Submitted:
11 December 2023
Posted:
12 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Population
Patient Inclusion and Exclusion Criteria
2.2. Study methodology and data extraction
2.3. Statistical Analysis
3. Results
3.1. Description of patient data
3.2. Cardiovascular events during follow up
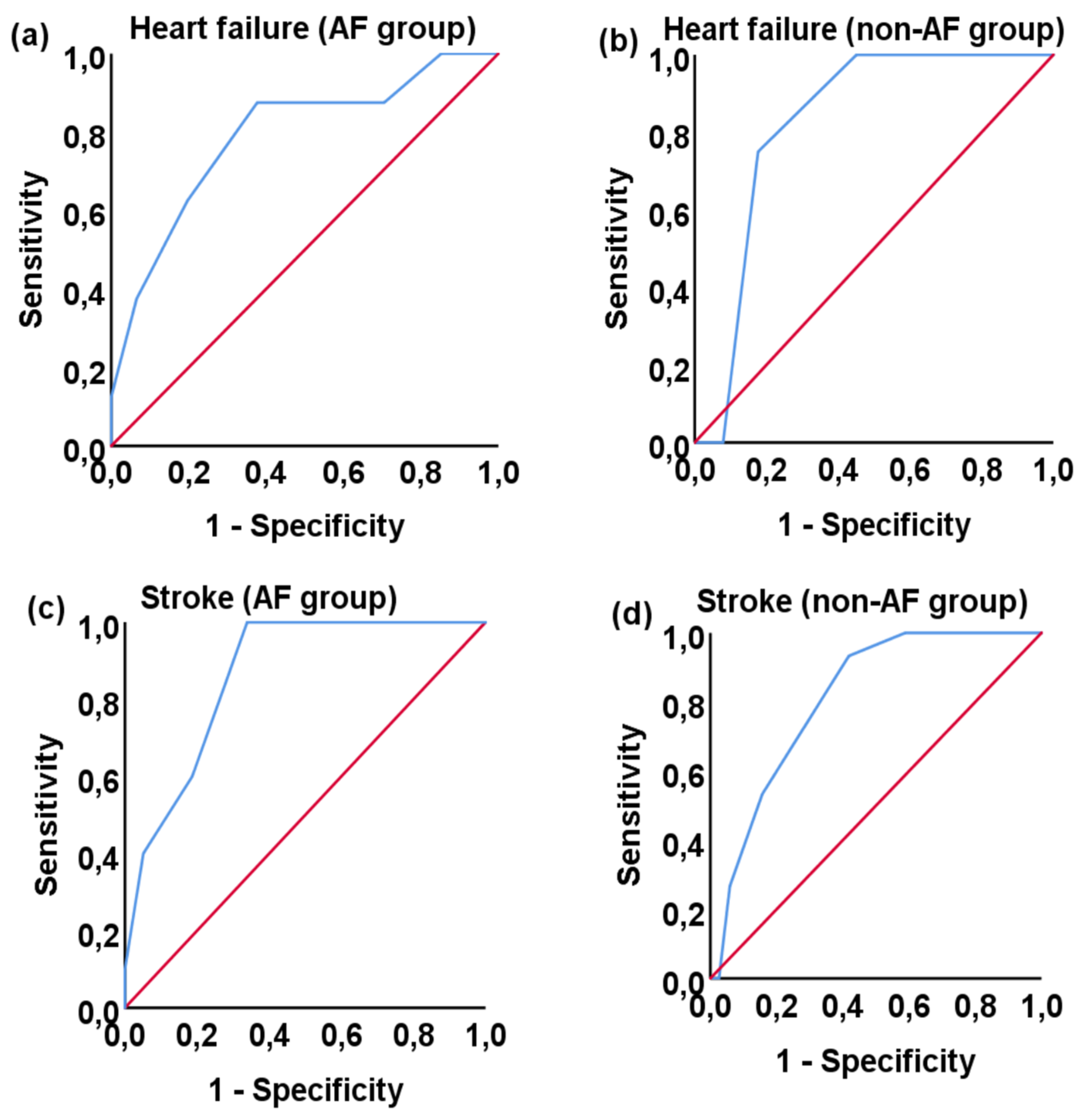
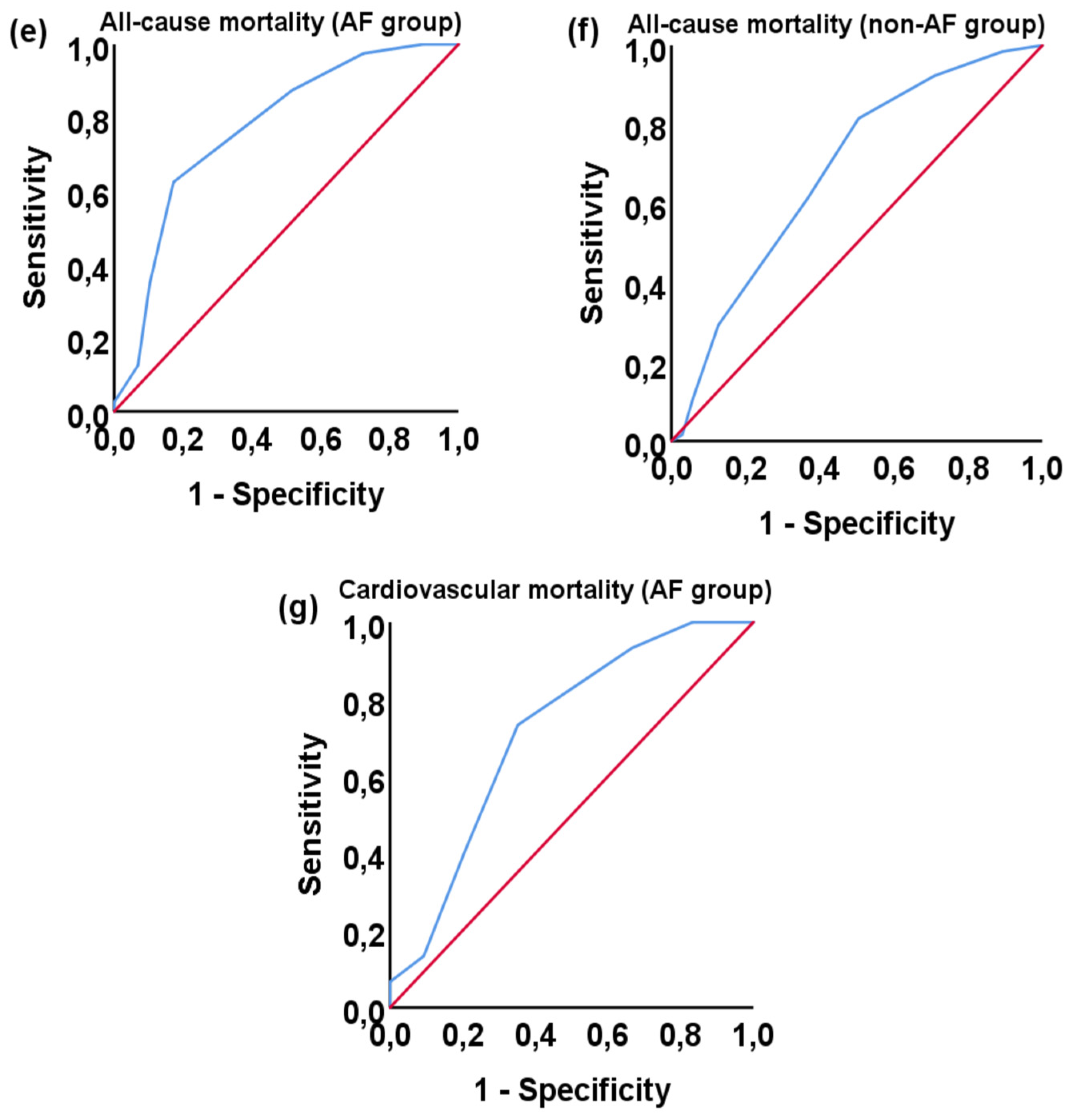
3.3. Correlation with morbidity and mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hermans:, W.R.; Foley, D.P.; Rensing, B.J.; Rutsch, W.; Heyndrickx, G.R.; Danchin, N.; Mast, G.; Hanet, C.; Lablanche, J.M.; Rafflenbeul, W. Usefulness of Quantitative and Qualitative Angiographic Lesion Morphology, and Clinical Characteristics in Predicting Major Adverse Cardiac Events during and after Native Coronary Balloon Angioplasty. CARPORT and MERCATOR Study Groups. Am J Cardiol 1993, 72, 14–20. [Google Scholar] [CrossRef]
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 4 September 2022).
- Katan, M.; Luft, A. Global Burden of Stroke. Semin Neurol 2018, 38, 208–211. [Google Scholar] [CrossRef]
- Leung, K.C.-W.; MacRae, J.M. Anticoagulation in CKD and ESRD. J Nephrol 2019, 32, 719–731. [Google Scholar] [CrossRef]
- Joundi, R.A.; Cipriano, L.E.; Sposato, L.A.; Saposnik, G. Stroke Outcomes Research Working Group Ischemic Stroke Risk in Patients With Atrial Fibrillation and CHA2DS2-VASc Score of 1: Systematic Review and Meta-Analysis. Stroke 2016, 47, 1364–1367. [Google Scholar] [CrossRef]
- Peterson, D.; Geison, E. Pharmacist Interventions to Reduce Modifiable Bleeding Risk Factors Using HAS-BLED in Patients Taking Warfarin. Fed Pract 2017, 34, S16–S20. [Google Scholar] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int 2020, 98, S1–S115. [CrossRef] [PubMed]
- Levey, A.S.; Becker, C.; Inker, L.A. Glomerular Filtration Rate and Albuminuria for Detection and Staging of Acute and Chronic Kidney Disease in Adults: A Systematic Review. JAMA 2015, 313, 837–846. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Molnar, A.O.; Sood, M.M. Predicting in a Predicament: Stroke and Hemorrhage Risk Prediction in Dialysis Patients with Atrial Fibrillation. Semin Dial 2018, 31, 37–47. [Google Scholar] [CrossRef]
- Goudis, C.; Daios, S.; Korantzopoulos, P.; Liu, T. Does CHA2DS2-VASc Score Predict Mortality in Chronic Kidney Disease? Intern Emerg Med 2021, 16, 1737–1742. [Google Scholar] [CrossRef]
- Vodošek Hojs, N.; Ekart, R.; Bevc, S.; Piko, N.; Hojs, R. CHA2DS2-VASc Score as a Predictor of Cardiovascular and All-Cause Mortality in Chronic Kidney Disease Patients. Am J Nephrol 2021, 52, 404–411. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Lee, W.-H.; Chen, S.-C.; Tsai, Y.-C.; Chen, Y.-C.; Chu, C.-Y.; Lin, T.-H.; Voon, W.-C.; Lai, W.-T.; Sheu, S.-H.; et al. Using CHADS2 and CHA2DS2-VASc Scores for Mortality Prediction in Patients with Chronic Kidney Disease. Sci Rep 2020, 10, 18942. [Google Scholar] [CrossRef] [PubMed]
- Okubo, A.; Doi, T.; Morii, K.; Nishizawa, Y.; Yamashita, K.; Shigemoto, K.; Mizuiri, S.; Usui, K.; Arita, M.; Naito, T.; et al. Utility of CHA2DS2-VASc Score to Predict Mid-Term Clinical Outcomes in Hemodialysis Patients. Am J Nephrol 2022, 53, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Abrantes, A.M.; Marques da Silva, B.; Branco, C.; Costa, C.; Peres, N.; Cardoso, A.; Sant’Ana, M.; Fonseca, J.A.; Outerelo, C.; Resina, C.; et al. One-Year Mortality after Hemodialysis Initiation: The Prognostic Role of the CHA2DS2-VASc Score. J Clin Med 2023, 12, 1011. [Google Scholar] [CrossRef] [PubMed]
- Schamroth Pravda, M.; Cohen Hagai, K.; Topaz, G.; Schamroth Pravda, N.; Makhoul, N.; Shuvy, M.; Benchetrit, S.; Assali, A.; Pereg, D. Assessment of the CHA2DS2-VASc Score in Predicting Mortality and Adverse Cardiovascular Outcomes of Patients on Hemodialysis. Am J Nephrol 2020, 51, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Sab, M.; Chelala, D.; Aoun, M.; Azar, R.; Abdel Massih, T. Stroke in Hemodialysis Patients and Its Association with CHA2DS2-VASC and HAS-BLED Scores: A Retrospective Study. Clin Kidney J 2022, 16, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Bel-Ange, A.; Itskovich, S.Z.; Avivi, L.; Stav, K.; Efrati, S.; Beberashvili, I. Prior Ischemic Strokes Are Non-Inferior for Predicting Future Ischemic Strokes than CHA2DS2-VASc Score in Hemodialysis Patients with Non-Valvular Atrial Fibrillation. BMC Nephrol 2021, 22, 179. [Google Scholar] [CrossRef]
- Hiyamuta, H.; Yamada, S.; Taniguchi, M.; Nakano, T.; Tsuruya, K.; Kitazono, T. Causes of Death in Patients Undergoing Maintenance Hemodialysis in Japan: 10-Year Outcomes of the Q-Cohort Study. Clin Exp Nephrol 2021, 25, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, H.; Diao, Z.; Guo, W.; Huang, H.; Zuo, L.; Liu, W. Epidemiological Analysis of Death among Patients on Maintenance Hemodialysis: Results from the Beijing Blood Purification Quality Control and Improvement Center. BMC Nephrol 2023, 24, 236. [Google Scholar] [CrossRef]
- Hsieh, H.; Hsu, S.; Cheng, H.; Chen, C.; Huang, W.; Sue, Y.; Lin, F.; Shih, C.; Chen, J.; Lin, S.; et al. The Influence of Atrial Fibrillation on the Mortality of Incident ESRD Patients Undergoing Maintenance Hemodialysis. PLoS One 2020, 15, e0228405. [Google Scholar] [CrossRef]
- Kang, Y.; Choi, H.Y.; Kwon, Y.E.; Shin, J.H.; Won, E.M.; Yang, K.H.; Oh, H.J.; Ryu, D.-R. Clinical Outcomes among Hemodialysis Patients with Atrial Fibrillation: A Korean Nationwide Population-Based Study. Kidney Res Clin Pract 2021, 40, 99–108. [Google Scholar] [CrossRef]
- Masson, P.; Webster, A.C.; Hong, M.; Turner, R.; Lindley, R.I.; Craig, J.C. Chronic Kidney Disease and the Risk of Stroke: A Systematic Review and Meta-Analysis. Nephrol Dial Transplant 2015, 30, 1162–1169. [Google Scholar] [CrossRef]
- Boonpheng, B.; Thongprayoon, C.; Cheungpasitporn, W. The Comparison of Risk of Stroke in Patients with Peritoneal Dialysis and Hemodialysis: A Systematic Review and Meta-Analysis. J Evid Based Med 2018, 11, 158–168. [Google Scholar] [CrossRef]
- Findlay, M.; MacIsaac, R.; MacLeod, M.J.; Metcalfe, W.; Sood, M.M.; Traynor, J.P.; Dawson, J.; Mark, P.B. The Association of Atrial Fibrillation and Ischemic Stroke in Patients on Hemodialysis: A Competing Risk Analysis. Can J Kidney Health Dis 2019, 6, 2054358119878719. [Google Scholar] [CrossRef]
- Shinya, Y.; Miyawaki, S.; Kumagai, I.; Sugiyama, T.; Takenobu, A.; Saito, N.; Teraoka, A. Risk Factors and Outcomes of Cerebral Stroke in End-Stage Renal Disease Patients Receiving Hemodialysis. J Stroke Cerebrovasc Dis 2020, 29, 104657. [Google Scholar] [CrossRef]
- Cohen-Hagai, K.; Nacasch, N.; Rozenberg, I.; Korzets, Z.; Einbinder, Y.; Zitman-Gal, T.; Benchetrit, S. Clinical Outcomes of Stroke in Hemodialysis Patients: A Retrospective Single-Center Study. Int Urol Nephrol 2019, 51, 1435–1441. [Google Scholar] [CrossRef]
- Wang, C.-J.; Hsieh, Y.-P.; Kor, C.-T.; Chiu, P.-F. The CHA2DS2-VASc Score Predicts Chronic Kidney Disease among Patients with Atrial Fibrillation. Int Urol Nephrol 2020, 52, 1523–1531. [Google Scholar] [CrossRef]
- Ocak, G.; Ramspek, C.; Rookmaaker, M.B.; Blankestijn, P.J.; Verhaar, M.C.; Bos, W.J.W.; Dekker, F.W.; van Diepen, M. Performance of Bleeding Risk Scores in Dialysis Patients. Nephrol Dial Transplant 2019, 34, 1223–1231. [Google Scholar] [CrossRef]
- Nopp, S.; Spielvogel, C.P.; Schmaldienst, S.; Klauser-Braun, R.; Lorenz, M.; Bauer, B.N.; Pabinger, I.; Säemann, M.; Königsbrügge, O.; Ay, C. Bleeding Risk Assessment in End-Stage Kidney Disease: Validation of Existing Risk Scores and Evaluation of a Machine Learning-Based Approach. Thromb Haemost 2022, 122. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Total | AF group | Non-AF group | p |
|---|---|---|---|---|
| n | 237 | 69 | 168 | |
| Primary cause of end stage kidney disease | ||||
| Diabetic nephropathy (%) | 69 (29.1) | 19 (27.5) | 50 (29.8) | |
| Arterial hypertension (%) | 22 (9.3) | 9 (13) | 13 (7.7) | |
| Glomerulonephritis or vasculitis (%) | 20 (8.4) | 3 (4.3) | 17 (10.1) | |
| Polycystic kidney disease (%) | 8 (3.4) | 1 (1.4) | 7 (4.2) | |
| Obstructive cause (%) | 12 (5.1) | 2 (2.9) | 10 (6) | |
| Cardiorenal syndrome (%) | 9 (3.8) | 8 (11.6) | 1 (0.6) | |
| Cancer (%) | 4 (1.7) | 1 (1.4) | 3 (1.8) | |
| Unknown cause (%) | 93 (39.2) | 26 (37.7) | 67 (39.9) | |
| At time starting on HD | ||||
| Age (years, IQR) | 76 (15) | 79 (12) | 75 (17.75) | 0.067 |
| Gender (female/male) | 99 (41.8)/138 (58.2) | 25 (36.2)/44 (63.8) | 74 (44)/ 94 (56) | 0.268 |
| At time of evaluation | ||||
| Time since staring on HD (months, IQR) | 36 (44) | 34 (37) | 37 (46.75) | 0.032 |
| Age (years, IQR) | 73 (16) | 76 (11.5) | 72 (18.5) | 0.384 |
| BMI (kg/m2, ± SD) | 24.99 (± 5.12) | 25.31 (± 5.34) | 24.86 (± 5.04) | 0.568 |
| BMI classification (underweight/physiological/overweight/type I obesity/type II obesity/type III obesity) (%) | 41 (17.3)/90 (38)/66 (27.8)/32(13.5)/7 (3)/1 (0.4) | 13 (18.8)/22 (31.9)/20 (29)/11 (15.9)/3 (4.3)/0 (0) | 28 (16.7)/68 (40.5)/46 (27.4)/21 (12.5)/4 (2.4)/1 (0.6) | 0.76 |
| Survival (alive/dead) (%) | 132 (55.7)/105 (44.3) | 29 (42)/40 (58) | 103 (61.3)/65 (38.7) | 0.007 |
| Comorbidities | ||||
| Diabetes mellitus (%) | 121 (51.1) | 43 (62.3) | 78 (46.4) | 0.026 |
| Arterial hypertension (%) | 212 (89.5) | 63 (91.3) | 149 (88.7) | 0.552 |
| Dyslipidemia (%) | 161 (67.9) | 47 (68.1) | 114 (67.9) | 0.969 |
| Cancer (%) | 66 (27.8) | 22 (31.9) | 44 (26.2) | 0.374 |
| Chronic obstructive pulmonary disease (%) | 35 (14.8) | 11 (15.9) | 24 (14.3) | 0.744 |
| Hypothyroidism (%) | 43 (18.1) | 7 (10.1) | 36 (21.4) | 0.041 |
| Secondary hyperparathyroidism (%) | 59 (24.9) | 16 (23.2) | 43 (25.6) | 0.697 |
| Cholesterol (mg/dl, IQR) | 152 (54.5) | 147 (63.5) | 152 (49.5) | 0.122 |
| HDL-C (mg/dl, IQR) | 42 (18) | 39 (18) | 43.5 (17) | 0.113 |
| HDL-C (mg/dl) (< 40 / ≥ 40) | 105 (44,3)/132 (55,7) | 40 (58)/29 (42) | 65 (38.7)/103 (61.3) | 0.007 |
| LDL-C (mg/dl, IQR) | 80 (47) | 80 (54.1) | 80.1 (43.75) | 0.346 |
| Triglycerides (mg/dl, IQR) | 140 (85.5) | 142 (91.5) | 139.5 (80) | 0.569 |
| TnT-hs (ng/l, IQR) | 63 (64.85) | 79.9 (63.25) | 59.05 (62.03) | 0.004 |
| Cardiovascular event | Total | AF group | Non-AF group | p |
|---|---|---|---|---|
| Acute myocardial infarction | 82 | 35 | 47 | 0.001 |
| Occurrence of acute myocardial infarction after start of dialysis | 15 | 4 | 11 | 0.829 |
| Heart failure | 50 | 30 | 20 | <0.001 |
| Occurrence of heart failure after start of dialysis | 12 | 8 | 4 | 0.003 |
| Peripheral arterial disease | 80 | 32 | 48 | 0.008 |
| Occurrence of peripheral arterial disease after start of dialysis | 31 | 16 | 15 | 0.003 |
| Stroke (ischemic/hemorrhagic) | 60/3 | 22/3 | 38/0 | 0.031 |
| Complication or neurological residual from stroke | 35 | 15 | 20 | 0.053 |
| Occurrence of stroke after start of dialysis | 25 | 10 | 15 | 0.207 |
| Venous thromboembolic disease | 14 | 4 | 10 | 0.963 |
| Pacemaker or defibrillator | 13 | 8 | 5 | 0.008 |
| Prosthetic valve | 4 | 3 | 1 | 0.042 |
| Parameter | All | AF (+) | AF (-) | p |
|---|---|---|---|---|
| CHA2DS2-VASc score | 5 (3) | 5 (2.5) | 4 (2) | <0.0001 |
| classification of CHA2DS2-VASc score (low/intermediate/high) | 2/11/224 | 0/1/68 | 2/10/156 | 0.209 |
| HAS-BLED score | 4 (1) | 4 (1) | 4 (1) | 0.204 |
| classification of HAS-BLED score (low/intermediate/high) | 0/16/221 | 0/2/67 | 0/14/154 | 0.13 |
| Parameter | Total | AF group | Non-AF group |
|---|---|---|---|
| cancer | 19 | 4 | 15 |
| infection | 4 | 1 | 3 |
| septicemia | 18 | 6 | 12 |
| surgical | 5 | 3 | 2 |
| cachexia | 1 | 0 | 1 |
| dementia | 1 | 1 | 0 |
| chronic obstructive pulmonary disease | 1 | 1 | 0 |
| other | 11 | 5 | 6 |
| unknown | 11 | 4 | 7 |
| cardiovascular (cardiac arrest/AMI/ stroke/HF/APE/ruptured aneurysm/PE) |
(13/6/11/1/1/1/1) | (6/2/6/1/0/0/0) | (7/4/5/0/1/1/1) |
| AF group | Non-AF group | |||||
|---|---|---|---|---|---|---|
| Event | Yes | No | p | Yes | No | p |
| Acute myocardial infarction | 7 (4-7.75) | 5 (4-6) | 0.218 | 4 (3-5) | 4 (3-5) | 0.516 |
| Heart failure | 7 (6-8) | 5 (4-6) | 0.007 | 6 (5.25-6) | 4 (3-5) | 0.024 |
| Peripheral arterial disease | 6 (5-7) | 5 (4-6) | 0.066 | 4 (3-5) | 4 (3-5) | 0.756 |
| Stroke | 7 (6-8) | 5 (4-6) | <0.0001 | 6 (5-7) | 4 (3-5) | <0.0001 |
| Cardiovascular mortality | 6 (5-7) | 6 (5-7) | 0.33 | 5 (4-5) | 5 (4-6) | 0.988 |
| All-cause mortality | 6 (5-7) | 5 (3-5) | <0.0001 | 5 (4-6) | 4 (2-5) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





