1. Introduction
The importance of aluminum-based alloys in the field of materials is associated with the great relevance that these systems have in the automotive industry. This is mainly a consequence of their low density, which, associated with the wide range of other properties of technological relevance that they present, make them very versatile systems suitable for use in a variety of applications. However, it has been found that many of these alloys used to make engine parts suffer a decrease in performance when used at elevated temperatures [
1]. Therefore, in recent years, interest in aluminum-based alloys that have good properties at high temperatures has grown. It has been established that suitable alloys could be those that present the eutectic transformation at temperatures above 600 °C [
2,
3] and whose second phase precipitates are stable at these temperatures.
Al-Ni alloys, which present
a eutectic at 639.9 ° C, are currently considered suitable candidates for use in high temperature services [
4,
5,
6]. The adequate performance of the Al-Ni system at high temperatures has been associated with the presence of Al
3Ni intermetallic particles resistant in high temperature environments [
2,
3,
5,
7]. It is also expected that the presence of such particles improves the mechanical resistance of alloys.
The development of Al-Ni alloys with a controlled microstructure is a topical topic [
1,
2,
4,
5,
7,
8,
9,
10,
11,
12,
13,
14]. However, studies that relate macro- and microstructures with the electro-chemical behavior of Al-Ni alloys are scarce [
15,
16,
17]. It is vitally important to consider that the development and optimization of new alloy systems also requires studies that evaluate the compatibility of the materials with the environment with which they will be in contact. Corrosion, that is, the degradation of metallic materials, is an inevitable problem that must be faced in almost all industrial and technological environments.
In general, the electrochemical response of the systems will be the result of the combination of the different structures and composition of the alloys. It has been shown that the corrosion behavior of aluminum-based alloys will depend on the nature of the secondary phases, their distribution as a result of the different microstructures, their electrochemical behavior and their interaction with the matrix [
12,
18,
19].
With the aim of obtaining a complete overview regarding the performance of Al-Ni alloys, in the present work the influence of thermal parameters on the structures resulting from the directional solidification process of Al-Ni alloys with different alloy content has been studied. It has also been evaluated how the resulting macrostructures and microstructures and the distribution of second phases affect the microhardness and corrosion behavior of the alloys.
2. Materials and Methods
2.1. Materials
The Al-Ni alloys were prepared by using commercially pure metals (Chemical composition of Al: 99.93 Al, 0.038 Fe, < 0.001 Pb, 0.033 Si and < 0.001 others. Chemical composition of Ni: 99.97 Ni, 0.015 Fe, 0.012 Pb, 0.003 Si and < 0.001 others).
The solidification set-up was designed in such a way that heat was extracted only through the water-cooled bottom, promoting vertical upward directional solidification. More details concerning this solidification set-up can be obtained in previous articles [
23,
24,
25,
26,
27].
2.2. Directional Solidification Process and Metallographic Characterization
The experiments were carried out with four concentrations of Al-Ni alloys (Al-1wt. %Ni, Al-3wt. %Ni, Al-5.7wt. %Ni and Al-8wt. %Ni). The directional solidification process began by pouring the molten metal at a temperature of 800 - 850 ° C (approximately 200°C above the fusion temperature of pure aluminum [
20]) into the furnace previously heated to the same temperature. The furnace has a heat extraction system using circulation of water at a temperature of 5 ° C. It also has a temperature acquisition system through 6 type K thermocouples coated with refractory ceramics located inside the metal in different positions (that is, every 2 cm from the bottom of the mold) that allowed the recording of the thermal evolution during the entire solidification process. Cooling is initiated by activating the cooling water at the bottom of the mold (cold base). Consequently, the directional solidification takes place in a vertically ascending manner.
The processing of the acquired thermal data allowed obtaining the characteristic temperature versus time curves and the analysis of the thermal parameters involved.
The solidified samples were cut longitudinally in half and roughened in water with emery papers (#60 to #1200) and then polished with 6 µm diamond paste (Prazis
®, Buenos Aires, Argentina) for 1 h on a cloth by using ethylene glycol as lubricant. To reveal the macrostructure, the samples were immersed in a solution of 50 ml of HCl and 500 ml of water for 10-15 minutes until the appearance of grains was observed [
21,
22]. Visual observation of the macrostructures of the samples allowed to identify the different grain structures obtained in a directional solidification process could be observed. That is, columnar at the base of the specimen and equiaxial at the top. Between both structures there is a transition zone which is from columnar-to-equiaxed (CET) grain structure. The position of the CET zone was measure with a ruler from the base of the sample [
23,
24,
25,
26,
27].
From the images obtained from the development of the macrostructure, the width of the average columnar grains was measured, as explained in a previous work [
28]. The size of the equiaxed grains of the equiaxed zone close to the CET, and zones close above it, was determined.
Each sample was divided into equal surfaces and the size of the equiaxed grains was determined at each interval of approximately 10 mm, according to the ASTM 112-96 standard [
29]. With these values, the average equiaxed grain diameter, D
Ge was calculated.
The development of the microstructure was carried out using a solution of 1.5 ml HCl; 1 ml of HF, 2.5 ml of HNO
3 and 95 ml of H
2O [
21,
22]. The chemical attack times were 10 seconds for Al-1wt. %Ni, 8 seconds for Al-3wt. %Ni, and 7 seconds for the other both alloys. After chemical attack, the samples were rinsed with plenty of water and dried with hot air flow.
The characterization of the surface of Al-Ni alloys was carried out using an FEI Quanta 200
® Scanning Electron Microscope (SEM) with an energy dispersive spectroscopy (EDS) detector of the Electron Microscopy and Microanalysis Service (SemFi-LIMF, Faculty of Engineering, UNLP, Argentina). Microhardness measurements were carried out using a Future Tech FM800
® microvickers durometer with load of 50 g
f and a residence time of 10 s [
30]. Ten measurements were carried out in each of the phases of each alloy.
2.3. Electrochemical tests
To carry out the electrochemical tests, the specimens were cut to obtain 1 cm2 working electrodes with the different grain structures. One electrode with columnar grains, and one with equiaxed grains, was obtained for each alloy composition.
Cyclic potentiodynamic curves were carried out using a velocity of 10 mV/min. The sweep of the curves was initiated about 300 mV below the open circuit potential towards the anodic zone, and was reversed upon reaching a current of 1 mA/cm2. After the tests, the affected areas were analyzed using an FEI Quanta200 SEM with an EDS detector of the Electron Microscopy and Microanalysis Service (SeMFi-LIMF) - Faculty of Engineering, UNLP, Argentina (of the Electronic Microscopy and Microanalysis Service (SeMFi- LIMF) -Faculty of Engineering, UNLP, Argentina).
Electrochemical Impedance Spectroscopy spectra were acquired in a frequency range of 105-10-1 Hz. The potential amplitude was set to 10 mVrms, around the OCP. All the EIS data was fitted by the Gamry Echem Analyst®.
The electrochemical tests were carried out in a 3-electrode cell, according to ASTM G-5 Standard [
31] with a platinum counter electrode, a saturated calomel reference electrode and the alloys-working electrodes.
All the potentials mentioned in the present research are vs SCE (saturated calomel electrode). Measurements were carried out in 0.5 M NaCl solution deaerated by bubbling with nitrogen at room temperature. The equipment used is a Gamry Reference 600® potentiostat.
3. Results and Discussion
3.1. Solidification process
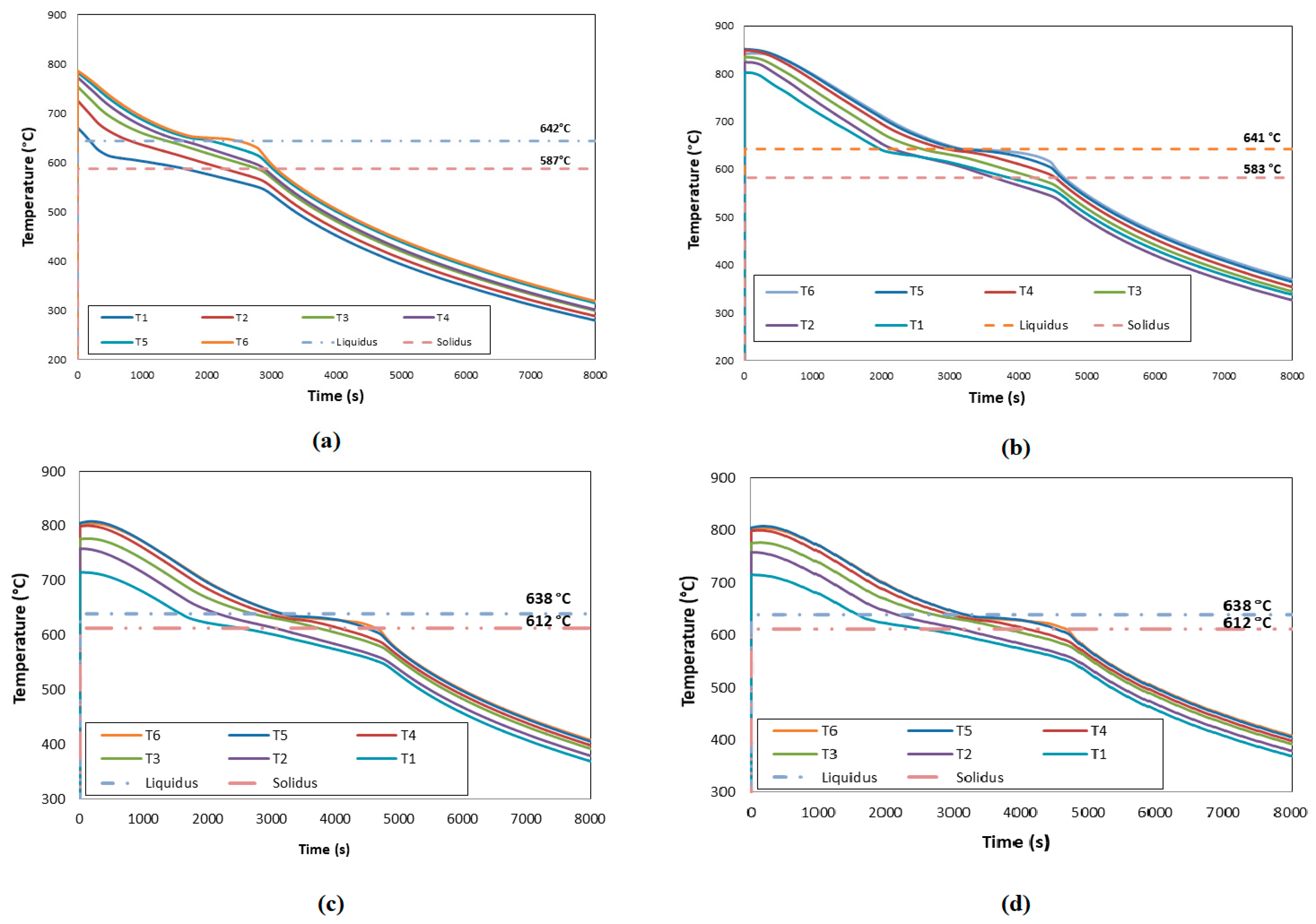
In
Figure 1 (a) to (d) the cooling curves obtained during the solidification of Al-1wt. %Ni, Al-3 wt. %Ni, Al-5.7 wt. %Ni and Al-8 wt. %Ni alloys are shown. The alloys begins to solidify at a temperature range of 665 ° C and 670 ° C, this is observed in Figures (a) to (d) due to a change in slope of curves, which corresponds to the
liquidus temperature (T
L). On the other hand, after the completion of solidification, the slope of the cooling curve alters again, allowing the
solidus temperature (T
S) to be determined.
The data obtained from the acquirer during directional solidifications were used to obtain values of the thermal parameters (cooling rate (V
e), thermal gradient (G
T), solidification rate (Vs)), which can be observed in
Table 1. The values next to the isotherms show good agreement with the equilibrium temperatures [
20].
3.2. Thermal gradients as a function of time
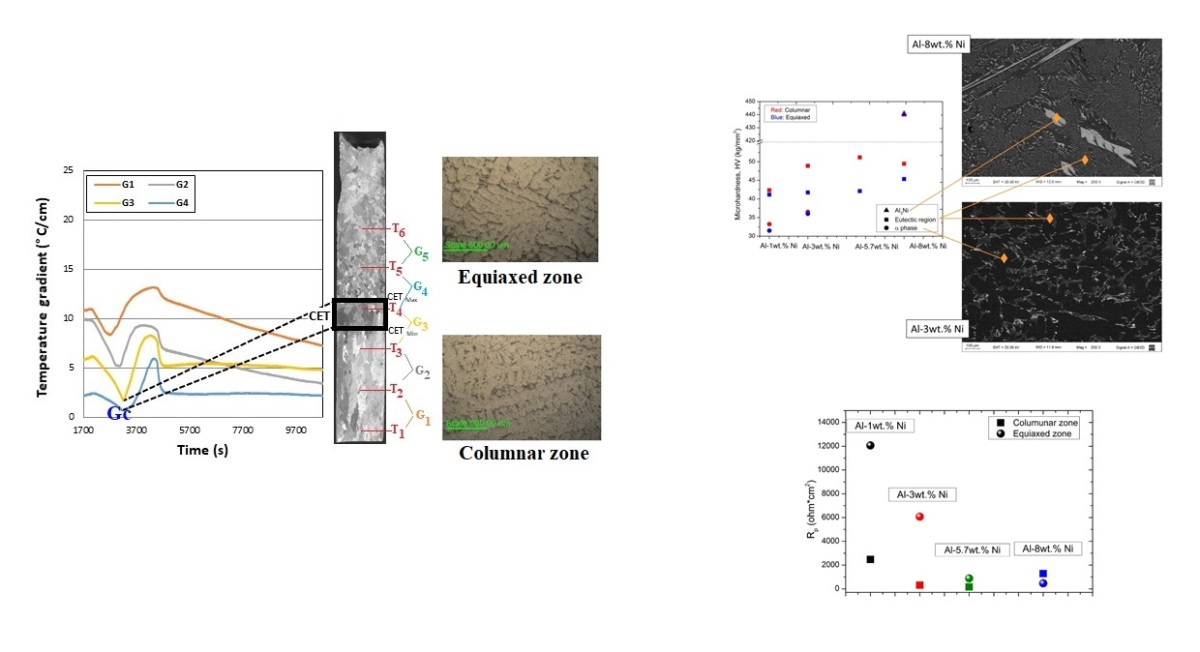
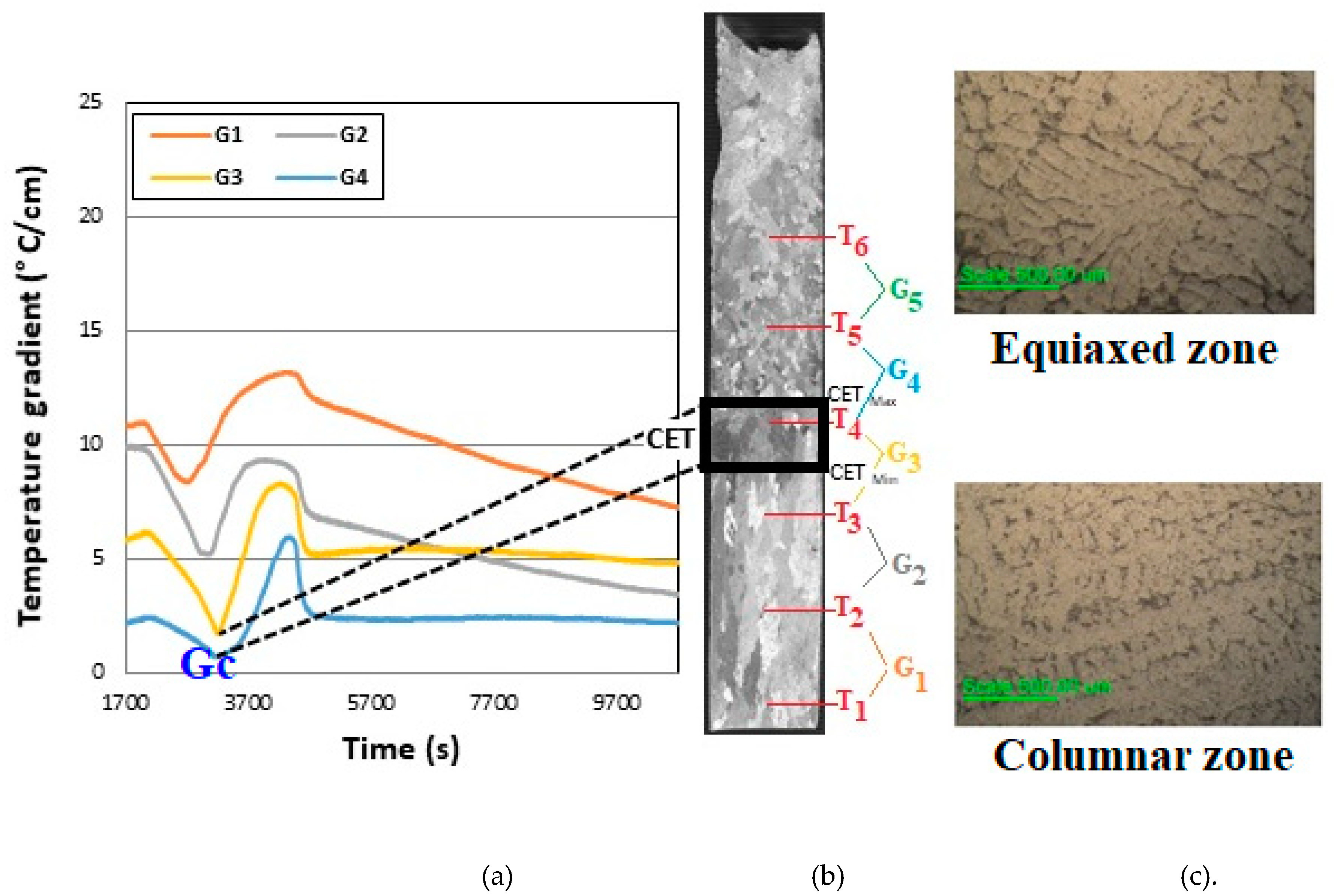
The thermal gradients for each adjacent thermocouple were calculated as a quotient between the difference in temperatures and the difference in distance between the two. In
Figure 2 can be observed the graph of gradients as a function of time for Al-3.wt %Ni alloy.
It is observed in the graph that the gradients at the beginning take higher values, both at the base and at the upper zone of the samples. Later, as the cooling progresses, it can be observed that the zone where the CET occurs, the gradient takes a minimum value and gives way to the subsequent growth of the equiaxed grains, hence the formation of the CET zone. From this graph, what is observed and given as a constant behavior in the different tests, is that where we see a minimum of the thermal gradient, G
c, the CET occurs. It can also be seen in
Figure 2 that CET does not occur in a line but in a transition zone of the order of 1 cm or greater, as was reported before [
23,
24,
25,
26,
27]. The values of the critical temperature gradients, Gc, are listed in
Table 1.
To verify what is shown in the thermal gradient graph, the zone where the CET take place in the macrograph of
Figure 2 was compared (corresponding to Al-3wt. %Ni) and related to the gradient graph. It is observed that when the CET occurs, the gradient takes a minimum corresponding to the thermocouple located at this position. For example, in the case of
Figure 2, the minimums are displayed between thermocouples #3 and #4, and in the macrograph the CET occurs right at this height of the sample.
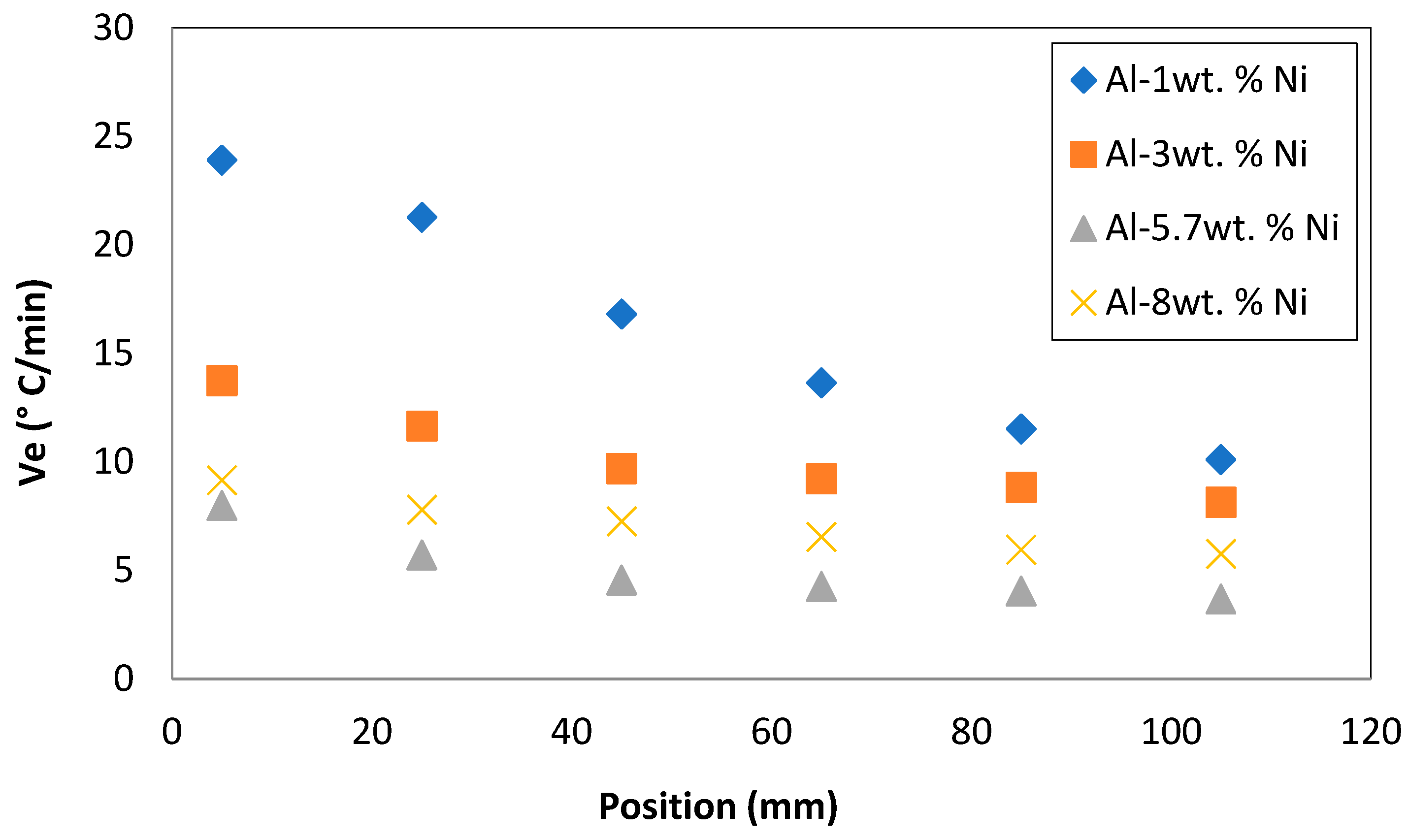
3.3. Cooling rates and macrostructure
The cooling rates, Ve, in the liquid were determined by taking the average values of the slopes of the temperature versus time curves in the zone before the plateau, for each thermocouple position. On the other hand, the solidification rates were obtained as the average value of the temperatures within the plateau, in the cooling curves. These thermal parameters are presented in
Table 1.
The temperatures decrease faster in the regions closest to the bottom of the water-cooled furnace. This caused that cooling rates gradually decrease towards the completion of local solidification located in the upper zone of the sample, see
Figure 3.
The analysis of macrostructures obtained allowed to determine the width of columnar grains, the height in the sample at which the CET zone occurr (positions of the CETMin and CETMax) and the average diameter of equiaxed grains, D
Ge (
Table 1). The CET transition zone is characterized due in such zone the columnar grains coexist with the equiaxed grains [
23,
24,
25,
26,
27].
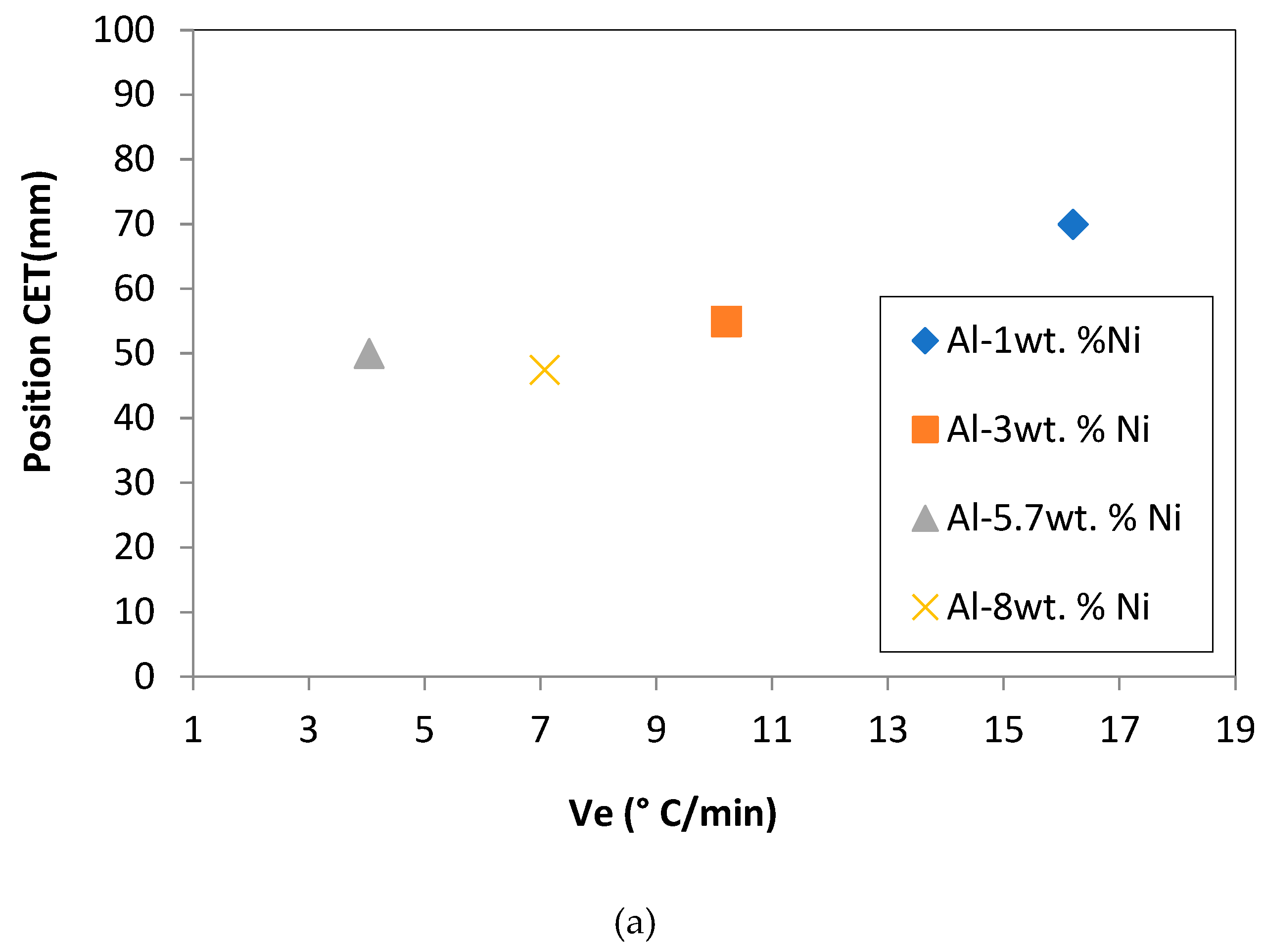
Figure 4 (a) and (b) represent the CET position in the sample and the columnar grain width, respectively, with respect to the cooling rate. In this work, a proportional relationship of the cooling rate with respect to the position in which the CET occurs was observed. At a higher cooling rate, the CET moves away from the base of the sample (considering the base the only point of heat extraction). It should be noted that this quasi-linear relationship is not fulfilled when the solute content comes into play, so that for a higher V
e, the CET changes its behavior, that is to say that the value of the distance with respect to the base decreases. This behavior could be attributed to the fact that there are more solute particles, these could generate greater solidification sites and favoring the nucleation of the equiaxed grains, despite the fact that the V
e is higher. This behavior was observed for the Al-8wt. %Ni alloy.
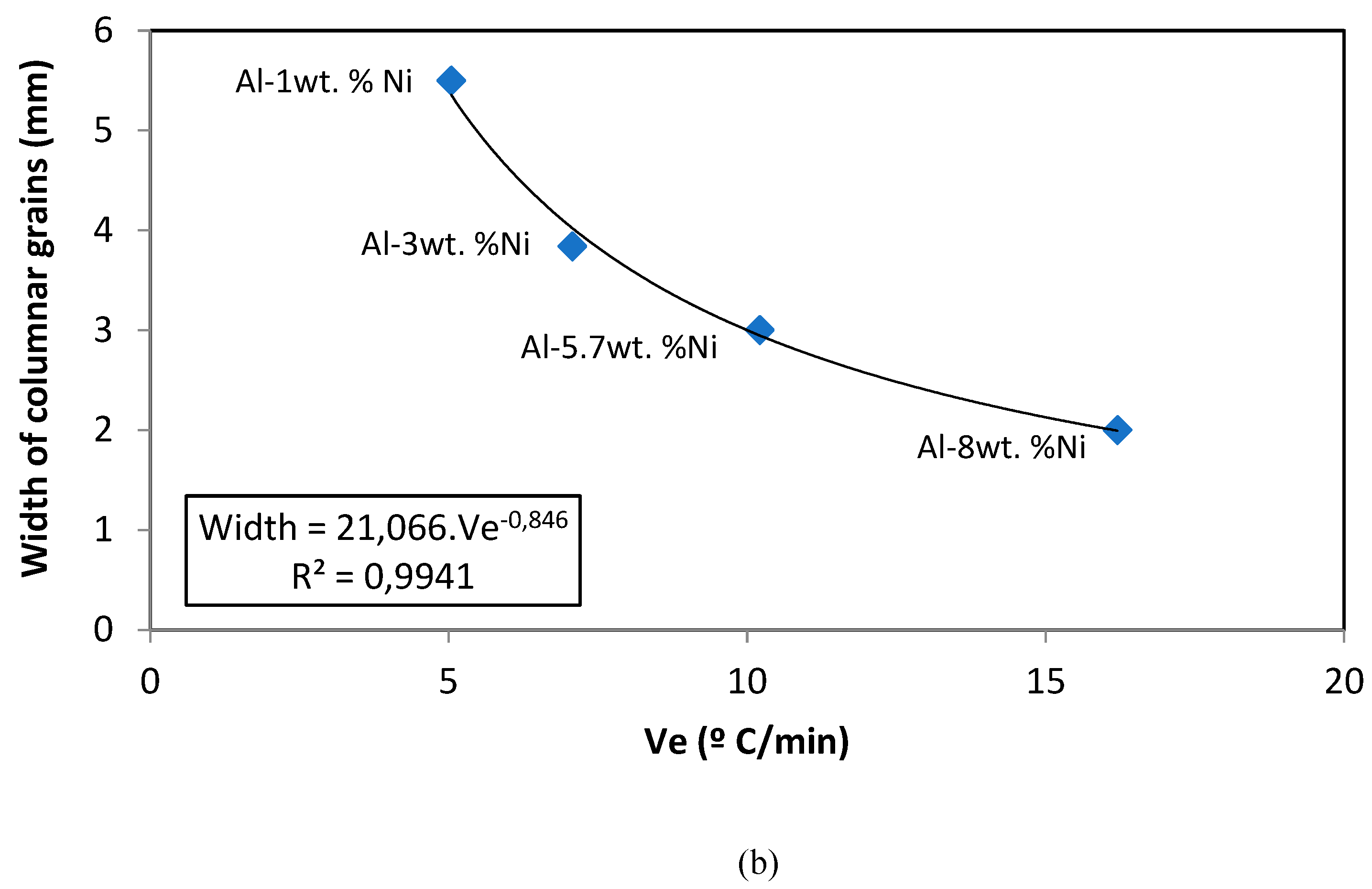
On the other hand, it is also evident that, at higher Ve values, a columnar grain refinement effect is generated. That is, higher cooling rates produce more refined columnar grains. A non-linear adjustment was made for this relationship, because it was observed that at very low velocities, very wide columns tend to form.
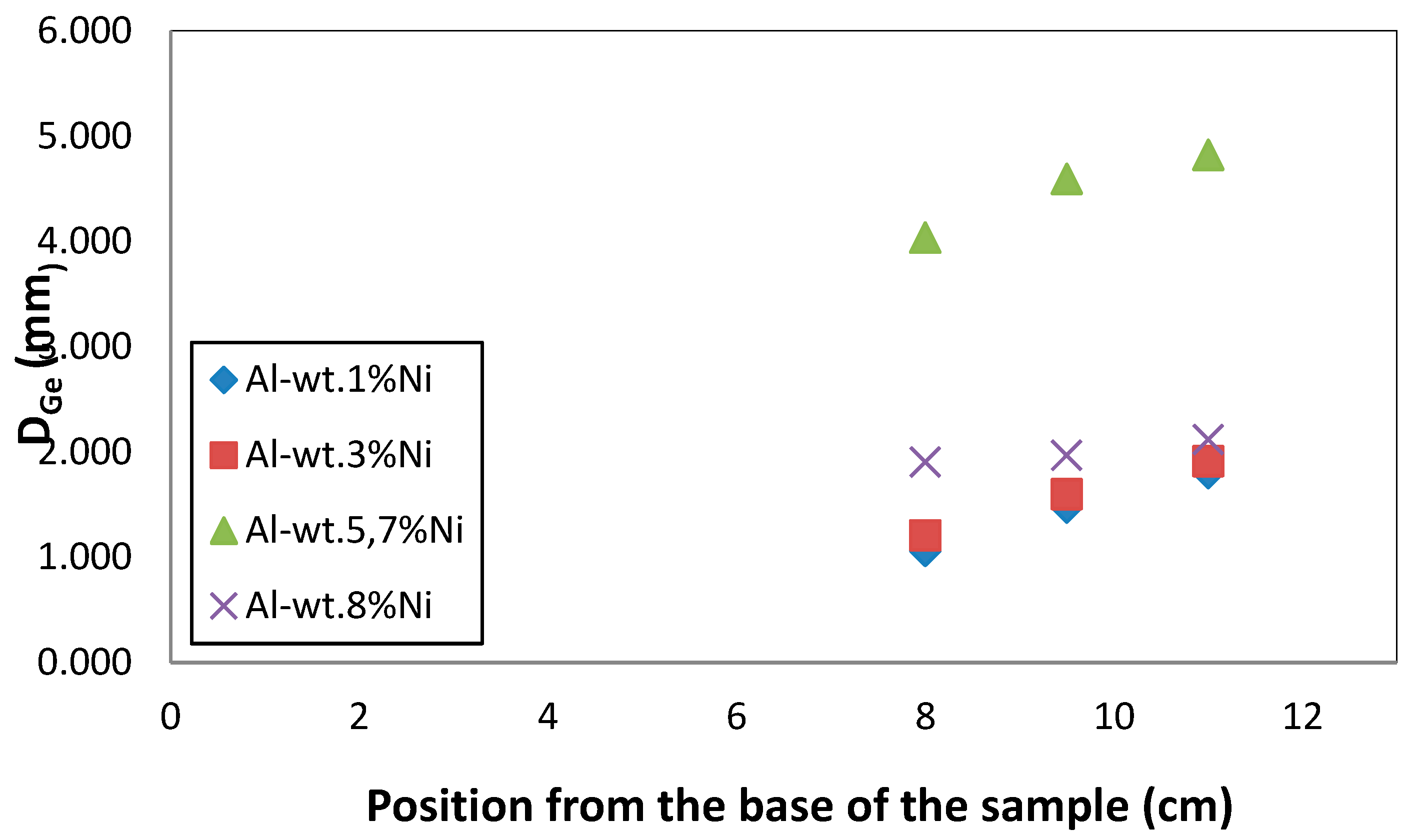
According to the data presented in
Table 1, it is possible to observe the effect of solidification velocity with respect to the D
Ge. It is observed that, at a lower solidification velocity, the equiaxed grains presented a higher D
Ge value. This is expected, because lower V
s generate longer solidification times, this allows the grains to increase their average size. As seen in
Figure 2, the heat extraction rate is lower in the areas furthest from the base of the sample. Consequently, it is expected that, being in a position further from the base, the D
Ge will increase. This behavior can be seen in
Figure 5, where the D
Ge values for the four alloys studied are shown, depending on the distance from the base of the samples.
3.4. Microstructure and microhardness
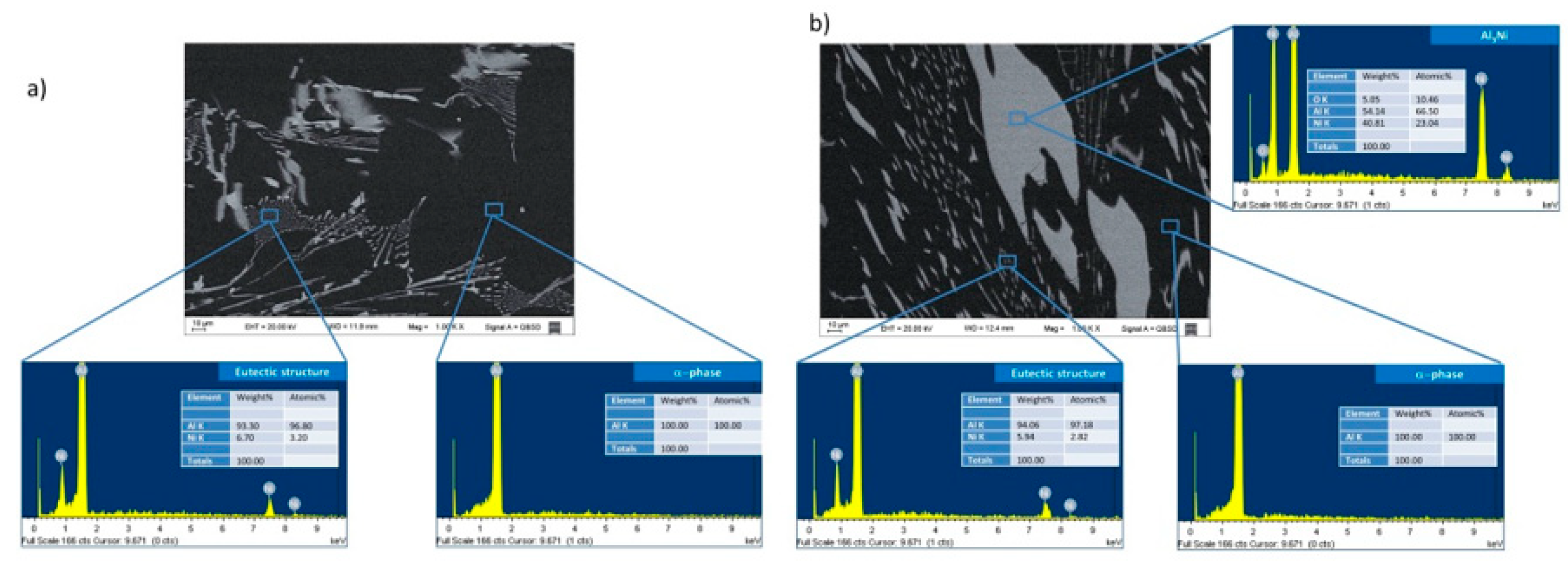
The microstructures of the Al-Ni alloys in the range of compositions studied are made up of three phases: the α-phase, rich in aluminum (with a nickel content of up to 0.24 % [
20]), the intermetallic compound Al
3Ni, and the eutectic structure consisting of thin Al
3Ni needles surrounded by the alpha phase [
32]. The compositions of these phases were determined by EDS and can be seen in
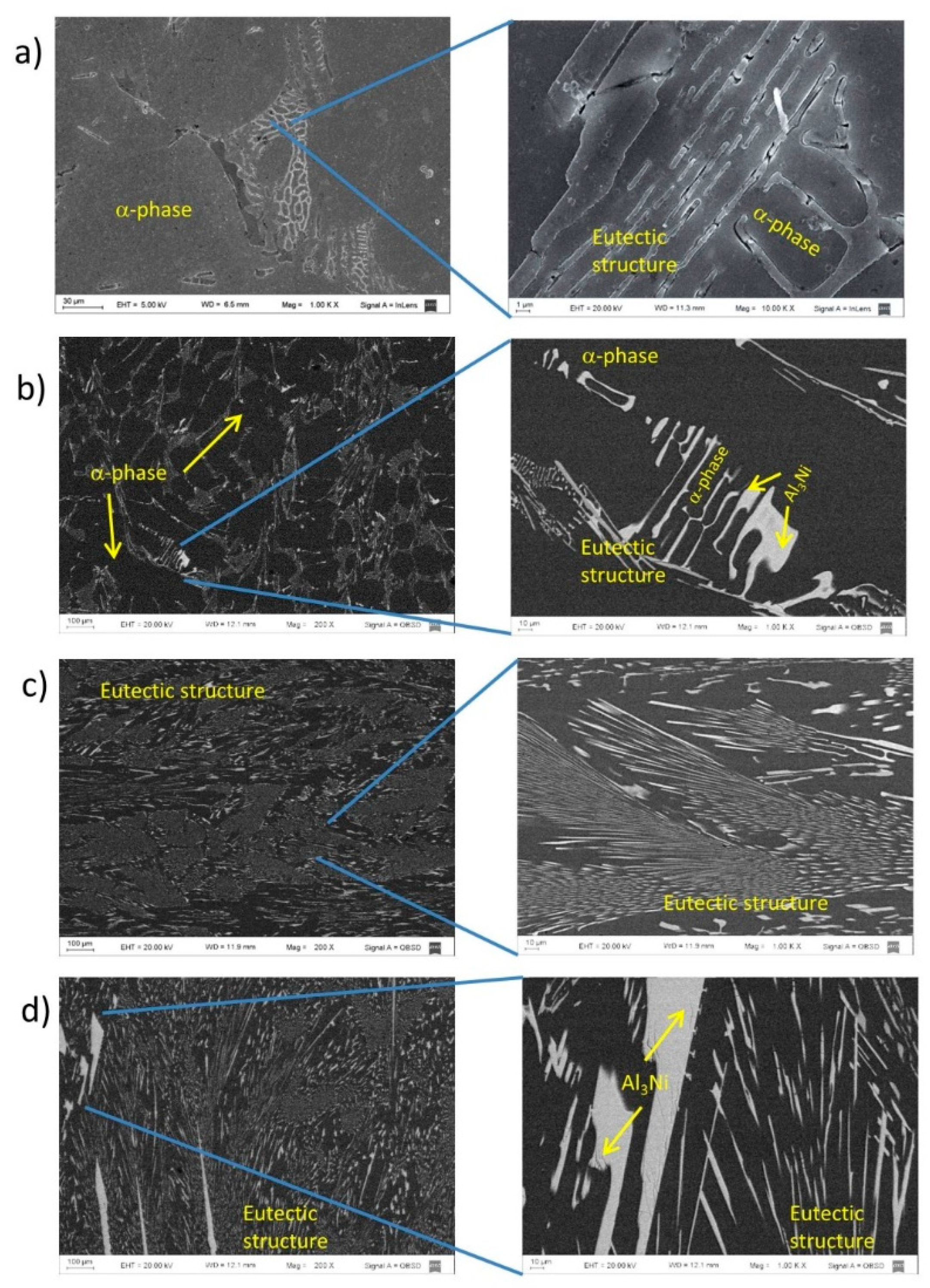
Figure 6, for (a) Al-3wt. %Ni hypoeutectic alloy and (b) for Al-8wt.%Ni hypereutectic alloy. The distribution of the phases in the microstructure depends on the nickel content of the alloy. In the hypoeutectic alloys, Al-1wt. %Ni and Al-3wt. %Ni, the microstructure is characterized by a α-phase (dendritic matrix), and the eutectic structure constituting the interdendritic region (
Figure 7 (a) and (b)). The Al-5.7wt. %Ni alloy presents the typical eutectic microstructure, as can be seen in
Figure 7 (c). The microstructure of the hypereutectic alloy, Al-8wt. %Ni is shown in
Figure 7 (d). It presents thick particles of Al
3Ni intermetallic, surrounded by the eutectic structure.
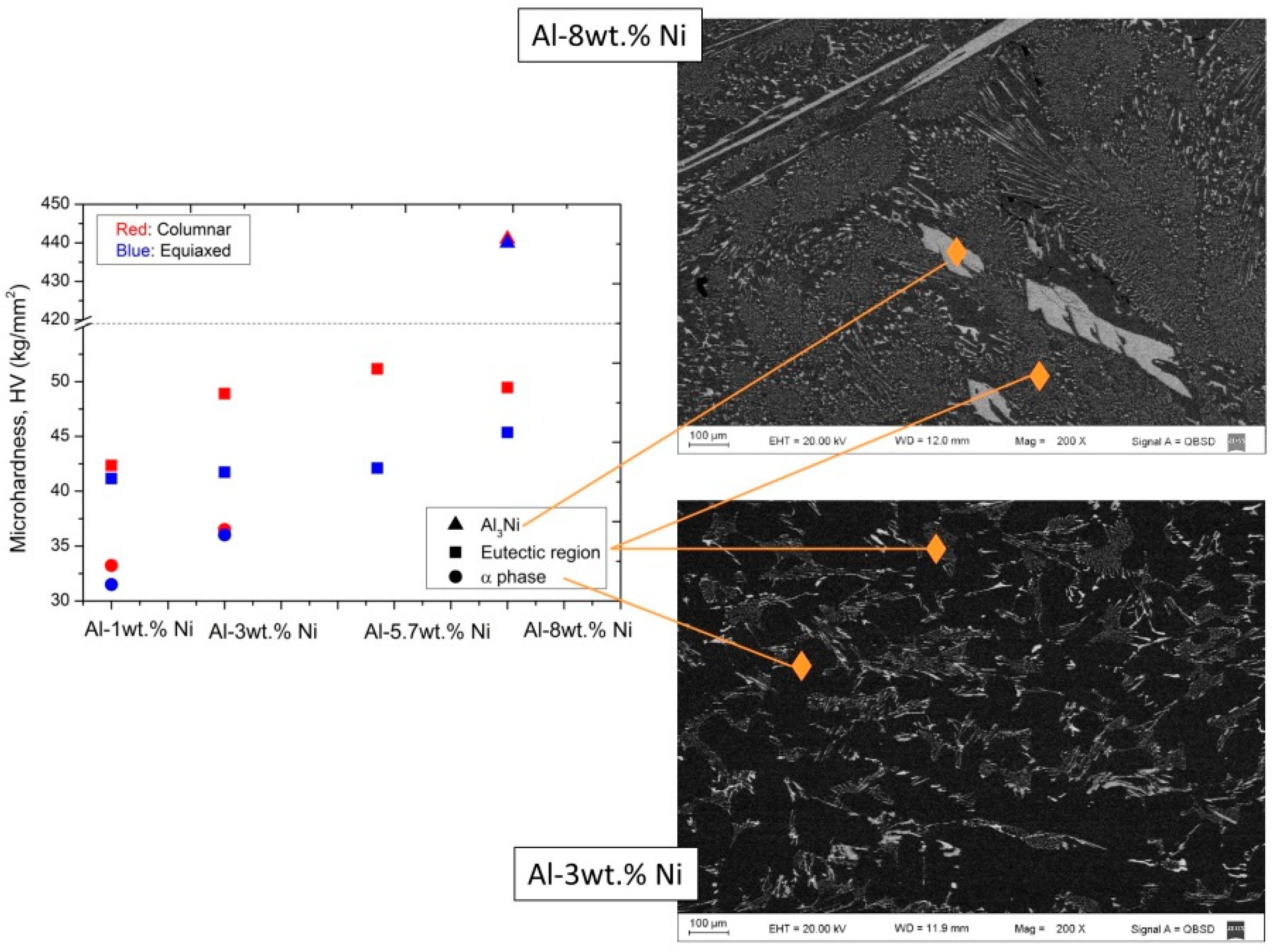
In
Figure 8 the microhardness values measured for each of the phases of the Al-Ni alloys is presented. It was found that the eutectic region has greater microhardness than the α-phase, logically associated with the higher Ni-content. It is also observed that the microhardness values increase in each region as the Ni content increases, until the eutectic composition is reached.
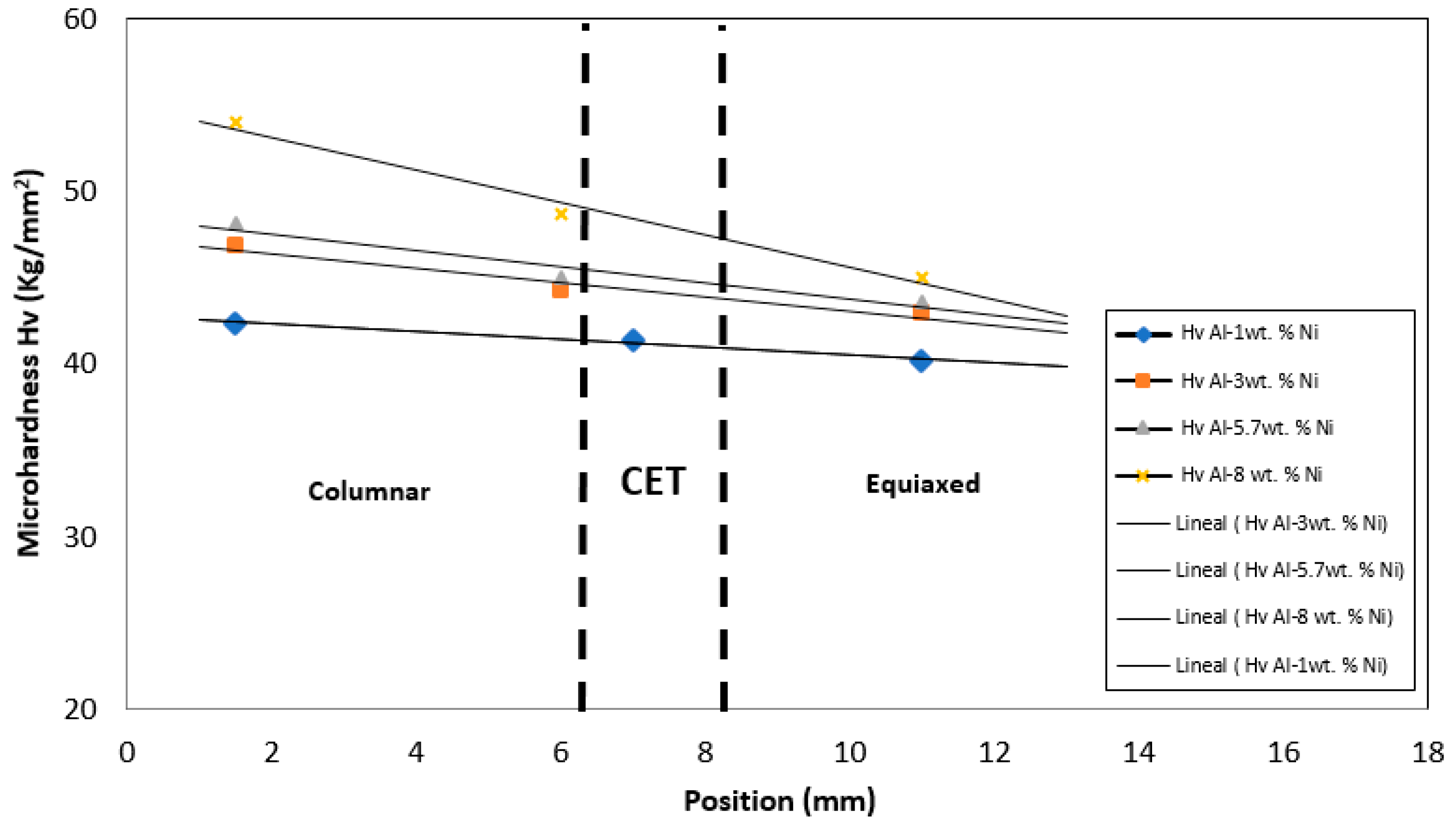
In order to obtain a relationship between the solidification parameters like cooling rate, Ve, and the microhardness (HV) for the directionally solidified Al-Ni alloys, a comparison of the HV obtained in the different grain zones of the samples was carried out.
In
Figure 9 it is observed that the HV values are smaller in the areas furthest from the base. This is attributed to the fact that in further areas the heat extraction rate is lower and the grains are less refined, similar values were found by other authors [
29].
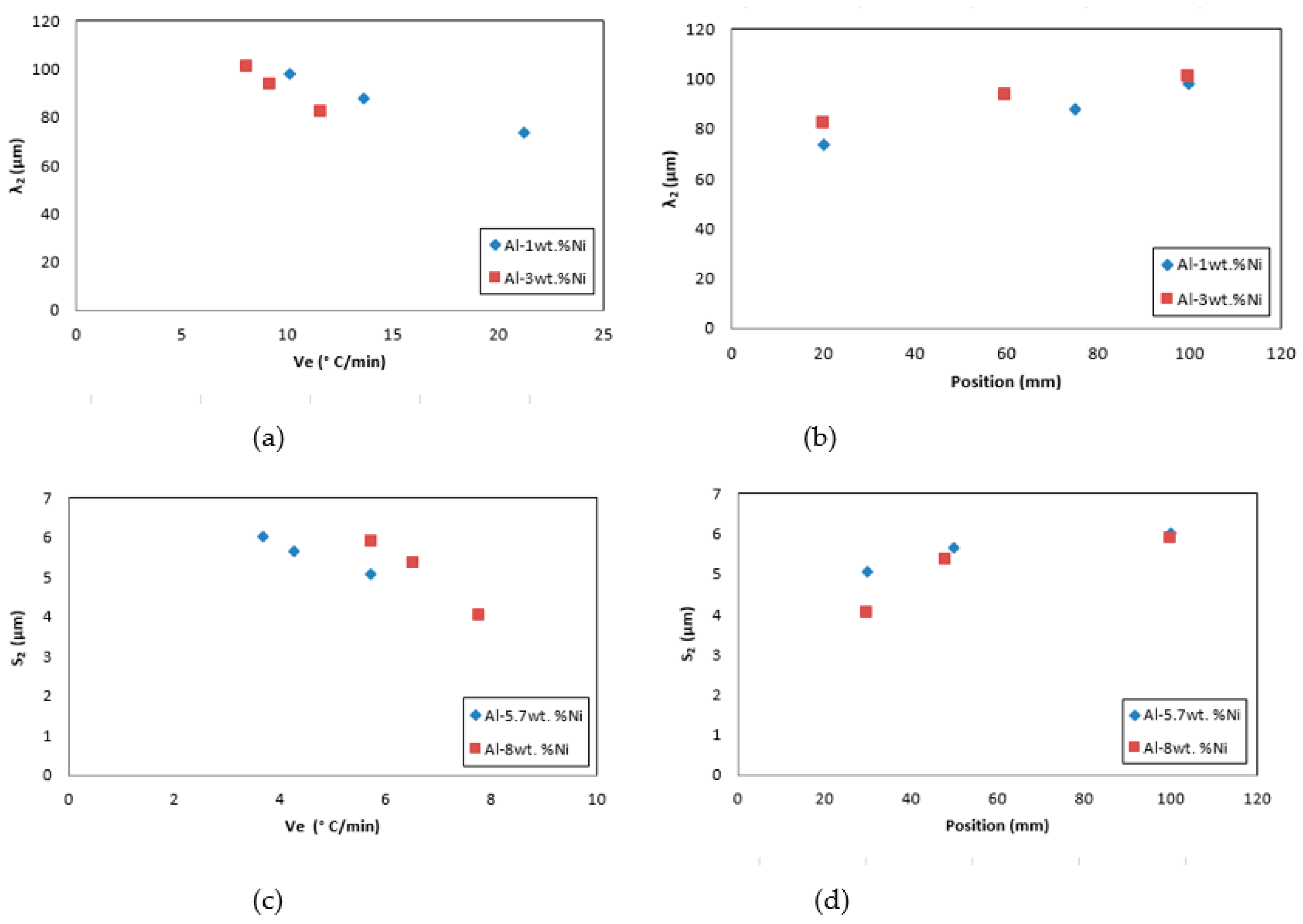
The highest values of cooling rate, V
e, generate refinement in the secondary dendritic spacing, λ
2, and lamellar spacing, S
2, obtaining greater microstructural refinement in the zones of the samples close to the base, as seen in
Figure 10 (a) to (d).
In
Figure 8 was presented a comparison of the HV values obtained in different positions of the sample for the eutectic phase. In this figure an increase in HV values was observed in the zone close to the base of the sample, where there are columnar grains. These values decrease linearly as we move away from the zone close to the heat extraction device (base of the furnace). This behavior is attributed to the fact that in zones further away from the base of the furnace, the V
e values are lower, as observed in
Figure 3. This decreasing behavior of the V
e generated a refinement of the grain size, distribution and morphology of the intermetallic compound, thus improving the mechanical property (HV).
The improvement in the HV values can be observed in
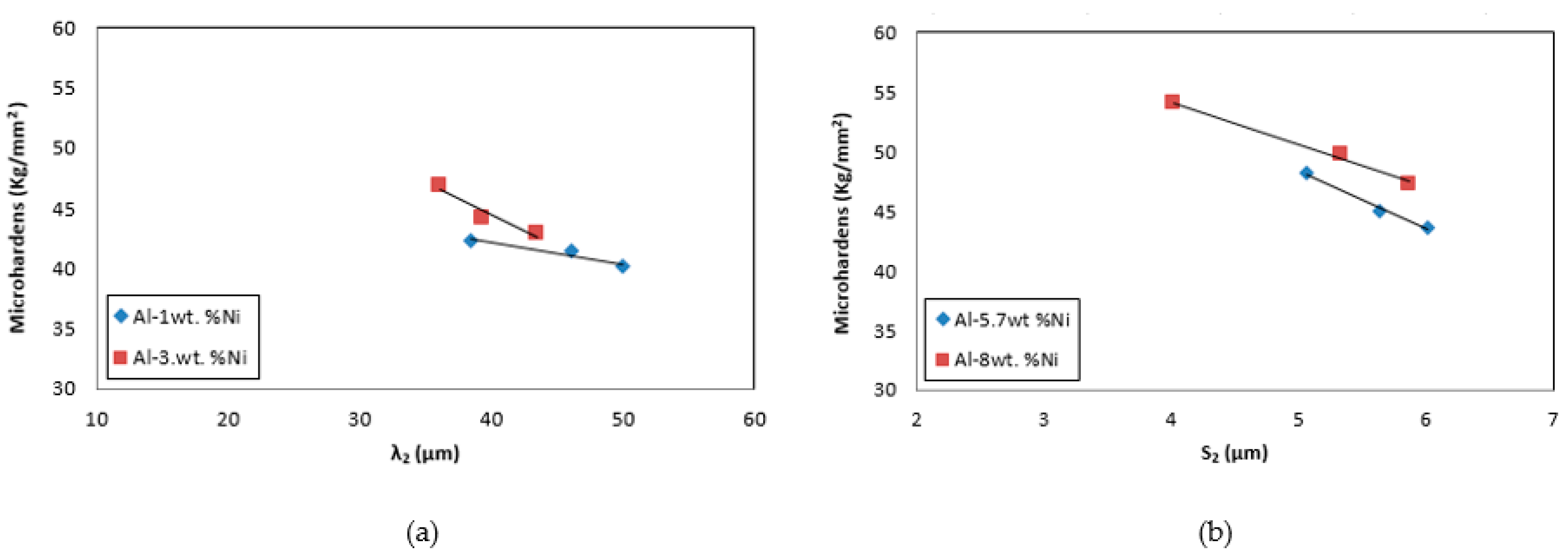
Figure 11 (a) where the variation of HV with respect to the secondary dendritic spacing, λ
2, is observed for Al-1wt. %Ni and Al-3wt. %Ni hypoeutectic alloys. In
Figure 11 (b) the variation of HV with respect to the lamellar spacing, S
2, for Al-5.7wt. %Ni and Al-8wt. %Ni eutectic and hypereutectic alloys, respectively. In both cases, it was observed that the decrease in the values of λ
2 and S
2 leads to an increase in the HV values. Finally, it is important to note that in all cases a small increase in the HV values was observed as a function of the Ni-content and also for each concentration it was found that as the cooling rates increased, the values of HV also increased linearly. Because the mechanical properties are linked to microstructural refinement (the values of λ
2 and S
2 were decreasing) and higher V
e.
Similarly, Martínez-Villalobos et al. [
32] found that by increasing the Ni-content in Al-Ni as cast alloys, a small increase in the hardness of the alloys was evident from a hypoeutectic to a hypereutectic composition. It should be noted that afore mentioned authors did not carry out differentiated microhardness measurements for the different phases of the alloys.
In the present study, for the alloy with a hypereutectic composition, the HV of the eutectic region in the columnar zone is lower than that for the eutectic alloy. This may be due to the decrease in the Ni-content corresponding to the eutectic region, at expense of the precipitation of the intermetallic compound Al
3Ni. The HV values measured for the Al-8wt. %Ni alloy in the phase corresponding to the Al
3Ni intermetallic are markedly higher than those measured with respect to the other areas of the alloys (see
Figure 8).
3.5. Potentiodynamic polarization curves
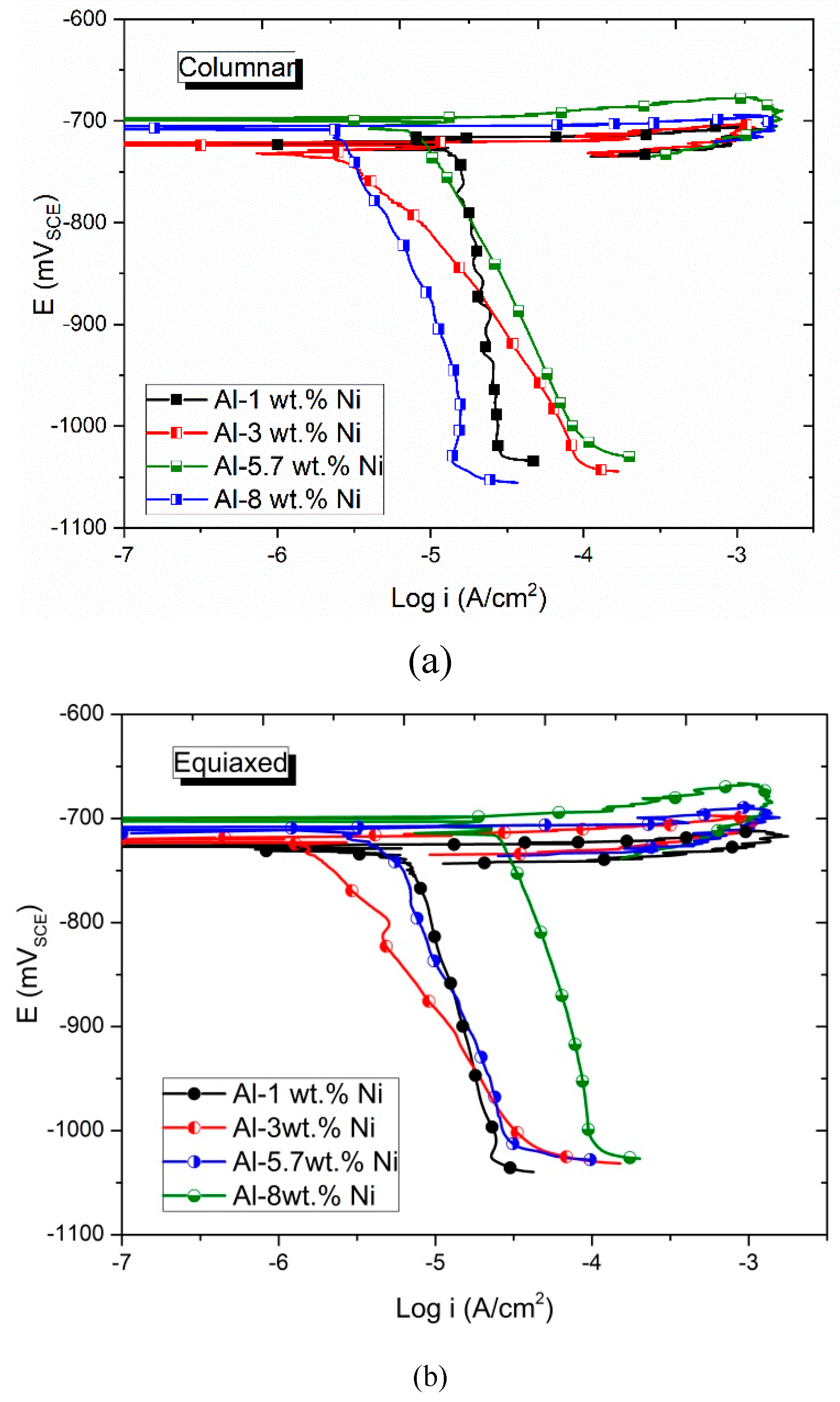
Figure 12 shows the cyclic potentiodynamic polarization curves obtained according to the type of grain for all the compositions of Al-Ni alloys studied. In
Figure 12 (a) and (b) the results obtained for both columnar and equiaxed grains zones are observed. The shape of the curves is similar for all compositions and grain zones. The direct dissolution of the material is evident after reaching the corrosion potential. Other authors [
16,
17] have already reported the beginning of the pitting phenomenon when studying Al-Ni alloys, with a content of around 5 %Ni in solutions with much lower NaCl content than that used in this research. In
Table 2, the measured corrosion potentials, E
corr, are presented.
Considering the pitting potential, E
pit, as proposed by Zaid et al [
33], as the potential value at which the Tafel anodic slope is zero, it is possible to establish that the E
corr values presented in
Table 3 correspond to the E
pit of the Al-Ni alloys.
In
Table 3 can be seen that the Al-5.7wt. % Ni alloy, which corresponds to the eutectic composition, presents the noblest E
pit values, followed by the Al-8wt. %Ni alloy, with a hypereutectic composition. Hypoeutectic alloys have the least noble potential values that are very close to each other.
Osorio et al [
16] found that a higher fraction of the eutectic structure is associated with a nobler pitting potential. Based on the results of
Table 3, an analogy could be made to this result considering that the alloy with eutectic composition, Al-5.7wt. % Ni, presented the highest E
corr value, which we assimilate to E
pit, due to the shape of the curves. In line with these results, Zhang et al. [
17], studying the effect of the microstructure on the corrosion resistance of an Al-5.4wt. %Ni alloy in NaCl, found that the more homogeneous structures presented higher pitting potential values and lower corrosion densities.
Regarding the influence of grain structures, for the same alloy composition, the zone of columnar grains presents nobler Epit values than the zone of equiaxed grains. However, these differences between Epit values are less than 5 mV, so they are not considered significant differences.
Therefore, it is possible to establish that the grain structure does not influence the E
pit. This result is consistent with what was reported for aluminum and other alloy systems [
19,
34].
The shape of the curves indicates that they present a predisposition to localized corrosion. The pitting phenomenon occurs when reaching the E
corr. For this type of corrosion, it is not feasible to determine the corrosion current density, because the active area of the corrosion phenomenon is unknown, that is, the area inside the pits [
35].
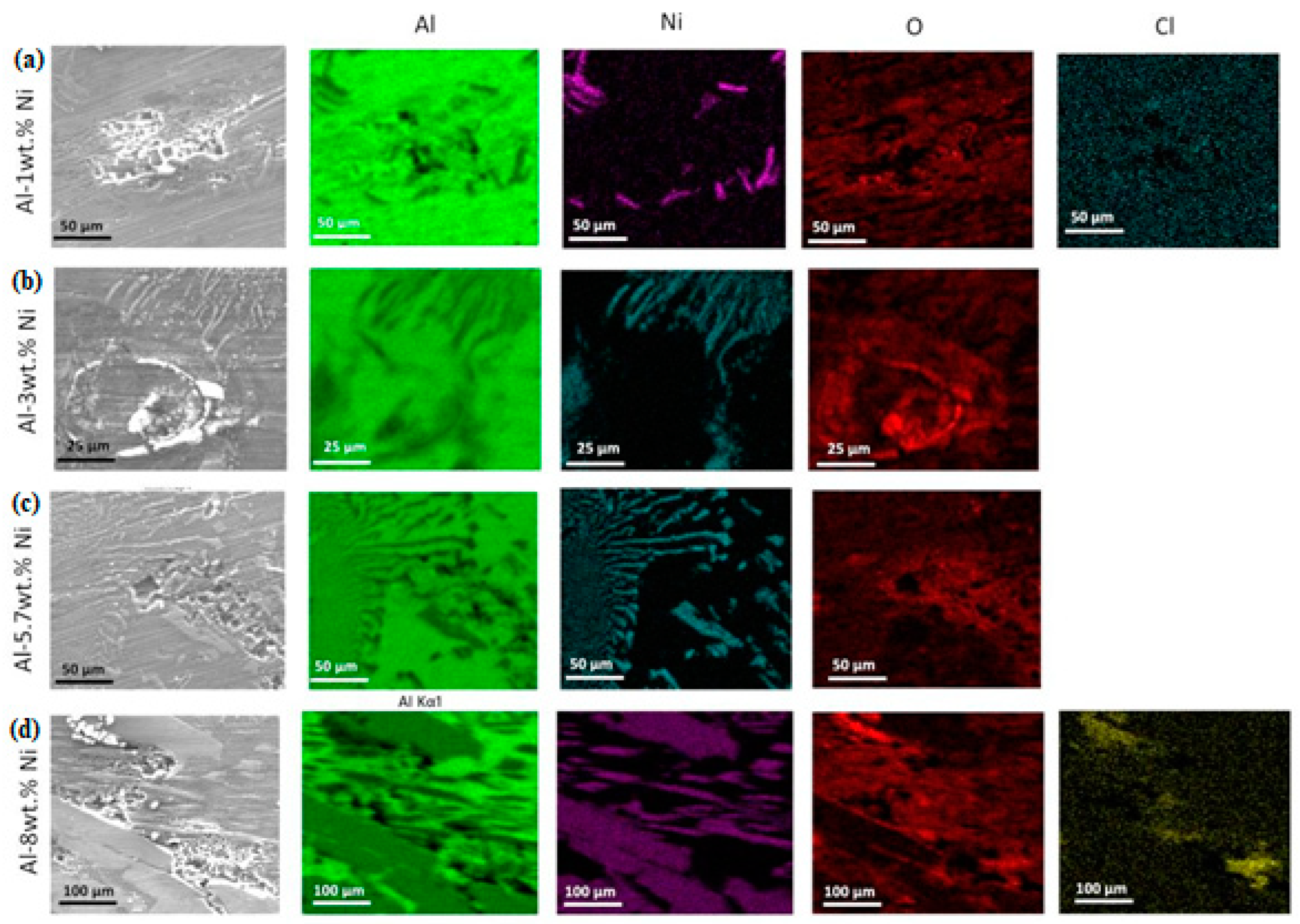
After making the potentiodynamic curves, the surface of the samples was analyze using the SEM microscope. The micrographs obtained can be seen in
Figure 13. Corrosion phenomena is evident on the α-phase, rich in aluminum, of the Al-1wt. % Ni alloy (
Figure 13 (a)).
In the samples with the highest Ni content, it is observed that corrosion is also located in the dendritic region, very close to areas with the presence of eutectic phase (
Figure 13 (b) and (c)). In the hypereutectic alloy, the corrosion effect is visible between the Al-3wt. %Ni precipitates (
Figure 13 (d)). The presence of pits in the matrix corresponding to the α-phase was already reported by other authors when studying the electrochemical behavior of Al-Ni alloys [
17]. In the EDS mapping the constituent elements of the alloy are identified. Aluminum is present in the dendritic matrix and Ni distributed as thin sheets, which correspond to the intermetallic Al
3Ni, a constituent of the eutectic phase. Element distribution mapping detected the presence of oxygen, suggesting the formation of an oxide due to the corrosion process. Since the color intensity of the images is greater in the aluminum-rich region. The oxide formed was Al
3O
2. The surface analysis allowed us to identify the presence of a fraction of Cl distributed uniformly. This would be explained by the adsorption of Cl
- ions on the oxide surface, a frequent phenomenon during the pitting process [
36,
37].
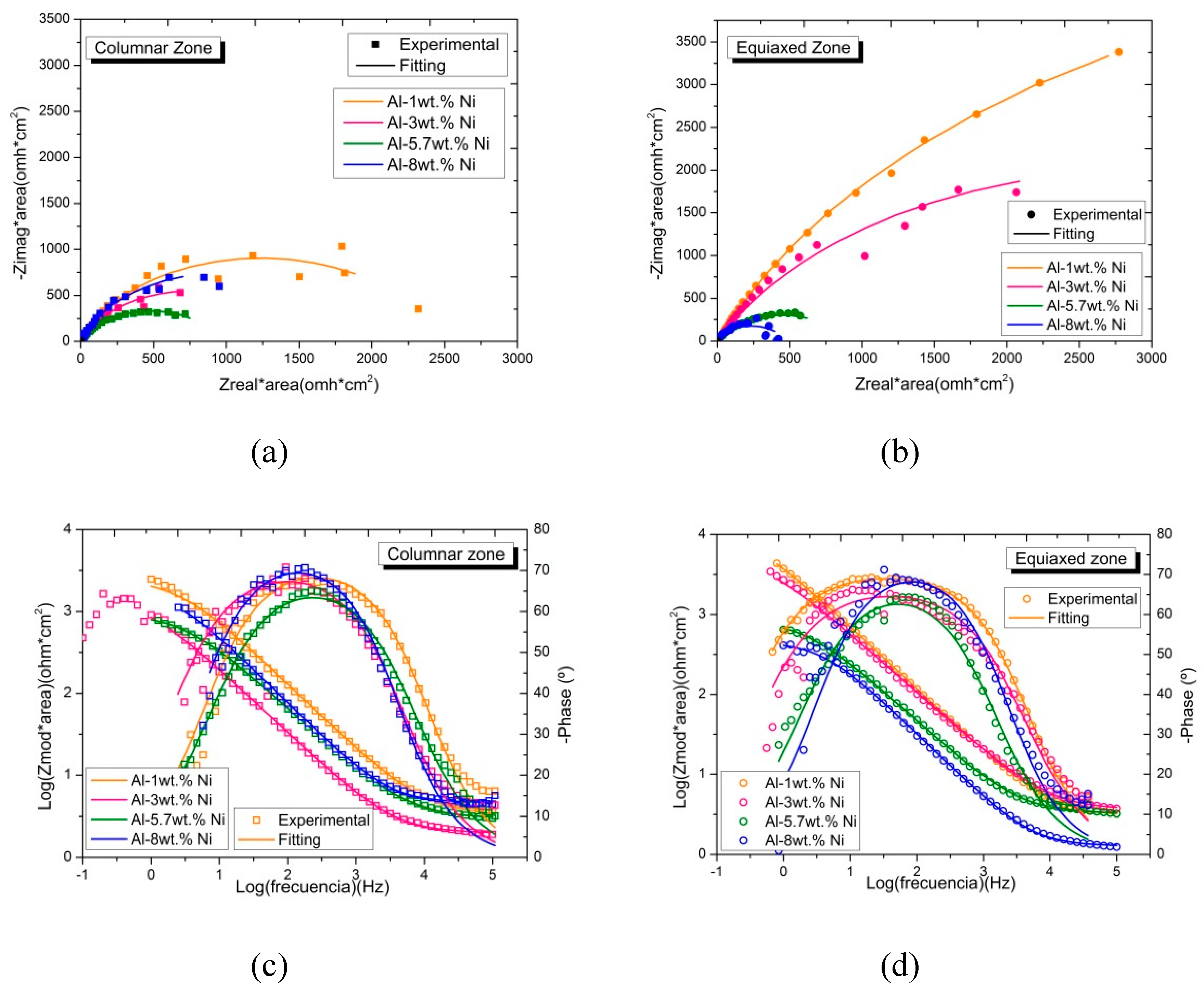
3.5. Electrochemical Impedance Spectroscopy
The electrochemical impedance spectra were obtained for the Al-Ni alloys in order to characterize the electrochemical phenomenon that takes place on the surface of the samples.
In
Figure 14 (a) and (b) the Nyquist diagrams can be seen and in
Figure 14 (c) and (d) the Bode diagrams are presented (
Figure 14 (a) and (c) for columnar grains and
Figure 14 (b) and (d) for equiaxed grains). The presence of a single capacitive contribution is clearly observed. To adjust the spectra, the Randles equivalent circuit was used (
Figure 15), commonly used to describe interfaces that are undergoing the initial stages of the corrosion process [
35,
38].
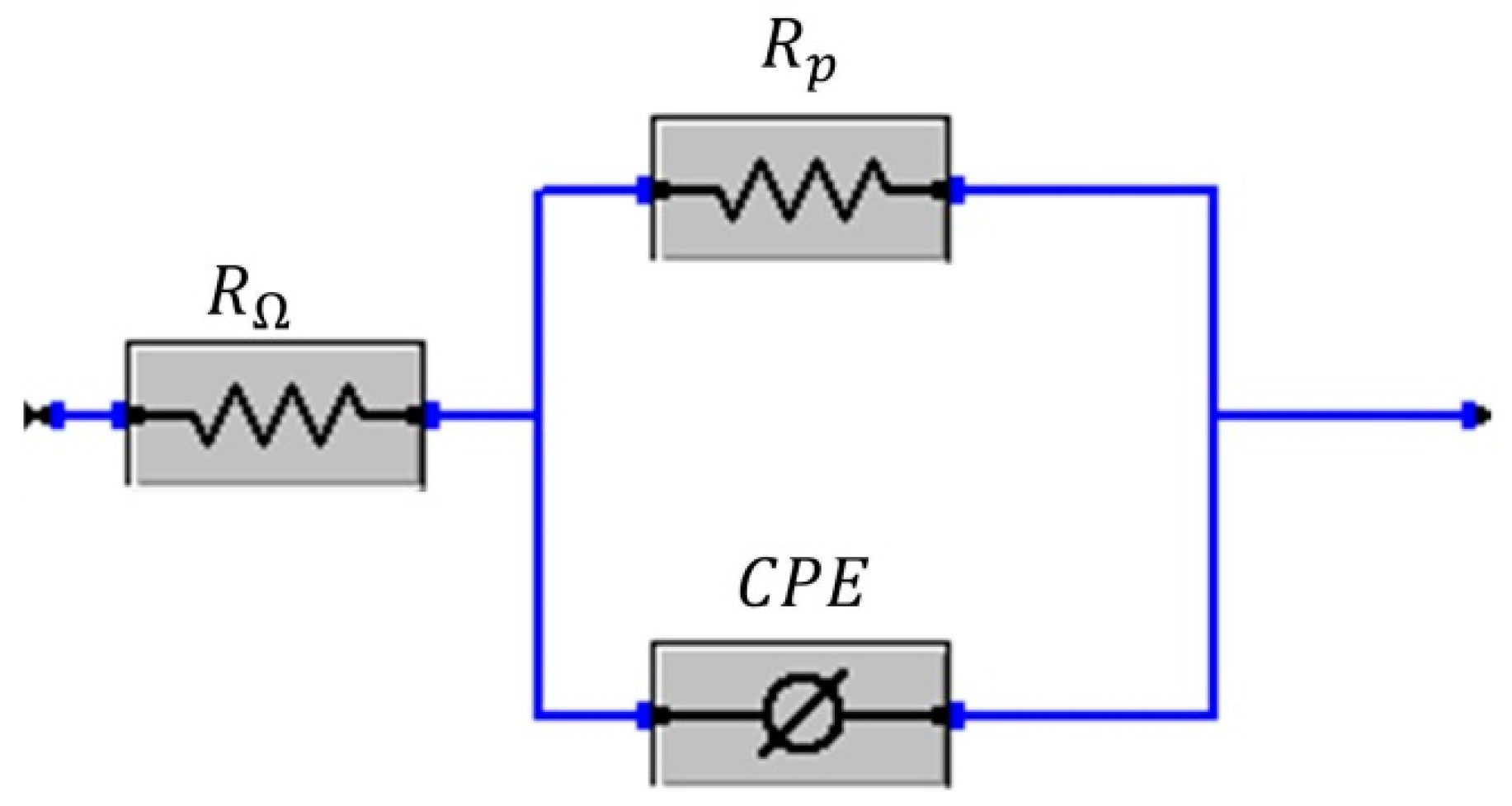
The physical meaning of the elements that constitute the equivalent electrical circuit of
Figure 15 is described below: R
Ω represents the resistance of the electrolyte, R
p, is the resistance to polarization due to the load resistance and CPE is the associated constant phase element. The use of a CPE to replace a capacitor is due to the need to represent the non-ideal behavior of the surface due to the lack of uniformity caused by roughness, porosity, roughness or other factors [
18,
19,
35,
39]. The parameter values obtained from the EIS fit are shown in
Table 3.
The parameter used as an indicator of non-ideal behavior will be n. According to Kelly et al. [
38], for surfaces that are suffering from pitting, this value ranges between 0.7 and 0.95. This is coincident with the values of n measured for the Al-Ni alloys.
R
p values can be associated with the corrosion resistance of the alloys. The higher the R
p value, the higher the corrosion resistance. [
18,
19,
28].
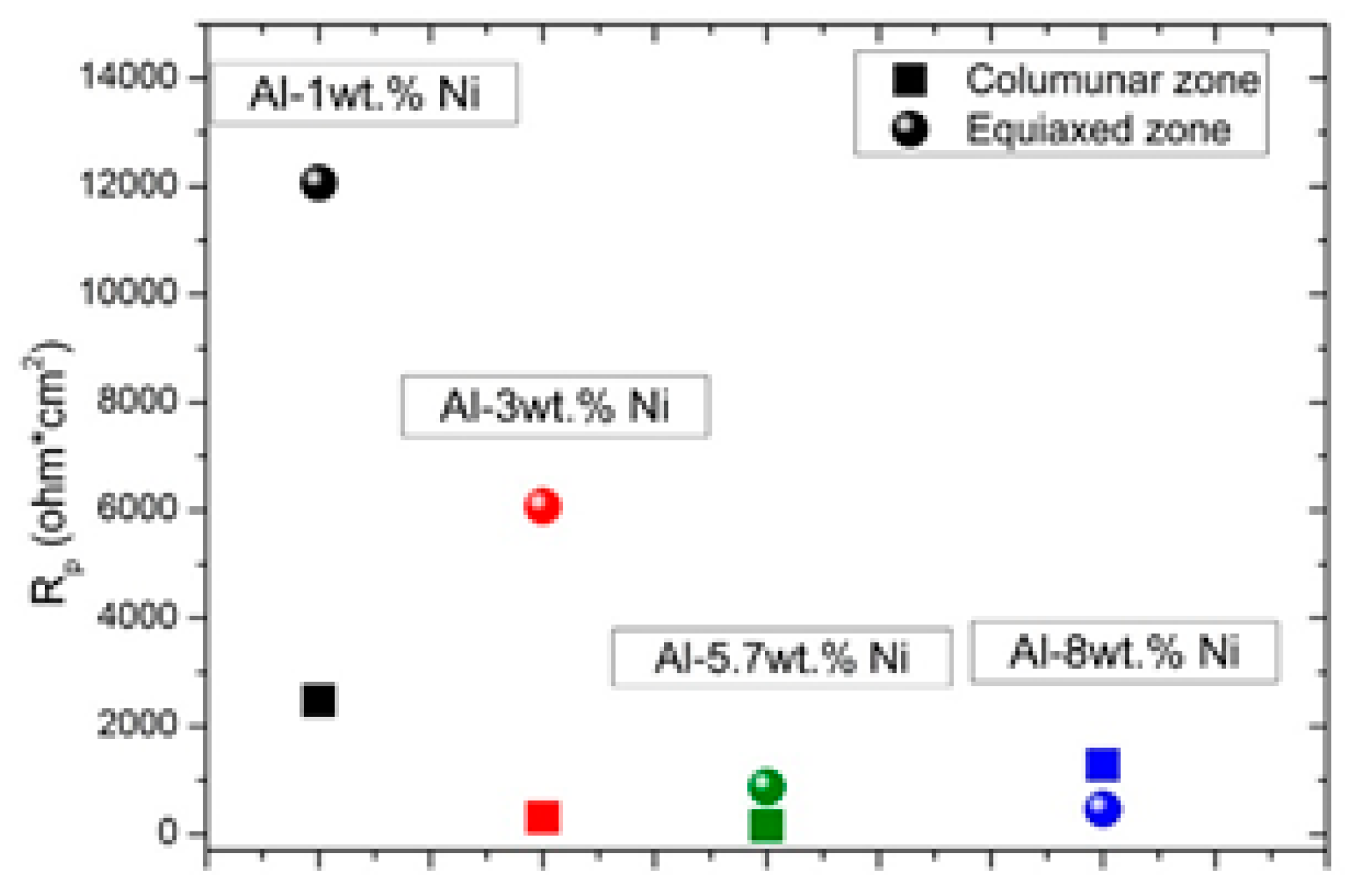
Figure 15 shows the R
p values depending on the composition of Al-Ni alloys, for the two grain structures. It is observed that by increasing the Ni content, R
p decreases for samples with equiaxed grain structure, throughout the range of compositions studied. Furthermore, the equiaxed grain structure presents higher R
p values than the columnar grain zone, for alloys with a composition equal to or lower than the eutectic composition.
It is evident that for Al-Ni alloys containing up to 5.7% Ni, the phase distribution generated by the equiaxed grain structure is more favorable. This may be due to the fact that the equiaxed grain structure, associated with higher values of λ
2, decreases the contact areas between the α-dendritic matrix and the second phase particles, Al
3Ni. This effect of microstructural morphology for alloys consisting of two phases had already been reported by the authors for the Al-Cu system [
19]. Zhang et al. [
17] reported that a homogeneous distribution of the eutectic mixture is essential to improve corrosion resistance, when studying an Al-5.4wt. % Ni alloy. Similarly, Osorio et al [
40] indicated that the distribution of Al
3Ni intermetallic particles play a determining role in the behavior of Al-Ni alloys against corrosion.
For the hypereutectic alloy, Al-8wt. % Ni, the trend is modified, with the zone of columnar grains being the one with the highest Rp. It is important to remember that for hypereutectic alloy, Al3Ni particles constitute the second phase particles precipitated in a eutectic matrix. Therefore, it can be considered that the columnar grain structure is favorable for alloys with a hypereutectic composition.
3.6. Correlation between electrochemical parameters and microhardness
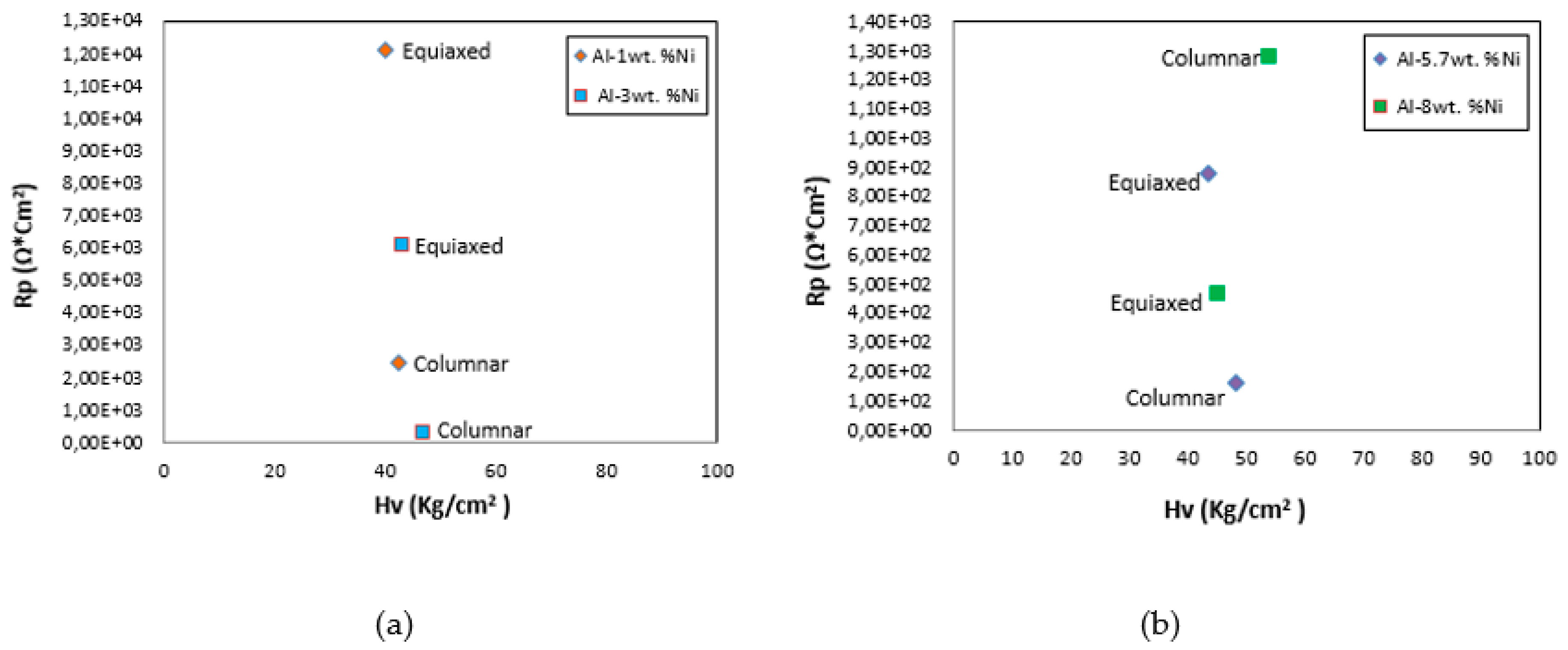
In
Figure 16 were correlated the values of polarization resistance, R
p, with microhardness, HV, for both types of grain structures (columnar and equiaxed). It is observed that the higher HV, the lower the value of R
p for hypoeutectic (Al-1wt. %Ni and Al-3wt. %Ni) and eutectic (Al-5.7wt. %Ni) alloys, and the trend for the hypereutectic (Al-8wt. %Ni) alloy changes.
This different behavior can be justified because the Al
3Ni intermetallic could play a dual role in the corrosion of an Al-Ni alloy. It can act as a barrier against corrosion or as a galvanic cathode that accelerates the corrosion process. The role that will dominate the corrosion process will depend largely on the quantity and distribution of intermetallic particles. This is in accordance with what was proposed by Osório et al. [
16].
5. Conclusions
From the results and discussion in the previous sections, the main conclusions of this investigation on the directional solidification of Al-Ni alloys are as follows.
The CET occurs in a zone were coexists columnar and equiaxed grains, and the position of the zone in the sample decreases as the Ni-content in the alloy increases.
The values of critical gradients (at the CET) were determined, and the vales are 1.3 to 2.9 ° C/cm.
The eutectic region has greater microhardness than the α-phase, reasonably associated with the higher Ni-content.
The microhardness values are smaller in the areas furthest from the base, due in further areas the heat extraction rate is lower and the grains are less refined.
The highest values of cooling rate generate refinement in the secondary dendritic arm spacing and lamellar spacing obtaining greater microstructural refinement in the zones of the samples close to the base.
A small increase in the microhardness values was observed as a function of the Ni-content. For each concentration, as the cooling rate increase the values of microhardness increase linearly.
The Al-5.7wt. %Ni alloy, which corresponds to the eutectic composition, presents the noblest pitting potential values, followed by the Al-8wt. %Ni alloy, with a hypereutectic composition. Hypoeutectic alloys have the least noble potential values that are very close to each other.
For the same alloy composition, the zone of columnar grains presents nobler pitting potential values than the zone of equiaxed grains. However, these differences between values are less than 5 mV, so they are not considered significant differences.
When the Ni content increase, the resistance to polarization decreases for samples with equiaxed grain structure, throughout the range of compositions studied. Furthermore, the equiaxed grain structure presents higher resistance to polarization values than the columnar grain zone, for alloys with a composition equal to or lower than the eutectic composition. For the hypereutectic alloy, Al-8wt. % Ni, the trend is modified, with the area of columnar grains being the one with the highest resistance to polarization.
With respect to the correlation between resistance to polarization and microhardness for Al-Ni alloys studied, when microhardness increase, the resistance to polarization decrease for hypoeutectic (Al-1wt. %Ni and Al-3wt. %Ni) and eutectic (Al-5.7wt. %Ni) alloys. The trend for the hypereutectic (Al-8wt. %Ni) alloy changes. The different behavior can be justified because the Al3Ni intermetallic could play a dual role in the corrosion of an Al-Ni alloy. It can act as a barrier against corrosion or as a galvanic cathode that accelerates the corrosion process.
Author Contributions
Conceptualization, A.E.A.; methodology, A.E.A.; formal analysis, A.E.A.; investigation, A.S.R., E.R.I., N.S.Z, and C.M.M.; resources, A.E.A.; data curation, A.E.A. and C.M.M.; writing—original draft preparation, A.E.A., A.S.R. and E.R.I.; writing—review and editing, A.E.A.; visualization, A.E.A.; supervision, A.E.A.; project administration, A.E.A. and C.M.M.; funding acquisition, A.E.A. All authors have read and agreed to the published version of the manuscript.
Funding
Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET): RES.2019-574- APN-DIR#CONICET; Agencia Nacional de Promoción Científica y Tecnológica: PICT-2017-0079.
Data Availability Statement
The data used in this study will be provided on request to the corresponding author.
Acknowledgments
All authors gratefully thanks the National Scientific CONICET of Argentina (Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina). Also, appreciate the support provided by the Universidad Nacional de Misiones (UNaM).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pandey P., Kumar S., Gault B. On the origin of a remarkable increase in the strength and stability of an Al rich Al-Ni eutectic alloy by Zr addition. Acta Mater 2019, 170, 205–217. [CrossRef]
- Suwanpreecha C., Pandee P., Patakham U., Limmaneevichitr C. New generation of eutectic Al-Ni casting alloys for elevated temperature services. Mater Sci Eng A 2018, 709, 46–54. [CrossRef]
- Fan Y., Makhlouf M.M. Castable aluminium alloys for high temperature applications. Mater Sci Forum 2013, 765, 8–12.
- Galenko P.K., Toropova L. V., Alexandrov D. V., Phanikumar G., Assadi H., Reinartz M., Paul P., Fang Y., Lippmann S. Anomalous kinetics, patterns formation in recalescence, and final microstructure of rapidly solidified Al-rich Al-Ni alloys. Acta Mater 2022, 241, 118384. [CrossRef]
- Zhang S., Li Y., Frederick A., Wang Y., Wang Y., Allard Jr., L., Koehler M., Shin S., Hu A., Feng Z. In-situ formation of Al3Ni nano particles in synthesis of Al 7075 alloy by friction stir processing with Ni powder addition. J Mater Process Technol 2023, 311, 117803. [CrossRef]
- Cosan K.A., Gunduz K.O., Tarakcı M., Gencer Y. Plasma electrolytic oxidation of as-cast and heat-treated binary Al-Ni alloys. Surf Coatings Technol 2022, 450, 128998. [CrossRef]
- Ding R., Deng J., Liu X., Wu Y., Geng Z., Li D., Zhang T., Chen C., Zhou K. Enhanced mechanical properties and thermal stability in additively manufactured Al-Ni alloy by Sc addition. J Alloys Compd 2023, 934, 167894. [CrossRef]
- Morando C., Fornaro O. Morphology and phase formation during the solidification of Al-Cu-Si and Al-Ag-Cu ternary eutectic system. Mater Res 2018, 21, 1–11.
- Wen Z., Zhao Y., Hou H., Tian J., Han P. First-principles study of Ni-Al intermetallic compounds under various temperature and pressure. Superlattices Microstruct 2017, 103, 9–18. [CrossRef]
- Babilas R., Młynarek K., Łoński W., Lis M, Łukowiec D., Kądziołka-Gaweł M., Warski T., Radoń A. Analysis of thermodynamic parameters for designing quasicrystalline Al-Ni-Fe alloys with enhanced corrosion resistance. J Alloys Compd 2021, 868, 159241. [CrossRef]
- Miyajima Y., Homma T., Takenaka S., Watanabe C., Adachi H., Ishikawa K. High strength and low electrical resistivity of Al-0.1 at%Ni alloys produced by accumulative roll bonding. Mater Today Commun 2022, 33, 104587.
- Esquivel J., Murdoch H.A., Darling K.A., Gupta R.K. Excellent corrosion resistance and hardness in Al alloys by extended solid solubility and nanocrystalline structure. Mater Res Lett 2017, 6, 79–83.
- Hu S.S.Q., Ding W.L.Z., Xia M.X.M. In situ study on the growth behavior of primary Al3Ni phase in solidifying Al–Ni alloy by synchrotron radiography. Acta Metall Sin (English Lett) 2018, 31, 668–672.
- Akopyan T.K., Belov N.A., Naumova E.A., Letyagin N. V. New in-situ Al matrix composites based on Al-Ni-La eutectic. Mater Lett 2019, 245, 110–113. [CrossRef]
- Osório W.R., Peixoto L.C., Canté M. V., Garcia A. Microstructure features affecting mechanical properties and corrosion behavior of a hypoeutectic Al-Ni alloy. Mater Des 2010, 31, 4485–4489. [CrossRef]
- Osório W.R., Peixoto L.C., Canté M. V., Garcia A. Electrochemical corrosion characterization of Al-Ni alloys in a dilute sodium chloride solution. Electrochim Acta 2010, 55, 4078–4085. [CrossRef]
- Zhang Z., Akiyama E., Watanabe Y. Effect of α-Al/Al3Ni microstructure on the corrosion behaviour of Al–5.4wt % Ni alloy fabricated by equal-channel angular pressing. Corros Sci 2007, 49, 2962–72.
- Román A.S., Ibañez E.R., Méndez C.M., Pedrozo M., Kramer G.R., Zadorozne N.S., Alonso P.R., Ares A.E. Electrochemical Properties of Diluted Al-Mg Alloys With Columnar-To-Equiaxed Transition. Front Mater 2022, 9, 1–15. doi:10.3389/fmats.2022.857671.
- Roman A.S., Mendez C.M., Gervasi C.A., Rebak R.B., Ares A.E. Corrosion resistance of aluminum-copper alloys with different grain structures. J Mater Eng Perform 2020, 30, 131–144.
- Okamoto H., Schlesinger M.E., Mueller E.M. Alloy Phase Diagrams, ASM Handbook, United States, 2016, 8; pp. 108-146.
- Petzow G. Metallographic Etching. 2nd ed. United States, 2008.
- Vander Voort G. Metallography and microstructures. ASM Handbook, United States, 2004, 9; #06044G.
- Gueijman S. F., Schvezov C. E., Ares A. E. Vertical and horizontal directional solidification of Zn-Al and Zn-Ag diluted alloys. Materials Transactions 2010, 51, 1861 – 1870.
- Ares A. E., Gueijman S. F., Schvezov C. E., Semi-empirical modeling for columnar and equiaxed growth of alloys. Journal of Crystal Growth 2002, 241, 235 – 240. [CrossRef]
- Ares A. E., Schvezov C. E. Influence of solidification thermal parameters on the columnar-to-equiaxed transition of aluminum-zinc and zinc-aluminum alloys. Metallurgical and Materials Transactions A 2007, 38, 1485 – 1499.
- Ares A. E., Schvezov C. E. Solidification parameters during the columnar-to-equiaxed transition in lead-tin alloys. Metallurgical and Materials Transactions A 2000, 31, 1611 – 1625.
- Kociubzyk A. I., Rozicki, R.S., Ares A. E. Movement of the interphases during the horizontal solidification of tin-zinc alloys. Revista Materia 2018, 23, e-11993. [CrossRef]
- Ares A. E., Gassa L. M., Corrosion susceptibility of Zn–Al alloys with different grains and dendritic microstructures in Nacl solutions. Corros Sci 2012, 59, 290–306.
- ASTM E 112 – 96e3. Standard Test Methods for Determining Average Grain Size, ASTM, United States, 2004.
- Kaya H., Böyük U., Çadırlı E., Maras N. Measurements of the microhardness , electrical and thermal properties of the Al – Ni eutectic alloy 2012, 34, 707–12. [CrossRef]
- ASTM G5-14. Standard Reference Test Method for Making Potentiodynamic Anodic Polarization Measurements, ASTM, United States, 2021.
- Martínez Villalobos M.A., Figueroa I.A., Suarez M.A., Angel G., Rodríguez L., Peralta O.N., González Reyes G., López I. A., Verduzco Martínez J., Trujillo C.D., Microstructural Evolution of Rapid Solidified Al-Ni Alloys 2016, 60, 67–72.
- Zaid B., Saidi D., Benzaid A., Hadji S. Effects of pH and chloride concentration on pitting corrosion of AA6061 aluminum alloy. Corros Sci 2008, 50, 1841–1847. [CrossRef]
- Ralston K.D., Fabijanic D., Birbilis N. Effect of grain size on corrosion of high purity aluminium. Electrochim Acta 2011, 56, 1729–1736. [CrossRef]
- Frankel G.S. Electrochemical Techniques in Corrosion: Status, Limitations, and Needs. Electrochem Tech Corros Status, Limitations, Needs 2008, 5, 1–27.
- Soltis J. Passivity breakdown, pit initiation and propagation of pits in metallic materials. Corros Sci 2015, 90, 5–22.
- Natishan P.M., O’Grady W.E. Chloride Ion Interactions with Oxide-Covered Aluminum Leading to Pitting Corrosion: A Review. J Electrochem Soc 2014, 161, C421–432.
- Kelly R.G., Scully J.R., Shoesmith D.W., Buchheit R.G. Electrochemical Techniques in Corrosion Science and Engineering. Routledge, Taylor and Francis Group, 2003. [CrossRef]
- Ma Y., Liu Y., Wang M. Microstructures and corrosion resistances of hypoeutectic Al-6.5Si-0.45Mg casting alloy with addition of Sc and Zr. Mater Chem Phys 2022, 276, 125321. [CrossRef]
- Osório W.R., Spinelli J.E., Afonso C.R.M., Peixoto L.C., Garcia A. Electrochemical corrosion behavior of gas atomized Al-Ni alloy powders. Electrochim Acta 2012, 69, 371–8. [CrossRef]
Figure 1.
(a) Cooling curves of (a) Al-1wt. %Ni, (b) Al-3wt. %Ni, (c) Al-5.7wt. %Ni and (d) Al-8wt. %Ni alloys.
Figure 1.
(a) Cooling curves of (a) Al-1wt. %Ni, (b) Al-3wt. %Ni, (c) Al-5.7wt. %Ni and (d) Al-8wt. %Ni alloys.
Figure 2.
(a) Thermal gradients indicating the position of the CET zone. b) Macrostructure and c) Microstructures corresponding to columnar and equiaxed zones of the samples. Al-3wt. %Ni.
Figure 2.
(a) Thermal gradients indicating the position of the CET zone. b) Macrostructure and c) Microstructures corresponding to columnar and equiaxed zones of the samples. Al-3wt. %Ni.
Figure 3.
Cooling rates, Ve, versus position for Al-Ni alloys.
Figure 3.
Cooling rates, Ve, versus position for Al-Ni alloys.
Figure 4.
(a) Position of CET (mm) versus cooling rate (Ve). (b) Width of columnar grains (mm) versus cooling rate (° C/min).
Figure 4.
(a) Position of CET (mm) versus cooling rate (Ve). (b) Width of columnar grains (mm) versus cooling rate (° C/min).
Figure 5.
Average diameter of the equiaxed grains, DGe, depending on the position, from the base of the sample.
Figure 5.
Average diameter of the equiaxed grains, DGe, depending on the position, from the base of the sample.
Figure 6.
Chemical composition analysis of Al-Ni alloys using SEM -EDX: (a) Al-3wt. %Ni. (b) Al-8wt.%Ni.
Figure 6.
Chemical composition analysis of Al-Ni alloys using SEM -EDX: (a) Al-3wt. %Ni. (b) Al-8wt.%Ni.
Figure 7.
Micrographs of the Al-Ni alloys: (a) Al-1wt. % Ni, (b) Al-3wt. % Ni, (c) Al-5.7wt. % Ni, (d) Al-8wt. % Ni.
Figure 7.
Micrographs of the Al-Ni alloys: (a) Al-1wt. % Ni, (b) Al-3wt. % Ni, (c) Al-5.7wt. % Ni, (d) Al-8wt. % Ni.
Figure 8.
Microhardness, HV, values of the different phases of Al-Ni alloys (Al-3wt. %Ni and Al-8wt. %Ni).
Figure 8.
Microhardness, HV, values of the different phases of Al-Ni alloys (Al-3wt. %Ni and Al-8wt. %Ni).
Figure 9.
Variation of microhardness, HV, depending on the different positions with respect to the base of the sample.
Figure 9.
Variation of microhardness, HV, depending on the different positions with respect to the base of the sample.
Figure 10.
Variation of secondary dendritic spacing, λ2, and lamellar spacing, S2, with: (a) and (c) cooling rate, Ve, and (b) and (d) Position.
Figure 10.
Variation of secondary dendritic spacing, λ2, and lamellar spacing, S2, with: (a) and (c) cooling rate, Ve, and (b) and (d) Position.
Figure 11.
(a) Variation of microhardness, HV, as a function of: (a) λ2 and (b) S2.
Figure 11.
(a) Variation of microhardness, HV, as a function of: (a) λ2 and (b) S2.
Figure 12.
Cyclic potentiodynamic curves of Al-Ni alloys in 0.5M NaCl solution: a) Columnar and b) Equiaxed zones.
Figure 12.
Cyclic potentiodynamic curves of Al-Ni alloys in 0.5M NaCl solution: a) Columnar and b) Equiaxed zones.
Figure 13.
EDS mapping of Al-Ni alloys after corrosion tests: (a) Al-1wt. %Ni (b) Al-3wt. %Ni (c) Al-5.7wt. %Ni and (d) Al-8wt. %Ni.
Figure 13.
EDS mapping of Al-Ni alloys after corrosion tests: (a) Al-1wt. %Ni (b) Al-3wt. %Ni (c) Al-5.7wt. %Ni and (d) Al-8wt. %Ni.
Figure 14.
Impedance Spectra for Al-Ni alloys in 0.5M NaCl solution. a) Nyquist diagram for the columnar zone, b) Nyquist diagram for the equiaxial zone, c) Bode diagram for the columnar zone, d) Bode diagram for the equiaxial zone.
Figure 14.
Impedance Spectra for Al-Ni alloys in 0.5M NaCl solution. a) Nyquist diagram for the columnar zone, b) Nyquist diagram for the equiaxial zone, c) Bode diagram for the columnar zone, d) Bode diagram for the equiaxial zone.
Figure 14.
Equivalent circuit used to fit the experimental data.
Figure 14.
Equivalent circuit used to fit the experimental data.
Figure 15.
Polarization resistances, Rp, for Al-Ni alloys with columnar and equiaxed structures, as a function of compositions.
Figure 15.
Polarization resistances, Rp, for Al-Ni alloys with columnar and equiaxed structures, as a function of compositions.
Figure 16.
Polarization resistance, Rp, versus microhardness, HV, for both types of grain structures (columnar and equiaxed) of Al-Ni alloys.
Figure 16.
Polarization resistance, Rp, versus microhardness, HV, for both types of grain structures (columnar and equiaxed) of Al-Ni alloys.
Table 1.
Values of thermal parameters obtained for different Al-Ni alloys.
Table 1.
Values of thermal parameters obtained for different Al-Ni alloys.
| Alloy |
Ve Liq.
(° C/min) |
VS
(mm/s) |
Gc (°C/cm) |
Solidification time
(s) |
CETMin
(mm) |
CETMax
(mm) |
Columnar grain width (mm) |
DGe
(mm)
|
| Al-1wt. %Ni |
16.20 |
1.25 |
1.9 |
743 |
65 |
75 |
2 |
1.45 |
| Al-3wt. %Ni |
10.26 |
0.79 |
1.3 |
814 |
50 |
60 |
3 |
1.60 |
| Al-5.7wt. %Ni |
5.04 |
0.67 |
2.9 |
905 |
45 |
55 |
5.5 |
6.86 |
| Al-8wt. %Ni |
6.52 |
1,18 |
2.5 |
864 |
43 |
52 |
4.6 |
1.99 |
Table 2.
Corrosion potential, Ecorr, of Al-Ni alloys in 0.5M NaCl.
Table 2.
Corrosion potential, Ecorr, of Al-Ni alloys in 0.5M NaCl.
| |
Ecorr, of Al-Ni alloys in 0.5M NaCl |
| |
(mV) |
| |
Al-1wt.% Ni |
Al-3wt.% Ni |
Al-5.7wt.% Ni |
Al-8wt.% Ni |
| Columnar zone |
-722 |
-721 |
-698 |
-707 |
| Equiaxed zone |
-726 |
-725 |
-701 |
-710 |
Table 3.
Electrochemical impedance spectroscopy fitting parameters.
Table 3.
Electrochemical impedance spectroscopy fitting parameters.
| |
|
RΩ
|
Rp
|
CPE |
n |
| |
|
Ω*cm2
|
Ω*cm2
|
Ω−1s-n*cm−2
|
|
| Al-1wt. % Ni |
Columnar zone |
3.60 |
2.47E+03 |
4.32E-05 |
0.81 |
| Equiaxed zone |
3.00 |
1.21E+04 |
5.45E-05 |
0.79 |
| Al-3wt. % Ni |
Columnar zone |
0.39 |
3.08E+02 |
9.40E-04 |
0.79 |
| Equiaxed zone |
3.56 |
6.08E+03 |
7.87E-05 |
0.74 |
| Al-5.7wt. % Ni |
Columnar zone |
2.67 |
1.58E+02 |
6.94E-04 |
0.70 |
| Equiaxed zone |
3.20 |
8.78E+02 |
1.55E-04 |
0.77 |
| Al-8wt. % Ni |
Columnar zone |
4.62 |
1.28E+03 |
5.43E-05 |
0.85 |
| Equiaxed zone |
1.26 |
4.68E+02 |
1.57E-04 |
0.82 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).