Submitted:
10 December 2023
Posted:
12 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
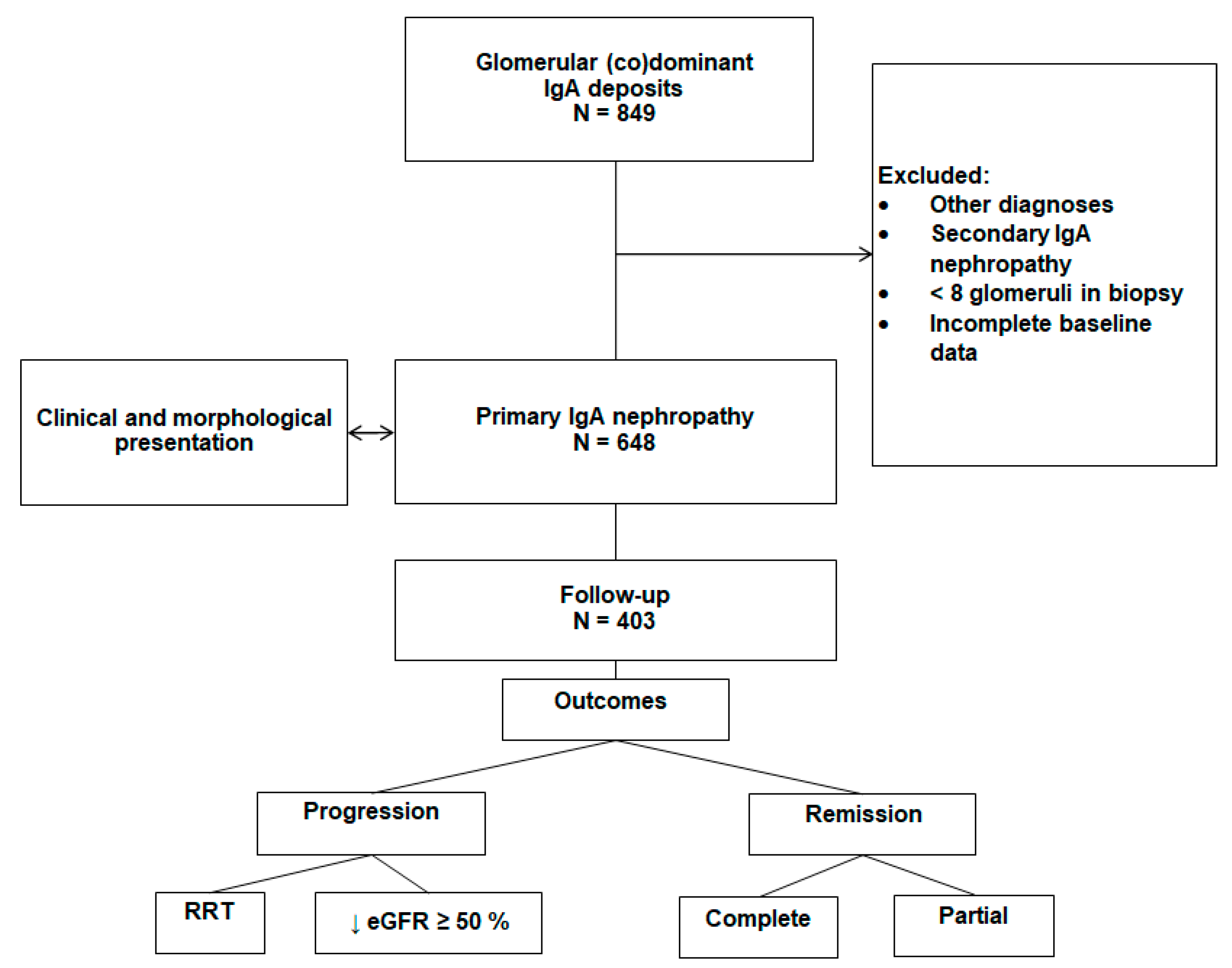
2.1. Study design
2.2. Baseline data at the time of kidney biopsy
Demographic and clinical data
Histologic data
2.3. Follow-up and outcomes
Dynamic clinical data
Therapy
Outcomes
2.4. Statistical analysis
3. Results
3.1. Clinical presentation
Histology of IgA nephropathy
Clinical and morphologic correlations
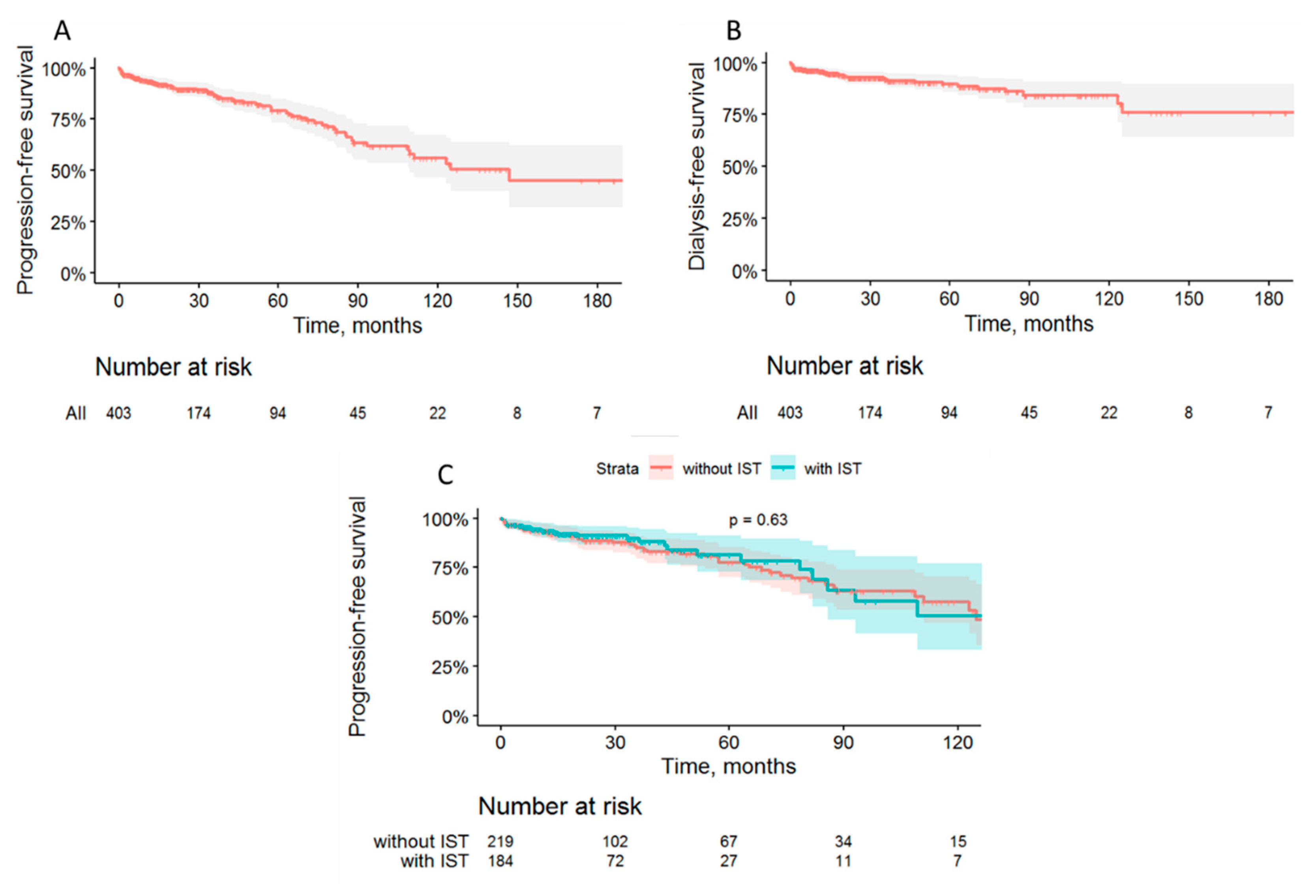
3.2. Follow-up and outcomes
Overall and renal survival
Treatment and remissions
3.3. Factors associated with prognosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schena FP, Nistor I. Epidemiology of IgA Nephropathy: A Global Perspective. Semin Nephrol 2018; 38: 435 – 442. [CrossRef]
- Reily C, Ueda H, Huang ZQ et al. Cellular Signaling and Production of Galactose-Deficient IgA1 in IgA Nephropathy, an Autoimmune Disease. Journal of Immunology Research 2014. [CrossRef]
- Hiki Y, Odani H, Takahashi M et al. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001 Mar; 59(3): 1077 – 1085. [CrossRef]
- Tomana M, Novak J, Julian B et al. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999 Jul; 104 (1): 73 - 81. [CrossRef]
- Boyaka, PN. Inducing Mucosal IgA: A Challenge for Vaccine Adjuvants and Delivery Systems. J Immunol 2017; 199: 9 - 16. [CrossRef]
- Muto M, Manfroi B, Suzuki H et al. Toll-Like Receptor 9 Stimulation Induces Aberrant Expression of a Proliferation-Inducing Ligand by Tonsillar Germinal Center B Cells in IgA Nephropathy. J Am Soc Nephrol 2017; 28 (4): 1227 – 1238. [CrossRef]
- Robert T, Berthelot L, Cambier A et al. Molecular Insights into the Pathogenesis of IgA Nephropathy. Trend Mol Med. 2015; 12: 762 – 775. [CrossRef]
- Ben Mkaddem S, Benhamou M, Monteiro RC. Understanding Fc Receptor Involvement in Inflammatory Diseases: From Mechanisms to New Therapeutic Tools. Front Immunol. 2019; 10: 1 - 12. [CrossRef]
- Novak J, Julian BA, Tomana M et al. IgA Glycosylation and IgA Immune Complexes in the Pathogenesis of IgA Nephropathy. Semin Nephrol 2008; 28 (1): 78 – 87. [CrossRef]
- Wyatt RJ, Julian BA. IgA Nephropathy. N Engl J Med 2013; 368: 2402 - 2414. [CrossRef]
- Novak J, Tomana M, Matousovic K, et al. IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int. 2005; 67: 504–513. [CrossRef]
- Kiryluk K, Li Y, Sanna-Cherchi S et al. Geographic Differences in Genetic Susceptibility to IgA Nephropathy: GWAS Replication Study and Geospatial Risk Analysis. PLoS Genet 8(6): e1002765. [CrossRef]
- Moriyama T, Tanaka K, Iwasaki C et al. Prognosis in IgA Nephropathy: 30-Year Analysis of 1,012 Patients at a Single Center in Japan. PLOS ONE 2014; 9 (3). [CrossRef]
- Lee H, Kim DK, Oh KH et al. Mortality of IgA Nephropathy Patients: A Single Center in Korea. Experience over 30 Years. PLoS ONE 7(12): e51225. [CrossRef]
- Le W, Liang S, Hu Y et al. Long-term renal survival and related risk factors in patients with IgA nephropathy: results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant 2012; 27: 1479–1485. [CrossRef]
- Coppo R, Troyanov S, Bellur S et al. Validation of the Oxford classification of IgA-nephropathy in cohorts with different presentations and treatments. Kidney Int. 2014; 86: 828–836. [CrossRef]
- Roberts, IS. Pathology of IgA nephropathy. Nat. Rev. Nephrol 2014; 10: 445 – 454. [CrossRef]
- Cattran DC, Coppo R, Cook HT et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. A Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. Kidney Int. 2009; 76(5): 534 - 545. [CrossRef]
- Trimarchi H, Barratt J, Cattran DC et al. IgAN Classification Working Group of the International IgA Nephropathy Network and the Renal Pathology Society; Conference Participants. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017; 91(5): 1014 - 1021. [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021 Oct;100(4S):S1-S276. [CrossRef]
- Yeo SC, Goh SM, Barratt J. Is immunoglobulin A nephropathy different in different ethnic populations? Nephrology (Carlton). 2019 Sep;24(9):885-895. [CrossRef]
- Zhang Z, Zhang Y, Zhang H. IgA Nephropathy: A Chinese Perspective. Glomerular Dis. 2021 Oct 12;2(1):30-41. PMID: 36751266; PMCID: PMC9677733.). [CrossRef]
- Lee M, Suzuki H, Nihei Y, Matsuzaki K, Suzuki Y. Ethnicity and IgA nephropathy: worldwide differences in epidemiology, timing of diagnosis, clinical manifestations, management and prognosis. Clin Kidney J. 2023 Dec 4;16(Suppl 2):ii1-ii8. [CrossRef]
- Stamellou E, Seikrit C, Tang SCW, Boor P, Tesař V, Floege J, Barratt J, Kramann R. IgA nephropathy. Nat Rev Dis Primers. 2023 Nov 30;9(1):67. [CrossRef] [PubMed]
- Chang JH, Kim DK, Kim HW, et al. Changing prevalence of glomerular diseases in Korean adults: a review of 20 years of experience. Nephrol Dial Transplant. 2009; 24(8): 2406 - 2410. [CrossRef]
- Alexander S, Varughese S, Franklin R, Roy S, Rebekah G, David VG, Mohapatra A, Valson AT, Jacob S, Koshy PM, Rajan G, Daha MR, Feehally J, Barratt J, John GT. Epidemiology, baseline characteristics and risk of progression in the first South-Asian prospective longitudinal observational IgA nephropathy cohort. Kidney Int Rep. 2020 Dec 7;6(2):414-428. [CrossRef]
- Coppo, R. The Gut-Renal Connection in IgA Nephropathy. Semin Nephrol 2018; 38(5): 504 - 512. [CrossRef]
- Zhu TT, Wang L, Wang HL et al. Helicobacter pylori participates in the pathogenesis of IgA nephropathy. Ren Fail. 2016; 38(9): 1398 – 1404. [CrossRef]
- Floege J, Feehally J. The mucosa-kidney axis in IgA nephropathy. Nat Rev Nephrol. 2016; 12(3): 147 - 156. [CrossRef]
- Heybeli C, Oktan MA et al. Clinical significance of mesangial IgM deposition in patients with IgA nephropathy. Clinical and Experimental Nephrology 2018; 23(3): 371 – 379. [CrossRef]
- Dong J, Peng T, Gao J et al. A pilot and comparative study between pathological and serological levels of immunoglobulin and complement among three kinds of primary Glomerulonephritis. BMC Immunology 2018; 19: 18. [CrossRef]
- Dobronravov VA, Smirnov AV. Etiology and clinic-morphological presentation of membranoproliferative glomerulonephritis in Russian population. Ter arh 2018; 12: 39 – 47. (In Russ.)]. [CrossRef]
- Alvarado AS, Andeen NK, Brodsky S et al. Location of glomerular immune deposits, not codeposition of immunoglobulin G, influences definitive renal outcomes in immunoglobulin A nephropathy. Nephrol Dial Transplant 2018; 33: 1168–1175. [CrossRef]
- Akio Koyama, Masaki Kobayashi. Treatment of IgA Nephropathy: Present and Future. Nephrology 1997; 3: 633 – 799.
- Zeng CH, Le W, Ni Z et al. A Multicenter Application and Evaluation of the Oxford Classification of IgA Nephropathy in Adult Chinese Patients. Am J Kidney Dis. 2012; 60: 812 - 820. [CrossRef]
- Li M, Yu X. Genetic study of immunoglobulin A nephropathy: From research to clinical application. Nephrology (Carlton) 2018; 23: 26 – 31. [CrossRef]
- Feehally J, Barratt J. The Genetics of IgA Nephropathy: An Overview from Western Countries. Kidney Dis (Basel) 2015; 1: 33 - 41. [CrossRef]
- Berthoux F, Mohey H, Laurent B et al. Predicting the Risk for Dialysis or Death in IgA Nephropathy. J Am Soc Nephrol 2011; 22: 752 - 761. [CrossRef]
- Reich HN, Troyanov S, Scholey JW et al. Remission of Proteinuria Improves Prognosis in IgA Nephropathy. J Am Soc Nephrol 2007; 18: 3177 - 3183. [CrossRef]
- Shi SF, Wang SX, Jiang L et al. Pathologic predictors of renal outcome and therapeutic efficacy in IgA nephropathy: validation of the oxford classification. Clin J Am Soc Nephrol. 2011; 6(9): 2175 – 2184. [CrossRef]
- Liu LJ, Li GT, Zhou Y et al. Clinicopathologic Features and Outcomes in Endocapillary Proliferative IgA Nephropathy. Nephron Clin Pract 2010; 115: 161 – 167. [CrossRef]
- Sevillano AM, Gutiérrez E, Yuste C et al. Remission of Hematuria Improves Renal Survival in IgA Nephropathy J Am Soc Nephrol 2017; 28(10):3089-3099. [CrossRef]
- Alamartine E, Sauron C, Laurent B et al. The Use of the Oxford Classification of IgA Nephropathy to Predict Renal Survival. Clin J Am Soc Nephrol 2011; 6: 2384 – 2388. [CrossRef]
- Yau T, Korbet SM, Schwartz MM et al. The Oxford Classification of IgA Nephropathy: A Retrospective Analysis. Am J Nephrol 2011; 34: 435 – 444. [CrossRef]
- Lee H, Sul Hee Yi, Mi Seon Seo et al. Validation of the Oxford Classification of IgA Nephropathy: A Single-Center Study in Korean Adults. Korean J Intern Med 2012; 27: 293 – 300. [CrossRef]
- Tanaka S, Ninomiya T, Katafuchi R et al. Development and Validation of a Prediction Rule Using the Oxford Classification in IgA Nephropathy. Clin J Am Soc Nephrol 2013; 8: 2082 – 2090. [CrossRef]
- Kang SH, Choi SR, Park HS et al. The Oxford classification as a predictor of prognosis in patients with IgA nephropathy. Nephrol Dial Transplant 2012; 27: 252 – 258. [CrossRef]
- Bellur SS, Roberts ISD, Troyanov S et al. Reproducibility of the Oxford classification of immunoglobulin A nephropathy, impact of biopsy scoring on treatment allocation and clinical relevance of disagreements: evidence from the Validation of IGA study cohort. Nephrol Dial Transplant 2019; 34(10): 1681 - 1690. [CrossRef]
- Rankin AJ, Kipgen D, Geddes CC et al. Assessment of active tubulointerstitial nephritis in non-scarred renal cortex improves prediction of renal outcomes in patients with IgA nephropathy. Clin Kidney J. 2018; 12: 348 - 354. [CrossRef]
- Sato R, Joh K, Komatsuda A et al. Validation of the Japanese histologic classification 2013 of immunoglobulin A nephropathy for prediction of long-term prognosis in a Japanese single-center cohort. Clin Exp Nephrol 2015; 19: 411 – 418. [CrossRef]
- Okonogi H, Kawamura T, Joh K et al. A grading system that predicts the risk of dialysis induction in IgA nephropathy patients based on the combination of the clinical and histological severity. Clin Exp Nephrol 2019; 23: 16 – 25. [CrossRef]
- Katafuchi R, Ninomiya T, Nagata M et al. Validation Study of Oxford Classification of IgA Nephropathy: The Significance of Extracapillary Proliferation. Clin J Am Soc Nephrol 2011; 6: 2806 – 2813. [CrossRef]
- Barbour SJ, Coppo R, Zhang H et al. Evaluating a New International Risk-Prediction Tool in IgA Nephropathy. JAMA Intern Med 2019; 179: 942 – 952. [CrossRef]
- Chen T, Li X, Li Y et al. Prediction and Risk Stratification of Kidney Outcomes in IgA Nephropathy. Am J Kidney Dis 2019; 74: 300 - 309. [CrossRef]
- Xie J, Kiryluk K, Wang W et al. Predicting Progression of IgA Nephropathy: New Clinical Progression Risk Score. PLoS ONE 2012; 7: e38904. [CrossRef]
- Goto M, Wakai K, Kawamura T et al. A scoring system to predict renal outcome in IgA nephropathy: a nationwide 10-year prospective cohort study. Nephrol Dial Transplant 2009; 24: 3068 – 3074. [CrossRef]
- Bartosik LP, Lajoie G, Sugar L et al. Predicting progression in IgA nephropathy. Am J Kidney Dis 2001; 38: 728 – 735. [CrossRef]
- Soares MFS, Roberts ISD. Histologic Classification of IgA Nephropathy: Past, Present, and Future. Seminars in Nephrology 2018; 38: 477 – 484. [CrossRef]
- Coppo, R. Treatment of IgA nephropathy: Recent advances and prospects. Nephrol Ther 2018; 1: 13 – 21. [CrossRef]
- Hotta O, Furuta T, Chiba S et al. Regression of IgA nephropathy: a repeat biopsy study. Am J Kidney Dis 2002; 39: 493 - 502. [CrossRef]
- Tumlin JA, Lohavichan V, Hennigar R. Crescentic, proliferative IgA nephropathy: clinical and histological response to methylprednisolone and intravenous cyclophosphamide. Nephrol Dial Transplant 2003; 18: 1321 – 1329. [CrossRef]
- McIntyre CW, Fluck RJ, Lambie SH. Steroid and cyclophosphamide therapy for IgA nephropathy associated with crescentic change: an effective treatment. Clin Nephrol 2001; 56: 193 - 198.
- Tan L, Tang Y, Peng W et al. Combined Immunosuppressive Treatment May Improve Short-Term Renal Outcomes in Chinese Patients with Advanced IgA Nephropathy. Kidney Blood Press Res 2018; 43: 1333 - 1343. [CrossRef]
- Lv J, Wong MG, Hladunewich MA, Jha V, Hooi LS, Monaghan H, Zhao M, Barbour S, Jardine MJ, Reich HN, Cattran D, Glassock R, Levin A, Wheeler DC, Woodward M, Billot L, Stepien S, Rogers K, Chan TM, Liu ZH, Johnson DW, Cass A, Feehally J, Floege J, Remuzzi G, Wu Y, Agarwal R, Zhang H, Perkovic V; TESTING Study Group. Effect of Oral Methylprednisolone on Decline in Kidney Function or Kidney Failure in Patients With IgA Nephropathy: The TESTING Randomized Clinical Trial. JAMA. 2022 May 17;327(19):1888‐1898. PMID: 35579642; PMCID: PMC9115617. [CrossRef]
- Yang YZ, Chen P, Liu LJ et al. Comparison of the effects of hydroxychloroquine and corticosteroid treatment on proteinuria in IgA nephropathy: a case-control study. BMC Nephrology 2019; 20: 297. [CrossRef]
- Rauen T, Fitzner C, Eitner F et al. Effects of Two Immunosuppressive Treatment Protocols for IgA Nephropathy. J Am Soc Nephrol 2018; 29: 317 – 325. [CrossRef]
- Hou JH, Le WB, Chen N et al. Mycophenolate Mofetil Combined With Prednisone Versus Full-Dose Prednisone in IgA Nephropathy With Active Proliferative Lesions: A Randomized Controlled Trial. Am J Kidney Dis 2017; 69: 788 – 795. [CrossRef]
- Chen S, Qing Yin, Song Ren et al. A comparison of the effectiveness of cyclophosphamide, leflunomide, corticosteroids, or conservative management alone in patients with IgA nephropathy: a retrospective observational study. Scientific Reports 2018; 8: 13663. [CrossRef]
- Tatematsu M, Yasuda Y, Morita Y et al. Complete remission within 2 years predicts a good prognosis after methylprednisolone pulse therapy in patients with IgA nephropathy. Clin Exp Nephrol 2012; 16: 883 – 891. [CrossRef]
| Indicator | Value* |
|---|---|
| Age, years | 34±12 |
| male gender, % | 55 |
| BMI, kg/m2 | 25.5±5 |
| Months from first IgAN manifestations to biopsy | 41 (10;116) |
| Proteinuria, g/24h | 2.5 (1.5-5.0) |
| Proteinuria >1 g/24h, % | 76 |
| Proteinuria>3.5 g/24h, % | 30 |
| Serum albumin, g/L | 35.7±5.6 |
| Nephrotic syndrome, % | 9.3 |
| Macrohematuria before biopsy, % | 41 |
| Erythrocyturia, cells per field of view | 9 (4;19) |
| Creatinine, mmol/L | 0.106 (0.082;0.140) |
| eGFR, ml/min/1.73 m2 | 71±32 |
| CKD stages 1/2/3AB/4/5, % | 31/32/25/7/4 |
| Total serum IgA, g/L | 3.5±1.3 |
| Serum IgA elevation, % | 10.6 |
| Serum C3, g/L | 1.08±0.24 |
| Serum C4, g/L | 0.24±0.07 |
| Mean ABP (maximal), mm Hg | 116±20 |
| Mean ABP (at admission), mm Hg | 99±20 |
| Arterial hypertension, % | 75 |
| Inflammatory diseases of upper respiratory tract, % | 55 |
| Tonsillitis, % | 49 |
| Inflammatory diseases of lower respiratory tract, % | 8 |
| Inflammatory diseases of gastrointestinal tract, % | 48 |
| Inflammatory diseases of lower urinary tract, % | 10 |
| Vaginitis/cervicitis,% | 7** |
| Indices | Values* |
|---|---|
|
Oxford classification (MEST-C): Mesangial proliferation (M1),% Endocapillary hypercellularity (E1) , % Segmental sclerosis or adhesions (S1) , % Tubular atrophy/interstitial fibrosis (T1+T2),% T0, % T1, % T2, % Cellular/fibro-cellular crescents, % C1, % C2, % |
40.5 22.9 70.2 31 69 22 9 16,7 12,3 4,4 |
|
Other changes: Global sclerosis, % of glomeruli Segmental sclerosis, % of glomeruli Fibrous crescents, % Interstitial infiltration, % >25% of interstitial area, % Peritubular capillaritis, % |
13 (5;30) 9 (0;17) 7.7 50.5 14.8 30.1 |
| Indices | Frequency, % |
|---|---|
| IgA in the mesangium | 100.0 |
| IgA n glomerular capillary wall | 17.1 |
| IgA in atreries | 4.0 |
| IgA in in peritubular capillaries | 5.7 |
| IgM in mesangium | 71.1 |
| IgG in mesangium | 9.6 |
| C3 in mesangium | 98.0 |
| C3 in glomerular capillary wall | 13.8 |
| C3 in arteries | 4.5 |
| C3 in peritubular capillaries | 6.6 |
| Fibrinogen in mesangium | 21.4 |
| Fibrinogen n glomerular capillary wall | 6.2 |
| Fibrinogen in peritubular capillaries | 12.2 |
| Histologic indices, units | Mean ABP | eGFR | Daily proteinuria | Hematuria |
|---|---|---|---|---|
| Global glomerular sclerosis, % | 0.29 p<0.001 |
-0.47 p<0.001) |
0.39 p <0.001 |
-0.03 NS |
| Segmental glomerular sclerosis, % | 0.16 p <0.001 |
-0.20 p<0.001 |
0.35 p<0.001 |
0.00 NS |
| Crescents (any), % | 0.04 NS |
-0.10 p=0.024 |
0.25 p<0.001 |
0.15 p<0.001 |
| Cellular/fibrocellular crescents,% | 0.05 NS |
-0.11 p<0.001 |
0.28 p<0.001 |
0.17 p<0.001 |
| IFTA, grades | 0.36 p<0.001 |
-0.57 p <0.001 |
0.39 p <0.001 |
-0.02 NS |
| Interstitial infiltration, grades | 0.29 p<0.001 |
-0.51 p <0.001 |
0.35 p <0.001 |
0.118 p =0.005 |
| Mesangium proliferation, grades | 0.07 NS |
-0.15 p <0.001 |
0.19 p <0.001 |
0.14 p =0.001 |
| Endocapillary hypercellularity, no vs yes | 0.15 p<0.001 |
-0.25 p<0.001 |
0.30 p<0.001 |
0.12 p=0.004 |
| PTC, grades | 0.20 p<0.001 |
-0.41 p<0.001 |
0.37 p<0.001 |
0.11 p=0.009 |
| IgA glomerular capillaries, grades | 0.13 p<0.001 |
-0.10 p=0.009 |
0.16 p <0.001 |
0.01 NS |
| IgA mesangium, grades | -0.12 p =0.002 |
0.06 NS |
0.00 NS |
0.04 NS |
| IgA peritubular capillaries, grades | 0.07 NS |
-0.20 p<0.001) |
0.16 p<0.001 |
0.01 NS |
| IgM mesangium, grades | -0.04 NS |
0.03 NS |
0.12 p=0.002 |
0.01 NS |
| IgG mesangium, grades | -0.07 p=0.042 |
0.05 NS |
-0.04 NS |
-0.01 NS |
| C3 glomerular capillaries, grades | 0.15 p<0.001 |
-0.11 p=0.005 |
0.18 p<0.001 |
0.02 NS |
| C3 mesangium, grades | -0.054 NS |
0.01 NS |
-0.01 NS |
0.01 NS |
| Models | Factors (unit change) | Expβ (95% CI) | Р-value |
|---|---|---|---|
| Clinical parametersa | Age (1 year) | 0.974 (0.949-0.999) | 0.041 |
| Male sex (vs female) | 1.956 (1.131-3.382) | 0.016 | |
| Mean BP maximal (1 mmHg) | 1.015 (1.002-1.029) | 0.025 | |
| eGFR (1 ml/min/1.73m2) | 0.950 (0.937-0.963) | <0.001 | |
| Ln (Hematuria) (1 unit) | 1.406 (1.121-1.762) | 0.003 | |
| Ln (Proteinuria) (1 unit) | 1.237 (0.893-1.714) | 0.20 | |
| Morphological parameters | M (M1 vs М0) b | 1.679 (0.984-2.864) | 0.06 |
| Е (Е1 vs Е0) b | 0.920 (0.502-1.685) | 0.67 | |
| S (S1 vs S0) b | 1.472 (0.746-2.905) | 0.26 | |
| Т (Т1 vs Т0) b | 1.021 (0.452-2.309) | 0.95 | |
| Т (Т2 vs Т0) b | 4.579 (1.835-11.462) | 0.001 | |
| С (С1 vs С0) b | 1.851 (0.960-3.569) | 0.07 | |
| С (С2 vs С0) b | 1.068 (0.400-2.855) | 0.89 | |
| Global glomerular sclerosis (1%) b | 1.014 (1.001-1.027) | 0.029 | |
| Any crescents (vs no crescents) b | 1.939 (1.110-3.389) | 0.02 | |
| Interstitial infiltration (1-24% vs 0%) b | 1.418 (0.624-3.222) | 0.40 | |
| Interstitial infiltration (≥25% vs <25%) b | 2.465 (1.097-5.539) | 0.029 | |
| PTC (moderate vs none) b | 1.321 (0.630-2.770) | 0.46 | |
| PTC (severe vs none) b | 2.590 (1.206-5.560) | 0.015 | |
| Clinical and morphological parameters с | Age (1 year) | 0.964 (0.939-0.990) | 0.006 |
| Male gender (vs. female) | 2.566 (1.448-4.548) | 0.001 | |
| eGFR (1 ml/min/1.73m2) | 0.966 (0.953-0.979) | <0.001 | |
| Ln(Hematuria) (1 unit) | 1.482 (1.166-1.884) | 0.001 | |
| Mean BP (maximal) (1 mm Hg) | 1.024 (1.010-1.040) | 0.001 | |
| PTC (severe vs none/moderate) | 2.422 (1.262-4.648) | 0.008 | |
| Tubular atrophy/interstitial fibrosis (T2 vs T0-1) | 6.738 (3.542-12.817) | <0.001 | |
| Any crescents (vs no crescents) | 2.078 (1.200-3.596) | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





