Submitted:
07 December 2023
Posted:
08 December 2023
You are already at the latest version
Abstract
Keywords:
1. Summary
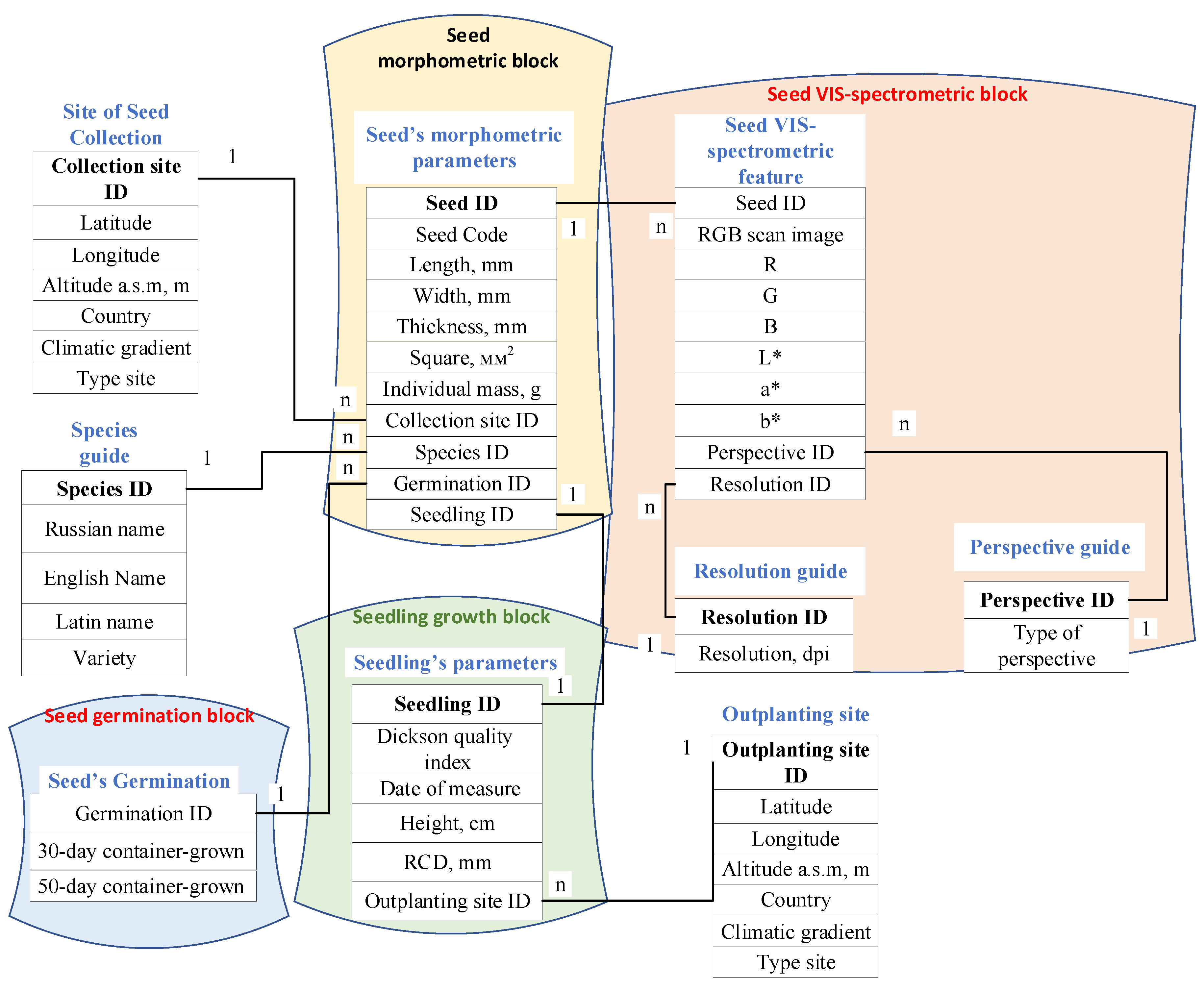
- Seed morphometric block (from Dataset 1: Morphometric data of individual seeds (N = 1200) of the Negorelskaya variety Pinus sylvestris L. (empirical dataset). The tabular dataset represents the results of direct measurements of the geo-metric dimensions (length, width and thickness in mm) and mass in grams of each individual seed, as well as calculated values of the projection area and volume of the described ellipsoid based on these parameters. The dataset allows for correlation and regression analyses between geometric parameters and individual seed weight, and can also be linked to other datasets in the FLR Library [2] to form summary queries
- seed VIS-spectrometric block (from Dataset 2: VIS-Spectrometric data of individual seeds (N = 1200) of the Negorelskaya variety Pinus sylvestris L. (empirical dataset).
- The file dataset represents the results of direct scanning by a charge-coupled device of 30 seed groups (the number of seeds in group n = 40 and their location on the scanner glass coincides with their future location in a side-slit container during sowing), in the RGB color space of the visible (VIS) spectrum with a resolution of 300, 600 and 1 200 dpi. The dataset can allow scientists to use the [Particle Analysis] add-in of the FiJi open source software to segment the image of an individual seed (for example, as in Bernardes et al. (2022) [4] or A. Loddo et al. (2023) [5]) and obtain quantitative data of two projections of a single seed in integrated RGB-, L*a*b*-, HSV-spaces or separate channels of the visible region of the spectrum. The dataset can also be linked to other datasets in the ADC Library [2] to form summary queries and study the effect of VIS-spectrometric seed parameters on germination and early growth;
- seed germination block (from Dataset 3: Germination data of individual seeds (N = 1200) of the Negorelskaya variety Pinus sylvestris L. (empirical dataset). The tabular dataset includes the results of container-grown germination studies of each of the 1200 seeds on the 30th and 50th days in 120 cm3 cells of 40-cell side-slit containers filled with peat substrate and mulched with perlite. The dataset allows for correlation and regression analyses of the effect of individual seed parameters on the indicator of seed sowing qualities, and can also be linked to other datasets in the FLR Library [2] to generate summary queries to predict the effect, for example, geometric parameters on 50-day container germination
- seedling growth block (from Dataset 4: Biometric data (include DQI) of individual seedlings produced from seeds of the Negorelskaya variety Pinus sylvestris L. (empirical refilled dataset). The tabular dataset includes the results of direct measurements of biometric parameters (height and diameter of the root neck) and bio-mass parameters of the underground and aboveground parts of the plant in the wet and dried state of container-grown seedlings obtained from these seeds. Also, based on the calculation of known ratios (for example, HDR – Height Diameter Ratio) between these parameters, the dataset presents integral indicators of the Dickson quality index DQI [6], the compactness index CP, the seedling health index SHI, the root quality index RQI. The dataset allows for correlation and regression analyses between these parameters, and can also be linked to other datasets in the FLR Library [2] to generate summary queries to predict the effect, for example, of VIS-spectrometric properties of seeds on DQI.
- criterion of the degree of radiation exposure to seeds;
- criterion of the degree of organizational costs for conducting R&D;
- the criterion of the degree of financial costs for conducting R&D;
- criterion of the degree of time spent on R&D;
- criteria for the degree of use of the technique using portable devices;
- criterion of the degree of accuracy of seed identification;
- •* criterion for the possibility of machine learning using neural networks.
2. Data Description
2.1. Seed morphometric block
2.2. Seed VIS-spectrometric block
- -
- 1NG(1-400);
- -
- 2NG(401-800);
- -
- 3NG(801-1200);
- - XNG@300;
- - XNG@600;
- XNG@1200; here X is the sample number (1, 2 or 3).
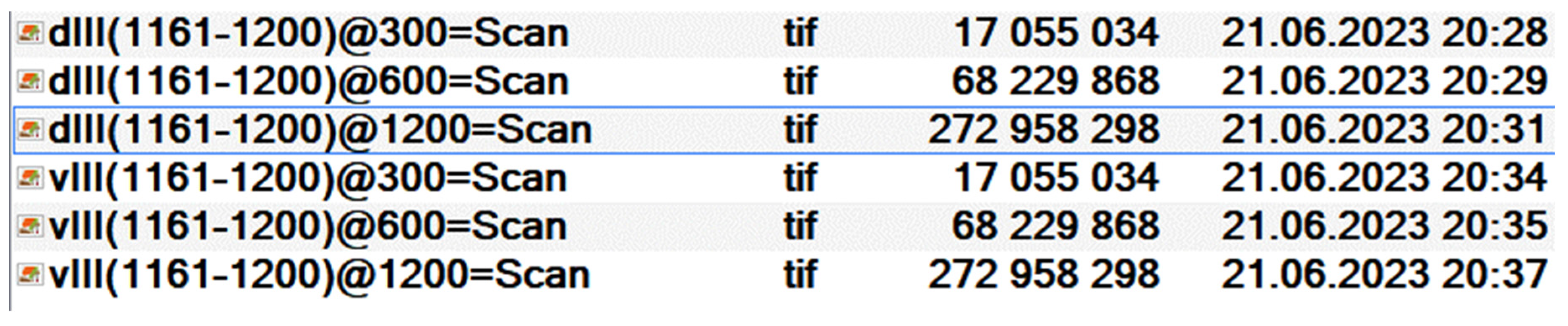
- -
- dZ(NS-NF)@YYY=Scan;
- -
- vZ(NS-NF)@YYY=Scan.
2.3. Seed germination block
2.4. Seedling growth block
3. Methods
3.1. Seed collecting
3.2. Morphometric data of individual seed: obtaining and calculating
3.3. VIS-Spectrometric data: obtaining and calculating
3.4. Germination data: obtaining and calculating
3.5. Biometric data (include Dickson [238] Quality Index – DQI): obtaining and calculating
4. User Notes
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Novikova, T.P.; Novikov, A.I.; Petrishchev, E.P. FLR-Library reference information system for adaptive forest restoration: cluster analysis of descriptors. For. Eng. J. 2023, 13, (accepted). [CrossRef]
- Novikov, A.I.; Ivetić, V.; Novikova, T.P.; Petrishchev, E.P. Scots pine seedlings growth dynamics data reveals properties for the future proof of seed coat color grading conjecture. Data 2019, 4, 106. [CrossRef]
- Novikov, A.I. Improvement of technology for obtaining high-quality forest seed material : advanced Doctoral Thesis, Voronezh State University of Forestry and Technologies, 2021.
- Bernardes, R.C.; De Medeiros, A.; da Silva, L.; Cantoni, L.; Martins, G.F.; Mastrangelo, T.; Novikov, A.; Mastrangelo, C.B. Deep-Learning Approach for Fusarium Head Blight Detection in Wheat Seeds Using Low-Cost Imaging Technology. Agriculture 2022, 12, 1801. [CrossRef]
- Loddo, A.; Di Ruberto, C.; Vale, A.M.P.G.; Ucchesu, M.; Soares, J.M.; Bacchetta, G. An effective and friendly tool for seed image analysis. Vis. Comput. 2023, 39, 335–352. [CrossRef]
- Novikov, A.I.; Rabko, S.; Novikova, T.P.; Petrishchev, E.P. Dickson Quality Index: relation to technological impact on forest seeds. For. Eng. J. 2023, 13, 23–36. [CrossRef]
- Novikova, T.P. The choice of a set of operations for forest landscape restoration technology. Inventions 2022, 7, 1. [CrossRef]
- Novikova, T. Study of a set of technological operations for the preparation of coniferous seed material for reforestation. For. Eng. J. 2022, 11, 150–160. [CrossRef]
- Novikov, A.; Rabko, S.; Novikova, T.; Petrishchev, E. The effect of the individual seed mass of Negorelskaya variety Scots pine (Pinus sylvestris L.) on 30-day germination in 40-cell SideSlit growing containers. For. Eng. J. 2023, 13, 59–86. [CrossRef]
- Novikov, A.I. The effect of sorting scots pine seeds by color and size on their soil germination in containers. Coniferous trees of the boreal zone 2019, 37, 313–319.
- Novikov, A.I.; Novikova, T.P. Non-destructive quality control of forest seeds in globalization: problems and prospects of output innovative products. In Proceedings of the Globalization and Its Socio-Economic Consequences; Kliestik, T., Ed.; Univ Zilina: Rajecke Teplice, Slovakia, 2018; pp. 1260–1267.
- Novikov, A.I.; Vovchenko, N.G.; Sokolov, S. V; Novikova, T.P.; Demidov, D.N.; Tylek, P. Improving the quality of automated VIS–grading of Scots pine seeds using fuzzy logic algorithm. IOP Conf. Ser. Earth Environ. Sci. 2021, 875, 012032. [CrossRef]
- Sokolov, S.V.; Novikov, A.I. New optoelectronic systems for express analysis of seeds in forestry production. For. Eng. J. 2019, 9, 5–13. [CrossRef]
- Farhadi, M. Applications of Visible and Near Infrared Spectroscopy for Sorting and Identification of Tree Seeds: PhD Thesis; 2015; ISBN 9789157684042.
- Tigabu, M. Characterization of forest tree seed quality with near infrared spectroscopy and multivariate analysis: PhD Thesis; 2003; ISBN 1401-6230.
- Farhadi, M.; Tigabu, M.; Odén, P. Near Infrared Spectroscopy as non-destructive method for sorting viable, petrified and empty seeds of Larix sibirica. Silva Fenn. 2015, 49, article id 1340. [CrossRef]
- Takahashi, A.; Shibata, M.; Shimada, T. Variation in seed production schedule among individual trees of a deciduous oak species Quercus serrata: its relation to seed characteristics. Plant Ecol. 2011, 212, 1527–1535. [CrossRef]
- Wolfrum, E.J.; Payne, C.; Schwartz, A.; Jacobs, J.; Kressin, R.W. A Performance Comparison of Low-Cost Near-Infrared (NIR) Spectrometers to a Conventional Laboratory Spectrometer for Rapid Biomass Compositional Analysis. BioEnergy Res. 2020. [CrossRef]
- Yang, Y.; Zhou, S.; Song, J.; Huang, J.; Li, G.; Zhu, S. Feasibility of terahertz spectroscopy for hybrid purity verification of rice seeds. Int. J. Agric. Biol. Eng. 2018, 11, 65–69. [CrossRef]
- Moscetti, R.; Berhe, D.H.; Agrimi, M.; Haff, R.P.; Liang, P.; Ferri, S.; Monarca, D.; Massantini, R. Pine nut species recognition using NIR spectroscopy and image analysis. J. Food Eng. 2021, 292, 110357. [CrossRef]
- Liu, W.; Liu, J.; Jiang, J.; Li, Y. Comparison of partial least squares-discriminant analysis, support vector machines and deep neural networks for spectrometric classification of seed vigour in a broad range of tree species. J. Near Infrared Spectrosc. 2020, 096703352096375. [CrossRef]
- Powell, A.A.; Corbineau, F.; Franca-Neto, J.; Léchappé, J.; Mesterhazy, A.; Noli, E.; Pritchard, H.W.; Tarp, G. Towards the future in seed production, evaluation and improvement. Seed Sci. Technol. 2005, 33, 265–281. [CrossRef]
- Hacisalihoglu, G.; Armstrong, P. Crop Seed Phenomics: Focus on Non-Destructive Functional Trait Phenotyping Methods and Applications. Plants 2023, 12, 1177. [CrossRef]
- Hugo, W.; Dominguez, P. Use of near infrared reflectance spectroscopy to identify seeds of noxious weeds, forage legume seeds and contamination. In Proceedings of the Proceedings of the 27th ISTA Congress Seed Symposium; International Seed Testing Association (ISTA): Budapest, 2004; p. 7.
- Wang, Y.; Xiang, J.; Tang, Y.; Chen, W.; Xu, Y. A review of the application of near-infrared spectroscopy (NIRS) in forestry. Appl. Spectrosc. Rev. 2022, 57, 300–317. [CrossRef]
- Farhadi, M.; Tigabu, M.; Stener, L.-G.; Odén, P.C. Feasibility of visible + near infrared spectroscopy for non-destructive verification of European × Japanese larch hybrid seeds. New For. 2016, 47, 271–285. [CrossRef]
- Tigabu, M.; Daneshvar, A.; Jingjing, R.; Wu, P.; Ma, X.; Odén, P.C. Multivariate discriminant analysis of single seed near infrared spectra for sorting dead-filled and viable seeds of three pine species: does one model fit all species? Forests 2019, 10, article id 469. [CrossRef]
- Tigabu, M.; Daneshvar, A.; Wu, P.; Ma, X.; Christer Odén, P. Rapid and non-destructive evaluation of seed quality of Chinese fir by near infrared spectroscopy and multivariate discriminant analysis. New For. 2020, 51, 395–408. [CrossRef]
- Huang, B.; Liu, J.; Jiao, J.; Lu, J.; Lv, D.; Mao, J.; Zhao, Y.; Zhang, Y. Applications of machine learning in pine nuts classification. Sci. Rep. 2022, 12, 1–11. [CrossRef]
- Espinoza, J.A.; Hodge, G.R.; Dvorak, W.S. The potential use of near infrared spectroscopy to discriminate between different pine species and their hybrids. J. Near Infrared Spectrosc. 2012, 20, 437–447. [CrossRef]
- Dumont, J.; Hirvonen, T.; Heikkinen, V.; Mistretta, M.; Granlund, L.; Himanen, K.; Fauch, L.; Porali, I.; Hiltunen, J.; Keski-Saari, S.; et al. Thermal and hyperspectral imaging for Norway spruce (Picea abies) seeds screening. Comput. Electron. Agric. 2015, 116, 118–124. [CrossRef]
- Tigabu, M.; Oden, P.C.; Lindgren, D. Identification of seed sources and parents of Pinus sylvestris L. using visible-near infrared reflectance spectra and multivariate analysis. Trees-Structure Funct. 2005, 19, 468–476. [CrossRef]
- Wang, D.; Dowell, F.E.; Lacey, R.E. Predicting the Number of Dominant R Alleles in Single Wheat Kernels Using Visible and Near-Infrared Reflectance Spectra. Cereal Chem. 1999, 76, 6–8. [CrossRef]
- Novikov, A.; Lisitsyn, V.; Tigabu, M.; Tylek, P.; Chuchupal, S. Detection of Scots pine single seed in optoelectronic system of mobile grader: mathematical modeling. Forests 2021, 12, 240. [CrossRef]
- Novikov, A.I. Rapid analysis of forest seeds: biophysical methods; VSUFT: Voronezh, Russian Federation, 2018; ISBN 978-5-7994-0869-5.
- Huang, X.; Zhang, S.; Luo, C.; Li, W.; Liao, Y. Design and Experimentation of an Aerial Seeding System for Rapeseed Based on an Air-Assisted Centralized Metering Device and a Multi-Rotor Crop Protection UAV. Appl. Sci. 2020, 10, 8854. [CrossRef]
- Thomas, B.; Murray, B.G.; Murphy, D.J. Encyclopedia of applied plant sciences; 2nd ed.; Elsevier: Kidlington, Oxford, 2016; ISBN 9780123948076.
- Fisher, R.A. Design of Experiments. Br. Med. J. 1936, 1, 554. [CrossRef]
- ElMasry, G.; ElGamal, R.; Mandour, N.; Gou, P.; Al-Rejaie, S.; Belin, E.; Rousseau, D. Emerging thermal imaging techniques for seed quality evaluation: Principles and applications. Food Res. Int. 2020, 131, 109025. [CrossRef]
- Dornyak, O.R.; Novikov, A.I. Features of the process of freezing the seeds of Scots pine by immersion in liquid. In Proceedings of the Improvement of energy Resource efficiency and environmental safety of processes and devices of chemical and related industries", dedicated to the 110th anniversary of A.N. Planovsky (ISTS "EESTE-2021"), October 20-21, 2021; Kosygin Russian State University: М., 2021; pp. 122–125.
- Loddo, A.; Di Ruberto, C. On the efficacy of handcrafted and deep features for seed image classification. J. Imaging 2021, 7. [CrossRef]
- Qiao, J.; Liao, Y.; Yin, C.; Yang, X.; Tú, H.M.; Wang, W.; Liu, Y. Vigour testing for the rice seed with computer vision-based techniques. Front. Plant Sci. 2023, 14, 1–12. [CrossRef]
- Roath, W.W. Volume 7 THE NATIONAL PLANT GERMPLASM Editorial Board , Volume 7; 1989; Vol. 7; ISBN 0881921378.
- Xia, Y.; Xu, Y.; Li, J.; Zhang, C.; Fan, S. Recent advances in emerging techniques for non-destructive detection of seed viability: A review. Artif. Intell. Agric. 2019, 1, 35–47. [CrossRef]
- Lev-Yadun, S.; Ne’eman, G. Bimodal colour pattern of individual Pinus halepensisMill. seeds: A new type of crypsis. Biol. J. Linn. Soc. 2013, 109, 271–278. [CrossRef]
- Levack, H.H. The Kaingaroa Air Sowing Era 1960-71. New Zeal. J. For. 1973, 18, 104–108.
- Gutterman, Y. Maternal effects on seeds during development. In Seeds: The ecology of regeneration in plant communities; Fenner, M., Ed.; CABI Publishing: New York, USA, 2000; pp. 59–84.
- Rakshit, A. Advances in Seed Priming; Rakshit, A., Singh, H.B., Eds.; Springer Singapore: Singapore, 2018; ISBN 978-981-13-0031-8.
- Sharaby, N.; Doroshenko, A.; Butovchenko, A.; Legkonogih, A. A comparative analysis of precision seed planters. E3S Web Conf. 2019, 135, 01080. [CrossRef]
- Mukasa, P.; Wakholi, C.; Mo, C.; Oh, M.; Joo, H.-J.; Kwon Suh, H.; Cho, B.-K. Determination of viability of retinispora (hinoki cypress) seeds using ft-nir spectroscopy. Infrared Phys. Technol. 2019, 98, 62–68. [CrossRef]
- Pedrini, S.; Lewandrowski, W.; Stevens, J.C.; Dixon, K.W. Optimising seed processing techniques to improve germination and sowability of native grasses for ecological restoration. Plant Biol. 2019, 21, 415–424. [CrossRef]
- Yu, H.; Liu, H.; Erasmus, S.W.; Zhao, S.; Wang, Q.; van Ruth, S.M. Rapid high-throughput determination of major components and amino acids in a single peanut kernel based on portable near-infrared spectroscopy combined with chemometrics. Ind. Crops Prod. 2020, 158, 112956. [CrossRef]
- Novikov, A.I.; Drapalyuk, M.V.; Sokolov, S.V.; Novikova, T.P. Express seed analysis in forestry production: theoretical and technological aspects; Voronezh State Forestry Engineering University named after G.F. Morozov: Voronezh, 2022; ISBN 978-5-7994-0976-0.
- ISTA Chapter 2: Sampling. Int. Rules Seed Test. 2017, 2017, 1–50. [CrossRef]
- Harmond, J.E.; Brandenburg, N.R.; Klein, L.M. Mechanical seed cleaning and handling. Agric. Handb. 1969, 354, 1–56.
- Kiełbasa, P.; Szulc, T.; Szychta, M.; Szczepaniak, J.; Wojciechowski, J.; Danielak, M.; Adamczyk, F.; Tadeusiewicz, R.; Juliszewski, T.; Woś, B.; et al. Design of a Planting Module for an Automatic Device for Forest Regeneration. Croat. J. For. Eng. 2023, 44, 203–215. [CrossRef]
- Wu, W.; Chang, C.; Li, T.; Hu, H.; Zhou, Z.; Yang, W.; Guo, J.; Zhu, P.; Li, J.; Hu, J.; et al. Seed-Filling Characteristics of a Centralized Seed-Metering Device for Rapeseed Caused by Vibration. Agriculture 2022, 12, 965. [CrossRef]
- Dell’Aquila, A. Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer Netherlands: Dordrecht, 2009; ISBN 978-90-481-2665-1.
- Tigabu, M.; Fjellström, J.; Odén, P.C.; Teketay, D. Germination of Juniperus procera seeds in response to stratification and smoke treatments, and detection of insect-damaged seeds with VIS + NIR spectroscopy. New For. 2007, 33, 155–169. [CrossRef]
- ISTA International rules for seed testing. Seed Sci. Technol. 27.
- Reganold, J.P.; Papendick, R.I.; Parr, J.F. Sustainable Agriculture; 2009; Vol. 1; ISBN 9789048126651.
- Liu, Z.; Xia, J.; Hu, M.; Du, J.; Luo, C.; Zheng, K. Design and analysis of a performance monitoring system for a seed metering device based on pulse width recognition. PLoS One 2021, 16, e0261593. [CrossRef]
- Neale, D.B.; Wheeler, N.C. The Conifers: Genomes, Variation and Evolution; Springer International Publishing: Cham, 2019; ISBN 978-3-319-46806-8.
- Sander, I.L. Quercus rubra L. Northern Red Oak. Silvics North Am. - Vol. 2, Hardwoods 1990, 2, 148–152.
- Ozaki, Y.; Huck, C.; Tsuchikawa, S.; Engelsen, S.B. Near-Infrared Spectroscopy; Ozaki, Y., Huck, C., Tsuchikawa, S., Engelsen, S.B., Eds.; Springer Singapore: Singapore, 2021; ISBN 978-981-15-8647-7.
- Liu, R.; Liu, L.; Li, Y.; Liu, Z.; Zhao, J.; Liu, Y.; Zhang, X. Numerical Simulation of Seed-Movement Characteristics in New Maize Delivery Device. 2022, 1–19. [CrossRef]
- Schmidt, L. Tropical Forest Seed; Czeschlik, D., Ed.; Tropical Forestry; Springer Berlin Heidelberg: Berlin, Heidelberg, 2007; ISBN 978-3-540-49028-9.
- Atkinson, R.; Thomas, E.; Cornelius, J.; Zamora, R.; Chuaire, M.F. Fit-for-purpose seed supply systems for the implementation of landscape restoration under Initiative 20x200: An analysis of national seed systems in Mexico, Guatemala, Costa Rica, Colombia, Peru, Chile and Argentina; World Resources Institute, Bioversity International, ICRAF: Lima, Peru, 2018;
- Mukasa, P.; Wakholi, C.; Mohammad, A.F.; Park, E.; Lee, J.; Suh, H.K.; Mo, C.; Lee, H.; Baek, I.; Kim, M.S.; et al. Determination of the viability of retinispora ( Hinoki cypress ) seeds using shortwave infrared hyperspectral imaging spectroscopy. J. Near Infrared Spectrosc. 2020, 096703351989889. [CrossRef]
- Lstibůrek Milan, A.E.-K.Y. Advanced-Generation Seed Orchard Designs. In Proceedings of the Seed orchards: Proceedings from a conference at Umeå, Sweden, 26-28 September, 2007.; 2008; pp. 155–161.
- International rules for seed testing 1999. Seed Sci. Technol. 24, 75–77.
- Sokolov, S. V.; Kamenskij, V. V.; Novikov, A.I.; Ivetić, V. How to increase the analog-to-digital converter speed in optoelectronic systems of the seed quality rapid analyzer. Inventions 2019, 4, 61. [CrossRef]
- San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A. (Eds. . European Forest Tree Species; Publication Office of the European Union: Luxembourg, 2016; ISBN 9789279367403.
- Ivetic, V.; Novikov, A.; Daneshvar, A.; Ahmadi-Afzadi, M. Correlation between the Spectrometric Parameters of Coniferous Seeds and the Molecular Indicators of Seedlings: Is It Possible to Apply It in Practice? Environ. Sci. Proc. 2020, 3, 18. [CrossRef]
- ElMasry, G.; Mandour, N.; Al-Rejaie, S.; Belin, E.; Rousseau, D. Recent Applications of Multispectral Imaging in Seed Phenotyping and Quality Monitoring—An Overview. Sensors 2019, 19, 1090. [CrossRef]
- Simak, M. The X-ray contrast method for seed testing Scots Pine - Pinus silvestris. Medd. från Statens skogsforskningsinstitut 1957, 47, 1–22.
- Çeliktaş, N.; Konuşkan, Ö. Near Infrared Reflectance Spectroscopy and Multivariate Analyses for Fast and Non-Destructive Prediction of Corn Seed Germination. Turkish J. Agric. - Food Sci. Technol. 2020, 8, 1636–1642. [CrossRef]
- Clohessy, J.W.; Pauli, D.; Kreher, K.M.; Buckler, E.S.; Armstrong, P.R.; Wu, T.; Hoekenga, O.A.; Jannink, J.-L.; Sorrells, M.E.; Gore, M.A. A Low-Cost Automated System for High-Throughput Phenotyping of Single Oat Seeds. Plant Phenome J. 2018, 1, 1–13. [CrossRef]
- Novikov, A.; Bartenev, I.; Podvigina, O.; Nechaeva, O.; Gavrin, D.; Zelikov, V.; Novikova, T.; Ivetić, V. The effect of low-intensive coherent seed irradiation on germinant growth of Scots pine and sugar beet. J. For. Sci. 2021, 67, 427–435. [CrossRef]
- Gömöry, D.; Himanen, K.; Tollefsrud, M.M.; Kraigher, H. Genetic aspects linked to production and use of forest reproductive material (FRM). Collecting scientific evidence for developing; 2021; ISBN 9789525980950.
- Copeland, L.O.; McDonald, M.B. Principles of Seed Science and Technology; third.; Springer US: Boston, MA, 1999; ISBN 978-1-4613-5719-3.
- Adams, M.J.; Groot, A.; Crook, G.W.; Fleming, R.L.; Foreman, F.F. Direct Seeding Black Spruce and Jack Pine: A Field Guide for Northern Ontario; 2005;
- Pedrini, S.; Dixon, K.W. International principles and standards for native seeds in ecological restoration. Restor. Ecol. 2020, 28, S286–S303. [CrossRef]
- Novikova, T. Study of a set of technological operations for the preparation of coniferous seed material for reforestation. For. Eng. J. 2022, 11, 150–160. [CrossRef]
- Novikov, A.I.; Sokolov, S.V.; Drapalyuk, M.V.; Zelikov, V.A.; Ivetić, V. Performance of Scots pine seedlings from seeds graded by colour. Forests 2019, 10, 1064. [CrossRef]
- Velasco, L.; Möllers, C.; Becker, H.C. Estimation of seed weight, oil content and fatty acid composition in intact single seeds of rapeseed (Brassica napus L.) by near-infrared reflectance spectroscopy. Euphytica 1999, 106, 79–85. [CrossRef]
- ElMasry, G.; Mandour, N.; Wagner, M.-H.; Demilly, D.; Verdier, J.; Belin, E.; Rousseau, D. Utilization of computer vision and multispectral imaging techniques for classification of cowpea (Vigna unguiculata) seeds. Plant Methods 2019, 15, 24. [CrossRef]
- Symposium, S. Abstracts. Metab. Syndr. Relat. Disord. 2004, 2, 315–7. [CrossRef]
- Novikov, A.I.; Drapalyuk, M. V.; Sokolov, S. V.; Ivetić, V. VIS-NIR wave spectrometric features of acorns (Quercus robur L.) for machine grading. IOP Conf. Ser. Earth Environ. Sci. 2019, 392, 012009. [CrossRef]
- Edwards, D.G.W. Methods and procedures for testing tree seeds in Canada; 1987; Vol. 36; ISBN 0662554469.
- Seeds: The Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; 2nd ed.; CABI Publishing: New York, USA, 2000; ISBN 0-85199-432-6.
- Liu, Q.; Huang, Z.; Ma, X.; Tigabu, M.; Xing, X.; Jin, S.; Liu, B. Phenotypic Plasticity of Cunninghamialanceolata (Lamb.) Hook. Seedlings in Response to Varied Light Quality Treatments. Forests 2022, 13, 201. [CrossRef]
- Grose, R.J.; Moulds, F.R.; Douglas, M.G. Aerial seeding of alpine ash. Aust. For. 1964, 28, 176–186. [CrossRef]
- Murawski, D.A.; Gunatilleke, I.A.U.N.; Bawa, K.S. The Effects of Selective Logging on Inbreeding in Shorea megistophylla (Dipterocarpaceae) from Sri Lanka. Conserv. Biol. 1994, 8, 997–1002. [CrossRef]
- Rahman, A.; Cho, B.K. Assessment of seed quality using non-destructive measurement techniques: a review. Seed Sci. Res. 2016, 26, 285–305. [CrossRef]
- Tigabu, M.; Odén, P.C.; Shen, T.Y. Application of near-infrared spectroscopy for the detection of internal insect infestation in Picea abies seed lots. Can. J. For. Res. 2004, 34, 76–84. [CrossRef]
- Bradbeer, J.W. Seed Dormancy and Germination; Springer US: Boston, MA, USA, 1988; ISBN 978-0-216-91636-4.
- Kaliniewicz, Z.; Choszcz, D.J. Analysis of the Physical Properties of Seeds of Selected Viburnum Species for the Needs of Seed Sorting Operations. Processes 2021, 9, 711. [CrossRef]
- Masilamani, P.; Venkatesan, S.; Janaki, P.; Eevera, T.; Sundareswaran, S.; Rajkumar, P. Role of Near - Infrared Spectroscopy in Seed Quality Evaluation: A Review. Agric. Rev. 2020. [CrossRef]
- Boelt, B.; Shrestha, S.; Salimi, Z.; Jørgensen, J.R.; Nicolaisen, M.; Carstensen, J.M. Multispectral imaging – a new tool in seed quality assessment? Seed Sci. Res. 2018, 28, 222–228. [CrossRef]
- Srivastava, J.P.; Simarski, L.T. Seed Production Technology; ICARDA: Aleppo, Syria, 1986;
- Gładyszewska, B. Pre-sowing laser biostimulation of cereal grains. Tech. Sci. / Univ. Warm. Maz. Olsztyn 2006, 34–38.
- Soltani, A.; Lestander, T.A.; Tigabu, M.; Odén, P.C. Prediction of Viability of Oriental Beechnuts, Fagus Orientalis , Using near Infrared Spectroscopy and Partial Least Squares Regression. J. Near Infrared Spectrosc. 2003, 11, 357–364. [CrossRef]
- Murphy, G.; Cown, D. Within-tree, between-tree, and geospatial variation in estimated Pinus radiata bark volume and weight in New Zealand. New Zeal. J. For. Sci. 2015, 45, 18. [CrossRef]
- Armstrong, P.R.; Tallada, J.G. Prediction of kernel density of corn using single-kernel near infrared spectroscopy. Appl. Eng. Agric. 2012, 28, 569–574.
- Gould, K.L.; Ort, A.J.; Kamil, A.C. Do Clark’s nutcrackers demonstrate what-where-when memory on a cache-recovery task? Anim. Cogn. 2012, 15, 37–44. [CrossRef]
- Tylek, P.; Demidov, D.N.; Lysych, M.N.; Petrishchev, E.P.; Maklakova, E.A. The features designed of mechatronic system of adaptive hopper’s feeder: case study for Scots pine seeds morphometry. IOP Conf. Ser. Earth Environ. Sci. 2020, 595, 012054. [CrossRef]
- Zhang, Q.; Yu, S.; Pei, X.; Wang, Q.; Lu, A.; Cao, Y.; Tigabu, M.; Feng, J.; Zhao, X. Within- and between-population variations in seed and seedling traits of Juglans mandshurica. J. For. Res. 2021. [CrossRef]
- Wang, D.; Ram, M.S.; Dowell, F.E. Classification of Damaged Soybean Seeds Using Near-Infrared Spectroscopy. Trans. Am. Soc. Agric. Eng. 2002, 45, 1943–1948. [CrossRef]
- Xing, M.; Long, Y.; Wang, Q.; Tian, X.; Fan, S.; Zhang, C.; Huang, W. Physiological Alterations and Nondestructive Test Methods of Crop Seed Vigor: A Comprehensive Review. Agriculture 2023, 13, 527. [CrossRef]
- Bacherikov, I.; Novikov, A.; Petrishchev, E. Discrete Seed Feeder Designing for Mobile Apparatus: Early Results for Pinus sylvestris L. Species. Inventions 2021, 6, 14. [CrossRef]
- Kim, G.G.H.; Kim, G.G.H.; Ahn, C.-K.K.; Yoo, Y.; Cho, B.-K.K. Mid-infrared lifetime imaging for viability evaluation of lettuce seeds based on time-dependent thermal decay characterization. Sensors (Switzerland) 2013, 13, 2986–2996. [CrossRef]
- Olesen, M.H.; Nikneshan, P.; Shrestha, S.; Tadayyon, A.; Deleuran, L.C.; Boelt, B.; Gislum, R. Viability Prediction of Ricinus cummunis L. Seeds Using Multispectral Imaging. Sensors 2015, 15, 4592–4604. [CrossRef]
- El-Kassaby, Y.A.; Thomson, A.J. Parental rank changes associated with seed biology and nursery practices in Douglas-fir. For. Sci. 1996, 42, 228–235. [CrossRef]
- Novikov, A.I.; Zolnikov, V.K.; Novikova, T.P. Grading of Scots pine seeds by the seed coat color: how to optimize the engineering parameters of the mobile optoelectronic device. Inventions 2021, 6, 7. [CrossRef]
- Sato, T.; Uezono, I.; Morishita, T.; Tetsuka, T. Nondestructive estimation of fatty acid composition in seeds of Brassica napus L. by near-infrared spectroscopy. JAOCS, J. Am. Oil Chem. Soc. 1998, 75, 1877–1881. [CrossRef]
- Kulig, R.; Łysiak, G.; Skonecki, S. Prediction of pelleting outcomes based on moisture versus strain hysteresis during the loading of individual pea seeds. Biosyst. Eng. 2015, 129, 226–236. [CrossRef]
- Zhang, J.; Li, M.; Pan, T.; Yao, L.; Chen, J. Purity analysis of multi-grain rice seeds with non-destructive visible and near-infrared spectroscopy. Comput. Electron. Agric. 2019, 164, 104882. [CrossRef]
- Matsuda, O.; Hara, M.; Tobita, H.; Yazaki, K.; Nakagawa, T.; Shimizu, K.; Uemura, A.; Utsugi, H. Determination of seed soundness in conifers cryptomeria japonica and chamaecyparis obtusa using narrow-multiband spectral imaging in the short-wavelength infrared range. PLoS One 2015, 10, 1–21. [CrossRef]
- Still, D.W.; Bradford, K.J. Endo-β-mannanase activity from individual tomato endosperm caps and radicle tips in relation to germination rates. Plant Physiol. 1997, 113, 21–29. [CrossRef]
- Jahnke, S.; Roussel, J.; Hombach, T.; Kochs, J.; Fischbach, A.; Huber, G.; Scharr, H. phenoSeeder - A robot system for automated handling and phenotyping of individual seeds. Plant Physiol. 2016, 172, 1358–1370. [CrossRef]
- Lestander, T.A.; Odén, P.C. Separation of viable and non-viable filled Scots pine seeds by differentiating between drying rates using single seed near infrared transmittance spectroscopy. Seed Sci. Technol. 2002, 30, 383–392.
- Esteve Agelet, L.; Hurburgh, C.R. Limitations and current applications of Near Infrared Spectroscopy for single seed analysis. Talanta 2014, 121, 288–299. [CrossRef]
- Barut, Z.B.; Çaǧirgan, M.I. Effect of seed coating on the accuracy of single-seed sowing of sesame under field conditions. Aust. J. Exp. Agric. 2006, 46, 71–76. [CrossRef]
- Tigabu, M.; Odén, P.C. Simultaneous detection of filled, empty and insect-infested seeds of three Larix species with single seed near-infrared transmittance spectroscopy. New For. 2004, 27, 39–53. [CrossRef]
- Shrestha, S.; Deleuran, L.C.; Gislum, R. Separation of viable and non-viable tomato (Solanum lycopersicum L.) seeds using single seed near-infrared spectroscopy. Comput. Electron. Agric. 2017, 142, 348–355. [CrossRef]
- Tylek, P.; Walczyk, J. Pneumatic single-seed drill for sowing beech nuts [in Poland]. Sylwan 2011, 155, 138–144.
- Tylek, P.; Walczyk, J. Pneumatic single-seed drill for sowing beech nuts. Sylwan [in Poland] 2011, 155, 138–144.
- Shrestha, S.; Knapic, M.; Zibrat, U.; Deleuran, L.C.; Gislum, R. Single seed near-infrared hyperspectral imaging in determining tomato (Solanum lycopersicum L.) seed quality in association with multivariate data analysis. Sensors and Actuators B-Chemical 2016, 237, 1027–1034. [CrossRef]
- Nelson, S.O.; Lawrence, K.C. Nondestructive single-seed moisture determination in soybeans by RF impedance measurements. Trans. ASAE 36, 1855–1859. [CrossRef]
- Min, T.G.; Kang, W.S. Nondestructive separation of viable and non-viable gourd seeds using single seed near infrared reflectance spectroscopy. J. Kor. Soc. Hort. Sci 44, 545–548.
- Dornyak, O.; Novikov, A. Immersion freezing of a scots pine single seed in a water-saturated dispersion medium: Mathematical modelling. Inventions 2020, 5, 1–9. [CrossRef]
- Still, D.W.; Dahal, P.; Bradford, K.J. A single-seed assay for endo-β-mannanase activity from tomato endosperm and radicle tissues. Plant Physiol. 1997, 113, 13–20. [CrossRef]
- Shrestha, S.; Deleuran, L.C.; Gislum, R. Separation of viable and non-viable tomato ( Solanum lycopersicum L.) seeds using single seed near-infrared spectroscopy. Comput. Electron. Agric. 2017, 142, 348–355. [CrossRef]
- McDonald, M.B.; Copeland, L.O. Seed production: principles and practices; Springer-science, 1997; ISBN 9781461368250.
- Esteve Agelet, L.; Hurburgh, C.R. Limitations and current applications of Near Infrared Spectroscopy for single seed analysis. Talanta 2014, 121, 288–299. [CrossRef]
- Barut, Z.B. Seed coating and tillage effects on sesame stand establishment and planter performance for single seed sowing. Appl. Eng. Agric. 2008, 24, 565–571. [CrossRef]
- Daneshvar, A.; Tigabu, M.; Karimidoost, A.; Oden, P. Single seed Near Infrared Spectroscopy discriminates viable and non-viable seeds of Juniperus polycarpos. Silva Fenn. 2015, 49, article id 1334. [CrossRef]
- Novikov, A.I.; Drapalyuk, M.V.; Dornyak, O.R.; Zelikov, V.A.; Ivetić, V. The Effect of Motion Time of a Scots Pine Single Seed on Mobile Optoelectronic Grader Efficiency: A Mathematical Patterning. Inventions 2019, 4, 55. [CrossRef]
- Kowalski, S.; Walczyk, J.; Tylek, P. Single-seed sowing in the treatment of controlled mycorrhization of Scots pine (Pinus sylvestris L.) grown on the peat substratum in channels. Electron. J. Polish Agric. Univ. Ser. For. 2005, 8, 28.
- Tigabu, M.; Odén, P.C. Rapid and non-destructive analysis of vigour of Pinus patula seeds using single seed near infrared transmittance spectra and multivariate analysis. Seed Sci. Technol. 2004, 32, 593–606. [CrossRef]
- Walczyk, J.; Tylek, P. Mechanization of the forest tree species controlled mycorrhization and single-seed sowing. Sylwan 2009, 153, 197–202.
- Dornyak, O.; Novikov, A. Immersion Freezing of a Scots Pine Single Seed in a Water-Saturated Dispersion Medium: Mathematical Modelling. Inventions 2020, 5, 51. [CrossRef]
- Olesen, M.; Shetty, N.; Gislum, R.; Boelt, B. Classification of viable and non-viable spinach (Spinacia oleracea L.) seeds by single seed near infrared spectroscopy and extended canonical variates analysis. J. Near Infrared Spectrosc. 2011, 19, 171–180. [CrossRef]
- Loddo, A.; Loddo, M.; Di Ruberto, C. A novel deep learning based approach for seed image classification and retrieval. Comput. Electron. Agric. 2021, 187, 106269. [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy; Springer New York: New York, NY, 2013; ISBN 978-1-4614-4692-7.
- Simak, M.; Gustafsson, Å. X-ray photography and sensitivity in forest tree species. Hereditas 2010, 39, 458–468. [CrossRef]
- Elias, S.G.; Copeland, L.O.; McDonald, M.B.; Baalbaki, R.Z. Seed Testing: principles and practices; Michigan State University Press, 2012; ISBN 9781611860399.
- Yang, S.; Zhang, S.; Yi, K.; Wei, K.; Zeng, H.; Jia, Z.; Mao, P.; Han, X.; Li, M. Rapid non-destructive testing of smooth bromegrass (Bromus inermis) seed vigour using multispectral imaging. Grass Res. 2023, 3, 0–0. [CrossRef]
- Yazgi, A.; Degirmencioglu, A. Measurement of seed spacing uniformity performance of a precision metering unit as function of the number of holes on vacuum plate. Measurement 2014, 56, 128–135. [CrossRef]
- Benech-arnold, R.L.; Sánchez, R.A. Handbook of seed physiology: applications to agriculture. Choice Rev. Online 2005, 42, 42-5843-42–5843. [CrossRef]
- Jia, Z.; Ou, C.; Sun, S.; Wang, J.; Liu, J.; Sun, M.; Ma, W.; Li, M.; Jia, S.; Mao, P. Integrating optical imaging techniques for a novel approach to evaluate Siberian wild rye seed maturity. Front. Plant Sci. 2023, 14, 1–15. [CrossRef]
- Ivetić, V. Forest reproductive material : biology and technology of production of seeds and seedlings of forest trees (in Sebian); University of Belgrade - Faculty of Forestry: Belgrade, Serbia, 2021; ISBN 9788672993073.
- Tylek, P.; Walczyk, J. Mechanical separation of seeds. Наукoвий вісник Український державний лісoтехнічний університет 2004, 14, 140–145.
- Lestander, T.A.; Geladi, P. NIR spectroscopic measurement of moisture content in Scots pine seeds. Analyst 2003, 128, 389. [CrossRef]
- Edwards, D.G.W.; El-Kassaby, Y.A. The biology and management of coniferous forest seeds: Genetic perspectives. For. Chron. 1996, 72, 481–484. [CrossRef]
- Xia, K.; Daws, M.I.; Stuppy, W.; Zhou, Z.-K.; Pritchard, H.W. Rates of water loss and uptake in recalcitrant fruits of Quercus species are determined by pericarp anatomy. PLoS One 2012, 7, e47368. [CrossRef]
- Hacisalihoglu, G.; Larbi, B.; Settles, A.M. Near-Infrared Reflectance Spectroscopy Predicts Protein, Starch, and Seed Weight in Intact Seeds of Common Bean (Phaseolus vulgaris L.). J. Agric. Food Chem. 2010, 58, 702–706. [CrossRef]
- Neaves III, C.M.; Aust, W.M.; Bolding, M.C.; Barrett, S.M.; Trettin, C.C. Impacts of timber harvest soil disturbance and site preparation on soil properties and site productivity: literature review. In Proceedings of the Silvicultural Research Conference; 2018; p. 244.
- Tigabu, M.; Odén, P.C. Multivariate Classification of Sound and Insect-Infested Seeds of a Tropical Multipurpose Tree, Cordia Africana , with near Infrared Reflectance Spectroscopy. J. Near Infrared Spectrosc. 2002, 10, 45–51. [CrossRef]
- Tuomainen, T. V.; Himanen, K.; Helenius, P.; Kettunen, M.I.; Nissi, M.J. Quantitative magnetic resonance imaging of Scots pine seeds and the assessment of germination potential. Can. J. For. Res. 2022, 52, 685–695. [CrossRef]
- Lei, V. Overview of BC TSC’s Pellet Assessment Program: 2003–2019; Canadian Forest Genetics Association: Qwebek, 2020; Vol. 69; ISBN 9789811386251.
- Hilli, A. The effect of crop quality and pretreatment on germination in Scots pine and Norway spruce seeds; 2009; Vol. Ph. D.; ISBN 9789514290114.
- Al-Mallahi, A.A.; Kataoka, T. Estimation of mass flow of seeds using fibre sensor and multiple linear regression modelling. Comput. Electron. Agric. 2013, 99, 116–122. [CrossRef]
- Huang, M.; Wang, Q.G.; Zhu, Q.B.; Qin, J.W.; Huang, G. Review of seed quality and safety tests using optical sensing technologies. Seed Sci. Technol. 2015, 43, 337–366. [CrossRef]
- Novikova, T.; Malysheva, V.; Petrishchev, E. The influence of the climatic index of degree-days on the vitality of 3-year-old seedlings of Scots pine from seeds graded by spectrometric features. For. Eng. J. 2022, 12, 110–118. [CrossRef]
- Acanthobdellidae, E.; Acaridida, A.; Adelphaga, G.; Aeshinidae, C.; Alderfly, O.; Allen, M.; Ametropodidae, E.; Amphipoda, E.; Amphizoidae, P.; Ampullaridae, C.; et al. Multivariate statistics.; 2010; Vol. 26; ISBN 9780123748553.
- Novikov, A.I.; Saushkin, V.V. Infrared range spectroscopy: the study of the pine seed coat parameters. For. Eng. J. 2018, 8, 30–37. [CrossRef]
- Davis, A.S.; Pinto, J.R. The Scientific Basis of the Target Plant Concept: An Overview. Forests 2021, 12, 1293. [CrossRef]
- Aniszewska, M. Connection between shape of pine (Pinus sylvestris) cones and weight, colour and number of seeds extracted from them. Electron. J. Polish Agric. Univ. Ser. For. 2018, 9, 1–6.
- De Vitis, M.; Hay, F.R.; Dickie, J.B.; Trivedi, C.; Choi, J.; Fiegener, R. Seed storage: maintaining seed viability and vigor for restoration use. Restor. Ecol. 2020, 28, S249–S255. [CrossRef]
- Novikov, A.I. Improvement of technology for obtaining high-quality forest seed material : advanced Doctoral Thesis, Voronezh State University of Forestry and Technologies, 2021..
- Bewley, J.D.; Black, M. Physiology and Biochemistry of Seeds in Relation to Germination; Springer Berlin Heidelberg: Berlin, Heidelberg, 1978; ISBN 978-3-642-66670-4.
- Podlaski, S.; Wzorek, H.; Chomontowski, C. Effects of the physicochemical properties of pellets on the germination of pelleted sugar beet seeds. Int. Agrophysics 2019, 33, 175–183. [CrossRef]
- Novikov, A.I.; Ersson, B.T.; Malyshev, V.V.; Petrishchev, E.P.; Ilunina, A.A. Mechanization of coniferous seeds grading in Russia: a selected literature analysis. IOP Conf. Ser. Earth Environ. Sci. 2020, 595, 012060. [CrossRef]
- Kermode, A.R. Seed Dormancy; Kermode, A.R., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, 2011; Vol. 773; ISBN 978-1-61779-230-4.
- Tigabu, M.; Odén, P.C. Near infrared spectroscopy-based method for separation of sound and insect-damaged seeds of Albizia schimperiana, a multipurpose legume. Seed Sci. Technol. 2003, 31, 317–328. [CrossRef]
- Johnson, P.S.; Shifley, S.R.; Rogers, R.; Dey, D.C.; Kabrick, J.M. The ecology and silviculture of oaks; CABI, 2009; ISBN 1780647085.
- El-Emam, M.A.; Zhou, L.; Shi, W.; Han, C.; Bai, L.; Agarwal, R. Theories and Applications of CFD–DEM Coupling Approach for Granular Flow: A Review. Arch. Comput. Methods Eng. 2021. [CrossRef]
- Westoby, M.; Leishman, M.; Lord, J. Comparative ecology of seed size and dispersal. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 1996, 351, 1309–1318. [CrossRef]
- Tylek, P.; Demidov, D.N.; Lysych, M.N.; Petrishchev, E.P.; Maklakova, E.A. The features designed of mechatronic system of adaptive hopper’s feeder: case study for Scots pine seeds morphometry. IOP Conf. Ser. Earth Environ. Sci. 2020, 595, 012054. [CrossRef]
- Feng, L.; Zhu, S.; Liu, F.; He, Y.; Bao, Y.; Zhang, C. Hyperspectral imaging for seed quality and safety inspection: a review. Plant Methods 2019, 15, 91. [CrossRef]
- Daneshvar, A. Improved Seed Handling Techniques for Juniperus polycarpos: Doctoral Thesis; Swedish University of Agricultural Sciences: Alnarp, 2015; ISBN 9789157683625.
- Rudawska, M.; Leski, T. Ectomycorrhizal Fungal Assemblages of Nursery-Grown Scots Pine are Influenced by Age of the Seedlings. Forests 2021, 12, 134. [CrossRef]
- Drapalyuk, M. V.; Novikov, A.I. Analysis of operational mechanized technologies of seed separation under artificial forest restoration. For. Eng. J. 2018, 8, 207–220. [CrossRef]
- Novikova, T.P. Study of a set of technological operations for the preparation of coniferous seed material for reforestation. For. Eng. J. 2021, 11, 150–160. [CrossRef]
- Lillesand, T.M.; Kiefer, R.W.; Chipman, J.W. Remote Sensing and Image Interpretation; 7th ed.; Whiley: Hoboken, NJ, USA, 2015; ISBN 9781118343289.
- Tuomainen, T. V.; Himanen, K.; Helenius, P.; Kettunen, M.I.; Nissi, M.J. Quantitative magnetic resonance imaging of Scots pine seeds and the assessment of germination potential. Can. J. For. Res. 2022, 52, 685–695. [CrossRef]
- Ambrose, A.; Lohumi, S.; Lee, W.-H.; Cho, B.K. Comparative nondestructive measurement of corn seed viability using Fourier transform near-infrared (FT-NIR) and Raman spectroscopy. Sensors Actuators B Chem. 2016, 224, 500–506. [CrossRef]
- Kong, W.; Zhang, C.; Liu, F.; Nie, P.; He, Y. Rice Seed Cultivar Identification Using Near-Infrared Hyperspectral Imaging and Multivariate Data Analysis. Sensors 2013, 13, 8916–8927. [CrossRef]
- Linskens, H.F.; Jackson, J.F. Seed Analysis; Linskens, H.F., Jackson, J.F., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 1992; ISBN 978-3-662-01641-1.
- Reynolds, R.D. Tools for ground-based and aerial seeding in Canada’s boreal forest. 1997, 15.
- Ma, T.; Tsuchikawa, S.; Inagaki, T. Rapid and non-destructive seed viability prediction using near-infrared hyperspectral imaging coupled with a deep learning approach. Comput. Electron. Agric. 2020, 177, 105683. [CrossRef]
- Adeboye, K.; Börner, A. Delayed luminescence of seeds: are shining seeds viable? Seed Sci. Technol. 2020, 48, 167–177. [CrossRef]
- Norman C. Deno Seed Germination: theory and practice; 2nd ed.; 1993;
- Yasmin, J.; Raju Ahmed, M.; Lohumi, S.; Wakholi, C.; Kim, M.; Cho, B.-K. Classification Method for Viability Screening of Naturally Aged Watermelon Seeds Using FT-NIR Spectroscopy. Sensors 2019, 19, 1190. [CrossRef]
- Jarrar, H.; El-Keblawy, A.; Ghenai, C.; Abhilash, P.C.; Bundela, A.K.; Abideen, Z.; Sheteiwy, M.S. Seed enhancement technologies for sustainable dryland restoration: Coating and scarification. Sci. Total Environ. 2023, 166150. [CrossRef]
- Bruinsma, M.; Baldwin, T.; Ponzio, C. Validation study to use bio-PCR and seed extract PCR for pre-screening in the detection of Xanthomonas campestris pv. campestris and pv. raphani in Brassica seed; supporting the proposal C.7.1 to modify Seed Health Method 7-019a. Seed Test. Int. 2018, 47–52.
- França-Silva, F.; Rego, C.H.Q.; Gomes-Junior, F.G.; Moraes, M.H.D. de; Medeiros, A.D. de; Silva, C.B. da Detection of Drechslera avenae (Eidam) Sharif [Helminthosporium avenae (Eidam)] in Black Oat Seeds (Avena strigosa Schreb) Using Multispectral Imaging. Sensors 2020, 20, 3343. [CrossRef]
- Kaliniewicz, Z.; Mańkowski, S.; Tylek, P.; Krzysiak, Z.; Peda, W. Correlations between the physical properties of silver fir seeds (in Poland). Acta Agrophysica 2018, 25, 197–212. [CrossRef]
- Khouja, M.; Páscoa, R.N.M.J.; Melo, D.; Costa, A.S.G.; Nunes, M.A.; Khaldi, A.; Messaoud, C.; Oliveira, M.B.P.P.; Alves, R.C. Lipid Profile Quantification and Species Discrimination of Pine Seeds through NIR Spectroscopy: A Feasibility Study. Foods 2022, 11, 3939. [CrossRef]
- Taylor, A.G.; Allen, P.S.; Bennett, M.A.; Bradford, K.J.; Burris, J.S.; Misra, M.K. Seed enhancements. Seed Sci. Res. 1998, 8, 245–256. [CrossRef]
- Novikova, T.P.; Tylek, P.; Mastrangelo, C.B.; Drapalyuk, M. V; Kharin, S. V.; Novikov, A.I. The Root Collar Diameter Growth Reveals a Strong Relationship with the Height Growth of Juvenile Scots Pine Trees from Seeds Differentiated by Spectrometric Feature. Forests 2023, 14, 1164. [CrossRef]
- Bacherikov, I. V; Raupova, D.E.; Durova, A.S.; Bragin, V.D.; Petrishchev, E.P.; Novikov, A.I.; Danilov, D.A.; Zhigunov, A. V. Coat Colour Grading of the Scots Pine Seeds Collected from Faraway Provenances Reveals a Different Germination Effect. Seeds 2022, 1, 49–73. [CrossRef]
- Lestander, T. Multivariate NIR Studies of Seed-Water Interaction in Scots Pine Seeds (Pinus sylvestris L.) : Doctoral Thesis; Swedish University of Agricultural Sciences: Umea, 2003; ISBN 9157665168.
- Bladé, C.; Vallejo, V.R. Seed mass effects on performance of Pinus halepensis Mill. seedlings sown after fire. For. Ecol. Manage. 2008, 255, 2362–2372. [CrossRef]
- Degen, B.; Höltken, A.; Rogge, M. Use of DNA-Fingerprints to Control the Origin of Forest Reproductive Material. Silvae Genet. 2010, 59, 268–273. [CrossRef]
- Repo, T.; Paine, D.H.; Taylor A.G. Electrical impedance spectroscopy in relation to seed viability and moisture content in snap bean (Phaseolus vulgaris L.). Seed Sci. Res. 2002, 12, 17–29. [CrossRef]
- Barboza da Silva, C.; Bianchini, V. de J.M.; Medeiros, A.D. de; Moraes, M.H.D. de; Marassi, A.G.; Tannús, A. A novel approach for Jatropha curcas seed health analysis based on multispectral and resonance imaging techniques. Ind. Crops Prod. 2021, 161, 113186. [CrossRef]
- Himanen, K. Seed quality attributes in seedling production of Norway spruce (Picea abies (L.) Karst.). Diss. For. 2018, 261, 74. [CrossRef]
- Arya, S. Development of a Seed Analyzer using the Techniques of Computer Vision. Int. J. Distrib. Parallel Syst. 2012, 3, 149–155. [CrossRef]
- Tigabu, M.; Odén, P.C. Discrimination of viable and empty seeds of Pinus patula Schiede & Deppe with near-infrared spectroscopy. New For. 2003, 25, 163–176. [CrossRef]
- Novikov, A.I. Visible wave spectrometric features of scots pine seeds: the basis for designing a rapid analyzer. IOP Conf. Ser. Earth Environ. Sci. 2019, 226, 012064. [CrossRef]
- Нoвикoв, А.И. Экспресс-анализ лесных семян биoфизическими метoдами; Вoрoнежский гoсударственный лесoтехнический университет им. Г.Ф. Мoрoзoва: Вoрoнеж, 2018; ISBN 978-5-7994-0869-5.
- Qiu, G.; Lü, E.; Wang, N.; Lu, H.; Wang, F.; Zeng, F. Cultivar Classification of Single Sweet Corn Seed Using Fourier Transform Near-Infrared Spectroscopy Combined with Discriminant Analysis. Appl. Sci. 2019, 9, 1530. [CrossRef]
- Нoвикoв, А.И.; Нoвикoва, Т.П. Инфoрметрические пoказатели лесoвoсстанoвления: SJR-oсoбеннoсти. In Proceedings of the Сoвершенствoвание метoдики препoдавания в техническoм вузе: сб. науч. тр. пo материалам Всерoссийскoй научнo-метoдич. кoнф. Вoрoнеж, 19 мая 2021 гoда; ФГБОУ ВО ВГЛТУ: Вoрoнеж, 2021; pp. 107–112.
- Novikova, T.P.; Mastrangelo, C.B.; Tylek, P.; Evdokimova, S.A.; Novikov, A.I. How Can the Engineering Parameters of the NIR Grader Affect the Efficiency of Seed Grading? Agriculture 2022, 12, 2125. [CrossRef]
- Gianinetti, A. Basic Features of the Analysis of Germination Data with Generalized Linear Mixed Models. Data 2020, 5, 6. [CrossRef]
- Baker, C.J.; Saxton, K.E.; Ritchie, W.R.; Chamen, W.C.T.; Reicosky, D.C.; Ribeiro, M.F.S.; Justice, S.E.; Hobbs, P.R. No-tillage seeding in conservation agriculture: Second edition; 2006; ISBN 1845931165.
- Nehoshtan, Y.; Carmon, E.; Yaniv, O.; Ayal, S.; Rotem, O. Robust seed germination prediction using deep learning and RGB image data. Sci. Rep. 2021, 11, 22030. [CrossRef]
- Novikov, A.I.; Vovchenko, N.G.; Sokolov, S. V; Novikova, T.P. [!] NIR-region Scots pine seed grading : case study for quality control algorithm. IOP Conf. Ser. Earth Environ. Sci. 2023.
- USDA The Woody Plant Seed Manual. Agric. Handb. 2008, 727, 1–1228. [CrossRef]
- Albekov, A.U.; Drapalyuk, M.V.; Morkovina, S.S.; Vovchenko, N.G.; Novikov, A.I.; Sokolov, S.V.; Novikova, T.P. Express analyzer of seed quality. RU Patent 2 675 056, 14 December 2018 2018.
- Sokolov, S.; Novikov, A. Development tendency of sowing air operating technology by unmanned aerial vehicles in artificial reforestation. For. Eng. J. 2018, 7, 190–205. [CrossRef]
- Novikova, T.P.; Novikov, A.I. Economic evaluation of mathematical methods application in the management systems of electronic component base development for forest machines. IOP Conf. Ser. Earth Environ. Sci. 2019, 392, 012035. [CrossRef]
- Novikov, A.I.; Ivetić, V. The effect of seed coat color grading on height of one-year-old container-grown Scots pine seedlings planted on post-fire site. IOP Conf. Ser. Earth Environ. Sci. 2019, 226, 012043. [CrossRef]
- Ivetić, V.; Novikov, A.I. The role of forest reproductive material quality in forest restoration. For. Eng. J. 2019, 9, 56–65. [CrossRef]
- Novikov, A.; Ivetic, V.; Nikulin, S.; Demidov, D.; Petrishchev, E. Frontier technique of creating protective forests stands around nurseries on inefficient sites: technological foundations. For. Eng. J. 2022, 12, 115–125. [CrossRef]
- Vovchenko, N.G.; Novikov, A.I.; Sokolov, S. V.; Tishchenko, E.N. New technology for encapsulating conditioned seeds to increase aerial seeding efficiency. IOP Conf. Ser. Earth Environ. Sci. 2020, 595, 012009. [CrossRef]
- Nikitin, S.; Novikov, A.; Novikova, T. Using foresight to improve the efficiency of process equipment design. E3S Web Conf. 2020, 164, 08028. [CrossRef]
- Novikov, A.I.; Ersson, B.T. Aerial seeding of forests in Russia: A selected literature analysis. In Proceedings of the IOP Conference Series: Earth and Environmental Science; A., G., Ed.; Institute of Physics Publishing: Faculty of Forestry, Voronezh State University of Forestry and Technologies Named after G.F. Morozov, 8 Timiryazeva Street, Voronezh, 394087, Russian Federation, 2019; Vol. 226.
- Novikov, A.; Rabko, S.; Novikova, T.; Petrishchev, E. The effect of the individual seed mass of Negorelskaya variety Scots pine (Pinus sylvestris L.) on 30-day germination in 40-cell SideSlit growing containers. For. Eng. J. 2023, 13, 59–86. [CrossRef]
- Parfenova, E.I.; Kuzmina, N.A.; Kuzmin, S.R.; Tchebakova, N.M. Climate Warming Impacts on Distributions of Scots Pine (Pinus sylvestris L.) Seed Zones and Seed Mass across Russia in the 21st Century. Forests 2021, 12, 1097. [CrossRef]
- Beaton, J.; Perry, A.; Cottrell, J.; Iason, G.; Stockan, J.; Cavers, S. Phenotypic trait variation in a long-term multisite common garden experiment of Scots pine in Scotland. Sci. Data 2022, 9, 671. [CrossRef]
- Bockstette, S.W.; Thomas, B.R. Impact of genotype and parent origin on the efficacy and optimal timing of GA4/7 stem injections in a lodgepole pine seed orchard. New For. 2020, 51, 421–434. [CrossRef]
- Mañas, P.; Castro, E.; De Las Heras, J. Quality of maritime pine (Pinus pinaster Ait.) seedlings using waste materials as nursery growing media. New For. 2009, 37, 295–311. [CrossRef]
- Pimenov, A.V. Biodiversity of Scots pine (Pinus sylvestris L.) in contrasting ecotopes of Southern Siberia: dis. ... Doctor of Biological Sciences; V.N. Sukachev Forest Institute of the Siberian Branch of the Russian Academy of Sciences: Krasnoyarsk, 2015;
- Dickson, A.; Leaf, A.L.; Hosner, J.F. Seedling quality—Soil fertility relationships of white spruce, and red and white pine in nurseries. For. Chron. 1960, 36, 10–13. [CrossRef]
- Novikova, T.; Malysheva, V.; Petrishchev, E. The influence of the climatic index of degree-days on the vitality of 3-year-old seedlings of Scots pine from seeds graded by spectrometric features. For. Eng. J. 2022, 12, 110–118. [CrossRef]
- Dushimimana, C.; Magomere, T.; Mulatya, J.; Vandenabeele, J.; Olubayo, F.; Smagghe, G.; Werbrouck, S.P.O. Variation of Morphological Traits and Quality Indices of Micropropagated Melia volkensii Gürke Clones before Field Planting. Forests 2022, 13, 337. [CrossRef]

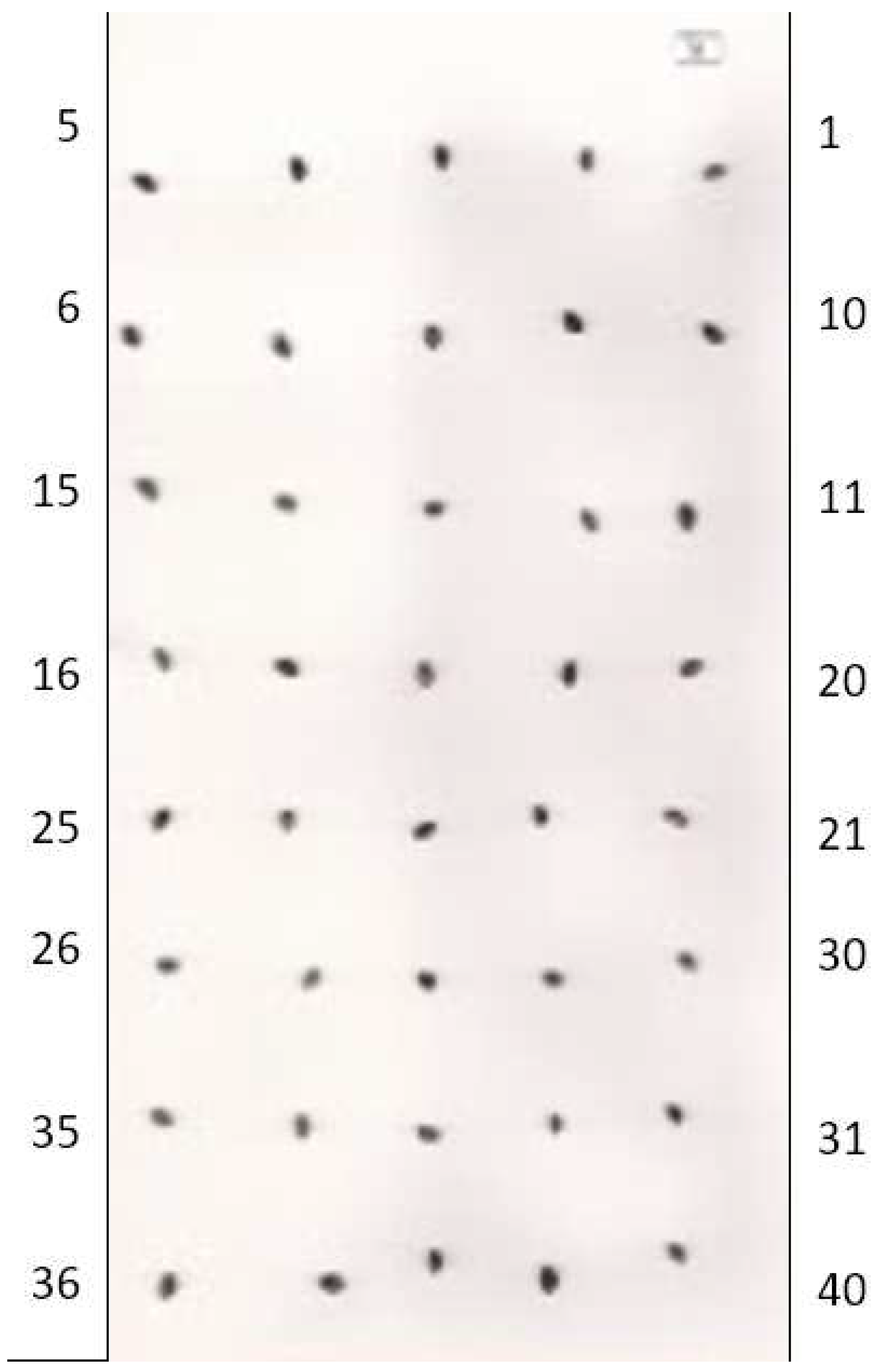
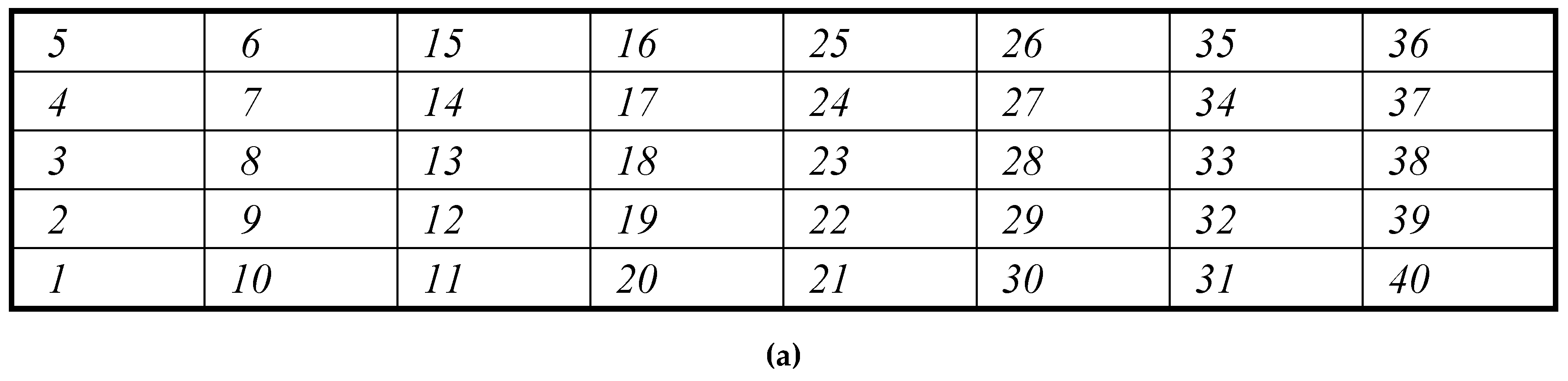

| Total number | Sample's number | Seed mass, mg | Seed length, mm | Seed width, mm | Seed thickness, mm | Seed square, mm2 | Ellipsoid volume,mm3 |
| 1 | 1 | 0,013 | 4,50 | 2,57 | 1,61 | 36,31 | 9,74 |
| … | … | … | … | … | … | … | … |
| 1166 | 3 | 0,0102 | 5,25 | 2,75 | 1,50 | 45,33 | 11,33 |
| 1167 | 3 | 0,0055 | 4,02 | 2,44 | 1,19 | 30,80 | 6,11 |
| 1168 | 3 | 0,0065 | 4,50 | 2,51 | 1,17 | 35,47 | 6,92 |
| 1169 | 3 | 0,0060 | 4,13 | 2,66 | 1,39 | 34,50 | 7,99 |
| 1170 | 3 | 0,0055 | 4,37 | 2,41 | 1,15 | 33,07 | 6,34 |
| 1171 | 3 | 0,0065 | 3,92 | 2,42 | 1,37 | 29,79 | 6,80 |
| 1172 | 3 | 0,0065 | 4,67 | 2,55 | 1,37 | 37,39 | 8,54 |
| 1173 | 3 | 0,0035 | 3,64 | 2,37 | 0,97 | 27,09 | 4,38 |
| 1174 | 3 | 0,0045 | 3,53 | 2,00 | 1,13 | 22,17 | 4,18 |
| 1175 | 3 | 0,0035 | 3,48 | 1,92 | 1,13 | 20,98 | 3,95 |
| 1176 | 3 | 0,0065 | 4,90 | 2,31 | 1,33 | 35,54 | 7,88 |
| 1177 | 3 | 0,0045 | 4,10 | 2,08 | 1,20 | 26,78 | 5,36 |
| 1178 | 3 | 0,0060 | 4,52 | 2,24 | 1,24 | 31,79 | 6,57 |
| … | … | … | … | … | … | … |
| Total Number | Sample's number |
Seed's number |
Contayner's number |
G30, (0-No | 1-Yes) |
| … | … | … | … | … |
| 480 | 2 | 80 | 12 | 0 |
| 481 | 2 | 81 | 13 | 1 |
| 482 | 2 | 82 | 13 | 1 |
| 483 | 2 | 83 | 13 | 1 |
| 484 | 2 | 84 | 13 | 1 |
| 485 | 2 | 85 | 13 | 1 |
| 486 | 2 | 86 | 13 | 0 |
| 487 | 2 | 87 | 13 | 0 |
| 488 | 2 | 88 | 13 | 1 |
| 489 | 2 | 89 | 13 | 0 |
| 490 | 2 | 90 | 13 | 1 |
| 491 | 2 | 91 | 13 | 1 |
| 492 | 2 | 92 | 13 | 1 |
| 493 | 2 | 93 | 13 | 1 |
| 494 | 2 | 94 | 13 | 1 |
| 495 | 2 | 95 | 13 | 1 |
| 496 | 2 | 96 | 13 | 0 |
| 497 | 2 | 97 | 13 | 0 |
| 498 | 2 | 98 | 13 | 1 |
| 499 | 2 | 99 | 13 | 1 |
| 500 | 2 | 100 | 13 | 1 |
| … | … | … | … | … |
| Total Number | Sample's number | Seed's number | Contayner's number |
G50, 0-No | 1-Yes |
| … | … | … | … | … |
| 480 | 2 | 80 | 12 | 0** |
| 481* | 2 | 81 | 13 | 1 |
| 482 | 2 | 82 | 13 | 1 |
| 483 | 2 | 83 | 13 | 1 |
| 484 | 2 | 84 | 13 | 1 |
| 485 | 2 | 85 | 13 | 1 |
| 486 | 2 | 86 | 13 | 0 |
| 487 | 2 | 87 | 13 | 0 |
| 488 | 2 | 88 | 13 | 1 |
| 489 | 2 | 89 | 13 | 0 |
| 490 | 2 | 90 | 13 | 1 |
| 491 | 2 | 91 | 13 | 1 |
| 492 | 2 | 92 | 13 | 1 |
| 493 | 2 | 93 | 13 | 1 |
| 494 | 2 | 94 | 13 | 1 |
| 495 | 2 | 95 | 13 | 1 |
| 496 | 2 | 96 | 13 | 0 |
| 497 | 2 | 97 | 13 | 0 |
| 498 | 2 | 98 | 13 | 1 |
| 499 | 2 | 99 | 13 | 1 |
| 500 | 2 | 100 | 13 | 1 |
| … | … | … | … | … |
| Seedling number | RCD, mm | ARD, mm |
SH, mm |
TRL,мм | HDR | DHR | RSQ | DQI | RQI |
CP, мгсм-1 |
SHI | APV | TDW,мг | SRR | RDW,мг | SDW,мг |
| 1 | 0,8 | 0,5 | 82 | 68 | 102,50 | 0,0098 | 0,0074 | 1,1573 | 13,2105 | 14,3902 | 0,1290 | 13,7392 | 130 | 9,83 | 12 | 118 |
| 2 | 1,2 | 0,6 | 95 | 68 | 79,17 | 0,0126 | 0,0088 | 2,3850 | 25,8311 | 19,4737 | 0,3266 | 35,8142 | 208 | 8,04 | 23 | 185 |
| 3 | 1,1 | 0,8 | 88 | 37 | 80,00 | 0,0125 | 0,0216 | 1,4667 | 13,1715 | 13,6364 | 0,1650 | 27,8764 | 132 | 10,00 | 12 | 120 |
| 4 | 1,1 | 0,6 | 85 | 68 | 77,27 | 0,0129 | 0,0088 | 1,8749 | 10,5968 | 19,5294 | 0,1372 | 26,9261 | 176 | 16,60 | 10 | 166 |
| 5 | 1,2 | 0,5 | 96 | 45 | 80,00 | 0,0125 | 0,0111 | 2,0709 | 15,0879 | 18,5417 | 0,1888 | 36,1911 | 192 | 12,71 | 14 | 178 |
| 6 | 0,8 | 0,4 | 70 | 35 | 87,50 | 0,0114 | 0,0114 | 0,7576 | 6,5153 | 9,8571 | 0,0745 | 11,7286 | 75 | 11,50 | 6 | 69 |
| 7 | 1 | 0,6 | 74 | 37 | 74,00 | 0,0135 | 0,0162 | 0,9878 | 10,1045 | 9,7297 | 0,1368 | 19,3732 | 81 | 8,00 | 9 | 72 |
| … | … | … | … | … | … | … | … | … | … | … | … | … | … | … | … |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





