1. Introduction
Glioblastoma (GBM) is the most aggressive primary brain tumor, with a dismal median survival rate of approximately 15 months despite the current standard of care that includes surgery, radiotherapy, and chemotherapy1–4. The 5-year survival rate for GBM patients after diagnosis is a dismal 5-10%.5,6 According to the Central Brain Tumor Registry of the United States (CBTRUS) Statistical Report7, 2015-2019, GBM accounted for 50.1% of primary malignant brain tumors.7 GBM is notorious for its radioresistant and chemo-resistant nature8–11, hindering curative therapy with existing techniques. The lethality and invasiveness of this tumor make it imperative to explore innovative treatment options.8,12,13. The recent success of immune checkpoint inhibitors (ICSs)14,15 in treating metastatic melanoma and non-small-cell lung cancer16, inter alia, has led to their evaluation in various clinical trials for potential use against glioblastoma and other forms of brain cancer. Three different groups of ICIs, including PD-1 inhibitors (Nivolumab, Pembrolizumab, and Cemiplimab), PD-L1 inhibitors (Atezolimumab, Durvalumab and Avelumab), and CTLA-4 inhibitor (Ipilimumab) have been approved by the US Food and Drug Administration (FDA) for the treatment of various types of cancer.17–19. Results from recent clinical trials using ICIs alone against GBM are disappointing20–22. Unsurprisingly, GBM has become a prime target for combination therapies, including radioimmunotherapy (RIT), which involves integrating radiation therapy and immunotherapy23–27. Phase I/II clinical trials involving ICIs in RIT for glioblastoma are currently underway and the cellular level impacts of these ICI-based RIT on GBM are still unknown, since the ICIs were adopted for clinical trials based on their safety profiles in treating other cancers.23,27–30 Of course, immunotherapies in general focus on system-level outcomes, especially those involving the immune system. In the context of RIT and combination therapies against GBM, an underlying question often neglected but relevant for full understanding, therefore presents itself: namely, what are the molecular and cellular-level effects of immunotherapeutic agents alone and in combination with radiotherapy on the for glioblastoma cells themselves?
This question reveals an urgent need for the development of new assays and platforms that provide deeper insights into the mechanisms of action of effective or ineffective cancer immunotherapies and RITs, both at the cellular level and at the system level. Here, we present in vitro assays we developed, which rapidly quantify biophysical markers of potential therapeutic efficacy for two immunotherapeutic drugs, in combination with radiotherapy. Our novel platform aims at providing cellular and molecular level insights for evaluation of efficacy or otherwise.
We use a Faxitron CellRad cell irradiator for applying clinically relevant doses31 to cancer cells following exposure to the immune checkpoint inhibitors, durvalumab and pembrolizumab. Through fluorescence microscopy and in-house developed morphometric routines32,33, we measure nuclear-lacunarity and mitotic cell ratio within 24-48 hours of treatment as early molecular readouts for cell repair/death. We utilize an Electric Cell Impedance Sensor (ECIS)3435,36 for measuring cell migration in real time (for 7 days following treatment) and extract from the data, a barrier function that reflects cell-cell adhesion37, an important indicator of local invasion and metastatic competence. We use cloud-based automated clonogenic assays (CytoSMART Omni) and more traditional colony counting techniques to quantify cell death and produce survival curves38 within 14-21 days. Thus, when optimized to systematically include more in vivo tissue microenvironment (TME) parameters, cells of the immune system and ultimately samples from patient biopsies39,40, our assays should tell within one month, the best options and the likely course of treatment (prognosis). In this work, we present results using these methods at the in vitro level, emphasizing the biophysical markers themselves, with respect to radioimmunotherapy (RIT) using immune checkpoint inhibitors (ICS).
The immune checkpoint blockers, durvalumab (anti-PD-L1)41, and pembrolizumab (anti-PD-1)42 in our in vitro assays, lead to several cellular and molecular level differences38 in their direct action on GBM cell lines, T98G and U87, even in the absence of immune cells. Interestingly, radiation alone as well as pembrolizumab with radiation, both reduced (p < 0.05) cell-cell adhesion. This reduction is indicative of increased potential for local invasion. These methods have the potential of providing a framework for patient-specific targeted cancer therapy. We envisage our in vitro assays to be applicable to cells from patient biopsies, to evaluate the potential efficacy of treatment strategies for the overall improvement of treatment outcomes against aggressive cancers such as glioblastomas.
2. Results
2.1. Cell Migration and Cell-Cell Adhesion
2.1.1. RIT Using Pembrolizumab, Has No Significant Effect on Cell Migration Compared to Radiation Alone or Pembrolizumab Alone
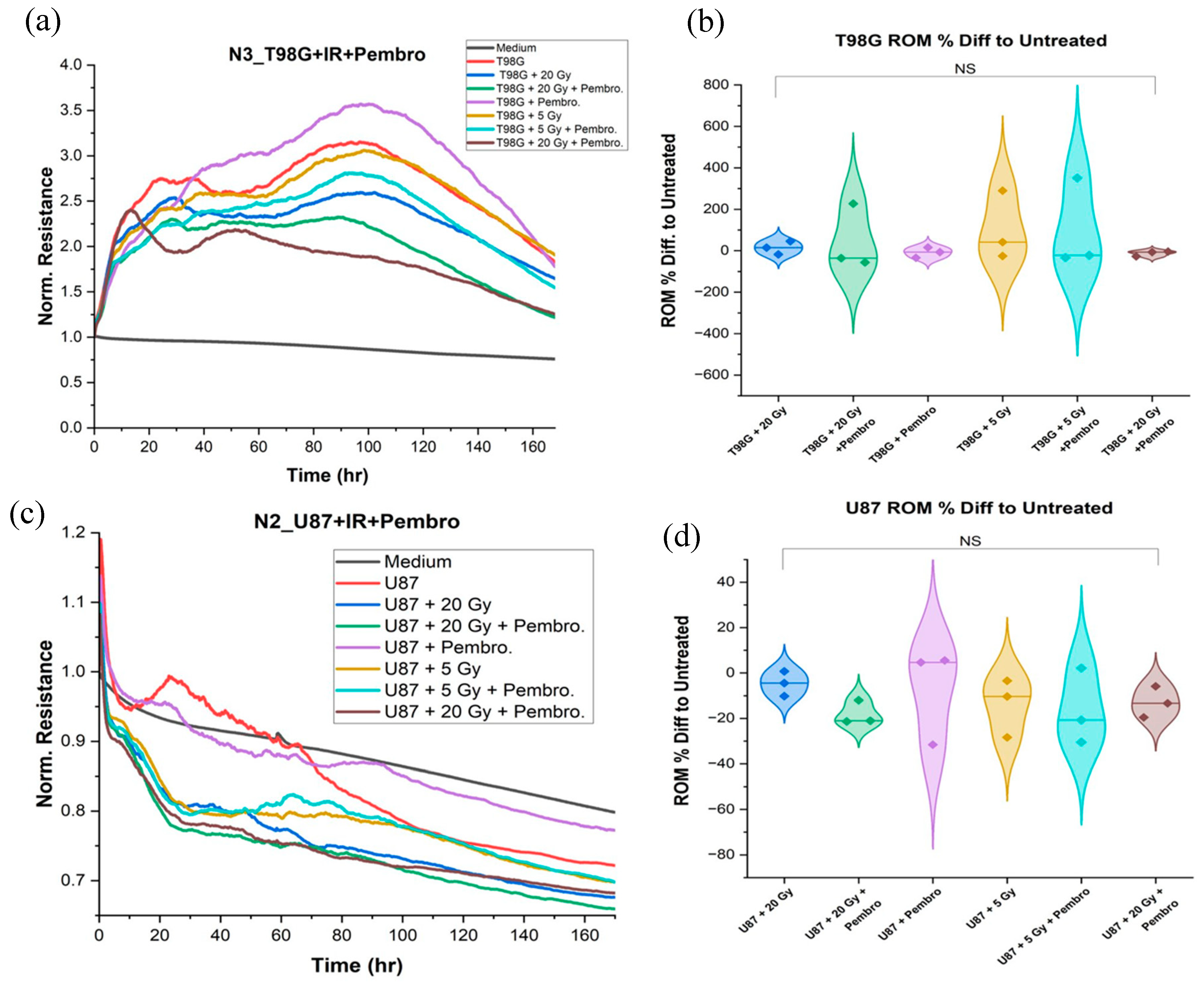
Using ECIS, we analyze the effect of treatment with immune checkpoint agent, pembrolizumab, in combination with radiation treatment doses of 0, 5, and 20 Gy, on cell migration in T98G and U87 cells. The analysis of the rate of migration (ROM) from the ECIS impedance curves after treatment, using one-way ANOVA hypothesis testing, indicates that there are no statistically significant differences in cell migration resulting between the group treated with the immune checkpoint inhibitor, pembrolizumab, and radiation doses at 5 Gy and 20 Gy (the RIT group), and the group treated with radiation alone or pembrolizumab alone for both cell lines, as presented in
Figure 1.
However, while there is no significant alteration to cell migration using the ANOVA method, the rate of migration analysis comparing the mean percentage difference from the three trials reveals a reduction of about 25% in cell migration for treatment condition with concurrent 20 Gy of irradiation and pembrolizumab (RIT), compared to untreated T98G while showing the highest possible reduction relative to other treatment conditions. A similar trend was observed in U87 cells where the rate of migration (ROM) shows no significant difference among treatment conditions versus radiation alone or pembrolizumab alone; however, mere evaluation of the percentage difference graph again shows that 20 Gy radiation dose with concurrent pembrolizumab (RIT) treatment led to reduction in cell migration to the tune of about 25% relative to untreated cells compared to other treatments conditions.
2.1.2. Decrease in Cell-Cell Adhesion at 20 Gy Irradiation with and without Pembrolizumab
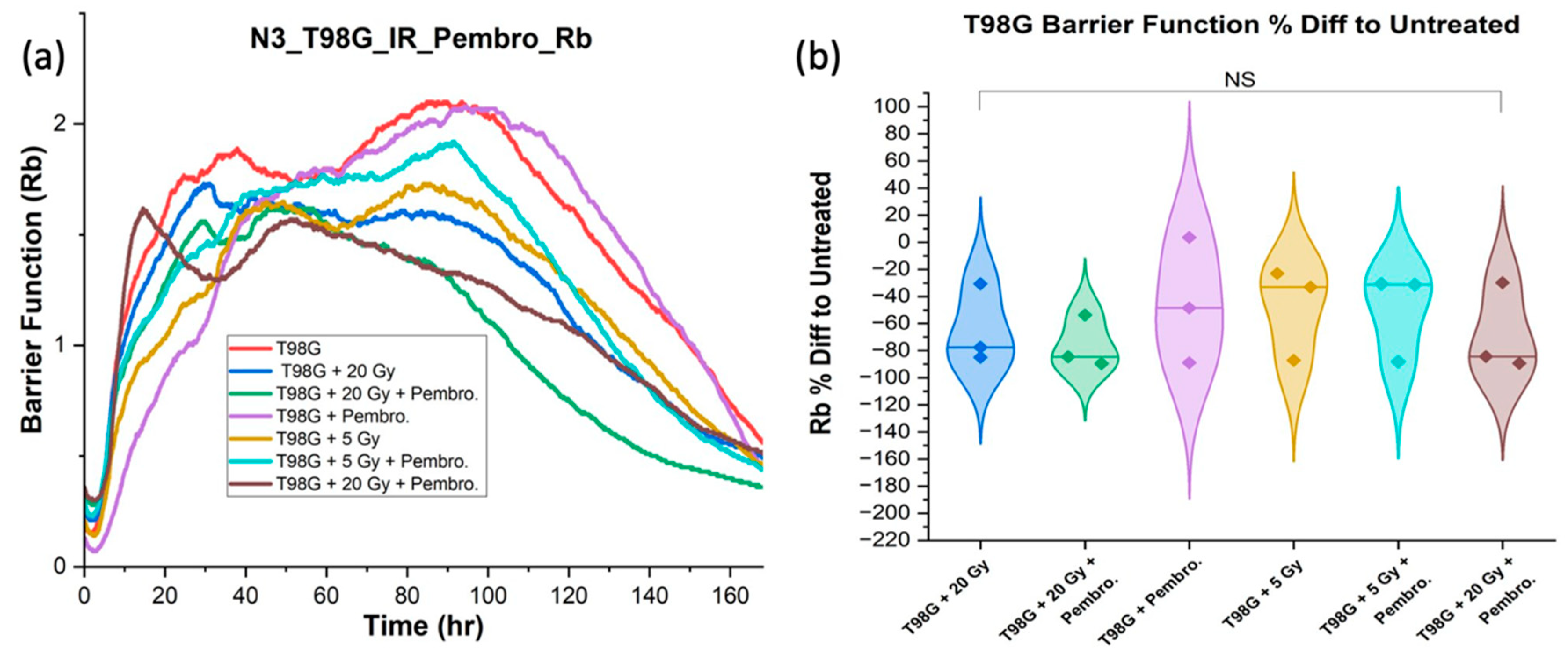
Maintaining adhesion is crucial to impede the metastatic cascade. Any reduction in this function may lead to increased metastasis and poorer long-term treatment outcomes. We hypothesized that the combination of pembrolizumab and radiation will improve cell-cell adhesion in glioblastoma cells. By direct modeling of ECIS data, we extracted the barrier function
Rb as a readout of cell-cell adhesion. The
Rb for comparisons and statistical testing were acquired at average range of 80 hours to 168 hours post-treatment, the late
Rb period in the ECIS data, to isolate the effects of treatment on cell-cell adhesion after three to seven days of treatment (
Figure 2).
The ANOVA analysis conducted on T98G barrier function did not reveal any statistically significant differences between the group receiving the combined treatment using pembrolizumab and radiation, and the group treated with radiation alone or pembrolizumab only. However, the barrier function (Rb) of T98G treated with 20 Gy radiation dose together with or without the addition of pembrolizumab yielded a significant difference relative to untreated T98G cells (p < 0.05). No significant differences were found at 5 Gy radiation dose (with or without the addition of pembrolizumab). This further reinforce the need for anti-metastatic strategies especially for high radiation dose use.
Barrier function (Rb) results were also analyzed for U87 cells, although they are not exclusively monolayer-forming cells at high density, barrier function calculations frequently produced rapidly fluctuating results, as clusters of cells moved over or away from electrodes.
2.2. Cell Survival from Clonogenic Assays
2.2.1. Radiation Most Significantly Determined Decrease in Cell Survival
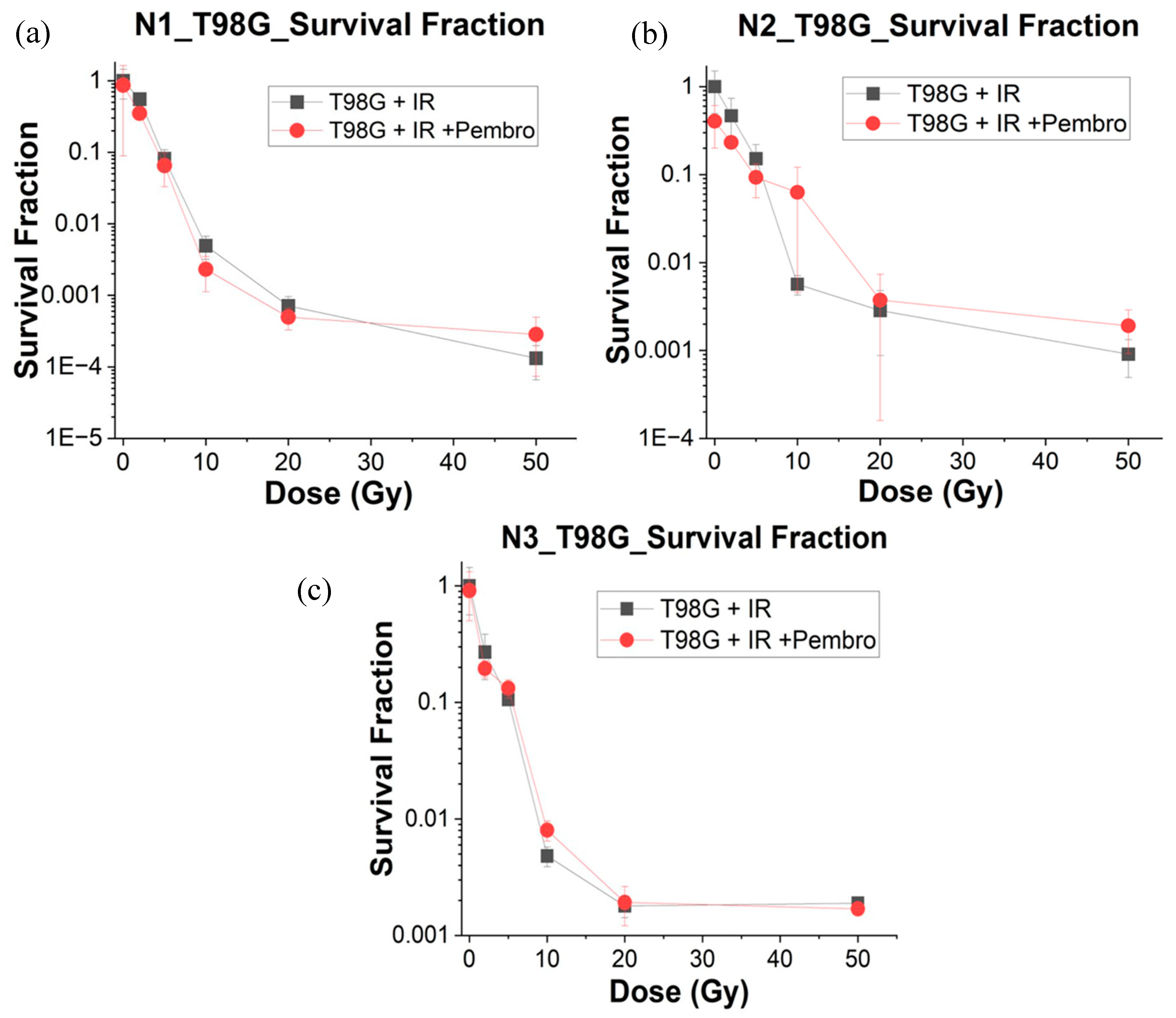
The Survival Fraction (SF) is a measure of the proportion of cells that survive after exposure to radiation and other treatment agent. A lower SF indicates greater cell killing or reduced cell survival. We carried out ANOVA analysis using the percentage difference of the SF at different treatment conditions compared to the untreated glioblastoma cells.
The SF for T98G cells alone without any treatment was 1 (obviously without radiation and pembrolizumab treatment). As the radiation dose increases, the SF decreases, which is expected (
Figure 3).
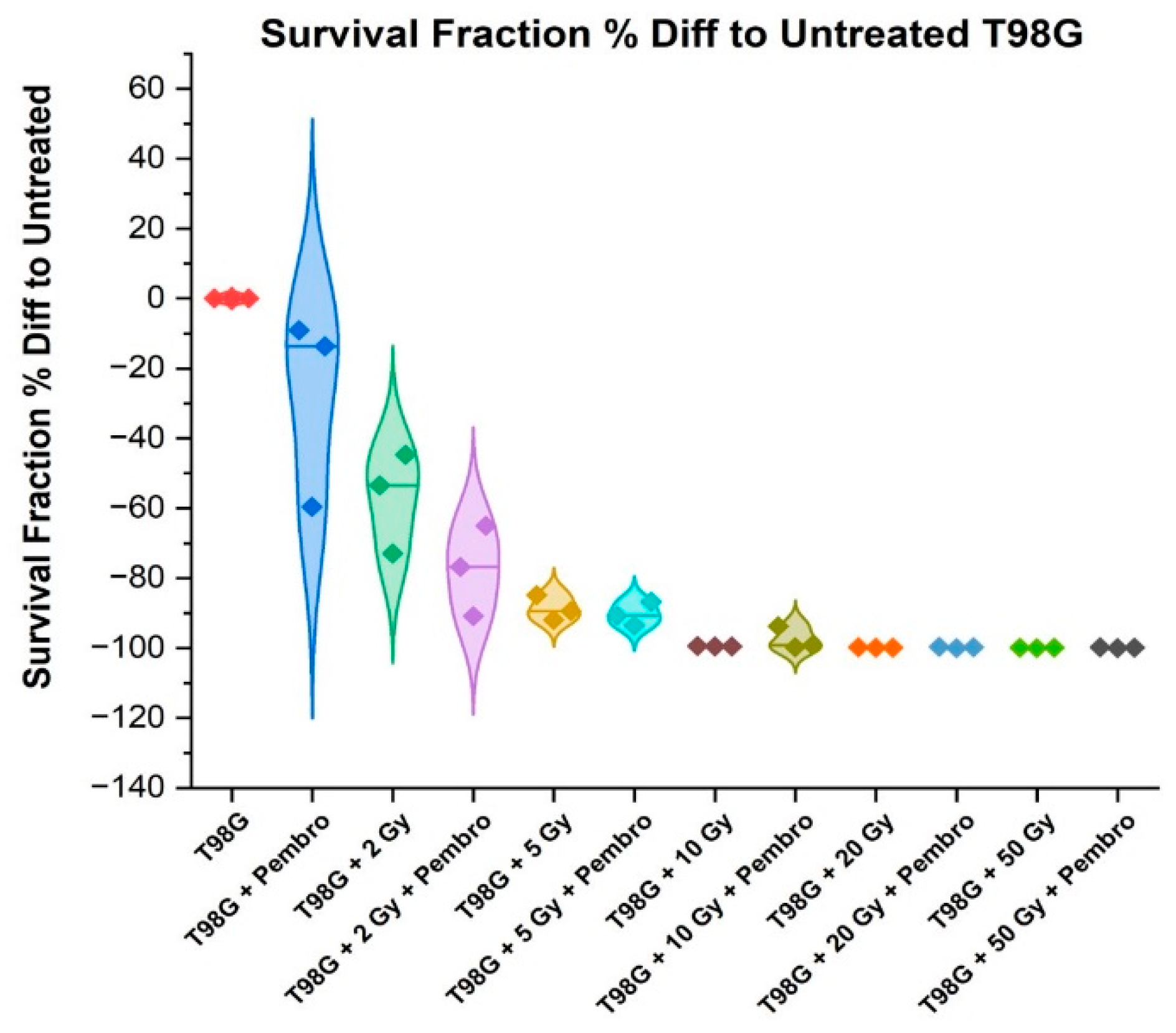
As the radiation dose increases (T98G + 2 Gy, 5 Gy, 10 Gy, 20 Gy, 50 Gy), the SF decreases significantly, indicating that radiation is effective at reducing cell survival in the T98G cell line. This dose-response relationship is consistent with the known effects of radiation on cell viability. Statistically significant difference (
p < 0.05) was found for treatment with 2 Gy radiation dose while with 5 Gy radiation dose treatment, a more statistically significant difference at p < 0.0001 was observed, indicating more effectiveness in cell killing than 2 Gy radiation treatment. Treatment with higher radiation doses at 10 Gy (
), 20 Gy (
), and 50 Gy (
), indicated extremely high levels of statistical significance (
Figure 4). This further justifies the use of higher radiation in clinical treatment technique such as stereotactic radiosurgery (SRS) for the treatment of glioblastoma.
At the p < 0.05 level of testing, there was no significant difference between treatment with radiation alone and treatment with radiation and pembrolizumab at the different radiation doses tested in this experiment. This suggests that the combination of pembrolizumab and radiation even at higher doses adds no significant effect on cell killing for glioblastoma. Furthermore,
Table S1 and S2 show the α/β data obtained through the clonogenic assay SF for the different conditions. The α/β showed no significant changes with the addition of pembrolizumab to radiation, suggesting little effect of these agents as radiosensitizers for T98G glioblastoma cells.
2.3. Molecular Readouts of Cell Death from Fluorescence-Guided Morphometry
2.3.1. Evidence of Increased DNA Damage with Pembrolizumab-Based RIT
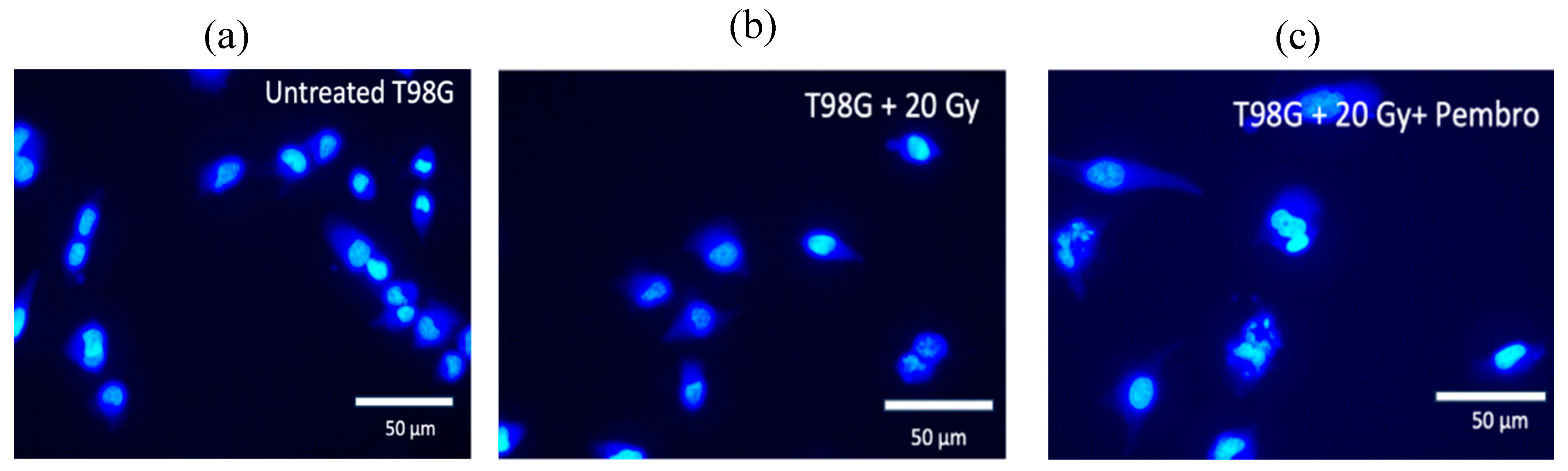
In fluorescence experiments, the dyes Calcein AM and Hoechst 33342 were utilized. The Hoechst dye stains DNA, while Calcein dye stains the cytoplasm, allowing for the selective examination of cellular constituents. The results of morphometric studies provide information regarding cell death and related pathways. Images and analysis of cells at about the time of treatment (0 hour,
Figure S1) and 36 hours after treatment are shown. Compared to untreated cells, T98G cells treated with 20 Gy radiation alone and the combination of 20 Gy radiation dose with concurrent pembrolizumab showed increased damage to cell nuclei. The average size of the nuclei in treated cells, and the cases of DNA mis-segregation that produced micronuclei and lost symmetry indicating DNA damage increased
43–45 (
Figure 5 and
Figure 6).
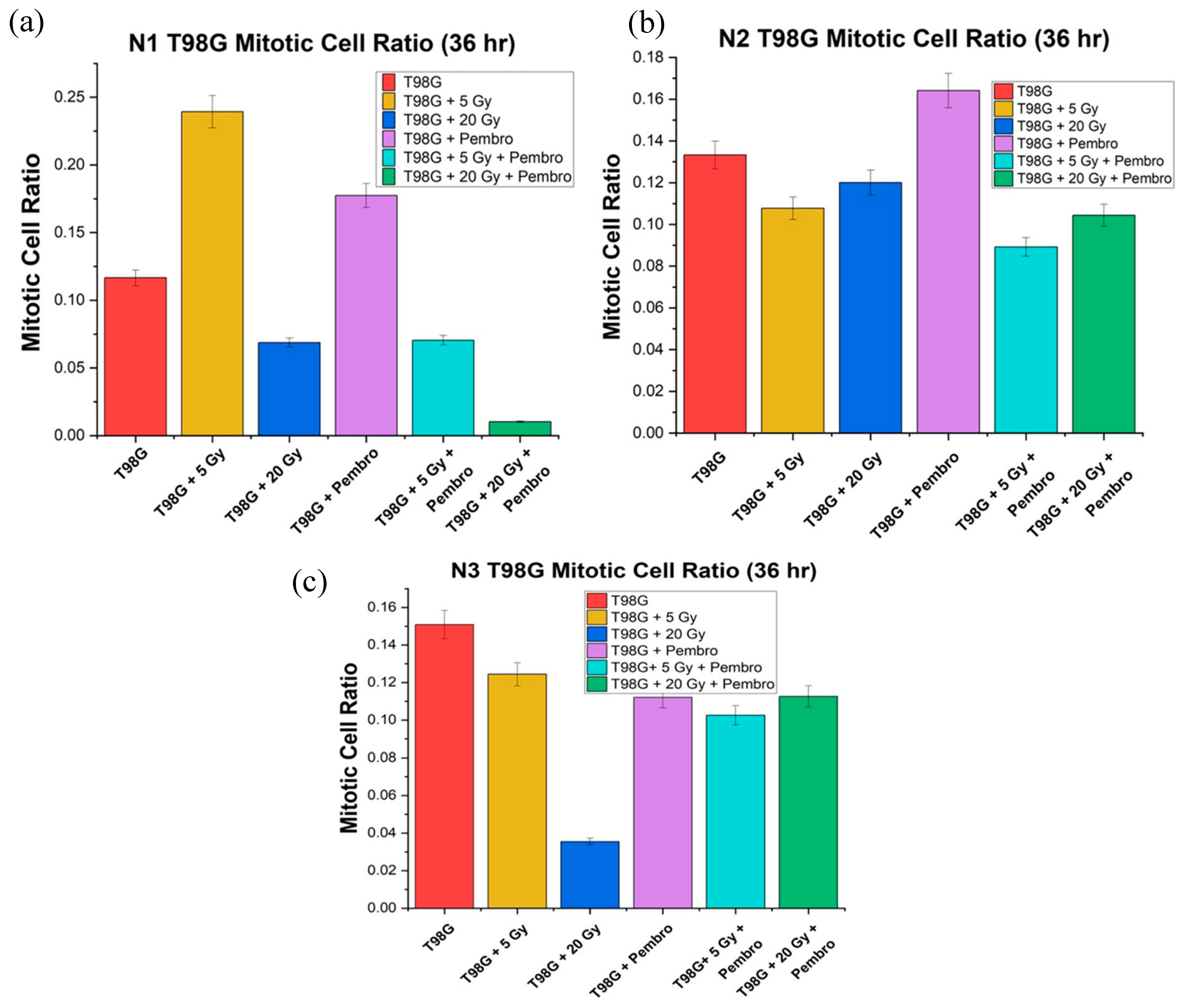
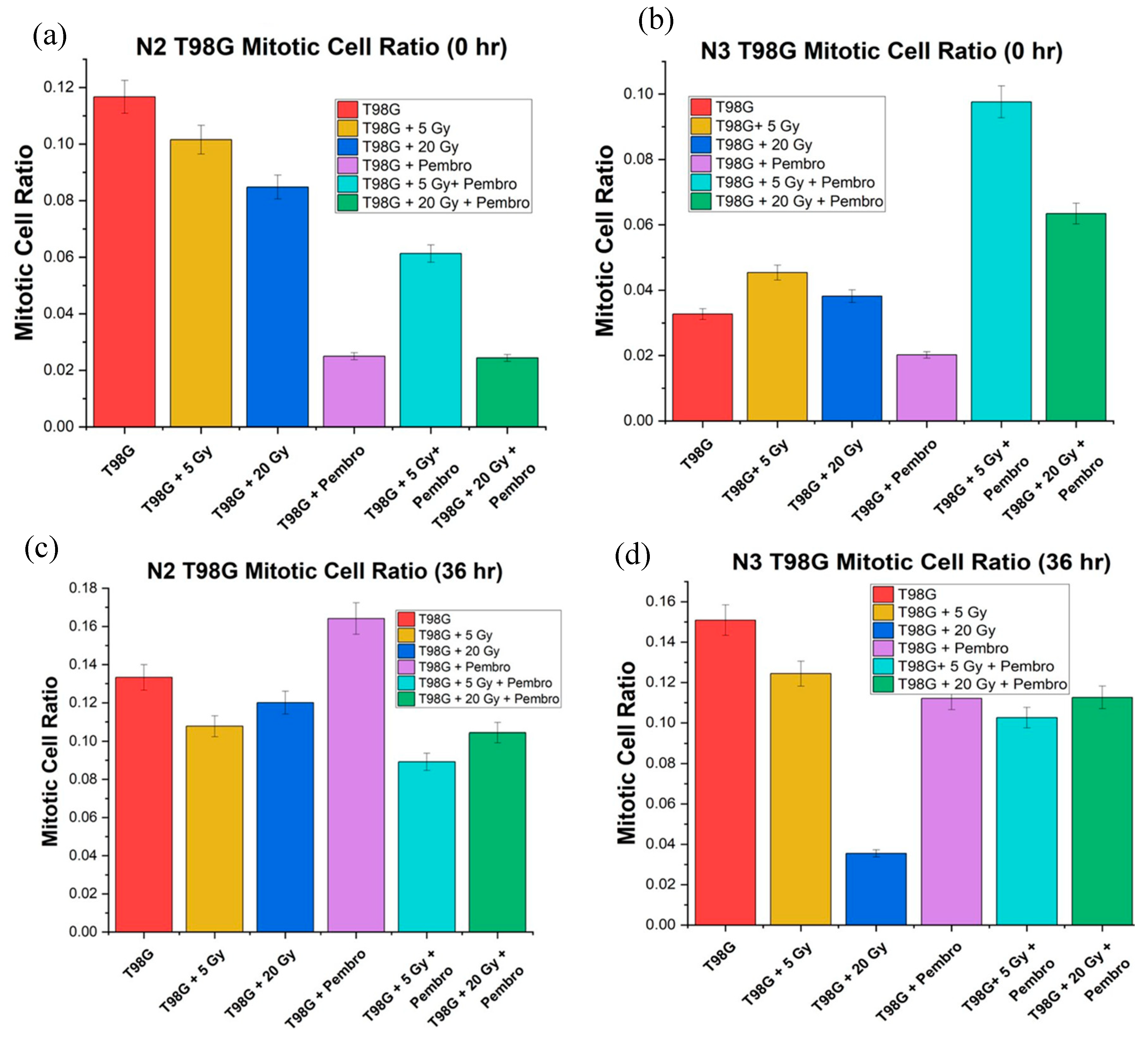
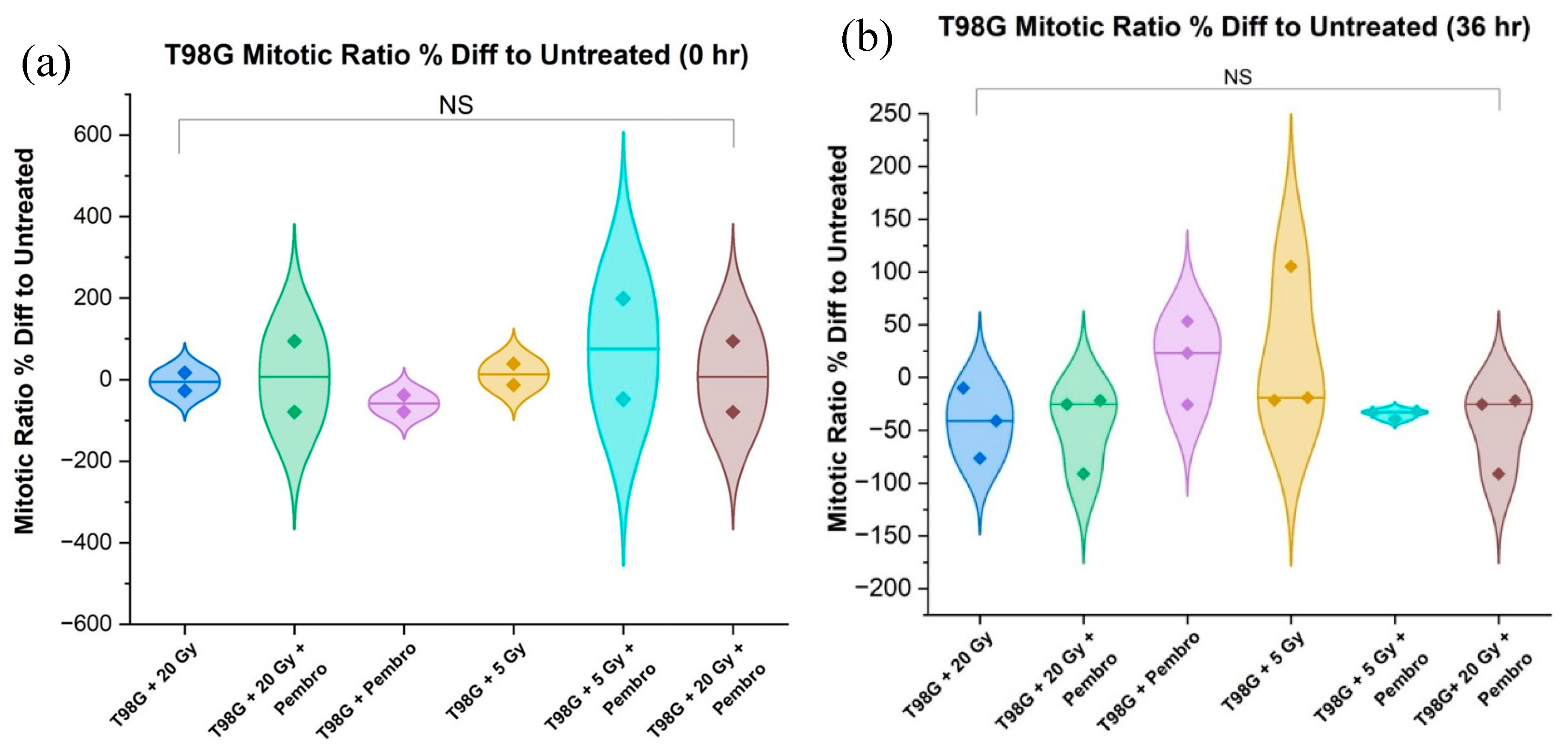
We directly compared the mitotic cell ratio for T98G immediately after treatment, (0 hour) and at 36 hours posttreatment in view of developing early molecular-level signals of potential treatment efficacy. As expected, there was an increased mitotic ratio observed in all conditions between 0 hr and 36 hr (
Figure 7). Treatment outcome at 36 hours was more stable than at 0 hours. T98G cells treated at the 20 Gy radiation dose alone and the combination of 20 Gy radiation dose and pembrolizumab had the lowest mitotic cell ratio relative to untreated T98G cells, signaling lower metabolic activity at higher radiation doses.
Treatments with 20 Gy radiation alone, 5 Gy plus pembrolizumab, and 20 Gy with pembrolizumab showed a decrease compared to untreated T98G cells. However, for the three trials, ANOVA showed no significant difference between all the all the conditions (
Figure 8).
Finally, for U87 cells, although cells treated with radiation alone and concurrent pembrolizumab combination showed some damage to cell nuclei (
Figure S1), no significant cellular or nuclear morphology changes were observed in U87 treated with radiation alone or concurrent pembrolizumab when used together 36 hours after treatment.
3. Discussion
The chemoresistance and radioresistance of GBM are not enough reasons for the poor outcome of clinical trials involving ICIs against GBM. New discoveries about the complexity of GBM continue to emerge. For instance, it has recently been reported that tumors from recurrent GBM cases have significantly more tumor-infiltrating lymphocytes and macrophages, as well as higher PD-L1 and PD-1 expression than tumors at primary diagnosis and benign brain specimens from epilepsy surgery46. Another new and startling complexity is the discovery that in GBM patients, PD-1, independent of PD-L1 ligation, can promote tumor growth47. About 8% of cells within the human GBM microenvironment were found to coexpress PD-1 and the marker for brain tumor-initiating cells which drive GBM growth. In fact, the tumor intrinsic promoting effects of PD-1 in GBM occurred even in the absence of T and B cells, pointing to a critical and yet nonimmune system resistance mechanism of patients with GBM to PD-1 and PD-L1 therapies47. These new findings accentuate the importance of our main question in this work: namely, what are the molecular and cellular-level effects of immunotherapeutic agents alone and in combination with radiotherapy on glioblastoma cells themselves? This question does not undermine but can help provide a more comprehensive picture regarding the roles of various components of the immune system when these components are also considered.
To address this question, we have developed and published in vitro assays which use biophysical parameters to quantify changes to cell behavior and cell death for both standard-of-care therapy, namely, temozolomide, (TMZ) against GBM, and radioimmunotherapy with durvalumab48, which is undergoing phase I/II clinical trials. Durvalumab, a PD-L1 inhibitor, did not alter cell migration as both radiation and TMZ did for the two glioblastoma cell lines (T98G and U87) tested. Cell–cell adhesion increased (p < 0.05) with durvalumab at a low radiation dose (5 Gy), unlike TMZ and radiation, which lowered cell–cell adhesion, in T98G cells. Thus, durvalumab shows a potential antimetastatic or anti-invasion effect at sublethal radiation doses. In the present work, we have carried out similar molecular and cellular level investigations using pembrolizumab, a PD-1 inhibitor. Motivated by previous research indicating that certain chemotherapeutic agents and radiation may inadvertently promote metastasis49–52 and having seen some of the cellular implications from research on the combination of immunotherapeutic agent, durvalumab52, and radiation, we conducted rigorous in-vitro assays to assess the cellular-level impact of pembrolizumab within the context of radioimmunotherapy for glioblastoma. Our study enabled us to simultaneously evaluate changes in various cellular characteristics, including cell death, motility, adhesion, and morphology, in real-time, following treatment. These findings lay the foundation for developing patient-adaptive therapeutic strategies, addressing an urgent need for more effective glioblastoma treatments.
An interesting aspect of our study lies in the observed trend shared by the responses of the two glioblastoma cell lines, T98G with its fibroblastic morphology53,54 and U87 with its epithelial morphology55, to the combination treatment of radiation and pembrolizumab. We found a 25% reduction in cell migration in both cell lines when subjected to concurrent 20 Gy radiation and pembrolizumab. Despite the lack of statistical significance in the one-way ANOVA analysis, this consistent response suggests an intriguing commonality between these cell lines: intriguing largely because they are known to respond differently10,56 to TMZ, the only FDA approved chemotherapy against GBM. The shared reduction in cell migration observed in both T98G and U87 cells when subjected to concurrent 20 Gy radiation and pembrolizumab is a notable finding that merits further investigation. This is because several reports have shown that radiation alone, in general, causes increase in migration of several cancer cells including GBM12,50–52. Since cell migration is a step in metastasis12,50–52,57,58 treatment strategies that reduce migration may be anti-metastatic as well, contributing to developing effective and targeted treatment strategies for glioblastoma and potentially other cancers.
Furthermore, the barrier function, Rb, in the Electric Cell Impedance Sensing (ECIS) model is useful in understanding potential pro- or anti-metastatic effects of therapeutic interventions since it is a readout of cell-cell adhesion. The loss of cell-cell adhesion, often associated with compromised tight junction integrity, is a fundamental step in the metastatic cascade, enabling invasive behavior as cancer cells detach from the primary tumor and infiltrate surrounding tissues. The absence of significant changes in barrier function following combined treatment with radiation and pembrolizumab suggests that this therapeutic approach does not markedly impact the integrity of tight junctions in T98G and U87 cells. Interesting, this result for pembrolizumab, an anti-PD-1 ICI, contrasts with our previous result for durvalumab, an anti-PD-L1 ICI, in which there was a reduction in barrier function (p < 0.05). This work, along with our previous RIT study using durvalumab, therefore presents a novel immune-system independent readout for characterizing RIT with various ICIs. This gives scientific rationale for similar investigations using other ICIs, such as the anti-CTLA-4 ICI, ipilimumab.
Regarding cell death, we found no statistically significant differences in cell death between the treatment groups. Whether exposed to pembrolizumab and 20 Gy radiation or 20 Gy radiation alone, the observed cell death rates did not significantly differ. This implies that pembrolizumab alone may not contribute significantly to cell death. Since pembrolizumab-induced radiosensitization has been reported in the case of clinical and system level RIT against skin cancer59, our work produces a novel insight: namely, that pembrolizumab is not an intrinsic radiosensitizer, at least against GBM.
Although radiation had the most significant reduction in cell survival (p < 0.0001) in our testing, in vitro assays that include the immune system in co-cultures will better reveal the cancer cell killing and tumor control abilities of the immune-modulating and immunotherapeutic agents we have tested, as well as similar agents under clinical trials. These methods have the potential of providing a framework for patient-specific targeted cancer therapy. Our assays provide biophysical markers by quantifying molecular level effects via morphometry in about 36-48 hours, cell migration within 7 days and clonogenic death between 14-21 days. These assays are potentially applicable to cells from patient biopsies and could rapidly evaluate the efficacy of treatment strategies for the overall improvement of treatment outcomes against aggressive cancers such as glioblastomas.
4. Materials and Methods
All the methods used in this work and most of the materials, are described in detail in our earlier durvalumab-based RIT work48 as well as previous chemo- and radiotherapy reports60–63. We briefly describe these methods again and provide details about the new materials employed in this work.
4.1. Cell Culture Materials, Drugs and Methods
We utilized T98G (ATCC CRL-1690) and U87 MG (ATCC HTB-14) glioblastoma multiforme cell lines for this research study. Both cell lines were purchased from the American Type Cell Culture Collection (ATCC, Manassas, VA, USA). We cultured the two cell lines following the same general cell culture protocol from ATCC, as described in detail in our previous work
48. The main steps are illustrated in
Figure S2.
Pembrolizumab (SigmaMAb Pembrolizumab MSQC24-0.5MG, SLCH2041) with a concentration of 10 µg/mL was used in this research as an immunotherapeutic agent. The drug was purchased from Sigma-Aldrich, USA. Pembrolizumab (anti-PD-1) is a potent, highly selective, fully humanized immunoglobulin (Ig) G4-kappa monoclonal antibody against PD-1 with potential immune checkpoint inhibitory and antineoplastic activities. The purity is 99.06%, protein concentration is 9.97 mg/ml, and Endotoxin Level is ≤1 EU/mg. The appropriate in vitro doses of pembrolizumab that are both effective and relevant in a clinical setting are currently not uniformly established. This is due to ongoing clinical trials to identify the appropriate dosage for patients under different conditions64–66. The in vitro concentration of pembrolizumab used in this experiment was determined to be 10 g/mL, as observed for similar experiments in two well-reviewed previous studies67–69 that used pembrolizumab within the range of 2 to 10 g/mL, and by following the guide provided by Liston and Davis, which lists the in vivo dosage corresponding to a standard clinical dose of anticancer drug.70 The half maximal effective concentration (EC50) for pembrolizumab is 39.90 ng/ml (34.01–46.80 g/ml)68 and a typical standard dose for GBM patients is 2 mg/kg patient, which in-vitro, corresponds to a maximum plasma concentration of 0.44090mol/L.70
4.2. Cell Irradiation Migration Measurements
The Faxitron CellRad compact x-ray device was used to deliver radiation at clinically relevant doses
48. We used a commercially available Electric Cell-Substrate Impedance Sensing (ECIS) device from Applied Biophysics (NY) for electrically assessing cell characteristics, including morphology, proliferation, migration, and behavior
48. The procedure for loading and running the ECIS is illustrated in
Figure S3.
4.3. Fluorescence Microscopy, Clonogenic Assays and Statistical Analyses
Cell fluorescence microscopy was performed with a Zeiss Vert.A1 AXIO fluorescence microscope equipped with an inserted USB microscope camera (AmScope MU300) and captured with the AmScope (86x) image capture program. Hoechst 33342 (ThermoFisher Scientific) and Calcein AM (Invitrogen by ThermoFisher Scientific) fluorescent dyes were used to stain the cells for imaging (
Figure S1). The imaging procedures and morphometric analyses were done as already described
48. Clonogenic assays were also performed, and results analyzed as previously reported
48. Some of these procedures are illustrated in
Figure S4. The equations for the extraction of parameters, all statistical analyses procedures for the determination of statistical significance and for comparisons were done as previously presented elaborately
48, including analysis of variance (ANOVA).
5. Conclusions
Our multimodal in vitro testing serves as a platform that could be upgraded by addition of immune system components to provide useful molecular and cellular level insights into therapeutic windows for improved brain tumor treatment outcomes. The methods established in this research quantify molecular and cellular level biophysical parameters that evaluate the performance of ICIs. They offer the potential for the development of patient-specific targeted therapies. Advancements in this direction can empower healthcare providers to assess the effectiveness of different agents and therapies against a patient's specific cancer before treatment. This personalized approach may significantly improve therapeutic outcomes by aiding in selecting optimal treatment strategies especially against primary brain tumors such as glioblastoma.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Green fluorescing Calcein and blue-fluorescing Hoechst-stained U87 cells; Figure S2: Cell culture protocol; Figure S3: Loading and preparation of the ECIS; Figure S4: Preparation of clonogenic assays.
Author Contributions
Conceptualization, A.E.; methodology, A.E., B.I., K.M.,A.K, J.H., M.S., A.B., E.J., A.H., Y.W.; software, B.I.,Y.W.; validation, A.E. and B.I.; formal analysis, B.I.; investigation, B.I., K.M.,A.K, J.H., M.S., A.B., E.J., A.H., Y.W.; resources, A.E.; data curation, A.E.; writing—original draft preparation, B.I., A.E.; writing—review and editing, A.E.; visualization, B.I.,K.M.,A.K.; supervision, A.E.; project administration, A.E.; funding acquisition, A.E.. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded a Creighton University Startup grant (to AEE) and a CURAS Faculty Research Award, MIRA, to AEE.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are already contained in the article itself and also in
Supplementary Materials. Raw data are available upon reasonable request.
Acknowledgments
We thank Dr Chi Zhang, MD, PhD, of the University of Nebraska College of Medicine for very helpful discussions on the state of clinical trials involving ICIs and GBM.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Tamimi, A. F. & Juweid, M. Epidemiology and Outcome of Glioblastoma. In: De Vleeschouwer S, Editor. Glioblastoma. Codon Publications (Codon Publications, 2017).
- Karachi, A., Dastmalchi, F., Mitchell, D. A. & Rahman, M. Temozolomide for immunomodulation in the treatment of glioblastoma. Neuro Oncol 20, 1566–1572 (2018). [CrossRef]
- Dhermain, F. Radiotherapy of high-grade gliomas: current standards and new concepts, innovations in imaging and radiotherapy, and new therapeutic approaches. Chin J Cancer 33, 16–24 (2014). [CrossRef]
- Stupp, R. et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. New England Journal of Medicine 352, 987–996 (2005). [CrossRef]
- Zhu, P., Du, X. L., Lu, G. & Zhu, J.-J. Survival benefit of glioblastoma patients after FDA approval of temozolomide concomitant with radiation and bevacizumab: A population-based study. Oncotarget 8, 44015–44031 (2017). [CrossRef]
- Stupp, R. et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma. JAMA 318, 2306 (2017). [CrossRef]
- Ostrom, Q. T. et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro Oncol 24, v1–v95 (2022). [CrossRef]
- Gzell, C., Back, M., Wheeler, H., Bailey, D. & Foote, M. Radiotherapy in Glioblastoma: the Past, the Present and the Future. Clin Oncol 29, 15–25 (2017). [CrossRef]
- Mann, J., Ramakrishna, R., Magge, R. & Wernicke, A. G. Advances in Radiotherapy for Glioblastoma. Front Neurol 8, (2018). [CrossRef]
- Lee, S. Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis 3, 198–210 (2016). [CrossRef]
- Lehtipuro, S., Nykter, M. & Granberg, K. J. Modes of immunosuppression in glioblastoma microenvironment. Oncotarget 10, 920–921 (2019). [CrossRef]
- Chaffer, C. L. & Weinberg, R. A. A Perspective on Cancer Cell Metastasis. Science (1979) 331, 1559–1564 (2011). [CrossRef]
- Wu, W. et al. Glioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacol Res 171, 105780 (2021). [CrossRef]
- Shiravand, Y. et al. Immune Checkpoint Inhibitors in Cancer Therapy. Current Oncology vol. 29 3044–3060 Preprint at https://doi.org/10.3390/curroncol29050247 (2022). [CrossRef]
- Varayathu, H., Sarathy, V., Thomas, B. E., Mufti, S. S. & Naik, R. Combination Strategies to Augment Immune Check Point Inhibitors Efficacy - Implications for Translational Research. Front Oncol 11, 1844 (2021). [CrossRef]
- Borghaei, H. et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N Engl J Med 373, 1627–1639 (2015). [CrossRef]
- Liebl, M. C. & Hofmann, T. G. Identification of responders to immune checkpoint therapy: which biomarkers have the highest value? J. Eur. Acad. Dermatol. Venereol 33, 52–56 (2019).
- Shiravand, Y. et al. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol 29, 3044–3060 (2022). [CrossRef]
- Huang, Q. et al. Comparative Efficacy and Safety of PD-1/PD-L1 Inhibitors for Patients with Solid Tumors: A Systematic Review and Bayesian Network Meta-analysis. J Cancer 12, 1133–1143 (2021).
- Wang, E. J. et al. Immunotherapy Resistance in Glioblastoma. Front Genet 12, 1–22 (2021). [CrossRef]
- Arrieta, V. A. et al. Immune checkpoint blockade in glioblastoma: from tumor heterogeneity to personalized treatment. Journal of Clinical Investigation 133, (2023). [CrossRef]
- Rocha Pinheiro, S. L. et al. Immunotherapy in glioblastoma treatment: Current state and future prospects. World J Clin Oncol 12, 138–159 (2023). [CrossRef]
- McGranahan, T., Therkelsen, K. E., Ahmad, S. & Nagpal, S. Current State of Immunotherapy for Treatment of Glioblastoma. Curr Treat Options Oncol 20, 24 (2019). [CrossRef]
- Karachi, A., Dastmalchi, F., Mitchell, D. A. & Rahman, M. Temozolomide for immunomodulation in the treatment of glioblastoma. Neuro Oncol 20, 1566–1572 (2018). [CrossRef]
- Derer, A., Frey, B., Fietkau, R. & Gaipl, U. S. Immune-modulating properties of ionizing radiation: rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitors. CANCER IMMUNOL IMMUN 65, 779–786 (2016). [CrossRef]
- Chakravarti, A. et al. Temozolomide-Mediated Radiation Enhancement in Glioblastoma: A Report on Underlying Mechanisms. Clin. Cancer Res. 12, 4738–4746 (2006). [CrossRef]
- Pouessel, D. et al. OS4.4 Hypofractionated stereotactic radiotherapy and anti-PDL1 Durvalumab combination in recurrent glioblastoma: Results of the phase I part of the phase I/II STERIMGLI trial. Neuro Oncol 21, iii10–iii11 (2019). [CrossRef]
- Welsh, J. et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: a randomized phase I/II trial. J Immunother Cancer 8, e001001 (2020). [CrossRef]
- Clinical Trials. https://clinicaltrials.gov/study/NCT03018288 (2022).
- Ahluwalia, M. S. et al. Phase II study of pembrolizumab plus SurVaxM for glioblastoma at first recurrence. J. Clin. Oncol. 38, TPS2581–TPS2581 (2020). [CrossRef]
- Merrick, M. et al. In vitro radiotherapy and chemotherapy alter migration of brain cancer cells before cell death. Biochem Biophys Rep 27, 101071 (2021). [CrossRef]
- Asuquo, M. I. et al. Microfluidic Microcirculation Mimetic as a Tool for the Study of Rheological Characteristics of Red Blood Cells in Patients with Sickle Cell Anemia. Applied Sciences 12, 4394 (2022). [CrossRef]
- Abraham, A. et al. Microfluidic Microcirculation Mimetic for Exploring Biophysical Mechanisms of Chemotherapy-Induced Metastasis. Micromachines (Basel) 14, 1653 (2023). [CrossRef]
- J Hong, K. K. M. M. C. C. S. K. et al. Electrical cell-substrate impedance sensing as a non-invasive tool for cancer cell study. Analyst vol. 136 237–245 (Royal Society of Chemistry, 2011).
- Robilliard, L. D. et al. Can ECIS Biosensor Technology Be Used to Measure the Cellular Responses of Glioblastoma Stem Cells? Biosensors (Basel) 11, 498 (2021).
- Das, D., Kamil, F. A., Biswas, K. & Das, S. Electrical characterization of suspended HeLa cells using ECIS based biosensor. in Proceedings of the International Conference on Sensing Technology, ICST 734–737 (2012). [CrossRef]
- Wirtz, D., Konstantopoulos, K. & Searson, P. C. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat Rev Cancer 11, 512–22 (2011). [CrossRef]
- Walter, Y. et al. Development of In Vitro Assays for Advancing Radioimmunotherapy against Brain Tumors. Biomedicines 10, 1796 (2022). [CrossRef]
- Ooft, S. N. et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci Transl Med 11, 2574 (2019). [CrossRef]
- Ratliff, M. et al. Patient-Derived Tumor Organoids for Guidance of Personalized Drug Therapies in Recurrent Glioblastoma. Int J Mol Sci 23, 6572 (2022). [CrossRef]
- Gray, J. E., Villegas, A. & Daniel, D. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC—Update from PACIFIC. Journal of Thoracic Oncology 15,. [CrossRef]
- Dang, T. O., Ogunniyi, A., Barbee, M. S. & Drilon, A. Pembrolizumab for the treatment of PD-L1 positive advanced or metastatic non-small cell lung cancer. Expert Rev Anticancer Ther 16, 13–20 (2016). [CrossRef]
- Garribba, L. et al. Short-term molecular consequences of chromosome mis-segregation for genome stability. Nat Commun 14, 1353 (2023). [CrossRef]
- Durante, M. & Formenti, S. C. Radiation-Induced Chromosomal Aberrations and Immunotherapy: Micronuclei, Cytosolic DNA, and Interferon-Production Pathway. Front Oncol 8, (2018). [CrossRef]
- Walter, Y. Advancing Radioimmunotherapy for Brain Tumors using In Vitro Assays. (2022).
- Wang, F. et al. Comparison of tumor immune environment between newly diagnosed and recurrent glioblastoma including matched patients. J Neurooncol 159, 163–175 (2022). [CrossRef]
- Mirzaei, R. et al. PD-1 independent of PD-L1 ligation promotes glioblastoma growth through the NFKB pathway. Sci Adv 7, 1–17 (2021). [CrossRef]
- Walter, Y. et al. Development of In Vitro Assays for Advancing Radioimmunotherapy against Brain Tumors. Biomedicines 10, 1796 (2022). [CrossRef]
- Karagiannis, G. S., Condeelis, J. S. & Oktay, M. H. Chemotherapy-induced metastasis: mechanisms and translational opportunities. Clin Exp Metastasis 35, 269–284 (2018). [CrossRef]
- Prathivadhi-Bhayankaram, S. V. et al. Chemotherapy impedes in vitro microcirculation and promotes migration of leukemic cells with impact on metastasis. Biochem Biophys Res Commun 479, 841–846 (2016). [CrossRef]
- Merrick, M. et al. In vitro radiotherapy and chemotherapy alter migration of brain cancer cells before cell death. Biochem Biophys Rep 27, 101071 (2021). [CrossRef]
- Walter, Y. et al. Development of In Vitro Assays for Advancing Radioimmunotherapy against Brain Tumors. Biomedicines 10, 1796 (2022). [CrossRef]
- Kiseleva, L. N., Kartashev, A. V., Vartanyan, N. L., Pinevich, A. A. & Samoilovich, M. P. A172 and T98G cell lines characteristics. Cell tissue biol 10, 341–348 (2016). [CrossRef]
- T98G [T98-G] Intended Use. . www.atcc.org.
- U-87 MG HTB-14 TM. www.atcc.org .
- Poon, M. T. C., Bruce, M., Simpson, J. E., Hannan, C. J. & Brennan, P. M. Temozolomide sensitivity of malignant glioma cell lines – a systematic review assessing consistencies between in vitro studies. BMC Cancer 21, 1240 (2021). [CrossRef]
- Hanahan, D. & Weinberg, R. A. Hallmarks of Cancer: The Next Generation. Cell 144, 646–674 (2011). [CrossRef]
- Hanahan, D. & Weinberg, R. A. The Hallmarks of Cancer. Cell 100, 57–70 (2000).
- Sibaud, V. et al. Acute skin reaction suggestive of pembrolizumab-induced radiosensitization. Melanoma Res 25, 555–558 (2015). [CrossRef]
- Merrick, M. et al. In vitro radiotherapy and chemotherapy alter migration of brain cancer cells before cell death. Biochem Biophys Rep 27, 101071 (2021). [CrossRef]
- Djam, K. H., Lee, B. H., Suresh, S. & Ekpenyong, A. E. Quantum Dots for Assessment of Reactive Oxygen Species Accumulation During Chemotherapy and Radiotherapy. in Methods in Molecular Biology vol. 2135 293–303 (Humana Press Inc., 2020).
- Lee, B. H., Suresh, S. & Ekpenyong, A. Fluorescence intensity modulation of CdSe/ZnS quantum dots assesses ROS during chemotherapy and radiotherapy for cancer cells. J Biophotonics 12, e201800172 (2018).
- Prathivadhi-Bhayankaram, S. V. S. V. S. V. et al. Chemotherapy impedes in vitro microcirculation and promotes migration of leukemic cells with impact on metastasis. Biochem Biophys Res Commun 479, 841–846 (2016).
- Study of Pembrolizumab Plus SurVaxM for Glioblastoma at First Recurrence. https://www.clinicaltrials.gov/ct2/show/NCT04013672.
- de Groot, J. et al. Window-of-opportunity clinical trial of pembrolizumab in patients with recurrent glioblastoma reveals predominance of immune-suppressive macrophages. Neuro Oncol 22, 539–549 (2020).
- Efficacy and Safety of Pembrolizumab (MK-3475) Plus Lenvatinib (E7080/MK-7902) in Previously Treated Participants With Select Solid Tumors (MK-7902-005/E7080-G000-224/LEAP-005). https://www.clinicaltrials.gov/ct2/show/NCT03797326.
- Toor, S. M., Syed Khaja, A. S., Alkurd, I. & Elkord, E. In-vitro effect of pembrolizumab on different T regulatory cell subsets. Clin Exp Immunol 191, 189–197 (2018).
- De Sousa Linhares, A. et al. Therapeutic PD-L1 antibodies are more effective than PD-1 antibodies in blocking PD-1/PD-L1 signaling. Sci Rep 9, 11472 (2019).
- Walker, J. W., Xu, L., Smylie, M. & Elahi, S. Effect of high-dose corticosteroids on CD4+ or CD8+ lymphocyte proliferation or IL-2 production after stimulation with pembrolizumab. Journal of Clinical Oncology 35, e14587–e14587 (2017). [CrossRef]
- Liston, D. R. & Davis, M. Clinically Relevant Concentrations of Anticancer Drugs: A Guide for Nonclinical Studies. Clin. Cancer Res. 23, 3489–3498 (2017). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).