Submitted:
12 December 2023
Posted:
13 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
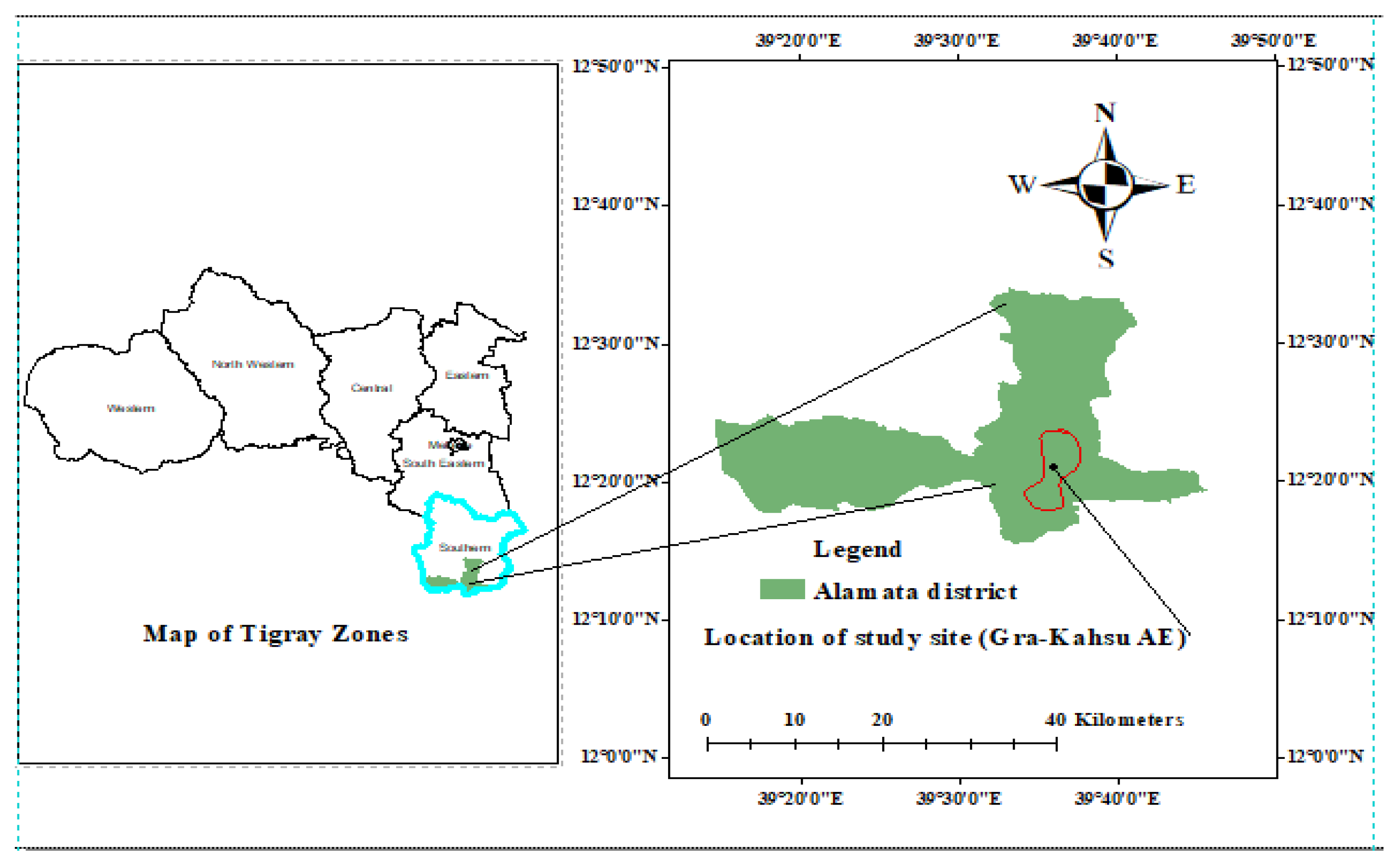
2.1. Description of study area
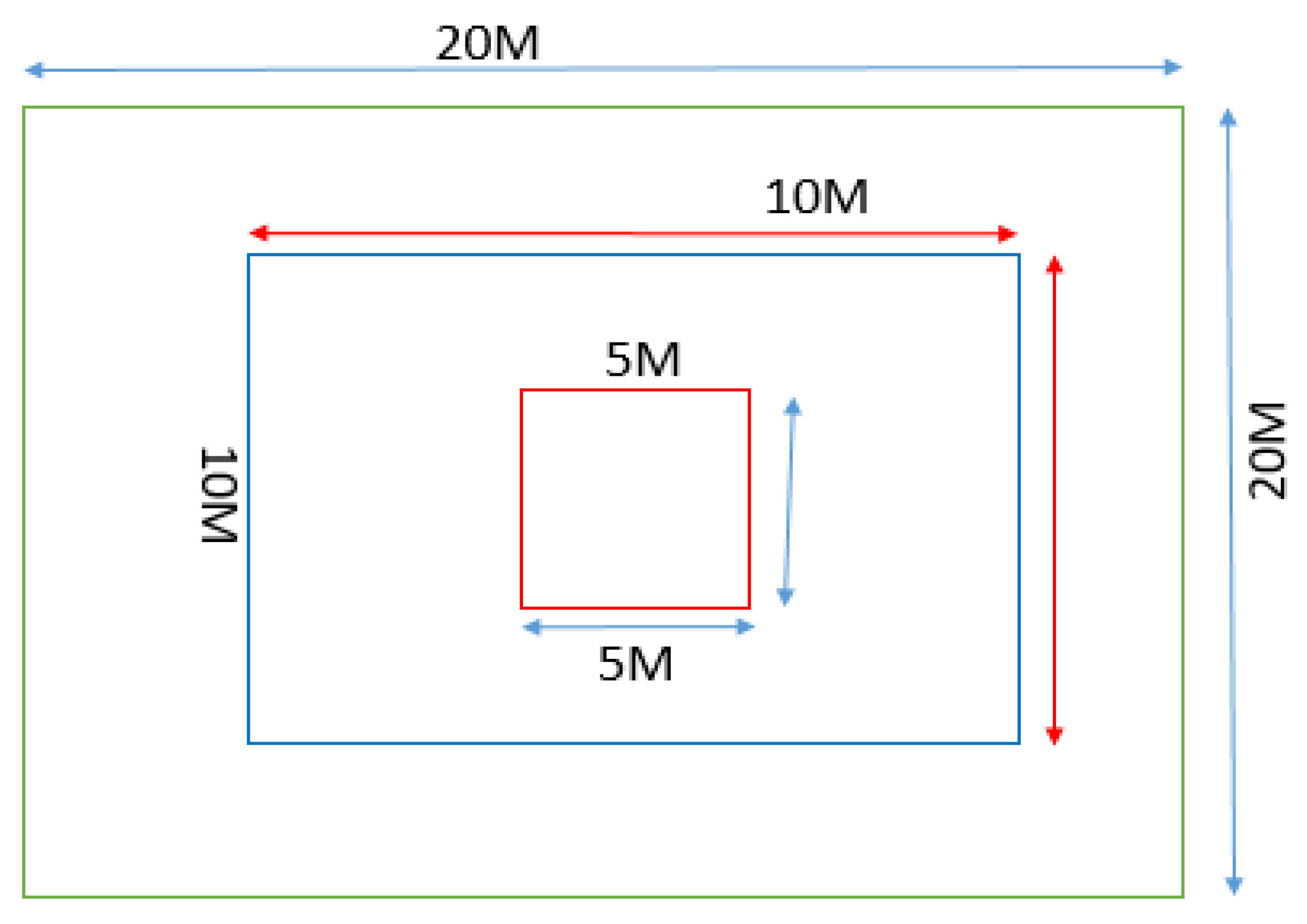
2.2. Field Survey Design and Vegetation Sampling
2.3. Preference Ranking
2.4. Data Analysis
3. Results and discussion
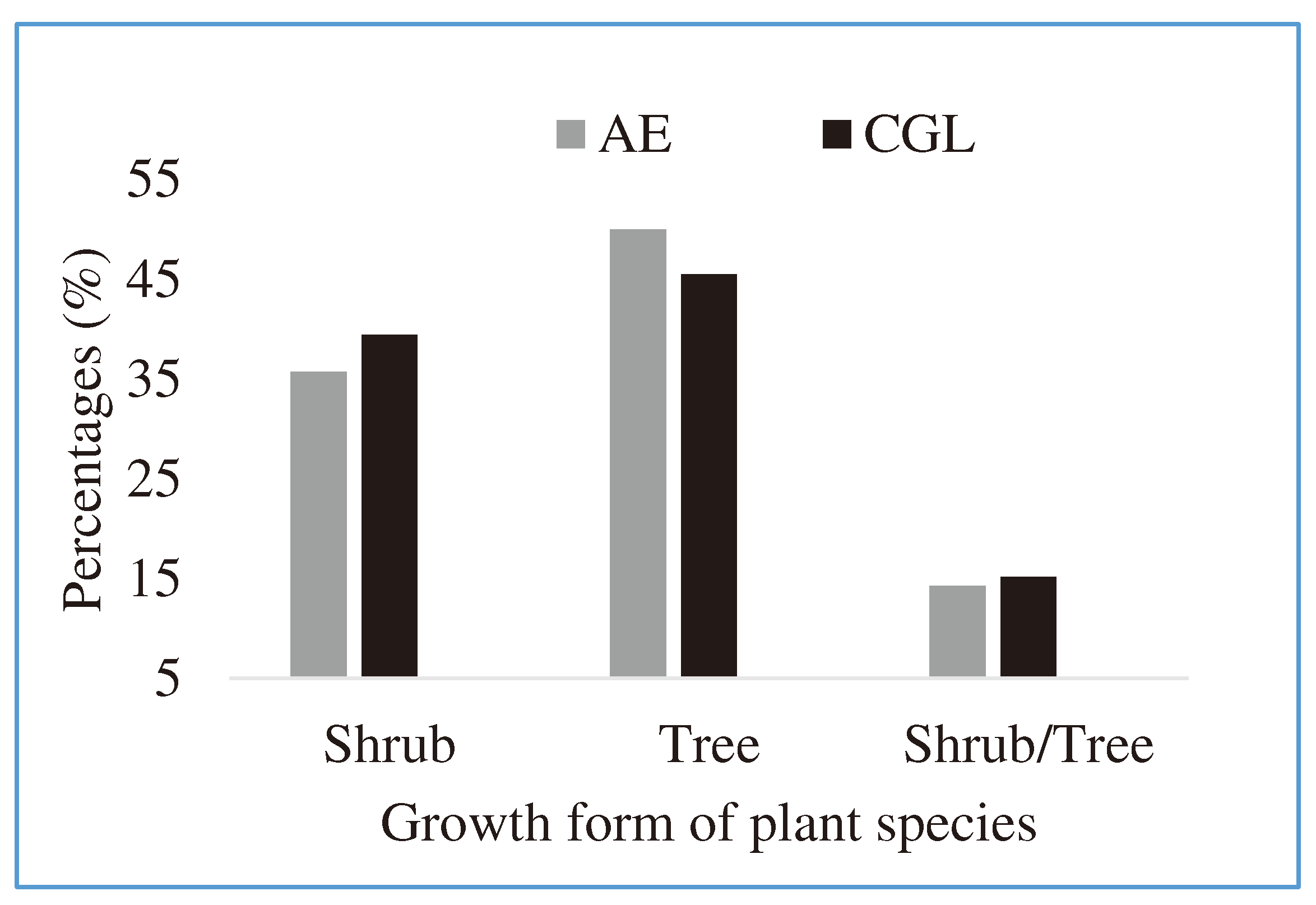
3.1. Major honey bee plants
3.2. Species composition
3.3. Density and abundance
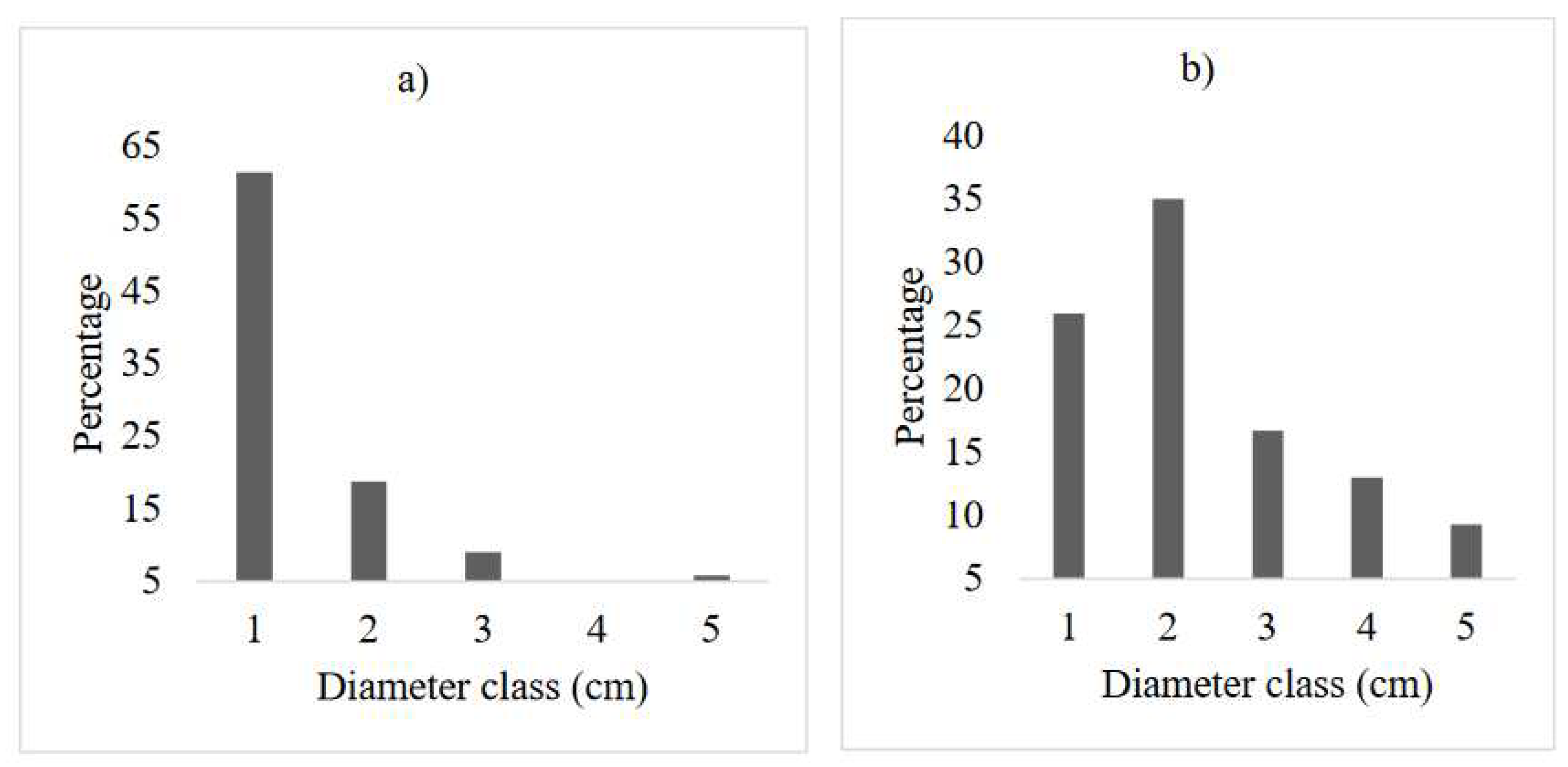
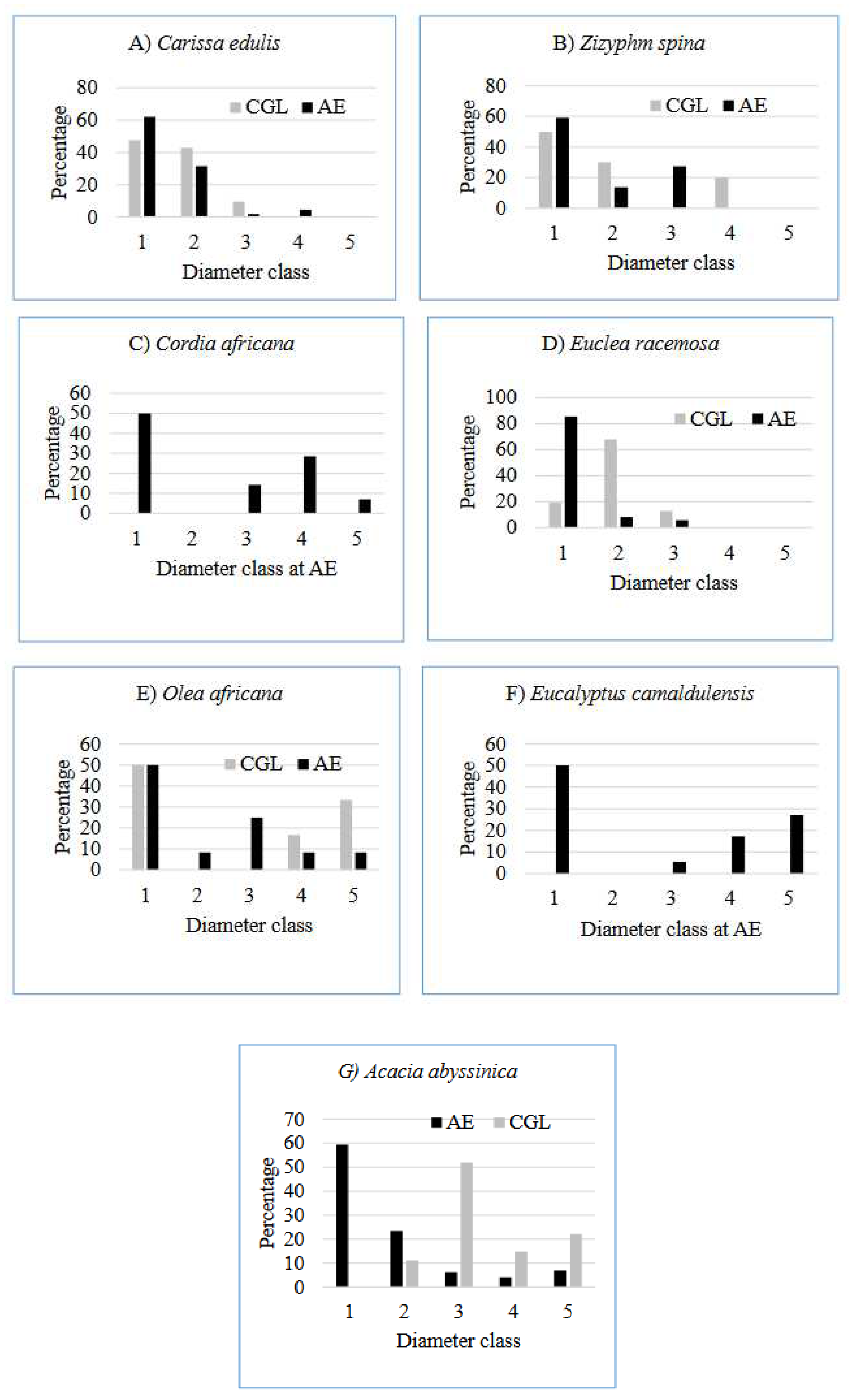
3.4. Population structure
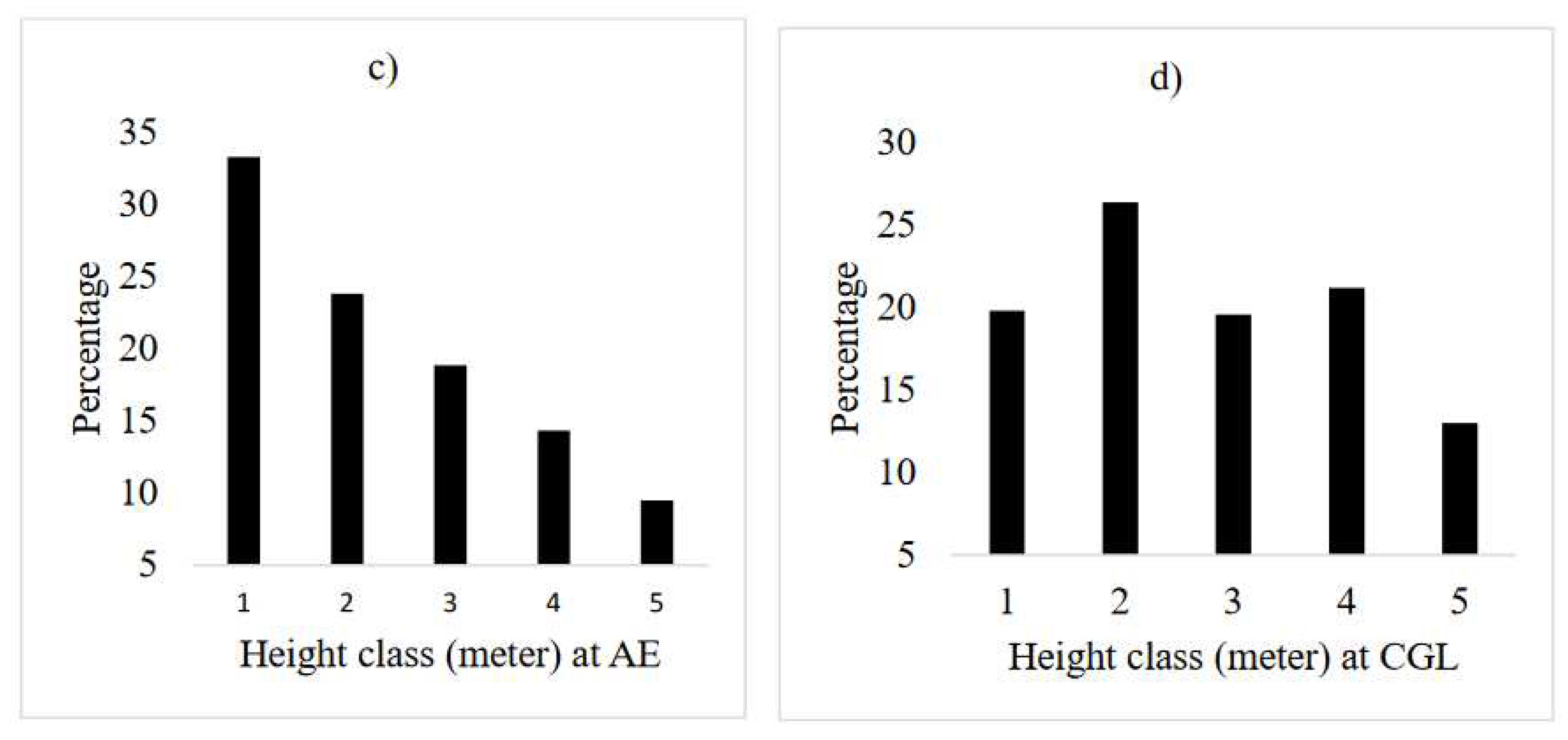
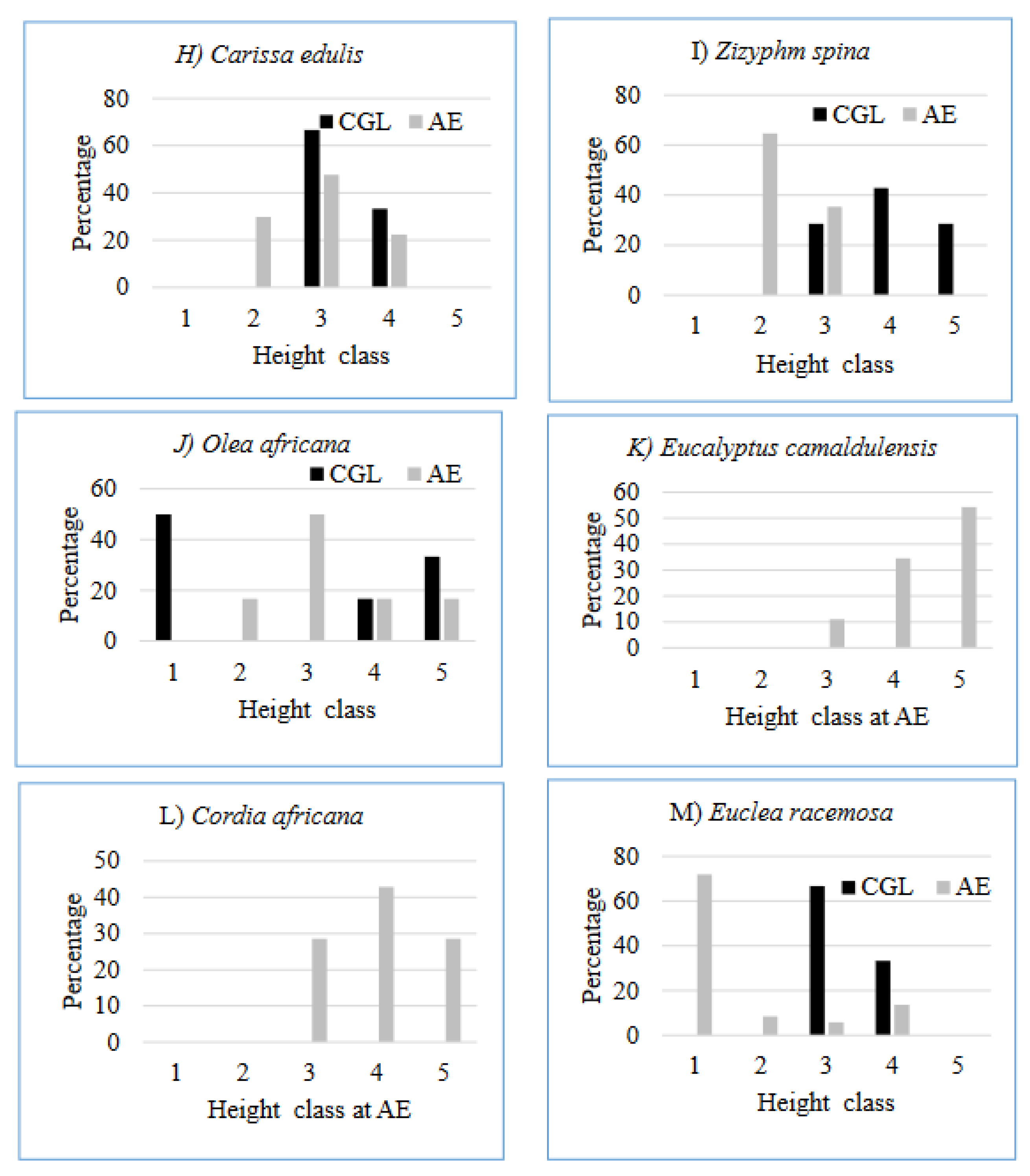
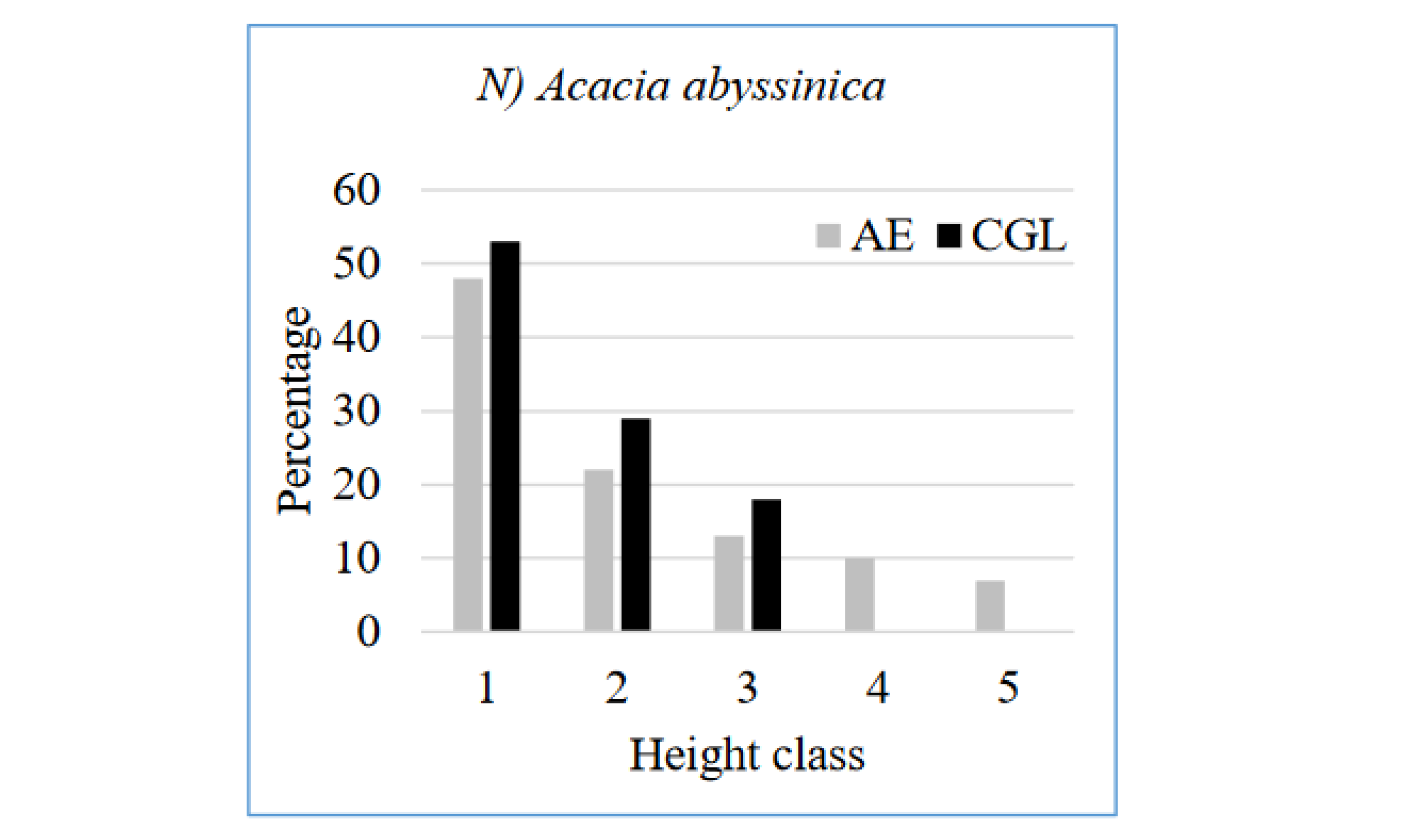
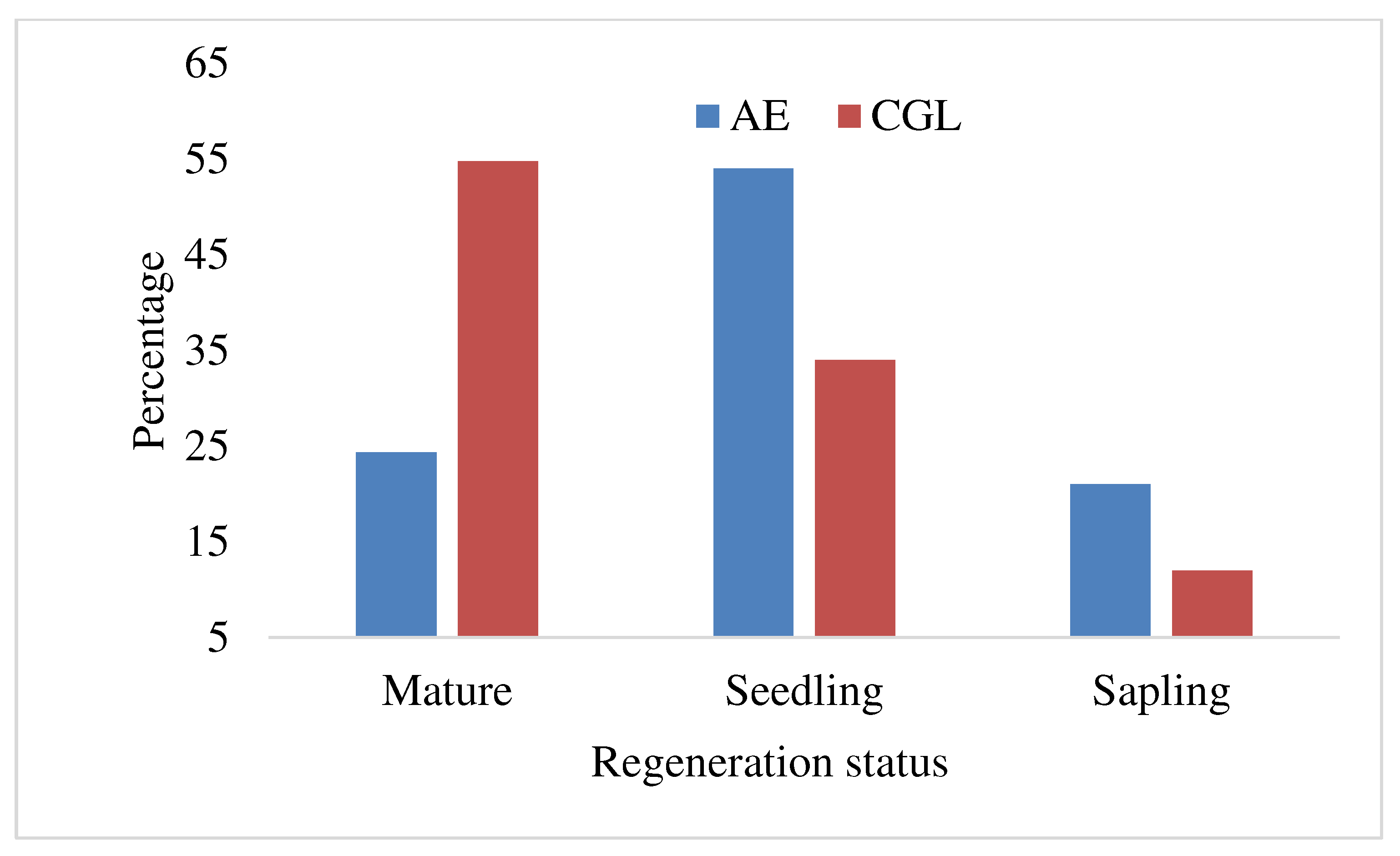
3.5. Regeneration status of honey bee floras
3.6. Species diversity, richness and evenness in the study land uses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Vernacular name | Scientific name of the plant | Life form/Habit | Land use system | |
| Tsada-grar | Acacia abyssinica Hochst. ex Benth | Tree | B | |
| Wancho | Acacia seyal | Tree | B | |
| Karora | Acacia tortilis (Forssk.) Hayne | Tree | B | |
| Sabansa | Accacia asak | Tree | B | |
| Seraw | Accacia etbaica | Tree | B | |
| Morez | Acokanthera schimperi | Shrub/Tree | AE | |
| Tetem-agazen | Astragalus atropilosulus | Shrub | B | |
| Bedeno | Balanites aegpytiaca | Tree | B | |
| Tebeb | Becium grandiflorum (Lam.) | Shrub | B | |
| Htsawts | Calpurnia aurea (Ait) Benth | Shrub | B | |
| Agam | Carissa edulis (Forssk.) Vahl | Shrub | B | |
| Keyh–om | Cassipourea malosana (Baker) Alston | Tree | AE | |
| Moto-koma | Celtis africana Burm. f. | Tree | AE | |
| Tsaeda-kotsilo | Conyza hypoleuca A.Rich | Shrub | B | |
| Awhi | Cordia Africana | Tree | AE | |
| Tahses | Dodonoae angustifoha | Shrub | AE | |
| Mongolhats | Dovyalis abyssinica (A.Rich.) Warb. | Shrub/Tree | B | |
| Key-bahrzaf | Eucalyptus camaldulensis Dehnh | Tree | AE | |
| Tsaeda-Bahrzaf | Eucalyptus globulus Labill | Tree | AE | |
| Kuleow | Euclea racemosa subsp. schimperi (A.DC.) Dandly | Shrub | B | |
| Kinchib | Euphorbia tirucalli | Tree | B | |
| Oda | Ficus sycomorus L | Tree | B | |
| Anchie | Gomphocarpus fruticosus | Shrub | B | |
| Meleglega | Grewia ferruginea Hochst.exA.Rich | Shrub/Tree | B | |
| Rowey | Grewia mollis juss | Shrub/Tree | B | |
| Swakerni | Lucas Abissinica | Shrub | B | |
| Atsats | Maytenus undata (Thunb.) Blakelock | Tree | B | |
| Kachmo | Myrsin Africana | Shrub | B | |
| Awlie | Olea Africana | Tree | B | |
| Kerets | Osyris quadripartite | Tree | B | |
| Chiendog | Otostegia integrifolia Benth. | Shrub | B | |
| Mayliho | Pittosporum viridiflorum Sims | Tree | B | |
| Tsehag | Psydrax schimperiana (A.Rich.) Bridson | Shrub | B | |
| Konteftefe | Ptrollobium stellatum | Shrub | B | |
| Tetaelo | Rhus glutinosa A.Rich. | Shrub/Tree | B | |
| Atami | Rhus natalensis Krauss | Shrub/Tree | B | |
| Hohot | Rumex nervosus Vahl | Shrub | B | |
| Agam-kinchil | Sageretia thea (Osbeck) M. C. Johnston | Shrub | B | |
| Tikur-berbere | Shinus molle | Tree | AE | |
| Girawa | Vernonia amygdalina Del. | Tree | B | |
| Kunkura/Gaba | Zizyphm spina christi | Tree | B | |
| Kunkurahdo | Zizyphus mucronata | Tree | AE |
References
- Aerts, R., Wagendrop, T., November, E., Mintesinot B., Deckers, J., Muys, B. 2004. Ecosystem thermal buffer capacity as an indicator of the restoration status of protected areas in the Northern Ethiopian Highlands. Restoration Ecology, 12: 586-596. [CrossRef]
- Alemayehu Wassie. 2002. Opportunities, constraints and prospective of Ethiopian orthodox tewahido churches in conserving forest and resources: the case of churches in South Gonder, Northern Ethiopia. Master’s Thesis, Swedish University of Agricultural.
- Alemtsehay Teklay. 2011. Seasonal Availability of Common Bee Flora in Relation to Land Use and Colony Performance in Gergera Watershed Atsbi Wenberta District Eastern zone of Tigrai, Ethiopia, MSc Thesis, Hawassa University, Wondo Genet College Of Forestry And Natural Resources, Wondo Genet, Ethiopia.
- Amsalu Bezabeh, Nuru Adgaba and Radloff, H. 2003. Multivariate morphometric analysis of honeybees in the Ethiopian region Apidolgie 35: 71-81. [CrossRef]
- Atsbaha H, Taye T and Kebede D. 2015. Assessment of honey production system, constraints and opportunities in three selected Districts of Tigray Region, Ethiopia. Basic Research Journal of Agricultural Science and Review, 4(10), 304-315.
- Ayalew Kassaye. 2006. The loss of some natural plant species in Tigrai and the concern to the living conditions of honeybees. Loss of natural plants: Proceedings of the 5th Annual National Conference of Ethiopian Beekeepers Association. 8-15.
- Ayalew Sebsibe, Feleke Woldeyes and Simon Shibru. 2018. Woody Vegetation Composition, Structure, and Community Types of Doshke Forest in Chencha, Gamo Gofa Zone, Ethiopia, International Journal of Biodiversity. [CrossRef]
- Birhane, E., Mengistu, T., Seyoum, Y., Hagazi, N., Putzel, L., Rannestad, M. M., and Kassa, H. 2017. Exclosures as forest and landscape restoration tools: lessons from Tigrai Region, Ethiopia. International Forestry Review. 8-14. [CrossRef]
- Blay, D. 2002. Rehabilitation of degraded lands in humid zones of Africa. Forest research Institute of Ghana University. Kumasi, Ghana.
- Bot, A. & Benites, J. 2005. The Importance of soil organic matter: key to drought-resistant soil and sustainable food production. FAO soils Bulletin, Rome, Italy. Pp 5-48.
- Central Statistical Agency. 2019. Agricultural Sample Survey 2018/19 [2011 E.C.], Report on livestock and livestock characteristics. Addis Ababa, Ethiopia.
- Couralet, C. Sass-klaessen, U., Sterck, F.J., Tesfaye Bekele, & Zuidema P.A. 2005. Combining dendrochonology and matrix modeling in demographic studies: an evaluation for Juniperus procera in Ethiopia. Forest Ecology and Management, 216:317-330. [CrossRef]
- Dhaulkhandi, M., Dobhal, A., Bhatt, S and Kumar, M. 2008. Community structure and regeneration potential of natural forest site in Gangotri, India. Journal of Basic and Applied Sciences, 4: 49-52.
- Edwards, S., Mesfin Tadesse, Sebsebe Demissew and Hedberg, I. (eds.) 2000. Flora of Ethiopia and Eritrea, Vol. 2. Part 1. The National Herbarium, Addis Ababa University, Addis Ababa and Department of Systematic Botany, Uppsala University, Uppsal.
- Emiru Birhane, Demel Teketay and Barklund, P. 2007. Enclosures to Enhance Woody Species Diversity in The Dry Lands of Eastern Tigray, Ethiopia, East African Journal of Sciences, 1 (2) 136-147. [CrossRef]
- Equar Gebru, Abraha Berhanu, Lemma Hayal, Amare Solomon and Asmelash Tsehaye. 2016. Honey bee flora diversity and their impact on honey production in Tigrai region of Ethiopia, Livestock Research for Rural Development 28 (7).
- Feyera Senbeta, Tadesse Woldemariam, Sebsebe Demissew and Denich Manfred. 2007. Floristic diversity and composition of Sheko forest, Southwest Ethiopia. Ethiopian Journal of Biological Sciences, 6: 11-42.
- Fichtl R and Admasu A.1994. Honeybee flora of Ethiopia. The national herbarium, Addis Ababa university, Deutscher Entwicklungsdienst (DED). Margraf Verlag, Germany.
- Fisaha G, Hundera K & Dalle G. 2013. Woody plants’ diversity, structural analysis and regeneration status of Wof Washa natural forest, north-east Ethiopia. African Journal of Ecology 51: 599-608. [CrossRef]
- Gebrewahd Amha. 2014. Herbaceous vegetation restoration potential and soil physical condition in a mountain grazing land of Eastern Tigrai, Ethiopia. Journal of Agriculture and Environment for International Development, 108(1): 81 – 106.
- Girmay Tesfay, Girmay Gebresamual, Alem Gebretsadik and Hailemariam Tekie. 2014. Participatory rural appraisal report: Raya-Alamata Wereda, Tigray region. CASCAPE working paper 2.6.4.
- Gurmessa F, Soromessa T & Kelbessa E. 2012. Structure and regeneration status of Komto Afromontane moist forest, East Wollega Zone, west Ethiopia. Journal of Forestry Research 23(2): 205−216.
- Haftom G, Zelealem Tesfay, Girmay Murutse and Awet Estifanos A 2013: Seasonal honeybee forage availability, swarming, absconding and honey harvesting in Debrekidan and Begasheka Watersheds of Tigray, Northern Ethiopia. Livestock Research for Rural Development 25(61). http://www.lrrd.org/lrrd25/4/haft25061.htm.
- Haftom Gebremedhn and Yaynishet Tesfay. 2012. Identification and evaluation propagation techniques of Hypoestes forskaolii (Grbia) as bee fodder for smallholder farmers, Livestock Research for Rural Development 24.
- Haftom Gebremedhn, Girmay Darcha and Kinfe Mezgebe. 2017. Distribution and abundance of Hypoestes forskaolii (Vahl) in the exclosures of Tigrai, northern Ethiopia, Livestock Research for Rural Development 29 (8).
- Hundera K & Gadissa T. 2008. Vegetation composition and structure of the Belete forest, Jimma zone, south western Ethiopia. Ethiop. J. Biol. Sci. 7(1): 1-15.
- Jimenez, J., Jurado, E., Aguirre, O. and Estrada, E. 2005. Effect of Grazing on Restoration of Endemic Dwarf Pine (Pinus culminicola Andresen et Beaman) Populations in Northeastern Mexico. Restoration Ecology, 13, 103-107. [CrossRef]
- Kebede T and Lemma T. 2007. Study of honey production system in Adami tulu jido Kombolcha district in mild rift valley of Ethiopia. Adami tulu Agricultural Research center, Zeway, Ethiopia. Retrieved from:. http://www.ird.org/irrd/giii/kebe/9162.htm19(11).
- Keller I, Fluri P and Imdorf A. 2005. Pollen nutrition and colony development in honey bees, Part II, Bee World 86: 27-34. [CrossRef]
- idane Welde, Tesfay Atsbha, Yemane Nega, Adehanom Baraki, Hagos kidane, Ykaelo Teklay, Hagos Hailu, Dawit Hadera, G/giwergis Aredahegn, Gebre Hadgu and Desalegn Emuru. (2016). Survey Report On: Participatory Agricultural Production Constraints Appraisal in AGP-I Districts of Sothern Tigray. Alamata Agricultural Research Center.
- Kikoti, I.A., Mligo, C. and Kilemo, D.B. 2015. The Impact of Grazing on Plant Natural Regeneration in Northern Slopes of Mount Kilimanjaro, Tanzania. Open Journal of Ecology, 5, 266-273. [CrossRef]
- Krebs, C.J. 1999. Ecological methodology. 2nd edition. University of British Colombia, Harper Collins, New York.
- Lulekal E, Kelbessa E, Bekele T & Yineger H. 2008. Plant species composition and structure of the Mana Angetu moist Montane forest, south-eastern, Ethiopia. Journal of East African Natural History 97(2): 165–185. [CrossRef]
- MacDicken, K.G. 1997. A guide to monitoring carbon storage in forestry and agro-forestry projects in forest carbon monitoring program: Winrock international institute for agricultural development, Arlington, Virginia.
- Magurran, A.E. 1996. Ecological diversity and its measurement: Chapman and Hall. London.
- Martin G J. 1995. Ethnobotany A method manua Royal botanical garden, Chapman and Hall, Kew, London 116-120.
- Mastewal, Y., Kindeya, G., M. Stein, & Wolde, M. 2006. Impact of Area Enclosures on Density, Diversity, and Population Structure of Woody Species: the Case of May Ba’ati-Douga Tembien, Tigray, Ethiopia. Ethiopian Journal of Natural Resources 8 (1): 99 – 121.
- Mekuanint Lewoyehu, Meareg Amare. 2019. Comparative Assessment on Selected Physicochemical Parameters and Antioxidant and Antimicrobial Activities of Honey Samples from Selected Districts of the Amhara and Tigray Regions, Ethiopia, International Journal of Food Science. [CrossRef]
- Mewcha Berhe and Yemane G.Egziabher. 2016. Quality honey forage sources in the highlands of semi-arid zone, northern Ethiopia, Journal of the drylands, 6(2): 546 – 559.
- Michener, C.D. 2007. The bees of the world, 2nd edition, The Johns Hopkins University Press, Baltimore and London, USA and UK 12-19.
- Misra, K.C. 1974. Manual of plant ecology. Oxford and IBH publishing Co., New Delhi. 376p.
- Nyssen J, Descheemaeker K, Nigussie H, Mitiku H, Deckers J and Poesen J (eds). 2007. Lessons learnt from 10 years research on soil erosion and soil and water conservation in Tigrai, Tigrai Livelihood papers no.7, Mekelle: Zala-Daget Project, Mekelle University, K.U.Leuven, Relief Society of Tigrai, Africamuseum and Tigrai Bureau of Agriculture and Rural Development, 53 p. ISBN 978-90-8826-027-8.
- Porto, R. G., de Almeida, R. F., Cruz-Neto, O., Tabarelli, M., Viana, B. F., Peres, C. A., & Lopes, A. V. 2020. Pollination ecosystem services: A comprehensive review of economic values, research funding and policy actions. Food Security, 12(6), 1425–1442. [CrossRef]
- R Development Core Team. 2021. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Austria, URL. http://www.Rproject.org/.
- Said F, Inayatullah M, Ahmad S. 2015. Foraging behavior of the Himalayan Honeybee, Apis cerana (Hymenoptera: Apidae) associated with sunflower (Helianthus annuus L.) at Peshawar District of Khyber Pakhtunkhwa (KP). J Entomol Zool Stud; 3(3): 203-7.
- Sarkar, M., and Devi, A. 2014. Assessment of diversity, population structure and regeneration status of tree species in Hollongapar Gibbon Wildlife Sanctuary, Assam, Northeast India. Tropical Plant Research, 1, 26-36.
- SER (Society for Ecological Restoration International Science and policy working group). 2008. Opportunities for integrating ecological restoration and biological conservation within the ecosystem approach. Society for Ecological Restoration International.
- Smit, C., Gusberti, M. and Müller-Schärer, H. 2006. Safe for Saplings; Safe for Seeds? Forest Ecology and Management, 237, 471-477. [CrossRef]
- Southern Zone BOARD. 2018. Natural resources core process, annual report. Southern Zone of Office of Agriculture and Rural Development, Unpublished, Tigrigna version.
- Stern, M., Quesada, M. and Stoner, K.E. 2002. Changes in Composition and Structure of a Tropical Dry Forest Following Intermittent Cattle Grazing. International Journal of Tropical Biology, 50, 1021-1034.
- Sultan M and Berhanu A. 2013. Floristic composition and structure of Yegof mountain forest, South Wollo, Ethiopia. Ethiopian Journal of. Sciences and Technology, I (1): 33-45.
- Tadele D, Lulekal E, Damtie D & Assefa A. 2014. Floristic diversity and regeneration status of woody plant in Zengena forest, a remnant montane forest patch in northwestern Ethiopia. Journal of Forestry Research 25(2): 329-336. [CrossRef]
- Tefera Mengistu, Demel Teketay, Hulten H. and Yonas Yemshaw. 2005. The role of enclosure in the recovery of woody vegetation in degraded dry land hillsides of central and northern Ethiopia. Journal of Arid Environments, 60(2): 259-281. [CrossRef]
- Teich, I., Cingolani, A.M., Renison, D., Hensen, I. and Giorgis, M.A. 2005. Do Domestic Herbivores Retard Polylepis australis Bitt. Woodland Recovery in the Mountains of Cordoba Argentina? Forest Ecology and Management, 219, 229-241. [CrossRef]
- Tesfay Atsbha and Solomon Wayu. 2020. Utilization of indigenous tree and shrub species as animal feed resources in south Tigray, north Ethiopia, and implication for sustainable livestock production, Amaz. Jour. of Plant Resear. 4(3):594-608. [CrossRef]
- Tesfay Atsbha, Anteneh Belayneh Desta, Tessema Zewdu. 2019. Woody species diversity, population structure, and regeneration status in the Gra-Kahsu natural vegetation, southern Tigrai of Ethiopia. Heliyon, 5 (2019) e01120. [CrossRef]
- Tesfay Atsbha. 2018. Socioeconomic implication of protecting natural vegetation: The case of Gra-Kahsu protecting natural vegetation In Southern Tigray, Northern Ethiopia, International Journal of Biodiversity and Conservation, 10(11), 486-496. [CrossRef]
- Teshome Gemechu, Teshome Soromessa and Ensermu Kelbessa. 2015. Structure and regeneration of Gendo Moist Montane forest, East Wellega Zone, Western Ethiopia. Journal of Environment and Earth Science, 5:15.
- Tewelde G. 2007. Study on identification and establishment floral calendar of honeyplants in Atakilty Kebele, Tigray, Ethiopia. School of Graduate Studies Thesis Degree. Addis Ababa University, Ethiopia. 72pag.
- Tiwari, G.P.K., Tadele, K., Aramde, F and Tiwari, S.C. 2010. Community structure and regeneration potential of ShorearobustaForest in sub-tropical Submontane Zone of Garhwal Himalaya, India. Nature and Science, 8:70-74.
- Tsegay G, Tessema ZK, Negasi S, Emiru B. 2019. Carbon sequestration and soil restoration potential of grazing lands under exclosure management in a semi-arid environment of northern Ethiopia. Ecol Evol 9:6468–6479. [CrossRef]
- Wolde M, Aynekulu E. 2011a. Enclosure land management for restoration of the soils in degraded communal grazing lands in northern Ethiopia. Land Degradation and Development, 24:528-538. [CrossRef]
- Wolde M, Veldkamp E, Mesfin T, Olschewski R. 2011c. Economic valuation of land restoration: The case of exclosures established on communal grazing lands in Tigrai, Ethiopia. Land degradation and development, 22:334-344. [CrossRef]
- Yayneshet Tesfay. 2011. Restoration of degraded semi-arid communal grazing land vegetation using the enclosure model. Mekelle University, Mekelle, Ethiopia. International Journal of Water Resources and Arid Environments, 1(5): 382-386.
- Yetimwork Gebremeskel, Berhan Tamir and Desalegn Begna. 2015. Honeybee production trend, potential and constraints in Eastern Zone of Tigray, Ethiopia Agric. Biol. J. N. Am., 6(1): 22-29. [CrossRef]
- Yirga G and Teferi M. 2010. Participatory Technology and Constraints Assessment to Improve the Livelihood of Beekeepers in Tigray Region, northern Ethiopia. MEJS 2: 76-92. [CrossRef]
- Zegeye H, Teketay D & Kelbessa E. 2011. Diversity and regeneration status of woody species in Tara Gedam and Abebaye forests, northwestern Ethiopia. Journal of Forestry Research 22(3): 315-328. [CrossRef]
| Species | Respondents | Weight | Index (%) | Rank | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||
| Becium grandiflorum | 7 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 94 | 15.36 | 1 |
| Leucas abyssinica | 3 | 4 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 87 | 14.22 | 2 |
| Cordia africana | 1 | 2 | 3 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 75 | 12.25 | 3 |
| Ziziphus spina | 2 | 2 | 1 | 0 | 1 | 2 | 1 | 0 | 1 | 0 | 68 | 11.11 | 4 |
| Eucalyptus camaldulensis | 0 | 0 | 2 | 3 | 1 | 1 | 0 | 0 | 1 | 2 | 52 | 8.50 | 6 |
| Carissa edulis | 0 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 0 | 1 | 52 | 8.50 | 6 |
| Euclea schimperi | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 2 | 1 | 2 | 47 | 7.68 | 8 |
| Olea africana | 1 | 0 | 2 | 0 | 0 | 1 | 2 | 2 | 1 | 1 | 48 | 7.84 | 7 |
| Schinus mole | 2 | 1 | 0 | 2 | 1 | 0 | 2 | 0 | 2 | 0 | 61 | 9.97 | 5 |
| Acacia abyssinica | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 2 | 3 | 28 | 4.58 | 9 |
| Scientific name | Family | Abundance | Frequency | Relative frequency | Density | Relative Density | |||||
| AE | CGL | AE | CGL | AE | CGL | AE | CGL | AE | CGL | ||
| Acacia abyssinica | Fabaceae | 58 | 32 | 44.44 | 41.67 | 2.99 | 5.59 | 90.63 | 80.00 | 4.41 | 7.24 |
| Acacia seyal | Fabaceae | 32 | 13 | 52.78 | 16.67 | 3.54 | 2.23 | 42.11 | 81.25 | 2.43 | 2.94 |
| Acacia tortilis | Fabaceae | 45 | 28 | 50.00 | 37.50 | 3.36 | 5.03 | 62.50 | 77.78 | 3.42 | 6.33 |
| Acacia asak | Fabaceae | 42 | 15 | 50.00 | 12.50 | 3.36 | 1.68 | 58.33 | 125.00 | 3.19 | 3.39 |
| Acacia etbaica | Fabaceae | 46 | 29 | 52.78 | 33.33 | 3.54 | 4.47 | 60.53 | 90.63 | 3.50 | 6.56 |
| Acokanthera schimperi | Apocynaceae | 19 | - | 19.44 | - | 1.31 | - | 67.86 | - | 1.44 | - |
| Astragalus atropilosulus | Fabaceae | 38 | 8 | 30.56 | 25.00 | 2.05 | 3.35 | 86.36 | 33.33 | 2.89 | 1.81 |
| Balanites aegpytiaca | Balanitaceae | 25 | 5 | 47.22 | 8.33 | 3.17 | 1.12 | 36.76 | 62.50 | 1.90 | 1.13 |
| Becium grandiflorum | Lamiaceae | 6 | 3 | 8.33 | 33.33 | 0.56 | 4.47 | 50.00 | 9.38 | 0.46 | 0.68 |
| Calpurnia aurea | Fabaceae | 27 | 4 | 33.33 | 12.50 | 2.24 | 1.68 | 56.25 | 33.33 | 2.05 | 0.90 |
| Carissa edulis | Apocynaceae | 67 | 28 | 63.89 | 50.00 | 4.29 | 6.70 | 72.83 | 58.33 | 5.09 | 6.33 |
| Cassipourea malosana | Rhizophoracea | 38 | - | 55.56 | - | 3.73 | - | 47.50 | - | 2.89 | - |
| Celtis africana | Ulmaceae | 7 | - | 27.78 | - | 1.87 | - | 17.50 | - | 0.53 | - |
| Conyza hypoleuca | Asteraceae | 17 | 12 | 22.22 | 50.00 | 1.49 | 6.70 | 53.13 | 58.33 | 1.29 | 6.33 |
| Cordia Africana | Boraginaceae | 7 | - | 22.22 | - | 1.49 | - | 21.88 | - | 0.53 | - |
| Dodonoae angustifoha | Sapindaceae | 51 | - | 69.44 | - | 4.66 | - | 51.00 | - | 3.88 | - |
| Dovyalis abyssinica | Flacourtiaceae | 15 | 6 | 38.89 | 16.67 | 2.61 | 2.23 | 26.79 | 37.50 | 1.14 | 1.36 |
| Eucalyptus camaldulensis | Myrtaceae | 72 | - | 47.22 | - | 3.17 | - | 105.88 | - | 5.47 | - |
| Eucalyptus globulus | Myrtaceae | 17 | - | 19.44 | - | 1.31 | - | 60.71 | - | 1.29 | - |
| Euclea racemosa | Ebenaceae | 132 | 39 | 80.56 | 62.50 | 5.41 | 8.38 | 113.79 | 65.00 | 10.03 | 8.82 |
| Euphorbia tirucalli | Euphorbiaceae | 31 | 6 | 55.56 | 8.33 | 3.73 | 1.12 | 38.75 | 75.00 | 2.36 | 1.36 |
| Ficus sycomorus | Moraceae | 15 | 13 | 13.89 | 16.67 | 0.93 | 2.23 | 75.00 | 81.25 | 1.14 | 2.94 |
| Gomphocarpus fruticosus | Asclepiadaceae | 28 | 19 | 33.33 | 8.33 | 2.24 | 1.12 | 58.33 | 237.50 | 2.13 | 4.30 |
| Grewia ferruginea | Tiliaceae | 9 | 14 | 8.33 | 12.50 | 0.56 | 1.68 | 75.00 | 116.67 | 0.68 | 3.17 |
| Grewia mollis juss | Tiliaceae | 26 | 20 | 33.33 | 45.83 | 2.24 | 6.15 | 54.17 | 45.45 | 1.98 | 4.52 |
| Lucas Abissinica | Lamiaceae | 25 | 15 | 19.44 | 25.00 | 1.31 | 3.35 | 89.29 | 62.50 | 1.90 | 3.39 |
| Maytenus undata | Celastraceae | 24 | 18 | 41.67 | 16.67 | 2.80 | 2.23 | 40.00 | 112.50 | 1.82 | 4.07 |
| Myrsine Africana | Myricinaceae | 16 | 3 | 19.44 | 8.33 | 1.31 | 1.12 | 57.14 | 37.50 | 1.22 | 0.68 |
| Olea africana | Oleaceae | 12 | 3 | 19.44 | 8.33 | 1.31 | 1.12 | 42.86 | 37.50 | 0.91 | 0.68 |
| Osyris quadripartite | Santalaceae | 18 | 17 | 25.00 | 12.50 | 1.68 | 1.68 | 50.00 | 141.67 | 1.37 | 3.85 |
| Otostegia integrifolia | Lamiaceae | 32 | 9 | 30.56 | 29.17 | 2.05 | 3.91 | 72.73 | 32.14 | 2.43 | 2.04 |
| Pittosporum viridiflorum | Pittosporaceae | 16 | 4 | 25.00 | 33.33 | 1.68 | 4.47 | 44.44 | 12.50 | 1.22 | 0.90 |
| Psydrax schimperiana | Rubiaceae | 34 | 8 | 30.56 | 16.67 | 2.05 | 2.23 | 77.27 | 50.00 | 2.58 | 1.81 |
| Ptrollobium stellatum | Fabaceae | 50 | 18 | 16.67 | 29.17 | 1.12 | 3.91 | 208.33 | 64.29 | 3.80 | 4.07 |
| Rhus glutinosa | Anacardiaceae | 32 | 19 | 44.44 | 29.17 | 2.99 | 3.91 | 50.00 | 67.86 | 2.43 | 4.30 |
| Rhus natalensis | Anacardiaceae | 45 | 7 | 52.78 | 12.50 | 3.54 | 1.68 | 59.21 | 58.33 | 3.42 | 1.58 |
| Rumex nervosus | Polygonaceae | 5 | 3 | 8.33 | 8.33 | 0.56 | 1.12 | 41.67 | 37.50 | 0.38 | 0.68 |
| Sageretia thea | Rhamnaceae | 43 | 7 | 44.44 | 20.83 | 2.99 | 2.79 | 67.19 | 35.00 | 3.27 | 1.58 |
| Shinus molle | Anacardiaceae | 5 | - | 5.56 | - | 0.37 | - | 62.50 | - | 0.38 | - |
| Vernonia amygdalina | Asteraceae | 42 | - | 33.33 | - | 2.24 | - | 87.50 | - | 3.19 | - |
| Zizyphm spina christi | Rhamnaceae | 13 | 13 | 36.11 | 29.17 | 2.43 | 3.91 | 25.00 | 46.43 | 0.99 | 2.94 |
| Zizyphus mucronata | Rhamnaceae | 34 | 4 | 55.56 | 12.50 | 3.73 | 168 | 42.50 | 33.33 | 2.58 | 0.90 |
| 1316 | 442 | ||||||||||
| Land use system | Maximum | Minimum | Mean | Standard deviation | t-Statistic | P value | |
| Diversity index | AE | 2.83 | 0.64 | 2.12a | 0.51 | 4.63 | <0.001 |
| CGL | 2.19 | 0.81 | 1.56b | 0.38 | |||
| Species richness | AE | 20 | 2 | 11.11a | 4.83 | 4.88 | <0.001 |
| CGL | 12 | 3 | 5.96b | 2.24 | |||
| Evenness | AE | 0.99 | 0.85 | 0.93a | 0.03 | 2.72 | 0.009 |
| CGL | 0.98 | 0.73 | 0.91b | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





