Submitted:
12 December 2023
Posted:
13 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Degree of Esterification (DE)
2.3. Sample Preparation
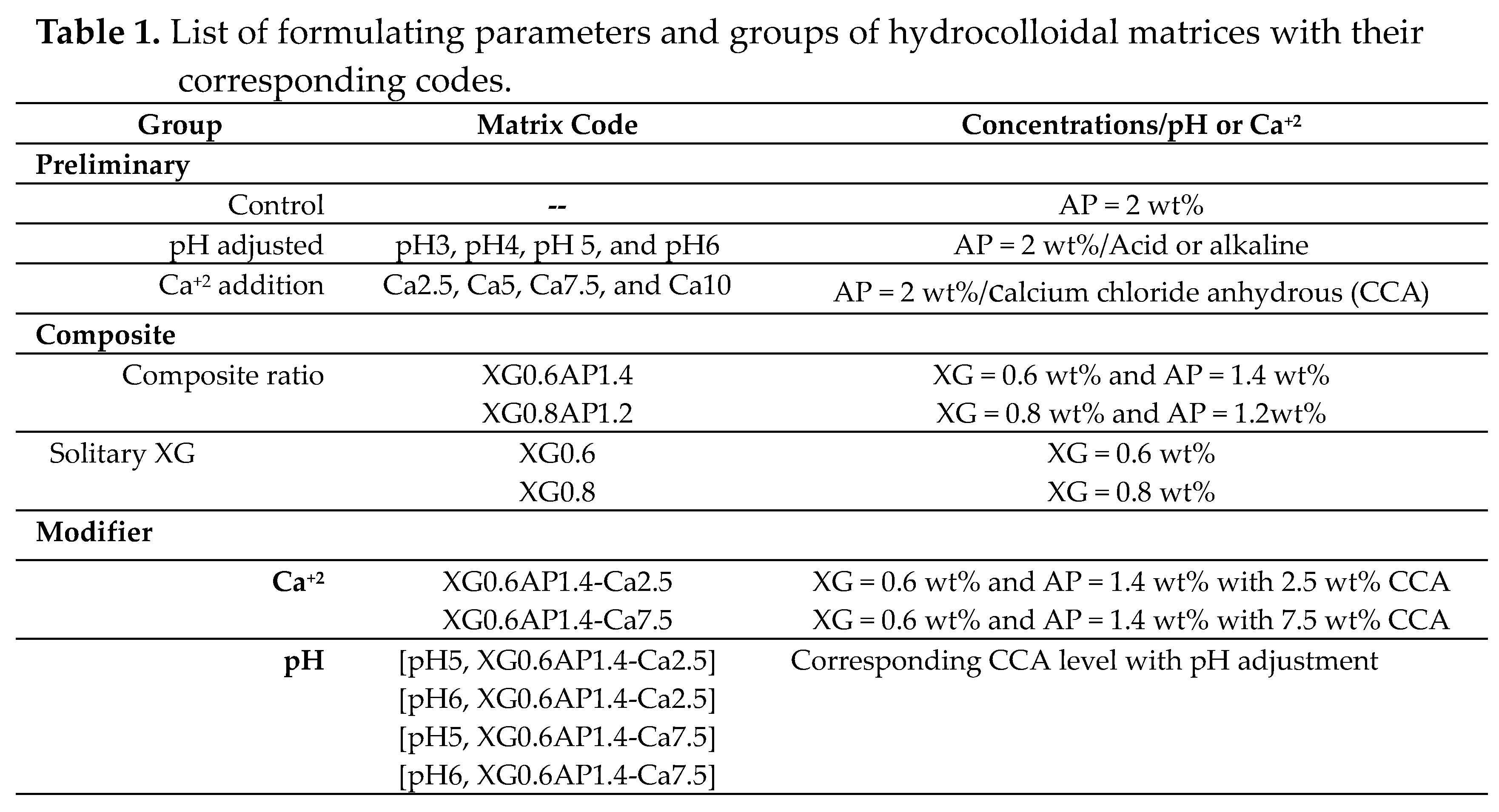
2.4. Thermal Processes
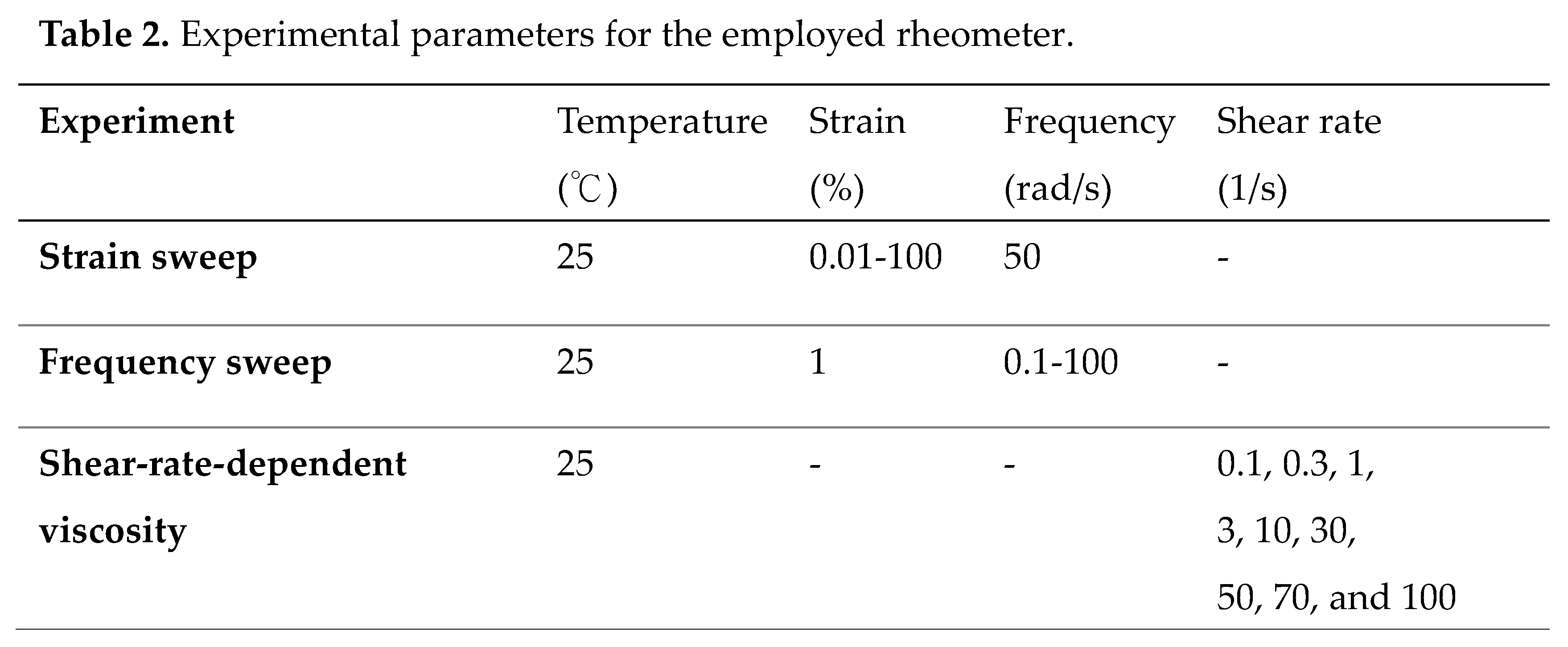
2.5. Rheological Measurements and Flow Behavior Characterization
2.6. Texture Profile Analysis (TPA)
2.7. Data Manipulation and Statistical Analysis
3. Results and Discussion
3.1. DE Evaluation of Apple Pectin Sample Powder
3.2. Thermal Stability Analysis of Thickened Composite Matrices (TCM)
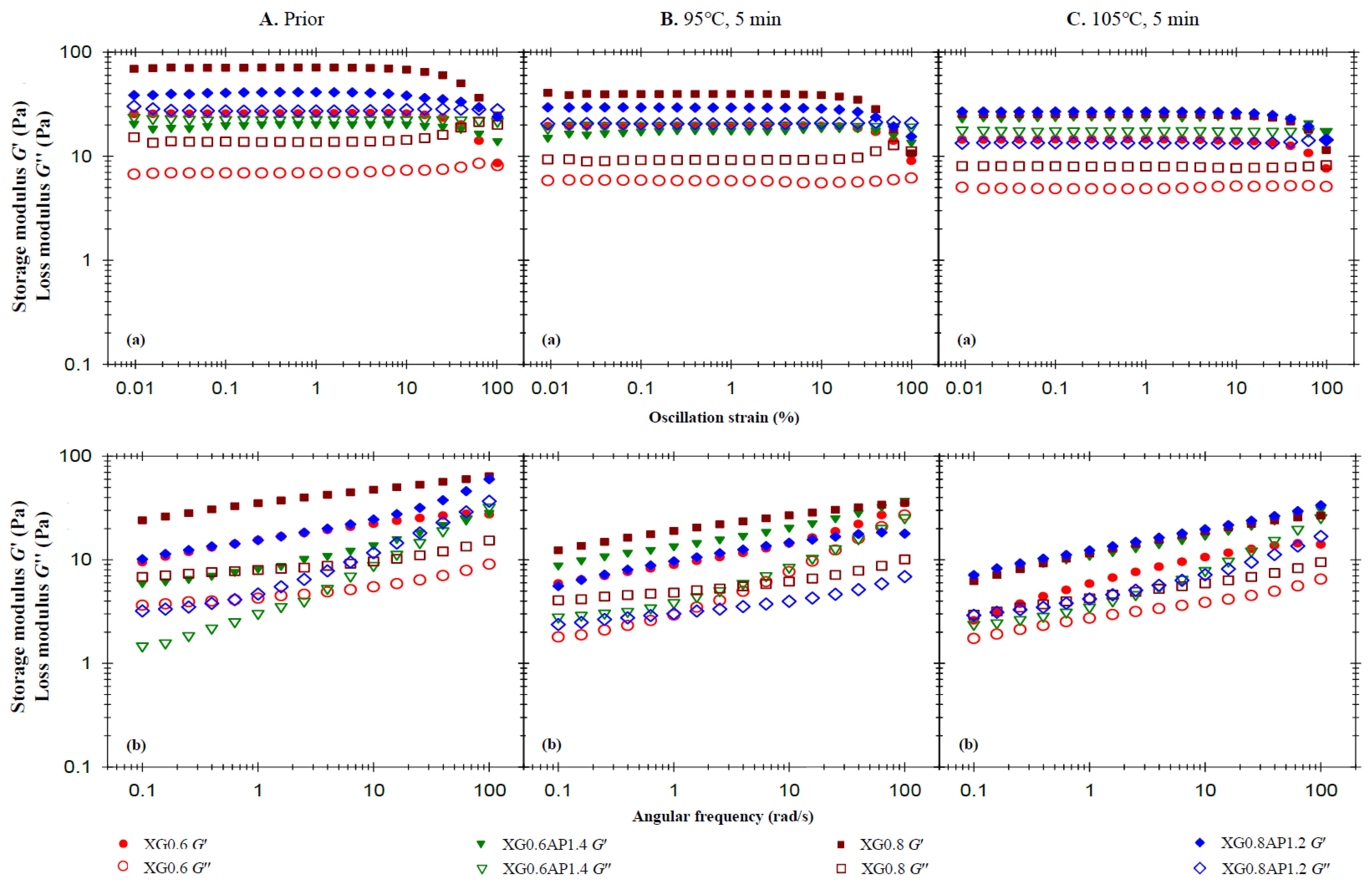
3.2.1. Effects of Xanthan Gum (XG) and High-Methoxy Apple Pectin (AP) Composite Ratio on Rheological Behavior
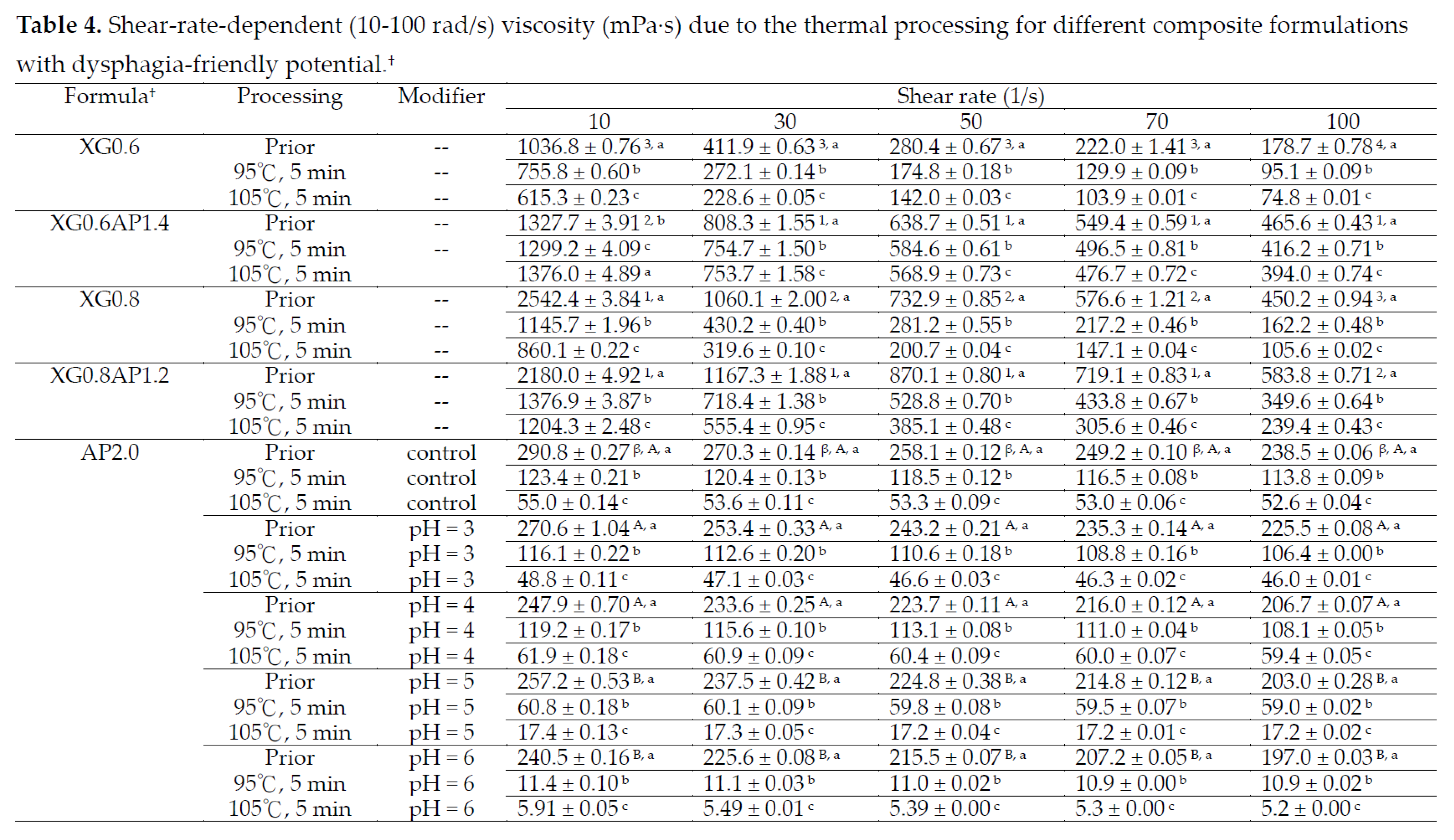
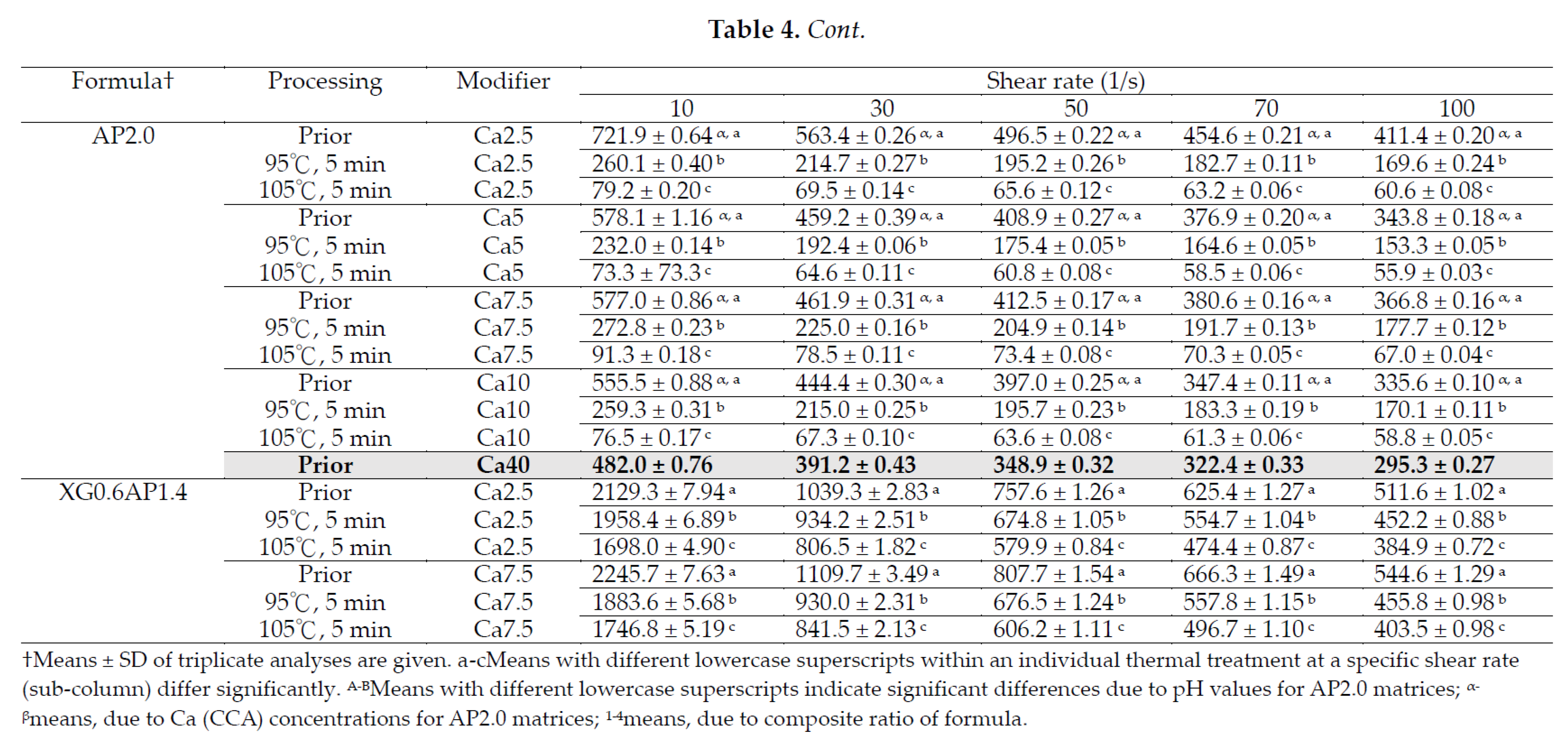
3.2.2. Modification Effects of pH and CCA on the Rheological Behavior of Thickened XG-AP Matrices
- Preliminary tests
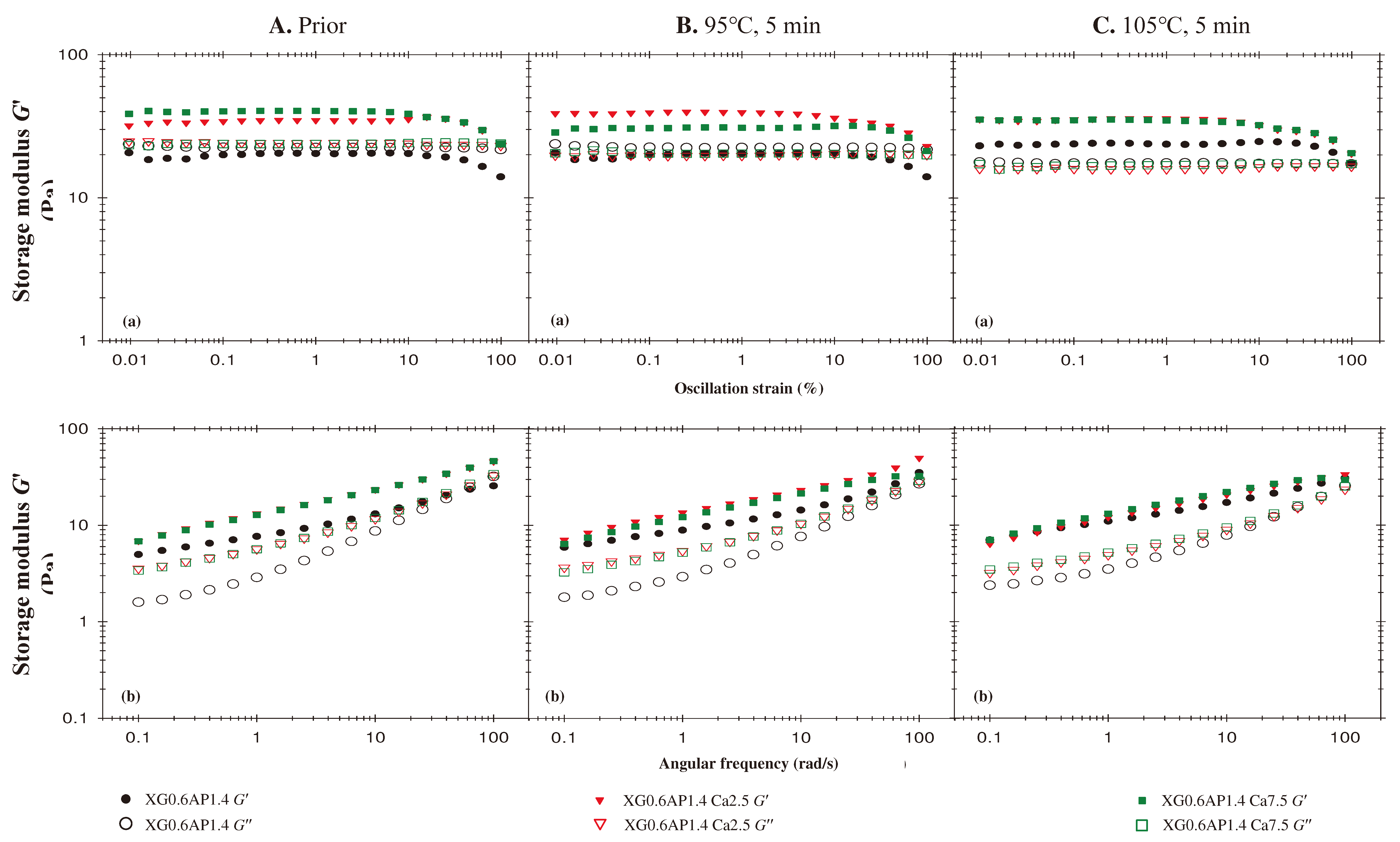
- Effects of CCA Concentration
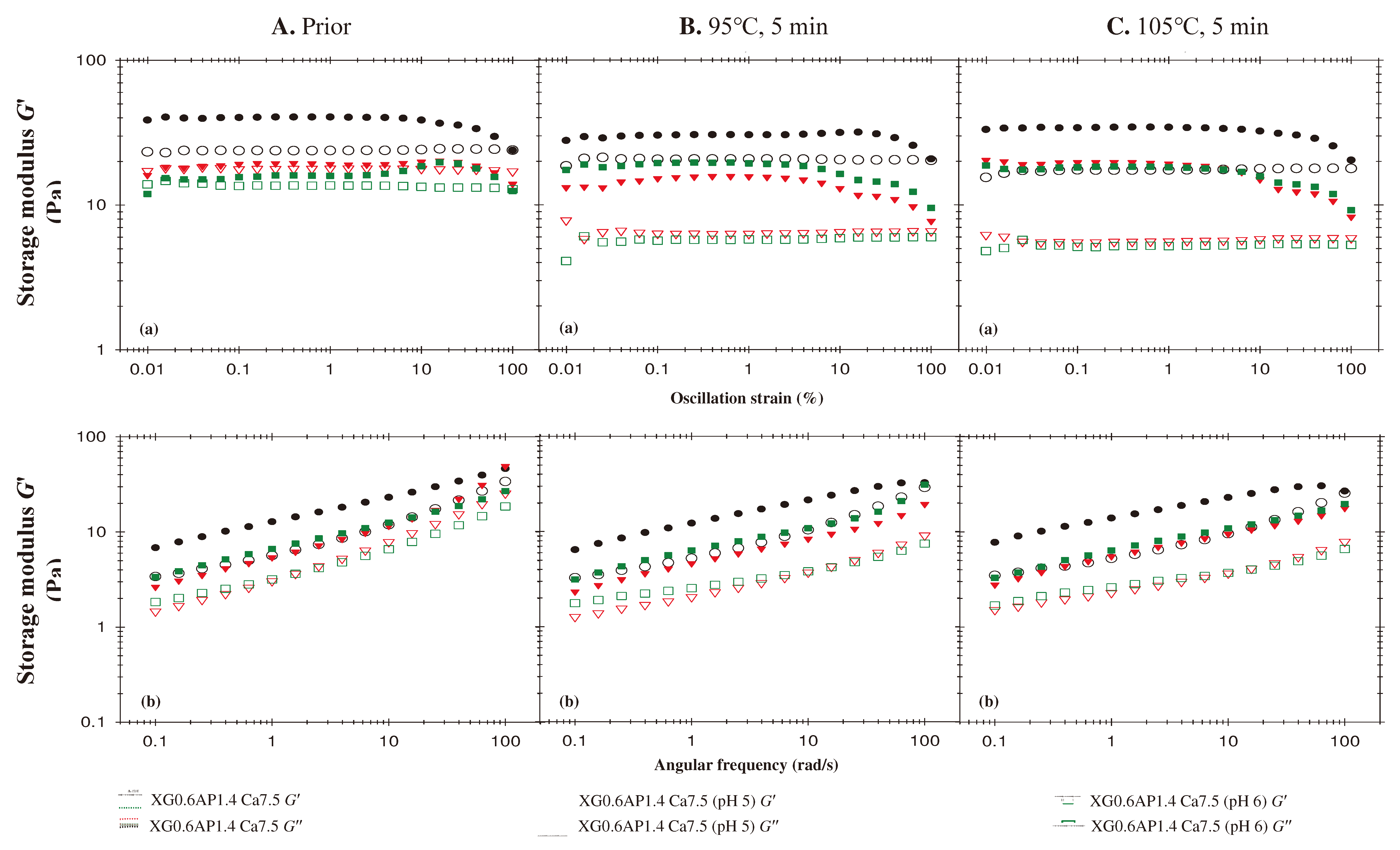
- Effects of pH
3.3. Summary of Rheological Behavior of Sample Matrices
3.4. Texture Analysis
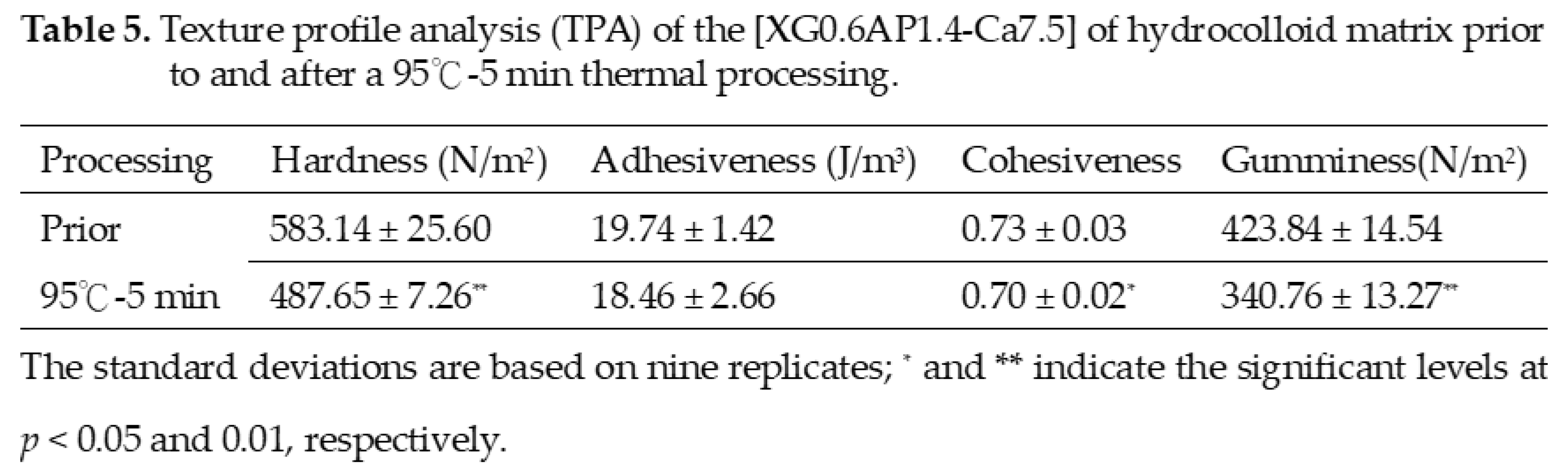
4. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Methacanon, P.; Gamonpilas, C.; Kongjaroen, A.; Buathongjan, C. Food polysaccharides and roles of rheology and tribology in the rational design of thickened liquids for oropharyngeal dysphagia: A review. Comprehensive Reviews in Food Science and Food Safety 2021, 20(4), 4101–19. [Google Scholar] [CrossRef]
- Dodds, W. J. The physiology of swallowing. Dysphagia 1989, 3(4), 171–8. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Vaezi, M. F. Dysphagia in the elderly. Gastroenterology & hepatology 2013, 9(12), 784. [Google Scholar] [CrossRef]
- Hudson, H. M.; Daubert, C.R.; Mills, R. H. The interdependency of protein-energy malnutrition, aging, and dysphagia. Dysphagia 2000, 15(1), 31–8. [Google Scholar] [CrossRef]
- Newman, R.; Vilardell, N.; Clavé, P.; Speyer, R. Effect of bolus viscosity on the safety and efficacy of swallowing and the kinematics of the swallow response in patients with oropharyngeal dysphagia: white paper by the European Society for Swallowing Disorders (ESSD). Dysphagia 2016, 31(2), 232–49. [Google Scholar] [CrossRef]
- Funami, T. Next target for food hydrocolloid studies: Texture design of foods using hydrocolloid technology. Food Hydrocolloids 2011, 25(8), 1904–14. [Google Scholar] [CrossRef]
- Cichero, J. A.; Lam, P.; Steele, C. M.; Hanson, B.; Chen, J.; Dantas, R. O.; et al. Development of international terminology and definitions for texture-modified foods and thickened fluids used in dysphagia management: the IDDSI framework. Dysphagia 2017, 32(2), 293–314. [Google Scholar] [CrossRef] [PubMed]
- Hadde, E. K.; Prakash, S.; Chen, W.; Chen, J. Instrumental texture assessment of IDDSI texture levels for dysphagia management. Part 1: Thickened fluids. Journal of Texture Studies 2022, 53(5), 609–16. [Google Scholar] [CrossRef]
- National Dysphagia Diet Task Force. In National Dysphagia Diet:Standardization for Optimal Care; American Dietetic Association: Chicago, IL, USA, 2002.
- The dysphagia diet committee of the Japanese Society of Dysphagia Rehabilitation. The Japanese Dysphagia diet 2013. Jpn J Dysphagia Rehabil. 2013, 17, 255–67. (In Japanses)
- Watanabe, E.; Yamagata, Y.; Fujitani. J.; Fujishima, I.; Takahashi, K.; Uyama, R.; et al. The criteria of thickened liquid for dysphagia management in Japan. Dysphagia 2018, 33(1), 26–32. [Google Scholar] [CrossRef]
- 12. Dietitians Association of Australia, and Speech Pathology Association of Australia Limited. Texture-modified foods and thickened fluids as used for individuals with dysphagia: Australian standardised labels and definitions. Nutrition & Dietetics 2007, 64, S53–S76. [CrossRef]
- Steele, C. M.; Alsanei, W. A. ; Ayanikalath. S.; Barbon, C. E. A.; Chen, J.; Cichero, J. A. Y.; et al., The Influence of Food Texture and Liquid Consistency Modification on Swallowing Physiology and Function: A Systematic Review. Dysphagia 2015, 30(1), 2–26. [Google Scholar] [CrossRef]
- Vickers, Z.; Damodhar, H.; Grummer, C.; Mendenhall, H.; Banaszynski, K.; Hartel, R.; et al. Relationships among rheological, sensory texture, and swallowing pressure measurements of hydrocolloid-thickened fluids. Dysphagia 2015, 30(6), 702–13. [Google Scholar] [CrossRef]
- Funami, T.; Ishihara, S.; Nakauma, M.; Kohyama, K.; Nishinari, K. Texture design for products using food hydrocolloids. Food Hydrocolloids 2012, 26(2), 412–20. [Google Scholar] [CrossRef]
- Garcia, J. M.; Chambers, E.; Matta, Z; Clark, M. Viscosity measurements of nectar-and honey-thick liquids: product, liquid, and time comparisons. Dysphagia 2005, 20(4), 325–35. [Google Scholar] [CrossRef] [PubMed]
- Sopade, P.; Halley, P.; Cichero, J.; Ward, L. Rheological characterisation of food thickeners marketed in Australia in various media for the management of dysphagia. I: Water and cordial. Journal of Food Engineering 2007, 79(1), 69–82. [Google Scholar] [CrossRef]
- Sopade, P.; Halley, P.; Cichero, J.; Ward, L.; Hui, L.; Teo, K. Rheological characterisation of food thickeners marketed in Australia in various media for the management of dysphagia. II. Milk as a dispersing medium. Journal of Food Engineering 2008, 84(4), 553–62. [Google Scholar] [CrossRef]
- Sopade, P.; Halley, P.; Cichero, J.; Ward, L.; Liu, J.; Varliveli, S. Rheological characterization of food thickeners marketed in Australia in various media for the management of dysphagia. III. Fruit juice as a dispersing medium. Journal of Food Engineering 2008, 86(4), 604–15. [Google Scholar] [CrossRef]
- Hadde, E. K.; Nicholson, T. M.; Cichero, J. A. Y. Rheological characterisation of thickened fluids under different temperature, pH and fat contents. Nutrition & Food Science 2015, 45(2), 270–85. [Google Scholar] [CrossRef]
- Mackley, M.; Tock, C.; Anthony, R.; Butler, S.; Chapman, G.; Vadillo, D. The rheology and processing behavior of starch and gum-based dysphagia thickeners. Journal of Rheology 2013, 57(6), 1533–53. [Google Scholar] [CrossRef]
- Payne, C.; Methven, L.; Fairfield, C.; Gosney, M.; Bell, A. E. Variability of starch-based thickened drinks for patients with dysphagia in the hospital setting. Journal of texture studies 2012, 43(2), 95–105. [Google Scholar] [CrossRef]
- Yang, H.-W.; Dai, H.-D.; Huang, W.-C.; Sombatngamwilai, T. Formulations of dysphagia-friendly food matrices with calorie-dense starchy thickeners and their stability assessments. Journal of Food Measurement and Characterization 2020, 14(6), 3089–102. [Google Scholar] [CrossRef]
- Hanson, B.; O’Leary, M. T.; Smith, C. H. The effect of saliva on the viscosity of thickened drinks. Dysphagia 2012, 27(1), 10–19. [Google Scholar] [CrossRef]
- Rofes, L.; Arreola, V.; Mukherjee, R.; Swanson, J.; Clavé, P. The effects of a xanthan gum-based thickener on the swallowing function of patients with dysphagia. Alimentary pharmacology & therapeutics 2014, 39(10), 1169–79. [Google Scholar] [CrossRef]
- Marik, P. E.; Kaplan, D. Aspiration pneumonia and dysphagia in the elderly. Chest 2003, 124(1), 328–36. [Google Scholar] [CrossRef]
- Petri, D. F. Xanthan gum: A versatile biopolymer for biomedical and technological applications. Journal of Applied Polymer Science 2015, 132(23), 42035. [Google Scholar] [CrossRef]
- Yoon, S.-N.; Yoo, B. Rheological behaviors of thickened infant formula prepared with xanthan gum-based food thickeners for dysphagic infants. Dysphagia 2017, 32(3), 454–62. [Google Scholar] [CrossRef]
- Ortega, O.; Bolívar-Prados, M.; Arreola, V.; Nascimento, W. V.; Tomsen, N.; Gallegos, C.; Brito-de La Fuente, E.; Clavé, P. Therapeutic effect, rheological properties and α-amylase resistance of a new mixed starch and xanthan gum thickener on four different phenotypes of patients with oropharyngeal dysphagia. Nutrients 2020, 12(6), 1873. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lin, Y. J. P. Effect of thermal processing on flow properties and stability of thickened fluid matrices formulated by tapioca starch, hydroxyl distarch phosphate (E-1442), and xanthan gum associating dysphagia-friendly potential. Polymers 2021, 13(1), 162. [Google Scholar] [CrossRef] [PubMed]
- Alexandri, M.; Kachrimanidou, V.; Papapostolou, H.; Papadaki, A.; Kopsahelis, N. Sustainable Food Systems: The Case of Functional Compounds towards the Development of Clean Label Food Products. Foods 2022, 11(18), 2796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Waterhouse, G. I.; Xu, F.; He, Z.; Du, Y.; Lian, Y.; et al. Recent advances in utilization of pectins in biomedical applications: a review focusing on molecular structure-directing health-promoting properties. Critical Reviews in Food Science and Nutrition 2021, 63(19), 1–34. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhao, C.; Feng, L.; Han, Y.; Du, H.; Xiao, H.; et al. Pectins from fruits: Relationships between extraction methods, structural characteristics, and functional properties. Trends in Food Science & Technology 2021, 110, 39–54. [Google Scholar] [CrossRef]
- Thibault, J. -F.; Ralet, M, -C., Physico-chemical properties of pectins in the cell walls and after extraction. Advances in pectin and pectinase research: Springer; 2003. p. 91-105.
- Yang, H.; Tsai, C. -C.; Jiang, J. -S.; Hua, C. -C., Rheological and textural properties of apple pectin-based composite formula with xanthan gum modification for preparation of thickened matrices with dysphagia-friendly potential. Polymers 2021, 13(6), 873. [Google Scholar] [CrossRef] [PubMed]
- Wicker, L.; Kim, Y.; Kim, M. -J.; Thirkield, B.; Lin, Z.; Jung, J., Pectin as a bioactive polysaccharide–Extracting tailored function from less. Food Hydrocolloids 2014, 42, 251–9. [Google Scholar] [CrossRef]
- Makarova, E.; Górnaś, P.; Konrade, I.; Tirzite, D.; Cirule, H.; Gulbe, A.; et al. Acute anti-hyperglycaemic effects of an unripe apple preparation containing phlorizin in healthy volunteers: a preliminary study. Journal of the Science of Food and Agriculture 2015, 95(3), 560–8. [Google Scholar] [CrossRef]
- Gunness, P.; Gidley, M. J. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food & Function 2010, 1(2), 149–55. [Google Scholar] [CrossRef]
- Gómez, B.; Gullon, B.; Remoroza, C.; Schols, H. A.; Parajo, J, C. ; Alonso, J. L., Purification, characterization, and prebiotic properties of pectic oligosaccharides from orange peel wastes. Journal of Agricultural and Food Chemistry 2014, 62(40), 9769–82. [Google Scholar] [CrossRef] [PubMed]
- Gómez, B.; Gullón, B.; Yáñez, R.; Schols, H.; Alonso, J. L. Prebiotic potential of pectins and pectic oligosaccharides derived from lemon peel wastes and sugar beet pulp: A comparative evaluation. Journal of Functional Foods. 2016, 20, 108–21. [Google Scholar] [CrossRef]
- Chung, W. S. F.; Meijerink, M.; Zeuner, B.; Holck, J.; Louis, P.; Meyer, A. S.; et al. Prebiotic potential of pectin and pectic oligosaccharides to promote anti-inflammatory commensal bacteria in the human colon. FEMS Microbiology Ecology 2017, 93(11), fix127. [Google Scholar] [CrossRef]
- Patel, J.; Maji, B.; Moorthy, N. H. N.; Maiti, S. Xanthan gum derivatives: Review of synthesis, properties and diverse applications. RSC Advances 2020, 10(45), 27103–36. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rao, K. M.; Han, S. S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydrate Polymers 2018, 180, 128–44. [Google Scholar] [CrossRef] [PubMed]
- Singthong, J.; Cui, S. W.; Ningsanond, S.; Goff, H. D. Structural characterization, degree of esterification and some gelling properties of Krueo Ma Noy (Cissampelos pareira) pectin. Carbohydrate polymers 2004, 58(4), 391–400. [Google Scholar] [CrossRef]
- Naji, S.; Razavi, S. M.; Karazhiyan, H. Effect of thermal treatments on functional properties of cress seed (Lepidium sativum) and xanthan gums: A comparative study. Food Hydrocolloids 2012, 28(1), 75–81. [Google Scholar] [CrossRef]
- Thakur, B. R.; Singh, R. K.; Handa, A. K.; Rao, M. Chemistry and uses of pectin—a review. Critical Reviews in Food Science & Nutrition 1997, 37(1), 47–73. [Google Scholar] [CrossRef]
- Fraeye, I.; De Roeck, A.; Duvetter, T.; Verlent, I.; Hendrickx, M.; Van Loey, A. Influence of pectin properties and processing conditions on thermal pectin degradation. Food Chemistry 2007, 105(2), 555–63. [Google Scholar] [CrossRef]
- Norziah, M.; Kong, S.; Abd Karim, A.; Seow, C. Pectin–sucrose–Ca2+ interactions: effects on rheological properties. Food Hydrocolloids 2001, 15(4), 491–8. [Google Scholar] [CrossRef]
- Löfgren, C.; Guillotin, S.; Evenbratt, H.; Schols, H.; Hermansson, A.-M. Effects of calcium, pH, and blockiness on kinetic rheological behavior and microstructure of HM pectin gels. Biomacromolecules 2005, 6(2), 646–652. [Google Scholar] [CrossRef]
- Neidhart, S.; Hannak, C.; Gierschner, K. Investigations of the influence of various cations on the rheological properties of high-esterified pectin gels. Progress in Biotechnology 1996, 14(2), 583–590. [Google Scholar] [CrossRef]
- Morris, E. R.; Powell, D. A.; Gidley, M. J.; Rees, D. A. Conformations and interactions of pectins: I. Polymorphism between gel and solid states of calcium polygalacturonate. Journal of Molecular Biology 1982, 155(4), 507–516. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, G.; Hong, Y.; Gu, Z.; Fang, F. Calcium cation triggers and accelerates the gelation of high methoxy pectin. Food Hydrocolloids 2013, 32(2), 228–234. [Google Scholar] [CrossRef]
- Cichero, J. A.; Steele, C.; Duivestein, J.; Clavé, P.; Chen, J.; Kayashita, J.; Dantas, R.; Lecko, C.; Speyer, R.; Lam, P. The need for international terminology and definitions for texture-modified foods and thickened liquids used in dysphagia management: foundations of a global initiative. Current physical medicine and rehabilitation reports 2013, 1(4), 280–91. [Google Scholar] [CrossRef] [PubMed]
- Burnip, E.; Cichero, J. A. Y., Head; Surgery, N. Review of the effect of amylase-resistant dysphagia products on swallowing safety. Current Opinion in Otolaryngology & Head and Neck Surgery 2022, 30(3), 169–176. [Google Scholar] [CrossRef]
- Leonard, R. J.; White, C.; McKenzie, S.; Belafsky, P. C. Effects of bolus rheology on aspiration in patients with dysphagia. Journal of the Academy of Nutrition and Dietetics 2014, 114(4), 590–4. [Google Scholar] [CrossRef] [PubMed]
- Hanson, B.; O’Leary, M. T.; Smith, C. H. J. D. The effect of saliva on the viscosity of thickened drinks. Dysphagia 2012, 27, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Bolivar-Prados, M.; Rofes, L.; Arreola, V.; Guida, S.; Nascimento, W. V.; Martin, A.; Vilardell, N.; Ortega Fernandez, O.; Ripken, D.; Lansink, M. J. N. Effect of a gum-based thickener on the safety of swallowing in patients with poststroke oropharyngeal dysphagia. Neurogastroenterology & Motility 2019, 31(11), e13695. [Google Scholar] [CrossRef]
- Diaz, J. V.; Anthon, G. E.; Barrett, D. M. Nonenzymatic degradation of citrus pectin and pectate during prolonged heating: effects of pH, temperature, and degree of methyl esterification. Journal of Agricultural and Food Chemistry 2007, 55(13), 5131–6. [Google Scholar] [CrossRef] [PubMed]
- Krall, S. M.; McFeeters, R. F. Pectin hydrolysis: effect of temperature, degree of methylation, pH, and calcium on hydrolysis rates. Journal of Agricultural and Food Chemistry 1998, 46(4), 1311–1315. [Google Scholar] [CrossRef]
- Kiss, J. β-Eliminative degradation of carbohydrates containing uronic acid residues. In Advances in carbohydrate chemistry and biochemistry, Elsevier: 1974; Vol. 29, pp 229-303. [CrossRef]
- Renard, C. M.; Thibault, J. F. Degradation of pectins in alkaline conditions: kinetics of demethylation. Carbohydrate Research 1996, 286, 139–50. [Google Scholar] [CrossRef]
- Albersheim, P.; Neukom, H.; Deuel, H. Splitting of pectin chain molecules in neutral solutions. Archives of Biochemistry and Biophysics 1960, 90(1), 46–51. [Google Scholar] [CrossRef] [PubMed]
- Sila, D. N.; Smout, C.; Elliot, F.; Loey, A. V.; Hendrickx, M. J. Non-enzymatic depolymerization of carrot pectin: toward a better understanding of carrot texture during thermal processing. Journal of Food Science 2006, 71(1), E1–E9. [Google Scholar] [CrossRef]
- Tiwari, P.; Joshi, A.; Varghese, E.; Thakur, M. Process standardization and storability of calcium fortified potato chips through vacuum impregnation. Journal of Food Science and Technology 2018, 55(8), 3221–31. [Google Scholar] [CrossRef]
- Márquez, A. L.; Wagner, J. R. Rheology of double (W/O/W) emulsions prepared with soybean milk and fortified with calcium. Journal of texture studies 2010, 41(5), 651–71. [Google Scholar] [CrossRef]
- Liu, X. T.; Zhang, H.; Wang, F.; Luo, J.; Guo, H. Y.; Ren, F. Z. Rheological and structural properties of differently acidified and renneted milk gels. Journal of Dairy Science 2014, 97(6), 3292–99. [Google Scholar] [CrossRef] [PubMed]
- Ross-Murphy, S. B, Small deformation measurements. In: Blanshard, J. M.V.; Mitchel, J. R. (Ed). Food Sturcture-Its Creation and Evaluation, Butterworths Ltd, London, pp 387-400.
- Kobori, T.; Matsumoto, A.; Sugiyama, S. PH-dependent interaction between sodium caseinate and xanthan gum. Carbohydrate Polymers. 2009, 75, 719–723. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




