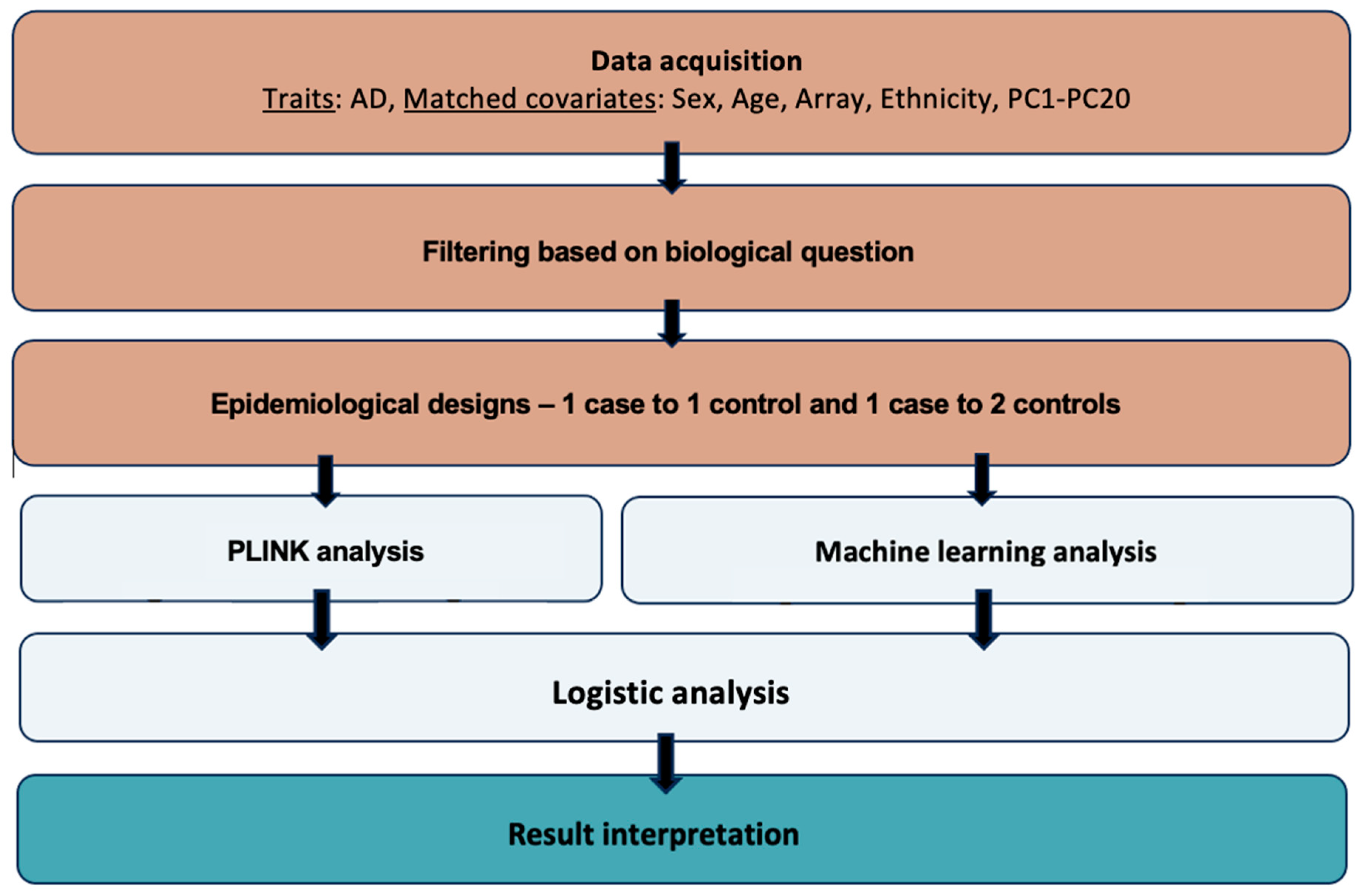
Introduction
Alzheimer’s disease (AD) is the most common type of dementia, affecting more than 32 million people worldwide (Breijyeh & Karaman, 2020; Gustavsson et al., 2022). It is characterized by progressive neurodegeneration, exhibiting neuritic plaques and neurofibrillary tangles, after accumulation of amyloid-beta peptide (Aβ) in the medial temporal lobe and neocortical structures, and the hyperphosphorylation of microtubule-associated Tau protein in neurons (De-Paula et al., 2012; Hampel et al., 2021). The incidence of AD seems to become more prominent in people over the age of 65. It is currently the sixth leading cause of death in the United States of America (Alzheimer's Association, 2022). Its symptoms include memory loss, decline of linguistic and execution abilities, as well as hallucinations, which are often accompanied by neuropsychiatric symptoms, such as depression and apathy (Kumar et al., 2022). There have been 152 studies catalogued by the GWAS Catalog and 2588 genetic variants associated with the disease so far (Sollis et al., 2023). The genetic variants with the largest effects are the ones linked to the APOE region, but other genes, such as BIN1, TNIP1, and CLNK, have also been associated with the disease (Wightman et al., 2021). The association of APOE to AD is so striking that the E4 allele of the APOE locus alone has an area under the curve (AUC) of approximately 0.68 (Escott-Price et al., 2018). Even though AD has a big genetic component, environmental factors, such as air pollution and alcohol consumption, are also believed to contribute to the disease (Eid, Mhatre & Richardson, 2019).
Traditional genome-wide association studies (GWAS) have identified associations between more than 400,000 genetic variants and hundreds of human phenotypes, providing insights into the pathophysiology of complex diseases and traits (Andreassen et al., 2023; Fabo & Khavari, 2023). Despite these successes, there are still several limitations to be considered (Fabo & Khavari, 2023). One of the most significant ones is the fact that the GWAS approach relies on statistical modelling, testing each single nucleotide polymorphism (SNP) separately, and the identified variants only account for a small proportion of the heritability (Mieth et al., 2021). Furthermore, genetic risk scores (GRS) calculated as risk predictors in precision medicine do not account for genetic interactions (Slunecka et al., 2021). In addition to that, GWAS analysis usually requires a large sample size to achieve the necessary statistical power (Korte & Farlow, 2013). Due to excessive multiple testing, a stringent threshold of significance is necessary to avoid false positive findings at the cost of losing some important variants with smaller effects. Finally, GWAS findings often lack direct biological interpretation and post-GWAS analyses integrating diverse types of data are necessary for identifying the causal genes and achieving triangulation (Tam et al., 2019; Uffelmann et al., 2021).
To overcome the aforementioned issues, machine learning algorithms can potentially be used (Doust et al., 2022; Mieth et al., 2021). Such algorithms can increase performance and power in gene discovery and outcome prediction. Although several machine learning tools have been developed, in this study we focus on a specific type of algorithms that aim to “explain” the decisions made by the machine learning model, hence allowing the easier understanding and interpretation of the results. This is referred to as “explainable artificial intelligence” and when applied on GWAS data, it can not only aid the identification of novel loci, but also provide information on correlation structures and even interactions. Explainable machine learning can unravel which locus and how much it contributes to a phenotype (Mieth et al., 2021).
A promising machine learning model is called GenNet developed by van Hilten et al. (2021). GenNet is a tool created to tackle the lack of interpretable results and address computational issues related to traditional GWAS. GenNet uses neural networks, as well as prior biological knowledge to create groups of nodes that are connected among layers, reducing the sum of learnable parameters that a fully connected neural network would need. The biological knowledge can be, for example, information on gene annotation, local and global pathways, exon annotation, chromosome annotation, as well as cell and tissue type expression. This way, in the model, the neurons are the biological entities and the weights the effects between the neurons, leading to a biologically interpretable network. An advantage of this method is that it allows the combination of machine learning methods with human biological input (van Hilten et al., 2021).
The aim of this study was to use AD data from the UK Biobank and a machine learning model to discover new genetic loci associated with the disease, as well as compare the model’s performance to the traditional GWAS approach (
Figure 1).
Materials and Methods
Phenotypes: Data from the UK Biobank was used for the current analysis. The UK Biobank is a large database with medical and genetic data from around 500,000 participants aged from 40 to 69 when recruited, from the United Kingdom (Bycroft et al., 2018). This database facilitates research on the prevention, diagnosis, and treatment of a variety of diseases (UK Biobank, 2023). Initial data collection and publications were done during the years 2006 to 2010 with more data and information being recorded since then as follow-ups for different health-related outcomes (Sudlow et al., 2015).
The UK Biobank medical dataset used for the current study was the hospital data for Alzheimer’s disease (ID: 131036, ICD10: G30*). The reported number of UK Biobank participants was 4,473 for AD. Age (ID: 21022), sex (ID: 31), genotyping array (ID: 263), ethnicity (ID: 21000), and the first 20 principal components (ID: 22009.0.1 – 2.0) were also extracted to be used as covariates to match on cases and controls in the analysis. The data was published after written consent of the participants and in accordance with the UK Biobank Ethics Advisory Committee. The participants were all registered with the UK National Health Service (NHS) (Sudlow et al., 2015).
Matching between AD cases and controls was conducted using the MatchIt package in R (Greifer, 2023). We matched on sex, age, array, ethnicity, and PC1-PC20. A 1 case to 1 control and a 1 case to 2 controls study design ratios were implemented. The final dataset of the 1 case to 1 control study design included 5,528 individuals and the 1 case to 2 controls study design included 8,292 individuals (
Table 1).
Genotypes: Two arrays were used for the genotyping of the individuals. 438,195 participants were genotyped with the UK Biobank Axiom Array and 49,932 were genotyped with the UK BiLEVE Axiom array. Around 850,000 variants were typed directly (Bycroft et al., 2018; UK Biobank, 2023). The genotypic data underwent quality control, phasing, and imputation of approximately 96M genotypes. The latter was achieved by using computationally efficient methods, alongside the use of the HRC and UK10K reference panels. Information on the quality control, phasing, and imputation of the data is available in the paper of Bycroft et al. (2018) titled "The UK Biobank resource with deep phenotyping and genomic data" (UK Biobank, 2023; Bycroft et al., 2018).
The imputed genotypic data collected from the UK Biobank initially consisted of 96 million SNPs from 22 chromosomes from half a million individuals, summing up to 2.5 terabytes. SNPs with minor allele frequency <0.05 were excluded as for these SNPs imputation might not be accurate. Pruning of data was done in PLINK using the --indep-pairwise 50 5 0.05 command. This way, only independent genetic variants were kept, leading to data redundancy and reduction of the computational expense for ANN analyses.
An additive model was implemented in PLINK 1.07 to test for association (“-assoc”) with AD (binary trait) (“-logistic”). SNPs that achieved a p-value lower than 5 × 10–8 were considered as statistically significant (Purcell, 2010). After running the association analysis for each autosomal chromosome separately, files were merged and QQ and Manhattan plots were generated. As the lambda (λ) factor showed slight inflation of 1.02 for the 1 case to 1 control design and 1.02 for the 1 case to 2 controls design the chi2 and corresponding p-values were corrected by these genomic inflation factors.
To run GenNet, three files were required: a genotype.h5 file, a subject.csv file and a topology file, as instructed by van Hilten et al. (2021). The genotype file is a genotype matrix, with each row showing participant ID. Each column is a feature, for instance a genetic variant. These files were generated by direct conversion of the genotype fam files from PLINK, using the –convert function built in which GenNet which operates in Python (3.9). The subjects file consisted of a minimum of three columns. The first one was named as “patient_id” and included the participant IDs. The second column was named as “labels” and indicated the phenotype. The third column was named as “set”, indicating which subjects would be used for training, validation, test, and which ones should be ignored (1 = training set, 2 = validation set, 3 = test, 4 = ignored). The split of the training, validation and test set was 60/20/20 respectively. Finally, the topology file is the description of the network. The rows indicate the path from the input to the output node, hence the connections of the network (van Hilten et al., 2021). For such connections to be built we first connected all SNPs in the plink .bim file to genes (layer 1) using two approaches. First, we annotated each SNP with its most implicated gene reported by Open Target Genetics and identified by integrating genetic and functional genomics features. Second, for variants not annotated by this approach, the nearest gene given by ANNOVAR annotation was hypothesized to be the most implicated one (Ghoussaini et al., 2021; Wang, Li & Hakonarson, 2010). A mapping between genes and pathways (layer 2) was made using GeneSCF and the KEGG databases (Subhash & Kanduri, 2016; Kanehisa & Goto, 2000).
To identify the genes that are potentially linked to the SNPs identified from the two different approaches, Open Targets Genetics was used for the SNPs that had an rsID (Mountjoy et al., 2021; Open Targets Genetics, 2023). The Genome Data Viewer by the National Library of Medicine was used to detect the closest genes to the SNPs that had no assigned rsID or had an rsID not found on Open Targets Genetics (Rangwala et al., 2020; NCBI, 2023; Mountjoy et al., 2021). For such SNPs, it was hypothesized that the closest genes by distance were implicated by the detected variant (Brodie, Azaria & Ofran, 2016). Open Targets Genetics use a variant-to-gene pipeline that integrates evidence from different data types and sources, such as the literature, the UK Biobank, quantitative trait loci experiments, in silico functional prediction, chromatin interaction experiments and distance. Statistical fine-mapping is used to extract an evidence score and detect the association signals and the genes each variant is linked to (Mountjoy et al., 2021).
Results
PLINK analyses showed 4 SNPs being significantly associated with AD (p < 5 × 10−8) for the 1 control to 1 case epidemiological design and 6 SNPs for the 1 case to 2 controls epidemiological design. Their p-values were corrected to account for inflation. Interestingly, all SNPs that reached this threshold are located on chromosome 19, which contains loci, such as apolipoprotein E (APOE) on its long arm. APOE is known to be the strongest risk factor for AD (Moreno-Grau et al., 2018).
At the 1 control to 1 case study design, four SNPs were found to be significantly associated with AD (
Table 2). From these, two of them, rs10410835 and rs11666329, are functionally linked to nectin cell adhesion molecule 2 (
NECTIN2), a gene found in the
APOE region (Kulminski
et al., 2018).
NECTIN2 is expressed in regions of the brain, having multiple functions, such as contribution to homeostasis of astrocytes and neurons, as well as the formation of synapses. Other SNPs in this genetic region have been previously shown to be associated with AD (Mizutani
et al., 2022). The rs10629382 variant is associated with B-cell CLL/lymphoma 3 (
BCL3), a proto-oncogene candidate that has been associated with late-onset familial AD and other cognitive impairment-related diseases (Nho
et al., 2017). It is upregulated by 27% in the brains of AD patients (Li
et al., 2018). The 19:45328407_GAC_G variant, based on its distance from the nearest genes, is probably linked either to
NECTIN2 or the basal cell adhesion molecule (Lutheran blood group) (
BCAM) gene. Both genes are found in the
APOE locus and are associated with AD (Kulminski
et al., 2018).
From the study design with the 1 case to 2 controls ratio, 6 SNPs were identified (
Table 3). Three of them 19:45328407_GAC_G, rs10629382 and rs10410835 were also found in the 1 case to 1 control study design. The top variant was rs405509, which is known to be located at the
APOE promoter and is a widely reported risk factor for AD (Ma
et al., 2016). It is linked to cognitive impairment of the elderly and can affect brain structure as well (Ye
et al., 2023; Ma
et al., 2016). Another variant linked to
NECTIN2 was rs57537848. The rs8106813 variant has been previously associated to AD and is linked to the apolipoprotein C1 pseudogene 1 (
APOC1P1) (Marioni
et al., 2018). The
APOE haplotype region is physically interacting with
APOC1P1 (Zhou
et al., 2019). The latter is associated with the development of late-onset AD (Shigemizu
et al., 2023).
To test the associations using GenNet we initially used a 1 case to 4 controls study design. A three-layered model with SNPs, gene annotation and pathway annotation and a two-layered model only with SNPs and gene annotation were implemented.
The three-layered model performed very well for the binary trait. The predictive performance for AD was very good, despite its highly polygenic nature, with an area under the curve (AUC) of 0.80 for the validation set and 0.79 for the test set. The specificity of the test was 0.99, and the sensitivity was 0.028, showing that the model predicted well the negative samples, but not the positive results.
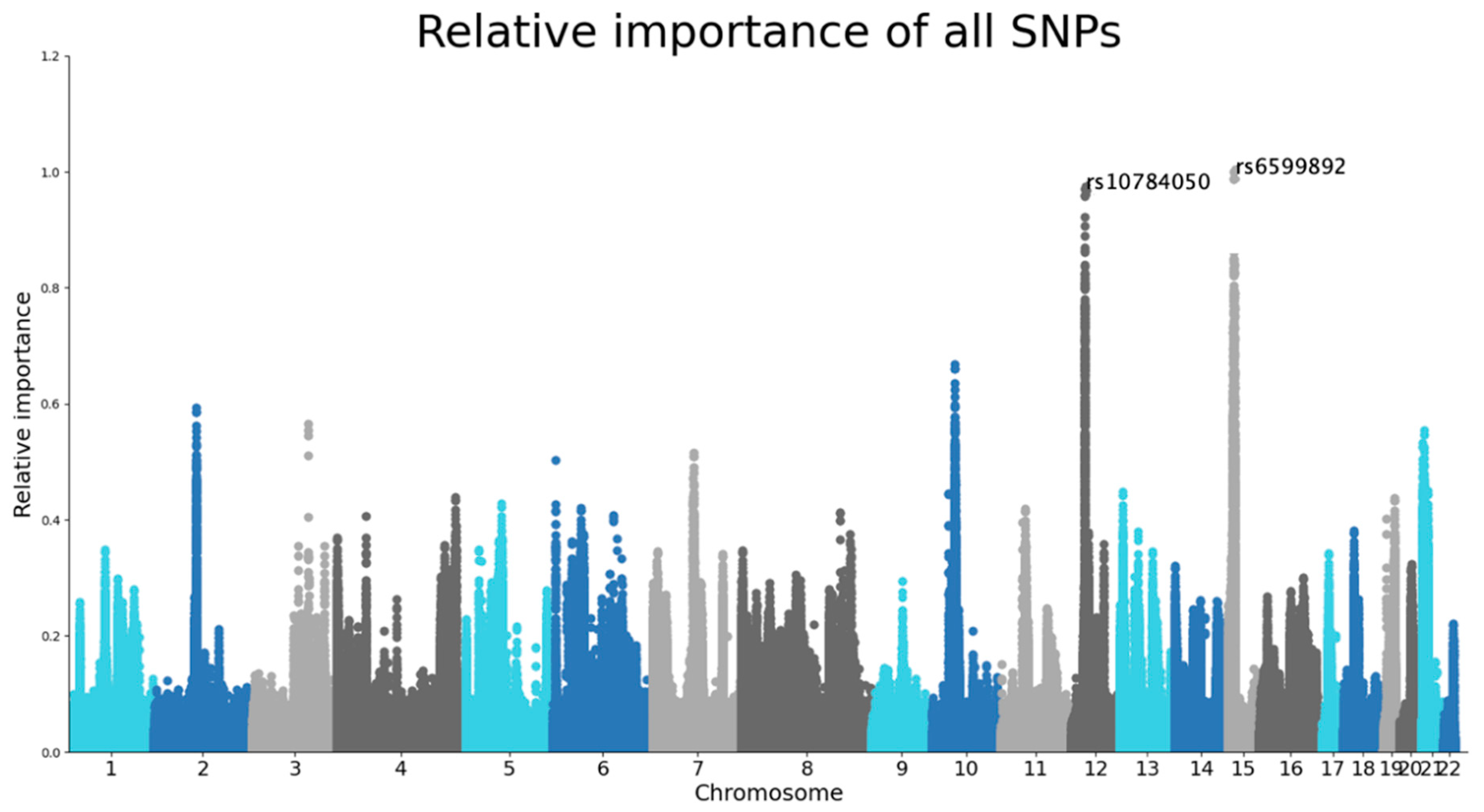
The SNPs with the highest importance values were rs6599892 at chromosome 15 and rs10784050 at chromosome 12 (
Figure 2). Although the genes and pathways for those were not identified by the model, the rs10784050 variant is functionally linked to ALG10 Alpha-1,2-glucosyltransferase B (
ALG10B) (Open Target Genetics, 2023).
ALG10B encodes a microglial Aβ Response protein. Molecular alterations in microglia, especially because of significant loss of microglial phagocytic function, is a very common characteristic of AD.
ALG10B might be indicating microglial changes in
early stages of Aβ deposition (Monasor
et al., 2020). The rs6599892 variant is located on the rhophilin Rho GTPase binding protein 2 pseudogene 1 (
RHPN2P1).
RHPN2P1 is expressed in the hippocampus and given the fact that RhoA GTPases play a role in neuron and glial cell signaling, it is hypothesized that it might also have a role in AD (Parcerisas
et al., 2014; Schmidt
et al., 2022).
Based on the sunburst figure, CUB and Sushi multiple domains 1 (CSMD1) and RNA binding Fox-1 homolog 1 (RBFOX1) genes seem to be significant. CSMD1 is a gene that is linked with cognitive functions of elderly people. Naturally, it regulates development, connection, and plasticity of circuits of the brain, and is expressed in the central nervous system and epithelial tissues. Several variants in this gene have been reported to be associated with AD, especially in individuals of older age (Stepanov et al., 2017). RBFOX1 has also been previously associated with AD. It is believed to play a role in neuronal development, and in people with AD, it is found around plaques and dystrophic neurites. When the expression of RBFOX1 is lower than normal, the β-amyloid burden increases (Raghavan et al., 2020). Also, RBFOX1 is linked to loss of gray matter in individuals with AD (Vaht et al., 2020).
When using only two layers, the AUC remained relatively high, with 0.73 for the validation set and 0.71 for the training set. The specificity remained high at 0.90 and the sensitivity increased to 0.24.
Ten SNPs were identified from this analysis, at chromosomes 7, 9, 14, 15 and 16. Six of them, rs28488717, rs7186857, rs79654388, rs61711771, rs3901280 and rs4786214, were located at chromosome 16 and were associated with the gene RBFOX1, that was identified at the analysis including both layers of genes and pathways. For the rest of the SNPs, the model did not manage to assign a gene. The rs4720976 is associated with PHD finger protein 14 (PHF14) (Open Targets Genetics, 2023). There have been no indications that this gene is linked to AD, but it has been hypothesized that age-related diseases of the retina, where PHF14 is sometimes overexpressed, might be associated with neurodegenerative diseases, such as AD, since they undergo similar changes in the neurons and microglia (Öhman et al., 2018). Based on its location to the nearest gene, the family with sequence similarity 74 member A4 (FAM74A4) is a potential target gene for rs28670889 (NCBI, 2023). It has been computationally predicted that FAM74A4 plays a role in neurodegenerative diseases, such as Parkinson’s disease and amyotrophic lateral sclerosis (Haenig et al., 2020). Two genes are close to the rs72488234 variant that are of interest (NCBI, 2023). One of them is the olfactory receptor family 11 subfamily H member 12 (OR11H12) gene, which is likely associated with differentially hydroxymethylated regions. These are associated with AD pathology, especially through neuritic plaques, which are a histological trait of AD (Zhao et al., 2017). The other one is the BCL2 interacting protein 3 pseudogene 6 (BNIP3P6) gene (NCBI, 2023). BNIP3P6 interacts with BCL2, which in turn modulates Ca+ signaling in the neurons. The dysregulation of this signaling contributes to the pathogenesis of AD (Callens et al., 2021). The rs12594050 variant potentially interacts with RHPN2P1 based on their distance, a gene that was also identified in the three-layered analysis (NCBI, 2023).
Discussion
In this study, the performance of traditional GWAS using PLINK was compared to a novel machine learning method, GenNet, which is based on deep neural networks. Genotypic and phenotypic data from the UK Biobank was used to assess the tools. The methods were tested on AD, a binary phenotype. This study was also able to identify potential genes linking new variants to AD.
Through traditional GWAS, four SNPs were found to be significantly associated with AD with the 1 case to 1 control study design and six with the 1 case to 2 controls study design. These variants were located on a chromosomal region that is highly relevant to AD, including genes such as APOE and NECTIN2. Our analysis also highlighted another gene, BCL3, which has also been previously associated with late-onset familial AD and other cognitive impairment-related diseases (Nho et al., 2017). The fact that the GWAS analysis using PLINK identified highly plausible genes, indicated that the cohort and epidemiological model used were appropriate and that the comparison with GenNet results would be possible.
The GenNet model trains its neural networks based on prior biological knowledge, reducing the number of trainable parameters (van Hilten et al., 2021). This model performed well in the AD binary phenotype, identifying a total of twelve SNPs on seven different biologically plausible genes, while obtaining an AUC of 0.80 when using the SNP, gene annotation and pathway layers, and an AUC of 0.73 when using only the SNP and gene annotation layers.
For the three-layered analysis of the binary trait, GenNet indicated two SNPs associated with two genes which were not previously linked to AD, but with potential biological plausibility. The two genes that were identified, ALG10B and RHPN2P1 are very likely to contribute to AD, as they play important functional roles in cells, such as microglia and neurons (Monasor et al., 2020; Parcerisas et al., 2014; Schmidt et al., 2022). Ten SNPs and five more genes were detected to play a role in AD when using only the SNP and gene annotation layers in the model. Even genes that were not previously identified for AD, such as BNIP3P6, are biologically plausible (Callens et al., 2021).
As there was no independent set available to run a classification analysis for AD results derived from PLINK to extract an AUC, GenNet results were compared to AUCs reported in the bibliography. AUC from conventional GWAS is reported to be around 75% to 84% (Baker & Escott-Price, 2020). GenNet achieved an AUC of 80% for AD with three layers, and 73% for AD with two layers.
Although the study yielded results, there are several limitations. Firstly, this study model did not take into account the age of AD onset. This is important as AD patients with different onset, for example, early or late, tend to have slightly different genes contributing to their pathology (Hoogmartens, Cacace & Van Broeckhoven, 2021). Moreover, this study did not account for medication. Such modification of the covariates could have increased the explained variance of the models.
An important aspect of GenNet is the freedom for the researcher to assign the network layers. While this introduces ample amount of biological knowledge and flexibility to the models, there is no standard way of doing it, as one may use different algorithms to assign a SNP to a gene and a gene to pathway or use GO terms of different hierarchical levels. It should also be mentioned that several SNPs had no gene linked to them and were left as singletons. When this information is lacking, a gene can be assigned to a SNP based on distance. This method is not very accurate, as SNPs in non-coding regions may interact with genes very far away from where they are located. In fact, they can be up to 2 Mbps apart (Brodie, Azaria & Ofran, 2016).
Another limitation of this study is the way the two approaches were compared. The comparison was mainly based on finding common SNPs and implicated genes between the two methods, and on looking at performance scores of studies of different sample sizes from existing bibliography. However, it would have been more accurate if another comparison method was followed that would, for example, allow us to compare directly AUC scores after also running a classification analysis in PLINK, or use genetic risk scores instead.
To infer associations between the SNPs identified in this study and the phenotype studied, repetition and validation of the analysis is needed. This study should be repeated with, preferably, more data, and taking into account important parameters, such as age of AD onset, that were not considered in the current study. Moreover, the two models should be compared in a more systematic way, such as having an independent set and running classification for AD results derived from PLINK to generate a polygenic risk score and corresponding AUC, and be able to do a direct comparison.
The study identified significant associations between AD and seven different SNPs linked to four different genes from the two study designs using PLINK, and twelve SNPs on seven different genes using GenNet. GenNet obtained an AUC of 0.80 for the three-layered model and of 0.73 for the two-layered model. This is one of the first studies attempting to compare the traditional GWAS approach to machine learning methods using the same data. Overall, this research is one of the few of its kind and more studies are expected in the future performing systematic comparisons to evaluate the advantages and disadvantages of machine learning methods for prediction and understanding of the genetic architecture of complex diseases.
Acknowledgments
This research has been conducted using data from UK Biobank, a major biomedical database (
www.ukbiobank.ac.uk).
References
- Alzheimer's Association (2022). 2022 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, 18(4). [CrossRef]
- Andreassen, O.A., Hindley, G.F.L., Frei, O. and Smeland, O.B. (2023). New insights from the last decade of research in psychiatric genetics: discoveries, challenges and clinical implications. World Psychiatry, [online] 22(1), pp.4–24. [CrossRef]
- Baker, E. and Escott-Price, V. (2020). Polygenic Risk Scores in Alzheimer’s Disease: Current Applications and Future Directions. Frontiers in Digital Health, 2. [CrossRef]
- Breijyeh, Z. and Karaman, R. (2020). Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules, [online] 25(24), p.5789. [CrossRef]
- Brodie, A., Azaria, J.R. and Ofran, Y. (2016). How far from the SNP may the causative genes be? Nucleic Acids Research, 44(13), pp.6046–6054. [CrossRef]
- Bycroft, C., Freeman, C., Petkova, D., Band, G., Elliott, L.T., Sharp, K., Motyer, A., Vukcevic, D., Delaneau, O., O’Connell, J., Cortes, A., Welsh, S., Young, A., Effingham, M., McVean, G., Leslie, S., Allen, N., Donnelly, P. and Marchini, J. (2018). The UK Biobank resource with deep phenotyping and genomic data. Nature, 562(7726), pp.203–209. [CrossRef]
- Callens, M., Kraskovskaya, N., Derevtsova, K., Annaert, W., Bultynck, G., Bezprozvanny, I. and Vervliet, T. (2021). The role of Bcl-2 proteins in modulating neuronal Ca2+ signaling in health and in Alzheimer’s disease. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1868(6), p.118997. [CrossRef]
- De-Paula, V.J., Radanovic, M., Diniz, B.S. and Forlenza, O.V. (2012). Alzheimer’s Disease. Protein Aggregation and Fibrillogenesis in Cerebral and Systemic Amyloid Disease, [online] 65, pp.329–352. [CrossRef]
- Eid, A., Mhatre, I. and Richardson, J.R. (2019). Gene-environment interactions in Alzheimer’s disease: A potential path to precision medicine. Pharmacology & Therapeutics, 199, pp.173–187. [CrossRef]
- Escott-Price, V., Myers, A., Huentelman, M., Shoai, M. and Hardy, J. (2018). POLYGENIC RISK SCORE ANALYSIS OF ALZHEIMER’S DISEASE IN CASES WITHOUT APOE4 OR APOE2 ALLELES. The Journal Of Prevention of Alzheimer’s Disease, pp.1–4. [CrossRef]
- Fabo, T. and Khavari, P. (2023). Functional characterization of human genomic variation linked to polygenic diseases. 39(6), pp.462–490. [CrossRef]
- Ghoussaini, M., Mountjoy, E., Carmona, M., Peat, G., Schmidt, E., Hercules, A., Fumis, L., Miranda, A., Carvalho-Silva, D., Buniello, A., Burdett, T., Hayhurst, J., Baker, J., Ferrer, J., Gonzalez-Uriarte, A., Jupp, S., Karim, M., Koscielny, G., Machlitt-Northen, S. and Malangone, C. (2020). Open Targets Genetics: systematic identification of trait-associated genes using large-scale genetics and functional genomics. Nucleic Acids Research, 49(D1), pp.D1311–D1320. [CrossRef]
- Greifer, N. (2023). MatchIt. Available at: https://cran.r-project.org/web/packages/MatchIt/vignettes/MatchIt.html.
- Gustavsson, A., Norton, N., Fast, T., Frölich, L., Georges, J., Holzapfel, D., Kirabali, T., Krolak-Salmon, P., Rossini, P.M., Ferretti, M.T., Lanman, L., Chadha, A.S. and van der Flier, W.M. (2022). Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. [online]. [CrossRef]
- Haenig, C., Atias, N., Taylor, A.K., Mazza, A., Schaefer, M.H., Russ, J., Riechers, S.-P., Jain, S., Coughlin, M., Fontaine, J.-F., Freibaum, B.D., Brusendorf, L., Zenkner, M., Porras, P., Stroedicke, M., Schnoegl, S., Arnsburg, K., Boeddrich, A., Pigazzini, L. and Heutink, P. (2020). Interactome Mapping Provides a Network of Neurodegenerative Disease Proteins and Uncovers Widespread Protein Aggregation in Affected Brains. Cell Reports, [online] 32(7), p.108050. [CrossRef]
- Hampel, H., Hardy, J., Blennow, K., Chen, C., Perry, G., Kim, S.H., Villemagne, V.L., Aisen, P., Vendruscolo, M., Iwatsubo, T., Masters, C.L., Cho, M., Lannfelt, L., Cummings, J.L. and Vergallo, A. (2021). The Amyloid-β Pathway in Alzheimer’s Disease. Molecular Psychiatry, [online] 26(10). [CrossRef]
- Hoogmartens, J., Cacace, R. and Van Broeckhoven, C. (2021). Insight into the genetic etiology of Alzheimer’s disease: A comprehensive review of the role of rare variants. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 13(1). [CrossRef]
- Kanehisa, M., & Goto, S. (2000). KEGG: kyoto encyclopedia of genes and genomes. Nucleic acids research, 28(1), 27–30. [CrossRef]
- Kulminski, A.M., Huang, J., Wang, J., He, L., Loika, Y. and Culminskaya, I. (2018). Apolipoprotein E region molecular signatures of Alzheimer’s disease. Aging Cell, 17(4), p.e12779. [CrossRef]
- Korte, A. and Farlow, A. (2013). The advantages and limitations of trait analysis with GWAS: a review. Plant Methods, 9(1), p.29. [CrossRef]
- Kumar, A. Sidhu, J., Goyal, A., Tsao, J.W. (2022). Alzheimer Disease. [online] Nih.gov. Available at: https://www.ncbi.nlm.nih.gov/books/NBK499922/.
- Li, X., Wang, H., Long, J., Pan, G., He, T., Anichtchik, O., Belshaw, R., Albani, D., Edison, P., Green, E.K. and Scott, J. (2018). Systematic Analysis and Biomarker Study for Alzheimer’s Disease. Scientific Reports, [online] 8(1), p.17394. [CrossRef]
- Ma, C., Zhang, Y., Li, X., Zhang, J., Chen, K., Liang, Y., Chen, Y., Liu, Z., & Zhang, Z. (2016). Is there a significant interaction effect between apolipoprotein E rs405509 T/T and ε4 genotypes on cognitive impairment and gray matter volume?. European journal of neurology, 23(9), 1415–1425. [CrossRef]
- Marioni, R. E., Harris, S. E., Zhang, Q., McRae, A. F., Hagenaars, S. P., Hill, W. D., Davies, G., Ritchie, C. W., Gale, C. R., Starr, J. M., Goate, A. M., Porteous, D. J., Yang, J., Evans, K. L., Deary, I. J., Wray, N. R., & Visscher, P. M. (2018). GWAS on family history of Alzheimer's disease. Translational psychiatry, 8(1), 99. [CrossRef]
- Mieth, B., Rozier, A., Rodriguez, J.A., Höhne, M.M.C., Görnitz, N. and Müller, K.-R. (2021). DeepCOMBI: explainable artificial intelligence for the analysis and discovery in genome-wide association studies. NAR Genomics and Bioinformatics, [online] 3(3), p.lqab065. [CrossRef]
- Mizutani, K., Miyata, M., Shiotani, H., Kameyama, T. and Takai, Y. (2022). Nectin-2 in general and in the brain. Molecular and Cellular Biochemistry, 477(1), pp.167–180. [CrossRef]
- Monasor, S.L., Müller, S.A., Colombo, A.V., Tanrioever, G., König, J., Roth, S., Liesz, A., Berghofer, A., Piechotta, A., Prestel, M., Saito, T., Saido, T.C., Herms, J., Willem, M., Haass, C., Lichtenthaler, S.F. and Tahirovic, S. (2020). Fibrillar Aβ triggers microglial proteome alterations and dysfunction in Alzheimer mouse models. eLife, 9. [CrossRef]
- Moreno–Grau, S., Rodriguez-Gomez, O., Sanabria, A., Pérez-Cordón, A., Sánchez-Ruiz, D., Abdelnour, C., Valero, S., Hernández, I., Maitée Rosende-Roca, Mauleón, A., Vargas, L., Lafuente, A., Gil, S., Santos-Santos, M.A., Montserrat Alegret, Espinosa, A., Ortega, G., Guitart, M., Gailhajanet, A. and Itziar de Rojas (2017). Exploring APOE genotype effects on Alzheimer’s disease risk and amyloid β burden in individuals with subjective cognitive decline: The FundacioACE Healthy Brain Initiative (FACEHBI) study baseline results. Alzheimers & Dementia, 14(5), pp.634–643. [CrossRef]
- Mountjoy, E., Schmidt, E.M., Carmona, M., Schwartzentruber, J., Peat, G., Miranda, A., Fumis, L., Hayhurst, J., Buniello, A., Karim, M.A., Wright, D., Hercules, A., Papa, E., Fauman, E.B., Barrett, J.C., Todd, J.A., Ochoa, D., Dunham, I. and Ghoussaini, M. (2021). An open approach to systematically prioritize causal variants and genes at all published human GWAS trait-associated loci. Nature Genetics, 53(11), pp.1527–1533. [CrossRef]
- NCBI (2023). Genome Data Viewer. National Center for Biotechnology Information. [online] Nih.gov. Available at: https://www.ncbi.nlm.nih.gov/.
- Nho, K., Kim, S., Horgusluoglu, E., Risacher, S.L., Shen, L., Kim, D., Lee, S., Foroud, T., Shaw, L.M., Trojanowski, J.Q., Aisen, P.S., Petersen, R.C., Jack, C.R., Weiner, M.W., Green, R.C., Toga, A.W. and Saykin, A.J. (2017). Association analysis of rare variants near the APOE region with CSF and neuroimaging biomarkers of Alzheimer’s disease. BMC Medical Genomics, 10(S1). [CrossRef]
- Öhman, T., Fitsum Tamene, Helka Göös, Sirpa Loukovaara and Markku Varjosalo (2018). Systems pathology analysis identifies neurodegenerative nature of age-related vitreoretinal interface diseases. Aging Cell, [online] 17(5), pp.e12809–e12809. [CrossRef]
- Open Targets. (2023). Open Targets Genetics. [online] Available at: https://genetics.opentargets.org.
- Parcerisas, A., Rubio, S., Ashraf Muhaisen, Gómez-Ramos, A., Lluís Pujadas, Montserrat Puiggròs, Rossi, D., Jesús Ureña, Ferran Burgaya, Pascual, M., Torrents, D., Rábano, A., Avila, J. and Soriano, E. (2014). Somatic Signature of Brain-Specific Single Nucleotide Variations in Sporadic Alzheimer’s Disease. 42(4), pp.1357–1382. [CrossRef]
- Purcell, S. (2010). PLINK (1.07) Documentation. [online] Available at: https://zzz.bwh.harvard.edu/plink/dist/plink-doc-1.07.pdf.
- Raghavan, N.S., Dumitrescu, L., Mormino, E., Mahoney, E.R., Lee, A.J., Gao, Y., Bilgel, M., Goldstein, D., Harrison, T., Engelman, C.D., Saykin, A.J., Whelan, C.D., Liu, J.Z., Jagust, W., Albert, M., Johnson, S.C., Yang, H.-S., Johnson, K., Aisen, P. and Resnick, S.M. (2020). Association Between Common Variants in RBFOX1, an RNA-Binding Protein, and Brain Amyloidosis in Early and Preclinical Alzheimer Disease. JAMA Neurology, 77(10), p.1288. [CrossRef]
- Rangwala, S.H., Kuznetsov, A., Ananiev, V., Asztalos, A., Borodin, E., Evgeniev, V., Joukov, V., Lotov, V., Pannu, R., Rudnev, D., Shkeda, A., Weitz, E.M., Schneider, V.A. (2020) Accessing NCBI data using the NCBI Sequence Viewer and Genome Data Viewer (GDV). Genome Res. January 2021 31: 159-169. [CrossRef]
- Schmidt, S.I., Blaabjerg, M., Freude, K. and Meyer, M. (2022). RhoA Signaling in Neurodegenerative Diseases. Cells, 11(9), p.1520. [CrossRef]
- Shigemizu, D., Akiyama, S., Suganuma, M., Furutani, M., Yamakawa, A., Nakano, Y., Ozaki, K. and Shumpei Niida (2023). Classification and deep-learning–based prediction of Alzheimer disease subtypes by using genomic data. Translational Psychiatry, [online] 13(1). [CrossRef]
- Slunecka, J.L., van der Zee, M.D., Beck, J.J., Johnson, B.N., Finnicum, C.T., Pool, R., Hottenga, J.-J., de Geus, E.J.C. and Ehli, E.A. (2021). Implementation and implications for polygenic risk scores in healthcare. Human Genomics, 15(1). [CrossRef]
- Sollis, E., Mosaku, A., Abid, A., Buniello, A., Cerezo, M., Gil, L., Groza, T., Güneş, O., Hall, P., Hayhurst, J., Ibrahim, A., Ji, Y., John, S., Lewis, E., MacArthur, J. A. L., McMahon, A., Osumi-Sutherland, D., Panoutsopoulou, K., Pendlington, Z., Ramachandran, S., … Harris, L. W. (2023). The NHGRI-EBI GWAS Catalog: knowledgebase and deposition resource. Nucleic acids research, 51(D1), D977–D985. [CrossRef]
- Stepanov, V., Marusin, A., Vagaitseva, K., Bocharova, A. and Makeeva, O. (2017). Genetic Variants in CSMD1 Gene Are Associated with Cognitive Performance in Normal Elderly Population. Genetics Research International, 2017, pp.1–5. [CrossRef]
- Subhash, S., & Kanduri, C. (2016). GeneSCF: a real-time based functional enrichment tool with support for multiple organisms. BMC bioinformatics, 17(1), 365. [CrossRef]
- Sudlow, C., Gallacher, J., Allen, N., Beral, V., Burton, P., Danesh, J., Downey, P., Elliott, P., Green, J., Landray, M., Liu, B., Matthews, P., Ong, G., Pell, J., Silman, A., Young, A., Sprosen, T., Peakman, T. and Collins, R. (2015). UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLOS Medicine, [online] 12(3), p.e1001779. [CrossRef]
- Tam, V., Patel, N., Turcotte, M., Bossé, Y., Paré, G. and Meyre, D. (2019). Benefits and limitations of genome-wide association studies. Nature Reviews Genetics, 20(8), pp.467–484. [CrossRef]
- Uffelmann, E., Huang, Q.Q., Munung, N.S., de Vries, J., Okada, Y., Martin, A.R., Martin, H.C., Lappalainen, T. and Posthuma, D. (2021). Genome-wide association studies. Nature Reviews Methods Primers, 1(1). [CrossRef]
- UK Biobank (2023). Learn more about UK Biobank. [online] Available at: https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank.
- Vaht, M., Laas, K., Fernàndez-Castillo, N., Kurrikoff, T., Kanarik, M., Faraone, S.V., Tooding, L.-M., Veidebaum, T., Franke, B., Reif, A., Cormand, B. and Harro, J. (2020). Variants of the Aggression-Related RBFOX1 Gene in a Population Representative Birth Cohort Study: Aggressiveness, Personality, and Alcohol Use Disorder. Frontiers in Psychiatry, 11. [CrossRef]
- van Hilten, A., Kushner, S.A., Kayser, M., Ikram, M.A., Adams, H.H.H., Klaver, C.C.W., Niessen, W.J. and Roshchupkin, G.V. (2021). GenNet framework: interpretable deep learning for predicting phenotypes from genetic data. Communications Biology, 4(1). [CrossRef]
- Wang, K., Li, M. and Hakonarson, H. (2010). ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Research, 38(16), pp.e164–e164. [CrossRef]
- Wightman, D.P., Jansen, I.E., Savage, J.E., Shadrin, A.A., Bahrami, S., Holland, D., Rongve, A., Børte, S., Winsvold, B.S., Drange, O.K., Martinsen, A.E., Skogholt, A.H., Willer, C., Bråthen, G., Bosnes, I., Nielsen, J.B., Fritsche, L.G., Thomas, L.F., Pedersen, L.M. and Gabrielsen, M.E. (2021). A genome-wide association study with 1,126,563 individuals identifies new risk loci for Alzheimer’s disease. Nature Genetics, 53(9), pp.1276–1282. [CrossRef]
- Ye, Z., Tan, D., Luo, T., Gou, R., Cai, J., Wei, Y., He, K., Xiao, S., Mai, T., Tang, X., Liu, Q., Mo, X., Lin, Y., Huang, S., Li, Y., Qin, J., & Zhang, Z. (2023). ApoE gene polymorphisms and metals and their interactions with cognitive function. BMC medical genomics, 16(1), 206. [CrossRef]
- Zhao, J., Zhu, Y., Yang, J., Li, L., Wu, H., De Jager, P.L., Jin, P. and Bennett, D.A. (2017). A genome-wide profiling of brain DNA hydroxymethylation in Alzheimer’s disease. Alzheimer’s & Dementia, 13(6), pp.674–688. [CrossRef]
- Zhou, X., Chen, Y., Mok, K.Y., Kwok, T.C.Y., Mok, V.C.T., Guo, Q., Ip, F.C., Chen, Y., Mullapudi, N., Giusti-Rodríguez, P., Sullivan, P.F., Hardy, J., Fu, A.K.Y., Li, Y. and Ip, N.Y. (2019). Non-coding variability at the APOE locus contributes to the Alzheimer’s risk. Nature Communications, 10(1). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).