Submitted:
11 December 2023
Posted:
12 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant material
2.2. Extraction and isolation
2.3. In vitro anti-inflammatory activities
2.3.1. Cell Viability Assay (RAW 264.7)
2.3.2. Stimulation of RAW 264.7 Cells with LPS
2.3.3. Determination of NO concentration
2.3.4. Analysis of Pro-Inflammatory Cytokine IL-6
2.4. Treatment of RAW-Blue Cells with LPS
2.5. Statistical analysis
3. Results and discussion
3.1. Chemical Composition
3.2. Inhibition of LPS-induced NO production by compounds 1-11
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- McVaugh, R. Compositae. Flora Novo-Galiciana. A Descriptive Account of the Vascular Plants of Western Mexico; The University of Michigan Press: Ann Arbor, MI, USA, 1984; Volume 12.
- GBFI. Ageratina pichinchensis. Available online: https://www.gbif.org/es/species/5400249 (accessed on 01 December 2023).
- Villaseñor, R.; Espinosa, G.F.J. Catálogo de Malezas de México; Universidad Nacional Autónoma de México: México City, México, 1998; pp. 1-448.
- Villaseñor, J.L. Diversidad y distribución de la familia Asteraceae en México. Bot. Sci. 2018, 96, 332-358.
- INAH. Jardín Etnobotánico y Museo de la Medicina Tradicional. 2023. Available online: https://lugares.inah.gob.mx/es/museos-inah/colecciones/piezas/12915-12915-axihuitl.html?lugar_id=389 (accessed on 1 December 2023).
- Argueta, A.; Cano, L.; Rodarte, M. Atlas de la Medicina Tradicional Mexicana, Tomo 1-3; Instituto Nacional Indigenista: Mexico City; Mexico, 1994; p. 1786.
- Avilés, M.; Suárez, G. Catálogo de Plantas Medicinales. Jardín Etnobotánico; Centro INAH: Cuernavaca, Mexico, 1994; p. 47.
- Ríos, M.Y.; Aguilar-Guadarrama, B.; Navarro, V. Two new benzofurans from Eupatorium aschenbornianum and their antimicrobial activity. Planta Med. 2003, 69, 967-970. [CrossRef]
- Navarro-García, V.M.; Gonzalez, A.; Fuentes, M.; Aviles, M.; Ríos, M.Y.; Zepeda, G.; Rojas, M.G. Antifungal activities of nine traditional Mexican medicinal plants. J. Ethnopharmacol. 2003, 87, 85-88. [CrossRef]
- Sánchez-Mendoza, M.E.; Reyes-Trejo, B.; Sánchez-Gómez, P.; Rodriguez-Silverio, J.; Castillo-Henkel, C.; Cervantes-Cuevas, H.; Arrieta, J. Bioassay-guided isolation of an anti-ulcer chromene from Eupatorium aschembornianum: Role of nitric oxide, prostaglandins and sulfydryls. Fitoterapia 2010, 81, 66-71. [CrossRef]
- Sánchez-Mendoza, M.; Rodriguez-Silverio, J.; Rivero-Cruz, J.F. Rocha-González, H.; Pineda-Farías, J.; Arrieta, J. Antinociceptive effect and gastroprotective mechanisms of 3,5-diprenyl-4-hydroxyacetophenone from Ageratina pichinchensis. Fitoterapia 2013, 87, 11-19. [CrossRef]
- Aguilar-Guadarrama, B.; Navarro, V.; León-Rivera, I.; Ríos, M.Y.; Active compounds against tinea pedis dermatophytes from Ageratina pichinchensis var. bustamenta. Nat. Prod. Res. 2009, 23, 1559-1565. [CrossRef]
- Romero-Cerecero, O.; Zamilpa, A.; Jiménez, E.; Tortoriello, J. Effect on the wound healing process and in vitro cell proliferation by the medical Mexican plant Ageratina pichinchensis. Planta Med. 2011, 77, 979-983. [CrossRef]
- Torres-Barajas, L.; Rojas-Vera, J.; Morales-Méndez, A.; Rojas-Fermín, L.; Lucena, M.; Buitrago, A. Chemical composition and evaluations of antibacterial activity of oils of Ageratina jahnii and Ageratina pichinchensis collected in Mérida, Venezuela. Bol. Latinioam. Caribe Plant. Med. Aromat. 2013, 12, 92-98.
- Romero-Cerecero, O.; Zamilpa, A.; González-Cortazar, M.; Alonso-Cortés, D.; Jiménez-Ferrer, E.; Nicasio-Torres, P.; Aguilar-Santamaría, L.; Tortoriello, J. Pharmacological and chemical study to identify wound-healing active compounds in Ageratina pichinchensis. Planta Med. 2013, 79, 622-627. [CrossRef]
- White, M.M.D. Mediators of inflammation and the inflammatory process. Journal of Allergy and Clinical Immunology. 1999, 103, S978-S981. [CrossRef]
- Duleba, M.D.A.J.; Dokras, M.D.A. Is PCOS an inflammatory process? Fertility and Sterility. 2012, 97, 7-12. [CrossRef]
- Lugrin, J.; Rosenblantt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biological Chemistry 2014, 395, 203-230. [CrossRef]
- Adbulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: a review. Veterinary World 2018, 11, 627-635. [CrossRef]
- Sugimoto, M.A.; Vago, J.P.; Perretti, M.; Teixeira M.M. Mediators of the resolutions of the inflammatory response. Trends in Immunology 2019, 1545, 1-16. [CrossRef]
- Veríssimo, F.J.; Oliveira, C.R.N.; Nunes, J.C.A.; Araújo, M.F.T.A.; Veríssimo, A.J.W.; Galvao, A.J.M. The role of the mediators of inflammation in cancer development. Pathol. Onco. Res. 2015, 21, 527-534. [CrossRef]
- Salgado, A.; Bóveda, J.L.; Monasterio, J.; Segura, R.M.; Mourelle, M.; Gómez-Jiménez, J.; Peracaula, R. Inflammatory mediators and their influence on haemostasis. Pathophysiology of Haemostasis and Trombosis 1994, 24, 132-138. [CrossRef]
- Schlag, G.; Redl, H. Mediators of injury and inflammation. World J. Sug. 1996, 20, 406-410. [CrossRef]
- Bradley, T.S.; Geller, D.A. Molecular regulation of the human inducible nictric oxidase synthase (iNOS) gene. Shock 2000, 13, 413-424. [CrossRef]
- Krishna, R.K.M. Molecular mechanisms regulating iNOS expression in various cell types. J Toxicol Environ Health B Crit Rev 2000, 3, 27-58. [CrossRef]
- Chiou, W.-F.; Chen, C.-F.; Lin, J.-J. Mechanisms of suppression of inducible nitric oxide synthase (iNOS) expression in RAW 264.7 cells by andrographolide. Br. J. Pharmacol. 2000, 129, 1553-1560. [CrossRef]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639-653. [CrossRef]
- Panaro, M.A.; Brandonisio, O.; Acquafredda, A.; Sisto, M.; Mitolo, V. Evidences for iNOS Expression and Nitric Oxide Production in the Human Macrophages. Curr Drug Targ Immune, Endocrine & Metabolic Disorders 2003, 3, 210-221. [CrossRef]
- Poteser, M.; Wakabayashi, I. Serum albumin induces iNOS expression and NO production in RAW 264.7 macrophages. Br. J. Pharmacol. 2004, 143, 143-151. [CrossRef]
- Gosgnach, W.; Messika-Zeitoun, D.; Gonzalez, W.; Philipe, M.; Michel, J.B. Shear stress induces iNOS expression in cultured smooth muscle cells: role of oxidative stress. Am J Physiol Cell Physiol 2000, 279, C1880-C1888. [CrossRef]
- Anavi, S.; Tirosh, O. iNOS as a metabolic enzyme under stress conditions. Free Radic. Biol. Med. 2020, 146, 16-35. [CrossRef]
- Weinberg, J.B.; Misukonis, M.A.; Shami, P.J.; Mason, S.N.; Sauls, D.L.; Dittman, W.A.; Wood, E.R.; Smith, G.K.; McDonald, B.; Bachus, K.E. Human molecular phagocyte inducible nitric oxide synthase (iNOS): analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood 1995, 83, 1184-1195.
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on immune cells and its role in diseases. Int. J. Mol. Sci. 2018, 19, 3805. [CrossRef]
- Xu, W.; Liu, L.Z.; Loizidou, M.; Ahmed, M.; Charles, I.G. The role of nitric oxide in cancer. Cell Research 2002, 12, 311-320. [CrossRef]
- Xie, Q.-W.; Nathan, C. The high-output nitric oxide pathway: role and regulation. J. Leuk. Biol. 1994, 56, 576-582. [CrossRef]
- Bruckdorfer, R. The basics about nitric oxide. Mol. Asp. Med. 2005, 26, 3-31. [CrossRef]
- Nagy, G.; Clark, J.M.; Buzás, E.I.; Gorman, C.L.; Cope, A.P. Nitric oxide, chronic inflammation and autoimmune. Immunol Let 2007, 111, 1-5. [CrossRef]
- Laroux, F.S.; Pavlick, K.P.; Hines, I.N.; Kawachi, S.; Harada, H.; Bharwani, S.; Hoffman, J.M.; Grisham, M.B. Role of nitric oxide in inflammation. Acta Physiol Scand 2001, 173, 113-118. [CrossRef]
- Papi, S.; Ahmadizar, F.; Hasanvand, A. The role of nitric oxide in inflammation and oxidative stress. Immunopathol Persa 2019, 5, e08. [CrossRef]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. Pathog. Dis. 2007, 51, 443-452. [CrossRef]
- Zamora, R.; Vodovotz, Y.; Billiar, T.R. Inducible nitric oxide synthase and inflammatory diseases. Molecular Medicine 2000, 6, 347-373. [CrossRef]
- Maniscalco, M.; Sofia, M.; Pelaia, G. Nitric oxide in upper airways inflammatory diseases. Inflamm. Res. 2007, 56, 58-69. [CrossRef]
- Soufli, I.; Toumi, R.; Rafa, H.; Touil-Boukoffa, C. Overview of cytokines and nitric oxide involvement in immune-pathogenesis of inflammatory bowel diseases. World J Gatrointest Pharmacol Ther 2016, 7, 353-360. [CrossRef]
- Rask-Madsen, P. Review article: the potential role of nitric oxide in chronic inflammatory bowel disorders. AP&T 2001, 13, 135-144. [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of the oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252-259. [CrossRef]
- Blantz, R.C.; Munger, K. Role of nitric oxide in inflammatory conditions. Nephron 2002, 90, 373-378. [CrossRef]
- Amirghofran, Z.; Malek-Hosseini, S.; Golmoghaddam, H.; Kalantar, F.; Shabani, M. Inhibition of nitric oxide production and proinflammatory cytokines by several medicinal plants. Iran. J. Immunol. 2011, 8, 159-169.
- Perera, H.D. S. M.; Samarasekera, J.K.R.R.; Handunnetti, S.M.; Weerasena, D.S.J.; Weeratunga, H.D.; Jabeen, A.; Choudhary, M.I. In vitro pro-inflammatory enzyme inhibition and anti-oxidant potential of selected Sri Lankan medicinal plants. BMC 2018, 18, 271. [CrossRef]
- Jae-Ha, R.; Han-Na, A.; Hwa-Jin, L.; Li, F.; Wen-He, Q.; Yong-Nam, H.; Byung-Hoon, H. Inhibitory activity of Chinese medicinal plants on nitric oxide synthesis in lipopolysaccharide activated macrophages. Biomolecules & Therapeutics 2001, 9, 183-187.
- Achenbach, H.; Waibel, R.; Addae-Mensah, I. Constituents of West African medicinal plants. Part 17. Sesquiterpenes from Carissa edulis. Phytochemistry 1985, 24, 2325-8.
- You, C.X.; Yang, K.; Wang, C.F.; Zhang, W.J.; Wang, Y.; Han, J.; Fan, L.; Du, S.S.; Geng, Z.F.; Deng, Z.W. Cytotoxic compounds isolated from Murraya tetramera huang. Mol. 2014, 19, 13225-13234. [CrossRef]
- Dupre, S.; Bohlmann, F.; Knox, E. Prenylated p-hydroxyacetophenone derivatives from the giant Senecio johnstonii. Biochem. Syst. Ecol. 1990, 18, 149-50. [CrossRef]
- Bohlmann, F.; Rao, N. New hydroxyacetophenone derivatives from Espeletia Schultzii. Chem. Ber. 1973, 106, 3035-8.
- Becerra, J.; Silva, M.; Delle-Monache, G.; Delle-Monache, F.; Botta, M. Two new chromenes from Eupatorium glechonophyllum Less. Rev. Lat. Quím. 1983, 14, 92-4.
- Merfort, I.; Wendisch, D. Flavonoid glycosides from Arnica montana and Arnica chamissonis. Planta Med. 1987, 53, 434-7. [CrossRef]
- Juergens, U.R. Anti-inflammatory properties of the monoterpene 1.8-cineole: Current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014, 64, 638–646. [CrossRef]
- Schwartz, M.A. Syntheses of (+)-α- and (+)-β-eudesmol and their diastereomers by intramolecular nitrone-olefin cycloaddition. J. Org. Chem. 1985, 50, 1359–1365. [CrossRef]
- Ismail, M.; Javed, S.; Kazim, M.; Razaq, A.; Hussain, E.; Attia-tul-Wahab; Ali, S.; Choudhary, Muhammad Iqbal. Phenylpropanoids from Tanacetum baltistanicum with Nematocidal and Insecticidal Activities. Chem. Nat. Compd. 2022, 58, 637-643. [CrossRef]
- Bohlmann, F.; Vorwerk, E. Synthesis of naturally occurring p-hydroxyacetophenone derivatives. II. Chem. Ber. 1980, 113, 261-6. [CrossRef]
- Bjeldanes, L.; Geissman, T. Euparinoid constituents of Encelia californica. Phytochemistry. 1969, 8, 1293–1296. [CrossRef]
- Sfikakis P.P. The first decade of biologic TNF antagonists in clinical practice: lessons learned, unresolved issues and future directions. Curr Dir Autoimmun. 2010, 11, 180–210. [CrossRef]
- Owona, B.A.; Njayou, N.F.; Laufer, S.; Moundipa, P.F.; Schluesener, H.J. A fraction of stem bark extract of Entada africana suppresses lipopolysaccharide-induced inflammation in RAW 264.7 cells. J. Ethnopharmacol. 2013, 149, 162–168. [CrossRef]
- Almeida, J.R.G.S.; Souza, G.R.; Silva, J.C.; Saraiva, S.R.G.D.; Junior, R.G.O.; Quintans, J.S.S.; Barreto, R.S.S.; Bonjardim, L.R.; Cavalcanti, S.C.H.; Junior, L.J.Q. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. Sci. World J. 2013, 808460. [CrossRef]
- Choi, Y.K.; Cho, G.S.; Hwang, S.; Kim, B.W.; Lim, J.H.; Lee, J.C.; Kim, H.C.; Kim, W.K.; Kim, Y.S. Methyleugenol reduces cerebral ischemic injury by suppression of oxidative injury and inflammation. Free Radical. Res. 2010, 44, 925–935. [CrossRef]
- Kim, D.S.; Lee, H.J.; Jeon, Y.D.; Han, Y.H.; Kee, J.Y.; Kim, H.J.; Shin, H.J.; Kang, J.W.; Lee, B.S.; Kim, S.H.; Kim, S.J.; Park, S.H.; Choi, B.M.; Park, S.J.; Um, J.Y.; Hong, S.H. Alpha-pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-kB pathway in mouse peritoneal macrophages. Am. J. Chin. Med. 2015, 43, 731–742. [CrossRef]
- Mankhong, S.; Iawsipo, P.; Srisook, E.; Srisook, K. 4-methoxycinnamyl p-coumarate isolated from Etlingera pavieana rhizomes inhibits inflammatory response via suppression of NF-κB, Akt and AP-1 signaling in LPS-stimulated RAW 264.7 macrophages. Phytomedicine. 2019, 54, 89-97. [CrossRef]
- Wu, Y.L.; Han, F.; Luan, S.S.; Ai, R.; Zhang, P.; Li, H.; Chen, L.X. Triterpenoids from Ganoderma lucidum and Their Potential Anti-inflammatory Effect. J. Agric. Food Chem. 2019, 67, 5147-5158. [CrossRef]
- Iloki, S.B.; Gil-Salido, A.A.; Lewis, L.M.; Rosas-Durazo, A.; Acosta-Silva, A.L.; Rivera-Castañeda, E.G.; Rubio-Pino, J.L. Cell growth curves for different cell lines and their relationship with biological activities. Int. J. Biotechnol. Mol. Biol. Res. 2013, 4, 60−70.
- Merfort, I.; Wendisch, D. New flavonoid glycosides from Arnicae flos DAB 91. Planta Med. 1992, 58, 355-7. [CrossRef]
- Lyss, G.; Schmidt, T.J.; Merfort, I.; Pahl, H.L. Helenalin, an anti-inflammatory sesquiterpene lactone from Arnica, selectively inhibits transcription factor NF-κB. Biol. Chem. 1997, 378, 951-961. [CrossRef]
- Nam, S.Y.; Kim, H.Y.; Kim, H.M.; Jeong, H.J. Βeta-eudesmol reduces stem cell factor-induced mast cell migration. Int. Immunopharmacol. 2017, 48, 1–7. [CrossRef]
- Ma, E.L.; Li, Y.C.; Tsuneki, H.; Xiao, J.F.; Xia, M.; Wang, M.W.; Kimura, I. β-Eudesmol suppresses tumour growth through inhibition of tumour neovascularisation and tumour cell proliferatio. J. Asian Nat. Prod. Res. 2008, 10, 159–167. [CrossRef]
- Seo, M.J.; Kim, S.J.; Kang, T.H.; Rim, H.K.; Jeong, H.J.; Um, J.Y.; Hong, S.H.; Kim, H.M. The regulatory mechanism of β-eudesmol is through the suppression of caspase-1 activation in mast cell–mediated inflammatory response. Immunopharmacol Immunotoxicol. 2011, 33, 178–185. [CrossRef]
- Yang G, Lee K, Lee M, Ham I, Choi H-Y. Inhibition of lipopolysaccharide induced nitric oxide and prostaglandin E2 production by chloroform fraction of Cudrania tricuspidata in RAW 264.7 macrophages. BMC 2012, 12, 250. [CrossRef]
- Brice Ayissi Owona, B.; Frederic Njayou, N.; Laufer, S.; Fewou Moundip, P.; Schluesener, H.J. A fraction of stem bark extract of Entada africana suppresses lipopolysaccharide-induced inflammation in RAW264.7cells. J. Ethnopharmacol 2013, 149, 162–168. [CrossRef]
- An, X.; Gil Lee, S.; Kang, H.; Jin Heo, H.; Cho, Y.S: and Ok Kim, D. Antioxidant and Anti-Inflammatory Effects of Various Cultivars of Kiwi Berry (Actinidia arguta) on Lipopolysaccharide-Stimulated RAW 264.7 Cells. J. Microbiol. Biotechnol. 2016, 26, 1367–1374. [CrossRef]
- Lim JY, Won TJ, Hwang BY, Kim HR, Hwang KW, Sul D, et al. The new diterpene isodojaponin D inhibited LPS- induced microglial activation through NF-kβ and MAPK signaling pathways. Eur J Pharmacol 2010, 642, 10–8. [CrossRef]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Sethi, G.; Aggarwal, B.B.; Sainis, K.B. Potent anti-inflamatory activity of ursolic acid, a triterpenoid antioxidant, is mediated throgh suppresion of NF-kB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [CrossRef]
- Shishodia, S.; Majumdar, S.; Banerjee, S.; Aggarwal, B.B. Ursolic acid inhibits nuclear factor-kB activation induced by carcinogenic agents through suppression of IƙBα kinase and p65 phosphorylation: Correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003, 63, 4375–4383.
- Sánchez-Ramos, M.; Marquina, B.S.; Romero-Estrada, A.; Bernabé-Antonio, B.; Cruz-Sosa, F.; González-Christen, J.; Acevedo-Fernández, J.J.; Perea-Arango, I.; Alvarez, L. Establishment and phytochemical analysis of a callus cultures from Ageratina pichinchensis (Asteraceae) and its anti-inflammatory activity. Molecules 2018, 23, 1258. [CrossRef]
- Schwartz, M.A. Syntheses of (+)-α- and (+)-β-eudesmol and their diastereomers by intramolecular nitrone-olefin cycloaddition. J. Org. Chem. 1985, 50, 1359–1365. [CrossRef]
- Shamsuddin, K.M.; Musharraf, M.A.; Zobairi, M.O.; Ali, N. Demethylacetovanillochromene from Tithonia diversifolia (Hemes1.) A. Gray. Indian J. Chem.- Sect. B Org. Med. Chem. 2001, 8, 751-752.
- Zhai, H.L.; Zhao, G.J.; Yang, G.J.; Sun, H.; Yi, B.; Sun, L.N.; Chen, W.S.; Zheng, S.Q. A new chromene glycoside from Tithonia diversifolia. Chem. Nat. Compd. 2010, 46, 198-200. [CrossRef]
- Aguilar, M.I.; Delgado, Guillermo; Bye, R.; Linares, E. Bisabolenes, polycyclic diterpenoids and other constituents from the roots of Iostephane heterophylla. Phytochemistry. 1993, 33, 1161-3. [CrossRef]
- Bohlmann, F.; Zdero, C.; Franke, H. Naturally occurring coumarin derivatives. IX. Constituents of the genus Gerbera. Chem. Ber. 1973, 106, 382-7. [CrossRef]
- Castañeda, P.; Gómez, L.; Mata, R.; Lotina-Hennsen, B.; Anaya, A.L.; Bye, R. Phytogrowth-Inhibitory and Antifungal Constituents of Helianthella quinquenervis. J. Nat. Prod. 1996, 59, 323–326. [CrossRef]
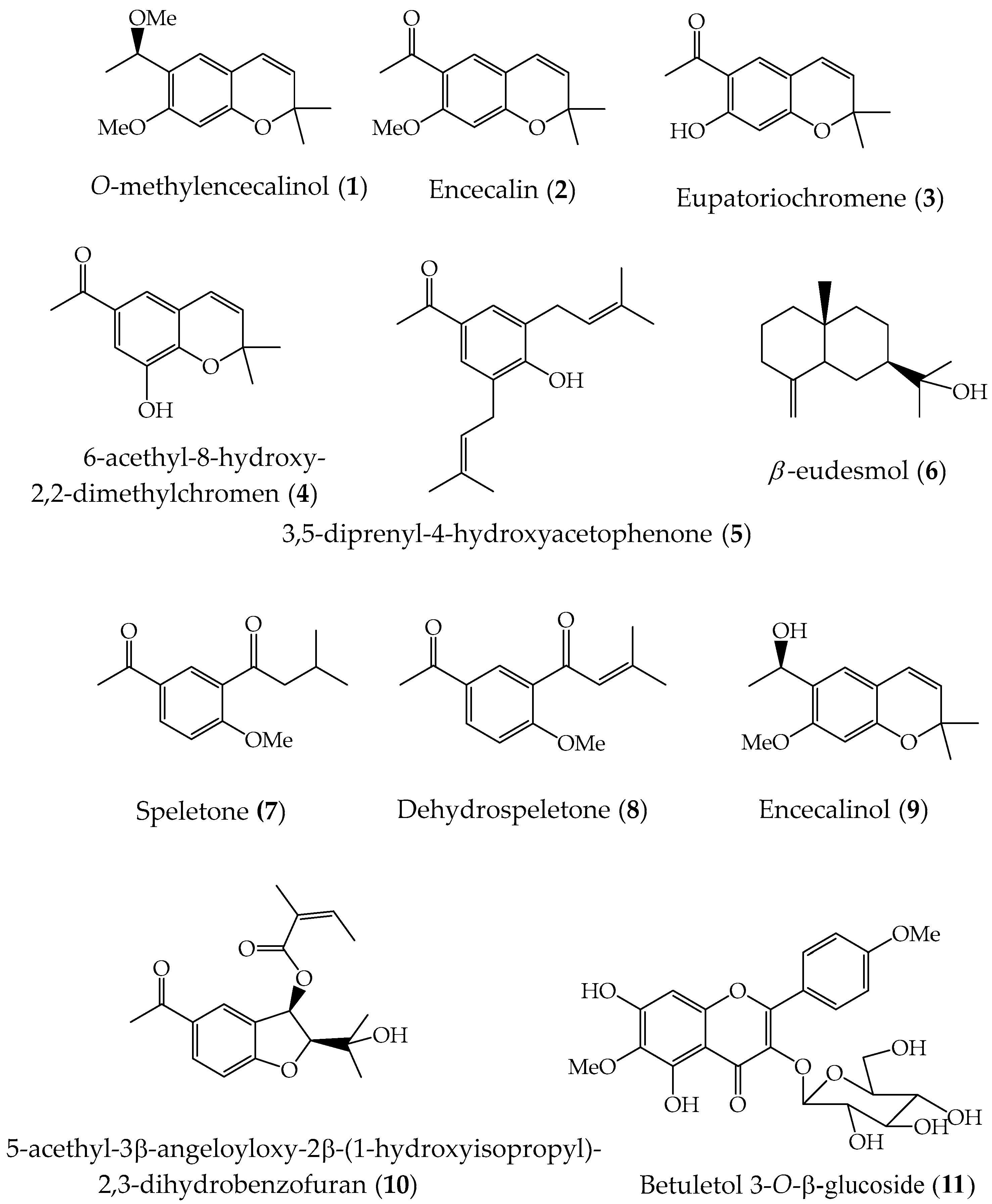

| Compounds | Cell viability (%)a | NO Inhibition (%) | NO inhibition (IC50, µM) |
|---|---|---|---|
| 1 | 109.9 ± 7.16 | 0.95 ± 1.35 | > 75 |
| 2 | 112.4 ± 24.08 | 16.75 ± 5.36 | > 75 |
| 3 | 115.6 ± 1.58 | 11.98 ± 7.85 | > 75 |
| 4 | 99.33 ± 12.39 | 22.63 ± 10.38 | > 75 |
| 5 | 100.20 ± 2.95 | 29.77 ± 18.27 | ˃ 75 |
| 6 | 61.14 ± 6.31 | ----- | ----- |
| 7 | 104.7 ± 1.82 | 5.90 ± 8.35 | > 75 |
| 8 | 103.9 ± 3.83 | 36.73 ± 16.93 | > 75 |
| 9 | 110.9 ± 8.3 | 29.77 ± 9.37 | ˃ 75 |
| 10 | 121.2 ± 10.20 | 5.98 ± 5.22 | > 75 |
| 11 | 101.3 ± 1.62 | 75.08 ± 3.07 | 20.55 ± 0.27 |
| DMSOb | ----- | ----- | ----- |
| Indomethacinc (84 µM) | ----- | 65.93 ± 6.03 | 54.69 ± 10.34 |
| Etoposided (68 µM) | 42.02 ± 4.23 | ----- | ----- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





