1. Introduction
Influenza viruses are the principal human respiratory pathogens that cause seasonal epidemics as well as unpredictable outbreaks and pandemics [
1]. Seasonal influenza occurs globally and is estimated to affect 1 in 5 unvaccinated children and 1 in 10 unvaccinated adults [
2]. At present, vaccination remains the most effective method for counteracting seasonal infections and is essential to ensure preparedness for annual influenza epidemics [
3,
4,
5]. In the manufacture of the vast majority of flu vaccines, a traditional technology is used—cultivation of the virus in chicken embryos—which was developed more than half a century ago [
6,
7]. Although the technology involving chicken embryos is very reliable and safe, there are still some unsolvable problems associated with this production system, in particular, an occasional antigenic mismatch between egg-adapted viruses and circulating viruses as well as allergic reactions to egg whites when a ready-made vaccine is administered to a patient [
8,
9,
10]. The speed of technological process is also crucial. The full production cycle of a vaccine involving chicken embryos is 6 months; this state of affairs substantially reduces manufacture flexibility and makes the task of a rapid response to an epidemiological threat impossible [
11].
In the last two decades, research into vaccine manufacturing approaches based on cell cultures has been gaining popularity among scientific laboratories and biotech companies [
12,
13]. Such production systems partially eliminate the above disadvantages and offer several potential advantages, including the ability to efficiently produce sufficient amounts of a wider range of strains, rapid scalability, and high antigenic specificity of a vaccine virus to seasonal strains [
14,
15]. In addition, the more isolated process for the production of cell-based vaccines and the possibility of detailed testing of cell banks for contamination by random agents considerably reduce the risk of contamination in finished vaccines [
16,
17].
The main criterion for choosing a cell line for the production of inactivated influenza vaccines is the sensitivity and ability of the cells to support propagation of a wide variety of influenza viruses at high titers. To date, the mammalian Madin–Darby canine kidney (MDCK) cell line and the African green monkey kidney (Vero) cell line have found the largest number of applications in this field [
18,
19,
20]. Nonetheless, a virus produced in cell lines of animal origin can accumulate adaptive mutations and may have an altered glycosylation profile; these factors negatively affect immunological properties of the resulting vaccines [
21,
22].
The technology for producing vaccine strains of an influenza virus in cell lines of human origin makes sense and is promising [
23]. On the other hand, practical use of such cell lines is limited because they generate only low titers of vaccine strains. The limiting factor is the system of innate immune responses functioning within human cells [
24,
25]. One of possible ways to eliminate this phenomenon is a targeted knockout of genes encoding components of the innate immune response [
26]. Therefore, in the present work, by means of genome-editing system CRISPR/Cas9, monoclonal cell lines with an
IFITM3 knockout were created from cells of human origin.
2. Results
2.1. The Choice of a Target Gene and Cell Line for the CRISPR/Cas9 Editing
The
IFITM3 gene, which is a member of the family of interferon-induced transmembrane proteins (IFITMs) [
27], was chosen as the target gene for the knockout. IFITM3, along with proteins IFITM1 and IFITM2, has an antiviral activity [
28]. IFITM3 inhibits the fusion of viral and cellular membranes, thereby limiting the penetration of the virus into the cytoplasm and virus replication [
29,
30]. It has been shown that IFITM3 depletion profoundly decreases the antiviral processes in virus-infected cells, and overexpression of IFITM3 increases resistance to infection with influenza A viruses [
31].
To knock out
IFITM3, immortalized human embryonic lung fibroblast line WI-38 VA13 was chosen (subline 2RA from the Collection of Cultures of Vertebrate Cells at the Institute of Cytology, the Russian Academy of Sciences). This cell line was derived from WI-38 cells, which have been employed to design vaccines against several viral diseases [
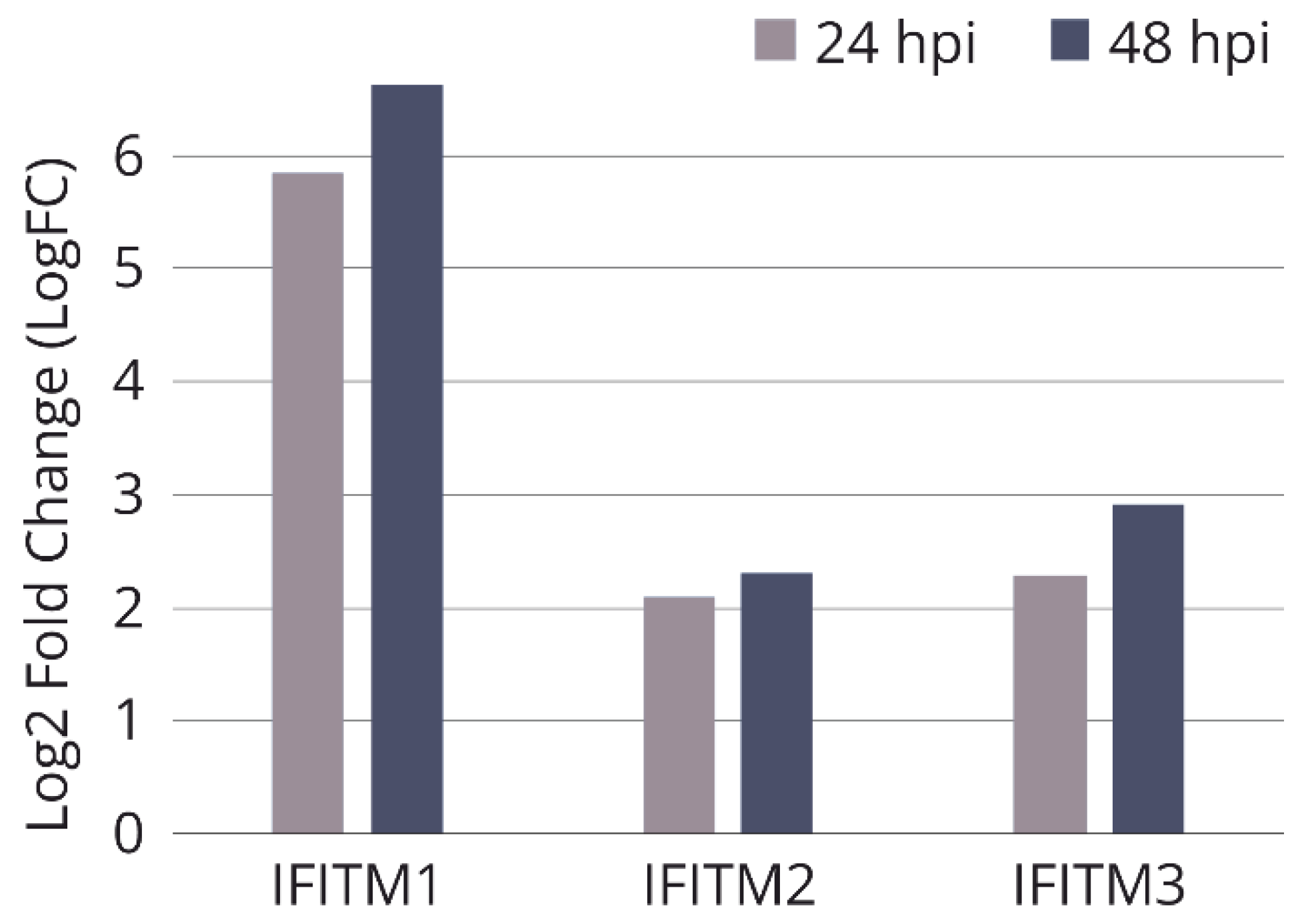
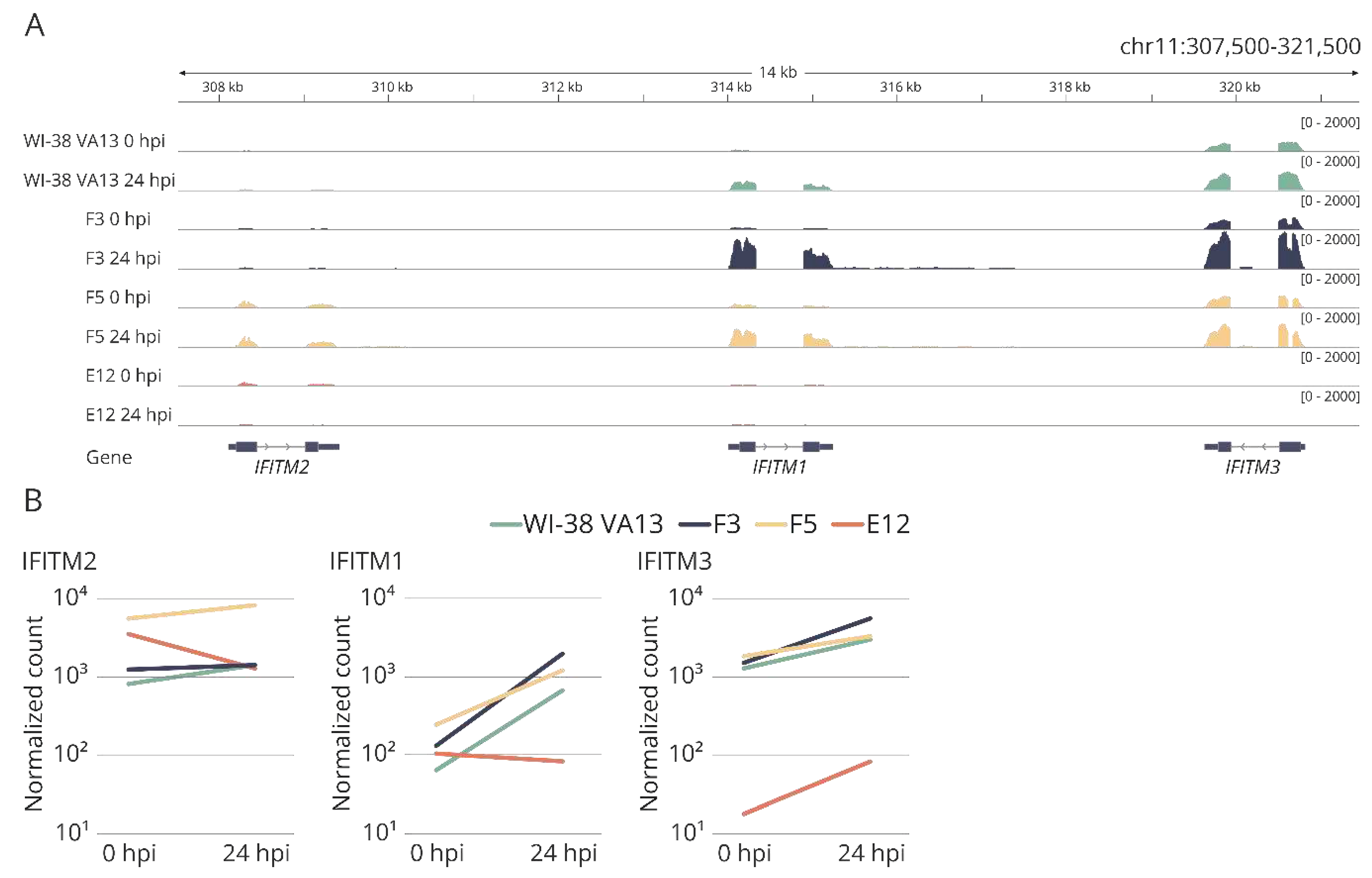
32]. Cell subline WI-38 VA13 is characterized by a known transformation history, which allows for its use as a substrate for vaccine production. According to results of our transcriptomic analysis, at 24 and 48 h after infection of WI-38 VA13 cells with influenza virus A/PR/8/34 (H1N1), the expression level of the
IFITM1,
2 and
3 genes increases relative to uninfected cells (
Figure 1).
2.2. Construction of Cell Lines with Knocked out IFITM3
The procedure for obtaining monoclonal cell lines by means of the CRISPR/Cas9 system included standard stages: transfection of WI-38 VA13 cells with vectors constructed based on plasmid pX458 (Addgene, cat. # 48138), sorting of GFP-positive cells, subcloning of a cell population, selection of monoclonal cell lines with mutations in a target region of
IFITM3, determination of the mutations in each allele by TA-cloning of PCR products and Sanger sequencing, and a comparison of proliferation rates and selection of monoclonal cell lines using xCELLigence (ACEA Biosciences) technology [
33]. Thus, three monoclonal lines of viable actively dividing cells were obtained with mutations in the target region of the
IFITM3 gene (
Figure 2).
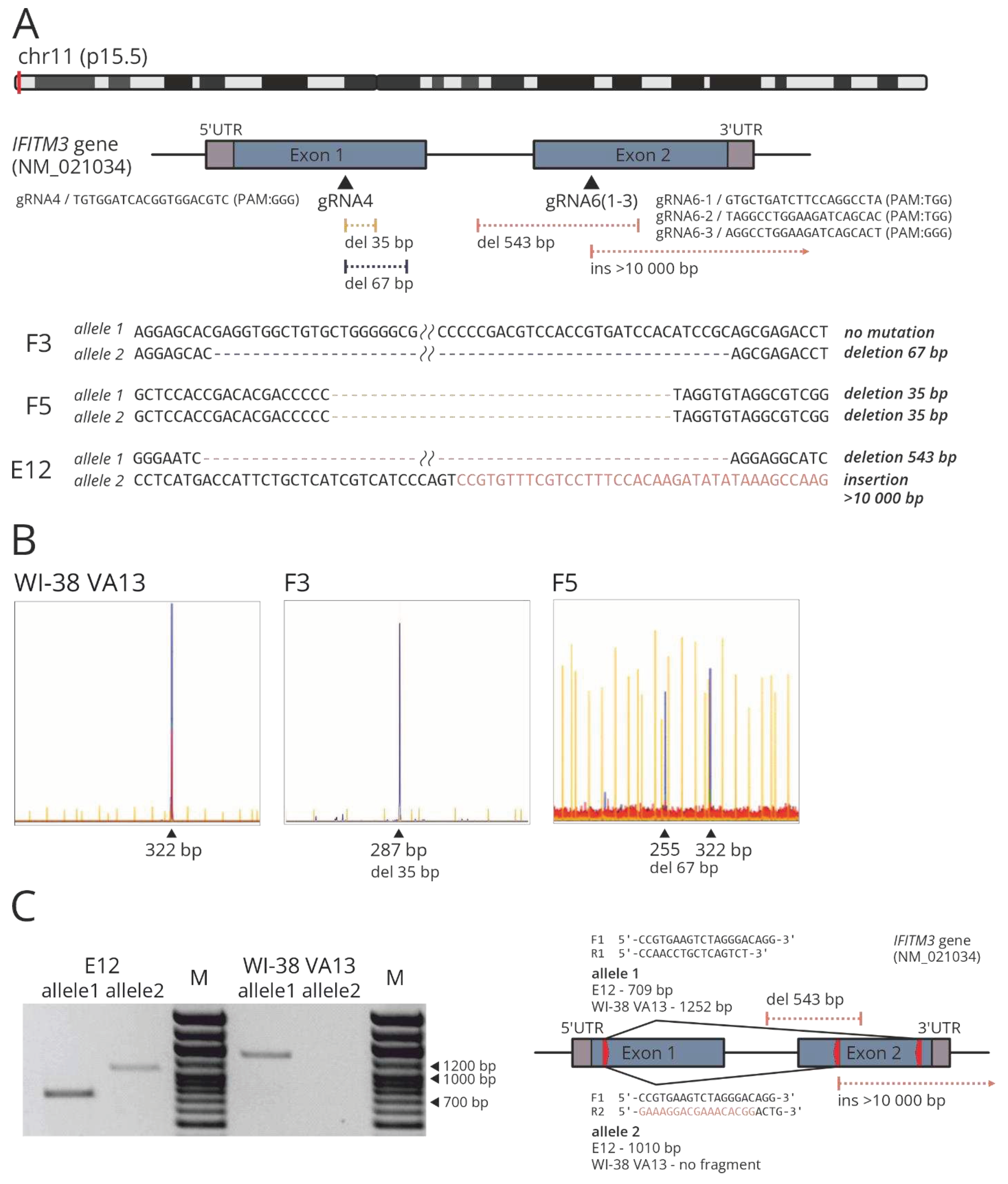
Clone F5 was found to carry two mutant alleles with 35-nucleotide (nt) deletions, which may indicate a frame shift in both copies of the target gene (
Figure 2A). Clone F3 carries only one allele with the mutation (namely, a 67-nt deletion), and the second allele corresponds to the wild type. Clone E12 proved to be a carrier of a 543-nt deletion and of an insertion of a >10 kbp part of the PX458 plasmid. Mutations in clones F3 and F5 were confirmed via indel detection by amplicon analysis (IDAA) (
Figure 2B) [
34]. The presence of an extended deletion in clone E12 was corroborated by PCR analysis of genomic DNA with flanking primers (
Figure 2C). To confirm the insertion, a reverse primer was utilized that was partly complementary to the
IFITM3 gene and partly to the plasmid PX458 region integrated into the genome (
Figure 2C).
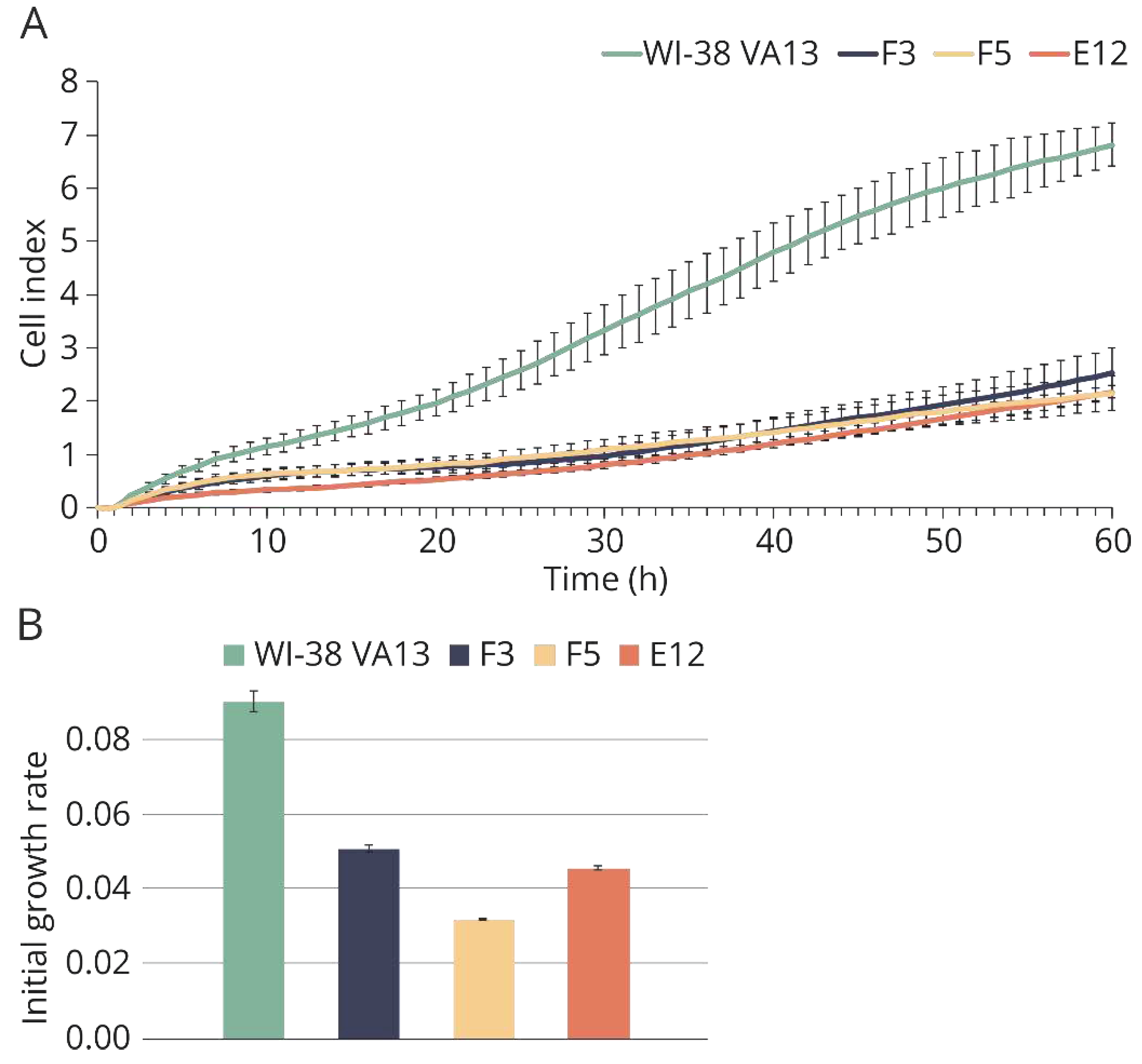
Using XCelligence technology, the selected monoclonal cell lines were proven to be viable, but their proliferation rate was lower than that of the control cell line (
Figure 3).
2.3. Evaluation of IFITM3 mRNA Expression
To assess the level of mRNA expression, real-time reverse transcription (RT) PCR was carried out (
Figure 4A). It was demonstrated that the mRNA level of the
IFITM3 gene is significantly reduced in clone E12 compared to the original cell line, likely because of the presence of the extensive mutations in both alleles. The high mRNA level of
IFITM3 in clones F3 and F5 may be explained by the fact that the presence of mutations detected in the clones did not interfere with the synthesis of full-size, but incorrect
IFITM3 gene mRNA or its short forms.
Western blot analysis was performed to determine the presence or absence of the target protein in the selected clones (
Figure 4B). In the original cell line WI-38 VA13, a high level of IFITM3 expression was confirmed. Mutations in clones F5 and E12 caused the absence of the protein product in the cells. In clone F3, synthesis of the IFITM3 protein was observed, apparently due to the presence of a the nonmutant allele, but the protein level was lower than that in the control cell line.
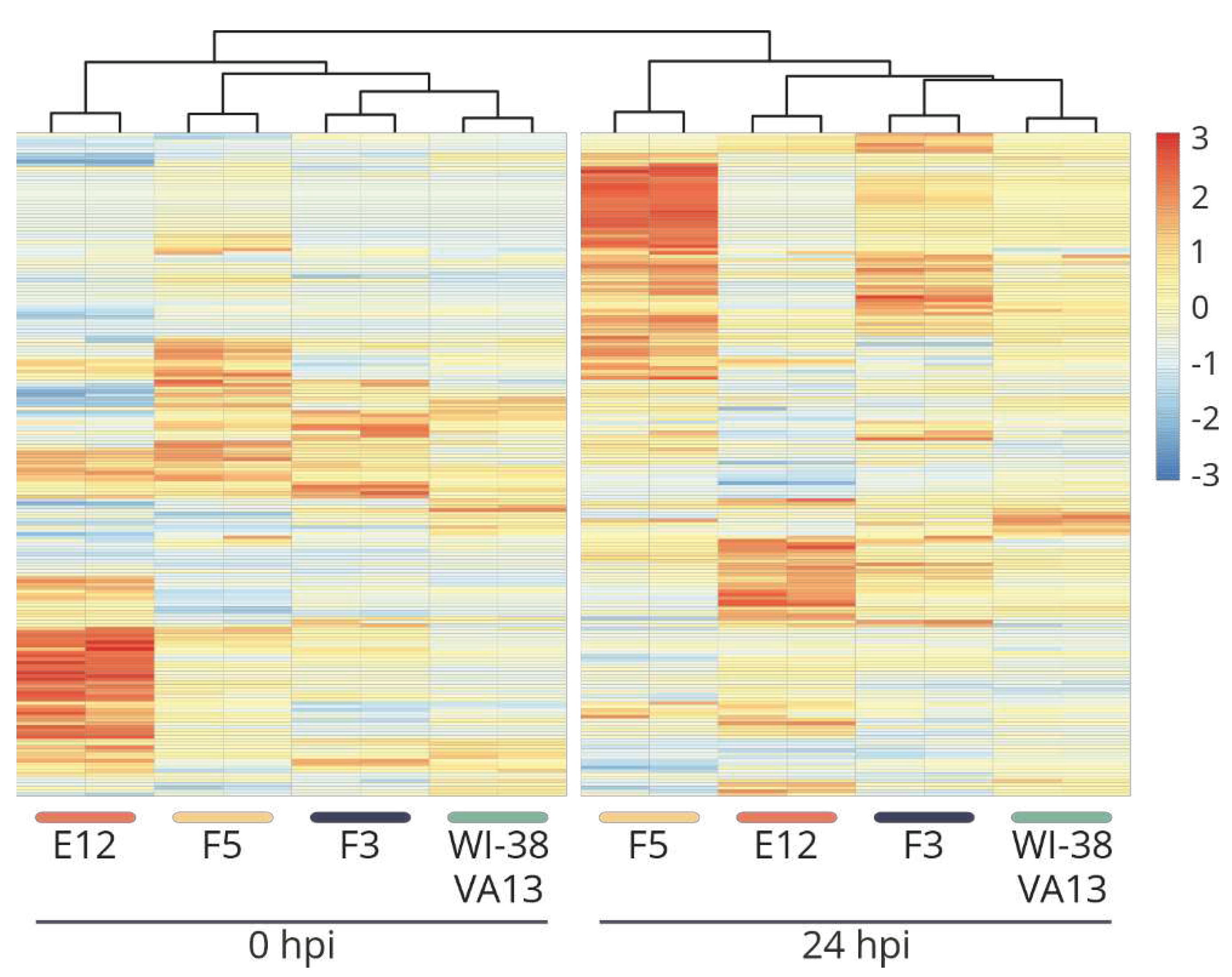
Mutations in the clones were corroborated by an analysis of transcriptome sequencing data (
Figure 5). A decrease in the
IFITM3 mRNA level in clones with mutations was successfully verified. Additionally, mutual regulation of transcriptional activity was demonstrated among genes within the IFITM family. For instance, in clone E12, along with the knockout of
IFITM3, almost complete suppression of the transcriptional activity of the
IFITM1 gene was observed (
Figure 5B).
The magnitude of the observed changes in gene expression at 24 h after influenza A virus infection of the cells is summarized in
Table 1. The most significant alterations as compared to parental cells were noted in the E12 cell line.
In the cell clones with mutations in
IFITM3, functional analysis of the genes that are most differentially expressed (relative to uninfected cells) revealed that in cell lines F3 and F5, the enrichment with functional terms was similar to that in the original cell line WI-38 VA13; these functional terms are characteristic of a viral infection. On the other hand, in the set of differentially expressed genes of monoclonal cell line E12, there was enrichment with terms not directly related to the antiviral response: biosynthesis of macromolecules and transcription processes (
Figure 6). This result was suggestive of a disturbance in the activation of the innate-immune-response cascade specifically in the clone with impaired expression of the two genes of the IFITM family.
Examination of the “response to virus” functional group (GO:0009615) allowed us to confirm this supposition: results of the examination, presented in the form of a heat map, indicated significant differences of cell clones F5 and E12 from the control cell line (
Figure 7). At the same time, in monoclonal cell line F5 (a knockout of only
IFITM3), a tendency toward overexpression of some antiviral-response genes was documented, whereas in cell line E12 (combined suppression of both
IFITM3 and
IFITM1), we noted reduced activity of the genes of this category.
A detailed analysis of the most differentially expressed genes within obtained cell lines identified a gene set directly related to the Jak–STAT signaling pathway:
SOCS3,
STAT1,
STAT2, and
IRF9 (
Figure 8). In monoclonal cell line E12, the results of transcriptomic analysis revealed disturbances in the activation of a group of genes of the Jak–STAT cascade as compared with control WI-38 VA13 cells (
Figure 8B). For several other key genes in the pathways of response to influenza A virus infection, aberrations (from the control) in regulation were also observed in both uninfected monoclonal cell lines and in virus-infected cells (
Figure 8C). From these results, we can conclude that a knockout of stand-alone genes of the innate immune response leads to dysregulation of the antiviral response. The combined downregulation (of the activity of genes from the IFITM family) seen in the E12 monoclonal cells induces substantial disturbances of the innate-immune-response cascade.
2.4 Sensitivity of WI-38 VA13 IFITM3–/– Cell Lines to Influenza Infection
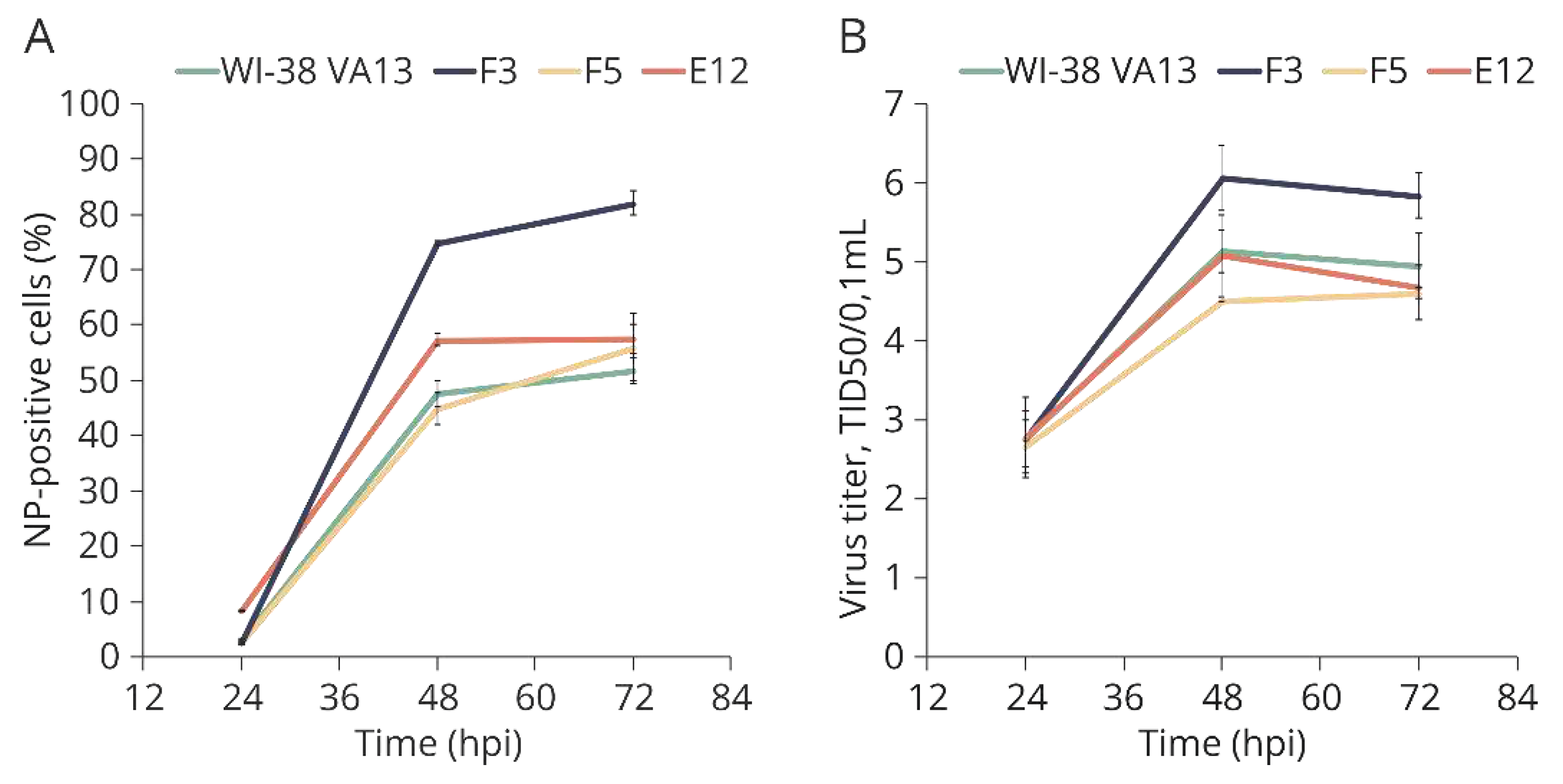
To assess the permissiveness of the original and obtained cell lines, cells were infected with a model influenza virus—A/PR/8/34 (H1N1)—and the dynamics of its replication were assessed. Every 24 h, the number of infected cells was evaluated by staining with fluorescently labeled antibodies against virus protein NP, followed by cytometry, and the number of newly formed viral particles in the culture medium was determined by titration in highly permissive cells (MDCK). The percentage of infected cells (
Figure 9A) was found to be elevated in cell clones F3 and E12. Nonetheless, the titer of newly formed viral particles in the culture medium of the monoclonal cell lines did not differ significantly from the control (
Figure 9B).
In cell lines F3 and E12, sensitivity to epidemiologically relevant wild-type influenza viruses was assessed too: strains A/California/07/2009 (H1N1pdm09) and A/Hong Kong/4801/2014 (H3N2). The results indicated an increased percentage of infected cells in the modified cell lines, and the percentage was higher as compared to infection with strain A/PR/8/34(H1N1) (
Figure 10). Differences in the prevalence of infection between the modified cell lines can be explained by a concomitant impairment of the activation of not only IFITM3 but also IFITM1; this difference was also observed when the cells were infected with influenza virus strain A/PR/8/34(H1N1).
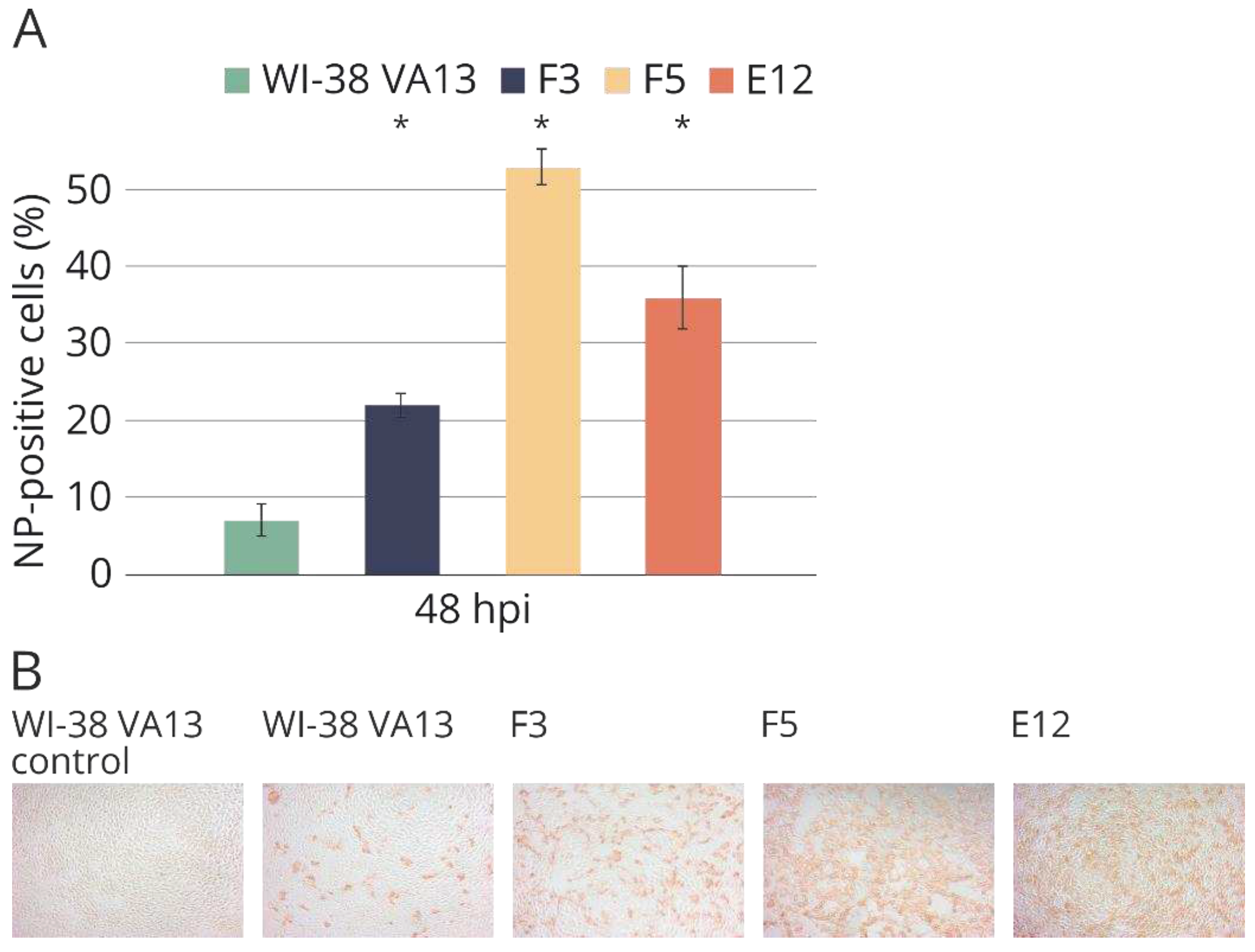
To estimate the permissiveness of the cell lines, we also employed a technique for assessing the accumulation of viral protein NP by means of the number of focus-forming units. Infection with influenza virus A/WSN/33 (H1N1) was carried out at 0.01 TCID
50 (median tissue culture infectious dose) per cell. It was shown that the knockout of the
IFITM3 gene increases the sensitivity of WI-38 VA13 cells to the influenza A virus; the percentage of infected cells in clones E12 and F5 was higher 6- and 8-fold, respectively, relative to wild-type cells (
Figure 11).
3. Discussion
The study shows that mutations in the
IFITM3 gene lead to elevated sensitivity of human embryonic lung fibroblast subline WI-38 VA13 to infection with an influenza virus. At the same time, differences were observed depending on the virus strain: the cells had the highest sensitivity to infection with strains A/California/07/2009 (H1N1pdm09) and A/WSN/33 (H1N1). Meanwhile, the knockout of
IFITM3 did not significantly affect the number of newly formed viral particles in the culture medium after infection of the cell lines (
Figure 9B). It is likely that a knockout of one gene is not enough to effectively suppress the antiviral response system. As a way to overcome this problem, a simultaneous knockout can be performed on several target genes that act at different stages of cell infection with an influenza virus, and this approach will implement comprehensive suppression of innate immune response.
It is also interesting that cell lines F5 and E12, in which the IFITM3 protein is undetectable, differ in the level of sensitivity to the influenza virus. This phenomenon is likely due to the nature of the mutations obtained with the help of the CRISPR/Cas9 system. Transcriptomic analysis of alterations of gene expression revealed that in clone E12, together with downregulation of mRNA of target gene
IFITM3, there is downregulation of
IFITM1 mRNA. On the other hand, in clone F5, upregulation of
IFITM2 mRNA was observed, but the mRNA levels of genes
IFITM1 and
IFITM3 did not differ significantly from control cells (
Figure 5). The combined changes in expression may be caused by dysregulation of transcription of genes of the
IFITM family; however, the mechanisms of this transcriptional regulation are currently poorly understood. It is known that there is
cis-regulatory enhancer E2-3 located in a region located 35 kbp away from the
IFITM3 gene, and this enhancer is capable of regulating the expression of the
IFITM1–3 gene group [
35]. A mutation at the locus containing
IFITM genes, such as extended insertions or deletions (as in clone E12), may affect the interaction of the regulatory region with promoters and diminish the mRNA level. Because it is known that partial deletion of enhancer E2-3 does not completely repress the genes of the
IFITM family, the existence of other enhancers that regulate the transcription of these genes is possible [
35]. Nonetheless, their location with respect to genomic locus
IFITM is currently unknown.
It is also worth noting that in clone E12, significant underexpression of genes of the Jak–STAT pathway was observed, thereby confirming the suppression of innate immune response in these cells (
Figure 8). In clone F5, this effect was not well-pronounced. Differences in the antiviral response between cell lines E12 and F5 are also well illustrated by the heat map based on normalized expression levels of genes in the “response to virus” group (GO:0009615) (
Figure 7). The alterations seen in the F5 cell line correlate more with changes in the F3 cell line, in which downregulation of the target protein was demonstrated after mutations in one allele. These findings suggest that reducing the activity of one member of the IFITM family is insufficient for altering the expression of antiviral-response genes. For this alteration to occur, comprehensive repression of several genes is necessary, as observed in the E12 cell line.
In this study, a total of 92 monoclonal cell lines were analyzed into which the genome-editing system was introduced; among them, only three cell lines with mutations in the IFITM3 gene were detected, and only two cell lines were homozygous for mutant alleles. Furthermore, the proliferation rate of the mutant cell lines was significantly lower than that of the control, and many cell lines even died after several passages. This may mean that this protein affects cell viability and probably participates not only in the regulation of the antiviral response but also (according to the results of our transcriptomic analysis) in the modulation of the processes of cell adhesion, proliferation, and cell-to-cell contacts.
4. Materials and Methods
4.1. Construction of Plasmids Expressing Components of CRISPR/Cas9 Systems Directed to IFITM3 Gene Knockout
Analysis of gene sequences for the presence of protospacer-associated motifs (PAMs) and the selection of protospacers depending on the parameters of specific and nonspecific activity were performed using the Benchling online tool (available at
https://benchling.com/, accessed on 3 February 2021). Protospacers were chosen according to the specificity criterion: the 20-nt sequence was completely complementary only to a region of the
IFITM3 gene; by contrast, during modeling of the interaction of single guide RNA with sequences of other homologous members of the
IFITM family (primarily
IFITM2), only imperfect duplexes formed, with 2–3 mismatches in the seed sequence area. In total, 8 protospacers were selected to construct sgRNAs (Table S1). After that, genetic constructs based on the pSpCas9(BB)-2A-GFP (PX458) vector (Addgene, cat. # 48138) were obtained; they encoded single guide RNA with required protospacers (as described in the original article [
33]).
4.2. Cell Transfection and Cloning
WI-38 VA13 subline 2RA (ATCC #CCL-75.1, derived from the Collection of Vertebrate Cell Cultures, Institute of Cytology, Russian Academy of Sciences, Russia) cells were maintained in DMEM/F12 (Thermo Fisher Scientific, United States) supplemented with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, United States) at 37°C with CO2. The cells were seeded into the wells of 6-well plate in 1.5 mL of complete culture medium a day before the transfection. The cell density was 50–80% on the day of transfection. Transfection of cells was carried out using the Lipofectamine® 3000 (Thermo Fisher Scientific, United States) reagent in a serum-free growth medium DMEM/F12 (Thermo Fisher Scientific, United States). For the transfection, 4 μL of the Lipofectamine reagent was mixed with 1 μg of plasmid constructs based on vector pSpCas9(BB)-2A-GFP (PX458), and the total volume of the mixture was brought to 20 μL with sodium phosphate buffer (pH 7.4). The transfection mixture was incubated for 20 min at room temperature, after which it was added to the cells in the serum-free growth medium. The cells were incubated for 6 h in a CO2 incubator at 37 ℃. Then, the medium was changed to 1.5 mL of fresh DMEM/F12 supplemented with 10% of FBS. The cells were left in the CO2 incubator at 37 ℃ overnight. The transfection outcome was assessed with the help of the ZOE™ fluorescent cell imaging system (Bio-Rad, United States). GFP-positive cells were collected 48 h after the transfection on a cell sorter S3e Cell Sorter (Bio-Rad Laboratories, United States). The cell suspension was diluted to approximately 100 cells/mL and seeded on 96-well plates (1 cell/well). The clones of individual cells were trypsinized and replicated in 96-well plates for the analysis.
4.3. Analysis of the monoclonal cell lines for IFITM3 gene mutations
For some monoclonal lines the effectiveness of the genetic modifications introduced by the CRISPR/Cas9 genome-editing system in the IFITM3 gene was examined by means of endonuclease I of bacteriophage T7. Protospacers 3 and 4 were selected so that the site of the expected break introduced by the CRISPR/Cas9 system coincided with the site cleaved by restriction endonucleases ZraI (SibEnzyme, Russia) and AatII (SibEnzyme, Russia), respectively. The presence of a mutation meant disruption of a restriction site; in this case, DNA is not cut by the restriction endonuclease. This approach enabled us to determine the presence/absence of mutations in the monoclonal cell lines obtained via the transfection with plasmid vectors carrying protospacer 3 or 4. For the restriction analysis, a 20 μL reaction mixture was prepared consisting of 1 μL of an amplicon, 2 μL of restriction endonuclease ZraI for clones with putative mutations in the region of protospacer 3 (or restriction endonuclease AatII for clones with putative mutations in the region of protospacer 4), 2 μL of 10X restriction enzyme buffer, and nuclease-free water up to the final volume. The samples were incubated for 1 h at 37 ℃. The reaction products were analyzed by electrophoresis in a 1.5% agarose gel.
4.4. Detection of Deletions and Insertions in Monoclonal Cell Lines
To determine the presence/absence of deletions and insertions in DNA samples from monoclonal cell lines, the indel detection by amplicon analysis (IDAA) was applied [
34]. PCR was conducted with 6-FAM-5′-labeled primers, which implemented one-step fluorophore labeling of amplicons. Next, the amplicons were purified on ExtraGen magnetic particles (GenTerra, Russia). PCR products were visualized by electrophoresis in a 1.5% agarose gel. After that, DNA (1–5 ng) was dried on a vacuum evaporator and dissolved in formamide for assessment on genome analyzer ABI Genetic Analyser 3500 (ABI / Life Technologies, United States). The data were examined in the Thermo Fisher Cloud Dashboard software (Thermo Scientific, United States).
4.5. Real-Time Cell Growth Analysis
To evaluate the proliferation rate of the cells carrying mutations in the IFITM3 gene, a proliferation assay was performed as compared to the original WI-38 VA13 cells with the help of xCelligence technology on the cell analyzer RTCA xCelligence DP (ACEA Biosciences, United States) equipped with E-plate 16 chips (ACEA Biosciences, United States). The results were analyzed using a RTCA Software version 1.2 software (ACEA Bioscience, United States). The number of live cells was determined beforehand on a LUNA-II automatic cell counter (Logos Biosystems, South Korea) by staining with trypan blue (Bio-Rad Laboratories, United States). The cells with viability at least 90% in three biological replicates obtained by independent defrosting of the cell bank were used for the experiments. Cells were seeded at a density of 20,000/well in the DMEM/F12 medium supplemented with 10% of fetal bovine serum and monitored in real time for 60 h.
4.6. Quantification of mRNA and Viral RNA Expression by RT-PCR
Total RNA from WI-38 VA13 cells and modified monoclonal lines were isolated using the Kit for Isolation of Total RNA and MicroRNA from Cells and Tissues (Biolabmix, Russia) according to the manufacturer’s protocol. RT-PCR was carried out with the BioMaster RT-PCR SYBR Blue (2×) kit (Biolabmix, Russia). The analysis of the results was conducted using a Light Cycler 96 Software 1.1 software package (Roche, Switzerland) and qBase+ (Biogazelle, Belgium). To assess the expression of the IFITM3 gene, primers IFITM3-F1 5′-CCGTGAAGTCTAGGGACAGG-3′ and IFITM3-R1 5′-CCTGGAAGATCAGCACTGG-3′ were used. As reference signals, GAPDH mRNA (GAPDH-F: 5′-GAAGATGGTGATGGGATTTC-3′, GAPDH-R: 5′-GAAGGTGAAGGTCGGAGT-3′) and of 18S rRNA (18S-F: 5′-GATGGTAGTCGCCGTGCC-3′, 18S-R: 5′-GCCTGCTGCCTTCCTTGG-3′) were quantitated. To estimate the level of viral mRNA, the primers allowing to detect fragment of the M gene of the influenza A were used: FluA-F 5′-CCCTCAAAGCCGAGATCGC-3′ and FluA-R 5′-AGGTGACARRATTGGTCTTGTCTTTA-3′. The primers for IFITM3 mRNA and Influenza A RNA detection contained phosphoryl guanidine groups.
4.7. Detection of the IFITM3 Protein in the Monoclonal Cell Lines by Western Blotting
Proteins were isolated from a pool of cells (2.5 × 105) via the addition of 50 μL of RIPA lysis buffer to a cell pellet, incubation for 30 min on ice, then centrifugation for 20 min at 4 ℃ and 13000 relative centrifugal field units, and the pellet was discarded. The cell lysates were stored at −20 ℃. Proteins were separated by protein electrophoresis in a 10% polyacrylamide gel; 10 μL of Spectra™ Multicolor Broad Range Protein Ladder (Thermo Scientific, United States) served as molecular-weight markers. After that, the proteins were electrotransferred onto a nitrocellulose membrane, and western blotting was performed in an IBind system (Thermo Scientific, United States). Primary antibodies: Rabbit anti Human IFITM3 antibody (1:200) (VPA00312, Bio-Rad Laboratories, United States), Rabbit anti GAPDH antibody (1:1000) (ab181602, Abcam, United Kingdom); secondary antibodies: Rabbit IgG Horseradish Peroxidase-conjugated Antibody (1:200) (HAF008, R&D Systems, United States). To visualize the results, the membrane was incubated in a luminol solution. Next, the membrane was washed with deionized water. Imaging was implemented on an Amersham Imager 600 device (GE Healthcare Life Sciences, United States).
4.8. Infection of Cells with the Influenza A Virus
Influenza viruses A/PR/8/34 (H1N1) from the Collection of Influenza and Acute-Respiratory-Disease Viruses at the Smorodintsev Research Institute of Influenza (St Petersburg, Russia) and A/California/07/2009 (H1N1pdm09), and A/Hong Kong/4801/2014 (H3N2), obtained from CDC (Atlanta, United States) were used in this work. The viruses were cultured in 10–12-day-old chicken embryos. Infectious activity of viruses was determined by titration in MDCK cells. A monolayer of cells in a 96-well plate was infected with serial 10-fold dilutions of a virus (in rows, 4 wells per dilution) in the serum-free medium supplemented with 2.5 μg/mL TPCK trypsin (Sigma, United States) and was incubated at 37 °C and 5% of CO2 for 3 days. Virus replication was assessed by hemagglutination reaction with a 0.5% suspension of chicken erythrocytes. Infectious activity was calculated by the Reed–Muench method and expressed as TCID50/mL.
To determine sensitivity to influenza virus infection, WI-38 VA13 cells were grown in 12-well plates until a dense monolayer formed. Before infection, the cells were washed with the serum-free medium supplemented with 1 μg/mL TPCK trypsin. A virus dilution was prepared in the above medium so that multiplicity of infection was 0.01 TCID50/cell, considering a monolayer density of 1.3 × 105 cells/cm2, and an inoculum volume of 250 μL/well. The inoculum was added to cells, incubated for 1 h at 37 °C, 5% of CO2, and then removed. Serum-free media with trypsin was added in a volume of 2 mL/well and plates were incubated at 37 °C and 5% of CO2. The experiment was conducted with three biological replicates. At 24, 48, and 72 h after the infection, aliquots of the culture supernatant were collected, in which either the number of viral particles was determined via titration with the TCID50 assay in MDCK cells. Infected cells were also collected, fixed, permeabilized (BD Cytofix/Cytoperm), and stained with fluorescently labeled antibodies to the NP protein of influenza A virus (clone 6D11-FITC, PPDP Ltd., St Petersburg, Russia; dilution 1:1000). Uninfected cells served as a control in the staining procedure. The percentage of infected cells was determined on a Cytoflex flow cytometer (Beckman Coulter, United States).
To assess the permissiveness of cells to infection by focus assay, based on accumulation of viral NP protein (nucleoprotein), cells were grown in a 96-well plate in quadruplicate to a density of 50 × 103 cells/well. Before infection, the cells were placed in the serum-free growth medium supplemented with 1 μg/mL trypsin. The virus dilution was prepared so that infection dose was 0.01 TCID50/cell. After addition of the virus, the cells were incubated for 24 h at 37 °C, 5% of CO2. Next, the cells were fixed with acetone, after which 50 μL (1:2500 dilution) of a primary antibody against the NP protein of the influenza A virus [mouse anti-influenza A monoclonal antibody, MAB8258 (Millipore, United States)] was added to the cells and incubated at 37 °C for 1 h. After that, cells were washed twice with PBS, and 50 μL (1/4000 dilution) of a biotinylated secondary antibody was added into the wells [Anti-Mouse IgG (Fab specific)–Biotin antibody, B7151 (Sigma-Aldrich, United States)] and incubated at 37 °C for 30 min. The cells were washed twice with PBS, and 50 μL (1:4000 dilution) of streptavidin conjugated with horseradish peroxidase (Sigma-Aldrich, United States) was added and incubated at 37 °C for 30 min. The cells were washed twice with PBS, and 50 μL of AEC buffer was introduced; the multiwell plates were left in a dark place at room temperature for 10–15 min until coloring appeared. The number of focus-forming units (number of stained cells) was estimated in the ImageJ software.
4.9. Analysis of Transcriptome-Sequencing data
Construction of cDNA libraries was conducted according to a standard protocol based on the NEBNext Ultra II Directional library preparation kit (New England Biolabs, United States) and a module for enrichment of the poly(A) fraction of RNA (New England Biolabs, United States), followed by mass parallel sequencing on the NextSeq Illumina platform at the Institute of Fundamental Medicine and Biology at Kazan Federal University (Kazan, Russia). Paired-end reads of 75 nucleotides each were obtained by means of the NextSeq 500/550 High Output v2.0 Kit (150 cycles) (Illumina, United States).
The raw data were saved as FASTQ format files. The quality control of the raw and trimmed reads was performed using FastQC (v0.11.9) and MultiQC (v1.9) [
36,
37]. Trimming of the adapter content and quality trimming was performed using fastp (v0.21.0) [
38]. The reads complementary to ribosomal RNA were filtered out from the trimmed reads using SortMeRNA (v2.1b) [
39]. The filtered reads were aligned via STAR (v2.7.7a) to a combined genomic reference of the human genome (GRCh38) and the genome of influenza A/Puerto Rico/8/1934 (H1N1) [
40]. Mapped reads were visualized using the Integrative Genomics Viewer (IGV) (v.2.8.0) [
41].The counting reads was performed with the featureCounts function from the R package Rsubread (v2.4.3) [
42]. Differentially expressed RNAs were identified using R package DESeq2 (v1.30.1) with a FDR-adjusted p -value < 0.05 and the absolute value of a log2(FC) > 0.58 [
43]. RNA-seq data have been deposited in the ArrayExpress database at EMBL-EBI (
https://www.ebi.ac.uk/biostudies/arrayexpress) under accession number E-MTAB-9511 (virus infected original WI-38 VA-13) and E-MTAB-13582 (virus infected original WI-38 VA-13 and clones with mutations in the
IFITM3 gene (F3, F5, Е12)) [
44]
Author Contributions
Conceptualization, G.S., A.K. and M.S.; methodology, G.S., M.S., N.E., K.K., E.G. and E.Z.; software, E.Z.; validation, M.S. and E.Z.; formal analysis, E.Z.; investigation, G.S., M.S., N.E., K.K., E.G. and E.Z.; resources, A.K. and G.S.; data curation, G.S., A.K. and M.S.; writing—original draft preparation, N.E. and E.Z.; writing—review and editing, G.S., A.K. and M.S.; visualization, E.Z.; supervision, G.S., A.K. and M.S; project administration, G.S.; funding acquisition, G.S. and E.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation (project no. 23-25-00450).
Data Availability Statement
RNA-seq data have been deposited in the ArrayExpress database at EMBL-EBI (
https://www.ebi.ac.uk/biostudies/arrayexpress) under accession number E-MTAB-9511 (virus infected original WI-38 VA-13) and E-MTAB-13582 (virus infected original WI-38 VA-13 and clones with mutations in the
IFITM3 gene (F3, F5, Е12)).
Acknowledgments
The English language was corrected by shevchuk-editing.com.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Prim. 2018, 4, 1–21. [Google Scholar] [CrossRef]
- Somes, M.P.; Turner, R.M.; Dwyer, L.J.; Newall, A.T. Estimating the annual attack rate of seasonal influenza among unvaccinated individuals: A systematic review and meta-analysis. Vaccine 2018, 36, 3199–3207. [Google Scholar] [CrossRef]
- Vaccines against influenza: WHO position paper – May 2022. Available online: https://www.who.int/publications/i/item/who-wer9719 (accessed on 4 October 2023).
- Ott, J.J.; Breteler, J.K.; Tam, J.S.; Hutubessy, R.C.W.; Jit, M.; De Boer, M.R. Influenza vaccines in low and middle income countries: a systematic review of economic evaluations. Hum. Vaccin. Immunother. 2013, 9, 1500–1511. [Google Scholar] [CrossRef]
- Jefferson, T.; Rivetti, A.; Di Pietrantonj, C.; Demicheli, V. Vaccines for preventing influenza in healthy children. Cochrane database Syst. Rev. 2018, 2. [Google Scholar] [CrossRef] [PubMed]
- Burnet, F. Influenza Virus Infections of the Chick Embryo by the Amniotic Route. Aust. J. Exp. Biol. Med. Sci. 1940, 18, 353–360. [Google Scholar] [CrossRef]
- Burnet, F. Growth of influenza virus in the allantoic cavity of the chick embryo. Aust. J. Exp. Biol. Med. Sci. 1941; 19, 291–295. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Janjua, N.Z.; De Serres, G.; Sabaiduc, S.; Eshaghi, A.; Dickinson, J.A.; Fonseca, K.; Winter, A.L.; Gubbay, J.B.; Krajden, M.; et al. Low 2012–13 Influenza Vaccine Effectiveness Associated with Mutation in the Egg-Adapted H3N2 Vaccine Strain Not Antigenic Drift in Circulating Viruses. PLoS One 2014, 9, e92153. [Google Scholar] [CrossRef]
- Zost, S.J.; Parkhouse, K.; Gumina, M.E.; Kim, K.; Perez, S.D.; Wilson, P.C.; Treanor, J.J.; Sant, A.J.; Cobey, S.; Hensley, S.E. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 12578–12583. [Google Scholar] [CrossRef]
- Coop, C.A.; Balanon, S.K.; White, K.M.; Whisman, B.A.; Rathkopf, M.M. Anaphylaxis from the influenza virus vaccine. Int. Arch. Allergy Immunol. 2008, 146, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.W.; Reichl, U. Downstream Processing: From Egg to Cell Culture-Derived Influenza Virus Particles. Chem. Eng. Technol. 2008, 31, 846–857. [Google Scholar] [CrossRef]
- Lamb, Y.N. Cell-Based Quadrivalent Inactivated Influenza Virus Vaccine (Flucelvax® Tetra/Flucelvax Quadrivalent®): A Review in the Prevention of Influenza. Drugs 2019, 79, 1337–1348. [Google Scholar] [CrossRef]
- Nolan, T.; Fortanier, A.C.; Leav, B.; Põder, A.; Bravo, L.C.; Szymański, H.T.; Heeringa, M.; Vermeulen, W.; Matassa, V.; Smolenov, I.; et al. Efficacy of a Cell-Culture-Derived Quadrivalent Influenza Vaccine in Children. N. Engl. J. Med. 2021, 385, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.S.; Cook, P.; Attwell, A.M.; Williams, S.P. Replicative advantage in tissue culture of egg-adapted influenza virus over tissue-culture derived virus: implications for vaccine manufacture. Vaccine 1995, 13, 1583–1588. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhou, H.; Chan, W.; Kemble, G.; Jin, H. Single amino acid substitutions in the hemagglutinin of influenza A/Singapore/21/04 (H3N2) increase virus growth in embryonated chicken eggs. Vaccine 2006, 24, 6691–6693. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.R. Adventitious Agents and Vaccines - Volume 7, Number 7—June 2001 - Emerging Infectious Diseases journal - CDC. Emerg. Infect. Dis. 2001, 7, 562. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, J.P. A quantitative risk assessment of exposure to adventitious agents in a cell culture-derived subunit influenza vaccine. Vaccine 2008, 26, 3332–3340. [Google Scholar] [CrossRef] [PubMed]
- Genzel, Y.; Dietzsch, C.; Rapp, E.; Schwarzer, J.; Reichl, U. MDCK and Vero cells for influenza virus vaccine production: a one-to-one comparison up to lab-scale bioreactor cultivation. Appl. Microbiol. Biotechnol. 2010, 88, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, J.P.; Schmitt, H.J.; Trusheim, H.; Bröker, M. Safety of MDCK cell culture-based influenza vaccines. Future Microbiol. 2011, 6, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.J.; Berezuk, G.; Fritsch, S.; Aichinger, G.; Singer, J.; Portsmouth, D.; Hart, M.K.; El-Amin, W.; Kistner, O.; Barrett, P.N. Clinical development of a Vero cell culture-derived seasonal influenza vaccine. Vaccine 2012, 30, 4377–4386. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, J.; Rapp, E.; Hennig, R.; Genzel, Y.; Jordan, I.; Sandig, V.; Reichl, U. Glycan analysis in cell culture-based influenza vaccine production: influence of host cell line and virus strain on the glycosylation pattern of viral hemagglutinin. Vaccine 2009, 27, 4325–4336. [Google Scholar] [CrossRef]
- Hütter, J.; Rödig, J. V.; Höper, D.; Seeberger, P.H.; Reichl, U.; Rapp, E.; Lepenies, B. Toward animal cell culture-based influenza vaccine design: viral hemagglutinin N-glycosylation markedly impacts immunogenicity. J. Immunol. 2013, 190, 220–230. [Google Scholar] [CrossRef]
- Global Influenza Strategy 2019–2030. Available online: https://www.who.int/publications/i/item/9789241515320 (accessed on 10 October 2023).
- Iwasaki, A.; Pillai, P.S. Innate immunity to influenza virus infection. Nat. Rev. Immunol. 2014, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Flerlage, T.; Boyd, D.F.; Meliopoulos, V.; Thomas, P.G.; Schultz-Cherry, S. Influenza virus and SARS-CoV-2: pathogenesis and host responses in the respiratory tract. Nat. Rev. Microbiol. 2021, 19, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Prokhorova, D.; Zhukova, N.; Lemza, A.; Sergeeva, M.; Amirkhanov, R.; Stepanov, G. Application of the CRISPR/Cas9 System to Study Regulation Pathways of the Cellular Immune Response to Influenza Virus. Viruses 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Hickford, D.; Frankenberg, S.; Shaw, G.; Renfree, M.B. Evolution of vertebrate interferon inducible transmembrane proteins. BMC Genomics 2012, 13, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, J.; Winkler, C.A.; An, P.; Guo, J.T. IFITM genes, variants, and their roles in the control and pathogenesis of viral infections. Front. Microbiol. 2019, 10, 3228. [Google Scholar] [CrossRef] [PubMed]
- SUN, Q.; LEI, N.; LU, J.; GAO, R.B.; LI, Z.; LIU, L.Q.; SUN, Y.; GUO, J.F.; WANG, D.Y.; SHU, Y.L. Interferon-induced Transmembrane Protein 3 Prevents Acute Influenza Pathogenesis in Mice. Biomed. Environ. Sci. 2020, 33, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Munguía, I.; Beaven, A.H.; Blank, P.S.; Sodt, A.J.; Zimmerberg, J. Interferon-induced transmembrane protein 3 (IFITM3) and its antiviral activity. Curr. Opin. Struct. Biol. 2022, 77, 102467. [Google Scholar] [CrossRef]
- Brass, A.L.; Huang, I.C.; Benita, Y.; John, S.P.; Krishnan, M.N.; Feeley, E.M.; Ryan, B.J.; Weyer, J.L.; van der Weyden, L.; Fikrig, E.; et al. The IFITM Proteins Mediate Cellular Resistance to Influenza A H1N1 Virus, West Nile Virus, and Dengue Virus. Cell 2009, 139, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- J. Olshansky, S.; Hayflick, L. The Role of the WI-38 Cell Strain in Saving Lives and Reducing Morbidity. AIMS public Heal. 2017, 4, 127–138. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013 811 2013, 8, 2281–2308. [Google Scholar] [CrossRef]
- Yang, Z.; Steentoft, C.; Hauge, C.; Hansen, L.; Thomsen, A.L.; Niola, F.; Vester-Christensen, M.B.; Frödin, M.; Clausen, H.; Wandall, H.H.; et al. Fast and sensitive detection of indels induced by precise gene targeting. Nucleic Acids Res. 2015, 43, e59. [Google Scholar] [CrossRef]
- Li, P.; Shi, M.L. Coordinated regulation of IFITM1, 2 and 3 genes by an IFN-responsive enhancer through long-range chromatin interactions. Biochim. Biophys. Acta - Gene Regul. Mech. 2017, 1860, 885–893. [Google Scholar] [CrossRef]
- Andrews, S. Babraham Bioinformatics - FastQC A Quality Control tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 6 August 2020).
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: an ultra-fast all-in-one FASTQ preprocessor. [CrossRef]
- Kopylova, E.; Noé, L.; Lè Ne Touzet, H. SortMeRNA: fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 2012, 28, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. Sequence analysis STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer, 2011.
- Liao, Y.; Smyth, G.K.; Shi, W. Sequence analysis featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Athar, A.; Füllgrabe, A.; George, N.; Iqbal, H.; Huerta, L.; Ali, A.; Snow, C.; Fonseca, N.A.; Petryszak, R.; Papatheodorou, I.; et al. ArrayExpress update – from bulk to single-cell expression data. Nucleic Acids Res. 2019, 47, D711–D715. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Relative expression of IFITM genes in influenza A infected WI-38 VA-13 cells after 24 h (24 hpi) and 48 h (48 hpi), according to results of our transcriptomic analysis. Log2 Fold Change (LogFC) values were generated for transcriptome sequencing samples by comparing the average RPKM values of genes at each timepoint of infection vs. uninfected cells.
Figure 1.
Relative expression of IFITM genes in influenza A infected WI-38 VA-13 cells after 24 h (24 hpi) and 48 h (48 hpi), according to results of our transcriptomic analysis. Log2 Fold Change (LogFC) values were generated for transcriptome sequencing samples by comparing the average RPKM values of genes at each timepoint of infection vs. uninfected cells.
Figure 2.
(A) Scheme of the location of protospacers and identified CRISPR/Cas9-mediated mutations in target region of the IFITM3 gene. (B) IDAA analysis of modified (F3, F5) and control WI-38 VA13 cells. Amplicon and indel sizes determined (in bp) are shown above the most prominent peaks. (C) PCR products obtained from the gDNA extracted from modified (E12) and control WI-38 VA13 cells, with the primers flanking the extended deletion in clone E12 (F1_R1 / allele 1) and with the reverse primer that was partly complementary to the IFITM3 gene and partly to the plasmid PX458 region integrated into the genome (F1_R2 / allele 2). M, DNA molecular weight marker. The scheme shows the location of the primers (red arrows) and the expected length of the products for modified (E12) and control WI-38 VA13 cells.
Figure 2.
(A) Scheme of the location of protospacers and identified CRISPR/Cas9-mediated mutations in target region of the IFITM3 gene. (B) IDAA analysis of modified (F3, F5) and control WI-38 VA13 cells. Amplicon and indel sizes determined (in bp) are shown above the most prominent peaks. (C) PCR products obtained from the gDNA extracted from modified (E12) and control WI-38 VA13 cells, with the primers flanking the extended deletion in clone E12 (F1_R1 / allele 1) and with the reverse primer that was partly complementary to the IFITM3 gene and partly to the plasmid PX458 region integrated into the genome (F1_R2 / allele 2). M, DNA molecular weight marker. The scheme shows the location of the primers (red arrows) and the expected length of the products for modified (E12) and control WI-38 VA13 cells.
Figure 3.
Growth characteristics of modified and control WI-38 VA13 cells. (A) Growth curves of cells defective in IFITM3 gene (F3, F5, E12) and control WI-38 VA13 cells according to xCELLigence data. (B) Values of initial growth rate of cell lines during first 48 hours.
Figure 3.
Growth characteristics of modified and control WI-38 VA13 cells. (A) Growth curves of cells defective in IFITM3 gene (F3, F5, E12) and control WI-38 VA13 cells according to xCELLigence data. (B) Values of initial growth rate of cell lines during first 48 hours.
Figure 4.
(A) Relative basic level of IFITM3 mRNA in original WI-38 VA13 cells and in the clones with depressed IFITM3 gene activity (F3, F5, Е12). Average value of a change in the expression level relative to reference GAPDH and 18S rRNA is presented, standard deviation was calculated from three independent experiments by data on three independent experiments. *Difference between modified lines and WI-38 VA13 cells is statistically significant at p < 0.05 (unpaired Student’s t-test) (B) Western blot analysis of IFITM3 protein level in original WI-38 VA13 cells and monoclonal lines with detected mutations in the IFITM3 gene.
Figure 4.
(A) Relative basic level of IFITM3 mRNA in original WI-38 VA13 cells and in the clones with depressed IFITM3 gene activity (F3, F5, Е12). Average value of a change in the expression level relative to reference GAPDH and 18S rRNA is presented, standard deviation was calculated from three independent experiments by data on three independent experiments. *Difference between modified lines and WI-38 VA13 cells is statistically significant at p < 0.05 (unpaired Student’s t-test) (B) Western blot analysis of IFITM3 protein level in original WI-38 VA13 cells and monoclonal lines with detected mutations in the IFITM3 gene.
Figure 5.
(A) The coverage tracks of the genome fragment encoding IFITM2, IFITM1 and IFITM3 genes for the original WI-38 VA13 cells and clones with mutations (F3, F5, Е12) before infection (0 hpi) and at 24 h (24 hpi) after influenza A virus infection. (B) Expression of IFITM2, IFITM1, IFITM3 genes in original WI-38 VA13 cells and clones with mutations (F3, F5, Е12) before infection (0 hpi) and at 24 h (24 hpi) after influenza A virus infection. There are shown the normalized counts of mapped reads averaged for two biological repeats.
Figure 5.
(A) The coverage tracks of the genome fragment encoding IFITM2, IFITM1 and IFITM3 genes for the original WI-38 VA13 cells and clones with mutations (F3, F5, Е12) before infection (0 hpi) and at 24 h (24 hpi) after influenza A virus infection. (B) Expression of IFITM2, IFITM1, IFITM3 genes in original WI-38 VA13 cells and clones with mutations (F3, F5, Е12) before infection (0 hpi) and at 24 h (24 hpi) after influenza A virus infection. There are shown the normalized counts of mapped reads averaged for two biological repeats.
Figure 6.
Gene ontology analysis of the most differentially expressed genes in original WI-38 VA13 cells and clones with mutations (F3, F5, Е12) at 24 h after influenza A virus infection (relative to uninfected cells). Bar charts showing the top 10 GO terms for biological process, ranked by fold enrichment following analysis of the most differentially expressed genes.
Figure 6.
Gene ontology analysis of the most differentially expressed genes in original WI-38 VA13 cells and clones with mutations (F3, F5, Е12) at 24 h after influenza A virus infection (relative to uninfected cells). Bar charts showing the top 10 GO terms for biological process, ranked by fold enrichment following analysis of the most differentially expressed genes.
Figure 7.
Heat map of scaled normalized expression values of genes of the "response to the virus" group (GO: 0009615) for two biological repeats of original WI-38 VA13 cells and clones with mutations (F3, F5, Е12) before infection (0 hpi) and at 24 h (24 hpi) after influenza A virus infection.
Figure 7.
Heat map of scaled normalized expression values of genes of the "response to the virus" group (GO: 0009615) for two biological repeats of original WI-38 VA13 cells and clones with mutations (F3, F5, Е12) before infection (0 hpi) and at 24 h (24 hpi) after influenza A virus infection.
Figure 8.
(A) Fragment of the scheme of molecular interactions during influenza A virus infection involved in the regulation of Jak-STAT signaling pathway. (B) Relative expression of genes involved in the Jak-STAT signaling pathway in influenza A infected WI-38 VA-13 cells at 24 h after influenza A virus infection, according to results of our transcriptomic analysis. Log2 Fold Change (LogFC) values were generated for transcriptome sequencing samples by comparing the average RPKM values of genes at 24 of infection vs. uninfected cells. (C) Expression of genes involved in the Jak-STAT signaling pathway in original WI-38 VA13 cells and clones with mutations (F3, F5, Е12) before infection (0 hpi) and at 24 h (24 hpi) after influenza A virus infection. There are shown the normalized counts of mapped reads averaged for two biological repeats.
Figure 8.
(A) Fragment of the scheme of molecular interactions during influenza A virus infection involved in the regulation of Jak-STAT signaling pathway. (B) Relative expression of genes involved in the Jak-STAT signaling pathway in influenza A infected WI-38 VA-13 cells at 24 h after influenza A virus infection, according to results of our transcriptomic analysis. Log2 Fold Change (LogFC) values were generated for transcriptome sequencing samples by comparing the average RPKM values of genes at 24 of infection vs. uninfected cells. (C) Expression of genes involved in the Jak-STAT signaling pathway in original WI-38 VA13 cells and clones with mutations (F3, F5, Е12) before infection (0 hpi) and at 24 h (24 hpi) after influenza A virus infection. There are shown the normalized counts of mapped reads averaged for two biological repeats.
Figure 9.
(A) Accumulation of influenza virus nucleoprotein in the original WI-38 VA13 cells (WI-38 VA13) and clones with mutations (F3, F5, Е12) after influenza A/PR/8/34 (H1N1) virus infection. The amount of NP-positive (infected) cells was measured at specified time points using a flow cytometry method and staining with antibodies against influenza A virus NP. (B) Growth curves of influenza virus in original WI-38 VA13 cells (WI-38 VA13) and clones with mutations (F3, F5, Е12) after influenza A/PR/8/34 (H1N1) virus infection. Infectious titer was measured at specified time points in supernatant of cells infected at a dose of 0.001 TID50/cell.
Figure 9.
(A) Accumulation of influenza virus nucleoprotein in the original WI-38 VA13 cells (WI-38 VA13) and clones with mutations (F3, F5, Е12) after influenza A/PR/8/34 (H1N1) virus infection. The amount of NP-positive (infected) cells was measured at specified time points using a flow cytometry method and staining with antibodies against influenza A virus NP. (B) Growth curves of influenza virus in original WI-38 VA13 cells (WI-38 VA13) and clones with mutations (F3, F5, Е12) after influenza A/PR/8/34 (H1N1) virus infection. Infectious titer was measured at specified time points in supernatant of cells infected at a dose of 0.001 TID50/cell.
Figure 10.
Accumulation of influenza virus nucleoprotein inthe original WI-38 VA13 cells (WI-38 VA13) and clones with mutations (F3, F5, Е12) after influenza A/California/07/2009 (H1N1pdm09) and influenza A/Hong Kong/4801/2014 (H3N2) virus infection. The amount of NP-positive (infected) cells was measured at specified time points using a flow cytometry method and staining with antibodies against influenza A virus NP. *Difference between clone and original cells is statistically significant at p < 0.05 (two-factor ANOVA analysis with a posteriori Bonferroni criterion).
Figure 10.
Accumulation of influenza virus nucleoprotein inthe original WI-38 VA13 cells (WI-38 VA13) and clones with mutations (F3, F5, Е12) after influenza A/California/07/2009 (H1N1pdm09) and influenza A/Hong Kong/4801/2014 (H3N2) virus infection. The amount of NP-positive (infected) cells was measured at specified time points using a flow cytometry method and staining with antibodies against influenza A virus NP. *Difference between clone and original cells is statistically significant at p < 0.05 (two-factor ANOVA analysis with a posteriori Bonferroni criterion).
Figure 11.
(A) Accumulation of influenza virus nucleoprotein inthe original WI-38 VA13 cells (WI-38 VA13) and clones with mutations (F3, F5, Е12) after influenza A/WSN/33 (H1N1) virus infection. The amount of NP-positive (infected) cells was measured at specified time points using a flow cytometry method and staining with with 6D11 monoclonal antibodies against influenza A virus NP. *Difference between clone and original cells is statistically significant at p < 0.05 (two-factor ANOVA analysis with a posteriori Bonferroni criterion). (B) Immunostaining of original WI-38 VA13 cells (WI-38 VA13) and clones with mutations (F3, F5, Е12) after influenza A/WSN/33 (H1N1) virus infection. The influenza virus NP protein was stained with MAB8258 monoclonal antibody and visualized with HRP-conjugate.
Figure 11.
(A) Accumulation of influenza virus nucleoprotein inthe original WI-38 VA13 cells (WI-38 VA13) and clones with mutations (F3, F5, Е12) after influenza A/WSN/33 (H1N1) virus infection. The amount of NP-positive (infected) cells was measured at specified time points using a flow cytometry method and staining with with 6D11 monoclonal antibodies against influenza A virus NP. *Difference between clone and original cells is statistically significant at p < 0.05 (two-factor ANOVA analysis with a posteriori Bonferroni criterion). (B) Immunostaining of original WI-38 VA13 cells (WI-38 VA13) and clones with mutations (F3, F5, Е12) after influenza A/WSN/33 (H1N1) virus infection. The influenza virus NP protein was stained with MAB8258 monoclonal antibody and visualized with HRP-conjugate.
Table 1.
The number of genes differentially expressed in original WI-38 VA13 cells and clones with mutations (F3, F5, Е12) at 24 h after influenza A virus infection (relative to uninfected cells) (FDR-adjusted p-value < 0.05, absolute value of a LogFC > 0.58).
Table 1.
The number of genes differentially expressed in original WI-38 VA13 cells and clones with mutations (F3, F5, Е12) at 24 h after influenza A virus infection (relative to uninfected cells) (FDR-adjusted p-value < 0.05, absolute value of a LogFC > 0.58).
| Sample |
Regulation |
Number |
| WI-38 VA13 (24 hpi vs. 0 hpi) |
Up-regulated |
177 |
| Down-regulated |
3 |
| F3 (24 hpi vs. 0 hpi) |
Up-regulated |
364 |
| Down-regulated |
54 |
| F5 (24 hpi vs. 0 hpi) |
Up-regulated |
397 |
| Down-regulated |
43 |
| E12 (24 hpi vs. 0 hpi) |
Up-regulated |
965 |
| Down-regulated |
334 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).