Submitted:
10 December 2023
Posted:
11 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study participants
2.3. Patient survey
2.2. MRI data acquisition
- − Magnetization-transfer-weighted: TR = 20 ms, echo time (TE) = 4.76 ms, flip angle (FA) = 8°, scan time 5 min 40 s;
- − T1-weighted: TR =16 ms, TE = 4.76 ms, FA =18°, scan time 4 min 32 s;
- In addition, the following sequences were included in the protocol:
- − 3D FLAIR: TR = 5000 ms, TE = 390 ms, TI = 1800 ms;
- − 3D T1-weighted: TR = 16 ms, TE = 4.76 ms;
- − 3D T2-weighted: TR=3000ms, TE=335ms.
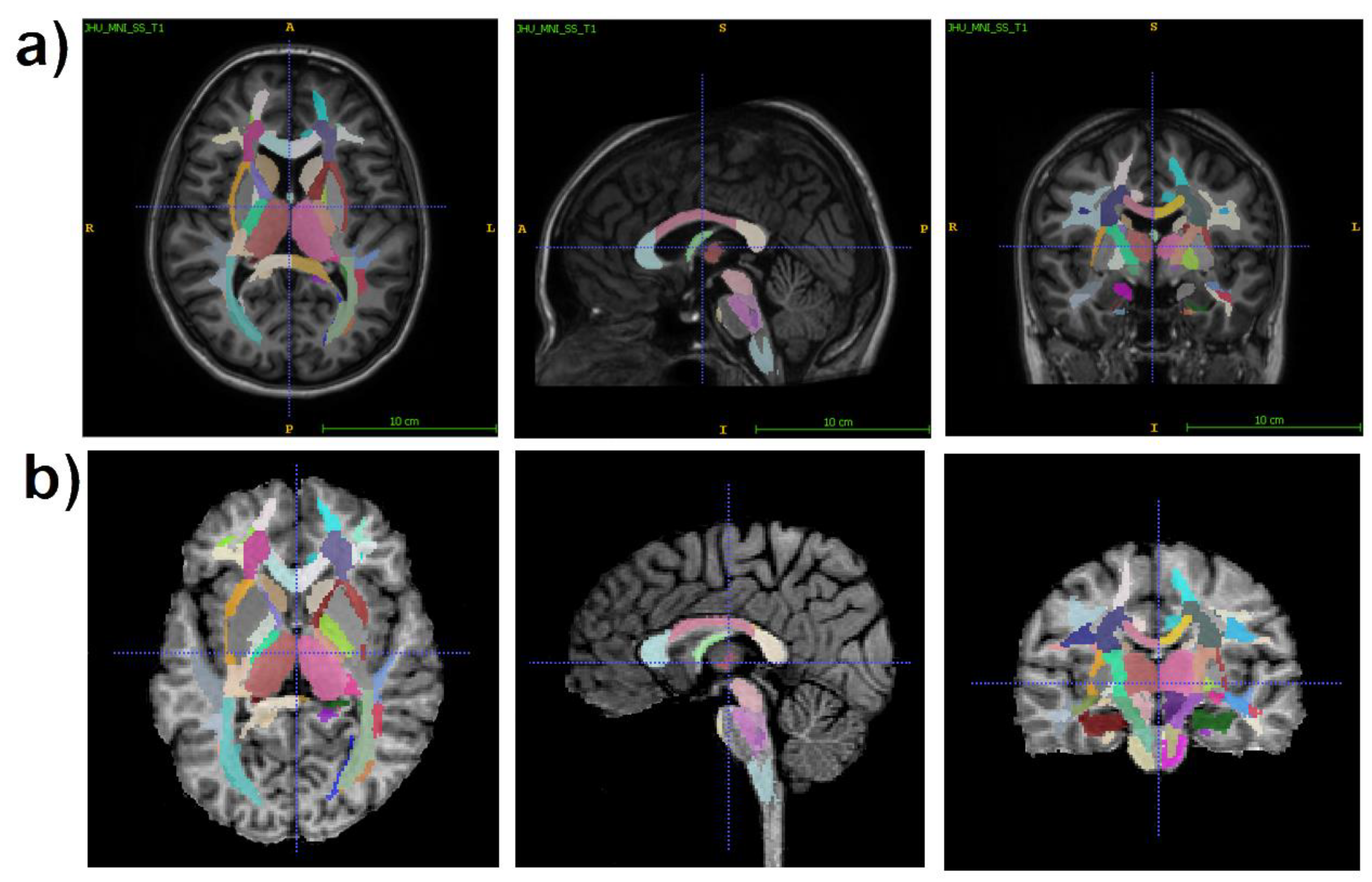
2.3. Image processing
- 1)
- juxtacortical (superficial) WM: superior parietal, superior, middle, and inferior frontal; precentral; postcentral; angular; pre-cuneus; cuneus; lingual; fusiform; superior, inferior, and middle occipital; superior, inferior, and middle temporal; lateral and middle fronto-orbital, supramarginal, rectus, cingulum (parts of cingulate gyrus and hippocampus);
- 2)
- WM pathways and fasciculi: corticospinal tract (CST); medial lemniscus; anterior limb, posterior limb, and retrolenticular part of internal capsule (IC); inferior, superior, and middle cerebellar peduncles (CP); cerebral peduncles; posterior thalamic radiation; anterior, superior, and posterior corona radiata (CR); fornix (FX) (stria terminalis, column and body); superior longitudinal (SL) fasciculus; superior (SFO) and inferior fronto-occipital (IFO) fasciculi; uncinate fasciculus; sagittal stratum; external capsule; pontine crossing tract; genu, body, and splenium of corpus callosum (CC); tapetum;
- 3)
- subcortical and allocortical GM structures: amygdala; hippocampus; entorhinal area; caudate nucleus; putamen; globus pallidus; thalamus;
- 4)
- brainstem structures: midbrain; pons; medulla.
2.5. Statistical analysis
3. Results
3.1. Acute and post-COVID symptoms
3.2. Neuropsychological results
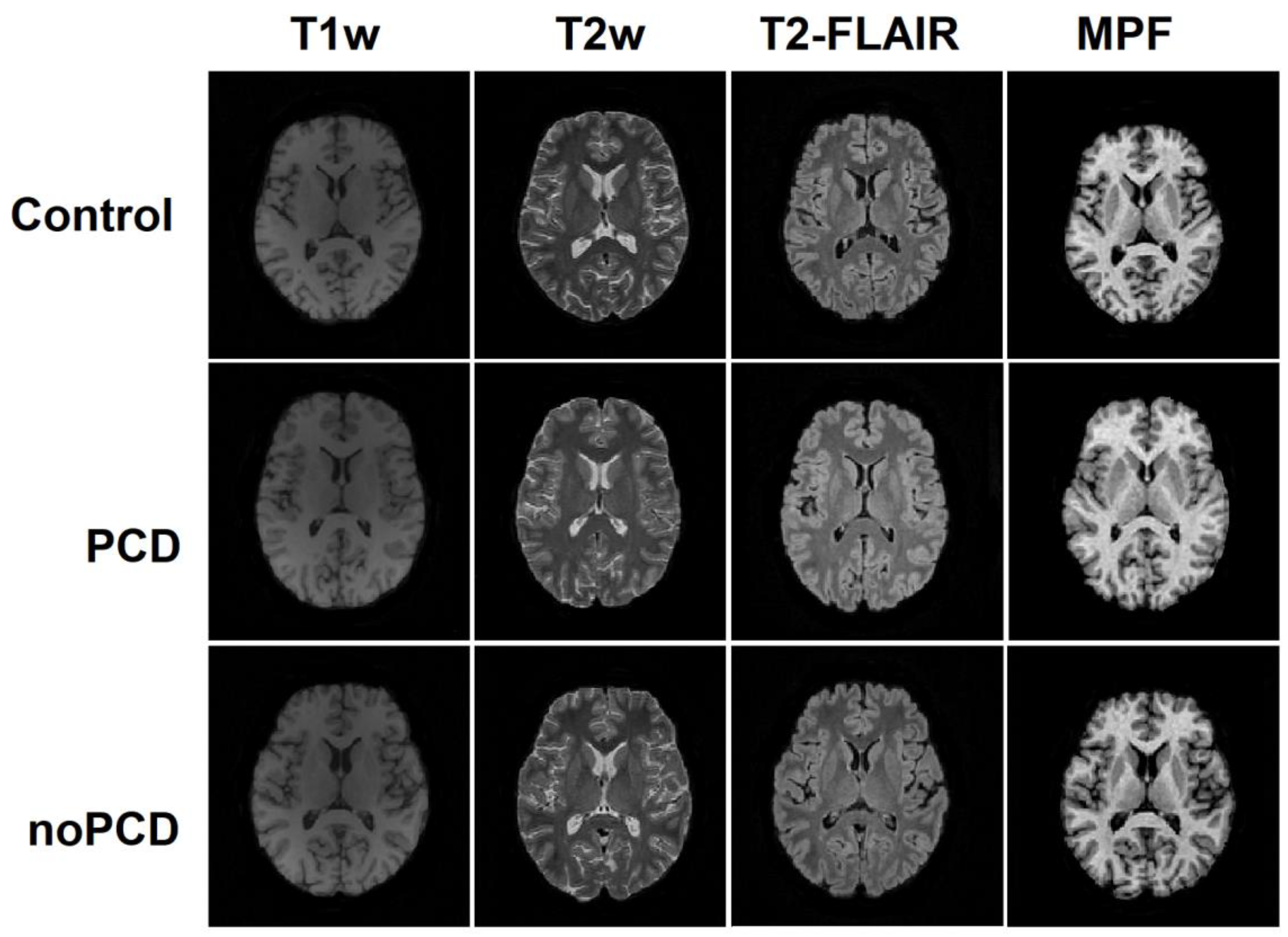
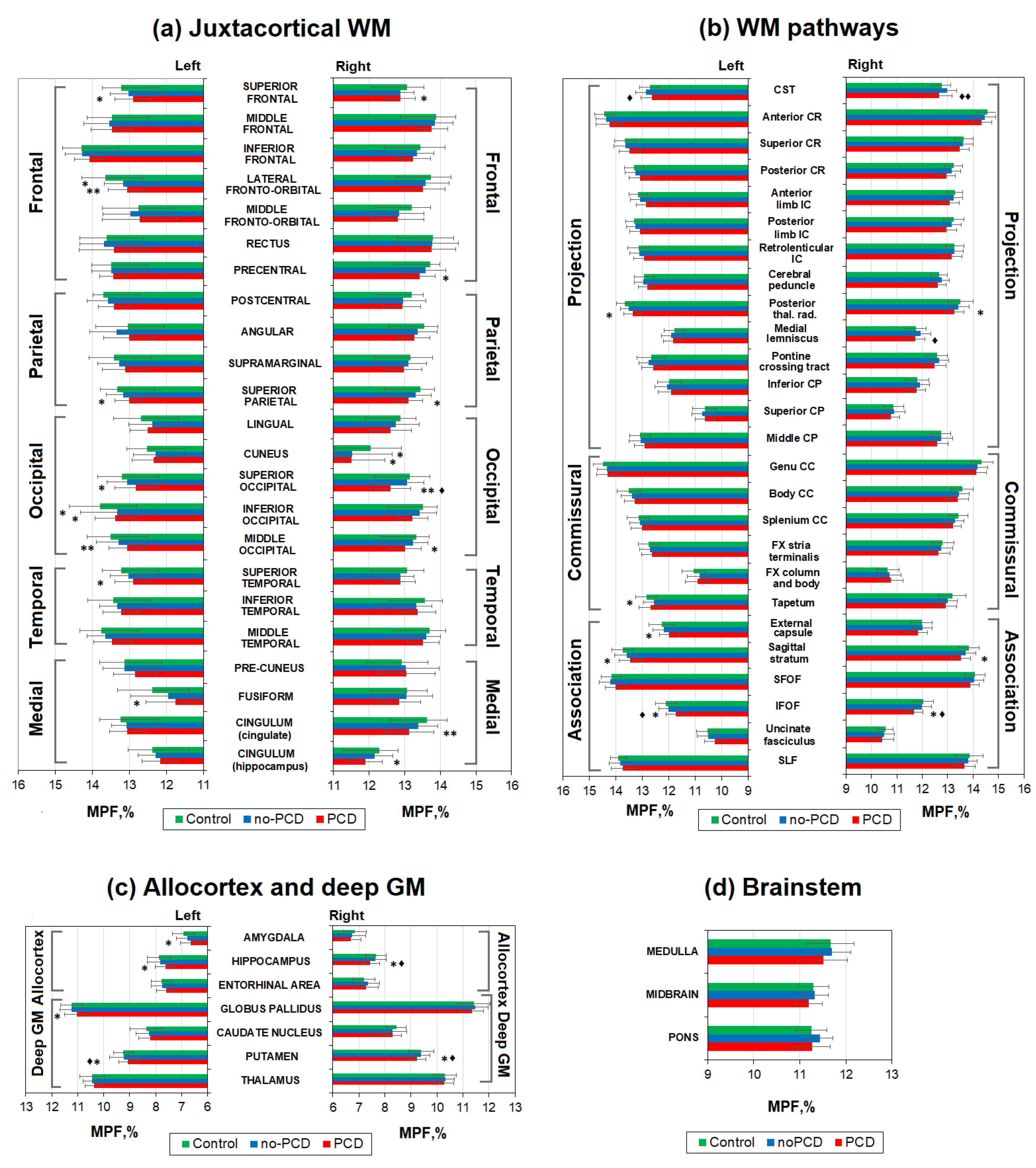
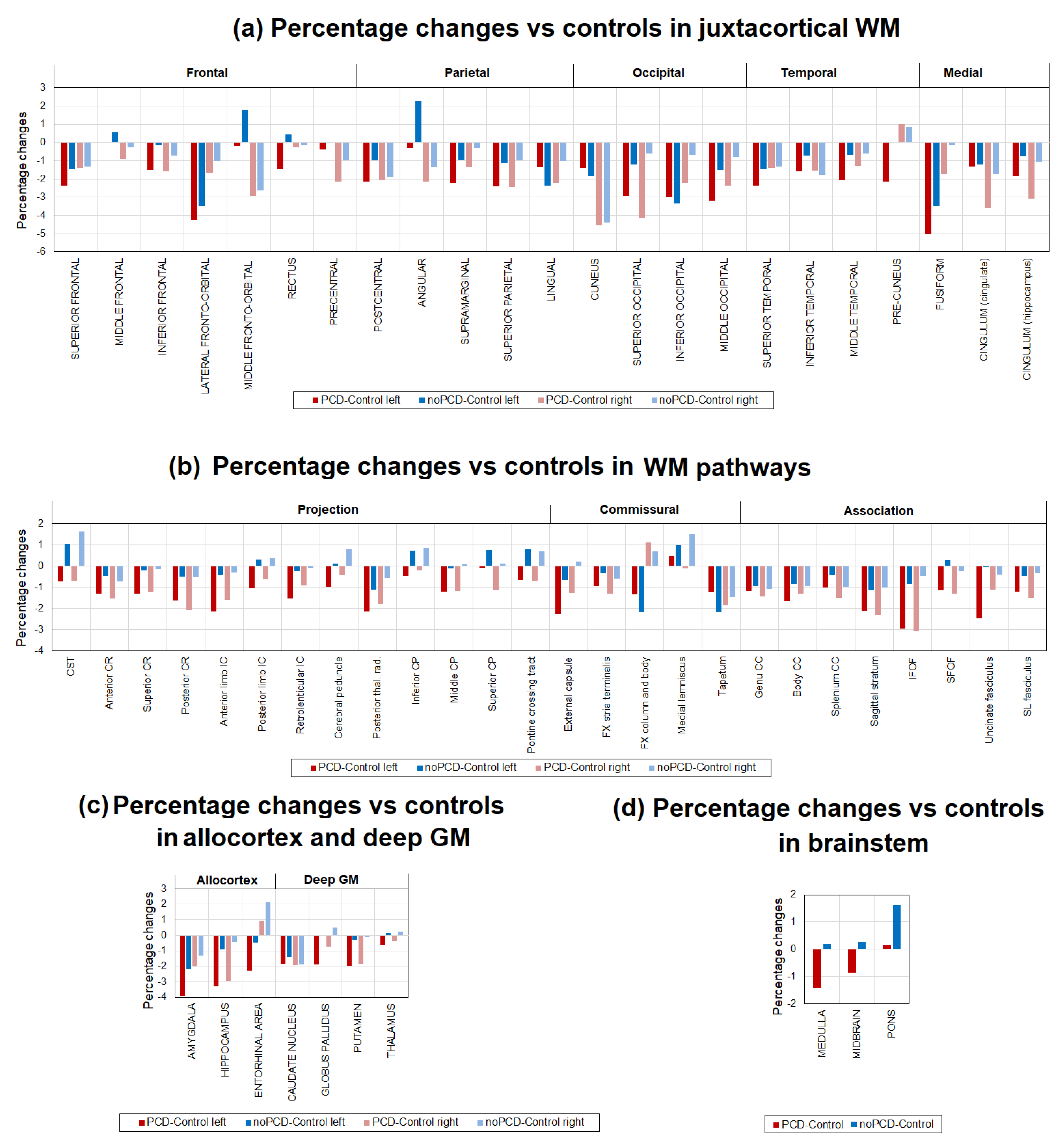
3.3. Brain demyelination in the patients with post-COVID depression
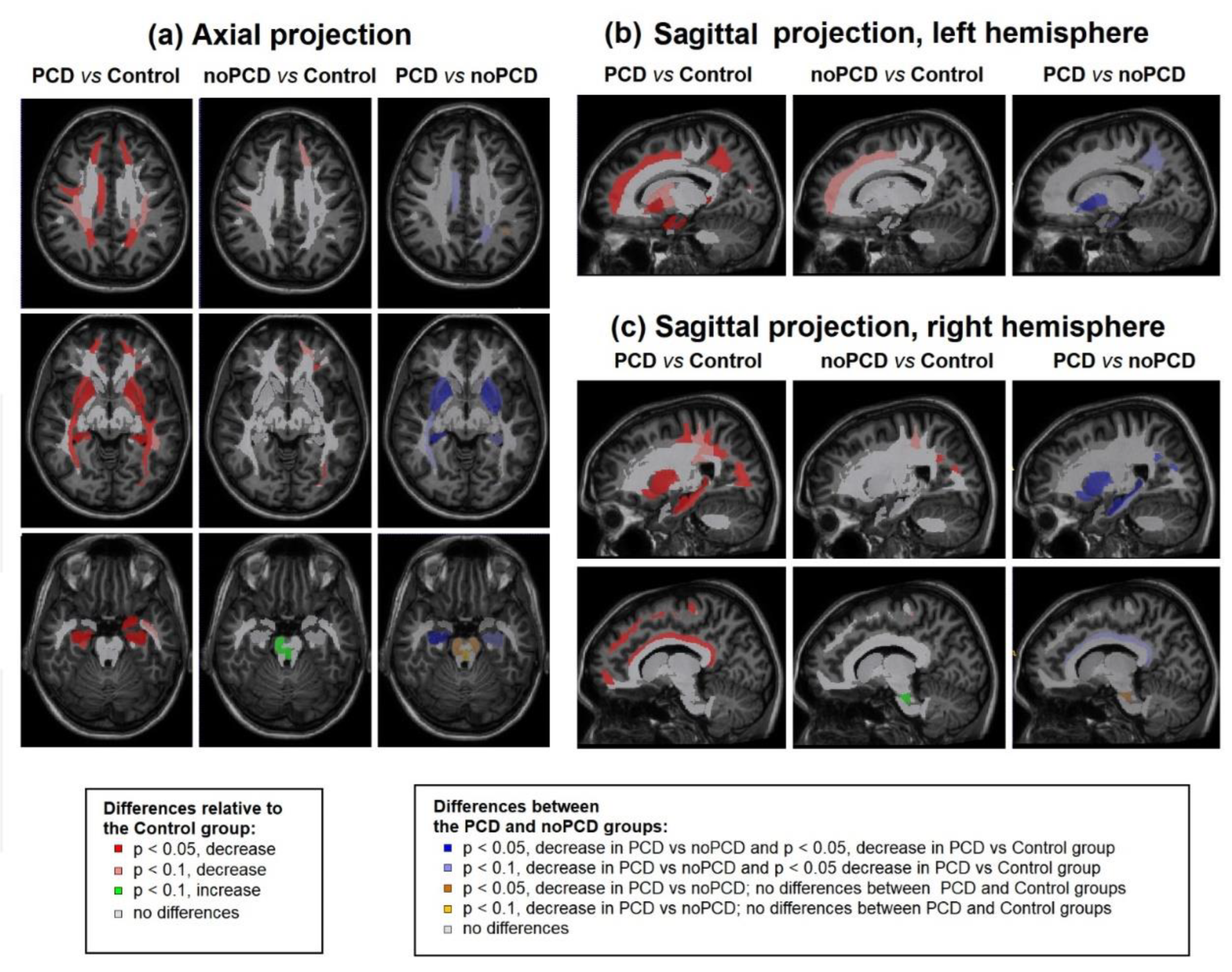
3.4. Specifiсity of demyelination in the patients with post-COVID depression
4. Discussion
5. Conclusions
6. Study limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M. V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-Acute COVID-19 Syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Nasserie, T.; Hittle, M.; Goodman, S.N. Assessment of the Frequency and Variety of Persistent Symptoms among Patients with COVID-19: A Systematic Review. JAMA Netw. Open 2021, 4, 1–19. [Google Scholar] [CrossRef]
- Huang, Y.; Pinto, M.D.; Borelli, J.L.; Mehrabadi, M.A.; Dutt, N.; Lambert, N.; Nurmi, E.L.; Chakraborty, R.; Amir, M. COVID Symptoms, Symptom Clusters, and Predictors for Becoming a Long-Hauler: Looking for Clarity in the Haze of the Pandemic. Clin. Nurs. Res. 2022, 31, 1390–1398. [Google Scholar] [CrossRef]
- de Erausquin, G.A.; Snyder, H.; Carrillo, M.; Hosseini, A.A.; Brugha, T.S.; Seshadri, S. The Chronic Neuropsychiatric Sequelae of COVID-19: The Need for a Prospective Study of Viral Impact on Brain Functioning. Alzheimer’s Dement. 2021, 17, 1056–1065. [Google Scholar] [CrossRef]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent Neurologic Symptoms and Cognitive Dysfunction in Non-Hospitalized Covid-19 “Long Haulers. ” Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.; Kamaeva, D.A.; Naumova, A. V. Role of Demyelination in the Persistence of Neurological and Mental Impairments after COVID-19. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Varatharaj, A.; Thomas, N.; Ellul, M.A.; Davies, N.W.S.; Pollak, T.A.; Tenorio, E.L.; Sultan, M.; Easton, A.; Breen, G.; Zandi, M.; et al. Neurological and Neuropsychiatric Complications of COVID-19 in 153 Patients: A UK-Wide Surveillance Study. The Lancet Psychiatry 2020, 7, 875–882. [Google Scholar] [CrossRef]
- Miskowiak, K.W.; Johnsen, S.; Sattler, S.M.; Nielsen, S.; Kunalan, K.; Rungby, J.; Lapperre, T.; Porsberg, C.M. Cognitive Impairments Four Months after COVID-19 Hospital Discharge: Pattern, Severity and Association with Illness Variables. Eur. Neuropsychopharmacol. 2021, 46, 39–48. [Google Scholar] [CrossRef]
- Daroische, R.; Hemminghyth, M.S.; Eilertsen, T.H.; Breitve, M.H.; Chwiszczuk, L.J. Cognitive Impairment After COVID-19—A Review on Objective Test Data. Front. Neurol. 2021, 12. [Google Scholar] [CrossRef]
- Woo, M.S.; Malsy, J.; Pöttgen, J.; Seddiq Zai, S.; Ufer, F.; Hadjilaou, A.; Schmiedel, S.; Addo, M.M.; Gerloff, C.; Heesen, C.; et al. Frequent Neurocognitive Deficits after Recovery from Mild COVID-19. Brain Commun. 2020, 2, 1–9. [Google Scholar] [CrossRef]
- Stefanou, M.I.; Palaiodimou, L.; Bakola, E.; Smyrnis, N.; Papadopoulou, M.; Paraskevas, G.P.; Rizos, E.; Boutati, E.; Grigoriadis, N.; Krogias, C.; et al. Neurological Manifestations of Long-COVID Syndrome: A Narrative Review. Ther. Adv. Chronic Dis. 2022, 13, 1–21. [Google Scholar] [CrossRef]
- Peterson, C.J.; Sarangi, A.; Bangash, F. Neurological Sequelae of COVID-19: A Review. Egypt. J. Neurol. Psychiatry Neurosurg. 2021, 57. [Google Scholar] [CrossRef]
- Efstathiou, V.; Stefanou, M.-I.; Demetriou, M.; Siafakas, N.; Makris, M.; Tsivgoulis, G.; Zoumpourlis, V.; Kympouropoulos, S.; Tsoporis, J.; Spandidos, D.; et al. Long COVID and Neuropsychiatric Manifestations (Review). Exp. Ther. Med. 2022, 23, 1–12. [Google Scholar] [CrossRef]
- Bhola, S.; Trisal, J.; Thakur, V.; Kaur, P.; Kulshrestha, S.; Bhatia, S.K.; Kumar, P. Neurological Toll of COVID-19. Neurol. Sci. 2022, 43, 2171–2186. [Google Scholar] [CrossRef]
- Naphade, P.; Singh, P.; Rao, P.; Rohatgi, S.; Chaudhury, S.; Nirhale, S. Psychiatric Symptoms and Fatigue in COVID-19 Survivors. Cureus 2023, 15, 0–7. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Al-Aly, Z. Risks of Mental Health Outcomes in People with Covid-19: Cohort Study. BMJ 2022, 376, 1–13. [Google Scholar] [CrossRef]
- Simonetti, A.; Bernardi, E.; Margoni, S.; Catinari, A.; Restaino, A.; Ieritano, V.; Palazzetti, M.; Mastrantonio, F.; Janiri, D.; Tosato, M.; et al. Mixed Depression in the Post-COVID-19 Syndrome: Correlation between Excitatory Symptoms in Depression and Physical Burden after COVID-19. Brain Sci. 2023, 13. [Google Scholar] [CrossRef]
- Lorkiewicz, P.; Waszkiewicz, N. Biomarkers of Post-COVID Depression. J. Clin. Med. 2021, 10, 4142. [Google Scholar] [CrossRef]
- Li, Z.; Ruan, M.; Chen, J.; Fang, Y. Major Depressive Disorder: Advances in Neuroscience Research and Translational Applications. Neurosci. Bull. 2021, 37, 863–880. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Braga, J.; Lepra, M.; Kish, S.J.; Rusjan, P.M.; Nasser, Z.; Verhoeff, N.; Vasdev, N.; Bagby, M.; Boileau, I.; Husain, M.I.; et al. Neuroinflammation after COVID-19 with Persistent Depressive and Cognitive Symptoms. JAMA Psychiatry 2023, 80, 787–795. [Google Scholar] [CrossRef]
- Sriwastava, S.; Tandon, M.; Podury, S.; Prasad, A.; Wen, S.; Guthrie, G.; Kakara, M.; Jaiswal, S.; Subedi, R.; Elkhooly, M.; et al. COVID-19 and Neuroinflammation: A Literature Review of Relevant Neuroimaging and CSF Markers in Central Nervous System Inflammatory Disorders from SARS-COV2. J. Neurol. 2021, 268, 4448–4478. [Google Scholar] [CrossRef]
- Chapman, T.W.; Hill, R.A. Myelin Plasticity in Adulthood and Aging. Neurosci. Lett. 2020, 715, 134645. [Google Scholar] [CrossRef]
- Sams, E.C. Oligodendrocytes in the Aging Brain. Neuronal Signal. 2021, 5, 1–24. [Google Scholar] [CrossRef]
- Kohama, S.G.; Rosene, D.L.; Sherman, L.S. Age-Related Changes in Human and Non-Human Primate White Matter: From Myelination Disturbances to Cognitive Decline. Age (Omaha). 2012, 34, 1093–1110. [Google Scholar] [CrossRef]
- Faro, S.H.; Manmatharayan, A.; Leiby, B.; Jain, N.; Mohamed, F.B.; Talekar, K.S.; Doshi, A.; Jambor, I.; Chang, S.; Finkelstein, M.; et al. Neuroimaging Findings in 4342 Hospitalized COVID-19 Subjects: A Multicenter Report from the United States and Europe. J. Neuroimaging 2023, 11–14. [Google Scholar] [CrossRef]
- Erdoğan, T.; Koçer, B.; Şen, S.; Petek Balci, B.; Terzi, M. Newly Diagnosed Tumefactive Demyelinating Lesion and Multiple Sclerosis After COVID-19 Infection. Noropsikiyatri Ars. 2023, 60, 223–230. [Google Scholar] [CrossRef]
- Bezold, A.; Hussain, H.; Nwanze, J.; Roberts, J.T.; Hsieh, K.J. Radiologic-Pathologic Correlation of COVID-19-Associated Acute Disseminated Encephalomyelitis. Cureus 2023, 15. [Google Scholar] [CrossRef]
- Sharma, V.; Chhabra, T.; Singh, T.G. Correlation of Covid-19 and Guillain-Barré Syndrome: A Mechanistic Perspective. Obes. Med. 2023, 40. [Google Scholar] [CrossRef]
- Malekpour, M.; Khanmohammadi, S.; Meybodi, M.J.E.; Shekouh, D.; Rahmanian, M.R.; Kardeh, S.; Azarpira, N. COVID-19 as a Trigger of Guillain-Barré Syndrome: A Review of the Molecular Mechanism. Immunity, Inflamm. Dis. 2023, 11. [Google Scholar] [CrossRef]
- Sklinda, K.; Dorobek, M.; Wasilewski, P.G.; Dreżewski, K.; Dȩbicka, M.; Walecki, J.; Mruk, B. Radiological Manifestation of Neurological Complications in the Course of SARS-CoV-2 Infection. Front. Neurol. 2021, 12. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Mishra, H.P.; Goel, A.; Gupta, A. COVID-19 – a Potential Trigger for MOGAD-Associated Optic Neuritis: A Case Report and Literature Review. Ther. Adv. Ophthalmol. 2023, 15, 1–9. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, X.; Zhao, W.; Du, Y.; Yang, D.; Huang, Y.; Chen, Y.; Zhang, H.; Yang, G.; Liu, J.; et al. Dynamic White Matter Changes in Recovered COVID-19 Patients: A Two-Year Follow-up Study. Theranostics 2023, 13, 724–735. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, Z.; Yang, D.; Zhao, W.; Zeng, M.; Xie, X.; Du, Y.; Jiang, Y.; Zhou, X.; Yang, W.; et al. Persistent White Matter Changes in Recovered COVID-19 Patients at the 1-Year Follow-Up. Brain 2022, 145, 1830–1838. [Google Scholar] [CrossRef]
- Bispo, D.D. de C.; Brandão, P.R. de P.; Pereira, D.A.; Maluf, F.B.; Dias, B.A.; Paranhos, H.R.; von Glehn, F.; de Oliveira, A.C.P.; Regattieri, N.A.T.; Silva, L.S.; et al. Brain Microstructural Changes and Fatigue after COVID-19. Front. Neurol. 2022, 13. [Google Scholar] [CrossRef]
- Benedetti, F.; Palladini, M.; Paolini, M.; Melloni, E.; Vai, B.; De Lorenzo, R.; Furlan, R.; Rovere-Querini, P.; Falini, A.; Mazza, M.G. Brain Correlates of Depression, Post-Traumatic Distress, and Inflammatory Biomarkers in COVID-19 Survivors: A Multimodal Magnetic Resonance Imaging Study. Brain, Behav. Immun. - Heal. 2021, 18, 100387. [Google Scholar] [CrossRef]
- Qin, Y.; Wu, J.; Chen, T.; Li, J.; Zhang, G.; Wu, D.; Zhou, Y.; Zheng, N.; Cai, A.; Ning, Q.; et al. Long-Term Microstructure and Cerebral Blood Flow Changes in Patients Recovered from COVID-19 without Neurological Manifestations. J. Clin. Invest. 2021, 131. [Google Scholar] [CrossRef]
- Tian, T.; Wu, J.; Chen, T.; Li, J.; Yan, S.; Zhou, Y.; Peng, X.; Li, Y.; Zheng, N.; Cai, A.; et al. Long-Term Follow-up of Dynamic Brain Changes in Patients Recovered from COVID-19 without Neurological Manifestations. JCI Insight 2022, 7. [Google Scholar] [CrossRef] [PubMed]
- Boito, D.; Eklund, A.; Tisell, A.; Levi, R.; Özarslan, E.; Blystad, I. MRI with Generalized Diffusion Encoding Reveals Damaged White Matter in Patients Previously Hospitalized for COVID-19 and with Persisting Symptoms at Follow-Up. Brain Commun. 2023, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; Palladini, M.; Poletti, S.; Benedetti, F. Post-COVID-19 Depressive Symptoms: Epidemiology, Pathophysiology, and Pharmacological Treatment. CNS Drugs 2022, 36, 681–702. [Google Scholar] [CrossRef] [PubMed]
- Wise, T.; Marwood, L.; Perkins, A.M.; Herane-Vives, A.; Joules, R.; Lythgoe, D.J.; Luh, W.M.; Williams, S.C.R.; Young, A.H.; Cleare, A.J.; et al. Instability of Default Mode Network Connectivity in Major Depression: A Two-Sample Confirmation Study. Transl. Psychiatry 2017, 7. [Google Scholar] [CrossRef]
- van Velzen, L.S.; Kelly, S.; Isaev, D.; Aleman, A.; Aftanas, L.I.; Bauer, J.; Baune, B.T.; Brak, I. V.; Carballedo, A.; Connolly, C.G.; et al. White Matter Disturbances in Major Depressive Disorder: A Coordinated Analysis across 20 International Cohorts in the ENIGMA MDD Working Group. Mol. Psychiatry 2020, 25, 1511–1525. [Google Scholar] [CrossRef]
- Hou, G.; Lai, W.; Jiang, W.; Liu, X.; Qian, L.; Zhang, Y.; Zhou, Z. Myelin Deficits in Patients with Recurrent Major Depressive Disorder: An Inhomogeneous Magnetization Transfer Study. Neurosci. Lett. 2021, 750, 135768. [Google Scholar] [CrossRef]
- Sacchet, M.D.; Gotlib, I.H. Myelination of the Brain in Major Depressive Disorder: An in Vivo Quantitative Magnetic Resonance Imaging Study. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Lai, C.H.; Wu, Y. Te The White Matter Microintegrity Alterations of Neocortical and Limbic Association Fibers in Major Depressive Disorder and Panic Disorder: The Comparison. Med. (United States) 2016, 95, 1–6. [Google Scholar] [CrossRef]
- Liao, Y.; Huang, X.; Wu, Q.; Yang, C.; Kuang, W.; Du, M.; Lui, S.; Yue, Q.; Chan, R.C.K.; Kemp, G.J.; et al. Is Depression a Disconnection Syndrome? Meta- Analysis of Diffusion Tensor Imaging Studies in Patients with MDD. J. Psychiatry Neurosci. 2013, 38, 49–56. [Google Scholar] [CrossRef]
- Coloigner, J.; Batail, J.M.; Commowick, O.; Corouge, I.; Robert, G.; Barillot, C.; Drapier, D. White Matter Abnormalities in Depression: A Categorical and Phenotypic Diffusion MRI Study. NeuroImage Clin. 2019, 22. [Google Scholar] [CrossRef]
- Trujillo, P.; Summers, P.E.; Ferrari, E.; Zucca, F.A.; Sturini, M.; Mainardi, L.T.; Cerutti, S.; Smith, A.K.; Smith, S.A.; Zecca, L.; et al. Contrast Mechanisms Associated with Neuromelanin-MRI. Magn. Reson. Med. 2017, 78, 1790–1800. [Google Scholar] [CrossRef]
- Li, K.; Li, H.; Zhang, X.Y.; Stokes, A.M.; Jiang, X.; Kang, H.; Quarles, C.C.; Zu, Z.; Gochberg, D.F.; Gore, J.C.; et al. Influence of Water Compartmentation and Heterogeneous Relaxation on Quantitative Magnetization Transfer Imaging in Rodent Brain Tumors. Magn. Reson. Med. 2016, 76, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Yarnykh, V.L.; Krutenkova, E.P.; Aitmagambetova, G.; Henson, L.K.J.; Piedmont, H.; Repovic, P.; Mayadev, A.; Qian, P.; Gangadharan, B. Iron-Insensitive Quantitative Assessment of Subcortical Gray Matter Demyelination in Multiple Sclerosis Using Macromolecular Proton Fraction. Am. J. Neuroradiol. 2018, 39, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Kisel, A.A.; Naumova, A. V.; Yarnykh, V.L. Macromolecular Proton Fraction as a Myelin Biomarker: Principles, Validation, and Applications. Front. Neurosci. 2022, 16, 1–10. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.Y.; Sorokina, I.V.V.; Glazacheva, V.Y.Y.; Akulov, A.E.E.; Nemirovich-Danchenko, N.M.M.; Romashchenko, A.V. V.; Tolstikova, T.G.G.; Mustafina, L.R.R.; Yarnykh, V.L.L. Histological Validation of Fast Macromolecular Proton Fraction Mapping as a Quantitative Myelin Imaging Method in the Cuprizone Demyelination Model. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.; Pishchelko, A.O.; Glazacheva, V.Y.; Pan, E.S.; Akulov, A.E.; Svetlik, M.V.; Tyumentseva, Y.A.; Anan’ina, T.V. ; Yarnykh Vasily Leonidovich Quantitative Imaging of White and Gray Matter Remyelination in the Cuprizone Demyelination Model Using the Macromolecular Proton Fraction. Cells 2019, 8, 1204. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.; Gubskiy, I.L.; Kudabaeva, M.S.; Namestnikova, D.D.; Kisel, A.A.; Anan’ina, T. V.; Tumentceva, Y.A.; Mustafina, L.R.; Yarnykh, V.L. Long-Term Monitoring of Chronic Demyelination and Remyelination in a Rat Ischemic Stroke Model Using Macromolecular Proton Fraction Mapping. J. Cereb. Blood Flow Metab. 2021, 41, 2856–2869. [Google Scholar] [CrossRef]
- Khodanovich, M.Y.; Kisel, A.A.; Akulov, A.E.; Atochin, D.N.; Kudabaeva, M.S.; Glazacheva, V.Y.; Svetlik, M. V.; Medvednikova, Y.A.; Mustafina, L.R.; Yarnykh, V.L. Quantitative Assessment of Demyelination in Ischemic Stroke in Vivo Using Macromolecular Proton Fraction Mapping. J. Cereb. Blood Flow Metab. 2018, 38, 919–931. [Google Scholar] [CrossRef]
- Naumova, A.V.; Akulov, A.E.; Khodanovich, M.Y.; Yarnykh, V.L. High-Resolution Three-Dimensional Macromolecular Proton Fraction Mapping for Quantitative Neuroanatomical Imaging of the Rodent Brain in Ultra-High Magnetic Fields. Neuroimage 2017, 147, 985–993. [Google Scholar] [CrossRef]
- Anisimov, N.V.; Pavlova, O.S.; Pirogov, Y.A. Yarnykh Vasily Leonidovich Three-Dimensional Fast Single-Point Macromolecular Proton Fraction Mapping of the Human Brain at 0.5 Tesla. Quant. Imaging Med. Surg. 2020, 10, 1441–1449. [Google Scholar] [CrossRef]
- Smirnova, L.P.; Yarnykh, V.L.; Parshukova, D.A.; Kornetova, E.G.; Semke, A. V.; Usova, A. V.; Pishchelko, A.O.; Khodanovich, M.Y.; Ivanova, S.A. Global Hypomyelination of the Brain White and Gray Matter in Schizophrenia: Quantitative Imaging Using Macromolecular Proton Fraction. Transl. Psychiatry 2021, 11, 365. [Google Scholar] [CrossRef]
- Yarnykh, V.L.L.; Prihod’ko, I.Y.Y.; Savelov, A.A.A.; Korostyshevskaya, A.M.M. Quantitative Assessment of Normal Fetal Brain Myelination Using Fast Macromolecular Proton Fraction Mapping. Am. J. Neuroradiol. 2018, 39, 1341–1348. [Google Scholar] [CrossRef]
- Korostyshevskaya, A.M.M.; Prihod’ko, I.Y.Y.; Savelov, A.A.A.; Yarnykh, V.L.L. Direct Comparison between Apparent Diffusion Coefficient and Macromolecular Proton Fraction as Quantitative Biomarkers of the Human Fetal Brain Maturation. J. Magn. Reson. Imaging 2019, 50, 52–61. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snalth, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. [Revista En Internet] 2014 [Acceso 28 de Noviembre de 2019]; 64(5): 361-370. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Hamilton, M. A Rating Scale for Depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Hamilton, M. Development of a Rating Scale for Depressive Illness. Br. J. Soc. Clin. Psychol. 1967, 6, 278–296. [Google Scholar] [CrossRef]
- Chan Sui Ko, A.; Candellier, A.; Mercier, M.; Joseph, C.; Schmit, J.L.; Lanoix, J.P.; Andrejak, C. Number of Initial Symptoms Is More Related to Long COVID-19 than Acute Severity of Infection: A Prospective Cohort of Hospitalized Patients. Int. J. Infect. Dis. 2022, 118, 220–223. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Pellicer-Valero, O.J.; Navarro-Pardo, E.; Rodríguez-Jiménez, J.; Martín-Guerrero, J.D.; Cigarán-Méndezd, M. The Number of Symptoms at the Acute COVID-19 Phase Is Associated with Anxiety and Depressive Long-Term Post-COVID Symptoms: A Multicenter Study. J. Psychosom. Res. 2021, 150, 110625. [Google Scholar] [CrossRef]
- Durstenfeld, M.S.; Peluso, M.J.; Peyser, N.D.; Lin, F.; Knight, S.J.; Djibo, A.; Khatib, R.; Kitzman, H.; O’Brien, E.; Williams, N.; et al. Factors Associated with Long COVID Symptoms in an Online Cohort Study. Open Forum Infect. Dis. 2023, 10, 1–10. [Google Scholar] [CrossRef]
- Asadi-Pooya, A.A.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Nemati, H.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; Farjoud-Kouhanjani, M.; et al. Risk Factors Associated with Long Covid Syndrome: A Retrospective Study. Iran. J. Med. Sci. 2021, 46, 428–436. [Google Scholar] [CrossRef]
- Yarnykh, V.L. Time-Efficient, High-Resolution, Whole Brain Three-Dimensional Macromolecular Proton Fraction Mapping. Magn. Reson. Med. 2016, 75, 2100–2106. [Google Scholar] [CrossRef]
- Yarnykh, V.L.; Tartaglione, E. V.; Ioannou, G.N. Fast Macromolecular Proton Fraction Mapping of the Human Liver in Vivo for Quantitative Assessment of Hepatic Fibrosis. NMR Biomed. 2015, 28, 1716–1725. [Google Scholar] [CrossRef]
- Avants, B.B.; Tustison, N.J.; Song, G.; Cook, P.A.; Klein, A.; Gee, J.C. NeuroImage A Reproducible Evaluation of ANTs Similarity Metric Performance in Brain Image Registration. Neuroimage 2011, 54, 2033–2044. [Google Scholar] [CrossRef]
- Avants, B.B.; Yushkevich, P.; Pluta, J.; Minkoff, D.; Korczykowski, M.; Detre, J.; Gee, J.C. NeuroImage The Optimal Template Effect in Hippocampus Studies of Diseased Populations. Neuroimage 2010, 49, 2457–2466. [Google Scholar] [CrossRef]
- Oishi, K.; Faria, A.; Jiang, H.; Li, X.; Akhter, K.; Zhang, J.; Hsu, J.T.; Miller, M.I.; Zijl, P.C.M. Van; Albert, M.; et al. NeuroImage Atlas-Based Whole Brain White Matter Analysis Using Large Deformation Diffeomorphic Metric Mapping : Application to Normal Elderly and Alzheimer ’ s Disease Participants. Neuroimage 2009, 46, 486–499. [Google Scholar] [CrossRef]
- Khodanovich, M.; Svetlik, M.; Naumova, A.; Kamaeva, D.; Usova, A.; Kudabaeva, M.; Wasserlauf, I.; Pashkevich, V.; Moshkina, M.; Obukhovskaya, V.; et al. Age-Related Decline in Brain Myelination : Quantitative Macromolecular Proton Fraction Mapping, T2-FLAIR Hyperintensity Volume, and Anti-Myelin Antibodies 7 Years Apart. Biomedicines 2023, Preprint. [Google Scholar] [CrossRef]
- Caverzasi, E.; Papinutto, N.; Amirbekian, B.; Berger, M.S.; Henry, R.G. Q-Ball of Inferior Fronto-Occipital Fasciculus and Beyond. PLoS One 2014, 9. [Google Scholar] [CrossRef]
- Conner, A.K.; Briggs, R.G.; Sali, G.; Rahimi, M.; Baker, C.M.; Burks, J.D.; Glenn, C.A.; Battiste, J.D.; Sughrue, M.E. A Connectomic Atlas of the Human Cerebrum-Chapter 13: Tractographic Description of the Inferior Fronto-Occipital Fasciculus. Oper. Neurosurg. 2018, 15, 5436–5443. [Google Scholar] [CrossRef]
- Hausman, H.K.; Hardcastle, C.; Albizu, A.; Kraft, J.N.; Evangelista, N.D.; Boutzoukas, E.M.; Langer, K.; O’Shea, A.; Van Etten, E.J.; Bharadwaj, P.K.; et al. Cingulo-Opercular and Frontoparietal Control Network Connectivity and Executive Functioning in Older Adults. GeroScience 2022, 44, 847–866. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, D.; Wang, Y.; Wang, Y. Subcomponents and Connectivity of the Inferior Fronto-Occipital Fasciculus Revealed by Diffusion Spectrum Imaging Fiber Tracking. Front. Neuroanat. 2016, 10, 1–13. [Google Scholar] [CrossRef]
- Agosta, F.; Pievani, M.; Sala, S.; Geroldi, C.; Galluzzi, S.; Frisoni, G.B.; Filippi, M. White Matter Damage in Alzheimer Disease and Its Relationship to Gray Matter Atrophy. Radiology 2011, 258, 853–863. [Google Scholar] [CrossRef]
- Waller, R.; Dotterer, H.L.; Murray, L.; Maxwell, A.M.; Hyde, L.W. White-Matter Tract Abnormalities and Antisocial Behavior: A Systematic Review of Diffusion Tensor Imaging Studies across Development. NeuroImage Clin. 2017, 14, 201–215. [Google Scholar] [CrossRef]
- Peng, Z.W.; Lui, S.S.Y.; Cheung, E.F.C.; Jin, Z.; Miao, G.D.; Jing, J.; Chan, R.C.K. Brain Structural Abnormalities in Obsessive-Compulsive Disorder: Converging Evidence from White Matter and Grey Matter. Asian J. Psychiatr. 2012, 5, 290–296. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, J.; Yu, H.; Nie, B.; Li, N.; Luo, C.; Li, H.; Liu, F.; Bai, Y.; Shan, B.; et al. Delineation of Early and Later Adult Onset Depression by Diffusion Tensor Imaging. PLoS One 2014, 9. [Google Scholar] [CrossRef]
- Liang, S.; Wang, Q.; Kong, X.; Deng, W.; Yang, X.; Li, X.; Zhang, Z.; Zhang, J.; Zhang, C.; Li, X. min; et al. White Matter Abnormalities in Major Depression Biotypes Identified by Diffusion Tensor Imaging. Neurosci. Bull. 2019, 35, 867–876. [Google Scholar] [CrossRef]
- Williams, M.R.; Sharma, P.; Macdonald, C.; Pearce, R.K.B.; Hirsch, S.R.; Maier, M. Axonal Myelin Decrease in the Splenium in Major Depressive Disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 387–395. [Google Scholar] [CrossRef]
- Baranger, D.A.A.; Halchenko, Y.O.; Satz, S.; Ragozzino, R.; Iyengar, S.; Swartz, H.A.; Manelis, A. Aberrant Levels of Cortical Myelin Distinguish Individuals with Depressive Disorders from Healthy Controls. NeuroImage Clin. 2021, 32, 102790. [Google Scholar] [CrossRef]
- Reppermund, S.; Zhuang, L.; Wen, W.; Slavin, M.J.; Trollor, J.N.; Brodaty, H.; Sachdev, P.S. White Matter Integrity and Late-Life Depression in Community-Dwelling Individuals: Diffusion Tensor Imaging Study Using Tract-Based Spatial Statistics. Br. J. Psychiatry 2014, 205, 315–320. [Google Scholar] [CrossRef]
- Hollocks, M.J.; Lawrence, A.J.; Brookes, R.L.; Barrick, T.R.; Morris, R.G.; Husain, M.; Markus, H.S. Differential Relationships between Apathy and Depression with White Matter Microstructural Changes and Functional Outcomes. Brain 2015, 138, 3803–3815. [Google Scholar] [CrossRef]
- Wu, J.Y.; Zhang, Y.; Wu, W.B.; Hu, G.; Xu, Y. Impaired Long Contact White Matter Fibers Integrity Is Related to Depression in Parkinson’s Disease. CNS Neurosci. Ther. 2018, 24, 108–114. [Google Scholar] [CrossRef]
- Shen, X.; Reus, L.M.; Cox, S.R.; Adams, M.J.; Liewald, D.C.; Bastin, M.E.; Smith, D.J.; Deary, I.J.; Whalley, H.C.; McIntosh, A.M. Subcortical Volume and White Matter Integrity Abnormalities in Major Depressive Disorder: Findings from UK Biobank Imaging Data. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and Predictors of Long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-Month Consequences of COVID-19 in Patients Discharged from Hospital: A Cohort Study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Bahmer, T.; Borzikowsky, C.; Lieb, W.; Horn, A.; Krist, L.; Fricke, J.; Scheibenbogen, C.; Rabe, K.F.; Maetzler, W.; Maetzler, C.; et al. Severity, Predictors and Clinical Correlates of Post-COVID Syndrome (PCS) in Germany: A Prospective, Multi-Centre, Population-Based Cohort Study. EClinicalMedicine 2022, 51, 101549. [Google Scholar] [CrossRef]
- Pilotto, A.; Cristillo, V.; Cotti Piccinelli, S.; Zoppi, N.; Bonzi, G.; Sattin, D.; Schiavolin, S.; Raggi, A.; Canale, A.; Gipponi, S.; et al. Long-Term Neurological Manifestations of COVID-19: Prevalence and Predictive Factors. Neurol. Sci. 2021, 42, 4903–4907. [Google Scholar] [CrossRef]
- Ramasubbu, R.; Konduru, N.; Cortese, F.; Bray, S.; Gaxiola-Valdez, I.; Goodyear, B. Reduced Intrinsic Connectivity of Amygdala in Adults with Major Depressive Disorder. Front. Psychiatry 2014, 5, 1–11. [Google Scholar] [CrossRef]
- Krutenkova, E.P.; Khodanovich, M.; Bowen, J.; Gangadharan; Henson, L.K.J.; Mayadev, A.; Repovic, P.; Qian, P.Q.; Yarnykh, V.L. Demyelination and Iron Accumulation in Subcortical Gray Matter (GM) in Multiple Sclerosis (MS). Ann. Neurol. 2015, 78. [Google Scholar]
- Giannouli, V. Predictive Factors of Depressive Symptoms of Elderly Patients with Cancer: A Critical Comment. Psychooncology. 2017, 26, 870. [Google Scholar] [CrossRef]
| Parameter | PCD | No-PCD | Control |
|---|---|---|---|
| Sample size | 25 | 38 | 19 |
| Male (%) | 4(16) | 14(29) | 8(42.1) |
| Female (%) | 21(84) | 24(71) | 11(57.9) |
| Age, years±SD | 36.96±13.7 | 42.05±9.17 | 38.53±10.57 |
| Age, median (min-max) | 42 (19-59) | 42(21-58) | 37(20-56) |
| Parameter | PCD | noPCD | Statistics |
|---|---|---|---|
| Severity, mild/moderate/severe (%) | 88/8/4 | 73/24/3 | F(2, 79)=1.74, p=0.18 |
| Number of COVID-19 episodes, mean±SD | 1.6±0.7 | 1.5±0.7 | F(1, 61)=0.33, p=0.56 |
| Time after the first COVID-19, months±SD | 20.3±8.2 | 19.7±9.8 | F(1, 61)=0.06, p=0.81 |
| Time after last COVID-19, months±SD | 13.1±10.3 | 13.8±9.9 | F(1, 61)=0.07, p=0.79 |
| Acute symptoms | |||
| Anosmia, n (%) | 22(88%) | 29(76%) | Chi sq, p= 0.25 |
| Ageusia, n (%) | 19(76%)* | 19(50%) | Chi sq, p= 0.04 |
| Fever, n (%) | 22(88%) | 36(95%) | Chi sq, p= 0.33 |
| Difficulty breathing, n (%) | 14 (56%) | 17(45%) | Chi sq, p= 0.38 |
| Cough, n (%) | 22(88%)* | 24(65%) | Chi sq, p= 0.04 |
| Muscle weakness, n (%) | 24(96%) | 35(92%) | Chi sq, p= 0.53 |
| Myalgia, n (%) | 20 (80%) | 22(58%) | Chi sq, p= 0.07 |
| Headache, n (%) | 22(88%)* | 25(66%) | Chi sq, p= 0.047 |
| Dizziness, n (%) | 14 (56%) | 15(39%) | Chi sq, p= 0.20 |
| Number of acute symptoms | 7.24± 1.85** | 5.82±2.13 | F(1, 61)=7.45, p=0.008 |
| Post-COVID symptoms | |||
| Headache, n (%) | 7 (28%) | 4 (11%) | Chi sq, p= 0.07 |
| Dizziness, n (%) | 10 (40%) | 13 (34%) | Chi sq, p= 0.64 |
| Brain fog, n (%) | 14 (56%) | 16 (42%) | Chi sq, p= 0.28 |
| Anosmia, n (%) | 16 (64%)** | 11(29%) | Chi sq, p= 0.006 |
| Ageusia, n (%) | 14 (56%)** | 8(21%) | Chi sq, p= 0.004 |
| Sensitivity, n (%) | 3 (12%) | 4(11%) | Chi sq, p= 0.86 |
| Hypertensia/hypotensia, n (%) | 7 (28%) | 15(39%) | Chi sq, p= 0.35 |
| Insomnia, n (%) | 20 (80%)* | 19(50%) | Chi sq, p= 0.02 |
| Fatigue, n (%) | 24(96%)** | 25(66%) | Chi sq, p= 0.005 |
| Attention deficit, n (%) | 23(92%)*** | 19(50%) | Chi sq, p= 0.0005 |
| Memory deficit, n (%) | 19(76%) | 22(58%) | Chi sq, p= 0.14 |
| Myalgia, n (%) | 15(60%) | 14(37%) | Chi sq, p= 0.07 |
| Depression1, n (%) | 24(96%)*** | 13(34%) | Chi sq, p= 0.000 |
| Panic attacks, n (%) | 5(20%)* | 1(3%) | Chi sq, p= 0.03 |
| Number of post-COVID symptoms | 8.04±2.23*** | 4.84±3.50 | F(1, 61)=16.45, p=0.000 |
| Test | Parameter | Control | PCD | noPCD |
|---|---|---|---|---|
| HDRS | Hamilton score | 4.0±3.40 | 18.36±3.66 *** ### | 6.11±3.52 * |
| HADS | Anxiety | 4.42±2.41 | 10.84±3.25 *** ### | 5.32±3.59 *** |
| Depression | 3.47±2.44 | 10.36±4.78 *** ### | 4.05±2.89 *** | |
| Total score | 7.89±3.75 | 21.04±7.40 *** ### | 9.18±4.73 *** |
| Factor | Eigenvalue | % Total Variance | Cumulative % | Brain structures with scores > 0.7 |
| Factor 1 | 55.77 | 48.50 | 48.50 | Anterior, Superior, and Posterior CR (L+R); Genu, Body, and Splenium of CC (L+R); Posterior thal. rad.(L+R); Tapetum (L+R); SLF (L+R); Superior Frontal WM (L+R); Inferior Frontal WM (L); Superior Parietal WM (L+R); Middle Occipital WM (R); Superior Occipital (L); Middle Temporal WM (R) |
| Factor 2 | 8.84 | 7.69 | 56.19 | CST (L+R); Cerebral peduncle (L+R); Medial lemniscus (L+R); Pontine crossing tract (L+R); Inferior, Superior, and Middle CP (L+R); Midbrain, Pons, Medulla |
| Factor 3 | 4.92 | 4.28 | 60.46 | Middle Fronto-Orbital (R); Rectus (L+R) |
| Factor 4 | 4.20 | 3.65 | 64.12 | Amygdala (L+R) |
| Factor 5 | 3.26 | 2.83 | 66.95 | Cuneus (R) |
| Factor 6 | 2.85 | 2.48 | 69.42 | Globus Pallidus (R); Putamen (R) |
| Factor 7 | 2.49 | 2.16 | 71.584 | IFOF (L+R); Uncinate fasciculus (L+R) |
| Factor 8 | 2.22 | 1.93 | 73.52 | Entorhynal area (L) |
| Factor 9 | 2.01 | 1.75 | 75.27 | Caudate Nucleus (L+R) |
| Factor 10 | 1.94 | 1.68 | 76.95 | Fusiform WM (L) |
| Factor 11 | 1.75 | 1.52 | 78.48 | Pre-cuneus WM (R) |
| Factor 12 | 1.60 | 1.39 | 79.87 | Lingual WM (L) |
| Factor 13 | 1.40 | 1.21 | 81.08 | Postcentral WM (L+R) |
| Factor 14 | 1.31 | 1.14 | 82.23 | Hippocampus (R+L) |
| Factor 15 | 1.28 | 1.11 | 83.34 | - |
| Factor 16 | 1.20 | 1.04 | 84.38 | FX column and body (L) |
| Factor 17 | 1.16 | 1.01 | 85.39 | Middle Fronto-Orbital (R) |
| Factor 18 | 1.09 | 0.95 | 86.34 | - |
| Factor 19 | 1.08 | 0.94 | 87.28 | - |
| Parameter | Total | PCD | noPCD | |||
|---|---|---|---|---|---|---|
| Multiple R | 0.64 | 0.82 | 0.36 | |||
| Multiple R2 | 0.41 | 0.68 | 0.123 | |||
| F | 20.53 | 10.53 | 5.24 | |||
| p | 0.0000 | 0.0000 | 0.0281 | |||
| Variables in the model | β coefficient | p | β coefficient | p | β coefficient | p |
| Number of acute symptoms | 0.48 | 0.0016 | ||||
| Number of post-COVID symptoms | 0.56 | 0.0000 | 0.36 | 0.0281 | ||
| Factor 7 | -0.34 | 0.0010 | -0.58 | 0.0002 | ||
| Factor 15 | -0.40 | 0.0068 | ||||
| Factor 12 | 0.29 | 0.0393 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





