Submitted:
08 December 2023
Posted:
08 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Provision of Energy and Macronutrients in Pediatric Patients on PN
3. PN with SMOFlipid in Newborn Infants, Infants/Toddlers, Children, and Adolescents – Clinical Experience
4. Crystalline Amino Acid Solutions for Newborn Infants, Infants/Toddlers, Children, and Adolescents – Clinical Experience
5. Summary and Conclusion
Funding
Acknowledgments
Abbreviations
- -
- AA: amino acid
- -
- ALA: alpha-linolenic acid
- -
- ALT: alanine aminotransferase
- -
- ARA: arachidonic acid
- -
- AST: aspartate aminotransferase
- -
- BW: body weight
- -
- CAAS: crystalline amino acids solution
- -
- CBil: conjugated bilirubin
- -
- DBil: direct bilirubin
- -
- DHA: docosahexaenoic acid
- -
- EFA: essential fatty acid
- -
- EFAD: essential fatty acid deficiency
- -
- EPA: eicosapentaenoic acid
- -
- FA: fatty acid
- -
- FO: fish oil
- -
- GGT: gamma-glutamyl transferase
- -
- HPN: home parenteral nutrition
- -
- IF: intestinal failure
- -
- IFALD: intestinal failure associated liver disease
- -
- ILE: intravenous lipid emulsion
- -
- LA: linoleic acid
- -
- LCPUFA: long-chain polyunsaturated fatty acid
- -
- LNA: linolenic acid
- -
- MCB: multi-chamber bags
- -
- MCT: medium-chain triglyceride
- -
- NPEI: non-protein-energy intake
- -
- OO: olive oil
- -
- PN: parenteral nutrition
- -
- PNDI: parenteral nutrition dependency index
- -
- PUFA: polyunsaturated fatty acid
- -
- RBC: red blood cell
- -
- RDA: recommended dietary allowance
- -
- REE: resting energy expenditure
- -
- SBS: short bowel syndrome
- -
- SO: soybean oil
- -
- TBil: total bilirubin
- -
- VLBW: very low birth weight
References
- Worthington, P.; Balint, J.; Bechtold, M.; Bingham, A.; Chan, L.-N.; Durfee, S.; Jevenn, A.K.; Malone, A.; Mascarenhas, M.; Robinson, D.T. When Is Parenteral Nutrition Appropriate? JPEN J Parenter Enteral Nutr 2017, 41, 324-377. [CrossRef]
- Koletzko, B.; Goulet, O.; Hunt, J.; Krohn, K.; Shamir, R. 1. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), Supported by the European Society of Paediatric Research (ESPR). J Pediatr Gastroenterol Nutr 2005, 41 Suppl 2, S1-87. [CrossRef]
- Joosten, K.; Embleton, N.; Yan, W.; Senterre, T. ESPGHAN/ESPEN/ESPR guidelines on pediatric parenteral nutrition: Energy. Clin Nutr 2018, 37, 2309-2314. [CrossRef]
- Ayers, P.; Boullata, J.; Sacks, G. Parenteral nutrition safety: the story continues. Nutr Clin Pract 2018, 33, 46-52. [CrossRef]
- Singer, P. Advances in Medical Nutrition Therapy: Parenteral Nutrition. Nutrients 2020, 12, 717. [CrossRef]
- Keady, S.; Morgan, C.; Ozzard, A.; Chauhan, B. Effect of a neonatal standard aqueous parenteral nutrition formulation on aseptic unit capacity planning. e-SPEN 2010, 5, e14-e17. [CrossRef]
- Goulet, O.; Ruemmele, F. Causes and management of intestinal failure in children. Gastroenterology 2006, 130, S16-S28. [CrossRef]
- Vlug, L.E.; Nagelkerke, S.C.J.; Jonkers-Schuitema, C.F.; Rings, E.; Tabbers, M.M. The Role of a Nutrition Support Team in the Management of Intestinal Failure Patients. Nutrients 2020, 12, 172. [CrossRef]
- Goulet, O.; Breton, A.; Coste, M.E.; Dubern, B.; Ecochard-Dugelay, E.; Guimber, D.; Loras-Duclaux, I.; Abi Nader, E.; Marinier, E.; Peretti, N.; et al. Pediatric Home Parenteral Nutrition in France: A six years national survey. Clin Nutr 2021, 40, 5278-5287. [CrossRef]
- Elia, M. Changing concepts of nutrient requirements in disease: implications for artificial nutritional support. Lancet 1995, 345, 1279-1284. [CrossRef]
- Schofield, W.N. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 1985, 39 Suppl 1, 5-41.
- Goulet, O.; Abi Nader, E.; Pigneur, B.; Lambe, C. Short Bowel Syndrome as the Leading Cause of Intestinal Failure in Early Life: Some Insights into the Management. Pediatr Gastroenterol Hepatol Nutr 2019, 22, 303-329. [CrossRef]
- Abi Nader, E.; Lambe, C.; Talbotec, C.; Pigneur, B.; Lacaille, F.; Garnier-Lengliné, H.; Petit, L.M.; Poisson, C.; Rocha, A.; Corriol, O.; et al. Outcome of home parenteral nutrition in 251 children over a 14-y period: report of a single center. Am J Clin Nutr 2016, 103, 1327-1336. [CrossRef]
- Abi Nader, E.; Lambe, C.; Talbotec, C.; Dong, L.; Pigneur, B.; Goulet, O. A new concept to achieve optimal weight gain in malnourished infants on total parenteral nutrition. JPEN J Parenter Enteral Nutr 2018, 42, 78-86. [CrossRef]
- Goulet, O.; Lamazière, A.; Abi Nader, E.; Talbotec, C.; Wolf, C.; Lambe, C. Erythrocyte fatty acid membrane composition in children on long-term parenteral nutrition enriched with ω-3 fatty acids. Am J Clin Nutr 2022, 115, 422-431. [CrossRef]
- Mehta, N.M.; Compher, C. A.S.P.E.N. Clinical Guidelines: nutrition support of the critically ill child. JPEN J Parenter Enteral Nutr 2009, 33, 260-276. [CrossRef]
- Mesotten, D.; Joosten, K.; van Kempen, A.; Verbruggen, S. ESPGHAN/ESPEN/ESPR guidelines on pediatric parenteral nutrition: Carbohydrates. Clin Nutr 2018, 37, 2337-2343. [CrossRef]
- Bresson, J.L.; Narcy, P.; Putet, G.; Ricour, C.; Sachs, C.; Rey, J. Energy substrate utilization in infants receiving total parenteral nutrition with different glucose to fat ratios. Pediatr Res 1989, 25, 645-648. [CrossRef]
- Salas, J.; Girardet, J.P.; De Potter, S.; Martí-Henneberg, C.; Goulet, O.; Ricour, C. Glucose versus glucose-fat mixture in the course of total parenteral nutrition: effects on substrate utilisation and energy metabolism in malnourished children. Clin Nutr 1991, 10, 272-278. [CrossRef]
- Preissig, C.M.; Rigby, M.R. Hyperglycaemia results from beta-cell dysfunction in critically ill children with respiratory and cardiovascular failure: a prospective observational study. Crit Care 2009, 13, R27. [CrossRef]
- Hirshberg, E.; Larsen, G.; Van Duker, H. Alterations in glucose homeostasis in the pediatric intensive care unit: Hyperglycemia and glucose variability are associated with increased mortality and morbidity. Pediatr Crit Care Med 2008, 9, 361-366. [CrossRef]
- Dasarathy, S.; Dodig, M.; Muc, S.M.; Kalhan, S.C.; McCullough, A.J. Skeletal muscle atrophy is associated with an increased expression of myostatin and impaired satellite cell function in the portacaval anastamosis rat. Am J Physiol Gastrointest Liver Physiol 2004, 287, G1124-1130. [CrossRef]
- Lapillonne, A.; Fidler Mis, N.; Goulet, O.; van den Akker, C.H.P.; Wu, J.; Koletzko, B. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Lipids. Clin Nutr 2018, 37, 2324-2336. [CrossRef]
- Goulet, O.J.; Cai, W.; Seo, J.M. Lipid Emulsion Use in Pediatric Patients Requiring Long-Term Parenteral Nutrition. JPEN J Parenter Enteral Nutr 2020, 44 Suppl 1, S55-S67. [CrossRef]
- Martindale, R.G.; Berlana, D.; Boullata, J.I.; Cai, W.; Calder, P.C.; Deshpande, G.H.; Evans, D.; Garcia-de-Lorenzo, A.; Goulet, O.J.; Li, A.; et al. Summary of Proceedings and Expert Consensus Statements From the International Summit “Lipids in Parenteral Nutrition”. JPEN J Parenter Enteral Nutr 2020, 44 Suppl 1, S7-S20. [CrossRef]
- Salas, J.S.; Dozio, E.; Goulet, O.J.; Marti-Henneberg, C.; Moukarzel, E.; Ricour, C. Energy expenditure and substrate utilization in the course of renutrition of malnourished children. JPEN J Parenter Enteral Nutr 1991, 15, 288-293. [CrossRef]
- Deshpande, G.C.; Cai, W. Use of Lipids in Neonates Requiring Parenteral Nutrition. JPEN J Parenter Enteral Nutr 2020, 44 Suppl 1, S45-s54. [CrossRef]
- Klek, S. Omega-3 Fatty Acids in Modern Parenteral Nutrition: A Review of the Current Evidence. J Clin Med 2016, 5, 34. [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest 2018, 128, 2657-2669. [CrossRef]
- Calder, P.C.; Waitzberg, D.L.; Klek, S.; Martindale, R.G. Lipids in Parenteral Nutrition: Biological Aspects. JPEN J Parenter Enteral Nutr 2020, 44 Suppl 1, S21-S27. [CrossRef]
- Wanten, G.J.; Calder, P.C. Immune modulation by parenteral lipid emulsions. Am J Clin Nutr 2007, 85, 1171-1184. [CrossRef]
- Lien, E.L.; Richard, C.; Hoffman, D.R. DHA and ARA addition to infant formula: Current status and future research directions. Prostaglandins Leukot Essent Fatty Acids 2018, 128, 26-40. [CrossRef]
- Hoffman, D.R.; Boettcher, J.A.; Diersen-Schade, D.A. Toward optimizing vision and cognition in term infants by dietary docosahexaenoic and arachidonic acid supplementation: a review of randomized controlled trials. Prostaglandins Leukot Essent Fatty Acids 2009, 81, 151-158. [CrossRef]
- Qawasmi, A.; Landeros-Weisenberger, A.; Bloch, M.H. Meta-analysis of LCPUFA supplementation of infant formula and visual acuity. Pediatrics 2013, 131, e262-272. [CrossRef]
- Goulet, O.; De Potter, S.; Antebi, H.; Driss, F.; Colomb, V.; Bereziat, G.; Alcindor, L.G.; Corriol, O.; Le Brun, A.; Dutot, G.; et al. Long-term efficacy and safety of a new olive oil-based intravenous fat emulsion in pediatric patients: a double-blind randomized study. Am J Clin Nutr 1999, 70, 338-345. [CrossRef]
- Goulet, O.; Joly, F.; Corriol, O.; Colomb-Jung, V. Some new insights in intestinal failure-associated liver disease. Curr Opin Organ Transplant 2009, 14, 256-261. [CrossRef]
- Lacaille, F.; Gupte, G.; Colomb, V.; D'Antiga, L.; Hartman, C.; Hojsak, I.; Kolacek, S.; Puntis, J.; Shamir, R. Intestinal failure-associated liver disease: a position paper of the ESPGHAN Working Group of Intestinal Failure and Intestinal Transplantation. J Pediatr Gastroenterol Nutr 2015, 60, 272-283. [CrossRef]
- Gura, K.M.; Duggan, C.P.; Collier, S.B.; Jennings, R.W.; Folkman, J.; Bistrian, B.R.; Puder, M. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics 2006, 118, e197-e201. [CrossRef]
- Nandivada, P.; Baker, M.A.; Mitchell, P.D.; O'Loughlin, A.A.; Potemkin, A.K.; Anez-Bustillos, L.; Carlson, S.J.; Dao, D.T.; Fell, G.L.; Gura, K.M.; et al. Predictors of failure of fish-oil therapy for intestinal failure-associated liver disease in children. Am J Clin Nutr 2016, 104, 663-670. [CrossRef]
- Hojsak, I.; Colomb, V.; Braegger, C.; Bronsky, J.; Campoy, C.; Domellof, M.; Embleton, N.; Fidler Mis, N.; Hulst, J.M.; Indrio, F.; et al. ESPGHAN Committee on Nutrition Position Paper. Intravenous Lipid Emulsions and Risk of Hepatotoxicity in Infants and Children: a Systematic Review and Meta-analysis. J Pediatr Gastroenterol Nutr 2016, 62, 776-792. [CrossRef]
- Dupont, I. Peroxidation of lipid emulsions: effects of changes in fatty acid pattern and α-tocopherol content on the sensitivity to peroxidative damage. Clin Nutr 1999, 18, 113-116. [CrossRef]
- Goulet, O.; Girot, R.; Maier-Redelsperger, M.; Bougle, D.; Virelizier, J.L.; Ricour, C. Hematologic disorders following prolonged use of intravenous fat emulsions in children. JPEN J Parenter Enteral Nutr 1986, 10, 284-288. [CrossRef]
- Hukkinen, M.; Mutanen, A.; Nissinen, M.; Merras-Salmio, L.; Gylling, H.; Pakarinen, M.P. Parenteral Plant Sterols Accumulate in the Liver Reflecting Their Increased Serum Levels and Portal Inflammation in Children With Intestinal Failure. JPEN J Parenter Enteral Nutr 2017, 41, 1014-1022. [CrossRef]
- Pereira-Fantini, P.M.; Lapthorne, S.; Gahan, C.G.M.; Joyce, S.A.; Charles, J.; Fuller, P.J.; Bines, J.E. Farnesoid X Receptor Agonist Treatment Alters Bile Acid Metabolism but Exacerbates Liver Damage in a Piglet Model of Short-Bowel Syndrome. Cell Mol Gastroenterol Hepatol 2017, 4, 65-74. [CrossRef]
- Cao, Y.; Xiao, Y.; Zhou, K.; Yan, J.; Wang, P.; Yan, W.; Cai, W. FXR agonist GW4064 improves liver and intestinal pathology and alters bile acid metabolism in rats undergoing small intestinal resection. Am J Physiol Gastrointest Liver Physiol 2019, 317, G108-G115. [CrossRef]
- Bach, A.C.; Babayan, V.K. Medium-chain triglycerides: an update. Am J Clin Nutr 1982, 36, 950-962. [CrossRef]
- Goulet, O.; Antebi, H.; Wolf, C.; Talbotec, C.; Alcindor, L.G.; Corriol, O.; Lamor, M.; Colomb-Jung, V. A new intravenous fat emulsion containing soybean oil, medium-chain triglycerides, olive oil, and fish oil: a single-center, double-blind randomized study on efficacy and safety in pediatric patients receiving home parenteral nutrition. JPEN J Parenter Enteral Nutr 2010, 34, 485-495. [CrossRef]
- ASPEN. Guidelines for the use of parenteral and enteral nutrition in adult and paediatric patients. JPEN J Parenter Enteral Nutr 2002, 26, 1SA-138SA. [CrossRef]
- van Goudoever, J.B.; Carnielli, V.; Darmaun, D.; Sainz de Pipaon, M. ESPGHAN/ESPEN/ESPR guidelines on pediatric parenteral nutrition: Amino acids. Clin Nutr 2018, 37, 2315-2323. [CrossRef]
- Goulet, O.; DePotter, S.; Salas, J.; Robert, J.J.; Rongier, M.; Ben Hariz, M.; Koziet, J.; Desjeux, J.F.; Ricour, C.; Darmaun, D. Leucine metabolism at graded amino acid intakes in children receiving parenteral nutrition. Am J Physiol 1993, 265, E540-546. [CrossRef]
- Vlaardingerbroek, H.; Vermeulen, M.J.; Carnielli, V.P.; Vaz, F.M.; van den Akker, C.H.; van Goudoever, J.B. Growth and fatty acid profiles of VLBW infants receiving a multicomponent lipid emulsion from birth. J Pediatr Gastroenterol Nutr 2014, 58, 417-427. [CrossRef]
- Dudrick, S.J.; Wilmore, D.W.; Vars, H.M.; Rhoads, J.E. Long-term total parenteral nutrition with growth, development, and positive nitrogen balance. Surgery 1968, 64, 134-142.
- Holt, L.E., Jr.; Snyderman, S.E. The amino acid requirements of infants. Jama 1961, 175, 100-103. [CrossRef]
- Elango, R.; Pencharz, P.B.; Ball, R.O. The branched-chain amino acid requirement of parenterally fed neonatal piglets is less than the enteral requirement. J Nutr 2002, 132, 3123-3129. [CrossRef]
- Kien, C.L.; Horswill, C.A.; Zipf, W.B.; McCoy, K.S.; Denne, S.C. Splanchnic uptake of leucine in healthy children and in children with cystic fibrosis. Pediatr Res 1999, 45, 680-683. [CrossRef]
- Heird, W.C. Amino acids in pediatric and neonatal nutrition. Curr Opin Clin Nutr Metab Care 1998, 1, 73-78. [CrossRef]
- Beganović, N.; Kok, K.; de Leeuw, R.; de Vries, I.J.; Schutgens, R. Amino acids in parenteral nutrition of preterm infants. Comparison of oral and parenteral supply. Acta Paediatr Scand 1983, 72, 421-425. [CrossRef]
- Andersen, G.E.; Bucher, D.; Friis-Hansen, B.; Nexø, E.; Olesen, H. Plasma amino acid concentrations in newborn infants during parenteral nutrition. JPEN J Parenter Enteral Nutr 1983, 7, 369-373. [CrossRef]
- Bürger, U.; Wolf, H.; Fritsch, U.; Bauer, M. Parenteral nutrition in preterm infants: influence of respiratory treatment and effect of different amino acid compositions. J Pediatr Gastroenterol Nutr 1983, 2, 644-652.
- Bell, E.F.; Filer, L.J., Jr.; Wong, A.P.; Stegink, L.D. Effects of a parenteral nutrition regimen containing dicarboxylic amino acids on plasma, erythrocyte, and urinary amino acid concentrations of young infants. Am J Clin Nutr 1983, 37, 99-107. [CrossRef]
- Coran, A.G.; Drongowski, R.A. Studies on the toxicity and efficacy of a new amino acid solution in pediatric parenteral nutrition. JPEN J Parenter Enteral Nutr 1987, 11, 368-377. [CrossRef]
- Sankaran, K.; Berscheid, B.; Verma, V.; Zakhary, G.; Tan, L. An evaluation of total parenteral nutrition using Vamin and Aminosyn as protein base in critically ill preterm infants. JPEN J Parenter Enteral Nutr 1985, 9, 439-442. [CrossRef]
- Chessex, P.; Zebiche, H.; Pineault, M.; Lepage, D.; Dallaire, L. Effect of amino acid composition of parenteral solutions on nitrogen retention and metabolic response in very-low-birth weight infants. J Pediatr 1985, 106, 111-117. [CrossRef]
- Meurling, S.; Grotte, G. Total parenteral nutrition in pediatric surgery using a new amino acid solution (Vaminolac). Acta Chir Scand Suppl 1983, 517, 79-87.
- Pineault, M.; Chessex, P.; Lepage, D.; Dallaire, L.; Brisson, G.; Qureshi, I. Total parenteral nutrition in very low birth weight infants with Travasol 10% blend C. JPEN J Parenter Enteral Nutr 1986, 10, 296-299. [CrossRef]
- Vlaardingerbroek, H.; Roelants, J.A.; Rook, D.; Dorst, K.; Schierbeek, H.; Vermes, A.; Vermeulen, M.J.; van Goudoever, J.B.; van den Akker, C.H. Adaptive regulation of amino acid metabolism on early parenteral lipid and high-dose amino acid administration in VLBW infants - a randomized, controlled trial. Clin Nutr 2014, 33, 982-990. [CrossRef]
- Vinton, N.E.; Laidlaw, S.A.; Ament, M.E.; Kopple, J.D. Taurine concentrations in plasma, blood cells, and urine of children undergoing long-term total parenteral nutrition. Pediatr Res 1987, 21, 399-403. [CrossRef]
- Laidlaw, S.A.; Kopple, J.D. Newer concepts of the indispensable amino acids. Am J Clin Nutr 1987, 46, 593-605. [CrossRef]
- Fitzgerald, K.A.; MacKay, M.W. Calcium and phosphate solubility in neonatal parenteral nutrient solutions containing TrophAmine. Am J Hosp Pharm 1986, 43, 88-93.
- Fitzgerald, K.A.; MacKay, M.W. Calcium and phosphate solubility in neonatal parenteral nutrient solutions containing Aminosyn PF. Am J Hosp Pharm 1987, 44, 1396-1400.
- Koletzko, B.; Rodriguez-Palmero, M.; Demmelmair, H.; Fidler, N.; Jensen, R.; Sauerwald, T. Physiological aspects of human milk lipids. Early Hum Dev 2001, 65 Suppl, S3-S18. [CrossRef]
- Gil, A.; Ramirez, M.; Gil, M. Role of long-chain polyunsaturated fatty acids in infant nutrition. Eur J Clin Nutr 2003, 57 Suppl 1, S31-S34. [CrossRef]
- Oliveira, O.R.; Santana, M.G.; Santos, F.S.; Conceição, F.D.; Sardinha, F.L.; Veiga, G.V.; Tavares do Carmo, M.G. Composition of fatty acids in the maternal and umbilical cord plasma of adolescent and adult mothers: relationship with anthropometric parameters of newborn. Lipids Health Dis 2012, 11, 157. [CrossRef]
- Agostoni, C.; Galli, C.; Riva, E.; Risé, P.; Colombo, C.; Giovannini, M.; Marangoni, F. Whole blood fatty acid composition at birth: from the maternal compartment to the infant. Clin Nutr 2011, 30, 503-505. [CrossRef]
- Koletzko, B. Human Milk Lipids. Ann Nutr Metab 2016, 69 Suppl 2, 28-40. [CrossRef]
- Koletzko, B.; Goulet, O.; Hunt, J.; Krohn, K.; Shamir, R. 1. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), Supported by the European Society of Paediatric Research (ESPR). J Pediatr Gastroenterol Nutr 2005, 41 Suppl 2, S1-87. [CrossRef]
- Tomsits, E.; Pataki, M.; Tölgyesi, A.; Fekete, G.; Rischak, K.; Szollár, L. Safety and efficacy of a lipid emulsion containing a mixture of soybean oil, medium-chain triglycerides, olive oil, and fish oil: a randomised, double-blind clinical trial in premature infants requiring parenteral nutrition. J Pediatr Gastroenterol Nutr 2010, 51, 514-521. [CrossRef]
- Skouroliakou, M.; Konstantinou, D.; Koutri, K.; Kakavelaki, C.; Stathopoulou, M.; Antoniadi, M.; Xemelidis, N.; Kona, V.; Markantonis, S. A double-blind, randomized clinical trial of the effect of omega-3 fatty acids on the oxidative stress of preterm neonates fed through parenteral nutrition. Eur J Clin Nutr 2010, 64, 940-947. [CrossRef]
- Skouroliakou, M.; Konstantinou, D.; Agakidis, C.; Kaliora, A.; Kalogeropoulos, N.; Massara, P.; Antoniadi, M.; Panagiotakos, D.; Karagiozoglou-Lampoudi, T. Parenteral MCT/omega-3 Polyunsaturated Fatty Acid-Enriched Intravenous Fat Emulsion Is Associated With Cytokine and Fatty Acid Profiles Consistent With Attenuated Inflammatory Response in Preterm Neonates: A Randomized, Double-Blind Clinical Trial. Nutr Clin Pract 2016, 31, 235-244. [CrossRef]
- Rayyan, M.; Devlieger, H.; Jochum, F.; Allegaert, K. Short-term use of parenteral nutrition with a lipid emulsion containing a mixture of soybean oil, olive oil, medium-chain triglycerides, and fish oil: a randomized, double-blind study in preterm infants. JPEN J Parenter Enteral Nutr 2012, 36, 81S-94S. [CrossRef]
- Deshpande, G.; Simmer, K.; Deshmukh, M.; Mori, T.A.; Croft, K.D.; Kristensen, J. Fish Oil (SMOFlipid) and olive oil lipid (Clinoleic) in very preterm neonates. J Pediatr Gastroenterol Nutr 2014, 58, 177-182. [CrossRef]
- Navaratnarajah, N.; Girard, G.; Sant'Anna, G.; Langlois, H.; Sant'Anna, A.M. The impact of a lipid injectable emulsion (SMOF) on conjugated bilirubin levels in children receiving prolonged parenteral nutrition: A large single center experience. Clin Nutr ESPEN 2022, 49, 289-294. [CrossRef]
- Rumore, S.; McGrath, K.; Scott, A.; Sexton, E.; Wong, T. Fat soluble vitamin status in children on home parenteral nutrition in a tertiary paediatric intestinal rehabilitation unit. Clin Nutr ESPEN 2021, 46, 240-245. [CrossRef]
- Wassef, J.; Lipkin, E.; Hardigan, P.; Duro, D. Trends in liver profile and nutrition outcomes in children undergoing intestinal rehabilitation using a mixed lipid injectable emulsion. Nutr Clin Pract 2021, 37, 1180-1189. [CrossRef]
- Daniel, S.; Svoboda, L.; Chen, J. Liver Function in Pediatric Recipients: A Comparison of Intralipid and Smoflipid. J Pediatr Pharmacol Ther 2021, 26, 258-264. [CrossRef]
- Lezo, A.; D'Onofrio, V.; Puccinelli, M.P.; Capriati, T.; De Francesco, A.; Bo, S.; Massarenti, P.; Gandullia, P.; Marin, M.; Derevlean, L.; et al. Plasma and Red Blood Cell PUFAs in Home Parenteral Nutrition Paediatric Patients-Effects of Lipid Emulsions. Nutrients 2020, 12, 3748. [CrossRef]
- Nagelkerke, S.C.J.; Draijer, L.G.; Benninga, M.A.; Koot, B.G.P.; Tabbers, M.M. The prevalence of liver fibrosis according to non-invasive tools in a pediatric home parenteral nutrition cohort. Clin Nutr 2021, 40, 460-466. [CrossRef]
- Ho, B.E.; Chan, S.C.; Faino, A.V.; Mortensen, M.; Williamson, N.; Javid, P.J.; Horslen, S.P.; Wendel, D. Evaluation of SMOFlipid in Pediatric Intestinal-Failure Patients and Its Effects on Essential Fatty Acid Levels. JPEN J Parenter Enteral Nutr 2021, 45, 546-552. [CrossRef]
- Huff, K.A.; Breckler, F.; Cruse, W.; Szeszycki, E.; Vanderpool, C. Pediatric Smoflipid Therapy: Patient Response and Safety Concerns. JPEN J Parenter Enteral Nutr 2021, 45, 792-799. [CrossRef]
- Hanindita, M.H.; Widjaja, N.A.; Irawan, R.; Hidajat, B. Influence of intravenous fish oil-enriched lipid emulsion on the inflammatory response in children post gastrointestinal surgery. Pak J Nutr 2019, 18, 1036-1041. [CrossRef]
- Hanindita, M.H.; Widjaja, N.A.; Irawan, R.; Hidayat, B.; Hariastawa, I.A. Impact of Intravenous Omega-3-Enriched Lipid Emulsion on Liver Enzyme and Triglyceride Serum Levels of Children Undergoing Gastrointestinal Surgery. Pediatr Gastroenterol Hepatol Nutr 2020, 23, 98-104. [CrossRef]
- Danko, M.; Żyła-Pawlak, A.; Książyk, J.; Olszewska-Durkacz, K.; Sibilska, M.; Żydak, J.; Popińska, K. A Retrospective Analysis of the Effect of Combination of Pure Fish Oil with Third Generation Lipid Emulsion on Liver Function in Children on Long-Term Parenteral Nutrition. Nutrients 2019, 11, 2495. [CrossRef]
- Casson, C.; Nguyen, V.; Nayak, P.; Channabasappa, N.; Berris, K.; Panczuk, J.; Bhiladvala, C.; Dasgupta, T.; Piper, H.G. A Comparison of Smoflipid® and Intralipid® in the Early Management of Infants with Intestinal Failure. J Pediatr Surg 2020, 55, 153-157. [CrossRef]
- Belza, C.; Wales, J.C.; Courtney-Martin, G.; de Silva, N.; Avitzur, Y.; Wales, P.W. An Observational Study of Smoflipid vs Intralipid on the Evolution of Intestinal Failure-Associated Liver Disease in Infants With Intestinal Failure. JPEN J Parenter Enteral Nutr 2020, 44, 688-696. [CrossRef]
- Jiang, W.; Chen, G.; Zhang, J.; Lv, X.; Lu, C.; Chen, H.; Li, W.; Li, H.; Geng, Q.; Xu, X.; et al. The effects of two mixed intravenous lipid emulsions on clinical outcomes in infants after gastrointestinal surgery: a prospective, randomized study. Pediatr Surg Int 2019, 35, 347-355. [CrossRef]
- Lam, C.K.L.; Church, P.C.; Haliburton, B.; Chambers, K.; Martincevic, I.; Vresk, L.; Courtney-Martin, G.; Bandsma, R.; Avitzur, Y.; Wales, P.C.; et al. Long-term Exposure of Children to a Mixed Lipid Emulsion Is Less Hepatotoxic Than Soybean-based Lipid Emulsion. J Pediatr Gastroenterol Nutr 2018, 66, 501-504. [CrossRef]
- Olszewska, K.; Ksiazyk, J.; Kozlowski, D.; Pajdowska, M.; Janusz, M.; Jaworski, M. Nutritional therapy complications in children with ultra-short bowel syndrome include growth deficiency but not cholestasis. Acta Paediatr 2018, 107, 1088-1093. [CrossRef]
- Pereira-da-Silva, L.; Nóbrega, S.; Rosa, M.L.; Alves, M.; Pita, A.; Virella, D.; Papoila, A.L.; Serelha, M.; Cordeiro-Ferreira, G.; Koletzko, B. Parenteral nutrition-associated cholestasis and triglyceridemia in surgical term and near-term neonates: A pilot randomized controlled trial of two mixed intravenous lipid emulsions. Clin Nutr ESPEN 2017, 22, 7-12. [CrossRef]
- Diamond, I.R.; Grant, R.C.; Pencharz, P.B.; de Silva, N.; Feldman, B.M.; Fitzgerald, P.; Sigalet, D.; Dicken, B.; Turner, J.; Marchand, V.; et al. Preventing the Progression of Intestinal Failure-Associated Liver Disease in Infants Using a Composite Lipid Emulsion: A Pilot Randomized Controlled Trial of SMOFlipid. JPEN J Parenter Enteral Nutr 2017, 41, 866-877. [CrossRef]
- Pichler, J.; Watson, T.; McHugh, K.; Hill, S. Prevalence of Gallstones Compared in Children With Different Intravenous Lipids. J Pediatr Gastroenterol Nutr 2015, 61, 253-259. [CrossRef]
- De Cunto, A.; Paviotti, G.; Travan, L.; Bua, J.; Cont, G.; Demarini, S. Impact of Surgery for Neonatal Gastrointestinal Diseases on Weight and Fat Mass. J Pediatr 2015, 167, 568-571. [CrossRef]
- Pichler, J.; Simchowitz, V.; Macdonald, S.; Hill, S. Comparison of liver function with two new/mixed intravenous lipid emulsions in children with intestinal failure. Eur J Clin Nutr 2014, 68, 1161-1167. [CrossRef]
- Hoffmann, K.M.; Grabowski, M.; Rodl, S.; Deutschmann, A.; Schwantzer, G.; Sovinz, P.; Strenger, V.; Urban, C.; Muntean, W.; Hauer, A.C. Short-term intravenous fish-oil emulsions in pediatric oncologic patients--effect on liver parameters. Nutr Cancer 2014, 66, 1070-1076. [CrossRef]
- Wong, R.S.; Walker, K.; Halliday, R.; Trivedi, A. Influence of Soybean Oil or Non-Soybean Oil Based Lipid Emulsions on Parenteral Nutrition Associated Liver Disease in Late Preterm and Term Infants. International Journal of Child Health and Nutrition 2014, 3, 179-184. [CrossRef]
- Ariyawangso, U.; Puttilerpong, C.; Ratanachu-ek, S.; Anuntkosol, M. Short-term safety and efficacy of fish-oil emulsions on the prevention of parenteral nutrition-associated liver disease in surgical neonates: a randomized controlled trial. Thai Journal of Pharmaceutical Sciences 2014, 38.
- Bishay, M.; Pichler, J.; Horn, V.; MacDonald, S.; Ellmer, M.; Eaton, S.; Hill, S.; Pierro, A. Intestinal failure-associated liver disease in surgical infants requiring long-term parenteral nutrition. J Pediatr Surg 2012, 47, 359-362. [CrossRef]
- Muhammed, R.; Bremner, R.; Protheroe, S.; Johnson, T.; Holden, C.; Murphy, M.S. Resolution of parenteral nutrition-associated jaundice on changing from a soybean oil emulsion to a complex mixed-lipid emulsion. J Pediatr Gastroenterol Nutr 2012, 54, 797-802. [CrossRef]
- Anez-Bustillos, L.; Dao, D.T.; Fell, G.L.; Baker, M.A.; Gura, K.M.; Bistrian, B.R.; Puder, M. Redefining essential fatty acids in the era of novel intravenous lipid emulsions. Clin Nutr 2018, 37, 784-789. [CrossRef]
- WHO. Protein and amino acid requirements in human nutrition. WHO Technical Report Series 2007, 935.
- Stapleton, P.P.; Charles, R.P.; Redmond, H.P.; Bouchier-Hayes, D.J. Taurine and human nutrition. Clin Nutr 1997, 16, 103-108. [CrossRef]
- Chesney, R.W.; Helms, R.A.; Christensen, M.; Budreau, A.M.; Han, X.; Sturman, J.A. An updated view of the value of taurine in infant nutrition. Adv Pediatr 1998, 45, 179-200.
- Lourenco, R.; Camilo, M.E. Taurine: a conditionally essential amino acid in humans? An overview in health and disease. Nutr Hosp 2002, 17, 262-270.
- Lima, L.; Obregon, F.; Cubillos, S.; Fazzino, F.; Jaimes, I. Taurine as a micronutrient in development and regeneration of the central nervous system. Nutr Neurosci 2001, 4, 439-443. [CrossRef]
- Kumpf, V.J. Parenteral nutrition-associated liver disease in adult and pediatric patients. Nutr Clin Pract 2006, 21, 279-290. [CrossRef]
- Norsa, L.; Artru, S.; Lambe, C.; Talbotec, C.; Pigneur, B.; Ruemmele, F.; Colomb, V.; Capito, C.; Chardot, C.; Lacaille, F. Long term outcomes of intestinal rehabilitation in children with neonatal very short bowel syndrome: Parenteral nutrition or intestinal transplantation. Clin Nutr 2019, 38, 926-933. [CrossRef]
- Struijs, M.-C.; Schaible, T.; van Elburg, R.M.; Debauche, C.; te Beest, H.; Tibboel, D. Efficacy and safety of a parenteral amino acid solution containing alanyl-glutamine versus standard solution in infants: A first-in-man randomized double-blind trial. Clin Nutr 2013, 32, 331-337. [CrossRef]
- Ong, E.; Eaton, S.; Wade, A.; Horn, V.; Losty, P.; Curry, J.; Sugarman, I.; Klein, N.; Pierro, A. Randomized clinical trial of glutamine-supplemented versus standard parenteral nutrition in infants with surgical gastrointestinal disease. Br J Surg 2012, 99, 929-938. [CrossRef]
- Mohamad Ikram, I.; Quah, B.; Noraida, R.; Djokomuljanto, S.; Faris Irfan, C.; Van Rostenberghe, H. A randomised controlled trial of glutamine-enriched neonatal parenteral nutrition in Malaysia. Singapore Med J 2011, 52, 356-360.
- Albers, M.J.; Steyerberg, E.W.; Hazebroek, F.W.; Mourik, M.; Borsboom, G.J.; Rietveld, T.; Huijmans, J.G.; Tibboel, D. Glutamine Supplementation of Parenteral Nutrition Does Not Improve Intestinal Permeability, Nitrogen Balance, or Outcome in Newborns and Infants Undergoing Digestive-Tract Surgery: Results From a Double-Blind, Randomized, Controlled Trial. Ann Surg 2005, 241, 599-606. [CrossRef]
- Cruccetti, A.; Pierro, A.; Uronen, H.; Klein, N. Surgical infants on total parenteral nutrition have impaired cytokine responses to microbial challenge. J Pediatr Surg 2003, 38, 138-142. [CrossRef]
- Guimber, D.; Michaud, L.; Ategbo, S.; Turck, D.; Gottrand, F. Experience of parenteral nutrition for nutritional rescue in children with severe liver disease following failure of enteral nutrition. Pediatr Transplant 1999, 3, 139-145. [CrossRef]
- Thornton, L.; Griffin, E. Evaluation of a taurine containing amino acid solution in parenteral nutrition. Arch Dis Child 1991, 66, 21-25. [CrossRef]
- Puntis, J.; Ball, P.; Preece, M.; Green, A.; Brown, G.; Booth, I. Egg and breast milk based nitrogen sources compared. Arch Dis Child 1989, 64, 1472-1477. [CrossRef]
- Puntis, J.W.; Edwards, M.A.; Green, A.; Morgan, I.; Booth, I.W.; Ball, P.A. Hyperphenylalaninaemia in parenterally fed newborn babies. Lancet 1986, 2, 1105-1106. [CrossRef]
- Riskin, A.; Picaud, J.C.; Shamir, R. ESPGHAN/ESPEN/ESPR guidelines on pediatric parenteral nutrition: Standard versus individualized parenteral nutrition. Clin Nutr 2018, 37, 2409-2417. [CrossRef]
- Ferreira, M.; Guerra, P.; Ferreras, C.; Espinheira, M.D.C.; Trindade, E.; Dias, J.A. Could Commercial Formulations Replace Individualized Prescription in Pediatric Home Parenteral Nutrition? J Pediatr Gastroenterol Nutr 2021, 73, 548-554. [CrossRef]
- Meyer, R.; Timmermann, M.; Schulzke, S.; Kiss, C.; Sidler, M.; Furlano, R. Developing and implementing all-in-one standard paediatric parenteral nutrition. Nutrients 2013, 5, 2006-2018. [CrossRef]
- Hermanspann, T.; Schoberer, M.; Robel-Tillig, E.; Hartel, C.; Goelz, R.; Orlikowsky, T.; Eisert, A. Incidence and Severity of Prescribing Errors in Parenteral Nutrition for Pediatric Inpatients at a Neonatal and Pediatric Intensive Care Unit. Front Pediatr 2017, 5, 149. [CrossRef]
- Riskin, A.; Shiff, Y.; Shamir, R. Parenteral nutrition in neonatology--to standardize or individualize? Isr Med Assoc J 2006, 8, 641-645.
- Mena, K.D.R.; Espitia, O.L.P.; Vergara, J.A.D. Management of Ready-to-Use Parenteral Nutrition in Newborns: Systematic Review. JPEN J Parenter Enteral Nutr 2018, 42, 1123-1132. [CrossRef]
- Simmer, K.; Rakshasbhuvankar, A.; Deshpande, G. Standardised parenteral nutrition. Nutrients 2013, 5, 1058-1070. [CrossRef]
- Adamkin, D.H.; Radmacher, P.G. Current trends and future challenges in neonatal parenteral nutrition. J Neonatal Perinatal Med 2014, 7, 157-164. [CrossRef]
- Lapillonne, A.; Berleur, M.P.; Brasseur, Y.; Calvez, S. Safety of parenteral nutrition in newborns: Results from a nationwide prospective cohort study. Clin Nutr 2018, 37, 624-629. [CrossRef]
- Kumpf, V.J. Challenges and Obstacles of Long-Term Home Parenteral Nutrition. Nutr Clin Pract 2019, 34, 196-203. [CrossRef]
- Holcombe, B.; Mattox, T.W.; Plogsted, S. Drug shortages: effect on parenteral nutrition therapy. Nutr Clin Pract 2018, 33, 53-61. [CrossRef]
- Hill, S.; Ksiazyk, J.; Prell, C.; Tabbers, M. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Home parenteral nutrition. Clin Nutr 2018, 37, 2401-2408. [CrossRef]
- Neelis, E.; de Koning, B.; van Winckel, M.; Tabbers, M.; Hill, S.; Hulst, J. Wide variation in organisation and clinical practice of paediatric intestinal failure teams: an international survey. Clin Nutr 2018, 37, 2271-2279. [CrossRef]
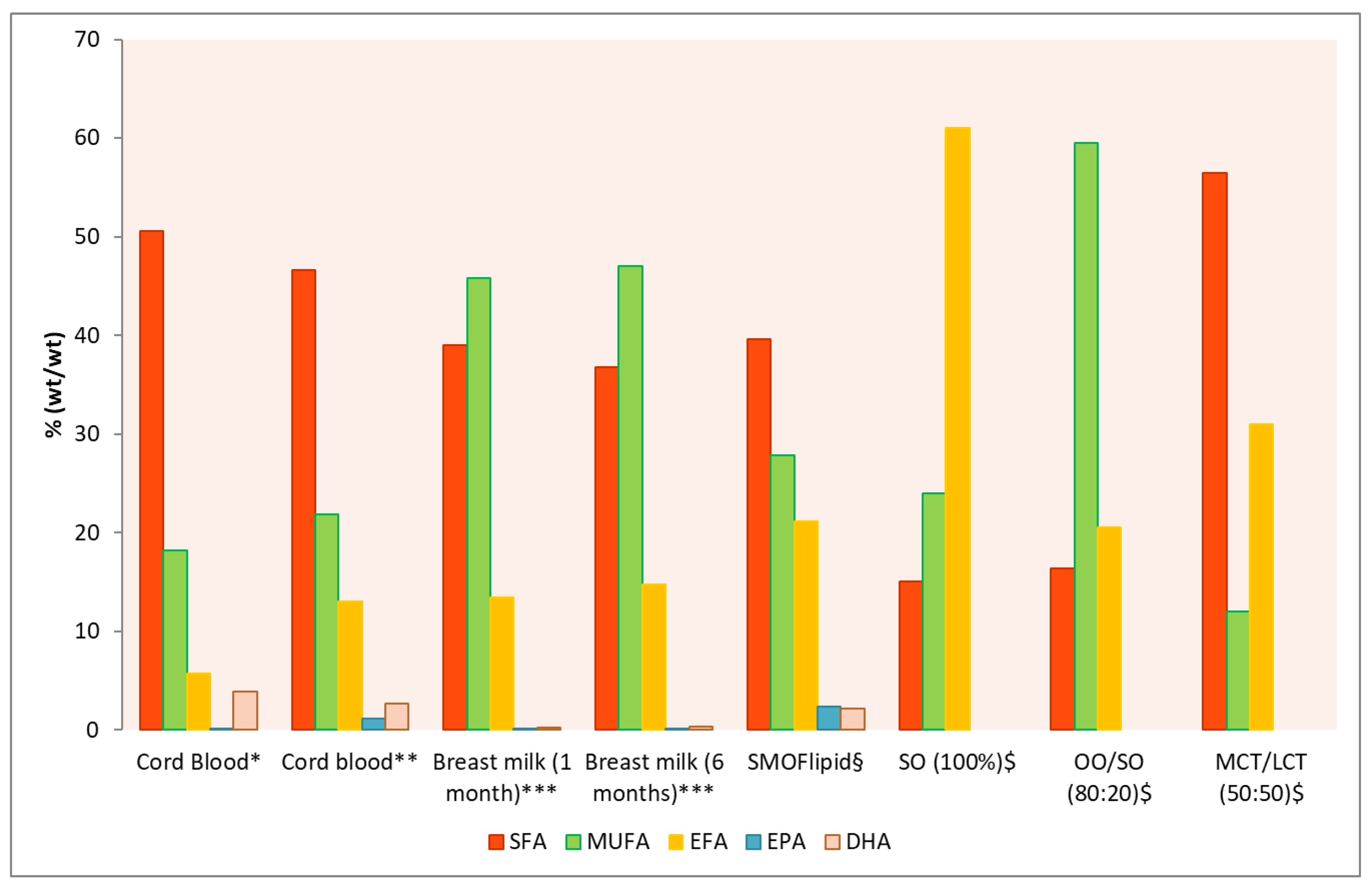
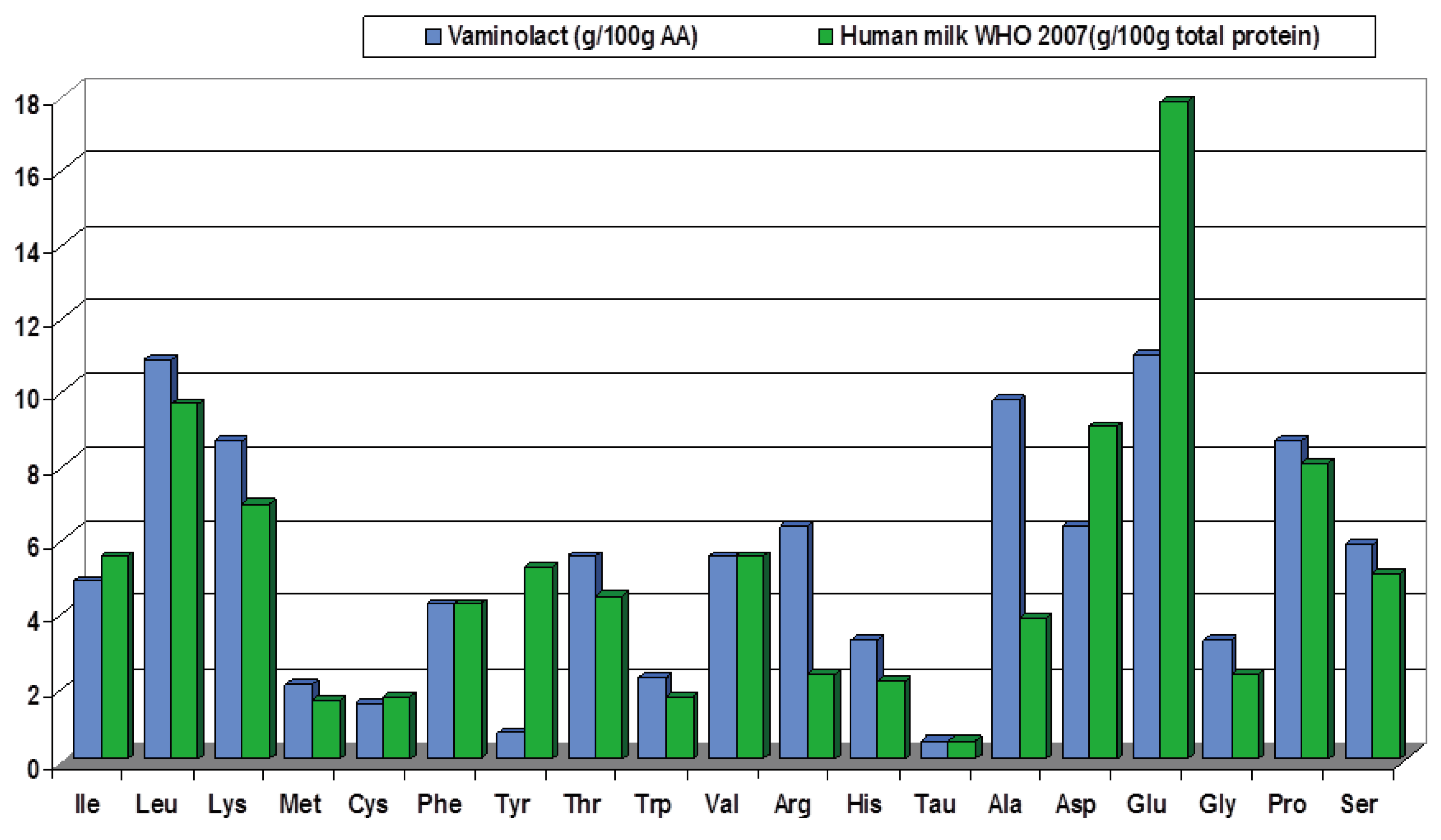
| Study | Design Number of patients (N) |
Patients Age at inclusion |
Intervention | Control | PN Lipid dose (g/kg BW/day) |
In line with ESPGHAN | PN Duration | Main outcomes and conclusions |
|---|---|---|---|---|---|---|---|---|
| Navaratnarajah et al. 2022 [82] | Retrospective chart review N=160 |
Hospitalized infants/toddlers on prolonged PN < 1 year |
SMOF (n=88) | SO (n=72) |
SMOF: 2.5 [1.7-2.8] SO 2.5 [1.9-2.8] |
☑ | ≥28 days | SMOF vs. SO: Cholestasis/liver function Lower incidence of cholestasis (CBil >3.0 µmol/L): 4.5% vs. 20% during the study period Lower log-transformed CBil at the end of the ILE administration (p<0.02) Growth No significant differences (NSD) |
| Goulet et al. 2022 [15] | Prospective cross-sectional N=70 |
Children on HPN SMOF: 5.9 [4.1–8.4] years Weaned: 6.9 [4.0–8.7] years |
SMOF (n=46) 25 children remained PN dependent and were assessed a 2° time 2.4 years later (SMOF 2, n=25). |
Weaned off PN (n=24) |
SMOF: 1.9 [1.4-2.0] |
☑ | 2.4 ± 0.9 years | SMOF vs. weaned: Growth/nutritional status NSD Cholestasis/liver function Higher CBil (p<0.0001) and liver enzymes (all p<0.01), but close to the normal FA profile Higher proportions of EPA and DHA but lower proportions of MA and ARA incorporated in RBC (all p<0.0001) Indices of EFAD No significant difference in Holman index |
| Goulet et al. 2021 [9] | Retrospective cross-sectional Multicenter N=268 in 2014 N=385 in 2019 |
Children on HPN Median age per year over the 5 year observation period: 62.5 – 84.1 months |
HPN with different types of ILEs (OO/SO, SO, SMOF, MCT/SO or pure FO in case of cholestasis) | - | 1.02 -1.5 | ☑ | 5 years | 2014 vs. 2019: The use of a SMOF increased from 67.4% to 88.3% (p < 0.001) Blood stream infections CRBSIs dropped from 1.04 CRSBIs per 1000 days HPN to 0.61 (p<0.001) Cholestasis/liver function Prevalence of cholestasis (CBil ≥ 20 μmol/l) low and stable between 4.1 and 5.9% during the study period. |
| Rumore et al. 2021 [83] | Retrospective N=92 |
Infants/toddlers and children on HPN Range 3-223 months, (median 11 months) |
SMOF (n=79) Switch to SMOF (n=13) |
Non-SMOF (SO or OO/SO) (n=19) | n.a. To be deleted ??? |
- | n.a. | SMOF vs. non-SMOF Clinical and biological outcome Lower overall transplantation rate (7.6% vs. 21.9%; p<0.05) Lower mortality rate (14.1% vs. 21.9% n.s.) Higher vitamin E level and vitamin E:lipid ratio, both p<0.001 Higher vitamin D level (p<0.001) |
| Wassef et al. 2021 [84] | Prospective observational N=16 |
Infants/toddlers, children and adolescents with IF | SMOF (n=16) | - | Mean dose at initiation: 1.5 (range 1-2.5) Mean dose at study end: 1.6 (range 1-2.5) |
☑ | 16.4 [4-33] months | End of study vs. baseline: Cholestasis/liver function Decrease in mean TBil by 67.08% (p<0.05) normalization of DBil in all patients No significant changes in AST or ALT Significant change in mean TBil after 4-5 months (p<0.01; stable TBil at around 0.6 mg/dL thereafter) No new cases of IFALD EFAD No EFAD |
| Daniel et al. 2021 [85] | Retrospective N=101 |
Hospitalized newborn infants (preterm/term), infants/toddlers, children and adolescents SMOF: 300 [0- 1095] days SO: 31 [0-795] days |
SMOF (n=60) | SO (n=41) | Initiated: 0.5-1.0 Increased up to 2.0-3.0 |
☑ | SMOF: 28.5 [20.75, 44] days SO: 32 [23, 55] days |
SMOF vs. SO Cholestasis/liver function Significantly lower incidence of IFALD (12% vs. 32%; p<0.05) No significant difference ay onset of IFALD Subgroup of patients with IFALD (n=20), over 6 months: Significantly lower bilirubin (p<0.05) |
| Lezo et al. 2020 [86] | Prospective observational Multicenter N=38 |
Infants/toddlers, children and adolescents on HPN SMOF; 3.3 [0.9–16.9] years OO/SO: 8.4 [1.6–18.6] years |
SMOF (n=23) | OO/SO (n=15) | SMOF: 1.3 [0.5-2.5] OO/SO: 1.3 [0.5-1.7] |
☑ | SMOF: 22.2 [9.8–202] months OO/SO: 21.1 [7.0–104.0] months |
FA pattern SMOF vs. OO/SO and vs. healthy reference range: - Higher EPA and DHA and lower MA in plasma and RBC membranes (all p<0.01) - Lower ARA in plasma (both both is strange ??? p<0.01) With both ILEs vs. healthy reference range: - Lower ARA in RBC membranes (both p<0.01); n.s. between ILEs Indices of EFAD With both ILEs vs. healthy reference range: - NoNSD in Holman index; no EFAD with both ILEs Cholestasis/liver function Absence of liver fibrosis with both ILEs Growth/nutritional status SMOF vs. OO/SO: - Higher proportion of patients malnourished according to BMI or WFL z-scores (≤2) (5/23) vs. (0/15) (p<0.05) - NSD between groups for Median Z-score of weight, height or BMI for age were |
| Nagelkerke et al. 2020 [87] | Prospective cross-sectional N=32 |
Infants/toddlers, children and adolescents on HPN Median 8.0 (range 0.3-17.8) years |
SMOF (n=23) or OO/SO (n=6) |
- | Median 1.0 (range 0-2.6) | ☑ | Median 45 months |
Cholestasis/liver function Transient elastography (TE), ASAT-to platelet-ratio-index (APRI) and enhanced liver fibrosis (ELF) score showed a varying, but substantial proportion of subjects with fibrosis in the cohort: - Significant fibrosis (TE and ELF): n=6 and 10, respectively - Fibrosis (APRI): n=12 - Moderate fibrosis (ELF): n=17 |
| Ho 2020 [88] | Retrospective N=20 |
Children on HPN Median 6.2 years |
SMOF | Pre-SMOF (SO or SO/FO combination) |
2.0 [1.6-2.0] | ☑ | 1.5 years | From SMOF initiation to 1.5 years post SMOF initiation: FA pattern Increases in ARA, LA, DHA and ALA, all p<0.01 Cholestasis/liver function Decreases in ALT and GGT; both p<0.005 Growth/nutritional status Slight increases in BMI z-score; n.s. EFAD NSD in MA or Homan ratio |
| Huff 2020 [89] | Retrospective N=47 |
Newborn infants, infants/toddlers and children with IF and cholestasis (n=42) 45 [4–1623] days |
SMOF | - | Median 2.1 (range 0.8-3.0) | ☑ | Median 53 days (range 1-432 days) |
Cholestasis/liver function 16/42 cholestatic patients (38%) had resolution with SMOF, 7/42 (17%) improved, 19/42 (45%) showed no response Patients with resolution of cholestasis were older at start of SMOF therapy (p=0.010), treated with SMOF longer (p=0.001), and had lower DBil at SMOF initiation (p=0.035) EFAD Biochemical signs of EFAD (T:T ratio range 0.05-0.151) were observed in 15/28 patients with measurements available; EFAD was mild in all patients; without clinical symptoms of EFAD |
| Hanindita 2019 [90], 2020 [91] | RCT N=14 |
Newborn infants post surgery SMOF: 14.1±17.1 days MCT/SO: 14.0±12.1 days |
SMOF (n not reported) | SO/MCT (n not reported) | 1.0-4.0 | ☑ | SMOF: 29.0±34.8 days MCT/SO: 30.0±20.3 (18.2±15.7 days reported in the 2019 study) |
SMOF vs. SO/MCT group: Inflammation Decrease with SMOF vs. increase of serum IL-6 from baseline to POD 3 Significant differences in IL-6 levels before surgery (p=0.048), on POD 3 (p=0.013), and in changes within 3 days (p=0.003) NSD in TNF-α levels between groups |
| Danko 2019 [92] | Retrospective N=40 |
Children on long-term PN Median 38 months (range 1.5–200) |
SMOF + FO (Omegaven) To be deleted because of this combination that does not fit with our aim |
- | SMOF: median 1.0 (range 0.5-2.0) Omegaven dose not reported |
☑ | Median 149 days (range 28-418) | End of treatment vs baseline: Cholestasis/liver function Significant reduction in TBil, CBil, ALT, AST, and GGT in the total group and in cholestatic patients (n=13; all p<0.05) 11/40 patients had increasing or unchanged bilirubin levels (nonresponders), but no patient with initial CBil <34.2 µmol/L (<2 mg/dL) became cholestatic |
| Casson et al. 2019 [93] | Retrospective Two centers N=44 |
Newborn infants and infants/toddlers during the first 8 weeks of intestinal rehabilitation SMOF: mean 7 (range 4–50) days SO: mean 8 (range 4–47) days |
SMOF (n=21) | SO (n=23) | SMOF: week 1: 3.0 [2.0-3.0] week 4: 3.0 [1.3-3.0] week 8: 2.0 [1.5-3.0] SO: week 1: 2.0 [1.5-3.0] week 4: 2.0 [1.5-3.0] week 8: 1.5 [1.0-3.4] |
☑ | > 8 weeks | SMOF vs. SO: Cholestasis/liver function: During the 8 weeks observation period, cholestasis occurred in 76% vs. 91% of infants (p=0.18) CBil levels normalized more quickly (p=0.04) Subset of infants without any EN tolerance: Lower incidence of cholestasis (78% vs. 92%, p=0.057) |
| Belza 2019 [94] | Retrospective N=37 |
Infants/toddlers with IF < 12 months |
SMOF (n=17) | SO (n=20) | 2.0-3.0 | ☑ | SMOF: 421 [203-822] days SO: 213 [104-364] days |
SMOF vs. SO: Cholestasis/liver function Lower likelihood to reach a serum CBil of 34 µmol/L or 50 µmol/L; both p≤0.05) With SMOF: no need for Omegaven to resolve IFALD (0% vs. 30%, p=0.014) Lower median CBil 3 months after PN initiation (p=0.023) Growth /nutritional status Improved weight z-scores at 3 and 6 months (both p<0.05) Anthropometrics (weight, height, head circumference) remained within the normal range in both groups |
| Jiang 2019 [95] | RCT N=160 |
Newborn infants after GI surgery Mean 4-5 days |
SMOF (n=74) | SO/MCT (n=86) | 1.0-3.0 | ☑ | >2 weeks (22/24 patients >4 weeks) | SMOF vs. SO/MCT: Cholestasis/liver function NSD in liver enzymes and DBil at end of weeks 1 and 2 Infants who received lipids for >4 weeks: -Lower ALT, AST and DBil levels at end of week 4 (all p<0.05) Growth/nutritional status NSD in weight gain or nutrition indices at end of weeks 2 and 4 months |
| Lam 2018 [96] | Retrospective N=40 |
Hospitalized newborn infants and infants/toddlers/children SMOF: 0.6 (0.1–28) months SO: 0.8 (0.1–33) months |
SMOF (n=20) | SO (n=20) | SMOF: 2.2 (1.8-2.5] SO: 2.1 [1.6-2.3] |
☑ | SMOF: 9 [5-13] weeks SO: 6 [4-13] weeks |
SMOF vs. SO: Cholestasis/liver function Significantly lower trajectory of CBil (p<0.001) NSDin AST, ALT, or ALP trajectories (sensitivity analysis excluding outliers showed lower AST and ALT trajectories for the SMOF group (both p<0.001) Growth/nutritional status NSD between groups |
| Olszewska 2018 [97] | Prospective observational N=17 |
Infants, children and adolescents with ultra-short bowel syndrome Range 0.8-14.2 years |
SMOF (n=10) or SMOF/FO (Omegaven) (n=5) or SO/MCT (n=2) |
- | n.a. | - | median duration of PN 6.6 years (range 0.8-14.2 years) | During the 1 year observation period: Growth/nutritional status Body mass deficits were found in the cohort with a median standard deviation score (SDS) of -1.2 for body mass according to chronological age, -1.72 according to height and -0.59 according to height for age Cholestasis/liver function None of the patients had elevated CBil levels above 34.2 µmol/L |
| Pereira-da-Silva 2018 [98] | RCT N=49 |
Newborn infants (term/preterm) undergoing major surgery <48 hours |
SMOF (n=22) | SO/MCT (n=27) | Median cumulative dose: SMOF: 14.7 g/kg SO/MCT: 12.5 g/kg Such high doses should be verified | ☑ | SMOF: median 16 days SO/MCT: median 18 days |
SMOF vs. SO/MCT: Cholestasis/liver function Similar cumulative incidence rates of CBil >1 mg/dL or >20% of TBil between groups Triglycerides Cumulative incidence of hypertriglyceridemia >250 mg/dL was lower (p=0.0024) Mean serum TG increase was lower (p=0.013) |
| Diamond 2017 [99] | RCT Multicenter N=24 |
Infants/toddlers with early IFALD SMOF: mean 6.5 weeks (range 4.3-8.7) SO: mean 5.3 weeks (range 3.5-7.2) |
SMOF (n=11) | SO (n=13) | 2.0-3.0 | ☑ | SMOF: 8 [5.5-10.5] weeks SO: 8 [5.7-10.7] weeks |
SMOF vs. SO: Cholestasis/liver function Lower serum CBil at trial completion (primary endpoint, p=0.001) Higher likeliness to have a decrease in serum CBil to 0 µmol/L over the entire study period (p = 0.006) Less patients with serum CBil >50 µmol/L at primary endpoint (p = 0.04) Higher GGT at trial completion (p=0.04) |
| Pichler 2015 [100] | Controlled trial non-randomized N=67 |
Newborn infants (preterm/term), infants/toddlers, children and adolescents with IF 0.7 [0.01-15.1] years |
Mixed ILE: SMOF or OO/SO (n=27) | SO or SO/MCT (n=40) | n.a. | - | 2-3 times per week Duration n.a. |
Mixed ILE vs. SO: Cholestasis/liver function Lower frequency of sludge and/or gallstones (p=0.05). Lower incidence of liver echogenicity (p=0.003). Overall, in 7 (10%) children, sludge and/or gallstones resolved spontaneously without further intervention. Five of the 7 children were receiving mixed ILE. |
| De Cunto 2015 [101] | Retrospective N=42 |
Newborn infants (preterm/term) undergoing GI surgery 1-82 days Matched controls (n=21, thereof 5 receiving PN due to prematurity) |
SMOF (surgical group, n=21) | 0.5-3.0 | ☑ | Mean 40 days | Surgical infants vs. matched controls: Growth/nutritional status Postsurgical infants were shorter (p=0.001), lighter (p<0.001), and had lower fat mass content (p<0.0001) than their peers at similar corrected age (43 [4] weeks). Cholestasis/liver function Nine infants in the surgical group and 1 in the control group had PN-associated cholestasis. |
|
| Pichler 2014 [102] | Retrospective N=177 |
Hospitalized infants/toddlers, children and adolescents changing to or starting PN with a FO-ILE Median 0.6 (range 0-16) years |
SMOF (n=71) | SO/MCT (n=56) | SMOF: 2.3±0.8 SO/OO/FO: 2.2±0.9 |
☑ | SMOF: median 41 (range 3-311) days SO/OO/FO: median 30 (range 3-436) days SO: median 73 (range 19-154) days |
Cholestasis/liver function With SMOF (baseline vs. end of treatment): - Reduced ALT (p=0.006), ALP (p=0.008) and GGT (p=0.01) - Hyperbilirubinaemia incidence decreased from 34% to 24% (p<0.05). SMOF vs. SO/MCT: - Lower ALT at end of treatment (p=0.01) Growth/nutritional status No adverse effects were detected and significant weight gain was achieved with both FO-ILEs (p<0.05) |
| Hoffmann 2014 [103] | Retrospective N=30 |
Children with haemato-oncologic disease during CT 10.69±7.11 years (mean±SD) |
SMOF (n=15) | SO (n=15) | SMOF: 0.9 SO: 1.0 |
☑ | > 14 days |
Cholestasis/liver function No significant changes vs. baseline at day 7 and day 14 in TBil, GGT, AST, ALT, and AP in the SMOF group GGT increased in the SO group (p<0.05) No patient in either group developed cholestasis Other Decrease in lactate dehydrogenase levels (marker of cell damage) with SMOF vs increase in the SO group (p=0.016) |
| Wong 2014 [104] | Retrospective N=208 | Newborn infants (preterm/term) and infants Age not reported |
Mixed ILEs: SMOF or SO/OO (n=54) | SO (n=154) | n.a. | - | Mixed ILEs: mean 19 days SO: mean 21 days |
Cholestasis/liver function Mixed ILEs vs. SO: Lower prevalence of IFALD (17% vs. 21%; p=0.315) |
| Ariyawangso 2014 [105] | RCT N=42 |
Surgical newborn infants (preterm/term) Age not reported |
SMOF (n=21) | SO (n=21) | SMOF: 2.6 ± 0.3 SO: 2.6 ± 0.2 |
☑ | SMOF: 22.5 ± 8.5 days SO: 20.9 ± 5.5 days |
SMOF vs. SO: Cholestasis/liver function Lower plasma TBil (p<0.001)] and DBil (p<0.001)] Increase in TBil and DBil in the control group(p=0.02 and p<0.001) Decrease in TBil (p<0.001) and unchanged DBil with SMOF Growth/nutritional status NSD at day 22 Other Laboratory safety parameters (liver enzymes, lipid profiles, renal function and hematological parameters): n.s. between groups |
| Bishay 2012 [106] | Retrospective N=87 | Infants post surgery with and without IFALD Non-IFALD: mean 19 (range: 1-347) days IFALD: mean 45 (range: 4-270) days |
SMOF or SO/OO/FO |
- | n.a. | - | Non-IFALD: mean 48 (range 28-310) days IFALD: mean 77 (range 30-276) days |
Cholestasis/liver function IFALD occurred in 33% (n=29) whatever they received SMO or SO/OO/FO IFALD was associated with Ionger PN duration (p=0.002) female sex (overall [p=0.04] and trend for increasing severity [p=0.006]) Other 61 children receiving long-term PN (70%) have achieved enteral autonomy, whereas 12 (14%) required HPN |
| Muhammed 2012 [107] | Retrospective N=17 |
Infants/toddlers and children with PN-associated jaundice SMOF: 12-164 weeks SO: 8-64 weeks |
SMOF (n=8) | SO (n=9) | SMOF: 0.6–3.5 SO: 2.5-3.5 |
☑ | SMOF: 12-148 weeks SO: 8-64 weeks |
Cholestasis/liver function After 6 months, 5 of 8 children in the SMOF and 2 of 9 children in the SO group had total resolution of jaundice Median TBil decreased in the SMOF group and increased in the Intralipid group (p=0.02) |
| Goulet 2010 [47] | RCT N=28 |
Infants/toddlers and children on HPN SMOF: 30.3±23.9 months SO: 38.8 ± 35.5 months |
SMOF (n=15) | SO (n=13) | SMOF: 1.4 ± 0.5 SO: 1.4 ± 0.5 |
☑ | SMOF: 27.3±0.6 days SO: 27.5±0.5 days |
Baseline vs. end of study: Cholestasis/liver function TBil values were significantly decreased with SMOF (p<0.01) FA pattern In RBC and in plasma phospholipids, EPA and DHA were significantly increased in the SMOF group N3-/n6-FA ratio in plasma and RBC was significantly elevated with SMOF compared to SO (both p<0.01) Other Plasma α-tocopherol levels increased significantly more with SMOF vs. SO (p<0.05). |
| Age group | Term newborn infants up to 1 month |
Infants/ Toddlers 1 month - 3 years |
Children and adolescents 3-18 years |
|---|---|---|---|
|
Recommended total amino acid dose range acc. to ESPGHAN (g/kg BW/day) |
1.5-3 | 1-2.5 | 1-2 |
| Cysteine/cysteine (mg/kg/day) | 23-46 | 15-38 | 15-31 |
| Tyrosine(mg/kg/day) | 11-23 | 8-19 | 8-15 |
| Taurine(mg/kg/day) | 7-14 | 5-14 | 5-9 |
| Arginine(mg/kg/day) | 94-188 | 63-157 | 63-126 |
| Phenylalanine(mg/kg/day) | 62-124 | 41-103 | 41-83 |
| Valine(mg/kg/day) | 83-165 | 55-138 | 55-110 |
| Isoleucine(mg/kg/day) | 71-142 | 47-119 | 47-95 |
| Study | Design Number of patients (N) |
Patients Age at inclusion |
Intervention | Control | Amino acid dose (g/kg BW/day) |
In line with ESPGHAN* | PN Duration | Main outcomes |
|---|---|---|---|---|---|---|---|---|
| Norsa et al. 2019 [115] | Retrospective Cross-sectional N=36 |
Infants/toddlers and children with neonatal short bowel syndrome Median 9 (3-73) months |
PN with Vaminolact or Primene (n=16) Remained on long-term PN (n=16) or Received intestinal transplantation after 2.5 -9 years (n=20) |
- | n.a. | - | Follow-up: median 17 (9-20) years |
Clinical outcomes 16 children remained on long-term PN - 6 were weaned off PN after a mean of 8 years - All children were alive at a mean age of 16 years (9-20) 20 children underwent intestinal transplantation and outcome - 8 died 29 months (0-127) - 12 were weaned off PN 73 days (13-330) after transplantation. Growth/nutritional status At latest follow-up NSD in Z-score for height, weight and BMI in alive patients between both groups |
| Abi Nader et al. 2017 [14] | Retrospective N=17 |
Infants/toddlers with severe malnutrition treated with PN for IF 4.5±2.2 (range 2–9) months |
PN with Vaminolact or Primene | - | 2.9±0.3 | ☑ | 1.9±0.4 years |
Growth/nutritional status Weight gain after 28 days of PN was 110 ± 5% of optimal weight gain for age The mean NPEI from PN was 104.3±8.0 kcal/kg/d The mean ratio of NPEI over REE was 2.1±0.2 |
| Abi Nader et al. 2016 [13] | Retrospective N=251 |
Infants/toddlers on HPN Age 8.4±3.6 months |
HPN with Vaminolact or Primene or Vintene | - | n.a. | - | Mean duration: 1.9±0.4 years |
Clinical outcomes 19 children with primary digestive diseases underwent intestinal transplantation, thereof 3 children died because of uncontrollable acute rejection 24 children died while receiving HPN (10%) 91 children with SBS were weaned off HPN at a mean age of 2.9±0.6 years The major complications of HPN were CRBSIs: 1.7/1000 days of PN and cholestasis (51 children; 20% of cohort) Growth/nutritional status Patients weaned off HPN: - NSD in weight for-age and height-for-age z scores at weaning off HPN and follow-up visits (P = 0.23 and 0.16, respectively) Children with SBS still receiving HPN at the end of the study period (n = 45): - Weight for-age and height-for-age z scores were not significantly different from those of weaned patients - The level of PN dependency assessed by the NPEI:REE ratio was 1.31±0.2 |
| Struijs et al. 2013 [116] | RCT N=23 |
Surgical newborn infants Gln-AA:1 [1-3] days Standard-AA: 2 [1-3] days |
GLN-AA: Pediatric AA solution with glycyl-tyrosine, Ala-Gln and acetyl-cysteine, higher in arginine and taurine vs. control (n=17) | Standard-AA: Vaminolact (n=6) | GLN-AA: 2.1±0.5 Standard-AA: 2.1±0.2 Requirements based on ESPGHAN 2005 |
☑ | GLN-AA: 7.5±3.1 days Standard-AA: 8.9±2.1 days |
Gln-AA vs. standard AA: Growth/nutritional status NSD for body weight, head circumference and pre-albumin AA profiles Plasma AA-profiles were closer to the normal ranges Clinical laboratory and safety No significant differences were found between groups for hematological and biochemical laboratory values, adverse events, and safety parameters |
| Ong et al. 2012 [117] | RCT Multicenter N=164 |
Surgical newborn infants and infants/toddlers Ala-Gln group: 5 (1–47) days Control: 5 (1-51) days |
PN + Ala-Gln (n=82) AA solution not specified |
Standard PN (n=82) Vaminolact in 12 centers and Primene in 2 centers |
1.5 | ☑ | Control:15.0 (13.3–16.8) days Ala-Gln: 19.0 (14.6–23.4) days |
PN with Ala-Gln vs. standard PN: Clinical outcomes During exclusive PN: significantly decreased incidence of sepsis (p=0.005) No effect on the overall incidence of sepsis or septicaemia Growth/nutritional status NSD in weight centile change between groupsHead circumference centiles were maintained in each group Clinical laboratory and safety No side-effects were reported in either group No documented abnormal levels of serum ammonia Other NSD in time to full enteral feeding or time to first enteral feeding between groups |
| Ikram et al. 2011 [118] | RCT N=132 |
Newborn infants (preterm/term) requiring PN Age <72 hours |
PN + Ala-Gln (n=132) Prepared with Vaminolact |
Standard PN (n=138) Prepared with Vaminolact |
Ala-Gln 0.6 Vaminolact dose not reported |
- | n.a. | PN with Ala-Gln vs. standard PN: Clinical outcomes NSD for Clinical outcomes: (NEC, clinical sepsis and culture-proven sepsis, duration of ventilation, duration of NICU stay) Other NSD in the median time to reach full enteral nutrition (6 days |
| Goulet et al. 2010 [47] | RCT N=28 |
Infants/toddlers and children on HPN SMOF: 30.3±23.9 months SO: 38.8 ± 35.5 |
SMOF (n=15) Vaminolact for children <3 years (n=8) |
SO (n=13) Vaminolact for children <3 years (n=7) |
1.8 | ☑ | SMOF: 27.3±0.6 days SO: 27.5±0.5 days |
Clinical laboratory and safety Treatment groups were comparable with respect to clinical indices, vital signs, biological safety parameters, including BUN, glucose, creatinine, and plasma electrolytes and growth parameters |
| Albers et al. 2005 [119] | RCT N=80 |
Newborn infants (preterm/term) and infants/toddlers Standard PN: 14.0 (2.3–54.0) days Gln-PN: 8.0 (2.8–94.0) days |
Gln-PN (n=41) AA solution not specified Article to be deleted ?? |
Standard PN (n=31) 2CB with Vaminolact |
1.5-2.5 | ☑ | n.a. | Gln-AA vs. standard AA: Protein metabolism No significant differences in nitrogen excretion and 3MH-creatinine excretion Serum ammonia levels were higher at study end (p=0.008) (not clinically relevant) Clinical outcomes No significant differences in mortality rate, length of stay in the ICU/hospital and in septic episodes Other No significant differences in intestinal permeability between groups |
| Cruccetti et al. 2003 [120] | Controlled study (not randomized) N=24 |
(a) newborn infants and infants/toddlers post surgery on PN Age: 101 (14-168) days (b) newborn infants and infants/toddlers on enteral formula 53.5 (13-172) days |
PN with Vaminolact (a) (n=9) | Formula (b) (n=8) Formula means that the two groups are not on PN To be deleted ??? |
0.5-3 | ☑/ ⊠ (for intakes <1.5) |
Mean 20 (10-106) days | PN with Vaminolact vs. formula group: Inflammation PS stimulation ex vivo: - Impaired cytokine response - Lower percentage of monocytes producing IL-6 and TNF-α (p<0.05) indicating reduced inflammatory response |
| Guimber et al. 1999 [121] | Retrospective N=7 |
Newborn infants, infants/toddlers and children with severe liver disease 2.5 years (4 days - 11.5 years) |
PN with Vaminolact or Primene | - | 1.7±0.5 | ☑/ ⊠ (for intakes <1.5) |
105 days (1 day-6 months) |
After PN vs. before PN: Growth/nutritional status Weight/height Z-score increased significantly (p<0.05) Height/age Z-score showed a trend to worsen (ns) Clinical laboratory and safety No significant differences in serum transaminases, serum albumin, serum ammonia, prothrombin time, and Factor V were noted |
| Thornton et al. 1991 [122] | Controlled study (not randomized) N=25 |
Critically ill newborn infants requiring PN 0-4 days |
PN with Vaminolact (n=15) | PN with Vamin glucose (n=10) Without taurine |
Vaminolact: 1.8±0.2 at day 1 2.3±0.2 from day 3 Vamin glucose: 1.9±0.1 at day 1 2.3±0.1 from day 3 |
☑ | Vaminolact: 12±5 days Vamin glucose: 10±3 days |
Vaminolact vs. Vamin glucose: AA profiles No serious abnormalities in AA concentrations in either group Taurine levels recovered more rapidly (n.s.) Phenylalanine levels were significantly lower at day 1 and day 3 Protein metabolism Nitrogen retention on day 1 was significantly higher Measurements n.a. on day 3 Growth With Vaminolact infants regained their birth weight faster than with Vamin glucose Clinical laboratory and safety Metabolic acidosis, which occurred in several subjects in each group, was not a serious problem Liver function tests remained satisfactory in both groups |
| Puntis et al. 1989 [123] | RCT N=14 |
Surgical newborn infants and infants/toddlers Vaminolact group: 36 days (11-84) Vamin 9 glucose group: 16 days (11-29) |
Vaminolact (n=7) | Vamin 9 glucose (n=7) Without taurine |
0.5-2.5 | ☑/⊠ (for intakes <1.5) | 6 days | Vaminolact vs. Vamin 9 glucose: AA profiles Lower PHE, TYR and total cystine/cysteine concentrations (p=0.0028, p=0.0004, p<0.05, respectively) Mean concentrations of all AA except THRE, LYS, HIST, and CYST closer to mean of the target range Growth and protein metabolism No significant difference in growth or nitrogen retention Clinical laboratory and safety No haematological or biochemical measurements changed significantly. Neither evidence of liver dysfunction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




