Submitted:
11 December 2023
Posted:
11 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample Characteristics
2.2. Immunohistochemistry
2.3. Statistical Analysis
| Antibody | Product number |
Source | Clone | Treatment | Dilution |
|---|---|---|---|---|---|
| CD56/NCAM | M730429-2 | Agilent | 123C3 | HIER | 1:100 |
| CD117/c-kit | A450229-2 | Agilent | polyclonal | HIER | 1:50 |
| DSP | sc-73632 | Santa Cruz Biotechnology | polyclonal | Digestion | 1:4000 |
| HES1 | #11988 | Cell Signalling Technology | D6P2U | HIER* | 1:200 |
| Nestin | HPA007007 | Sigma-Aldrich | polyclonal | HIER* | 1:5000 |
3. Results
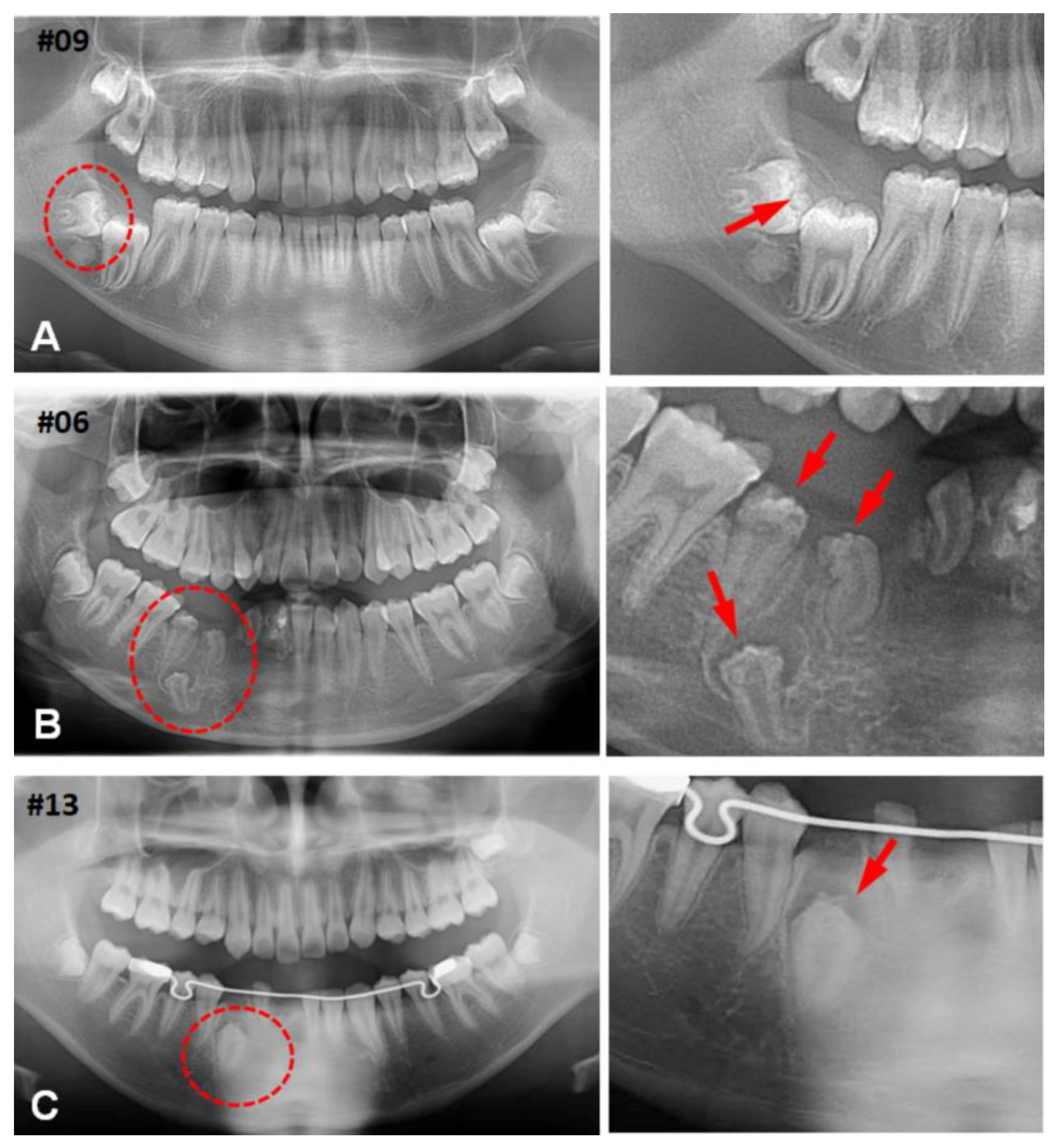
3.1. Clinical Characteristics of (CHDFs)
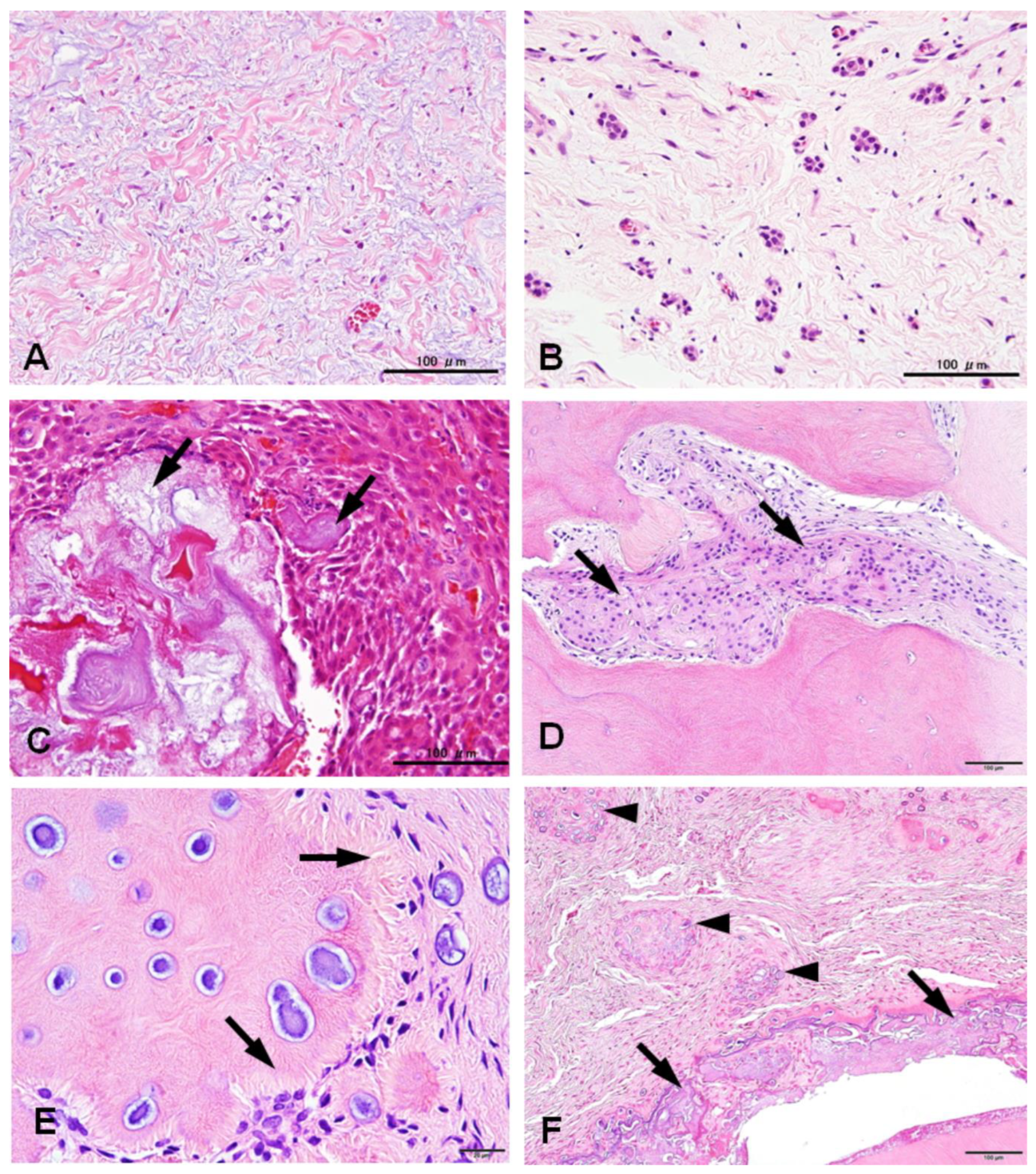
3.2. Histological Changes of HDFs
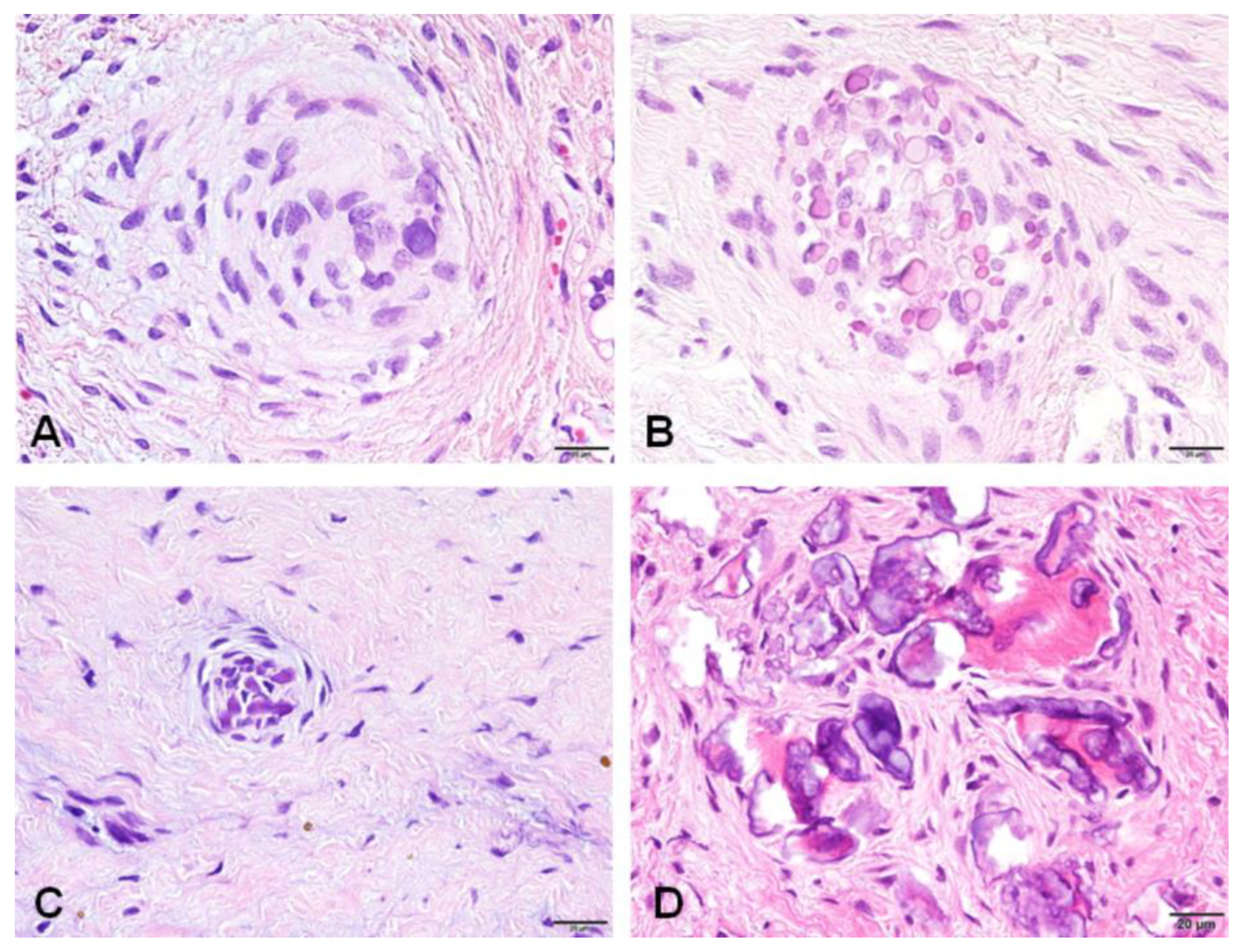
3.2. Immunohistochemical Profiles of CWNs in CHDFs
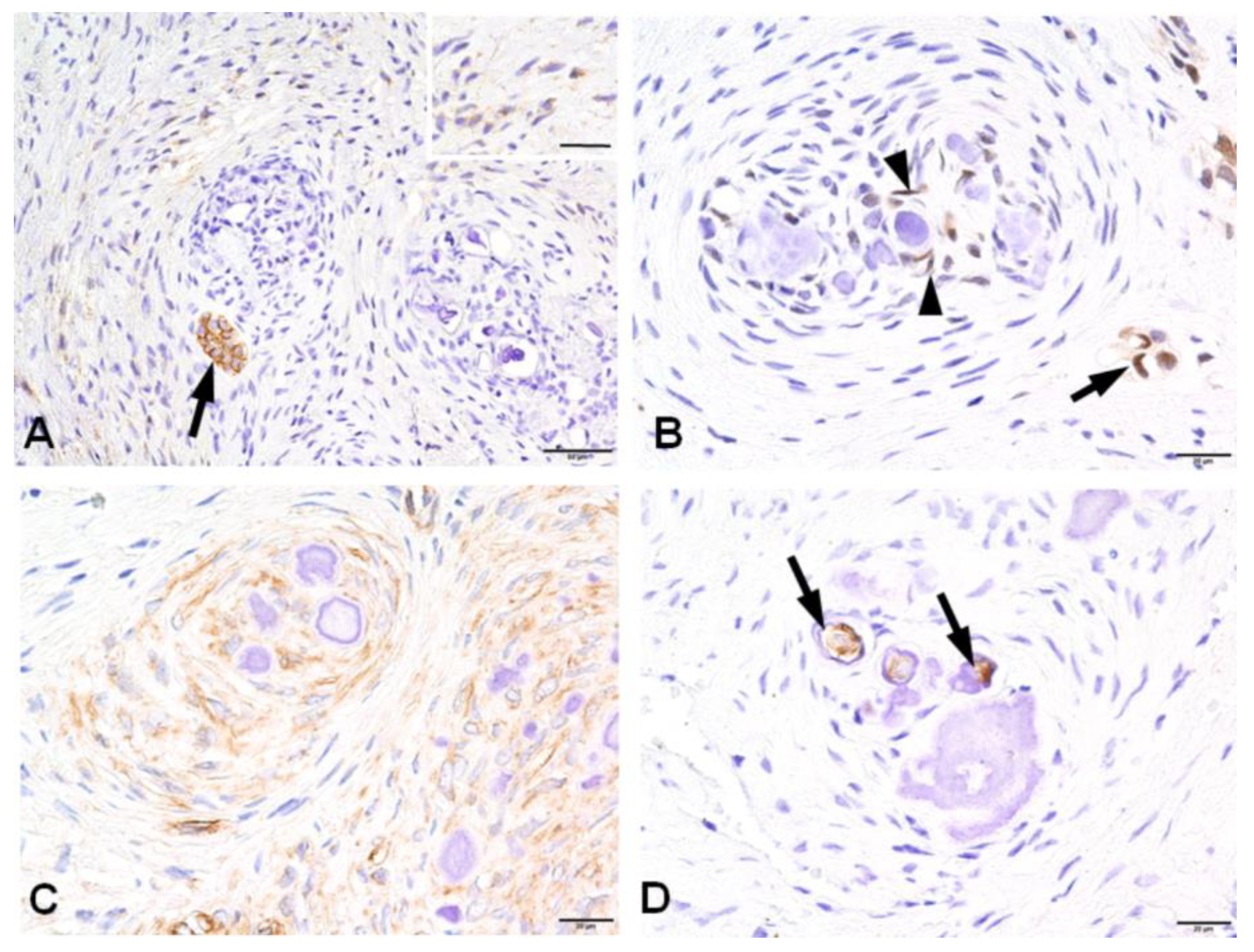
| Components | CD56 | Nestin | HES1 | CD117 | DSP |
|---|---|---|---|---|---|
| Stroma | + | + | + | - | - |
| Epithelial islands | + | - | + | - | - |
| CWNs | - | + | + | - | + |
| Psammoma bodies | - | - | - | - | - |
| Dentinoid | - | - | - | - | - |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karabas, H.C.; Ozcan, I.; Tekkesin, M.S.; Tasyapan, S.A.; Guray, B.; Atapek, M.M. Evaluation of Radiolucent Lesions Associated with Impacted Teeth: A Retrospective Study. Curr Med Imaging 2020, 16, 1332–1339, . [CrossRef]
- Hirschberg, A.; Buchner, A.; Dayan, D. The Central Odontogenic Fibroma and the Hyperplastic Dental Follicle: Study with Picrosirius Red and Polarizing Microscopy. J. Oral Pathol. Med. 1996, 25, 125–127, . [CrossRef]
- Fukuta, Y.; Totsuka, M.; Takeda, Y.; Yamamoto, H. Pathological Study of the Hyperplastic Dental Follicle. J Nihon Univ Sch Dent 1991, 33, 166–173.
- Yonemochi, H.; Noda, T.; Saku, T. Pericoronal Hamartomatous Lesions in the Opercula of Teeth Delayed in Eruption: An Immunohistochemical Study of the Extracellular Matrix. J. Oral Pathol. Med. 1998, 27, 441–452, . [CrossRef]
- Ide, F.; Obara, K.; Yamada, H.; Mishima, K.; Saito, I.; Horie, N.; Shimoyama, T.; Kusama, K. Hamartomatous Proliferations of Odontogenic Epithelium within the Jaws: A Potential Histogenetic Source of Intraosseous Epithelial Odontogenic Tumors. J. Oral Pathol. Med. 2007, 36, 229–235, . [CrossRef]
- Sandler, H.J.; Nersasian, R.R.; Cataldo, E.; Pochebit, S.; Dayal, Y. Multiple Dental Follicles with Odontogenic Fibroma-like Changes (WHO Type). Oral Surg. Oral Med. Oral Pathol. 1988, 66, 78–84.
- Gardner, D.G.; Radden, B. Multiple Calcifying Hyperplastic Dental Follicles. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995, 79, 603–606.
- Gomez, R.S.; Silva, E.C.; Silva-Filho, E.C.; Castro, W.H. Multiple Calcifying Hyperplastic Dental Follicles. J. Oral Pathol. Med. 1998, 27, 333–334.
- Cho, Y.-A.; Yoon, H.-J.; Hong, S.-P.; Lee, J.-I.; Hong, S.-D. Multiple Calcifying Hyperplastic Dental Follicles: Comparison with Hyperplastic Dental Follicles. J. Oral Pathol. Med. 2011, 40, 243–249, . [CrossRef]
- Aydin, U.; Baykul, T.; Yildirim, B.; Yildirim, D.; Bozdemir, E.; Karaduman, A. Multiple Calcifying Hyperplastic Dental Follicles: A Case Report. Imaging Sci Dent 2013, 43, 303–308, . [CrossRef]
- Jamshidi, S.; Zargaran, M.; Mohtasham, N. Multiple Calcifying Hyperplastic Dental Follicle (MCHDF): A Case Report. J Dent Res Dent Clin Dent Prospects 2013, 7, 174–176, . [CrossRef]
- Desai, R.S.; Momin, Y.N.A.; Bansal, S.; Karjodkar, F.R. Multiple Calcifying Hyperplastic Dental Follicles: A Case Report and Literature Review. J. Oral Maxillofac. Surg. 2017, 75, 1702–1705, . [CrossRef]
- Davari, D.; Arzhang, E.; Soltani, P. Multiple Calcifying Hyperplastic Dental Follicles: A Case Report. J. Oral Maxillofac. Surg. 2019, 77, 757–761, . [CrossRef]
- Ulutürk, H.; Yücel, E.; Akinci, H.O.; Calisan, E.B.; Yildirim, B.; Gizli, A. Multiple Calcifying Hyperplastic Dental Follicles. J Stomatol Oral Maxillofac Surg 2019, 120, 77–79, . [CrossRef]
- Jussila, M.; Thesleff, I. Signaling Networks Regulating Tooth Organogenesis and Regeneration, and the Specification of Dental Mesenchymal and Epithelial Cell Lineages. Cold Spring Harb Perspect Biol 2012, 4, a008425, . [CrossRef]
- Rusu, M.C.; Loreto, C.; Sava, A.; Mănoiu, V.; Didilescu, A.C. Human Adult Dental Pulp CD117/c-Kit-Positive Networks of Stromal Cells. Folia Morphol. (Warsz) 2014, 73, 68–72, . [CrossRef]
- Pisciotta, A.; Carnevale, G.; Meloni, S.; Riccio, M.; De Biasi, S.; Gibellini, L.; Ferrari, A.; Bruzzesi, G.; De Pol, A. Human Dental Pulp Stem Cells (hDPSCs): Isolation, Enrichment and Comparative Differentiation of Two Sub-Populations. BMC Dev. Biol. 2015, 15, 14, . [CrossRef]
- Lima, R.L.; Holanda-Afonso, R.C.; Moura-Neto, V.; Bolognese, A.M.; DosSantos, M.F.; Souza, M.M. Human Dental Follicle Cells Express Embryonic, Mesenchymal and Neural Stem Cells Markers. Arch. Oral Biol. 2017, 73, 121–128, . [CrossRef]
- Kanao, S.; Ogura, N.; Takahashi, K.; Ito, K.; Suemitsu, M.; Kuyama, K.; Kondoh, T. Capacity of Human Dental Follicle Cells to Differentiate into Neural Cells In Vitro. Stem Cells Int 2017, 2017, 8371326, . [CrossRef]
- Quispe-Salcedo, A.; Ida-Yonemochi, H.; Nakatomi, M.; Ohshima, H. Expression Patterns of Nestin and Dentin Sialoprotein during Dentinogenesis in Mice. Biomed. Res. 2012, 33, 119–132, . [CrossRef]
- Nakatomi, M.; Quispe-Salcedo, A.; Sakaguchi, M.; Ida-Yonemochi, H.; Okano, H.; Ohshima, H. Nestin Expression Is Differently Regulated between Odontoblasts and the Subodontoblastic Layer in Mice. Histochem. Cell Biol. 2018, 149, 383–391, . [CrossRef]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software “EZR” for Medical Statistics. Bone Marrow Transplant 2013, 48, 452–458, . [CrossRef]
- Al-Tuwirqi, A.; Lambie, D.; Seow, W.K. Regional Odontodysplasia: Literature Review and Report of an Unusual Case Located in the Mandible. Pediatr Dent 2014, 36, 62–67.
- Schmitd, L.B.; Bravo-Calderón, D.M.; Soares, C.T.; Oliveira, D.T. Hyperplastic Dental Follicle: A Case Report and Literature Review. Case Rep Dent 2014, 2014, 251892, . [CrossRef]
- Angiero, F.; Rossi, C.; Ferri, A.; Seramondi, R.; Magistro, S.; Farronato, D.; Benedicenti, S.; Farronato, G.; Fini, M.; Carpi, A.; et al. Stromal Phenotype of Dental Follicle Stem Cells. Front Biosci (Elite Ed) 2012, 4, 1009–1014.
- Bernal, A.; Arranz, L. Nestin-Expressing Progenitor Cells: Function, Identity and Therapeutic Implications. Cell Mol Life Sci 2018, 75, 2177–2195, . [CrossRef]
- Morsczeck, C.; Ernst, W.; Florian, C.; Reichert, T.E.; Proff, P.; Bauer, R.; Müller-Richter, U.; Driemel, O. Gene Expression of Nestin, Collagen Type I and Type III in Human Dental Follicle Cells after Cultivation in Serum-Free Medium. Oral Maxillofac Surg 2008, 12, 89–92, . [CrossRef]
- Fujita, S.; Hideshima, K.; Ikeda, T. Nestin Expression in Odontoblasts and Odontogenic Ectomesenchymal Tissue of Odontogenic Tumours. J Clin Pathol 2006, 59, 240–245, . [CrossRef]
- Pan, H.; Yang, Y.; Xu, H.; Jin, A.; Huang, X.; Gao, X.; Sun, S.; Liu, Y.; Liu, J.; Lu, T.; et al. The Odontoblastic Differentiation of Dental Mesenchymal Stem Cells: Molecular Regulation Mechanism and Related Genetic Syndromes. Front Cell Dev Biol 2023, 11, 1174579, . [CrossRef]
- Li, W.; Chen, L.; Chen, Z.; Wu, L.; Feng, J.; Wang, F.; Shoff, L.; Li, X.; Donly, K.J.; MacDougall, M.; et al. Dentin Sialoprotein Facilitates Dental Mesenchymal Cell Differentiation and Dentin Formation. Sci Rep 2017, 7, 300, . [CrossRef]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch Signaling Pathway: Architecture, Disease, and Therapeutics. Signal Transduct Target Ther 2022, 7, 95, . [CrossRef]
- Kopan, R.; Ilagan, M.X.G. The Canonical Notch Signaling Pathway: Unfolding the Activation Mechanism. Cell 2009, 137, 216–233, . [CrossRef]
- Liu, Z.-H.; Dai, X.-M.; Du, B. Hes1: A Key Role in Stemness, Metastasis and Multidrug Resistance. Cancer Biol. Ther. 2015, 16, 353–359, . [CrossRef]
- Kobayashi, T.; Kageyama, R. Hes1 Regulates Embryonic Stem Cell Differentiation by Suppressing Notch Signaling. Genes Cells 2010, 15, 689–698, . [CrossRef]
- Mustonen, T.; Tümmers, M.; Mikami, T.; Itoh, N.; Zhang, N.; Gridley, T.; Thesleff, I. Lunatic Fringe, FGF, and BMP Regulate the Notch Pathway during Epithelial Morphogenesis of Teeth. Dev. Biol. 2002, 248, 281–293.
- Felszeghy, S.; Suomalainen, M.; Thesleff, I. Notch Signalling Is Required for the Survival of Epithelial Stem Cells in the Continuously Growing Mouse Incisor. Differentiation 2010, 80, 241–248, . [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





