1. Introduction
Neurodegenerative diseases significantly affect the health of the human population and place a burden on economies of countries around the world. Among these diseases, Parkinson's disease (PD) is the second most common disorder and is associated with the death of dopaminergic motor neurons. At present, the disease is not curable, however, the motor symptoms of PD are effectively treated with levodopa, the metabolic precursor of dopamine. To enhance the therapeutic action of levodopa, this drug is frequently combined with monoamine oxidase (MAO) B inhibitors, compounds that reduce the central metabolism of dopamine [
1]. While MAO-B inhibitors may allow for a reduction of the effective levodopa dosage, these compounds have also been studied as neuroprotective agents [
2], candidates for reducing neuroinflammation [
3], as well as compounds with potential value in the therapy of oncological diseases [
4]. Possible mechanisms by which MAO-B inhibitors may exert neuroprotective effects include enhancement of the levels of brain-derived neurotrophic factor (BDNF) [
5] and molecular adhesion of L1CAM (L1) nerve cells, which can also promote axonal regrowth and increase neuronal survival, synaptic plasticity and remyelination [
6]. Thus, the development of a new generation of neuroprotective agents that act by inhibiting MAO might have relevance in the future treatment of neurodegenerative disorders.
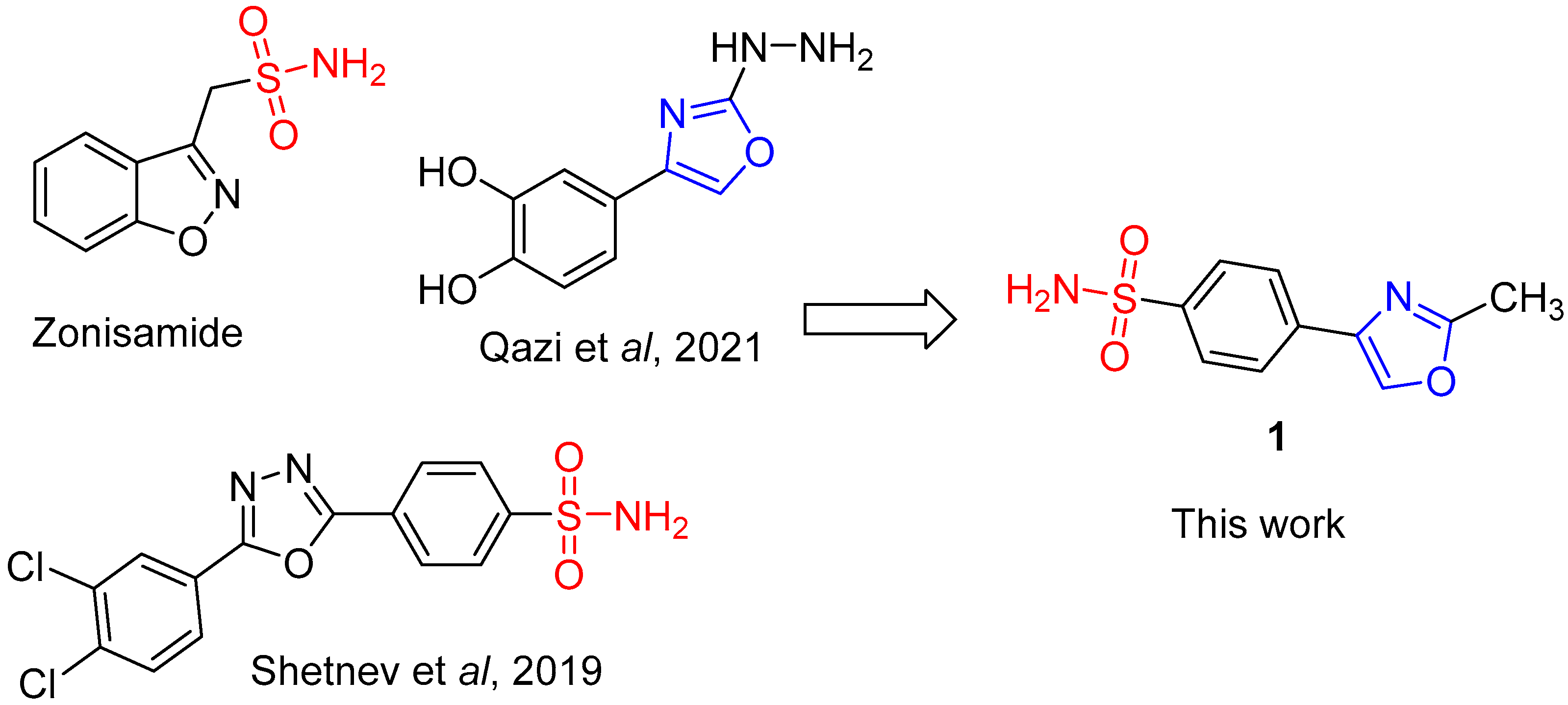
Recently, benzenesulfonamide compounds have been identified as potent and isoform-specific inhibitors of MAO-B, with come compounds exhibiting potencies in the nanomolar range. Such compounds might represent promising candidates for the future treatment of PD [
7,
8]. Also, it has been established that 1,3-oxazole derivatives also exhibit potent and specific inhibition of the MAO-B isoform [
9]. This work reports a successful attempt to combine both these structural features into a single molecule (
Figure 1).
Recently our research group reported a variety of new lead compounds for the development of isoform specific MAO-B inhibitors such as 2.1-benzisoxazoles [
10], indazoles [
11,
12] and pyrazolo[1,5-
a]quinoxalin-4-ones [
13]. Continuing the search of novel MAO inhibitors, in this work we used a simple convergent approach to synthesize a new 1,3-isoxazole compound substituted with a primary benzene sulfonamide functionality.
2. Results
2.1. Chemistry
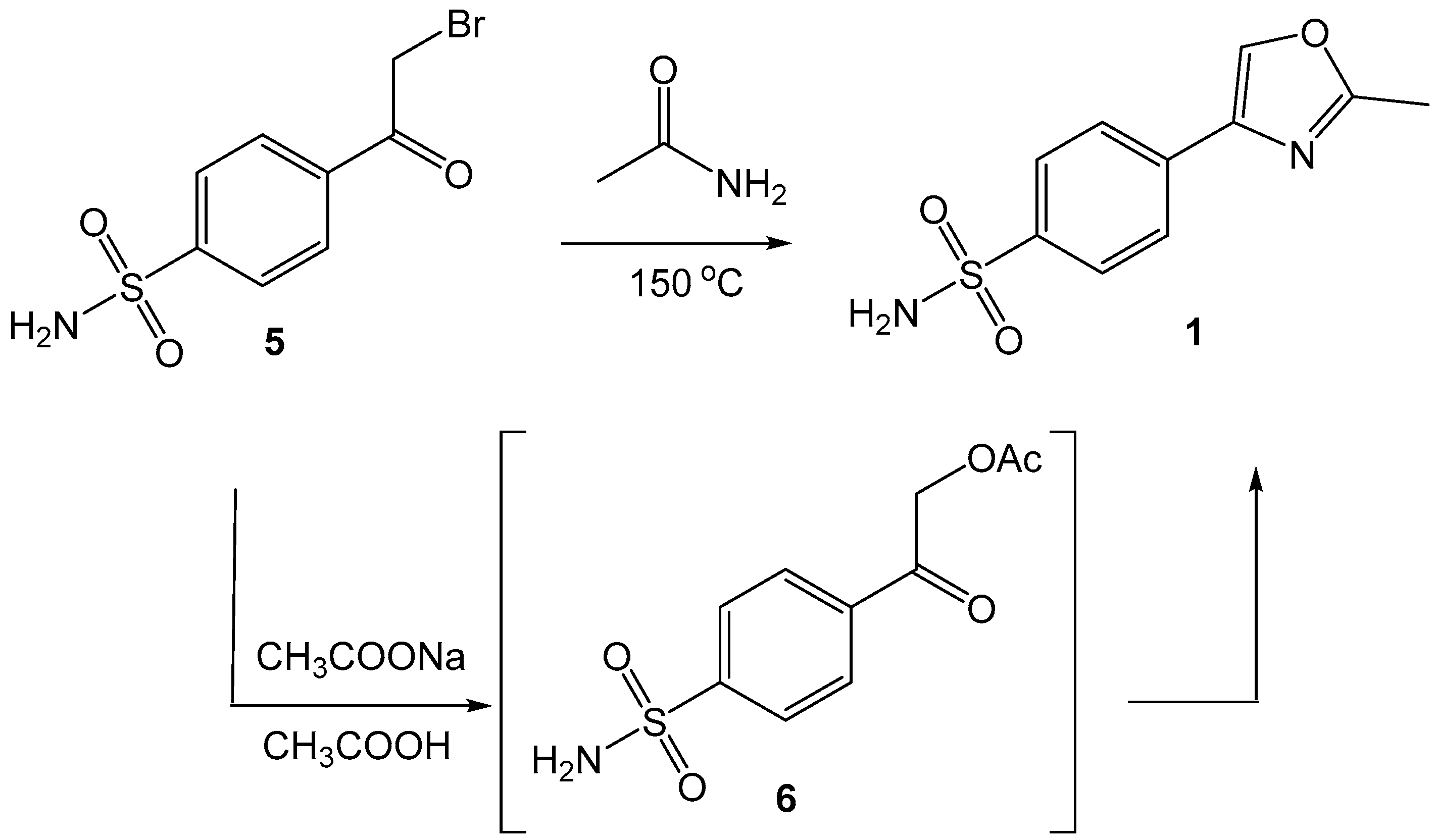
We examined the possibility of synthesizing the target oxazole
1 by the reaction of sulfonamide-containing phenacyl bromide
5 with acetamide or ammonium acetate.
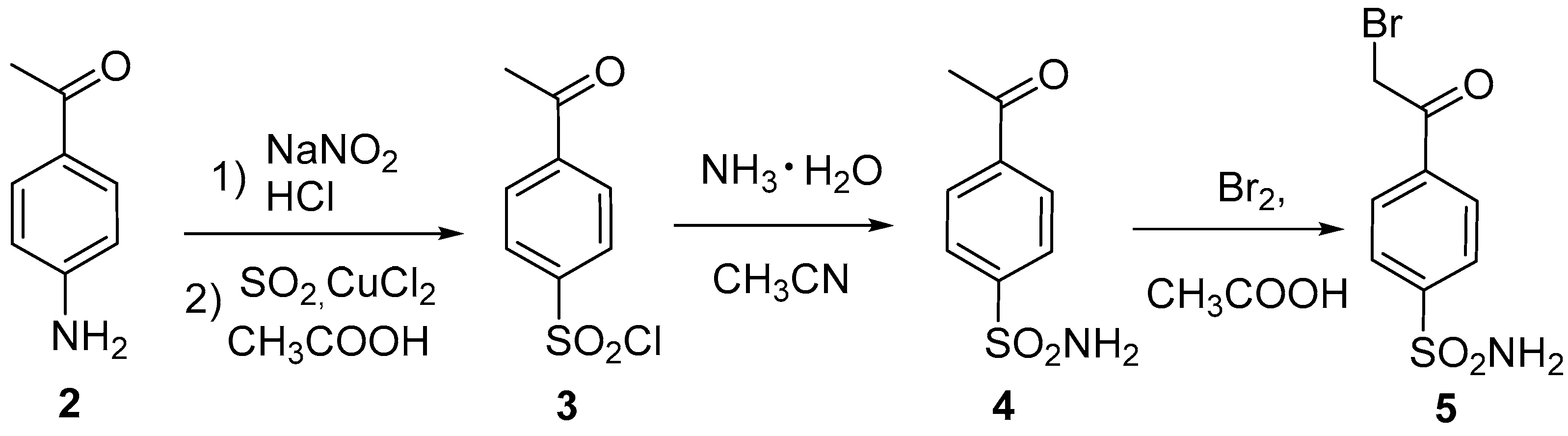
Scheme 1.
Synthesis of 4-(2-bromoacetyl)benzenesulfonamide (5).
Scheme 1.
Synthesis of 4-(2-bromoacetyl)benzenesulfonamide (5).
The starting material, phenacyl bromide
5, was obtained according to a well-known three-step procedure using 4-acetylaniline (
2) as key reagent [
14]. The best yield (63%) of the target oxazole
1 was obtained by fusing phenacyl bromide
5 with excess acetamide at 150 °С. When using ammonium acetate in acetic acid [
15], the target oxazole
1 was obtained in much lower yield (24%).
Scheme 1.
Synthesis of 4-(2-methyloxazol-4-yl)benzenesulfonamide (1).
Scheme 1.
Synthesis of 4-(2-methyloxazol-4-yl)benzenesulfonamide (1).
2.2. MAO inhibition
The MAO inhibition potency of 4-(2-methyloxazol-4-yl)benzenesulfonamide (
1) was evaluated using recombinant human MAO-A and MAO-B, following the protocol described in literature [
15]. The results of the MAO inhibition studies are presented in
Table 1. Compound
1 inhibited MAO-B with an IC
50 value of 3.47 μM, whereas weak inhibition of the MAO-A isoform was recorded.
3. Discussion
This study reports the MAO inhibition potency of 4-(2-methyloxazol-4-yl)benzenesulfonamide (1). This compound inhibited MAO-B with an IC50 value of 3.47 μM while the MAO-A isoform was inhibited with an IC50 value of 43.3 μM. The discovery of this active MAO-B inhibitor paves the way for the future discovery of MAO-B inhibitors among 1,3-isoxazole derivatives substituted with a primary benzene sulfonamide. Such compounds may find application in the treatment of neurodegenerative disorders such as PD.
4. Materials and Methods
4.1. General
All reagents and solvents were obtained from commercial sources (Aldrich, Merck, Aladdin) and were used without purification. Reactions were monitored by analytical thin layer chromatography (TLC) using Merck TLC sheets. Visualization of the developed sheets was performed by fluorescence quenching at 254 nm. 1H NMR and 13C NMR spectra were recorded on a Varian 400 Unity Plus instrument (400 MHz for 1H and 100 MHz for 13C, respectively). Chemical shifts (δ) are given in parts per million (ppm) and were referenced to the solvent signal for DMSO-d6 (2.50 ppm for proton and 39.52 ppm for carbon), while the coupling constants (J) are reported in hertz (Hz). Multiplicities are abbreviated as follows: s = singlet, d = doublet, dd = doublet of doublets, t = triplet, q = quartet, m = multiplet. Melting points were determined on an Electrothermal IA 9300 series digital melting point apparatus. Mass spectra were recorded on a microTOF spectrometer (ESI ionization).
4.2. Procedure for the preparation of 4-(2-bromoacetyl)benzenesulfonamide (5)
4-Acetylbenzenesulfonamide (4) (1g, 4.5 mmol, 1 equiv.) was dissolved in acetic acid (22 ml), while bromine (0.752 g, 4.7 mmol, 1.05 equiv) was mixed separately with acetic acid (5 ml). The first portion of bromine was added to the reaction mixture and the solution became colorless. The remaining bromine solution was subsequently added dropwise. The reaction mixture was stirred at 40 °C for 1 h, cooled to room temperature and the solvent was evaporated under reduced pressure. The resulting oil was diluted with cold water (25 ml) and the precipitate that formed was collected by filtration, washed with water (15 ml) and air-dried at 25 °C. 4-(2-Bromoacetyl)benzenesulfonamide (5) 1.22 g (98%) isolated as the beige crystalline solid. m.p. 154-155 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.17 (d, J = 8.2 Hz, 2H), 7.96 (d, J = 8.2 Hz, 2H), 7.57 (s, 2H), 4.98 (s, 2H) (Scobie et al, 2018).
4.3. Synthesis and characterization of 4-(2-methyloxazol-4-yl)benzenesulfonamide (1)
Acetamide (0,12 g, 0.002 mol, 3 equiv.) and 4-(2-bromoacetyl)benzenesulfonamide (5; 0,19 g, 0.00068 mol, 1 equiv.) were mixed together. The reaction mixture was melted, stirred at 150 °C for 20 min and subsequently diluted with cold water (30 ml). The resulting precipitate was collected by filtration, washed with water (10 ml) and air-dried at 50 °C. Yield 0.101 g, 63%, beige solid, mp 237-239 °C; 1H NMR (400 MHz, DMSO-d6) δ 8.61 (s, 1H), 7.93 (d, J = 8.4 Hz, 2H), 7.85 (d, J = 8.4 Hz, 2H), 7.38 (s, 2H), 2.48 (s, 3H); 13C NMR (101 MHz, DMSO) δ 162.58, 143.67, 139.18, 136.95, 134.91, 126.93, 125.92, 14.21; MS (ESI+): m/z [M+H]+. Anal. Calcd for C10H10N2O3: 239.0485. Found: 239.0489.
4.6. MAO inhibition studies
The measurement of IC
50 values for the inhibition of human MAO-A and MAO-B was carried out according to a previously reported protocol [
8,
16]. Recombinant human MAO-A and MAO-B were obtained from Sigma-Aldrich and fluorescence measurements were recorded with a SpectraMax iD3 multi-mode microplate reader (Molecular Devices). The measurement of MAO activity was based on the fluorescence signal generated when the substrate, kynuramine, is oxidized by the MAOs to yield 4-hydroxyquinoline.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Copies of 1H and 13C NMR spectra.
Author Contributions
Conceptualization of the study was done by Anton Shetnev; formal analysis and investigation - Julia Efimova and Anél Petzer; writing—original draft preparation was done by Anton Shetnev; writing—review and editing was done by Mikhail Korsakov and Jacobus P. Petzer. All authors have read and agreed to the published version of the manuscript.
Funding
The chemical section of this work was supported by Russian Science Foundation (project 22-13-20085). MAO inhibition studies were funded by the National Research Foundation of South Africa [grant specific unique reference numbers (UID) 137997 (JPP) and 132168 (AP)]. The Grantholders acknowledge that opinions, findings and conclusions or recommendations expressed in any publication generated by the NRF supported research are that of the authors, and that the NRF accepts no liability whatsoever in this regard.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no competing interests.
References
- Tan, Y.Y.; Jenner, P.; Chen, S.D. Monoamine Oxidase-B Inhibitors for the Treatment of Parkinson's Disease: Past, Present, and Future. J Parkinsons Dis. 2022;12(2):477-493. [CrossRef]
- Szökő, É.; Tábi, T.; Riederer, P.; Vécsei, L.; Magyar, K. Pharmacological aspects of the neuroprotective effects of irreversible MAO-B inhibitors, selegiline and rasagiline, in Parkinson’s disease. J Neural Transm. 2018, 125, 1735–1749. [Google Scholar] [CrossRef] [PubMed]
- Ostadkarampour, M.; Putnins, E.E. Monoamine Oxidase Inhibitors: A Review of Their Anti-Inflammatory Therapeutic Potential and Mechanisms of Action. Front Pharmacol. 2021, 12, 676239. [Google Scholar] [CrossRef] [PubMed]
- Zarmouh, N.O.; Messeha, S.S.; Mateeva, N.; Gangapuram, M.; Flowers, K.; Eyunni, S.V.K.; Zhang, W.; Redda, K.K.; Soliman, K.F.A. The Antiproliferative Effects of Flavonoid MAO Inhibitors on Prostate Cancer Cells. Molecules. 2020, 25(9), 2257. [Google Scholar] [CrossRef] [PubMed]
- Balu, D.T.; Hoshaw, B.A.; Malberg, J.E.; Rosenzweig-Lipson, S.; Schechter, L.E.; Lucki, I. Brain Res. 2008, 1211, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Sahu, S.; Schachner, M. Pharmacol Biochem Behav. 2018, 171, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Grover, N.D.; Limaye, R.P.; Gokhale, D.V.; Patil, T.R. Zonisamide: a review of the clinical and experimental evidence for its use in Parkinson's disease". Indian Journal of Pharmacology. 2013, 45(6), 547–55. [Google Scholar] [CrossRef] [PubMed]
- Shetnev, A.; Shlenev, R.; Efimova, J.; Ivanovskii, S.; Tarasov, A.; Petzer, A.; Petzer, J.P. 1,3,4-Oxadiazol-2-ylbenzenesulfonamides as privileged structures for the inhibition of monoamine oxidase B. Bioorg. Med. Chem. Lett. 2019, 29, 126677. [Google Scholar] [CrossRef] [PubMed]
- Qazi, S.U.; Naz, A.; Hameed, A.; Osra, F.A.; Jalil, S.; Iqbal, J.; Shah, S.A.; Mirza, A.Z. Semicarbazones, thiosemicarbazone, thiazole and oxazole analogues as monoamine oxidase inhibitors: Synthesis, characterization, biological evaluation, molecular docking, and kinetic studies. Bioorg Chem. 2021, 115, 105209. [Google Scholar] [CrossRef] [PubMed]
- Shetnev, A.; Kotov, A.; Kunichkina, A.; Proskurina, I.; Baykov, S.; Korsakov, M.; Petzer, A.; Petzer, J.P. Monoamine oxidase inhibition properties of 2,1-benzisoxazole derivatives. Mol Divers 2023. [Google Scholar] [CrossRef] [PubMed]
- Tzvetkov, N.T.; Antonov, L. Subnanomolar indazole-5-carboxamide inhibitors of monoamine oxidase B (MAO-B) continued: indications of iron binding, experimental evidence for optimised solubility and brain penetration. J. Enzyme Inhib. Med. Chem. 2017, 32, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Efimova, J.A.; Shetnev, A.A.; Baykov, S.V.; Petzer, A.; Petzer, J.P. 3-(3,4-Dichlorophenyl)-5-(1H-indol-5-yl)-1,2,4-oxadiazole. Molbank 2023, M1552. [Google Scholar]
- Panova, V.A.; Filimonov, S.I.; Chirkova, Z.V.; Kabanova, M.V.; Shetnev, A.A.; Korsakov, M.K.; Petzer, A.; Petzer, J.P.; Suponitsky, K.Y. Investigation of pyrazolo[1,5-a]quinoxalin-4-ones as novel monoamine oxidase inhibitors. Bioorg. Chem. 2021, 108, 104563. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.W.; Leadbetter, M.R.; Bell, N.; Koo-McCoy, S.; Carreras, C.W.; He, L.; Kohler, J.; Kozuka, K.; Labonté, E.D.; Navre, M.; Spencer, A.G.; Charmot, D. Discovery of tenapanor: A first-in-class minimally systemic inhibitor of intestinal na+/h+ exchanger isoform 3. ACS Med. Chem. Lett 2022, 13(7), 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Denney, D.B.; Pastor, S.D. Structures in solution of adducts of hexamethylphosphorus triamide and substituded benzils. Phosphorus and Sulfur and the Related Elements. 1983, 16(3), 239–246. [Google Scholar] [CrossRef]
- Mostert, S.; Petzer, A.; Petzer, J.P. Indanones as high-potency reversible inhibitors of monoamine oxidase. Chem. Med. Chem. 2015, 10, 862–873. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).