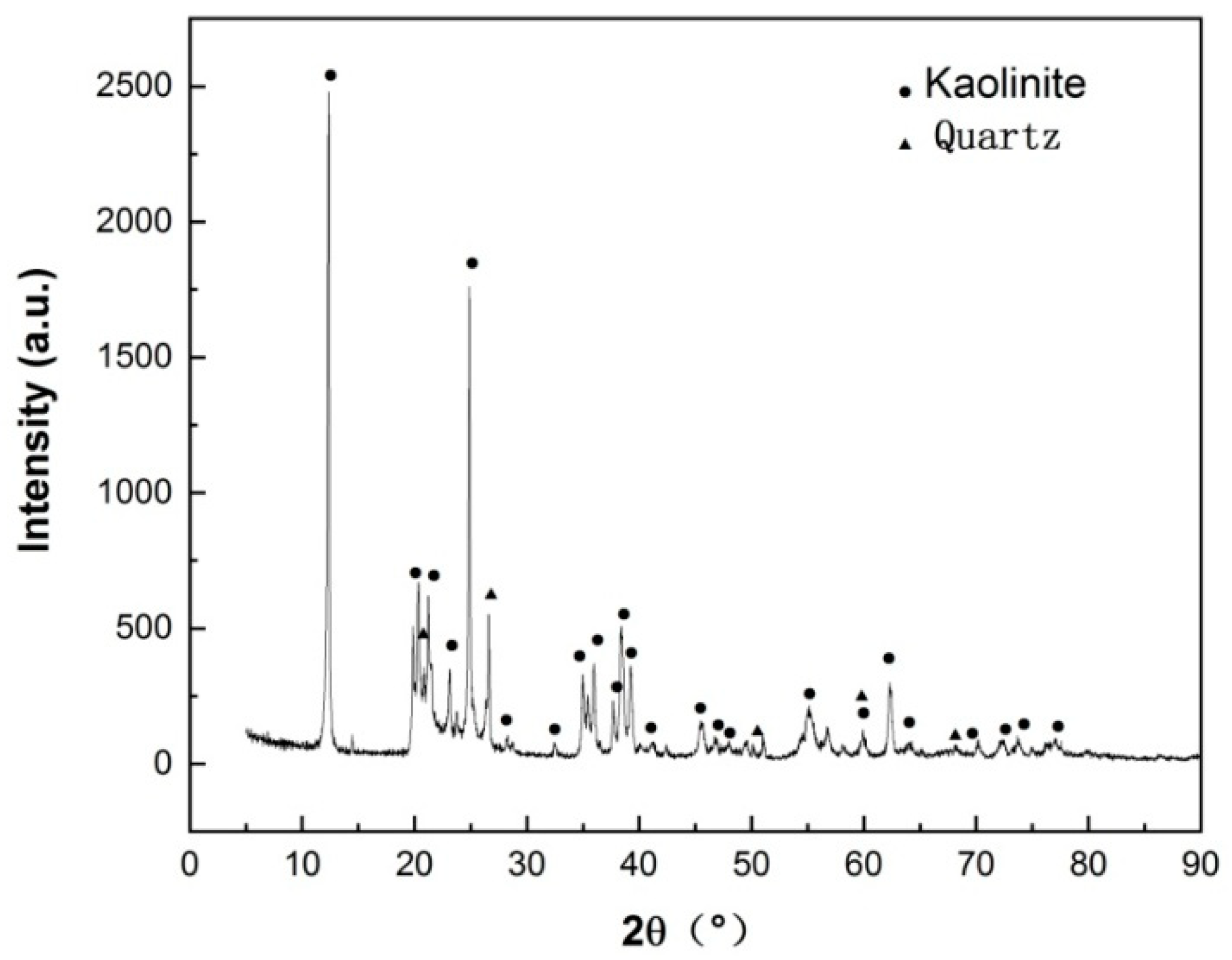
3.1. Thermal transformation of HACG-CH mixture
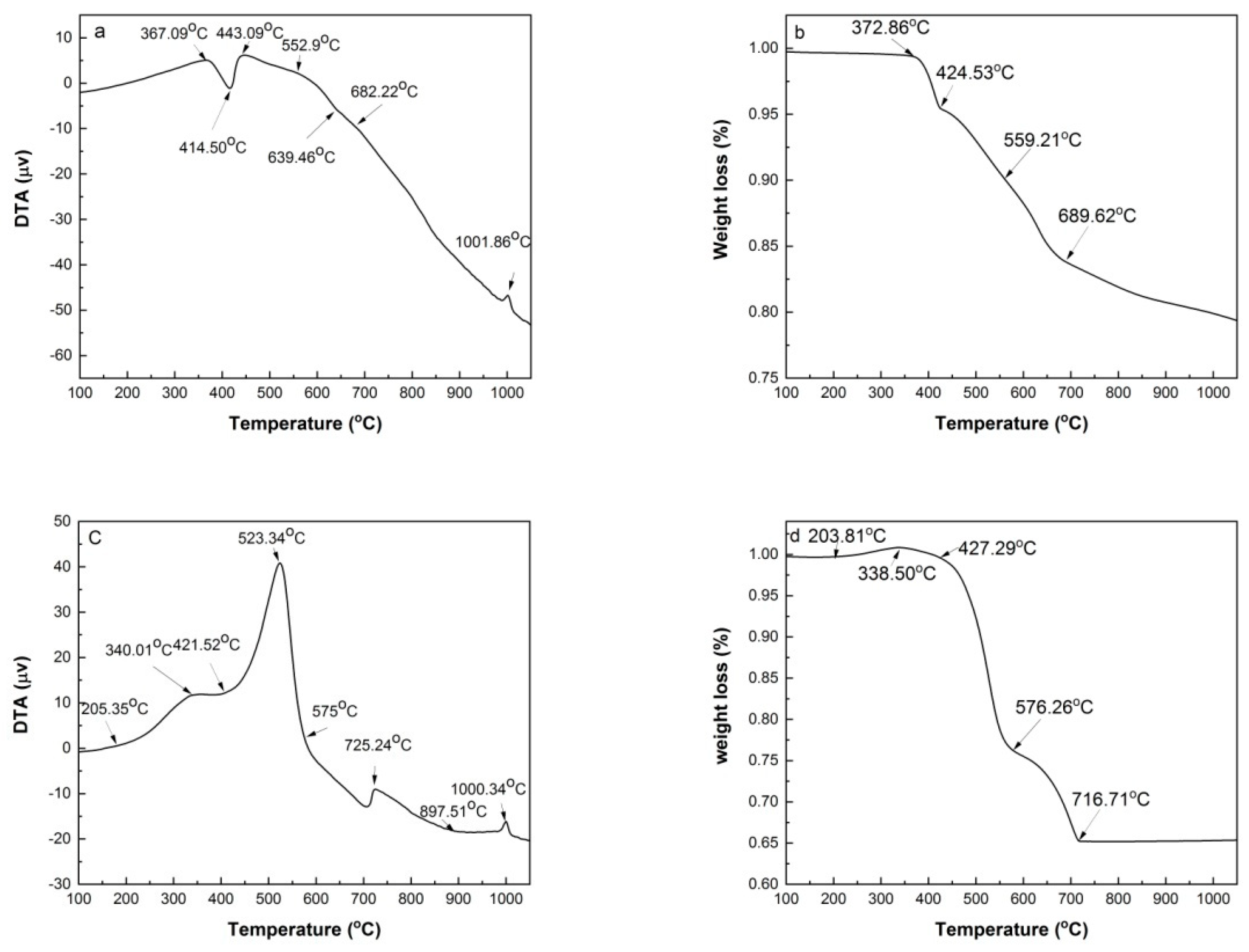
Figure 4 shows DTA and TG curves of HACG powder mixed with CH obtained under Ar atmosphere and air conditions. The reason for conducting the measurement under Ar gas atmosphere is to mainly prohibit many influence factors, such as H
2O and CO
2 in air and combustion of carbon in HACG, from involving in the thermal transformation process, and thus the analysis of thermal transformation of HACG-CH mixture would be simple and clear. Under Ar atmosphere, as seen from
Figure 4(a), there are an evident endothermic peak at 414.50
oC and a small exothermic peak at 1001.86
oC on DTA curve. The former corresponds to the dehydroxylation of CH, and its onset and finish temperatures are 367.09
oC and 443.09
oC respectively, while the latter represents the formation of mullite [
23]. Moreover, there is an almost imperceptible endothermic peak between 552.97 and 682.22
oC, at 639.46
oC exactly, which derives from the dehydroxylation of kaolinite. This deduction was made based on the fact that raw coal gangue was found to be dehydrated to form metakaolin (Al
2O
3•2SiO
2) in the temperature range of 555.73-689.90
oC by DTA analysis under Ar atmosphere (not shown here). It is noted that the dehydroxylation temperature for kaolinite in the studied HACG is much higher than that of CGs discharged in other regions in China. This might be attributed to a high degree of crystallinity in kaolinite for the studied HACG. The CGs including kaolinite-type CG discharged in Hebei Province and Jiangsu Province in China, were reported to be dehydrated around a lower temperature between 510
oC and 550
oC [
24,
25]. Similarly, kaolinite in HACG produced in Taiyuan coalfield in Shanxi Province, China, was also reported to undergo a dehydroxylation process in a higher temperature range of 550-700
oC [
26]. The thermal transformations such as dehydroxylation of CH and kaolinite were directly reflected on TG curve, as shown in
Figure 4(b). Almost no weight loss was observed before 372.86
oC, but a rapid weight loss occurred in the range of 372.86-424.53
oC due to dehydroxylation of CH, thereafter weight loss proceeded moderately before 559.21
oC, and then became a little significant in the temperature range of 559.21
oC-689.62
oC due to dehydroxylation of kaolinite, and finally turned to be mild until 1050
oC due to volatilization of some matters in HACG.
Under air condition, the thermal transformation and weight loss variation were more complicated than under Ar gas protection condition. As seen from
Figure 4(c), there are four exothermic peaks in DTA curve. They are at 340.01
oC, 523.34
oC, 725.24
oC and 1000.34
oC, respectively. The first three are not observed on the DTA curve measured under Ar atmosphere. The first one was resulted from the formation of a trace of CaCO
3, i.e. CH reacted with CO
2 in air to produce CaCO
3 and H
2O, as expressed by Eq.(3). The second one originated from combustion of carbon in HACG, namely the burning of a large amount of carbon in HACG led to an intense and sharp exothermic peak. The third one was associated with the formation of CaCO
3 due to a large amount of CO
2 produced from the combustion of carbon in HACG and its reaction with CaO generated from dehydroxylation of CH, as expressed by Eq.(4). The fourth one corresponded to the formation of mullite. In addition, there were two endothermic peaks, the obvious one was at 421.52
oC, and the weak one was at 897.51
oC. The two endothermic peaks were attributed to the dehydroxylation of CH and decomposition of CaCO
3, respectively.
These thermal transformations were also reflected on TG curve, as illustrated in
Figure 4(d). It was noted that a little weight gain occurred in the temperature range of 203.81-338.50
oC due to formation of a trace of CaCO
3 and H
2O. The weight loss was mild in the temperature range of 338.50-427.29
oC owing to dehydroxylation of CH, and became more intense until 576.26
oC owing to carbon combustion in HACG, thereafter it entered into a moderate to rapid stage until 716.77
oC owing to the effect of dehydroxylation process of kaolinite and the opposite effect of CaCO
3 formation. The evidences regarding CH dehydroxylation into CaO, and CaCO
3 generating form the reaction between CaO and carbon combustion-induced CO
2 were further confirmed by the XRD and SEM analysis in
Section 3.2 and
Section 3.3.
3.2. Calcination products of HACG –CH mixtures
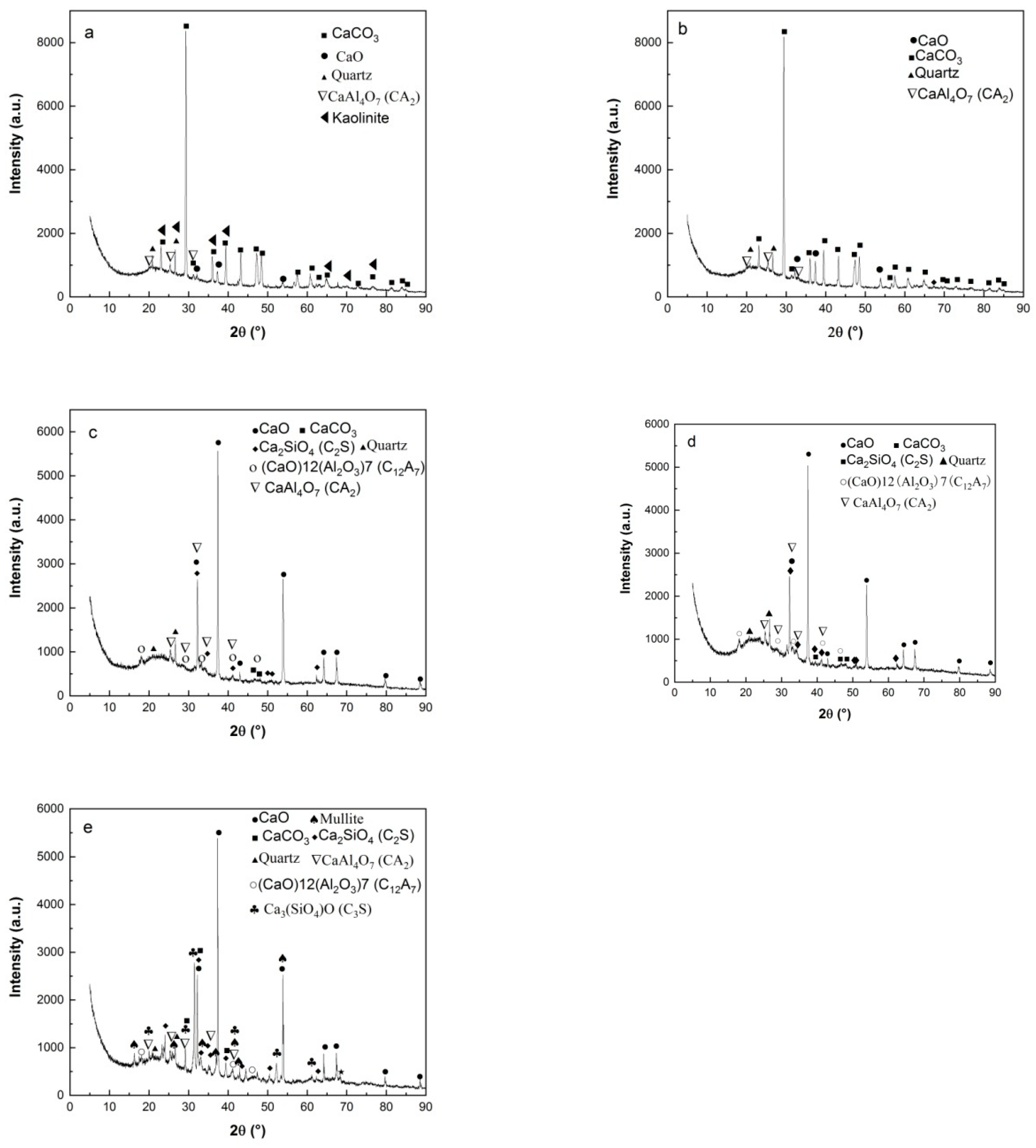
The calcination products of HACG powders mixed with CH calcined at different temperatures was analyzed by XRD, as shown in
Figure 5. At 600
oC, the typically strong diffraction peaks of kaolinite at 2
θ=12.35
o and 24.85
o found in raw HACG disappeared, and a low intensity hump emerged within 15
o to 25
o, suggesting that kaolinite began to transform into amorphous metakaolin (
Figure 5(a)). Metakaolin is reported to have excellent pozzolanic properties for containing silica and alumina in an active form [
27,
28]. Meanwhile, a little amount of CaO and CaAl
4O
7 (CaO•2Al
2O
3 (CA
2)) were found since their diffraction peaks were relatively weak. However, a great amount of CaCO
3 was formed unexpectedly at this calcining temperature, since the strongest diffraction peak at 2
θ=29.36
o belong to CaCO
3. Apparently, CH was totally dehydroxylated to form CaO at 600
oC, and a little part of CaO reacted with Al
2O
3 in metakaolin to form CA
2 accordingly, as expressed by Eq.(5). As for the formation of a large amount of CaCO
3, C element in CaCO
3 can only come from CO
2 produced during combustion of carbon in HACG, as a rapid and significant carbon combustion-induced weight loss occurred in the temperature range of 427.29-576.26
oC on TG curve in air. Therefore, a large amount of CO
2 reacted with the most part of CaO, resulting in the massive formation of CaCO
3. At 700
oC, kaolinite was essentially transformed into metakaolin and the diffraction peaks of CaCO
3 was still the most pronounced, while the other main constituent phases maintained their presence unchanged (
Figure 5(b)).
As temperature was increased to 800
oC, the diffraction peaks of CaCO
3 almost disappeared due to decomposition, while CaO demonstrated the most prominent diffraction peaks (
Figure 5(c)). The formation of large amount of CaO is actually a turning point in improvement of the reactivity HACG. On the one hand, the transformation can facilitate the chemical reactions between CaO and SiO
2 or Al
2O
3, namely the formation of hydratable mineral products such as Ca
2SiO
4 (2CaO•SiO (C
2S)) and (CaO)12(Al
2O
3)7 (12CaO•7Al
2O
3(C
12A
7)), as expressed by Eq.(6) and (7). On the other hand, large amount of CaO can be transformed into quite amount of CH during hydration process, which helps the hydration reaction among CH, active SiO
2 or Al
2O
3 in metakaolin and H
2O. Therefore, the presence of a large amount of CaO in HACG-CH mixture could favors the enhancement in mechanical strength for HACG-cement mortar. At 850
oC, the main mineral products were almost the same as those formed at 800
oC, but the diffraction peaks of CA
2, C
2S and C
12A
7 became a little stronger (
Figure 5(d)), indicating a little increase in their amounts. However, At the highest temperature of 900
oC, the most significant change was the formation of crystallized mullite and (Ca
3SiO
4)O (3CaO•SiO (C
3S)) (
Figure 5(e)). Mullite was transformed from metakaolin as expressed by Eq.(8) [
3], and C
3S was formed by the reaction between CaO and SiO
2, as expressed by Eq.(9). C
3S is well known to be an effective hydratable product in cement, but mullite has almost no pozzolanic activity and consequently reduces the reactivity of CG [
29,
30,
31]. Mullite has never been detected in HACG or other CGs by XRD analysis after calcined at a temperature below 950
oC, according to the literatures available [
24,
25,
26,
30,
31]. In the present study, the presence of mullite at 900
oC could be promoted by a series of reactions between CaO and Al
2O
3 or SiO
2 in metakaolin. These reactions damage the metakaolin structure greatly, and accelerate the formation of mullite. Although the amount of mullite was little at this temperature, the presence of mullite in HACG-CH mixture implies an increase in crystallinity degree for the remained metakaolin, which could decrease the reactivity of metakaolin and bring about a negative effect on mechanical properties of HACG-cement mortar.
3.3. Surface structures of HACG- CH mixtures calcined at different temperatures
Figure 6 shows the morphologies of HACG powders mixed with CH calcined at different temperatures. As compared with original smashed HACG powder (
Figure 6(a)), at 600
oC, a lot of laminar products were formed on HACG particles (
Figure 6(b)). They appeared to be a weathered surface layer with wide spacing between each piece, exposing the HACG substrate underneath. Such morphology could be brought by dehydroxylation and combustion of carbon in HACG or by the chemical reaction between HACG and CH. At 700
oC, most part of laminar products became tiny and dense, covering almost entire surfaces of HACG particles, and the rest evolved into fine granules (
Figure 6(c)). This morphological characteristic was associated with formation of large amount of CaCO
3 on the original HACG particles as identified by latter EDS composition analysis. At 800
oC, the surface products were essentially granular due to a large scale decomposition of CaCO
3 into CaO (
Figure 6(d)), while at 850
oC, some plate-like products occurred besides granular particles, and they connected together (
Figure 6(e)), which means that CaO reacted with metakaolin, producing more calcium aluminates and calcium silicates such as CA
2, C
12A
7 and C
2S as reveal by latter EDS analysis. At the highest temperature 900
oC, the morphology of surface products changed significantly, some fine powders appeared besides more connected plate-like products (
Figure 6(f)), indicating certain different reaction products generating.
The chemical composition of surface products formed on HACG powders mixed with CH calcined at different temperatures was analyzed by EDS point scanning. The resulting products were deduced to include CaO, CaCO
3, metakaolin, CA
2, C
2S and C
12A
7 according to their respective compositional characteristics at locations indicated by numbers, as summarized in
Table 2. The products emerged at different temperatures were as follows: CaO, CaCO
3 and metakaolin at 600
oC, CA
2 at 700
oC, C
2S at 800
oC, C
12A
7 at 850
oC and 900
oC.
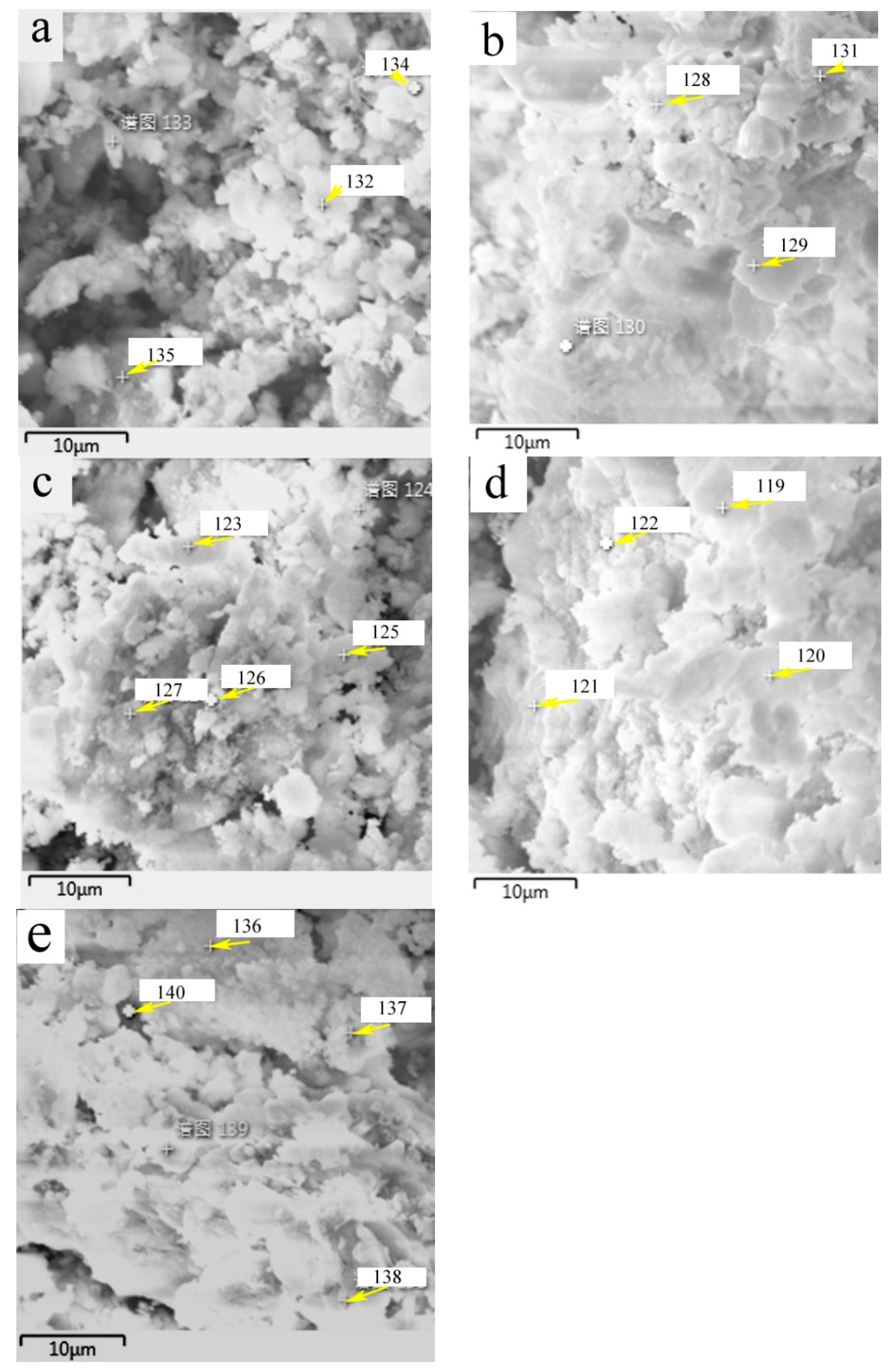
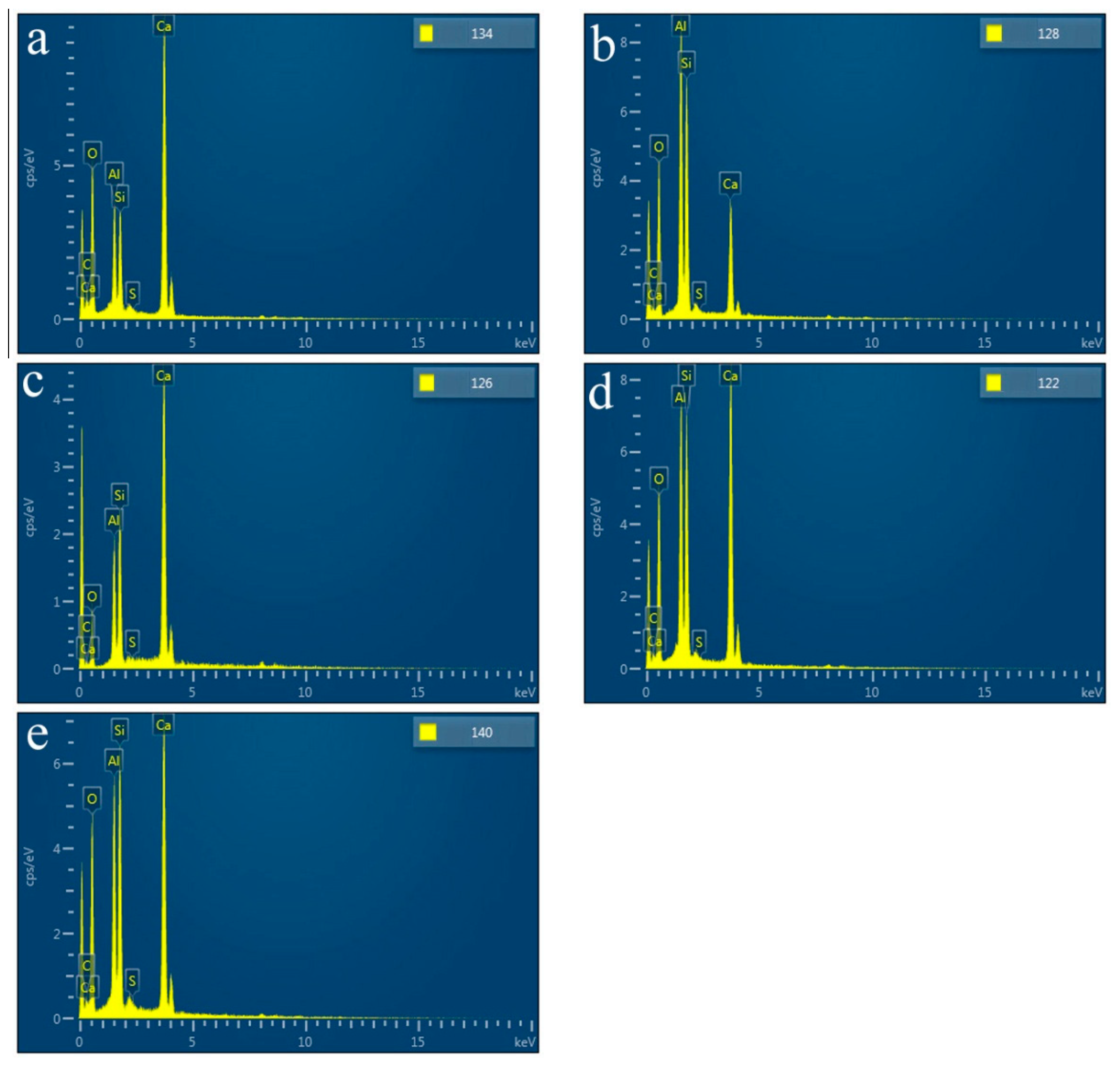
SEM images of HACG powders mixed with CH calcined at different temperatures are shown in
Figure 7, in which EDS point scanning locations are indicated by numbers. EDS patterns at certain locations are shown in
Figure 8. In order to identify calcining products as accurately as possible, two analysis methods were used. The first one was that if the composition at a location was close to that of a certain type of product, the product could be thus identified. For example, the product at location 135 had 22.35wt.%Al, 22.61wt.%Si, 53.78 wt.%O and only 1.14wt.%Ca, suggesting that it originated from HACG. The contents of Al and Si elements were similar to nominal composition of metakaolin: 21.26 wt.% Al and 22.05 wt.%Si, only the content of O element was higher than 44.09 wt.%O in metakaolin. The product at location 123 was also identified as CaCO
3 in such a method, the content of O and Ca elements are similar to nominal 48wt.%O and 40wt.%Ca in CaCO
3. The second one was taken by considering metakaolin composition involvement in the product reaction, which included two cases. In the first case, thin product layers could be formed on the metakaolin, such as CaCO
3 and CaO. The composition of the product should be handled by reduction of the contents of O, Al and Si elements according to the proportions of O, Al and Si elements in metakaolin. That is, the content of O element is about twice the content of Al or Si elements in metakaolin. For instance, the product at location 129 had about 38wt.%O and 36wt.%Ca after reduction of 12wt.%O according to the contents of 7wt.%Al and 6wt.%Si in metakaolin. The product with 38wt.%O and 36wt.%Ca roughly agreed with normal chemical contents of O and Ca elements in CaCO
3, i.e. 48wt.%O and 40wt.%Ca, and was thus deduced to be CaCO
3 formed on the metakaolin substrate. In the second case, when Al
2O
3 or SiO
2 in metakaolin reacted with CaO to form products such as CA
2, C
12A
7 and C
2S, the composition of products such as CA
2, C
12A
7 and C
2S should be evaluated by taking metakaolin as an ensemble using following molecular formulas: CaO•2Al
2O
3•4SiO
2, 12CaO•7Al
2O
3•14SiO
2 and 2CaO•SiO•0.5Al
2O
3. The deducted products by EDS analysis in
Table 2 were also previously identified from XRD patterns in
Figure 5, but mullite and C
3S were not found by EDS analysis, perhaps owing to the limited number of selected locations for analysis.
3.4. hydration products generated from HACG auxiliary cementitious admixture and cement
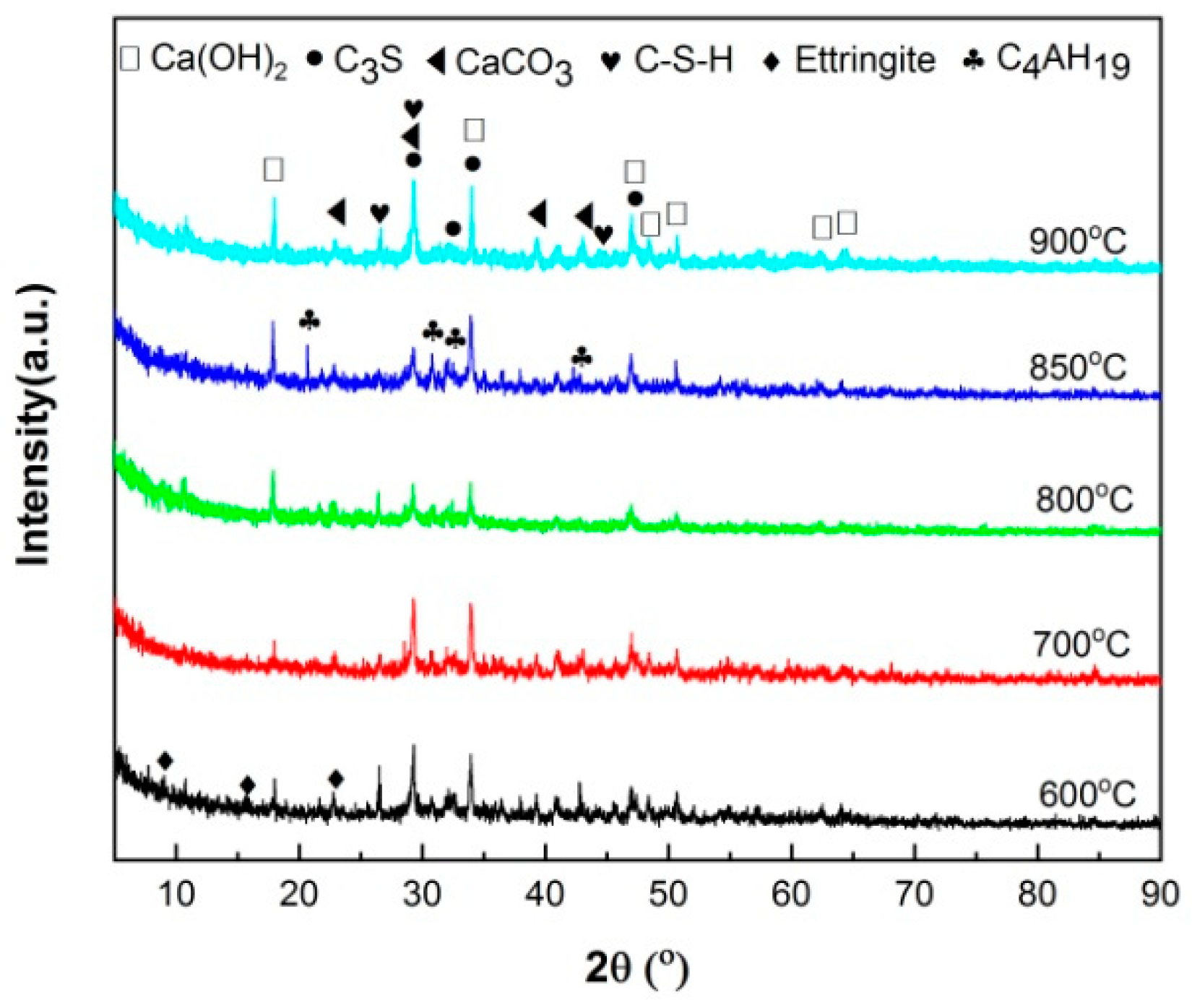
The XRD patterns of hydrated HACG-cement powders are shown in
Figure 9. The mineral phases were found to include C
3S in cement, CaCO
3 in calcined HACG auxiliary admixtures and several types of hydration products. The hydration products were CH, ettringite (Aft (3CaO•Al
2O
3•3CaSO
4•32H
2O)), C-S-H and 4CaO•Al
2O
3•32H
2O (C
4AH
19), in which CH was orignated from both cement and HACG auxiliary cementitious admixture, ettringite and C-S-H were produced from cement, but C
4AH
19 mainly came from HACG auxiliary cementitious admixture. The dependence of hydration products on the calcining temperature can be clearly observed in
Figure 9. At low calcining temperatures of 600 and 700
oC, the hydration products were ettringite, CH and C-S-H. However, as calcining temperature above 700
oC, the diffraction peaks of CH became stronger than that at 600 and 700
oC. Apparently, it is associated with formation of more CaO in HACG auxiliary cementitious admixture. The most noteworthy is that the diffraction peaks of C
4AH
19 emerge at the calcining temperature of 800
oC, and become more significant at 850
oC, suggesting that some of calcination products containing Al
2O
3 in HACG auxiliary cementitious admixture react with CH to form C
4AH
19. However, at the highest calcining temperature of 900
oC, those diffraction peaks from C
4AH
19 dissaperared immediatly. This could be due to the decrease in reactivity of metakaolin and formation of mullite.
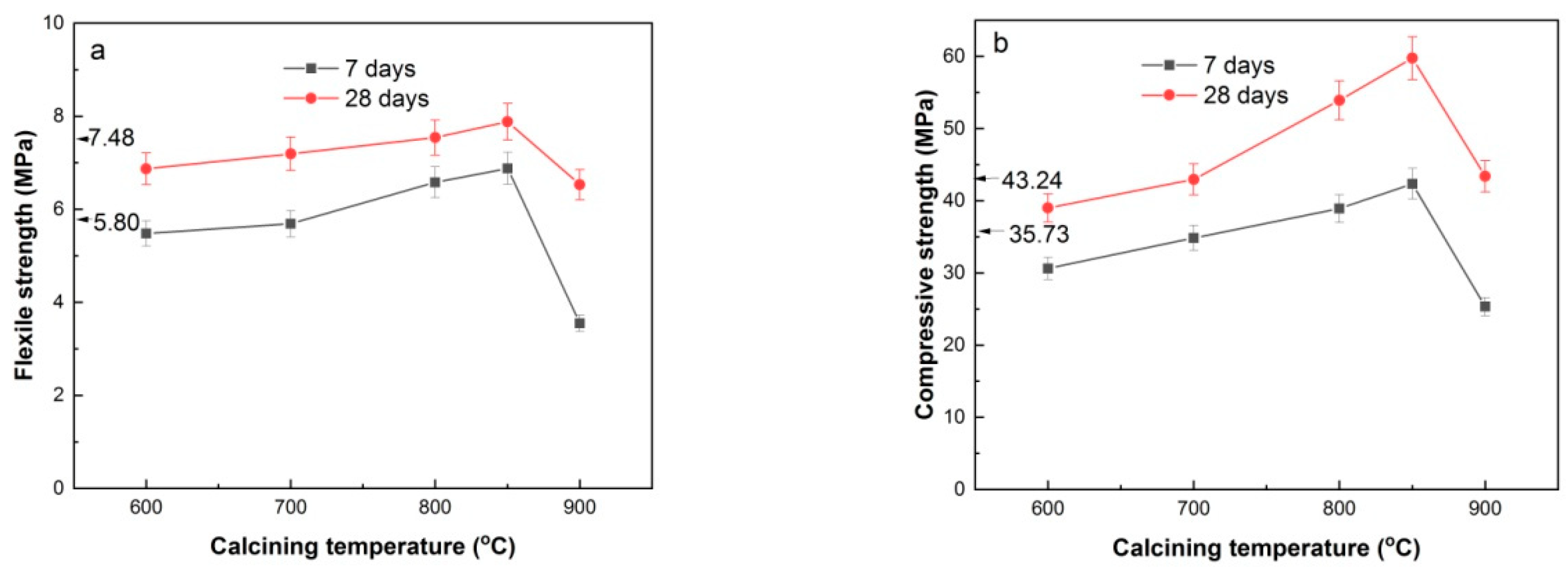
3.5. Flexural and compressive strength of HACG-cement mortar specimens
Figure 10 shows the flexural and compressive strength of HACG-cement mortar specimens. The pure cement mortar specimens had flexural strength of 5.80 MPa and 7.48 MPa from 7 days curing and 28 days curing respectively, and compressive strength of 35.73 MPa and 43.24 MPa form 7 days curing and 28 days curing, respectively. With increasing the calcining temperature, 7-d and 28-d flexural strength of HACG-cement mortar specimens increased until 850
oC, and then decreased rapidly at 900
oC (
Figure 10(a)). At 800 and 850
oC, HACG-cement mortar specimens had higher 7-d and 28-d flexural strength than pure cement mortar specimens. Similarly, 7-d and 28-d compressive strength of HACG-cement mortar specimens went up with increasing the calcining temperature and reached the maximum at 850
oC, and then went down considerably at 900
oC (
Figure 10(b)). The maximal 28-d flexural and compressive strength of HACG-cement mortar specimens were increased by 5.4% and 38.2% respectively, as compared with that of pure cement mortar specimens.
The variation trend for flexural and compressive strength with calcining temperature can be essentially elucidated on the basis of XRD, EDS and hydration products results mentioned above. Even though hydratable CA2 was found to form at 600 and 700oC, the mechanical properties of HACG-cement mortar were not increased significantly, since quite amount of undecomposed kaolinite and carbon were still remained at 600oC, and massive formation of CaCO3 was originated from the combustion of carbon in HACG at 700oC. These substances have low activity and could exert negative effect on flexural and compressive strength. At higher calcining temperatures such as 800 and 850oC, kaolinite transformed into active metakaolin thoroughly and a great amount of CaCO3 was discomposed into CaO, which promotes the reaction between metakaolin and CaO, and thus a variety of hydratable products such C2S, CA2 and C12A7 were formed. These products can significantly increase the flexural and compressive strength of HACG-cement mortar during hydration process. In addition, the excessive CaO can take part in hydration reaction. However, at the highest temperature of 900oC, the amorphous metakaolin began to be crystallized and partly transformed into mullite, which weakened the activity of HACG auxiliary cement admixture and thus resulted in a considerable decrease in flexural and compressive strength.
3.6. Fracture surfaces of HACG-cement mortar specimens
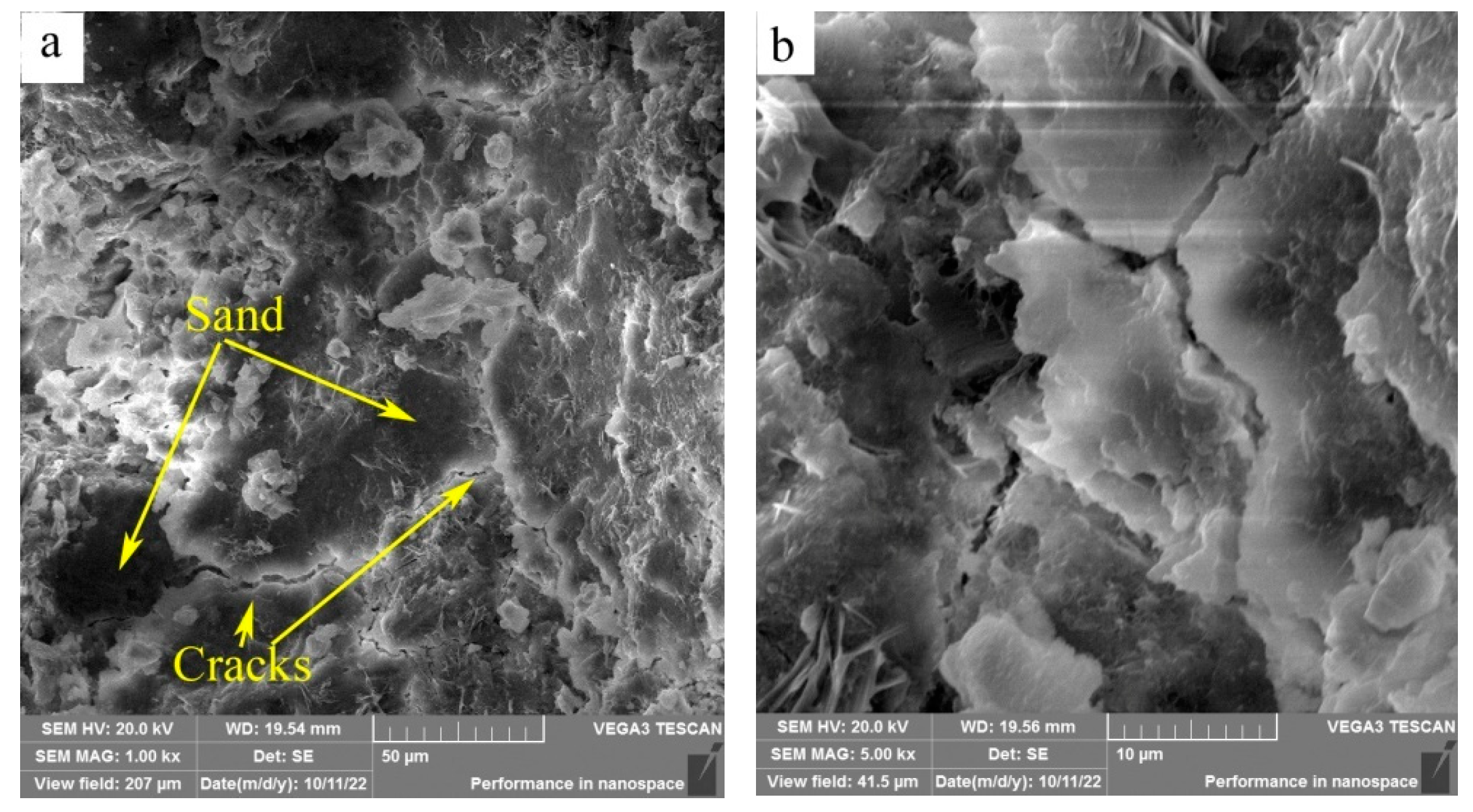
SEM images of the fracture surfaces of HACG-cement mortar specimens after 28-d flexural strength testing are shown in
Figure 11. At the calcining temperature of 600
oC, the surface presented typical features of cleavage fracture that the surface appeared flat and consisted of a number of large or small planes parallel to the fracture surface (
Figure 11(a)). The large dark planes were parts of sand particles as identified by EDS element mapping. Meanwhile, a few of cracks were observed on the boundaries between sand particles and cementious material, and also in cementitious material region as seen from the high magnification photograph (
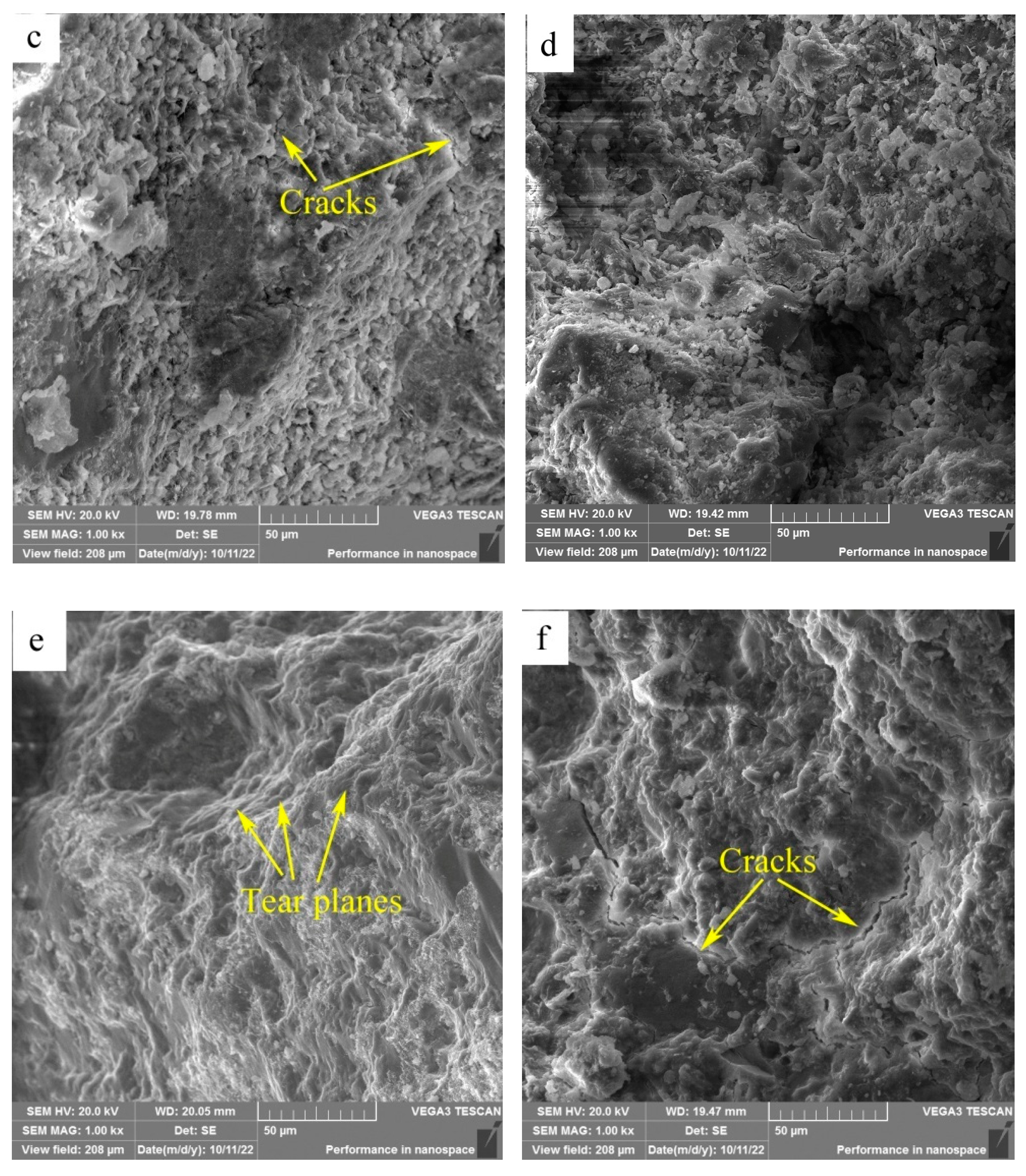
Figure 11(b)), suggesting a weak bonding between sand particle and cementitious material regions, and a low strength in the cementitious material region. At the calcining temperature of 700
oC, the surface looked a little rough and consisted of a large amount of lamellas and particles, meanwhile cracks were also formed in the cementitious material region (
Figure 11(c)), suggesting that constituents in HACG auxiliary cementitious material were not well hydrated and integrated. As the calcining temperature was increased to 800
oC, the surface consisted of dark sand particles and grey cementitious material region (
Figure 11(d)). No obvious cracks were found on the boundaries between sand and cementitious material region besides few microcracks in cementitious material region, and there were still a few of lamellas and particles in cementitious material region, suggesting that constituents in cement and HACG auxiliary cementious admixture were still not bonded compactly. At the calcining temperature of 850
oC, the surface demonstrated a typical feature of tear fracture that the surface consisted of a series of tear planes of different heights, and almost no crack was observed on the surface (
Figure 11(e)). Such a tear fracture mode indicates that every part in HACG-cement mortar was bonded firmly. However, at the highest calcining temperature of 900
oC, the surface again showed the features of cleavage fracture. Meanwhile, a few of large cracks were formed on the boundaries between sand and cementious material region, and also found in the cementious material region (
Figure 11(f)), suggesting a weak bonding between sand and cementious material regions as well as among the constituents in cementious material. The variation of fracture surface morphology with calcining temperature agrees well with the trend of flexural and compressive strength of HACG-cement mortar, i.e. when a good integrated boundary between sand particle and cementious material region is formed, and the constituents in the cementious material is fully hydrated, the flexural and compressive strength of HACG-cement mortar reach their respective maximum values. That is the reason for the mortar specimens using HACG auxiliary cementitious admixture calcined at 850
oC to present the maximum mechanical properties.
The elemental mappings were conducted on the fracture surfaces by EDS (not all shown here). The chemical compositions of the surfaces are listed in
Table 3. It is noted that the content of Ca element reaches a high level above 20.1wt.% on the surfaces of mortar specimens with HACG auxiliary cementitious admixtures calcined at 800 and 850
oC, which is much higher than that on other mortar specimens surfaces. Meanwhile, the content of C element descends to a low level below 13.4% on the surfaces of mortar specimens with HACG auxiliary cementitious admixtures calcined at 800 and 850
oC, which is lower than that on other mortar specimens’ surfaces. The phenomenon of high-Ca and low-C in the mortar specimens with HACG auxiliary cementitious admixtures calcined at 800 and 850
oC, indicates not only the significant burning loss of C in HACG, but also a large loss of CO
2 due to decomposition of CaCO
3 into CaO and subsequent formation of many hydratable products such as C
2S, CA
2 and C
12A
7 during calcining process. On the other hand, high level of CaO in the HACG auxiliary cementitious admixtures is conducive to formation of more CH during hydration process, so CH can reacts with H
2O and active Al
2O
3 or SiO
2 in metakaolin to form more hydration products such as C-A-H and C-S-H gel. Apparently, these advantages contribute greatly to the higher flexural and compressive strength shown by mortar specimens with HACG auxiliary cementitious admixtures calcined at 800 and 850
oC.
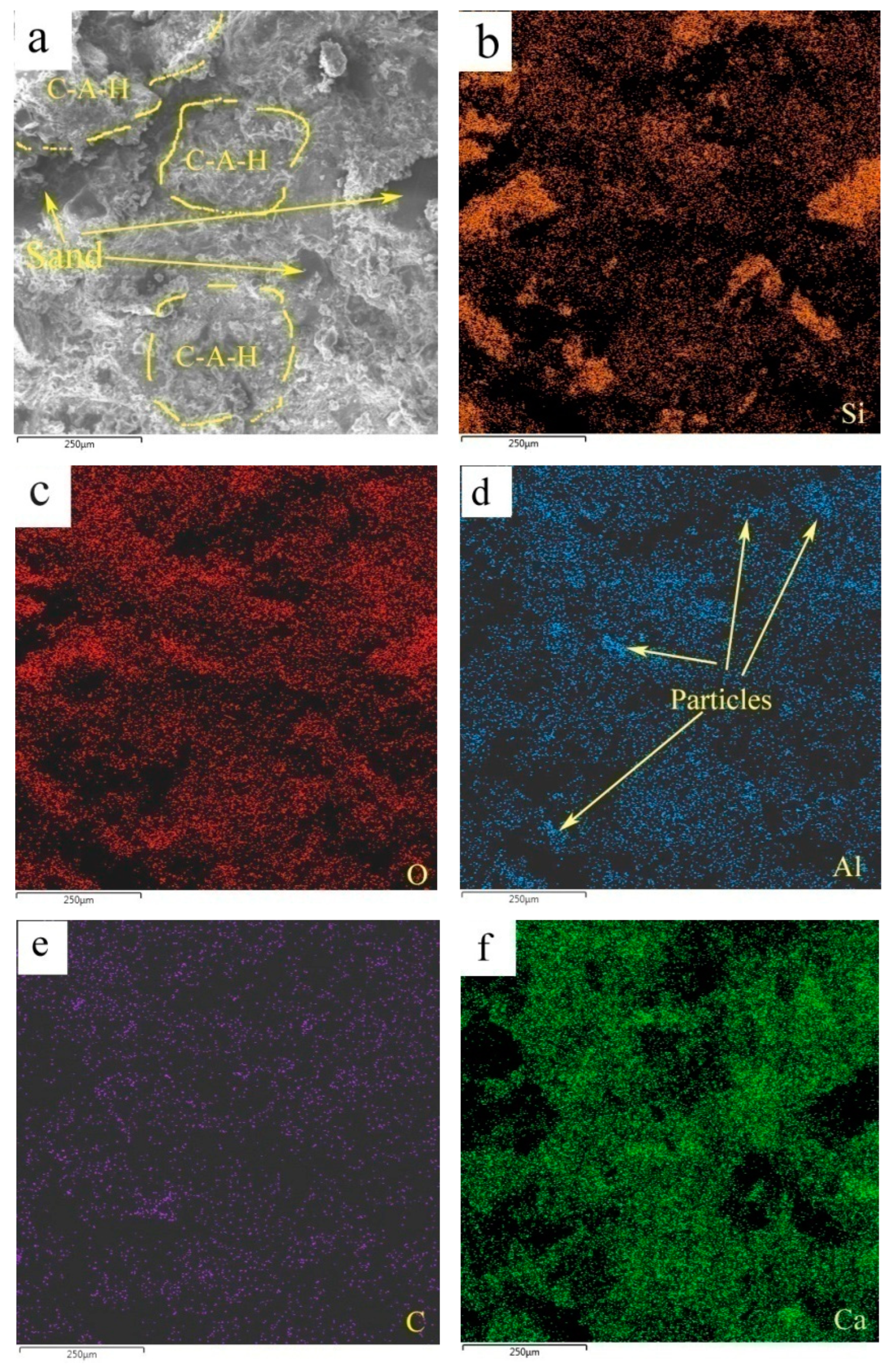
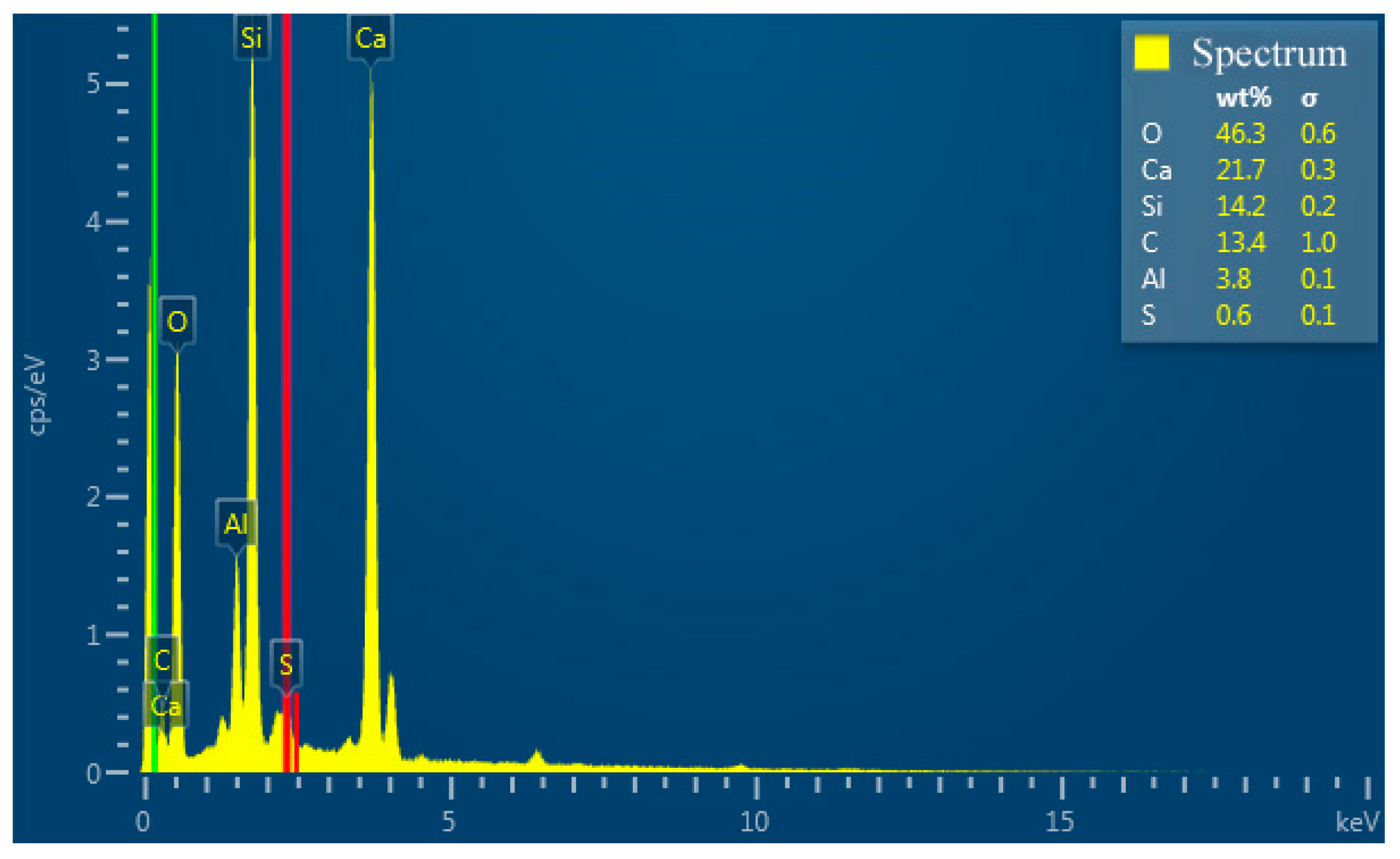
Figure 12 shows the elemental mappings of the mortar specimen fracture surface with HACG auxiliary cementitious admixture calcined at 850oC. The corresponding EDS pattern of the fracture surface is shown in
Figure 13. The dark flat areas indicated by yellow arrows on the SEM image were apparently occupied by sand particles (
Figure 12(a)), which can be easily confirmed by the mappings for Si and O elements (
Figure 12(b) and (c)). The mapping for Al and C elements told the locations of big and small calcined HACG particles (
Figure 12(d) and (e)), as indicated by yellow arrows, since these particles exhibited the feature of high contents of Al and C elements in HACG. However, Ca element was found almost absent at these locations (
Figure 12(f)), suggesting that the HACG particles hardly involved in the hydration reaction, and made almost no contribution to the strengthening effect on mortar. Obviously, the number and size of HACG particles without involvement in the hydration reaction were less, and most Al-enriched areas were found to be distributed with Ca elements homogeneously, as indicated by yellow circles in
Figure 12(a). This proves that HACG auxiliary cementitious admixture calcined at 850oC can be well hydrated to form C-A-(S)-H gel.