1. Introduction
One of the possible ways to create drug delivery systems is to use nanoscale cavi-tands capable of host-guest complexation with drugs. Drug complexes can be obtained with various cavitand molecules: cyclodextrins, calixarenes, cucurbiturils, crown ethers, cryptophanes, pillararenes, etc [
1,
2,
3]. Among cavitands, cucurbiturils have advantages, such as the ability to form strong complexes with various compounds [
4,
5,
6,
7]. The average value of binding capacity (the concept is used in characterizing guest-host-guest interaction and receptor-drug, enzyme-substrate interactions) for cucurbit[
6]uril( CB[
6]) when studying the connection with 56 guest molecules is Ka = 10
3.8 ± 1.5 M
-1 [
8,
9]. A comparison of the complexes of CB [
6] and α-cyclodextrin, also capable of “host-guest” complexation, showed that CB[
6] forms stronger noncovalent bonds (with the exception of hexanol) with molecules having a positive charge [
10,
11]. Complexation of CB[
6] has a number of characteristics that are similarly applicable to the entire family. Since the portals of the molecule have some negative charge, the formation of associates with positively charged particles occurs in these areas [
12]. In the cavity area, binding of hydrophobic molecules and their particles occurs. The relative mobility and proximity of three binding regions, two for positively charged groups and one for hydrophobic residues, imparts high selectivity when binding to cucurbiturils to form host-guest complexes. When studying the binding constants of a number of alkylammonium ions with CB[
6] in a solution of HCO
2H/H
2O (1:1), values from 10
1 to 10
7 M
-1 were obtained [
8]. Similar features of complex formation will be characteristic of other cucurbiturils due to their similar structure.
Since cucurbit[n]urils are a series of homologues that differ in the number of glyco-luril fragments that form this molecule, and hence in the size of the cavity[
12], cucur-bit[
6]uril, cucurbit[
7]uril, and cucurbit[
8]uril (CB [
6], CB[
7] and CB[
8], respectively) have the most suitable sizes for complexation and most often used to create drug delivery sys-tems.
The bioavailability of many drugs is low due to poor solubility. CB7 has good solubility in aqueous media, while CB6 and CB8 are poorly soluble. However, in the presence of a number of ions, which are abundant in biological media, the solubility of CB6 increases significantly [
13].
Guest-host complexes of CB[
7] and CB[
8] with a guest dye molecule can cross the cell membrane [
14]. It is likely that other CB[n] guest-host complexes will be similarly capable of passing through the cell membrane, and therefore may be effective carriers for drug transport. Thus, the assessment of the biological safety of cucurbiturils is relevant. It is now known that cucurbiturils have demonstrated low toxicity in a lot of in vitro and in vivo studies [
15,
16,
17,
18,
19,
20]. The use of cucurbiturils at very high doses may cause myotoxicity and neurotoxicity, but there are no signs of toxicity at standard concentrations used in complexation with drugs.
However, in addition to immunotoxicity, it is also necessary to investigate the possible immunomodulatory properties of delivery systems, since the immunostimulatory or immunosuppressive effects of the system can either enhance or weaken the effect of the delivered drug. Recent studies have shown that the closest analogues of cucurbiturils, cyclodextrins, have anti-inflammatory properties [
21] On the other hand, it is currently known that cyclodextrin can act as an immunostimulant, with prolonged exposures (rats taking 0.4 g/kg/day for three months) causing increased monocyte and overall white blood cells counts [
22], and a single co-administration with an antigen (albumin) increasing proliferation of T helpers 2 (Th2) and T follicular helpers lymphocytes [
23]. Investigating the possible immunomodulatory effect of nanosized cavitands is important for the development of new delivery systems and should be considered when assessing indications and contraindications for the treatment of various diseases.
In previous studies, we evaluated the possible immunomodulatory effect of cucurbi-turils [
24,
25,
26,
27], it was shown that during cultivation in the presence of CB[n], the prolifera-tive activity of cells increased and the expression of HLA-DR on lymphocytes increased, which indicates an immunostimulatory effect. It was shown that cucurbiturils had prac-tically no immunosuppressive effect under in vitro conditions, except for a slight decrease in HLA-DR expression and the production of reactive oxygen species by T-helpers in stimulated cultures [
24,
27]. However, for macrocyclic cavitands and for cucurbiturils it has been demonstrated that some properties only manifest themselves under in vivo conditions. Therefore, it seems relevant to study the immunosafety and immunomodulatory properties of cucurbiturils in vivo.
2. Materials and Methods
Materials
CB[
6], CB[
7] and CB[
8] were synthesized at the Nikolaev Institute of Inorganic Chemistry SB RAS (Novosibirsk, Russia) according to the standard protocol [
28]. The structures of CB[
6] and CB[
7] were verified with 1H NMR in D
2O at 25 °C using a 500 MHz Bruker Avance III spectrometer. For this study, cucurbiturils were diluted in phosphate-buffered saline and administered intraperitoneally to laboratory animals. It should be noted that CB[
6] and CB[
8] have poor solubility in water, however, the media and buffer solutions used in assessing the immunomodulatory properties contain various ions, including Na
+, that can increase the solubility of cucurbiturils. However, the solubility limit of CB[
8] did not allow obtaining a solution with a concentration higher than 0.2 mM, which made it difficult to comprehensively assess the immunomodulatory properties of this cavitand.
Mice
BALB/c male mice aged 2–3 months were used in the work. The mice were kept in standard vivarium conditions (free access to food and water and a 12 h day/night cycle). The animals were divided into groups of 5-7 mice, which were injected intraperitoneally three times during a week: the first group - 0.25 ml of phosphate-buffered saline (PBS), the second group - 4M CB[
6] solution, the third - the second group 4M CB[
7] solution, the fourth - the second group of 4M solution of CB[
8].
Blood sampling was carried out one day after the third injection. The indicators of the cellular composition of the blood were assessed, namely the number of leukocytes, erythrocytes, platelets, granulocytes (neutrophils, eosinophils, basophils), lymphocytes, as well as the level of hemoglobin, the average concentration of hemoglobin in the erythrocyte and hematocrit. On the next day after the third injection, the animals were sacrificed by decapitation, after which a set of samples (blood, spleen, bone marrow) was taken. Isolation of blood mononuclear cells was performed by centrifugation on a ficoll-urografin density gradient. Isolation of bone marrow and spleen cells was carried out using standard techniques. Bone marrow was washed from mouse femurs with phosphate buffer and resuspended with a syringe. To obtain a homogeneous suspension, the cells were broken up by passing the suspension repeatedly through needles of different diameters, and then passed through a cell filter (30 μm). The spleen was crushed with a homogenizer, the resulting suspension was filtered, then the erythrocytes were lysed using a lysis buffer, after which cells were washed twice with phosphate buffer. Next, cells were counted.
Cytotoxicity assay
3 million cell suspension was used for LDH, the study was carried out according to the instructions specified in the kit. LDH-Cytox™ Assay Kit (BioLegend, San Diego, CA, USA) Cells (splenocytes, bone marrow cells) at a concentration of 0.5 million/100 µl were plated in a 96-well plate (3 wells positive control, 3 wells sample). Next, lysis buffer was added to the wells to evaluate the positive control. After 30 min incubation with lysis buffer and 30 min incubation with assay buffer, the reaction was stopped by adding stop solution and absorbance was measured using an Infinite F50 microplate reader (Tecan, Grödig, Austria) at 490 nm. The average optical density of each set of wells was calculated in triplicate and the value of the background control was subtracted. The percentage of cytotoxicity was calculated using the following equation:
Cytotoxicity (%) = ((A - C) / (B - C)) × 100
A: test substance, B: positive control, C: negative control.
Flow cytometry
Samples of blood, spleen, and bone marrow cells were stained with fluorescently la-beled monoclonal antibodies. The following anti-mouse antibodies were used: FITC-anti CD45 (clone S18009F), APC-anti-CD3 (clone 17A2), PerCP/Cyanine5.5 anti CD4 (clone GK1.5), PE/Cy7 – anti CD16 (clone S17014E) and PE- anti-CD19 (clone 1D3/CD19), from Biolegend, USA. Monoclonal antibodies were added to 100 μL of cell suspension containing at least 2 × 106 cells. After 30 min incubation at room temperature in the dark , cells were washed and analyzed. Analyses were performed using a six-color FACSCanto II (Becton Dickinson, USA) and FACSDiva software (Becton Dickinson, USA).
IgM–Plaque–Forming Cell (IgM–PFC) and Delayed-Type Hypersensitivity (DTH) Assay
To assess the possible effect of CB[n] on cellular and humoral immunity in vivo, mice were immunized with thymus-dependent antigen - 0.25% solution of sheep red blood cells (SRBCs) (ZAO ECOLab, Russia) intraperitoneally, immunization was carried out the next day after the second injection and 2 days before the third injection of cucurbiturils. The results were evaluated on the eighth day after the first administration of the drug.
The humoral immune response was assessed at the peak of the response, on the fourth day (IgM-PFC), by the number of plaques (i.e., clear areas of hemolysis around each antibody-forming cell). First, a single cell suspension was prepared from each spleen. Next, an incubation mixture was prepared: 500 µl of cell suspension, 500 µl of comple-ment (ZAO ECOLab, Russia), and 500 µl of SRBC suspension (12 × 108 SRBCs/ml). The components were mixed, the mixture was poured into glass chambers made of two glass slides glued together along the longitudinal sides using a mixture of wax and paraffin. When filling with a mixture, the volume of the chamber was taken into account. The chambers were incubated at 37°C for 90 minutes. The plaques were counted under a bin-ocular loupe according to the formula: Absolute PFC= (A×B×C)/D, where A is the number of plaques per chamber, B is the volume of the cell suspension, C is the dilution of the cell suspension, D is the volume of the chamber.
The cellular immune response was assessed by the severity of the DTH reaction, namely, paw edema after administration of a second dose of SRBCs to previously sensi-tized animals. After four days, animals were challenged with 50 µl 50% SRBC suspension in the left hind foot pad. The right foot pad was injected with the same volume of PBS to serve as control for nonspecific swelling. The footpad thickness was measured with mi-crocaliper 24 h after the challenge. The ratio of the thickness between left foot pad and right foot pad was used as a measure of DTH reaction.
Statistical analysis
All experimental data were expressed as means ± standard error of the mean (SEM) or median with interquartile range. Differences between groups were evaluated for statistical significance using a Student’s t-test or Mann–Whitney test when the data were not normally distributed. A p-value < 0.05 was regarded as the minimum criteria for statistical significance.
3. Results
3.1. Immunosafety Findings
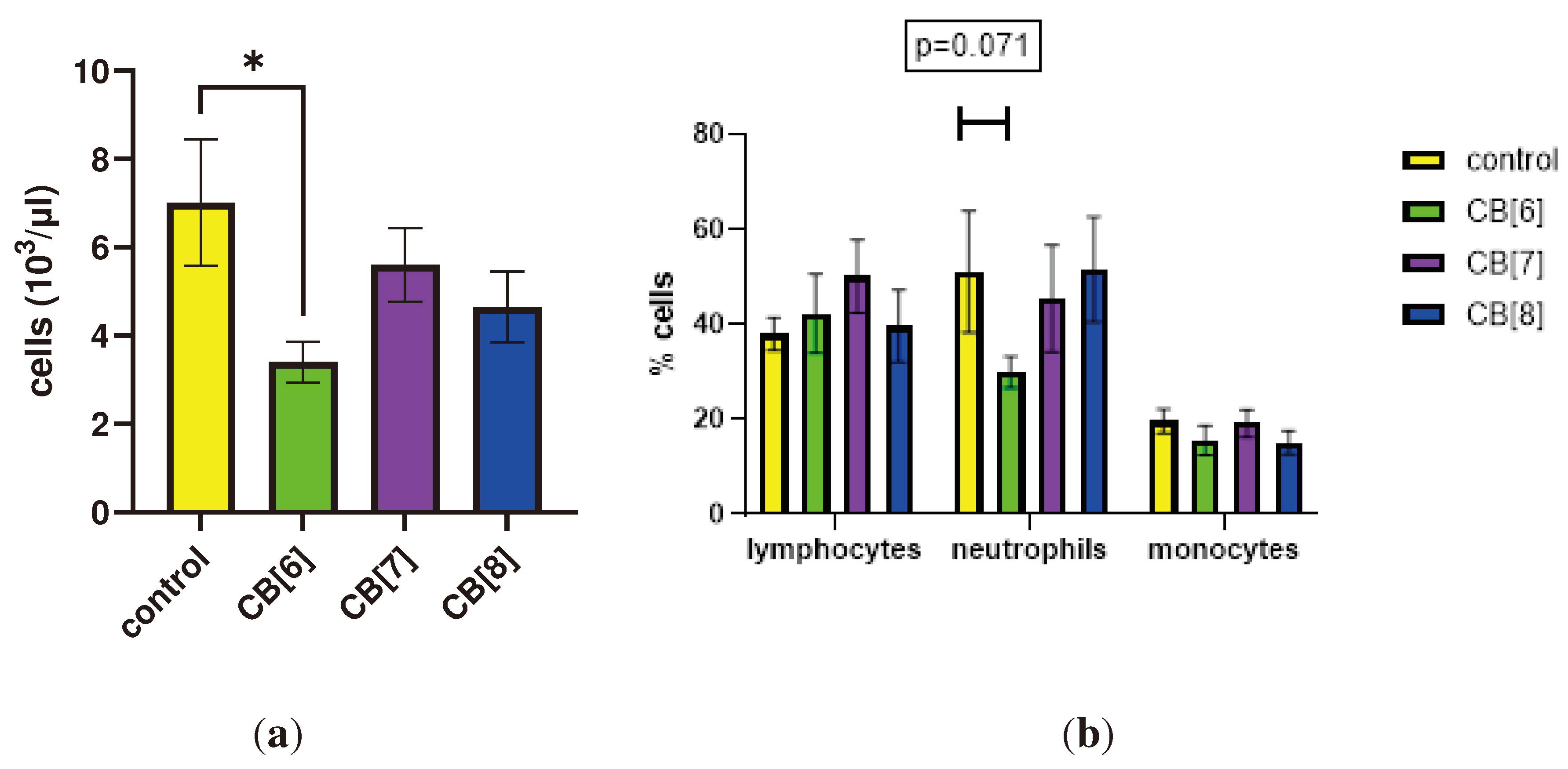
First, we evaluated the effect of cucurbit[n]urils administered parenterally on the blood parameters of laboratory animals of the BALB/c line. When assessing the effect of cucurbit[
7]uril on blood parameters after three intraperitoneal injections within a week in laboratory animals, a decrease in white blood cells was found in mice after injections of CB[
6] from 7013±1437 to 3400±461 cells/μl (Figure 1). The typical leykocytes count in mice is 2000 to 10,000 per μl [
29]. Therefore, the observed decrease in the number of white blood cells is within the normal range. CB[
6] did not cause a statistically significant decrease in the relative number of leukocyte subpopulations such as lymphocytes, monocytes and neutrophils. However, trends were observed for a decrease in the number of neutrophils after the third injection in animals that were injected with CB[
6]. CB[
7] and CB[
8] did not affect the leukocyte counts of mice after three injections. Changes in the number of platelets, erythrocytes, monocytes, as well as in several other indicators, such as hematocrit or erythrocyte volumetric dispersion, were not detected (Figure S1).
Figure 1.
Effect of intraperitoneal administration of cucurbiturils on white blood cells count. (a) The number of white blood cells; (b) Lymphocytes, neutrophils and monocytes measurements. Data are presented as the median with interquartile range. . * Indicates a significant difference (p < 0.05) vs. the control.
Figure 1.
Effect of intraperitoneal administration of cucurbiturils on white blood cells count. (a) The number of white blood cells; (b) Lymphocytes, neutrophils and monocytes measurements. Data are presented as the median with interquartile range. . * Indicates a significant difference (p < 0.05) vs. the control.
The assessment of the immunotoxic effect of cucurbiturils on the central and peripheral organs of the immune system was carried out by the method of determining the activity of LDH. It was found that three times intraperitoneal administration of cucurbiturils at high concentrations did not have a toxic effect on mouse bone marrow cells (Figure 1a). However, it has been shown that the administration of CB[
7] can lead to toxic damage to spleen cells, since a mild toxic effect on splenocytes was found (Figure 1b). However, CB[
6] and CB[
8] did not have a toxic effect on spleen cells. At the same time, the total level of LDH in the serum of mice was increased after administration of CB[
6], CB[
7] and CB[
8] (Figure S2), which indicates a possible toxic effect of high doses of cucurbituril on other organs and tissues. It is known that cucurbiturils have myotoxicity and neurotoxicity [
18], so the increase in LDH in the blood serum is more likely associated with a toxic effect on muscles and nervous system, rather than the immune system.
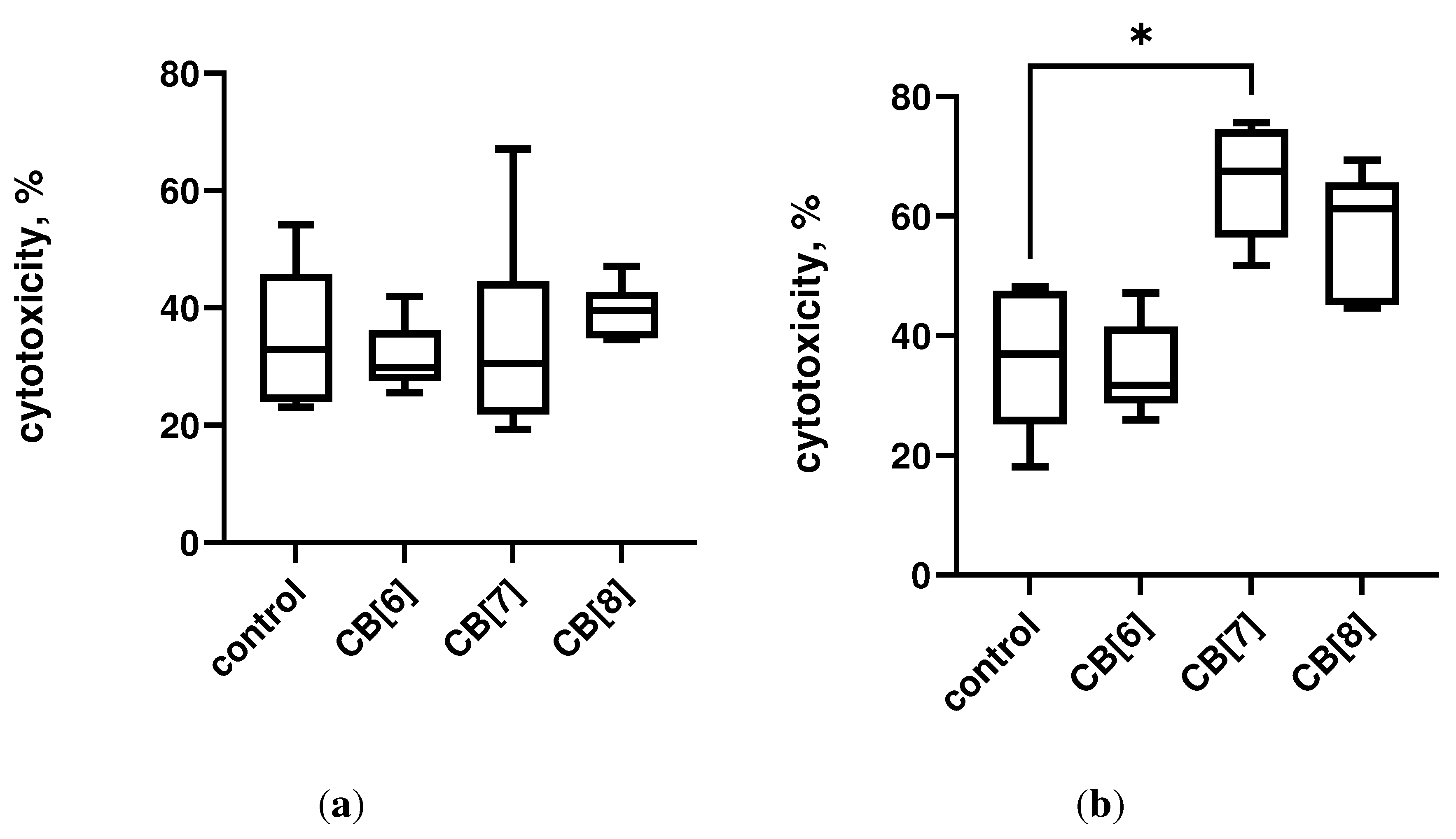
Figure 1. Lactate dehydrogenase (LDH) leakage in cells after intraperitoneal cucurbituril administration. (a) Bone marrow; (b) Spleen. Data are presented as box-and-whisker plots, with boxes extending from the 25th to the 75th percentile, with a horizontal line at the median, while the whiskers extend to the lowest and highest data points. * Indicates a significant difference (p < 0.05) vs. the control.
3.2. Lymphocytes
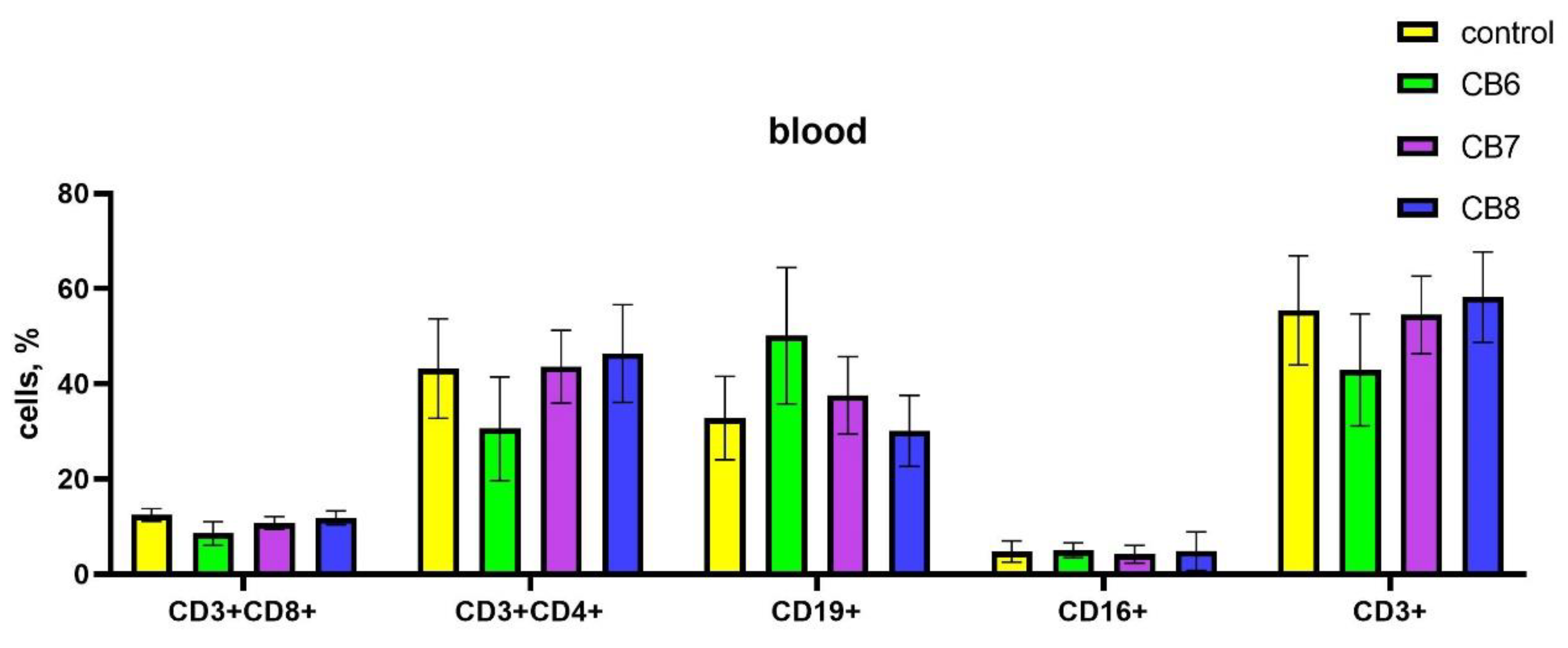
The impact of cucurbiturils on the subpopulation composition of peripheral blood, spleen and bone marrow lymphocytes of laboratory animals was assessed. It was found that after the injections of CB[
6] in the peripheral blood there was a decrease in CD4+ T-helpers, and at the same time an increase in the proportion of CD19+ B-lymphocytes compared with the control (
Figure 2). CB[
7] and CB[
8] did not affect the subpopulation composition of peripheral blood lymphocytes.
Figure 2.
Evaluation of peripheral blood subsets percentage in response to intraperitoneal administration of cucurbiturils. Data are presented as the median with interquartile range.
Figure 2.
Evaluation of peripheral blood subsets percentage in response to intraperitoneal administration of cucurbiturils. Data are presented as the median with interquartile range.
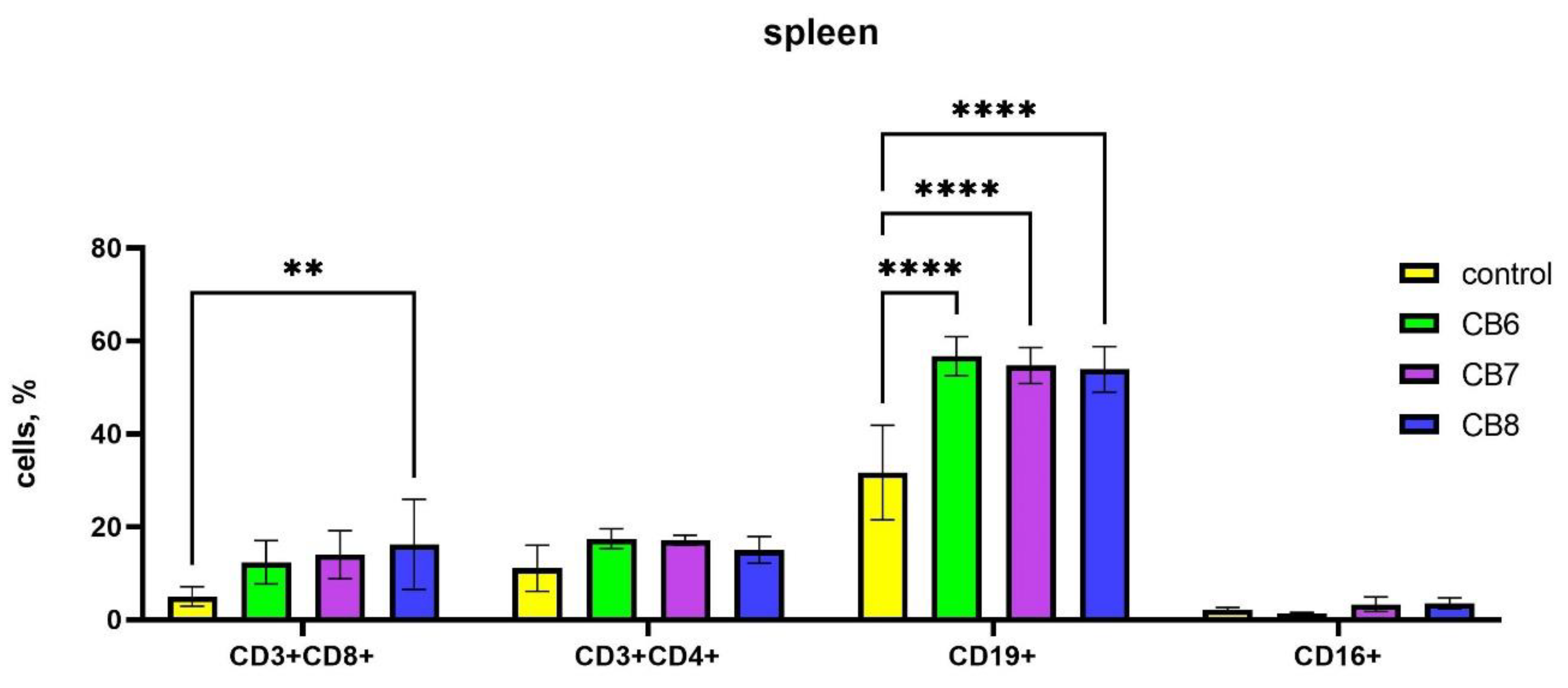
Since the peripheral blood contains only a small proportion of the body's lymphocytes, the next step was to assess the subpopulation composition of lymphocytes in the spleen, where lymphocytes are found in large numbers in the white pulp. In the case of assessing the influence of the subpopulation composition of spleen cells, CB[
6] and CB[
7] increased the number of B-lymphocytes, and CB[
8] increased the amount of B-cells and cytotoxic T-lymphocytes (
Figure 3).
Figure 3.
Evaluation of spleen subsets percentage in response to intraperitoneal administration of cucurbiturils. Data are presented as the median with interquartile range. (** Indicates a significant difference (p < 0.01) vs. the control. **** Indicates a significant difference (p < 0.0001) vs. the control.).
Figure 3.
Evaluation of spleen subsets percentage in response to intraperitoneal administration of cucurbiturils. Data are presented as the median with interquartile range. (** Indicates a significant difference (p < 0.01) vs. the control. **** Indicates a significant difference (p < 0.0001) vs. the control.).
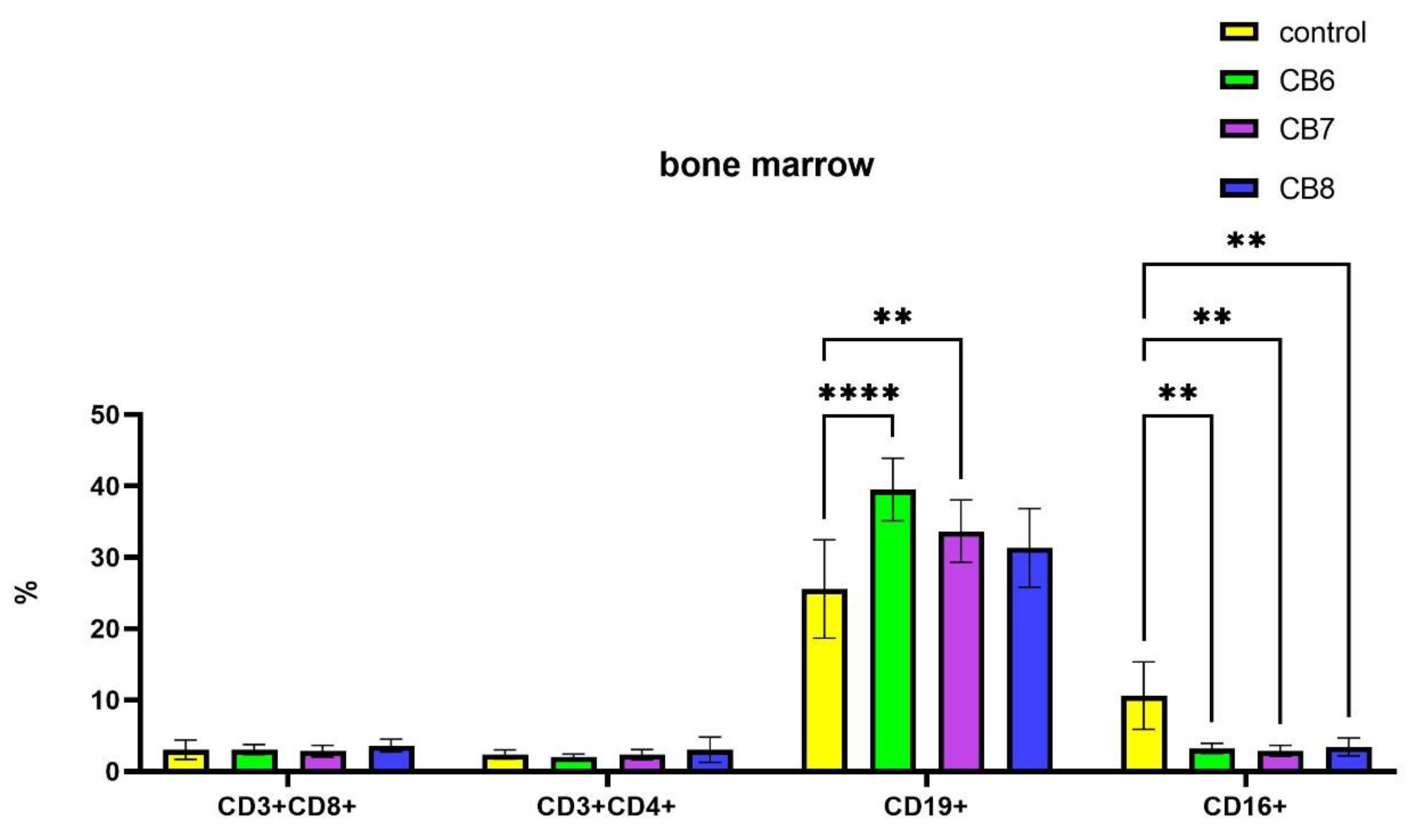
It is known that the formation of new naive B lymphocytes occurs in the bone marrow. In bone marrow CB[
6] and CB[
7] led to an increase in the relative number of B-lymphocytes, while the number of CD16+ NK cells decreased proportionally (
Figure 4). CB[
8] also reduces the relative number of NK cells.
Thus, our studies have shown that CB[
6] and CB[
7] can increase the percentage of B cells in the organs of the immune system, both in the spleen and in the bone marrow. The treatment with CB[
8] leads to increase the relative number of B cells and cytotoxic T-lymphocytes in mouse spleen.
Figure 4.
Evaluation of bone marrow lymphocyte subsets percentage in response to intraperitoneal administration of cucurbiturils. Data are presented as the median with interquartile range. (** Indicates a significant difference (p < 0.01) vs. the control. **** Indicates a significant difference (p < 0.0001) vs. the control.).
Figure 4.
Evaluation of bone marrow lymphocyte subsets percentage in response to intraperitoneal administration of cucurbiturils. Data are presented as the median with interquartile range. (** Indicates a significant difference (p < 0.01) vs. the control. **** Indicates a significant difference (p < 0.0001) vs. the control.).
3.3. Immune Responses Findings
Next, we have explored the effect of cucurbiturils on different types of immune responses after SRBCs antigen administration in mice. (Figure 5). Treatment with CB[
6] reduced the level of PFC in the spleen of laboratory animals compared to control. While CB[
7] and CB[
8] did not affect the number of PFCs, the observed slight decrease was not significant. On the contrary, the severity of the DTH reaction increased with the administration of cucurbiturils, while significant changes were observed with the administration of CB[
7] and CB[
8].
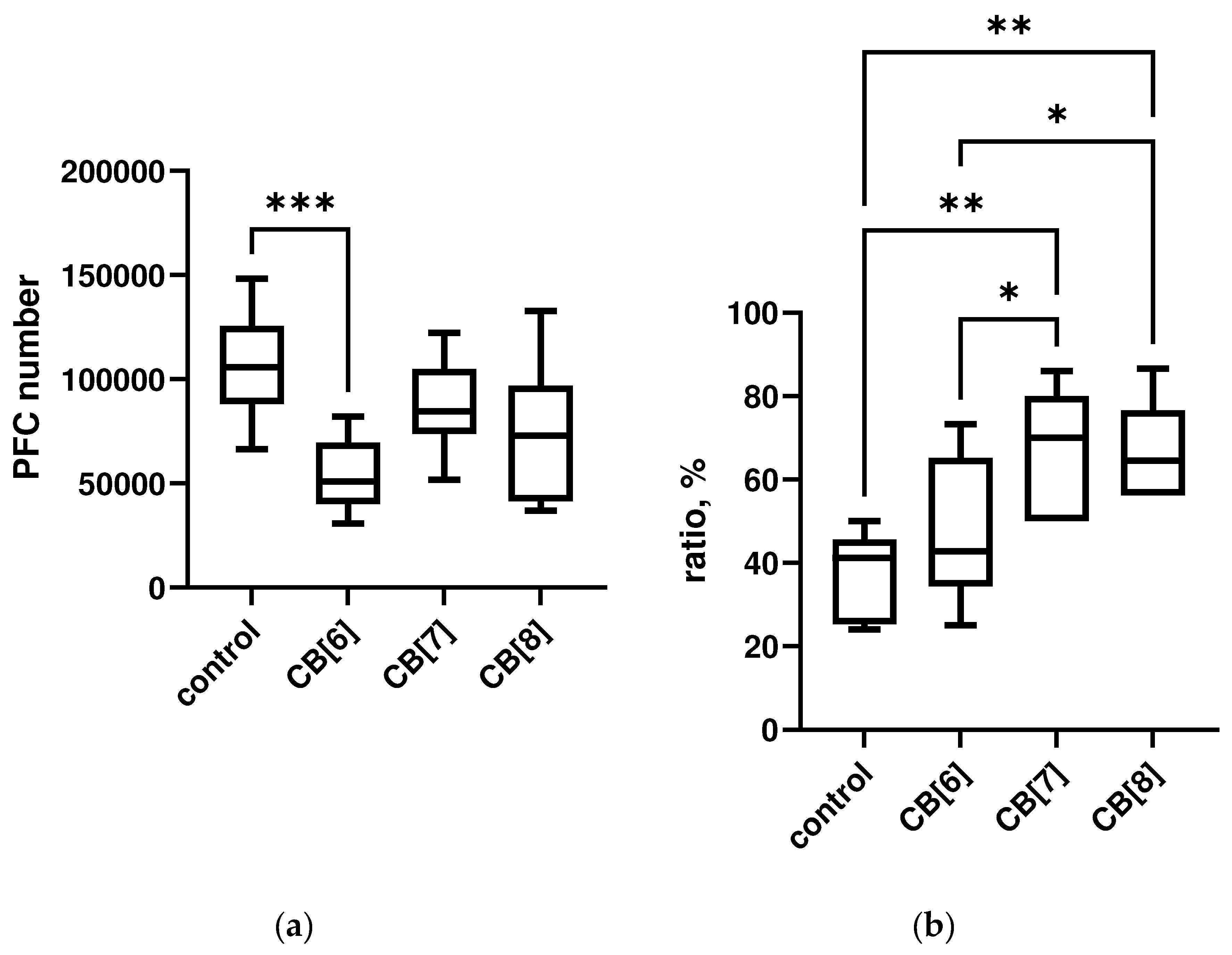
Figure 1. Evaluation of immune response after intraperitoneal cucurbituril administration. (a) IGM-PFC; (b) DTH. Data are presented as box-and-whisker plots, with boxes extending from the 25th to the 75th percentile, with a horizontal line at the median, while the whiskers extend to the lowest and highest data points. * Indicates a significant difference (p < 0.05) vs. the control.
Thus, CB[
6] can suppress the severity of the humoral immune response to the antigen. However, the treatment with CB[
7] and CB[
8] does not suppress the immune response, but stimulates the severity of the DTH reaction.
4. Discussion
The present study was conducted to explore the immunotoxicity and immunomodulation properties of CB[n] in vivo. Intraperitoneal administration of cucurbirturils to mice did not lead to changes in blood parameters, and did not cause the death of immune cells, with the exception of spleen cells when treated with CB[
7]. It should be noted that high concentrations of cucurbiturils were used in this study; when used for drug delivery, these high concentrations are unlikely to be required.
In previous in vitro studies, cucurbiturils did not show immunotoxicity [
24,
25]. In the present animal study, the low immunotoxicity of cucurbiturils was confirmed. Therefore, cucurbiturils have practically no immunotoxicity, except for the cytotoxic effect on spleen cells in vivo when CB[
7] is administered at a high dose, and the trend towards an increase in the number of apoptotic cells in cultures cultivated in the presence of CB[
8] [
25]. The results may indicate the immunosafety of using cucurbiturils for drug delivery.
We also evaluated the influence of the immunomodulatory action of cucurbiturils. It was demonstrated that different homologues have different effects on the immune system. Based on the obtained data in vitro, CB[
8] causes a decrease in the level of IFN-gamma [
26], which indicates its possible immunosuppressive effect. Since СB[
8] is able to suppress cytokine production, a possible immunosuppressive effect requires further study. If immunosuppressive activity is confirmed, CB[
8] can be used as a basis for delivery systems for various immunosuppressive drugs. However, when assessing the cellular and humoral immune response in animals after i.p. administration of CB[
8], no immunosuppression was observed.
In the case of CB[
6], its stimulating effect on the B cells and humoral immune response was demonstrated, since it increased the expression of HLA-DR molecules on B-lymphocytes [
15], increased the level of spontaneous production of IL-4 in 1.5 times [
26], and also increased in vivo the relative number of B cells in the organs of the immune system. At the same time, the severity of the humoral immune response to a certain antigen after the introduction of CB[
6] was reduced, which requires studying the mechanisms of this process. Perhaps the strong nonspecific stimulation of B-lymphocytes did not allow the cells to respond to antigen stimulation.
CB[
7] showed mild immunostimulatory properties, enhancing the DTH response in vivo, increasing the relative number of B cells in mice and suppressing the production of IL-10 in PBMC stimulated culture in vitro [
26]. These properties make CB[
7] an excellent basis for drug delivery systems for the treatment of infectious and tumor diseases. . Complexation of drugs with CB[
7] enchanced their antitumor activities [
30,
31]. It is known that antitumor drugs can have a direct effect on tumor cells, leading to their death. However, in recent years, due to the spread of immunotherapy, more and more information is emerging about the importance of the role of the immune system in protecting against cancer cells, including during chemotherapy. Extensive evidence suggests that the clinical success of chemotherapy is due not only to toxicity to tumor cells, but also to the restoration of immunosurveillance, which has been sorely neglected in previous preclinical and clinical studies [
32,
33,
34]. Immune cells are an important component of the tumor microenvironment, promoting immunosuppression in relation to tumor structures, and also influencing the effectiveness of therapy [
35]. Therefore, when developing new drugs for chemotherapy, it is necessary to evaluate the restoration of the antitumor response and immunological surveillance of the tumor, which will allow a comprehensive assessment of the antitumor effect of the studied constructs even at the stage of preclinical studies. We assume that the use of CB[7} as a drug delivery system can enhance the antitumor effect due to its effect on immune cells in tumor tissue.
Thus, at high concentrations, cucurbiturils may effect on the immune system in vivo. At the same time, the action of various cucurbiturils differs in different homologues, which, apparently, is associated with different interactions with components the internal environment of the body. Since homologues differ in their effects on the immune system in vivo, further research into the mechanisms of such action is required. It is believed that broader and deeper exploration of the possible immune properties of cucurbiturils could facilitate the further development of new drugs and novel treatment strategies.
Author Contributions
Conceptualization, V.K. and E.P.; methodology, E.P. and E.Ga.; investigation and experiments, O.B., M.B., A.A. and E.Go.; validation, L.G. and E.P.; formal analysis, E.P.; resources, E.K.; writing—original draft preparation, E.P.; writing—review and editing, E.K., E.P., N.S.; supervision, L.G.; project administration, E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Russian Science Foundation according to research project No. 19-15-00192.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Local Ethical Committee of Research Institute of Fundamental and Clinical Immunology (protocol No. 130; 30 March 2021).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Rodell C.B., Mealy J..E, Burdick J.A. Supramolecular Guest-Host Interactions for the Preparation of Biomedical Materials. Bioconjug Chem. 2015, 26(12), 2279-89. [CrossRef]
- Li W., Xu W.., Zhang S., Li J, Zhou J., Tian D., Cheng J., Li H. Supramolecular Biopharmaceutical Carriers Based on Host-Guest Interactions. J Agric Food Chem. 2022, 70(40), 12746-12759. [CrossRef]
- Saji V.S. Recent Updates on Supramolecular-Based Drug Delivery - Macrocycles and Supramolecular Gels. Chem Rec. 2022, 22(7), e202200053. [CrossRef]
- Barrow SJ, Kasera S, Rowland MJ, del Barrio J, Scherman OA. Cucurbituril-Based Molecular Recognition. Chem Rev. 2015 Nov 25;115(22):12320-406. [CrossRef]
- Das D., Assaf K.I., Nau W.M. Applications of cucurbiturils in medicinal chemistry and chemical biology. Front. Chem. 2019;7:619–631. [CrossRef]
- Barooah N, Mohanty J, Bhasikuttan AC. Cucurbituril-Based Supramolecular Assemblies: Prospective on Drug Delivery, Sensing, Separation, and Catalytic Applications. Langmuir. 2022 May 24;38(20):6249-6264. [CrossRef]
- Shukla S, Sagar B, Sood AK, Gaur A, Batra S, Gulati S. Supramolecular Chemotherapy with Cucurbit[n]urils as Encapsulating Hosts. ACS Appl Bio Mater. 2023, 6(6), 2089-2101. [CrossRef]
- Mock W. L., Shih N.-Y. Structure and selectivity in host-guest complexes of cucurbituril. J. Org. Chem. 1986, 51, 23, 4440–4446. [CrossRef]
- Wheate NJ, Buck DP, Day AI, Collins JG. Cucurbit[n]uril binding of platinum anticancer complexes. Dalton Trans,. 2006 Jan 21;(3):451-458. [CrossRef]
- Buschmann H.J., Mutihac L., Mutihac R.C., Schollmeyer E. Complexation behavior of cucurbit[6]uril with short polypeptides. Therm. Acta, 2005, 430, 79-82. [CrossRef]
- Fujiwara H., Arakawa H., Murata S., Sasaki Y. Entropy Changes in the Inclusion Complex Formation of α-Cyclodextrin with Alcohols as Studied by the Titration Calorimetry. Bull. Chem. Soc. Jpn. 1987, 60, 3891 – 3894.
- Lagona J., Mukhopadhyay P., Chacrabarti S., Isaacs L. The Cucurbit[n]uril Family. Angew. Chem. Int. Ed. 2005, 44, 4844-4870. [CrossRef]
- Walker S., Kaur R., McInnesF.J., WheateN.J.Synthesis, processing and solid state excipient interactions of cucurbit[6]uril and its formulation into tablets for oral drug delivery. Mol. Pharm. 2010, 7, 2166-2172. [CrossRef]
- Montes-Navajas P., González-Béjar M., Scaiano J.C., García H. Cucurbituril complexes cross the cell membrane. // PhotochemPhotobiol Sci., 2009, 8(12), 1743-7. [CrossRef]
- Uzunova V.D., Cullinane C., Brix K., Nau W.M., Day A.I. Toxicity of cucurbit[7]uril and cucurbit[8]uril: An exploratory in vitro and in vivo study. Org. Biomol. Chem. 2010;8:2037–2042. [CrossRef]
- Chen H., Chan J.Y.W., Yang X., Wyman I.W., Macartney D.H., Bardelang D., Lee S.M.Y., Wang R. Developmental and organspecific toxicity of cucurbit[7]uril: In vivo study on zebrafish models. RSC Adv. 2015;5:30067–30074. [CrossRef]
- Chen H., Chan J.Y.W., Li S., Liu J.J., Wyman I.W., Lee S.M.Y., Macartney D.H., Wang R. In vivo reversal of general anesthesia by cucurbit[7]uril with zebrafish models. RSC Adv. 2015;5:63745–63752. [CrossRef]
- Oun R., Floriano R.S., Isaacs L., Rowana E.G., Wheate N.J. The ex vivo neurotoxic, myotoxic and cardiotoxic activity of cucurbiturilbased macrocyclic drug delivery vehicles. Toxicol. Res. 2014;3:447–455. [CrossRef]
- Zhang X., Xu X., Li S., Wang L.H., Zhang J., Wang R. A systematic evaluation of the biocompatibility of cucurbit[7]uril in mice. Sci. Rep. 2018;8:8819–8825. [CrossRef]
- Pejchal J., Jošt P., Múčková L., Andrýs R., Lísa M., Zdarova Karasova J. A systematic evaluation of the cucurbit[7]uril pharmacokinetics and toxicity after a single dose and short-term repeated administration in mice. Arch Toxicol. 2022 May;96(5):1411-1421. [CrossRef]
- Lucia Appleton S, Navarro-Orcajada S, Martínez-Navarro FJ, Caldera F, López-Nicolás JM, Trotta F, Matencio A. Cyclodextrins as Anti-inflammatory Agents: Basis, Drugs and Perspectives. Biomolecules. 2021;11(9):1384. [CrossRef]
- Braga, S.S.; Barbosa, J.S.; Santos, N.E.; El-Saleh, F.; Paz, F.A.A. Cyclodextrins in Antiviral Therapeutics and Vaccines. Pharmaceutics 2021, 13, 409. [CrossRef]
- Onishi, M.; Ozasa, K.; Kobiyama, K.; Ohata, K.; Kitano, M.; Taniguchi, K.; Homma, T.; Kobayashi, M.; Sato, A.; Katakai, Y.; et al. Hydroxypropyl-β-cyclodextrin spikes local inflammation that induces Th2 cell and T follicular helper cell responses to the coadministered antigen. J. Immunol. 2015, 194, 2673–2682. [CrossRef]
- Pashkina E., Aktanova A., Blinova E., Mirzaeva I., Kovalenko E., Knauer N., Ermakov A., Kozlov V. Evaluation of the Immunosafety of Cucurbit[n]uril on Peripheral Blood Mononuclear Cells In Vitro. Molecules. 2020;25:3388. [CrossRef]
- Aktanova A., Abramova T., Pashkina E., Boeva O., Grishina L., Kovalenko E., Kozlov V. Assessment of the Biocompatibility of Cucurbiturils in Blood Cells. Nanomaterials. 2021;11:1356. [CrossRef]
- Aktanova A.A., Kovalenko E.A., Pashkina E.A. Effect of cucurbiturils on cytokine production by peripheral blood mononuclear cells of healthy donors // Russian Journal of Immunology. – 2022, 25, 369-374. [CrossRef]
- Aktanova A.A., Boeva O.S., Barkovskaya M.S., Kovalenko E.A., Pashkina E.A. Influence of Cucurbiturils on the Production of Reactive Oxygen Species by T- and B-Lymphocytes, Platelets and Red Blood Cells. Int J Mol Sci. 2023, 24(2), 1441. [CrossRef]
- Day A., Arnold A.P., Blanch R.J., Snushall B. Controlling factors in the synthesis of cucurbituril and its homologues. J. Org. Chem. 2001;66:8094–8100. [CrossRef]
- O'Connell K.E., Mikkola A.M., Stepanek A.M., Vernet A, Hall C.D.., Sun C., Yildirim E., Staropoli J.F., Lee J.T., Brown D.E. Practical murine hematopathology: a comparative review and implications for research. Comp Med. 2015, 65(2), 96-113.
- Pashkina E.A., Grishina L.V., Aktanova A.A., Kozlov V.A. Antitumor activity of supramolecular complexes of cucurbituril with platinum(II) compounds. Inorg. Chim. Acta. 2021;522:120370. [CrossRef]
- Li Y, Su Y, Li Z, Chen Y. Supramolecular Combination Cancer Therapy Based on Macrocyclic Supramolecular Materials. Polymers. 2022; 14(22):4855. [CrossRef]
- Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell. 2015 Dec 14;28(6):690-714. [CrossRef]
- Zhang L, Zhou C, Zhang S, Chen X, Liu J, Xu F, Liang W. Chemotherapy reinforces anti-tumor immune response and enhances clinical efficacy of immune checkpoint inhibitors. Front Oncol. 2022 Aug 8;12:939249. [CrossRef]
- Merlano MC, Denaro N, Galizia D, Ruatta F, Occelli M, Minei S, Abbona A, Paccagnella M, Ghidini M, Garrone O. How Chemotherapy Affects the Tumor Immune Microenvironment: A Narrative Review. Biomedicines. 2022 Jul 28;10(8):1822. [CrossRef]
- Binnewies, M., Roberts, E.W., Kersten, K. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med 24, 541–550 (2018). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).