Submitted:
11 December 2023
Posted:
12 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
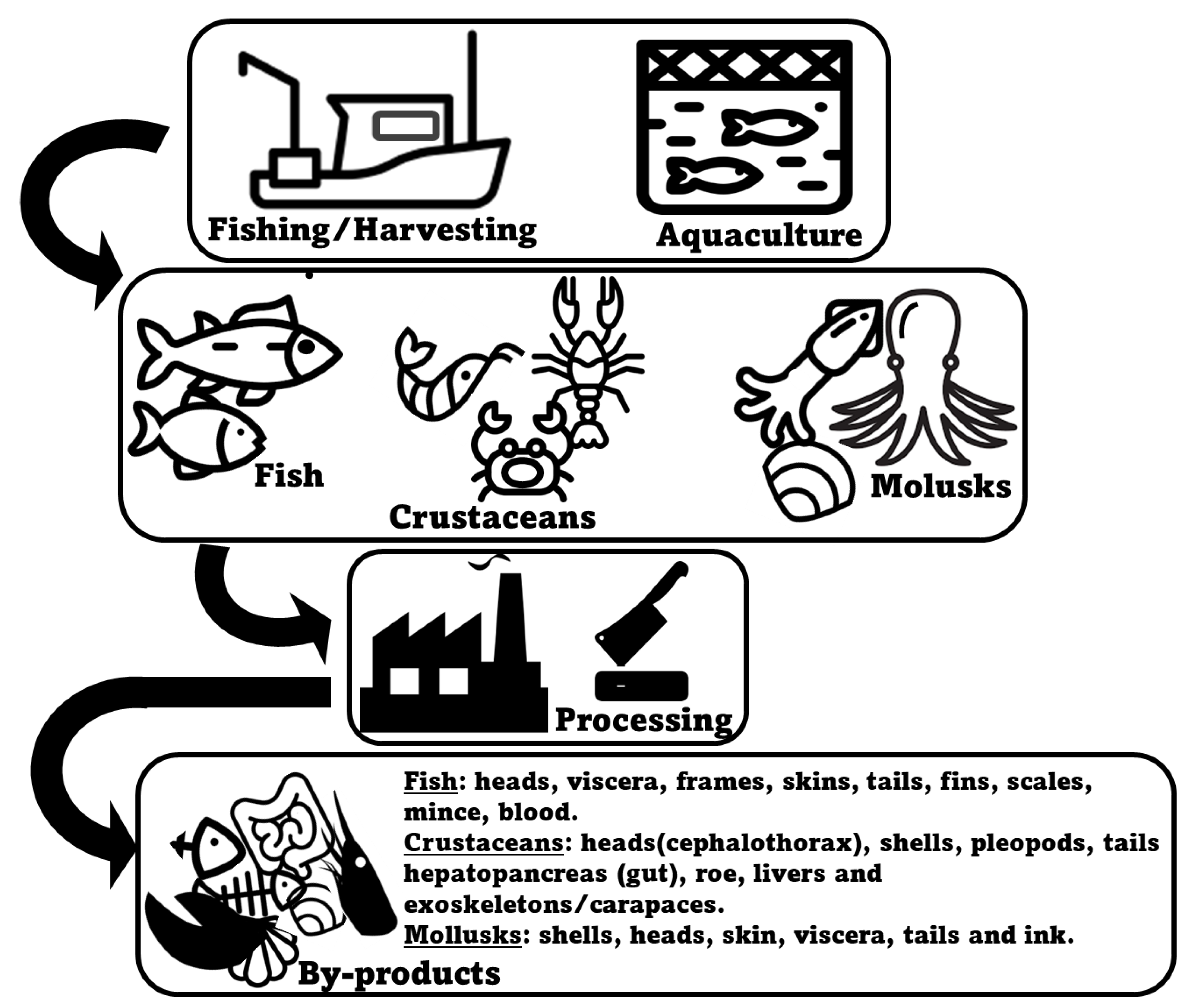
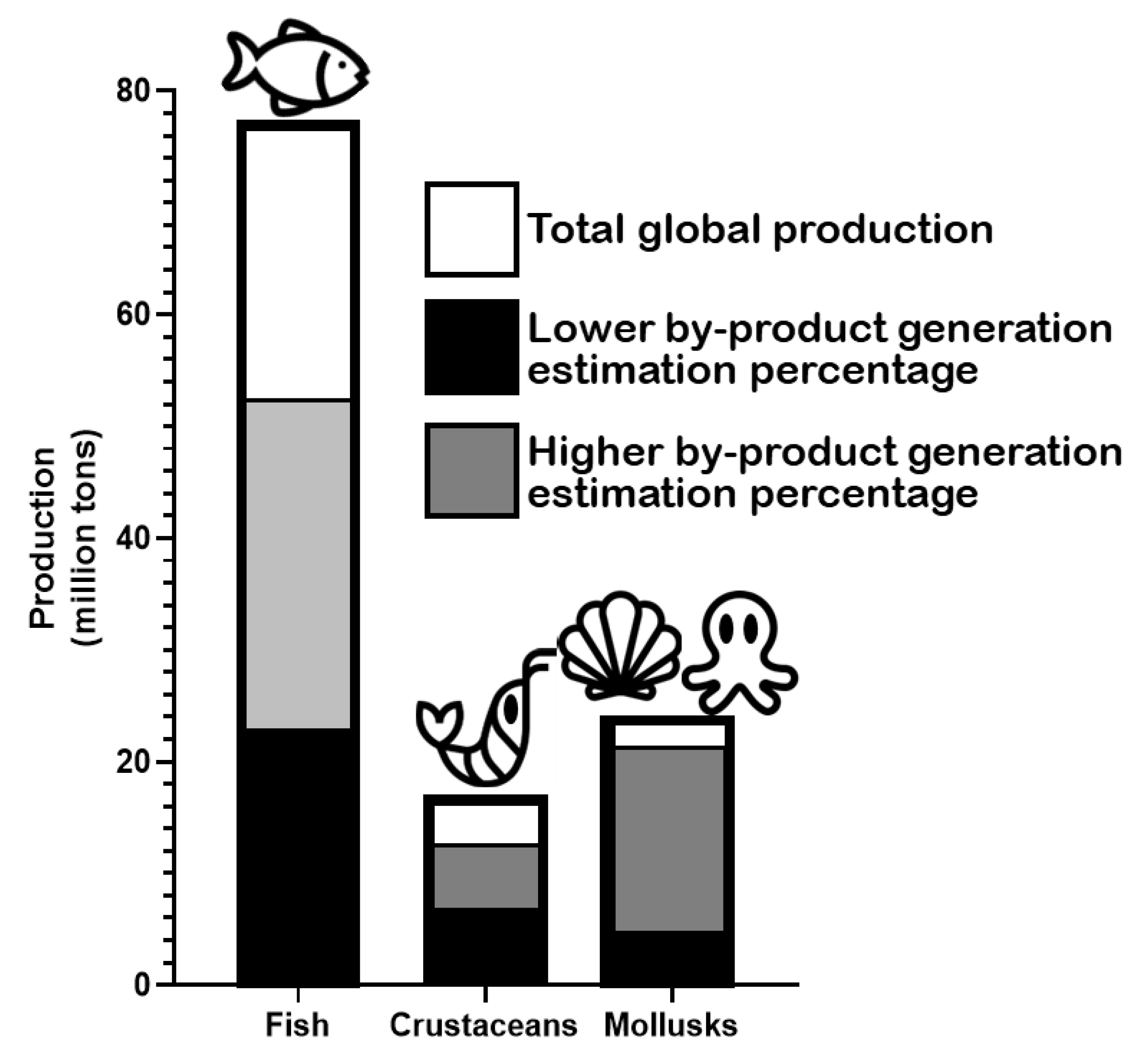
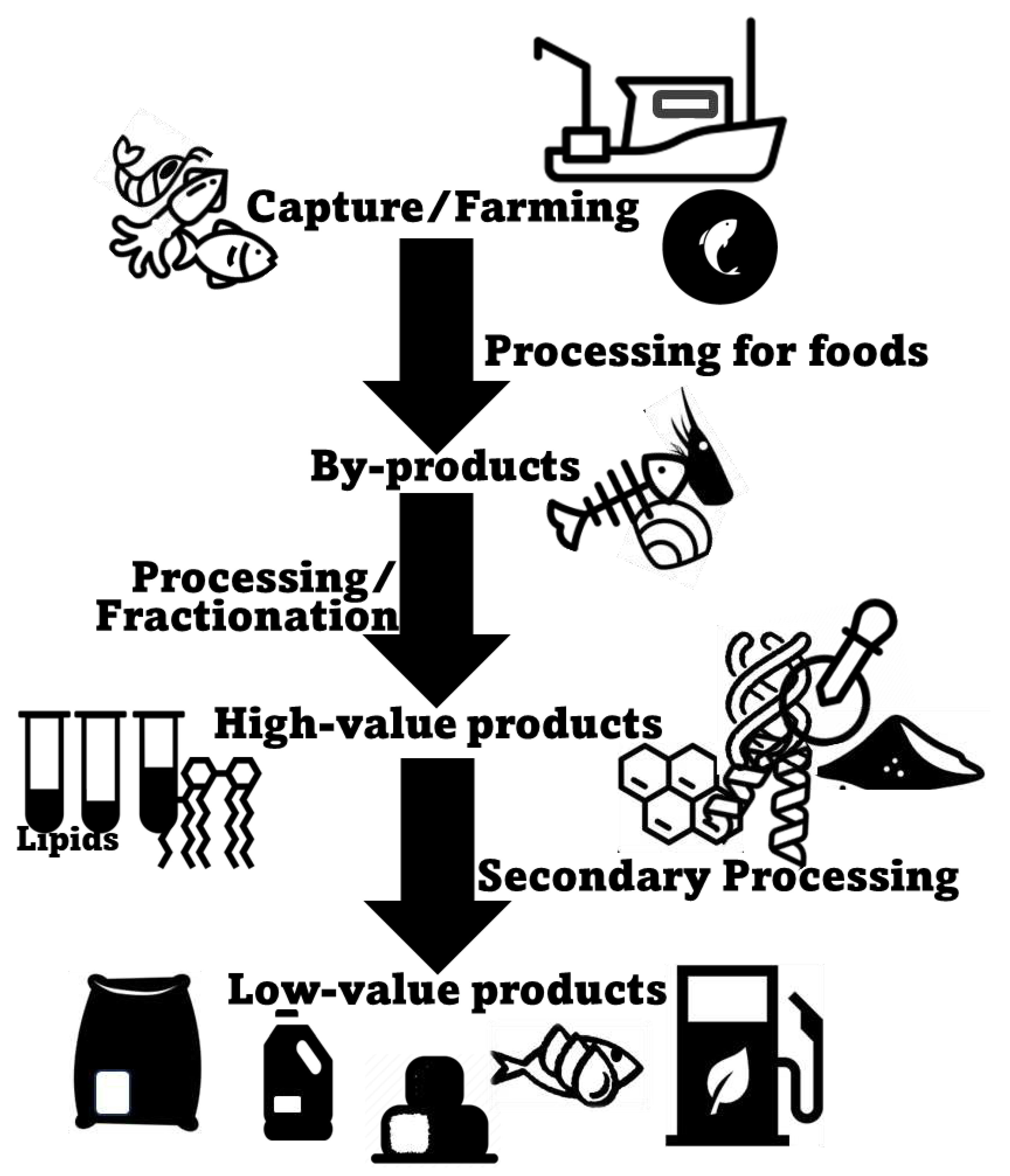
2. Marine Animal Co-Products
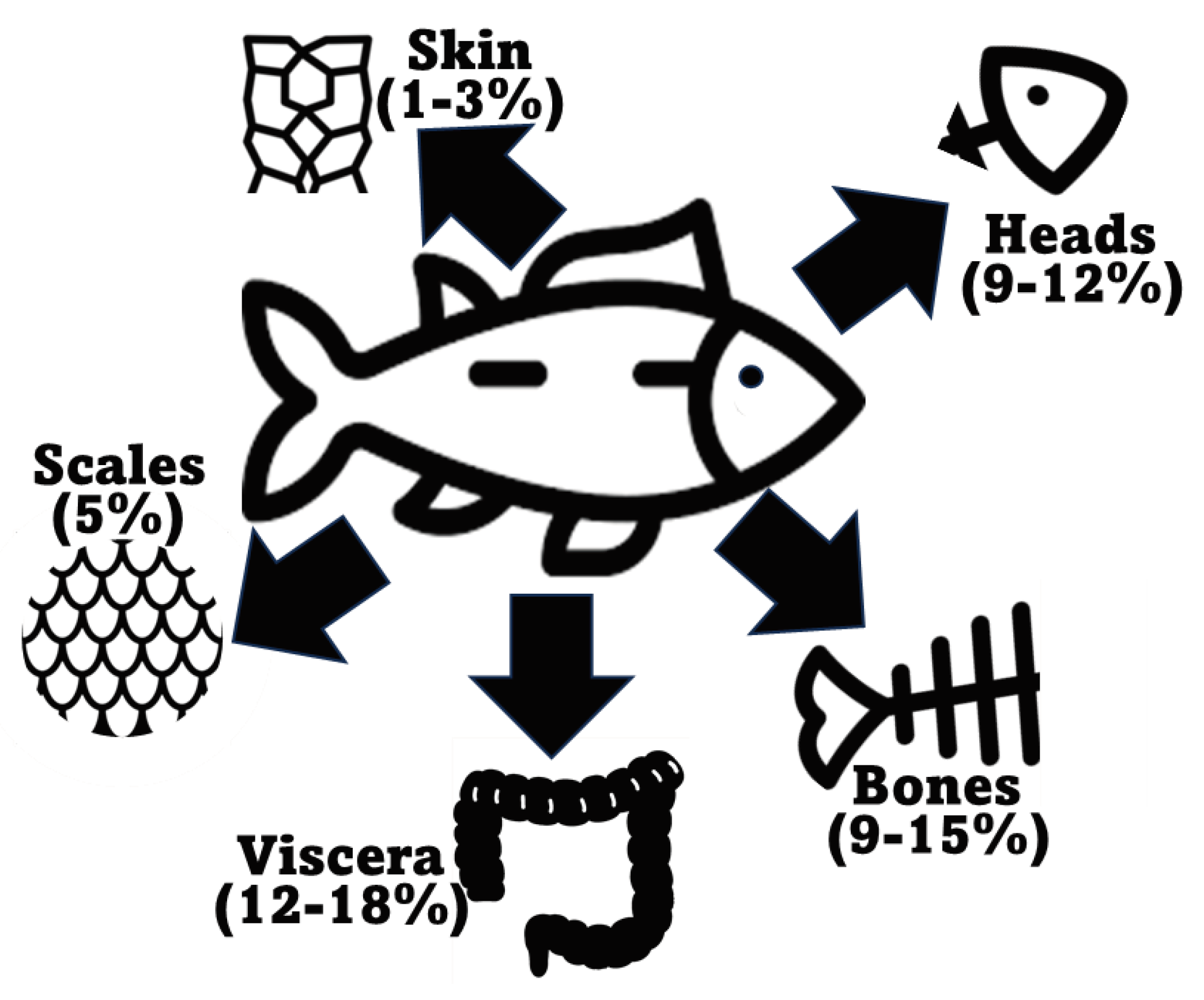
2.1. Fish
2.2. Crustaceans
2.3. Mollusks
3. Marine Animal Co-Products as a Source Of Healthy Lipids
3.1. Fish
3.2. Crustaceans
3.3. Mollusks
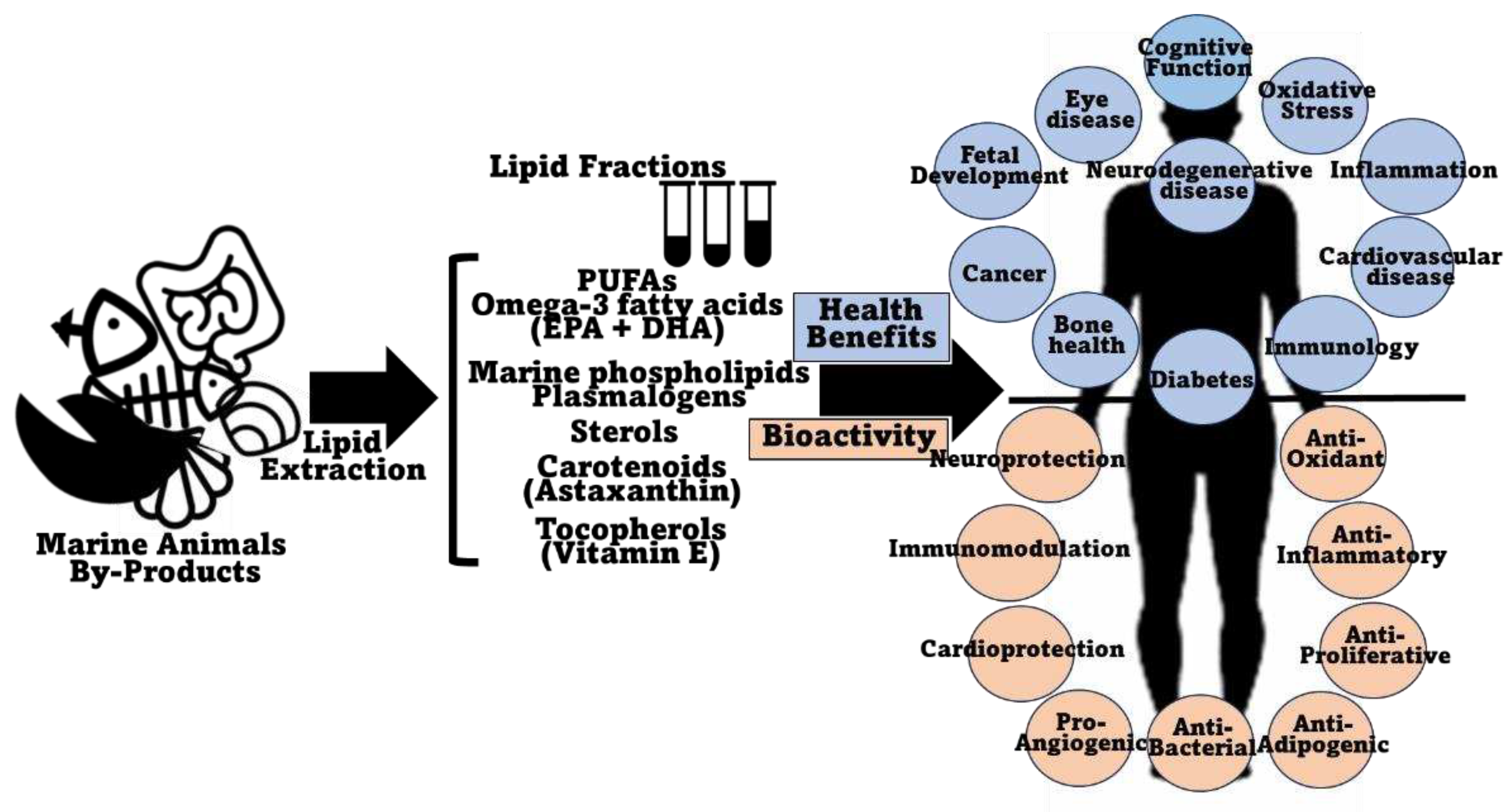
4. The Value of Marine Animal Co-Product Lipids for Human Health
5. The Value of Marine Animal Co-Product Lipids for Industry
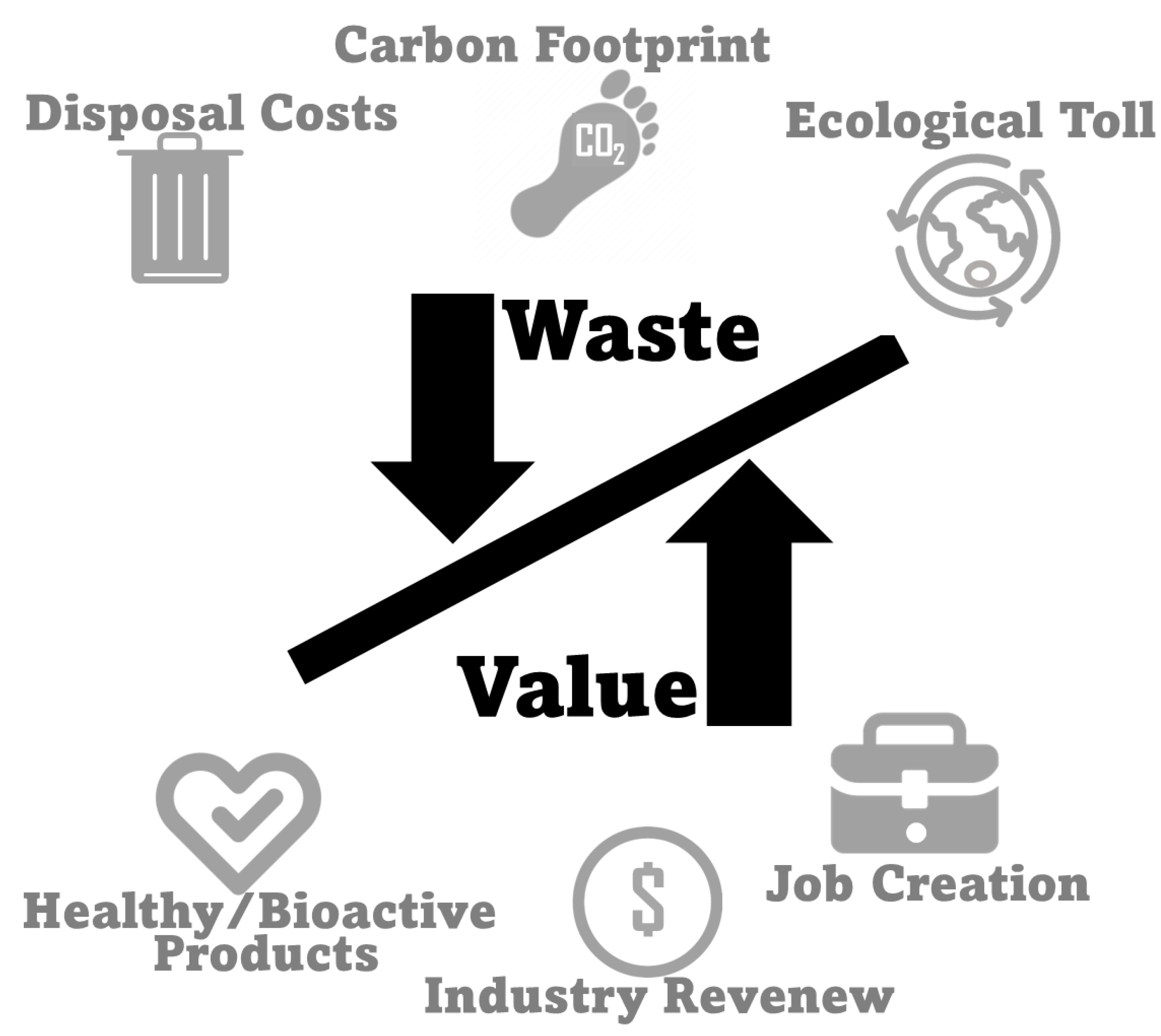
6. Sustainability and Environmental Impact
7. Challenges and Future Directions
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shahidi, F., Maximising the value of marine by-products. Woodhead Publishing: 2006.
- Rustad, T.; Storrø, I.; Slizyte, R. Possibilities for the utilisation of marine by-products. International Journal of Food Science & Technology 2011, 46, 2001–2014. [CrossRef]
- Ferraro, V.; Cruz, I. B.; Jorge, R. F.; Malcata, F. X.; Pintado, M. E.; Castro, P. M. L. Valorisation of natural extracts from marine source focused on marine by-products: A review. Food Research International 2010, 43, 2221–2233. [Google Scholar] [CrossRef]
- Vieira, H.; Leal, M. C.; Calado, R. Fifty Shades of Blue: How Blue Biotechnology is Shaping the Bioeconomy. Trends in Biotechnology 2020, 38, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, H.; Newton, R.; Auchterlonie, N.; Müller, D. Systems approach to quantify the global omega-3 fatty acid cycle. Nature 2020, 1, 59–62. [Google Scholar] [CrossRef]
- Cardoso, C.; Afonso, C.; Bandarra, N. M. Seafood lipids and cardiovascular health. Nutrire 2016, 41, 7. [Google Scholar] [CrossRef]
- Dyerberg, J., Fats from marine animals in human nutrition and prevention of cardiovascular diseases. In Atherosclerosis and Cardiovascular Diseases: Proceedings of the Sixth International Meeting on Atherosclerosis and Cardiovascular Diseases held in Bologna, Italy, October 27–29,1986, Lenzi, S.; Descovich, G. C., Eds. Springer Netherlands: Dordrecht, 1987; pp 261-267.
- Innes, J. K.; Calder, P. C. Marine Omega-3 (N-3) Fatty Acids for Cardiovascular Health: An Update for 2020. International Journal of Molecular Sciences 2020, 21. [Google Scholar] [CrossRef] [PubMed]
- Mori, T. A. Marine OMEGA-3 fatty acids in the prevention of cardiovascular disease. Fitoterapia 2017, 123, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, Y. A.; Portois, L.; Malaisse, W. J. n−3 Fatty acids and the metabolic syndrome 23. The American Journal of Clinical Nutrition 2006, 83, 1499S–1504S. [Google Scholar] [CrossRef] [PubMed]
- Tørris, C.; Småstuen, M. C.; Molin, M. Nutrients in Fish and Possible Associations with Cardiovascular Disease Risk Factors in Metabolic Syndrome. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Guo, X. F.; Li, X.; Shi, M.; Li, D. n-3 Polyunsaturated Fatty Acids and Metabolic Syndrome Risk: A Meta-Analysis. Nutrients 2017, 9, (7). [CrossRef]
- Damaiyanti, D. W.; Tsai, Z. Y.; Masbuchin, A. N.; Huang, C. Y.; Liu, P. Y. Interplay between fish oil, obesity and cardiometabolic diabetes. Journal of the Formosan Medical Association 2023, 122, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Calder, P. C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2015, 1851, 469–484. [CrossRef]
- Lordan, R.; Nasopoulou, C.; Tsoupras, A.; Zabetakis, I., The Anti-inflammatory Properties of Food Polar Lipids. In Bioactive Molecules in Food, Mérillon, J.-M.; Ramawat, K. G., Eds. Springer International Publishing: Cham, 2018; pp 1-34.
- Mendivil, C. O. Dietary Fish, Fish Nutrients, and Immune Function: A Review. Frontiers in Nutrition 2020, 7, 617652. [Google Scholar] [CrossRef]
- Hans, S.; Karadimou, A.; Mulvihill, J. J. E.; Grabrucker, A. M.; Zabetakis, I. The Role of Dietary Lipids in Cognitive Health: Implications for Neurodegenerative Disease. Biomedicines 2022, 10, 3250. [Google Scholar] [CrossRef]
- Rahman, M. A.; Dash, R.; Sohag, A. A. M.; Alam, M.; Rhim, H.; Ha, H.; Moon, I. S.; Uddin, M. J.; Hannan, M. A. Prospects of Marine Sterols against Pathobiology of Alzheimer's Disease: Pharmacological Insights and Technological Advances. Marine Drugs 2021, 19. [Google Scholar] [CrossRef]
- Assisi, A.; Banzi, R.; Buonocore, C.; Capasso, F.; Di Muzio, V.; Michelacci, F.; Renzo, D.; Tafuri, G.; Trotta, F.; Vitocolonna, M.; Garattini, S. Fish oil and mental health: the role of n-3 long-chain polyunsaturated fatty acids in cognitive development and neurological disorders. International Clinical Psychopharmacology 2006, 21, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Ivanovs, K.; Blumberga, D. Extraction of fish oil using green extraction methods: a short review. Energy Procedia 2017, 128, 477–483. [Google Scholar] [CrossRef]
- Thirukumaran, R.; Anu Priya, V. K.; Krishnamoorthy, S.; Ramakrishnan, P.; Moses, J. A.; Anandharamakrishnan, C. Resource recovery from fish waste: Prospects and the usage of intensified extraction technologies. Chemosphere 2022, 299, 134361. [Google Scholar] [CrossRef] [PubMed]
- Krueck, N. C.; Ahmadia, G. N.; Possingham, H. P.; Riginos, C.; Treml, E. A.; Mumby, P. J. Marine Reserve Targets to Sustain and Rebuild Unregulated Fisheries. PLoS Biology 2017, 15, e2000537. [Google Scholar] [CrossRef] [PubMed]
- Issifu, I.; Alava, J. J.; Lam, V. W. Y.; Sumaila, U. R. Impact of Ocean Warming, Overfishing and Mercury on European Fisheries: A Risk Assessment and Policy Solution Framework. Frontiers in Marine Science 2022, 8. [Google Scholar] [CrossRef]
- zu Ermgassen, P. S. E.; Thurstan, R. H.; Corrales, J.; Alleway, H.; Carranza, A.; Dankers, N.; DeAngelis, B.; Hancock, B.; Kent, F.; McLeod, I.; Pogoda, B.; Liu, Q.; Sanderson, W. G. The benefits of bivalve reef restoration: A global synthesis of underrepresented species. Aquatic Conservation: Marine and Freshwater Ecosystems 2020, 30, 2050–2065. [CrossRef]
- Kyzar, T.; Safak, I.; Cebrian, J.; Clark, M. W.; Dix, N.; Dietz, K.; Gittman, R. K.; Jaeger, J.; Radabaugh, K. R.; Roddenberry, A.; Smith, C. S.; Sparks, E. L.; Stone, B.; Sundin, G.; Taubler, M.; Angelini, C. Challenges and opportunities for sustaining coastal wetlands and oyster reefs in the southeastern United States. Journal of Environmental Management 2021, 296, 113178. [Google Scholar] [CrossRef] [PubMed]
- FAO, The State of World Fisheries and Aquaculture, 2022. Food & Agriculture Org.: 2022; Vol. 3.
- Hicks, D. T. Seafood Safety and Quality: The Consumer’s Role. Foods 2016, 5, 71. [Google Scholar] [CrossRef]
- Ozogul, F.; Cagalj, M.; Šimat, V.; Ozogul, Y.; Tkaczewska, J.; Hassoun, A.; Kaddour, A. A.; Kuley, E.; Rathod, N. B.; Phadke, G. G. Recent developments in valorisation of bioactive ingredients in discard/seafood processing by-products. Trends in Food Science & Technology 2021, 116, 559–582. [CrossRef]
- Venugopal, V. Valorization of Seafood Processing Discards: Bioconversion and Bio-Refinery Approaches. Frontiers in Sustainable Food Systems 2021, 5. [Google Scholar] [CrossRef]
- Cooney, R.; de Sousa, D. B.; Fernández-Ríos, A.; Mellett, S.; Rowan, N.; Morse, A. P.; Hayes, M.; Laso, J.; Regueiro, L.; Wan, A. H. L.; Clifford, E. A circular economy framework for seafood waste valorisation to meet challenges and opportunities for intensive production and sustainability. Journal of Cleaner Production 2023, 392, 136283. [Google Scholar] [CrossRef]
- Mutalipassi, M.; Esposito, R.; Ruocco, N.; Viel, T.; Costantini, M.; Zupo, V. Bioactive Compounds of Nutraceutical Value from Fishery and Aquaculture Discards. Foods 2021, 10, 1495. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R. L.; Toppe, J.; Karunasagar, I. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends in Food Science & Technology 2014, 36, 144–151. [CrossRef]
- Ideia, P.; Pinto, J.; Ferreira, R.; Figueiredo, L.; Spínola, V.; Castilho, P. C. Fish Processing Industry Residues: A Review of Valuable Products Extraction and Characterization Methods. Waste and Biomass Valorization 2020, 11, 3223–3246. [Google Scholar] [CrossRef]
- Saravanan, A.; Yuvaraj, D.; Senthil Kumar, P.; Karishma, S.; Rangasamy, G. Fish processing discards: A plausible resource for valorization to renewable fuels production, optimization, byproducts and challenges. Fuel 2023, 335, 127081. [Google Scholar] [CrossRef]
- Coppola, D.; Lauritano, C.; Palma Esposito, F.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish Waste: From Problem to Valuable Resource. Marine Drugs 2021, 19. [Google Scholar] [CrossRef] [PubMed]
- Samat, A. F.; Muhamad, N. A. S.; Abd Rasib, N. A.; Mohd Hassan, S. S., Khairunissa Syairah Aminah; Ahmad; Iberahim, N. I., The Potential of Biodiesel Production derived from Fish Waste. IOP Conference Series: Materials Science and Engineering 2018, 318, (1), 012017. [CrossRef]
- Comission, E. Oceans and fisheries - Consumption. https://oceans-and-fisheries.ec.europa.eu/facts-andfigures/facts-and-figures-common-fisheries-policy/consumption_en.
- Siddiqui, S. A.; Schulte, H.; Pleissner, D.; Schönfelder, S.; Kvangarsnes, K.; Dauksas, E.; Rustad, T.; Cropotova, J.; Heinz, V.; Smetana, S. Transformation of Seafood Side-Streams and Residuals into Valuable Products. Foods 2023, 12, 422. [Google Scholar] [CrossRef] [PubMed]
- Toppe, J.; Olsen, R. L.; Peñarubia, O. R.; James, D. G. Production and Utilization of Fish Silage: a manual on how to turn fish waste into profit and a valuable feed ingredient or fertilizer. Food and Agriculture Organization of the United Nations: 2018.
- Villamil, O.; Váquiro, H.; Solanilla, J. F. Fish viscera protein hydrolysates: Production, potential applications and functional and bioactive properties. Food Chemistry 2017, 224, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Boronat, Ò.; Sintes, P.; Celis, F.; Díez, M.; Ortiz, J.; Aguiló-Aguayo, I.; Martin-Gómez, H. Development of added-value culinary ingredients from fish waste: Fish bones and fish scales. International Journal of Gastronomy and Food Science 2023, 31, 100657. [Google Scholar] [CrossRef]
- Pinheiro, A. C. D. A. S.; Martí-Quijal, F. J.; Barba, F. J.; Tappi, S.; Rocculi, P. Innovative Non-Thermal Technologies for Recovery and Valorization of Value-Added Products from Crustacean Processing By-Products—An Opportunity for a Circular Economy Approach. Foods 2021, 10, 2030. [Google Scholar] [CrossRef] [PubMed]
- Saima, M. K.; Roohi, I. Z. A. Isolation of novel chitinolytic bacteria and production optimization of extracellular chitinase. Journal of Genetic Engineering and Biotechnology 2013, 11, 39–46. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J. M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends in Food Science & Technology 2016, 48, 40–50. [CrossRef]
- Cretton, M.; Malanga, G.; Mazzuca Sobczuk, T.; Mazzuca, M. Lipid Fraction from Industrial Crustacean Waste and Its Potential as a Supplement for the Feed Industry: A Case Study in Argentine Patagonia. Waste and Biomass Valorization 2021, 12, 2311–2319. [Google Scholar] [CrossRef]
- Sachindra, N. M.; Bhaskar, N.; Mahendrakar, N. S. Carotenoids in different body components of Indian shrimps. Journal of the Science of Food and Agriculture 2005, 85, 167–172. [Google Scholar] [CrossRef]
- Heu, M.-S.; Kim, J.-S.; Shahidi, F. Components and nutritional quality of shrimp processing by-products. Food Chemistry 2003, 82, 235–242. [Google Scholar] [CrossRef]
- Takeungwongtrakul, S.; Benjakul, S.; H-kittikun, A. Lipids from cephalothorax and hepatopancreas of Pacific white shrimp (Litopenaeus vannamei): Compositions and deterioration as affected by iced storage. Food Chemistry 2012, 134, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Phadtare, I.; Vaidya, H.; Hawboldt, K.; Cheema, S. K. Shrimp Oil Extracted from Shrimp Processing By-Product Is a Rich Source of Omega-3 Fatty Acids and Astaxanthin-Esters, and Reveals Potential Anti-Adipogenic Effects in 3T3-L1 Adipocytes. Marine Drugs 2021, 19. [Google Scholar] [CrossRef] [PubMed]
- Cahú, T. B.; Santos, S. D.; Mendes, A.; Córdula, C. R.; Chavante, S. F.; Carvalho, L. B.; Nader, H. B.; Bezerra, R. S. Recovery of protein, chitin, carotenoids and glycosaminoglycans from Pacific white shrimp (Litopenaeus vannamei) processing waste. Process Biochemistry 2012, 47, 570–577. [Google Scholar] [CrossRef]
- Albalat, A.; Nadler, L. E.; Foo, N.; Dick, J. R.; Watts, A. J. R.; Philp, H.; Neil, D. M.; Monroig, O. Lipid Composition of Oil Extracted from Wasted Norway Lobster (Nephrops norvegicus) Heads and Comparison with Oil Extracted from Antarctic Krill (Euphasia superba). Marine Drugs 2016, 14, 219. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T. T.; Barber, A. R.; Corbin, K.; Zhang, W. Lobster processing by-products as valuable bioresource of marine functional ingredients, nutraceuticals, and pharmaceuticals. Bioresources and Bioprocessing 2017, 4, 27. [Google Scholar] [CrossRef]
- Naik, A. S.; Hayes, M. Bioprocessing of mussel by-products for value added ingredients. Trends in Food Science & Technology 2019, 92, 111–121. [CrossRef]
- Tokeshi, M.; Ota, N.; Kawai, T. A comparative study of morphometry in shell-bearing molluscs. Journal of Zoology 2000, 251, 31–38. [Google Scholar] [CrossRef]
- Morris, J. P.; Backeljau, T.; Chapelle, G. Shells from aquaculture: a valuable biomaterial, not a nuisance waste product. Reviews in Aquaculture 2019, 11, 42–57. [Google Scholar] [CrossRef]
- Uzcátegui, L. U. M.; Vergara, K.; Martínez Bordes, G. Sustainable alternatives for by-products derived from industrial mussel processing: A critical review. Waste Management & Research 2022, 40, 123–138. [CrossRef]
- Méndez, L.; Rodríguez, A.; Aubourg, S. P.; Medina, I. Low-Toxicity Solvents for the Extraction of Valuable Lipid Compounds from Octopus (Octopus vulgaris) Waste. Foods 2023, 12, 3631. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Trigo, M.; Aubourg, S. P.; Medina, I. Optimisation of Low-Toxicity Solvent Employment for Total Lipid and Tocopherol Compound Extraction from Patagonian Squid By-Products. Foods 2023, 12, 504. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.-Y.; Ahn, D.-H.; Wilkinson, G. T.; Chun, B.-S. Extraction of lipids and cholesterol from squid oil with supercritical carbon dioxide. Korean Journal of Chemical Engineering 2005, 22, 399–405. [Google Scholar] [CrossRef]
- Fitahia, E. M.; Croyal, M.; Raheriniaina, C. E.; Ferchaud-Roucher, V.; Nazih, H. High-Resolution Mass Spectrometry Unravels a Broad Range of Bioactive Lipid Species in Octopus cyanea and Loligo sp. By-products from Southwestern Madagascar. Waste and Biomass Valorization 2018, 9, 1787–1793. [CrossRef]
- Wang, C.-H.; Doan, C. T.; Nguyen, V. B.; Nguyen, A. D.; Wang, S.-L. Reclamation of Fishery Processing Waste: A Mini-Review. Molecules 2019, 24, 2234. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Di Lena, G.; Gabrielli, P.; Santini, A.; Lombardi-Boccia, G.; Lucarini, M. Nutrients and Bioactive Compounds in Seafood: Quantitative Literature Research Analysis. Fishes 2022, 7, 132. [Google Scholar] [CrossRef]
- Durmus, M. Fish oil for human health: omega-3 fatty acid profiles of marine seafood species. Food Science and Technology 2019, 39. [Google Scholar] [CrossRef]
- Rincón-Cervera, M. Á.; González-Barriga, V.; Romero, J.; Rojas, R.; López-Arana, S. Quantification and Distribution of Omega-3 Fatty Acids in South Pacific Fish and Shellfish Species. Foods 2020, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Oliver, L.; Dietrich, T.; Marañón, I.; Villarán, M. C.; Barrio, R. J. Producing Omega-3 Polyunsaturated Fatty Acids: A Review of Sustainable Sources and Future Trends for the EPA and DHA Market. Resources 2020, 9, 148. [Google Scholar] [CrossRef]
- Olgunoglu, İ. Review on Omega-3 (n-3) Fatty Acids in Fish and Seafood. Journal of Biology, Agriculture and Healthcare 2017, 7, 37–45.
- Ceyda, I. L., The Effects of Shellfish Consumption Frequency for Human Health. In Update on Malacology, Sajal, R.; Soumalya, M., Eds. IntechOpen: Rijeka, 2021; p Ch. 1.
- Carboni, S.; Kaur, G.; Pryce, A.; McKee, K.; Desbois, A. P.; Dick, J. R.; Galloway, S. D. R.; Hamilton, D. L. Mussel Consumption as a "Food First" Approach to Improve Omega-3 Status. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annual Review of Food Science and Technology 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Li, J.; Pora, B. L. R.; Dong, K.; Hasjim, J. Health benefits of docosahexaenoic acid and its bioavailability: A review. Food Science & Nutrition 2021, 9, 5229–5243. [CrossRef]
- Dempsey, M.; Rockwell, M. S.; Wentz, L. M. The influence of dietary and supplemental omega-3 fatty acids on the omega-3 index: A scoping review. Frontiers in Nutrition 2023, 10, 1072653. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chemical Society Reviews 2010, 39, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of Animal and Marine Origin: Structure, Function, and Anti-Inflammatory Properties. Molecules 2017, 22, (11). [CrossRef]
- Tran, Q. T.; Le, T. T. T.; Pham, M. Q.; Do, T. L.; Vu, M. H.; Nguyen, D. C.; Bach, L. G.; Bui, L. M.; Pham, Q. L. Fatty Acid, Lipid Classes and Phospholipid Molecular Species Composition of the Marine Clam Meretrix lyrata (Sowerby 1851) from Cua Lo Beach, Nghe An Province, Vietnam. Molecules 2019, 24, 895. [Google Scholar] [CrossRef] [PubMed]
- Kizmaz, V. Analysis of lipid classes and the fatty acid composition of fresh and the salted fish, Alburnus tarichi. Cogent Food & Agriculture 2022, 8, 2126052. [CrossRef]
- Sushchik, N. N.; Makhutova, O. N.; Rudchenko, A. E.; Glushchenko, L. A.; Shulepina, S. P.; Kolmakova, A. A.; Gladyshev, M. I. Comparison of Fatty Acid Contents in Major Lipid Classes of Seven Salmonid Species from Siberian Arctic Lakes. Biomolecules 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Biandolino, F.; Prato, E. A preliminary investigation of the lipids and fatty acids composition of Gammarus aequicauda (Crustacea: Amphipoda) and its main food source. Journal of the Marine Biological Association of the United Kingdom 2006, 86, 345–348. [Google Scholar] [CrossRef]
- Laudicella, V. A.; Beveridge, C.; Carboni, S.; Franco, S. C.; Doherty, M. K.; Long, N.; Mitchell, E.; Stanley, M. S.; Whitfield, P. D.; Hughes, A. D. Lipidomics analysis of juveniles' blue mussels (Mytilus edulis L. 1758), a key economic and ecological species. PLoS One 2019, 768937. [CrossRef]
- Medina, I.; Aubourg, S. P.; Martín, R. P. Composition of phospholipids of white muscle of six tuna species. Lipids 1995, 30, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Al Khawli, F.; Pateiro, M.; Domínguez, R.; Lorenzo, J. M.; Gullón, P.; Kousoulaki, K.; Ferrer, E.; Berrada, H.; Barba, F. J. Innovative Green Technologies of Intensification for Valorization of Seafood and Their By-Products. Marine Drugs 2019, 17, 689. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Munekata, P. E. S.; Domínguez, R.; Wang, M.; Barba, F. J.; Bermúdez, R.; Lorenzo, J. M. Nutritional Profiling and the Value of Processing By-Products from Gilthead Sea Bream (Sparus aurata). Marine Drugs 2020, 18, 101. [Google Scholar] [CrossRef]
- Kundam, D.; Acham, I. O.; Girgih, A. Bioactive Compounds in Fish and Their Health Benefits. Asian Food Science Journal 2018, 4, 1–14. [Google Scholar] [CrossRef]
- Malcorps, W.; Newton, R. W.; Sprague, M.; Glencross, B. D.; Little, D. C. Nutritional Characterisation of European Aquaculture Processing By-Products to Facilitate Strategic Utilisation. Frontiers in Sustainable Food Systems 2021, 5. [Google Scholar] [CrossRef]
- Kandyliari, A.; Mallouchos, A.; Papandroulakis, N.; Golla, J. P.; Lam, T. T.; Sakellari, A.; Karavoltsos, S.; Vasiliou, V.; Kapsokefalou, M. Nutrient Composition and Fatty Acid and Protein Profiles of Selected Fish By-Products. Foods 2020, 9. [Google Scholar] [CrossRef] [PubMed]
- Messina, C. M.; Renda, G.; La Barbera, L.; Santulli, A. By-products of farmed European sea bass (Dicentrarchus labrax L.) as a potential source of n-3 PUFA. Biologia 2013, 68, 288–293. [CrossRef]
- Messina, C. M.; Arena, R.; Manuguerra, S.; La Barbera, L.; Curcuraci, E.; Renda, G.; Santulli, A. Valorization of Side Stream Products from Sea Cage Fattened Bluefin Tuna (Thunnus thynnus): Production and In Vitro Bioactivity Evaluation of Enriched ω-3 Polyunsaturated Fatty Acids. Marine Drugs 2022, 20. [Google Scholar] [CrossRef] [PubMed]
- Kacem, M.; Sellami, M.; Kammoun, W.; Frikha, F.; Miled, N.; Ben Rebah, F. Seasonal Variations in Proximate and Fatty Acid Composition of Viscera of Sardinella aurita, Sarpa salpa, and Sepia officinalis from Tunisia. Journal of Aquatic Food Product Technology 2011, 20, 233–246. [Google Scholar] [CrossRef]
- Ovissipour, R.; Abedian Kenari, A.; Motamedzadegan, A.; Nazari, R. Optimization of Enzymatic Hydrolysis of Visceral Waste Proteins of Yellowfin Tuna (Thunnus albacares). Food and Bioprocess Technology 2010, 5, 696–705. [Google Scholar] [CrossRef]
- Aidos, I.; Lourenclo, S.; van der Padt, A.; Luten, J. B.; Boom, R. Stability of Crude Herring Oil Produced from Fresh Byproducts: Influence of Temperature during Storage. Journal of Food Science 2002, 67, 3314–3320. [Google Scholar] [CrossRef]
- Khoddami, A. Quality and fatty acid profile of the oil extracted from fish waste (head, intestine and liver) (Euthynnus affinis). African Journal of Biotechnology 2012, 11. [Google Scholar] [CrossRef]
- Khoddami, A.; Ariffin, A.; Bakar, J.; Mohd Ghazali, H. Fatty acid profile of the oil extracted from fish waste (head, intestine and liver) (Sardinella lemuru). World Applied Science Journal 2009, 7. [Google Scholar]
- Shahidi, F.; Naczk, M.; Pegg, R. B.; Synowiecki, J. Chemical composition and nutritional value of processing discards of cod (Gadus morhua). Food Chemistry 1991, 42, 145–151. [Google Scholar] [CrossRef]
- Ahmmed, M. K.; Carne, A.; Ahmmed, F.; Stewart, I.; Sabrina Tian, H.; Bekhit, A. E. A. Positional distribution of fatty acids and phospholipid composition in King salmon (Oncorhynchus tshawytscha) head, roe and skin using nuclear magnetic resonance spectroscopy. Food Chemistry 2021, 363, 130302. [Google Scholar] [CrossRef] [PubMed]
- Ahmmed, M. K.; Ahmmed, F.; Stewart, I.; Carne, A.; Tian, H. S.; Bekhit, A. E. A. Omega-3 phospholipids in Pacific blue mackerel (Scomber australasicus) processing by-products. Food Chemistry 2021, 353, 129451. [Google Scholar] [CrossRef] [PubMed]
- Guiry, E. J.; Szpak, P.; Richards, M. P. Effects of lipid extraction and ultrafiltration on stable carbon and nitrogen isotopic compositions of fish bone collagen. Rapid Communications in Mass Spectrometry 2016, 30, 1591–1600. [Google Scholar] [CrossRef]
- Rosidi, W. N. T. M.; Arshad, N. M.; Mohtar, N. F. Characterization of Sardinella fimbriata and Clarias gariepinus bones. Biodiversitas Journal of Biological Diversity 2021, 22. [Google Scholar] [CrossRef]
- During, A.; Penel, G.; Hardouin, P. Understanding the local actions of lipids in bone physiology. Progress in Lipid Research 2015, 59, 126–146. [Google Scholar] [CrossRef]
- Mularchuk, P.; Boskey, A. Lipids in bone: Optimal conditions for tissue storage prior to lipid analyses. Calcified Tissue International 1990, 46, 57–59. [Google Scholar] [CrossRef]
- Aitta, E.; Marsol-Vall, A.; Damerau, A.; Yang, B. Enzyme-Assisted Extraction of Fish Oil from Whole Fish and by-Products of Baltic Herring (Clupea harengus membras). Foods 2021, 10, 1811. [Google Scholar] [CrossRef]
- Lee, S.; Koo, M. H.; Han, D. W.; Kim, I. C.; Lee, J. H.; Kim, J. H.; Sultana, R.; Kim, S. Y.; Youn, U. J.; Kim, J. H. Comparison of Fatty Acid Contents and MMP-1 Inhibitory Effects of the Two Antarctic Fish, Notothenia rossii and Champsocephalus gunnari. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Jiang, W.; Yan, X. Proximate Composition and Nutritional Profile of Rainbow Trout (Oncorhynchus mykiss) Heads and Skipjack tuna (Katsuwonus Pelamis) Heads. Molecules 2019, 24, 3189. [Google Scholar] [CrossRef]
- Hu, Z.; Chin, Y.; Liu, J.; Zhou, J.; Li, G.; Hu, L.; Hu, Y. Optimization of fish oil extraction from Lophius litulon liver and fatty acid composition analysis. Fisheries and Aquatic Sciences 2022, 25, 76–89. [Google Scholar] [CrossRef]
- Gbogouri, G.; Linder, M.; Fanni, J.; Parmentier, M. Analysis of lipids extracted from salmon (Salmo salar) heads by commercial proteolytic enzymes. European Journal of Lipid Science and Technology 2006, 108, 766–775. [Google Scholar] [CrossRef]
- Abiona, O. O.; Awojide, S. H.; Anifowose, A. J.; Adegunwa, A. O.; Agbaje, W. B.; Tayo, A. S. Quality characteristics of extracted oil from the head and gills of Catfish and Titus fish. Bulletin of the National Research Centre 2021, 45, 101. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, R.; Zhao, X.; Lu, H.; Zhang, P.; Dong, X.; Wang, Y. Comparison of the Effect of Phospholipid Extracts from Salmon and Silver Carp Heads on High-Fat-Diet-Induced Metabolic Syndrome in C57BL/6J Mice. Marine Drugs 2023, 21. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A. P.; Meireles, M. Â. A.; Lopes, B. L. F.; Cabral, F. A. Proximate composition and extraction of carotenoids and lipids from Brazilian redspotted shrimp waste (Farfantepenaeus paulensis). Journal of Food Engineering 2011, 102, 87–93. [Google Scholar] [CrossRef]
- Ahmadkelayeh, S.; Hawboldt, K. Extraction of lipids and astaxanthin from crustacean by-products: A review on supercritical CO2 extraction. Trends in Food Science & Technology 2020, 103, 94–108. [CrossRef]
- Limam, Z.; Arafa, S.; Sadok, S.; El Abed, A. Lipids and fatty acids composition in the tissues and by-products of two Tunisian shrimp species from the north and south regions. Nutrition and Health 2008, 19, 215–220. [Google Scholar] [CrossRef]
- Toyes-Vargas, E.; Robles-Romo, A.; Méndez, L.; Palacios, E.; Civera, R. Changes in fatty acids, sterols, pigments, lipid classes, and heavy metals of cooked or dried meals, compared to fresh marine by-products. Animal Feed Science and Technology 2016, 221, 195–205. [Google Scholar] [CrossRef]
- Takeungwongtrakul, S.; Benjakul, S.; Santoso, J.; Trilaksani, W.; Nurilmala, M. Extraction and Stability of Carotenoid-Containing Lipids from Hepatopancreas of Pacific White Shrimp (Litopenaeus vannamei). Journal of Food Processing and Preservation 2015, 39, 10–18. [Google Scholar] [CrossRef]
- Senphan, T.; Benjakul, S. Compositions and yield of lipids extracted from hepatopancreas of Pacific white shrimp (Litopenaeus vannamei) as affected by prior autolysis. Food Chemistry 2012, 134, 829–835. [Google Scholar] [CrossRef]
- Trang Si, T.; Pham Thi Dan, P. Bioactive Compounds from By-Products of Shrimp Processing Industry in Vietnam. Journal of Food and Drug Analysis 2012, 20, 194–197. [Google Scholar] [CrossRef]
- O'Leary, C. D.; Matthews, A. D. Lipid class distribution and fatty acid composition of wild and farmed prawn, Penaeus monodon (Fabricius). Aquaculture 1990, 89, 65–81. [Google Scholar] [CrossRef]
- Miniadis-Meimaroglou, S.; Kora, L.; Sinanoglou, V. J. Isolation and identification of phospholipid molecular species in α wild marine shrimp Penaeus kerathurus muscle and cephalothorax. Chemistry and Physics of Lipids 2008, 152, 104–112. [Google Scholar] [CrossRef]
- Ravichandran, S.; Rameshkumar, G.; Prince, A. R. Biochemical composition of shell and flesh of the Indian white shrimp Penaeus indicus (H. milne Edwards 1837). American-Eurasian Journal of Scientific Research 2009, 4, 191–194.
- Tsvetnenko, E.; Kailis, S.; Evans, L.; Longmore, R. Fatty acid composition of lipids from the contents of rock lobster (Panulirus cygnus) cephalothorax. Journal of the American Oil Chemists' Society 1996, 73, 259–261. [CrossRef]
- Nguyen, T. T.; Zhang, W.; Barber, A. R.; Su, P.; He, S. Significant Enrichment of Polyunsaturated Fatty Acids (PUFAs) in the Lipids Extracted by Supercritical CO2 from the Livers of Australian Rock Lobsters (Jasus edwardsii). Journal of Agricultural and Food Chemistry 2015, 63, 4621–4628. [Google Scholar] [CrossRef]
- Beaulieu, L.; Thibodeau, J.; Bryl, P.; Carbonneau, M.-É. Characterization of enzymatic hydrolyzed snow crab (Chionoecetes opilio) by-product fractions: A source of high-valued biomolecules. Bioresource Technology 2009, 100, 3332–3342. [Google Scholar] [CrossRef]
- Lv, S.; Xie, S.; Liang, Y.; Xu, L.; Hu, L.; Li, H.; Mo, H. Comprehensive lipidomic analysis of the lipids extracted from freshwater fish bones and crustacean shells. Food Science & Nutrition 2022, 10, 723–730. [CrossRef]
- Muriana, F. J.; Ruiz-Gutierrez, V.; Gallardo-Guerrero, M. L.; Mínguez-Mosquera, M. I. A study of the lipids and carotenoprotein in the prawn, Penaeus japonicus. Journal of Biochemistry 1993, 114, 223–229. [Google Scholar] [CrossRef]
- Shen, Q.; Song, G.; Wang, H.; Zhang, Y.; Cui, Y.; Xie, H.; Xue, J.; Wang, H. Isolation and lipidomics characterization of fatty acids and phospholipids in shrimp waste through GC/FID and HILIC-QTrap/MS. Journal of Food Composition and Analysis 2021, 95, 103668. [Google Scholar] [CrossRef]
- Sinthusamran, S.; Benjakul, S.; Kijroongrojana, K.; Prodpran, T.; Agustini, T. W. Yield and chemical composition of lipids extracted from solid residues of protein hydrolysis of Pacific white shrimp cephalothorax using ultrasound-assisted extraction. Food Bioscience 2018, 26, 169–176. [Google Scholar] [CrossRef]
- Gulzar, S.; Benjakul, S. Ultrasound Waves Increase the Yield and Carotenoid Content of Lipid Extracted From Cephalothorax of Pacific White Shrimp (Litopenaeus vannamei). European Journal of Lipid Science and Technology 2018, 120, 1700495. [Google Scholar] [CrossRef]
- Zhang, M.; Li, P.; Wang, F.; Zhang, S.; Li, H.; Zhang, Y.; Wang, X.; Liu, K.; Li, X. Separation, identification and cardiovascular activities of phospholipid classes from the head of Penaeus vannamei by lipidomics and zebrafish models. Food & Function 2021, 12, 2282–2291. [CrossRef]
- Sriket, P.; Benjakul, S.; Visessanguan, W.; Kijroongrojana, K. Comparative studies on chemical composition and thermal properties of black tiger shrimp (Penaeus monodon) and white shrimp (Penaeus vannamei) meats. Food Chemistry 2007, 103, 1199–1207. [Google Scholar] [CrossRef]
- Guary, J.-C. Lipid class distribution and fatty acid composition of prawn, Penaus japonicus bate. Bulletin of the Japanese Society of Scientific Fisheries 1974, 40, 1027–1032. [Google Scholar] [CrossRef]
- Sowmya, R.; Sachindra, N. M. Evaluation of antioxidant activity of carotenoid extract from shrimp processing byproducts by in vitro assays and in membrane model system. Food Chemistry 2012, 134, 308–314. [Google Scholar] [CrossRef]
- Sachindra, N. M.; Bhaskar, N.; Siddegowda, G. S.; Sathisha, A. D.; Suresh, P. V. Recovery of carotenoids from ensilaged shrimp waste. Bioresource Technology 2007, 98, 1642–1646. [Google Scholar] [CrossRef]
- Li, J.; Sun, W.; Ramaswamy, H. S.; Yu, Y.; Zhu, S.; Wang, J.; Li, H. High Pressure Extraction of Astaxanthin from Shrimp Waste (Penaeus Vannamei Boone): Effect on Yield and Antioxidant Activity. Journal of Food Process Engineering 2017, 40, e12353. [Google Scholar] [CrossRef]
- Shahidi, F.; Synowiecki, J. Isolation and characterization of nutrients and value-added products from snow crab (Chionoecetes opilio) and shrimp (Pandalus borealis) processing discards. Journal of Agricultural and Food Chemistry 1991, 39, 1527–1532. [Google Scholar] [CrossRef]
- Sachindra, N. M.; Bhaskar, N.; Mahendrakar, N. S. Carotenoids in crabs from marine and fresh waters of India. LWT - Food Science and Technology 2005, 38, 221–225. [CrossRef]
- Sánchez-Camargo, A.; Martinez-Correa, H.; Paviani, L.; Cabral, F. Supercritical CO2 extraction of lipids and astaxanthin from Brazilian redspotted shrimp waste (Farfantepenaeus paulensis). Journal of Supercritical Fluids 2011, 56, 164–173. [Google Scholar] [CrossRef]
- Sachindra, N. M.; Bhaskar, N.; Mahendrakar, N. S. Recovery of carotenoids from shrimp waste in organic solvents. Waste Management 2006, 26, 1092–1098. [Google Scholar] [CrossRef]
- Holanda, H.; Netto, F. Recovery of Components from Shrimp (Xiphopenaeus kroyeri) Processing Waste by Enzymatic Hydrolysis. Journal of Food Science 2006, 71, C298–C303. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F. M. Astaxanthin: a review of its chemistry and applications. Critical Reviews in Food Science and Nutrition 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Montoya, J.; Mata, S.; Acosta, J.; Cabrera, B.; López-Valdez, L.; Cesar, R.; Barrales-Cureño, H. Obtaining of Astaxanthin from Crab Exosqueletons and Shrimp Head Shells. Biointerface Research in Applied Chemistry 2021, 11. [Google Scholar] [CrossRef]
- López-Cervantes, J.; Sánchez-Machado, D. I.; Ríos-Vázquez, N. J. High-performance liquid chromatography method for the simultaneous quantification of retinol, α-tocopherol, and cholesterol in shrimp waste hydrolysate. Journal of Chromatography A 2006, 1105, 135–139. [Google Scholar] [CrossRef]
- Subra-Paternault, P.; ThongDeng, H.; Grélard, A.; Cansell, M. Extraction of phospholipids from scallop by-product using supercritical CO2/alcohol mixtures. LWT - Food Science and Technology 2015, 60, (2, Part 1), 990-998. [CrossRef]
- Savoire, R.; Subra-Paternault, P.; Bardeau, T.; Morvan, E.; Grélard, A.; Cansell, M. Selective extraction of phospholipids from food by-products by supercritical carbon dioxide and ethanol and formulating ability of extracts. Separation and Purification Technology 2020, 238, 116394. [Google Scholar] [CrossRef]
- Aubourg, S. P.; Trigo, M.; Prego, R.; Cobelo-García, A.; Medina, I. Nutritional and Healthy Value of Chemical Constituents Obtained from Patagonian Squid (Doryteuthis gahi) By-Products Captured at Different Seasons. Foods 2021, 10. [Google Scholar] [CrossRef]
- Rodríguez, A.; Trigo, M.; Aubourg, S. P.; Medina, I. Optimisation of Healthy-Lipid Content and Oxidative Stability during Oil Extraction from Squid (Illex argentinus) Viscera by Green Processing. Marine Drugs 2021, 19, (11). [CrossRef]
- Aubourg, S. P.; Trigo, M.; González, M. J.; Lois, S.; Medina, I. Comparative Study of Bioactive Lipid Extraction from Squid (Doryteuthis gahi) by-Products by Green Solvents. Foods 2022, 11, 2188. [Google Scholar] [CrossRef]
- Aubourg, S. P.; Rodríguez, A.; Trigo, M.; Medina, I. Yield Enhancement of Valuable Lipid Compounds from Squid (Doryteuthis gahi) Waste by Ethanol/Acetone Extraction. Foods 2023, 12, 2649. [Google Scholar] [CrossRef]
- Uddin, M. S.; Kishimura, H.; Chun, B. S. Isolation and characterization of lecithin from squid (Todarodes pacificus) viscera deoiled by supercritical carbon dioxide extraction. Journal of Food Science 2011, 76, C350–C354. [Google Scholar] [CrossRef]
- Hayashi, K. Composition and distribution of lipids in different tissues of the arrow squid Loligo bleekeri. Fisheries science 1996, 62, 84–87. [Google Scholar] [CrossRef]
- Hayashi, K.; Kishimura, H. Amount and Composition of Diacyl Glyceryl Ethers in Various Tissue Lipids of the Deep-sea Squid Berryteuthis magister. Journal of Oleo Science 2002, 51, 523–529. [Google Scholar] [CrossRef]
- Saito, H.; Sakai, M.; Wakabayashi, T. Characteristics of the lipid and fatty acid compositions of the Humboldt squid, Dosidicus gigas: The trophic relationship between the squid and its prey. European Journal of Lipid Science and Technology 2014, 116, 360–366. [Google Scholar] [CrossRef]
- Azam, K.; Basher, Z.; Asaduzzaman, M.; Hossain, M.; Ali, M. Biochemical assessment of fourteen selected dried fish. University Journal of Zoology Rajshahi University 2003, 23–26. [Google Scholar] [CrossRef]
- Pongsetkul, J.; Benjakul, S.; Vongkamjan, K.; Sumpavapol, P.; Osako, K. Changes in lipids of shrimp (Acetes vulgaris) during salting and fermentation. European Journal of Lipid Science and Technology 2017, 119, 1700253. [Google Scholar] [CrossRef]
- Kommuri, P. K.; Mugada, N.; Kondamudi, R. B. Proximate Analysis and Mineral Composition of Commercially Important Spiny Lobsters from Visakhapatnam Coast, Andhra Pradesh, India. Asian Journal of Fisheries and Aquatic Research 2021, 14, 39–47. [Google Scholar] [CrossRef]
- Rosa, R.; Pereira, J.; Nunes, M. Biochemical composition of cephalopods with different life strategies, with special reference to a giant squid, Architeuthis sp. Marine Biology 2005, 146, 739–751. [Google Scholar] [CrossRef]
- Ahmad, T. B.; Rudd, D.; Kotiw, M.; Liu, L.; Benkendorff, K. Correlation between Fatty Acid Profile and Anti-Inflammatory Activity in Common Australian Seafood by-Products. Marine Drugs 2019, 17. [Google Scholar] [CrossRef]
- Tocher, D. R.; Betancor, M. B.; Sprague, M.; Olsen, R. E.; Napier, J. A. Omega-3 Long-Chain Polyunsaturated Fatty Acids, EPA and DHA: Bridging the Gap between Supply and Demand. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Takahashi, K.; Inoue, Y. Marine by-product phospholipids as booster of medicinal compounds. Advances in Food and Nutrition Research 2012, 65, 31–46. [Google Scholar] [CrossRef]
- Burri, L.; Hoem, N.; Banni, S.; Berge, K. Marine omega-3 phospholipids: metabolism and biological activities. International Journal of Molecular Sciences 2012, 13, 15401–15419. [Google Scholar] [CrossRef]
- Nollet, L. M.; Toldrá, F. Handbook of seafood and seafood products analysis. CRC Press: 2009.
- von Schacky, C. Omega-3 index in 2018/19. Proceedings of the Nutrition Society 2020, 79, 381–387. [Google Scholar] [CrossRef]
- Arterburn, L. M.; Hall, E. B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. The American Journal of Clinical Nutrition 2006, 83 (6 Suppl), 1467s–1476s. [Google Scholar] [CrossRef]
- Baker, E. J.; Miles, E. A.; Burdge, G. C.; Yaqoob, P.; Calder, P. C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Progress in Lipid Research 2016, 64, 30–56. [Google Scholar] [CrossRef]
- Kuipers, R. S.; Luxwolda, M. F.; Janneke Dijck-Brouwer, D. A.; Eaton, S. B.; Crawford, M. A.; Cordain, L.; Muskiet, F. A. J. Estimated macronutrient and fatty acid intakes from an East African Paleolithic diet. British Journal of Nutrition 2010, 104, 1666–1687. [Google Scholar] [CrossRef]
- Simopoulos, A. P.; DiNicolantonio, J. J. The importance of a balanced ω-6 to ω-3 ratio in the prevention and management of obesity. Open Heart 2016, 3, e000385. [Google Scholar] [CrossRef]
- Harris, W. S.; Mozaffarian, D.; Lefevre, M.; Toner, C. D.; Colombo, J.; Cunnane, S. C.; Holden, J. M.; Klurfeld, D. M.; Morris, M. C.; Whelan, J. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. Journal of Nutrition 2009, 139, 804s–19s. [Google Scholar] [CrossRef]
- Kris-Etherton, P. M.; Grieger, J. A.; Etherton, T. D. Dietary reference intakes for DHA and EPA. Prostaglandins Leukot Essent Fatty Acids 2009, 81, 99–104. [Google Scholar] [CrossRef]
- Siscovick, D. S.; Barringer, T. A.; Fretts, A. M.; Wu, J. H. Y.; Lichtenstein, A. H.; Costello, R. B.; Kris-Etherton, P. J., M. ; A., T.; Engler, M. B.; Alger, H. M.; Appel, L. J.; Mozaffarian, D., Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease. Circulation 2017, 135, (15), e867-e884. [CrossRef]
- Lenighan, Y. M.; McNulty, B. A.; Roche, H. M. Dietary fat composition: replacement of saturated fatty acids with PUFA as a public health strategy, with an emphasis on α-linolenic acid. Proceedings of the Nutrition Society 2019, 78, 234–245. [Google Scholar] [CrossRef]
- Weylandt, K. H.; Serini, S.; Chen, Y. Q.; Su, H.-M.; Lim, K.; Cittadini, A.; Calviello, G. Omega-3 Polyunsaturated Fatty Acids: The Way Forward in Times of Mixed Evidence. BioMed Research International 2015, 2015, 143109. [Google Scholar] [CrossRef]
- Saini, R. K.; Prasad, P.; Sreedhar, R. V.; Akhilender Naidu, K.; Shang, X.; Keum, Y. S. Omega-3 Polyunsaturated Fatty Acids (PUFAs): Emerging Plant and Microbial Sources, Oxidative Stability, Bioavailability, and Health Benefits-A Review. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S. A. Omega-3 fatty acids EPA and DHA: health benefits throughout life. Advances in Nutrition 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Bae, J. H.; Lim, H.; Lim, S. The Potential Cardiometabolic Effects of Long-Chain ω-3 Polyunsaturated Fatty Acids: Recent Updates and Controversies. Advances in Nutrition 2023, 14, 612–628. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P. C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- DiNicolantonio, J. J.; O'Keefe, J. H. The Importance of Marine Omega-3s for Brain Development and the Prevention and Treatment of Behavior, Mood, and Other Brain Disorders. Nutrients 2020, 12. [Google Scholar] [CrossRef]
- Liao, J.; Xiong, Q.; Yin, Y.; Ling, Z.; Chen, S. The Effects of Fish Oil on Cardiovascular Diseases: Systematical Evaluation and Recent Advance. Frontiers in Cardiovascular Medicine 2021, 8, 802306. [Google Scholar] [CrossRef]
- Mone, P.; Varzideh, F.; Kansakar, U.; Infante, C.; Lombardi, A.; de Donato, A.; Frullone, S.; Santulli, G. Omega-3 fatty acids coordinate glucose and lipid metabolism in diabetic patients. Lipids in Health and Disease 2022, 21, 31. [Google Scholar] [CrossRef]
- Abdelhamid, A. S.; Brown, T. J.; Brainard, J. S.; Biswas, P.; Thorpe, G. C.; Moore, H. J.; Deane, K. H.; Summerbell, C. D.; Worthington, H. V.; Song, F.; Hooper, L. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Systematic Reviews 2020, 3, Cd003177. [Google Scholar] [CrossRef]
- Sherratt, S. C. R.; Libby, P.; Budoff, M. J.; Bhatt, D. L.; Mason, R. P. Role of Omega-3 Fatty Acids in Cardiovascular Disease: the Debate Continues. Current Atherosclerosis Reports 2023, 25, 1–17. [Google Scholar] [CrossRef]
- Jo, S.-H.; Han, S. H.; Kim, S.-H.; Eckel, R. H.; Koh, K. K. Cardiovascular effects of omega-3 fatty acids: Hope or hype? Atherosclerosis 2021, 322, 15–23. [Google Scholar] [CrossRef]
- Toth, P. P.; Chapman, M. J.; Parhofer, K. G.; Nelson, J. R. Differentiating EPA from EPA/DHA in cardiovascular risk reduction. American Heart Journal Plus: Cardiology Research and Practice 2022, 17, 100148. [CrossRef]
- Liput, K. P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C. S.; Czarnik, U.; Pierzchała, M. Effects of Dietary n-3 and n-6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. International Journal of Molecular Sciences 2021, 22. [Google Scholar] [CrossRef]
- Mukhametov, A.; Yerbulekova, M.; Aitkhozhayeva, G.; Tuyakova, G.; Dautkanova, D. Effects of ω-3 fatty acids and ratio of ω-3/ω-6 for health promotion and disease prevention. Food Science and Technology 2022, 42, e58321. [Google Scholar] [CrossRef]
- Ferdosh, S.; Sarker, M. Z.; Jinap, S.; Saari, N.; Akanda, M. J.; Abbas, K. A.; Norulaini, N. PUFAs in Fish: Extraction, Fractionation, Importance in Health. Comprehensive Reviews in Food Science and Food Safety 2009, 8, 59–74. [Google Scholar] [CrossRef]
- Simopoulos, A. P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomedicine & Pharmacotherapy 2002, 56, 365–379. [CrossRef]
- Zhang, T.-T.; Xu, J.; Wang, Y.-M.; Xue, C.-H. Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Progress in Lipid Research 2019, 75, 100997. [Google Scholar] [CrossRef]
- Shen, Q.; Li, L.; Song, G.; Feng, J.; Li, S.; Wang, Y.; Ma, J.; Wang, H. Development of an intelligent surgical knife rapid evaporative ionization mass spectrometry based method for real-time differentiation of cod from oilfish. Journal of Food Composition and Analysis 2020, 86, 103355. [Google Scholar] [CrossRef]
- Ramprasath, V. R.; Eyal, I.; Zchut, S.; Shafat, I.; Jones, P. J. Supplementation of krill oil with high phospholipid content increases sum of EPA and DHA in erythrocytes compared with low phospholipid krill oil. Lipids in Health and Disease 2015, 14, 142. [Google Scholar] [CrossRef]
- Ferreira, I.; Rauter, A. P.; Bandarra, N. M. Marine Sources of DHA-Rich Phospholipids with Anti-Alzheimer Effect. Marine Drugs 2022, 20, 662. [Google Scholar] [CrossRef]
- Sehl, A.; Couëdelo, L.; Chamekh-Coelho, I.; Vaysse, C.; Cansell, M. In vitro lipolysis and lymphatic absorption of n-3 long-chain PUFA in the rat: influence of the molecular lipid species as carrier. British Journal of Nutrition 2019, 122, 639–647. [Google Scholar] [CrossRef]
- Cansell, M. Marine phospholipids as dietary carriers of long-chain polyunsaturated fatty acids. Lipid Technology 2010, 22, 223–226. [Google Scholar] [CrossRef]
- Ahmmed, M. K.; Ahmmed, F.; Tian, H. S.; Carne, A.; Bekhit, A. E. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Comprehensive Reviews in Food Science and Food Safety 2020, 19, 64–123. [Google Scholar] [CrossRef]
- Wang, C.; Wang, D.; Xu, J.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. DHA enriched phospholipids with different polar groups (PC and PS) had different improvements on MPTP-induced mice with Parkinson’s disease. Journal of Functional Foods 2018, 45, 417–426. [Google Scholar] [CrossRef]
- Ahmmed, M.; Ahmmed, F.; Tian, H.; Carne, A.; Bekhit, A. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Comprehensive Reviews in Food Science and Food Safety 2019, 19. [Google Scholar] [CrossRef]
- Grey, A.; Bolland, M. Clinical Trial Evidence and Use of Fish Oil Supplements. JAMA Internal Medicine 2014, 174, 460–462. [Google Scholar] [CrossRef]
- de Magalhães, J. P.; Müller, M.; Rainger, G. E.; Steegenga, W. Fish oil supplements, longevity and aging. Aging (Albany NY) 2016, 8, 1578–1582. [CrossRef]
- Wymann, M. P.; Schneiter, R. Lipid signalling in disease. Nature Reviews Molecular Cell Biology 2008, 9, 162–176. [Google Scholar] [CrossRef]
- Deckelbaum, R. J.; Torrejon, C. The omega-3 fatty acid nutritional landscape: health benefits and sources. Journal of Nutrition 2012, 142, 587s–591s. [Google Scholar] [CrossRef]
- Gebauer, S. K.; Psota, T. L.; Harris, W. S.; Kris-Etherton, P. M. n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. The American Journal of Clinical Nutrition 2006, 83 (6 Suppl), 1526s–1535s. [Google Scholar] [CrossRef]
- Gogus, U.; Smith, C. n-3 Omega fatty acids: a review of current knowledge. International Journal of Food Science & Technology 2010, 45, 417–436. [CrossRef]
- Nigam, D.; Yadav, R.; Tiwari, U., Omega-3 Fatty Acids and Its Role in Human Health. In Functional Food and Human Health, Rani, V.; Yadav, U. C. S., Eds. Springer Singapore: Singapore, 2018; pp 173-198.
- Hama, S.; Ogino, C.; Kondo, A. Enzymatic synthesis and modification of structured phospholipids: recent advances in enzyme preparation and biocatalytic processes. Applied Microbiology and Biotechnology 2015, 99, 7879–7891. [Google Scholar] [CrossRef]
- Braverman, N. E.; Moser, A. B. Functions of plasmalogen lipids in health and disease. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 2012, 1822, 1442–1452. [CrossRef]
- Vítová, M.; Palyzová, A.; Řezanka, T. Plasmalogens - Ubiquitous molecules occurring widely, from anaerobic bacteria to humans. Progress in Lipid Research 2021, 83, 101111. [Google Scholar] [CrossRef]
- Hossain, M. S.; Mawatari, S.; Fujino, T. Biological Functions of Plasmalogens. Advances in Experimental Medicine and Biology 2020, 1299, 171–193. [Google Scholar] [CrossRef]
- Goodenowe, D. B.; Haroon, J.; Kling, M. A.; Zielinski, M.; Mahdavi, K.; Habelhah, B.; Shtilkind, L.; Jordan, S. Targeted Plasmalogen Supplementation: Effects on Blood Plasmalogens, Oxidative Stress Biomarkers, Cognition, and Mobility in Cognitively Impaired Persons. Frontiers in Cell and Developmental Biology 2022, 10, 864842. [Google Scholar] [CrossRef]
- Yamashita, S.; Miyazawa, T.; Higuchi, O.; Kinoshita, M.; Miyazawa, T. Marine Plasmalogens: A Gift from the Sea with Benefits for Age-Associated Diseases. Molecules 2023, 28, 6328. [Google Scholar] [CrossRef]
- Bozelli, J. C.; Azher, S.; Epand, R. M. Plasmalogens and Chronic Inflammatory Diseases. Frontiers in Physiology 2021, 12. [Google Scholar] [CrossRef]
- Chakraborty, K.; Joy, M. High-value compounds from the molluscs of marine and estuarine ecosystems as prospective functional food ingredients: An overview. Food Research International 2020, 137, 109637. [Google Scholar] [CrossRef]
- Joy, M.; Chakraborty, K.; Raola, V. K. New sterols with anti-inflammatory potentials against cyclooxygenase-2 and 5-lipoxygenase from Paphia malabarica. Natural Product Research 2017, 31, 1286–1298. [Google Scholar] [CrossRef]
- Šimat, V.; Rathod, N. B.; Čagalj, M.; Hamed, I.; Mekinić, I. G. Astaxanthin from Crustaceans and Their Byproducts: A Bioactive Metabolite Candidate for Therapeutic Application. Marine Drugs 2022, 20. [Google Scholar] [CrossRef]
- Ambati, R. R.; Phang, S. M.; Ravi, S.; Aswathanarayana, R. G. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications--a review. Marine Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Elbandy, M. Anti-Inflammatory Effects of Marine Bioactive Compounds and Their Potential as Functional Food Ingredients in the Prevention and Treatment of Neuroinflammatory Disorders. Molecules 2022, 28. [Google Scholar] [CrossRef]
- Tilami, S. K.; Sampels, S. Nutritional Value of Fish: Lipids, Proteins, Vitamins, and Minerals. Reviews in Fisheries Science & Aquaculture 2018, 26, 243–253. [CrossRef]
- Samia Ben, M.-G.; Dhouha, S.-N., Vitamin E: Natural Antioxidant in the Mediterranean Diet. In Vitamin E in Health and Disease, Pınar, E.; Júlia Scherer, S., Eds. IntechOpen: Rijeka, 2021; p Ch. 8.
- Rizvi, S.; Raza, S. T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of vitamin e in human health and some diseases. Sultan Qaboos University Medical Journal 2014, 14, e157–e165. [Google Scholar] [PubMed]
- Gamna, F.; Spriano, S. Vitamin E: A Review of Its Application and Methods of Detection When Combined with Implant Biomaterials. Materials (Basel) 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Li, P.; Zhu, Y.; He, J.; Zhang, M.; Liu, K.; Lin, H.; Zhai, H.; Li, X.; Ma, Y. Comparative bioactivity profile of phospholipids from three marine byproducts based on the zebrafish model. Journal of Food Biochemistry 2022, 46, e14229. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhang, L.; Zhao, H.; Li, J.; You, H.; Jiang, L.; Hu, J. Activation of Macrophages in vitro by Phospholipids from Brain of Katsuwonus pelamis (Skipjack Tuna). Journal of Oleo Science 2018, 67, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiao, G.; Song, C.; Gu, S.; Brown, R. E.; Zhang, J.; Zhang, P.; Gagnon, J.; Locke, S.; Stefanova, R.; Pelletier, C.; Zhang, Y.; Lu, H. An Extract from Shrimp Processing By-Products Protects SH-SY5Y Cells from Neurotoxicity Induced by Aβ(25-35). Marine Drugs 2017, 15. [Google Scholar] [CrossRef]
- Gómez-Guillén, M. C.; Montero, P.; López-Caballero, M. E.; Baccan, G. C.; Gómez-Estaca, J. Bioactive and technological functionality of a lipid extract from shrimp (L. vannamei) cephalothorax. LWT 2018, 89, 704–711. [CrossRef]
- López-Saiz, C.; Coronado-Aceves, E.; Tavera-Hernández, R.; Espitia-Pinzón, C.; Jiménez-Estrada, M.; Morán-Corrales, P.; Hernández-Zazueta, M. Antibacterial and antimycobacterial activity of white shrimp (Litopenaeus vannamei) exoskeleton and cephalothorax by-products extracts: fatty acids profile of the active hexanic shrimp cephalothorax extract. Biotecnia 2022, 24, 45–52. [Google Scholar] [CrossRef]
- Fitahia, E. M.; Raheriniaina, C. E.; Bazin, M. A.; Huvelin, J.-M.; Logé, C.; Ranaivoson, E.; Nazih, H. Anti-proliferative and Pro-apoptotic Effect of Dichloromethane Extract of Octopus vulgaris By-Products on Human Breast Cancer Cell Lines. Waste and Biomass Valorization 2015, 6, 237–242. [Google Scholar] [CrossRef]
- Toda, Y.; Sato, T.; Hosomi, R.; Harumatsu, S.; Fukuda, S.; Matsuda, Y.; Yoshida, M.; Fukunaga, K. Lipid Components Prepared from an Oyster-extract By-product Decreases Triacylglycerol Contents by Suppressing Acetyl-CoA Carboxylase Activity and Lowering the Stearoyl-CoA Desaturase Index in Rat Livers. Trace Nutrients Research 2015, 32, 20–26. [Google Scholar] [CrossRef]
- Tran, N. K.; Kwon, J. E.; Kang, S. C.; Shim, S. M.; Park, T. S. Crassaostrea gigas oyster shell extract inhibits lipogenesis via suppression of serine palmitoyltransferase. Natural Product Communications 2015, 10, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M. V.; Zanwar, A. A.; Adekar, S. P., Nutrition, Life, Disease, and Death. In Omega-3 Fatty Acids: Keys to Nutritional Health, Hegde, M. V.; Zanwar, A. A.; Adekar, S. P., Eds. Springer International Publishing: Cham, 2016; pp 1-10.
- Patel, A.; Desai, S. S.; Mane, V. K.; Enman, J.; Rova, U.; Christakopoulos, P.; Matsakas, L. Futuristic food fortification with a balanced ratio of dietary ω-3/ω-6 omega fatty acids for the prevention of lifestyle diseases. Trends in Food Science & Technology 2022, 120, 140–153. [CrossRef]
- Mohebi-Nejad, A.; Bikdeli, B. Omega-3 supplements and cardiovascular diseases. Tanaffos 2014, 13, 6–14. [Google Scholar] [PubMed]
- Bauer, I.; Hughes, M.; Rowsell, R.; Cockerell, R.; Pipingas, A.; Crewther, S.; Crewther, D. Omega-3 supplementation improves cognition and modifies brain activation in young adults. Human Psychopharmacology 2014, 29, 133–144. [Google Scholar] [CrossRef]
- Sørensen, A.-D. M.; Nielsen, N. S.; Jacobsen, C. Oxidative stability of fish oil-enriched mayonnaise-based salads. European Journal of Lipid Science and Technology 2010, 112, 476–487. [Google Scholar] [CrossRef]
- Let, M. B.; Jacobsen, C.; Meyer, A. S. Lipid Oxidation in Milk, Yoghurt, and Salad Dressing Enriched with Neat Fish Oil or Pre-Emulsified Fish Oil. Journal of Agricultural and Food Chemistry 2007, 55, 7802–7809. [Google Scholar] [CrossRef]
- Gomez-Estaca, J.; Comunian, T. A.; Montero, P.; Ferro-Furtado, R.; Favaro-Trindade, C. S. Encapsulation of an astaxanthin-containing lipid extract from shrimp waste by complex coacervation using a novel gelatin–cashew gum complex. Food Hydrocolloids 2016, 61, 155–162. [Google Scholar] [CrossRef]
- Montero, P.; Calvo, M. M.; Gómez-Guillén, M. C.; Gómez-Estaca, J. Microcapsules containing astaxanthin from shrimp waste as potential food coloring and functional ingredient: Characterization, stability, and bioaccessibility. LWT 2016, 70, 229–236. [Google Scholar] [CrossRef]
- Venugopalan, V. K.; Gopakumar, L. R.; Kumaran, A. K.; Chatterjee, N. S.; Soman, V.; Peeralil, S.; Mathew, S.; McClements, D. J.; Nagarajarao, R. C. Encapsulation and Protection of Omega-3-Rich Fish Oils Using Food-Grade Delivery Systems. Foods 2021, 10, 1566. [Google Scholar] [CrossRef]
- Srigley, C.; Rader, J. I. T. Content and Composition of Fatty Acids in Marine Oil Omega-3 Supplements. Journal of Agricultural and Food Chemistry 2014, 62, 7268–7278. [Google Scholar] [CrossRef]
- Erkan, O. N.; Tunçelli, İ. C. A. N.; Özden, Ö. Content and economic evaluation of omega-3 fatty acid nutritional supplements. Journal of Food and Nutrition Research 2023, 62, 14–25. [Google Scholar]
- yahoo!finance Global Lipid Nutrition Market Report to 2031 - by Product, Source, Form, Application, Distribution and Region. https://finance.yahoo.com/news/global-lipid-nutrition-market-report-102800783.html.
- Laganà, P.; Avventuroso, E.; Romano, G.; Gioffré, M. E.; Patanè, P.; Parisi, S.; Moscato, U.; Delia, S.; Laganà, P.; Avventuroso, E. The Codex Alimentarius and the European legislation on food additives. Chemistry and hygiene of food additives 2017, 23–32. [Google Scholar] [CrossRef]
- Bandarra, N. M.; Campos, R. M.; Batista, I.; Nunes, M. L.; Empis, J. M. Antioxidant synergy of α-tocopherol and phospholipids. Journal of the American Oil Chemists' Society 1999, 76, 905–913. [CrossRef]
- Hidalgo, F. J.; León, M. M.; Zamora, R. Antioxidative Activity of Amino Phospholipids and Phospholipid/Amino Acid Mixtures in Edible Oils As Determined by the Rancimat Method. Journal of Agricultural and Food Chemistry 2006, 54, 5461–5467. [Google Scholar] [CrossRef]
- Robert, C.; Couëdelo, L.; Vaysse, C.; Michalski, M. C. Vegetable lecithins: A review of their compositional diversity, impact on lipid metabolism and potential in cardiometabolic disease prevention. Biochimie 2020, 169, 121–132. [Google Scholar] [CrossRef]
- Haq, M.; Suraiya, S.; Ahmed, S.; Chun, B.-S. Phospholipids from marine source: Extractions and forthcoming industrial applications. Journal of Functional Foods 2021, 80, 104448. [Google Scholar] [CrossRef]
- Drescher, S.; van Hoogevest, P. The Phospholipid Research Center: Current Research in Phospholipids and Their Use in Drug Delivery. Pharmaceutics 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian Journal of Pharmaceutical Sciences 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Mundargi, R. C.; Taneja, N.; Hadia, J. J.; Khopade, A. J. Liposomes as Targeted Drug-Delivery Systems. In Targeted Drug Delivery, 2022; pp 69-125.
- Brunt, E. G.; Burgess, J. G. The promise of marine molecules as cosmetic active ingredients. International Journal of Cosmetic Science 2018, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, L.; Guagni, M. Zooceuticals and Cosmetic Ingredients Derived from Animals. Cosmetics 2022, 9, 13. [Google Scholar] [CrossRef]
- Gulzar, S.; Raju, N.; Ravishankar, C.; Benjakul, S. Oil and pigments from shrimp processing by-products: Extraction, composition, bioactivities and its application- A review. Trends in Food Science & Technology 2020, 100. [CrossRef]
- Nag, M.; Lahiri, D.; Dey, A.; Sarkar, T.; Pati, S.; Joshi, S.; Bunawan, H.; Mohammed, A.; Edinur, H. A.; Ghosh, S.; Ray, R. R. Seafood Discards: A Potent Source of Enzymes and Biomacromolecules With Nutritional and Nutraceutical Significance. Frontiers in Nutrition 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Zaffarin, A. S. M.; Ng, S. F.; Ng, M. H.; Hassan, H.; Alias, E. Pharmacology and Pharmacokinetics of Vitamin E: Nanoformulations to Enhance Bioavailability. International Journal of Nanomedicine 2020, 15, 9961–9974. [Google Scholar] [CrossRef]
- Fiume, M. M.; Bergfeld, W. F.; Belsito, D. V.; Hill, R. A.; Klaassen, C. D.; Liebler, D. C.; Marks, J. G., Jr.; Shank, R. C.; Slaga, T. J.; Snyder, P. W.; Andersen, F. A.; Heldreth, B. Safety Assessment of Tocopherols and Tocotrienols as Used in Cosmetics. International Journal of Toxicology 2018, 37, (2_suppl), 61s-94s. [CrossRef]
- Bohnes, F. A.; Hauschild, M. Z.; Schlundt, J.; Laurent, A. Life cycle assessments of aquaculture systems: a critical review of reported findings with recommendations for policy and system development. Reviews in Aquaculture 2019, 11, 1061–1079. [Google Scholar] [CrossRef]
- Colombo, S.; Beheshti, M.; Parrish, C. Fats and Oils in Aquafeed Formulations. 2020; pp 1-28.
- Santigosa, E.; Olsen, R. E.; Madaro, A.; Søfteland, L.; Carr, I. The impact of varying EPA:DHA ratio on Atlantic salmon health and welfare. Aquaculture 2023, 576, 739868. [Google Scholar] [CrossRef]
- Alhazzaa, R.; Nichols, P. D.; Carter, C. G. Sustainable alternatives to dietary fish oil in tropical fish aquaculture. Reviews in Aquaculture 2019, 11, 1195–1218. [Google Scholar] [CrossRef]
- Turchini, G. M.; Torstensen, B. E.; Ng, W.-K. Fish oil replacement in finfish nutrition. Reviews in Aquaculture 2009, 1, 10–57. [Google Scholar] [CrossRef]
- Kok, B.; Malcorps, W.; Tlusty, M. F.; Eltholth, M. M.; Auchterlonie, N. A.; Little, D. C.; Harmsen, R.; Newton, R. W.; Davies, S. J. Fish as feed: Using economic allocation to quantify the Fish In: Fish Out ratio of major fed aquaculture species. Aquaculture 2020, 528, 735474. [Google Scholar] [CrossRef]
- Zou, Y.; Heyndrickx, M.; Debode, J.; Raes, K.; de Pascale, D.; Behan, P.; Giltrap, M.; O’Connor, C.; Solstad, R. G.; Lian, K.; Altintzoglou, T.; Dragøy, R.; Scheers, N.; Undeland, I.; Robbens, J. Valorisation of crustacean and bivalve processing side streams for industrial fast time-to-market products: A review from the European Union regulation perspective. Frontiers in Marine Science 2023, 10. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M. d. L.; Christensen, H.; Dusemund, B.; Kouba, M.; Kos Durjava, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; Mayo, B.; Pechová, A.; Petkova, M.; Ramos, F.; Sanz, Y.; Villa, R. E.; Woutersen, R.; Bories, G.; Brantom, P.; Renshaw, D.; Schlatter, J. R.; Ackerl, R.; Holczknecht, O.; Steinkellner, H.; Vettori, M. V.; Gropp, J. Safety and efficacy of astaxanthin-dimethyldisuccinate (Carophyll® Stay-Pink 10%-CWS) for salmonids, crustaceans and other fish. EFSA Panel on Additives Products or Substances used in Animal Feed 2019, 17, e05920. [Google Scholar] [PubMed]
- Dmytrów, I.; Szymczak, M.; Szkolnicka, K.; Kamiński, P. Development of Functional Acid Curd Cheese (Tvarog) with Antioxidant Activity Containing Astaxanthin from Shrimp Shells Preliminary Experiment. Foods 2021, 10, (4). [CrossRef]
- Racioppo, A.; Speranza, B.; Campaniello, D.; Sinigaglia, M.; Corbo, M. R.; Bevilacqua, A. Fish Loss/Waste and Low-Value Fish Challenges: State of Art, Advances, and Perspectives. Foods 2021, 10, (11). [CrossRef]
- Guillen, J.; Holmes, S. J.; Carvalho, N.; Casey, J.; Dörner, H.; Gibin, M.; Mannini, A.; Vasilakopoulos, P.; Zanzi, A. A Review of the European Union Landing Obligation Focusing on Its Implications for Fisheries and the Environment. Sustainability 2018, 10, 900. [Google Scholar] [CrossRef]
- Affairs, U. N.-D. o. E. a. S. Sustainable Development Goals. https://sdgs.un.org/goals.
- Dwivedi, S. L.; Lammerts van Bueren, E. T.; Ceccarelli, S.; Grando, S.; Upadhyaya, H. D.; Ortiz, R. Diversifying Food Systems in the Pursuit of Sustainable Food Production and Healthy Diets. Trends in Plant Science 2017, 22, 842–856. [Google Scholar] [CrossRef]
- Rudovica, V.; Rotter, A.; Gaudêncio, S. P.; Novoveská, L.; Akgül, F.; Akslen-Hoel, L. K.; Alexandrino, D. A. M.; Anne, O.; Arbidans, L.; Atanassova, M.; Bełdowska, M.; Bełdowski, J.; Bhatnagar, A.; Bikovens, O.; Bisters, V.; Carvalho, M. F.; Catalá, T. S.; Dubnika, A.; Erdoğan, A.; Ferrans, L.; Haznedaroglu, B. Z.; Setyobudi, R. H.; Graca, B.; Grinfelde, I.; Hogland, W.; Ioannou, E.; Jani, Y.; Kataržytė, M.; Kikionis, S.; Klun, K.; Kotta, J.; Kriipsalu, M.; Labidi, J.; Lukić Bilela, L.; Martínez-Sanz, M.; Oliveira, J.; Ozola-Davidane, R.; Pilecka-Ulcugaceva, J.; Pospiskova, K.; Rebours, C.; Roussis, V.; López-Rubio, A.; Safarik, I.; Schmieder, F.; Stankevica, K.; Tamm, T.; Tasdemir, D.; Torres, C.; Varese, G. C.; Vincevica-Gaile, Z.; Zekker, I.; Burlakovs, J. Valorization of Marine Waste: Use of Industrial By-Products and Beach Wrack Towards the Production of High Added-Value Products. Frontiers in Marine Science 2021, 8. [Google Scholar] [CrossRef]
- Cadavid-Rodríguez, L. S.; Vargas-Muñoz, M. A.; Plácido, J. Biomethane from fish waste as a source of renewable energy for artisanal fishing communities. Sustainable Energy Technologies and Assessments 2019, 34, 110–115. [Google Scholar] [CrossRef]
- Lopes, C.; Antelo, L. T.; Franco-Uría, A.; Alonso, A. A.; Pérez-Martín, R. Valorisation of fish by-products against waste management treatments--Comparison of environmental impacts. Waste Management 2015, 46, 103–112. [Google Scholar] [CrossRef]
- Monteiro, A.; Paquincha, D.; Martins, F.; Queirós, R. P.; Saraiva, J. A.; Švarc-Gajić, J.; Nastić, N.; Delerue-Matos, C.; Carvalho, A. P. Liquid by-products from fish canning industry as sustainable sources of ω3 lipids. Journal of Environmental Management 2018, 219, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Stoll, J. S.; Bailey, M.; Jonell, M. Alternative pathways to sustainable seafood. Conservation Letters 2020, 13, e12683. [Google Scholar] [CrossRef]
- Anderson, C. M.; Himes-Cornell, A.; Pita, C.; Arton, A.; Favret, M.; Averill, D.; Stohs, S.; Longo, C. S. Social and Economic Outcomes of Fisheries Certification: Characterizing Pathways of Change in Canned Fish Markets. Frontiers in Marine Science 2021, 8. [Google Scholar] [CrossRef]
- Caruso, G.; Floris, R.; Serangeli, C.; Di Paola, L. Fishery Wastes as a Yet Undiscovered Treasure from the Sea: Biomolecules Sources, Extraction Methods and Valorization. Marine Drugs 2020, 18, 622. [Google Scholar] [CrossRef]
- Granito, R. N.; Renno, A. C. M.; Yamamura, H.; de Almeida, M. C.; Menin Ruiz, P. L.; Ribeiro, D. A. Hydroxyapatite from Fish for Bone Tissue Engineering: A Promising Approach. International Journal of Molecular and Cellular Medicine 2018, 7, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Wang, Z.; Hao, Y.; Zhang, W. Marine Products As a Promising Resource of Bioactive Peptides: Update of Extraction Strategies and Their Physiological Regulatory Effects. Journal of Agricultural and Food Chemistry 2022, 70, 3081–3095. [Google Scholar] [CrossRef] [PubMed]
- Adeoti, I. A.; Hawboldt, K. A review of lipid extraction from fish processing by-product for use as a biofuel. Biomass and Bioenergy 2014, 63, 330–340. [Google Scholar] [CrossRef]
- Jaiswal, K. K.; Dutta, S.; Banerjee, I.; V.P, M.; Bhushan, M., Chapter ten - Lipid Extraction From Fish Processing Residues for Sustainable Biofuel Production. In Sustainable Fish Production and Processing, Galanakis, C. M., Ed. Academic Press: 2022; pp 293-319.
- Vicente, F. A.; Ventura, S. P. M.; Passos, H.; Dias, A. C. R. V.; Torres-Acosta, M. A.; Novak, U.; Likozar, B. Crustacean waste biorefinery as a sustainable cost-effective business model. Chemical Engineering Journal 2022, 442, 135937. [Google Scholar] [CrossRef]
- Hülsey, M. J. Shell biorefinery: A comprehensive introduction. Green Energy & Environment 2018, 3, 318–327. [CrossRef]
- Fiori, L.; Volpe, M.; Lucian, M.; Anesi, A.; Manfrini, M.; Guella, G. From Fish Waste to Omega-3 Concentrates in a Biorefinery Concept. Waste and Biomass Valorization 2017, 8, 2609–2620. [Google Scholar] [CrossRef]
- Commission, E. Circular economy action plan - The EU’s new circular action plan paves the way for a cleaner and more competitive Europe. https://environment.ec.europa.eu/strategy/circular-economyaction-plan_en.
- Commission, E. Bioeconomy Strategy. https://knowledge4policy.ec.europa.eu/bioeconomy/bioeconomystrategy_en.
- Commission, E. EU Biorefinery Outlook to 2030. https://research-and-innovation.ec.europa.eu/news/allresearch-and-innovation-news/eu-biorefinery-outlook-2030-2021-06-02_en.
- Gilman, E.; Roda, A. P.; Huntington, T.; Kennelly, S. J.; Suuronen, P.; Chaloupka, M.; Medley, P. A. H. Benchmarking global fisheries discards. Scientific Reports 2020, 10, 14017. [Google Scholar] [CrossRef] [PubMed]
- Alfio, V. G.; Manzo, C.; Micillo, R. From Fish Waste to Value: An Overview of the Sustainable Recovery of Omega-3 for Food Supplements. Molecules 2021, 26, 1002. [Google Scholar] [CrossRef]
- Sachindra, N. M.; Mahendrakar, N. S. Stability of carotenoids recovered from shrimp waste and their use as colorant in fish sausage. Journal of Food Science and Technology 2010, 47, 77–83. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, D.-Y.; Liu, Z.-Y.; Yin, F.-W.; Liu, Z.-Q.; Li, D.-Y.; Shahidi, F. Hydrolysis and oxidation of lipids in mussel Mytilus edulis during cold storage. Food Chemistry 2019, 272, 109–116. [Google Scholar] [CrossRef]
- Calvo-Flores, F. G.; Martin-Martinez, F. J. Biorefineries: Achievements and challenges for a bio-based economy. Frontiers in Chemistry 2022, 10. [Google Scholar] [CrossRef]
- Rombaut, N.; Tixier, A.-S.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuels, Bioproducts and Biorefining 2014, 8, 530–544. [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M. T. Biorefineries as a driver for sustainability: Key aspects, actual development and future prospects. Journal of Cleaner Production 2023, 418, 137925. [Google Scholar] [CrossRef]
- Bligh, E. G.; Dyer, W. J. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G. H. S. A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Council, E. P. a. o. t. Directive 2009/32/EC of the European Parliament and of the Council. Official Journal of the European Union 2009, L 141, 3–11. [Google Scholar]
- de Jesus, S. S.; Filho, R. M. Recent advances in lipid extraction using green solvents. Renewable and Sustainable Energy Reviews 2020, 133, 110289. [Google Scholar] [CrossRef]
- Patil, P. D.; Patil, S. P.; Kelkar, R. K.; Patil, N. P.; Pise, P. V.; Nadar, S. S. Enzyme-assisted supercritical fluid extraction: An integral approach to extract bioactive compounds. Trends in Food Science & Technology 2021, 116, 357–369. [CrossRef]
- Quitério, E.; Grosso, C.; Ferraz, R.; Delerue-Matos, C.; Soares, C. A Critical Comparison of the Advanced Extraction Techniques Applied to Obtain Health-Promoting Compounds from Seaweeds. Marine Drugs 2022, 20, (11). [CrossRef]
- Mwaurah, P. W.; Kumar, S.; Kumar, N.; Attkan, A. K.; Panghal, A.; Singh, V. K.; Garg, M. K. Novel oil extraction technologies: Process conditions, quality parameters, and optimization. Comprehensive Reviews in Food Science and Food Safety 2020, 19, 3–20. [Google Scholar] [CrossRef]
- Marsol-Vall, A.; Aitta, E.; Guo, Z.; Yang, B. Green technologies for production of oils rich in n-3 polyunsaturated fatty acids from aquatic sources. Critical Reviews in Food Science and Nutrition 2022, 62, 2942–2962. [Google Scholar] [CrossRef]
- Rubio-Rodríguez, N.; de Diego, S. M.; Beltrán, S.; Jaime, I.; Sanz, M. T.; Rovira, J. Supercritical fluid extraction of fish oil from fish by-products: A comparison with other extraction methods. Journal of Food Engineering 2012, 109, 238–248. [Google Scholar] [CrossRef]
- Zhou, J.; Gullón, B.; Wang, M.; Gullón, P.; Lorenzo, J. M.; Barba, F. J. The Application of Supercritical Fluids Technology to Recover Healthy Valuable Compounds from Marine and Agricultural Food Processing By-Products: A Review. Processes 2021, 9, 357. [Google Scholar] [CrossRef]
- Amar, K.; Hind, M., Valorization Technologies of Marine By-Products. In Innovation in the Food Sector Through the Valorization of Food and Agro-Food By-Products, Ana Novo de, B.; Irene, G., Eds. IntechOpen: Rijeka, 2020; p Ch. 3.
- Ahmed, R.; Haq, M.; Cho, y. j.; Chun, B.-S. Quality Evaluation of Oil Recovered from By-products of Bigeye Tuna Using Supercritical Carbon Dioxide Extraction. Turkish Journal of Fisheries and Aquatic Sciences 2017, 14, 663–672. [Google Scholar] [CrossRef]
- Sprick, B.; Linghu, Z.; Amamcharla, J. K.; Metzger, L. E.; Smith, J. S. Selective extraction of phospholipids from whey protein phospholipid concentrate using supercritical carbon dioxide and ethanol as a co-solvent. Journal of Dairy Science 2019, 102, 10855–10866. [Google Scholar] [CrossRef]
- Huss, H. H. Quality and quality changes in fresh fish. Rome, 1995.
- Hossain, M. B.; Bhuiyan, N. Z.; Kasem, A.; Hossain, M. K.; Sultana, S.; Nur, A.-A. U.; Yu, J.; Albeshr, M. F.; Arai, T. Heavy Metals in Four Marine Fish and Shrimp Species from a Subtropical Coastal Area: Accumulation and Consumer Health Risk Assessment. Biology 2022, 11, 1780. [Google Scholar] [CrossRef]
- Worm, B.; Lotze, H. K.; Jubinville, I.; Wilcox, C.; Jambeck, J. Plastic as a Persistent Marine Pollutant. Annual Review of Environment and Resources 2017, 42, 1–26. [Google Scholar] [CrossRef]
- Maes, J.; De Meulenaer, B.; Van Heerswynghels, P.; De Greyt, W.; Eppe, G.; De Pauw, E.; Huyghebaert, A. Removal of dioxins and PCB from fish oil by activated carbon and its influence on the nutritional quality of the oil. Journal of the American Oil Chemists' Society 2005, 82, 593–597. [CrossRef]
- Karp, W. A.; Breen, M.; Borges, L.; Fitzpatrick, M.; Kennelly, S. J.; Kolding, J.; Nielsen, K. N.; Viðarsson, J. R.; Cocas, L.; Leadbitter, D., Strategies Used Throughout the World to Manage Fisheries Discards – Lessons for Implementation of the EU Landing Obligation. In The European Landing Obligation: Reducing Discards in Complex, Multi-Species and Multi-Jurisdictional Fisheries, Uhlmann, S. S.; Ulrich, C.; Kennelly, S. J., Eds. Springer International Publishing: Cham, 2019; pp 3-26.
- Council, E. P. a. o. t. Regulation (EC) No 999/2001 of the European Parliament and of the Council. Official Journal of the European Union 2001, 147, 1–40. [Google Scholar]
- Council, E. P. a. o. t. Commission Regulation (EU) No 142/2011. Official Journal of the European Union 2011, L 54, 1–254. [Google Scholar]
- Meijer, N.; Van Raamsdonk, L. W. D.; Gerrits, E. W. J.; Appel, M. J. The use of animal by-products in a circular bioeconomy: Time for a TSE road map 3? Heliyon 2023, 9, e14021. [Google Scholar] [CrossRef]
- Commission, E. Communication from the commission to the European parliament, the European council, the council, the European economic and social committee and the committee of the regions. A new Circular Economy Action Plan. For a cleaner and more competitive Europe. /TXT/?uri=COM%3A2020%3A98%3AFIN, 11/March/2020. European Commission Brussels, 2020.
| Species | Co-product | Extraction | PUFAs | Omega-3 | n-6/3 | EPA | DHA | Ref. |
|---|---|---|---|---|---|---|---|---|
| Champsocephalus gunnari | Brain | Solvent (hexane) | 0% | 0% | ------ | 0% | 0% | [100] |
| Champsocephalus gunnari | Liver | Solvent (hexane) | 2.29% | 1.34% | 0 | 1.34% | 0% | [100] |
| Champsocephalus gunnari | Stomach | Solvent (hexane) | 35.6% | 32.7% | 0.02 | 15.9% | 14.9% | [100] |
| Champsocephalus gunnari | Skin | Solvent (hexane) | 27.8% | 25.9% | 0 | 15.6% | 8.0% | [100] |
| Clupea harengus | Co-product mix (heads, fins, tails and viscera) | Bligh & Dyer | 35.5% | 26.4% | 0.34 | 5.6% | 9.2% | [99] |
| Clupea harengus | Minced co-product (heads, frames, skin and viscera) | Bligh & Dyer | 21.9% | ------ | ------ | 6.4% | 9.4% | [89] |
| Dicentrarchus labrax | Heads | Folch | 28.0% | 12.4% | 1.21 | 3.0% | 5.1% | [83] |
| Dicentrarchus labrax | Frames | Folch | 29.4% | 13.1% | 1.19 | 3.2% | 5.2% | [83] |
| Dicentrarchus labrax | Skin | Folch | 33.8% | 16.6% | 1.00 | 4.0% | 7.5% | [83] |
| Dicentrarchus labrax | Trimmings | Folch | 27.3% | 11.0% | 1.43 | 2.6% | 4.3% | [83] |
| Dicentrarchus labrax | Viscera | Folch | 27.9% | 10.7% | 1.55 | 2.4% | 4.0% | [83] |
| Euthynnus affinis | Head | Bligh & Dyer | 28.8% | 17.2% | 0.67 | 1.5% | 15.7% | [90] |
| Euthynnus affinis | Intestine | Bligh & Dyer | 27.4% | 17.0% | 0.61 | 2.7% | 14.3% | [90] |
| Euthynnus affinis | Liver | Bligh & Dyer | 24.0% | 15.9% | 0.51 | 1.7% | 14.2% | [90] |
| Gadus morhua | Offal (head, viscera and skeletal frames) |
Bligh & Dyer | 32.1% | ------ | ------ | 8.9% | 13.3% | [92] |
| Gadus morhua | Liver | Bligh & Dyer | 24.7% | ------ | ------ | 7.7% | 11.4% | [92] |
| Katsuwonus Pelamis | Heads | Soxhlet | 12.7% | 9.6% | 0.32 | 1.3% | 6.3% | [101] |
| Lophius litulon | Liver | Soxhlet | 46.6% | ------ | ------ | 1.2% | 8.1% | [102] |
| Notothenia rossii | Brain | Solvent (hexane) | 32.9% | 32.2% | 0.02 | 9.8% | 22.0% | [100] |
| Notothenia rossii | Liver | Solvent (hexane) | 26.0% | 21.8% | 0.13 | 8.4% | 11.5% | [100] |
| Notothenia rossii | Stomach | Solvent (hexane) | 41.6% | 30.8% | 0.32 | 11.3% | 18.6% | [100] |
| Notothenia rossii | Skin | Solvent (hexane) | 35.0% | 31.0% | 0.09 | 16.2% | 10.7% | [100] |
| Salmo salar | Heads | Bligh & Dyer | 35.4% | 27.7% | 0.28 | 8.4% | 12.1% | [103] |
| Salmo salar | Heads | Folch | 31.9% | 16.3% | 0.93 | 3.2% | 4.8% | [83] |
| Salmo salar | Frames | Folch | 31.9% | 15.9% | 0.98 | 3.0% | 4.6% | [83] |
| Salmo salar | Skin | Folch | 31.9% | 15.4% | 1.05 | 2.8% | 4.0% | [83] |
| Salmo salar | Trimmings | Folch | 32.0% | 15.9% | 0.98 | 3.0% | 4.0% | [83] |
| Salmo salar | Viscera | Folch | 25.0% | 10.4% | 1.37 | 1.6% | 2.3% | [83] |
| Sardinella lemuru | Head | Bligh & Dyer | 26.4% | 17.8% | 0.54 | 1.8% | 16.0% | [91] |
| Sardinella lemuru | Intestine | Bligh & Dyer | 24.9% | 13.6% | 0.83 | 1.7% | 11.9% | [91] |
| Sardinella lemuru | Liver | Bligh & Dyer | 22.7% | 15.7% | 0.44 | 2.8 | 13.0% | [91] |
| Sardinella aurita | Viscera | Bligh & Dyer | 30.5% | 26.1% | 0.15 | 7.4% | 13.6% | [87] |
| Sarpa salpa | Viscera | Bligh & Dyer | 34.8% | 20.4% | 0.71 | 4.1% | 6.0% | [87] |
| Scomber australasicus | Head | EtOH:hexane | 39.9% | 36.6% | 0.09 | 9.1% | 21.9% | [94] |
| Scomber australasicus | Skin | EtOH:hexane | 38.1% | 34.8% | 0.09 | 9.6% | 19.5% | [94] |
| Scomber australasicus | Roe | EtOH:hexane | 47.0% | 44.4% | 0.06 | 11.3% | 27.5% | [94] |
| Scomber australasicus | Male gonads | EtOH:hexane | 44.7% | 42.5% | 0.05 | 12.1% | 24.7% | [94] |
| Scomber scombrus | Head | Soxhlet | 25.4% | ------ | ------ | 3.6% | 9.3% | [104] |
| Scomber scombrus | Gills | Soxhlet | 12.3% | ------ | ------ | 1.0% | 1.7% | [104] |
| Scophthalmus maximus | Heads | Folch | 36.8% | 22.5% | 0.61 | 4.4% | 11.6% | [83] |
| Scophthalmus maximus | Frames | Folch | 36.5% | 21.7% | 0.64 | 5.2% | 7.9% | [83] |
| Scophthalmus maximus | Skin | Folch | 37.4% | 22.6% | 0.62 | 5.2% | 8.9% | [83] |
| Scophthalmus maximus | Trimmings | Folch | 37.5% | 22.8% | 0.61 | 4.9% | 9.8% | [83] |
| Scophthalmus maximus | Viscera | Folch | 33.3% | 17.7% | 0.86 | 2.7% | 7.6% | [83] |
| Sparus aurata | Fishbone | Bligh & Dyer | 33.8% | 13.6% | 1.48 | 2.8% | 4.6% | [81] |
| Sparus aurata | Frames | Folch | 28.5% | 12.3% | 1.27 | 2.2% | 4.8% | [83] |
| Sparus aurata | Gills | Bligh & Dyer | 31.2% | 11.9% | 1.62 | 1.9% | 4.1% | [81] |
| Sparus aurata | Guts | Bligh & Dyer | 33.1% | 12.1% | 1.75 | 1.8% | 3.5% | [81] |
| Sparus aurata | Head | Bligh & Dyer | 33.8% | 14.0% | 1.41 | 2.8% | 5.0% | [81] |
| Sparus aurata | Head | Folch | 28.4% | 12.7% | 1.20 | 2.2% | 5.2% | [83] |
| Sparus aurata | Liver | Bligh & Dyer | 32.2% | 13.6% | 1.38 | 1.9% | 4.9% | [81] |
| Sparus aurata | Skin | Bligh & Dyer | 33.2% | 12.9% | 1.57 | 2.0% | 4.0% | [81] |
| Sparus aurata | Skin | Folch | 29.9% | 13.2% | 1.21 | 2.3% | 5.5% | [83] |
| Sparus aurata | Trimmings | Folch | 29.6% | 13% | 1.23 | 2.2% | 5.4% | [83] |
| Sparus aurata | Viscera | Folch | 28.8% | 12.9% | 1.20 | 1.7% | 5.9% | [83] |
| Thunnus thynnus | Minced side streams | Folch | 33.2% | 29.9% | 0.06 | 9.9% | 13.6% | [86] |
| Species | Co-product | Extraction | PUFAs | Omega-3 | n-6/3 | EPA | DHA | Ref. |
|---|---|---|---|---|---|---|---|---|
| Chionoecetes opilio | Co-products mix (cephalothorax, digestive systems and physiological liquid) | Bligh & Dyer | 24.4% | 21.1% | 0.10 | 9.9% | 8.9% | [118] |
| Commercial crab (no specified species) |
Shells | Folch | 35.9% | 23.2% | 0.52 | 12.5% | 9.9% | [119] |
| Commercial shrimp (no specified species) |
Shells | Folch | 40.9% | 12.3% | 2.2 | 6.3% | 4.1% | [119] |
| Jasus edwardsii | Hepatopancreas | Soxhlet | 7.8% | 3.1% | 1.52 | 0.9% | 0.9% | [117] |
| Lithodes santolla | Exoskeleton | Bligh & Dyer | 40.0% | 40.0% | 0 | 20.5% | 14.4% | [45] |
| Metapenaeus monoceros | Minced co-product (heads, tails, shells) | Folch | 34.5% | ------ | ------ | 8.9% | 6.9% | [108] |
| Nephrops norvegicus | Heads | Folch | 36.2% | 27.6% | 0.26 | 15.5% | 8.4% | [51] |
| Pandalus borealis | Co-product mix (heads, tails, shells) | Bligh & Dyer | 43.9% | 24.2% | 0.57 | 8.9% | 10.7% | [47] |
| Pandalus borealis | Processing co-product | Soxhlet | 41.1% | 37.1% | 0.11 | 21.1% | 13.9% | [49] |
| Panulirus cygnus | Cephalothorax | Folch | 38.2% | 13.5% | 0.64 | 5.6% | 4.2% | [116] |
| Penaeus japonicus | Hepatopancreas | Folch | 37.2% | 20.0% | 0.82 | 8.4% | 6.1% | [120] |
| Penaeus kerathurus | Cephalothorax | Bligh & Dyer | 44.5% | 28.7% | 0.55 | 14.5% | 13.4% | [114] |
| Penaeus kerathurus | Minced co-product (heads, tails, shells) | Folch | 38.8% | ------ | ------ | 12.2% | 16.1% | [108] |
| Penaeus monodon | Heads | Bligh & Dyer | 44.8% | 29.8% | 0.50 | 15.4% | 13.3% | [113] |
| Penaeus paulensis | Minced co-product (heads, tails, shells) | Bligh & Dyer | 34.6% | 26.0% | 0.30 | 11.7% | 12.2% | [106] |
| Penaeus vannamei | Cephalothorax | Bligh & Dyer | 43.0% | 12.2% | ------ | 5.0% | 7.2% | [121] |
| Penaeus vannamei | Cephalothorax | Folch | 42.5% | 10.5% | ------ | 4.1% | 6.4% | [121] |
|
Penaeus vannamei |
Cephalothorax | Bligh & Dyer | 39.3% | 14.7% | 1.67 | 4.6% | 8.3% | [48] |
| Penaeus vannamei | Cephalothorax | “Typical solvent extraction” | 48.5% | 24.5% | 0.95 | 9.6% | 13.3% | [122] |
| Penaeus vannamei | Cephalothorax | “Solvent extraction” | 37.5% | 18.1% | 1.08 | 9.2% | 8.1% | [123] |
|
Penaeus vannamei |
Hepatopancreas | Bligh & Dyer | 37.4% | 10.6% | 2.53 | 2.2% | 6.2% | [48] |
|
Penaeus vannamei |
Hepatopancreas | Bligh & Dyer | 38.1% | 16.2% | 1.35 | 3.3% | 10.4% | [111] |
| Pleoticus muelleri | Shells | Bligh & Dyer | 52.0% | 50.3% | 0.03 | 21.5% | 22.3% | [45] |
| Pleoticus muelleri | Shells + heads | Bligh & Dyer | 43.9% | 42.3% | 0.04 | 14.9% | 22.0% | [45] |
| Trachypena curvirostris | Co-product mix (heads, tails, shells) | Bligh & Dyer | 48.3% | 26.1% | 0.44 | 10.7% | 10.9% | [47] |
| Hard-shelled mollusks | Co-product | Extraction | PUFAs | Omega-3 | n-6/3 | EPA | DHA | Ref. |
|---|---|---|---|---|---|---|---|---|
|
Pecten maximus |
Pooled mantle, gill, liver, digestive gland, kidney |
Supercritical extraction | 42.1% | 40.7% | 0.03 | 20.0% | 12.3% | [139] |
| Pinna rugosa | Viscera | Folch | 41.8% | 37.5% | ------ | 17.0% | 20.0% | [109] |
| Cephalopods | Co-product | Extraction | PUFAs | Omega-3 | n-6/3 | EPA | DHA | Ref. |
| Commercial squid (no specified species) |
Squid viscera oil | Supercritical extraction | 44.7% | ------ | ------ | 15.1% | 24.9% | [59] |
| Doryteuthis gahi | Pooled viscera, heads, skin | Bligh & Dyer | 52.6% | 48.6% | 0.08 | 17.2% | 30.8% | [140] |
| Dosidicus gigas | Viscera | Folch | 36.6% | 34.0% | ------ | 15.5% | 17.8% | [109] |
| Illex argentinus | Viscera | Wet pressing | ------ | ------ | 0.75 | 9.3% | 16.4% | [141] |
| Octopus vulgaris | Pooled co-products | Bligh & Dyer | 49.3% | 36.8% | 0.34 | 12.9% | 22.2% | [57] |
| Sepia officinalis | Viscera | Bligh & Dyer | 44.0% | 26.0% | 0.69 | 11.6% | 6.3% | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





