Submitted:
05 December 2023
Posted:
08 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
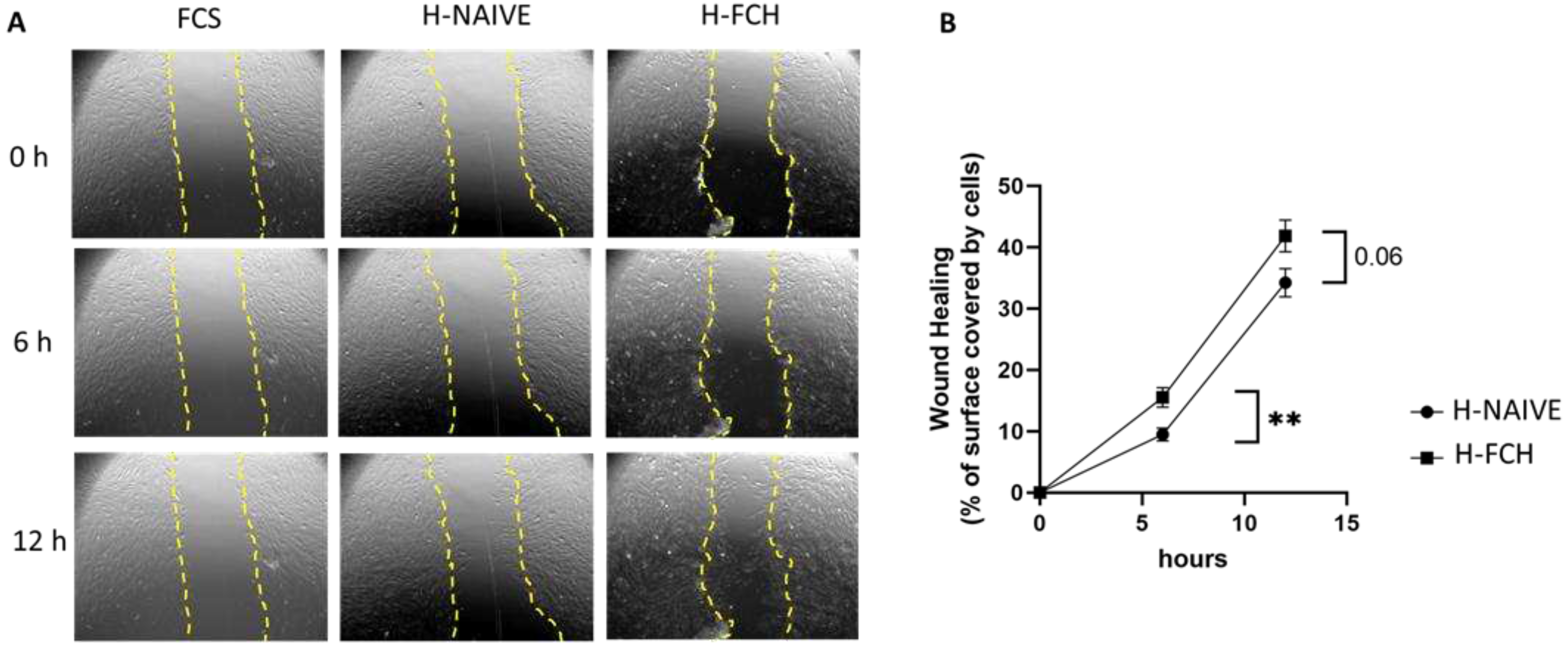
2.1. FCH-Enriched Serum Improved HDF Migration during Wound Healing Assay
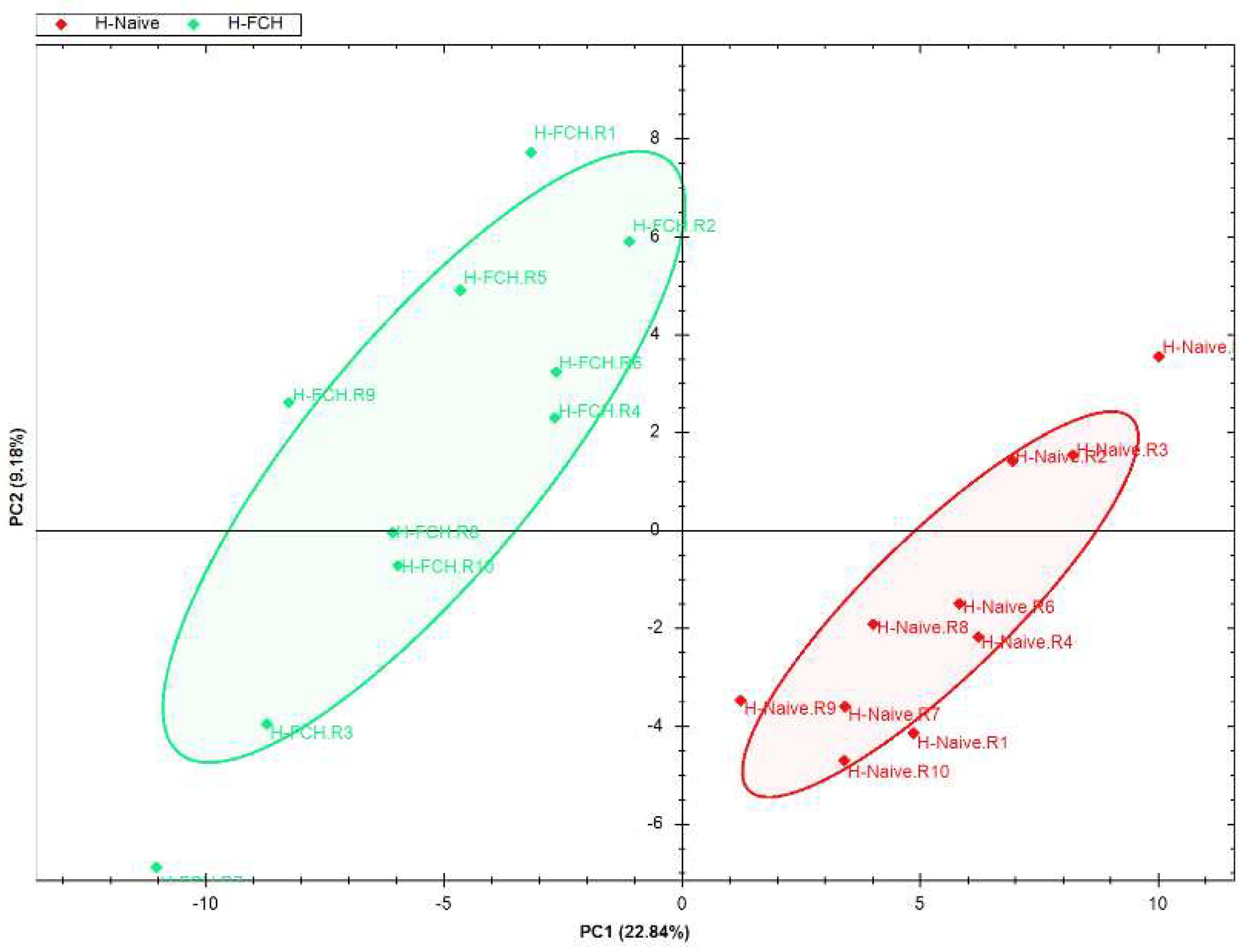
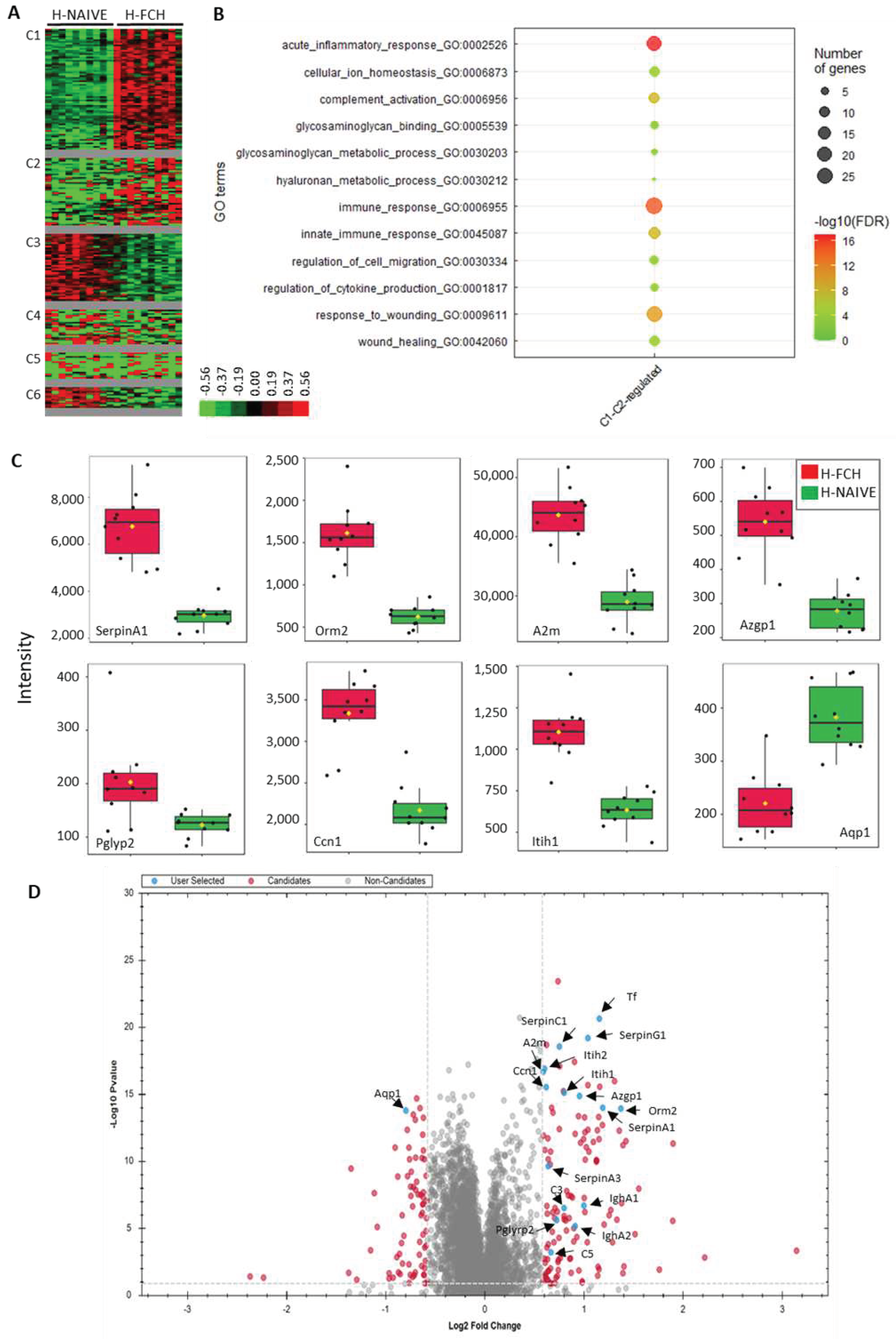
2.2. FCH-Enriched Serum Modulates Protein Expression Involved in Healing
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.1.1. Ethics and Clinical Trial
4.1.2. Product Tested
4.1.3. Human Study Protocol
4.1.4. Human Primary Dermal Fibroblasts (HDFs) Cultures
4.2. Wound Healing
4.2.1. Cell Treatment
4.2.2. Statistics
4.3. DiaPASEF Proteomic Analysis
4.3.1. Cultures for diaPASEF Proteomic Analysis
4.3.2. Sample Preparation for diaPASEF Analysis
4.3.3. Sample Preparation for Spectral Library
4.3.4. Nanoliquid Chromatography Coupled with Tandem Mass Spectrometry (NanoLC-MS/MS) Acquisition of Fractions for Spectral Library–DDA-PASEF
4.3.5. NanoLC-MS/MS Acquisition of Samples in diaPASEF Mode
4.3.6. MS Data Processing
4.3.7. Data Analysis–Library Generation
4.3.8. Library Search of DIA Data
4.3.9. Quantification and Statistical Analyses of Proteomics Data
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elias, P.M. The Skin Barrier as an Innate Immune Element. Semin Immunopathol 2007, 29, 3–14. [CrossRef]
- Shpichka, A.; Butnaru, D.; Bezrukov, E.A.; Sukhanov, R.B.; Atala, A.; Burdukovskii, V.; Zhang, Y.; Timashev, P. Skin Tissue Regeneration for Burn Injury. Stem Cell Res Ther 2019, 10, 94. [CrossRef]
- Quondamatteo, F. Skin and Diabetes Mellitus: What Do We Know? Cell Tissue Res 2014, 355, 1–21. [CrossRef]
- Boguniewicz, M.; Leung, D.Y.M. Atopic Dermatitis: A Disease of Altered Skin Barrier and Immune Dysregulation. Immunological Reviews 2011, 242, 233–246. [CrossRef]
- Paller, A.S.; Kong, H.H.; Seed, P.; Naik, S.; Scharschmidt, T.C.; Gallo, R.L.; Luger, T.; Irvine, A.D. The Microbiome in Patients with Atopic Dermatitis. Journal of Allergy and Clinical Immunology 2019, 143, 26–35. [CrossRef]
- Elder, J.T.; Bruce, A.T.; Gudjonsson, J.E.; Johnston, A.; Stuart, P.E.; Tejasvi, T.; Voorhees, J.J.; Abecasis, G.R.; Nair, R.P. Molecular Dissection of Psoriasis: Integrating Genetics and Biology. Journal of Investigative Dermatology 2010, 130, 1213–1226. [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. European Surgical Research 2016, 58, 81–94. [CrossRef]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Marine Drugs 2022, 20, 61. [CrossRef]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen—Sources and Applications. Molecules 2019, 24, 4031. [CrossRef]
- Hu, Z.; Yang, P.; Zhou, C.; Li, S.; Hong, P. Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis Niloticus): Characterization and Wound Healing Evaluation. Mar Drugs 2017, 15, 102. [CrossRef]
- Yang, T.; Zhang, K.; Li, B.; Hou, H. Effects of Oral Administration of Peptides with Low Molecular Weight from Alaska Pollock (Theragra Chalcogramma) on Cutaneous Wound Healing. Journal of Functional Foods 2018, 48, 682–691. [CrossRef]
- Wang, J.; Xu, M.; Liang, R.; Zhao, M.; Zhang, Z.; Li, Y. Oral Administration of Marine Collagen Peptides Prepared from Chum Salmon (Oncorhynchus Keta) Improves Wound Healing Following Cesarean Section in Rats. Food & Nutrition Research 2015, 59, 26411. [CrossRef]
- Zhang, Z.; Wang, J.; Ding, Y.; Dai, X.; Li, Y. Oral Administration of Marine Collagen Peptides from Chum Salmon Skin Enhances Cutaneous Wound Healing and Angiogenesis in Rats. Journal of the Science of Food and Agriculture 2011, 91, 2173–2179. [CrossRef]
- Valcarcel, J.; Novoa-Carballal, R.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A. Glycosaminoglycans from Marine Sources as Therapeutic Agents. Biotechnology Advances 2017, 35, 711–725. [CrossRef]
- Campo, G.M.; Avenoso, A.; Campo, S.; D’Ascola, A.; Traina, P.; Samà, D.; Calatroni, A. Glycosaminoglycans Modulate Inflammation and Apoptosis in LPS-Treated Chondrocytes. Journal of Cellular Biochemistry 2009, 106, 83–92. [CrossRef]
- Tan, G.-K.; Tabata, Y. Chondroitin-6-Sulfate Attenuates Inflammatory Responses in Murine Macrophages via Suppression of NF-κB Nuclear Translocation. Acta Biomaterialia 2014, 10, 2684–2692. [CrossRef]
- Henrotin, Y.; Herman, J.; Uebelhoer, M.; Wauquier, F.; Boutin-Wittrant, L.; Donneau, A.-F.; Monseur, J.; Fotso, V.M.; Duquenne, M.; Wagner, M.; et al. Oral Supplementation with Fish Cartilage Hydrolysate in an Adult Population Suffering from Knee Pain and Function Discomfort: Results from an Innovative Approach Combining an Exploratory Clinical Study and an Ex Vivo Clinical Investigation. BMC Musculoskeletal Disorders 2023, 24, 748. [CrossRef]
- Krichen, F.; Ghlissi, Z.; Abdallah, R.B.; Kallel, R.; Martinez-Alvarez, O.; Carmen Gómez-Guillén, M.; Sila, A.; Boudawara, T.; Sahnoun, Z.; Bougatef, A. Glycosaminoglycans from Grey Triggerfish and Smooth Hound Skins: Rheological, Anti-Inflammatory and Wound Healing Properties. International Journal of Biological Macromolecules 2018, 118, 965–975. [CrossRef]
- Wauquier, F.; Boutin-Wittrant, L.; Bouvret, E.; Le Faouder, J.; Roux, V.; Macian, N.; Pickering, G.; Wittrant, Y. Benefits of Circulating Human Metabolites from Fish Cartilage Hydrolysate on Primary Human Dermal Fibroblasts, an Ex Vivo Clinical Investigation for Skin Health Applications. Nutrients 2022, 14, 5027. [CrossRef]
- Maia Campos, P.M.B.G.; Franco, R.S.B.; Kakuda, L.; Cadioli, G.F.; Costa, G.M.D.; Bouvret, E. Oral Supplementation with Hydrolyzed Fish Cartilage Improves the Morphological and Structural Characteristics of the Skin: A Double-Blind, Placebo-Controlled Clinical Study. Molecules 2021, 26, 4880. [CrossRef]
- Wauquier, F.; Daneault, A.; Granel, H.; Prawitt, J.; Fabien Soulé, V.; Berger, J.; Pereira, B.; Guicheux, J.; Rochefort, G.Y.; Meunier, N.; et al. Human Enriched Serum Following Hydrolysed Collagen Absorption Modulates Bone Cell Activity: From Bedside to Bench and Vice Versa. Nutrients 2019, 11, 1249. [CrossRef]
- Wauquier, F.; Mevel, E.; Krisa, S.; Richard, T.; Valls, J.; Hornedo-Ortega, R.; Granel, H.; Boutin-Wittrant, L.; Urban, N.; Berger, J.; et al. Chondroprotective Properties of Human-Enriched Serum Following Polyphenol Extract Absorption: Results from an Exploratory Clinical Trial. Nutrients 2019, 11, 3071. [CrossRef]
- Kleinnijenhuis, A.J.; van Holthoon, F.L.; Maathuis, A.J.H.; Vanhoecke, B.; Prawitt, J.; Wauquier, F.; Wittrant, Y. Non-Targeted and Targeted Analysis of Collagen Hydrolysates during the Course of Digestion and Absorption. Anal Bioanal Chem 2020, 412, 973–982. [CrossRef]
- Wauquier, F.; Boutin-Wittrant, L.; Viret, A.; Guilhaudis, L.; Oulyadi, H.; Bourafai-Aziez, A.; Charpentier, G.; Rousselot, G.; Cassin, E.; Descamps, S.; et al. Metabolic and Anti-Inflammatory Protective Properties of Human Enriched Serum Following Artichoke Leaf Extract Absorption: Results from an Innovative Ex Vivo Clinical Trial. Nutrients 2021, 13, 2653. [CrossRef]
- Wauquier, F.; Boutin-Wittrant, L.; Pourtau, L.; Gaudout, D.; Moras, B.; Vignault, A.; Monchaux De Oliveira, C.; Gabaston, J.; Vaysse, C.; Bertrand, K.; et al. Circulating Human Serum Metabolites Derived from the Intake of a Saffron Extract (Safr’InsideTM) Protect Neurons from Oxidative Stress: Consideration for Depressive Disorders. Nutrients 2022, 14, 1511. [CrossRef]
- Demichev, V.; Szyrwiel, L.; Yu, F.; Teo, G.C.; Rosenberger, G.; Niewienda, A.; Ludwig, D.; Decker, J.; Kaspar-Schoenefeld, S.; Lilley, K.S.; et al. Dia-PASEF Data Analysis Using FragPipe and DIA-NN for Deep Proteomics of Low Sample Amounts. Nat Commun 2022, 13, 3944. [CrossRef]
- Guergues, J.; Wohlfahrt, J.; Stevens, S.M.Jr. Enhancement of Proteome Coverage by Ion Mobility Fractionation Coupled to PASEF on a TIMS–QTOF Instrument. J. Proteome Res. 2022, 21, 2036–2044. [CrossRef]
- Sodhi, H.; Panitch, A. Glycosaminoglycans in Tissue Engineering: A Review. Biomolecules 2021, 11, 29. [CrossRef]
- Cheng, F.; Shen, Y.; Mohanasundaram, P.; Lindström, M.; Ivaska, J.; Ny, T.; Eriksson, J.E. Vimentin Coordinates Fibroblast Proliferation and Keratinocyte Differentiation in Wound Healing via TGF-β–Slug Signaling. Proceedings of the National Academy of Sciences 2016, 113, E4320–E4327. [CrossRef]
- Liarte, S.; Bernabé-García, Á.; Nicolás, F.J. Role of TGF-β in Skin Chronic Wounds: A Keratinocyte Perspective. Cells 2020, 9, 306. [CrossRef]
- Law, R.H.; Zhang, Q.; McGowan, S.; Buckle, A.M.; Silverman, G.A.; Wong, W.; Rosado, C.J.; Langendorf, C.G.; Pike, R.N.; Bird, P.I.; et al. An Overview of the Serpin Superfamily. Genome Biology 2006, 7, 216. [CrossRef]
- Park, D.J.; Duggan, E.; Ho, K.; Dorschner, R.A.; Dobke, M.; Nolan, J.P.; Eliceiri, B.P. Serpin-Loaded Extracellular Vesicles Promote Tissue Repair in a Mouse Model of Impaired Wound Healing. Journal of Nanobiotechnology 2022, 20, 474. [CrossRef]
- Grimstein, C.; Choi, Y.-K.; Satoh, M.; Lu, Y.; Wang, X.; Campbell-Thompson, M.; Song, S. Combination of Alpha-1 Antitrypsin and Doxycycline Suppresses Collagen-Induced Arthritis. The Journal of Gene Medicine 2010, 12, 35–44. [CrossRef]
- Janciauskiene, S.M.; Nita, I.M.; Stevens, T. A1-Antitrypsin, Old Dog, New Tricks: A1-Antitrypsin Exerts in Vitro Anti-Inflammatory Activity in Human Monocytes by Elevating cAMP. Journal of Biological Chemistry 2007, 282, 8573–8582. [CrossRef]
- Congote, L.F.; Temmel, N.; Sadvakassova, G.; Dobocan, M.C. Comparison of the Effects of Serpin A1, a Recombinant Serpin A1-IGF Chimera and Serpin A1 C-Terminal Peptide on Wound Healing. Peptides 2008, 29, 39–46. [CrossRef]
- Hoffmann, D.C.; Textoris, C.; Oehme, F.; Klaassen, T.; Goppelt, A.; Römer, A.; Fugmann, B.; Davidson, J.M.; Werner, S.; Krieg, T.; et al. Pivotal Role for A1-Antichymotrypsin in Skin Repair*. Journal of Biological Chemistry 2011, 286, 28889–28901. [CrossRef]
- Kirschfink, M.; Nürnberger, W. C1 Inhibitor in Anti-Inflammatory Therapy: From Animal Experiment to Clinical Application. Molecular Immunology 1999, 36, 225–232. [CrossRef]
- Begieneman, M.P.V.; Kubat, B.; Ulrich, M.M.W.; Hahn, N.E.; Stumpf-Stolker, Y.; Tempelaars, M.; Middelkoop, E.; Zeerleder, S.; Wouters, D.; van Ham, M.S.; et al. Prolonged C1 Inhibitor Administration Improves Local Healing of Burn Wounds and Reduces Myocardial Inflammation in a Rat Burn Wound Model. Journal of Burn Care & Research 2012, 33, 544–551. [CrossRef]
- Schraufstatter, I.U.; Khaldoyanidi, S.K.; DiScipio, R.G. Complement Activation in the Context of Stem Cells and Tissue Repair. World J Stem Cells 2015, 7, 1090–1108. [CrossRef]
- Sinno, H.; Malholtra, M.; Lutfy, J.; Jardin, B.; Winocour, S.; Brimo, F.; Beckman, L.; Watters, K.; Philip, A.; Williams, B.; et al. Topical Application of Complement C3 in Collagen Formulation Increases Early Wound Healing. Journal of Dermatological Treatment 2013, 24, 141–147. [CrossRef]
- Sinno, H.; Malhotra, M.; Lutfy, J.; Jardin, B.; Winocour, S.; Brimo, F.; Beckman, L.; Watters, K.; Philip, A.; Williams, B.; et al. Accelerated Wound Healing with Topical Application of Complement C5. Plastic and Reconstructive Surgery 2012, 130, 523. [CrossRef]
- Man, R.C.; Idrus, R.B.H.; Ibrahim, W.I.W.; Saim, A.B.; Lokanathan, Y. Secretome Analysis of Human Nasal Fibroblast Identifies Proteins That Promote Wound Healing. In; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham; pp. 1–18.
- Hochepied, T.; Berger, F.G.; Baumann, H.; Libert, C. A1-Acid Glycoprotein: An Acute Phase Protein with Inflammatory and Immunomodulating Properties. Cytokine & Growth Factor Reviews 2003, 14, 25–34. [CrossRef]
- Vandooren, J.; Itoh, Y. Alpha-2-Macroglobulin in Inflammation, Immunity and Infections. Frontiers in Immunology 2021, 12. [CrossRef]
- Nishio, N.; Ito, S.; Suzuki, H.; Isobe, K.-I. Antibodies to Wounded Tissue Enhance Cutaneous Wound Healing. Immunology 2009, 128, 369–380. [CrossRef]
- Noh, J.Y.; Shin, J.U.; Kim, J.H.; Kim, S.H.; Kim, B.-M.; Kim, Y.H.; Park, S.; Kim, T.-G.; Shin, K.-O.; Park, K.; et al. ZAG Regulates the Skin Barrier and Immunity in Atopic Dermatitis. Journal of Investigative Dermatology 2019, 139, 1648-1657.e7. [CrossRef]
- Haque, M.; Siegel, R.J.; Fox, D.A.; Ahmed, S. Interferon-Stimulated GTPases in Autoimmune and Inflammatory Diseases: Promising Role for the Guanylate-Binding Protein (GBP) Family. Rheumatology 2021, 60, 494–506. [CrossRef]
- Bowcock, A.M.; Shannon, W.; Du, F.; Duncan, J.; Cao, K.; Aftergut, K.; Catier, J.; Fernandez-Vina, M.A.; Menter, A. Insights into Psoriasis and Other Inflammatory Diseases from Large-Scale Gene Expression Studies. Human Molecular Genetics 2001, 10, 1793–1805. [CrossRef]
- Park, S.Y.; Gupta, D.; Hurwich, R.; Kim, C.H.; Dziarski, R. Peptidoglycan Recognition Protein Pglyrp2 Protects Mice from Psoriasis-Like Skin Inflammation by Promoting Treg and Limiting Th17 Responses. J Immunol 2011, 187, 5813–5823. [CrossRef]
- Chen, C.-C.; Mo, F.-E.; Lau, L.F. The Angiogenic Factor Cyr61 Activates a Genetic Program for Wound Healing in Human Skin Fibroblasts *. Journal of Biological Chemistry 2001, 276, 47329–47337. [CrossRef]
- Bost, F.; Diarra-Mehrpour, M.; Martin, J.-P. Inter-α-Trypsin Inhibitor Proteoglycan Family. European Journal of Biochemistry 1998, 252, 339–346. [CrossRef]
- Yamashita, T.; Asano, Y.; Saigusa, R.; Taniguchi, T.; Nakamura, K.; Miura, S.; Toyama, T.; Takahashi, T.; Ichimura, Y.; Hirabayashi, M.; et al. Increased Expression of Aquaporin-1 in Dermal Fibroblasts and Dermal Microvascular Endothelial Cells Possibly Contributes to Skin Fibrosis and Edema in Patients with Systemic Sclerosis. Journal of Dermatological Science 2019, 93, 24–32. [CrossRef]
- Chmilewsky, F.; Jeanneau, C.; Laurent, P.; About, I. Pulp Fibroblasts Synthesize Functional Complement Proteins Involved in Initiating Dentin-Pulp Regeneration. Am J Pathol 2014, 184, 1991–2000. [CrossRef]
- Kulics, J.; Circolo, A.; Strunk, R.C.; Colten, H.R. Regulation of Synthesis of Complement Protein C4 in Human Fibroblasts: Cell- and Gene-Specific Effects of Cytokines and Lipopolysaccharide. Immunology 1994, 82, 509–515.
- Katz, Y.; Strunk, R.C. Synovial Fibroblast-like Cells Synthesize Seven Proteins of the Complement System. Arthritis & Rheumatism 1988, 31, 1365–1370. [CrossRef]
- Loo, A.E.K.; Halliwell, B. Effects of Hydrogen Peroxide in a Keratinocyte-Fibroblast Co-Culture Model of Wound Healing. Biochemical and Biophysical Research Communications 2012, 423, 253–258. [CrossRef]
- Hong, Z.-X.; Zhu, S.-T.; Li, H.; Luo, J.-Z.; Yang, Y.; An, Y.; Wang, X.; Wang, K. Bioengineered Skin Organoids: From Development to Applications. Military Med Res 2023, 10, 40. [CrossRef]
- Hijnen, D.; Knol, E.F.; Gent, Y.Y.; Giovannone, B.; Beijn, S.J.P.; Kupper, T.S.; Bruijnzeel-Koomen, C.A.F.M.; Clark, R.A. CD8+ T Cells in the Lesional Skin of Atopic Dermatitis and Psoriasis Patients Are an Important Source of IFN-γ, IL-13, IL-17, and IL-22. Journal of Investigative Dermatology 2013, 133, 973–979. [CrossRef]
- Yilmaz, O.; Com, E.; Lavigne, R.; Pineau, C.; Bobe, J. Liquid Chromatography and Tandem Mass Spectrometry in Label-Free Protein QuantificationProtein Quantification of Zebrafish (Danio Rerio)Zebrafish (Danio Rerio)Eggs. In Germline Development in the Zebrafish: Methods and Protocols; Dosch, R., Ed.; Methods in Molecular Biology; Springer US: New York, NY, 2021; pp. 277–290 ISBN 978-1-07-160970-5.
- Banliat, C.; Tsikis, G.; Labas, V.; Teixeira-Gomes, A.-P.; Com, E.; Lavigne, R.; Pineau, C.; Guyonnet, B.; Mermillod, P.; Saint-Dizier, M. Identification of 56 Proteins Involved in Embryo–Maternal Interactions in the Bovine Oviduct. Int J Mol Sci 2020, 21, 466. [CrossRef]
- Bruderer, R.; Bernhardt, O.M.; Gandhi, T.; Xuan, Y.; Sondermann, J.; Schmidt, M.; Gomez-Varela, D.; Reiter, L. Optimization of Experimental Parameters in Data-Independent Mass Spectrometry Significantly Increases Depth and Reproducibility of Results *. Molecular & Cellular Proteomics 2017, 16, 2296–2309. [CrossRef]
- Callister, S.J.; Barry, R.C.; Adkins, J.N.; Johnson, E.T.; Qian, W.-J.; Webb-Robertson, B.-J.M.; Smith, R.D.; Lipton, M.S. Normalization Approaches for Removing Systematic Biases Associated with Mass Spectrometry and Label-Free Proteomics. J Proteome Res 2006, 5, 277–286. [CrossRef]
- Achcar, F.; Camadro, J.-M.; Mestivier, D. AutoClass@IJM: A Powerful Tool for Bayesian Classification of Heterogeneous Data in Biology. Nucleic Acids Res 2009, 37, W63–W67. [CrossRef]
- Maere, S.; Heymans, K.; Kuiper, M. BiNGO: A Cytoscape Plugin to Assess Overrepresentation of Gene Ontology Categories in Biological Networks. Bioinformatics 2005, 21, 3448–3449. [CrossRef]
- Bonnot, T.; Gillard, M.; Nagel, D. A Simple Protocol for Informative Visualization of Enriched Gene Ontology Terms. BIO-PROTOCOL 2019, 9. [CrossRef]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A Web Server for Metabolomic Data Analysis and Interpretation. Nucleic Acids Research 2009, 37, W652–W660. [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE Database Resources in 2022: A Hub for Mass Spectrometry-Based Proteomics Evidences. Nucleic Acids Research 2022, 50, D543–D552. [CrossRef]
| Pathway | Protein IDs | Gene name | Protein name |
|---|---|---|---|
| Response to wounding | P01023 | A2M | Alpha-2-macroglobulin |
| P02743 | APCS | Serum amyloid P-component | |
| P02652 | APOA2 | Apolipoprotein A-II | |
| P02749 | APOH | Beta-2-glycoprotein 1 | |
| P02746 | C1QB | Complement C1q subcomponent subunit B | |
| P02747 | C1QC | Complement C1q subcomponent subunit C | |
| P09871 | C1S | Complement C1s subcomponent | |
| P06681 | C2 | Complement C2 | |
| P01024 | C3 | Complement C3 | |
| P01031 | C5 | Complement C5 | |
| P02748 | C9 | Complement component C9 | |
| P21926 | CD9 | CD9 antigen | |
| P00751 | CFB | Complement factor B | |
| P08603 | CFH | Complement factor H | |
| P00742 | F10 | Coagulation factor X | |
| P02763 | ORM1 | Alpha-1-acid glycoprotein 1 | |
| P19652 | ORM2 | Alpha-1-acid glycoprotein 2 | |
| P35542 | SAA4 | Serum amyloid A-4 protein | |
| P01009 | SERPINA1 | Alpha-1-antitrypsin | |
| P01011 | SERPINA3 | Alpha-1-antichymotrypsin | |
| P01008 | SERPINC1 | Antithrombin-III | |
| P05155 | SERPING1 | Plasma protease C1 inhibitor | |
| P02787 | TF | Serotransferrin | |
| Immune response | P25311 | AZGP1 | Zinc-alpha-2-glycoprotein |
| P02746 | C1QB | Complement C1q subcomponent subunit B | |
| P02747 | C1QC | Complement C1q subcomponent subunit C | |
| P09871 | C1S | Complement C1s subcomponent | |
| P06681 | C2 | Complement C2 | |
| P01024 | C3 | Complement C3 | |
| P01031 | C5 | Complement C5 | |
| P02748 | C9 | Complement component C9 | |
| P00751 | CFB | Complement factor B | |
| P08603 | CFH | Complement factor H | |
| Q92989 | CLP1 | Polyribonucleotide 5'-hydroxyl-kinase Clp1 | |
| P32456 | GBP2 | Guanylate-binding protein 2 | |
| P01876 | IGHA1 | Immunoglobulin heavy constant alpha 1 | |
| P01877 | IGHA2 | Immunoglobulin heavy constant alpha 2 | |
| P01857 | IGHG1 | Immunoglobulin heavy constant gamma 1 | |
| P01859 | IGHG2 | Immunoglobulin heavy constant gamma 2 | |
| P01860 | IGHG3 | Immunoglobulin heavy constant gamma 3 | |
| P01861 | IGHG4 | Immunoglobulin heavy constant gamma 4 | |
| P01871 | IGHM | Immunoglobulin heavy constant mu | |
| P01834 | IGKC | Immunoglobulin kappa constant | |
| P01602 | IGKV1-5 | Immunoglobulin kappa variable 1-5 | |
| P04433 | IGKV3D-11 | Immunoglobulin kappa variable 3-11 | |
| A0A0C4DH25 | IGKV3D-20 | Immunoglobulin kappa variable 3D-20 | |
| P06312 | IGKV4-1 | Immunoglobulin kappa variable 4-1 | |
| Q96PD5 | PGLYRP2 | N-acetylmuramoyl-L-alanine amidase | |
| Q9Y535 | POLR3H | DNA-directed RNA polymerase III subunit RPC8 | |
| P05155 | SERPING1 | Plasma protease C1 inhibitor | |
| Glycosaminoglycan binding |
Q96PD5 | PGLYRP2 | N-acetylmuramoyl-L-alanine amidase |
| P01008 | SERPINC1 | Antithrombin-III | |
| P02749 | APOH | Beta-2-glycoprotein 1 | |
| O00622 | CCN1 | CCN family member 1 | |
| P47914 | RPL29 | 60S ribosomal protein L29 | |
| Glycosaminoglycan metabolic process |
Q96PD5 | PGLYRP2 | N-acetylmuramoyl-L-alanine amidase |
| P19823 | ITIH2 | Inter-alpha-trypsin inhibitor heavy chain H2 | |
| P19827 | ITIH1 | Inter-alpha-trypsin inhibitor heavy chain H1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





