).
2.2. Grape Pomace
Cabernet Sauvignon (Vitis vinifera L.) variety grape pomace (GP) was collected from a local winery (Erdut Winery, Croatia, 2018 harvest). The GP was air-dried (48 h, 25 – 27 °C) to reduce the moisture content from 48.60 % to < 10 % and prevent spoilage, and the dried pomace was stored at room temperature. Before use for preparation biscuits or for extraction, the GP was ground to a particle size of up to 1 mm using an ultracentrifugal mill (Retsch ZM200, Haan, Germany).
2.3. Extraction of Phenolic Compounds from Grape Pomace
The GP was extracted with 50 % aqueous ethanol at a liquid-to-solid ratio of 40 mL/g in laboratory flasks, which were placed in a shaking water bath preheated to 80 °C (Julabo SW 23, Labortechnik GmbH, Seelbach, Germany) and shaken at 200 rpm for 120 minutes. After completion of the extraction, the suspension of sample and solvent was centrifuged at 11,000 x g for 10 minutes (Hermle Z326K, Hermle Labortechnink, Wehngen, Germany). After centrifugation, the supernatant was separated from the precipitate and concentrated to dryness on a rotary evaporator (Büchi, R -210, Flawil, Switzerland) at 50 °C and 48 mbar. The obtained extract was used in two forms for the preparation of functional biscuits, as powder (GPE) and as encapsulated extracts in the form of alginate beads (BGPE).
2.4. Grape Pomace Extract Encapsulation
The GPE (0.66-1.31 g) was dissolved on a magnetic stirrer in 10 mL of 96 % ethanol and, after dissolution, 40 mL of redistilled water was added. After brief stirring, 1.5 g of sodium alginate was added to the solution. The mixture was stirred on a magnetic stirrer for 24 hours, and encapsulation was performed. A syringe (25 mL) with a 20-gauge needle was used for encapsulation. A mixture of sodium alginate and extract was encapsulated in 300 mL of 0.25 mol/L calcium chloride, and the resulting hydrogel allowed to solidify in the calcium chloride solution for 10 minutes. After solidification, the hydrogel beads were filtered on filter paper and washed twice with 200 mL of redistilled water to remove residual calcium ions from the surface of the beads. The washed hydrogel beads were dried at room temperature for 48 hours (BGPE) and used for the preparation of functional biscuits.
2.5. Preparation of Biscuits
The preparation of the functional biscuits is based on the standard AACC interna-tional method 10–50.05 [
31], which contains wheat flour, distilled water, shortening, su-crose, sodium chloride, and sodium bicarbonate. In addition to the control sample (AACC standard formulation), 13 different functional biscuit formulations were prepared such that a specific substitution and/or addition was made to the AACC standard formulation. In the substitution, a certain amount of wheat flour (10 %, 20 %, or 30 %) was replaced by GP and/or distilled water was replaced by 50 % ethanol, while GPE or BGPE were used as additives (
Table 1). GPE and BGPE were added in amounts equivalent to GP replacing 10%, 20%, and 30% of wheat flour, respectively, in terms of TPC content. The replacement of distilled water with 50 % ethanol was made because the dry extract of grape pomace was not readily soluble in water, which would prevent homogeneous distribution of the additive in the dough. To determine whether this change in the formulation with 50 % ethanol had any effect on the tested biscuit properties, a special biscuit sample (eC) was prepared with ethanol but without the addition of GP, GPE or BGPE.
An electronic mixer (Gorenje MMC800W, Slovenia) was used to mix the ingredients into a dough. The firm dough was rolled out to a thickness of 7 mm and cut with a 60 mm round biscuit cutter. The prepared biscuits were baked for 10 minutes at 205 °C in a convection oven (Wiesheu Minimat Zibo, Wiesheu GmbH, Germany). The baked biscuits were cooled to room temperature prior to analysis. The biscuits were made in triplicate batches.
2.6. Two-Stage Extraction Phenolic Compounds from Biscuits
For the first extraction step, crushed biscuits (2 g) were mixed with 20 mL of 70 % DMSO in a Falcon tube. The mixture was subjected to an ultrasonic bath (15 min at 50 °C) in sweep mode (37 kHz) and 60 % power (Elma Elmasonic P 120 H, Elma Schmidbauer GmbH, Germany), followed by centrifugation at 10,000 x g for 10 min. The supernatant was separated and a second extraction was performed with the remaining precipitate. The precipitate was mixed with 20 mL of 70 % DMSO before being immersed again in the ultrasonic bath and then centrifuged under the same conditions as described previously. The supernatants from both extractions were pooled and used for further analysis (phenolic compound content, antioxidant activity, and antiproliferative activity).
2.7. Determination of the Phenolic Compound Content of Biscuits
TPC and total flavonoid content (TF) were determined spectrophotometrically (UV/VIS spectrophotometer UV-1800, Shimadzu, Japan). Results were expressed as the mean of replicates ± standard deviation (SD).
TPC was determined according to the Folin-Ciocalteu micro-method described by Waterhouse [
32] with minor modifications. To 3160 µL of distilled water, 40 µL of sample was added and then mixed with 200 µL of Folin-Ciocalteu reagent. After 8 minutes, 600 µL of sodium carbonate (20 %, w/v) was added and then incubated at 40 °C for 30 minutes. The absorbance of the samples was measured at 765 nm compared to the blank sample and the results were expressed as gallic acid equivalents per dry weight of biscuits (mg
GAE/g
db). The blank sample was prepared with the extraction solvent instead of the sample.
TF was determined according to the aluminum chloride assay described by Marinova et al. [
33] with some modifications. To 2 mL of water and 500 µL of the sample, 150 µL of sodium nitrite (5 %, w/v) was added. After 5 minutes, 150 µL of aluminum chloride (10 %, w/v) was added and after exactly 6 minutes, 1 mL of sodium hydroxide (1 mol/L) was added. Then 1.2 mL of distilled water was added and after shaking the mixture, the absorbance was measured at 510 nm compared to a blank containing water instead of the sample. Results were expressed as (+)-catechin equivalents per dry weight of biscuits (mg
CE/g
db).
2.8. Determination of Antioxidant Activity of Biscuits
The antioxidant activity of biscuits was evaluated spectrophotometrically using FRAP and the ABTS assay described by Martinović et al. [
30]. Trolox (an analogue of vitamin E), which is a potent antioxidant, was used as a positive control. All assays were performed in triplicate, and final results were expressed in Trolox equivalents per dry weight of biscuits (mg
TE/g
db) as the mean of replicates ± standard deviation (SD).
To measure the ferric reducing power (FRAP), the method of Benzie and Strain [
34] was used with minor modifications. 150 µL of the sample was mixed with 270 µL of distilled water and with 2.7 mL of FRAP working reagent, shaken, and incubated at 37 °C for 40 min. The absorbance was measured at 592 nm. The blank sample was prepared in the same way, but distilled water was used instead of extract.
The protocol of Re et al. [
35] was used with minor modifications to determine the antioxidant activity of the sample using the ABTS assay. Briefly, 950 µL of a diluted ABTS•+ radical solution prepared fresh daily was added to 50 µL of the extracts. The mixtures of sample and reagent were allowed to stand in the dark for 10 min and then the absorbance was measured at 734 nm. The control sample was prepared in the same way, but absolute ethanol was used instead of the sample.
2.9. Determination of Antiproliferative Activity of Biscuits
Three human tumor cell lines (Caco-2; SW 620 and Hep G2) were cultured in DMEM medium with the addition of 10 % fetal bovine serum and 2 mM glutamine until they reached 90 % confluence in a humidified atmosphere under the conditions of 37 °C/5% CO2 gas in a CO2 incubator (Shell Lab, Sheldon Manufacturing, USA). The trypan blue exclusion method was used to assess the viability of the cells.
The biscuit extracts were filtered through a 0.45 μm syringe filter (Sarsted, Germany) before use. Working concentrations (50 mg/mL; 5 mg/mL; 0.5 mg/mL) were prepared in DMEM medium before treatment. The antiproliferative effect of the biscuit extracts was determined using the MTT assay (cell viability test) [
36]. Cells were seeded in 96-well flat-bottomed plates (Greiner, Frickenhausen, Austria) at a concentration of 2 × 40
4 cells/mL and left overnight in the CO
2 incubator to attach to the plate surface. 72 hours after addition of the samples, the growth medium was discarded and 5 mg/mL MTT was added. After 4 hours of incubation at 37 °C, the water-insoluble MTT formazan crystals were dissolved in DMSO. Absorbance was measured at 595 nm using an Elisa microplate reader (iMark, BIO RAD, Hercules, CA, USA). Control cells were grown under the same conditions.
2.10. Determination of Physical and Textural Characteristics of Biscuits
Biscuits were characterized on physical (width, thickness, spread factor, color) and textural characteristic (snapping force, breaking distance, bending force index).
The dimensions of the biscuits, width (W) and thickness (T), were determined using the instructions of given in the AACC international method 10 50.05 (AACC, 2010). The spread factor was calculated as W/T multiplied by 10. Six biscuits from each batch were measured. The color of the biscuit samples was measured at 6 different measuring points on the sample surface. It is specified with the colorimeter Chroma Meter CR-400 (Konica Minolta, Tokyo, Japan) and expressed in CIE L* a* b* system.
The TA.XT2i Texture Analyzer (Stable Microsystems Ltd., Surrey, UK) was used to perform the three-point bend/break test. The distance between two lower supports was 40 mm, and the test speed of the knife blade was 1 mm/s. The snapping force (N) and the breaking distance (mm) were obtained from the test curve. By dividing these two parameters, the bending force index (N/mm) was calculated. This index served as an indicator of the hardness/softness of the biscuits [
37].
2.11. Determination of Sensory Characteristics of Biscuits
Sensory properties were evaluated according to the method of Yamsaengsung et al. [
38] by members of a trained sensory team from the Faculty of Food Technology Osijek, Croatia. The evaluation team assessed the taste, odour, colour, texture (in the mouth), and overall sensory impression of 14 different biscuit samples. The 9-point hedonic scale was used to assess sensory properties. Scores from 1 to 9 were: extremely dislike (1), very much dislike (2), moderately dislike (3), slightly dislike (4), neither dislike nor like (5), slightly like (6), moderately like (7), very much like (8), and extremely like (9). The evaluated biscuit samples can be seen in Figure 6.
2.12. Statistical analysis
Statistica 14.0.015 (TIBCO Software Inc., Tulsa, USA) was used for statistical analysis of the results. All results were expressed as mean values. To test the significance level of the difference between the means of the samples representing the population (phenolic compounds, antioxidant activities, sensory parameters, physical and textural parameters of biscuits), a one-way analysis of variance (ANOVA) was performed. After statistically significant differences were found, an additional post-hoc test (Duncan´s test for multiple ranges) was performed to determine the specific populations that showed significant differences (p < 0.05). Samples belonging to the same population were labelled with the same letter of the alphabet in the figures or tables. Nonparametric Mann-Whitney U-test were used to compare MTT data. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Content of Total Phenolic Compounds and Total Flavonoid in Biscuits
The reference formulation of the AACC biscuit (standard, i.e., control sample) [
31] served as the basis for the preparation of the functional biscuit and was enriched with functional ingredients, which were essentially solid wastes from wine production. The functional ingredient consisted of ground grape pomace (GP), grape pomace extract (GPE) or encapsulated grape pomace extract with sodium alginate in the form of beads (BGPE) were used. The preparation of each functional ingredient is described in subsections 2.2 – 2.4.
The chemical composition of the GP used, including the total phenolic compound (TPC) content (53.55 mg
GAE/g
db) is known from our previous study [
30] and provides an important starting point for adding a known content of TPC to each functional biscuit formulation.
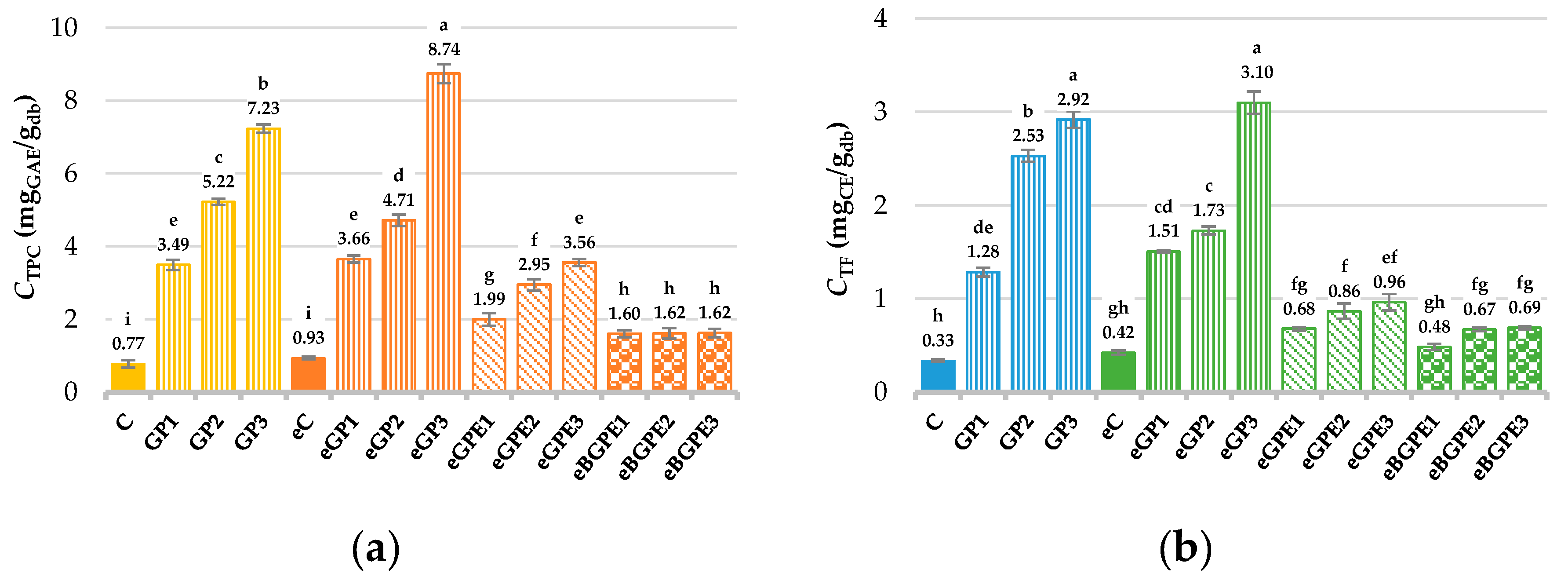
After baking and grinding, the TPC and total flavonoid content (TF) was determined for all biscuit samples, and the results are shown in
Figure 1.
The TPC content in the biscuits varied from 0.77 mg
GAE/g
db (control sample, C) to 8.74 mg
GAE/g
db (eGP3) (
Figure 1a). An increase in TPC content was observed with increasing addition of all tested functional ingredients, being least pronounced with the addition of BGPE. This observation is consistent with the results of previous studies [
17,
23,
39], which only investigated the effects of different percentages of GP on the TPC content of functional biscuits. Their TPC values were lower (< 4.03 mg
GAE/g) than those obtained in this work due to differences in the GP varieties used, the percentage of GP in biscuits, and the extraction process for TPC, among other factors. The TPC content of standard control biscuit prepared with water (C) and modified sample prepared with 50 % ethanol (eC) was generally not statistically significant different. Biscuits prepared with the addition of BGPE had the lowest TPC content among all prepared functional biscuits and were statistically significant (
p < 0.05) different from the other samples. This is probably a consequence of the different distribution of BGPE in the biscuit compared to the corresponding additions of GP and GPE and the fact that TPC is protected by the coating used, which affects the low availability of the phenolic compounds to solvent during extraction.
On the other hand, biscuits prepared with GP and GPE addition showed more homogeneous distribution in the biscuits, due to the larger proportion (especially in the case of GP) in the biscuit dough, which allowed easier extractability of TPC from GPE and GP biscuits. The content of GP- and GPE-enriched biscuits were statistically significantly different (p < 0.05) from the control biscuits and eBGPE biscuits, but in addition, a statistically significant difference (p < 0.05) was observed within each observed data group as well as between groups, with the exception of samples GP1, eGP1, and eGPE3, which were not statistically significantly different in detected TPC content.
The total flavonoid content (TF) (Figure 1b) of the biscuits ranged from 0.33 mgCE/gdb (C) to 3.10 mgCE/gdb (eGP3) with similar trends for all samples studied as the TPC, which was confirmed by the calculated high correlation (R > 0.97) between them. Briefly, the highest yield of TF was observed in biscuits with the addition of GP regardless of the water or 50 % ethanol used for the dough mixture, followed by biscuits containing GPE and BGPE biscuits.
3.2. Antioxidant Activity of Biscuits
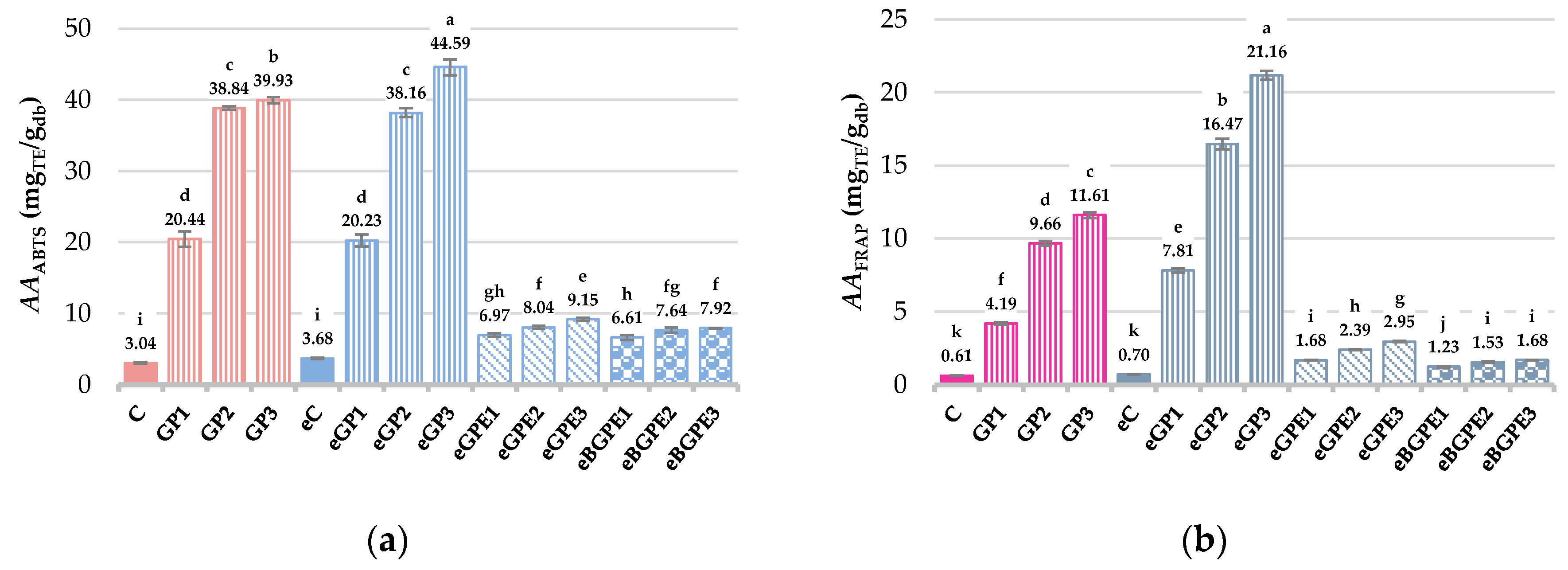
The antioxidant activity (AA) of biscuits was measured by two methods to allow a more accurate interpretation of the results. According to the ABTS method (
Figure 2a), AA ranged from 3.04 mg
TE/g
db to 44.59 mg
TE/g
db, with the highest value obtained for sample eGP3, which was statistically significantly different (
p < 0.05) from the other samples. Lower values (0.61 – 21.16 mg
TE/g
db) of AA were obtained when the method FRAP (
Figure 2b) was used, but the high positive correlation (
R > 0.93) was found between the methods of
AA determination.
In overall, AA was the highest for biscuits prepared with GP regardless on C or eC biscuits, followed with samples enriched with GPE and finally with BGPE. The high positive correlation (R = 0.89 – 0.96) between AA and TPC and TF, regardless of the antioxidant assay used, proves that the above compounds contribute to the total AA of the biscuits.
3.3. Antiproliferative Activity of Biscuits
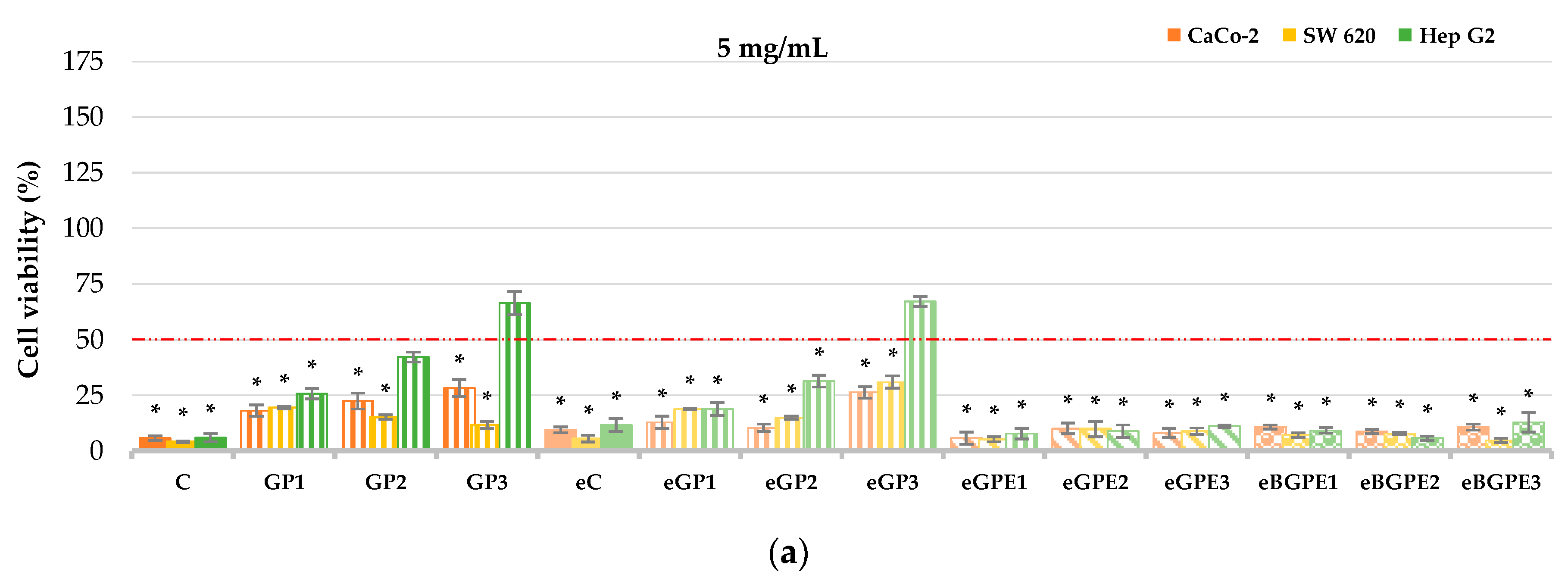
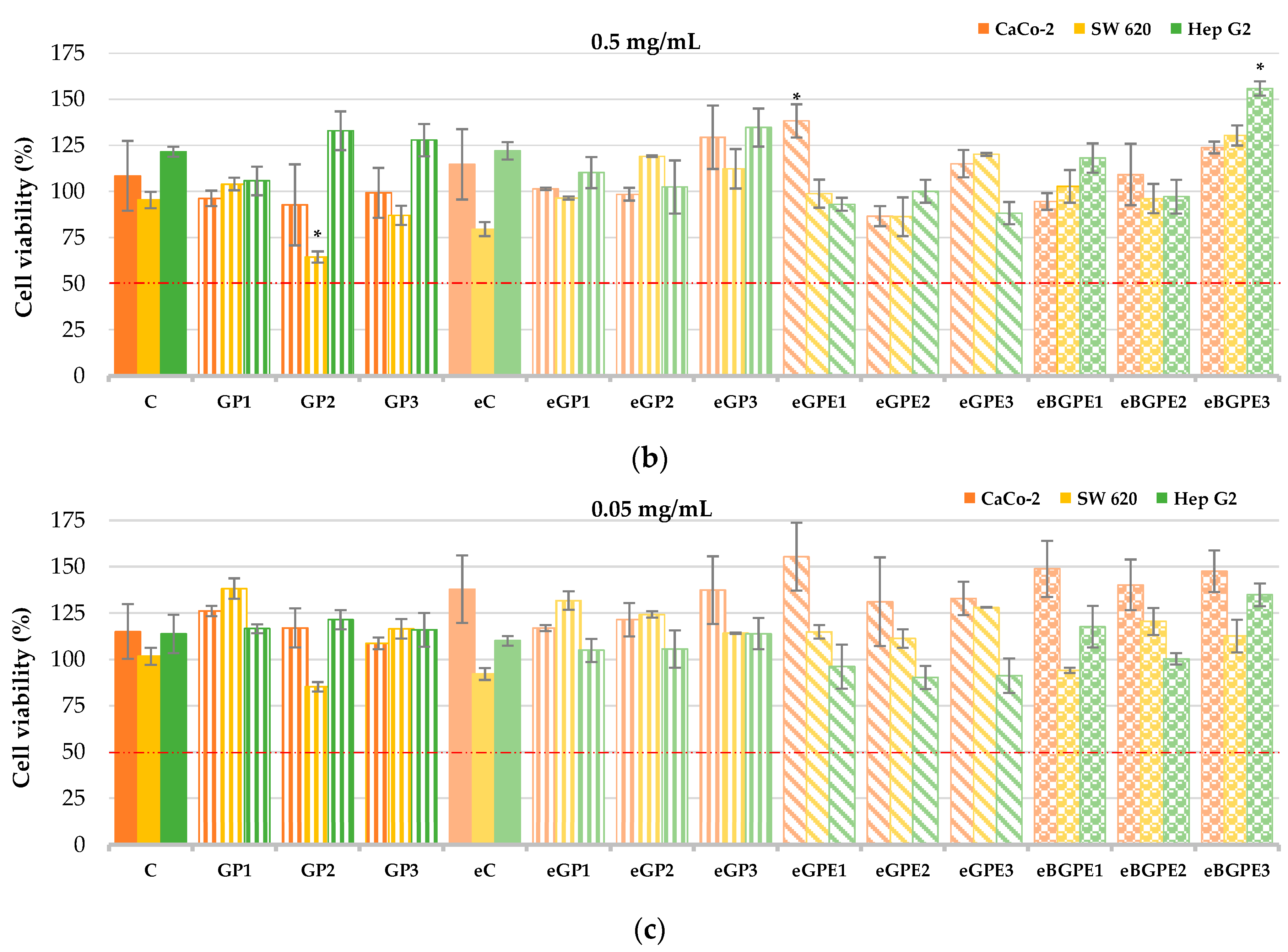
It is known that phenolic compounds have antiproliferative activity, and our previous work demonstrated that phenol-rich grape pomace extract from Cabernet Sauvignon affects the reduction of cell viability of Caco-2 and SW 620 cancer cell lines [
40]. In the present study, the human liver cell line HepG2 and the colon cell lines Caco-2 and SW620 were used to evaluate cell viability in the presence of grape pomace-enriched biscuits (
Figure 3). Among three evaluated concentrations of biscuit extracts (5.0; 0.5; 0.05 mg/mL) the most effective was 5.0 mg/mL reducing the cell viability of SW620 cells from 69.07 % to 95.82 % (
p < 0.05), Caco-2 cells from 71.79 % to 94.24 % (
p < 0.05), and Hep G2 cells from 32.77 % to 94.01 % (
p < 0.05).
The highest cell viability was observed in Hep G2 cells when the eGP3 and GP3 samples were tested with a concentration of biscuit extract of 5.0 mg/mL (
Figure 3a), and was 66.49 % and 67.23 %, respectively. These samples had no statistically significant (
p < 0.05) effect on the reduction of Hep G2 cells in contrast to all other samples tested. Generally, addition of GPE (eGPE1 - eGPE3) and BGPE (eBGPE1 - eBGPE3) in biscuit formulation indicate smaller viability of the tested cells than biscuits enrichment with GP.
At a biscuit extract concentration of 0.5 mg/mL, a statistically significant (
p < 0.05) reduction in cell viability of SW 620 (for 35.54 %) was observed in the GP2 sample compared with the control. Conversely, eGPE1 and eBGPE3 samples showed a statistically significant (
p < 0.05) increase in cell viability for Caco-2 and Hep G2, respectively, compared to control cells (
Figure 3b). At a biscuit extract concentration of 0.05 mg/mL, none of the samples tested had a statistically significant effect on cell viability compared to the control (
Figure 3c).
Fernandez et al. [
41] and all have shown that the viability of Hep G2 cells was affected by grape skin extracts but not by grape seed extracts, suggesting that the bioactivity of the grape pomace extract may be derive from the compounds in the skin. Our results regarding the sensitivity of Caco-2 cells in the presence of GP, which is rich in flavonoids and phenolic acids, are consistent with those previously reported by Oliviera et al. [
42]. Similar results were reported by Perez et al. [
25] on the potent antiproliferative effect of GP on Caco-2 and HT-29 cancer cell lines which is in agreement with our results on Caco-2 and SW620 cells. The biological activity of GP and GPE is obviously present in the enriched biscuits and more effective in the colorectal cell lines (Caco-2, SW620) than in the hepatocellular cell line (HepG2). Another study conducted by Caponio et al. [
43] reported that the effects of GP on Caco-2 cell proliferation is a dose-dependent action with a significant cell proliferation inhibition effect at concentrations of 25, 50, and 100 μg/mL of grape pomace. All reported studies are conducted on the elementary grape pomace or grape pomace extracts not as GP incorporated in complex food matrix.
Overall, the antiproliferative activities of the biscuit extracts were strongly negatively correlated with TPC (
R = -(0.81 – 0.95)) and with AA (
R = (-(0.71 – 0.92)). Thus, the antiproliferative activities of the extracts cannot be explained by the total phenolic content of the biscuits tested and the results suggest that specific individual phenolic compounds or other bioactive substances in GPE and BGPE may be responsible for the antiproliferative activities of the biscuits. Therefore, identification of individual phenolic or other phytochemicals will be included in further studies. Similar results on the relationship between polyphenols, antioxidant and antiproliferative activities on Hep G2 cells were published by Sun et al. [
44] when they investigated the bioactivity of different fruit extracts.
3.4. Physical and Textural Characteristics
From the results presented above and taking into account the tested biological activity, it is evident that the addition of GP, BGPE and BGPE in standard biscuits results in functional biscuits. However, the formulation of a functional biscuit must meet certain technological and sensory properties to be accepted by consumers. Therefore, the physicochemical properties, texture, and sensory characteristics of the biscuits were determined.
3.4.1. Dimensions of Biscuits
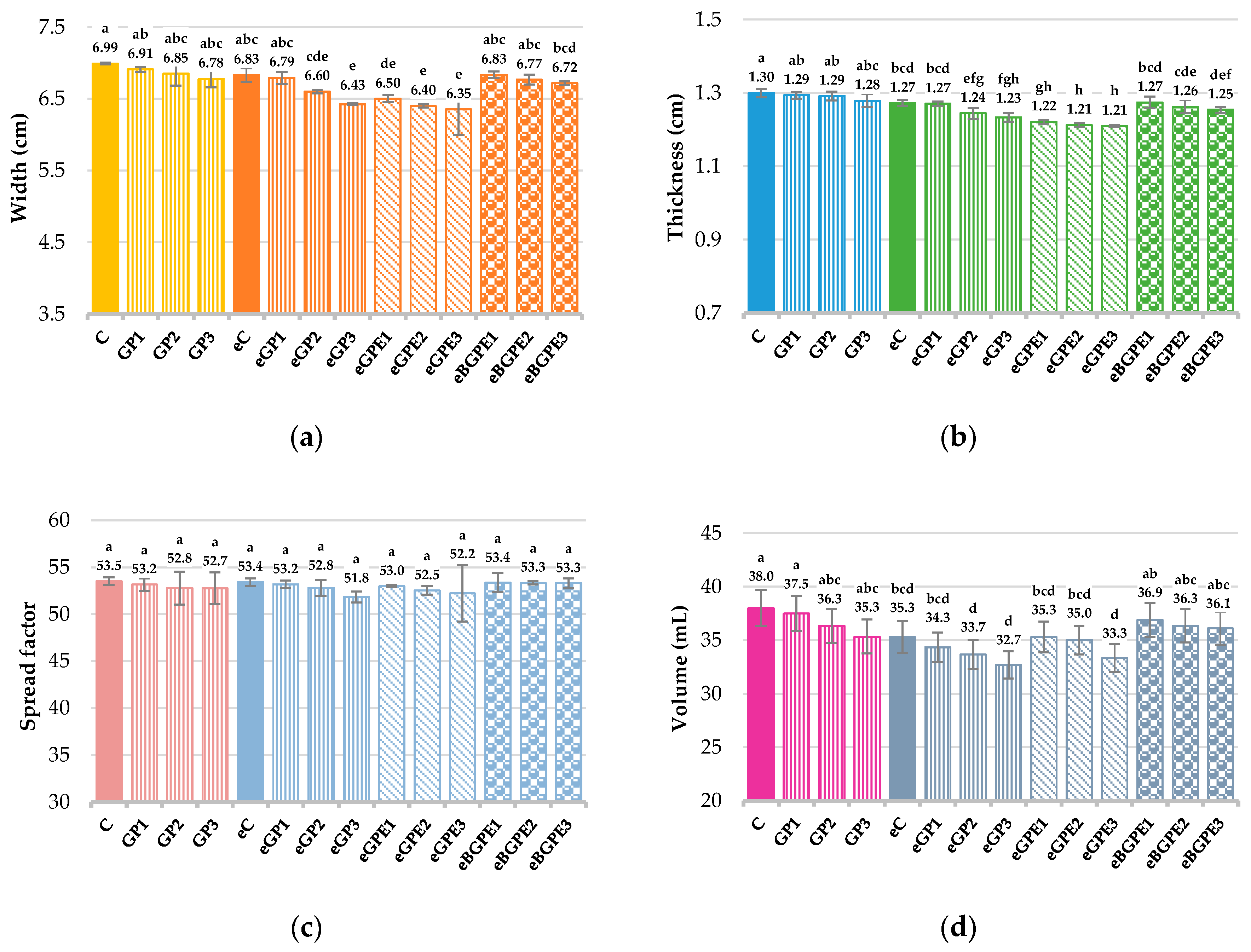
Figure 4 shows the effects of the addition GP, GPE or BGPE and ethanol on the dimensions (width and thickness), spread factor and volume of the biscuits.
When comparing the width of the two control biscuits (C and eC), the width of biscuit C prepared with water was slightly higher than the width of biscuit eC where ethanol was used instead of water. Increasing the ingredients content in all samples with GP, GPE or BGPE decreases the width, thickness and spread factor of the biscuits compared to the control sample (C), but no significant difference was found between the control sample (C) and the samples GP1-GP3 (
Figure 4a – c). For the biscuits with ethanol, a statistically significant difference (
p < 0.05) in the width and thickness of the samples is only observed when adding 30 % GP for width (width decreased from 6.83 cm for eC to 6.43 cm for eGP3) and when adding ≥ 20% GP for thickness of the biscuits (thickness decreased from 1.27 for eC to 1.24 for eGP2, i.e. to 1.23 for eGP3) as shown in
Figure 4a and 4b. Acun and Gül [
45] reached a similar conclusion in their study on the effects of whole grape pomace flour on biscuit quality and found no significant difference between the control sample and the samples with an addition of up to 15% whole GP flour in the determination of width, thickness and spread factor. In the same paper, the authors showed that the addition of pomace flour without seeds does not change the width and spread factor, but had a statistically significant effect on the reduction of thickness. In agreement with our results are also the investigations of Molnar et al. [
46], who optimised a wholemeal biscuit formulation with grape and aronia pomace as a partial substitute for cocoa powder and found that the biscuit thickness and spread factor were not significantly affected by the composition of the mixture. However, Molnar et al. [
46] found that grape and aronia pomace had a significant negative effect on biscuit width.
The biscuit volume is highest for control sample C and decreases when the content of additives GP, GPE or BGPE is increased (
Figure 4d). Although the volume is 7.1% lower for sample GP3 and 5 % lower for eBGPE3, the reduction was not statistically significant for samples GP1 – GP3 and eBGPE1 – eBGPE3 compared to control sample C. The reduction in volume was also not significant for the eGP1 – eGP3, eGPE1 – eGPE3 and eBGPE1 - eBGPE3 formulations compared to the eC biscuit (the reduction was up to 7.36%). Kuchtová et al. [
47], who studied the effect of incorporation of red grape skins and red grape seeds on the rheological properties of wheat dough and on the qualitative parameters of biscuits, reported a significant decrease in the volume of biscuits with an increase in the content of functional additives. A significant decrease in biscuit volume with an increase in apple pomace powder was found by Kohajdová et al. [
48], who followed the addition of high-fibre powders from two apple varieties on the farinographic properties of wheat dough and biscuit quality.
The composition, type, and amount of functional ingredients, as well as all other raw materials used in the production of biscuits, have a decisive influence on the differences in the results of studies on the influence of grape pomace and other additives on the dimensions of biscuits (width, thickness, and volume) [
46,
49]. The decrease in biscuit width and thickness is probably due to the higher viscosity of the dough in the samples with added functional ingredients, and similarly Mancebo et al. [
50] concluded in a study on the influence of different flours on the quality of gluten-free sugar biscuits. In addition, the samples with the addition of GP have a higher fibre content and thus a higher water retention capacity. In these samples, less water is available to dissolve the sugar, which is an important factor for the expansion of the biscuits during baking [
51]. A similar influence could be assumed for samples with added ethanol. Ethanol has a lower boiling point than water and first evaporates, so can be assumed that the amount of liquid available for dissolving the sugar is reduced. In this case, ethanol affects the expansion of the biscuits and could also explain the deteriorated textural properties of the samples with added ethanol.
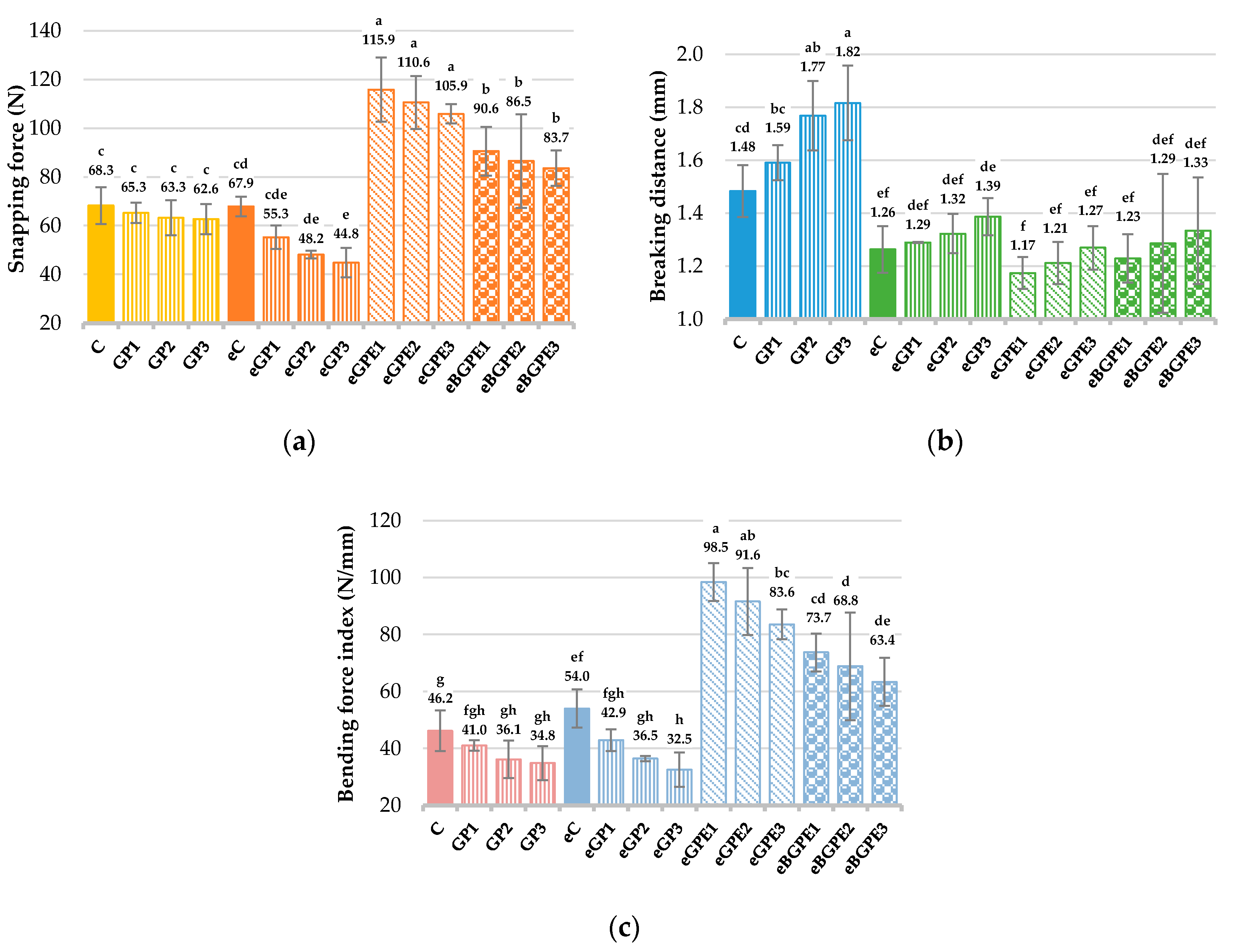
3.4.2. Texture of Biscuits
The determination of the textural properties of biscuits prepared without and with functional ingredients (GP, GPE and BGPE) was performed using the three-point bend/break test and the results are shown in
Figure 5.
It was found (
Figure 5a) that with increasing addition of GP the snapping force decreases (from 68.3 N for the control sample C to 44.8 N for the sample eGP3 prepared with addition of 30 % GP and ethanol). The braking distance is significantly lower for all samples with ethanol addition (
Figure 5b). From the bending force index results (
Figure 5c), addition of GP softens the texture of the biscuits, and increasing the content of GP results in biscuits with a softer, crumbly texture and increased graininess. Biscuits prepared with GPE (eGPE1 – eGPE3) or BGPE (eBGPE1 – eBGPE3) were significantly the hardest, as shown by the value of the bending force index (
Figure 5c), which increases significantly (
p < 0.05) from 46.2 N/mm in the control sample C to almost double the value for biscuits enriched with GPE (83.6 – 98.5 N/mm) or by about 50 % for samples enriched with BGPE (63.4 – 73.7 N/mm). It can be assumed that the reason for this is the easily evaporating ethanol, which has a negative effect on the sucrose-water matrix and thus on the texture of the biscuit. Davidov-Pardo et al. [
52] studied biscuits made with bulk and microencapsulated grape seed extract. Their results are the opposite of those in this paper. They reported that the hardness of the biscuits was not significantly different, with the addition of grape seed extract slightly decreased the hardness of the biscuits. However, Davidov-Pardo et al. [
52] used water, whereas in this paper 50 % ethanol was used to prepare the dough with GPE and BGPE. The decrease in hardness due to the addition of GP was found by Theagarajan et al. [
23], who used GP (cv. Muscat) for the development of GP biscuits, as a functional snack. Kuchtová et al. [
47] observed lower fracturability and hardness of biscuits with different content of grape skins and grape seeds. Replacing wheat flour with GP, grape seeds or grape skins leads to a decrease in gluten content in the biscuit dough, slows down the formation of gluten matrices and results in a decrease in hardness [
47,
53].
3.4.3. Color of Biscuits
The color of the biscuits is not only influenced by the amount of sugar, the temperature and the baking time, but also by the content of GP used and the amount of pigments it contains. The colour of the different biscuits determined by the CIELab coordinates is shown in
Table 2.
From the results it can be seen that an increase in the content of GP results in a significant decrease (p < 0.05) in all three color parameters: lightness L* (from 67.61 for sample C to 36.32 for GP3 sample and from 66.68 for sample eC to 36.66 for sample eGP3), redness a* (from 6.23 for sample C to 4.91 for GP3sample and from 6.42 for sample eC to 4.73 for sample eGP3) and the colour parameter for yellowness b* (from 33.99 for C to 9.40 for S GP3 and from 33.64 for eC sample to 8.93 for the eGP3 biscuit sample).
Kuchtová et al. [
47] also reported a decrease in lightness and yellowness in biscuits with grape skins and seeds, while the color parameter a* decreased for grape skins and increased for grape seeds compared to the control sample. The results of Acun and Gül [
45] are similar, according to which the biscuits became darker and their colour parameter b* decreased with an increase in the content of whole grape pomace flour, pomace flour without seeds and seed flour, while the values of the color parameter a* decreased with an increase in the content of grape pomace flour and pomace flour without seeds and increased with the addition of grape seed flour.
The darkening of the biscuits during baking is a result of the Maillard reaction and the caramelization of the sugar, and the differences in the color of the samples when GP is added are primarily due to this addition. The reason for this is anthocyanins, which occur naturally in grape berries [
54] and are the cause of the purple-blue coloring of the GP. The overall result of the color change due to the addition of GP was a darker color and a convergence of green and blue colors, so that biscuits with this addition had lighter or darker shades of dark green-blue color, depending on the content of GP. The addition of GPE also reduced the lightness, while the addition of BGPE did not significantly (
p < 0.05) affect the lightness of the biscuits compared to the control samples. The values of the color parameters for redness and yellowing decrease with the addition of GPE, while they increase with the addition of BGPE compared to the control sample. The results on the decrease of lightness are in agreement with the results published by Davidov-Pardo et al. [
52]. According to their results, the lightness decreased with bulk and microencapsulated grape seed extract was added to biscuits, and the values for the parameters a* and b* increased compared to the control sample, which corresponds to the results of this study for the addition of BGPE to biscuits and the opposite of the results in the case of the addition of GPE. The total change in the color of biscuits calculated in comparison with sample C was very faintly perceptible to the human eye in biscuit samples eBGPE2 and eBGPE3 (0.2 – 1), in samples eC and eBGPE1 this change was faintly perceptible (1 – 3), while in the remaining samples the color change was very clearly perceptible to the human eye (ΔE
ab > 6), which can also be seen in the photographs of representative samples from each series of biscuits (
Figure 6).
3.4.4. Sensory Evaluation of Biscuits
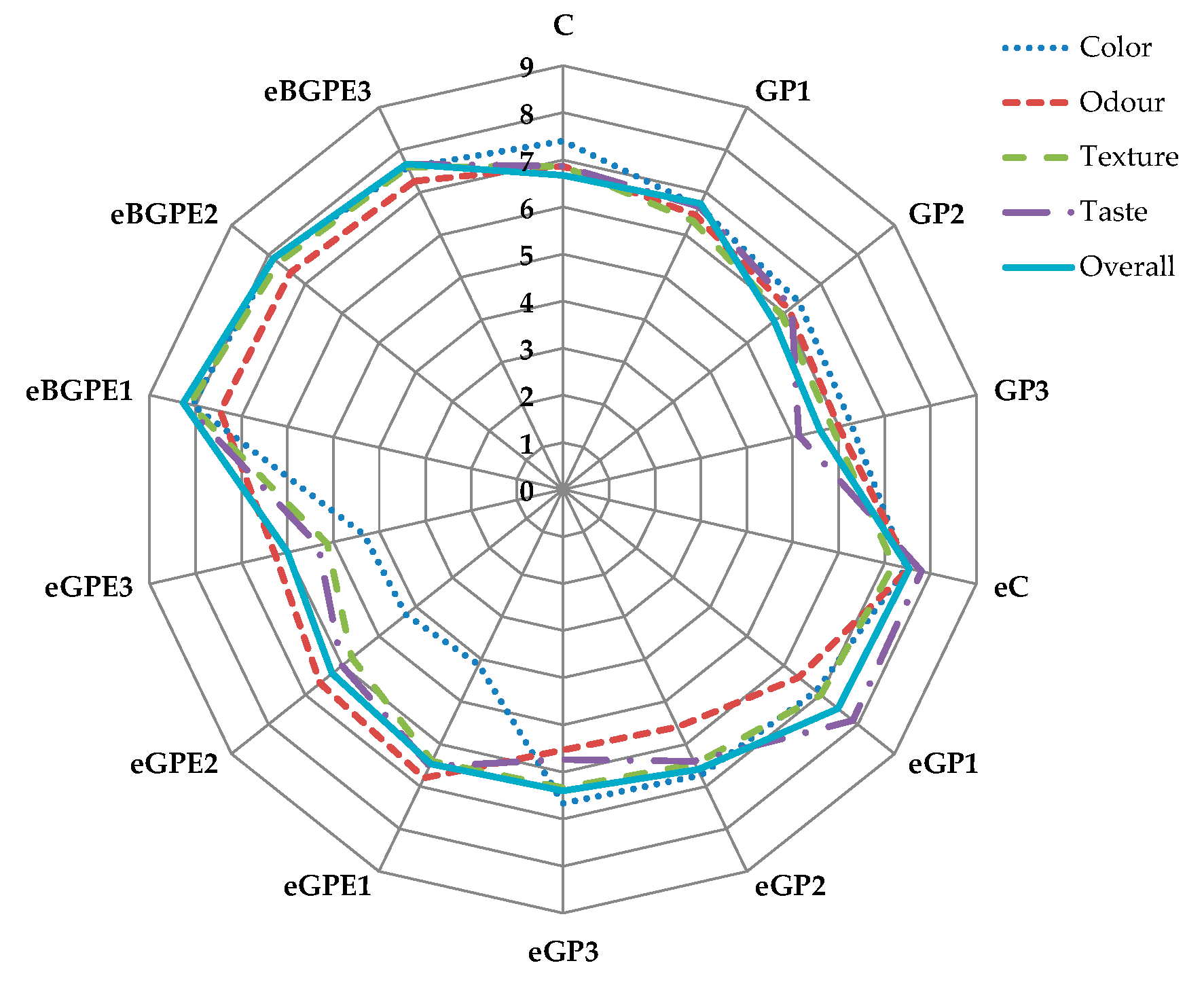
The sensory evaluation of the biscuits prepared with and without addition of GP, GPE and BGPE, as determined by sensory analysis using a 9-point hedonic scale, is shown in
Figure 7.
The results show that the color rating decreases (ranging from 6.3 to 6.9) as the content of GP increases. Considering at the other ratings, it is clear that dark green-blue shades are not particularly attractive to panelists. Biscuits with BGPE (average 7.9) and the control samples C (7.4), as well as eC (7.5) received the highest ratings for color. The color of the samples with GPE (from 4.1 to 4.3) was evaluated the worst. Comparing these results with the instrumental determinations, it can be seen that the best evaluated samples are the lightest with the highest values for the color parameters a* and b*, i.e. with the most pronounced red and yellow tones.
The best odour scores were obtained for the eC (7.5) and the samples with the addition of BGPE (from 7.3 to 7.5). Odour scores decreased with increasing GP content. This is in agreement with the results of Molnar et al. [
46], who reported that odour ratings decreased with increasing GP addition, as well as aronia pomace content. The most significant differences between biscuits prepared with GP addition and control biscuits were found in texture and taste. The texture ratings decreased significantly with increasing content of GP and were significantly higher in the samples with BGPE addition. The panelists found that the sample with the addition of GP was softer, which is consistent with the instrumental texture determination, and that its texture was grainy. The grainy texture is related to the size of the particles of GP, especially those derived from the grape seeds contained in GP. During using GP in biscuits and similar products, the size of the particles should be taken into account, especially because of the grape seeds. Kuchtová et al. [
47] reported that the score for texture gradually decreases with increasing amount of grape seeds in biscuits. Comparing the obtained results with the instrumental measurements, it is found that the samples with the addition of BGPE are softer than the samples with GPE, but harder than the samples with the addition of GP, which was rated highly by the panellists. The sample with the least addition of BGPE has the highest taste score (8.3), and the biscuit GP3 has the lowest score (5.1). For comparison, the taste of the control biscuit prepared with water (C) was rated 6.9, and that of the biscuit prepared with ethanol (eC) was rated 7.8. The lowest taste ratings of biscuits with the highest content of GP are explained by Davidov-Pardo et al. [
52] as being due to the presence of phenolic compounds in grape seeds and grape pomace, which contribute to the astringent taste of the product.
The overall acceptability score is highest for biscuits with BGPE added (from 7.7 to 8.3), and these scores are on a nine-point hedonic scale between "very much like" and "extremely like". The overall rating for biscuits with the addition of GP is in the range of 5.6 to 6.7 and can be described as a rating between "slightly like" and "moderately like". Biscuits with GPE added can be described as "moderately like" as their overall rating is between 6.3 and 6.4, and the same applies to the control sample C with an overall score of 6.7.
4. Conclusions
In this work, the physical, textural, sensory and functional properties of biscuits enriched with phenolic compounds from grape pomace (GP) were studied. The results show that the prepared biscuit formulation have the higher content of phenolic compounds and antioxidant activity compared with control sample and exhibit antiproliferative activity against cancer cell lines SW 620, Caco-2 and Hep G2, which places it in the category of potential functional products. Biscuits enriched with encapsulated grape pomace extracts (BGPE) and grape pomace extract (GPE) were significantly harder than control biscuits and GP - enriched samples. Color, odor, texture and taste of biscuits with BGPE addition received the best sensory evaluation by panelists, indicating that these biscuits could be well acceptable from consumers.
Considering the functionality, phenolic compounds often interact with other components of the complex food matrix during digestion and undergo numerous transformations related to the changes in the digestive tract, including temperature, pH, ionic strength, and other metabolic factors, so it is necessary to investigate the release behavior of phenolic compounds from biscuits during in vitro digestion simulation. Future research will therefore focus on evaluating the bioaccessibility of phenolic compounds from biscuits in the intestines, which is a prerequisite for their absorption and subsequent health effects. Since the biscuits that contained the encapsulated extract were the best evaluated, part of the future research will focus on investigating the influence of the encapsulation method and different natural coatings on the functional properties of the biscuits and the quality and consumer acceptance of the biscuits.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Chemical composition of grape pomace Cabernet Sauvignon variety; Table S2: Content of individual phenolic compounds (C) in grape pomace Cabernet Sauvignon extract determined by UHPLC analysis.
Author Contributions
Conceptualization, D.K.K., A.B.-K.; investigation, D.K.K., M.J., J.L., V.V., K.M.Š, formal analysis, G.P, J.M., G.Š.; writing—original draft preparation, D.K.K., G.P., A.B.-K.; data curation, J.L., M.J., M.P., K.M.Š., writing—review and editing, D.K.K., M.P., A.B.-K.; supervision, A.B.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the CROATIAN SCIENCE FOUNDATION, grant number: IP-2018-01-1227 (“Development of a sustainable integrated process for the production of bioactive isolates from food industry residues”, POPI-WinCEco).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the winery of the company Erdutski vinogradi d.o.o., Croatia, for the donation of grape pomace samples
Conflicts of Interest
The authors declare no conflict of interest.
References
- Luvián-Morales, J.; Varela-Castillo, F.O.; Flores-Cisneros, L.; Cetina-Pérez, L.; Castro-Eguiluz, D. Functional Foods Modulating Inflammation and Metabolism in Chronic Diseases: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2022, 62, 4371–4392. [Google Scholar] [CrossRef]
- Temple, N.J. A Rational Definition for Functional Foods: A Perspective. Front. Nutr. 2022, 9, 957516. [Google Scholar] [CrossRef]
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current Trends and Possibilities for Exploitation of Grape Pomace as a Potential Source for Value Addition. Environ. Pollut. 2021, 278, 116796. [Google Scholar] [CrossRef]
- Tan, E.C.D.; Lamers, P. Circular Bioeconomy Concepts—A Perspective. Front. Sustain. 2021, 2. [Google Scholar] [CrossRef]
- Ubando, A.T.; Felix, C.B.; Chen, W.-H. Biorefineries in Circular Bioeconomy: A Comprehensive Review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef]
- Baumgartner, B.; Özkaya, B.; Saka, I.; Özkaya, H. Functional and Physical Properties of Cookies Enriched with Dephytinized Oat Bran. J. Cereal Sci. 2018, 80, 24–30. [Google Scholar] [CrossRef]
- Onacik-Gür, S.; Żbikowska, A.; Majewska, B. Effect of spirulina (Spirulina Platensis) addition on textural and quality properties of cookies. Ital. J. Food Sci. 2018, 30. [Google Scholar] [CrossRef]
- Turksoy, S.; Özkaya, B. Pumpkin and Carrot Pomace Powders as a Source of Dietary Fiber and Their Effects on the Mixing Properties of Wheat Flour Dough and Cookie Quality. Food Sci. Technol. Res 2011, 17, 545–553. [Google Scholar] [CrossRef]
- Loza, A.; Quispe, M.; Villanueva, J.; Peláez, P.P. Development of Functional Cookies with Wheat Flour, Banana Flour (Musa Paradisiaca), Sesame Seeds (Sesamum Indicum) and Storage Stability. Sci. Agropecu. 2017, 8, 315–325. [Google Scholar] [CrossRef]
- Antarkar, S.; Sharma, A.; Bhargava, A.; Gupta, H.; Tomar, R.; Srivastava, S. Physico-Chemical and Nutritional Evaluation of Cookies with Different Levels of Rosehip and Hibiscus Powder Substitution. Arch. Curr. Res. Int. 2019, 1–10. [Google Scholar] [CrossRef]
- Karnopp, A.R.; Figueroa, A.M.; Los, P.R.; Teles, J.C.; Simões, D.R.S.; Barana, A.C.; Kubiaki, F.T.; Oliveira, J.G.B. de; Granato, D. Effects of Whole-Wheat Flour and Bordeaux Grape Pomace (Vitis Labrusca L.) on the Sensory, Physicochemical and Functional Properties of Cookies. Food Sci. Technol. 2015, 35, 750–756. [Google Scholar] [CrossRef]
- Curutchet, A.; Cozzano, S.; Tárrega, A.; Arcia, P. Blueberry Pomace as a Source of Antioxidant Fibre in Cookies: Consumer’s Expectations and Critical Attributes for Developing a New Product. Food Sci. Technol. Int. 2019, 25, 642–648. [Google Scholar] [CrossRef]
- Alongi, M.; Melchior, S.; Anese, M. Reducing the Glycemic Index of Short Dough Biscuits by Using Apple Pomace as a Functional Ingredient. LWT-Food Sci. Technol. 2019, 100, 300–305. [Google Scholar] [CrossRef]
- Difonzo, G.; Troilo, M.; Squeo, G.; Pasqualone, A.; Caponio, F. Functional Compounds from Olive Pomace to Obtain High-Added Value Foods – a Review. J. Sci. Food 2021, 101, 15–26. [Google Scholar] [CrossRef]
- Abdo, E.M.; Shaltout, O.E.-S.; El-Sohaimy, S.; Abdalla, A.E.M.; Zeitoun, A.M. Effect of Functional Beetroot Pomace Biscuit on Phenylhydrazine Induced Anemia in Albino Rats: Hematological and Blood Biochemical Analysis. J. Funct. Foods 2021, 78, 104385. [Google Scholar] [CrossRef]
- Nakov, G.; Brandolini, A.; Hidalgo, A.; Ivanova, N.; Stamatovska, V.; Dimov, I. Effect of Grape Pomace Powder Addition on Chemical, Nutritional and Technological Properties of Cakes. LWT-Food Sci. Technol. 2020, 134, 109950. [Google Scholar] [CrossRef]
- Lou, W.; Zhou, H.; Li, B.; Nataliya, G. Rheological, Pasting and Sensory Properties of Biscuits Supplemented with Grape Pomace Powder. Food Sci. Technol. 2021, 42, e78421. [Google Scholar] [CrossRef]
- Mohammadi, M.; Khorshidian, N.; Yousefi, M.; Khaneghah, A.M. Physicochemical, Rheological, and Sensory Properties of Gluten-Free Cookie Produced by Flour of Chestnut, Date Seed, and Modified Starch. J. Food. Qual. 2022, 2022, e5159084. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M.; Arlorio, M.; Coisson, J.D. Spent Grape Pomace as a Still Potential By-Product. Int. J. Food Sci. 2015, 50, 2022–2031. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#home (accessed on 16 October 2023).
- Tolun, A.; Artik, N.; Altintas, Z. Effect of Different Microencapsulating Materials and Relative Humidities on Storage Stability of Microencapsulated Grape Pomace Extract. Food Chem. 2020, 302, 125347. [Google Scholar] [CrossRef]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2019, 9, 35. [Google Scholar] [CrossRef]
- Theagarajan, R.; Malur Narayanaswamy, L.; Dutta, S.; Moses, J.A.; Chinnaswamy, A. Valorisation of Grape Pomace (Cv. Muscat) for Development of Functional Cookies. Int. J. Food Sci. Technol. 2019, 54, 1299–1305. [Google Scholar] [CrossRef]
- Balea, Ş.S.; Pârvu, A.E.; Pârvu, M.; Vlase, L.; Dehelean, C.A.; Pop, T.I. Antioxidant, Anti-Inflammatory and Antiproliferative Effects of the Vitis Vinifera L. Var. Fetească Neagră and Pinot Noir Pomace Extracts. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Pérez-Ortiz, J.M.; Alguacil, L.F.; Salas, E.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; González-Martín, C. Antiproliferative and Cytotoxic Effects of Grape Pomace and Grape Seed Extracts on Colorectal Cancer Cell Lines. Food Sci. Nutr. 2019, 7, 2948–2957. [Google Scholar] [CrossRef]
- De Sales, N.F.F.; Silva da Costa, L.; Carneiro, T.I.A.; Minuzzo, D.A.; Oliveira, F.L.; Cabral, L.M.C.; Torres, A.G.; El-Bacha, T. Anthocyanin-Rich Grape Pomace Extract (Vitis Vinifera L.) from Wine Industry Affects Mitochondrial Bioenergetics and Glucose Metabolism in Human Hepatocarcinoma HepG2 Cells. Molecules 2018, 23, 611. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of Grape Pomace: An Approach That Is Increasingly Reaching Its Maturity – a Review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Zam, W.; Bashour, G.; Abdelwahed, W.; Khayata, W. Alginate-Pomegranate Peels’ Polyphenols Beads: Effects of Formulation Parameters on Loading Efficiency. Braz. J. Pharm. Sci. 2014, 50, 741–748. [Google Scholar] [CrossRef]
- Liang, J.; Li, F.; Fang, Y.; Yang, W.; An, X.; Zhao, L.; Xin, Z.; Cao, L.; Hu, Q. Synthesis, Characterization and Cytotoxicity Studies of Chitosan-Coated Tea Polyphenols Nanoparticles. Colloids Surf. B Biointerfaces 2011, 82, 297–301. [Google Scholar] [CrossRef]
- Martinović, J.; Lukinac, J.; Jukić, M.; Ambrus, R.; Planinić, M.; Šelo, G.; Klarić, A.-M.; Perković, G.; Bucić-Kojić, A. Physicochemical Characterization and Evaluation of Gastrointestinal In Vitro Behavior of Alginate-Based Microbeads with Encapsulated Grape Pomace Extracts. Pharmaceutics 2023, 15, 980. [Google Scholar] [CrossRef]
- AACC (American Association of Cereal Chemists). Official Method 10-50.05. In Approved methods of analysis, 11th ed.; Baking quality of cookie flour; Cereals & Grains Association: St. Paul, MN, U.S.A, 2000. [Google Scholar]
- Waterhouse, A.L. Determination of total phenolics. In Current protocols in food analytical chemistry; Wrolstad, R.E., Ed.; John Wiley & Sons Inc.: New York, NY, USA, 2001; pp. I1.1.1–I1.1.8. [Google Scholar] [CrossRef]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Jukić, M.; Nakov, G.; Komlenić, D.K.; Vasileva, N.; Šumanovac, F.; Lukinac, J. Quality Assessment of Cookies Made from Composite Flours Containing Malted Barley Flour and Wheat Flour. Plants 2022, 11, 761. [Google Scholar] [CrossRef]
- Yamsaengsung, R.; Berghofer, E.; Schoenlechner, R. Physical Properties and Sensory Acceptability of Cookies Made from Chickpea Addition to White Wheat or Whole Wheat Flour Compared to Gluten-Free Amaranth or Buckwheat Flour. Int. J. Food Sci. Technol. 2012, 47, 2221–2227. [Google Scholar] [CrossRef]
- Maner, S.; Sharma, A.K.; Banerjee, K. Wheat Flour Replacement by Wine Grape Pomace Powder Positively Affects Physical, Functional and Sensory Properties of Cookies. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 2017, 87, 109–113. [Google Scholar] [CrossRef]
- Mišković Špoljarić, K.; Šelo, G.; Pešut, E.; Martinović, J.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Antioxidant and Antiproliferative Potentials of Phenolic-Rich Extracts from Biotransformed Grape Pomace in Colorectal Cancer. BMC Complement Altern. Med. 2023, 23, 29. [Google Scholar] [CrossRef]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive Compounds in Wine: Resveratrol, Hydroxytyrosol and Melatonin: A Review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- de Oliveira, M.F.; Amoêdo, N.D.; Rumjanek, F.D. Energy and Redox Homeostasis in Tumor Cells. Int. J. Biochem. Cell Biol. 2012, 2012, e593838. [Google Scholar] [CrossRef]
- Caponio, G.R.; Minervini, F.; Tamma, G.; Gambacorta, G.; De Angelis, M. Promising Application of Grape Pomace and Its Agri-Food Valorization: Source of Bioactive Molecules with Beneficial Effects. Sustainability 2023, 15, 9075. [Google Scholar] [CrossRef]
- Sun, J.; Chu, Y.-F.; Wu, X.; Liu, R.H. Antioxidant and Antiproliferative Activities of Common Fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef]
- Acun, S.; Gül, H. Effects of Grape Pomace and Grape Seed Flours on Cookie Quality. Qual. Assur. Saf. Crops Foods 2014, 6, 81–88. [Google Scholar] [CrossRef]
- Molnar, D.; Novotni, D.; Krisch, J.; Bosiljkov, T.; Scetar, M. The Optimisation of Biscuit Formulation with Grape and Aronia Pomace Powders as Cocoa Substitutes. Hrvat. čas. preh. tehnol. biotehnol. nutr. 2020, 15, 38–44. [Google Scholar] [CrossRef]
- Kuchtova, V.; Kohajdova, Z.; Karovicova, J.; Laukova, M. Physical, Textural and Sensory Properties of Cookies Incorporated with Grape Skin and Seed Preparations. Polish J. Food Nutr. 2018, 68. [Google Scholar] [CrossRef]
- Kohajdová, V.K.Z.; Karovičová, J.; Lauková, M. Physical, Textural and Sensory Properties of Cookies Incorporated with Grape Skin and Seed Preparations. Pol. J. Food Nutr. Sci. 2018, 68, 309–317. [Google Scholar] [CrossRef]
- Mamat, H.; Hill, S. Structural and Functional Properties of Major Ingredients of Biscuit. Int. Food Res. J. 2018, 25, 462–471. [Google Scholar]
- Mancebo, C.M.; Picón, J.; Gómez, M. Effect of Flour Properties on the Quality Characteristics of Gluten Free Sugar-Snap Cookies. LWT - Food Sci. Technol. 2015, 64, 264–269. [Google Scholar] [CrossRef]
- Nakov, G.; Jukić, M.; Šimić, G.; Šumanovac, F.; Komlenić, D.K.; Lukinac, J. Effect of the Addition of Hulless Barley Flour on the Quality of Short-Dough Cookies. Foods 2022, 11, 2428. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; Moreno, M.; Arozarena, I.; Marín-Arroyo, M.R.; Bleibaum, R.N.; Bruhn, C.M. Sensory and Consumer Perception of the Addition of Grape Seed Extracts in Cookies. J. Food Sci. 2012, 77, S430–438. [Google Scholar] [CrossRef]
- Chauhan, A.; Saxena, D.C.; Singh, S. Physical, Textural, and Sensory Characteristics of Wheat and Amaranth Flour Blend Cookies. Cogent Food Agric. 2016, 2, 1125773. [Google Scholar] [CrossRef]
- Boff, J.M.; Strasburg, V.J.; Ferrari, G.T.; de Oliveira Schmidt, H.; Manfroi, V.; de Oliveira, V.R. Chemical, Technological, and Sensory Quality of Pasta and Bakery Products Made with the Addition of Grape Pomace Flour. Foods 2022, 11, 3812. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Content of (a) total phenolic compounds (TPC); (b) total flavonoid (TF) in biscuits. (C) – control sample, standard AACC biscuit formulation; (GP) – biscuit with grape pomace; (e) – biscuit in which water has been replaced by 50 % ethanol; (GPE) – biscuit with grape pomace extract; (BGPE) – biscuit with beads of encapsulated grape pomace extract.
Figure 1.
Content of (a) total phenolic compounds (TPC); (b) total flavonoid (TF) in biscuits. (C) – control sample, standard AACC biscuit formulation; (GP) – biscuit with grape pomace; (e) – biscuit in which water has been replaced by 50 % ethanol; (GPE) – biscuit with grape pomace extract; (BGPE) – biscuit with beads of encapsulated grape pomace extract.
Figure 2.
Antioxidant activity of biscuits according: (a) ABTS method; (b) FRAP method. (C) – control sample, standard AACC biscuit formulation; (GP) – biscuit with grape pomace; (e) – biscuit in which water has been replaced by 50 % ethanol; (GPE) – biscuit with grape pomace extract; (BGPE) – biscuit with beads of encapsulated grape pomace extract.
Figure 2.
Antioxidant activity of biscuits according: (a) ABTS method; (b) FRAP method. (C) – control sample, standard AACC biscuit formulation; (GP) – biscuit with grape pomace; (e) – biscuit in which water has been replaced by 50 % ethanol; (GPE) – biscuit with grape pomace extract; (BGPE) – biscuit with beads of encapsulated grape pomace extract.
Figure 3.
CaCo-2, SW 620 and Hep G2 cell viability at different concentration of biscuit extract: (a) 5 mg/mL; (b) 0.5 mg/mL; (c) 0.05 mg/mL. (C) – control sample, standard AACC biscuit formulation; (GP) – biscuit with grape pomace; (e) – biscuit in which water has been replaced by 50 % ethanol; (GPE) – biscuit with grape pomace extract; (BGPE) – biscuit with beads of encapsulated grape pomace extract.
Figure 3.
CaCo-2, SW 620 and Hep G2 cell viability at different concentration of biscuit extract: (a) 5 mg/mL; (b) 0.5 mg/mL; (c) 0.05 mg/mL. (C) – control sample, standard AACC biscuit formulation; (GP) – biscuit with grape pomace; (e) – biscuit in which water has been replaced by 50 % ethanol; (GPE) – biscuit with grape pomace extract; (BGPE) – biscuit with beads of encapsulated grape pomace extract.
Figure 4.
Dimension properties of biscuits: (a) width; (b) thickness; (c) spread factor; (d) volume. (C) – control sample, standard AACC biscuit formulation; (GP) – biscuit with grape pomace; (e) – biscuit in which water has been replaced by 50 % ethanol; (GPE) – biscuit with grape pomace extract; (BGPE) – biscuit with beads of encapsulated grape pomace extract. .
Figure 4.
Dimension properties of biscuits: (a) width; (b) thickness; (c) spread factor; (d) volume. (C) – control sample, standard AACC biscuit formulation; (GP) – biscuit with grape pomace; (e) – biscuit in which water has been replaced by 50 % ethanol; (GPE) – biscuit with grape pomace extract; (BGPE) – biscuit with beads of encapsulated grape pomace extract. .
Figure 5.
Textural properties of biscuits: a) snapping force; (b) breaking distance; (c) breaking force index. (C) – control sample, standard AACC biscuit formulation; (GP) – biscuit with grape pomace; (e) – biscuit in which water has been replaced by 50 % ethanol; (GPE) – biscuit with grape pomace extract; (BGPE) – biscuit with beads of encapsulated grape pomace extract.
Figure 5.
Textural properties of biscuits: a) snapping force; (b) breaking distance; (c) breaking force index. (C) – control sample, standard AACC biscuit formulation; (GP) – biscuit with grape pomace; (e) – biscuit in which water has been replaced by 50 % ethanol; (GPE) – biscuit with grape pomace extract; (BGPE) – biscuit with beads of encapsulated grape pomace extract.
Figure 6.
Images of representative biscuits from each batch. (C) – control sample, standard AACC biscuit formulation; (GP) – biscuit with grape pomace; (e) – biscuit in which water has been re-placed by 50 % ethanol; (GPE) – biscuit with grape pomace extract; (BGPE) – biscuit with beads of encapsulated grape pomace extract.
Figure 6.
Images of representative biscuits from each batch. (C) – control sample, standard AACC biscuit formulation; (GP) – biscuit with grape pomace; (e) – biscuit in which water has been re-placed by 50 % ethanol; (GPE) – biscuit with grape pomace extract; (BGPE) – biscuit with beads of encapsulated grape pomace extract.
Figure 7.
Sensory evaluation score by 9-point hedonic scale. (C) – control sample, standard AACC biscuit formulation; (GP) – biscuit with grape pomace; (e) – biscuit in which water has been replaced by 50 % ethanol; (GPE) – biscuit with grape pomace extract; (TPC) – total phenolic compounds; (BGPE) – biscuit with beads of encapsulated grape pomace extract.
Figure 7.
Sensory evaluation score by 9-point hedonic scale. (C) – control sample, standard AACC biscuit formulation; (GP) – biscuit with grape pomace; (e) – biscuit in which water has been replaced by 50 % ethanol; (GPE) – biscuit with grape pomace extract; (TPC) – total phenolic compounds; (BGPE) – biscuit with beads of encapsulated grape pomace extract.
Table 1.
Biscuit formulation scheme. Standard AACC biscuit formulation (C) and modified biscuit formulations with substituted/added ingredients (GP1 – eBGPE3).
Table 1.
Biscuit formulation scheme. Standard AACC biscuit formulation (C) and modified biscuit formulations with substituted/added ingredients (GP1 – eBGPE3).
| SAMPLE 1 |
Substitution |
Addition |
| C |
- |
- |
| GP1 |
10 % wheat flour replaced by GP |
- |
| GP2 |
20 % wheat flour replaced by GP |
- |
| GP3 |
30 % wheat flour replaced by GP |
- |
| eC |
distilled water replaced by 50 % ethanol |
- |
| eGP1 |
distilled water replaced by 50 % ethanol;
10 % wheat flour replaced by GP |
- |
| eGP2 |
distilled water replaced by 50 % ethanol;
20 % wheat flour replaced by GP |
- |
| eGP3 |
distilled water replaced by 50 % ethanol;
30 % wheat flour replaced by GP |
- |
| eGPE1 |
distilled water replaced by 50 % ethanol |
GPE equivalent to GP replacing 10 % of wheat flour (in terms of TPC content) |
| eGPE2 |
distilled water replaced by 50 % ethanol |
GPE equivalent to GP replacing 20 % of wheat flour (in terms of TPC content) |
| eGPE3 |
distilled water replaced by 50 % ethanol |
GPE equivalent to GP replacing 30 % of wheat flour (in terms of TPC content) |
| eBGPE1 |
distilled water replaced by 50 % ethanol |
BGPE equivalent to GP replacing 10 % of wheat flour (in terms of TPC content) |
| eBGPE2 |
distilled water replaced by 50 % ethanol |
BGPE equivalent to GP replacing 20 % of wheat flour (in terms of TPC content) |
| eBGPE3 |
distilled water replaced by 50 % ethanol |
BGPE equivalent to GP replacing 30 % of wheat flour (in terms of TPC content) |
Table 2.
Color parameters of the biscuits: lightness (L*), redness (a*), yellowness (b*) and total color change (ΔEab) of the biscuit compared to the color of the control sample of the biscuit.
Table 2.
Color parameters of the biscuits: lightness (L*), redness (a*), yellowness (b*) and total color change (ΔEab) of the biscuit compared to the color of the control sample of the biscuit.
| SAMPLE 1
|
L* |
a* |
b* |
ΔEab
|
| C |
67.61 ± 2.5 a
|
6.23 ± 1.5 b
|
33.99 ± 1.0 b
|
- |
| GP1 |
49.85 ± 0.6 e
|
5.03 ± 0.3 c
|
18.14 ± 0.9 de
|
23.83 |
| GP2 |
41.13 ± 0.7 f
|
4.98 ± 0.3 c
|
13.73 ± 0.6 g
|
33.36 |
| GP3 |
36.32 ± 3.4 g
|
4.91 ± 1.5 c
|
9.40 ± 2.0 h
|
39.82 |
| eC |
66.68 ± 0.9 a
|
6.42 ± 0.4 b
|
33.64 ± 0.8 b
|
1.01 |
| eGP1 |
50.89 ± 0.9 e
|
4.86 ± 0.3 c
|
17.51 ± 0.8 ef
|
23.52 |
| eGP2 |
42.19 ± 0.9 f
|
4.76 ± 0.4 c
|
13.15 ± 1.2 g
|
32.90 |
| eGP3 |
36.66 ± 0.9 g
|
4.73 ± 0.8 c
|
8.93 ± 0.8 h
|
39.85 |
| eGPE1 |
62.87 ± 8.4 b
|
4.45 ± 1.1 c
|
24.83 ± 1.5 c
|
10.47 |
| eGPE2 |
57.70 ± 0.7 c
|
2.82 ± 0.6 d
|
18.75 ± 1.4 d
|
18.50 |
| eGPE3 |
53.08 ± 0.8 d
|
2.65 ± 1.0 d
|
16.56 ± 2.0 f
|
22.97 |
| eBGPE1 |
68.12 ± 1.1 a
|
7.41 ± 1.0 a
|
35.30 ± 0.6 a
|
1.84 |
| eBGPE2 |
67.76 ± 2.0 a
|
6.93 ± 0.9 ab
|
34.46 ± 0.9 ab
|
0.86 |
| eBGPE3 |
66.92 ± 2.5 a
|
6.58 ± 0.8 b
|
34.21 ± 0.9 b
|
0.80 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).