Submitted:
12 December 2023
Posted:
13 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Mechanism of ncRNA Action in Astrocytes under HI Conditions
2.1. MiRNAs
2.2. LncRNAs and circRNAs
3. Non-Coding RNAs Modulate Astrocytes in Neuroinflammation
3.1. MiRNAs
3.2. LncRNAs and circRNAs
4. Regulatory Effect of Non-Coding RNAs in Astrocytes for Treatment Strategy
4.1. Cell Therapy: Potential in Treatment Targeting Astrocyte
4.2. The Use of miRNAs in Nanotherapeutics
4.3. The Neurogenic Potential of Astrocytes Contributes to Nerve after Brain Injury
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Perin, J.; Mulick, A.; Yeung, D.; Villavicencio, F.; Lopez, G.; Strong, K.L.; Prieto-Merino, D.; Cousens, S.; Black, R.E.; Liu, L. Global, regional, and national causes of under-5 mortality in 2000-19: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc. Health 2022, 6, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Amer, R.; Moddemann, D.; Seshia, M.; Alvaro, R.; Synnes, A.; Lee, K.-S.; Lee, S.K.; Shah, P.S.; Canadian Neonatal Network, C. Neurodevelopmental Outcomes of Infants Born at < 29 Weeks of Gestation Admitted to Canadian Neonatal Intensive Care Units Based on Location of Birth. J. Pediatr. 2018, 196, 31–+. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X.; Chen, X.; Wei, Y. Neuronal injuries in cerebral infarction and ischemic stroke: From mechanisms to treatment (Review). Int. J. Mol. Med. 2022, 49. [Google Scholar] [CrossRef] [PubMed]
- Orellana-Urzua, S.; Rojas, I.; Libano, L.; Rodrigo, R. Pathophysiology of Ischemic Stroke: Role of Oxidative Stress. Curr. Pharm. Des. 2020, 26, 4246–4260. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.D.; McCullough, L.D. Inflammatory responses in hypoxic ischemic encephalopathy. Acta Pharmacol. Sin. 2013, 34, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Kulesh, A.; Drobakha, V.; Kuklina, E.; Nekrasova, I.; Shestakov, V. Cytokine Response, Tract-Specific Fractional Anisotropy, and Brain Morphometry in Post-Stroke Cognitive Impairment. J. Stroke Cerebrovasc. Dis. 2018, 27, 1752–1759. [Google Scholar] [CrossRef]
- Urday, S.; Kimberly, W.T.; Beslow, L.A.; Vortmeyer, A.O.; Selim, M.H.; Rosand, J.; Simard, J.M.; Sheth, K.N. Targeting secondary injury in intracerebral haemorrhage-perihaematomal oedema. Nat. Rev. Neurol. 2015, 11, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.R. Specification and Morphogenesis of Astrocytes. Science 2010, 330, 774–778. [Google Scholar] [CrossRef]
- Disdier, C.; Stonestreet, B.S. Hypoxic-ischemic-related cerebrovascular changes and potential therapeutic strategies in the neonatal brain. J. Neurosci. Res. 2020, 98, 1468–1484. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Astroglial cradle in the life of the synapse. Philos. Trans. R. Soc. B-Biol. Sci. 2014, 369. [Google Scholar] [CrossRef] [PubMed]
- Mari, C.; Odorcyk, F.K.; Sanches, E.F.; Wartchow, K.M.; Martini, A.P.; Nicola, F.; Zanotto, C.; Wyse, A.T.; Gonçalves, C.A.; Netto, C.A. Arundic acid administration protects astrocytes, recovers histological damage and memory deficits induced by neonatal hypoxia ischemia in rats. Int. J. Dev. Neurosci. 2019, 76, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Farina, C. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 2016, 37, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.P.; Fang, G.; Rozowsky, J.; Snyder, M.; Gerstein, M.B. Annotating non-coding regions of the genome. Nat. Rev. Genet. 2010, 11, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Slack, F.J.; Chinnaiyan, A.M. The Role of Non-coding RNAs in Oncology. Cell 2019, 179, 1033–1055. [Google Scholar] [CrossRef] [PubMed]
- Marangon, D.; Castro, E.S.J.H.; Lecca, D. Neuronal and Glial Communication via Non-Coding RNAs: Messages in Extracellular Vesicles. Int. J. Mol. Sci. 2022, 24. [Google Scholar] [CrossRef] [PubMed]
- Douglas-Escobar, M.; Weiss, M.D. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 2015, 169, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Huo, J. A1/A2 astrocytes in central nervous system injuries and diseases: Angels or devils? Neurochem. Int. 2021, 148, 105080. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, P.; Salman, M.M.; Halsey, A.M.; Clarke-Bland, C.; MacDonald, J.A.; Ishida, H.; Vogel, H.J.; Almutiri, S.; Logan, A.; Kreida, S.; et al. Targeting Aquaporin-4 Subcellular Localization to Treat Central Nervous System Edema. Cell 2020, 181, 784. [Google Scholar] [CrossRef]
- Salman, M.M.; Kitchen, P.; Halsey, A.; Wang, M.X.; Tornroth-Horsefield, S.; Conner, A.C.; Badaut, J.; Iliff, J.J.; Bill, R.M. Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain 2022, 145, 64–75. [Google Scholar] [CrossRef]
- Fu, X.; Li, Q.; Feng, Z.; Mu, D. The roles of aquaporin-4 in brain edema following neonatal hypoxia ischemia and reoxygenation in a cultured rat astrocyte model. Glia 2007, 55, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Sepramaniam, S.; Armugam, A.; Lim, K.Y.; Karolina, D.S.; Swaminathan, P.; Tan, J.R.; Jeyaseelan, K. MicroRNA 320a Functions as a Novel Endogenous Modulator of Aquaporins 1 and 4 as Well as a Potential Therapeutic Target in Cerebral Ischemia. J. Biol. Chem. 2010, 285, 29223–29230. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-C.; Li, W.-J.; Li, L.-Z. Regulatory effect of miRNA 320a on expression of aquaporin 4 in brain tissue of epileptic rats. Asian Pac. J. Trop. Med. 2015, 8, 791–796. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, L.; Chen, M.; Pei, A.; Xie, L.; Zhu, S. Upregulation of miR-130b protects against cerebral ischemic injury by targeting water channel protein aquaporin 4 (AQP4). Am. J. Transl. Res. 2017, 9, 3452–3461. [Google Scholar] [PubMed]
- Zheng, L.F.; Cheng, W.; Wang, X.J.; Yang, Z.G.; Zhou, X.L.; Pan, C.L. Overexpression of MicroRNA-145 Ameliorates Astrocyte Injury by Targeting Aquaporin 4 in Cerebral Ischemic Stroke. Biomed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-N.; Sun, X.-L.; Gao, L.; Fan, Y.; Ding, J.-H.; Hu, G. Aquaporin-4 deficiency down-regulates glutamate uptake and GLT-1 expression in astrocytes. Mol. Cell. Neurosci. 2007, 34, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8. [Google Scholar] [CrossRef]
- Pajarillo, E.; Rizor, A.; Lee, J.; Aschner, M.; Lee, E. The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics. Neuropharmacology 2019, 161. [Google Scholar] [CrossRef]
- Morken, T.S.; Brekke, E.; Håberg, A.; Widerøe, M.; Brubakk, A.M.; Sonnewald, U. Altered astrocyte-neuronal interactions after hypoxia-ischemia in the neonatal brain in female and male rats. Stroke 2014, 45, 2777–2785. [Google Scholar] [CrossRef]
- Benediktsson, A.M.; Marrs, G.S.; Tu, J.C.; Worley, P.F.; Roth-Stein, J.D.; Bergles, D.E.; Dailey, M.E. Neuronal activity regulates glutamate transporter dynamics in developing astrocytes. Glia 2012, 60, 175–188. [Google Scholar] [CrossRef]
- Johnston, M.V.; Fatemi, A.; Wilson, M.A.; Northington, F. Treatment advances in neonatal neuroprotection and neurointensive care. Lancet Neurol. 2011, 10, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Zhou, H.; Zhao, Q.; Xue, L.; Al-Hawwas, M.; He, J.; Wu, M.; Zou, Y.; Yang, M.; Dai, J.; et al. Overexpression of miR-124 Protects Against Neurological Dysfunction Induced by Neonatal Hypoxic-Ischemic Brain Injury. Cell. Mol. Neurobiol. 2020, 40, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Jiang, C.; Ye, H.-B.; Jiao, J.-T.; Cheng, C.; Huang, J.; Liu, J.; Zhang, R.; Shao, J.-F. miR-124 upregulates astrocytic glutamate transporter-1 via the Akt and mTOR signaling pathway post ischemic stroke. Brain Res. Bull. 2019, 149, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Hoye, M.L.; Regan, M.R.; Jensen, L.A.; Lake, A.M.; Reddy, L.V.; Vidensky, S.; Richard, J.P.; Maragakis, N.J.; Rothstein, J.D.; Dougherty, J.D.; et al. Motor neuron-derived microRNAs cause astrocyte dysfunction in amyotrophic lateral sclerosis. Brain 2018, 141, 2561–2575. [Google Scholar] [CrossRef] [PubMed]
- Brkic, J.; Dunk, C.; O'Brien, J.; Fu, G.; Nadeem, L.; Wang, Y.-l.; Rosman, D.; Salem, M.; Shynlova, O.; Yougbare, I.; et al. MicroRNA-218-5p Promotes Endovascular Trophoblast Differentiation and Spiral Artery Remodeling. Mol. Ther. 2018, 26, 2189–2205. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Mei, L.; Xie, L. The Clinical Features of 101 Cases with Early-Onset Severe Preeclampsia. J. Pract. Obstet. Gynecol. 2013, 29, 63–66. [Google Scholar]
- Mo, Y.; Sun, Y.-Y.; Liu, K.-Y. Autophagy and inflammation in ischemic stroke. Neural Regen. Res. 2020, 15, 1388–1396. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Liu, Y.; Pan, S.; Zhang, H.; Fang, M.; Jiang, H.; Yin, J.; Zou, S.; Li, Z.; et al. Melatonin reduces hypoxic-ischaemic (HI) induced autophagy and apoptosis: An in vivo and in vitro investigation in experimental models of neonatal HI brain injury. Neurosci. Lett. 2017, 653, 105–112. [Google Scholar] [CrossRef]
- Mao, L.; Liu, S.; Hu, L.; Jia, L.; Wang, H.; Guo, M.; Chen, C.; Liu, Y.; Xu, L. miR-30 Family: A Promising Regulator in Development and Disease. Biomed Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Chang, T.-C.; Yu, D.; Lee, Y.-S.; Wentzel, E.A.; Arking, D.E.; West, K.M.; Dang, C.V.; Thomas-Tikhonenko, A.; Mendell, J.T. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat. Genet. 2008, 40, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, W.Q.; Zhu, H.; Qian, Y.Y.; Zhou, L.; Ren, Y.J.; Ren, X.C.; Zhang, L.; Liu, X.P.; Liu, C.G.; et al. Regulation of autophagy by miR-30d impacts sensitivity of anaplastic thyroid carcinoma to cisplatin. Biochem. Pharmacol. 2014, 87, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Qu, Y.; Wang, H.; Huang, L.; Zhu, J.; Li, S.; Tong, Y.; Zhang, L.; Li, J.; Mu, D. The effect of miR-30d on apoptosis and autophagy in cultured astrocytes under oxygen-glucose deprivation. Brain Res. 2017, 1671, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Prerna, K.; Dubey, V.K. Beclin1-mediated interplay between autophagy and apoptosis: New understanding. Int. J. Biol. Macromol. 2022, 204, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Qu, Y.; Zhu, J.; Zhang, L.; Huang, L.; Liu, H.; Li, S.; Mu, D. miR-30d-5p Plays an Important Role in Autophagy and Apoptosis in Developing Rat Brains After Hypoxic-Ischemic Injury. J. Neuropathol. Exp. Neurol. 2017, 76, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, S.; Yin, Y.; Yu, J.; Liu, Y.; Gao, H. Dexmedetomidine suppresses hippocampal astrocyte pyroptosis in cerebral hypoxic-ischemic neonatal rats by upregulating microRNA-148a-3p to inactivate the STAT/JMJD3 axis. Int. Immunopharmacol. 2023, 121, 110440. [Google Scholar] [CrossRef] [PubMed]
- Gaughwin, P.; Ciesla, M.; Yang, H.; Lim, B.; Brundin, P. Stage-Specific Modulation of Cortical Neuronal Development by Mmu-miR-134. Cereb. Cortex 2011, 21, 1857–1869. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Kadkhoda, S.; Ghafouri-Fard, S. Synaptic plasticity and depression: the role of miRNAs dysregulation. Mol. Biol. Rep. 2022, 49, 9759–9765. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Larsen, L.A.; Kauppinen, S.; Schratt, G. Recombinant adeno-associated virus-mediated microRNA delivery into the postnatal mouse brain reveals a role for miR-134 in dendritogenesis in vivo. Front. Neural Circuits 2010, 3. [Google Scholar] [CrossRef]
- Chen, W.B.; Zhang, L.X.; Zhao, Y.K.; Li, J.; Jiao, Y. C/EBP alpha-mediated transcriptional activation of miR-134-5p entails KPNA3 inhibition and modulates focal hypoxic-ischemic brain damage in neonatal rats. Brain Res. Bull. 2020, 164, 350–360. [Google Scholar] [CrossRef]
- Guan, X.H.; Zhou, W.; Li, L.; Peng, Q.X. Dexmedetomidine Alleviates Hypoxic-Ischemic Brain Damage in Neonatal Rats Through Reducing MicroRNA-134-5p-Mediated NLRX1 Downregulation. J. Stroke Cerebrovasc. Dis. 2022, 31. [Google Scholar] [CrossRef] [PubMed]
- Numakawa, T.; Nakajima, S.; Yamamoto, N.; Ooshima, Y.; Odaka, H.; Hashido, K.; Adachi, N.; Kunugi, H. Basic fibroblast growth factor induces miR-134 upregulation in astrocyte for cell maturation. Biochem. Biophys. Res. Commun. 2015, 456, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Gutschner, T.; Haemmerle, M.; Eissmann, M.; Hsu, J.; Kim, Y.; Hung, G.; Revenko, A.; Arun, G.; Stentrup, M.; Gross, M.; et al. The Noncoding RNA MALAT1 Is a Critical Regulator of the Metastasis Phenotype of Lung Cancer Cells. Cancer Res. 2013, 73, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Michalik, K.M.; You, X.; Manavski, Y.; Doddaballapur, A.; Zoernig, M.; Braun, T.; John, D.; Ponomareva, Y.; Chen, W.; Uchida, S.; et al. Long Noncoding RNA MALAT1 Regulates Endothelial Cell Function and Vessel Growth. Circ. Res. 2014, 114, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Michalik, K.M.; You, X.; Manavski, Y.; Doddaballapur, A.; Zörnig, M.; Braun, T.; John, D.; Ponomareva, Y.; Chen, W.; Uchida, S.; et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ. Res. 2014, 114, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, X.; Jin, J.; Zheng, L.; Guan, T.; Huo, Y.; Xie, S.; Wu, Y.; Chen, W. LncRNA MALAT1 silencing protects against cerebral ischemia-reperfusion injury through miR-145 to regulate AQP4. J. Biomed. Sci. 2020, 27. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.; Wang, Q.; Mao, Y.; Cao, Q.; Li, S.; Qiao, C.; Zhang, D.; Zhou, G. Knockdown of LncRNA MALAT1 contributes to cell apoptosis via regulating NF-kappa B/CD80 axis in neonatal respiratory distress syndrome. Int. J. Biochem. Cell Biol. 2018, 104, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wu, J.; Yu, X.; Zhou, J.; Yu, H.; Ma, L. Long non-coding RNA MALAT1 enhances the apoptosis of cardiomyocytes through autophagy inhibition by regulating TSC2-mTOR signaling. Biol. Res. 2019, 52. [Google Scholar] [CrossRef] [PubMed]
- Pachnis, V.; Belayew, A.; Tilghman, S.M. LOCUS UNLINKED TO ALPHA-FETOPROTEIN UNDER THE CONTROL OF THE MURINE RAF AND RIF GENES. Proc. Natl. Acad. Sci. USA 1984, 81, 5523–5527. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Esmaeili, M.; Taheri, M. H19 lncRNA: Roles in tumorigenesis. Biomed. Pharmacother. 2020, 123. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, L.; Chen, J.; Shen, H.; Chen, Z. Mechanisms Underlying Abnormal Expression of lncRNA H19 in Neonatal Hypoxic-Ischemic Encephalopathy. Am. J. Perinatol. 2022, 39, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, B.; Gao, Y.; Chen, Y.H.; Feng, J. Exosome-transported lncRNA H19 regulates insulin-like growth factor-1 via the H19/let-7a/insulin-like growth factor-1 receptor axis in ischemic stroke. Neural Regen Res. 2023, 18, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Zhu, X.; Zhuo, C. H19/miR-130a-3p/DAPK1 axis regulates the pathophysiology of neonatal hypoxic-ischemia encephalopathy. Neurosci. Res. 2021, 163, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zou, Q.; Lv, D.; Raza, M.A.; Wang, X.; Li, P.; Chen, Y.; Xi, X.; Wen, A.; Zhu, L.; et al. Comprehensive transcriptional profiling of porcine brain aging. Gene 2019, 693, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, E.; Cairns, M.J. Circular RNAs are temporospatially regulated throughout development and ageing in the rat. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, H.J.; Fan, Z.M.; Zhao, R.B.; Xia, Z.K. Circular RNA expression profiles in neonatal rats following hypoxic-ischemic brain damage. Int. J. Mol. Med. 2019, 43, 1699–1708. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Zhang, Y.; Zhang, Y.; Bai, Y.; Chen, X.; Huang, R.; Wu, F.; Leng, S.; Chao, J.; Zhang, J.H.; et al. Novel insight into circular RNA <i>HECTD1</i> in astrocyte activation via autophagy by targeting <i>MIR142</i>-TIPARP: implications for cerebral ischemic stroke. Autophagy 2018, 14, 1164–1184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Huang, Z.; Zhu, X.; Hong, T.; Zhao, Y. Circular RNA 0025984 Ameliorates Ischemic Stroke Injury and Protects Astrocytes Through miR-143-3p/TET1/ORP150 Pathway. Mol. Neurobiol. 2021, 58, 5937–5953. [Google Scholar] [CrossRef]
- Guo, X.X.; He, Q.Z.; Li, W.; Long, D.X.; Pan, X.Y.; Chen, C.; Zeng, H.C. Brain-Derived Neurotrophic Factor Mediated Perfluorooctane Sulfonate Induced-Neurotoxicity via Epigenetics Regulation in SK-N-SH Cells. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Meng, X.; Zhong, J.; Zeng, C.; Yung, K.K.L.; Zhang, X.; Wu, X.; Qu, S. MiR-30a-5p Regulates GLT-1 Function via a PKCα-Mediated Ubiquitin Degradation Pathway in a Mouse Model of Parkinson's Disease. ACS Chem. Neurosci. 2021, 12, 1578–1592. [Google Scholar] [CrossRef]
- Reichenstein, I.; Eitan, C.; Diaz-Garcia, S.; Haim, G.; Magen, I.; Siany, A.; Hoye, M.L.; Rivkin, N.; Olender, T.; Toth, B.; et al. Human genetics and neuropathology suggest a link between miR-218 and amyotrophic lateral sclerosis pathophysiology. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, M. Astrocyte-derived extracellular vesicles inhibit the abnormal activation of immune function in neonatal mice with hypoxic-ischemic brain damage by carrying miR-124-3p. Neurol. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, B.; Sun, S.; Wang, B. Overexpression of long non-coding RNA H19 relieves hypoxia-induced injury by down-regulating microRNA-107 in neural stem cells. Neurosci. Lett. 2021, 753. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, G.; Sabato, C.; Russo, M.; Rosa, A.; Abballe, L.; Besharat, Z.M.; Po, A.; Miele, E.; Bellavia, D.; Chiacchiarini, M.; et al. Loss of miR-107, miR-181c and miR-29a-3p Promote Activation of Notch2 Signaling in Pediatric High-Grade Gliomas (pHGGs). Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-B.; Luo, X.-J.; Ren, K.-D.; Peng, J.-J.; Tan, B.; Liu, B.; Lou, Z.; Xiong, X.-M.; Zhang, X.-J.; Ren, X.; et al. Beneficial effect of magnesium lithospermate B on cerebral ischemia-reperfusion injury in rats involves the regulation of miR-107/glutamate transporter 1 pathway. Eur. J. Pharmacol. 2015, 766, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Wang, L.Q.; Chen, M.L.; Pei, A.J.; Xie, L.W.; Zhu, S.M. Upregulation of miR-130b protects against cerebral ischemic injury by targeting water channel protein aquaporin 4 (AQP4). Am. J. Transl. Res. 2017, 9, 3452–3461. [Google Scholar]
- Winkler, I.; Heisinger, T.; Hammerl, M.; Huber, E.; Urbanek, M.; Kiechl-Kohlendorfer, U.; Griesmaier, E.; Posod, A. MicroRNA Expression Profiles as Diagnostic and Prognostic Biomarkers of Perinatal Asphyxia and Hypoxic-Ischaemic Encephalopathy. Neonatology 2022, 119, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimy, N.; Gasterich, N.; Behrens, V.; Amini, J.; Fragoulis, A.; Beyer, C.; Zhao, W.Y.; Sanadgol, N.; Zendedel, A. Neuroprotective effect of the Nrf2/ARE/miRNA145-5p signaling pathway in the early phase of spinal cord injury. Life Sci. 2022, 304. [Google Scholar] [CrossRef]
- Zhao, H.P.; Li, G.W.; Wang, R.L.; Tao, Z.; Zhang, S.J.; Li, F.F.; Han, Z.P.; Li, L.Z.; Liu, P.; Luo, Y.M. MiR-424 prevents astrogliosis after cerebral ischemia/reperfusion in elderly mice by enhancing repressive H3K27me3 via NFIA/DNMT1 signaling. Febs J. 2019, 286, 4926–4936. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, X.; Yan, W. LncRNA-MALAT1, as a biomarker of neonatal BPD, exacerbates the pathogenesis of BPD by targeting miR-206. Am. J. Transl. Res. 2021, 13, 462–479. [Google Scholar]
- Jin, R.; Yang, G.; Li, G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J. Leukoc. Biol. 2010, 87, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Yap, V.; Perlman, J.M. Mechanisms of brain injury in newborn infants associated with the fetal inflammatory response syndrome. Semin. Fetal Neonatal Med. 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Galinsky, R.; Polglase, G.R.; Hooper, S.B.; Black, M.J.; Moss, T.J.M. The consequences of chorioamnionitis: preterm birth and effects on development. J. Pregnancy 2013, 2013, 412831–412831. [Google Scholar] [CrossRef] [PubMed]
- Arayici, S.; Simsek, G.K.; Oncel, M.Y.; Eras, Z.; Canpolat, F.E.; Oguz, S.S.; Uras, N.; Zergeroglu, S.; Dilmen, U. The effect of histological chorioamnionitis on the short-term outcome of preterm infants <= 32 weeks: a single-center study. J. Matern. -Fetal Neonatal Med. 2014, 27, 1129–1133. [Google Scholar] [CrossRef]
- Disdier, C.; Awa, F.; Chen, X.; Dhillon, S.K.; Galinsky, R.; Davidson, J.O.; Lear, C.A.; Bennet, L.; Gunn, A.J.; Stonestreet, B.S. Lipopolysaccharide-induced changes in the neurovascular unit in the preterm fetal sheep brain. J. Neuroinflammation 2020, 17. [Google Scholar] [CrossRef]
- Leonardo, C.C.; Eakin, A.K.; Ajmo, J.M.; Collier, L.A.; Pennypacker, K.R.; Strongin, A.Y.; Gottschall, P.E. Delayed administration of a matrix metalloproteinase inhibitor limits progressive brain injury after hypoxia-ischemia in the neonatal rat. J. Neuroinflammation 2008, 5. [Google Scholar] [CrossRef]
- Revuelta, M.; Elicegui, A.; Moreno-Cugnon, L.; Buehrer, C.; Matheu, A.; Schmitz, T. Ischemic stroke in neonatal and adult astrocytes. Mech. Ageing Dev. 2019, 183. [Google Scholar] [CrossRef]
- Colombo, E.; Farina, C. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 2016, 37, 608–620. [Google Scholar] [CrossRef]
- Ye, Y.; Hao, J.; Hong, Z.; Wu, T.; Ge, X.; Qian, B.; Chen, X.; Zhang, F. Downregulation of MicroRNA-145-5p in Activated Microglial Exosomes Promotes Astrocyte Proliferation by Removal of Smad3 Inhibition. Neurochem. Res. 2022, 47, 382–393. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Yang, S.-H.; Tzeng, S.-F. MicroRNA-145 as One Negative Regulator of Astrogliosis. Glia 2015, 63, 194–205. [Google Scholar] [CrossRef]
- Capece, D.; Verzella, D.; Flati, I.; Arboretto, P.; Cornice, J.; Franzoso, G. NF-κB: blending metabolism, immunity, and inflammation. Trends Immunol 2022, 43, 757–775. [Google Scholar] [CrossRef]
- Kang, Z.; Altuntas, C.Z.; Gulen, M.F.; Liu, C.; Giltiay, N.; Qin, H.; Liu, L.; Qian, W.; Ransohoff, R.M.; Bergmann, C.; et al. Astrocyte-Restricted Ablation of Interleukin-17-Induced Act1-Mediated Signaling Ameliorates Autoimmune Encephalomyelitis. Immunity 2010, 32, 414–425. [Google Scholar] [CrossRef]
- Shih, R.H.; Wang, C.Y.; Yang, C.M. NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front. Mol. Neurosci. 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, R.; Bracchi-Ricard, V.; Hu, W.H.; Frydel, B.; Bramwell, A.; Karmally, S.; Green, E.J.; Bethea, J.R. Inhibition of astroglial nuclear factor kappa B reduces inflammation and improves functional recovery after spinal cord injury. J. Exp. Med. 2005, 202, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Chibowska, K.; Korbecki, J.; Gutowska, I.; Metryka, E.; Tarnowski, M.; Goschorska, M.; Barczak, K.; Chlubek, D.; Baranowska-Bosiacka, I. Pre- and Neonatal Exposure to Lead (Pb) Induces Neuroinflammation in the Forebrain Cortex, Hippocampus and Cerebellum of Rat Pups. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- Lee, H.-G.; Wheeler, M.A.; Quintana, F.J. Function and therapeutic value of astrocytes in neurological diseases. Nat. Rev. Drug Discov. 2022, 21, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Li, Q.; Kang, L.; Jia, W.; Yan, Y. MicroRNA-155 intensifies hypoxic-ischemic brain injury in neonatal rats by regulating nucleatide-binding oligomerizaton domains 1 / nuclear facter kappaB signaling pathway. Acta Anat. Sin. 2020, 51, 848–854. [Google Scholar]
- He, J.; Zhao, J.; Peng, X.; Shi, X.; Zong, S.; Zeng, G. Molecular Mechanism of MiR-136-5p Targeting NF-kappa B/A20 in the IL-17-Mediated Inflammatory Response after Spinal Cord Injury. Cell. Physiol. Biochem. 2017, 44, 1224–1241. [Google Scholar] [CrossRef]
- Ma, Q.; Dasgupta, C.; Shen, G.; Li, Y.; Zhang, L. MicroRNA-210 downregulates TET2 and contributes to inflammatory response in neonatal hypoxic-ischemic brain injury. J. Neuroinflammation 2021, 18, 6. [Google Scholar] [CrossRef]
- Jiang, D.; Gong, F.; Ge, X.; Lv, C.; Huang, C.; Feng, S.; Zhou, Z.; Rong, Y.; Wang, J.; Ji, C.; et al. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J. Nanobiotechnology 2020, 18. [Google Scholar] [CrossRef]
- Tian, Y.; Tian, Y.; Tu, Y.; Zhang, G.; Zeng, X.; Lin, J.; Ai, M.; Mao, Z.; Zheng, R.; Yuan, Y. microRNA-124 inhibits stem-like properties and enhances radiosensitivity in nasopharyngeal carcinoma cells via direct repression of expression of JAMA. J. Cell. Mol. Med. 2020, 24, 9533–9544. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.-C.; Pastrana, E.; Tavazoie, M.; Doetsch, F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat. Neurosci. 2009, 12, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Zhang, T.; Duan, H.; Pan, Y.; Zhang, X.; Yang, G.; Wang, J.; Deng, Y.; Yang, Z. MiR-124 contributes to M2 polarization of microglia and confers brain inflammatory protection via the C/EBP-alpha, pathway in intracerebral hemorrhage. Immunol. Lett. 2017, 182, 1–11. [Google Scholar] [CrossRef]
- Periyasamy, P.; Liao, K.; Kook, Y.H.; Niu, F.; Callen, S.E.; Guo, M.-L.; Buch, S. Cocaine-Mediated Downregulation of miR-124 Activates Microglia by Targeting KLF4 and TLR4 Signaling. Mol. Neurobiol. 2018, 55, 3196–3210. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Wang, J.; Luo, F.; He, Y. Role of microRNA-124-3p/Bax axis in neonatal hypoxic-ischaemic encephalopathy. Biotechnol. Biotechnol. Equip. 2020, 34, 163–170. [Google Scholar] [CrossRef]
- Hur, J.Y. gamma-Secretase in Alzheimer's disease. Exp. Mol. Med. 2022, 54, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, J.; Zhao, C.; Ren, K.; Xia, Z.; Yu, H.; Jiang, K. Acute Blockage of Notch Signaling by DAPT Induces Neuroprotection and Neurogenesis in the Neonatal Rat Brain After Stroke. Transl. Stroke Res. 2016, 7, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; He, K.; Wang, F.W.; Li, X.; Liu, D.J. Notch-1 signaling regulates astrocytic proliferation and activation after hypoxia exposure. Neurosci. Lett. 2015, 603, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Aslani, M.; Mortazavi-Jahromi, S.S.; Mirshafiey, A. Efficient roles of miR-146a in cellular and molecular mechanisms of neuroinflammatory disorders: An effectual review in neuroimmunology. Immunol. Lett. 2021, 238, 1–20. [Google Scholar] [CrossRef]
- Zhong, X.; Jiang, Y.Z.; Liu, P.; He, W.; Xiong, Z.; Chang, W.; Zhu, J.; Cui, Q. Toll-like 4 receptor /NFκB inflammatory/miR-146a pathway contributes to the ART-correlated preterm birth outcome. Oncotarget 2016, 7, 72475–72485. [Google Scholar] [CrossRef]
- Liu, X.S.; Chopp, M.; Pan, W.L.; Wang, X.L.; Fan, B.Y.; Zhang, Y.; Kassis, H.; Zhang, R.L.; Zhang, X.M.; Zhang, Z.G. MicroRNA-146a Promotes Oligodendrogenesis in Stroke. Mol. Neurobiol. 2017, 54, 227–237. [Google Scholar] [CrossRef]
- Rigg, E.; Wang, J.; Xue, Z.; Lunavat, T.R.; Liu, G.; Hoang, T.; Parajuli, H.; Han, M.; Bjerkvig, R.; Nazarov, P.V.; et al. Inhibition of extracellular vesicle-derived miR-146a-5p decreases progression of melanoma brain metastasis via Notch pathway dysregulation in astrocytes. J. Extracell Vesicles 2023, 12, e12363. [Google Scholar] [CrossRef]
- Li, H.; Tang, C.; Wang, D. LncRNA H19 promotes inflammatory response induced by cerebral ischemia-reperfusion injury through regulating the miR-138-5p-p65 axis. Biochem Cell Biol. 2020, 98, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, Y.; Dai, Y.; Wang, B.; Li, L.; Jiang, B.; Wu, P.; Xu, J. The LncRNA H19/miR-1-3p/CCL2 axis modulates lipopolysaccharide (LPS) stimulation-induced normal human astrocyte proliferation and activation. Cytokine 2020, 131. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, M.Z. Igf2-H19, an imprinted tandem gene, is an important regulator of embryonic development, a guardian of proliferation of adult pluripotent stem cells, a regulator of longevity, and a 'passkey' to cancerogenesis. Folia Histochem. Et Cytobiol. 2012, 50, 171–179. [Google Scholar] [CrossRef]
- Zhao, W.; Lin, X.Y.; Han, H.; Zhang, H.X.; Li, X.L.; Jiang, C.M.; Feng, M. Long noncoding RNA H19 contributes to the proliferation and autophagy of glioma cells through mTOR/ULK1 pathway. Neuroreport 2021, 32, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.H.; Zhang, B.H.; Fang, C.Z.; Yan, C.X.; Lai, F.F.; Chen, S.; Wang, G.H. Long non-coding RNA CRNDE deteriorates intrauterine infection-induced neonatal brain injury. Mol. Cell Probes. 2020, 52, 101565. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.L.; Hiranita, T.; Hong, W.C.; Job, M.O.; McCurdy, C.R. A Role for Sigma Receptors in Stimulant Self-Administration and Addiction. In Sigma Proteins: Evolution of the Concept of Sigma Receptors; Handbook of Experimental Pharmacology; Kim, F.J., Pasternak, G.W., Eds.; 2017; Volume 244, pp. 177–218. [Google Scholar]
- Martin, P.; Reeder, T.; Sourbron, J.; de Witte, P.A.M.; Gammaitoni, A.R.; Galer, B.S. An Emerging Role for Sigma-1 Receptors in the Treatment of Developmental and Epileptic Encephalopathies. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Loranca, B.E.; Garces-Ramirez, L.; Rosales, A.A.M.; Ramirez, C.L.; Hernandez, G.V.; Morales-Dionisio, O.; Gonzalez-Elizalde, K.; Flores, G.; Zamudio, S.; De la Cruz-Lopez, F. The Sigma Agonist 1,3-Di-o-tolyl-guanidine Reduces the Morphological and Behavioral Changes Induced by Neonatal Ventral Hippocampus Lesion in Rats. Synapse 2015, 69, 213–225. [Google Scholar] [CrossRef]
- Huang, R.R.; Zhang, Y.; Han, B.; Bai, Y.; Zhou, R.B.; Gan, G.M.; Chao, J.; Hu, G.; Yao, H.H. Circular RNA HIPK2 regulates astrocyte activation via cooperation of autophagy and ER stress by targeting MIR124-2HG. Autophagy 2017, 13, 1722–1741. [Google Scholar] [CrossRef]
- Zheng, Q.; Bao, C.; Guo, W.; Li, S.; Chen, J.; Chen, B.; Luo, Y.; Lyu, D.; Li, Y.; Shi, G.; et al. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yan, X.F.; Si, Y.; Chen, X.Z. CTGF Triggers Rat Astrocyte Activation and Astrocyte-Mediated Inflammatory Response in Culture Conditions. Inflammation 2019, 42, 1693–1704. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, H.; Zhong, J.; Yang, J.; Darwazeh, R.; Tian, X.; Huang, Z.; Jiang, L.; Cheng, C.; Wu, Y.; et al. Significant changes in circular RNA in the mouse cerebral cortex around an injury site after traumatic brain injury. Exp. Neurol. 2019, 313, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.L.; Sun, L.L.; Xiao, C.Y.; You, W.J.; Sun, L.; Wang, S.Y.; Zhang, Z.J.; Liu, S. Circular RNA METTL9 contributes to neuroinflammation following traumatic brain injury by complexing with astrocytic SND1. J. Neuroinflammation 2023, 20. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Zurolo, E.; Prabowo, A.; Fluiter, K.; Spliet, W.G.M.; van Rijen, P.C.; Gorter, J.A.; Aronica, E. MicroRNA-146a: A Key Regulator of Astrocyte-Mediated Inflammatory Response. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Gomes, C.; Sequeira, C.; Gonçalves-Ribeiro, J.; Pina, C.C.; Carvalho, L.A.; Moreira, R.; Vaz, S.H.; Vaz, A.R.; Brites, D. Recovery of Depleted miR-146a in ALS Cortical Astrocytes Reverts Cell Aberrancies and Prevents Paracrine Pathogenicity on Microglia and Motor Neurons. Front. Cell Dev. Biol. 2021, 09. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Chang, Q.; Zhang, Y.; Guan, X.; Ma, Z.; Chen, X.; Liu, W.; Li, Y.; Feng, H. MiR-155-5p Aggravated Astrocyte Activation and Glial Scarring in a Spinal Cord Injury Model by Inhibiting Ndfip1 Expression and PTEN Nuclear Translocation. Neurochem. Res. 2023, 48, 1912–1924. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.Y.; Gao, Y.B.; Cen, Z.X.; He, J.C.; Cao, B.C.; Zeng, G.F.; Zong, S.H. miR-136-5p Regulates the Inflammatory Response by Targeting the IKK/NF-B/A20 Pathway After Spinal Cord Injury. Cell. Physiol. Biochem. 2018, 50, 512–524. [Google Scholar] [CrossRef]
- Fu, C.H.; Lai, F.F.; Chen, S.; Yan, C.X.; Zhang, B.H.; Fang, C.Z.; Wang, G.H. Silencing of long non-coding RNA CRNDE promotes autophagy and alleviates neonatal hypoxic-ischemic brain damage in rats. Mol. Cell Biochem. 2020, 472, 1–8. [Google Scholar] [CrossRef]
- Huang, R.; Cai, L.; Ma, X.; Shen, K. Autophagy-mediated circHIPK2 promotes lipopolysaccharide-induced astrocytic inflammation via SIGMAR1. Int. Immunopharmacol. 2023, 117. [Google Scholar] [CrossRef]
- Chen, Z.H.; Wang, H.; Zhong, J.J.; Yang, J.Q.; Darwazeh, R.; Tian, X.C.; Huang, Z.J.; Jiang, L.; Cheng, C.J.; Wu, Y.; et al. Significant changes in circular RNA in the mouse cerebral cortex around an injury site after traumatic brain injury. Exp. Neurol. 2019, 313, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Dallerac, G.; Chever, O.; Rouach, N. How do astrocytes shape synaptic transmission? Insights from electrophysiology. Front. Cell. Neurosci. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Xu, D.; Luo, Y.; Li, X.; Gu, Y.; Wang, L. Homeostatic Regulation of Astrocytes by Visual Experience in the Developing Primary Visual Cortex. Cereb Cortex 2022, 32, 970–986. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, X.; Chen, L.; Lenahan, C.; Fu, Z.; Fang, Y.; Yu, W. Crosstalk Between the Oxidative Stress and Glia Cells After Stroke: From Mechanism to Therapies. Front. Immunol. 2022, 13, 852416. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic Analysis of Reactive Astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Ikegaya, Y.; Koyama, R. The effects of microglia- and astrocyte-derived factors on neurogenesis in health and disease. Eur. J. Neurosci. 2021, 54, 5880–5901. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.M.; Miller, R.H.; Silver, J. CHANGING-ROLE OF FOREBRAIN ASTROCYTES DURING DEVELOPMENT, REGENERATIVE FAILURE, AND INDUCED REGENERATION UPON TRANSPLANTATION. J. Comp. Neurol. 1986, 251, 23–43. [Google Scholar] [CrossRef]
- Kliot, M.; Smith, G.M.; Siegal, J.D.; Silver, J. ASTROCYTE POLYMER IMPLANTS PROMOTE REGENERATION OF DORSAL-ROOT FIBERS INTO THE ADULT MAMMALIAN SPINAL-CORD. Exp. Neurol. 1990, 109, 57–69. [Google Scholar] [CrossRef]
- Becerra-Calixto, A.; Cardona-Gómez, G.P. Neuroprotection Induced by Transplanted CDK5 Knockdown Astrocytes in Global Cerebral Ischemic Rats. Mol. Neurobiol. 2017, 54, 6681–6696. [Google Scholar] [CrossRef]
- Becerra-Calixto, A.; Posada-Duque, R.; Cardona-Gómez, G.P. Recovery of Neurovascular Unit Integrity by CDK5-KD Astrocyte Transplantation in a Global Cerebral Ischemia Model. Mol. Neurobiol. 2018, 55, 8563–8585. [Google Scholar] [CrossRef] [PubMed]
- Merienne, N.; Delzor, A.; Viret, A.; Dufour, N.; Rey, M.; Hantraye, P.; Deglon, N. Gene transfer engineering for astrocyte-specific silencing in the CNS. Gene Ther. 2015, 22, 830–839. [Google Scholar] [CrossRef]
- Nicaise, C.; Mitrecic, D.; Falnikar, A.; Lepore, A.C. Transplantation of stem cell-derived astrocytes for the treatment of amyotrophic lateral sclerosis and spinal cord injury. World J. Stem Cells 2015, 7, 380–398. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Chang, Y.S.; Sung, D.K.; Sung, S.I.; Park, W.S. Hypothermia broadens the therapeutic time window of mesenchymal stem cell transplantation for severe neonatal hypoxic ischemic encephalopathy. Sci. Rep. 2018, 8, 7665. [Google Scholar] [CrossRef]
- Corcelli, M.; Hawkins, K.; Vlahova, F.; Hunjan, A.; Dowding, K.; De Coppi, P.; David, A.L.; Peebles, D.; Gressens, P.; Hagberg, H.; et al. Neuroprotection of the hypoxic-ischemic mouse brain by human CD117(+)CD90(+)CD105(+) amniotic fluid stem cells. Sci. Rep. 2018, 8, 2425. [Google Scholar] [CrossRef]
- Mora, S.; Muniz-Garcia, A.; Zorzano, A. Molecular Mechanisms of Hypoxia-Induced Extracellular Vesicle Release. Faseb J. 2020, 34. [Google Scholar] [CrossRef]
- Urabe, F.; Kosaka, N.; Ito, K.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am. J. Physiol. Cell Physiol. 2020, 318, C29–c39. [Google Scholar] [CrossRef]
- Li, Y.; Tan, J.; Miao, Y.; Zhang, Q. MicroRNA in extracellular vesicles regulates inflammation through macrophages under hypoxia. Cell Death Discov. 2021, 7. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Xian, P.; Hei, Y.; Wang, R.; Wang, T.; Yang, J.; Li, J.; Di, Z.; Liu, Z.; Baskys, A.; Liu, W.; et al. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics 2019, 9, 5956–5975. [Google Scholar] [CrossRef]
- Buller, B.; Liu, X.; Wang, X.; Zhang, R.L.; Zhang, L.; Hozeska-Solgot, A.; Chopp, M.; Zhang, Z.G. MicroRNA-21 protects neurons from ischemic death. Febs J. 2010, 277, 4299–4307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Lv, Q.; Gao, J.; Hu, L.; He, Z. MicroRNA-21 Overexpression Promotes the Neuroprotective Efficacy of Mesenchymal Stem Cells for Treatment of Intracerebral Hemorrhage. Front. Neurol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Xin, D.-Q.; Zhao, Y.-J.; Li, T.-T.; Ke, H.-F.; Gai, C.-C.; Guo, X.-F.; Chen, W.-Q.; Liu, D.-X.; Wang, Z. The delivery of miR-21a-5p by extracellular vesicles induces microglial polarization via the STAT3 pathway following hypoxia-ischemia in neonatal mice. Neural Regen. Res. 2022, 17, 2238–2246. [Google Scholar] [CrossRef]
- Qiu, L.; Cai, Y.; Geng, Y.; Yao, X.; Wang, L.; Cao, H.; Zhang, X.; Wu, Q.; Kong, D.; Ding, D.; et al. Mesenchymal stem cell-derived extracellular vesicles attenuate tPA-induced blood-brain barrier disruption in murine ischemic stroke models. Acta Biomater. 2022, 154, 424–442. [Google Scholar] [CrossRef] [PubMed]
- Li, L.C.; Li, M.C. Astrocyte-derived extracellular vesicles inhibit the abnormal activation of immune function in neonatal mice with hypoxic-ischemic brain damage by carrying miR-124-3p. Neurol. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.-Y.D.; Shetty, A.K. Treating Parkinson's disease by astrocyte reprogramming: Progress and challenges. Sci. Adv. 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.A.; Jaronen, M.; Covacu, R.; Zandee, S.E.J.; Scalisi, G.; Rothhammer, V.; Tjon, E.C.; Chao, C.C.; Kenison, J.E.; Blain, M.; et al. Environmental Control of Astrocyte Pathogenic Activities in CNS Inflammation. Cell 2019, 176, 581. [Google Scholar] [CrossRef] [PubMed]
- Buffo, A.; Rite, I.; Tripathi, P.; Lepier, A.; Colak, D.; Horn, A.-P.; Mori, T.; Goetz, M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc. Natl. Acad. Sci. USA 2008, 105, 3581–3586. [Google Scholar] [CrossRef] [PubMed]
- Robel, S.; Berninger, B.; Goetz, M. The stem cell potential of glia: lessons from reactive gliosis. Nat. Rev. Neurosci. 2011, 12, 88–104. [Google Scholar] [CrossRef]
- Blum, R.; Heinrich, C.; Sanchez, R.; Lepier, A.; Gundelfinger, E.D.; Berninger, B.; Goetz, M. Neuronal Network Formation from Reprogrammed Early Postnatal Rat Cortical Glial Cells. Cerebral Cortex 2011, 21, 413–424. [Google Scholar] [CrossRef]
- Berninger, B.; Costa, M.R.; Koch, U.; Schroeder, T.; Sutor, B.; Grothe, B.; Gotz, M. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J. Neurosci. 2007, 27, 8654–8664. [Google Scholar] [CrossRef]
- Aravantinou-Fatorou, K.; Ortega, F.; Chroni-Tzartou, D.; Antoniou, N.; Poulopoulou, C.; Politis, P.K.; Berninger, B.; Matsas, R.; Thomaidou, D. CEND1 and NEUROGENIN2 Reprogram Mouse Astrocytes and Embryonic Fibroblasts to Induced Neural Precursors and Differentiated Neurons. Stem Cell Rep. 2015, 5, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Cates, K.; McCoy, M.J.; Kwon, J.-S.; Liu, Y.; Abernathy, D.G.; Zhang, B.; Liu, S.; Gontarz, P.; Kim, W.K.; Chen, S.; et al. Deconstructing Stepwise Fate Conversion of Human Fibroblasts to Neurons by MicroRNAs. Cell Stem Cell 2021, 28, 127. [Google Scholar] [CrossRef] [PubMed]
- Drouin-Ouellet, J.; Legault, E.M.; Nilsson, F.; Pircs, K.; Bouquety, J.; Petit, F.; Shrigley, S.; Birtele, M.; Pereira, M.; Storm, P.; et al. Age-related pathological impairments in directly reprogrammed dopaminergic neurons derived from patients with idiopathic Parkinson's disease. Stem Cell Rep. 2022, 17, 2203–2219. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, E.; Koutsoudaki, P.N.; Thanou, I.; Karagkouni, D.; Karamitros, T.; Chroni-Tzartou, D.; Gaitanou, M.; Gkemisis, C.; Margariti, M.; Xingi, E.; et al. A miR-124-mediated post-transcriptional mechanism controlling the cell fate switch of astrocytes to induced neurons. Stem Cell Rep. 2023, 18, 915–935. [Google Scholar] [CrossRef]
- Diaz-Castro, B.; Bernstein, A.M.; Coppola, G.; Sofroniew, M.V.; Khakh, B.S. Molecular and functional properties of cortical astrocytes during peripherally induced neuroinflammation. Cell Rep. 2021, 36. [Google Scholar] [CrossRef]
- Zhang, P.J.; Wu, W.Y.; Chen, Q.; Chen, M. Non-Coding RNAs and their Integrated Networks. J. Integr. Bioinform. 2019, 16. [Google Scholar] [CrossRef]
- Drommelschmidt, K.; Serdar, M.; Bendix, I.; Herz, J.; Bertling, F.; Prager, S.; Keller, M.; Ludwig, A.K.; Duhan, V.; Radtke, S.; et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav. Immun. 2017, 60, 220–232. [Google Scholar] [CrossRef]
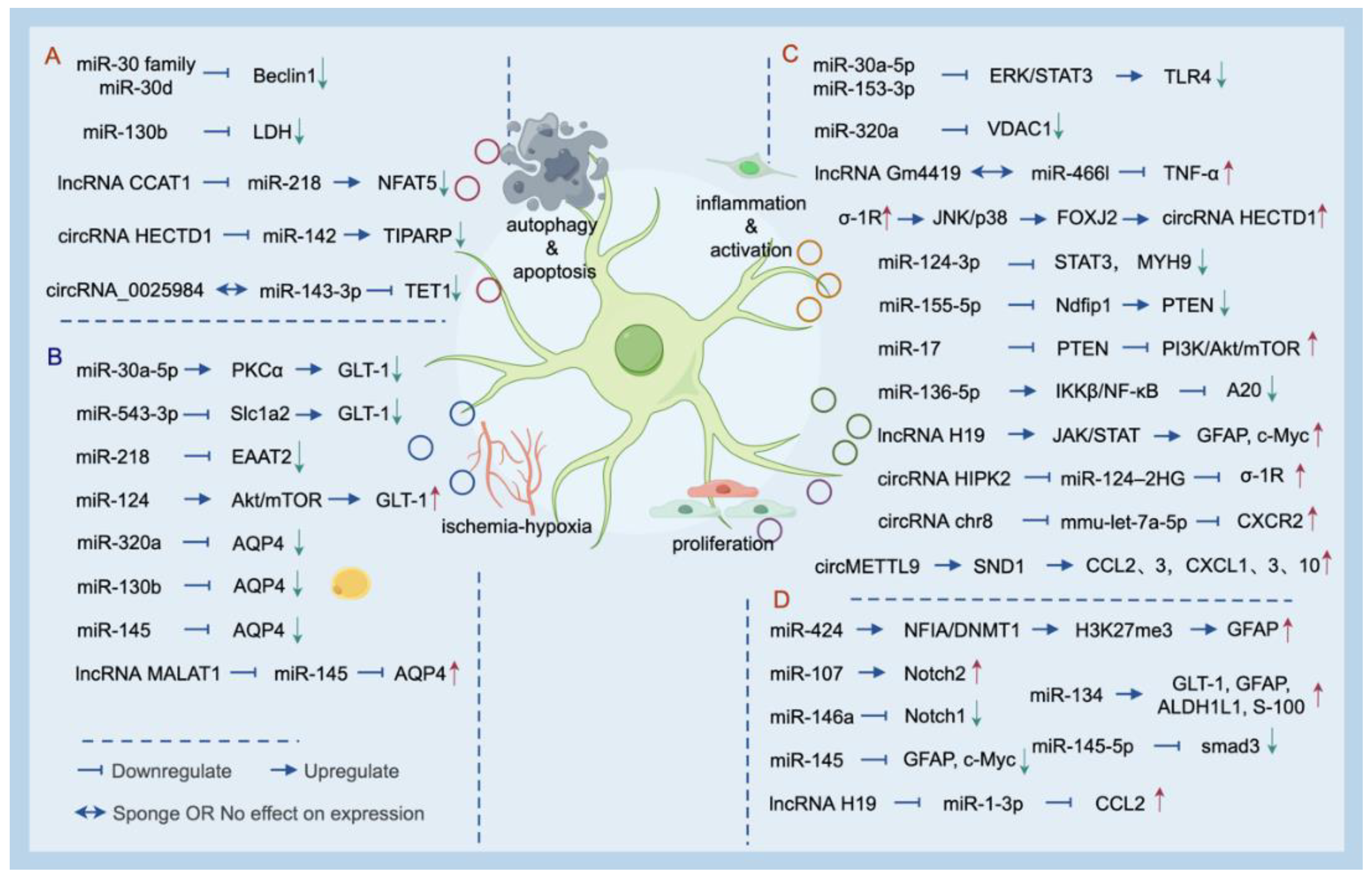
| miR-30 family | miR-30a-5p | Regulation of reactive astrocyte or microglia proliferation | Neonatal exposure[69]; PD[70]; |
| miR-30d | Promotion of autophagy in astrocytes | Neonatal HIE[45]; OGD-induced autophagy[43] |
|
| miR-148a-3p | Inhibition of pyroptosis in astrocytes | Neonatal HIE[46] | |
| miR-218 | miR-218-5p | Inhibition of GLT-1 expression in astrocyte; Promotion of vascular remodeling | PE[35]; ALS[71]; |
| miR-124 | Inhibit of GLT-1 expression; Biomarkers of cerebral ischemia |
Perinatal Asphyxia and HIE[72]; Ischemic Stroke[33]; |
|
| miR-107 | Inhibit of GLT-1 expression; promotion of tumor cell proliferation | Neonatal HIE[73]; Pediatric High-grade glioma[74]; Ischemic Stroke[75]; |
|
| miR-320a | Reduction of cellular edema | Ischemic Injury; Neonatal Epilepsy[23] |
|
| miR-130b | Protection against astrocytes and nerve damage | OGD- induced brain injury; I/RI[76] |
|
| miR-145 | hsa-miR-145-5p | Protection against astrocytes damage; Inhibition of astrocyte proliferation | Neonatal HIE[77]; SCI[78] |
| miR-424 | Inhibition of reactive astrocyte proliferation | I/RI[79] | |
| miR-134 | miR-134-5p | Promotion of astrocyte maturation | Ischemic Injury; Neonatal HIE[52] |
| lncRNA MALAT1 | Influence of AQP4 expression in astrocytes | Neonatal BPD[80]; I/RI[56]; |
|
| lncRNA H19 | Downregulation of IGF-1 in astrocyte | Neonatal HIE[62] | |
| circRNA HECTD1 | Inhibition of astrocyte activation | Ischemic Stroke[67] | |
| circRNA 0025984 | Inhibition of astrocyte autophagy and apoptosis | Ischemic Stroke[68] | |
| Non-Coding RNA | Function | Disease | |
|---|---|---|---|
| miR-145 | miR-145-5p | Influence of inflammation-induced astrocyte proliferation | Neonatal HIE[90]; Ischemic Stroke; |
| miR-146a | Negative regulators of astrocyte-mediated inflammatory responses | Epilepsy[126]; ALS[127] | |
| miR-124 | miR-124-3p | Inhibition of astrocyte activation | Neonatal HIE[105]; SCI[100] |
| miR-155 | miR-155-5p | Promotion of astrocyte activation | Neonatal HIE[97]; SCI[128] |
| miR-136 | miR-136-5p | Promotion of astrocyte activation | SCI[129] |
| lncRNA H19 | Promotion of astrocyte activation and proliferation | I/R[113] | |
| lncRNA CRNDE | Inhibition of astrocyte activation | intrauterine infection-induced injury[117]; HIBD[130] |
|
| circRNA HIPK2 | Promotion of astrocyte activation by autophagy and ER stress | TBI[131] | |
| circRNA_chr8 | Promotion of astrocyte activation and neuroinflammation | TBI[132] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





