Submitted:
12 December 2023
Posted:
12 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
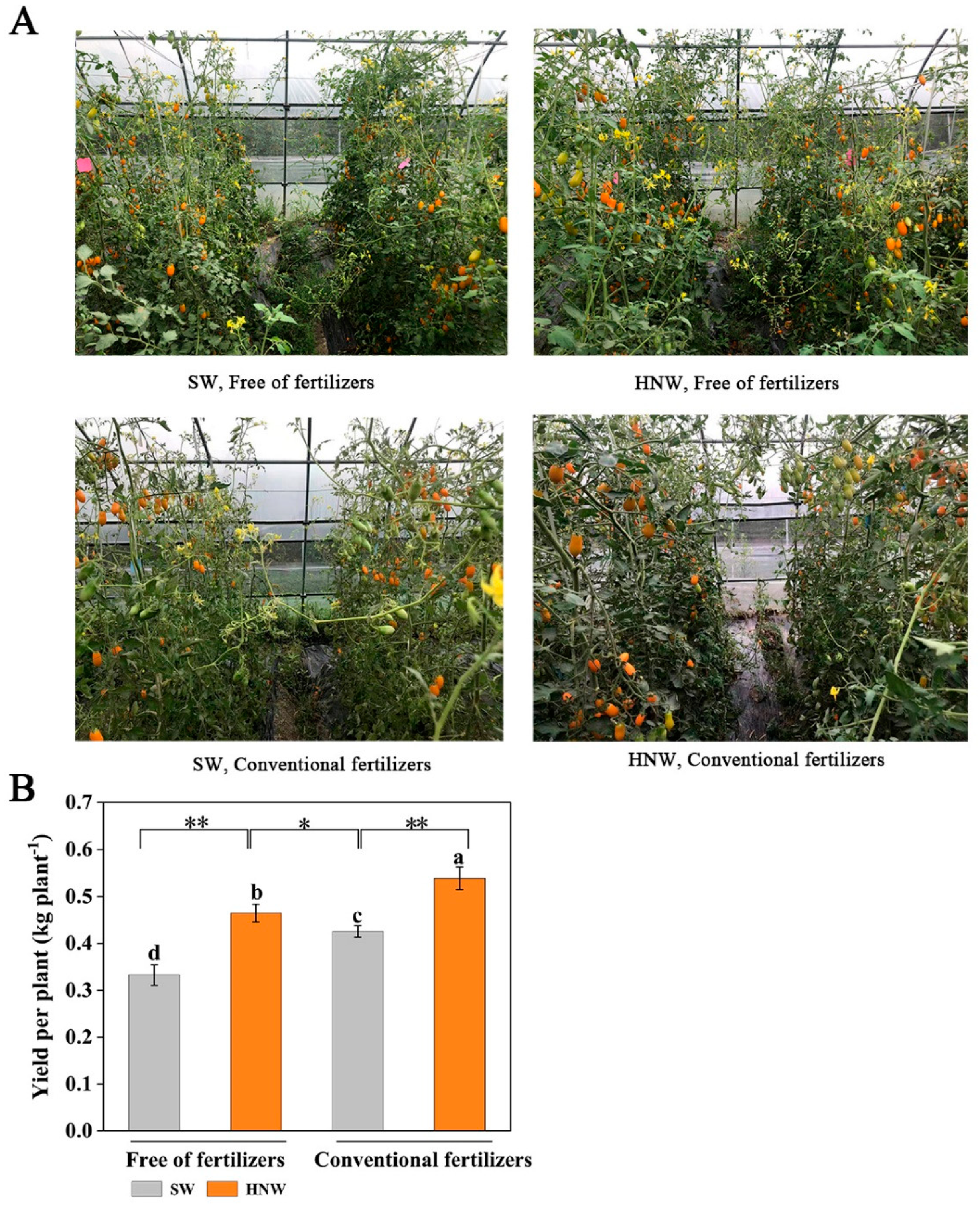
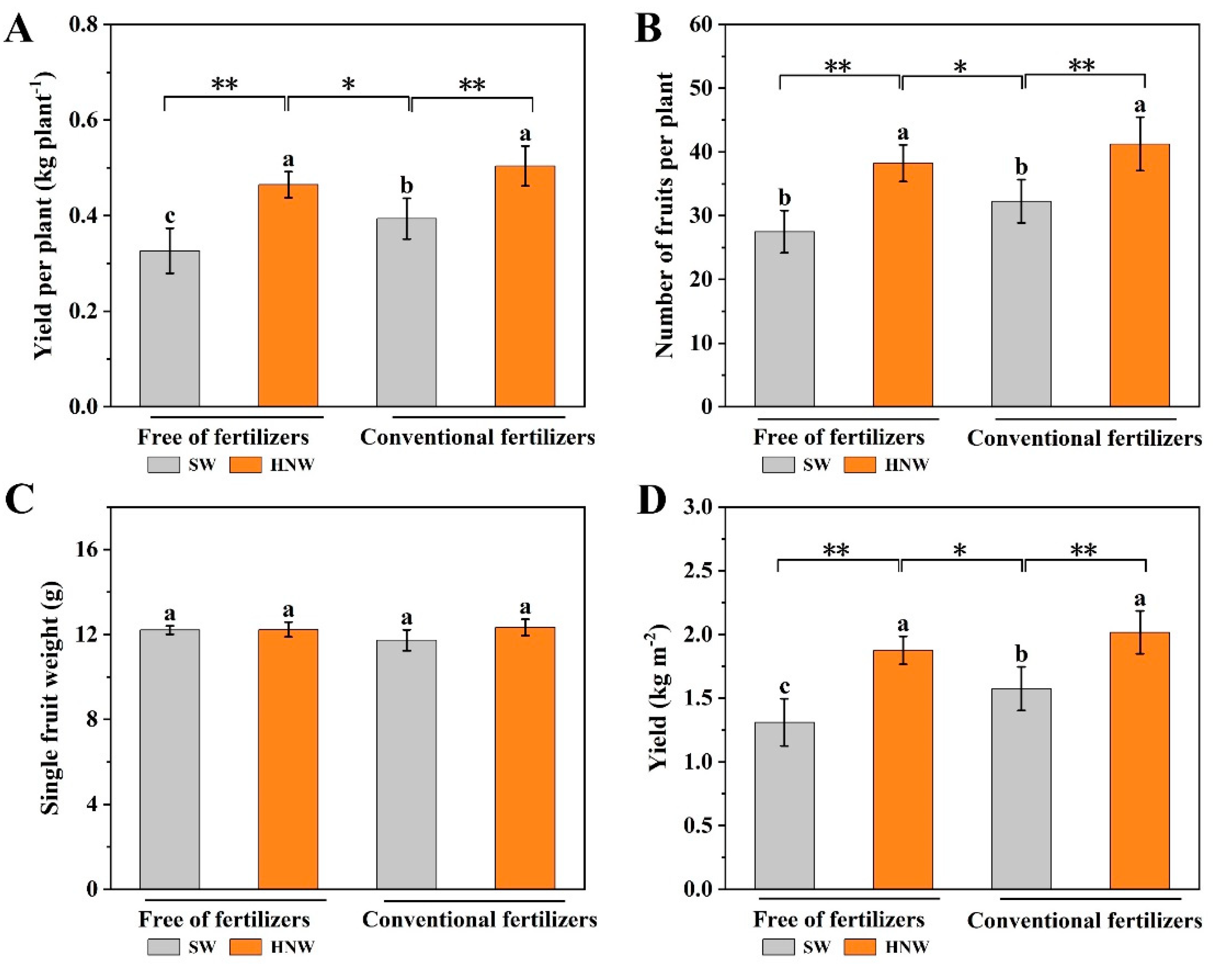
2.1. Preharvest Application of HNW Improves Cherry Tomato Yield
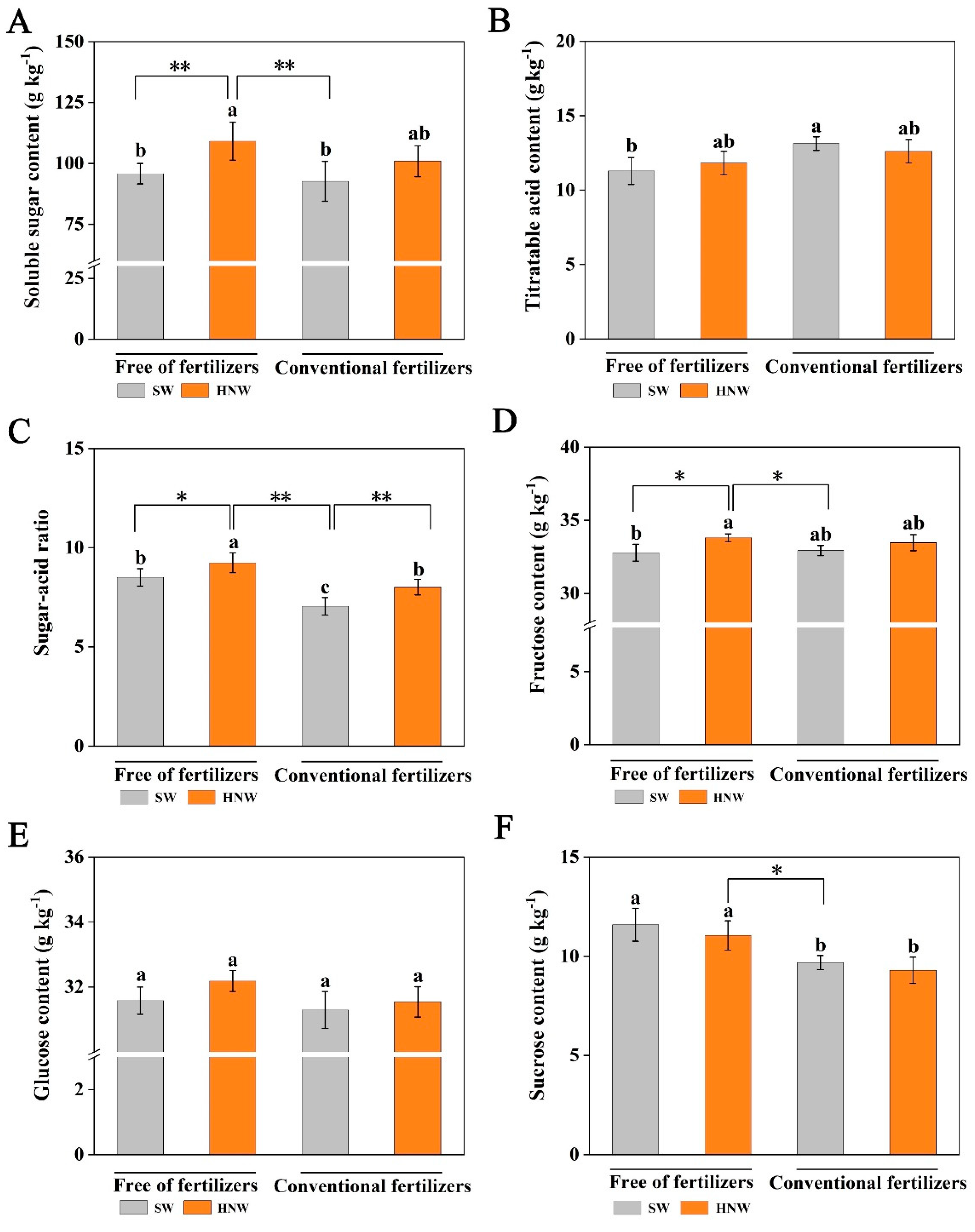
2.2. Effects of HNW on the Balance of Sugars and Acids in Cherry Tomatoes
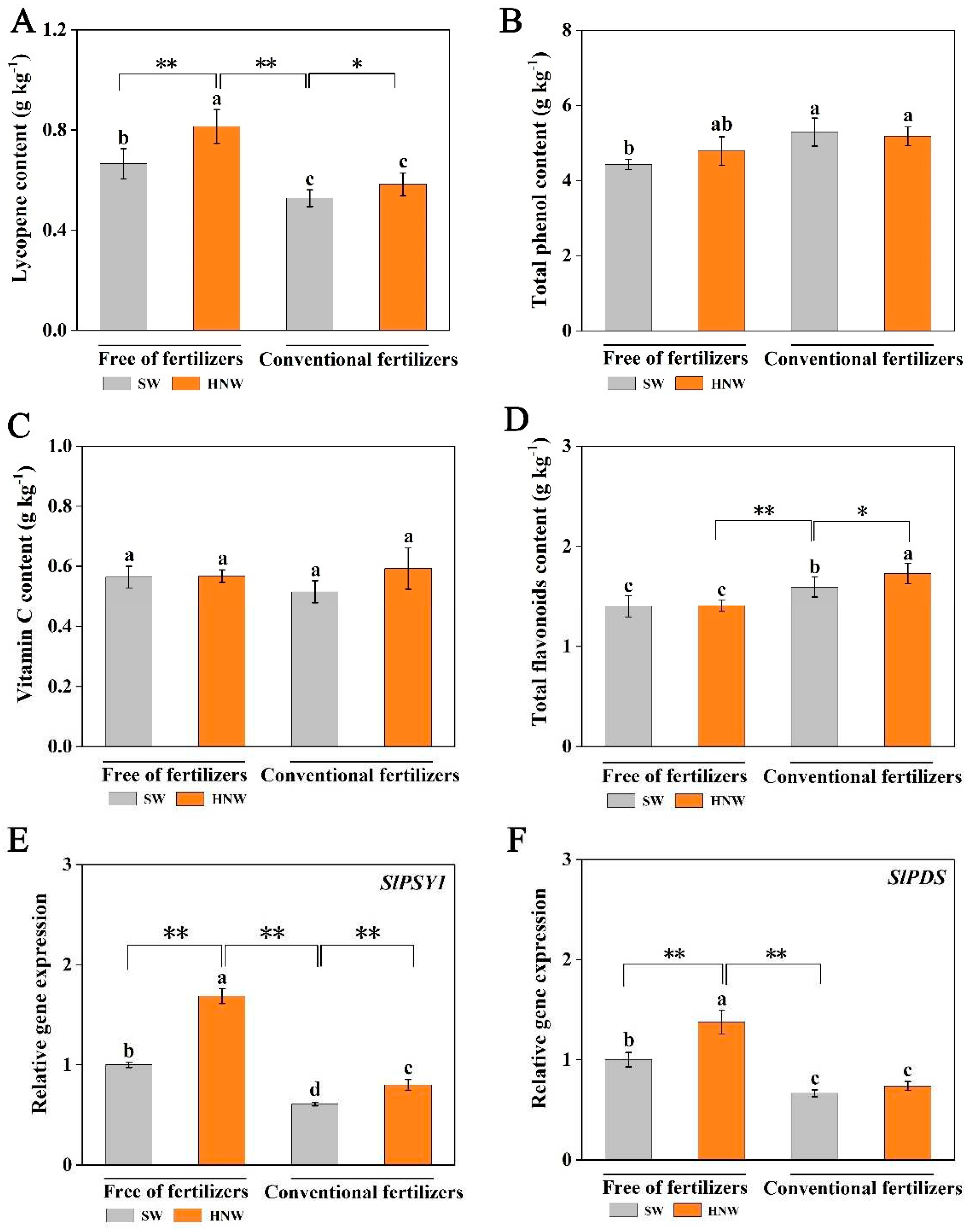
2.3. Antioxidant Compounds Accumulation in Response to HNW
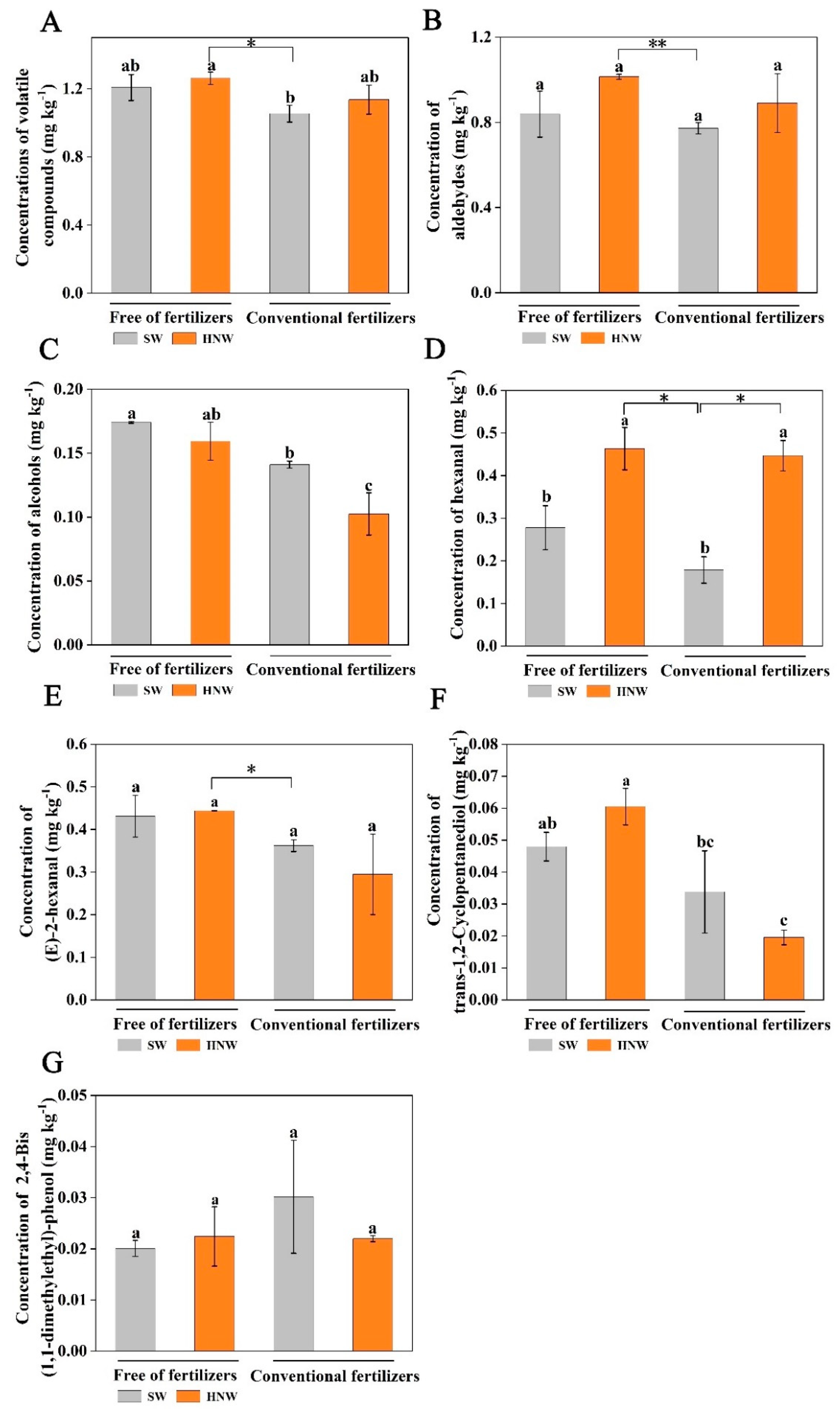
2.4. Modulation of the Aromatic Profiles in Cherry Tomatoes Achieved by HNW
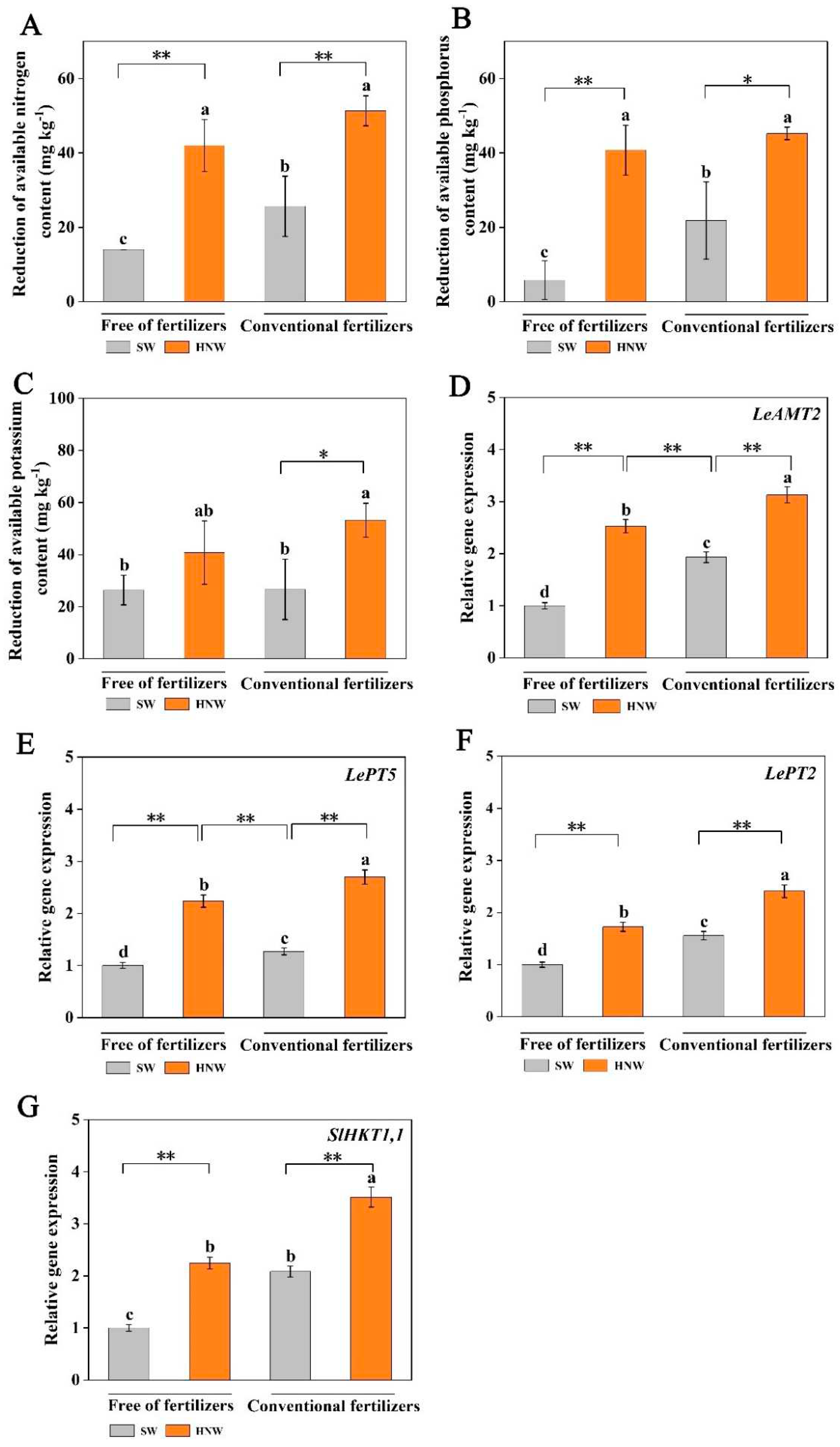
2.5. The Absorption of Soil Elements was Influenced by HNW
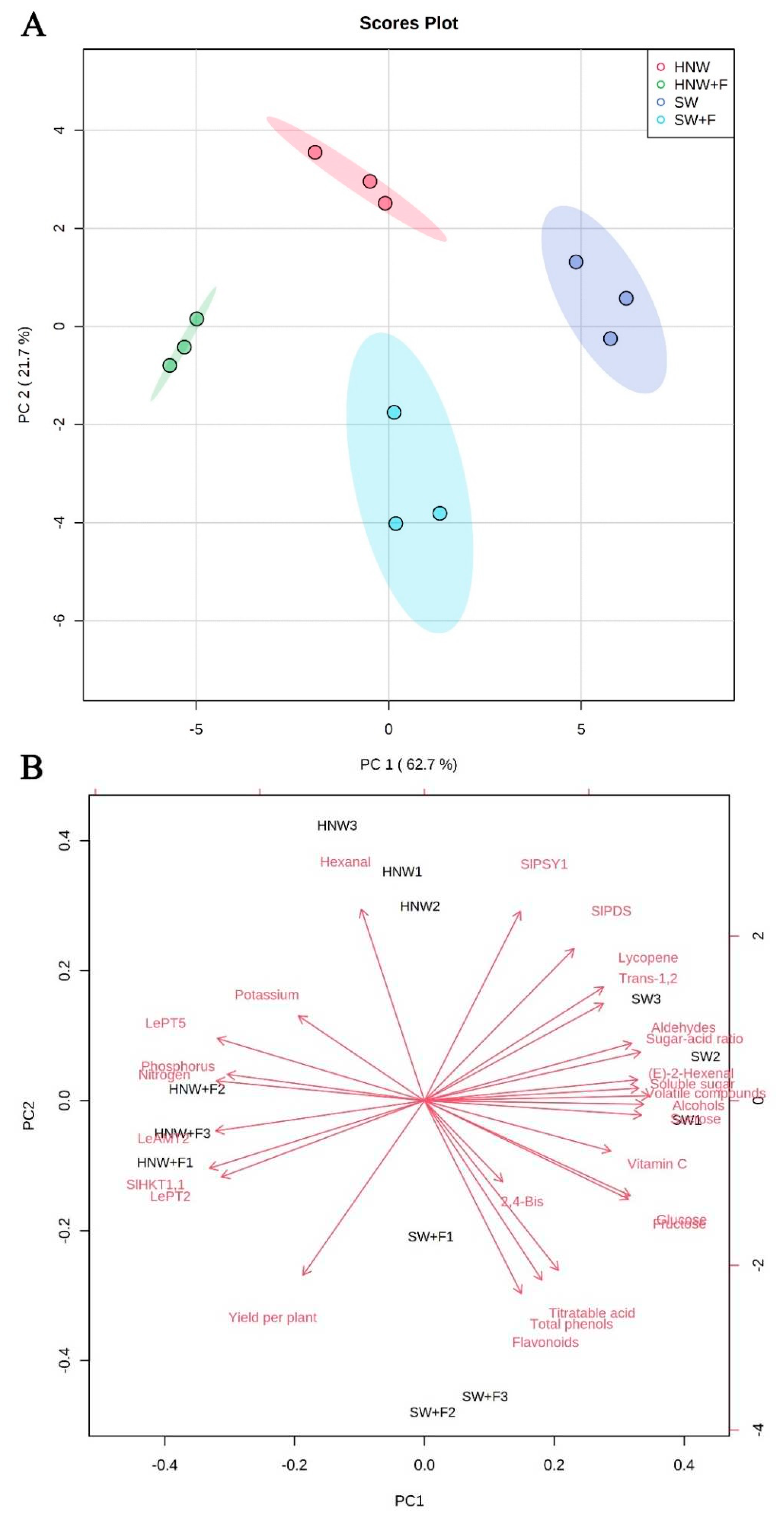
2.6. Principal Component Analysis
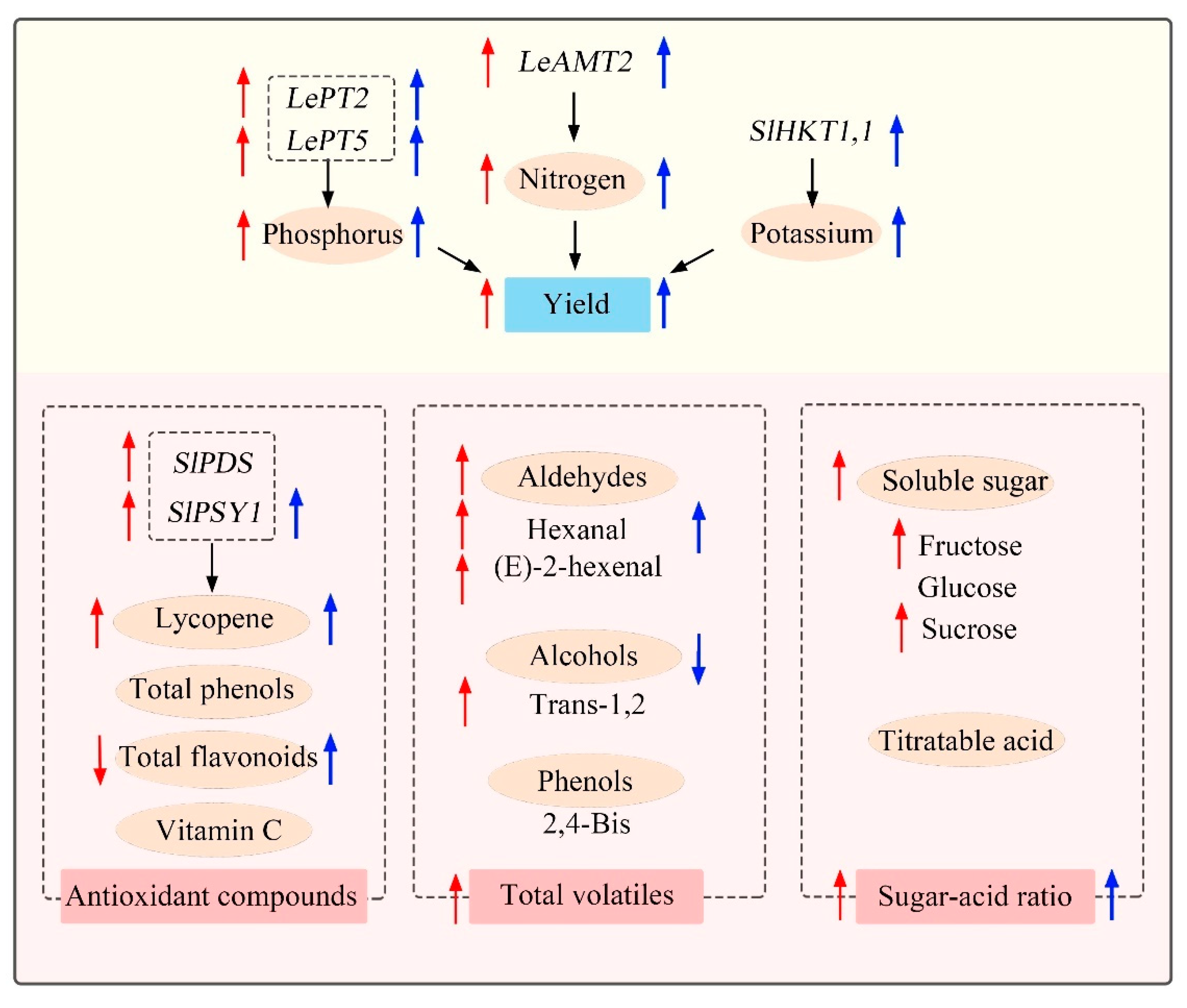
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Experimental Design
4.2. Preparation of HNW
4.3. Determination of Cherry Tomato Yield
4.4. Evaluation of Fructose, Glucose, Sucrose, Titratable Acid, and Soluble Sugar Contents
4.5. Determination of Lycopene, Vitamin C, Total Phenols, and Flavonoids Contents
4.6. Extraction and Analyses of Aromatic Compounds
4.7. Determination of Soil Available Nitrogen (N), Potassium (K), and Phosphorus (P) Contents
4.8. Real-time Fluorescence Quantitative PCR Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.P.; Lutts, S. Tomato fruit development and metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef] [PubMed]
- Perveen, R.; Suleria, H.A.R.; Anjum, F.M.; Butt, M.S.; Pasha, I.; Ahmad, S. Tomato (Solanum lycopersicum) carotenoids and lycopenes chemistry; metabolism, absorption, nutrition, and allied health claims—a comprehensive review. Crit. Rev. Food Sci. 2015, 55, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.C.; Koron, D.; Jakopič, J.; Veberič, R.; Hudina, M.; Česnik, H.B. Influence of nitrogen, calcium and nano-fertilizer on strawberry (Fragaria × ananassa Duch.) fruit inner and outer quality. Agronomy-Basel. [CrossRef]
- Zhong, W.; Hu, C.; Wang, M. Nitrate and nitrite in vegetables from north China: Content and intake. Food Addit. Contam. 2002, 19, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.F.; Dou, Z.X.; He, P.; Ju, X.T.; Powlson, D.; Chadwick, D.; Norse, D.; Lu, Y.L; Zhang, Y; Wu, L; Chen, X.P; Cassman, K.G; Zhang, F.S. New technologies reduce greenhouse gas emissions from nitrogenous fertilizer in China. Proc. Natl Acad. Sci. USA 2013, 110, 8375–8380. [Google Scholar] [CrossRef] [PubMed]
- Matei, N.; Camara, R.; Zhang, J.H. Emerging mechanisms and novel applications of hydrogen gas therapy. Med. Gas Res. 2018, 8, 98–102. [Google Scholar] [CrossRef]
- Jin, Q.; Zhu, K.; Cui, W.; Xie, Y.; Han, B.; Shen, W. Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat-induced oxidative stress via the modulation of heme oxygenase-1 signaling system. Plant Cell Environ. 2013, 36, 956–969. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Duan, X.; Yao, P.; Cui, W.; Cheng, D.; Zhang, J.; Jin, Q.; Chen, J.; Dai, T.; Shen, W. Hydrogen gas is involved in auxin-induced lateral root formation by modulating nitric oxide synthesis. Int. J. Mol. Sci. 2017, 18, 2084. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Kuang, Y.; Feng, L.; Liu, Y.; Wang, S.; Du, H.; Shen, W. Molecular hydrogen maintains the storage quality of Chinese chive through improving antioxidant capacity. Plants 2021, 10, 1095. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, S.; Li, P.; Shen, W. Hydrogen gas prolongs the shelf life of kiwifruit by decreasing ethylene biosynthesis. Postharvest Biol. Technol. 2018, 135, 123–130. [Google Scholar] [CrossRef]
- Li, L.; Yin, Q.; Zhang, T.; Cheng, P.; Xu, S.; Shen, W. Hydrogen nanobubble water delays petal senescence and prolongs the vase life of cut carnation (Dianthus caryophyllus L.) flowers. Plants 2021, 10, 1662. [CrossRef]
- Dong, Z.; Wu, L.; Kettlewell, B.; Caldwell, C.D.; Layzell, D.B. Hydrogen fertilization of soils-is this a benefit of legumes in rotation? Plant Cell Environ. 2003, 26, 1875–1879. [Google Scholar] [CrossRef]
- Golding, A.L.; Dong, Z. Hydrogen production by nitrogenase as a potential crop rotation benefit. Environ. Chem. Lett. 2010, 8, 101–121. [Google Scholar] [CrossRef]
- Hu, H.; Li, P.; Shen, W. Preharvest application of hydrogen-rich water not only affects daylily bud yield but also contributes to the alleviation of bud browning. Sci. Hortic. 2021, 287, 110267. [Google Scholar] [CrossRef]
- Xia, G.; Zhang, L.; Chen, X.; Huang, Y.; Sun, D.; Fang, F.; Guo, Z.; Yu, X. Carbon hollow nanobubbles on porous carbon nanofibers: An ideal host for high-performance sodium-sulfur batteries and hydrogen storage. Energy Storage Mater. 2018, 14, 314–323. [Google Scholar] [CrossRef]
- Zhao, G.; Cheng, P.; Zhang, T.; Abdalmegeed, D.; Xu, S.; Shen, W. Hydrogen-rich water prepared by ammonia borane can enhance rapeseed (Brassica napus L.) seedlings tolerance against salinity, drought or cadmium. Ecotox. Environ. Safe. 2021, 224, 112640. [CrossRef]
- Wang, Y.; Lv, P.; Kong, L.; Shen, W.; He, Q. Nanomaterial-mediated sustainable hydrogen supply induces lateral root formation via nitrate reductase-dependent nitric oxide. Chem. Eng. J. 2021, 405, 126905. [Google Scholar] [CrossRef]
- Temesgen, T.; Bui, T.T.; Han, M.; Kim, T.I.; Park, H. Micro and nanobubble technologies as a new horizon for water-treatment techniques: A review. Adv. Colloid Interfac. 2017, 246, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhang, Y.; Liu, S.; Li, X.; Li, J. Alleviation of copper toxicity in Daphnia magna by hydrogen nanobubble water. J. Hazard. Mater. 2020, 389, 122155. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Ding, X.W.; Jiang, R.; Ouyang, P.L.; Gui, J.; Feng, L.; Yang, L.; Song, L.H. Effects of hydrogen-rich water on the nutrient composition and antioxidative characteristics of sprouted black barley. Food Chem. 2019, 299, 125095. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Wang, J.; Zhao, Z.; Kong, L.; Lou, W.; Zhang, T.; Jing, D.; Yu, J.; Shu, Z.; Huang, L.; Zhu, W.; Yang, Q.; Shen, W. Molecular hydrogen increases quantitative and qualitative traits of rice grain in field trials. Plants 2021, 10, 2331. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, J.; Jiang, K.; Kuang, Y.; Zeng, Y.; Cheng, X.; Liu, Y.; Wang, S.; Shen, W. Preharvest application of hydrogen nanobubble water enhances strawberry flavor and consumer preferences. Food Chem. 2022, 377, 131953. [Google Scholar] [CrossRef]
- Beckles, D.M. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2012, 63, 129–140. [CrossRef]
- Yelle, S.; Hewitt, J.D.; Robinson, N.L.; Damon, S.; Bennett, A.B. Sink metabolism in tomato fruit: III. Analysis of carbohydrate assimilation in a wild species. Plant Physiol. 1988, 87, 737–740. [Google Scholar] [CrossRef]
- Navarro-González, I.; García-Valverde, V.; García-Alonso, J.; Periago, M.J. Chemical profile, functional and antioxidant properties of tomato peel fiber. Food Res. Int. 2011, 44, 1528–1535. [Google Scholar] [CrossRef]
- Arathi, B.P.; Sowmya, P.R.R.; Kuriakose, G.C.; Shilpa, S.; Shwetha, H.J.; Kumar, S.; Raju, M.; Vallikannan, B.; Lakshminarayana, R. Fractionation and characterization of lycopene oxidation products by LC-MSMS (ESI)+: Elucidation of chemoprevention potency of oxidized lycopene in breast cancer cell lines. J. Agric. Food Chem. 2018, 66, 11362–11371. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Saia, S.; Rappa, V.; Ruisi, P.; Abenavoli, M.R.; Sunseri, F.; Giambalvo, D.; Frenda, A.S.; Martinelli, F. Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front. Plant Sci. 2015, 815, 6. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Raddatz, N.; Pardo, J.M.; Yun, D.J. HKT sodium and potassium transporters in Arabidopsis thaliana and related halophyte species. Physiol. Plantarum 2021, 171, 546–558. [Google Scholar] [CrossRef]
- Li, L.; Lou, W.; Kong, L.; Shen, W. Hydrogen commonly applicable from medicine to agriculture: From molecular mechanisms to the field. Curr. Pharm. Design 2021, 27, 747–759. [Google Scholar] [CrossRef]
- Weldegebriel, R.; Araya, T.; Egziabher, Y.G. Effect of NPK and blended fertilizer application on nutrient uptake and use efficiency of selected sorghum (Sorghum bicolor (L.) Moench) varieties under rain-fed condition in Sheraro District, Northern Ethiopia. Momona Ethiop. J. Sci. 2018, 10, 140156. [CrossRef]
- Maimaiti, J.; Zhang, Y.; Yang, J.; Cen, Y.P.; Layzell, D.B.; Peoples, M.; Dong, Z. Isolation and characterization of hydrogen-oxidizing bacteria induced following exposure of soil to hydrogen gas and their impact on plant growth. Environ. Microbiol. 2007, 9, 435–444. [Google Scholar] [CrossRef]
- Dong, Z.; Layzell, D.B. H2 oxidation, O2 uptake and CO2 fixation in hydrogen treated soils. Plant Soil 2001, 229, 1–12. [Google Scholar] [CrossRef]
- Liu, H.; Wang, W.; Cao, G.; Tang, M. Effect of hydrogen on microbial population and enzyme activity in Robinia pseudoacacia rhizosphere soil. Chin. J. Appl. Environ. Biol. 2010, 16, 515–518. [Google Scholar] [CrossRef]
- Shukla, S.K.; Sharma, L.; Jaiswal, V.P.; Pathak, A.D.; Tiwari, R.; Awasthi, S.K.; Gaur, A. Soil quality parameters vis-a-vis growth and yield attributes of sugarcane as influenced by integration of microbial consortium with NPK fertilizers. Sci. Rep-UK. 2020, 10, 1-17. [CrossRef]
- Zhu, F.; Wen, W.; Cheng, Y.; Fernie, A.R. The metabolic changes that effect fruit quality during tomato fruit ripening. Mol. Hortic. 2022, 2, 1–19. [Google Scholar] [CrossRef]
- Liao, L.; Dong, T.; Qiu, X.; Rong, Y.; Wang, Z.; Zhu, J. Nitrogen nutrition is a key modulator of the sugar and organic acid content in citrus fruit. Plos One 2019, 14, e0223356. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, W.; Duan, X.; Dai, C.; Zhang, Y.; Cui, W.; Wang, R.; Shen, W. Hydrogen-rich water-alleviated ultraviolet-B-triggered oxidative damage is partially associated with the manipulation of the metabolism of (iso)flavonoids and antioxidant defence in Medicago sativa. Funct. Plant Biol. 2015, 42, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, G.; Cheng, P.; Yan, X.; Li, Y.; Cheng, D.; Wang, R.; Chen, J.; Shen, W. Nitrite accumulation during storage of tomato fruit as prevented by hydrogen gas. Int. J. Food Prop. 2019, 22, 1425–1438. [Google Scholar] [CrossRef]
- Yun, Z.; Gao, H.; Chen, X.; Chen, Z.; Zhang, Z.; Li, T.; Qu, H.; Jiang, Y. Effects of hydrogen water treatment on antioxidant system of litchi fruit during the pericarp browning. Food Chem. 2021, 336, 127618. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Kai, W.; Zhao, B.; Sun, Y.; Yuan, B.; Dai, S.; Li, Q.; Chen, P.; Wang, Y.; Pei, Y.; Wang, H.; Guo, Y.; Leng, P. SlNCED1 and SlCYP707A2: key genes involved in ABA metabolism during tomato fruit ripening. J. Exp. Bot. 2014, 65, 5243–5255. [Google Scholar] [CrossRef]
- Lomelí-Martín, A.; Martínez, L.M.; Welti-Chanes, J.; Escobedo-Avellaneda, Z. Induced changes in aroma compounds of foods treated with high hydrostatic pressure: A review. Foods 2021, 10, 878. [Google Scholar] [CrossRef] [PubMed]
- Tandon, K.S.; Baldwin, E.A.; Shewfelt, R.L. Aroma perception of individual volatile compounds in fresh tomatoes (Lycopersicon esculentum, Mill.) as affected by the medium of evaluation. Postharvest Biol. Technol. 2000, 20, 261–268. [CrossRef]
- Fan, Z.; Hasing, T.; Johnson, T.S.; Garner, D.M.; Schwieterman, M.L.; Barbey, C.R.; Colquhoun, T.A.; Sims, C.A.; Resende, M.F.R.; Whitaker, V.M. Strawberry sweetness and consumer preference are enhanced by specific volatile compounds. Hortic. Res. 2021, 8, 66. [CrossRef]
- Bood, K.G.; Zabetakis, I. The biosynthesis of strawberry flavor (II): Biosynthetic and molecular biology studies. J. Food Sci. 2002, 67, 2–8. [Google Scholar] [CrossRef]
- Ubeda, C.; San-Juan, F.; Concejero, B.; Callejón, R.M.; Troncoso, A.M.; Morales, M. L.; Ferreira, V.; Hernández-Orte, P. Glycosidically bound aroma compounds and impact odorants of four strawberry varieties. J. Agric. Food Chem. 2012, 60, 6095–6102. [Google Scholar] [CrossRef]
- Booth, D.A.; Kendal-Reed, M.S.; Freeman, R.P.J. A strawberry by any other name would smell as sweet, green, fruity and buttery. Multisensory cognition of a food aroma. Appetite 2010, 55, 738–741. [Google Scholar] [CrossRef]
- Yan, J.W.; Ban, Z.J.; Lu, H.Y.; Li, D.; Poverenov, E.; Luo, Z.S.; Li, L. The aroma volatile repertoire in strawberry fruit: A review. J. Sci. Food Agric. 2018, 98, 4395–4402. [Google Scholar] [CrossRef]
- Choi, S.T.; Park, D.S.; Kang, S.M.; Kang, S.K. Influence of leaf-fruit ratio and nitrogen rate on fruit characteristics, nitrogenous compounds, and nonstructural carbohydrates in young persimmon trees. Hortscience 2012, 47, 410–413. [Google Scholar] [CrossRef]
- Davis, D.R. Declining fruit and vegetable nutrient composition: What is the evidence? Hortic. Sci. 2009, 44, 15–19. [Google Scholar] [CrossRef]
- Liu, L.; Ji, M.L.; Chen, M.; Sun, M.Y.; Fu, X.L.; Li, L.; Gao, D.S.; Zhu, C.Y. The flavor and nutritional characteristic of four strawberry varieties cultured in soilless system. Food Sci. Nutr. 2016, 4, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Buckman, E.S.; Oduro, I.; Plahar, W.A.; Tortoe, C. Determination of the chemical and functional properties of yam bean (Pachyrhizus erosus (L.) Urban) flour for food systems. Food Sci. Nutr 2018, 6, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Gao, J.; Gu, L.; Wang, S.; Zeng, R. Effects of agaro-oligosaccharide treatment on postharvest quality of cherry tomatoes during cold storage. J. Food Process. Preserv. 2015, 39, 949–955. [Google Scholar] [CrossRef]
- Liu, Y.J.; Wang, G.L.; Ma, J.; Xu, Z.S.; Wang, F.; Xiong, A.S. Transcript profiling of sucrose synthase genes involved in sucrose metabolism among four carrot (Daucus carota L.) cultivars reveals distinct patterns. BMC Plant Biol. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Yamashita, I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Nippon Shokuhin Kogy. 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Giovanelli, G.; Zanoni, B.; Lavelli, V.; Nani, R. Water sorption, drying and antioxidant properties of dried tomato products. J. Food Eng. 2002, 52, 135–141. [Google Scholar] [CrossRef]
- Musa, K.H.; Abdullah, A.; Jusoh, K.; Subramaniam, V. Antioxidant activity of pink-flesh guava (Psidium guajava L.): Effect of extraction techniques and solvents. Food Anal. Method. 2011, 4, 100–107. [Google Scholar] [CrossRef]
- Hasperué, J.H.; Rodoni, L.M.; Guardianelli, L.M.; Chaves, A.R.; Martínez, G.A. Use of LED light for Brussels sprouts postharvest conservation. Sci. Hortic. 2016, 213, 281–286. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, J.; Tian, J.; Li, N.; Jia, L.; Shen, W.; Cui, J. Enhanced anthocyanin accumulation of immature radish microgreens by hydrogen-rich water under short wavelength light. Sci. Hortic. 2019, 247, 75–85. [Google Scholar] [CrossRef]
- Lu, B.; Liu, N.; Wang, X.; Hu, C.; Tang, X. A feasibility quantitative analysis of NIR spectroscopy coupled Si-PLS to predict coco-peat available nitrogen from rapid measurements. Comput. Electron. Agr. 2020, 173, 105410. [Google Scholar] [CrossRef]
- Alexander, D.; Ellerby, R.; Hernandez, A.; Wu, F.; Amarasiriwardena, D. Investigation of simultaneous adsorption properties of Cd, Cu, Pb and Zn by pristine rice husks using ICP-AES and LA-ICP-MS analysis. Microchem. J. 2017, 135, 129–139. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, Y.; Zhang, G.; Li, L.; Shen, W. Molecular hydrogen positively influences lateral root formation by regulating hydrogen peroxide signaling. Plant Sci. 2022, 325, 111500. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, S.; Liu, Z.; Chen, G.; Cheng, P.; Li, L.; Xu, S.; Shen, W. H2 supplied via ammonia borane stimulates lateral root branching via phytomelatonin signaling. Plant Physiol. 2023. [Google Scholar] [CrossRef]
- Cheng, P.; Wang, Y.; Cai, C.; Li, L.; Zeng, Y.; Cheng, X.; Shen, W. Molecular hydrogen positively regulates nitrate uptake and seed size by targeting nitrate reductase. Plant Physiol. 2023, 193, 2734–2749. [Google Scholar] [CrossRef]
- BloombergNEF. Hydrogen economy outlook. 2020. Available online: https://data.bloomberglp.com/professional/sites/24/BNEF-Hydrogen-Economy-Outlook-Key-Messages-30-Mar-2020.pdf (accessed on 12 Nov 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




