Submitted:
09 December 2023
Posted:
12 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
- Wastes of agriculture, most abundant ones are wheat straw, beet pulp, sugar cane pulp, corn cobs, grain husks.
- Wastes of wood, paper industries.
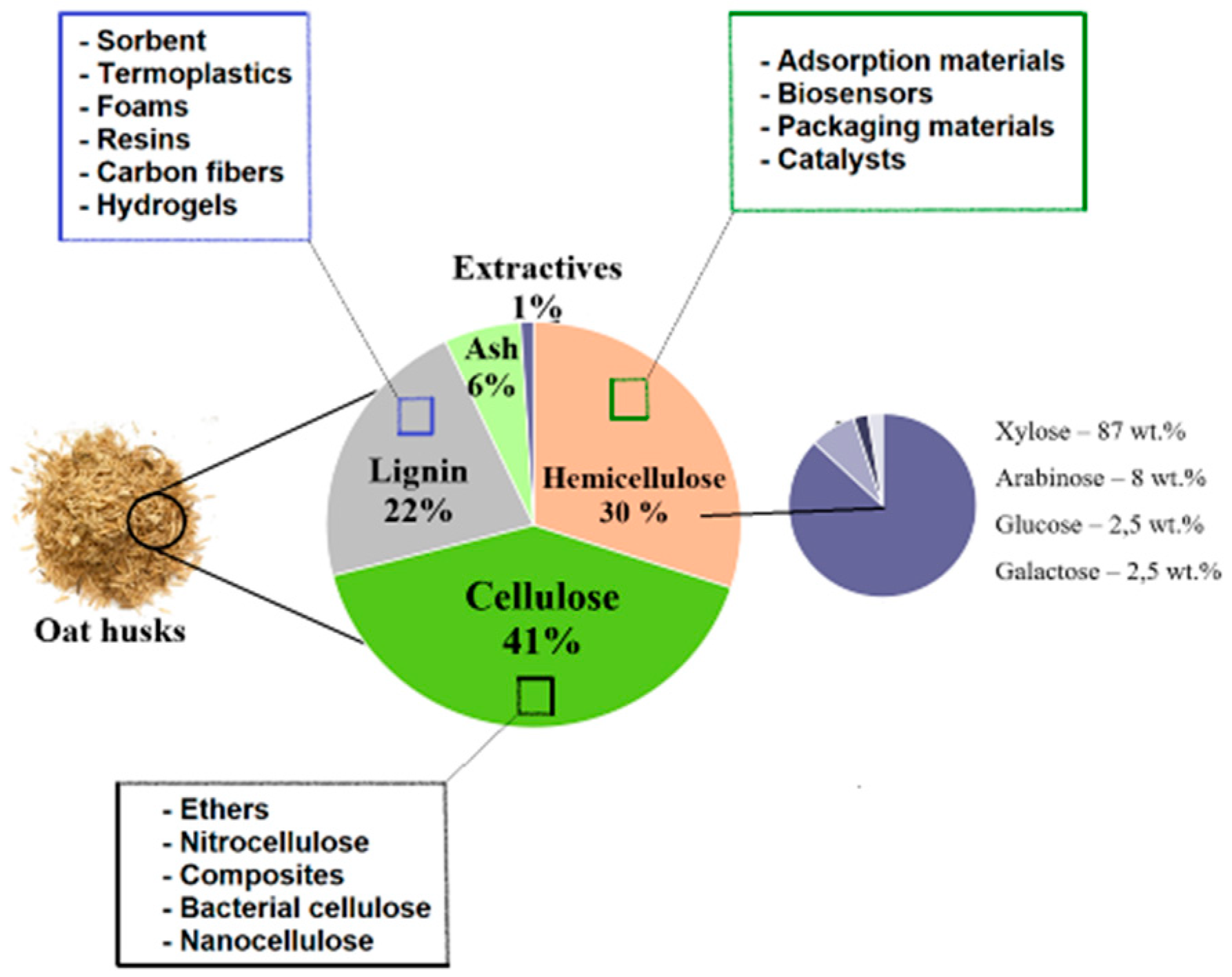
2. Composition of lignocellulose of oat hulls and the main challenges of its processing
3. Pretreatment and delignification of oat husks
3.1. Acid, alkaly and physical treatment
3.1.1. Acid treatment
3.1.2. Alkali treatment
3.1.3. Combination of chemical and physical treatment approaches
- -
- alkali pretreatment with sodium hydroxide solutions (0.5%, 1%, 2%, 3% or 4% (w/v)) spent at S:L of 1:10 (w/v), 121 °C, 0.5 h;
- -
- alkali pretreatment with H2O2 solution (7.5% (v/v), pH = 11.5, S:L of 1:25 (w/v), 25 °C, 1 h);
- -
- alkali treatment using ultrasonication (1% (w/v) NaOH solution, S:L of 1:7 (w/v), power 90 W, frequency 20 kHz, 20 min);
- -
- sequential alkaline (1% NaOH solution (w/v), S:L of 1:10 (w/v), 121 °C, 0.5 h) and ultrasonic pretreatment (water, S:L of 1:7 (w/v), power 90 W, frequency 20 kHz, 20 min).
3.2. Organosolv treatment
3.3. Hydrotropic method
4. Materials based on cellulose isolated of oat husks
4.1. Cellulose ethers
4.2. Nitrocellulose
4.3. Cellulose biodegradable composites
4.4. Nanocellulose
4.4.1. Bacterial nanocellulose
4.4.2. Nano fibrillated cellulose
4.4.3. Nanocrystal cellulose
4.5. Hydrogels and aerogels
5. Conclusions
- -
- world production of oats (Avena sativa) reached eight million tons, it generates upto four million tons of hulls as it takes almost 50% of grain wait,
- -
- high content of fiber in the oat husk (90 g per 100 g of the feedstock),
- -
- cellulose amount in oat husks can be upto 45%.
- Cellulose isolated from oat wastes can be used for the production of different materials such as hydro and aerogels, bacterial, nanocrystal and nanofibrillated cellulose, cellulose based composites and ethers. All these materials can be spent in different industries: production of pharmaceuticals, medicine (including targeted delivery of drugs, sponges etc.), agriculture (controlled delivery of pesticides or fertilizers), electronics, production of films, membranes, plastics, coatings, paints, biodegradable packages, and plenty of others.
- Pretreatment of the oat husk biomass should be carried out in most cases. When deriving cellulose, one should take into account the following parameters of the product: the portion of impurities, the crystallinity degree and the polymerization.
- One-step acid or alkaline treatments of oat husks at low temperatures allow one to reduce the lignin content in the composition of the cellulose product to 5-13% only. The combination of acid and alkaline treatment results in a reduction in residual non-cellulosic components in the final product. Such cellulose can be used for the synthesis of ethanol or for the production of composites based on it. Reiteration of treatment (acid-base and/or peroxide-base) combined with bleaching by bases or hydrogen peroxide produces cellulose being suitable for the synthesis of bacterial cellulose, composites, hydrogels, cellulose esters.
- To our knowledge, only a few works are devoted to organosolv or hydrotropic cooking of oat husks. It makes investigations in this field are perspective. Additional investigations are required to increase the purity of the target cellulose.
- A combination of chemical and physical (reactive extrusion, steam treatment) is perspective to produce pure cellulose.
- To obtain bacterial biomass with high yield, it is necessary to use a hydrolyzate which contain only a trace amount of lignin. Pretreatment methods such as single-step alkaline or acidic are apparently not suitable for using the resulting hydrolysates for the biosynthesis of bacterial cellulose in high yields. Oat husk can be considered as a perspective source for the production of cellulose nanocrystals being used in aerogels, water absorbers, food packaging
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Edgar, K.J.; Buchanan, C.M.; Debenham, J.S.; Rundquist, P.A.; Seiler, B.D.; Shelton, M.C.; Tindall, D. Advances in cellulose ester performance and application. Progress in Polymer Science 2001, 26, 1605–1688. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites – A review. Biotechnology Reports 2019, 21, e00316. [Google Scholar] [CrossRef]
- Jacometti, G.A.; Mello, L.R.P.F.; Nascimento, P.H.A.; Sueiro, A.C.; Yamashita, F.; Mali, S. The physicochemical properties of fibrous residues from the agro industry. LWT - Food Science and Technology 2015, 62, 138–143. [Google Scholar] [CrossRef]
- Ben Fradj, N.; Rozakis, S.; Borzęcka, M.; Matyka, M. Miscanthus in the European bio-economy: A network analysis. Industrial Crops and Products 2020, 148, 112281. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Banu, J.R.; Chang, J.-S. Chapter 10 - Biohydrogen Production From Renewable Biomass Resources. In Biohydrogen (Second Edition); Pandey, A., Mohan, S.V., Chang, J.-S., Hallenbeck, P.C., Larroche, C., Eds.; Elsevier, 2019; pp. 247–277. [Google Scholar]
- Chopda, R.; Ferreira, J.A.; Taherzadeh, M.J. Biorefining Oat Husks into High-Quality Lignin and Enzymatically Digestible Cellulose with Acid-Catalyzed Ethanol Organosolv Pretreatment. Processes 2020, 8, 435. [Google Scholar] [CrossRef]
- Galdeano, M.C.; Grossmann, M.V.E. Oat hulls treated with alkaline hydrogen peroxide associated with extrusion as fiber source in cookies. Food Science and Technology 2006, 26, 123–126. [Google Scholar] [CrossRef]
- Schmitz, E.; Nordberg Karlsson, E.; Adlercreutz, P. Warming weather changes the chemical composition of oat hulls. Plant Biology 2020, 22, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Neitzel, N.; Eder, M.; Hosseinpourpia, R.; Walther, T.; Adamopoulos, S. Chemical composition, particle geometry, and micro-mechanical strength of barley husks, oat husks, and wheat bran as alternative raw materials for particleboards. Materials Today Communications 2023, 36, 106602. [Google Scholar] [CrossRef]
- Skiba, E.A.; Budaeva, V.V.; Baibakova, O.V.; Zolotukhin, V.N.; Sakovich, G.V. Dilute nitric-acid pretreatment of oat hulls for ethanol production. Biochemical Engineering Journal 2017, 126, 118–125. [Google Scholar] [CrossRef]
- Murillo, H.A.; Díaz-Robles, L.A.; Santander, R.E.; Cubillos, F.A. Conversion of residual oat husk and pine sawdust by co-hydrothermal carbonization towards biofuel production for pellet stoves. Industrial Crops and Products 2021, 174, 114219. [Google Scholar] [CrossRef]
- Bulkan, G.; Ferreira, J.A.; Taherzadeh, M.J. Retrofitting analysis of a biorefinery: Integration of 1st and 2nd generation ethanol through organosolv pretreatment of oat husks and fungal cultivation. Bioresource Technology Reports 2021, 15, 100762. [Google Scholar] [CrossRef]
- Mesa, L.; Albernas, Y.; Morales, M.; Corsano, G.; González, E. Chapter 11 - Integration of Organosolv Process for Biomass Pretreatment in a Biorefinery. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Mussatto, S.I., Ed.; Elsevier: Amsterdam, 2016; pp. 229–254. [Google Scholar]
- Vásquez-Garay, F.; Carrillo-Varela, I.; Vidal, C.; Reyes-Contreras, P.; Faccini, M.; Teixeira Mendonça, R. A Review on the Lignin Biopolymer and Its Integration in the Elaboration of Sustainable Materials. Sustainability 2021, 13, 2697. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Taran, O.P.; Timofeeva, M.N.; Parmon, V.N. Hydrolysis of Cellulose in the Presence of Catalysts Based on Cesium Salts of Heteropoly Acids. Catalysis in Industry 2021, 13, 73–80. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Taran, O.P.; Bukhtiyarov, A.V.; Aymonier, C.; Prosvirin, I.P.; Parmon, V.N. Hydrothermal Solubilization–Hydrolysis–Dehydration of Cellulose to Glucose and 5-Hydroxymethylfurfural Over Solid Acid Carbon Catalysts. Topics in Catalysis 2018, 61, 1912–1927. [Google Scholar] [CrossRef]
- Sorokina, K.N.; Taran, O.P.; Medvedeva, T.B.; Samoylova, Y.V.; Piligaev, A.V.; Parmon, V.N. Cellulose Biorefinery Based on a Combined Catalytic and Biotechnological Approach for Production of 5-HMF and Ethanol. ChemSusChem 2017, 10, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Gromov, N.V.; Medvedeva, T.B.; Lukoyanov, I.A.; Panchenko, V.N.; Prikhod'ko, S.A.; Parmon, V.N.; Timofeeva, M.N. Hydrolysis-oxidation of cellulose to formic acid in the presence of micellar vanadium-containing molybdophosphoric heteropoly acids. Results in Engineering 2023, 17, 100913. [Google Scholar] [CrossRef]
- Gromov, N.V.; Zhdanok, A.A.; Medvedeva, T.B.; Lukoyanov, I.A.; Poluboyarov, V.A.; Taran, O.P.; Parmon, V.N.; Timofeeva, M.N. Self-Propagating High-Temperature Synthesis of Composite Materials Based on Tungsten Carbides: Effect of Phase Composition on the Yield of Ethylene and Propylene Glycols in the One-Pot Hydrolysis–Hydrogenolysis of Cellulose. Catalysis in Industry 2020, 12, 343–352. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Rodikova, Y.A.; Timofeeva, M.N.; Panchenko, V.N.; Taran, O.P.; Kozhevnikov, I.V.; Parmon, V.N. One-pot synthesis of sorbitol via hydrolysis-hydrogenation of cellulose in the presence of Ru-containing composites. Bioresource Technology 2021, 319, 124122. [Google Scholar] [CrossRef]
- Gromov, N.V.; Medvedeva, T.B.; Panchenko, V.N.; Timofeeva, M.N.; Parmon, V.N. Hydrolysis–Hydrogenation of Arabinogalactan Catalyzed by Ru/Cs3HSiW12O40. Catalysis in Industry 2021, 13, 81–89. [Google Scholar] [CrossRef]
- Nishanth, S.; Chikunov, A.S.; Thankappan, S.; Taran, O.P.; Parmon, V.N.; Uthandi, S. Lignin derived aromatic monomers from birch wood by laccase (LccH) pretreatment and Ru/C catalyst: a two-pot approach for sustainable biorefineries. Biomass Conversion and Biorefinery 2022. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Miroshnikova, A.V.; Baryshnikov, S.V.; Malyar, Y.N.; Skripnikov, A.M.; Fetisova, O.Y.; Yakovlev, V.A.; Taran, O.P. Hydrogenation of fir wood etanollignin with hydrogen in ethanol in the presence of a NiCuMo/SiO2 catalyst. Khimiya Rastitel'nogo Syr'ya [Chemistry of plant raw materials] (in Russian) 2022, 4, 89–98. [Google Scholar] [CrossRef]
- Mesfun, S.; Matsakas, L.; Rova, U.; Christakopoulos, P. Technoeconomic Assessment of Hybrid Organosolv–Steam Explosion Pretreatment of Woody Biomass. Energies 2019, 12. [Google Scholar] [CrossRef]
- Ajao, O.; Benali, M.; Faye, A.; Li, H.; Maillard, D.; Ton-That, M.T. Multi-product biorefinery system for wood-barks valorization into tannins extracts, lignin-based polyurethane foam and cellulose-based composites: Techno-economic evaluation. Industrial Crops and Products 2021, 167, 113435. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, H.; Zhang, X.; Liu, C. Ammonia-assisted hydrothermal carbon material with schiff base structures synthesized from factory waste hemicelluloses for Cr(VI) adsorption. Journal of Environmental Chemical Engineering 2021, 9, 106187. [Google Scholar] [CrossRef]
- Liu, X.; Yang, K.; Chang, M.; Wang, X.; Ren, J. Fabrication of cellulose nanocrystal reinforced nanocomposite hydrogel with self-healing properties. Carbohydrate Polymers 2020, 240, 116289. [Google Scholar] [CrossRef]
- Li, Z.; Pan, X. Strategies to modify physicochemical properties of hemicelluloses from biorefinery and paper industry for packaging material. Reviews in Environmental Science and Bio/Technology 2018, 17, 47–69. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, N.; Chen, M.; Wei, Y.; Liu, C. Functional packaging films originating from hemicelluloses laurate by direct transesterification in ionic liquid. Carbohydrate Polymers 2020, 229, 115336. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Han, G.; Cai, J.; Wang, X. Au@Carbon quantum Dots-MXene nanocomposite as an electrochemical sensor for sensitive detection of nitrite. Journal of Colloid and Interface Science 2022, 607, 1313–1322. [Google Scholar] [CrossRef]
- Rao, J.; Lv, Z.; Chen, G.; Peng, F. Hemicellulose: Structure, chemical modification, and application. Progress in Polymer Science 2023, 140, 101675. [Google Scholar] [CrossRef]
- Gromov, N.V.; Taran, O.P.; Sorokina, K.N.; Mishchenko, T.I.; Uthandi, S.; Parmon, V.N. New methods for the one-pot processing of polysaccharide components (cellulose and hemicelluloses) of lignocellulose biomass into valuable products. Part 1: Methods for biomass activation. Catalysis in Industry 2016, 8, 176–186. [Google Scholar] [CrossRef]
- Denisova, M.N.; Budaeva, V.V.; Pavlov, I.N. Pulps isolated from Miscanthus, oat hulls, and intermediate flax straw with sodium benzoate. Korean Journal of Chemical Engineering 2015, 32, 202–205. [Google Scholar] [CrossRef]
- Denisova, M.N.; Makarova, E.I.; Pavlov, I.N.; Budaeva, V.V.; Sakovich, G.V. Enzymatic Hydrolysis of Hydrotropic Pulps at Different Substrate Loadings. Applied Biochemistry and Biotechnology 2016, 178, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Anu, *!!! REPLACE !!!*; Kumar, A.; Rapoport, A.; Kunze, G.; Kumar, S.; Singh, D.; Singh, B. Multifarious pretreatment strategies for the lignocellulosic substrates for the generation of renewable and sustainable biofuels: A review. Renewable Energy 2020, 160, 1228–1252. [Google Scholar] [CrossRef]
- Singh nee’ Nigam, P.; Gupta, N.; Anthwal, A. Pre-treatment of Agro-Industrial Residues. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues; Singh nee’ Nigam, P., Pandey, A., Eds.; Springer Netherlands: Dordrecht, 2009; pp. 13–33. [Google Scholar]
- Kashcheyeva, E.I.; Gismatulina, Y.A.; Budaeva, V.V. Pretreatments of Non-Woody Cellulosic Feedstocks for Bacterial Cellulose Synthesis. Polymers 2019, 11, 1645. [Google Scholar] [CrossRef] [PubMed]
- Paschoal, G.B.; Muller, C.M.O.; Carvalho, G.M.; Tischer, C.A.; Mali, S. Isolation and characterization of nanofibrillated cellulose from oat hulls. Química Nova 2015, 38. [Google Scholar] [CrossRef]
- Pereira, J.F.; Marim, B.M.; Mali, S. Chemical Modification of Cellulose Using a Green Route by Reactive Extrusion with Citric and Succinic Acids. Polysaccharides 2022, 3, 292–305. [Google Scholar] [CrossRef]
- Gil Giraldo, G.A.; Mantovan, J.; Marim, B.M.; Kishima, J.O.F.; Mali, S. Surface Modification of Cellulose from Oat Hull with Citric Acid Using Ultrasonication and Reactive Extrusion Assisted Processes. Polysaccharides 2021, 2, 218–233. [Google Scholar] [CrossRef]
- Protopopov, A.V.; Bobrovskaya, S.A.; Voroshilova, A.V.; Klevtsova, M.V. Cellulose ethers with aromatic oxy acids from the oat hulls. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2016, 2, 171–176. [Google Scholar]
- Sakovich, G.V.; Budaeva, V.V.; Korchagina, A.A.; Gismatulina, Y.A. Prospects for cellulose nitrates from non-traditional raw materials for explosive compositions. Khimiya Rastitel'nogo Syr'ya [Chemistry of plant raw materials] (in Russian) 2019, 1, 259–268. [Google Scholar] [CrossRef]
- Sakovich, G.V.; Mikhailov, Y.M.; Budaeva, V.V.; Korchagina, A.A.; Gismatulina, Y.A.; Kozyrev, N.V. Cellulose Nitrates from Unconventional Feedstocks. Doklady Chemistry 2018, 483, 287–291. [Google Scholar] [CrossRef]
- Korchagina, A.A.; Budaeva, V.V.; Kukhlenko, A.A. Esterification of oat-hull cellulose. Russian Chemical Bulletin 2019, 68, 1282–1288. [Google Scholar] [CrossRef]
- Sakovich, G.V.; Budaeva, V.V.; Korchagina, A.А.; Gismatulina, Y.А.; Kozyrev, N.V.; Vakutin, А.G. Oat-hull cellulose nitrates for explosive compositions. Doklady Academii nauk [Reports of the Academy of Sciences] (in Russian) 2019, 487, 391–395. [Google Scholar] [CrossRef]
- Oliveira, J.P.d.; Bruni, G.P.; Lima, K.O.; Halal, S.L.M.E.; Rosa, G.S.d.; Dias, A.R.G.; Zavareze, E.d.R. Cellulose fibers extracted from rice and oat husks and their application in hydrogel. Food Chemistry 2017, 221, 153–160. [Google Scholar] [CrossRef]
- Valdebenito, F.; Pereira, M.; Ciudad, G.; Azocar, L.; Briones, R.; Chinga-Carrasco, G. On the nanofibrillation of corn husks and oat hulls fibres. Industrial Crops and Products 2017, 95, 528–534. [Google Scholar] [CrossRef]
- Cardoso, M.A.P.; Carvalho, G.M.; Yamashita, F.; Mali, S.; Eiras, D.; Demiate, I.M.; Grossmann, M.V.E. Oat hull fibers bleached by reactive extrusion with alkaline hydrogen peroxide in thermoplastic starch/poly(butylene adipate-co-terephthalate) composites. Polymer Composites 2018, 39, 1950–1958. [Google Scholar] [CrossRef]
- Giubilini, A.; Sciancalepore, C.; Messori, M.; Bondioli, F. Valorization of oat hull fiber from agri-food industrial waste as filler for poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Journal of Material Cycles and Waste Management 2021, 23, 402–408. [Google Scholar] [CrossRef]
- Peixoto, T.d.S.; Yamashita, F.; Bilck, A.P.; Carvalho, G.M.; Grossmann, M.V.E. Crosslinking starch/oat hull mixtures for use in composites with PLA. Polímeros 2019, 29, e2019040(1-8). [Google Scholar] [CrossRef]
- Debiagi, F.; Faria-Tischer, P.C.S.; Mali, S. A Green Approach Based on Reactive Extrusion to Produce Nanofibrillated Cellulose from Oat Hull. Waste and Biomass Valorization 2021, 12, 1051–1060. [Google Scholar] [CrossRef]
- Denisova, M.N. Study of the influence of hydrotropic washing cellulose on main characteristics final product. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2016, 2, 167–170. [Google Scholar]
- Gromov, N.V.; Denisova, M.N.; Medvedeva, T.B.; Yatsenko, D.A.; Taran, O.P. The Effect of Mechanical Activation and Lignin Impurities on the Hydrolysis-Dehydration of Cellulose in the Presence of Sibunit-4 Solid Acidic Carbon Catalysts. Journal of Siberian Federal University. Chemistry 2019, 12, 434–444. [Google Scholar] [CrossRef]
- Erbas Kiziltas, E.; Kiziltas, A.; Gardner, D.J. Synthesis of bacterial cellulose using hot water extracted wood sugars. Carbohydrate Polymers 2015, 124, 131–138. [Google Scholar] [CrossRef]
- Skiba, E.A.; Budaeva, V.V.; Ovchinnikova, E.V.; Gladysheva, E.K.; Kashcheyeva, E.I.; Pavlov, I.N.; Sakovich, G.V. A technology for pilot production of bacterial cellulose from oat hulls. Chemical Engineering Journal 2020, 383, 123128. [Google Scholar] [CrossRef]
- Li, W.; Shen, Y.; Liu, H.; Huang, X.; Xu, B.; Zhong, C.; Jia, S. Bioconversion of lignocellulosic biomass into bacterial nanocellulose: challenges and perspectives. Green Chemical Engineering 2023, 4, 160–172. [Google Scholar] [CrossRef]
- Korchagina, A.A.; Gismatulina, Y.A.; Budaeva, V.V.; Kukhlenko, A.A.; Vdovina, N.P.; Ivanov, P.P. Autoclaving cellulose nitrates obtained from oat hulls. izvestiya Vysshikh Uchebnykh Zavedenii Khimiya i Khimicheskaya Tekhnologiya [News of Higher Educational Institutions Chemistry and Chemical Technology] (in Russian) 2019, 63, 92–98. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresource Technology 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Denisova, M.N.; Kukhlenko, A.A.; Orlov, S.E. Investigation into the Process of Hydrotropic Delignification of Oat Straw in the Universal Thermobaric Unit. Theoretical Foundations of Chemical Engineering 2018, 52, 661–663. [Google Scholar] [CrossRef]
- Cheng, W.; Zhu, Y.; Jiang, G.; Cao, K.; Zeng, S.; Chen, W.; Zhao, D.; Yu, H. Sustainable cellulose and its derivatives for promising biomedical applications. Progress in Materials Science 2023, 138, 101152. [Google Scholar] [CrossRef]
- Gladysheva, E.K. Results of X-ray studies of bacterial cellulose. Fundamental`nye issledovaniya [fundamental research] (in Russian) 2015, 7, 240–244. [Google Scholar]
- Urbina, L.; Corcuera, M.Á.; Gabilondo, N.; Eceiza, A.; Retegi, A. A review of bacterial cellulose: sustainable production from agricultural waste and applications in various fields. Cellulose 2021, 28, 8229–8253. [Google Scholar] [CrossRef]
- Meleh, N.V. Natural and technical celluloses; LAP LAMBERT Academic Publishing, 2014; Volume 104. [Google Scholar]
- Schroeder, L.R.; Gentile, V.M.; Atalla, R.H. Nondegradative Preparation of Amorphous Cellulose. Journal of Wood Chemistry and Technology 1986, 6, 1–14. [Google Scholar] [CrossRef]
- van Zyl, E.M.; Coburn, J.M. Hierarchical structure of bacterial-derived cellulose and its impact on biomedical applications. Current Opinion in Chemical Engineering 2019, 24, 122–130. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnology for Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods – A review. Carbohydrate Polymers 2019, 219, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jiang, F.; Xu, X.; Kuang, Y.; Fu, K.; Hitz, E.; Hu, L. Super-Strong, Super-Stiff Macrofibers with Aligned, Long Bacterial Cellulose Nanofibers. Advanced Materials 2017, 29, 1702498. [Google Scholar] [CrossRef] [PubMed]
- Debiagi, F.; Faria-Tischer, P.C.S.; Mali, S. Cellulose and Nanocellulose Produced from Lignocellulosic Residues by Reactive Extrusion. In Biomass Extrusion and Reaction Technologies: Principles to Practices and Future Potential; ACS Symposium Series; American Chemical Society, 2018; Volume 1304, pp. 227–242. [Google Scholar]
- Ovchinnikova, E.V.; Mironova, G.F.; Banzaraktsaeva, S.P.; Skiba, E.A.; Budaeva, V.V.; Kovgan, M.A.; Chumachenko, V.A. Bioprocessing of Oat Hulls to Ethylene: Impact of Dilute HNO3 or NaOH-Pretreatment on Process Efficiency and Sustainability. ACS Sustainable Chemistry & Engineering 2021, 9, 16588–16596. [Google Scholar] [CrossRef]
- SJÖSTEDT, N. Isolation of cellulose fibres from agricultural waste. Production of dissolving-grade pulp from oat husk and wheat straw; Chalmers University of Technology: Gothenburg, Sweden, 2022. [Google Scholar]
- Demirel, F.; Germec, M.; Coban, H.B.; Turhan, I. Optimization of dilute acid pretreatment of barley husk and oat husk and determination of their chemical composition. Cellulose 2018, 25, 6377–6393. [Google Scholar] [CrossRef]
- Lawford, H.G.; Rousseau, J.D.; Tolan, J.S. Comparative ethanol productivities of different Zymomonas recombinants fermenting oat hull hydrolysate. Applied Biochemistry and Biotechnology 2001, 91, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Tabil, L. Evaluation of biocomposite-based supports for immobilized-cell xylitol production compared with a free-cell system. Biochemical Engineering Journal 2014, 82, 166–173. [Google Scholar] [CrossRef]
- Soleimani, M.; Tabil, L.G.; Niu, C. Delignification of intact biomass and cellulosic coproduct of acid-catalyzed hydrolysis. AIChE Journal 2015, 61, 1783–1791. [Google Scholar] [CrossRef]
- Baibakova, O.V.; Skiba, E.A.; Budaeva, V.V.; Sakovich, G.V. Preparing bioethanol from oat hulls pretreated with a dilute nitric acid: Scaling of the production process on a pilot plant. Catalysis in Industry 2017, 9, 257–263. [Google Scholar] [CrossRef]
- Patent RU2448118C1, Budaeva, V.V.; Obrezkova, M.V.; Zolotukhin, V.N.; Sakovich, G.V.; Sysoljatin, S.V. Method of producing cellulose from non-wood plant material with native cellulose content of not more than 50% and method of producing carboxymethyl cellulose therefrom. 2010. 2010.
- Makarova, E.I.; Budaeva, V.V.; Skiba, E.A. Enzymatic hydrolysis of cellulose from oat husks at different substrate concentrations. Russian Journal of Bioorganic Chemistry 2014, 40, 726–732. [Google Scholar] [CrossRef]
- Makarova, E.I.; Budaeva, V.V.; Skiba, E.A.; Sakovich, G.V. Enzymatic hydrolysis of celluloses obtained via the hydrothermal processing of Miscanthus and oat hulls. Catalysis in Industry 2014, 6, 67–71. [Google Scholar] [CrossRef]
- Skiba, E.A.; Momot, T.O.; Bychin, N.V.; Zolotukhin, V.N. Enzymative hydrolysis of lignocellulosic materials depending on the method of their preparation. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2013, 3, 197–202. [Google Scholar]
- Budaeva, V.; Makarova, E.I.; Gismatulina, Y.A. Integrated Flowsheet for Conversion of Non-Woody Biomass into Polyfunctional Materials. Key Engineering Materials 2016, 670, 202–206. [Google Scholar] [CrossRef]
- Budaeva, V.V.; Skiba, E.A.; Baibakova, O.V.; Makarova, E.I.; Orlov, S.E.; Kukhlenko, A.A.; Udoratina, E.V.; Shcherbakova, T.P.; Kuchin, A.V.; Sakovich, G.V. Kinetics of the enzymatic hydrolysis of lignocellulosic materials at different concentrations of the substrate. Catalysis in Industry 2016, 8, 81–87. [Google Scholar] [CrossRef]
- Budaeva, V.V.; Skiba, E.A.; Makarova, E.I.; Zolotukhin, V.N.; Sakovich, G.V.; Udoratina, E.; Kuvshinova, L.; Scherbakova, T.P.; Kuchin, A.V. Obtaining lignocellulosic materials from sub-wood raw materials and studing them as sub-strates of enzymative hydrolysis. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2013, 1, 215–219. [Google Scholar]
- Gismatulina, Y.A.; Budaeva, V.V. Nitric acid method for producing cellulose (review). Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2016, 4, 174–178. [Google Scholar]
- Makarova, E.I.; Budaeva, V.V.; Kukhlenko, A.A.; Orlov, S.E. Enzyme kinetics of cellulose hydrolysis of Miscanthus and oat hulls. 3 Biotech 2017, 7, 317. [Google Scholar] [CrossRef] [PubMed]
- Mironova, G.F.; Pavlov, I.N.; Kashcheyeva, E.I. Study of the possibility of increasing the yield of bioethanol from the production of alkaline delignification of oat hulls using the feeding method. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2018, 1, 111–116. [Google Scholar] [CrossRef]
- Skiba, E.A.; Baibakova, O.V.; Budaeva, V.V.; Pavlov, I.N.; Makarova, E.I.; Mironova, G.; Kriukov, Y.A.; Sakovich, G. Bioethanol from Oat Hulls Pretreated by Alkaline Delignification. II. Scaling of Alcoholic Fermentation up to Pilot Process. Biotekhnologiya 2017, 33, 47–56. [Google Scholar] [CrossRef]
- Skiba, E.A.; Budaeva, V.V.; Baibakova, O.V.; Udoratina, E.V.; Shakhmatov, E.G.; Shcherbakova, T.P.; Kuchin, A.V.; Sakovich, G.V. Enzymatic hydrolysis of lignocellulosic materials in aqueous media and the subsequent microbiological synthesis of bioethanol. Catalysis in Industry 2016, 8, 168–175. [Google Scholar] [CrossRef]
- Nepenin, N.N.; Nepenin, Y.N. Technologiia tsellulozy. Ochistka, sushka i otbelka tsellulozy. Prochie sposoby proizvodstva tsellulozy [Cellulose technology. Cleaning, drying and bleaching of cellulose. Other pulp production methods]; Ecologiia: Moscow, 1994; Volume 3, p. 592. [Google Scholar]
- Gismatulina, Y.A.; Budaeva, V.V.; Sakovich, G.V. Nitric acid preparation of cellulose from miscanthus as a nitrocellulose precursor. Russian Chemical Bulletin 2015, 64, 2949–2953. [Google Scholar] [CrossRef]
- Korchagina, A.A. Alternative raw materials for nitrocellulose. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2016, 4, 157–160. [Google Scholar]
- Fund, S.A.D.; Chemical, U.o.S.D.o.; Engineering, B. An Integrated Process for the Production of Xylitol and Biocomposites from Lignocellulosic Biomass (oat Hulls); Final Report; Agriculture Development Fund, 2012. [Google Scholar]
- Qazanfarzadeh, Z.; Kadivar, M. Properties of whey protein isolate nanocomposite films reinforced with nanocellulose isolated from oat husk. International Journal of Biological Macromolecules 2016, 91, 1134–1140. [Google Scholar] [CrossRef]
- Marim, B.M.; Mantovan, J.; Giraldo, G.A.; Mali, S. Environmentally friendly process based on a combination of ultrasound and peracetic acid treatment to obtain cellulose from orange bagasse. Journal of Chemical Technology & Biotechnology 2021, 96, 630–638. [Google Scholar] [CrossRef]
- Marim, B.M.; Mantovan, J.; Pereira, J.F.; Debiagi, F.; Mali, S. Sustainable process based on reactive extrusion to modify cellulose from oat hull with sodium trimetaphosphate and tartaric acid. Polymer Bulletin 2023. [Google Scholar] [CrossRef]
- Shahi, N.; Joshi, G.; Min, B. Potential Sustainable Biomaterials Derived from Cover Crops. BioResources 2020, 15, 5641–5652. Available online: https://bioresources.cnr.ncsu.edu/resources/potential-sustainable-biomaterialsderived-from-cover-crops/. [CrossRef]
- Baibakova, O.V. Full cycle of bioethanol production in production conditions. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2018, 1, 121–124. [Google Scholar] [CrossRef]
- Machado, T.B.; Corrêa Junior, L.C.S.; de Mattos, M.V.C.d.V.; Gautério, G.V.; Kalil, S.J. Sequential Alkaline and Ultrasound Pretreatments of Oat Hulls Improve Xylanase Production by Aureobasidium pullulans in Submerged Cultivation. Waste and Biomass Valorization 2021, 12, 5991–6004. [Google Scholar] [CrossRef]
- Makarova, E.I.; Budaeva, V.V. Study of enzymative hydrolysis of the fibrous production of oat hulls at different substrate concentranion. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2015, 3, 113–116. [Google Scholar]
- Skiba, E.A.; Baibakova, O.V.; Budaeva, V.V.; Pavlov, I.N.; Vasilishin, M.S.; Makarova, E.I.; Sakovich, G.V.; Ovchinnikova, E.V.; Banzaraktsaeva, S.P.; Vernikovskaya, N.V.; et al. Pilot technology of ethanol production from oat hulls for subsequent conversion to ethylene. Chemical Engineering Journal 2017, 329, 178–186. [Google Scholar] [CrossRef]
- Yin, X.; Wei, L.; Pan, X.; Liu, C.; Jiang, J.; Wang, K. The Pretreatment of Lignocelluloses With Green Solvent as Biorefinery Preprocess: A Minor Review. Frontiers in Plant Science 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Rasche, C. Impact of type and pretreatment of lignocellulosics on lignin and pulp properties. PhD Thesis, University of Dresden, Dresden, Germany, 2016. Available online: https://nbn-resolving.org/urn:nbn:de:bsz:14-qucosa-201345.
- Subhedar, P.B.; Gogate, P.R. Alkaline and ultrasound assisted alkaline pretreatment for intensification of delignification process from sustainable raw-material. Ultrasonics Sonochemistry 2014, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Sakovich, G.V.; Budaeva, V.V.; Skiba, E.A.; Makarova, E.I.; Pavlov, I.N.; Kortusov, A.N.; Zolotukhin, V.N. Experience in scaleing enzymative hydrolysis of technical cellulose from miscanthus and oat hulls. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2012, 4, 173–177. [Google Scholar]
- Kashcheyeva, E.I.; Mironova, G.F.; Budaeva, V.V.; Khan, H. Bioconversion of oat hull and miscanthus cellulose to glucose solutions. PROCEEDINGS OF UNIVERSITIES APPLIED CHEMISTRY AND BIOTECHNOLOGY 2019, 9, 654–664. [Google Scholar] [CrossRef]
- Dehkhoda, S.; Bagheri, M.; Heydari, M.; Rabieh, S. Extraction of carboxylated nanocellulose from oat husk: Characterization, surface modification and in vitro evaluation of indomethacin drug release. International Journal of Biological Macromolecules 2022, 212, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Tang, L.; Lu, Q.; Wang, S.; Chen, X.; Huang, B. Preparation of cellulose nanocrystals and carboxylated cellulose nanocrystals from borer powder of bamboo. Cellulose 2014, 21, 1611–1618. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.-W. Isolation of oxidized nanocellulose from rice straw using the ammonium persulfate method. Cellulose 2018, 25, 2143–2149. [Google Scholar] [CrossRef]
- Rozenberga, L.; Vikele, L.; Vecbiskena, L.; Sable, I.; Laka, M.; Grinfelds, U. Preparation of Nanocellulose Using Ammonium Persulfate and Method’s Comparison with other Techniques. Key Engineering Materials 2016, 674, 21–25. [Google Scholar] [CrossRef]
- Mitbumrung, W.; Rungraung, N.; Muangpracha, N.; Akanitkul, P.; Winuprasith, T. Approaches for Extracting Nanofibrillated Cellulose from Oat Bran and Its Emulsion Capacity and Stability. Polymers 2022, 14, 327. [Google Scholar] [CrossRef]
- Budaeva, V.V.; Makarova, E.I.; Skiba, E.A.; Sakovich, G.V. Enzymatic hydrolysis of the products of hydro-thermobaric processing of Miscanthus and oat hulls. Catalysis in Industry 2013, 5, 335–341. [Google Scholar] [CrossRef]
- Tsukanov, S.N.; Budaeva, V.V. Hydrothermobaric process for the production of cellulose from cereal wastes. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2011, 4, 236–239. [Google Scholar]
- Debiagi, F.; Madeira, T.B.; Nixdorf, S.L.; Mali, S. Pretreatment Efficiency Using Autoclave High-Pressure Steam and Ultrasonication in Sugar Production from Liquid Hydrolysates and Access to the Residual Solid Fractions of Wheat Bran and Oat Hulls. Applied Biochemistry and Biotechnology 2020, 190, 166–181. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.A.P.; Carvalho, G.M.; Yamashita, F.; Mali, S.; Olivato, J.B.; Grossmann, M.V.E. Oat fibers modification by reactive extrusion with alkaline hydrogen peroxide. Polímeros 2016, 26, 320–326. [Google Scholar] [CrossRef]
- Galdeano, M.C.; Grossmann, M.V.E. Effect of treatment with alkaline hydrogen peroxide associated with extrusion on color and hydration properties of oat hulls. Brazilian Archives of Biology and Technology 2005, 48, 63–72. [Google Scholar] [CrossRef]
- Shuai, L.; Luterbacher, J. Organic Solvent Effects in Biomass Conversion Reactions. ChemSusChem 2016, 9, 133–155. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Wu, R.; Liu, D. Organosolv fractionating pre-treatment of lignocellulosic biomass for efficient enzymatic saccharification: chemistry, kinetics, and substrate structures. Biofuels, Bioproducts and Biorefining 2017, 11, 567–590. [Google Scholar] [CrossRef]
- Zhou, Z.; Lei, F.; Li, P.; Jiang, J. Lignocellulosic biomass to biofuels and biochemicals: A comprehensive review with a focus on ethanol organosolv pretreatment technology. Biotechnology and Bioengineering 2018, 115, 2683–2702. [Google Scholar] [CrossRef]
- Obama, P.; Ricochon, G.; Muniglia, L.; Brosse, N. Combination of enzymatic hydrolysis and ethanol organosolv pretreatments: Effect on lignin structures, delignification yields and cellulose-to-glucose conversion. Bioresource Technology 2012, 112, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, J.; Hu, T.; Zhao, X.; Liu, D. A comparison of several organosolv pretreatments for improving the enzymatic hydrolysis of wheat straw: Substrate digestibility, fermentability and structural features. Applied Energy 2015, 150, 224–232. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Reith, J.H.; den Uil, H. Pretreatment and Fractionation of Wheat Straw by an Acetone-Based Organosolv Process. Industrial & Engineering Chemistry Research 2010, 49, 10132–10140. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Smit, A.T.; Reith, J.H.; Uil, H.d. Catalytic organosolv fractionation of willow wood and wheat straw as pretreatment for enzymatic cellulose hydrolysis. Journal of Chemical Technology & Biotechnology 2011, 86, 1428–1438. [Google Scholar] [CrossRef]
- Wen, J.-L.; Sun, S.-L.; Yuan, T.-Q.; Xu, F.; Sun, R.-C. Structural Elucidation of Lignin Polymers of Eucalyptus Chips during Organosolv Pretreatment and Extended Delignification. Journal of Agricultural and Food Chemistry 2013, 61, 11067–11075. [Google Scholar] [CrossRef] [PubMed]
- li, Z.; Jiang, Z.; Fei, B.; Pan, X.; Cai, Z.; Liu, X.e.; Yu, Y. Ethanol organosolv pretreatment of bamboo for efficient enzymatic saccharification. Bioresources 2012, 7. [Google Scholar] [CrossRef]
- Park, N.; Kim, H.-Y.; Koo, B.-W.; Yeo, H.; Choi, I.-G. Organosolv pretreatment with various catalysts for enhancing enzymatic hydrolysis of pitch pine (Pinus rigida). Bioresource Technology 2010, 101, 7046–7053. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Lin, H.; Zheng, Y.; Zhao, J.; Pelletier, A.; Li, K. Effects of solvents and catalysts in liquefaction of pinewood sawdust for the production of bio-oils. Biomass and Bioenergy 2013, 59, 158–167. [Google Scholar] [CrossRef]
- Vallejos, M.E.; Zambon, M.D.; Area, M.C.; Curvelo, A.A.d.S. Low liquid-solid ratio fractionation of sugarcane bagasse by hot water autohydrolysis and organosolv delignification. Industrial Crops and Products 2015, 65, 349–353. [Google Scholar] [CrossRef]
- Amiri, H.; Karimi, K.; Zilouei, H. Organosolv pretreatment of rice straw for efficient acetone, butanol, and ethanol production. Bioresource Technology 2014, 152, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Huang, W.; Huang, W.; Wang, K.; Chen, Q.; Wu, Y. Pretreatment of rice straw for ethanol production by a two-step process using dilute sulfuric acid and sulfomethylation reagent. Applied Energy 2015, 154, 190–196. [Google Scholar] [CrossRef]
- Ayusheev, A.; Taran, O.; Afinogenova, I.; Mishchenko, T.; Shashkov, M.; Sashkina, K.; Semeikina, V.; Parkhomchuk, E.; Parmon, V.; Agabekov, V. Depolymerization of Birch-Wood Organosolv Lignin Over Solid Catalysts in Supercritical Ethanol. Journal of Siberian Federal University. Chemistry 2016, 9, 353–370. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Miroshnikova, A.V.; Kazachenko, A.S.; Baryshnikov, S.V.; Malyar, Y.N.; Yakovlev, V.A.; Skripnikov, A.M.; Fetisova, O.Y.; Xu, Y.; Taran, O.P. Reductive Catalytic Fractionation of Abies Wood into Bioliquids and Cellulose with Hydrogen in an Ethanol Medium over NiCuMo/SiO2 Catalyst. Catalysts 2023, 13(2), 413. [Google Scholar] [CrossRef]
- Sar, T.; Arifa, V.H.; Hilmy, M.R.; Ferreira, J.A.; Wikandari, R.; Millati, R.; Taherzadeh, M.J. Organosolv pretreatment of oat husk using oxalic acid as an alternative organic acid and its potential applications in biorefinery. Biomass Conversion and Biorefinery 2022. [Google Scholar] [CrossRef]
- Kubo, S.; Kadla, J.F. Poly(Ethylene Oxide)/Organosolv Lignin Blends: Relationship between Thermal Properties, Chemical Structure, and Blend Behavior. Macromolecules 2004, 37, 6904–6911. [Google Scholar] [CrossRef]
- Lora, J.H.; Glasser, W.G. Recent Industrial Applications of Lignin: A Sustainable Alternative to Nonrenewable Materials. Journal of Polymers and the Environment 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Budaeva, V.V.; Denisova, M.N.; Pavlov, I.N.; Gismatulina, Y.A.; Sakovich, G.V. Study of physical and chemical features hydrotropic cellulose and structural-dimensional charachteristics of its fiber. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2015, 2, 95–98. [Google Scholar]
- Denisova, M.N. Neutral method for obtaining cellulose from oat hulls. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2011, 4, 239–243. [Google Scholar]
- Denisova, M.N. Production of Cellulose from Oat-Processing Waste by the Hydrotropic Method Followed by the Synthesis of Cellulose Nitrates. Theoretical Foundations of Chemical Engineering 2018, 52, 648–652. [Google Scholar] [CrossRef]
- Denisova, M.N.; Pavlov, I.N. Study of the hydrotropic process cooking pine sawdust. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2016, 4, 166–169. [Google Scholar]
- Makarova, E.I.; Denisova, M.N.; Budaeva, V.V.; Sakovich, G.V. Enzymatic hydrolysis of hydrotropic celluloses. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2013, 1, 219–222. [Google Scholar]
- Denisova, M.N. Hydrotropic cellulose from recycled agricultural raw materials. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2016, 1, 73–76. [Google Scholar]
- Denisova, M.N.; Kadulina, L.N. Study of cereal straw samples and hydrotropic cellulose isolated from them by IR spectroscopy method. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2016, 2, 155–158. [Google Scholar]
- Keldibekova, R.; Suleimenova, S.; Nurgozhina, G.; Kopishev, E. Interpolymer Complexes Based on Cellulose Ethers: Application. Polymers 2023, 15, 3326. [Google Scholar] [CrossRef] [PubMed]
- Mattar, H.; Baz, Z.; Saleh, A.; Shalaby, A.; Azzazy, A.; Salah, H.; Ismail, I. Nitrocellulose: Structure, Synthesis, Characterization, and Applications. Water, Energy, Food and Environment Journal, 2020, 3, 1–15. [Google Scholar]
- Saunders, C.W.; Taylor, L.T. A review of the synthesis, chemistry and analysis of nitrocellulose. Journal of Energetic Materials 1990, 8, 149–203. [Google Scholar] [CrossRef]
- Dong, Y.-D.; Zhang, H.; Zhong, G.-J.; Yao, G.; Lai, B. Cellulose/carbon Composites and their Applications in Water Treatment – a Review. Chemical Engineering Journal 2021, 405, 126980. [Google Scholar] [CrossRef]
- Omran, A.A.B.; Mohammed, A.A.B.A.; Sapuan, S.M.; Ilyas, R.A.; Asyraf, M.R.M.; Rahimian Koloor, S.S.; Petrů, M. Micro- and Nanocellulose in Polymer Composite Materials: A Review. Polymers 2021, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Ul-Islam, M.; Khattak, W.A.; Park, J.K. Overview of bacterial cellulose composites: A multipurpose advanced material. Carbohydrate Polymers 2013, 98, 1585–1598. [Google Scholar] [CrossRef] [PubMed]
- Shojaeiarani, J.; Bajwa, D.S.; Chanda, S. Cellulose nanocrystal based composites: A review. Composites Part C: Open Access 2021, 5, 100164. [Google Scholar] [CrossRef]
- Chu, Y.; Sun, Y.; Wu, W.; Xiao, H. Dispersion Properties of Nanocellulose: A Review. Carbohydrate Polymers 2020, 250, 116892. [Google Scholar] [CrossRef]
- Isogai, A. Emerging Nanocellulose Technologies: Recent Developments. Advanced Materials 2021, 33, 2000630. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resources Conversion 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Chen, C.; Xi, Y.; Weng, Y. Recent Advances in Cellulose-Based Hydrogels for Tissue Engineering Applications. Polymers 2022, 14, 3335. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Huo, X.; Chen, J.; El-Bahy, S.M.E.; El-Bahy, Z.M.E. An Overview of Cellulose Aerogel: Classification and Applications. ES Food & Agroforestry 2022, 10, 1–9. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Derval dos Santos, R.; Denise Maria, L. Biocomposites: Influence of Matrix Nature and Additives on the Properties and Biodegradation Behaviour. In Biodegradation, Rolando, C., Francisca, R., Eds.; IntechOpen: Rijeka, 2013; Ch. 6. [Google Scholar]
- Bobrovskaya, S.A.; Voroshilova, A.V.; Klevtsova, M.V.; Protopopov, A.V. Chemical modification of oats hulls with aromatic oxyacids; Moscow, 2016. [Google Scholar]
- Klevtsova, M.V.; Protopopov, A.V. Obtaining cellulose ethers with sulphosalicylic and benzoic acids from oat hulls. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2016, 2, 181–185. [Google Scholar]
- Kostag, M.; Gericke, M.; Heinze, T.; El Seoud, O.A. Twenty-five years of cellulose chemistry: innovations in the dissolution of the biopolymer and its transformation into esters and ethers. Cellulose 2019, 26, 139–184. [Google Scholar] [CrossRef]
- Gismatulina, Y.; Korchagina, A.; Budaeva, V.; Sakovich, G. Highly soluble cellulose nitrates from unconventional feedstocks. MATEC Web Conf. 2018, 243, 00005. [Google Scholar] [CrossRef]
- Gismatulina, Y.A.; Budaeva, V.V.; Sakovich, G.V. Cellulose nitrates from intermediate flax straw. Russian Chemical Bulletin 2016, 65, 2920–2924. [Google Scholar] [CrossRef]
- Gismatulina, Y.A.; Gladysheva, E.K.; Budaeva, V.V.; Sakovich, G.V. Synthesis of bacterial cellulose nitrates. Russian Chemical Bulletin 2019, 68, 2130–2133. [Google Scholar] [CrossRef]
- Korchagina, A.A.; Budaeva, V.V. Recearch of cellulose from non-conventional raw materials and its nitrates by the method of FTIR spectroscopy. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2018, 2, 111–116. [Google Scholar] [CrossRef]
- Romanova, S.M.; Fatykhova, L.A. Study of the interaction of cellulose nitric ethers with carboxylic acids chlorides. izvestiya Vysshikh Uchebnykh Zavedenii Khimiya i Khimicheskaya Tekhnologiya [News of Higher Educational Institutions Chemistry and Chemical Technology] (in Russian) 2021, 64, 30–34. [Google Scholar] [CrossRef]
- Sakovich, G.V.; Budaeva, V.V.; Korchagina, A.A.; Gismatulina, Y.A.; Kozyrev, N.V.; Vakutin, A.G. Oat-Hull Cellulose Nitrates for Explosive Compositions. Doklady Chemistry 2019, 487, 221–225. [Google Scholar] [CrossRef]
- Sabatini, J.J.; Johnson, E.C. A Short Review of Nitric Esters and Their Role in Energetic Materials. ACS Omega 2021, 6, 11813–11821. [Google Scholar] [CrossRef] [PubMed]
- Bekhouche, S.; Trache, D.; Abdelaziz, A.; Tarchoun, A.F.; Chelouche, S.; Boudjellal, A.; Mezroua, A. Preparation and characterization of MgAl-CuO ternary nanothermite system by arrested reactive milling and its effect on the thermocatalytic decomposition of cellulose nitrate. Chemical Engineering Journal 2023, 453, 139845. [Google Scholar] [CrossRef]
- Korchagina, A.A.; Budaeva, V.V.; Aleshina, L.A.; Lyukhanova, I.V.; Bychin, N.V.; Sakovich, G.V. Modification of plant cellulose and its synthetic analogues into low-substituted eterification products. izvestiya Vysshikh Uchebnykh Zavedenii Khimiya i Khimicheskaya Tekhnologiya [News of Higher Educational Institutions Chemistry and Chemical Technology] (in Russian) 2022, 65, 64–74. [Google Scholar] [CrossRef]
- Tang, R.; Alam, N.; Li, M.; Xie, M.; Ni, Y. Dissolvable sugar barriers to enhance the sensitivity of nitrocellulose membrane lateral flow assay for COVID-19 nucleic acid. Carbohydrate Polymers 2021, 268, 118259. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Xie, M.Y.; Li, M.; Cao, L.; Feng, S.; Li, Z.; Xu, F. Nitrocellulose Membrane for Paper-based Biosensor. Applied Materials Today 2022, 26, 101305. [Google Scholar] [CrossRef]
- Dai, J.; Xu, J.; Wang, F.; Tai, Y.; Shen, Y.; Shen, R.; Ye, Y. Facile formation of nitrocellulose-coated Al/Bi2O3 nanothermites with excellent energy output and improved electrostatic discharge safety. Materials & Design 2018, 143, 93–103. [Google Scholar] [CrossRef]
- Gismatulina, Y.A. Synthesis of cellulose nitrates from easily renewable non-wood raw materials. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2018, 1, 125–130. [Google Scholar] [CrossRef]
- Efanov, M.V.; Panchenko, O.A.; Zabelina, A.V. Oxidative nitration of flax seeds. Khimiya Rastitel'nogo Syr'ya [Chemistry of plant raw materials] (in Russian) 2004, 3, 95–97. [Google Scholar]
- Muvhiiwa, R.; Mawere, E.; Moyo, L.B.; Tshuma, L. Utilization of cellulose in tobacco (Nicotiana tobacum) stalks for nitrocellulose production. Heliyon 2021, 7, e07598. [Google Scholar] [CrossRef] [PubMed]
- Valishina, Z.T.; Kostochko, A.V.; Shipina, O.T.; Gatina, R.F.; Mikhailov, Y.M. Structure and properties of new types of cellulose nitrate ethers. Vestnik KGTU [Bulletin of KSTU] (in Russian) 2010, 9, 281–290. [Google Scholar]
- Panchenko, O.A.; Napilkova, O.A. Influence of pre-treatment of lignocellulosic raw materials on the properties of the cellulose nitrates. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2015, 4, 117–119. [Google Scholar]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Abdelaziz, A.; Bekhouche, S.; Boukeciat, H.; Sahnoun, N. Making progress towards promising energetic cellulosic microcrystals developed from alternative lignocellulosic biomasses. Journal of Energetic Materials 1–26. [CrossRef]
- Trache, D.; Khimeche, K.; Mezroua, A.; Benziane, M. Physicochemical properties of microcrystalline nitrocellulose from Alfa grass fibres and its thermal stability. Journal of Thermal Analysis and Calorimetry 2016, 124, 1485–1496. [Google Scholar] [CrossRef]
- Gismatulina, Y.A.; Budaeva, V.V. Chemical composition of five Miscanthus sinensis harvests and nitric-acid cellulose therefrom. Industrial Crops and Products 2017, 109, 227–232. [Google Scholar] [CrossRef]
- Gismatulina, Y.A.; Budaeva, V.V.; Sakovich, G.V. Nitrocellulose Synthesis from Miscanthus Cellulose. Propellants, Explosives, Pyrotechnics 2018, 43, 96–100. [Google Scholar] [CrossRef]
- Korchagina, A.; Gismatulina, Y.; Budaeva, V.; Zolotukhin, V.; Bychin, N.; Sakovich, G. Miscanthus × Giganteus var. KAMIS as a New Feedstock for Cellulose Nitrates. Journal of Siberian Federal University. Chemistry 2020, 565–577. [Google Scholar] [CrossRef]
- Yakusheva, A.A. Cellulose nitrates from a new source of cellulose -- oat hulls. fundamental`nye issledovaniya [fundamental research] (in Russian) 2014, 8, 360–364. [Google Scholar]
- Budaeva, V.V.; Pavlov, I.N.; Skiba, E.A.; Baibakova, O.V.; Vasilishin, M.S.; Gismatulina, Y.A.; Denisova, M.N.; Ivanov, O.S.; Makarova, E.I.; Zolotukhin, V.N. Fundamental bases of implementation of comprehensive processing of non-wood plant raw materials into demanded production in small-volume technological equipment. Polzunovski` vestnik [Polzunovski` bulletin] (in Russian) 2016, 2, 186–191. [Google Scholar]
- Zhukov, B. Energeticheskie Kondensirovannye Sistemy: Kratkii Entsiklopedicheskii Slovar'. 2000. [Google Scholar]
- Pandit, A.; Kumar, R. A Review on Production, Characterization and Application of Bacterial Cellulose and Its Biocomposites. Journal of Polymers and the Environment 2021, 29, 2738–2755. [Google Scholar] [CrossRef]
- Huang, X.; Luo, Q.; Zhu, J.; Li, Z.; Li, C.; Pei, C. The Preparation and Rheological Properties of Novel Energetic Composites TEGDN/NBC. Propellants, Explosives, Pyrotechnics 2020, 45, 101–110. [Google Scholar] [CrossRef]
- Gismatulina, Y.A.; Korchagina, A.A.; Budaeva, V.V. Synrhesis of nanostructured bacterial cellulose nitrates. PROCEEDINGS OF UNIVERSITIES. APPLIED CHEMISTRY AND BIOTECHNOLOGY (in Russian) 2023, 13, 38–49. [Google Scholar]
- Zayed, A.; Fouad, M.M.K.; Elnashaie, S.S.E.H. Advanced Integrated Bio-Refineries (IBRs) and Sustainable Development (SD): IBRs: Generation of Sustainable Alternatives. Water, Energy, Food and Environment Journal 2020, 1, 29–40. [Google Scholar]
- Yamamoto, H.; Horii, F.; Hirai, A. Structural studies of bacterial cellulose through the solid-phase nitration and acetylation by CP/MAS 13C NMR spectroscopy. Cellulose 2006, 13, 327–342. [Google Scholar] [CrossRef]
- Gismatulina, Y.A. Promising Energetic Polymers from Nanostructured Bacterial Cellulose. Polymers 2023, 15(9), 2213. [Google Scholar] [CrossRef] [PubMed]
- Jori Roslan, N.; Jamal, S.H.; Ong, K.K.; Wan Yunus, W.M.Z. Preliminary Study on the Effect of Sulphuric Acid to Nitric Acid Mixture Composition, Temperature and Time on Nitrocellulose Synthesis Based Nata de Coco. Solid State Phenomena 2021, 317, 312–319. [Google Scholar] [CrossRef]
- Luo, Q.; Zhu, J.; Li, Z.; Duan, X.; Pei, C.; Mao, C. The solution characteristics of nitrated bacterial cellulose in acetone. New Journal of Chemistry 2018, 42, 18252–18258. [Google Scholar] [CrossRef]
- Sun, D.-P.; Ma, B.; Zhu, C.-L.; Liu, C.-S.; Yang, J.-Z. Novel Nitrocellulose Made from Bacterial Cellulose. Journal of Energetic Materials 2010, 28, 85–97. [Google Scholar] [CrossRef]
- Grewal, R. Investigations on biocomposites from oat hull and biodegradable polymers; University of Saskatchewan: Canada, 2016. [Google Scholar]
- Soleimani, M.; Tabil, L.; Panigrahi, S.; Opoku, A. The Effect of Fiber Pretreatment and Compatibilizer on Mechanical and Physical Properties of Flax Fiber-Polypropylene Composites. Journal of Polymers and the Environment 2008, 16, 74–82. [Google Scholar] [CrossRef]
- Formela, K.; Hejna, A.; Haponiuk, J.; Tercjak, A. 8 - In situ processing of biocomposites via reactive extrusion. In Biocomposites for High-Performance Applications; Ray, D., Ed.; Woodhead Publishing, 2017; pp. 195–246. [Google Scholar]
- Formela, K.; Zedler, L.; Hejna, A.; Tercjak, A. Reactive extrusion of bio-based polymer blends and composites – Current trends and future developments. Express Polymer Letters 2018, 12(1), 24–57. [Google Scholar] [CrossRef]
- Reddy, J.P.; Misra, M.; Mohanty, A. Injection Moulded Biocomposites from Oat Hull and Polypropylene/Polylactide Blend: Fabrication and Performance Evaluation. Advances in Mechanical Engineering 2013, 5, 761840. [Google Scholar] [CrossRef]
- Tan, T.; Zhou, J.; Gao, X.; Tang, X.; Zhang, H. Synthesis, characterization and water-absorption behavior of tartaric acid-modified cellulose gel fromcorn stalk pith. Industrial Crops and Products 2021, 169, 113641. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: a review. Journal of Materials Research and Technology 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Pang, L.; Gao, Z.; Feng, H.; Wang, S.; Wang, Q. Cellulose based materials for controlled release formulations of agrochemicals: A review of modifications and applications. Journal of Controlled Release 2019, 316, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, P.G.; Sundaram, J.; Mani, S. Preparation and characterization of citric acid crosslinked chitosan-cellulose nanofibrils composite films for packaging applications. Journal of Applied Polymer Science 2022, 139, 52017. [Google Scholar] [CrossRef]
- Ma, X.; Liu, C.; Anderson, D.P.; Chang, P.R. Porous cellulose spheres: Preparation, modification and adsorption properties. Chemosphere 2016, 165, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.; Saravanan, S.; Al-Maleki, A.R.; Ramesh, M.; Vadivelu, J. A review of natural polysaccharides for drug delivery applications: Special focus on cellulose, starch and glycogen. Biomedicine & Pharmacotherapy 2018, 107, 96–108. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Gardner, D.J.; Shen, W. Contact Angles and Wettability of Cellulosic Surfaces: A Review of Proposed Mechanisms and Test Strategies. Bioresources 2015, 10, 8657–8749. Available online: https://bioresources.cnr.ncsu.edu/wp-content/uploads/2016/06/BioRes_10_4_8657_Review_Hubbe_GS_Contact_Angle_Wettabilty_Cellulos_Surf_Mechanisms_Test_Strategies_7907.pdf. [CrossRef]
- Hazwan Hussin, M.; Trache, D.; Chuin, C.T.H.; Nurul Fazita, M.R.; Mohamad Haafiz, M.K.; Hossain, M.S. Extraction of Cellulose Nanofibers and Their Eco-friendly Polymer Composites. In Sustainable Polymer Composites and Nanocomposites, Inamuddin, Thomas, S., Kumar Mishra, R., Asiri, A.M., Eds.; Springer International Publishing: Cham, 2019; pp. 653–691. [Google Scholar]
- Khalid, M.Y.; Al Rashid, A.; Arif, Z.U.; Ahmed, W.; Arshad, H. Recent advances in nanocellulose-based different biomaterials: types, properties, and emerging applications. Journal of Materials Research and Technology 2021, 14, 2601–2623. [Google Scholar] [CrossRef]
- Mohammed, L.; Ansari, M.N.M.; Pua, G.; Jawaid, M.; Islam, M.S. A Review on Natural Fiber Reinforced Polymer Composite and Its Applications. International Journal of Polymer Science 2015, 2015, 243947. [Google Scholar] [CrossRef]
- Shak, K.P.Y.; Pang, Y.L.; Mah, S.K. Nanocellulose: Recent advances and its prospects in environmental remediation. Beilstein Journal of Nanotechnology 2018, 9, 2479–2498. [Google Scholar] [CrossRef] [PubMed]
- Shavyrkina, N.A.; Budaeva, V.V.; Skiba, E.A.; Mironova, G.F.; Bychin, N.V.; Gismatulina, Y.A.; Kashcheyeva, E.I.; Sitnikova, A.E.; Shilov, A.I.; Kuznetsov, P.S.; et al. Scale-Up of Biosynthesis Process of Bacterial Nanocellulose. Polymers 2021, 13, 1920. [Google Scholar] [CrossRef] [PubMed]
- Zharikov, A.N.; Lubyansky, V.G.; Gladysheva, E.K.; Skiba, E.; Budaeva, V.; Semenova, E.N.; Motin, Y.G.; Zharikov, A. Prosthetic hernioplasty using bacterial nanocellulose: An experimental study. Clinical and Experimental Surgery 2018, 6, 59–66. [Google Scholar] [CrossRef]
- Faria, L.U.S.; Pacheco, B.J.S.; Oliveira, G.C.; Silva, J.L. Production of cellulose nanocrystals from pineapple crown fibers through alkaline pretreatment and acid hydrolysis under different conditions. Journal of Materials Research and Technology 2020, 9, 12346–12353. [Google Scholar] [CrossRef]
- Owoyokun, T.O.; Berumen, C.M.P.; Luévanos, A.M. ; Liliana; Cantú; Ceniceros, A. Cellulose Nanocrystals: Obtaining and Sources of a Promising Bionanomaterial for Advanced Applications. Biointerface Research in Applied Chemistry 2020, 11(4), 11797-11816. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Buldum, G.; Mantalaris, A.; Bismarck, A. More Than Meets the Eye in Bacterial Cellulose: Biosynthesis, Bioprocessing, and Applications in Advanced Fiber Composites. Macromolecular Bioscience 2014, 14, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Skiba, E.A.; Baibakova, O.V.; Gladysheva, E.K.; Budaeva, V.V. Study of the influence of Medusomyces gisevii Sa-12 inoculum dosage on bacterial cellulose yield and degree of polymerisatio. PROCEEDINGS OF UNIVERSITIES. APPLIED CHEMISTRY AND BIOTECHNOLOGY (in Russian) 2019, 9, 420–429. [Google Scholar] [CrossRef]
- Aleshina, L.A.; Gladysheva, E.K.; Budaeva, V.V.; Skiba, E.A.; Arkharova, N.A.; Sakovich, G.V. X-ray Diffraction Study of Bacterial Nanocellulose Produced by the Medusomyces gisevii Sa-12 Culture in Enzymatic Hydrolysates of Oat Hulls. Crystallography Reports 2018, 63, 955–960. [Google Scholar] [CrossRef]
- Jedrzejczak-Krzepkowska, M.; Kubiak, K.; Ludwicka, K.; Bielecki, S. Chapter 2 - Bacterial NanoCellulose Synthesis, Recent Findings. In Bacterial Nanocellulose, Gama, M., Dourado, F., Bielecki, S., Eds.; Elsevier: Amsterdam, 2016; pp. 19–46. [Google Scholar] [CrossRef]
- Lima, F.d.M.T.d.; Pinto, F.C.M.; Andrade-da-Costa, B.L.d.S.; Silva, J.G.M.d.; Campos Júnior, O.; Aguiar, J.L.d.A. Biocompatible bacterial cellulose membrane in dural defect repair of rat. Journal of Materials Science: Materials in Medicine 2017, 28, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cao, J.; Zhao, S.; Luo, H.; Yang, Z.; Gama, M.; Zhang, Q.; Su, D.; Wan, Y. Biocompatibility evaluation of bacterial cellulose as a scaffold material for tissue-engineered corneal stroma. Cellulose 2020, 27, 2775–2784. [Google Scholar] [CrossRef]
- Urbina, L.; Algar, I.; García-Astrain, C.; Gabilondo, N.; González, A.; Corcuera, M.; Eceiza, A.; Retegi, A. Biodegradable composites with improved barrier properties and transparency from the impregnation of PLA to bacterial cellulose membranes. Journal of Applied Polymer Science 2016, 133. [Google Scholar] [CrossRef]
- Zahan, K.A.; Azizul, N.M.; Mustapha, M.; Tong, W.Y.; Abdul Rahman, M.S.; Sahuri, I.S. Application of bacterial cellulose film as a biodegradable and antimicrobial packaging material. Materials Today: Proceedings 2020, 31, 83–88. [Google Scholar] [CrossRef]
- Gromovykh, T.I.; Pigaleva, M.A.; Gallyamov, M.O.; Ivanenko, I.P.; Ozerova, K.E.; Kharitonova, E.P.; Bahman, M.; Feldman, N.B.; Lutsenko, S.V.; Kiselyova, O.I. Structural organization of bacterial cellulose: The origin of anisotropy and layered structures. Carbohydrate Polymers 2020, 237, 116140. [Google Scholar] [CrossRef]
- Roman, M.; Haring, A.P.; Bertucio, T.J. The growing merits and dwindling limitations of bacterial cellulose-based tissue engineering scaffolds. Current Opinion in Chemical Engineering 2019, 24, 98–106. [Google Scholar] [CrossRef]
- Skiba, E.A.; Shavyrkina, N.A.; Budaeva, V.V.; Sitnikova, A.E.; Korchagina, A.A.; Bychin, N.V.; Gladysheva, E.K.; Pavlov, I.N.; Zharikov, A.N.; Lubyansky, V.G.; et al. Biosynthesis of Bacterial Cellulose by Extended Cultivation with Multiple Removal of BC Pellicles. Polymers 2021, 13, 2118. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, V.G. WOUND COVER WITH HEMOSTATIC ACTION, AND METHOD FOR ITS PRODUCTION. 2016. [Google Scholar]
- Silva, M.d.A.; Leite, Y.K.d.C.; Carvalho, C.E.S.d.; Feitosa, M.L.T.; Alves, M.M.d.M.; Carvalho, F.A.d.A.; Neto, B.C.V.; Miglino, M.A.; Jozala, A.F.; Carvalho, M.A.M.d. Behavior and biocompatibility of rabbit bone marrow mesenchymal stem cells with bacterial cellulose membrane. PeerJ 2018, 6, e4656. [Google Scholar] [CrossRef]
- Zharikov, A.N.; Lubyansky, V.G.; Gladysheva, E.K.; Skiba, E.A.; Budaeva, V.V.; Semyonova, E.N.; Zharikov, A.A.; Sakovich, G.V. Early morphological changes in tissues when replacing abdominal wall defects by bacterial nanocellulose in experimental trials. Journal of Materials Science: Materials in Medicine 2018, 29, 95. [Google Scholar] [CrossRef]
- Cichosz, S.; Masek, A. Cellulose Fibers Hydrophobization via a Hybrid Chemical Modification. Polymers 2019, 11, 1174. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, H.; Teymouri, M.; Saboor, S.; Khalili, A.; Goodarzi, V.; Poudineh Hajipoor, F.; Khonakdar, H.A.; Shojaei, S.; Asefnejad, A.; Bagheri, H. Challenge between sequence presences of conductive additives on flexibility, dielectric and supercapacitance behaviors of nanofibrillated template of bacterial cellulose aerogels. European Polymer Journal 2019, 115, 335–345. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, Y.; Li, W.; Xu, X.; Zhang, H.; Zhao, Y.; Lin, J.; Sun, D. Facile synthesis and light-induced antibacterial activity of ketoprofen functionalized bacterial cellulose membranes. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2019, 568, 231–238. [Google Scholar] [CrossRef]
- Zhuravleva, N.; Reznik, A.; Kiesewetter, D.; Stolpner, A.; Smirnova, E.; Budaeva, V. Improvement of properties of cellulose dielectrics by their structure modification with nanocellulose produced of wastes of agricultural crops. Journal of Physics: Conference Series 2019, 1410, 012068. [Google Scholar] [CrossRef]
- Goelzer, F.D.E.; Faria-Tischer, P.C.S.; Vitorino, J.C.; Sierakowski, M.R.; Tischer, C.A. Production and characterization of nanospheres of bacterial cellulose from Acetobacter xylinum from processed rice bark. Materials Science and Engineering: C 2009, 29, 546–551. [Google Scholar] [CrossRef]
- Qi, G.-X.; Luo, M.-T.; Huang, C.; Guo, H.-J.; Chen, X.-F.; Xiong, L.; Wang, B.; Lin, X.-Q.; Peng, F.; Chen, X.-D. Comparison of bacterial cellulose production by Gluconacetobacter xylinus on bagasse acid and enzymatic hydrolysates. Journal of Applied Polymer Science 2017, 134, 45066. [Google Scholar] [CrossRef]
- Chen, L.; Hong, F.; Yang, X.-x.; Han, S.-f. Biotransformation of wheat straw to bacterial cellulose and its mechanism. Bioresource Technology 2013, 135, 464–468. [Google Scholar] [CrossRef]
- Ye, J.; Zheng, S.; Zhang, Z.; Yang, F.; Ma, K.; Feng, Y.; Zheng, J.; Mao, D.; Yang, X. Bacterial cellulose production by Acetobacter xylinum ATCC 23767 using tobacco waste extract as culture medium. Bioresource Technology 2019, 274, 518–524. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, R.; Liu, X.; Liu, X.; Chen, H. Green synthesis of bacterial cellulose via acetic acid pre-hydrolysis liquor of agricultural corn stalk used as carbon source. Bioresource Technology 2017, 234, 8–14. [Google Scholar] [CrossRef]
- Rani, M.U.; Appaiah, K.A.A. Production of bacterial cellulose by Gluconacetobacter hansenii UAC09 using coffee cherry husk. Journal of Food Science and Technology 2013, 50, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-H.; Huang, C.-Y.; Shieh, C.-J.; Wang, H.-M.D.; Tseng, C.-Y. Hydrolysis of Orange Peel with Cellulase and Pectinase to Produce Bacterial Cellulose using Gluconacetobacter xylinus. Waste and Biomass Valorization 2019, 10, 85–93. [Google Scholar] [CrossRef]
- Fan, X.; Gao, Y.; He, W.; Hu, H.; Tian, M.; Wang, K.; Pan, S. Production of nano bacterial cellulose from beverage industrial waste of citrus peel and pomace using Komagataeibacter xylinus. Carbohydrate Polymers 2016, 151, 1068–1072. [Google Scholar] [CrossRef]
- Algar, I.; Fernandes, S.C.M.; Mondragon, G.; Castro, C.; Garcia-Astrain, C.; Gabilondo, N.; Retegi, A.; Eceiza, A. Pineapple agroindustrial residues for the production of high value bacterial cellulose with different morphologies. Journal of Applied Polymer Science 2015, 132. [Google Scholar] [CrossRef]
- Molina-Ramírez, C.; Castro, C.; Zuluaga, R.; Gañán, P. Physical Characterization of Bacterial Cellulose Produced by Komagataeibacter medellinensis Using Food Supply Chain Waste and Agricultural By-Products as Alternative Low-Cost Feedstocks. Journal of Polymers and the Environment 2018, 26, 830–837. [Google Scholar] [CrossRef]
- Urbina, L.; Hernández-Arriaga, A.M.; Eceiza, A.; Gabilondo, N.; Corcuera, M.A.; Prieto, M.A.; Retegi, A. By-products of the cider production: an alternative source of nutrients to produce bacterial cellulose. Cellulose 2017, 24, 2071–2082. [Google Scholar] [CrossRef]
- Cerrutti, P.; Roldán, P.; García, R.M.; Galvagno, M.A.; Vázquez, A.; Foresti, M.L. Production of bacterial nanocellulose from wine industry residues: Importance of fermentation time on pellicle characteristics. Journal of Applied Polymer Science 2016, 133. [Google Scholar] [CrossRef]
- Abdelraof, M.; Hasanin, M.S.; El -Saied, H. Ecofriendly green conversion of potato peel wastes to high productivity bacterial cellulose. Carbohydrate Polymers 2019, 211, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Guo, X.; Zhang, S.; Han, S.-f.; Yang, G.; Jönsson, L.J. Bacterial cellulose production from cotton-based waste textiles: Enzymatic saccharification enhanced by ionic liquid pretreatment. Bioresource Technology 2012, 104, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Sakovich, G.V.; Skiba, E.A.; Budaeva, V.V.; Gladysheva, E.K.; Aleshina, L.A. Technological fundamentals of bacterial nanocellulose production from zero prime-cost feedstock. Doklady Biochemistry and Biophysics 2017, 477, 357–359. [Google Scholar] [CrossRef]
- Budaeva, V.; Gladysheva, Е.; Shavyrkina, N.; Pavlov, I.N.; Golubev, D.; Mironova, G.; Kashcheyeva, Е.; Gismatulina, Y.; Korchagina, А.; Sakovich, G. Main technological stages of bacterial cellulose synthesis from easily renewable cellulosic feedstocks by Мedusomyces gisevii SA-12 symbiotic culture. Biotechnology: state of the art and perspectives. 2020, 290–292. [Google Scholar] [CrossRef]
- Gladysheva, E.K.; Budaeva, V.V.; Skiba, E.A.; Kashcheyeva, E.I.; Zolotukhin, V.N. Selection of herbaceous cellulose-containing raw materials for biotechnological processing. PROCEEDINGS OF UNIVERSITIES. APPLIED CHEMISTRY AND BIOTECHNOLOGY (in Russian) 2023, 13, 310–317. [Google Scholar] [CrossRef]
- Ferrer, A.; Salas, C.; Rojas, O.J. Physical, thermal, chemical and rheological characterization of cellulosic microfibrils and microparticles produced from soybean hulls. Industrial Crops and Products 2016, 84, 337–343. [Google Scholar] [CrossRef]
- Musyoka, S.M.; Ngila, J.C.; Mamba, B.B. Remediation studies of trace metals in natural and treated water using surface modified biopolymer nanofibers. Physics and Chemistry of the Earth, Parts A/B/C 2013, 66, 45–50. [Google Scholar] [CrossRef]
- Serrano, L.; Urruzola, I.; Nemeth, D.; Belafi-Bako, K.; Labidi, J. Modified cellulose microfibrils as benzene adsorbent. Desalination 2011, 270, 143–150. [Google Scholar] [CrossRef]
- Winuprasith, T.; Suphantharika, M. Microfibrillated cellulose from mangosteen (Garcinia mangostana L.) rind: Preparation, characterization, and evaluation as an emulsion stabilizer. Food Hydrocolloids 2013, 32, 383–394. [Google Scholar] [CrossRef]
- Xhanari, K.; Syverud, K.; Stenius, P. Emulsions Stabilized by Microfibrillated Cellulose: The Effect of Hydrophobization, Concentration and O/W Ratio. Journal of Dispersion Science and Technology 2011, 32, 447–452. [Google Scholar] [CrossRef]
- Jawaid, M.; Kian, L.K.; Fouad, H.; Saba, N.; Alothman, O.Y.; Hashem, M. Morphological, structural, and thermal analysis of three part of Conocarpus cellulosic fibres. Journal of Materials Research and Technology 2021, 10, 24–33. [Google Scholar] [CrossRef]
- Ramlee, N.A.; Jawaid, M.; Zainudin, E.S.; Yamani, S.A.K. Tensile, physical and morphological properties of oil palm empty fruit bunch/sugarcane bagasse fibre reinforced phenolic hybrid composites. Journal of Materials Research and Technology 2019, 8, 3466–3474. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Industrial Crops and Products 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Powell, L.C.; Khan, S.; Chinga-Carrasco, G.; Wright, C.J.; Hill, K.E.; Thomas, D.W. An investigation of Pseudomonas aeruginosa biofilm growth on novel nanocellulose fibre dressings. Carbohydrate Polymers 2016, 137, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Syverud, K.; Pettersen, S.R.; Draget, K.; Chinga-Carrasco, G. Controlling the elastic modulus of cellulose nanofibril hydrogels—scaffolds with potential in tissue engineering. Cellulose 2015, 22, 473–481. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and characterization of nanofibers from agricultural residues – Wheat straw and soy hulls. Bioresource Technology 2008, 99, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Jonoobi, M.; Harun, J.; Shakeri, A.; Misra, M.; Oksman, K. Chemical composition, crystallinity, and thermal degradation of bleached and unbleached kenaf bast (Hibiscus cannabinus) pulp and nanofibers. BioResources 2009, 4(2), 626–639. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresource Technology 2018, 262, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Qua, E.H.; Hornsby, P.R.; Sharma, H.S.S.; Lyons, G. Preparation and characterisation of cellulose nanofibres. Journal of Materials Science 2011, 46, 6029–6045. [Google Scholar] [CrossRef]
- Zimmermann, T.; Bordeanu, N.; Strub, E. Properties of nanofibrillated cellulose from different raw materials and its reinforcement potential. Carbohydrate Polymers 2010, 79, 1086–1093. [Google Scholar] [CrossRef]
- Zhou, Y. Effect of nanocellulose isolation techniques on the formation of reinforced poly(vinyl alcohol) nanocomposite films. Express Polymer Letters 2012, 6, 794–804. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G.; Yu, Y.; Diserud, O. Quantitative Electron Microscopy of Cellulose Nanofibril Structures from Eucalyptus and Pinus radiata Kraft Pulp Fibers. Microscopy and Microanalysis 2011, 17, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-Y.; Aitomäki, Y.; Berglund, L.A.; Oksman, K.; Bismarck, A. On the use of nanocellulose as reinforcement in polymer matrix composites. Composites Science and Technology 2014, 105, 15–27. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G. Optical methods for the quantification of the fibrillation degree of bleached MFC materials. Micron 2013, 48, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Chinga-Carrasco, G.; Averianova, N.; Kondalenko, O.; Garaeva, M.; Petrov, V.; Leinsvang, B.; Karlsen, T. The effect of residual fibres on the micro-topography of cellulose nanopaper. Micron 2014, 56, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Noremylia, M.B.; Hassan, M.Z.; Ismail, Z. Recent advancement in isolation, processing, characterization and applications of emerging nanocellulose: A review. International Journal of Biological Macromolecules 2022, 206, 954–976. [Google Scholar] [CrossRef] [PubMed]
- Bruni, G.P.; de Oliveira, J.P.; Fonseca, L.M.; da Silva, F.T.; Dias, A.R.G.; da Rosa Zavareze, E. Biocomposite Films Based on Phosphorylated Wheat Starch and Cellulose Nanocrystals from Rice, Oat, and Eucalyptus Husks. Starch - Stärke 2020, 72, 1900051. [Google Scholar] [CrossRef]
- de Oliveira, J.P.; Bruni, G.P.; el Halal, S.L.M.; Bertoldi, F.C.; Dias, A.R.G.; Zavareze, E.d.R. Cellulose nanocrystals from rice and oat husks and their application in aerogels for food packaging. International Journal of Biological Macromolecules 2019, 124, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Shankaran, D.R. Chapter 14 - Cellulose Nanocrystals for Health Care Applications. In Applications of Nanomaterials; Mohan Bhagyaraj, S., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Woodhead Publishing, 2018; pp. 415–459. [Google Scholar] [CrossRef]
- Blanco, A.; Monte, M.C.; Campano, C.; Balea, A.; Merayo, N.; Negro, C. Chapter 5 - Nanocellulose for Industrial Use: Cellulose Nanofibers (CNF), Cellulose Nanocrystals (CNC), and Bacterial Cellulose (BC). In Handbook of Nanomaterials for Industrial Applications; Mustansar Hussain, C., Ed.; Elsevier, 2018; pp. 74–126. [Google Scholar]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydrate Polymers 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Shahi, N.; Joshi, G.; Min, B. Effect of Regenerated Cellulose Fibers Derived from Black Oat on Functional Properties of PVA-Based Biocomposite Film. Processes 2020, 8, 1149. [Google Scholar] [CrossRef]
- Wang, Z.; Qiao, X.; Sun, K. Rice straw cellulose nanofibrils reinforced poly(vinyl alcohol) composite films. Carbohydrate Polymers 2018, 197, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Choo, K.; Ching, Y.C.; Chuah, C.H.; Julai, S.; Liou, N.-S. Preparation and Characterization of Polyvinyl Alcohol-Chitosan Composite Films Reinforced with Cellulose Nanofiber. Materials 2016, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Bamdad, F.; Goli, A.H.; Kadivar, M. Preparation and characterization of proteinous film from lentil (Lens culinaris): Edible film from lentil (Lens culinaris). Food Research International 2006, 39, 106–111. [Google Scholar] [CrossRef]
- Cho, M.-J.; Park, B.-D. Tensile and thermal properties of nanocellulose-reinforced poly(vinyl alcohol) nanocomposites. Journal of Industrial and Engineering Chemistry 2011, 17, 36–40. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L.; Zhou, J.; Zhang, L.; Kennedy, J.F. Structure and properties of hydrogels prepared from cellulose in NaOH/urea aqueous solutions. Carbohydrate Polymers 2010, 82, 122–127. [Google Scholar] [CrossRef]
- Vecino, X.; Devesa-Rey, R.; Villagrasa, S.; Cruz, J.M.; Moldes, A.B. Kinetic and morphology study of alginate-vineyard pruning waste biocomposite vs. non modified vineyard pruning waste for dye removal. Journal of Environmental Sciences 2015, 38, 158–167. [Google Scholar] [CrossRef]
- Hebeish, A.; Farag, S.; Sharaf, S.; Shaheen, T.I. Thermal responsive hydrogels based on semi interpenetrating network of poly(NIPAm) and cellulose nanowhiskers. Carbohydrate Polymers 2014, 102, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Lu, C.; Zhang, W. Cellulose hydrogels prepared from micron-sized bamboo cellulose fibers. Carbohydrate Polymers 2014, 114, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Luiz de Paula, E.; Mano, V.; Pereira, F.V. Influence of cellulose nanowhiskers on the hydrolytic degradation behavior of poly(d,l-lactide). Polymer Degradation and Stability 2011, 96, 1631–1638. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S.; Okano, T. Flow properties of microcrystalline cellulose suspension prepared by acid treatment of native cellulose. Colloids and Surfaces A: Physicochemical and Engineering Aspects 1998, 142, 75–82. [Google Scholar] [CrossRef]
| № | Method of pretreatment | Residual lignin, % |
Residual hemicellulose, % |
Nanocellulose | Composites | Nitrocellulose | Cellulose ethers | Hydrogels | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial cellulose | Nanocrystal cellulose | Cellulose nanofibrils | |||||||||
| 1 | Acid only | 7.2-12.1 | 6.9-7.4 | + | + | [39] | |||||
| 3.0-3.5 | 8 | + | + | [40,41] | |||||||
| 2 | Acid+H2O2 | 3 | 7-8 | + | [41,42] | ||||||
| 3 | Alkali only | 3.3-4 | 8.1-9.4 | + | [39] | ||||||
| 4 | Acid-alkali | 0.32-0.8 | 0.7-3 | + | + | + | [39,43,44,45,46,47] | ||||
| 5 | Multi alkali-acid | 1 | 1.4-26 | + | + | + | + | + | [39,48,49] | ||
| 6 | Multi acid-alkali | < 1-2% | 2-6.5 | + | [39] | ||||||
| 7 | Alkali peroxide and physical methods | 7.7-9.5 | 3-17 | + | [50,51,52] | ||||||
| 8 | Alkali-acid combined with physical methods | 7.2-12.6 | 3-26 | + | + | [53] | |||||
| 9 | Hydrotropic method | 5-9 | - | + | [54] | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





